Abstract
Amebiasis is a major cause of morbidity and mortality throughout the tropical world. Entamoeba histolytica is now recognized as a separate species from the morphologically identical E. dispar, which cannot invade. Cysteine proteinases are a key virulence factor of E. histolytica and play a role in intestinal invasion by degrading the extracellular matrix and circumventing the host immune response through cleavage of secretory immunoglobulin A (sIgA), IgG, and activation of complement. Cysteine proteinases are encoded by at least seven genes, several of which are found in E. histolytica but not E. dispar. A number of new animal models, including the formation of liver abscesses in SCID mice and intestinal infection in human intestinal xenografts, have proven useful to confirm the critical role of cysteine proteinases in invasion. Detailed structural analysis of cysteine proteinases should provide further insights into their biochemical function and may facilitate the design of specific inhibitors which could be used as potential chemotherapeutic agents in the future.
INTRODUCTION
Symptomatic and Asymptomatic Infections with E. histolytica and E. dispar
Amebiasis is defined as invasive intestinal or extraintestinal infection with the protozoan parasite Entamoeba histolytica. More than 50 million people worldwide are infected, and up to 110,000 of these die every year (126). Only malaria and schistosomiasis surpass amebiasis as parasitic causes of death. Based on biochemical, immunological, and genetic data, E. histolytica has been reclassified into two morphologically identical but genetically distinct species: E. histolytica, which is potentially invasive, and E. dispar, which is not (128). Together, E. histolytica and E. dispar infect about 10% of the world's population (128). Infection with the commensal E. dispar is much more common, so that the true prevalence of invasive E. histolytica is perhaps closer to 1% worldwide.
Infection by Entamoeba is initiated by the ingestion of cysts, which release motile trophozoites in the small intestine. If patients are infected with E. dispar, the trophozoites remain as harmless commensals in the bowel lumen. Local invasion does not occur, even in immunocompromised patients. No histologic or serologic evidence of infection could be detected in 55 homosexual men colonized with E. dispar (previously called nonpathogenic E. histolytica) (3). In addition, 19 patients with AIDS colonized with E. dispar did not develop an antibody response and spontaneously cleared their infection (85).
The first evidence of amebic pathology is local depletion of intestinal mucus and disruption of the epithelial barrier as a result of degradation of the extracellular matrix, which occurs in part from the action of cysteine proteinases. Trophozoites subsequently attach to colonic mucus and epithelial cells by a galactose-inhibitable lectin (70), invade between epithelial cells, and extend submucosally in a pattern that causes the formation of flask-shaped ulcers. Amebic colitis is characterized by bloody diarrhea with a paucity of neutrophils. From the intestine, E. histolytica trophozoites can disseminate to cause distant abscesses, particularly of the liver (reviewed in references 71a, 79, and 82). Prepatent or asymptomatic infection with E. histolytica does occur in some patients, who can be identified by a positive amebic serology (30). In a 1-year longitudinal study of untreated asymptomatic carriers of E. histolytica in South Africa, 10% of carriers developed amebic colitis (30). Therefore, not every patient infected with E. histolytica develops invasive amebiasis, and the ultimate outcome of infection depends on the balance between parasite virulence factors and the host response.
It is important to target treatment to patients infected with E. histolytica, whether they are symptomatic or not. E. histolytica can be differentiated microscopically from E. dispar in stool specimens only if hematophagous trophozoites are detected. If only cysts or trophozoites without ingested red cells are seen, the specimen must be reported as “E. histolytica/E. dispar.” E. histolytica and E. dispar can be separated by several laboratory tests that are still primarily research tools: isoenzymes of culture isolates (97), PCR based on rRNA genes (2), and monoclonal antibodies (88, 115, 116), particularly to the galactose-inhibitable lectin (36). The only commercially available means of separating the species is by an E. histolytica-specific stool enzyme-linked immunosorbent assay (37).
Proteinases as Virulence Factors
Evidence supporting the role of the extracellular cysteine proteinases of E. histolytica as virulence factors include the production and extracellular release of 10- to 1,000-fold more cysteine proteinase from lysates of E. histolytica cells than from lysates of noninvasive E. dispar (89). Cysteine proteinases purified from axenized E. histolytica cleave collagen, elastin, fibrinogen, and laminin, elements of the extracellular matrix that trophozoites must penetrate to cause invasive disease (46, 56). Cysteine proteinases are responsible for the detachment of tissue culture monolayers, the most widely used assay for amebic toxins and other virulence factors. The cytopathic effect on fibroblast monolayers as a result of supernatants of clinical E. histolytica strains is completely inhibited by Z-Phe-Arg-CH2F, a specific, irreversible cysteine proteinase inhibitor that is not toxic to host cells (84). In vitro cell lysis caused by E. histolytica is a more complex process, requiring attachment via the galactose-inhibitable lectin (70) and lysis by the amebapore (51).
In addition, cysteine proteinases interfere with the function of the host immune system. The cysteine proteinase purified from E. histolytica can specifically cleave C3 by a unique mechanism which enables E. histolytica to activate complement in the fluid phase (87). The proteinase also degrades immunoglobulin A (IgA) and the anaphylatoxins C3a and C5a, which may explain the relative paucity of neutrophils noted in amebic liver abscesses (86). The proteinases must be released during the course of invasive amebiasis, because more than 80% of infected patients make antibody to cysteine proteinases (89). E. histolytica also has several unique genes encoding cysteine proteinases (11, 84). In vivo studies by Stanley's and Mirelman's groups demonstrated that inhibition of cysteine proteinase activity with inhibitors or an antisense construct significantly decreased liver abscess formation in SCID mice (113) and hamsters (5). Taken together, the data supporting a key role of cysteine proteinases in virulence are extremely strong.
GENERAL PROPERTIES OF AMEBIC CYSTEINE PROTEINASES
Multiple Forms of Cysteine Proteinases
A number of cysteine proteinases with molecular masses of 16 to 96 kDa have been observed in various extracts of E. histolytica. Gelatin-polyacrylamide gels of trophozoite lysates usually reveal four to six bands of proteinase activity. The predominant forms of cysteine proteinases in Entamoeba are 27- to 30-kDa mature enzymes. Early investigators purified at least two similar but distinct cysteine proteinase activities from E. histolytica and characterized them as amoebapain (99) and histolysin (56) (Table 1). Two 27-kDa cysteine proteinases could also be purified by their affinity to laminin, amoebapain (EhCP1 or ACP3) and ACP2 (EhCP2) (54). The apparent differences in molecular mass of these and other preparations may reflect differences in purification protocols, resulting in multimer formation, autoproteolysis, or the secretion of higher-molecular-mass proenzymes.
TABLE 1.
 |
Reprinted from reference 74 with permission of the publisher.
Summary of the cysteine proteinase genes, mRNAs, and protein products of E. histolytica and E. dispar.
The different nomenclature for the gene products by Reed et al. (84) and Bruchhaus et al. (11) is listed in the first two columns.
Expression of the gene products is noted by mRNA, with question marks (?) for conflicting data.
The percent identity of the deduced amino acid sequence of the cysteine proteinases is presented in more detail in reference 11.
ND, not determined.
E. histolytica trophozoites release large amounts of cysteine proteinases into the culture medium. Recent clinical isolates of E. histolytica released 10- to 1,000-fold more cysteine proteinase activity into the supernatant than did E. dispar isolates, although there was significant day-to-day variability (89). Keene et al. detected a major neutral proteinase in secretions of axenically cultured E. histolytica trophozoites (46). This proteinase has a molecular mass of ∼56 kDa (by sodium dodecyl sulfate-polyacrylamide gel electrophoresis), a neutral pH optimum, and a pI of 6. The amino terminus of the 56-kDa enzyme was blocked, and so the sequence could not be directly determined.
An interesting question is whether the extracellular higher-molecular-mass forms of cysteine proteinases may represent proenzymes. There is significant precedence for the release of active proenzymes by metastatic tumors. Both E. histolytica and metastatic tumors release extracellular cysteine proteinases, which are linked to invasion. High-molecular-mass forms of cathepsin B (35 and 40 kDa) have been detected in the ascitic fluid and culture medium of cells from cancer patients (63, 64), spontaneous mammary tumors (80), and melanoma cell lines (109). The production and secretion of cathepsin B have been linked to the invasiveness of human carcinomas (94, 110). A membrane-linked form of the enzyme in melanoma cell lines is thought to contribute to the focal dissolution of extracellular matrices that precedes tissue invasion by the melanoma tumors (94, 109). Cathepsin B precursors released extracellularly by malignant tumors, however, usually do not have mannose-rich carbohydrates, suggesting a possible defect in posttranslational processing (108). Whether the release of proenzymes by tumors and amebae represents an evolutionarily conserved mechanism of metastasis awaits further characterization of the high-molecular-mass forms.
Two other classes of high-molecular-mass proteases of E. histolytica were also identified recently. One of the activities was attributed to the E. histolytica proteasome based on the electrophoretic mobility of subunits and on its reactivity with an antiproteasome antibody (101). An E. histolytica proteasome α-subunit gene has recently been cloned (77). The other activity was due to a complex of six subunits that had unique proteolytic activity, including the cleavage of substrates with aromatic P1 residues, and inhibition by chymostatin and a calpain inhibitor. The metallocollagenase of E. histolytica is a surface-bound proteinase and is expressed in lesser amounts than the thiol proteinase. It degrades type I and type III collagen, and its expression also correlates with pathogenicity of Entamoeba (52, 103). The gene encoding the metallocollagenase of Entamoeba has not yet been identified.
Substrate Specificity
The substrate specificity of E. histolytica proteinases is typical of cathepsin B-like proteases of the papain family (46, 56). Most of the cysteine proteinases are active against a synthetic peptide substrate, Z-Arg-Arg-AMC (benzyloxycarbonyl-arginine-arginine-4-amino-7-methylcoumarin), with arginine at the P1 and P2 positions. The amebic cysteine proteinases have little affinity for the cathepsin L substrate, Z-Phe-Arg-AMC, and the cathepsin H substrate, Z-Arg-AMC, although molecular analysis of proteinase genes has identified only cathepsin L-like sequences. Although the pH optimum of E. histolytica cysteine proteinases is broad, ranging from about pH 5 to 9, most of these enzymes are active in slightly acidic and neutral pH regions (8).
