Abstract
Physical dysfunction after discharge from the intensive care unit (ICU) is recognized as a common complication among ICU patients. Early mobilization (EM), defined as the ability to sit on the edge of the bed within 5 days, may help improve physical dysfunction. However, the barriers to, and achievement of, EM and their impact on physical dysfunction have not been fully investigated. This study aimed to investigate the achievement of EM and barriers to it and their impact on patient outcomes in mechanically ventilated ICU patients. We conducted this multicenter retrospective cohort study by collecting data from six ICUs in Japan. Consecutive patients who were admitted to the ICU between April 2019 and March 2020, were aged ≥ 18 years, and received mechanical ventilation for > 48 h were eligible. The primary outcome was the rate of independent activities of daily living (ADL), defined as a score ≥ 70 on the Barthel index at hospital discharge. Daily changes in barriers of mobilization, including consciousness, respiratory, circulatory, medical staff factors, and device factors (catheter, drain, and dialysis), along with the clinical outcomes were investigated. The association among barriers, mobilization, and Barthel index ≥ 70 was analyzed using multivariable logistic regression analysis. During the study period, 206 patients were enrolled. EM was achieved in 116 patients (68%) on the fifth ICU day. The primary outcome revealed that achieving EM was associated with a Barthel index ≥ 70 at hospital discharge [adjusted odds ratio (AOR), 3.44; 95% confidence interval (CI), 1.70–6.96]. Device factors (AOR, 0.31; 95% CI, 0.13–0.75, respectively) were significantly associated with EM achievement. EM was associated with independent ADL at hospital discharge. Time to first mobilization and barriers to achieving mobilization can be important parameters for achieving ADL independence at discharge. Further research is required to determine the most common barriers so that they can be identified and removed.
Subject terms: Disease prevention, Therapeutics, Geriatrics, Risk factors
Introduction
Dramatic developments and improvements in the technology, equipment, and educational systems used in intensive care units (ICU) have reduced mortality among critically ill patients over the past four decades1. However, the proportion of patients with severe physical disorders has also increased concomitantly2. Physical disability occurs in 40–70% of ICU survivors3–5 and can last for several months or years after hospital discharge6. Critically ill patients admitted to the ICU have poor general conditions and tend to be immobilized, especially with mechanical ventilation management7. Independence in activities of daily living (ADL) is considered one of the most important factors for returning home after an ICU stay8,9. Active physical rehabilitation during ICU stays, especially when initiated within the first 72 h, is recommended to prevent physical disabilities and improve the clinical outcomes of ICU patients10.
Previous studies have shown that initiating early mobilization (EM) after ICU admission reduces the incidence of ICU-acquired weakness (ICU-AW) and delirium4,11, length of ICU and hospital stays12,13, duration of mechanical ventilation4,14, and medical costs15,16 while improving quality of life17. Other studies have revealed that achieving mobilization, such as sitting on the edge of the bed, standing, or walking early during the ICU stay, may improve outcomes4,11–15,18. For example, delays in mobilization for > 5 days after admission to the ICU can be detrimental16. Therefore, it is necessary to develop an efficient method for achieving EM in ICU patients. Notably, the term “mobilization” indicates the ability to sit on the edge of the bed, and “EM” indicates the time to achieve mobilization within 5 days of ICU admission. These definitions were based on previous studies16,19.
EM in critically ill patients is expected to have many effects, but there are several barriers to its actual implementation20,21. The main barriers shown in previous studies are deep sedation, a lack of coordination with rehabilitation-related professionals and other rehabilitation staff and team leaders, and a lack of understanding of the benefits and knowledge of early rehabilitation20–23. Therefore, even if the sedative management is of good quality, it is difficult to conduct effective rehabilitation20–23. However, improving the barriers and achieving EM can improve the physical function of patients and shorten the length of stay in the ICU and hospital24.
EM benefits have not been fully evaluated in terms of day-to-day changes in barriers to the implementation of EM, especially in multicenter studies25. A multicenter study is warranted to reduce any bias generated by particular features of any one research institute and the likely unique background of its patients. Investigating changes in the rate of mobilization and associated barriers simultaneously may guide planning rehabilitation, allowing these patients to achieve EM and prevent delays in initiating EM. In this study, we hypothesized that achieving EM in the ICU would improve patient outcomes. The primary objective of this multicenter study was to investigate the association between achieving EM in the ICU and ADL independence at hospital discharge. The secondary objective of this multicenter study was to identify barriers to the mobilization of patients and assess the association of barriers to EM on a day-to-day basis.
Methods
Study design and setting
The medical records of patients admitted to the ICU of one of six Japanese tertiary hospitals between April 2019 and March 2020 were retrospectively reviewed. All these were mixed medical-surgical ICUs. This multicenter retrospective cohort study was conducted at the Nagoya Medical Center and five other participating hospitals (Tosei General Hospital, Kainan Hospital, Itinomiyanishi Hospital, Toyohashi Municipal Hospital, and Shizuoka Medical Center). Detailed characteristics of the institutions are listed in Supplementary Table 1.
We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines26 and all methods in this study were performed following the relevant guidelines and regulations. ICU patients who received mechanical ventilation for ≥ 48 h were screened for inclusion. The exclusion criteria were as follows: patients aged < 18 years, unable to walk independently before hospitalization3, neurologically impaired, incapable of communicating in Japanese, with a condition that limits mobilization, with a terminal/end-of-life status, or who died during ICU stay (Supplementary Table 2).
