Abstract
Viral infections are some of the most common sources of respiratory illness in pediatric and adult populations worldwide. Influenza and coronaviruses are viral pathogens that could lead to severe respiratory illness and death. More recently, respiratory illness from coronaviruses, accounts for more than 1 million deaths in the United States alone. This article will explore the epidemiology, pathogenesis, diagnosis, treatment, and prevention of severe acute respiratory syndrome caused by coronavirus-2, and Middle Eastern respiratory syndrome.
Keywords: Coronavirus, SARS-CoV2, MERS, Influenza, Severe acute respiratory syndrome
Key points
-
•
Despite advancements in vaccines and antimicrobial development, life-threatening pathogens continue to emerge and reemerge globally.
-
•
Globalization, climate change and human encroachment into nature accelerate emergence, and spread of infectious diseases.
-
•
Microbial adaptation and breakdown of public health measures are also major drivers for emergence and reemergence of human pathogens.
-
•
An integrated approach to global surveillance, vector control, early detection, drug and vaccine development, public hygiene and sanitation, public education and health policy interventions will be essential.
-
•
Pandemic preparedness plans should be developed at all levels to facilitate rapid responses, prevent spread, and reduce morbidity and mortality.
Abbreviations.
| ORFs | Open reading frames |
| NSPs | Nonstructural proteins |
| NP | Nasopharyngeal |
| OP | Oropharyngeal |
| CT | Computed tomography |
| CRP | C-reactive protein |
| CXR | Chest x-ray |
Introduction
Viral infections are a source of respiratory illness in pediatric and adult populations worldwide. Influenza and coronaviruses (CoV) are some of the most common viral pathogens leading to respiratory illness and death. Prevalence of mortality rates from influenza range from 291,243 to 645,832 per year worldwide1 , 2 and 12,000 to 52,000 in the United States (US).3 More recently, respiratory illness from CoV severe acute respiratory syndrome (CoV-SARS) accounts for more than 1 million deaths in the United States alone.4 Although infections with respiratory viral pathogens are not selective, they are most detrimental to vulnerable populations such as the very young, elderly, and those with comorbid conditions. Generally, viral pathogens cause respiratory symptoms such as cough and shortness of breath but other organ systems can become compromised. Treatment is primarily supportive with antivirals and anti-inflammatory pharmacotherapeutics. However, vaccination is the best method for preventing disease complications and death. This article will explore the epidemiology, pathogenesis, diagnosis, treatment, and prevention of severe acute respiratory syndrome caused by coronavirus 2 (SARS-CoV2), and Middle Eastern respiratory syndrome (MERS).
SEVERE ACUTE RESPIRATORY SYNDROME-CORONAVIRUS 2 (SARS-COV2)
Prevalence and Mortality
The global and US incidence and prevalence of SARS-CoV2 varies due to population demographics, individual comorbidities, as well as emergence of new viral strains. Currently, the US Centers for Disease Control and Prevention (CDC) reports a total of approximately 89.1 million cumulative cases since the initial occurrence of the virus in the US, with a daily average of more than 126,000 cases per 7-day moving average as of July 2022.4 , 5 Although death counts from SARS-CoV2 vary on a weekly basis, common risk factors include patient demographics such as age 60 years and older (85%), male gender (66%), history of smoking, as well as comorbid conditions including hypertension, diabetes, heart disease, chronic kidney or lung disease, and malignancy.6 Patients reporting a history of smoking are more likely to experience acute respiratory distress, which is a primary cause of death in hospitalized patients with SARS-CoV2. Social isolation among both community-dwelling and hospitalized individuals infected with SARS-CoV2 presents a significant psychological concern. Additionally, their loss from the workforce, and the resulting decline in productivity, negatively influences national and global economies.
Pathogenesis
SARS-CoV2 is a member of the Coronaviridae family. It is an enveloped positive-stranded RNA virus containing a large genome, ranging from 26 to 32 kilobases.7 , 8 The virion is spherical in shape with a core shell and luminous surface protein projections that give it the appearance of a solar crown (Latin: corona = crown). The genome contains 14-open reading frames (ORFs) that encode for 27-proteins.9 The 5′-terminus of the genome encodes for 15-nonstructural proteins (NSPs), which are responsible for the transcription and replication of the SARS-CoV2 genome.9 The 3′-terminus of the genome contains genes that encode for the 4-surface structural proteins, as well as 8-other proteins with unknown functional purpose.9 The function of the 4-structural proteins is as follows: the N-protein is responsible for the viral replication as well as the host’s response to the virus; the S-protein facilitates viral binding to susceptible host cells; the M-protein aids in creating networks within the host cell that produce viral particles; the E-protein assists with the production and release of viral particles into the host cell.7 , 9 , 10
SARS-CoV2 enters the host by attaching to receptors in cells that express angiotensin-converting enzyme 2 (ACE-2). SARS-CoV2 affinity for human ACE-2 cells is greater than that of SARS-COV.8 Cells that express ACE-2 can be found in the lungs, gastrointestinal (GI) tract, medullary sections of the brain, epithelial cells, as well as cardiovascular, and immune systems; therefore, symptoms can indicate single or multiple system compromise (Table 1 ). However, most of the pathologic findings indicate respiratory compromise. Early lung changes include pulmonary edema, protein exudation, vascular congestion, pneumocyte hyperplasia, and interstitial thickening. Late pulmonary changes may include injury of alveoli, formation of fibrous exudates, desquamation, and hyaline membrane formation (ie, acute respiratory distress syndrome [ARDS]).10 Respiratory failure without subjective perception of dyspnea (silent hypoxemia) has been reported, and is associated with hypocapnia caused by compensatory hyperventilation.11
Table 1.
| Organ System | Common Symptoms | Severe Symptoms |
|---|---|---|
| Systemic | Fever/chills Fatigue Myalgias |
|
| Respiratory | Cough Shortness of breath Sputum production Chest tightness Congestion or runny nose Sore throat |
Dyspnea Hemoptysis Hyperventilation Severe respiratory distress |
| Cardiovascular | Not commonly present | Disseminated thrombosis Acute ischemic stroke |
| Neurologic | Headache New loss of sense of taste or smell Foggy mentation (COVID brain) |
Altered consciousness Neurologic deficits |
| Gastrointestinal | Nausea/vomiting Diarrhea Anorexia |
Abdominal pain |
| Skin | Rash (purpuric vascular, urticarial, vesicular)14 |
The virus may also infiltrate occasional lymphocytes in the esophageal squamous epithelium, as well as plasma cells and lymphocytes in the lamina propria of the stomach, duodenum, and rectum.10 , 12
Risk Factors and Complications
Risk factors for SARS-CoV2 include older age, gender, and presence of comorbid conditions. Although most patients infected with the virus are reported to be aged between 30 and 79 years, poor prognosis and death is more common in the elderly (ie, aged 80 years or older).6 Being male has also been associated with greater risk for infection, although no causative link has been reported thus far. Additionally, comorbid conditions associated with greater risk for SARS-CoV2 infection include diabetes, chronic lung disease, cardiovascular disease, immunocompromising conditions, chronic kidney disease, and obesity.6 Careful history taking can identify patients at greatest risk and prevent complications.
