Abstract
Background and Aims
Mexico is among the countries with the highest estimated excess mortality rates due to the COVID–19 pandemic, with more than half of reported deaths occurring in adults younger than 65 years old. Although this behavior is presumably influenced by the young demographics and the high prevalence of metabolic diseases, the underlying mechanisms have not been determined.
Methods
The age–stratified case fatality rate (CFR) was estimated in a prospective cohort with 245 hospitalized COVID–19 cases, followed through time, for the period October 2020–September 2021. Cellular and inflammatory parameters were exhaustively investigated in blood samples by laboratory test, multiparametric flow cytometry and multiplex immunoassays.
Results
The CFR was 35.51%, with 55.2% of deaths recorded in middle–aged adults. On admission, hematological cell differentiation, physiological stress and inflammation parameters, showed distinctive profiles of potential prognostic value in patients under 65 at 7 days follow–up.
Pre–existing metabolic conditions were identified as risk factors of poor outcomes. Chronic kidney disease (CKD), as single comorbidity or in combination with diabetes, had the highest risk for COVID–19 fatality. Of note, fatal outcomes in middle–aged patients were marked from admission by an inflammatory landscape and emergency myeloid hematopoiesis at the expense of functional lymphoid innate cells for antiviral immunosurveillance, including NK and dendritic cell subsets.
Conclusions
Comorbidities increased the development of imbalanced myeloid phenotype, rendering middle–aged individuals unable to effectively control SARS–CoV–2. A predictive signature of high–risk outcomes at day 7 of disease evolution as a tool for their early stratification in vulnerable populations is proposed.
Key Words: Chronic comorbidities, Emergency hematopoiesis, COVID–19, Middle adulthood, Inflammation, Chronic kidney disease
Graphical abstract
Introduction
Despite increasing strategies to prevent severe COVID–19, the reduction in fatalities in some vulnerable world regions requires detailed understanding of the biology behind relationships between infection and comorbidities (1). Older age, male sex, obesity, diabetes and chronic kidney disease (CKD) have been highlighted as risk factors for adverse COVID–19 outcomes worldwide (2). Given the crucial influence of host metabolic health on COVID–19 evolution, Mexico faces a dramatic challenge: frequent pre–existing metabolic conditions in its population, hinder acute infection resolution, that largely depends on the prompt response of a functional hematopoietic system. Mexico is both one of the countries with more obese/overweight/diabetic population and one of the countries with the highest excess mortality during the pandemic so far.
One of the biggest challenges in this pandemic has been the early detection of individuals who will struggle to recover from COVID–19 and who would benefit the most from early interventions. Previous studies confirming the association between metabolic diseases and severe COVID–19, have underscored the need for stratification profiles for vulnerable population in Mexico and Latin America (1). Comorbidities and age are crucial components of such profiles, but it should not be taken for granted that Latin populations will behave the same as other more studied, higher income populations. COVID–19 fatalities and hospitalizations have shown important differences: in Mexico, 63% hospitalizations and 50% deaths have been in individuals younger than 65, with an important rise at ages 40–50. Plus, about 67% of individuals who died of COVID–19 in 2020–2022 had at least one preexisting chronic disease and almost 40% had two or more (3). Moreover, the relative risk of COVID–19 death (RRd) contributed by having any comorbidity in Mexicans decreased sharply with age, being substantial in middle–aged adults, but low in adults 65+ (3). In particular, middle aged–adults, who are a large portion of the labor–active population in Mexico, have been a group of concern, after elders, as they show much higher COVID–19 fatality rates, mortality and years of life lost, than similar age–groups in high–income countries. In Latin America, middle–aged adults have a large burden of pre–existing metabolic diseases, yet few prospective studies have focused on them or in the particularities of their COVID–19 pathophysiology.
Given the involvement of emergency hematopoiesis and of the innate immune response in COVID–19 clinical fate (4), connecting these biological mechanisms with the physiological conditions that put patients at risk in middle adulthood, is crucial for their stratification and the development of preventive strategies and targeted therapies. We studied a prospective cohort of COVID–19 hospitalized adults, living in an urban/semi–urban region in central Mexico (Atlixco–Puebla, Mexico), who had not received SARS–CoV–2 vaccination, and that were not considered for intensive care or assisted mechanical ventilation upon admission. We propose a laboratory and hematological panel of predictive value for populations in our region. Our observations suggest a spectrum of gradual health decline due to chronic diseases, that in Mexico undermines the chances of clearing acute infections and inflammatory pathologies in patients younger than 65 yo.
Materials and Methods
Patient Characteristics and Sample Collection
This Research was Performed in Accordance with the Declaration of Helsinki and was approved by the Ethics, Research and Biosafety Committees from the National Committee of Scientific Research at IMSS (R–2020–785–134). All samples were collected after informed consent from a relative. The study was conducted on a cohort of 245 COVID–19 patients (from here on called ‘Atlixco Cohort’), confirmed by a positive real time polymerase chain reaction (PCR) nasopharyngeal test for SARS–CoV–2 or COVID–19 Ag rapid test, who were treated and hospitalized from October 2020 to September 2021, at the Hospital General de Zona 5 or the Hospital General de Zona 20, the two IMSS–reference hospitals for COVID–19 in Puebla, Mexico (Supplementary Table 1). No patients needing invasive mechanical ventilation were included in the study. Patients who had received SARS–CoV–2 vaccination, or who had previous SARS–CoV–2 infections, were excluded.
At Respiratory Triage upon hospital admission, and before any pharmacological treatment, more than 200 clinical parameters were investigated by an exhaustive medical history collected through an epidemiologic survey. Peripheral blood specimens were collected by punction before any treatment and according to international and institutional guidelines. Complete laboratory tests, including blood cell count and indexes, inflammatory and metabolic markers, cytokines, arterial blood gases and immune cell populations, among others, were conducted (Supplementary Table 1). Conventional pneumonia severity scores CURB–65 (confusion, urea, respiratory rate, blood pressure and age ≥65), SOFA (Sequential Organ Failure Assessment), CALL (comorbidity, age, lymphocyte and LDH) and NEWS2 (National Early Warning Score), were used for clinical orientation.
Patient's blood samples with and without K2EDTA anticoagulant were analyzed at three different times: at admission, 7 days after hospital admission and 4 weeks after discharge (convalescence). Complete hemogram, creatinine, C–reactive protein (CRP), lactate dehydrogenase (LDH), fibrinogen, glucose and D–dimer were used as prognostic parameters. Control blood samples were obtained from healthy adults. All freshly isolated peripheral blood cells were immunophenotyped by flow cytometry, while serum samples were stored at –80°C until further analysis. The complete process was performed within the BSL–2+ facility at the Virology laboratory, Centro de Investigación Biomédica de Oriente (CIBIOR), IMSS. For analysis, the cohort was subclassified into four groups: survivors under 65 years old (A), non–survivors under 65 years old (B), survivors over 65 years old (C), non–survivors over 65 years old (D).
Multiparametric Flow Cytometry Analyses of Hematopoietic Cell Populations
White blood cell numbers were determined using Turk's solution to place 1×106 leukocytes per tube. Whole blood cells were incubated with 10% fetal bovine serum (FBS) in phosphate–buffered saline (PBS) during 10 min at 4°C and then washed with phosphate–buffered saline (PBS), before their 30 min incubation at room temperature with the following fluorochrome–coupled antibodies from BioLegend:
Tube 1: CD45–FITC (Clone HI30), CD33–PE (Clone P67.6), CD3–PerCP (Clone HIT3a), CD123–PE/Cyanine7 (Clone 6H6), CD19–APC (Clone HIB19), CD4–APC/Cyanine7 (Clone OKT4), HLA–DR–Pacific BlueTM (Clone L243), CD56–Brilliant Violet 510TM (Clone HCD56). Tube 2: CD45–FITC (Clone HI30), CD3–PerCP (Clone HIT3a), HLA–DR–Pacific BlueTM (Clone L243), TCRγδ–PE (Clone B1), CD8–PE/ Cyanine7 (Clone HIT8a), CD11c–APC (Clone 3.9), CD16–APC/Cyanine7 (Clone 3G8), CD14–Brilliant Violet 510TM (Clone 63D3). Red blood cell lysis buffer (BioLegend # 420301) was added and incubated for 15 min. Cells were centrifuged and washed with PBS. One million events were further acquired for immunophenotyping in a three laser BD FACSCanto II flow cytometer. Analysis of flow cytometry data was performed using the Infinicyt 1.8 software.