Early mobilization protocol
This study’s early goal-directed rehabilitation protocol3,10,13,15,16,25 was developed > 6 months prior to study initiation, and the details of the protocol were specifically arranged according to the participating hospital. This protocol has been used in routine practice in multiple centers, and validation of the protocol’s safety has already been reported27. In this study, we sought to mobilize all patients equally and daily under the protocol tailored to each participating hospital. Details of the EM protocol are presented in Supplementary Table 3. The protocol includes five rehabilitation levels (level 1, passive range of motion and respiratory physical therapy; level 2, active range of motion; level 3, sitting exercise; level 4, standing exercise; and level 5, walking exercise) based on the patient’s medical condition. At each participating site, ICU physicians or physiotherapists referred to the protocol and decided each patient’s rehabilitation level based on the patient’s condition. All patients received at least one rehabilitation session per weekday for 20 min by a physical therapist. In addition, for weekend rehabilitation, at least one 20-min rehabilitation session was performed by the attending nurse using a protocol similar to that for the weekdays. The target number of implementation units and frequency were determined by each hospital based on the patient's condition.
All participating hospitals followed the 2018 Clinical Practice Guidelines28 and the clinical practice guidelines for the management of acute respiratory distress syndrome29. The former concerns the management of pain, agitation, and delirium in adult patients in the ICU, and the latter concerns ventilator settings and drug therapy. There was no difference in weekend medical treatment among the participating hospitals. After ICU discharge, physical or occupational therapists provided rehabilitation, such as muscle strengthening, balance, walking, and stair exercises, for more than 20 min on weekdays to each patient according to the rehabilitation policy in the general ward of each hospital.
Data collection
Patient data were retrieved retrospectively from electronic medical records. Data on baseline characteristics of all enrolled patients were collected at the time of ICU admission, including age, sex, body mass index, Charlson comorbidity index, Barthel index before hospitalization, admission source, ICU admission diagnosis, Acute Physiology and Chronic Health Evaluation II (APACHE II) score, Sequential Organ Failure Assessment (SOFA) score, need for mechanical ventilation, continuous vasopressor use, continuous sedation, continuous analgesia, and hemodialysis. Barthel index before hospitalization was scored at the time of ICU admission based on the information obtained from the family or the patients if they were conscious. Vasopressors, sedation, and analgesia refer to those administered continuously beyond ICU admission and did not include intermittent administration.
Rehabilitation session data were recorded daily by a physical therapist or nurse following each rehabilitation session, including data on the highest activity level according to the ICU mobility scale30 and barriers preventing mobilization during that session. The ICU mobility scale is a sensitive 11-point ordinal scale, with scores ranging from 0 (no mobilization) to 10 (independent ambulation). We collected these data within the first 5 days of the ICU stay.
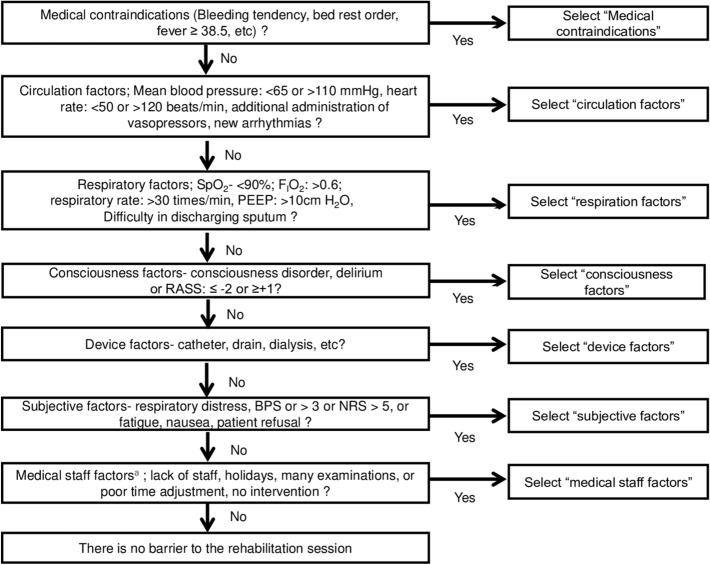
Perceived barriers included predefined barriers described in prior studies21,22,24,25,31. Barrier assessment was routinely used at each institution prior to this study’s initiation. These included consciousness; subjective symptoms; and respiratory, circulatory, device, subject, and medical staff factors25. Details of these factors are shown in Fig. 1. If several barriers were identified in one session, only the primary reason was recorded, and not the individual components of categories. During each rehabilitation session, a physical therapist or nurse in the ICU determined the primary barrier to preventing mobilization by the end of the session according to the algorithm shown in Fig. 1. The priority of this list was based on previous studies investigating barriers to mobilization, the consensus of early rehabilitation experts created in Japan, and our clinical experience24,25,32,33.
Figure 1.
