Abstract
Introduction: Bovine and ovine mucosa represent alternate anticoagulants to porcine mucosa for production of unfractionated heparin (UFH). Standardized heparins from various sources can be blended and potency adjusted, blended heparins exhibit comparable effects as single-sourced porcine UFH. This study evaluated the pharmacologic profile of blended heparin and compared their activities to that of single sourced porcine, ovine, and bovine heparins. Methods: The anticoagulant effects of gravimetric and potency-adjusted heparins were evaluated with aPTT, TT, anti-Xa, anti-IIa, ACT, and TGA studies. Protamine sulfate studies were used for neutralization potential of each of the individual heparins. Results: The potency-adjusted heparins demonstrated comparable aPTT, TT, anti-Xa, anti-IIa, and ACT values at all concentrations (U/mL). However, in gravimetric studies, bovine heparin consistently showed lower values with the exception of thrombin generation inhibition studies. The protamine sulfate neutralization studies demonstrated complete neutralization at all concentrations for the potency-adjusted heparins. However, at gravimetric concentrations, minor differences were noted in the neutralization profile in each of these heparins. Conclusion: These studies support the hypothesis that blended heparin from bovine, ovine, and porcine tissue, when standardized in unit-equivalent proportions, exhibits a comparable anticoagulant profile to the single species derived heparins.
Keywords: heparin, USP reference heparin, blended heparins, gravimetric, anticoagulant assays
Introduction
Heparin was first discovered in 1916 by medical student Jay McLean while working in William Henry Howell's laboratory at Johns Hopkins University.1 McLean originally extracted heparin from dog liver, but this source was characterized as unsuitable for mass production and public use.2 Since the initial extraction from dog liver, alternate sources of heparin have been identified and used and optimal purification practices have been developed. Primarily, heparin is sourced and used from porcine intestinal mucosa (PMH), but heparin has also been obtained from bovine (BMH) and ovine intestinal mucosa (OMH).3 The use of BMH is limited worldwide because of fears originating in the 1990s that bovine spongiform encephalopathy (BSE) may contaminate heparin sourced from bovine gut mucosa and lead to Creutzfeldt–Jakob disease in humans.4 New techniques have been developed to enhance the safety of BMH by removing BSE spike proteins, which significantly decrease infectivity and reduce harm from prion proteins.5 Despite advancements in the purification processes of BMH, and the comparable pharmacologic profile of OMH in relation to PMH, globally, PMH is almost solely relied upon for heparin usage.6
This global reliance on a single source of heparin can lead to adverse consequences when the supply chain is interrupted or compromised. Such an issue was first illustrated in 2008 when impurities in the Chinese pig population lead to 80 human deaths and hundreds of adverse events from use of PMH sourced from China.7 More recently, outbreaks of African Swine Fever have led to the death of an estimated 150–200 million Chinese pigs, severely threatening the global supply chain of heparin.8 Additionally, the reliance on PMH negatively impacts parts of the world that for various cultural and religious reasons, refrain from consuming pork products. This can lead to sub-optimal health care as heparin is relied upon for decreasing adverse outcomes associated with hypercoagulable pathology. These examples stress the importance of diversifying the global heparin supply chain and moving away from reliance on single-sourced PMH. Globally, there are an estimated 1.5 billion cattle, and an estimated 1.2 billion sheep.9 These represent a largely untapped alternative to pigs for potential sourcing of heparin.
Heparin is best known for its use as an anticoagulant. Specifically, heparin is the first-line drug of choice for anticoagulation during surgical and interventional procedures, hemodialysis, and surface coating of devices.6 Additionally, heparins clinical indications include usage to prevent and treat arterial and venous thromboembolism, thrombotic stroke, and unstable angina in coronary artery syndrome.10 More recently, heparin has been used for thromboprophylaxis in patients with high-risk COVID-19. Studies found that heparin usage in these patients significantly reduced risk of major thromboembolic events and death.11 The global heparin market was valued at 7.3 billion dollars in 2021 and is expected to reach 9 billion dollars by 2030.12 Together, these indications and usages make heparin a critically important drug in healthcare today.
Heparin primarily exerts its anticoagulant effects through binding and enhancement of the inhibitory plasma protein antithrombin (AT).13 AT inhibits two primary targets within the coagulation cascade, thrombin, and factor Xa. Endogenously, AT binds to thrombin in a 1:1 complex, however, when complexed with heparin, AT's rate of inactivation of thrombin is increased by 1000-fold.14 Additionally, heparin can exert anticoagulant effects via heparin cofactor II (HCII). Specifically, heparin stimulates the formation of the HCII – thrombin complex which similarly increases thrombin inactivation by 1000-fold.15 Heparin also exhibits anticoagulant properties through its effects on tissue factor pathway inhibitor (TFPI). Tissue factor pathway inhibitor is the primary inhibitor of the extrinsic coagulation pathway. Studies have demonstrated that heparin both releases TFPI and upregulates TFPI gene expression.16, 17
Platelet factor 4 (PF4) is a naturally occurring, positively charged protein stored in the alpha granules of platelets. PF4 binds with strong affinity to negatively charged glycosaminoglycans, such as heparin, and creates a prothrombotic environment. Therefore, PF4 is considered an endogenous antagonist of heparin. PF4 preferentially binds with heparin when compared to other glycosaminoglycans, leading to the formation of PF4/Heparin complexes. These complexes have the potential to trigger an immunogenic response in humans, with formation of autoantibodies to these complexes.18 Formation of autoantibodies to the complexes can lead to the serious complication of heparin induced thrombocytopenia (HIT). HIT leads to thrombocytopenia and a paradoxical prothrombotic state. The autoantibodies formed by the immunogenic complexes trigger platelet activation, leading to generation of procoagulant materials and eventually thrombin generation. While this contributes to the prothrombotic complications of HIT, thrombocytopenia results from the clearance of antibody marked platelets.19 All sources of heparin have the potential to perpetuate HIT. Importantly, the origin of heparin sources, such as BMH, PMH, OMH, does not impact the immunogenic potential.20 Therefore, there is no increased risk for development of HIT when comparing PMH, BMH, and OMH.