Neutrophils were identified as CD45+CD33loCD16+, basophils as CD45+HLA–DR–CD123+, monocytes as CD45+CD33hi CD14+/–CD16+/– and subclassified according to the expression of CD14 and CD16 as classical CD14+CD16–, intermediate CD14+CD16+ and non–classical CD14–CD16+; B cells as CD45+CD19+, T cells as CD45+CD3+ and then subclassified according to the expression of CD4+, CD8+ and TCRγδ+; NK cells as CD45+CD3–CD56+, NKT cells as CD45+CD3+CD56+, plasmacytoid dendritic cells (pDCs) as CD45+HLA–DRhiCD123+ and cDC2 as CD45+HLA–DRhiCD11c+CD14–CD16–.
Cytokines Detection: Chemokines’, cytokines’ and growth factors’ content in serum was investigated by Multiplex Milliplex Map Immunoassay (Merck Millipore), according to the manufacturer's instructions. The following analytes were included in the assay: transforming growth factor–beta (TGF– β), fibroblast growth factor–2 (FGF–2), Fms–related tyrosine kinase 3 ligand (FLT–3L), granulocyte colony–stimulating factor (G–CSF), interferon alpha (IFN–α), interferon gamma–induced protein/C–X–C motif chemokine ligand 10 (IP–10/CXCL-10), monocyte chemoattractant protein 1 (MCP–1), monokine induced by gamma interferon/C–X–C motif chemokine ligand 9 (MIG/CXCL–9), interferon gamma (IFN–γ), interleukin 10 (IL–10), interleukin 1–beta (IL–1β), interleukin 2 (IL–2), interferon–inducible T cell alpha chemoattractant/C–X–C motif chemokine ligand 11 (ITAC/CXCL-11), macrophage inflammatory protein 1–alpha (MIP–1α), stem cell factor (SCF), C–X–C motif chemokine ligand 12 (CXCL–12), interleukin 6 (IL–6), interleukin 8 (IL–8), macrophage migration inhibitory factor (MIF), tumor necrosis factor (TNF–α), vascular endothelial growth factor (VEGF).
Statistical analysis: Quantitative variables are presented as medians (interquartile ranges [IQR]), and nominal variables as absolute counts and proportions (N, %). Kruskal–Wallis or X2 tests were used to compare continuous and categorical variables, respectively. Analyses were conducted using GraphPad Prism version 9.3.0 for Windows (GraphPad Software, La Jolla, California USA). Case–fatality rates (CFR) were calculated as the percent of cases that died from COVID–19. Relative risk of death (RRd) and 95% confidence interval (CI) was calculated with Koopman asymptotic score. The non–parametric Kruskal–Wallis with Dunn's post–test was used to compare continuous variables. p values <0.05 were considered statistically significant.
Results
The Atlixco Cohort: Demographics, Clinical Spectrum and Outcomes Revealing Notable Differences According to Age
A total of 245 patients 21–94 years old, who were admitted for the first time to the participating IMSS hospitals were included in the Atlixco cohort. All clinical parameters, symptoms on admission, comorbidities, blood cell counts, inflammatory markers, hematological values, arterial blood gases and pneumonia severity scales, are shown in Supplementary Table 1 and Supplementary Figure 1. Infected individuals who had received any SARS–CoV–2 vaccination dose(s), as well as patients previously treated for COVID–19 or admitted to the intensive care unit or receiving mechanical ventilation assistance, were excluded.
The median age of the Atlixco cohort was 58 years (IQR 48–71 years) and 64.49% of the patients were males. The case fatality rate (CFR) of the whole cohort was 35.51%, with males showing the higher CFR values (38.61%). Accordingly, male participants showed a relative risk of COVID–19 death (RRd), 1.29, (95% CI: 0.90–1.90, p = 0.21) compared to females (Supplementary Figure 1, Supplementary Table 2).
Since age is a crucial factor in COVID–19 outcomes, the Atlixco cohort was divided in two groups for analysis: adults 21–64 years old (yo) (from here on called “under 65” or “middle–aged patients”) and adults 65–94 years old (“65+” or “elderly”). Strikingly, 60% of the patients were under 65 yo and 55.2% of the deaths were recorded in this group, similar to national frequencies. Both, the incidence of fatal and recovering cases peaked at ages 50–59 (Supplementary Figure 1, Supplementary Table 2).
As expected, the most frequent symptoms on admission were cough (77.55%), fever (75.51%), dyspnea (70.61%), myalgia (69.80%) and arthralgia (68.57%), whereas arterial hypertension (43.27%), diabetes (35.92%), obesity (23.67%), dyslipidemia (8.98%) and CKD (8.57%), were the most frequently reported comorbidities (Supplementary Table 1).
For stratification and follow–up, blood samples were taken in 3 occasions: at admission, after 7 days in hospital, and 4 weeks after discharge. This allowed us to search for potential signatures through time, among survivors (S) and non–survivors (NS), under and over 65 years (Figure 1 ). The cohort was thus sub–classified into 4 groups: A. Survivors under 65 yo (21–64 yo), B. Non–survivors under 65 yo (21–64 yo), C) Survivors 65+ (65–94 yo) and D) Non–survivors 65+ (65–94 yo).
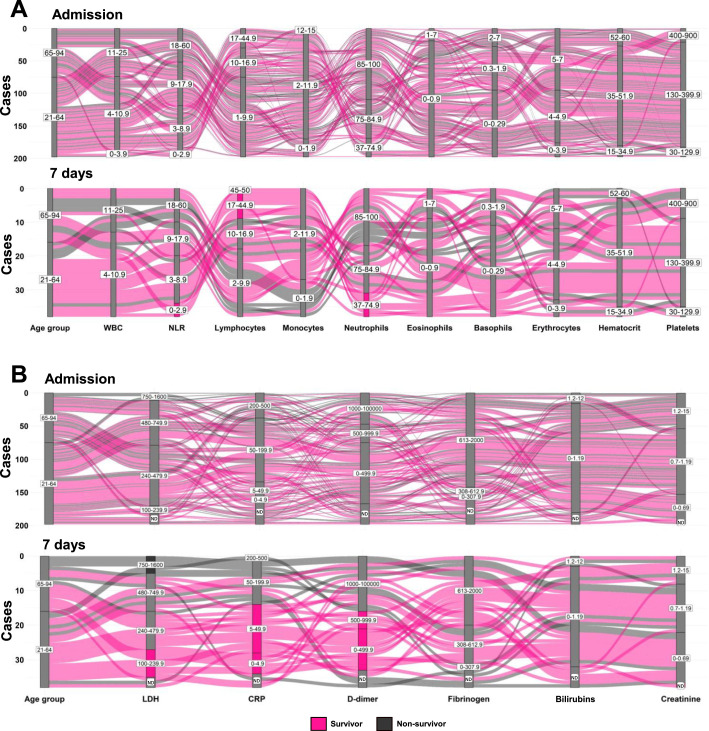
Figure 1.