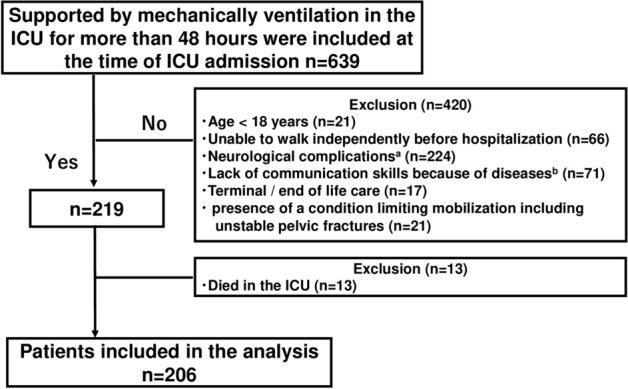
Flow chart of the patient selection process. ICU intensive care unit. Superscript a: neurological complications include cerebral infarction, cerebral hemorrhage, acute subdural hematoma, acute epidural hematoma, traumatic subarachnoid hemorrhage, and encephalitis. Superscript b: mental and cognitive diseases include depression, anxiety, schizophrenia, dementia, cerebral infarction, cerebral hemorrhage, dementia, and alcoholism. This includes cases where standard rehabilitation and assessment of outcomes are difficult to assess due to the inability to communicate in Japanese.
Study outcomes
The primary outcome measured in this study was ADL independence at hospital discharge, which was defined as a score of 70 or higher on the Barthel index4,7. This widely used and reliable scale measures a patient's ability to perform daily activities34.
The secondary outcomes included total medical costs, duration of mechanical ventilation, duration of ICU stay, duration of hospital stay, the incidence of ICU-AW at ICU discharge, incidence of delirium during ICU stay, and rate of discharge to home. Data on medical costs and discharge destinations were collected from the Medical Affairs Department.
Medical costs were calculated based on the diagnostic procedure combination/per-diem payment system and converted from Japanese Yen to United States dollars (USD) at an exchange rate of 108 Yen/USD35. Delirium was assessed using The Intensive Care Delirium Screening Checklist36. ICU-AW was defined as a Medical Research Council sum score (evaluated by a physical therapist) < 48 at ICU discharge37.
Statistical analysis
Continuous variables are presented as medians and interquartile ranges (IQRs), whereas categorical variables are presented as numbers and percentages. When appropriate, all patient outcomes were categorized as dichotomous data using median values16.
We performed multiple logistic regression analysis to evaluate the effect of EM on patient outcomes. The analysis took into account several factors such as age, Barthel index before hospitalization, planned operation, septic shock, APACHE II score, and use of continuous vasopressors. These variables were selected based on the results of previous studies2,4,11,24,28,38,39. We limited the number of covariates to six to prevent overfitting the model40. For the post hoc analysis, multiple logistic regression analysis was performed to examine the association between barriers and the achievement of EM; the results are reported as odds ratios (ORs) with 95% confidence intervals (CIs). In multiple logistic analysis, the explanatory variables included the following seven factors associated with mobilization barriers: medical contraindication and respiratory, circulatory, consciousness, device, subject, and medical staff factors from days 1 to 5. In our study, we used multivariable logistic regression analysis and Bonferroni correction to examine the relationship between mobilization barriers, primary and secondary outcomes, and achieving EM as measured by ADL independence at hospital discharge.
Three sensitivity analyses were performed. (1) The association between the achievement of mobilization and outcomes within 2, 3, 4, 6, or 7 days after ICU admission was examined using the same method (multiple logistic regression analysis) as the primary analysis. (2) We included those who died after ICU discharge in the ADL non-independent group because the association between EM and fatality is assumed to be bidirectional, and ADL non-independence can result from fatality. ADL non-independence may be associated with in-hospital mortality (3) To address other potential confounders of ADL independence, we selected different covariates to assess the robustness of our findings. In Model 2, sex, body mass index, and the Charlson comorbidity index were added. Model 3 incorporated institutions and SOFA scores. Variables in the model with P < 0.05 from the lack-of-fit test were excluded from the results of this study considering non-fitting41.
All analyses were performed using JMP software (version 13.0; SAS Institute Inc., Cary, NC, USA). Statistical tests were two-sided, and statistical significance was defined as P < 0.05.
Ethics approval and consent to participate
This multicenter retrospective cohort study was approved by the Ethics Committee of Nagoya Medical Center (approval number: 2021-012) and the respective ethics committees of the five other participating hospitals. It was conducted in accordance with Helsinki Declaration and the need for informed consent, according to national legislation, was waived by the IRB listed above because this was a retrospective cohort study. Human participants' names and other identifiers were not used during the study process and were not included in all sections of the manuscript, including Supplementary Information.
Results
Baseline patient characteristics
During the study period from April 2019 to March 2020, 639 consecutive patients were screened, and 13 died during hospitalization. Finally, 206 patients without deficiencies were enrolled in this study (Fig. 2). The baseline characteristics of the enrolled patients are shown in Table 1. There were 139 men (68%), with a median age of 70 (IQR, 62–77) years. The median APACHE II and SOFA scores at ICU admission were 23 (IQR, 17–28) and 8 (IQR, 6–10), respectively (Table 1). A total of 115 patients (56%) achieved mobilization on or before the fifth day of ICU stay. The rates of achieving each mobilization level are shown in Supplementary Fig. 1. In this study, all 42 patients who were discharged from the ICU within fifth days achieved mobilization during their ICU stay.
Figure 2.
