Abstract
Background
Amantadine has been proposed as a treatment for COVID-19 because it shows anti-SARS-CoV-2 activity in vitro. However, to date, no controlled study has assessed the safety and efficacy of amantadine in COVID-19.
Research question
Whether amantadine is effective and safe among patients with different COVID-19 severity classifications.
Study design
and Methods: This was multi-centre, randomised, placebo-controlled study.Patients with oxygen saturation ≤94% and no need for high-flow oxygen or ventilatory support were randomly allocated to receive oral amantadine or placebo (1:1) for 10 days in addition to standard care. The primary endpoint was time to recovery assessed over 28 days since randomisation, defined as discharge from hospital or no need for supplemental oxygen.
Results
The study was terminated early due to a lack of efficacy after an interim analysis. Final data from 95 patients who received amantadine (mean age, 60.2 years; 65% male; 66% with comorbidities) and 91 patients who received placebo (mean age, 55.8 years; 60% male; 68% with comorbidities) were obtained. The median (95% CI) time to recovery was 10 days both in the amantadine (9–11) and placebo arms (8–11; subhazard ratio = 0.94 [95%CI 0.7–1.3]). The percentage of deaths and percentage of patients who required intensive care at 14 and 28 days did not significantly differ between the amantadine and placebo groups.
Interpretation
Adding amantadine to standard care in patients hospitalised with COVID-19 did not increase the likelihood of recovery.
Clinical trial registration
ClinicalTrials.gov; No.: NCT04952519; www.clinicaltrials.gov.
Keywords: Amantadine, COVID- 19, Hospitalisation, Randomised controlled trial, SARS
1. Introduction
Numerous attempts have been made to repurpose existing medications for the treatment of COVID-19 (coronavirus disease 2019), particularly antiviral medications (e.g. remdesivir, lopinavir, ritonavir) and anti-inflammatory drugs (e.g. tocilizumab, dexamethasone, hydroxychloroquine) [1]. Emerging evidence has supported the use of some of these medications, whereas other treatments have proven inefficient or harmful (lopinavir plus ritonavir, hydroxychloroquine) [2].
Amantadine was first approved for the treatment of influenza A infections, but has been used in other indications, such as Parkinson's disease and fatigue in multiple sclerosis [[3], [4], [5]]. Amantadine has been proposed as a treatment for COVID-19 because it shows activity against SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) in vitro [[6], [7], [8]], and it may have anti-inflammatory properties [9]. Three uncontrolled studies (n < 60 each) reported that amantadine was associated with a less than 10% risk of hospitalisation due to COVID-19,10–12 and one retrospective study reported that chronic use of amantadine was associated with a lower risk of acquiring COVID-19 [13]. Another retrospective study assessed amantadine use in a real-world setting. The main findings were mutually contradictory and showed that amantadine could be associated with an increased risk of death in patients with COVID-19 but was beneficial as an addition to antibiotic treatment [14]. In Poland, the potential efficacy of amantadine in COVID-19 treatment was noticeable in the clinical practice of academic and non-academic settings during the first stage of the COVID-19 pandemic, which resulted in general interest in out-of-label amantadine use. This translated into significant sales of this drug in Poland: unpublished data from Polish registers of prescribed drugs indicate that sales of amantadine in the last quarter of 2021 reached 60,000 packages, at least 4 times higher than in the corresponding period 2 years earlier.
However, to date, no controlled study has assessed the safety and efficacy of amantadine in COVID-19. The level of evidence from previous studies is low, because of their poor-quality design and the small number of participants. Therefore 2 double-blind, placebo-controlled studies were designed to assess the efficacy of amantadine among patients with different COVID-19 severity classifications. Here, we present the results from a clinical study conducted among patients hospitalised with COVID-19. Based on the randomised study of remdesivir, which showed high efficacy among hospitalised patients with COVID-19,15 we assumed that some patients might achieve benefit from use of amantadine.
2. Study design and methods
We performed a randomised, double-blind, placebo-controlled trial in 21 centres (14 of them were actively recruiting) in Poland. The study protocol and all its amendments were approved by the Bioethics Committee of the Silesian Medical University, Katowice, Poland (PCN/0022/KB1/23/X/21). Funding was provided by the Medical Research Agency (grant no. 2021/ABM/COVID19/GCM). The funder had no role in designing or conducting the study, data collection or analysis, or in the preparation of this manuscript. The trial was registered with the number NCT04952519 (https://clinicaltrials.gov/ct2/show/NCT04952519).
2.1. Participants
Eligible participants comprised hospitalised adults (≥18 years) with COVID-19 diagnosed within 10 days and SARS-CoV-2 infection confirmed by PCR, who met all the following inclusion criteria: pneumonia confirmed on a chest X-ray or computed tomography; oxygen saturation <95%; and no need for high-flow oxygen or for invasive or non-invasive ventilatory support. The onset of COVID-19 was defined as the appearance of any of the following symptoms typical for COVID-19: fever, cough, dyspnoea, dysgeusia, dysosmia, myalgia, chest pain, diarrhoea, nausea, vomiting, throat pain, or hyperaemia of the nasal mucus membrane.