Inhibitor Specificity
Amebic cysteine proteinases are classified as cysteine (thiol) proteinases because their activities are inhibited by the cysteine proteinase-specific inhibitor l-trans-epoxysuccinyl-leucylamido-(4-guanidino)butane (E-64) and not by the serine proteinase-specific inhibitor phenylmethylsulfonyl fluoride. Amebic cysteine proteinases are also inhibited by sulfhydryl reagents (p-chloromercuribenzoate [PCMB]) and activated by dithiothreitol and 2-mercaptoethanol. Inhibition of protease activities is a new approach to anti-infectious therapy, which has been revolutionized by the therapeutic efficacy of synthetic protease inhibitors active specifically against the aspartyl proteinase of human immunodeficiency virus. New generations of cysteine proteinase-specific inhibitors, including diazomethanes, vinyl sulfones, and synthetic peptide inhibitors, have been active in the micromolar to nanomolar range against E. histolytica (84), as well as other parasites (reviewed in references 93 and 98). A specific vinyl sulfone cysteine proteinase inhibitor prevented lethal Trypanosoma cruzi infection in mice (25), supporting the potential of cysteine proteinase inhibitors as novel antiparasitic therapy.
MOLECULAR CLONING AND STRUCTURAL ASPECTS OF CYSTEINE PROTEINASE GENES
Structure and Processing of Amebic Cysteine Proteinases
E. histolytica has multiple cysteine proteinases, and at least seven distinct genes encoding typical prepro-forms of papain-family proteinases have been identified and sequenced. The groups of McKerrow and Reed identified three amebic cysteine proteinase genes (termed acp1, acp2, and acp3) (84). Tannich's group subsequently identified three more cysteine proteinase genes, designated ehcp4, ehcp5, and ehcp6, of which only ehcp5 is expressed in axenic E. histolytica (11). The majority of the proteinase activity detected in E. histolytica lysates can be attributed to the expression of four of these genes: acp1, acp2, acp3, and ehcp5. E. dispar has genes (edcp1 to edcp4) homologous to four of the six genes of E. histolytica (Fig. 1 and Table 1). There is debate as to which genes are present in E. histolytica but absent in E. dispar. acp1 (ehcp3) was initially reported to be present in E. histolytica alone (84), but a related homologue (edcp3) with 95% identity was found in E. dispar (12, 62). ehcp1 (acp3) is a second gene reported to be unique to E. histolytica (12), but sequences were detected in clinical strains of E. dispar (84). The third gene reported to be present only in E. histolytica is ehcp5, which encodes a surface-associated cysteine proteinase (43). Comparison of the deduced peptide sequences of the ehcp1 to ehcp6 genes indicated that the primary structures of the six ameba enzymes are similar and have sequence similarities of between 43 and 87% (11). All of the genes predict proteins with 34 to 39% identity to papain, 36 to 46% identity to cathepsin L, and complete conservation of all residues known to be critical for cysteine proteinase function. Orozco's group has recently described a novel surface cysteine proteinase which is fused with an adhesin (29). The cysteine proteinase has a transmembrane sequence and an RGD (integrin attachment) domain (29). Whether the increased cysteine proteinase activity and invasive potential of E. histolytica can be attributed to the expression of specific cysteine proteinase genes or an overall increased level of cysteine proteinase activity remains to be determined.
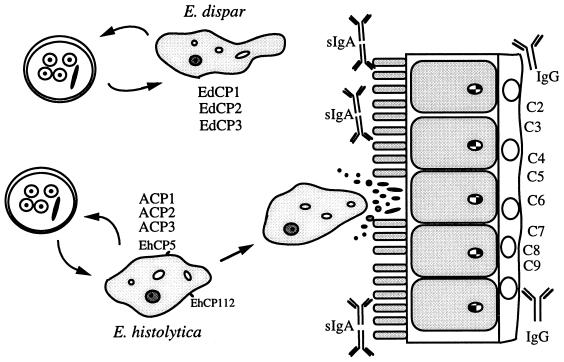
FIG. 1.
Model of cysteine proteinase expression during infection by E. histolytica and E. dispar. E. dispar expresses at least three cysteine proteinases (EdCP1, EdCP2, and EdCP3) but cannot invade. E. histolytica expresses at least five cysteine proteinases, ACP1, ACP2, ACP3, EhCP5, and EhCP112. Extracellular cysteine proteinases cleave sIgA, degrade the extracellular matrix, activate complement, and degrade IgG to circumvent the host immune response. Reprinted from reference 82a with permission of the publisher.
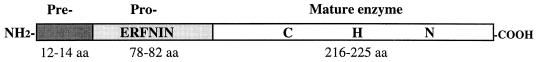
Amebic cysteine proteinases are synthesized as precursor proteins with a 12- to 14-amino-acid hydrophobic predomain signal peptide, a 78- to 82-amino-acid prodomain, a 216- to 225-amino-acid catalytic domain, and no C-terminal extension (Fig. 2). The preproenzymes are subsequently processed to the mature enzymes. The signal sequences consist of a N-terminal charged region (n-region), a central hydrophobic region (h-region) and a polar C-terminal region (c-region). A putative processing site between the signal peptide and propeptide region is predicted by the -3, -1 rule of von Heijne (125). The prodomains contain a stretch of sequence containing the ERFNIN motif [Glu-X3-Arg-X2-(Val/Ile)-Phe-X2-Asn-X3-Ile-X3-Asn] near residue -50 of all six proteinases. This motif has been identified at a similar position in all cathepsin H- or L-like proteinases but not in cathepsin B-like enzymes (44). The prodomain of eukaryotic cathepsins has two well-defined functions: (i) to maintain the enzyme in an inactive form (zymogen) until it reaches an appropriate site of protease function and (ii) to act as a structural template to ensure proper folding during translation (21). EhCP5 contains an Asn-X-(Ser/Thr) recognition sequences within the prosequence, which may be posttranslationally modified by glycosylation (43). The mature regions are homologous to cathepsin L-like cysteine proteinases and contain all conserved cysteine residues implicated in the maintenance of the three-dimensional structure.
FIG. 2.
Model of the cysteine proteinases of Entamoeba. The relative sizes of the preenzyme, proenzyme, and mature enzymes are depicted. The ERFNIN motif of cathepsin L in the prosequence is shown, along with the three residues of the active site, cysteine (C), histidine (H), and asparagine (N). aa, amino acids.
Eukaryotic Cysteine Proteinases
Eukaryotic cathepsins (lysosomal cysteine proteinases) are best known for their role in intracellular protein turnover (8). They are synthesized with an amino-terminal hydrophobic presequence (∼20 residues) that allows translocation of the nascent protein into the endoplasmic reticulum (ER), followed by a pro-amino-terminal extension (∼62 to 123 residues), which stabilizes the enzyme and may be essential for proper protein folding (19, 111). Cathepsins in most eukaryotic cells are glycosylated in the Golgi complex and targeted to lysosomes by mannose-6-phosphate receptors, which recognize phosphorylated asparagine-linked oligosaccharides of lysosomal proteins during their synthesis. Processing of the proenzymes to a mature enzyme of ∼27 to 30 kDa subsequently takes place in the acidic lysosomal environment (33).
Localization and Trafficking of Amebic Cysteine Proteinases
The trafficking of amebic cysteine proteinases must differ from the usual pathway of higher eukaryotes. The Golgi complex, ER, and lysosomes, the key organelles of protein transport, are only rudimentary structures in Entamoeba. The amebic Golgi apparatus does not form tightly packed lamellae; instead, it contains a few large vesicles adjacent to the nucleus (60). However, a number of proteins involved in eukaryotic secretory pathways have been identified from E. histolytica; these include the 54-kDa subunit of the signal recognition peptide, which mediates attachment to secreted proteins; an ER retention receptor (ERD2), which binds to C-terminal KDEL peptides (96); BiP, an ER Ig-binding protein that aids protein folding; and ADP-ribosylating factor (ARF), a Golgi-associate coatomer protein (32). Samuelson's group has also identified N-terminal signal sequences and C-terminal ER retention sequences in chitinase, a critical enzyme in encystation of Entamoeba which localizes to multiple vesicles (32). True lysosomes have not been identified in Entamoeba, although numerous acidic vesicles are present in the cytoplasm (78). Only one amebic cysteine proteinase, EhCP5, has a potential asparagine-linked glycosylation site, but it is not known if mannose-6-phosphate is present in the mature enzyme (43). Therefore, the mannose-6-phosphate-dependent pathway cannot be the major mechanism to target amebic cysteine proteinases. Several mannose-6-phosphate-independent pathways of protease trafficking have been identified. Cathepsin B precursors released extracellularly by malignant tumors usually do not have mannose-rich carbohydrates, suggesting a possible defect in posttranslational processing (108). A conserved prodomain sequence interacts with microsomal membrane receptors in mannose-6-phosphate-independent targeting of cathepsins in mammalian cells (61). A similar prodomain loop has been shown by McKerrow's group to target the cathepsins of Trypanosoma cruzi and Leishmania mexicana, which do not have carbohydrate modifications (39). Confirmation and further analysis of mannose-6-phosphate-independent pathways of lysosomal protease trafficking in amebic cells remains to be done.
The cathepsin L-like proteinases of T. cruzi, L. mexicana, and L. major were localized to lysosome-like organelles by subcellular fractionation, confocal fluorescence microscopy, and immunoelectron microscopy (24, 39). The existence of acidic vesicles in E. histolytica and conserved cathepsin L-like sequences in the proregions suggests that some of the mechanisms of synthesis, processing, and trafficking may be similar to those of higher eukaryotes. A low level of mature proteinase has been detected on the plasma membrane of trophozoites. Amoebapain (ACP3) has been localized to both the amebic cell surface and subcellular pinocytotic vesicles (100). EhCP5 is thought to associate with membranes of E. histolytica by virtue of a hydrophobic patch sequence (43). The most recently identified surface cysteine proteinase, the 112-kDa cysteine proteinase-adhesin fusion, does have a transmembrane domain and an RGD sequence (29). During phagocytosis of erythrocytes, the entire protein is translocated to phagocytic vesicles (29). Although E. histolytica readily releases cysteine proteinases extracellularly, it is not known if this is an active secretory process.