Algorithm to determine the primary barrier preventing mobilization. SpO2 oxygen saturation of the peripheral artery, FiO2 fraction of inspiratory oxygen, RASS Richmond agitation sedation scale, BPS behavioral pain scale, NRS numerical rating scale. The barrier to mobilization was determined by the intensivist in charge of the patient following this algorithm. In every rehabilitation session, only one selected barrier was recorded in the medical record.
Table 1.
Baseline characteristics at the time of intensive care unit admissions.
| Variables | All Patients, n = 206 |
|---|---|
| Age (years), median [IQR] | 70 [62–77] |
| Gender (male), n (%) | 139 (68) |
| BMI (kg/m2), median [IQR] | 22.7 [19.7–26.6] |
| Charlson Comorbidity Index, median [IQR] | 1 [0–3] |
| Barthel index before hospitalization, n (%)a | 100 [100–100] |
| Admission source, n (%) | |
| Emergency department | 126 (61) |
| General ward in hospital | 36 (18) |
| Planned operationb | 44 (21) |
| Sepsis shock at ICU admission, n (%) | 120 (58) |
| ICU admission diagnosis, n (%) | |
| Acute respiratory failure (including pneumonia) | 34 (16) |
| Cardiovascular disease | 87 (42) |
| Gastric or colonic surgery | 44 (21) |
| Sepsis, non-pulmonary | 29 (14) |
| Other diagnoses | 12 (6) |
| APACHE II score, median [IQR] | 23 [17–28] |
| SOFA at ICU admission, median [IQR] | 8 [6–10] |
| The use of continuous vasopressor during ICU stay, n (%) | 148 (72) |
| The use of continuous sedation during ICU stay, n (%) | 201 (98) |
| The use of continuous analgesia during ICU stay, n (%) | 183 (89) |
| The use of dialysis during ICU stay, n (%) | 43 (21) |
| The use of neuromuscular blocking agent during ICU stay, n (%) | 35 (17) |
Data are presented as median [interquartile range] or number (%).
IQR interquartile range, BMI body mass index; ICU intensive care unit, APACHE acute physiology and chronic health evaluation, SOFA sequential organ failure assessment.
aBarthel index before hospitalization was scored at the time of ICU admission based on the information from the family or the patients if they were conscious.
bBreakdown of post-operation: cardiovascular surgery, 27 (61%); Gastrointestinal surgery, 11 (25%); other surgery, 6 (14%).
Primary and secondary outcomes
The prevalence of ADL non-independence at hospital discharge was 30.1% (62/206) (Table 2). Table 2 shows the association between mobilization and outcomes. After adjusting for covariates, the primary outcome revealed that achieving EM was associated with ADL independence at hospital discharge [adjusted odds ratio (AOR), 3.44; 95% CI, 1.70–6.96]. Results regarding the secondary outcomes also showed a significant association with EM achievement and total medical costs (AOR, 3.05; 95% CI, 1.59–5.87), duration of ICU stay (AOR, 4.62; 95% CI, 2.47–8.66), and the duration of hospital stay (AOR, 2.98; 95% CI, 1.53–5.80). In the sensitivity analysis, our results were unaffected by altering the definition of EM (Supplementary Table 4), the inclusion of those who died after ICU discharge (Supplementary Table 5), or by developing other models using different covariates (Supplementary Table 6). In the multivariable logistic regression analysis when varying the definitions of EM within 2, 3, 4, or 6 days, the highest odds ratio between achieving EM within 5 days and ADL independence at hospital discharge was recorded (Supplementary Table 4).
Table 2.
Multivariable logistic regression analysis of the association between early mobilization achievement and outcomes.
| Outcomes | All (n = 206) | Adjusted OR (95% CI) | p-value |
|---|---|---|---|
| Primary outcomes | |||
| ADL independence at discharge, n (%) | 125 (61) | 3.44 (1.70–6.96) | < 0.001 |
| Secondary outcomes | |||
| Total medical costs < 2500-dollar, n (%) | 95 (49) | 3.05 (1.59–5.87) | < 0.001 |
| Duration of mechanical ventilation < 5 days, n (%) | 106 (52) | 1.38 (0.75–2.53) | 0.295 |
| ICU length of stay < 7 days, n (%) | 105 (51) | 4.62 (2.47–8.66) | < 0.001 |
| Hospital length of stay < 28 days, n (%) | 79 (38) | 2.98 (1.53–5.80) | 0.001 |
| ICU-AW at ICU discharge, n (%)a | 73 (45) | 0.39 (0.19–0.81) | 0.012 |
| Delirium during ICU stay, n (%)b | 78 (38) | 0.44 (0.22–0.88) | 0.023 |
| Discharge to home, n (%) | 144 (70) | 2.26 (1.14–4.46) | 0.019 |
Variables for the outcomes in the multivariable logistic regression included age, Barthel index before hospitalization, planned operation, Sepsis shock at ICU admission, APACHE II score, use of continuous vasopressor.
OR odds ratio, IQR interquartile range, ADL activity daily living, ICU intensive care unit, APACHE acute physiology and chronic health evaluation, ICU-AW ICU-acquired weakness.
aOf 206 patients, 44 were missing.
bOf 206 patients, 39 were missing.
Barriers to mobilization
Medical contraindications were most frequently described as barriers to EM on days 3 (20%), 4 (16%), and 5 (18%). On days 1 and 2, the most frequently described barrier was circulatory factors (35% and 25%, respectively) (Table 3).
Table 3.