Insulin is one of the most widely used drugs in the world for the management of diabetes. In 2021, insulin commanded a global market value of 18.5 billion dollars, with a projected growth to 30 billion dollars by 2031.21 Insulin, a drug of biologic origin, is manufactured and commercially available in blended preparations. This is made possible through implementation of standardized manufacturing practices to ensure equal pharmacologic and biologic efficacy across different sources. Heparin, much like insulin, is of biologic origin with multiple documented sources available. However, there is no evidence to date evaluating the potential for creating a standardized, blended heparin product from various sources. This study will seek to determine if heparin blended from bovine, ovine, and porcine mucosa can elicit comparable anticoagulant effects to that of single-sourced heparins.
Materials and Methods
Two sets of heparins were evaluated in this study, porcine, ovine, bovine, and the blended heparin in gravimetric measurements (ug/mL) and these same four in potency adjusted units following the US Pharmacopeia standard (U/mL). A single batch of each of the individual heparins was obtained from various suppliers in their active pharmaceutical ingredient (API) form as powders. The bovine heparin was obtained from Adeste Pharma (Sao Paolo, Brazil). The ovine heparin was obtained from Ronssi Pharmaceutical (Suzhou, China). The porcine heparin was obtained from Medefil Inc (Glendale Heights, IL, USA). The blended heparin was prepared by taking one-third proportion of PMH, OMH, and BMH, mixing and vortexing to generate the blended product. This was done for both the gravimetric blended heparin (ug/mL) and the potency-adjusted blended heparin (U/mL). The pharmacologic profiles of the heparins investigated in this study were elucidated via global anticoagulant assays and anti-protease assays performed in blood bank plasma, a pool of citrated plasma from healthy humans (BBP). Each heparin sample was subject to characterization via activated partial thromboplastin time (aPTT), thrombin time (TT), anti-Xa, anti-IIa, and thrombin generation assays (TGA) in BBP. Protamine sulfate neutralization (PS) studies were also carried out in BBP containing each of these heparins in both the gravimetric and potency-adjusted mixtures. Additionally, each sample was studied with activated clotting time (ACT) using whole blood from healthy volunteers.
Activated Clotting Time (ACT): The manufacturer's instructions were followed to perform this assay. Into individual syringes, 200 µL of 100 µg/mL gravimetric heparin and 10 U/mL potency-adjusted heparin was added. Blood from a healthy human volunteer was drawn into these syringes and was mixed with the heparin agent (final concentrations were 10 µg/mL and 1 U/mL, respectively). The heparinized blood was placed into a celite ACT tube and was vigorously shaken. The ACT tube was placed into the ACT machine (Hemachron; Edison, New Jersey, USA) and the time to clot was recorded.
Thrombin Time (TT): The TT assay was performed on the ACL-300-Plus fast kinetic coagulation analyzer (Beckman CoulterÒ; Fullerton, California, USA) using a human thrombin reagent obtained from Enzyme Research Laboratories (South Bend, Indiana, USA). The thrombin was reconstituted with 0.25 M CaCl2 to yield a 5 U/mL solution. The gravimetric heparin samples were prepared to a concentration of 100 µg/mL and the potency-adjusted heparin samples were prepared to a concentration of 10 U/mL in saline. Test agents were further diluted in pooled human plasma to achieve a six-point concentration range of 0–10 µg/mL or 0–1 U/mL, and then were assayed for anticoagulant activity.
Activated partial thromboplastin time (aPTT): An aliquot of 250 µL of each of the plasma samples was placed into individual test cups, which were then placed in an ACL test cup carousel in a defined sequence. BBP samples were included in every carousel as controls. The reagents were placed in their appropriate positions (TriniCLOT/Hemosil APTT S reagent in position 2 and CaCl2 in position 3). A clean ACL rotor was placed in the carousel. The appropriate parameters were set on the machine before running the test. An aliquot of 50 µL of each of the plasma samples and 50 µL of aPTT reagent were incubated at 37 °C for 5 min. After 5 min of incubation, pre-warmed calcium chloride was added to trigger coagulation, and the clotting times were then measured on an ACL Elite (Beckman-Coulter, Miami, FL). The measurement of clotting time was stopped at 300 s as clotting times beyond 300 s are outside of the linear range of the instrument. The gravimetric heparin samples were prepared to a concentration of 100 µg/mL and the potency-adjusted heparin samples were prepared to a concentration of 10 U/mL in saline. All agents were serial diluted in pooled human plasma to achieve a six-point concentration range of 0–10 µg/mL or 0–1 U/mL then were assayed for anticoagulant activity.
