Abstract
Objectives
This study aims to: (1) identify the information required by family caregivers of people with dementia to be targeted within our dementia family caregiver intervention and (2) test the feasibility of the intervention and methodology to underpin a fully powered randomized controlled trial.
Methods
The study setting will be the Department of Geriatrics at Gia Dinh People's Hospital in Ho Chi Minh City. Inclusion criteria will be the family caregivers of people with dementia living in the community, who attend the Department and use smartphones. In phase 1, we will identify the intervention content with family caregivers of people with dementia through 20 in-depth interviews to determine what information and skills they need. In phase 2, a pilot randomized control trial design will be conducted, with 60 family caregivers of people with dementia being assigned to the intervention or control group by the block randomization method with a ratio of 1:1. The intervention will include weekly, online, psycho-educational, group sessions hosted on the Zalo app. The participants will complete questionnaires at baseline, immediately postintervention, and 3-month postintervention. The feasibility of the intervention and methodology will be assessed, including the rates of recruitment, retention, completion of assessments, and acceptability of the intervention.
Results
The required information and skills in phase 1 may include dealing with worrying behavior changes in people with dementia, emotional support, and seeking support sources. The rates of recruitment, retention, completion of assessments, and acceptability of the intervention will be obtained in phase 2. The scores of symptoms of stress, depression, and anxiety in the intervention group may be lower than those in the control group at postintervention and 3-month postintervention.
Conclusion
The study will provide a foundation for a fully powered clinical trial for the smartphone app-based intervention to reduce stress, depression, and anxiety among family caregivers of people with dementia in Vietnam.
Keywords: Anxiety, caregiver, dementia, depression, intervention, pilot, randomized control trial, smartphone, stress
Introduction
The global prevalence of dementia among older people (≥ 65 years old) is 5% to 8%, and the total number of people with dementia is projected to reach 82 million in 2030 and 152 million by 2050.1 Vietnam is one of the most rapidly ageing countries in Asia with approximately 11.5 million people aged ≥60 years in 2019.2 According to World Health Organization (WHO), Vietnam became an ageing country (≥7% of the population aged 65 years or more) in 2015 and it will be an aged country (≥ 14% of the population aged 65 years or more) by 2035.3 Previous community-based studies in the North and South of Vietnam found that the prevalence of dementia was 4.8% to 5%4 while a study in Hue City (Central Vietnam) reported a prevalence of 9.4%.5 It was estimated that there were 660,000 people with dementia in Vietnam in 2015 and this figure would increase to 2.4 million people by 2050.6,7
Most people with dementia live at home and receive care from their family members.8 Therefore, the increasing number of people with dementia will lead to rising demands for family caregivers, who are often poorly equipped with the necessary knowledge and skills to provide care.8,9 Family caregivers of people with dementia often experience mental health problems such as symptoms of stress, depression, and anxiety besides economic costs and physical distress.10–12 When compared to the caregivers of people with other chronic illnesses, the family caregivers of people with dementia experienced a higher level of stress and more severe depressive symptoms.11,13,14 A meta-analysis found that the prevalence of depression and anxiety among family caregivers of people with dementia were 34% and 44%, respectively.15 The psychological distress of caring for people with dementia affects the caregiver's physical health and is associated with patient neglect and poor quality of care.16 Based on the Roy Adaptation Model17 and the Theory of Caregiver Stress,18 a psycho-educational intervention for the caregivers of people with dementia is essential to improve their mental and physical health outcomes as well as the quality of life for both the caregivers and care recipients.16,19
Information technology is a promising solution. According to a recent systematic review,20 mobile applications appear to provide an effective solution to reduce distress on caregivers, improve their quality of life, and avoid the negative physical and psychological consequences of caring. It can enable remote information dissemination including education on health issues, up-skilling in self-management, a communication forum, and social support.21 Tremont et al. found that the telephone-based intervention reduced psychosocial distress such as depression, anxiety, and stress for the caregivers of people with dementia, and improved w?>their ability to deal with changed behaviors of care recipients.22,23 In addition, according to Matthew, a 5-week intervention using a smartphone app was effective for caregivers of people with chronic physical or mental conditions experiencing high levels of stress.24 The intervention group reduced depressive and stress symptoms postintervention. Although there are many studies on psychoeducational interventions to reduce stress in caregivers of people with dementia in Asia, most studies were conducted in high-income countries such as Japan or Taiwan.25,26 In particular, digitally based interventions remain new in low-income and middle-income countries such as Vietnam. In Vietnam, Zalo is a mobile application used by approximately 80% of smartphone users.27 The group chat in this application is likely to be a familiar environment to provide educational information and communication as well as social support for caregivers. Potentially, a smartphone app-based intervention with codesigned content might provide a convenient, feasible, and effective solution to reduce psychological distress for family caregivers of people with dementia and improve the quality of life for them and their care recipients. Therefore, before conducting a full power efficacy trial of this intervention in Vietnam, we will perform a pilot randomized controlled trial (RCT) to evaluate the feasibility and acceptability of both the intervention and trial methodology.