Primary barriers preventing the achievement of early mobilization.
| Day 1 (n = 206) | Day 2 (n = 206) | Day 3 (n = 206) | Day 4 (n = 191) | Day 5 (n = 164) | |
|---|---|---|---|---|---|
| Medical contraindication | 67 (33) | 45 (22) | 41 (20) | 31 (16) | 29 (18) |
| Circulatory factor | 72 (35) | 51 (25) | 28 (13) | 15 (8) | 9 (5) |
| Respiratory factor | 17 (8) | 23 (11) | 23 (12) | 15 (8) | 13 (8) |
| Consciousness factor | 24 (12) | 32 (16) | 27 (13) | 27 (15) | 19 (12) |
| Device factor | 11 (5) | 13 (6) | 9 (4) | 15 (8) | 7 (4) |
| Subject factor | 6 (3) | 6 (3) | 7 (3) | 4 (2) | 6 (3) |
| Medical staff factor | 6 (3) | 11 (5) | 16 (8) | 11 (5) | 11 (7) |
| Achievement of EM | 3 (1) | 25 (12) | 55 (27) | 73 (38) | 70 (43) |
Number of patients (%).
EM early mobilization, ICU intensive care unit.
Significant values are in bold.
Among the seven major barriers detected within 5 days of ICU admission, we found that from days 1 to 5, device factors (AOR, 0.31; CI, 0.13–0.75) were significantly associated with EM achievement (Table 4). Supplementary Table 7 shows the number of components by category for each of the seven major barriers detected within 5 days of ICU admission.
Table 4.
Association between mobilization barriers and achieving early mobilization.
| Variable | Achieved EM | ||
|---|---|---|---|
| OR | 95% CI | P value | |
| Medical contraindication from day 1 to 5 | 0.54 | 0.28–1.05 | 0.070 |
| Circulatory factor from day 1 to 5 | 0.56 | 0.30–1.07 | 0.081 |
| Respiratory factor from day 1 to 5 | 0.44 | 0.21–0.92 | 0.029 |
| Consciousness factor from day 1 to 5 | 0.59 | 0.32–1.12 | 0.108 |
| Device factor from day 1 to 5 | 0.31 | 0.13–0.75 | < 0.001 |
| Subject factor from day 1 to 5 | 1.52 | 0.59–3.96 | 0.385 |
| Medical staff factor from day 1 to 5 | 0.69 | 0.34–1.40 | 0.298 |
We adjusted for multiple comparisons by using the Bonferroni correction and considered a result to be statistically significant if P < 0.007.
OR odds ratio, CI confidence interval, EM early mobilization, ADL activity daily living, ICU intensive care unit.
Significant values are in bold.
Discussion
In this multicenter retrospective cohort study conducted using data from patients treated in the ICUs of one of six hospitals in Japan, we investigated the achievement of EM in a mixed population of mechanically ventilated patients in the ICU as well as the association between EM and ADL independence at hospital discharge.
Moreover, this study reviewed the barriers preventing EM and ADL independence at multiple centers. This is the first multicenter study to investigate the relationship between day-to-day changes in barriers to EM and ADL independence at hospital discharge. Our findings suggest that within 5 days of ICU admission, removing barriers to mobilization for patients on mechanical ventilation and initiating mobilization are preferable in terms of preventing physical dysfunction in the ICU.
The underlying pathophysiological mechanisms of physical dysfunction at discharge are multifactorial, including age, altered level of consciousness, delirium, ICU-AW development, and immobility3,4,24. Of these, bed rest in the supine position is a risk factor that can be easily improved. Given these proposed mechanisms, EM against mechanical ventilation is a potentially efficient strategy, similar to how muscle strength exercise appears to prevent the development of ICU-AW28,37. However, some recent randomized studies have failed to detect significant improvements in the EM42, which may have been related to delayed EM initiation, beginning approximately after more than 1 week after ICU admission. The achievement of EM in this study was associated not only with ADL independence at hospital discharge but also with total medical costs, ICU stay duration, hospital stay duration, and the incidence of ICU-AW and delirium. Previous reports have also indicated that achieving EM within 5 days of ICU admission does not affect survival but is effective in improving functional outcomes16,19. Thus, achieving EM in the ICU, as shown in this study, might help prevent disuse syndrome and achieve independent ADL.
This study also showed daily changes in the rate of achieving mobilization, which was very low on ICU days 1 (1%) and 2 (12%) and increased from days 3 (27%) to 5 (43%). The overall rate of achieving mobilization (56%) was comparable with that in a prior study22. Medical restrictions and cardiovascular and respiratory factors were identified in more than half of the patients as the main barriers to achieving mobilization on days 1 and 2 in the ICU. Most patients were probably hemodynamically unstable upon admission to the ICU and required vasopressor support. However, in this study, medical and circulatory factors were not significantly associated with ADL independence at hospital discharge. Future studies should consider whether low and passive exercise43 or neuromuscular electrical stimulation44 can substitute for EM in patients who cannot achieve EM due to medical contraindications or cardiovascular factors. Previous studies have reported that factors related to the medical staff are a major barrier to mobilization21–23. However, in this study, its influence was very small, and as a result, the achievement rate of EM was high. Based on established protocols, assessing barriers to EM may reduce medical staff-related factors. The results of this study were drawn from data from a hospital that actively performs ICU rehabilitation in Japan, and may not be generalizable to all ICUs.