Patients under 65 and over 65 years of age differ in hematologic values that predict outcomes. A. Alluvial plots for hematological cell populations in patients under 65 or over 65. No apparent relation between hematological parameters and outcomes is recorded at admission, while after 7 days, case clusters are obvious for some conditions. In elderly, higher WBC counts and NLR, low levels of lymphocytes and monocytes, and high levels of neutrophils and eosinophils relates to poor or fatal outcomes. Meanwhile, survivors are more frequent at younger ages, concomitant to low WBC and NLR and high numbers of lymphocytes. B. Alluvial plots for inflammatory markers expression at admission and followed by 7 days in survivors and deceased patients. Prediction of outcomes according to inflammatory parameters is clearer in middle age than in older adults. Low values of LDH, CRP and D–dimer are associated with better outcomes. Reference values: white blood cells (WBC): 4–11×103 cells/µL, neutrophil/lymphocyte ratio (NLR): 1–3, Lymphocytes: 17–45%, Monocytes: 2–12%, Neutrophils: 37–75%, Eosinophils: 1–7%, Basophils: 0.3–2%, Erythrocytes: 4–5×106 cells/µL, Hematocrit: 35–52%, Platelets: 130–400×103 cells/µL, lactate dehydrogenase (LDH): 240–480 U/L, C–reactive protein (CRP): 0–5 mg/L, D–dimer: 0–500 U/L, Fibrinogen: 308–613 mg/dL, Bilirubin: 1–1.2 mg/dL, Creatinine: 0.7–1.2 mg/dL. ND, no data.
The identification of potential clinical patterns over time was addressed by alluvial plotting of the cellular laboratory parameters (Figure 1A and Supplementary Figure 2A) and inflammatory markers (Figure 1B and Supplementary Figure 2B) at admission and upon 7 days hospitalized. Alluvial representations allow the integrative comparison of parameters assigned as vertical axes and their values (in blocks), whose sizes vary according to the proportion of cases that pass through. We defined block length using reference values, framed by inferior or superior blocks, according to population behavior. At admission, no apparent relation of outcomes was detected in the cellular parameters, while after 7 days, an outcome trend was observed, where survivors (in pink) displayed normal WBC counts, lower NLR values, higher lymphocytes and near normal numbers of monocytes. Importantly, clustering profiles from patients show that after 7 days, three cellular parameters, independently or in combination, are associated with favorable outcomes: NLR value <3, lymphocytes ≥17% and/or neutrophils <75%. This suggests that the primitive differentiation pathway of hematopoietic cells at the stage of early multipotent progenitors of megakaryoblast, erythroid and granulocytic cells is overactivated, at the expense of the oligopotent monocytic/lymphoid differentiation pathway. Further investigations will be crucial to unravel the identity of the primitive cell involved in disease pathogenesis, as well as the early innate immunosurveillance precursor cell, for targeted therapeutic development. Moreover, the exhaustion of the myeloid compartment due to extended neutrophil production (emergency hematopoiesis) may contribute to the observed profile. Regarding inflammatory parameters, again a pattern that predicted outcomes was not obvious at admission, although we observed a big flow stream (high number of cases), consisting of both survivor and non–survivor, through the blocks CRP>5 and 613–2000 fibrinogen. Then, upon 7 days an outcome pattern is identified with survivors showing lower LDH, CRP and D–dimer than non–survivors. These three inflammatory parameters, independently or in combination, seem to mark fate decisions in hospitalized middle–aged adults with COVID–19. While good prognosis is associated to LDH <240, CRP <50 and/or D–dimer <1000, poor prognosis is observed in patients with LDH >750. Thus, an outcome profile in COVID–19 patients integrated by six laboratory parameters –NLR, lymphocytes, neutrophils, LDH, CRP and D–dimer– may help patient stratification and clinical management.
Risk of COVID–19 Fatality and Poor Prognosis Hematological Profiles is Increased by Pre–existing Metabolic Conditions
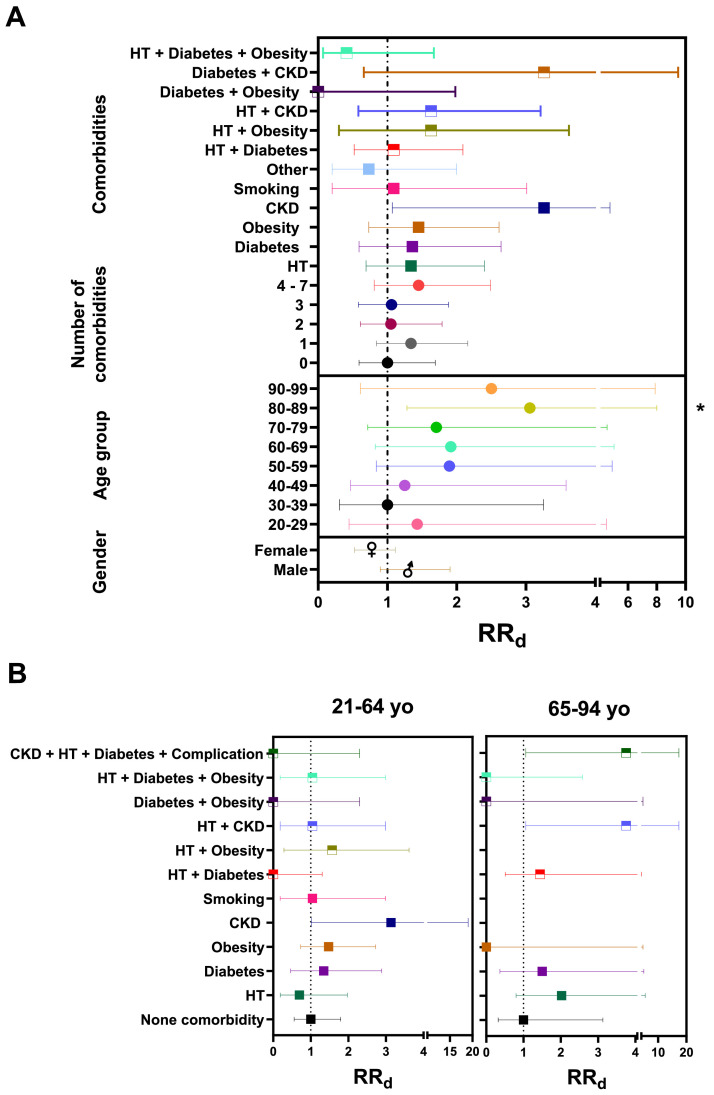
Of note, 74.69% patients and 78.16% of non–survivors in this cohort had at least one preexisting metabolic condition (comorbidity). By calculating the relative risk of COVID–19 death (RRd) we observed that RRd increases with age and with some comorbidities (Figure 2 A and Supplementary Table 2), with higher risk in patients with more than three comorbidities (RRd = 1.45, 95% CI: 0.81 – 2.49, p = 0.23) (Supplementary Table 2). CFR and RRd increased with age after 40 years old, with significant risk of death at ages 80–89 yo (RRd = 3.06, 95% CI: 1.28–8.00, p = 0.02) and more than a fourth of deaths were in the group 50–59 yo, which had RRd = 1.89 (95% CI: 0.84 – 4.90, p = 0.18), although this did not reach significance. A tendency for higher risk was observed with the combination of 4–7 pre–existing comorbidities (RRd = 1.45, 95% CI: 0.81 – 2.49, p = 0.23), with chronic kidney disease (CKD) presenting the highest RR for COVID–19 fatality (3.26, 95% CI: 1.07 – 4.75, p = 0.10) alone or in combination with diabetes or HT. Obesity, diabetes, and HT also present a RRd higher than 1 (1.45, 1.36 and 1.34, respectively). Hypertension in combination with CKD or obesity presented a higher RRd, than in combination with diabetes. Smoking did not increase risk of death in this cohort (RRd = 1.09, 95% CI: 0.20 – 3.01, p = >0.99) (Supplementary Table 2). As reported before (3), our findings confirm a higher risk in males (RRd 1.29, 95% IC: 0.90 – 1.90, p = 0.21), compared to female patients (0.77, 95% IC:0.53 – 1.11, p = 0.21).
Figure 2.