Patients who had used amantadine within 3 months were excluded. Additional exclusion criteria were as follows: clinically relevant renal or hepatic insufficiency; history of epileptic seizures; history of delirium, confusion, or psychosis regardless of the cause; relevant cardiovascular diseases (severe heart failure, cardiomyopathy, myocarditis, II-III degree atrioventricular block, bradycardia, QT elongation, visible U-wave, history of serious arrhythmia, including torsade de pointes); immunosuppression (transplantation of solid organs or haematopoietic stem cells, AIDS, use of immunosuppressants, including >20 mg of prednisolone equivalents daily); use of QT-elongating medications; hypokalaemia or hypomagnesemia; untreated angle-closure glaucoma; and pregnancy or lactation. All participants provided written informed consent before enrolment.
2.2. Randomisation and intervention
Eligible patients were randomly allocated (1:1) to receive oral amantadine tablets (100 mg, Parkadina, Laboratórios Basi, Portugal) or matching placebo tablets for 10 days in addition to standard care. Randomisation was stratified by remdesivir use or treatment with convalescent plasma. Randomisation was not stratified by age. Randomisation was done by a blinded investigator via an interactive web responsive system. The first dose of investigated product (IP) was 2 tablets for all participants. For the first 2 days, participants aged 18–65 years received 100 mg of IP every 6 h, those aged 66–85 years received IP every 8 h, and those aged ≥86 years received IP every 12 h. On days 3–10, all participants received 100 mg of IP every 12 h. Participants and investigators were blinded to the assigned treatment. Standard care complied with the COVID-19 treatment recommendations of the Polish Agency for Health Technology Assessment and Tariffs of 27/11/2020, with subsequent updates [16].
2.3. Outcomes
The primary endpoint was time to recovery assessed over 28 days since enrolment, defined as a score of 1–3 on an 8-point ordinal scale of clinical improvement (1 = not hospitalised, no limitations on activities; 2 = not hospitalised, limitation on activities and/or requiring home oxygen; 3 = hospitalised, not requiring supplemental oxygen and not requiring ongoing medical care; 4 = hospitalised, not requiring supplemental oxygen but requiring ongoing medical care; 5 = hospitalised, requiring supplemental oxygen; 6 = hospitalised, on non-invasive ventilation or high-flow oxygen; 7 = hospitalised, on invasive mechanical ventilation or extracorporeal mechanical oxygenation; 8 = death). Pre-specified secondary endpoints included frequency of participants dead or requiring intensive care on days 14 and 28. All endpoints were assessed daily in hospitalised participants and on days 14 and 28 in those discharged home with scores of 1–3 on the 8-point scale.
2.4. Sample size estimation and early study termination
Sample size was estimated based on the assumption that 126 events in the amantadine group and 193 events in the placebo group would provide 95% power with a two-sided significance level of 0.05 to detect a hazard ratio of 0.70 for the time to recovery with placebo vs. amantadine, assuming a median time to improvement of 8 days in the amantadine group and 12 days in the placebo group. The values of the median time to improvement in the 2 groups were taken from a randomised study of remdesivir [15] and an observational study of amantadine in COVID-19,11 respectively. Based on these calculations, we planned to enrol 250 patients each in the amantadine and placebo arms. However, the steering committee decided to terminate the trial early due to a lack of efficacy after the pre-planned interim analysis (∼150 patients). After the study was discontinued, we finished collecting the data for final analysis. This analysis is presented herein.
2.5. Statistical analysis
The primary endpoint, i.e. time to recovery, was analysed in the intention-to-treat population according to the treatment allocated at randomisation. To analyse the primary endpoint, we adopted a competing risk approach in which recovery and death were competing events. The Gray's non-parametric test was used to compare the cumulative incidence functions between the study arms. Fine-Gray subdistribution hazard models were used to estimate subhazard ratios (sHR). The mean time to recovery was estimated from cumulative incidence functions. The Wald method was used to calculate confidence intervals for pointwise estimates. Proportions of patients dead or requiring intensive care were compared with logistic regression models (unadjusted and adjusted for age as a continuous covariate). The chi-squared test was used to compare categorical variables between study arms at baseline. We performed sensitivity analyses among patients with symptom duration at randomisation of <7 days vs. ≥ 7 days and among patients who received or did not receive remdesivir. Analyses of secondary endpoints and subgroup analyses were exploratory, not adjusted for multiplicity. The R software was used for all analyses. The cmprsk package was used to fit the Fine-Gray subdistribution hazard models. A p < 0.05 was considered statistically significant.