SARS-CoV2 complications include multisystem organ failure including ARDS and respiratory failure, sepsis and septic shock, arrhythmia, acute cardiac injury, myocarditis, heart failure, cardiac tamponade, coagulopathy, and acute renal injury.15 Heart failure, myocardial infarction, arrhythmias, and cardiac arrest occur more frequently in patients with associated pneumonia. Coagulopathy such as acute venous thromboembolism, deep vein thrombosis, and pulmonary embolism have been reported in patients with SARS-CoV2 infection.15 Analysis suggests multifactorial causes of coagulopathy in these patients including older age, more severe illness, more chronic illness, stasis, and high thrombotic and inflammatory abnormalities.16 In severe COVID-19 disease, hypercoagulability can be stimulated by endothelial cell dysfunction, increased blood viscosity from hypoxia.15 Additionally, patients who concomitantly experienced an acute ischemic stroke were found to have an elevated d-Dimer, fibrinogen, and the presence of antiphospholipid antibodies, although the mechanism of action has yet to be determined.13
Screening and Diagnosis
The preferred standard method for detecting the presence of SARS-COV2 is the reverse transcriptase polymerase chain reaction test (RT-PCR).17 The RT-PCR is a version of nucleic acid amplification testing (NAAT) used to amplify the genetic material of the collected sample to detect presence of viral RNA.18 Recommendations for aspirate collection include nasopharyngeal (NP) and oropharyngeal (OP) swabs for asymptomatic persons, and bilateral anterior nares collection for those who are symptomatic. Other recommended aspirates for diagnosis include tracheal or bronchoalveolar lavage aspirates. However, because the use of bronchoscopy poses the risk for exposure to patients and health-care providers, it is recommended only for intubated patients and if diagnosis, using upper airway aspirate, is uncertain.11 Overall sensitivity (Sn) of RT-PCR tests for SARS-CoV2 is reported in the 45% to 60% for nasal and NP specimens.19 A major reason for the high rate of false-negative RT-PCR results is the timing of sampling relative to the onset of symptoms. Studies have shown that the median false-negative rate decreases from 67% 1 day before symptom onset to 38% on the day of symptom onset and to 20% by days 3 and 4 postonset of symptoms. Thereafter, Sn declines and the false-negative rate is 100% by day 8 to 14. Therefore, the optimal days for RT-PCR testing via nasal or NP swab are days 3 to 4 after symptom onset, and asymptomatic testing can be expected to have a significantly high rate of false-negative results. Thus, clinical and epidemiologic data should be factored into clinical decision-making, and if there is a high index of suspicion for SARS-CoV2, a negative RT-PCR alone cannot rule out infection. Repeat testing over several days is recommended to improve test sensitivity. According to the US Food and Drug Administration, the specificity (Sp) of available RT-PCR tests for SARS-CoV2 is 100%.18
Antigen tests are immunoassays that detect the presence of the viral antigen, indicating current infection with SARS-CoV2. Antigen tests currently include point-of-care, laboratory-based, and self-tests.17 Antigen tests can be used at point-of-care because they produce results in 15 to 30 minutes. Similar to RT-PCR and other NAAT tests, antigen tests require the collection of NP, nasal, or saliva specimens from symptomatic persons. However, antigen tests are less sensitive than NAAT tests, meaning they should be used with caution in asymptomatic individuals. The Sn of antigen tests for SARS-CoV2 varies considerably between asymptomatic and symptomatic persons (58.1% and 72%, respectively).18 Similarly, Sn is also highly variable between brands, ranging from 34.1% to 88.1%. Average Sp is high among all patients and brands at 99.6%.18 Studies have shown some antigen tests to be as effective as viral culture in detecting the presence of SARS-CoV2.20 Thus, they may be useful as a biomarker to detect contagiousness. However, they may provide false-negative results early in the disease process and are not used routinely for this purpose in clinical practice.
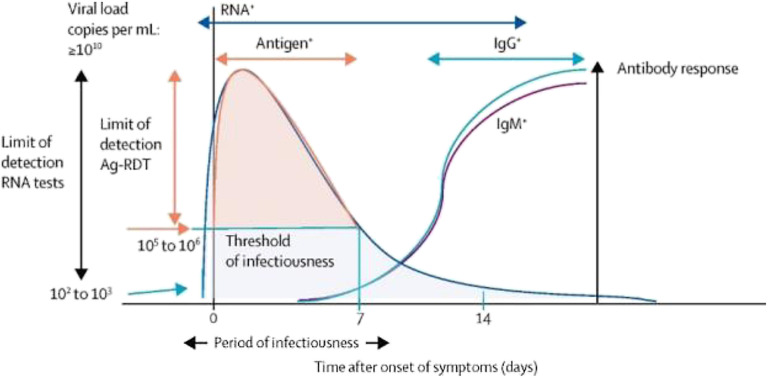
Antibody tests, such as the SARS-CoV2 rapid IgG–IgM combined antibody test, can be used to detect antibodies against the virus in persons who have been previously infected or vaccinated. Antibody tests are not recommended for the diagnosis of acute SARS-CoV2 but can be used to identify an earlier infection. However, antibody testing is not recommended to determine immunity to SARS-CoV2 due to either an earlier infection or vaccination. Indeed, new strains of the virus may predispose individuals to become infected or require booster vaccination to sustain immunity against the virus.21 Antibody testing may be used to support the presumptive diagnosis of COVID-19 in persons who present with SARS-CoV2 symptoms greater than 8 days after onset.21 Available antibody tests include chemiluminescence assay (CLIA), enzyme-linked immunosorbent assay (ELISA) and lateral flow immunoassay (LFIA). CLIA has the highest Sn/Sp (92% and 99%), followed by ELISA (86% and 99%), and then LFIA (78% and 98%).22 Table 2 reviews the advantages and disadvantages of tests for SARS-CoV2 infection. Figs. 1 and 2 illustrate the optimal timing of diagnostic tests for SARS-CoV2 infection and algorithms for SARS-CoV2 testing in symptomatic and asymptomatic persons.
Table 2.
Advantages and disadvantages of tests to detect the presence of severe acute respiratory syndrome caused by coronavirus11,17, 18, 19, 20, 21, 22, 23
| Test | Use | Advantages | Disadvantages |
|---|---|---|---|
| Molecular test (RT-PCR, NAAT) |
|
|
|
| Antigen rapid detection tests |
|
|
|
| Antibody test |
|
|
|
Fig. 1.
The viral dynamics of, and antibody response to, SARS-CoV-2 infection in a patient who is symptomatic, and the optimal timeframe for deployment of different types of tests.
Reprinted with permission from Elsevier. The Lancet, 2022, 399: 757-68.
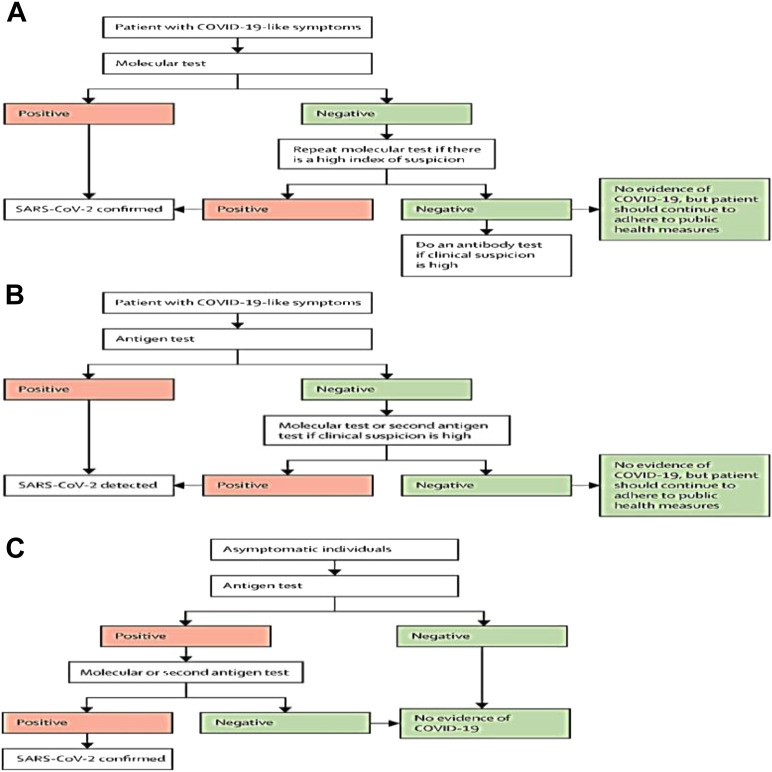
Fig. 2.
Algorithms for testing to detect SARS-CoV-2 in symptomatic and asymptomatic individuals. (A) Preferred testing algorithm for individuals with COVID-19-like symptoms. (B) Testing algorithm for individuals with COVID-19-like symptoms when molecular testing is not available, or results are delayed. (C) Testing algorithm for COVID-19 case finding among asymptomatic individuals.