Expression and Regulation of Amebic Cysteine Proteinase Genes
Specific factors regulating cysteine proteinase gene expression have not been identified. To further elucidate the molecular basis of E. histolytica pathogenicity, it would be of particular interest to find which of the various cysteine proteinase genes are responsible for the elevated cysteine proteinase expression in E. histolytica compared to E. dispar. Incubation of trophozoites with components of the extracellular matrix has been linked to release of proteolytic activity. Trophozoites incubated with immobilized fibronectin secrete cysteine proteinases at the site of attachment (119, 124). Exposure of axenic E. histolytica to collagen leads to the release of collagenolytic activity, which is mediated primarily by a metalloproteinase (65). Numerous reports have cited the increase virulence of E. histolytica strains following passage through animals or following association with cholesterol or bacteria, but the exact effects on specific virulence genes have not been delineated. Repeated passage of one axenic (bacterium-free) and five xenic (grown with bacteria) strains of E. histolytica through hamster livers led to progressive increases in cysteine proteinase activity of lysates as well as to increased enterotoxicity, as measured in gerbil intestinal segments in Ussing chambers (66), but the effect on expression of specific cysteine proteinase genes was not determined.
ROLES OF CYSTEINE PROTEINASES IN HOST-PARASITE INTERACTIONS
Nutrition Acquisition and Developmental Cycle
Studies of other protozoan parasites suggest that the functions of cysteine proteinases are diverse (reviewed in references 90 and 93) and include key roles in acquisition of nutrients and as part of the developmental cycle. Plasmodial proteases hydrolyze globin to free acids for the growth of intraerythrocytic parasites (92), and the protease inhibitors that block hemoglobin degradation block the development of cultured malaria parasites (95). Cysteine proteinase inhibitors also block the intracellular development of T. cruzi (23) and Leishmania (102). Although E. histolytica is not an intracellular parasite, when its trophozoites were incubated with a diazopeptidyl inhibitor, growth was decreased by 50% (22), suggesting that cysteine proteinases are important for the acquisition of nutrients, even from a liquid medium.
Cysteine proteinases also play a role in the development of many parasites. Specific inhibitors blocked the transformation from epimastigotes to trypomastigotes (28) and from amastigotes to trypomastigotes in T. cruzi (38). In T. brucei, cysteine proteinase activity increased during differentiation from long slender to short stumpy forms (68). Most recently, cysteine proteinases have been shown to be critical for excystation of Giardia (127). In E. invadens, which has been used as a model for encystation and excystation of E. histolytica, specific cysteine proteinase inhibitors significantly decreased the efficiency of encystation (105). Further studies are required to clarify whether cysteine proteinase play a direct role in the initiation of encystation or whether the effect of inhibition is secondary through decreased trophozoite multiplication.
Host Invasion
Cysteine proteinases are critical to host invasion in a number of parasites. Specific inhibitors block invasion in T. cruzi (28), Plasmodium falciparum (59), Cryptosporidium parvum (27), and Toxoplasma gondii (X. Que, D. S. Herdman, and S. L. Reed, Abstr. Fifth Toxoplasmosis Conf., 1999). The role of cysteine proteinases in tissue invasion has been best documented in E. histolytica. The purified proteinase degrades components of the extracellular matrix, including fibronectin, laminin, and collagen, as well as an extracellular matrix from vascular smooth muscle (46). Cysteine proteinases are primarily responsible for the cytopathic effect, an in vitro assay of virulence that measures monolayer detachment (46, 84). The cytopathic effect correlates with the amount of cysteine proteinase activity released into the medium by clinical isolates of E. histolytica (89) and can be inhibited by specific peptide inhibitors (84). Mutants of E. histolytica strain HM-1 which are deficient in both proteinase expression and cytopathic effect have been identified (45). The in vitro cytopathic effect correlates with the early pathology of invasion in animal models in which the intestinal epithelial cells separate before making direct contact with trophozoites, presumably from disruption of the extracellular matrix (118).
ROLES OF CYSTEINE PROTEINASES IN IMMUNE SYSTEM EVASION
Degradation of Human IgA
IgA is the predominant immunoglobulin defense at the mucosal surface (reviewed in reference 123). The biologic functions of IgA are not completely understood but include immobilization and prevention of adherence of microorganisms, binding of toxins, and inhibition of antigen absorption (48, 123). Most B lymphocytes at the external mucosa are dedicated to the production of IgA, which is released into the bowel as a dimer linked by disulfide bonds and carbohydrate-rich secretory component, which is necessary for transepithelial secretion (123). IgA may play an indirect role in antibody-mediated cytotoxicity through Fcα receptors and potentiation of the action of nonspecific antibacterial factors such as lactoferrin, lactoperoxidase, and lysozyme (48).
Immune secretory IgA (sIgA) has been demonstrated in patients with invasive amebiasis by measurement of fecal (57, 106), colostral (34), and salivary (1) antibodies. Ximenez's group has shown that carriers of E. dispar also develop a salivary sIgA response (76). Both human salivary sIgA (16) and anti-amebic monoclonal IgA (53) blocked the adherence of E. histolytica trophozoites to epithelial or colonic cell monolayers.
Mucosally invasive bacteria, including Haemophilus influenzae, Neisseria gonorrhoeae, and Neisseria meningitidis, produce IgA1 proteases (31, 49, 50). All IgA1 proteinases which have been characterized are extracellular, neutral endopeptidases that cleave the hinge region of IgA1 specifically to produce Fab and Fc (72). Cleavage of IgA1 might reduce its affinity for antigen, interfere with antigen disposal, or mask immunogenic determinants by coating them with Fab fragments (48). Quezada-Calvillo et al. (75) have demonstrated limited cleavage of IgA by whole amebae. Kelsall and Ravdin (47) found that the degradation of IgA appeared to be mediated predominantly by cysteine proteinases.
Disruption of Immune IgG
A number of parasites release proteinases which cleave IgG. Trypomastigotes of T. cruzi bind IgG through the Fab fragment and then cleave the Fc fraction with cruzipain, its major cysteine proteinase (9). Schistosoma mansoni binds Igs via an Fc receptor and then degrades the Fab portion of IgG (6). Cysteine proteinases of Tritrichomonas foetus (120), Trichomonas vaginalis (73), and Fasciola hepatica (15) also degrade IgG.
A systemic IgG response develops in patients who are colonized or have invasive infection with E. histolytica, in contrast to those colonized with E. dispar (42). A protective role for IgG in amebic disease has been difficult to establish, and antibody levels correlate with the length of disease, not with the clinical response to infection (42). In animal models, a serum antibody response did not protect hamsters but SCID mice were passively protected by rabbit polyclonal immune serum (20).
We found that both intact trophozoites and the purified extracellular cysteine proteinase cleaved the heavy chain of IgG (122). When a monoclonal antibody to the surface thiol-specific antioxidant was cleaved by purified proteinase, binding to trophozoites was decreased by more than 80% (122). These results suggest that cleavage of IgG by the extracellular cysteine proteinase may limit the effectiveness of the host humoral response.
Resistance to Complement-Mediated Lysis
To invade successfully, trophozoites must be able to circumvent multiple local and systemic host defenses, including the action of activated complement proteins. Trophozoites have a carbohydrate-rich surface and were shown to consume components of both the classical and alternative pathways (13, 81). The substrate specificity of the amebic cysteine proteinases for positively charged amino acids is similar to that of the proteinases generated during complement activation. We showed that E. histolytica trophozoites activate complement by a unique mechanism, cleavage of the α-chain of C3, generating functionally active C3b (83). Activated terminal complement components are also generated, which lyse E. dispar but not E. histolytica by reactive lysis (87). E. histolytica is resistant to lysis by the membrane attack complex by virtue of the galactose-inhibitable lectin which has antigenic cross-reactivity with CD59, a membrane inhibitor of C5b-9 in human blood cells (10). This may be one mechanism by which E. dispar is confined to the lumen of the bowel by complement-mediated killing in tissues or the bloodstream. Other groups have reported that E. dispar strains are resistant to complement-mediated lysis while E. histolytica strains are susceptible (35). It is unclear whether the conflicting results are due to different culture conditions, but clinically, only E. histolytica causes invasive disease.
Degradation of Anaphylatoxins C3a and C5a
The anaphylatoxins, C3a and C5a, are potent stimulators of the host inflammatory response. C3a and C5a are generated by cleavage of the α-chains of C3 and C5 by their respective convertases. The remaining portion of each molecule, C3b and C5b, participates in activation of the late-acting components, leading to formation of the membrane attack complex (117). C3a has multiple physiologic effects, including increasing vascular permeability and smooth muscle contraction, suppressing T-cell proliferation, and releasing histamine from mast cells and interleukin-1 (IL-1) from macrophages (40). C5a induces chemotaxis of neutrophils, activates macrophages, and stimulates the release of IL-1, IL-6, and IL-8 (40). The same extracellular cysteine proteinase from E. histolytica that activates complement to produce hemolytically active C3b (83) also degrades the C3a and C5a which are subsequently formed (86). Both C3a and C5a are susceptible to proteolytic destruction of their biologic activity in a dose-dependent fashion (86). Thus, the extracellular cysteine proteinase of E. histolytica, which is capable of activating complement, may also circumvent the normal host immune response by inactivating the anaphylatoxins, C3a and C5a.
ANIMAL MODELS FOR THE STUDY OF CYSTEINE PROTEINASES
Intestinal and Hepatic Amebiasis in Gerbils and Hamsters
One of the main drawbacks of experimental amebiasis is the lack of an animal model that closely mimics human disease, in which liver abscesses develop after intestinal infection. Early stages of amebic invasion have been observed when trophozoites were injected into the ceca of gerbils (18, 107) or guinea pigs (118). Two of the most widely used models of amebic liver abscess utilize direct inoculation of trophozoites into the livers of baby gerbils or hamsters (69), (17). The size of liver abscesses can be readily quantified, and these models have been useful in studies of potential vaccine candidates, including the galactose-inhibitable lectin (71), the serine-rich E. histolytica protein (129), and the 29-kDa thiol-specific antioxidant (112) (reviewed in reference 114). In vivo models have also been used to confirm that E. dispar could not form amebic liver abscesses, even when injected directly into the liver (26). A cysteine proteinase-deficient and phagocytosis-deficient mutant of E. histolytica strain HM-1 also did not form liver abscesses (67).