When examining the relationship between ADL independence at hospital discharge and the time to the first mobilization varied by 2, 3, 4, 6, or 7 days after ICU admission, the value at 5 days showed the highest odds ratio. Shortening the interval to achieve EM after overcoming barriers may be an important aspect of early rehabilitation to maximize the impact on mechanical ventilation outcomes. The significant association between EM achievement and the Barthel index at discharge under multiple conditions supports our theory.
The small sample size and comparability of the two groups are central limitations. These could limit the generalizability to other ICUs. There are unadjusted confounding factors such as nutrition and ventilator settings. However, to do our best, multivariable analysis using logistic regression analysis tuned by key clinical and potential confounding factors showed consistent results. However, in the logistic regression analysis adjusted for potential confounders, covariates may have exhibited overfitting, and these results should be interpreted with caution. In our study, the outcomes were limited within short-term outcomes. Additionally, in this study, whether the patient could receive rehabilitation at the level of sitting over the edge or higher were depending on the rehabilitation policy in each participating hospital. Furthermore, in this study, we were unable to investigate the number of implementation units and frequency of rehabilitation implemented at each hospital. Therefore, it was difficult to identify whether EM could not be provided due to poor general conditions or other factors. The algorithm for determining barriers to mobilization in this study was created based on our clinical experience, and its reliability and validity were not sufficiently verified. A multicenter, prospective, cohort study including all mechanically ventilated patients will likely validate the questions that remain unanswered.
The main barriers to mobilization were device factors. Our observations show that the initiation of physical rehabilitation with an intensity greater than that needed for sitting on the edge of the bed within 5 days of ICU admission appears to be the preferred strategy for improving ADL independence at hospital discharge. Overcoming the barrier of mobilization may also be necessary to improve the ADL independence rate at hospital discharge. Further research is required to validate our results.
Supplementary Information
Acknowledgements
The authors would like to thank the study coordinators, Dr. Shuichi Suzuki, PT Kaito Kochi, PT Keisuke Mizutani, Ns Ono Mika, PT Yoshinori Naito, PT Shintaro Yamamoto, PT Takehisa Ito, Yuji Mori, PT Naoki Takeshita, PT Yusuke Nagae, and PT Yuki Ito. The authors would also like to thank the entire ICU staff at all the participating hospitals. The authors thank Akiko Kada, a biostatistician at Nagoya Medical Center, for providing assistance in reviewing this manuscript. No funding was received for this work.
Author contributions
S.W., J.H., Y.M., and Y.I. designed this study. S.W., J.H., Y.N., M.M., A.U., S.N., Y.M., and Y.I. conducted the study and acquired data. S.W. and Y.I. assessed the quality of the study and performed the analysis and interpretation. S.W. and Y.I. wrote the manuscript, and the other authors made substantial revisions. All authors have read and approved the final manuscript.
Data availability
The data will be available with the corresponding author at demand.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Shinichi Watanabe, Email: billabonghonor@yahoo.co.jp.
Yuki Iida, Email: y2iida@na.commufa.jp.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-023-31459-1.
References
- 1.Bemis-Dougherty AR, Smith JM. What follows survival of critical illness? Physical therapists' management of patients with post-intensive care syndrome. Phys. Ther. 2013;93(2):179–185. doi: 10.2522/ptj.20110429. [DOI] [PubMed] [Google Scholar]
- 2.Inoue S, Nakanishi N, Nakamura K. Post-intensive care syndrome-10 years after its proposal and future directions. J. Clin. Med. 2022;11(15):4381. doi: 10.3390/jcm11154381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Watanabe S, Kotani T, Taito S, Ota K, Ishii K, Ono M, et al. Determinants of gait independence after mechanical ventilation in the intensive care unit: A Japanese multicenter retrospective exploratory cohort study. J. Intensive Care. 2019;7:53. doi: 10.1186/s40560-019-0404-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schweickert WD, Pohlman MC, Pohlman AS, Nigos C, Pawlik AJ, Esbrook CL, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: A randomized controlled trial. Lancet. 2009;373:1874–1882. doi: 10.1016/S0140-6736(09)60658-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehrholz J, Mückel S, Oehmichen F, Pohl M. First results about recovery of walking function in patients with intensive care unit-acquired muscle weakness from the General Weakness Syndrome Therapy (GymNAST) cohort study. BMJ Open. 2015;5(12):e008828. doi: 10.1136/bmjopen-2015-008828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marra A, Pandharipande PP, Girard TD, Patel MB, Hughes CG, Jackson JC, et al. Co-occurrence of post-intensive care syndrome problems among 406 survivors of critical illness. Crit. Care Med. 2018;46(9):1393–1401. doi: 10.1097/CCM.0000000000003218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watanabe S, Morita Y, Suzuki S, Kochi K, Ohno M, Liu K, et al. Effects of the intensity and activity time of early rehabilitation on activities of daily living dependence in mechanically ventilated patients. Prog. Rehabil. Med. 2021;29(6):20210054. doi: 10.2490/prm.20210054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burtin C, Clerckx B, Robbeets C, Ferdinande P, Langer D, Troosters T, et al. Early exercise in critically ill patients enhances short-term functional recovery. Crit. Care Med. 2009;37:2499–2505. doi: 10.1097/CCM.0b013e3181a38937. [DOI] [PubMed] [Google Scholar]