Reprinted with permission from Elsevier. The Lancet, 2022, 399: 757-68.
Other Diagnostic Testing
Laboratory testing, chest x-ray, and chest computed tomography (CT) are useful diagnostic tests in the evaluation of confirmed or suspected SARS-CoV2 infection.8 A study of almost 1100 hospitalized patients with SARS-CoV2 showed variable white blood cell count. Leukopenia (<4000/mm3) was identified in 33.7%, normal leukocyte counts (4000–10,000/mm3) in 60.4%, and leukocytosis (>10,000/mm3) in 5.9%. Lymphopenia (<1500/mm3) occurred in 83.2%. Thrombocytopenia was identified in 36.2% of the cases. The most common abnormal serologic findings in this study were elevated C-reactive protein (CRP; 60.7% of patients), elevated lactase dehydrogenase (41.0%), and increased d-Dimer (46.4%).24 A meta-analysis of 43 studies including 3600 patients showed the most common abnormal laboratory findings were elevated CRP (68.8%), lymphopenia (57.4%), and elevated lactate dehydrogenase (LDH) (51.6%).25 Leukopenia, lymphopenia, thrombocytopenia, elevated CRP, elevated LDH, and increased d-Dimer were all more prevalent in patients with severe disease versus patients with nonsevere disease.24 , 25
Chest CT has been found to have moderate-to-high Sn (67%–100%) but low Sp (25%–80%) for the diagnosis of SARS-CoV2. Chest x-ray is less sensitive than CT, with a reported Sn of 69%.26 However, CT is not available in all parts of the world, and the use of chest CT in cases of suspected SARS-CoV2 infection is associated with higher radiation exposure, infection control issues related to patient transport, and delays due to the need for CT room decontamination. Therefore, the American College of Radiology recommends that CT should not be used as a first-line test for the diagnosis of SARS-CoV2 and should be reserved for hospitalized, symptomatic patients with specific clinical indications for CT.27
Chest x-ray (CXR) remains the primary imaging modality used in the diagnosis and evaluation of patients with SARS-CoV2; and positive chest x-ray findings can preclude the need for chest CT.28 The most common chest x-ray and CT findings in SARS-CoV2 infection are lung consolidation and ground glass opacities. These findings typically have a bilateral, peripheral, and lower lung distribution.28 Ground glass densities observed on CT may often have a correlate that is difficult to detect on CXR (Fig. 3 ). One of the most specific features of SARS-CoV2 pneumonia is the high prevalence of peripheral lung opacities (present in 33%–86% of patients), which often are multifocal and can be readily seen on chest radiograph (Fig. 4 ). Lung opacities can rapidly evolve into a diffuse pattern within weeks after symptom onset, often peaking at around 6 to 12 days (Fig. 5 ) and may result in diffuse air space disease (ARDS; Fig. 6 ).28
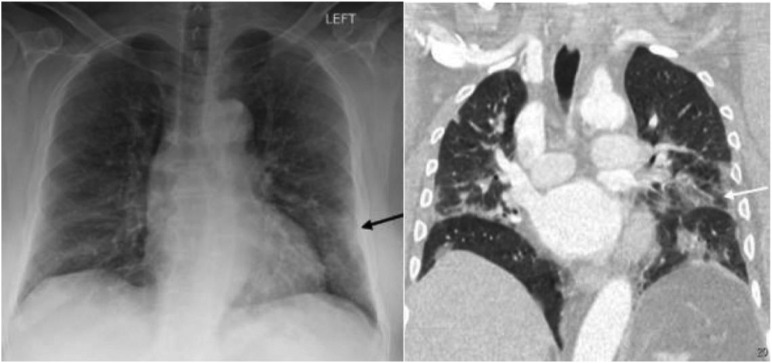
Fig. 3.
CXR (left) with patchy peripheral left mid to lower lung opacities (black arrow) corresponding to ground glass opacities (white arrow) on coronal image from contrast-enhanced the contemporaneous chest CT (right).
(Reprinted from Jacobi, et al.28)
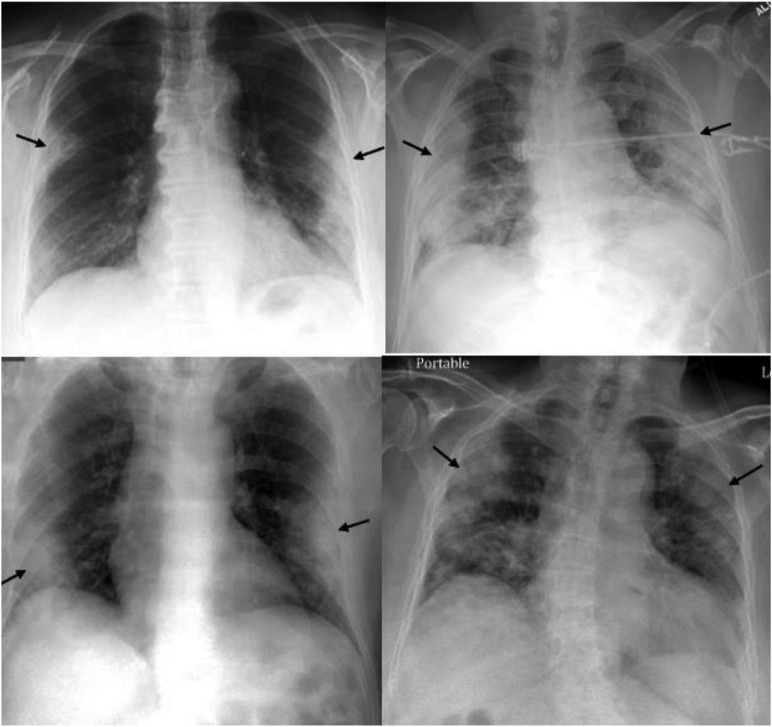
Fig. 4.
Four different patients with varying degrees of COVID-19 pneumonia on CXR predominantly involving the peripheral lungs bilaterally (black arrows).
(Reprinted from Jacobi, et al.28)
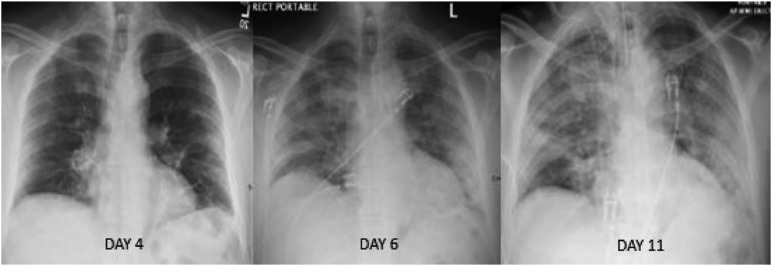
Fig. 5.
Serial radiographs during 7 days in a patient with COVID-19 infection depicting the progression of diffuse lung disease that ultimately required intubation.
(Reprinted from Jacobi, et al.28)
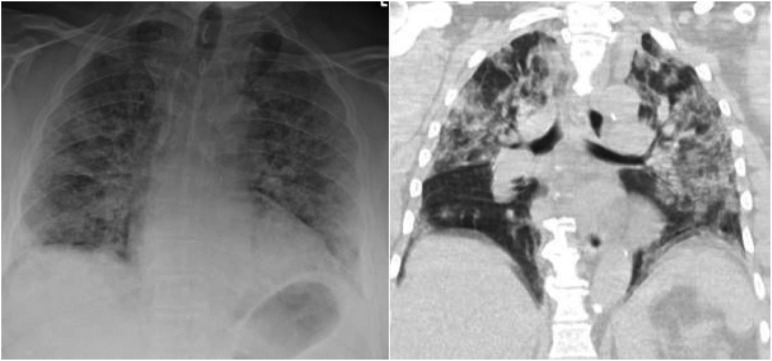
Fig. 6.
CXR (left) and subsequent coronal image from chest CT (right) performed in a patient with COVID-19 and diffuse ground glass and consolidative opacities throughout both lungs.