Liver Abscess in SCID Mice
Stanley's group has shown that liver abscesses are formed following direct injection of E. histolytica trophozoites into the livers of SCID mice (20). They were able to decrease the size of amebic liver abscesses significantly by incubating E. histolytica trophozoites with laminin, which binds and blocks the activity of cysteine proteinases (54). Affinity-purified antibodies against a recombinant cysteine proteinase of E. histolytica localized the proteinase in amebic trophozoites and extracellularly in amebic liver abscesses of infected SCID mice (113). Pretreatment of E. histolytica trophozoites with the specific cysteine proteinase inhibitor E-64 blocked or greatly decreased the size of liver abscesses at 48 h (104). This study suggests that cysteine proteinase plays an important role in amebic liver abscess formation.
Human Intestinal Xenografts
E. histolytica infects only humans and nonhuman primates, and there has been limited success at establishing reproducible animal models of intestinal amebiasis. Intestinal xenografts provide a sterile and biologically relevant animal model system for studying host-parasite interactions, particularly the first stages of invasion in the human intestine. Human fetal intestinal tissue is engrafted into the subcutaneous space on the backs of SCID mice (104). After 8 weeks, a functional human intestine develops. Amebiasis in this model closely mimicked the pathological findings reported in cases of human amebic colitis, with an early phase of mucosal damage and subsequent invasion of amebae into submucosal tissues with the formation of amebic ulcers (104). An early neutrophil response was detected, confirming previous findings in animal models (18, 107). Stanley and coworkers also demonstrated that human intestinal epithelial cells can produce inflammatory cytokines in vivo, including upregulation of IL-1β and IL-8, in response to amebic infection (104). These results suggest that signals produced by the intestinal epithelial cells may play an important role in inducing the early host inflammatory response to infection and raise the possibility that interventions that directly target intestinal epithelial cell production of inflammatory cytokines will alter the course of disease. The human xenograft model should prove useful to study the role of cysteine proteinases early in bowel invasion.
CYSTEINE PROTEINASE INHIBITORS AS ANTIPARASITIC CHEMOTHERAPY
Natural Cysteine Proteinase Inhibitors
Cysteine proteinases of E. histolytica play crucial roles in the interactions between parasite and host, including acquisition of nutrients, facilitation of tissue invasion, and defense against immune attack. Therefore, the amebic cysteine proteinases are important targets for novel chemotherapeutic strategies. Only a few reports have identified cysteine proteinase inhibitors produced by the parasites themselves. Inhibitors of papain were detected in a variety of parasitic protozoa, including Leishmania, Trichomonas, and Trypanosoma, suggesting that cystatin-like molecules may be widespread (41). A gene encoding a cystatin-like molecule has also been found in Schistosoma mansoni (14). It seems likely that the cystatins occurring within parasites may play a role in protecting the parasite from its own cysteine proteinases, and delineation of the structure of these inhibitors should provide important information on the binding requirements of the parasite enzymes.
The report that the proregions of cysteine proteinases can inhibit the corresponding mature enzyme may also be relevant to parasite cysteine proteinases (21). In particular, analysis of the structures of the proregions of the parasite cysteine proteinases could also provide valuable information on the inhibitor specificity of the enzymes themselves and aid in the design of specific inhibitors. To protect cells from uncontrolled degradation, almost all proteinases are synthesized as inactive precursors. The N-terminal propeptide extension of these precursors facilitates folding of the mature enzyme by acting as an intramolecular chaperone, maintains proteinase stability while the enzymes are trafficked through the secretory pathway, and acts as an intrinsic inhibitor of the enzyme. The mature, active enzyme is formed following release of the proregion by autoproteolytic cleavage under acidic conditions. Recently, a number of studies of mammalian and plant cysteine proteinases have demonstrated that free propeptides are potent and highly selective inhibitors for their corresponding mature enzymes (91, 121). Moreover, the propeptides exhibited the highest inhibition selectivity for the enzyme from which they originated; for example, cathepsin L propeptide was more selective for cathepsin L than for related members of the superfamily, such as cathepsin S, and showed no inhibitory activity against cathepsin B (21, 58). Roche et al. have investigated the specificity of the propeptide of liver fluke cathepsin L proteinase for its mature cognate enzyme (91). Recombinant propeptide of Fasciola hepatica cathepsin L1 (CL1), expressed in Escherichia coli, was purified and shown to be a potent inhibitor of the mature cathepsin L1 enzyme and, to a lesser extent, of F. hepatica cathepsin L2 (CL2) (91). Taylor et al. also showed that the specificity for inhibition of plant cysteine proteinases of the papain superfamily by papain propeptide correlated with the sequence identity (121). While the mechanism of inhibition of cysteine proteinases by their propeptides is beginning to be unraveled, more molecular information that defines the structural selectively of propeptides for their parent enzymes will undoubtedly lead to the design of more potent and selective antiparasitic drugs.
E-64 and Synthetic Peptide Inhibitors
E-64 [l-trans-epoxysuccinyl-leucylamido(4-guanidino)butane)], which was isolated from Aspergillus japonicum, is a broad inhibitor of cysteine proteinases (7). It has been a useful tool in experiments to block the activity of cysteine proteinases but is limited by an inability to penetrate cells. E-64 completely blocks the cysteine proteinase activity of cultured E. histolytica trophozoites and inhibits the destruction of mammalian cell monolayers (22, 84). In recent studies with infected SCID mice, Stanley and coworkers found that preincubation of E. histolytica trophozoites with E-64 markedly reduced liver abscess formation (104). Amebic cysteine proteinases have significant structural differences from their mammalian counterparts, and it should be possible to produce highly specific nonpeptide proteinase inhibitors (E64 analogues) by computer-aided secondary-structure analysis and modeling techniques.
A number of peptidyl chloromethyl ketone, diazomethyl ketone, and fluoromethyl ketone inhibitors block parasite proteinase activity when present at micromolar concentrations (reviewed in reference 93). Studies using biotinylated derivatives of these inhibitors verified that cysteine proteinases were likely targets (127). Specific peptide inhibitors against Trypanosoma cruzi were not concentrated in mammalian lysosomes, and no morphologic changes could be detected by electron microscopy of host cells (24). We have tested a number of synthetic peptide inhibitors (made by Prototek, Richmond, Calif.) which enter cells freely and have a 50% inhibitory concentration against E. histolytica cysteine proteinases of less than 5 μM (84). The new generations of inhibitors will be useful tools to establish definitively the role of cysteine proteinases in the pathogenesis of amebiasis and may be promising candidates for antiamebic drugs.
Effect of Antisense Inhibition of Cysteine Proteinases
Another approach to blocking the expression of a specific cysteine proteinase gene is through the use of antisense RNA. Mirelman's group has generated a transfectant of E. histolytica strain HM-1 with an episomally replicating plasmid in which the transcribed ehcp5 antisense RNA strongly reduces the expression of cysteine proteinase EhCP5 and probably cross-reacts with other cysteine proteinases (4). The total cysteine proteinase activity in lysates of the transfectant was approximately 10% of the level of cysteine proteinase activity in the controls. The transfected trophozoites had significantly lower erythrophagocytosis activity than did the parental strain, but they were not impaired in their ability to destroy tissue culture monolayers (cytopathic activity) (4). The lack of an effect of antisense inhibition on cytopathology is rather surprising, since many laboratories found that cysteine proteinase activity and cytopathic effect correlated well (29, 43, 46, 84, 89). The finding that monolayer destruction by the antisense transfectants could still be blocked by E-64 and that destruction was decreased in lysates but not intact trophozoites highlights the need for further studies looking at the importance of surface versus released cysteine proteinases. The effect of antisense inhibition on production of liver abscesses was dramatic, since no abscesses were detected in hamsters injected with transfected trophozoites in contrast to 100% of untreated controls (5).
Although the effects of episomally replicated antisense transcripts were dramatic, the technique requires transfection and long-term drug selection of trophozoites. As an alternative approach to antisense inhibition, we designed phosphorothioate-modified antisense oligodeoxynucleotides (S-oligonucleotides) specific for the first 21 nucleotides of acp1. The nucleotides, mixed with liposome-mediated tranfection reagents, are stable, rapidly taken up by trophozoites, and transported to the nucleus. After incubating E. histolytica trophozoites for 48 h with 5 μM antisense S-oligonucleotides, we detected more than a 60% decrease in ACP1-specific mRNA compared to sense controls (X. Que and S. L. Reed, unpublished observations). Stable modified oligonucleotides with appropriate lipid carriers could provide an alternative approach to antisense inhibition of specific cysteine proteinases.
CONCLUSIONS
Amebiasis is a major cause of morbidity and mortality throughout the tropical and subtropical world. E. histolytica and E. dispar were originally classified as identical species because they are morphologically indistinguishable, have the same life cycle, and occupy the same niche in the bowel. However, E. dispar is not associated with disease and is noninvasive. The World Health Organization has recently declared that they should be classified as distinct species based on genetic differences of multiple genes (128). Amebic proteins potentially associated with virulence include surface antigens (88, 116), galactose-inhibitable lectin (70), phospholipases (55), and amebapores (43), but equivalent genes for each have also been detected and expressed in E. dispar. Cysteine proteinases are the only putative virulence factors for which specific genes appear to be present or overexpressed in E. histolytica and absent or unexpressed in E. dispar.