This study aims to: (1) identify the information required by dementia family caregivers to be targeted within the intervention (phase 1); and (2) test the feasibility and acceptability of the intervention and the trial methodology to prepare for a fully powered RCT (phase 2).
Methods
The study has two phases: the identification of the intervention content (phase 1) and the pilot RCT phase (phase 2). The setting and the participants’ eligibility criteria are the same for the two phases.
Setting
The study will be conducted at the Department of Geriatrics at Gia Dinh People's Hospital, a public hospital located in Binh Thanh District, Ho Chi Minh City with approximately 500 beds. Family caregivers of patients with dementia admitted to the Department of Geriatrics will be screened for inclusion and exclusion criteria.
Participants
Potential participants will be the family caregivers of people with dementia, who have been (or are newly) diagnosed with dementia based on DSM-5, attend the Department of Geriatrics at Gia Dinh People's Hospital, and are living in the community with a caregiver. Inclusion criteria of the caregivers are: (1) aged ≥ 18 years and being the primary family caregiver of a person with dementia, defined as the family caregivers most involved in providing care for at least the past 6 months and is likely to continue to be for the next 6 months of the intervention; (2) being able to read and communicate in Vietnamese (at least primary education); (3) owning a smartphone that has the Zalo app or willing to have the Zalo app installed; and (4) having a moderate or higher level of distress over the past week based on the Distress thermometer (a score of ≥ 4, on a scale of 0–10).28,29 Exclusion criteria are: having any serious chronic diseases which would prevent completion of study requirements such as metastatic cancer, having vision or hearing impairment, or having a cognitive impairment (Mini-Cog score <4). The participants will be recruited by one geriatrician and all participants will be provided with a written informed consent form. Each participant will receive a reimbursement of approximately US$5 for participation in phase 1 and approximately US$10 for phase 2. Participants for phase 1 will not be invited to participate in phase 2.
Phase 1
Study design
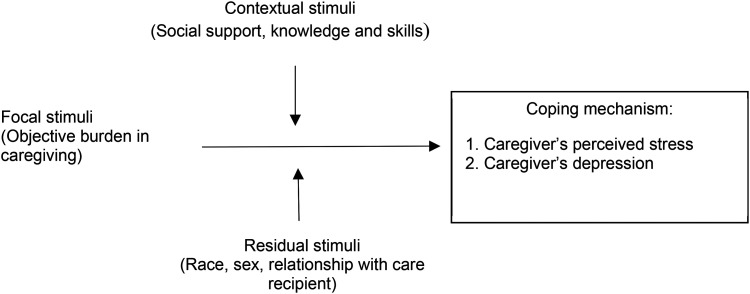
According to the Roy Adaptation Model17 and the Theory of Caregiver Stress,18 the objective burden in caregiving is considered as focal stimuli, and contextual and residual stimuli mediate the effect of focal stimuli on caregivers’ coping mechanism. Lack of social support and necessary knowledge and skills (contextual stimuli) to deal with changed behaviors of people with dementia (residual stimuli) will increase dementia caregivers’ stress and depression (Figure 1).18 Enhancing social support and equipping knowledge and skills that caregivers most require may reduce their stress and depression.30 Therefore, we will conduct in-depth semistructured interviews with caregivers of people with dementia. A semistructured interview guide has been developed, comprising open-ended questions and using prompts and probes to elicit more in-depth responses (see Supplemental Appendix 1). Each caregiver will be encouraged to share their thoughts freely.
Figure 1.
Concepts in the Roy adaptation and the theory of caregiver stress.
Sample size. We will recruit caregivers for the in-depth interviews until we reach data saturation,31 assuming around 20 participants.
Participant recruitment and procedure. A research assistant will screen potential participants against the inclusion/exclusion criteria including administering the distress thermometer then, describe the study to eligible caregivers and provide written consent forms to them if they are interested in participating in the study. A suitable time will be arranged for participants to be interviewed face-to-face by an interviewer, who is not providing medical care to participants’ family members, and will undertake the interviews in a private room in the Department of Geriatrics at Gia Dinh People’s Hospital. Interviews will be audio-recorded and fully transcribed into Vietnamese. The interviewer will receive training in qualitative interview techniques from an expert in qualitative research which will include both diastatic and experiential training using role play. After completing each interview, we will transcribe the audio-recording verbatim and commence analyzing the data to adjust the interview process. Emerging themes or new lines of inquiry will be investigated in subsequent interviews.