3. Results
3.1. Participants
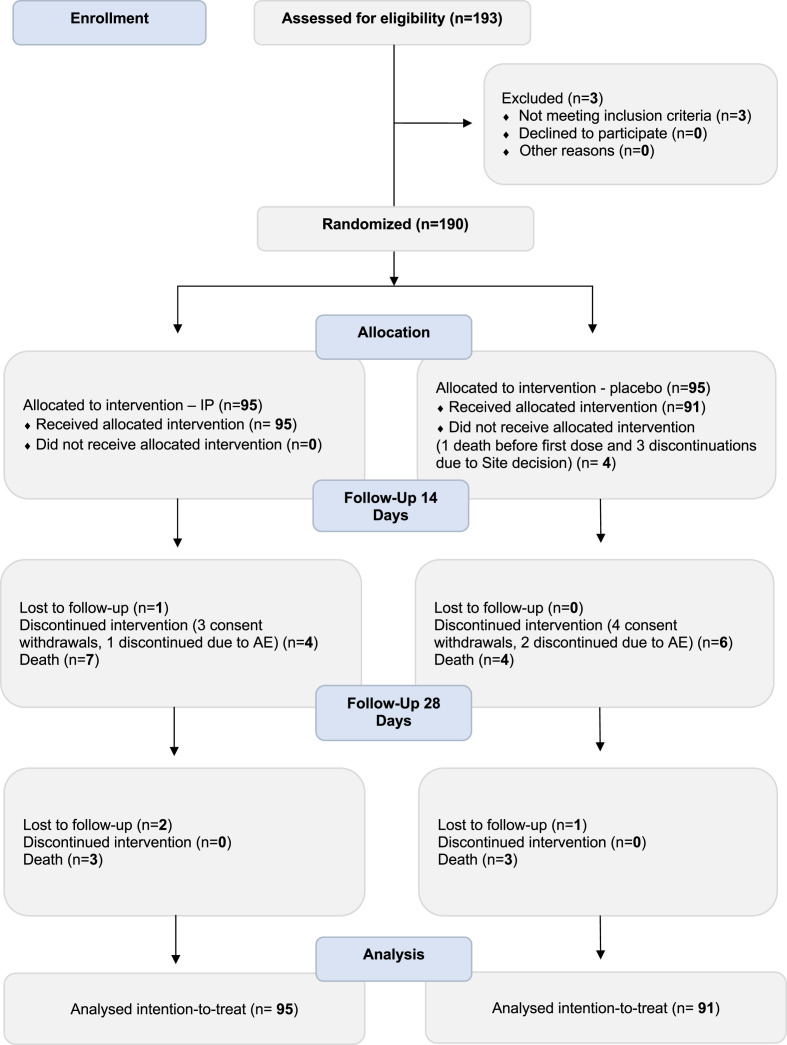
Between April 2021 and February 2022, 193 patients were assessed for eligibility. Of them, 190 met the inclusion criteria and were randomized: all 95 patients allocated to the amantadine arm received treatment, whereas of 95 patients in the placebo arm, 4 did not receive the intervention (1 died and 3 were discontinued by investigators before receiving the first dose). In total, 186 eligible participants were included in the intention-to-treat population. Fig. 1 shows the flow diagram of participants from screening to end of study for the two arms.
Fig. 1.
Consort flow chart.
The mean (SD) age was 60.2 (16.1) years in the amantadine arm and was 55.8 (15.2) years in the placebo arm (p = 0.055). The study arms were otherwise well balanced, with similar anthropometric and clinical characteristics (Table 1 ).
Table 1.
Baseline characteristics.
| Amantadine (n = 95) | Placebo (n = 91) | p-valuea | |
|---|---|---|---|
| Female | 33 (34.7%) | 36 (39.6%) | 0.545 |
| Age group | 0.122 | ||
| <45 years | 17 (17.9%) | 24 (26.4%) | n/a |
| 45–64 | 39 (41.1%) | 42 (46.2%) | |
| >65 | 39 (41.1%) | 25 (27.5%) | |
| BMI, mean (SD) | 27.8 (4.6) | 29 (5.7) | 0.198 |
| Remdesivir | 46 (48.4%) | 49 (53.8%) | 0.467 |
| Convalescent plasma | 1 (1.1%) | 0 (0%) | 1 |
| Symptoms at baseline (ordinal scale) | 0.682 | ||
| 4 | 4 (4.2%) | 2 (2.2%) | n/a |
| 5 | 91 (95.8%) | 89 (97.8%) | |
| Time from symptom onset to randomisation, mean (SD) | 6.7 (2.4) | 6.8 (2.1) | 0.731 |
| Fever | 65 (68.4%) | 66 (72.5%) | 0.650 |
| Cough | 56 (58.9%) | 61 (67%) | 0.322 |
| Dyspnoea | 70 (73.7%) | 67 (73.6%) | 1 |
| Abnormal smell or taste | 11 (11.7%) | 11 (12.1%) | 1 |
| Myalgia | 17 (18.1%) | 20 (22.2%) | 0.605 |
| Chest pain | 7 (7.4%) | 6 (6.6%) | 1 |
| Diarrhoea | 8 (8.5%) | 11 (12.1%) | 0.576 |
| Nausea | 3 (3.2%) | 6 (6.6%) | 0.463 |
| Vomiting | 2 (2.1%) | 5 (5.5%) | 0.415 |
| Sore throat | 5 (5.3%) | 5 (5.5%) | 1 |
| Nasal hyperaemia | 6 (6.4%) | 3 (3.3%) | 0.526 |
| Electrocardiography | 0.467 | ||
| Normal | 59 (63.4%) | 63 (69.2%) | n/a |