- 9.Verceles AC, Wells CL, Sorkin JD, Terrin ML, Beans J, Jenkins T, et al. A multimodal rehabilitation program for patients with ICU acquired weakness improves ventilator weaning and discharge home. J. Crit. Care. 2018;47:204–210. doi: 10.1016/j.jcrc.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watanabe S, Iida Y, Ito T, Mizutani M, Morita Y, Suzuki S, et al. Effect of early rehabilitation activity time on critically ill patients with intensive care unit-acquired weakness: A Japanese retrospective multicenter study. Prog. Rehabil. Med. 2018;9(3):20180003. doi: 10.2490/prm.20180003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hermans G, De Jonghe B, Bruyninckx F, Van den Berghe G. Interventions for preventing critical illness polyneuropathy and critical illness myopathy. Cochrane Database Syst. Rev. 2014;2014(1):CD006832. doi: 10.1002/14651858.CD006832.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morris PE, Goad A, Thompson C, Taylor K, Harry B, Passmore L, et al. Early intensive care unit mobility therapy in the treatment of acute respiratory failure. Crit. Care Med. 2008;36(8):2238–2243. doi: 10.1097/CCM.0b013e318180b90e. [DOI] [PubMed] [Google Scholar]
- 13.Watanabe S, Morita Y, Suzuki S, Someya F. Association between early rehabilitation for mechanically ventilated intensive care unit patients and oral ingestion. Prog. Rehabil. Med. 2018;3:20180009. doi: 10.2490/prm.20180009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kayambu G, Boots R, Paratz J. Physical therapy for the critically ill in the ICU: A systematic review and meta-analysis. Crit. Care Med. 2013;41(6):1543–1554. doi: 10.1097/CCM.0b013e31827ca637. [DOI] [PubMed] [Google Scholar]
- 15.Liu K, Ogura T, Takahashi K, Nakamura M, Ohtake H, Fujiduka K, et al. A progressive early mobilization program is significantly associated with clinical and economic improvement: A single-center quality comparison study. Crit. Care Med. 2019;47(9):e744–e752. doi: 10.1097/CCM.0000000000003850. [DOI] [PubMed] [Google Scholar]
- 16.Watanabe S, Liu K, Morita Y, Kanaya T, Naito Y, Suzuki S, et al. Effects of mobilization among critically ill patients in the intensive care unit: A single-center retrospective study. Prog. Rehabil. Med. 2022;7:20220013. doi: 10.2490/prm.20220013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alison JA, Kenny P, King MT, McKinley S, Aitken LM, Leslie GD, et al. Repeatability of the six-minute walk test and relation to physical function in survivors of a critical illness. Phys. Ther. 2012;92(12):1556–1563. doi: 10.2522/ptj.20110410. [DOI] [PubMed] [Google Scholar]
- 18.Adler J, Malone D. Early mobilization in the intensive care unit: A systematic review. Cardiopulm. Phys. Ther. J. 2012;23(1):5–13. doi: 10.1097/01823246-201223010-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Okada Y, Unoki T, Matsuishi Y, Egawa Y, Hayashida K, Inoue S. Early versus delayed mobilization for in-hospital mortality and health-related quality of life among critically ill patients: A systematic review and meta-analysis. J. Intensive Care. 2019;7:57. doi: 10.1186/s40560-019-0413-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harrold ME, Salisbury LG, Webb SA. Australia and Scotland ICU Physiotherapy Collaboration. Early mobilisation in intensive care units in Australia and Scotland: A prospective, observational cohort study examining mobilisation practises and barriers. Crit. Care. 2015;19(1):336. doi: 10.1186/s13054-015-1033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berney S, Harrold M, Webb S, Seppelt I, Patman S, Thomas PJ, et al. Intensive care unit mobility practices in Australia and New Zealand: A point prevalence study. Crit. Care Resusc. 2013;15(4):260–265. [PubMed] [Google Scholar]
- 22.Nydahl P, Ruhl AP, Bartoszek G, Dubb R, Filipovic S, Flohr HJ, Kaltwasser A, et al. Early mobilization of mechanically ventilated patients: A 1-day point-prevalence study in Germany. Crit. Care Med. 2014;42(5):1178–1186. doi: 10.1097/CCM.0000000000000149. [DOI] [PubMed] [Google Scholar]
- 23.Crozier TM, Pilcher DV, Bailey MJ, George C, Hart GK. Long-stay patients in Australian and New Zealand intensive care units: Demographics and outcomes. Crit. Care Resusc. 2007;9(4):327–333. [PubMed] [Google Scholar]
- 24.Ad Hoc Committee for Early Rehabilitation The Japanese Society of Intensive Care Medicine Evidence based expert consensus for early rehabilitation in the intensive care unit. J. Int. Care Med. 2017;24(2):255–303. [Google Scholar]