The multiple potential roles of cysteine proteinases in infection and invasion by the pathogenic ameba E. histolytica are well documented. At least five roles for cysteine proteinases can be envisioned during infection: (i) aiding attachment by degrading mucus and debris overlying the intestinal mucosa, (ii) aiding penetration of host tissue by digesting extracellular matrix, (iii) degrading host proteins to circumvent the immune response, (iv) activating host cell proteolytic cascades such as complement, and (v) aiding dissemination to produce metastatic lesions. Before bowel invasion, these cysteine proteinases degrade the host extracellular matrix and mucoproteins, dislodge epithelial cells, and degrade epithelial basement membrane (46). The enzyme released into the host bloodstream has also been proposed to contribute to pathogenesis more directly. These extracellular cysteine proteinases may also interfere with the immune response by degrading IgA (47) and IgG (122). They also activate the alternative complement pathway (83) while circumventing the inflammatory reaction by inactivating anaphylatoxins C3a and C5a (122). At least six genes have been cloned that encode typical papain-family proteins. These proteins have considerable structural homology to the cathepsin L enzymes and substrate specificity with the cathepsin B proteases (11, 84). Compared to noninvasive E. dispar, invasive E. histolytica strains expressed higher levels of cysteine proteinase-specific mRNA and released significantly greater amounts of active proteinases, both intracellularly and extracellularly. Therefore, E. dispar provides a unique comparative model to identify differences in cysteine proteinase regulation and processing in order to define their relationship to invasion and virulence. Finally, recent studies with SCID mice have firmly established the role of cysteine proteinases in the formation of amebic liver abscesses (113). The intestinal xenograft model will allow studies of early invasion of the human intestine for the first time (104). Methods to achieve stable transfection and alter levels of expression of specific cysteine proteinase genes in E. histolytica have recently been developed and should facilitate in vivo investigations of the role of each of these cysteine proteinases in virulence (4).
Because of the problems of drug toxicity and the theoretical risk of resistance, new drugs are needed for the treatment of amebiasis. Cysteine proteinases are attractive potential targets for the treatment of amebiasis because they are essential to pathogenesis. There is accumulating evidence that this approach may be successful. Protease inhibitors are now used to treat a number of diseases. Inhibitors of the human immunodeficiency virus protease have recently had an enormous impact on the treatment of AIDS. What are the best cysteine proteinase targets for inhibition? To answer this question, we must gain a better understanding of the functions of individual cysteine proteinases. The availability of recombinant enzymes should lead to more detailed studies on the inhibitor specificities of individual enzymes. Combined with detailed kinetic analysis of enzyme activity, this information should allow us to design inhibitors and substrates with high potency and specificity. Since cysteine proteinases play key roles in the pathogenesis of invasive amebiasis, their characterization should add important insights to our understanding of amebic invasion and allow us to develop new methods for the prevention, treatment, and control of intestinal and hepatic amebiasis.
ACKNOWLEDGMENTS
This work was supported in part by grants from NIH (AI-28035 and DK-35108).
We thank Charles Davis for helpful discussions.
REFERENCES
- 1.Abou-El-Magd I, Soon C G, El-Hawey A M, Ravdin J I. Humoral and mucosal IgA antibody response to a recombinant 52-kDa cysteine-rich portion of the Entamoeba histolytica galactose-inhibitable lectin correlates with detection of native 170-kDa lectin antigen in serum of patients with amebic colitis. J Infect Dis. 1966;174:157–162. doi: 10.1093/infdis/174.1.157. [DOI] [PubMed] [Google Scholar]
- 2.Acuna-Soto R, Samuelson J, De Girolami P, Zarate L, Millan-Velasco F, Schoolnick G, Wirth D. Application of the polymerase chain reaction to the epidemiology of pathogenic and nonpathogenic Entamoeba histolytica. Am J Trop Med Hyg. 1993;48:58–70. doi: 10.4269/ajtmh.1993.48.58. [DOI] [PubMed] [Google Scholar]
- 3.Allason-Jones E, Mindel A, Sargeaunt P G, Katz D. Outcome of untreated infection with Entamoeba histolytica in homosexual men with and without HIV antibody. Br Med J. 1988;297:654–657. doi: 10.1136/bmj.297.6649.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ankri S, Stolarsky T, Mirelman D. Antisense inhibition of expression of cysteine proteinases does not affect Entamoeba histolytica cytopathic or haemolytic activity but inhibits phagocytosis. Mol Microbiol. 1998;28:777–785. doi: 10.1046/j.1365-2958.1998.00837.x. [DOI] [PubMed] [Google Scholar]
- 5.Ankri S, Stolarsky T, Bracha R, Padilla-Vaca F, Mirelman D. Antisense inhibition of expression of cysteine proteinases affects Entamoeba histolytica-induced formation of liver abscess in hamsters. Infect Immun. 1999;67:421–322. doi: 10.1128/iai.67.1.421-422.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Auriault C, Ouaissi M A, Torpier G, Eisen H, Capron A. Proteolytic cleavage of IgG bound to the Fc receptor of Schistosoma mansoni schistosomula. Parasite Immunol. 1981;3:33–44. doi: 10.1111/j.1365-3024.1981.tb00383.x. [DOI] [PubMed] [Google Scholar]
- 7.Barrett A J, Kembhavi A A, Brown M A, Kirschke H, Knight C G, Tamai M, Hanada K. 1-trans-Epoxysuccinyl-leucylamido(4-guanidino)butane (E-64) and its analogues as inhibitors of cysteine proteinases including Cathepsins B, H, and L. Biochem J. 1982;201:189–198. doi: 10.1042/bj2010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrett A J, Kirschke H. Cathepsin B, cathepsin H, and cathepsin L. Methods Enzymol. 1981;80:535–561. doi: 10.1016/s0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
- 9.Bontempi E, Cazzulo J J. Digestion of human immunoglobulin G by the major cysteine proteinase (cruzipain) from Trypanosoma cruzi. FEMS Microbiol Lett. 1990;70:337–342. doi: 10.1111/j.1574-6968.1990.tb14000.x. [DOI] [PubMed] [Google Scholar]
- 10.Braga L, Ninomiya H, McCoy J J, Eacker S, Wiedmer T, Pham C, Wood S, Sims P J, Petri W A. Inhibition of the complement membrane attack complex by the galactose-specific adhesin of Entamoeba histolytica. J Clin Investig. 1992;90:1131–1137. doi: 10.1172/JCI115931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bruchhaus I, Jacobs T, Leippe M, Tannich E. Entamoeba histolytica and Entamoeba dispar: differences in numbers and expression of cysteine proteinase genes. Mol Microbiol. 1996;22:255–263. doi: 10.1046/j.1365-2958.1996.00111.x. [DOI] [PubMed] [Google Scholar]
- 12.Bruchhaus I, Tannich E. A gene highly homologous to ACP1 encoding cysteine proteinase 3 in Entamoeba histolytica is present and expressed in E. dispar. Parasitol Res. 1996;82:189–192. doi: 10.1007/s004360050093. [DOI] [PubMed] [Google Scholar]
- 13.Calderon J, Schreiber R D. Activation of the alternative and classical complement pathways by Entamoeba histolytica. Infect Immun. 1985;50:560–565. doi: 10.1128/iai.50.2.560-565.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao M, Chao H, Doughty B L. A cDNA from Schistosoma mansoni eggs sharing sequence features of mammalian cystatin. Mol Biochem Parasitol. 1993;57:175–6. doi: 10.1016/0166-6851(93)90256-w. [DOI] [PubMed] [Google Scholar]
- 15.Carmona C, Dowd A J, Smith A M, Dalton J P. Cathepsin L proteinase secreted by Fasciola hepatica in vitro prevents antibody-mediated eosinophil attachment to newly excysted juveniles. Mol Biochem Parasitol. 1993;62:9–18. doi: 10.1016/0166-6851(93)90172-t. [DOI] [PubMed] [Google Scholar]
- 16.Carrero J C, Diaz M Y, Viveros M, Espinoza B, Acosta E, Ortiz-Ortiz L. Human secretory immunoglobulin A anti-Entamoeba histolytica antibodies inhibit adherence of amebae to MDCK cells. Infect Immun. 1994;62:764–767. doi: 10.1128/iai.62.2.764-767.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chadee K, Meerovitch E. The pathogenesis of experimentally induced amebic liver abscess in the gerbil (Meriones unguiculatus) Am J Pathol. 1984;117:71–80. [PMC free article] [PubMed] [Google Scholar]
- 18.Chadee K, Meerovitch E. The pathology of experimentally induced cecal amebiasis in gerbils (Meriones unguiculatus) Am J Pathol. 1985;119:485–494. [PMC free article] [PubMed] [Google Scholar]