Anti-factor Xa assay: The inhibition of factor Xa in BBP was performed on the ACL Elite fast kinetics coagulation analyzer (Beckman-Coulter, Hialeah, FL), in the following manner. Test samples were prepared by placing 250 µL of test plasma into individual test cups, which were then placed in an ACL-test cup-carousel and their positions were recorded. BBP samples were included in every carousel as controls. Bovine factor Xa (1.0 mg/mL) (Enzyme Research Laboratories, South Bend, IN) was reconstituted with 4 mL (final factor Xa concentration of 6 µg/mL) of Xa buffer (50 mM Tris, 175 mM NaCL, 7.5 mM Na2 EDTA, 800 mL distilled H2O, pH = 8.4, 25°C) prior to use. The substrate, Spectrozyme FXa (5 µmole/vial) (Sekisui Diagnostics, Stamford, CT) was reconstituted by adding 2 mL of distilled water. The reagents were placed in their appropriate positions (bovine factor Xa in position 2 and Spectrozyme FXa in position 3). A clean ACL rotor was also placed in the instrument. Once the reagents and samples were in their proper places, the ACL keypad was used to set the appropriate specifications for the anti-Xa assay. An aliquot of 10 µL of plasma was incubated for 1 min at 37°C, followed by the addition of 100 µL of bovine factor Xa. After 5 min incubation at 37 °C, 75 µL of Spectrozyme FXa was added and the optical density change at 405 nm was measured for 30 s.
Anti-factor IIa assay: The inhibition of thrombin in human plasma samples was measured on the ACL Elite fast kinetics coagulation analyzer (Beckman-Coulter, Hialeah, FL), in the following manner. Test samples were prepared by placing 250 µL of test plasma into individual test cups, which were then placed in an ACL test cup carousel and their positions were recorded. BBP samples were included in every carousel as controls. Human thrombin (Enzyme Research Laboratories, South Bend, IN) was diluted to 5 U/mL in IIa buffer. The substrate, Spectrozyme TH (Sekisui Diagnostics, Stamford, CT) was reconstituted by adding 5 mL of distilled water (1 mM). The reagents were placed in their appropriate positions (thrombin in position 2 and Spectrozyme TH in position 3). A clean ACL rotor was also placed in the instrument. Once the reagents and samples were in their proper places the ACL keypad was used to set up the appropriate specifications for the anti-IIa assay. A 10 µL aliquot of plasma was incubated for 1 min at 37°C, followed by the addition of 100 µL of thrombin. Following 1 min incubation at 37°C, 40 µL of Spectrozyme TH was added, and the optical density change at 405 nm was measured for 30 s.
Protamine sulfate (PS) neutralization studies: Test agents were supplemented to BBP in a concentration range of 0.625–10 ug/mL and 0.0625–1 U/mL. Freshly prepared PS solution, at a fixed final concentration of 10 µg/mL, was added to each heparin-plasma mixture which was then assayed for aPTT, TT, anti-IIa, and anti-Xa activity. For comparison purposes, a parallel control using saline in place of PS was supplemented (using the same volume as used for protamine) to each heparin-plasma mixture. PS neutralization indices were calculated by comparing the PS supplemented and saline supplemented assays.
Thrombin generation assay (TGA): Inhibition of thrombin generation was measured using the Calibrated Automated Thrombogram Instrumentation (CAT, Diagnostica Stago, Parsippany, NJ) on a Fluoroskan Ascent fluorimeter. Fluo-substrate (amino-methylcoumarin, AMC) Fluo-buffer, tissue factor high reagent (mixture of tissue factor and phospholipids), and a thrombin calibrator were obtained from Diagnostica Stago, Parsippany, NJ, and used for determination of thrombin generation over time in BBP.
Statistical analyses were performed using Microsoft Excel, SigmaPlot Statistics, and Graph Pad Prism software. For each heparin sample, the mean and standard deviation was calculated for graphical representation purposes. Two Way Repeated Measures ANOVA tests were used to evaluate statistically significant differences between heparin samples. A P-value <.05 was considered statistically significant.
Results
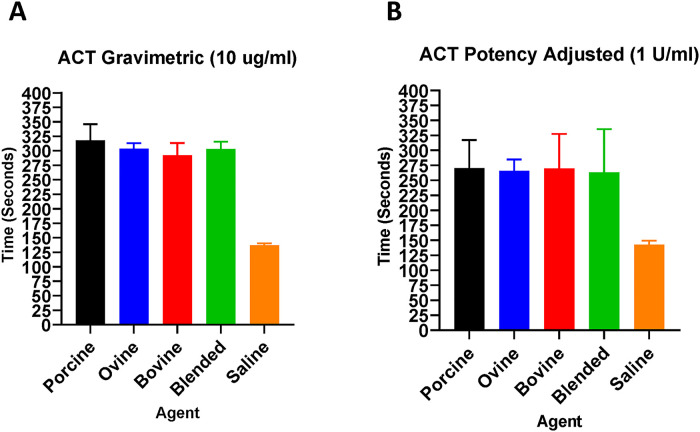
The ACT assay was used to demonstrate the anticoagulant effects of various heparins in whole human blood (Figure 1). In gravimetric concentrations (ug/mL), PMH prolonged the ACT compared to OMH, BMH, and blended heparin at 10 ug/mL. In potency-adjusted concentrations (U/mL), all heparins produced comparable ACT values at 1 U/mL.
Figure 1.
A comparison of heparins of various sources in the whole blood ACT assay. Gravimetric heparins (A) and potency-adjusted heparins (B) (n = 4).