(Reprinted from Jacobi, et al.28)
Pharmacologic Management of COVID-19
Clinical management of patients with COVID-19 is based on determination of outpatient versus inpatient treatment, disease severity, and the patient’s risk of disease progression. Disease severity is classified as asymptomatic/presymptomatic, mild, moderate, severe, and critical illness (Box 1 ).29
Box 1. Clinical spectrum of COVID-19 illness29.
| Severity | Criteria |
|---|---|
| Asymptomatic/presymptomatic | Positive test, no symptoms consistent with COVID-19 |
| Mild | Any COVID-19 symptoms without shortness of breath, dyspnea, or abnormal chest imaging |
| Moderate | Evidence of lower respiratory disease on imaging or examination and SpO2 ≥94% on room air |
| Severe | SpO2 < 94% on room air, Pao2/Fio2 <300 mm Hg, respiratory rate >30 breaths/min, or lung infiltrates >50% |
| Critical | Respiratory failure, septic shock, and/or multiorgan failure |
The clinical course is asymptomatic or mild in 80% to 90% of cases. Early in the COVID-19 pandemic (January 2019 to April 2020), the progression to severe disease occurred in about 10% of cases, and critical (life-threatening) illness developed in around 5% of cases. Vaccination is highly effective against COVID-19 associated hospitalization and death. During the first 11 months (December 2020 to October 2021) following the availability of SARS-CoV2 vaccines, the rates of severe COVID-19-associated outcomes and death dropped to 0.015% and 0.0033%, respectively.30 Death, when it occurs, is usually caused by the progression to ARDS and multiorgan failure.11 Early in the clinical course, the primary pathogenic process is driven by the replication of SARS-CoV2. In later stages and severe disease, a dysregulated immune/inflammatory response to SARS-CoV2 drives tissue damage. Based on this pathogenesis, therapies that directly target SARS-CoV2 are anticipated to have the greatest efficacy early in the course of the disease, and immunosuppressive/anti-inflammatory therapies are likely to be more useful in the later stages.31
Our scientific understanding of SARS-CoV2 continues to evolve and many of the therapeutic agents used to treat COVID-19 have not been compared side by side in clinical trials. Clinical guidelines for the management of patients with SARS-CoV2 infection have been promulgated and are under frequent revision as new data become available. Clinicians should refer to the most recent version of the guidelines for up-to-date information. For detailed information please visit https://www.covid19treatmentguidelines.nih.gov/.
Management of Nonhospitalized Patients
Most patients with COVID-19 have asymptomatic or mild illness and do not require targeted pharmacologic intervention. All symptomatic patients should receive supportive management, including the use of over-the-counter antipyretics, antitussives, and analgesics.31 Nonpharmacological treatments may also be used, including drinking fluids, pulmonary hygiene, and resting in a prone position.32 Patients who suffer from moderate illness and/or those who are at high risk of disease progression may require pharmacologic intervention. Considerations when selecting a pharmacologic agent are the potential for drug–drug interactions, the ability to administer intravenously (IV) medications, and the prevalent variants of concern in the region.
In nonhospitalized patients who do not require supplemental oxygen and are at high risk of disease progression, ritonavir-boosted nirmatrelvir or remdesivir is recommended as first-line therapy. Ritonavir-boosted nirmatrelvir is administered orally (PO) and is therefore preferred over remdesivir, which is administered IV. Ritonavir-boosted nirmatrelvir has significant drug–drug interactions and must be monitored carefully. Alternative therapy, such as molnupiravir should be reserved for cases where ritonavir-boosted nirmatrelvir and remdesivir are unavailable or their use is clinically inappropriate32 (note: bebtelovimab is no-longer authorized for use in the US due to poor infectiveness toward the major circulating Omicron subvariants in the country).33 The routine use of systemic corticosteroids is not recommended and should be reserved for cases where another indication warrants their use.32
Table 3 provides an overview of the mechanisms of actions, dosing, adverse drug reactions, monitoring, and clinical pearls for the use of pharmacologic agents used in the treatment of COVID-19.
Table 3.
Pharmacologic agents used in the treatment of severe acute respiratory syndrome caused by coronavirus
| Drug | Mechanism of Action | Dosing | Adverse Drug Reactions | Monitoring | Clinical Pearls |
|---|---|---|---|---|---|
| Antivirals | |||||
| Ritonavir-boosted Nirmatrelvir (Paxlovid)34 |
|
|
|
|
|
| Remdesivir35 | Adenosine analog inhibits RNA-dependent RNA polymerase causing premature termination of viral transcription |
|
|
|
|
| Molnupiravir36 | Prodrug Cytidine analog incorporated into viral RNA leading to accumulation of errors, resulting in inhibition of viral replication |
|
|
|
|
| Monoclonal Antibodies | |||||
| Bebtelovimab37 (NIH expert panel recommended against use of this drug for the treatment of COVID-19)33 |
Neutralizes the viral spike protein, blocking its attachment to human ACE2 receptors |
|
|
|
|
| Tixagevimab/cilgavimab (Evusheld)38 As of January 26, 2023, Evusheld is no longer authorized for emergency use due to its low effectiveness against the major variants circulating in the US39 |
Targets SARS-CoV2 spike protein-directed attachment |
|
|
|
|
| Sarilumab40,41 (NIH expert panel recommended against the use of this drug for the treatment of COVID-19)33 |
Binds IL-6, inhibits IL-6-mediated proinflammatory signaling |
|
|
|
|
| Tocilizumab42 |
|
|
|
|
|
| JAK Inhibitors | |||||
| Baricitinib43 |
|
|
|
|
|
| Tofacitinib44 | Blocks signaling from cytokines Inhibits inflammatory response |
|
|
|
|
| Corticosteroid | |||||
| Dexamethasone45 | Anti-inflammatory |
|
|
|
|
In previously hospitalized patients after discharge, the guidelines recommend against continuing treatment with remdesivir, baricitinib, or dexamethasone if the patient is stable and does not require oxygen supplementation. There is currently insufficient data to recommend for or against the use of these agents in patients requiring supplemental oxygenation postdischarge. In patients being discharged from the ED despite a new or increasing requirement for oxygen supplementation, it is recommended to start dexamethasone for a maximum duration of 10 days, until the patient no longer requires oxygen supplementation. Additionally, remdesivir can be used in this subset of patients; however, its use may be limited due to its parenteral administration, and availability of home infusion resources.32
Management of Hospitalized Patients
Patients with moderate illness who are at risk for progression to higher severity and those with severe disease (see Box 1) should be hospitalized. Inpatient treatment of COVID-19 involves the use of anti-inflammatory, immunosuppressive, and in certain cases, antithrombotic therapies. Treatment is determined based on the patient’s oxygen requirements. In hospitalized patients who do not require supplemental oxygen, corticosteroid therapy is not recommended based on the results from the Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial, showing no benefit in this population. Currently, there is insufficient evidence for or against the use of remdesivir in patients not requiring supplemental oxygen (level of evidence: BIII). The use of remdesivir in these patients should be determined using clinical judgment and reserved for patients at risk of progressing to severe disease.46
Patients requiring supplemental oxygenation are subdivided into 3 treatment groups:46
-
•
Patients requiring low-flow oxygen
-
•
Patients requiring high-flow oxygen or noninvasive ventilation (NIV)
-
•
Patients requiring mechanical ventilation (MV) or extracorporeal membrane oxygenation (ECMO)
Treatment for patients who require low flow supplemental oxygen consists of the use of one of the following therapies: remdesivir monotherapy, dexamethasone plus remdesivir, or dexamethasone monotherapy. Because early COVID-19 is characterized by rapid viral replication, the use of remdesivir is likely to be most effective in the first 10 days after symptom onset. In the later course of disease, corticosteroids may be beneficial in decreasing a potential systemic inflammatory response. Corticosteroid monotherapy may be used in certain cases. However, immunosuppressive effects of steroids may reduce viral clearance when used without an antiviral.46 In patients with rapidly increasing supplemental oxygen requirements, adding another immunomodulatory agent (eg, baricitinib, or tocilizumab) to dexamethasone, or remdesivir monotherapy, or combination therapy of remdesivir and dexamethasone is recommended. Overall, this group should be monitored closely for disease progression and increasing oxygen requirements, and the treatment should be adjusted accordingly. If disease progression occurs during remdesivir therapy, the full course of treatment should be completed.46 When selecting immunomodulatory agents, the clinical benefits versus the potential increased the risk of infection, including opportunistic infections must be considered. The level of evidence supporting the addition of an immunomodulatory agent to dexamethasone in this subgroup of patients is weak (ie, BIIa).46