- 19.Chan S J, Segundo B S, McCormick M B, Steiner D F. Nucleotide and predicted amino acid sequences of cloned human and mouse preprocathepsin B cDNAs. Proc Natl Acad Sci USA. 1986;83:7721–7725. doi: 10.1073/pnas.83.20.7721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cieslak P R, Virgin H W, Stanley S L. A severe combined immunodeficient (SCID) mouse model for infection with Entamoeba histolytica. J Exp Med. 1992;176:1605–1609. doi: 10.1084/jem.176.6.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coulombe R, Grochulski P, Sivaraman J, Menard R, Mort J S, Cygler M. Structure of human procathepsin L reveals the molecular basis of inhibition by the prosegment. EMBO J. 1996;15:5492–5503. [PMC free article] [PubMed] [Google Scholar]
- 22.De Meester F, Shaw E, Scholze H, Stolarsky T, Mirelman D. Specific labeling of cysteine proteinases in pathogenic and nonpathogenic Entamoeba histolytica. Infect Immun. 1990;58:1396–1401. doi: 10.1128/iai.58.5.1396-1401.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eakin A E, Mills A A, Harth G, McKerrow J H, Craik C S. The sequence, organization, and expression of the major cysteine proteinase (cruzain) from Trypanosoma cruzi. J Biol Chem. 1992;267:7411–7420. [PubMed] [Google Scholar]
- 24.Engel J C, Doyle P S, Palmer J, Hsieh I, Bainton D F, McKerrow J H. Cysteine protease inhibitors alter Golgi complex ultrastructure and function in Trypanosoma cruzi. J Cell Sci. 1998;111:597–606. doi: 10.1242/jcs.111.5.597. [DOI] [PubMed] [Google Scholar]
- 25.Engel J C, Doyle P S, Hsieh I, McKerrow J H. Cysteine protease inhibitors cure an experimental Trypanosoma cruzi infection. J Exp Med. 1998;188:725–734. doi: 10.1084/jem.188.4.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Espinosa M, Castanon G, Martinez-Palomo A. In vivo pathogenesis of Entamoeba dispar. Arch Investig Med. 1997;28:S204–S206. [PubMed] [Google Scholar]
- 27.Forney J R, Yang S, Healey M C. Protease activity associated with excystation of Cryptosporidium parvum oocysts. J Parasitol. 1996;82:889–892. [PubMed] [Google Scholar]
- 28.Franke de Cazzulo B M, Martinez J, North M, Coombs G H, Cazzulo J J. Effects of proteinase inhibitors on the growth and differentiation of Trypanosoma cruzi. FEMS Microbiol Lett. 1994;124:81–86. doi: 10.1111/j.1574-6968.1994.tb07265.x. [DOI] [PubMed] [Google Scholar]
- 29.Garcia-Rivera G, Rodriguez M A, Ocadiz R, Martinez-Lopez M C, Arroyo R, Gonzalez-Robles A, Orozco E. Entamoeba histolytica: a novel cysteine protease and an adhesin form the 112 kDa surface protein. Mol Microbiol. 1999;33:556–568. doi: 10.1046/j.1365-2958.1999.01500.x. [DOI] [PubMed] [Google Scholar]
- 30.Gathiram V, Jackson T F H G. A longitudinal study of asymptomatic carriers of pathogenic zymodemes of Entamoeba histolytica. S Afr Med J. 1987;72:669–672. [PubMed] [Google Scholar]
- 31.Genco R J, Plaut A G, Moellering R C. Evaluation of human oral organisms and pathogenic Streptococcus for production of IgA protease. J Infect Dis. 1975;131(Suppl. 1):s17–s21. doi: 10.1093/infdis/131.supplement.s17. [DOI] [PubMed] [Google Scholar]
- 32.Ghosh S K, Field J, Frisardi M, Rosenthal B, Mia Z, Rogers R, Samuelson J. Chitinase secretion by encysting Entamoeba invadens and transfected Entamoeba histolytica trophozoites: localization of secretory vesicles, endoplasmic reticulum, and Golgi apparatus. Infect Immun. 1999;67:3073–3081. doi: 10.1128/iai.67.6.3073-3081.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Griffiths G, Hoflack B, Simmons K, Mellman I, Kornfeld S. The mannose 6-phosphate receptor and the biogenesis of lysosomes. Cell. 1988;52:329–341. doi: 10.1016/s0092-8674(88)80026-6. [DOI] [PubMed] [Google Scholar]
- 34.Grundy M S, Cartwright-Taylor L, Lundin L. Antibodies against Entamoeba histolytica in human milk and serum in Kenya. J Clin Microbiol. 1983;17:753–758. doi: 10.1128/jcm.17.5.753-758.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamelmann C, Foerster B, Burchard G D, Horstmann R D. Lysis of pathogenic and nonpathogenic Entamoeba histolytica by human complement: methodological analysis. Parasite Immunol. 1992;14:23–35. doi: 10.1111/j.1365-3024.1992.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 36.Haque R, Neville L, Hahn P, Petri W A. Rapid diagnosis of Entamoeba infection by using Entamoeba and Entamoeba histolytica stool antigen detection kits. J Clin Microbiol. 1995;33:2558–2561. doi: 10.1128/jcm.33.10.2558-2561.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Haque R, Ali I K M, Akther S, Petri W A. Comparison of PCR, isoenzyme analysis, and antigen detection for diagnosis of Entamoeba histolytica infection. J Clin Microbiol. 1998;36:449–452. doi: 10.1128/jcm.36.2.449-452.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harth G, Andrews N, Mills A A, Engel J C, Smith R, McKerrow J H. Peptide-fluoromethyl ketones arrest intracellular replication and intercellular transmission of Trypanosoma cruzi. Mol Biochem Parasitol. 1993;58:17–24. doi: 10.1016/0166-6851(93)90086-d. [DOI] [PubMed] [Google Scholar]
- 39.Huete-Perez J A, Engel J C, Brinen L S, Mottram J C, McKerrow J H. Protease trafficking in two primitive eukaryotes is mediated by a prodomain protein motif. J Biol Chem. 1999;274:16249–16256. doi: 10.1074/jbc.274.23.16249. [DOI] [PubMed] [Google Scholar]
- 40.Hugli T E. Biochemistry and biology of anaphylatoxins. Complement. 1986;3:111–127. doi: 10.1159/000467889. [DOI] [PubMed] [Google Scholar]
- 41.Irvine J W, Coombs G H, North M J. Cystatin-like cysteine proteinase inhibitors of parasitic protozoa. FEMS Microbiol Lett. 1992;75:67–72. doi: 10.1016/0378-1097(92)90458-z. [DOI] [PubMed] [Google Scholar]
- 42.Jackson T F H G, Gathiram V, Simjee A E. Seroepidemiological study of antibody responses to the zymodemes of Entamoeba histolytica. Lancet. 1985;ii:716–719. doi: 10.1016/s0140-6736(85)91262-0. [DOI] [PubMed] [Google Scholar]
- 43.Jacobs T, Bruchhaus I, Dandekar T, Tannich E, Leippe M. Isolation and molecular characterization of a surface-bound proteinase of Entamoeba histolytica. Mol Microbiol. 1998;27:269–276. doi: 10.1046/j.1365-2958.1998.00662.x. [DOI] [PubMed] [Google Scholar]
- 44.Karrer K M, Peiffer S L, DiTomas M E. Two distinct gene subfamilies within the family of cystiene proteinase genes. Proc Natl Acad Sci USA. 1993;90:3063–3067. doi: 10.1073/pnas.90.7.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Keene W E, Hidalgo M E, Orozco E, McKerrow J H. Entamoeba histolytica: correlation of the cytopathic effect of virulent trophozoites with secretion of a cysteine proteinase. Exp Parasitol. 1990;71:199–206. doi: 10.1016/0014-4894(90)90022-5. [DOI] [PubMed] [Google Scholar]
- 46.Keene W E, Pettit M G, Allen S, McKerrow J H. The major neutral proteinase of Entamoeba histolytica. J Exp Med. 1986;163:536–549. doi: 10.1084/jem.163.3.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kelsall B L, Ravdin J I. Degradation of human immunoglobulin A by Entamoeba histolytica. J Infect Dis. 1993;168:1319–1322. doi: 10.1093/infdis/168.5.1319. [DOI] [PubMed] [Google Scholar]
- 48.Kilian M, Mestecky J, Russell M W. Defense mechanisms involving Fc-dependent functions of immunoglobulin A and their subversion by bacterial Immunoglobulin A proteases. Microbiol Rev. 1988;52:296–303. doi: 10.1128/mr.52.2.296-303.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kilian M, Mestecky J, Kulhavy R, Tomana M, Butler W T. IgA1 proteases from Haemophilus influenzae, Streptococcus pneumoniae, Neisseria meningitidis, and Streptococcus sanguis: comparative immunochemical studies. J Immunol. 1980;124:2596–2600. [PubMed] [Google Scholar]
- 50.Kornfeld S J, Plaut A G. Secretory immunity and the bacterial IgA proteases. Rev Infect Dis. 1981;3:521–534. doi: 10.1093/clinids/3.3.521. [DOI] [PubMed] [Google Scholar]
- 51.Leippe M, Sebastian E, Schoenberger O L, Horstmann R D, Muller-Eberhard H J. Pore-forming peptide of pathogenic Entamoeba histolytica. Proc Natl Acad Sci USA. 1991;88:7659–7663. doi: 10.1073/pnas.88.17.7659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Leon G, Fiori C, Das P, Moreno M, Tovar R, Sanchez-Salas J L, de Lourdes Munoz M. Electron probe analysis and biochemical characterization of electron-dense granules secreted by Entamoeba histolytica. Mol Biochem Parasitol. 1997;85:223–242. doi: 10.1016/s0166-6851(97)02833-8. [DOI] [PubMed] [Google Scholar]
- 53.Levya O, Rico G, Ramos F, Moran P, Melendro E I, Ximenez C. Entamoeba histolytica adherence: inhibition by IgA monoclonal antibodies. In: Mestecky J, editor. Advances in mucosal immunology. New York, N.Y: Plenum Press; 1995. pp. 681–683. [DOI] [PubMed] [Google Scholar]
- 54.Li E, Yang W-G, Zhang T, Stanley S L. Interaction of laminin with Entamoeba histolytica cysteine proteinases and its effect on amebic pathogenesis. Infect Immun. 1995;63:4150–4153. doi: 10.1128/iai.63.10.4150-4153.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Long-Krug S A, Fischer K J, Hysmith R M, Ravdin J I. Phospholipase A enzymes of Entamoeba histolytica: description and subcellular localization. J Infect Dis. 1985;152:536–541. doi: 10.1093/infdis/152.3.536. [DOI] [PubMed] [Google Scholar]
- 56.Luaces A L, Barrett A J. Affinity purification and biochemical characterization of histolysin, the major cysteine proteinase of Entamoeba histolytica. Biochem J. 1988;250:903–909. doi: 10.1042/bj2500903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mahajan R C, Agarwal S C, Chhuttani P N, Chitkara N L. Coproantibodies in intestinal amoebiasis. Ind J Med Res. 1972;60:547–550. [PubMed] [Google Scholar]