Morbi–mortality of the Atlixco cohort. A. Relative risk of death (RRd) by gender, age group, number and combination of comorbidities is depicted. Dotted vertical line marks RRd = 1. RRd were calculated by gender as male vs female; by age group as each age group vs 30–39 yo group (age group with <CFR); by the number of comorbidities and as a single or in multiple comorbidities combination vs the group with no comorbidity. B. RRd with the same parameters as A, according to age group. HT, hypertension; CKD, chronic kidney disease. *p <0.05.
The combination of four pre–existing conditions, CKD, hypertension, diabetes, and a diabetes complication, provided risk for patients over 65 years, but not under 65 yo. This might be due to the low frequency of this combination in the middle–aged group, who may have lower prevalence of diabetes complications since they have had less time to develop them or do not yet have them diagnosed. However, under age 65, a tendency for increased risk with comorbidities such as diabetes, obesity and obesity with hypertension (Figure 2B), suggests an induced aging of the hematopoietic system favored by the comorbidity or by lifestyle, that increases with age to generate inflammatory catastrophes. Furthermore, CKD was an important risk factor for death in middle–aged adults, followed by the combination of HT and obesity, then obesity and diabetes. While in the elderly group, the combination of HT and CKD or CKD/HT/Diabetes/Complication of diabetes had the highest risk of death followed by HT and diabetes. In our study, 75% of the deaths in 20–29 yo had CKD only. Thus, CKD is an important risk factor for attention from an early age in our regional population.
Next, we explored if abnormal laboratory parameters in patients with pre–existing metabolic conditions related to COVID–19 fatality (Supplementary Table 2). Strikingly, at hospital admission, patients with CKD and with NLR values ≥3 showed 2.69 risk of fatality (95% IC: 0.88–15.85, p = 0.15), higher than patients with the same NLR but no comorbidities. Furthermore, patients with diabetes, CKD, HT + Obesity or with CKD/HT/Diabetes/Complication of diabetes and with D–dimer ≥500, had RRd = 2.8. The risk of death increased three times in patients affected by CKD or HT + Obesity and CRP ≥5. After 7 days of hospitalization, patients with HT, diabetes, or obesity and with NLR ≥3 had 2.5 times higher risk of death, while patients with obesity and a D–dimer ≥500 or CRP ≥5 had five times higher risk of fatal outcomes.
When analyzing blood cell parameters in the context of comorbidities, at admission, we found that the combinations of neutrophils ≥75% and smoking, or NLR ≥3 and hypertension, increased the risk of death. This risk of death increased even more when we compared the same parameters 7 d after admission (Supplementary Table 2). Neutrophils ≥75% or NLR ≥3, increased the RRd with diabetes, obesity, and hypertension, while lymphocytes <17 only increased the risk with obesity and hypertension.
Chronic Comorbidities in Middle Adulthood Contribute to Inflammation, Hematopoietic Emergency of Clinical Risk and Death
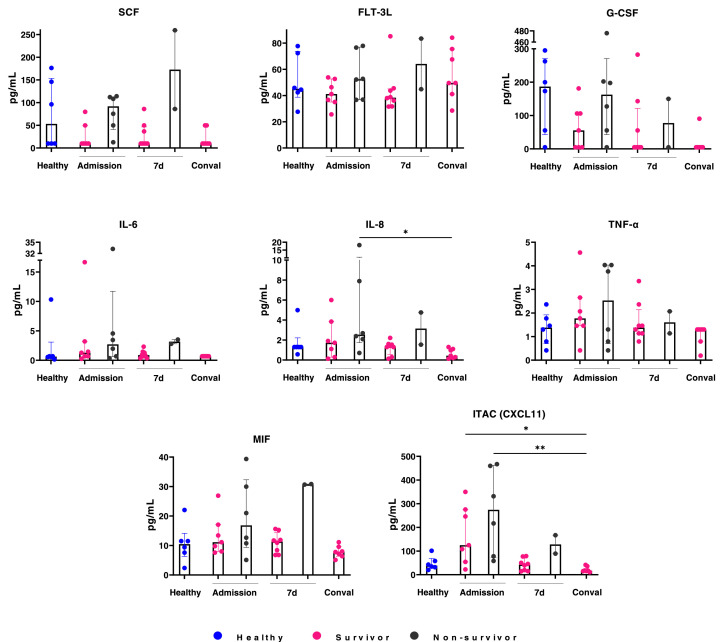
Cytokine storm has been reported in COVID–19 patients. We therefore evaluated a panel of chemokines, cytokines and growth factors in COVID–19 serum samples from the cohort at the three different time points (Figure 3 ). In the middle–aged group at admission, proinflammatory mediators such as IL–6, IL–8, TNF–α, MIF and CXCL-11 showed an increasing trend in COVID–19 patients compared to healthy individuals. Furthermore, SCF, FLT–3L, IL–6, IL–8, MIF, GCS–F, TNF–α and CXCL-11 levels were consistently higher in non–survivor than in survivor patients. Two distinct dynamics in the cytokine production throughout the study time were recorded in the non–survivor patients: a persistent increase of SCF, FLT–3L, IL–6, IL–8 and MIF levels, and at 7 days of hospitalization a decreased in GSC–F, TNF–α and CXCL11. An inflammatory environment is suggested by the results in middle–aged, non–survivor patients.
Figure 3.
An inflammatory profile is revealed in middle–aged non–survivors. Cytokine concentration at admission, 7 days and 4 weeks later were analyzed for patients under 65 years old. Conval, convalescence; d, days.