Data analysis. We will use Nvivo version 12 to manage the data and code the interview transcripts in Vietnamese. We will use content analysis,32 including coding data and category development, which will help us thoroughly explore caregivers’ perspectives and difficulties in providing dementia care as well as their required knowledge and skills. The analysis will begin by exploring emerging concepts during open codings. The principal investigator (PI) will complete the coding in collaboration with two other investigators. They will examine and code the data independently and then discuss and rework collaboratively until reaching an agreement. Categories will finally be formulated and all data will be re-read and grouped into relevant categories. Data will be analyzed by the Vietnamese research team and then reviewed by two experienced qualitative researchers (one is bilingual in Vietnamese and English) in Australia to achieve high-order categories and agree with the final analysis. The information, skills, and supports most commonly required by the caregiver participants will be determined to develop the intervention in phase 2.
Outcomes. The primary outcomes of phase 1 are the identification of the information and skills required by participants in providing dementia care, which may include dealing with worrying behavior changes of people with dementia, emotional support, and seeking support sources.33,34 We will use these outcomes to develop topics for the intervention in phase 2. The intervention content will be evidence-based and based on consultation with geriatricians, neurologists, and dementia experts to ensure its validity. Moreover, the content will be sent to 10 family caregivers to check its comprehension and acceptability and to suggest any revisions before the last version is established. Before conducting the pilot parallel-group RCT in phase 2, we will test the feasibility and acceptability of the intervention with a small group of five caregivers in a chat group on the Zalo app, who join phase 1 and are willing to participate in this stage.
Phase 2
Study design
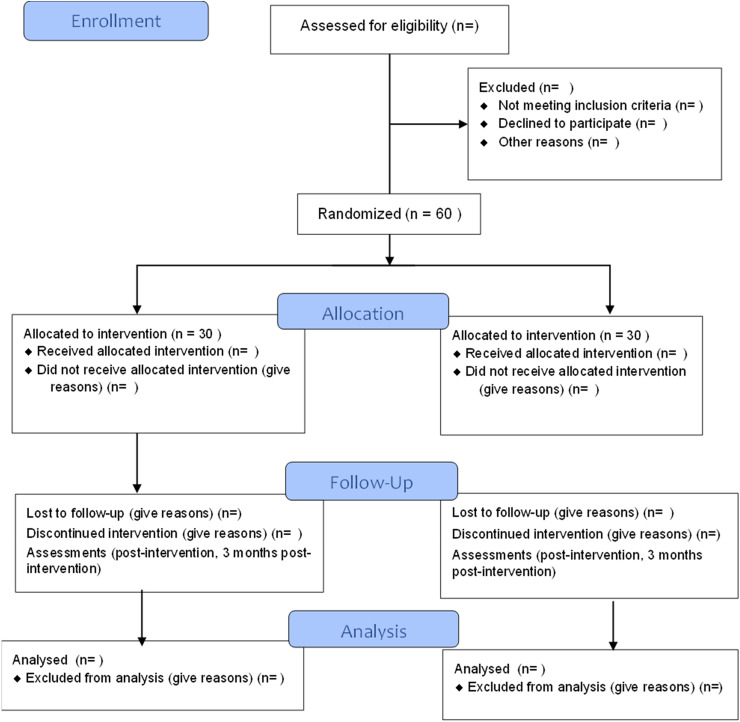
A pilot parallel-group RCT will be used with equal randomization of 1:1 to test the feasibility and acceptability of the smartphone app-based intervention. Sixty eligible dementia family caregivers will be enrolled in the RCT and will be allocated to the intervention or usual care group. After the intervention, the participants will be followed up for 3 months. Outcome measurements will be assessed face-to-face at baseline and via phone calls immediately postintervention, and 3-month follow-up postintervention. A Consolidated Standards of Reporting Trials (CONSORT; Figure 2) checklist for this study is available in Supplemantal Appendix 2.
Figure 2.
CONSORT flowchart of phase 2 of the study.
Participant recruitment
Caregivers of people with dementia admitted to the Department of Geriatrics will be screened for inclusion criteria; then, we will describe the study and provide written consent forms if they are interested in participating in the study. We will collect participants’ demographic information and phone numbers.
Informed consent
Written informed consent will be obtained from all participants. They will be informed about the study's purpose, procedures, risks, and benefits. Participants will be invited to participate in the study and they can withdraw from it at any point without impacting their families’ relationship with their care team or any other negative consequences. If a caregiver has a high level of stress or depression, we will refer him/her to a psychologist.