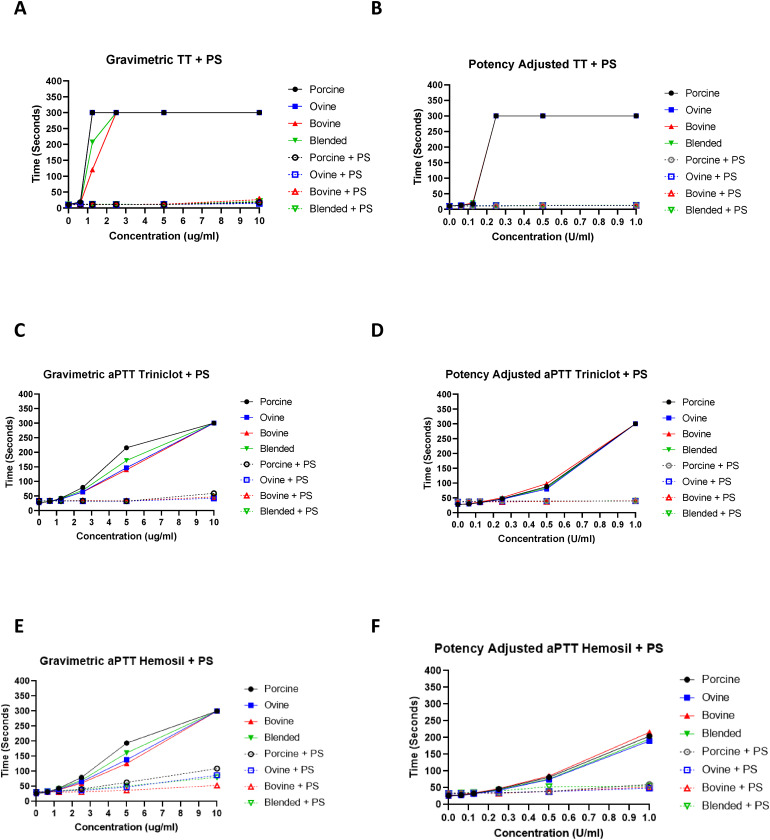
The TT assay was used to demonstrate the effects of various heparins on thrombin in BBP (Figure 2). In gravimetric concentrations (ug/mL), PMH and OMH prolonged the TT at 1.25 ug/mL compared to BMH and blended heparin. In potency-adjusted concentrations (U/mL), all heparins produced comparable TT values at all concentrations. The aPTT assay was used to demonstrate anticoagulant effects of various heparins in BBP (Figure 2). In gravimetric concentrations (ug/mL) PMH significantly (P < .001) prolonged the aPTT compared to OMH, BMH, and blended heparin at 5 ug/mL and 2.5 ug/mL. In potency-adjusted concentrations (U/mL), all heparins produced comparable aPTT values at all concentrations.
Figure 2.
A comparison of heparins of various sources in clot-based assays including TT and aPTT with additional PS neutralization. Anticoagulant effects were measured by the TT assay with additional PS neutralization of gravimetric heparins (A) and potency-adjusted heparins (B) (n = 3). Comparative anticoagulant effects measured by the aPTT Triniclot reagent assay with additional PS neutralization of gravimetric heparins (C) and potency-adjusted heparins (D) (n = 3). Comparative anticoagulant effects measured by the aPTT Hemosil reagent assay with additional PS neutralization of gravimetric heparins (E) and potency-adjusted heparins (F) (n = 3).
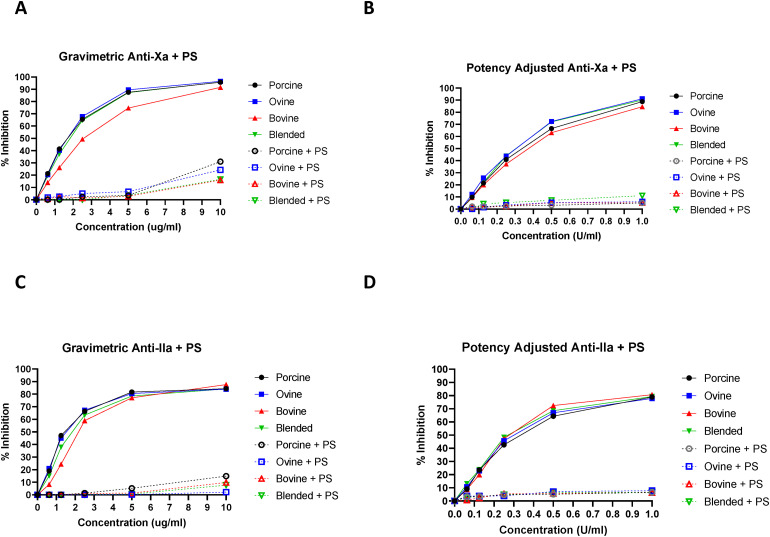
The anti-Xa assay was used to quantify factor Xa inhibition by the various heparins (Figure 3). In gravimetric concentrations (ug/mL), PMH, OMH, and blended heparin produced significantly (P < .001) stronger Xa inhibition than BMH at concentrations between 10 ug/mL and 0.625 ug/mL. In potency-adjusted concentrations (U/mL), all heparins produced comparable anti-Xa inhibition at all concentrations. The anti-IIa assay was used to quantify thrombin inhibition by the various heparins (Figure 3). In gravimetric concentrations (ug/mL) PMH and OMH produced significantly (P < .04) stronger IIa inhibition than BMH at 2.5, 1.25, and 0.625 ug/mL. In potency-adjusted concentrations (U/mL), all heparins produced comparable anti-IIa inhibition at all concentrations.
Figure 3.
A comparison of heparins of various sources in anti-protease assays including anti-Xa and anti-IIa with PS neutralization. The inhibition of factor Xa with additional PS neutralization by gravimetric heparins (A) and potency-adjusted heparins (B) (n = 3). The inhibition of factor IIa with additional PS neutralization by gravimetric heparins (C) and potency-adjusted heparins (D) (n = 3).