- 58.Maubach G, Schilling K, Rommerskirch W, Wenz I, Schultz J E, Weber E, Wiederanders B. The inhibition of cathepsin S by its propeptide-specificity and mechanism of action. Eur J Biochem. 1997;250:745–750. doi: 10.1111/j.1432-1033.1997.00745.x. [DOI] [PubMed] [Google Scholar]
- 59.Mayer R, Picard I, Lawton P, Grellier P, Barrault C, Monsigny M, and Schrevel J. Peptide derivatives specific for a Plasmodium falciparum proteinase inhibit the human erythrocyte invasion by merozoites. J Med Chem. 1991;34:3029–3035. doi: 10.1021/jm00114a011. [DOI] [PubMed] [Google Scholar]
- 60.Mazzuco A, Benchimol M, DeSouza W. Endoplasmic reticulum and Golgi-like elements in Entamoeba. Micron. 1997;28:241–247. doi: 10.1016/s0968-4328(97)00024-3. [DOI] [PubMed] [Google Scholar]
- 61.McIntyre G F, Godbold G D, Erickson A H. The pH-dependent membrane association of procathepsin L is mediated by a 9-residue sequence within the propeptide. J Biol Chem. 1994;269:567–572. [PubMed] [Google Scholar]
- 62.Mirelman D, Nucharnowitz Y, Bohm G B, Walderich B. A homologue of the cysteine proteinase gene (ACP1 or Eh-CPp3) of pathogenic Entamoeba histolytica is present in non-pathogenic E. dispar strains. Mol Biochem Parasitol. 1996;78:47–54. doi: 10.1016/s0166-6851(96)02603-5. [DOI] [PubMed] [Google Scholar]
- 63.Mort J S, Leduc M S, Recklies A D. Characterization of a latent cysteine proteinase from ascitic fluid as a high molecular weight form of cathepsin B. Biochim Biophys Acta. 1983;755:369–375. doi: 10.1016/0304-4165(83)90240-4. [DOI] [PubMed] [Google Scholar]
- 64.Mort J S, Leduc M, Recklies A D. A latent thiol proteinase from ascitic fluid of patients with neoplasia. Biochim Biophys Acta. 1981;662:173–180. doi: 10.1016/0005-2744(81)90027-9. [DOI] [PubMed] [Google Scholar]
- 65.Munoz M, Lamoyi E, Leon G, Tovar R, Perez-Garcia J, de la Torre M, Murueta E, Bernal R M. Antigens in electron-dense granules from Entamoeba histolytica as possible markers for pathogenicity. J Clin Microbiol. 1990;2418:2424. doi: 10.1128/jcm.28.11.2418-2424.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Navarro-Garcia F, Chavez-Duenas L, Tsutsumi V, Posadas del Rio F, Lopez-Revilla R. Entamoeba histolytica: increase of enterotoxicity and of 53- and 75-kDa cysteine proteinases in a clone of higher virulence. Exp Parasitol. 1995;80:361–372. doi: 10.1006/expr.1995.1048. [DOI] [PubMed] [Google Scholar]
- 67.Orozco E, Guarneros G, Martinez-Palomo A, Sanchez T. Entamoeba histolytica phagocytosis as a virulence factor. J Exp Med. 1983;158:1511–1521. doi: 10.1084/jem.158.5.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pamer E G, So M, Davis C E. Identification of a developmentally regulated cysteine protease of Trypanosoma brucei. Mol Biochem Parasitol. 1989;33:27–32. doi: 10.1016/0166-6851(89)90038-8. [DOI] [PubMed] [Google Scholar]
- 69.Perez-Tamayo R, Martinez R D, Montfort I, Becker I, Tello E, Perez-Montfort R. Pathogenesis of acute experimental amebic liver abscess in hamsters. J Parasitol. 1991;77:982–988. [PubMed] [Google Scholar]
- 70.Petri W A, Smith R D, Schlesinger P H, Murphy C F, Ravdin J I. Isolation of the galactose-binding lectin that mediates the in vitro adherence of Entamoeba histolytica. J Clin Investig. 1987;80:1238–1244. doi: 10.1172/JCI113198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Petri W A, Ravdin J I. Protection of gerbils from amebic liver abscess by immunization with the galactose-specific adherence lectin of Entamoeba histolytica. Infect Immun. 1990;59:97–101. doi: 10.1128/iai.59.1.97-101.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71a.Petri W A, Singh U. Diagnosis and management of amebiasis. Clin Infect Dis. 1999;29:11117–11125. doi: 10.1086/313493. [DOI] [PubMed] [Google Scholar]
- 72.Plaut A G. The IgA1 proteases of pathogenic bacteria. Annu Rev Microbiol. 1983;37:603–622. doi: 10.1146/annurev.mi.37.100183.003131. [DOI] [PubMed] [Google Scholar]
- 73.Provenzano D, Alderete J F. Analysis of human immunoglobulin-degrading cysteine proteinases of Trichomonas vaginalis. Infect Immun. 1995;63:3388–3395. doi: 10.1128/iai.63.9.3388-3395.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Que X, Reed S L. The role of extracellular cysteine proteinases in pathogenesis of Entamoeba histolytica invasion. Parasitol Today. 1997;13:190–194. doi: 10.1016/s0169-4758(97)01043-0. [DOI] [PubMed] [Google Scholar]
- 75.Quezada-Calvillo R, Lopez-Revilla R. IgA protease in Entamoeba histolytica trophozoites. Adv Exp Med Biol. 1986;216B:1283–1288. [PubMed] [Google Scholar]
- 76.Ramos F, Valenzuela O, Moran P, Gonzalez E, Ramiro M, Cedillo R, Martinez M, Gomez A, Munoz O, Melendro E, Ximenez C. Anti-E. histolytica IgA antibodies in saliva of E. histolytica or E. dispar infected individuals: longitudinal study of cohorts. Arch Med Res. 1997;28:S327–S329. [PubMed] [Google Scholar]
- 77.Ramos M A, Stock R P, Sanchez-Lopez, Rosana O, Felipe L, Paul A, and Alejandro M. The Entamoeba histolytica proteosome α-subunit gene. Mol Biochem Parasitol. 1997;84:131–135. doi: 10.1016/s0166-6851(96)02770-3. [DOI] [PubMed] [Google Scholar]
- 78.Ravdin J I, Schlesinger P H, Murphy C F, Gluzman I Y, Krogstad D J. Acid intracellular vesicles and the cytolysis of mammalian target cells by Entamoeba histolytica trophozoites. J Protozool. 1986;33:478–486. doi: 10.1111/j.1550-7408.1986.tb05646.x. [DOI] [PubMed] [Google Scholar]
- 79.Ravdin J I. Amebiasis. Clin Infect Dis. 1995;20:1453–1464. doi: 10.1093/clinids/20.6.1453. [DOI] [PubMed] [Google Scholar]
- 80.Recklies A D, Mort J S. Characterization of a cysteine proteinase secreted by mouse mammary gland. Cancer Res. 1985;45:2302–2307. [PubMed] [Google Scholar]
- 81.Reed S L, Curd J G, Gigli I, Gillin F D, Braude A I. Activation of complement by pathogenic and nonpathogenic Entamoeba histolytica. J Immunol. 1986;136:2265–2270. [PubMed] [Google Scholar]
- 82.Reed S L. Amebiasis: an update. Clin Infect Dis. 1992;14:385–393. doi: 10.1093/clinids/14.2.385. [DOI] [PubMed] [Google Scholar]
- 82a.Reed S L. New concepts regarding the pathogenesis of amebiasis. Clin Infect Dis. 1995;2(Suppl. 2):S182–S185. doi: 10.1093/clinids/21.supplement_2.s182. [DOI] [PubMed] [Google Scholar]
- 83.Reed S L, Keene W E, McKerrow J H, Gigli I. Cleavage of C3 by a neutral cysteine proteinase of Entamoeba histolytica. J Immunol. 1989;143:189–195. [PubMed] [Google Scholar]
- 84.Reed S L, Bouvier J, Pollack A S, Engel J C, Brown M, Hirata K, Que X, Eakin A, Hagblom P, Gillin F D, McKerrow J H. Cloning of a virulence factor of Entamoeba histolytica: pathogenic strains possess a unique cysteine proteinase gene. J Clin Investig. 1993;91:1532–1540. doi: 10.1172/JCI116359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Reed S L, Wessel D W, Davis C E. Entamoeba histolytica infection and AIDS. Am J Med. 1991;90:269–270. [PubMed] [Google Scholar]
- 86.Reed S L, Ember J A, Herdman D S, DiScipio R G, Hugli T E, Gigli I. The extracellular neutral cysteine proteinase of Entamoeba histolytica degrades anaphylatoxins C3a and C5a. J Immunol. 1995;155:266–274. [PubMed] [Google Scholar]
- 87.Reed S L, Gigli I. Lysis of complement-sensitive Entamoeba histolytica by activated terminal complement components. Initiation of complement activation by an extracellular neutral cysteine proteinase. J Clin Investig. 1990;86:1815–1822. doi: 10.1172/JCI114911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Reed S L, Flores B M, Batzer M A, Stein M A, Stroeher V L, Carlton J E, Diedrich D L, Torian B E. Molecular and cellular characterization of the 29-kDa peripheral membrane protein of Entamoeba histolytica: differentiation between pathogenic and nonpathogenic isolates. Infect Immun. 1992;60:542–549. doi: 10.1128/iai.60.2.542-549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Reed S L, Keene W E, McKerrow J H. Thiol proteinase expression correlates with pathogenicity of Entamoeba histolytica. J Clin Microbiol. 1989;27:2772–2777. doi: 10.1128/jcm.27.12.2772-2777.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Robertson C D, Coombs G H, North M J, Mottram J C. Parasite cysteine proteinases. Perspect Drug Discov Des. 1996;6:99–118. [Google Scholar]
- 91.Roche L, Tort J, Dalton J P. The propeptide of Fasciola hepatica cathepsin L is a potent and selective inhibitor of the mature enzyme. Mol Biochem Parasitol. 1999;98:271–277. doi: 10.1016/s0166-6851(98)00164-9. [DOI] [PubMed] [Google Scholar]
- 92.Rosenthal P J, McKerrow J H, Aikawa M, Nagasawa H, Leech J H. A malarial cysteine proteinase is necessary for hemoglobin degradation by Plasmodium falciparum. J Clin Investig. 1988;82:1560–1566. doi: 10.1172/JCI113766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rosenthal P J. Proteases of protozoan parasites. Adv Parasitol. 1999;43:106–139. doi: 10.1016/s0065-308x(08)60242-0. [DOI] [PubMed] [Google Scholar]