Intervention
The caregivers allocated to the intervention group will be added to a chat room in the Zalo app, which is named “The Caregiver Support group,” managed by one investigator. The Zao app is a free-cost app that is available on smartphones and computers. Users can text messages and make audio/video calls and share files or videos without any costs.27 Participants will have 5 days to introduce themselves within the chat group, then weekly, the administrator will post one topic which is developed in phase 1. Six days after posting the topic, one investigator will call the participants to ensure they read and understand the post by requesting them to answer. Multiple-choice questions relating to the posted topic will also be posted into the chat group before posting the new one to assess participants’ understanding of the intervention content. In addition, a chat group facilitator (another investigator) will facilitate group-based discussions about the topic (e.g. encourage participants to answer the multiple-choice questions and discuss their answers, to ask their own questions, or to share their feelings/experiences on the topic within the chat group. In case of no posts from participants, the facilitator will act as an icebreaker, posting relevant questions to encourage peer discussion. The chat group facilitator will collect the questions or comments from the participants, then post the summarized answers after following consultation with the experts. The planned recruitment date for the intervention phase will be September 1, 2023, and all participants will receive the intervention at the same time with the intended length of intervention of 6 to 8 weeks.
Enhanced usual care
The caregivers in the control group will be introduced to the website Alzheimer.org to search for eligible information (https://www.alz.org/asian/about/stages.asp?nL=VI&dL=VI).
Trial registration
A Mobile Phone-based Intervention on Dementia Patients’ Caregivers in Vietnam. ID: NCT04958707. URL: https://clinicaltrials.gov/ct2/show/NCT04958707?cond=A+Mobile+Phone-based+Intervention+on+Dementia+Patients%E2%80%99+Caregivers+in+Vietnam&draw=2&rank=1
Outcomes for methodology and intervention feasibility
(1) The recruitment rate will be calculated as the percentage of people who agree to participate in the study among all caregivers who are approached and meet eligibility criteria. (2) The retention rate will be calculated as the percentage of caregiver participants who complete the questionnaires at (a) postintervention and (b) 3-month postintervention out of those who consent. (3) Assessment completion will be calculated as the percentage of participants answering the entire questionnaire at each time point. Weekly, we will also collect the number of participants who read the post and the number of comments or questions, or interactions in the chat group. The acceptability of the intervention will also be evaluated using a structured questionnaire to measure the satisfaction of participants about the content, the format of the topics, the way of interactions within the chat group, and the length of intervention using the Likert 5 scales (from strongly disagree to strongly agree). To get feedback from participants on the intervention, we will conduct a focus group interview with 10 caregivers in the intervention group, using a focus group interview guide, to explore their perceptions of the intervention and potential improvements regarding intervention delivery and content.
Outcomes for preliminary intervention impact
To evaluate the preliminary impact of the intervention, we will use instruments to examine caregivers’ outcomes, which will be assessed at baseline, immediately postintervention, and 3-month postintervention in both the intervention and control groups. The questionnaires will be administered face-to-face at baseline and by phone interviews at postintervention and 3-month postintervention.
Primary outcomes. The primary outcomes are symptoms of depression, anxiety, and stress of caregivers, which will be evaluated using the Depression Anxiety Stress Scale 21 (DASS-21).35 DASS-21 scale contains 21 items, evaluating 3 subscales namely depression, anxiety, and stress states over the past week. The score of each subscale will range from 0 to 42. The minimum total score is 0 and the maximum total score is 126. Higher scores indicate more severity of emotional states. DASS-21 was translated and adapted into Vietnamese and was validated in a previous study.36
Secondary outcomes. Secondary outcomes include caregivers’ dementia understanding, perceived social support, perceived burden, and quality of life. The three instruments we will use to evaluate secondary outcomes were translated forward and backward and adapted into Vietnamese in previously reported studies.37–39 We have obtained permission from the authors of all questionnaires used in the study.
Dementia understanding of caregivers will be assessed using the questionnaire originating from Northern Ireland Life and Time Survey questionnaire which comprises 7 items.37,40 The score ranges from 0 to 7. A higher score indicates a higher understanding.
Perceived social support of caregivers will be assessed using the Multidimensional Scale of Perceived Social Support (MSPSS)38,41 comprising 12 self-report items assessing the perceived support from family, friends, or significant others. Each item is scored from 1 (very strongly disagree) to 7 (very strongly agree), based on a 7-point Likert scale. Higher scores indicate higher degrees of perceived social support.
Caregiving burden will be assessed using the Zarit 4-item burden interview.39,42 Each item is scored from 0 to 4, and higher scores indicate higher levels of burden.