Protamine sulfate studies were used to evaluate the reversal potential of anticoagulant effects of various heparins studied in the TT, aPTT, anti-Xa, and anti-IIa assays (Figures 1–3). In the gravimetric TT assay, PS was able to completely neutralize the anticoagulant effects of all heparins at all concentrations (ug/mL). In the gravimetric aPTT assays, PS was able to completely neutralize the anticoagulant effects of all heparins at concentrations 5 ug/mL and lower. In the gravimetric anti-Xa assay, PS was able to completely neutralize the anticoagulant effects of all heparins at concentrations 5 ug/mL and lower. In the gravimetric anti-IIa assay, PS was able to completely neutralize the anticoagulant effects of all heparins at concentrations 5 ug/mL and lower. In the potency-adjusted TT, aPTT, anti-Xa, and anti-IIa assays, PS was able to completely neutralize the anticoagulant effects of all heparins at all concentrations.
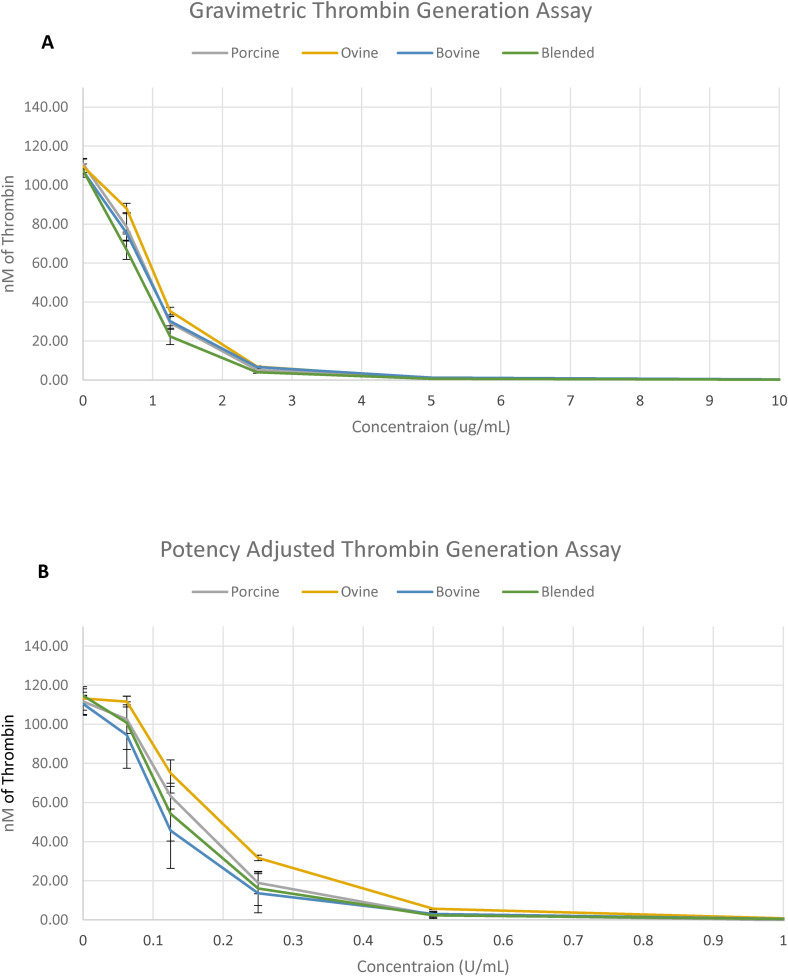
The TGA was used to demonstrate the inhibitory effects of various heparins on thrombin generation (Figure 4). In gravimetric concentrations (ug/mL), all heparins produced comparable TGA values at all concentrations. In potency-adjusted concentrations (U/mL), PMH, BMH, and blended heparin produced comparable TGA values at all concentrations, OMH demonstrated weaker TGA between concentrations 0.25 and 0.0625 U/mL.
Figure 4.
A comparison of heparins of various sources in the TGA. Inhibition of thrombin generation by gravimetric heparins (A) and potency-adjusted heparins (B) (n = 2).
Discussion
Heparin mediates its biological effects through the complexation with endogenous serpines such as AT and HCII along with the release of endogenous TFPI. Additionally heparin by virtue of its charge interacts with endothelial surface and modulates endogenous release of other mediators.22 Heparin is one of the most widely used drugs worldwide as it is used for anticoagulation during surgical procedures, hemodialysis, coating of devices, and for venous thromboembolism prophylaxis, with a recent increase in usage in COVID-19 cases.23 Globally, heparin is predominantly sourced from PMH, but heparin has also been obtained from BMH and OMH. Global reliance on PMH can lead to adverse consequences when the supply chain is interrupted or compromised. This is evident in the crisis in 2008 when impurities in the Chinese pig population lead to 80 human deaths and hundreds of adverse events from use of PMH sourced from China. Additionally, the presence of African Swine Fever has resulted in the death of an estimated 150–200 million Chinese pigs, straining the global supply chain of heparin.24 OMH and BMH represent viable alternative sources to that PMH and have been introduced in other parts of the world. Studies have shown that PMH, OMH, and BMH demonstrate comparable biochemical and pharmacological profiles when potency is adjusted.3 In this study, the biochemical and pharmacologic properties of blended heparin composed of 1/3 PMH, 1/3 OMH, and 1/3 BMH were investigated and compared to those of referenced, single sourced PMH, OMH, and BMH.
Whole blood ACT assays are regularly used during surgery to monitor the real-time anticoagulant effects of heparin to ensure anticoagulant efficacy.25 Heparin acts as an inhibitor in the ACT assay, such that prolongation of time to clot signifies strength of anticoagulation. Activated clotting time was used to measure the anticoagulation activity of heparins in freshly donated whole human blood from healthy volunteers. The gravimetric PMH prolonged the ACT compared to OMH, BMH, and blended heparin at 10 ug/mL. In potency-adjusted concentrations (U/mL), all heparins produced comparable ACT values at 1 U/mL. These results show that potency-adjusted heparins exhibit comparable anticoagulant effects as measured by the ACT assay. Furthermore, these results signify that potency-adjusted blended heparin, composed of 1/3 PMH, 1/3 OMH, and 1/3 BMH exhibits comparable ACT prolongation when compared to referenced, single sourced PMH, OMH, and BMH.