| Clinically irrelevant abnormalities | 33 (35.5%) | 28 (30.8%) | |
| Clinically relevant abnormalities | 1 (1.1%) | 0 (0%) | |
| Diabetes | 25 (26.3%) | 18 (19.8%) | 0.302 |
| Hypertension | 39 (41.1%) | 42 (46.2%) | 0.554 |
| Obesity | 25 (26.3%) | 31 (34.1%) | 0.321 |
| Chronic respiratory disease | 11 (11.6%) | 11 (12.1%) | 1 |
| Sleep apnoea | 2 (2.1%) | 0 (0%) | 0.496 |
| Coronary heart disease | 8 (8.4%) | 5 (5.5%) | 0.568 |
| Other cardiovascular disease | 11 (11.6%) | 7 (7.7%) | 0.459 |
| Dyslipidaemia | 9 (9.5%) | 5 (5.5%) | 0.407 |
| Hypothyroidism | 3 (3.2%) | 8 (8.8%) | 0.127 |
| Comorbidities | |||
| None | 38 (40%) | 31 (34.1%) | 0.224 |
| 1 | 19 (20%) | 22 (24.2%) | |
| 2 | 19 (20%) | 27 (29.7%) | |
| 3 or more | 19 (20%) | 11 (12.1%) |
Chi-squared test or Wilcoxon test; values show counts of patients (percentages) if not specified otherwise; BMI – body mass index; n/a – non applicable; SD, standard deviation.
3.2. Outcomes
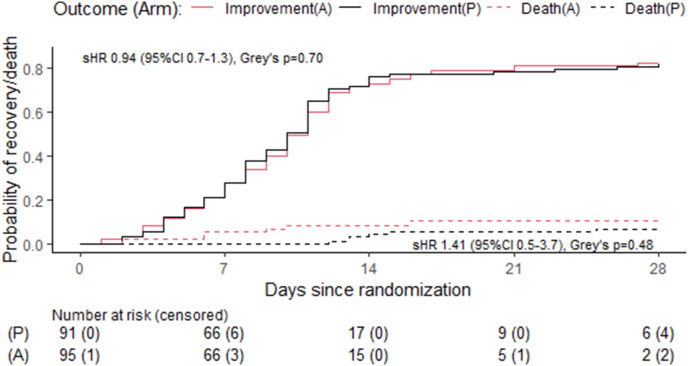
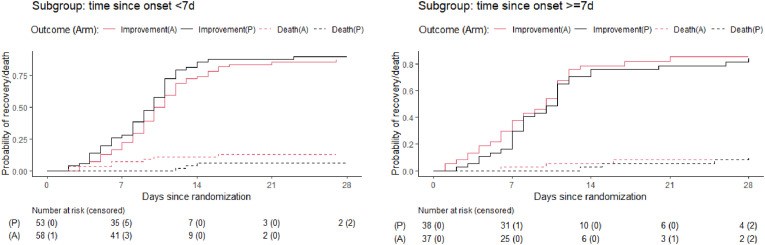
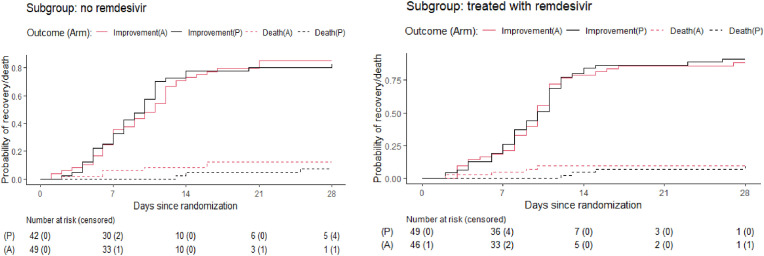
The median time to recovery was 10 days both in the amantadine (95% CI 9–11) and placebo arms (95%CI 8–11; p = 0.7, sHR = 0.94, 95%CI 0.7–1.3, Table 2 , Fig. 2 ). At 14 days, 8 patients (8.4%) in the amantadine arm and 4 patients (4.4%) in the placebo arm were dead (p = 0.374, OR 1.94, 95%CI 0.5–9.18; Table 1), whereas 8 patients (8.8%) in the amantadine arm and 9 patients (10.6%) in the placebo arm required intensive care (p = 0.800, OR = 0.81, 95%CI 0.26–2.52). At 28 days, 10 patients (11.1%) in the amantadine arm and 7 patients (8.2%) in the placebo arm were dead (p = 0.613; OR 1.39, 95% CI 0.45–4.54), whereas 8 patients (8.9%) in the amantadine arm and 9 patients (10.6%) in the placebo arm required intensive care (p = 0.802, OR = 0.82, 95%CI 0.26–2.55). The age-adjusted ORs for death in the amantadine arm vs. placebo were lower than the unadjusted ORs (see Table 2). Sensitivity analyses among patients differing in symptom duration at randomisation and among those receiving or not receiving remdesivir showed similar results as in the main analysis (Fig. 3, Fig. 4 ).