Treatment for patients requiring supplemental oxygen using a high-flow device or NIV consists of dexamethasone plus oral baricitinib, or plus intravenous tocilizumab, or monotherapy. Remdesivir may be added to one of these options in certain patients.47 Remdesivir monotherapy in this population may be clinically inadequate and should therefore not be used. Immunomodulatory agents may be added to dexamethasone in patients with rapidly increasing oxygen need because clinical benefit has been demonstrated in multiple trials for this patient subgroup. The Randomized, Embedded, Multi-factorial, Adaptive Platform Trial for Community-Acquired Pneumonia trial showed ∼22% reduction in mortality in patients receiving tocilizumab versus usual care. In the RECOVERY trial, there was a 14% reduction in all-cause mortality by day 28 in patients receiving tocilizumab plus dexamethasone versus usual care. A Study of Baricitinib in Participants With COVID-19 (COV-BARRIER) showed a 48% reduction in all-cause mortality in this subgroup.46
An open label, randomized controlled trial comparing baricitinib and tocilizumab in patients with COVID-19 showed that baricitinib was noninferior to tocilizumab for the time to hospital discharge within 28 days. The percentage of patients discharged alive for the baricitinib group was reported at 58.4% versus 52.4% for the tocilizumab group, with a P-value less than .001 for noninferiority.48 Therefore, the selection of these therapies is based on availability and patient-specific factors. In cases where baricitinib or tocilizumab are unavailable or their use is inappropriate, second-line options are oral tofacitinib (a JAK inhibitor) or IV sarilumab (an IL-6 receptor antagonist). JAK inhibitors and IL-6 receptor antagonists should not be used together due to an increased risk of serious infection and lack of supporting data.46
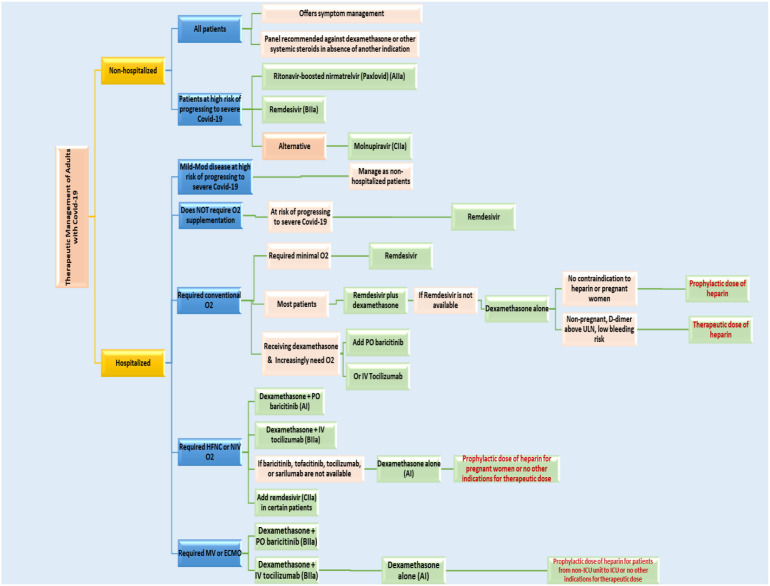
Treatment for patients requiring MV or ECMO must be provided in a critical care setting. At this stage of disease, patients are experiencing a systemic inflammatory response that can lead to multiorgan dysfunction syndrome. To limit damage due to inflammation, guidelines recommend starting dexamethasone plus IV tocilizumab or PO baricitinib is recommended. If baricitinib, tofacitinib, tocilizumab, or sarilumab is not available or not feasible to use, dexamethasone monotherapy should be started. Remdesivir monotherapy for critically ill patients is contraindicated due to studies showing possible increased mortality, and no improvement in recovery rate. However, for patients already receiving remdesivir who subsequently require MV or ECMO, remdesivir treatment should be continued until completion.46 Additional considerations in the treatment of hospitalized patients may be found in Box 2 . See Box 3 for additional consideration in the treatment of special populations with COVID-19. Fig. 7 provides an algorithm for the treatment of nonhospitalized and hospitalized patients with COVID-19.
Box 2. Additional considerations in the treatment of hospitalized patients with COVID-1946.
-
•
Equivalent dose of other corticosteroid such as prednisone, methylprednisolone, or hydrocortisone may be used if dexamethasone is not available.
-
•
Baricitinib or tocilizumab should only be given as dual therapy with dexamethasone (or another corticosteroid at an equivalent dose).
-
•
The combination of dexamethasone and a JAK inhibitor or IL-6 receptor antagonist increases the risk of opportunistic infections or reactivation of latent infections. For patients from Strongyloides endemic areas, antiparasitic prophylaxis (such as ivermectin) is indicated.
Box 3. Additional considerations in the treatment of special populations with COVID-1949.
| Pregnancy |
|
| Children |
|
| Cancer or immuno-compromised |
|
| Transplant |
|
| HIV |
|
| Influenza |
|
Fig. 7.
Algorithm for treatment of nonhospitalized and hospitalized patients with COVID-19.32,46,49,51
Antithrombotic therapy in patients with COVID-19
Acute thrombosis/thromboembolism may be a complication of SARS-CoV2 infection. Patients who experience sudden loss of peripheral perfusion, or rapid deterioration of pulmonary, cardiac, or neurologic function should be evaluated for thromboembolic disease. See Box 4 for key points in the evaluation and management of this population.
Box 4. Antithrombotic therapy in patients with COVID-1947,50.
-
•Prophylactic dose of heparin:
-
○Hospitalized patients with mild-to-moderate COVID-19 and at high risk of progressing to severe disease, unless contraindicated (AI).
-
○Pregnant patients (BIII)
-
○Patients without indication for therapeutic anticoagulation
-
○Patients transferred from a non-ICU unit to ICU and have no other indications for therapeutic anticoagulation (BIII)
-
○
-
•Therapeutic dose of heparin:
-
○Nonpregnant patients with d-Dimer level above the ULN without increased bleeding risk (CIIa)
-
○
-
•
Guidelines are continuously evolving based on current new data
-
•
Consult with a specialist for further guidance in the management of these populations
Prevention of COVID-19 infection
The most effective primary prevention of COVID-19 infection is vaccination. Mitigating the spread of COVID-19 can be achieved through hand hygiene, mask wearing, and social distancing. The outbreak of COVID-19 infection caused by the SARS-CoV2 virus is a unique pandemic with global impact. In December 2020, the United States implemented an Emergency Use Authorization (EUA) for a newly developed COVID-19 vaccine that boasted high efficacy. Since the introduction of COVID-19 vaccines, FDA has granted EUA to 4 vaccines, including the approval of 2 of them for use in specific age groups. This process continues to evolve and clinicians should find up-to-date information on FDA approval for COVID-19 vaccines at: https://www.fda.gov/emergency-preparedness-and-response/counterterrorism-and-emerging-threats/coronavirus-disease-2019-covid-19. As of September 2022, 4 countries have approved mucosal vaccines for SARS-CoV2. Phase III trial data for these vaccines have not been published as of this writing, although Phase II trial data of the inhaled vaccines (i.e from CanSino Biologics in Tianjin) showed significantly higher blood-serum antibody levels compared with booster by injection.52 Theoretically, mucosal vaccines could prevent viral transmission from person to person (so-called “sterilizing immunity”), which is not conferred by injected COVID-19 vaccines. Currently, there are more than 100 mucosal SARS-CoV2 vaccines in development, with more than 20 already in human trials. Mucosal COVID-19 vaccines could facilitate access to vaccines and reduce viral transmission. However, further research is needed to confirm their long-term efficacy.52 , 53
Mechanism of action
COVID-19 vaccines are available in several formulations: mRNA, protein subunit, and viral vector. Each of these will be discussed briefly here, and Table 4 outlines the vaccine platforms, dosing schedule, and common and severe adverse effects. Figs. 8 and 9 provide detailed dosing schedules for injectable COVID-19 vaccines available as of January 27, 2023. mRNA vaccines are lipid coated, which facilitate the delivery of nucleoside-modified mRNA into host cells to express the SARS-CoV2 spike (S) antigen (eg, Pfizer and Moderna). The expression of the S antigen elicits the host immune response, which protects against COVID-19. Protein subunit vaccines contain fragments of COVID-19 spike (S) protein together with an adjuvant that facilitates the immune response to the protein (eg, Novavax). Once the antigen has been recognized by the immune system, the host will then be able to respond quickly to the actual virus spike protein, which protects against COVID-19 infection. There is currently one viral vector vaccine for COVID-19, which uses a recombinant, replication-incompetent adenovirus (Ad26) vector (eg, Janssen). This vector delivers a stabilized variant of the SARS-CoV2 spike (S) protein that will safely generate an immune response to elicit host immunity to COVID-19.54
Table 4.