Health-related quality of life will be assessed using the 36-Item Short-Form Health Survey Questionnaire (SF-36), comprising 36 items assessing 8 domains of health: physical functioning, role limitations due to physical health, role limitations due to emotional problems, energy/fatigue, emotional wellbeing, social functioning, pain, and general health. Scores of each domain range from 0 to 100, and a higher score indicates a more favorable health state.43 The Vietnamese version of SF-36 was translated and adapted in 2016.44
Sample size. Phase 2 is the pilot trial to test the feasibility of the intervention and methodology to prepare for a fully powered RCT; thus, a power calculation is not needed. We will enroll 60 caregivers for 2 arms with an allocation ratio of 1:1.45
Randomization. The participants will be randomly assigned to the intervention or control group by an independent statistician, using the block randomization method with a block size of 4. This procedure will be concealed from participants and investigators, and all participants will commence at the same time.
Blinding. The investigator assessing outcomes at baseline, postintervention, and 3-month postintervention will be blind to participant allocation.
Statistical methods
We will use Stata version 14 (StataCorp, College Station, TX, USA) in the analysis. Continuous variables will be presented as mean and standard deviation when they are normal distribution, otherwise, as median and interquartile range. The rates of recruitment, retention and measurement completion will be presented as percentages. To assess the impact of the intervention, mean or median scores of DASS-21, dementia understanding, perceived social support, caregiving burden and health-related quality of life between the intervention and control groups will be compared using linear mixed-effects models with group × time interactions as the effect of interest and based on the intention-to-treat principle.
Results
The anticipated date of first participant enrollment will be September 1, 2023, and the recruitment will be completed by December 30, 2023. The baseline characteristics of the participants will be presented in Table 1 and the primary and secondary outcomes of the intervention and control groups will be presented in Table 2.
Table 1.
Baseline characteristics of the participants in the randomized controlled trial—phase 2.
| Characteristics | Overall | Intervention group | Control group |
|---|---|---|---|
| Age (years), mean (SD) | |||
| Sex | |||
| Marital status | |||
| Income status | |||
| Educational level | |||
| Employment status | |||
| Length of care (year) | |||
| Time spent caring per day (hour) | |||
| Stress level (point) | |||
| Dementia severity of care recipient | |||
| ADL impairment of care recipient |
Abbreviations: ADL, activities of daily living; SD, standard deviation.
Table 2.
Descriptive statistics by time point and group for primary and secondary outcomes.
| Items | Intervention group, mean (SD) | Control group, mean (SD) | ||||
|---|---|---|---|---|---|---|
| Baseline | Postintervention | 3-month postintervention | Baseline | Postintervention | 3-month postintervention | |
| DASS-21 score | ||||||
| Dementia understanding | ||||||
| Perceived social support | ||||||
| Caregiving burden | ||||||
| Health-related quality of life score | ||||||
Abbreviations: DASS, Depression Anxiety Stress Scale; SD, standard deviation.
Risks of the study, data, and safety monitoring plan
There might be some discomfort including emotional distress due to the intervention and/or having to answer study questionnaires. Participants may experience mild distress when discussing their caregiving experience or because of concerns about confidentiality. Interviewers and other research staff will be trained to be empathic listeners to create an emotionally comfortable environment for family caregivers and to remind participants about the voluntary nature of the study and the option of ending the session early (phase 1) or withdrawing from the study at any time (phase 2).
We have an independent safety officer (SO). During the study, the research team will meet the SO every 3 months to review the study progress and adverse events, and the confidentiality of data. The PI and research team will be responsible for data and safety monitoring. The Zalo group is a closed group where only the managing investigator and participating members can access the information in the group. The participants’ names in the chat group will be abbreviated. An investigator will be responsible for reporting the study's progress and participant safety in monthly meetings. Any adverse events will be reported to the SO within 24 h and an appropriate action plan will be agreed upon and implemented. Severe adverse events will be reported to the IRBs of the University of Medicine and Pharmacy at Ho Chi Minh City and the National Institute on Aging within 1 week.
Discussion
To the best of our knowledge, this is one of the first studies to evaluate the feasibility of a smartphone-based intervention to reduce stress, depression, and anxiety in caregivers of people with dementia in Vietnam.46 This is the first step toward a fully powered study to test the effectiveness of this type of intervention in Vietnam. During the COVID-19 pandemic, when face-to-face communication is restricted, digital communication provides a legible solution. Importantly, this intervention will be accessible to caregivers living in remote areas in Vietnam with smartphone access.