Table 2.
Efficacy and safety endpoints.
| Endpoint | Amantadine | Placebo | sHR or OR (95 %CI) | p-value |
|---|---|---|---|---|
| Median time to recovery in days (95%CI) | 10 (9–11) | 10 (8–11) | sHR 0.94 (0.7–1.3) | 0.7 |
| Median time to death in days (95%CI) | 9 (6–28) | 14 (13–28) | sHR 1.41 (0.5–3.7) | 0.48 |
| Fatalitiesa | ||||
| by day 14 | 8 (8.8%) | 4 (4.7%) | OR 1.94 (0.5–9.18) | 0.374 |
| aOR 1.47 (0.4–5.38) | 0.557 | |||
| by day 28 | 10 (11.1%) | 7 (8.2%) | OR 1.39 (0.45–4.54) | 0.613 |
| aOR 1.13 (0.4–3.23)) | 0.814 | |||
| Intensive carea | ||||
| by day 14 | 8 (8.8%) | 9 (10.6%) | OR 0.81 (0.26–2.52 | 0.800 |
| by day 28 | 8 (8.9%) | 9 (10.6%) | OR 0.82 (0.26–2.55) | 0.800 |
Proportions and odds ratios excluding patients lost to follow-up; CI, confidence interval; aOR, adjusted (for age as a linear covariate) odds ratio; OR, odds ratio; sHR, subhazard ratio.
Fig. 2.
Cumulative incidence of clinical improvement and death in the amantadine and placebo arms. A, amantadine; P, placebo; sHR, subhazard ratio.
Fig. 3.
Cumulative incidence of clinical improvement and death in the amantadine and placebo arms by duration of symptoms at randomisation.
Fig. 4.
Cumulative incidence of clinical improvement and death in the amantadine and placebo arms by remdesivir treatment at randomisation.
The reason why sensitivity analyses were carried out was the uncertainty whether the use of remdesivir in some recruited patients could have affected the lack of effectiveness of amantadine in the study population. However, the results of the sensitivity analysis indicate that whether or not remdesivir was used, the effect of amatadine compared to placebo on the primary and secondary endpoints was the same, leading to the conclusion that the failure to exclude patients on remdesivir from the study very likely did not affect on the study result.
3.3. Adverse events
Serious adverse events occurred in 23 patients from the amantadine arm and in 29 patients in the placebo arm. The proportions of serious adverse events according to system organ class were similar in the two arms (Table 3 ). Most adverse events were deemed unlikely related to treatment (87% in the amantadine arm; 82.8% in the placebo arm; Table 3). Of the 4 serious adverse events likely related to treatment, there were 2 cardiac disorders (1 in each study arm), 1 psychiatric disorder in the amantadine arm, and 1 gastrointestinal disorder in the placebo arm. None of the 17 deaths was deemed related to treatment. Most of the deaths were caused by COVID-19 deterioration.
Table 3.
Adverse events.
| Amantadine (N = 23) | Placebo (N = 29) | |
|---|---|---|
| Cardiac disorders | 3 (13%) | 2 (6.9%) |
| Gastrointestinal disorders | 0 (0%) | 1 (3.4%) |
| General disorders and administration site conditions | 4 (17.4%) | 1 (3.4%) |
| Infections and infestations | 1 (4.3%) | 3 (10.3%) |
| Injury, poisoning and procedural complication | 0 (0%) | 1 (3.4%) |
| Nervous system disorders | 0 (0%) | 1 (3.4%) |
| Psychiatric disorders | 1 (4.3%) | 0 (0%) |
| Renal and urinary disorders | 0 (0%) | 1 (3.4%) |
| Respiratory, thoracic and mediastinal disorders | 11 (47.8%) | 14 (48.3%) |
| Vascular disorders | 2 (8.7%) | 5 (17.2%) |
| No data | 1 (4.3%) | 0 (0%) |
| Major adverse cardiovascular events | 1 (5%) | 2 (6.9%) |
| Death | 10 (43.5%) | 7 (24.1%) |
| Association with treatment | ||
| Unlikely | 20 (87%) | 24 (82.8%) |
| Unknown | 1 (4.3%) | 3 (10.3%) |
| Likely | 2 (8.7%) | 2 (6.9%) |
| SAEs likely associated with treatment | ||
| Cardiac disorders | 1 (50%) | 1 (50%) |
| Gastrointestinal disorders | 0 (0%) | 1 (50%) |
| Psychiatric disorders | 1 (50%) | 0 (0%) |
| Major adverse cardiovascular events | 0 (0%) | 1 (50%) |
4. Discussion
To our knowledge, our study is the first double-blind, placebo-controlled study to use oral amantadine in addition to standard care in patients hospitalised with COVID-19 who did not need high-flow oxygen or mechanical ventilation on the day of entry to the study (moderate or severe COVID-19 according to WHO severity classification). We proved that amantadine did not show clinical efficacy in those patients.