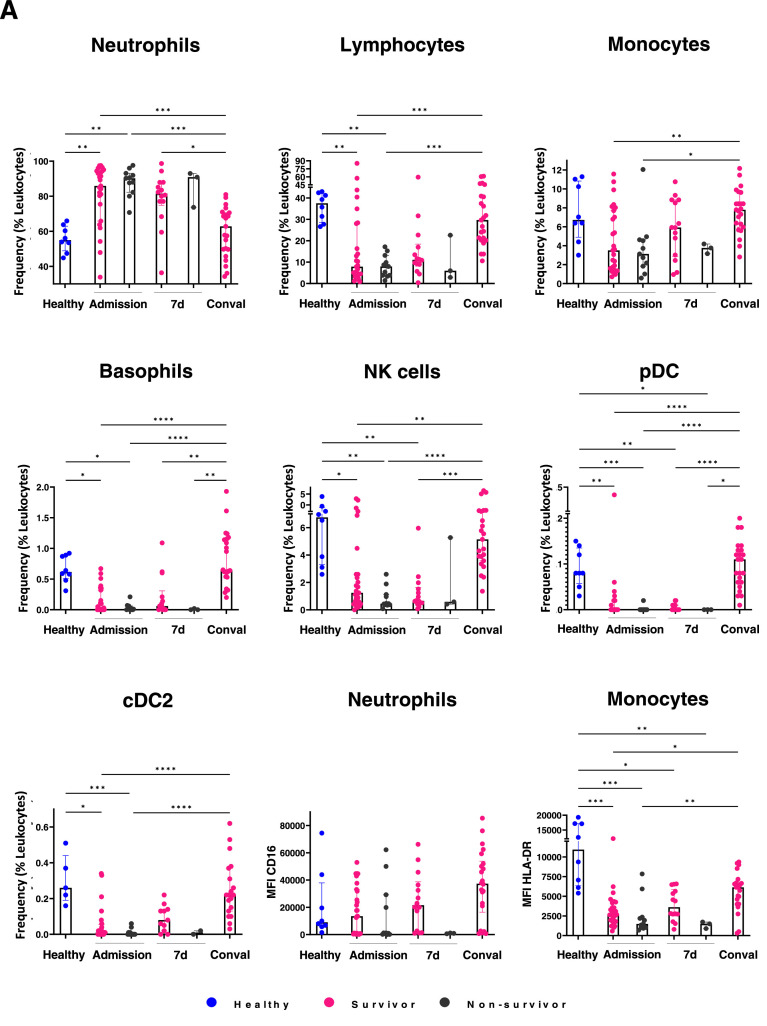
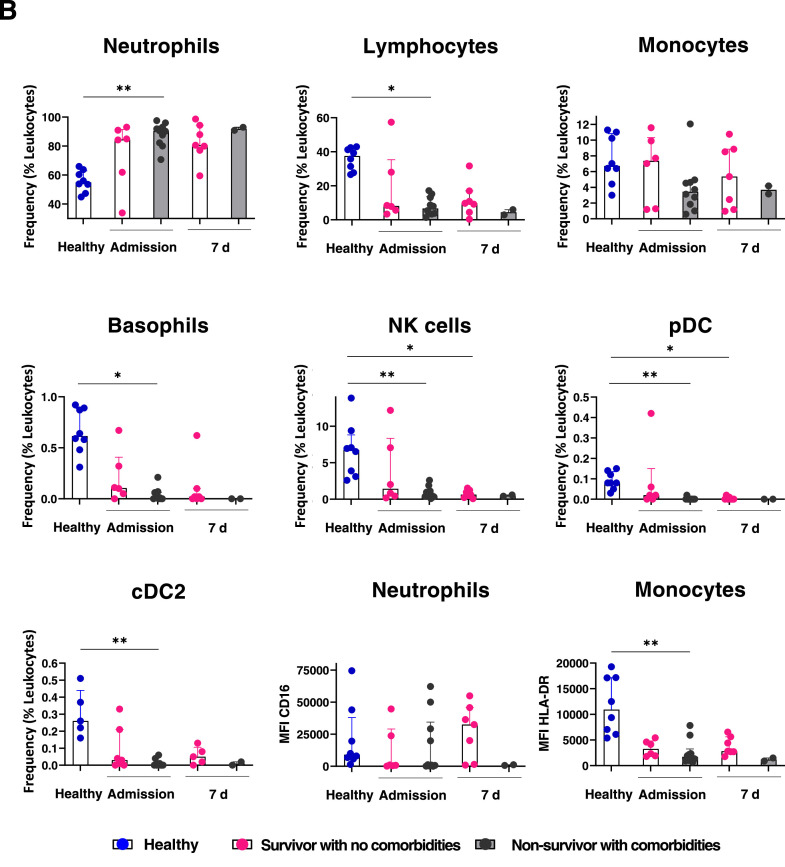
To investigate the alterations of COVID–19 in the immunological innate landscape, we evaluated the content of innate hematopoietic cell populations in COVID–19 blood samples by multiparametric flow cytometry. Cellular frequencies within the leukocyte compartment in the middle–aged group are shown (Figure 4 A). At admission, a significant increase in neutrophils concomitant to a substantial reduction in lymphocytes, NK cells, basophils, pDC (plasmacytoid dendritic cells) and CD11c+ cDC2 (type 2 dendritic cells) are observed in COVID–19 patients vs healthy individuals. Moreover, lymphoid lineage cells including NK and pDCs showed a pronounced reduction in non–survivors, suggesting that emergency lymphopoiesis in response to SARS–CoV–2 is not effectively established in those patients, driving to adverse outcomes. Myeloid–lineage cells potentially produced by effective emergency hematopoiesis are also severely reduced in non–survivors. Strikingly, the discrepancies in emergency cell frequencies between survivors and non–survivors are highest at day 7, when cell populations like cDC2 are close to normality. The depletion of pDC and cDC2 in non–survivors and their significant increase in convalescence, even higher than the values shown by healthy individuals, suggest the relevance of these populations for recovery (Figure 4A).
Figure 4.
Poor outcome in middle–aged patients is related to myeloid emergency hematopoiesis at the expense of innate immune response effector cells. Immune cell populations were evaluated in middle–aged patients by flow cytometry at admission, 7 days and 4 weeks later. In contrast to survivor patients, significant decrease in lymphocytes, monocytes, NK cells, pDC, CD11c+ DC (cDC2), basophils and functional neutrophils and monocytes is observed in non–survivors. CD16 and HLA–DR expression levels in neutrophils and monocytes, respectively, in poor outcome patients are shown A. Chronic comorbidities contribute to the pathogenic myeloid emergency hematopoiesis along to a defective lymphoid emergency compartment in middle aged COVID–19 patients. Hematological cell frequencies and CD16 and HLA–DR expression levels in neutrophils and monocytes, respectively, in COVID–19 survivors with no comorbidities are compared to their counterpart in non–survivors with comorbidities, at admission and upon 7 days of hospitalization. Healthy donor values are shown as control. Myeloid– and lymphoid– lineage cells related to emergency hematopoiesis are shown in B. MFI, median fluorescence intensity; d, days; Conval, convalescence.
The neutrophil subset from non–survivors barely displayed CD16 and low expression of HLA–DR were observed in monocytes (Figure 4A), strongly suggesting that myeloid linage emergency hematopoiesis is associated to COVID–19 immunopathogenesis in non–survivors and maintained by the high frequency of immature neutrophil precursors and HLA–DRlo monocytes (5). Both, CD16 and HLA–DR expression levels are lowest at day 7 upon admission, while at the convalescence phase, normal expression is apparent, as expected in a hematopoietic system with high regulatory capacity. Similar frequencies of emergency myelopoiesis–associated neutrophils were recorded in middle–aged non–survivor Mexican patients with chronic comorbidities (Figure 4B).
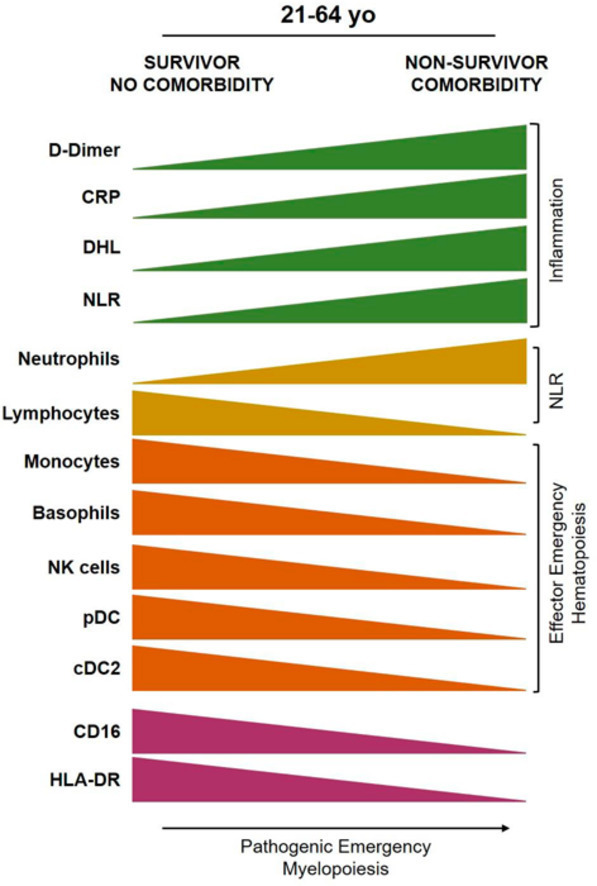
Finally, a pathogenic profile consisting of scarce or null innate NK cells, pDCs, cDC2, basophils and monocytes, was clearly shown in middle–aged non–survivor Mexican patients with chronic comorbidities (Figure 4B). Overall, our study demonstrates for the first time that ineffective lymphoid–lineage emergency hematopoiesis in parallel to a pathogenic mechanism of emergency myelopoiesis are associated to COVID–19 fatal outcomes of middle–aged patients with inflammatory chronic comorbidities. Based on clinical observations and the exhaustive cell immunophenotyping in the Atlixco cohort, with special emphasis on patients suffering from a pre–morbid condition, we propose a predictive signature of high–risk outcomes at day 7 of disease evolution (Graphical Abstract).