Our study fills an important gap in Vietnam, a rapidly ageing country in Asia, where family caregivers of people with dementia receive little attention. Moreover, smartphone app-based intervention may be a convenient, less time-consuming, and less costly solution. However, our study may have several limitations. First, this intervention is not available to caregivers who do not have access to a smartphone (15%) or do not speak Vietnamese. Second, participants will be recruited at a single site, so the sample may not nationally representative. In addition, the sample size selected for this study was not calculated and justified. Another limitation of the study is that we will use an existing app, and data in the chat group may be saved on the Zalo server. However, the posted files or videos will be automatically deleted after 3 months due to the information security policy of Zalo. Moreover, participants’ names in the chat group will be abbreviated to ensure confidentiality. After all, our study is the first step to developing an appropriate intervention for caregivers of people with dementia in Vietnam. This intervention has the potential to reduce psychological distress and improve the quality of life of caregivers of people with dementia in Vietnam, where approximately 700,000 people are living with dementia.47 Moreover, with a rigorous study protocol and the inclusion of target-end users’ perspectives in the design process, if the chat group works then this could be an economical, powerful, and scalable family caregiver intervention, which is based on a simple technology that already exists in Vietnam.
Conclusions
The study will provide a foundation for a fully powered clinical trial for the smartphone app-based intervention to reduce stress, depression, and anxiety among family caregivers of people with dementia in Vietnam. This could be an economical and scalable intervention in a lower-middle-income country such as Vietnam.
Supplemental Material
Supplemental material, sj-docx-1-dhj-10.1177_20552076231163786 for Smartphone app-based intervention for reducing stress, depression, and anxiety in caregivers of people with dementia in Vietnam: Study protocol for a pilot randomized controlled trial by The NH Than, Tran TT Nguyen, Tuan C Nguyen, Lan TD Vu, Phong T Vo, Khoa TTruong, Penelope Schofield and Tuan A Nguyen in Digital Health
Supplemental material, sj-doc-2-dhj-10.1177_20552076231163786 for Smartphone app-based intervention for reducing stress, depression, and anxiety in caregivers of people with dementia in Vietnam: Study protocol for a pilot randomized controlled trial by The NH Than, Tran TT Nguyen, Tuan C Nguyen, Lan TD Vu, Phong T Vo, Khoa TTruong, Penelope Schofield and Tuan A Nguyen in Digital Health
Acknowledgements
We would like to send special thanks to the National Geriatric Hospital and the University of Medicine and Pharmacy at Ho Chi Minh City for their great support in conducting the research.
Footnotes
Contributorship: THNT, TTTN, TCN, LTDV, PTV, and KTT conceived the study and drafted the initial protocol manuscript. TNHT, TTTN, PS, and TAN are responsible for the study design. THNT, TTTN, TCN, PTV, KTT, PS, and TAN revised the manuscript. All authors have read and approved the final manuscripts.
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: The study was approved by the Institue Review Board (IRB) of the University of Medicine and Pharmacy at Ho Chi Minh City (approval number: 299/HĐĐĐ-ĐHYD), according to the World Medical Association Declaration of Helsinki.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by the National Institute of Aging (NIA) of the National Institutes of Health (NIH) under award number R01AG064688 (Hinton/Nguyen MPI). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIA and the NIH.
Guarantors: TTTN and TAN.
ORCID iD: Lan TD Vu https://orcid.org/0000-0001-7952-4074
Tran TT Nguyen https://orcid.org/0000-0001-7225-5430
Khoa TTruong https://orcid.org/0000-0003-1275-7636
Trial registration: A Mobile Phone-based Intervention on Dementia Patients’ Caregivers in Vietnam. ID: NCT04958707. URL: https://clinicaltrials.gov/ct2/show/NCT04958707?cond=A+Mobile+Phone-based+Intervention+on+Dementia+Patients%E2%80%99+Caregivers+in+Vietnam&draw=2&rank=1
Supplemental material: Supplemental material for this article is available online.
References
- 1.World Health Organization. Dementia, https://www.who.int/news-room/fact-sheets/detail/dementia (2020, accessed 5 August 2021).
- 2.General Statistics Office. Population ageing and older persons in Viet Nam. July 2021.
- 3.The World Bank. Vietnam: adapting to an aging society, https://www.worldbank.org/en/country/vietnam/publication/vietnam-adapting-to-an-aging-society (2021, accessed 22 January 2023).