In this study, we planned to enrol 250 patients each in the amantadine and placebo arms. However, the interim analysis initiated when ∼150 patients were randomized into the study showed no trend for superiority of amantadine, and the steering committee decided to terminate the trial. The interim analysis process took several weeks, so eventually 186 patients hospitalised with COVID-19 were recruited for the study. The intention-to-treat population was representative for patients hospitalised with COVID-19: the mean age was nearly 60 years and about two-thirds had comorbidities, which is consistent with previous reports among hospitalised patients with COVID-19 [17].
Time to recovery, i.e. the primary endpoint, was similar in the amantadine and placebo arms (no significant difference, slight tendency to reduce the likelihood of recovery in the amantadine arm). Due to the discontinuation of the study, we carried out a post hoc sample size analysis (see Supplemental data) which showed that there was no chance of a significant difference in the primary endpoint when 500 participants were enrolled. Therefore, we assess the decision of early study termination as justified. Moreover, even if the study population could be increased to over 500 patients, there was no chance of showing the advantage of amantadine over placebo.
The risk of death was non-significantly increased in the amantadine arm at days 14 and 28. The results of post hoc sample size analysis showed that we would not obtain a significant difference in the risk of death after inclusion of 500 participants. The potential differences in death risk between the two arms in our study would have a chance to be significant only in a sample size of over 3200 participants at 28 days, which are considerably larger than the pre-specified sample size of 500 participants. Additionally, the non-significantly greater risk of death observed in the amantadine arm was, at least partly, due to a greater mean age in the placebo arm (the age-adjusted ORs for death in the amantadine arm vs. placebo were lower than the unadjusted ORs).
Data on the use of amantadine in patients with COVID-19 are scarce. Comparing our findings to those resulting from other studies might be difficult because of the different target groups and the dynamic flow of the pandemic with a predominance of various mutations of the virus. Our study does not support the claims from uncontrolled observational studies that amantadine could provide benefit to patients with COVID-19. In an observational study of 55 ambulatory patients with COVD-19 (mean age 56 years, 64% with comorbidities, 53% with pneumonia), who received 200–500 mg of amantadine daily for several days (exact number not given), at a median of 8 days since symptom onset, 50 (91%) achieved “clinical stabilisation” within 48 h (defined as no symptom progression), 4 (7%) required hospitalisation, and none died [11]. Those investigators concluded that amantadine may help reduce the risk of hospital admission, but the study was uncontrolled. In another study, of 15 ambulatory patients (mean age 48 years) with symptoms of COVID-19 (no PCR confirmation), who received amantadine for 14 days (2 × 100 mg), none required admission to hospital [10]. Among 10 patients with multiple sclerosis and 5 patients with Parkinson's disease, who tested positive for SARS-CoV-2 while on chronic amantadine treatment, none developed symptoms of COVID-19 [12]. The evidence available from these small, uncontrolled studies seems insufficient to support the claim that amantadine reduces the risk of acquiring SARS-CoV-2 infection of hospital admission due to COVID-19. The results of our study suggest that amantadine does not provide significant benefit to patients hospitalised with COVID-19.
The typical daily dose of oral amantadine in the approved indications varies from 100 mg in influenza A infections to 200 mg in Parkinson's disease or fatigue related to multiple sclerosis. We chose the dose of 200 mg daily on days 3–10 but used loading doses of up to 400 mg daily on days 1–2, which proved safe in a study among patients with severe traumatic brain injury [18]. The loading doses were lower in elderly patients, who have reduced amantadine clearance [19]. We chose to give amantadine for 10 days based on a previous study in which a 10-day treatment with remdesivir was able to significantly shorten the median time to discharge among patients hospitalised with COVID-19 (remdesivir, 10 days; placebo, 15 days) [15].
Although the data from subgroups with symptom duration at randomisation of <7 did not show the efficacy trend, still we cannot exclude that amantadine might be beneficial for patients with early or mild COVID-19 symptoms. A clinical study assessing the efficacy of amantadine among patients with mild COVID-19 and shorter symptom duration at randomisation is ongoing, with Prof. Rejdak as supervisor (ClinicalTrials.gov Identifier: NCT04854759).
Our study had limitations. We planned to enrol a total of 500 patients, but terminated the trial early due to a lack of efficacy after enrolling 186 patients. Another limitation is that there was a greater proportion of patients aged 65 years or older in the amantadine group, which could be a confounder and may have resulted in a non-significant proportion of deaths in the amantadine group and a trend of lack of efficacy. The imbalance in age groups results from the fact that randomisation was stratified by remdesivir use or treatment with convalescent plasma, and was not stratified by age. Conversely, we observed a non-significantly greater body mass index and a non-significantly greater proportion of patients with comorbidities in the placebo group.
In conclusion, adding amantadine to standard care in patients hospitalised with COVID-19 did not increase the likelihood of recovery.
Funding/support
Funding for this study was provided by the Polish Medical Research Agency (grant no. 2021/ABM/COVID19/GCM).