Discussion
Aging is the main risk factor for severe COVID–19. Mexico, along with Latin countries -such as Peru, Ecuador and Bolivia, have reported among the highest COVID–19 mortalities and excess deaths in the world (6), despite having populations younger than world average (3). In these countries, half of COVID–19 deaths have occurred in adults 20–64 yo, with mortality rates at those ages, several–fold higher than in high–income countries with similar large outbreaks (3). This has been attributed in part to younger population pyramids with smaller proportions of seniors and to poorer health systems. However, the pathobiology behind the phenomenon of younger COVID–19 deaths in Mexico and Latin America, has not been sufficiently explored. For the first time, we evaluated the contribution of chronic comorbidities to the emergency hematopoiesis response that determines COVID–19 outcomes, in a prospective cohort followed through time, to provide a tool for their early stratification.
The expectation that young populations will trigger an effective response against SARS–CoV–2, is based on the presumption of non–aged hematological and immune systems. However, myeloid emergency hematopoiesis, favored by pre–existing population health conditions, can impact COVID–19 outcomes even in the young and middle–aged (7). This impact has been disproportionate in Latin America (LA), where high rates of chronic metabolic diseases are disturbing, with >57% adults overweight, >19% obese, 8–13% with diabetes (8., 9., 10.) higher than global prevalence of hypertension (world, 31.1%; LA, around 40% in adults >35 yo) and a higher burden of CKD than expected for the level of development (11).
Mexico leads the world prevalence of overweight/obesity (71.3%) and diabetes (15.7%) (7,12,13). Additionally, the Mexican region of Puebla–Tlaxcala leads the world in CKD mortality rates, YLL (Years of Life Lost) and DALYs (Disability Adjusted Life Years) for CKD due to the growing burden of primary causes (diabetes, hypertension) and to potential environmental contributors from river water contamination (14., 15., 16., 17.). In our cohort, 8.6% patients suffer CKD, the single comorbidity that provided the most risk of fatal COVID–19 in patients under 65 yo. Furthermore, over a third of cohort patients had diabetes, and almost a quarter were obese, rendering it an optimal sample to explore how chronic metabolic and inflammatory conditions impact emergency hematopoiesis during severe COVID–19 at different ages. As in other world regions, studies in Mexico and Peru have associated these chronic diseases with the risk of severe COVID–19, finding more risk by the comorbidity in patients under 50–60 yo (1,3,18., 19., 20., 21., 22.), but the underlying biological mechanisms have not been determined in detail, in particular for middle–aged adults.
Emergency hematopoiesis, triggered by acute systemic infections, is required to control SARS–CoV–2. Through this seminal and demand–adapted mechanism, primitive undifferentiated populations respond to agonistic signals from toll–like receptor (TLR) ligands and pro–inflammatory elements, to differentiate toward innate myeloid or lymphoid cells (23). In aging, a bias toward myeloid populations is observed that can result in ineffective innate responses towards pathogens like SARS–CoV–2, but that had not been explored in young Latin COVID–19 cohorts in association to comorbidities. Inflammation exerts a profound effect across the entire hematopoietic hierarchy, activating and mobilizing effector cells, at the time of promoting hematopoietic stem and progenitor cells (HSPCs) development to replenish depleted lineages (24). However, along with altered immune–cell functions, inflammation is one of the key features shared by a number of chronic diseases (25). Obesity is characterized by secretion of inflammatory mediators like IL–6, IL–1, leptin, TNF–α and MCP–1, while patients with diabetes also present elevated circulating levels of acute phase proteins and inflammatory cytokines (26). Of note, a positive association between levels of neutrophils >3.6×109/L in patients with diabetes and CKD incidences has been recently observed by Zhang R et al. Neutrophils contribute to insulin resistance through neutrophil elastase, leading to a sequential degradation of insulin receptor substrate 1, hyperglycemia, neutrophil counts increase and their migration to the injury site of the glomerular basement membrane that ultimately trigger the inflammatory cascade and further chemotaxis of mononuclear macrophages. The neutrophil elastase contributes to CKD due to renal cell damage in patients with type 2 diabetes (27). Obesity may alter granulopoiesis and neutrophil maturation within the bone marrow, by stimulating myelopoiesis and myeloid cell–derived–ROS with adipose tissue and promoting progenitor cell expansion during emergency granulopoiesis. Strikingly, neutrophil–produced ROS, triggers the release of neutrophil extracellular DNA traps (NETs) and facilitates the influx of tumor cells from peripheral circulation due to loss of endothelial adhesions. Obesity is linked to several lung inflammatory conditions, including acute respiratory distress syndrome (ARDS), chronic obstructive pulmonary disease and asthma. In ARDS, obesity promote vascular injury trough the increment of leukocyte adhesion molecules (E–selectin and ICAM–1) and reduction of junctional adhesions (VE–cadherin and β–catenin) in the lung endothelium (28). The pro–inflammatory environment of individuals with chronic diseases exacerbate inflammation resulting in the activation of a demand–adapted hematopoiesis (26). This is true for diabetes and obesity, where the proinflammatory conditions aggravate infections, favor altered blood counts, and promote high IL–6, CRP, and ferritin levels in COVID–19 patients (29).
SARS–CoV–2 can be recognized by toll–like receptors. While TLR7 and TLR8 bind ssRNA, TLR3, RIG–like receptors and MDA–5 sense dsRNA, and TLR2, TLR4 and TLR6 sense the viral Spike protein (4). By conducting scRNAseq, Taniguchi–Ponciano et al found that critically ill COVID–19 patients developed emergency granulopoiesis with predominance of immature myeloid cells in the peripheral blood, with altered immune response and high Toll like receptor–2 and –4 (TLR2 and TLR4) expression, related to SARS–CoV–2 sensing (30). Interestingly, in metabolic diseases such as obesity, fatty acids and glucose can lead to macrophage activation by their interaction with TLR2 and TLR4 (31). Moreover, monocytes in obese donors have shown high TLR4 and TLR8 expression and IL–1β and TNF pro–inflammatory cytokine secretion, upon TLR ligand (LPS or ssRNA) stimulation (26). Obese patients also have higher total monocytes and a pro–inflammatory and immune–suppressive monocyte profile that contributes to low–grade–inflammation and infection susceptibility (32). Granulopoiesis and monopoiesis are induced in primitive hematopoietic populations upon TLR7, TLR8, TLR2, TLR3 and TLR4 stimulation. Severe COVID–19 derived monocytes have upregulated transcripts related to TLR responses such as CEBPβ, IRF1 and FOSL2, suggesting the development of trained immunity (33,34). TNF-α, IL–1β and M–CSF induce myeloid differentiation via up–regulation of Pu.1, while IFNs stimulate expression of Batf2 and Cebpβ, two key transcription factors also promoting myeloid differentiation. IL–6 acts primarily at the level of MPP4s, redirecting their output from lymphoid to myeloid linages (24). Thus, multiple of the mechanisms triggered by chronic inflammation and comorbidities, favor myeloid emergency hematopoiesis.