- 4.Pham T, Luong CT. Epidemiology of dementia among community-dwelling older people. Y Học Thực Hành 2010; 715: 53–55. [Google Scholar]
- 5.Doan K, Vo Van T, Ho D, et al. Prevalence of dementia among the elderly and health care needs for people living with dementia in an urban community of central VietNam. Vietnam J Public Health 2015; 3: 16–23. [Google Scholar]
- 6.Nguyen TA, Pham T, Dang TH, et al. Towards the development of Vietnam's national dementia plan—the first step of action. Australas J Ageing 2020; 39: 137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nguyen TA, Pham T, Vu HTT, et al. Use of potentially inappropriate medications in people with dementia in Vietnam and its associated factors. Am J Alzheimers Dis Other Demen 2018; 33: 423–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. Dementia—a public health priority. Geneva, Switzerland, 2015. [Google Scholar]
- 9.Ala TA, Berck LG, Popovich AM. Knowledge of personal information and caregiver awareness in Alzheimer's disease. Am J Alzheimers Dis Other Demen 2005; 20: 119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silvia S, Yeates C. Issues in dementia caregiving: effects on mental and physical health, intervention strategies, and research needs. Am J Geriatr Psychiatry 2011; 19: 491–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Victoria O, Ebrahim H, Shahla A. Depression in main caregivers of dementia patients: Prevalence and predictors. Adv Biomed Res 2018; 7: 34–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martin P, Anders W, Maëlenn G, et al. The global impact of dementia: An analysis of prevalence, incidence, cost and trends. London: Alzheimer's Disease International, 2015. [Google Scholar]
- 13.Pinquart M, Sörensen S. Differences between caregivers and noncaregivers in psychological health and physical health: A meta-analysis. Psychol Aging 2003; 18: 250–267. [DOI] [PubMed] [Google Scholar]
- 14.Li Q, Zhang H, Zhang M, et al. Prevalence and risk factors of anxiety, depression, and sleep problems among caregivers of people living with neurocognitive disorders during the COVID-19 pandemic. Front Psychiatry 2021; 11: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sallim AB, Sayampanathan AA, Cuttilan A, et al. Prevalence of mental health disorders among caregivers of patients with Alzheimer disease. J Am Med Dir Assoc 2015; 16: 1034–1041. [DOI] [PubMed] [Google Scholar]
- 16.Pickering C, Yefimova M, Maxwell C. Caregiver stress theory may explain elder abuse but not neglect in dementia family caregiving. Innovation Aging 2018; 2: 851–851. [Google Scholar]
- 17.Andrews HA, Roy C. Essentials of the Roy adaptation model. In: Andrews HA, Roy C. (eds) The Roy adaptation model: The definitive statement. Norwalk: Appleton and Lange, 1991, pp.3–26. [Google Scholar]
- 18.Tsai PF. A middle-range theory of caregiver stress. Nurs Sci Q 2003; 16: 137–145. [DOI] [PubMed] [Google Scholar]
- 19.Goren A, Montgomery W, Kahle-Wrobleski K, et al. Impact of caring for persons with Alzheimer’s disease or dementia on caregivers’ health outcomes: Findings from a community based survey in Japan. BMC Geriatr 2016; 16: 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sala-González M, Pérez-Jover V, Guilabert M, et al. Mobile apps for helping informal caregivers: A systematic review. Int J Environ Res Public Health 2021; 18: 1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iribarren S, Stonbraker S, Suero-Tejeda N, et al. Information, communication, and online tool needs of Hispanic family caregivers of individuals with Alzheimer's disease and related dementias. Inf Health Social Care 2019; 44: 115–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tremont G, Davis JD, Papandonatos GD, et al. Psychosocial telephone intervention for dementia caregivers: A randomized, controlled trial. Alzheimers Dement 2015; 11: 541–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tremont G, Davis JD, Bishop DS, et al. Telephone-delivered psychosocial intervention reduces burden in dementia caregivers. Dementia (London) 2008; 7: 503–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fuller-Tyszkiewicz M, Richardson B, Little K, et al. Efficacy of a smartphone app intervention for reducing caregiver stress: randomized controlled trial. JMIR Ment Health 2020; 7: e17541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tawfik NM, Sabry NA, Darwish H, et al. Psychoeducational program for the family member caregivers of people with dementia to reduce perceived burden and increase patient's quality of life: A randomized controlled trial. J Prim Care Community Health 2021; 12: 21501327211014088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsu C-C, Wang Y-M, Huang C-R, et al. Sustained benefit of a psycho-educational training program for dementia caregivers in Taiwan. Int J Gerontol 2017; 11: 31–35. [Google Scholar]
- 27.Statista. Leading active social media apps among internet users in Vietnam as of 2nd quarter of 2021 by generation, https://www.statista.com/statistics/1229529/vietnam-leading-social-media-platforms-by-generation/ (2021, accessed 22 September 2021).