Financial/nonfinancial disclosures
AB; MB; ŁB; SzC; JD; MD; RH; MH; SK; AN; JN; WN; GP; MSW; SS; MCM; KW; GZ have nothing to disclose; MF received lectures fee from: Astra Zeneca Poland, Chiesi Poland sp z o.o., Glaxo SmithKline, outside the submitted work;
PK reports personal fees from Adamed, personal fees from AstraZeneca, personal fees from Berlin Chemie Menarini, personal fees from FAES, personal fees from Glenmark, personal fees from Novartis, personal fees from Polpharma, personal fees from Boehringer Ingelheim, personal fees from Teva, personal fees from Zentiva, outside the submitted work.
Role of sponsors
The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions
We wish to thank Rafal Szot and Diana Wójcik from Proper Medical Writing (Warsaw, Poland) for their help in the preparation of this manuscript. We would like to express our deepest appreciation to Ewa Bachta, Dariusz Kogut from CRO company, Klaudia Rogowska.
Take-home points
Study Question: Whether amantadine is effective and safe among patients with different COVID-19 severity classifications.
Results: The median time to recovery and also the percentage of deaths and percentage of patients who required intensive care at 14 and 28 days did not significantly differ between the amantadine and placebo groups.
Interpretation: Adding amantadine to standard care in patients hospitalised with COVID-19 did not increase the likelihood of recovery.
CRediT authorship contribution statement
Adam Barczyk: Conceptualization, Methodology, Formal analysis, Investigation, Supervision, Project administration, Funding acquisition, Writing – original draft, Writing – review & editing. Małgorzata Czajkowska-Malinowska: Conceptualization, Methodology, Formal analysis, Investigation, Supervision, Project administration. Małgorzata Farnik: Conceptualization, Methodology, Formal analysis, Investigation, Supervision, Project administration. Marek Barczyk: Conceptualization, Methodology, Formal analysis, Supervision, Project administration. Łukasz Boda: Conceptualization, Methodology, Formal analysis, Supervision, Project administration. Szczepan Cofta: Conceptualization, Methodology, Formal analysis, Investigation, Supervision, Project administration. Jan Duława: Conceptualization, Methodology, Formal analysis, Investigation, Supervision, Project administration. Maciej Dyrbuś: Conceptualization, Methodology, Formal analysis, Investigation, Supervision, Project administration. Rafał Harat: Conceptualization, Methodology, Formal analysis, Investigation, Supervision, Project administration. Maciej Huk: Conceptualization, Methodology, Formal analysis, Supervision, Project administration. Sylwia Kotecka: Conceptualization, Methodology, Formal analysis, Supervision, Project administration. Artur Nahorecki: Conceptualization, Methodology, Formal analysis, Investigation, Supervision, Project administration. Jacek Nasiłowski: Conceptualization, Methodology, Formal analysis, Investigation, Supervision, Project administration. Wojciech Naumnik: Conceptualization, Methodology, Formal analysis, Investigation, Supervision, Project administration. Grzegorz Przybylski: Conceptualization, Methodology, Formal analysis, Investigation, Supervision, Project administration. Monika Słaboń-Willand: Conceptualization, Methodology, Formal analysis, Supervision, Project administration. Szymon Skoczyński: Conceptualization, Methodology, Formal analysis, Investigation, Supervision, Project administration. Krystian Wita: Conceptualization, Methodology, Formal analysis, Supervision, Project administration, Funding acquisition. Grzegorz Zioło: Conceptualization, Methodology, Formal analysis, Investigation, Supervision, Project administration. Piotr Kuna: Methodology, Formal analysis, Investigation, Supervision, Project administration.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.rmed.2023.107198.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Karlsen A.P.H., Wiberg S., Laigaard J., Pedersen C., Rokamp K.Z., Mathiesen O. A systematic review of trial registry entries for randomized clinical trials investigating COVID-19 medical prevention and treatment. PLoS One [Internet. 2020;15(8) doi: 10.1371/journal.pone.0237903. https://dx.plos.org/10.1371/journal.pone.0237903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Institue of Health https://www.ncbi.nlm.nih.gov/books/NBK570371/pdf/Bookshelf_NBK570371.pdf Coronavirus Disease 2019 (COVID-19) Treatment Guidelines [Internet]. [cited 2022 Apr 4];
- 3.Hay A.J., Wolstenholme A.J., Skehel J.J., Smith M.H. The molecular basis of the specific anti-influenza action of amantadine. EMBO J [Internet. 1985;4(11):3021–3024. doi: 10.1002/j.1460-2075.1985.tb04038.x. http://www.ncbi.nlm.nih.gov/pubmed/4065098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rascol O., Fabbri M., Poewe W. Amantadine in the treatment of Parkinson's disease and other movement disorders. Lancet Neurol [Internet. 2021;20(12):1048–1056. doi: 10.1016/S1474-4422(21)00249-0. https://linkinghub.elsevier.com/retrieve/pii/S1474442221002490 [DOI] [PubMed] [Google Scholar]