| Pfizer/BioNTech | Moderna | Johnson & Johnson/Janssen | Novavax | AstraZeneca | |
|---|---|---|---|---|---|
| FDA status | Approved | Approved | EUA only as of August 2022 | EUA only as of August 2022 | Not approved as of August 2022 |
| Vaccine platform | mRNA | mRNA | Recombinant DNA vector | rS viral protein plus Matrix-M™ adjuvant | Recombinant DNA vector |
| CDC recommended dosing schedule | See Figs. 8 and 9 | See Figs. 8 and 9 | See Figs. 8 and 9 | See Figs. 8 and 9 | See package insert |
| Common adverse effects |
|
|
|
|
|
| Serious adverse effects |
|
|
|
|
|
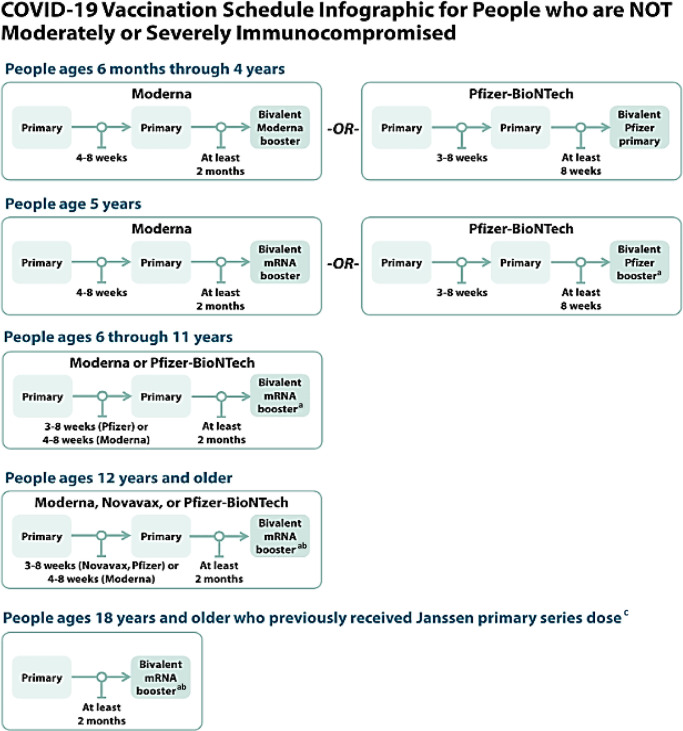
Fig. 8.
COVID-19 vaccine schedule for people who are not immunocompromised. aFor people who previously received a monovalent booster dose(s), the bivalent booster dose is administered at least 2 months after the last monovalent booster dose. bA monovalent novamax booster dose may be used in limited situations in people ages 18 years and older who completed a primary series using any COVID-19 vaccine, have not received any previous booster dose(s), and are able unable or unwilling to receive an mRNA vaccine. The monovalent Novavax booster dose is administered at least 6 months after completion of a primary series. cJanssen COVID-19 vaccine should only be used in certain limited situations. See https://www.cdc.gov/vaccine/covid-19/clinical-consideration/interlm-considerations-us-appendix,html#.appendix-a.
(Adapted from Centers for Disease Control and Prevention.61,62)
Fig. 9.
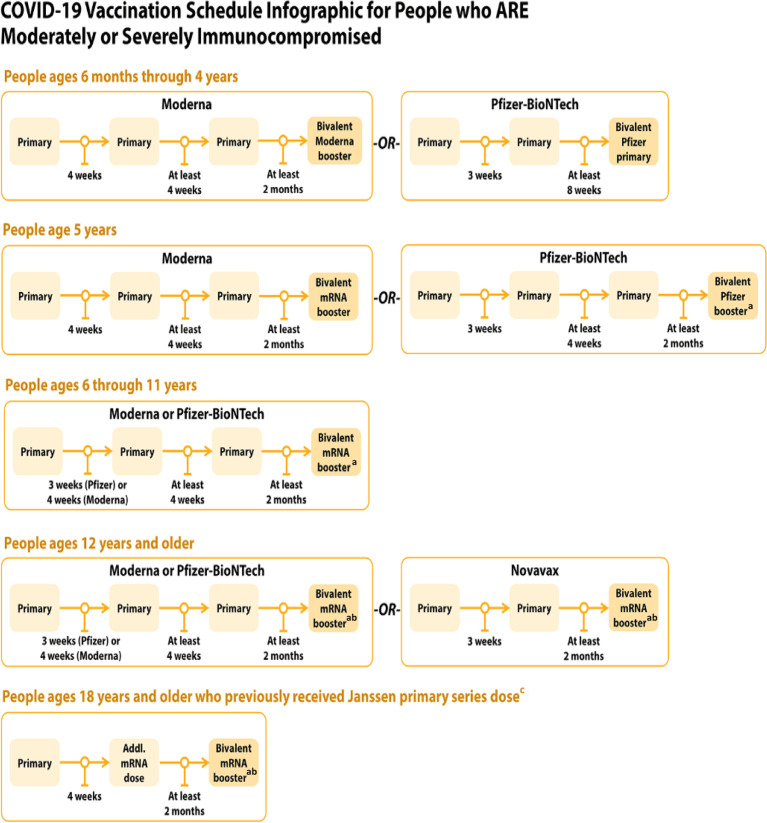
COVID-19 vaccine schedule for people who are moderately or severely immunocompromised. aFor people who previously received a monovalent booster dose is administered at least 2 months after the last monovalent booster dose. bA monovalent novamax booster dose may be used in limited situations in people ages 18 years and older who completed a primary series using any COVID-19 vaccine, have not received any previous booster dose(s), and are able unable or unwilling to receive an mRNA vaccine. The monovalent Novavax booster dose is administered at least 6 months after completion of a primary series. cJanssen COVID-19 vaccine should only be used in certain limited situations. See https://www.cdc.gov/vaccine/covid-19/clinical-consideration/interlm-considerations-us-appendix,html#.appendix-a.