- 28.Riba MB, Donovan KA, Andersen B, et al. Distress management, version 3.2019, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2019; 17: 1229–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zwahlen D, Hagenbuch N, Carley MI, et al. Screening cancer patients’ families with the distress thermometer (DT): A validation study. Psychooncology 2008; 17: 959–966. [DOI] [PubMed] [Google Scholar]
- 30.Zhong Y, Wang J, Nicholas S. Social support and depressive symptoms among family caregivers of older people with disabilities in four provinces of urban China: The mediating role of caregiver burden. BMC Geriatr 2020; 20: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moser A, Korstjens I. Series: Practical guidance to qualitative research. Part 3: Sampling, data collection and analysis. Eur J Gen Pract 2018; 24: 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bengtsson M. How to plan and perform a qualitative study using content analysis. NursingPlus Open 2016; 2: 8–14. [Google Scholar]
- 33.Khanassov V, Rojas-Rozo L, Sourial R, et al. Needs of patients with dementia and their caregivers in primary care: Lessons learned from the Alzheimer plan of Quebec. BMC Fam Pract 2021; 22: 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leocadie M-C, Morvillers J-M, Pautex S, et al. Characteristics of the skills of caregivers of people with dementia: Observational study. BMC Fam Pract 2020; 21: 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lovibond S, Lovibond P. The structure of negative emotional states: Comparison of the Depression Anxiety Stress Scales (DASS) with the beck depression and anxiety inventories. Behav Res Ther 1995; 33: 335–343. [DOI] [PubMed] [Google Scholar]
- 36.Tran TD, Tran T, Fisher J. Validation of the Depression Anxiety Stress Scales (DASS) 21 as a screening instrument for depression and anxiety in a rural community-based cohort of northern Vietnamese women. BMC Psychiatry 2013; 13: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nguyen T, The T, McFarland P, et al. Dementia prevalence among older hospitalized patients in Vietnam and dementia understanding of their caregivers. Aging Med Healthcare 2019; 10: 128–132. [Google Scholar]
- 38.Vo THM, Nakamura K, Seino K, et al. Fear of falling and cognitive impairment in elderly with different social support levels: Findings from a community survey in Central Vietnam. BMC Geriatr 2020; 20: 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen TA, Nguyen H, Pham T, et al. A cluster randomized controlled trial to test the feasibility and preliminary effectiveness of a family dementia caregiver intervention in Vietnam: The REACH VN study protocol. Medicine (Baltimore) 2018; 97: e12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McParland P, Devine P, Innes A, et al. Dementia knowledge and attitudes of the general public in Northern Ireland: An analysis of national survey data. Int Psychogeriatr 2012; 24: 1600–1613. Non-U S Gov't. [DOI] [PubMed] [Google Scholar]
- 41.Zimet GD, Dahlem NW, Zimet SG, et al. The multidimensional scale of perceived social support. J Pers Assess 1988; 52: 30–41. [Google Scholar]
- 42.Bédard M, Molloy DW, Squire L, et al. The Zarit Burden Interview: A new short version and screening version. Gerontologist 2001; 41: 652–657. [DOI] [PubMed] [Google Scholar]
- 43.Ware JE., Jr and Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992; 30: 473–483. [PubMed] [Google Scholar]
- 44.Vo TK, Nguyen TK. Translation, cultural adaptation and preliminary validity of the Vietnamese short form 36 (SF-36). Hội Nội Tiết và Đái Tháo Đường miền Trung 2016; 19: 1–8. [Google Scholar]
- 45.Browne RH. On the use of a pilot sample for sample size determination. Stat Med 1995; 14: 1933–1940. [DOI] [PubMed] [Google Scholar]
- 46.Nguyen TA, Tran K, Esterman A, et al. Empowering dementia carers with an iSupport Virtual Assistant (e-DiVA) in Asia-Pacific regional countries: Protocol for a pilot multisite randomized controlled trial. JMIR Res Protoc 2021; 10: e33572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nguyen TA, Dang TH, Tran K, et al. Dementia in Vietnam: A situational analysis. Alzheimers Dement 2020; 16: e039252. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-dhj-10.1177_20552076231163786 for Smartphone app-based intervention for reducing stress, depression, and anxiety in caregivers of people with dementia in Vietnam: Study protocol for a pilot randomized controlled trial by The NH Than, Tran TT Nguyen, Tuan C Nguyen, Lan TD Vu, Phong T Vo, Khoa TTruong, Penelope Schofield and Tuan A Nguyen in Digital Health
Supplemental material, sj-doc-2-dhj-10.1177_20552076231163786 for Smartphone app-based intervention for reducing stress, depression, and anxiety in caregivers of people with dementia in Vietnam: Study protocol for a pilot randomized controlled trial by The NH Than, Tran TT Nguyen, Tuan C Nguyen, Lan TD Vu, Phong T Vo, Khoa TTruong, Penelope Schofield and Tuan A Nguyen in Digital Health