- 5.Pucci E., Brañas Tato P., D'Amico R., Giuliani G., Solari A., Taus C. Cochrane Database Syst Rev [Internet; 2007. Amantadine for Fatigue in Multiple Sclerosis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toft-Bertelsen T.L., Jeppesen M.G., Tzortzini E., et al. Amantadine inhibits known and novel ion channels encoded by SARS-CoV-2 in vitro. Commun Biol [Internet. 2021;4(1):1347. doi: 10.1038/s42003-021-02866-9. https://www.nature.com/articles/s42003-021-02866-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fink K., Nitsche A., Neumann M., Grossegesse M., Eisele K.-H., Danysz W. Amantadine inhibits SARS-CoV-2 in vitro. Viruses. 2021;13(4):539. doi: 10.3390/v13040539. https://www.mdpi.com/1999-4915/13/4/539 [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butterworth R.F. Potential for the repurposing of adamantane antivirals for COVID-19. Drugs R. 2021;21(3):267–272. doi: 10.1007/s40268-021-00351-6. https://link.springer.com/10.1007/s40268-021-00351-6 [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiménez-Jiménez F.J., Alonso-Navarro H., García-Martín E., Agúndez J.A.G. Anti-inflammatory effects of amantadine and memantine: possible therapeutics for the treatment of Covid-19? J. Personalized Med. 2020;10(4):217. doi: 10.3390/jpm10040217. https://www.mdpi.com/2075-4426/10/4/217 [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aranda-Abreu G.E., Aranda-Martínez J.D., Araújo R., Hernández-Aguilar M.E., Herrera-Covarrubias D., Rojas-Durán F. Observational study of people infected with SARS-Cov-2, treated with amantadine. Pharmacol. Rep. 2020;72(6):1538–1541. doi: 10.1007/s43440-020-00168-1. https://link.springer.com/10.1007/s43440-020-00168-1 [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bodnar W., Aranda-Abreu G., Slabon-Willand M., Kotecka S., Farnik M., Bodnar J. The efficacy of amantadine hydrochloride in the treatment of COVID-19 - a single-center observation study. Pol Merkur Lekarski [Internet. 2021;49(294):389–393. http://www.ncbi.nlm.nih.gov/pubmed/34919079 [PubMed] [Google Scholar]
- 12.Rejdak K., Grieb P. Adamantanes might be protective from COVID-19 in patients with neurological diseases: multiple sclerosis, parkinsonism and cognitive impairment. Mult Scler Relat Disord. 2020;42 doi: 10.1016/j.msard.2020.102163. https://linkinghub.elsevier.com/retrieve/pii/S221103482030239X [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamel W.A., Kamel M.I., Alhasawi A., Elmasry S., AlHamdan F., Al-Hashel J.Y. Effect of pre-exposure use of amantadine on COVID-19 infection: a hospital-based Cohort study in patients with Parkinson's disease or multiple sclerosis. Front Neurol [Internet. 2021;12 doi: 10.3389/fneur.2021.704186. https://www.frontiersin.org/articles/10.3389/fneur.2021.704186/full [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mancilla-Galindo J., García-Méndez J.Ó., Márquez-Sánchez J., et al. vol. 20. 2021. pp. 199–222.http://www.ncbi.nlm.nih.gov/pubmed/33628159 (All-cause Mortality Among Patients Treated with Repurposed Antivirals and Antibiotics for COVID-19 in Mexico City: A Real-World Observational Study). EXCLI J [Internet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beigel J.H., Tomashek K.M., Dodd L.E., et al. Remdesivir for the treatment of Covid-19 — final report. N Engl J Med [Internet. 2020;383(19):1813–1826. doi: 10.1056/NEJMoa2007764. http://www.nejm.org/doi/10.1056/NEJMoa2007764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Polish Agency for Health Technology Assessment and Tariffs https://www.aotm.gov.pl/covid-19/zalecenia-w-covid-19/ Polish Agency for Health Technology Assessment and Tariffs of 27/11/2020 [Internet]
- 17.Kim L., Garg S., O'Halloran A., et al. Risk factors for intensive care unit admission and in-hospital mortality among hospitalized adults identified through the US coronavirus disease 2019 (COVID-19)-Associated hospitalization surveillance network (COVID-NET) Clin Infect Dis [Internet. 2021;72(9) doi: 10.1093/cid/ciaa1012. https://academic.oup.com/cid/article/72/9/e206/5872581 e206–e214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Giacino J.T., Whyte J., Bagiella E., et al. Placebo-controlled trial of amantadine for severe traumatic brain injury. N Engl J Med [Internet. 2012;366(9):819–826. doi: 10.1056/NEJMoa1102609. http://www.nejm.org/doi/abs/10.1056/NEJMoa1102609 [DOI] [PubMed] [Google Scholar]
- 19.Aoki F.Y., Sitar D.S. Amantadine kinetics in healthy elderly men: implications for influenza prevention. Clin Pharmacol Ther [Internet. 1985;37(2):137–144. doi: 10.1038/clpt.1985.25. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.