The impact of heparin on the coagulation cascade can be measured using both the aPTT and TT. These methods are used by hospital laboratories to monitor anticoagulation by heparin.26 In this study, TT was used to characterize the anticoagulant effects of heparins within BBP samples. The gravimetric PMH and OMH prolonged the TT at 1.25 ug/mL compared to BMH and blended heparin. In potency adjusted concentrations (U/mL), all heparins exhibited comparable TT values at all concentrations. These results demonstrate that potency adjusted heparins exhibit comparable anticoagulant activity as measured by the TT assay. Furthermore, these results signify that potency adjusted blended heparin, composed of 1/3 PMH, 1/3 OMH, and 1/3 BMH exhibits similar anticoagulant activity compared to the referenced single-sourced PMH, OMH, and BMH in the TT assay.
Activated partial thromboplastin time was used to measure the anticoagulant effects of heparins within BBP samples. The gravimetric PMH produced significantly stronger anticoagulant effects compared to the blended heparin, OMH, and PMH at 5 ug/mL and 2.5 ug/mL. In potency-adjusted concentrations (U/mL), all heparins exhibited comparable aPTT values at all concentrations. These results demonstrate that potency-adjusted heparins exhibit comparable anticoagulant activity as characterized by the aPTT assay. Furthermore, these results signify that potency-adjusted blended heparin, composed of 1/3 PMH, 1/3 OMH, and 1/3 BMH exhibits comparable anticoagulant activity compared to the referenced single-sourced PMH, OMH, and BMH in the aPTT assay. In the current studies, two different types of aPTT reagents were used to compare these heparins. These reagents, namely Triniclot and Hemosil are composed of different mixtures of activators. The Triniclot reagent contains a Platelet Factor 3 reagent (rabbit brain phospholipids) plus a particulate activator (micronized silica) in a suitable buffer. The Hemosil reagent contains lyophilized bovine brain cephalin and micronized silica with stabilizers and preservatives. Because of these composition differences, both reagents have different sensitivities to heparin concentrations. Both reagents are widely used in clinical practice. For this reason, these two reagents were included in the study. Despite the differential sensitivity of the reagents, the trends were similar in both reagents.
Antiprotease assays further characterize the ability of heparin to exert anticoagulant effects through quantifying inhibition of clotting factors. In this study, the anti-Xa assay was used to measure the % inhibition of Factor Xa by the various heparins. The gravimetric PMH, OMH, and blended heparin produced significantly stronger Xa inhibition than BMH at concentrations between 10 ug/mL, and 0.625 ug/mL. In potency-adjusted concentrations (U/mL), all heparins exhibited comparable Xa inhibition at all concentrations. These results demonstrate that while BMH at gravimetric concentrations demonstrates weaker Xa inhibition compared to PMH, OMH, and blended heparin, through potency adjustment, all heparins exhibit comparable Xa inhibition. Furthermore, these results signify that potency adjusted blended heparin, composed of 1/3 PMH, 1/3 OMH, and 1/3 BMH exhibits comparable Xa inhibition compared to the referenced single sourced PMH, OMH, and BMH.
The anti-IIa assay was used to evaluate the % inhibition of thrombin by the various heparins. The gravimetric PMH and OMH produced significantly stronger thrombin inhibition than BMH at 2.5 ug/mL, 1.25 ug/mL, and 0.625 ug/mL. In potency adjusted concentrations (U/mL), all heparins exhibited comparable thrombin inhibition at all concentrations. These results show that while BMH at gravimetric concentrations exhibits weaker thrombin inhibition compared to PMH and OMH, through potency adjustment, all heparins exhibit comparable thrombin inhibition. Furthermore, these results signify that potency-adjusted blended heparin, composed of 1/3 PMH, 1/3 OMH, and 1/3 BMH exhibits comparable thrombin inhibition compared to referenced single-sourced PMH, OMH, and BMH.
In these studies, USP standard reference was used to compare each of the individual heparins and the blended heparin mixture. The USP standard is a potency-designated reference heparin of porcine mucosal origin. This standard was diluted to obtain a calibration curve utilizing the USP compliant anti-Xa assay against which each of the heparin was compared in both the gravimetric concentration and the USP referenced unit potency concentrations.
Heparin is widely used as an anticoagulant, and therefore, requires the potential for reversal when anticoagulation is no longer needed, or in cases of unwanted bleeding. Protamine sulfate is the clinically used heparin antagonist for indications of anticoagulation reversal.27 This study evaluated the neutralization profile of each heparin with PS in the TT, aPTT, anti-Xa, and anti-IIa assays. In the gravimetric TT assay, PS was able to completely neutralize the anticoagulant effects of all heparins at all concentrations (ug/mL). In the gravimetric aPTT, anti-Xa, and anti-IIa assays, PS was able to completely neutralize the anticoagulant effects of all heparins at concentration 5 ug/mL and lower. In the potency adjusted (U/mL) TT, aPTT, anti-Xa, and anti-IIa assays, PS was able to completely neutralize the anticoagulant effects of all heparins at all concentrations. These results show that potency-adjusted heparins exhibit comparable reversal potential with PS. Furthermore, these results signify that potency-adjusted blended heparin, composed of 1/3 PMH, 1/3 OMH, and 1/3 BMH exhibits comparable reversal potential with PS compared to referenced single-sourced PMH, OMH, and BMH.