- 94.Rozhin J, Robinson D, Stevens M A, Lah T T, Honn K V, Ryan R E, Sloane B F. Properties of a plasma membrane-associated cathepsin B-like cysteine proteinase in metastatic B16 melanoma variants. Cancer Res. 1987;47:6620–6628. [PubMed] [Google Scholar]
- 95.Salas F, Fichmann J, Lee G K, Scott M D, Rosenthal P J. Functional expression of falcipain, a Plasmodium falciparum cysteine proteinase, supports its role as a malarial hemoglobinase. Infect Immun. 1995;63:2120–2125. doi: 10.1128/iai.63.6.2120-2125.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sanchez-Lopez R, Gama-Castro S, Ramos M A, Merino E, Lizardi P M, Alagon A. Cloning and expression of the Entamoeba histolytica ERD2 gene. Mol Biochem Parasitol. 1998;92:355–359. doi: 10.1016/s0166-6851(98)00017-6. [DOI] [PubMed] [Google Scholar]
- 97.Sargeaunt P G, Williams J E, Greene J D. The differentiation of invasive and noninvasive Entamoeba histolytica by isoenzyme electrophoresis. Trans R Soc Trop Med Hyg. 1978;72:519–521. doi: 10.1016/0035-9203(78)90174-8. [DOI] [PubMed] [Google Scholar]
- 98.Scheidt K A, Roush W R, McKerrow J H, Selzer P M, Hansell E, Rosenthal P J. Structure-based design, synthesis and evaluation of conformationally constrained cysteine protease inhibitors. Bioorg Med Chem. 1998;6:2477–2494. doi: 10.1016/s0968-0896(98)80022-9. [DOI] [PubMed] [Google Scholar]
- 99.Scholze H, Schulte W. On the specificity of a cysteine proteinase from Entamoeba histolytica. Biomed Biochim Acta. 1988;47:115–123. [PubMed] [Google Scholar]
- 100.Scholze H, Lohden-Bendinger U, Muller G, Bakker-Grunwald T. Subcellular distribution of amebapain, the major cysteine proteinase of Entamoeba histolytica. Arch Med Res. 1992;23:105–108. [PubMed] [Google Scholar]
- 101.Scholze H, Frey S, Cejka Z, Bakker-Grunwald T. Evidence for the existence of both proteasomes and a novel high molecular weight peptidase in Entamoeba histolytica. J Biol Chem. 1996;271:6212–6216. doi: 10.1074/jbc.271.11.6212. [DOI] [PubMed] [Google Scholar]
- 102.Selzer P M, Chen X, Chan V J, Cheng M, Kenyon G L, Kuntz I D, Sakanari J A, Cohen F E, McKerrow J H. Leishmania major: molecular modeling of cysteine proteases and prediction of new nonpeptide inhibitors. Exp Parasitol. 1997;87:212–221. doi: 10.1006/expr.1997.4220. [DOI] [PubMed] [Google Scholar]
- 103.Serrano J J, de la Garza M, Moreno M A, Tovar R, Leon G, Tsutsumi V, de Lourdes Munoz M. Entamoeba histolytica: electron-dense granule secretion, collagenase activity and virulence are altered in the cytoskeleton mutant BG-3. Mol Microbiol. 1994;11:787–792. doi: 10.1111/j.1365-2958.1994.tb00356.x. [DOI] [PubMed] [Google Scholar]
- 104.Seydel K B, Li E, Swanson P E, Stanley S L. Human intestinal epithelial cells produce proinflammatory cytokines in response to infection in a SCID mouse-human intestinal xenograft model of amebiasis. Infect Immun. 1997;65:1631–1639. doi: 10.1128/iai.65.5.1631-1639.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sharma M, Hirata K, Herdman S, Reed S. Entamoeba invadens: characterization of cysteine proteinases. Exp Parasitol. 1996;84:84–91. doi: 10.1006/expr.1996.0092. [DOI] [PubMed] [Google Scholar]
- 106.Sharma P, Das P, Dutta G P. Use of glutaraldehyde-treated sheep erythrocytes in indirect haemagglutination test for amoebic coproantibody. Indian J Med Res. 1981;74:215–218. [PubMed] [Google Scholar]
- 107.Shibayama M, Navarro-Garcia F, Lopez-Revilla R, Martinez-Palomo A, Tsutumi V. In vivo and in vitro experimental intestinal amebiasis in Mongolian gerbils (Meriones unguiculatus) Parasitol Res. 1997;83:170–176. doi: 10.1007/s004360050228. [DOI] [PubMed] [Google Scholar]
- 108.Sloane B F, Moin K, Krepela E, Rozhin J. Cathepsin B and its endogenous inhibitors: the role in tumor malignancy. Cancer Metastasis Rev. 1990;9:333–352. doi: 10.1007/BF00049523. [DOI] [PubMed] [Google Scholar]
- 109.Sloane B F, Rozhin J, Johnson K, Taylor H, Crissman J D, Honn K V. Cathepsin B: association with plasma membrane in metastatic tumors. Proc Natl Acad Sci USA. 1986;83:2483–2487. doi: 10.1073/pnas.83.8.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sloane B F, Dunn J R, Honn K V. Lysosomal cathepsin B: correlation with metastatic potential. Science. 1981;212:1151–1153. doi: 10.1126/science.7233209. [DOI] [PubMed] [Google Scholar]
- 111.Smith S M, Gottesman M M. Activity and deletion analysis of recombinant human cathepsin L expressed in Escherichia coli. J Biol Chem. 1989;264:20487–20495. [PubMed] [Google Scholar]
- 112.Soong C G, Torian B E, Abd-Alla M D, Jackson T F H G, Gatharim V, Ravdin J I. Protection of gerbils from amebic liver abscess by immunization with recombinant Entamoeba histolytica 29-kilodalton antigen. Infect Immun. 1995;63:472–377. doi: 10.1128/iai.63.2.472-477.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Stanley S L, Zhang T, Rubin D, Li E. Role of the Entamoeba histolytica cysteine proteinase in amebic liver abscess formation in severe combined immunodeficient mice. Infect Immun. 1995;63:1587–1589. doi: 10.1128/iai.63.4.1587-1590.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Stanley S L. Progress towards development of a vaccine for amebiasis. Clin Microbiol Rev. 1997;10:637–649. doi: 10.1128/cmr.10.4.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Strachan W D, Spice W M, Chiodini P L, Moody A H, Ackers J P. Immunological differentiation of pathogenic and non-pathogenic isolates of Entamoeba histolytica. Lancet. 1988;ii:561–563. doi: 10.1016/s0140-6736(88)91355-4. [DOI] [PubMed] [Google Scholar]
- 116.Tachibana H, Ihara S, Kobayashi S, Kaneda Y, Takeuchi T, Watanabe Y. Differences in genomic DNA sequences between pathogenic and nonpathogenic isolates of Entamoeba histolytica identified by polymerase chain reaction. J Clin Microbiol. 1991;29:2234–2239. doi: 10.1128/jcm.29.10.2234-2239.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tack B F, Janatova J, Thomas M L, Harrison R A, Hammer C H. The third, fourth, and fifth components of human complement: isolation and biochemical properties. Methods Enzymol. 1981;80:64–101. [Google Scholar]
- 118.Takeuchi A, Phillips B P. Electron microscope studies of experimental Entamoeba histolytica infections in the guinea pig. I. Penetration of the intestinal epithelium by trophozoites. Am J Trop Med Hyg. 1975;24:34–48. doi: 10.4269/ajtmh.1975.24.34. [DOI] [PubMed] [Google Scholar]
- 119.Talamas-Rohana P, Meza I. Interaction between pathogenic amebas and fibronectin: substrate degradation and changes in cytoskeleton organization. J Cell Biol. 1988;106:1787–1794. doi: 10.1083/jcb.106.5.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Talbot J A, Nielsen K, Corbeil L B. Cleavage of proteins of reproductive secretions by extracellular proteinases of Tritrichomonas foetus. Can J Microbiol. 1991;37:384–390. doi: 10.1139/m91-062. [DOI] [PubMed] [Google Scholar]
- 121.Taylor M A, Baker K C, Briggs G S, Connerton I F, Cummings N J, Pratt K A, Revell D F, Freedman R B, Goodenough P T. Recombinant pro-regions from papain and papaya proteinase IV are selective high affinity inhibitors of the mature papaya enzymes. Protein Eng. 1995;8:59–62. doi: 10.1093/protein/8.1.59. [DOI] [PubMed] [Google Scholar]
- 122.Tran V Q, Herdman D S, Torian B E, Reed S L. The neutral cysteine proteinase of Entamoeba histolytica degrades IgG and prevents its binding. J Infect Dis. 1998;177:508–511. doi: 10.1086/517388. [DOI] [PubMed] [Google Scholar]
- 123.Underdown B J, Schiff J M. Immunoglobulin A: strategic defense initiative at the mucosal surface. Annu Rev Immunol. 1986;4:389–417. doi: 10.1146/annurev.iy.04.040186.002133. [DOI] [PubMed] [Google Scholar]
- 124.Vazquez J, Franco E, Reyes G, Meza I. Characterization of adhesion plates induced by the interaction of Entamoeba histolytica trophozoites with fibronectin. Cell Motil Cytoskeleton. 1995;32:37–45. doi: 10.1002/cm.970320105. [DOI] [PubMed] [Google Scholar]
- 125.von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986;14:4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Walsh J A. Prevelence of Entamoeba histolytica infection. In: Ravdin J I, editor. Amebiasis. Human infection by Entamoeba histolytica. New York, N.Y: John Wiley & Sons, Inc.; 1988. pp. 93–105. [Google Scholar]
- 127.Ward W, Alvarado L, Rawlings N D, Engel J C, Franklin C, McKerrow J H. A primitive enzyme for a primitive cell: the protease required for excystation of Giardia. Cell. 1997;89:1–8. doi: 10.1016/s0092-8674(00)80224-x. [DOI] [PubMed] [Google Scholar]
- 128.World Health Organization/Pan America Health Organization Expert Consultation on Amoebiasis. Amoebiasis. W H O Weekly Epidem Rec. 1997;72:97–100. [Google Scholar]
- 129.Zhang T, Cieslak P R, Stanley S L. Protection of gerbils from amebic liver abscess by immunization with a recombinant Entamoeba histolytica antigen. Infect Immun. 1994;62:1166–1170. doi: 10.1128/iai.62.4.1166-1170.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]