(Adapted from Centers for Disease Control and Prevention.61,62)
Limitations of vaccine efficacy
Since the availability of COVID-19 vaccines, there has been varied acceptance among the general population. The initial efficacy of the mRNA vaccines against SARS-CoV2 was greater than 95%; however, over time the efficacy of these vaccines has significantly declined. With the emergence of the Omicron variant, a clinical study using test-negative case control design was developed to estimate the effectiveness of Pfizer-BioNTech (BNT162b2), Moderna (mRNA-1273), and AstraZeneca’s (ChAdOx1) vaccines against the Omicron and the Delta variants in England. Compared with the preclinical trials that displayed an initial 95% efficacy for individuals receiving a primary series of BNT162b2, this study reported vaccine efficacy of 65.5% at 2 to 4 weeks, with decrease to 8.8% after 10 weeks.55 In the population that received a booster dose, vaccine effectiveness was only 39.6% at an interval of 10 or more weeks.55 The best results were recorded when a primary course of BNT162b2 was followed by a booster of mRNA-1273 which reported vaccine effectiveness of 73.9% at 2 to 4 weeks, and 64.4% at 5 to 9 weeks.55
The methodology of using vaccines from multiple platforms in a vaccine series for the prevention of a specific disease is not novel. Current practice globally to address vaccine shortages includes using crossover of COVID-19 vaccine types/manufacturers based on availability.56 Mixing vaccines of different platforms has been demonstrated to result in a stronger cellular immune response, higher IgG and neutralizing antibodies.57, 58, 59, 60
COVID-19 vaccination schedule recommendations are published by the CDC and are determined by age, immune status, and the specific vaccine type (see Figs. 8 and 9).
In August 2022, the US FDA announced the EUA of bivalent formulations of the Moderna and Pfizer-BioNTech COVID-19 vaccines for use as a single booster dose at least 2 months following primary or booster vaccination. These “updated boosters” contain 2 mRNA components of SARS-CoV2, one from the original strain and one that is found in both the BA.4 and BA.5 lineages of the Omicron variant.
Summary
Global trends indicate that COVID-19 is transitioning from a pandemic to an endemic phase. As the SARS-CoV2 virus continues to evolve, our understanding and the body of literature will continue to rapidly expand. Clinicians are encouraged to review the most up-to-date information regarding variants of concern, vaccines, and treatment options.
Middle East Respiratory Syndrome
Prevalence, Mortality, and Economic Burden
The Middle East Respiratory Syndrome coronavirus (MERS-CoV) is one of the significant emerging viral infections in the twenty-first century. This infection is caused by a single-stranded positive-sense RNA virus,67 which was first officially reported in Saudi Arabia in September 2012. However, it was later traced back to the first case in Jordan in April of the same year.68 , 69 Since the identification of this new viral infection, it has spread through travel to many countries around the globe including the United States (US). Two confirmed cases in the US were reported in May 2014 from Indiana and Florida. These cases were among health-care workers who stayed and worked in Saudi Arabia.67
The original source of this viral infection has been linked to dromedary camels, from which the virus has jumped to humans perhaps during calving season.70 Studies have suggested that MERS coronavirus is exclusively maintained in camels and the spread of the virus between humans has been poor.70 In other words, the transmission from camels to humans has occurred many times, whereas the subsequent transmission from human to human has been limited.71 Unfortunately, in 2015, a widespread outbreak occurred in South Korea.71 This observed outbreak involved human-to-human transmission in a hospital setting, which led to 185 secondary cases from a single index case.71 According to the World Health Organization (WHO), the worldwide total of laboratory-confirmed cases of MERS as of July 2022 was reported at 2591, including 894 fatalities, which translated to a case fatality rate of 37.2%.72
The economic impact of MERS has been very significant. The estimated loss reported from the South Korea outbreak within 1 year was on the order of hundreds of millions to billions of dollars from various sectors such as tourism, accommodation, food, beverage, and transportation.73
Pathogenesis and complications
Progression of Infection
The pathogenesis and transmission of MERS-CoV have not been fully elucidated. There are many host factors, such as dipeptidyl peptidase-4 (DPP4), proteases, interferons, and sialic acids that have been identified in vitro to potentially affect the infection process.74 In particular the DPP4, also known as CD26,75 has been known to play an important role as the functional receptor for MERS-CoV.74 , 75 This enzyme could either be expressed on the cell surface or in aqueous solution, which is involved in many pathways cleaving various substrates, such as chemokines, neuropeptides, and hormones. The infection of MERS-CoV in human hosts is mediated by the interaction of the viral spike protein (S1) with DPP4 on the cell surface and the α2,3-sialic acids.74 There are other pathways that could potentially involve in virus–host interactions; however, they have been less studied. Once the virus has entered the host cell, it initiates transcription and translation of viral proteins within the host’s cytoplasm to generate new viral particles. In human hosts, MERS-CoV replication primarily occurs in the lower respiratory tract, especially in the epithelial cells of the bronchioles and alveoli.74 This is due to the significant prevalence of DPP4 in the lower pulmonary tract and its absence in the nasal epithelium.74
Transmission, Manifestations, and Risk Factors
MERS-CoV is an airborne infectious disease, which can be transmitted by inhaling the infectious respiratory droplets spread from infected individuals while coughing or sneezing.76 Many human-to-human transmissions have occurred in inpatient settings, such as intensive care (ICU), or other ambulatory care settings such as dialysis centers. Transmission may also occur through fomites such as contaminated surfaces, devices, or equipment. Once exposed, the incubation period averages 5 to 12 days.76
The symptoms of MERS-CoV infection include rhinorrhea, fever, cough, fatigue, nausea, vomiting, GI symptoms, myalgia, splenic atrophy, lymphadenopathy, seizures, hepatic dysfunction, shortness of breath, and respiratory failure.67 , 76 Nevertheless, the manifestation of the infection in human hosts may range from severe pneumonia to no significant symptoms.74 Risk factors that could contribute to more severe disease are chronic kidney disease, diabetes, pulmonary diseases, or immune compromise.76 Patients who have history of smoking or preexisting pneumonia were also found to have a higher rate of mortality.77 In addition, patients diagnosed with chronic obstructive pulmonary disease have been shown to express higher levels of DPP4 in their lung tissue, subsequently increasing the susceptibility of MERS-CoV infection.74 , 78 According to the autopsy data from human as well as animal models, the pathogenesis of severe MERS-CoV infection was highly associated with the upregulation of DPP4, especially in type 1 pneumocytes, which accounted for 95% of the total surface area of the alveoli. Damage of this pulmonary cell type caused by the viral infection eventually leads to significant morbidity.66 Other risk factors of more severe disease also include advanced age and the inadequate or delayed response of type 1 interferon, which is a cytokine that functions as an inhibitor of viral replication in susceptible cells.66
Laboratory testing and diagnosis
The diagnosis of MERS-CoV infection may be achieved by obtaining a complete clinical history and diagnostic laboratory tests.61 , 79 Clinical diagnosis involves a thorough medical history of clinical signs and symptoms as well as history of close contact and traveling to and from outbreak areas, which helps to rapidly screen and triage suspected cases. Since the symptomatic presentations of MERS are nonspecific, clinical history alone is not adequate. Further laboratory testing is required to confirm MERS-CoV infection. According to the CDC, these laboratory tests are categorized into either molecular or serology tests. The molecular tests are indicated for patients with active infection. These include real-time reverse-transcription polymerase chain reaction (rRT-PCR) assays. These molecular tests detect viral genetic material (ie, RNA) in collected specimens. As of this writing, there is no officially FDA-approved test available for MERS-CoV infection; however, there is one current rRT-PCR test available under EUA for the detection of MERS. When a suspected case of MERS-CoV presents, an rRT-PCR that detects at least 2 viral genetic targets should be ordered. The usual recommended genetic targets are the conserved domains ORF 1a and 1b or other targets including the regions upstream of gene E (upE), which is responsible for viral assembly; or gene S, which encodes for the spike protein that facilitates the binding of viral particles to the host cell; or gene N, which encodes the N protein that helps to regulate the viral replication.79 In fact, WHO has recommended rRT-PCR that targets both the upE and ORF-1a regions because this test can detect as low as 5 copies of RNA from MERS-CoV.80 In addition, the sensitivity and Sp of this test has also been reported at 95% and 100%, respectively.79 Meanwhile, the sensitivity may range from 55% to 100% and Sp of 33% to 100% for tests that used the N gene as a target.79
The CDC also recommends collection of samples from multiple locations, including the upper respiratory tract by using NP or OP swabs; or lower respiratory tract by collecting sputum and/or tracheal aspirates; or serum; or stool samples.67 Studies have suggested that the RNA of MERS-CoV may be detected in blood, stool, upper and lower pulmonary tracts, and urine.67 Viral loads from the pulmonary specimens usually peak during the second week after the onset of symptoms.67 Further, the positive rate from the cotton swab of the upper respiratory tract was up to 88.2%, which is lower than those samples from sputum or tracheal aspirate (ie, 100%).67 Serum samples show approximately 33% of positivity for MERS-CoV RNA at initial diagnosis. Seropositivity has been shown to be associated with poorer outcomes.81 The CDC recommends collection of collect samples of 2 to 3 mL of tracheal aspirate, or pleural fluid into a sterile collecting cup. The specimen may be stored at 2°C to 8°C up to 72 hours; otherwise, they may be stored at −70°C for a longer time. Of note, the NP and OP specimens may be collected and stored in the single vial under the same specified conditions.68 Clinicians should consult with local laboratories for specific specimen collection and handling guidelines. There is an increasing number of commercially available tests for MERS-CoV; however, their performance may have not been validated and compared with the reference PCR testing.71 In a patient who is under investigation for MERS-CoV, a single negative result of the rRT-PCR test confirms no current active MERS-CoV infection. Nevertheless, additional testing of other specimens should be completed to confirm negative results.67 In a patient with confirmed MERS-CoV infection, the CDC recommends 2 consecutive negative rRT-PCR tests on all specimens to ensure clearance of the infection.67 Fig. 10 provides an algorithm for laboratory testing in suspected MERS-CoV infection.