Thrombin generation inhibition assay serves as a useful tool for measuring thrombin generation potential in the presence of an inhibitor such as heparin. The critical value from the TGA is peak thrombin, and heparin acts in a concentration dependent manner to inhibit thrombin generation. This study used TGA to assess the inhibition of thrombin generation by the various heparins supplemented in BBP. In gravimetric concentrations (ug/mL), all heparins produced comparable TGA values at all concentrations. In potency adjusted concentrations (U/mL), PMH, BMH, and blended heparin produced comparable TGA values at all concentrations, OMH demonstrated weaker TGA between concentrations 0.25 and 0.0625 U/mL. This data indicates that thrombin generation inhibition profile of these heparins is dependent on both the non-AT and AT binding chains. Upon adjustments the proportions of non-AT binding chains are higher in the BMH and therefore it produces stronger inhibition in thrombin generation. Moreover, in the potency-adjusted heparins, the biologic actions which are mainly AT mediated are measured. Whereas, in the gravimetric amounts, the biologic activities of both the AT and HCII mediated thrombin generation inhibition is measured. Thus, the observed differences may be due to the presence of HCII binding chains. Additionally, the degree of sulfation also plays a role in the mediation of thrombin generation inhibition. Other stereochemical differences in the component chains in heparin of different sources may also contribute to non-AT mediated inhibition of thrombin and additional biological properties. In light of this, the potency adjusted OMH may exhibit weaker thrombin generation inhibition because most of its thrombin generation inhibitory effects are mediated through AT.
The findings reported in this study are limited by the number of batches used for each source of heparin, and the use of only one batch from each source to prepare the blended heparin. These studies did not take into account the endogenous release of TFPI which requires in-vivo studies in animals and human trials. Further studies characterizing the activity of blended heparin in non-human primates would be beneficial in validating the findings. Despite these limitations, this study demonstrated that while differences in anticoagulant activity exist among gravimetric PMH, OMH, BMH, and blended heparin, potency-adjusted heparins exhibit comparable biochemical and pharmacological profiles. This study also found that potency-adjusted blended heparin, comprised of 1/3 PMH, 1/3 OMH, and 1/3 BMH exhibits comparable biochemical and pharmacological profiles to that of referenced, single sourced PMH, OMH, and BMH. These findings clearly indicate that a blended heparin, composed of 1/3 potency adjusted PMH, 1/3 potency adjusted OMH, and 1/3 potency adjusted BMH can be used interchangeably with the widely used PMH for anticoagulation purposes. Use of blended heparin for anticoagulation would alleviate the strain placed on the global supply chain by providing viable alternatives to porcine mucosa. Additionally, the incorporation of ovine and bovine mucosa provides readily available resources to meet the demands of the continuously growing heparin market.
In view of the current supply chain challenges and other unforeseen circumstances, the potential shortage of porcine heparin possibility is imminent. Therefore, to avoid catastrophic events due to non-availability of porcine heparins, alternate sourcing has been addressed at both regulatory and industry level. Despite these discussions, no plans or recommendations are in place at this time. These studies underscore the importance of expanding alternate resourcing and provide evidence that blending heparins from various species is a feasible option to meet the demands. As evident in these studies, ovine and bovine heparin are very similar to porcine heparin in the potency adjusted concentrations and can be used interchangeably. These studies underscore the importance of potency adjustment against the USP standard or the international standard in mixtures of heparins from different sources. These mixtures of blended heparins are therefore a practical option to meet the demand and shortage of single sourced heparin. For this reason, the regulatory agencies and pharmacopeial organizations need to reconsider their requirements for the approval and standardization practices of resourced heparins including their blended versions.
Acknowledgements
The authors are thankful to the staff of the Hemostasis and Thrombosis Research laboratories for their assistance in completing the reported studies. We are also thankful to the Cardiovascular Institute for its support and partially funding these studies. A special thanks to Dr. Meharvan Singh, Provost of Research LUC, and Dr. Lowell Steen Director of the Division of Cardiology, Department of Medicine, LUMC for their guidance throughout these studies. We are also thankful to Mr. Jonas Kingo and Ms. Catherine Sandon for providing the reagents for some of the assays. The skillful assistance of Ms. Erin Healy-Erickson in preparing this manuscript is greatly appreciated.