- 25.Watanabe S, Liu K, Morita Y, Kanaya T, Naito Y, Arakawa R, et al. Changes in barriers to implementing early mobilization in the intensive care unit: A single center retrospective cohort study. Nagoya J. Med. Sci. 2021;83(3):443–464. doi: 10.18999/nagjms.83.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vandenbroucke JP, von Elm E, Altman DG, Gøtzsche PC, Mulrow CD, Pocock SJ, et al. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): Explanation and elaboration. Int. J. Surg. 2014;12(12):1500–1524. doi: 10.1016/j.ijsu.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 27.Katsukawa H, Ota K, Liu K, Morita Y, Watanabe S, Sato K, et al. Risk factors of patient-related safety events during active mobilization for intubated patients in intensive care units-a multi-center retrospective observational study. J. Clin. Med. 2021;10(12):2607. doi: 10.3390/jcm10122607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Devlin JW, Skrobik Y, Gélinas C, Needham DM, Slooter AJC, Pandharipande PP, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit. Care Med. 2018;46(9):e825–e873. doi: 10.1097/CCM.0000000000003299. [DOI] [PubMed] [Google Scholar]
- 29.Hashimoto S, Sanui M, Egi M, Ohshimo S, Shiotsuka J, Seo R, et al. ARDS clinical practice guideline committee from the Japanese Society of Respiratory Care Medicine and the Japanese Society of Intensive Care Medicine. The clinical practice guideline for the management of ARDS in Japan. J. Intensive Care. 2017;5:50. doi: 10.1186/s40560-017-0222-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hodgson C, Needham D, Haines K, Bailey M, Ward A, Harrold M, et al. Feasibility and inter-rater reliability of the ICU Mobility Scale. Heart Lung. 2014;43(1):19–24. doi: 10.1016/j.hrtlng.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 31.Taito S, Shime N, Yasuda H, Ota K, Sarada K, Lefor AK, et al. Out-of-bed mobilization of patients undergoing mechanical ventilation with orotracheal tubes: A survey study. J. Crit. Care. 2018;47:173–177. doi: 10.1016/j.jcrc.2018.06.022. [DOI] [PubMed] [Google Scholar]
- 32.Barber EA, Everard T, Holland AE, Tipping C, Bradley SJ, Hodgson CL. Barriers and facilitators to early mobilisation in Intensive Care: A qualitative study. Aust Crit Care. 2015;28(4):177–182. doi: 10.1016/j.aucc.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Dubb R, Nydahl P, Hermes C, Schwabbauer N, Toonstra A, Parker AM, et al. Barriers and strategies for early mobilization of patients in intensive care units. Ann. Am. Thorac. Soc. 2016;13(5):724–730. doi: 10.1513/AnnalsATS.201509-586CME. [DOI] [PubMed] [Google Scholar]
- 34.Ranhoff AH, Laake K. The Barthel ADL index: Scoring by the physician from patient interview is not reliable. Age Ageing. 1993;22(3):171–174. doi: 10.1093/ageing/22.3.171. [DOI] [PubMed] [Google Scholar]
- 35.Nakagawa Y, Takemura T, Yoshihara H, Nakagawa Y. A new accounting system for financial balance based on personnel cost after introduction of a DPC/DRG system. J. Med. Syst. 2011;35(2):251–264. doi: 10.1007/s10916-009-9361-y. [DOI] [PubMed] [Google Scholar]
- 36.Bergeron N, Dubois MJ, Dumont M, Dial S, Skrobik Y. Intensive Care Delirium Screening Checklist: Evaluation of a new screening tool. Intensive Care Med. 2001;27:859–864. doi: 10.1007/s001340100909. [DOI] [PubMed] [Google Scholar]
- 37.Patel BK, Pohlman AS, Hall JB, Kress JP. Impact of early mobilization on glycemic control and ICU-acquired weakness in critically ill patients who are mechanically ventilated. Chest. 2014;146:583–589. doi: 10.1378/chest.13-2046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Egi M, Ogura H, Yatabe T, Atagi K, Inoue S, Kakihana Y, Nishida O, et al. The Japanese Clinical Practice Guidelines for Management of Sepsis and Septic Shock 2020 (J-SSCG 2020) Acute Med Surg. 2020;25(9):53. doi: 10.1002/ams2.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolfe KS, Patel BK, MacKenzie EL, Giovanni SP, Pohlman AS, Churpek MM, et al. Impact of vasoactive medications on ICU-acquired weakness in mechanically ventilated patients. Chest. 2018;154(4):781–787. doi: 10.1016/j.chest.2018.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harrell FE., Jr . Regression Modeling Strategies: With Applications to linear Models, Logistic Regression, and Survival Analysis. Corrected. Springer; 2001. pp. 219–273. [Google Scholar]
- 41.Stephens MA. EDF statistics for goodness of fit and some comparisons. J. Am. Stat. Assoc. 1974;69:730–737. doi: 10.1080/01621459.1974.10480196. [DOI] [Google Scholar]
- 42.Morris PE, Berry MJ, Files DC, Thompson JC, Hauser J, Flores L, et al. Standardized rehabilitation and hospital length of stay among patients with acute respiratory failure: A randomized clinical trial. JAMA. 2016;315(24):2694–2702. doi: 10.1001/jama.2016.7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iida Y, Sakuma K. Skeletal muscle dysfunction in critical illness. INTECH. 2017;69051:142–168. [Google Scholar]
- 44.Routsi C, Gerovasili V, Vasileiadis I, Karatzanos E, Pitsolis T, Tripodaki E, et al. Electrical muscle stimulation prevents critical illness polyneuromyopathy: A randomized parallel intervention trial. Crit. Care. 2010;14(2):R74. doi: 10.1186/cc8987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data will be available with the corresponding author at demand.