Maturing neutrophil precursors gradually acquire surface membrane expression, including CD11b (myelocyte stage), CD16 (at transition from myelocyte to metamyelocyte stage), CD13, and CD10 (banded to segmented neutrophils), giving as a result the mature profile CD11bhi CD16hi CD13hi CD10pos. During inflammatory conditions, mature neutrophils and their immediate precursors (bands) reach the peripheral blood due to massive tissue demand (35). Patients with severe/critical COVID–19, present a high number of immature low–density granulocytes (LDG) due to emergency myelopoiesis (20), which are involved in neutrophil extracellular traps (NETs) production and a pro–inflammatory response in macrophages (36). Accordingly, low expression of CD16 in neutrophils, a highlight of immature LDG, corresponds with this phenomenon in our NS patients. Furthermore, low expression levels of HLA–DR in blood monocytes, a hallmark of severe disease, has been found in our cohort (Figure 4), suggesting an inadequate and prolonged monocyte activation (37). This phenomenon has been related to decreased capacity to kill pathogens and immunosuppression in sepsis, as well as exhausted T cells and dendritic cells with reduced capacity to present antigens (38). In obesity, it has been suggested that the pro–inflammatory cytokines may contribute to the loss of HLA–DR expression in monocytes (39). In addition, emergency myelopoiesis in severe COVID–19 is also characterized by mobilization of dysfunctional and immature myeloid cells including neutrophils with increased CD64 and CD274 (PD–L1) expression, and HLA–DRloCD14+ monocytes with high MAFB, PLBD1 and CD163 levels. This neutrophil profile is associated with T cell suppression, whereas the monocyte profile with anti–inflammatory macrophage functions. A profound depletion of non–classical monocytes is observed in all COVID–19 patients (data not shown) (5). As an immediate consequence of systemic inflammation, the CXCL12–CXCR4 axis, which strongly binds long and short–term hematopoietic stem cells to the bone marrow stroma, is disrupted, with seminal and progenitor cells being prompted to their migration to periphery, where they patrol and can conduct extramedullary hematopoiesis also may return to the bone marrow to reprogram the hematopoiesis from a steady state to emergency. Accordingly, high absolute numbers and cell frequencies of LDG neutrophil precursors were recorded in our cohort, indicating pathogenic emergency myelopoiesis concomitant to high production of pro–inflammatory mediators. Thus, the decrease in CD16 and HLA–DR expression levels on neutrophils and monocytes, respectively seems to be a predictor of unfavorable outcome in patients with COVID–19.
Natural killer (NK) cells have an important host protection role in antitumor and antiviral immunity, via perforins, granzymes, IFN–γ, TNF-α, IL–6 and GM–CSF production. NK cells induce self–destruction of virus–infected cells by apoptosis and set the maturation of dendritic and T cells (37). A reduction in NK cells and in their cytotoxic capabilities is reported in individuals with obesity (38). In severe COVID–19 patients, significantly reduced NK cells were reported in peripheral blood mononuclear cells as compared to age–matched non–severe COVID–19 patients (40). Dendritic cells (DCs) have been reported to be reduced during COVID–19, either as pDCs or cDCs, as well as deficiencies in these cells through the time after SARS–CoV–2 infection (41,42). Strikingly, our study demonstrates in such a vulnerable group of patients, a substantial reduction of all innate lymphoid cells previously reported as effectively promoted by emergency hematopoiesis upon viral infections, that is NK cells, pDC and cDCs (reviewed in 4, 23), a condition that may impair not only pathogen clearance but the effective establishment of physiological hematopoiesis and adaptive immune responses.
Conclusions
Overall, the harsh socioeconomic conditions and disparities in LA condition a large proportion of adults to develop (diagnosed or undiagnosed) chronic diseases early during adulthood, with profound effects on lifespan and DALYs and with early deterioration of overall health. In the Mexican population, the consequences of chronic metabolic disease, permeate hematopoietic health and contribute to high COVID–19 fatality rates in middle–aged adults. Fatal outcomes in middle–aged patients were marked from admission by emergency myeloid hematopoiesis at the expense of functional lymphoid innate cells for antiviral immunosurveillance. We showed that comorbidities increased the development of such imbalanced myeloid phenotype, rendering middle–aged individuals unable to effectively control SARS–CoV–2, and providing evidence for a gradual immune–health decline, associated to chronic diseases, that could be occurring earlier in Mexico than in other world populations. The dramatic impact of comorbidities as risk factors for severe COVID–19 (43,44), highlights the need for public health changes, not only limited to this pandemic, but centered in favoring healthy diets/lifestyles and in eliminating environmental harms (45).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
RRR and GZH acknowledge the scholarship provided by CONACYT. The authors acknowledge the Oncoimmunology and Cytomics Lab members for their critical assistance, and the professional staff from COVID–19 reference hospitals at IMSS. No external funding.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.arcmed.2023.03.003.
Appendix. Supplementary materials
References
- 1.Denova–Gutiérrez E, Lopez–Gatell H, Alomia–Zegarra JL, et al. The Association of Obesity, Type 2 Diabetes, and Hypertension with Severe Coronavirus Disease 2019 on Admission Among Mexican Patients. Obesity. 2020;28:1826–1832. doi: 10.1002/oby.22946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li Y, Ashcroft T, Chung A, et al. Risk factors for poor outcomes in hospitalised COVID–19 patients: A systematic review and meta–analysis. J Glob Health. 2021;11:1–11. doi: 10.7189/jogh.11.10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dominguez–Ramirez L, Rodriguez–Perez F, Sosa–Jurado F, et al. The role of metabolic comorbidity in COVID–19 mortality of middle–aged adults. The case of Mexico. medRxiv. 2020:1–30. https://www.medrxiv.org/content/10.1101/2020.12.15.20244160v2.full Available from. (Accessed December 17, 2020) [Google Scholar]
- 4.Balandrán JC, Zamora–Herrera G, Romo–Rodríguez R, et al. Emergency Hematopoiesis in the Pathobiology of COVID–19: The Dark Side of an Early Innate Protective Mechanism. J Interf Cytokine Res. 2022;42:393–405. doi: 10.1089/jir.2022.0028. [DOI] [PubMed] [Google Scholar]
- 5.Schulte–Schrepping J, Reusch N, Paclik D, et al. Severe COVID–19 Is Marked by a Dysregulated Myeloid Cell Compartment. Cell. 2020;182 doi: 10.1016/j.cell.2020.08.001. 1419–1440.e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karlinsky A, Kobak D. Tracking excess mortality across countries during the covid–19 pandemic with the world mortality dataset. Elife. 2021;10:1–21. doi: 10.7554/eLife.69336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shuchman M. Low– and middle–income countries face up to COVID–19. Nat Med. 2020;26:986–988. doi: 10.1038/d41591-020-00020-2. Available from. [DOI] [PubMed] [Google Scholar]
- 8.Avilés–Santa ML, Monroig–Rivera A, Soto–Soto A, et al. Current State of Diabetes Mellitus Prevalence, Awareness, Treatment, and Control in Latin America: Challenges and Innovative Solutions to Improve Health Outcomes Across the Continent. Curr Diab Rep. 2020;20 doi: 10.1007/s11892-020-01341-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lopez–Jaramillo P, Lopez–Lopez J, Cohen D, et al. Epidemiology of Hypertension and Diabetes Mellitus in Latin America. Curr Hypertens Rev. 2020;17:112–120. doi: 10.2174/1573402116999200917152952. [DOI] [PubMed] [Google Scholar]
- 10.Steenblock C, Schwarz PEH, Ludwig B, et al. COVID–19 and metabolic disease: mechanisms and clinical management. Lancet Diabetes Endocrinol. 2021;9:786–798. doi: 10.1016/S2213-8587(21)00244-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bikbov B, Purcell CA, Levey AS, et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pizuorno A, Fierro NA. Latin America and chronic diseases: A perfect storm during the COVID–19 pandemic. Ann Hepatol. 2021;22 doi: 10.1016/j.aohep.2021.100332. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Encuesta Nacional de Salud y Nutrición – ENSANUT. Available from: https://ensanut.insp.mx/encuestas/ensanut2018/index.php. (Accessed August 27, 2020).