Footnotes
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Guy Olson https://orcid.org/0000-0003-3454-2273
Fakiha Siddiqui https://orcid.org/0000-0002-2219-7049
Debra Hoppensteadt https://orcid.org/0000-0001-9342-4213
Ahmed Kouta https://orcid.org/0000-0001-7579-9572
Jawed Fareed https://orcid.org/0000-0003-3465-2499
References
- 1.Mclean J. The Discovery of Heparin. http://ahajournals.org
- 2.Couch NP, Roxbury W. From the New England Society for Vascular Surgery About Heparin, or… Whatever Jay McLean? doi: 10.1067/mva.1989.vs0100001. [DOI]
- 3.Kouta A, Jeske W, Hoppensteadt D, Iqbal O, Yao Y, Fareed J. Comparative pharmacological profiles of various bovine, ovine, and porcine heparins. Clin Appl Thromb. 2019;25:107602961988940. doi: 10.1177/1076029619889406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andrews O, Bett C, Shu Q, et al. Processing bovine intestinal mucosa to active heparin removes spiked BSE agent. Biologicals. 2020;67:56-61. doi: 10.1016/j.biologicals.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 5.Bett C, Andrews O, Asher DM, Pilant T, Keire D, Gregori L. Eliminating spiked bovine spongiform encephalopathy agent activity from heparin. Emerg Infect Dis. 2020;26(10):2478-2480. doi: 10.3201/EID2610.200142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fareed J, Jeske W, Ramacciotti E. Porcine mucosal heparin shortage crisis! What are the options? Clin Appl Thromb. 2019;25:107602961987878. doi: 10.1177/1076029619878786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vilanova E, Tovar AMF, Mourão PAS. Imminent risk of a global shortage of heparin caused by the African Swine Fever afflicting the Chinese pig herd. J Thromb Haemost. 2019;17(2):254-256. doi: 10.1111/jth.14372. [DOI] [PubMed] [Google Scholar]
- 8.Mass Pig Deaths in China Cause Short Supply of U.S. Blood Thinner - BNN Bloomberg. https://www.bnnbloomberg.ca/mass-pig-deaths-in-china-cause-short-supply-of-u-s-blood-thinner-1.1309438
- 9.Gilbert M, Nicolas G, Cinardi G, et al. Global distribution data for cattle, buffaloes, horses, sheep, goats, pigs, chickens and ducks in 2010. Sci Data. 2018;5(1). doi: 10.1038/SDATA.2018.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barritt DW, Jordan SC. Anticoagulant drugs in the treatment of pulmonary embolism. A controlled trial. Lancet. 1960;275(7138):1309-1312. doi: 10.1016/S0140-6736(60)92299-6. [DOI] [PubMed] [Google Scholar]
- 11.Spyropoulos AC, Goldin M, Giannis D, et al. Efficacy and safety of therapeutic-dose heparin vs standard prophylactic or intermediate-dose heparins for thromboprophylaxis in high-risk hospitalized patients with COVID-19: the HEP-COVID randomized clinical trial. JAMA Intern Med. 2021;181(12):1612. doi: 10.1001/JAMAINTERNMED.2021.6203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Global Heparin Market Size, Share & Trends Report, 2030. https://www.grandviewresearch.com/industry-analysis/heparin-market
- 13.Bleich HL, Boro ES, Rosenberg RD. Actions and interactions of antithrombin and heparin. N Engl J Med. 1975;292(3):146-151. doi: 10.1056/nejm197501162920307. [DOI] [PubMed] [Google Scholar]
- 14.Berry LR, Becker DL, Chan AKC. Inhibition of fibrin-bound thrombin by a covalent antithrombin-heparin complex. J Biochem. 2002;132(2):167-176. doi: 10.1093/oxfordjournals.jbchem.a003206. [DOI] [PubMed] [Google Scholar]
- 15.Kim YS, Linhardt RJ. Structural features of heparin and their effect on heparin cofactor II mediated inhibition of thrombin. Thromb Res. 1989;53(1):55-71. doi: 10.1016/0049-3848(89)90115-1. [DOI] [PubMed] [Google Scholar]
- 16.Crawley JTB, Lane DA. The haemostatic role of tissue factor pathway inhibitor. Arterioscler Thromb Vasc Biol. 2008;28(2):233-242. doi: 10.1161/ATVBAHA.107.141606. [DOI] [PubMed] [Google Scholar]
- 17.Sandset PM. Tissue Factor Pathway Inhibitor (Tfpi) - an update. Pathophysiol Haemost Thromb. 1996;26(Suppl 4):154-165. doi: 10.1159/000217293. [DOI] [PubMed] [Google Scholar]
- 18.Arepally GM. Heparin-induced thrombocytopenia. Blood. 2017;129(21):2864-2872. doi: 10.1182/BLOOD-2016-11-709873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chong BH. Heparin-induced thrombocytopenia. J Thromb Haemost. 2003;1(7):1471-1478. doi: 10.1046/J.1538-7836.2003.00270.X. [DOI] [PubMed] [Google Scholar]
- 20.Kouta A. Comparative Studies on Biochemical and Pharmacological Profiles of Unfractionated Heparins & Their Depolymerized Derivatives from Bovine, Ovine and Porcine Origins. Dissertations. https://ecommons.luc.edu/luc_diss/3845 [Google Scholar]
- 21.Global Insulin Market Size, Growth Report, 2022-2031. https://www.transparencymarketresearch.com/insulin-market.html
- 22.Onishi A, St Ange K, Dordick JS, Linhardt RJ. Heparin and anticoagulation. Front Biosci - Landmark. 2016;21(7):1372-1392. doi: 10.2741/4462/PDF. [DOI] [PubMed] [Google Scholar]
- 23.Beurskens DMH, Huckriede JP, Schrijver R, Hemker HC, Reutelingsperger CP, Nicolaes GAF. The anticoagulant and nonanticoagulant properties of heparin. Thromb Haemost. 2020;120(10):1371-1383. doi: 10.1055/S-0040-1715460. [DOI] [PubMed] [Google Scholar]
- 24.Baytas SN, Linhardt RJ. Advances in the preparation and synthesis of heparin and related products. Drug Discov Today. 2020;25(12):2095-2109. doi: 10.1016/J.DRUDIS.2020.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berkowitz O, Halabi M, Goldberg A, et al. Routine fixed-dose heparin vs. ACT-guided heparin administration for elective PCI and its influence on patient in-hospital outcome: a retrospective study. Coron Artery Dis. 2021;32(6):549-553. doi: 10.1097/MCA.0000000000001008. [DOI] [PubMed] [Google Scholar]
- 26.Marlar RA, Clement B, Gausman J. Activated partial thromboplastin time monitoring of unfractionated heparin therapy: issues and recommendations. Semin Thromb Hemost. 2017;43(3):253-260. doi: 10.1055/S-0036-1581128. [DOI] [PubMed] [Google Scholar]
- 27.Thachil J. Protamine-the journey from DNA to heparin neutralization to gene therapy. Semin Thromb Hemost. 2022;48(2):240-243. doi: 10.1055/S-0041-1736574. [DOI] [PubMed] [Google Scholar]