- 14.Villalvazo P, Carriazo S, Martin–Cleary C, Ortiz A. Aguascalientes: one of the hottest chronic kidney disease (CKD) hotspots in Mexico and a CKD of unknown aetiology mystery to be solved. Clin Kidney J. 2021;14:2285–2294. doi: 10.1093/ckj/sfab136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Castresana GP, Flores VT, Reyes LL, et al. Atoyac River pollution in the metropolitan area of Puebla. México. Water (Switzerland) 2018;10 [Google Scholar]
- 16.Mora A, García–Gamboa M, Sánchez–Luna MS, et al. A review of the current environmental status and human health implications of one of the most polluted rivers of Mexico: The Atoyac River. Puebla. Sci Total Environ. 2021;782 doi: 10.1016/j.scitotenv.2021.146788. Available from. [DOI] [PubMed] [Google Scholar]
- 17.Hernandez–Ramirez AG, Martinez–Tavera E, Rodriguez–Espinosa PF, et al. Detection, provenance and associated environmental risks of water quality pollutants during anomaly events in River Atoyac, Central Mexico: A real–time monitoring approach. Sci Total Environ. 2019;669:1019–1032. doi: 10.1016/j.scitotenv.2019.03.138. Available from. [DOI] [PubMed] [Google Scholar]
- 18.Cordero–Franco HF, De La Garza–Salinas LH, Gomez–Garcia S, et al. Risk Factors for SARS–CoV–2 Infection, Pneumonia, Intubation, and Death in Northeast Mexico. Front Public Heal. 2021;9:1–6. doi: 10.3389/fpubh.2021.645739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albarrán–Sánchez A, Ramírez–Rentería C, Anda–Garay JC, et al. Differences in mortality rate among patients hospitalized with severe COVID–19 according to their body mass index. Obes Sci Pract. 2021:1–10. doi: 10.1002/osp4.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Torres–Ruiz J, Pérez–Fragoso A, Maravillas–Montero JL, et al. Redefining COVID–19 Severity and Prognosis: The Role of Clinical and Immunobiotypes. Front Immunol. 2021;12:1–13. doi: 10.3389/fimmu.2021.689966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramírez–Soto MC, Alarcón–Arroyo M, Chilcon–Vitor Y, et al. Association between obesity and covid–19 mortality in peru: An ecological study. Trop Med Infect Dis. 2021;6 doi: 10.3390/tropicalmed6040182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oyelade T, Alqahtani J, Canciani G. Prognosis of COVID–19 in Patients with Liver and Kidney Diseases: An Early Systematic Review and Meta–Analysis. Trop Med Infect Dis. 2020;5 doi: 10.3390/tropicalmed5020080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Welner RS, Pelayo R, Kincade PW. Evolving views on the genealogy of B cells. Nat Rev Immunol. 2008;8:95–106. doi: 10.1038/nri2234. [DOI] [PubMed] [Google Scholar]
- 24.Collins A, Mitchell CA, Passegue E. Inflammatory signaling regulates hematopoietic stem and progenitor cell development and homeostasis. J Exp Med. 2021;218:1–11. doi: 10.1084/jem.20201545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bagatini MD, Cardoso AM, Reschke CR, Carvalho FB. Immune system and chronic diseases 2018. J Immunol Res. 2018:2018. doi: 10.1155/2018/8653572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kapellos TS, Bonaguro L, Gemünd I, et al. Human monocyte subsets and phenotypes in major chronic inflammatory diseases. Front Immunol. 2019;10:1–13. doi: 10.3389/fimmu.2019.02035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang R, Chen J, Xiong Y, et al. Increased neutrophil count Is associated with the development of chronic kidney disease in patients with diabetes. J Diabetes. 2022;14:442–454. doi: 10.1111/1753-0407.13292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McDowell SAC, Luo RBE, Arabzadeh A, et al. Neutrophil oxidative stress mediates obesity–associated vascular dysfunction and metastatic transmigration. Nat Cancer. 2021;2:545–562. doi: 10.1038/s43018-021-00194-9. [DOI] [PubMed] [Google Scholar]
- 29.Ahmad Malik J, Ahmed S, Shinde M, et al. The Impact of COVID–19 On Comorbidities: A Review Of Recent Updates For Combating It. Saudi J Biol Sci. 2022;29:3586–3599. doi: 10.1016/j.sjbs.2022.02.006. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Taniguchi–Ponciano K, Vadillo E, Mayani H, et al. Increased expression of hypoxia–induced factor 1α mRNA and its related genes in myeloid blood cells from critically ill COVID–19 patients. Ann Med. 2021;53:197–207. doi: 10.1080/07853890.2020.1858234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Resende DP, da Costa AC, de Souza Rosa LP, et al. Non–classical circulating monocytes in severe obesity and obesity with uncontrolled diabetes: A comparison with tuberculosis and healthy individuals. Tuberculosis. 2019;114:30–41. doi: 10.1016/j.tube.2018.11.003. Available from. [DOI] [PubMed] [Google Scholar]
- 32.Friedrich K, Sommer M, Strobel S, et al. Perturbation of the monocyte compartment in human obesity. Front Immunol. 2019;10:1–10. doi: 10.3389/fimmu.2019.01874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vadillo E, Taniguchi–Ponciano K, Lopez–Macias C, et al. A Shift Towards an Immature Myeloid Profile in Peripheral Blood of Critically Ill COVID–19 Patients. Arch Med Res. 2021;52:311–323. doi: 10.1016/j.arcmed.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Torres–Ruiz J, Absalón–Aguilar A, Nuñez–Aguirre M, al Pet. Neutrophil extracellular traps contribute to COVID–19 hyperinflammation and humoral autoimmunity. Cells. 2021;10 doi: 10.3390/cells10102545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Orfao A, Matarraz S, Pérez–Andrés M, et al. Immunophenotypic dissection of normal hematopoiesis. J Immunol Methods. 2019;475 doi: 10.1016/j.jim.2019.112684. Available from. [DOI] [PubMed] [Google Scholar]
- 36.Vázquez–Jiménez A, Avila–Ponce De León UE, Matadamas–Guzman M, et al. On Deep Landscape Exploration of COVID–19 Patients Cells and Severity Markers. Front Immunol. 2021;12:1–34. doi: 10.3389/fimmu.2021.705646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cruz–Zárate D, Cabrera–Rivera GL, Ruiz–Sánchez BP, et al. Innate Lymphoid Cells Have Decreased HLA–DR Expression but Retain Their Responsiveness to TLR Ligands during Sepsis. J Immunol. 2018;201:3401–3410. doi: 10.4049/jimmunol.1800735. [DOI] [PubMed] [Google Scholar]
- 38.O'Shea D, Hogan AE. Dysregulation of natural killer cells in obesity. Cancers (Basel) 2019;11:1–12. doi: 10.3390/cancers11040573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bao Y, Mo J, Ruan L, et al. Increased monocytic CD14+HLADRlow/– myeloid–derived suppressor cells in obesity. Mol Med Rep. 2015;11:2322–2328. doi: 10.3892/mmr.2014.2927. [DOI] [PubMed] [Google Scholar]
- 40.Mishra KP, Singh AK, Singh SB. Hyperinflammation and Immune Response Generation in COVID–19. Neuroimmunomodulation. 2021;27:80–86. doi: 10.1159/000513198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pérez–Gómez A, Vitallé J, Gasca–Capote C, et al. Dendritic cell deficiencies persist seven months after SARS–CoV–2 infection. Cell Mol Immunol. 2021;18:2128–2139. doi: 10.1038/s41423-021-00728-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Galati D, Zanotta S, Capitelli L, et al. A bird's eye view on the role of dendritic cells in SARS–CoV–2 infection: Perspectives for immune–based vaccines. Allergy Eur J Allergy Clin Immunol. 2022;77:100–110. doi: 10.1111/all.15004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bae SA, Kim SR, Kim MN. Impact of cardiovascular disease and risk factors on fatal outcomes in patients with COVID–19 according to age: A systematic review and meta–analysis. Heart. 2021;107:373–380. doi: 10.1136/heartjnl-2020-317901. [DOI] [PubMed] [Google Scholar]
- 44.Alqahtani JS, Oyelade T, Aldhahir AM, et al. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID–19: A rapid systematic review and meta–analysis. PLoS One. 2020;15:1–13. doi: 10.1371/journal.pone.0233147. Available from. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Halpern B, Louzada ML, da C, Aschner P, et al. Obesity and COVID–19 in Latin America: A tragedy of two pandemics—Official document of the Latin American Federation of Obesity Societies. Obes Rev. 2021;22:1–12. doi: 10.1111/obr.13165. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.