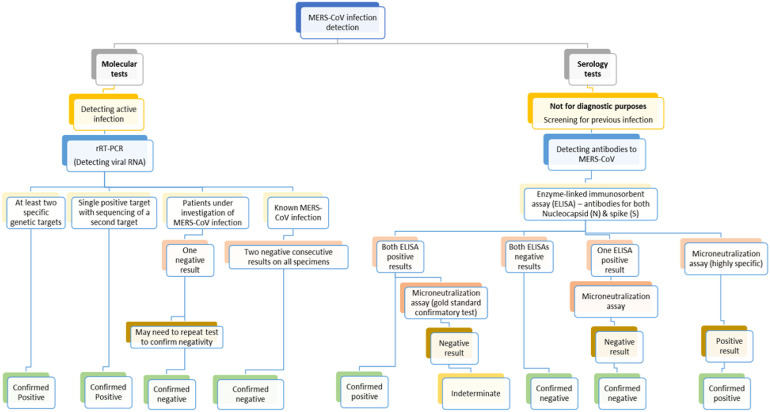
Fig. 10.
Algorithm of CDC recommendations for suspected MERS-CoV infection.67,79
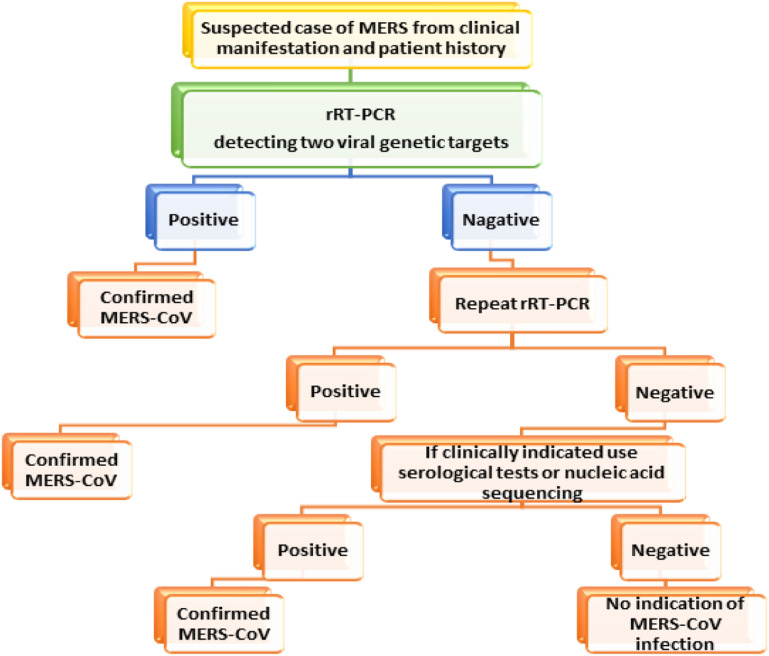
When the result of rRT-PCR test is positive, it confirms MERS-CoV infection. However, if the result is negative, then the test should be repeated. If the repeat test result is positive, it also confirms the diagnosis. However, if the result of the second rRT-PCR is negative, and the patient still shows clinical indication of suspected MERS-CoV infection, other serologic tests or nucleic acid sequencing are indicated. Positive result of either of these alternative tests confirm the diagnosis; otherwise, the patient has no indication of MERS-CoV infection (Fig. 11 ).79
Fig. 11.
Algorithm of suspected MERS-CoV infection using rRT-PCR for two viral genetic targets.67,79
Serology tests detect antibodies against MERS-CoV in patients who either have had an earlier infection or have been exposed to the virus. These tests are generally not for diagnostic purposes but for checking the development of immune response. The enzyme-linked immunosorbent assay (ELISA) was recommended as a screening test to detect the presence of antibodies against 2 viral targets including the nucleocapsid (N) and spike (S) proteins. When the ELISA result is positive, a microneutralization test is conducted to confirm the positive result. This microneutralization test detects the presence of antibodies that can neutralize the virus and inhibit viral entry. Although it is labor intensive and may take up to 5 days to turn around, this microneutralization test is considered as a gold standard for detecting antibodies in serum samples.60 According to current data, the protein-based tests are less sensitive than nucleic acid-based tests and provide higher risk of false-positive results.79 A decision algorithm similar to that used for the rRT-PCR test can be used for serology testing in patients with suspected MERS-CoV (see Fig. 10).
Pharmacologic Therapy
The treatment of MERS-CoV infection has been mostly supportive, which includes the administration of analgesics and antipyretics such as acetaminophen or nonsteroidal anti-inflammatory drugs (NSAIDs), as well as hydration and MV support. Other pharmacologic supportive therapies included chloroquine, nitazoxanide, silvestrol, steroids, and mycophenolate mofetil (MMF).82 In addition, antiviral agents such as ribavirin, ritonavir/lopinavir, alisporivir, and interferons have shown inhibitory activity against MERS-CoV in vitro.82 As of this writing, there is no highly effective therapeutic regimen that specifically treats MERS-CoV infection. However, various treatment strategies have been used with partial success. For example, immunotherapy from convalescent plasma and intravenous immunoglobulin (IVIG) has been exploited, whereas other combination therapies of antivirals together with an immunosuppressant such as cyclosporine, mycophenolic acid, or interferon-alpha have also been investigated. Interestingly, ribavirin and interferon-alpha were reported to have synergistic effects during early infection,76 whereas MMF showed synergistic effects with interferon-beta 1b.82 The combination of interferon-alpha together with lopinavir, or ribavirin, or MMF has also been implemented.82 Unfortunately, none of these studies has reduced the overall mortality rate in either human or animal models.82 , 83 The role of antibiotics has been limited to patients who develop bacterial coinfection. Various antibiotic treatments may be considered; however, there are limited empiric data to support improved survival outcomes in this viral infection.82 Until an effective prophylactic or therapeutic option becomes available, it is critically important to implement preventive measures by patient isolation, education, and supportive quarantine settings to prevent future outbreaks.
Vaccines
Despite several vaccine candidates under development, there is currently no vaccine licensed specifically for MERS.84 MERS-CoV vaccine candidates are based mainly on the viral spike (S) protein (as the vital role in the viral infectivity), as well as other viral proteins including nucleocapsid (N) protein, envelope (E) protein, and NSP16.85 There are currently 6 types of vaccines under investigation: viral vector-based vaccine, DNA vaccine, subunit vaccine, nanoparticle-based vaccine, inactivated-whole virus vaccine, and live-attenuated vaccine.85 The first clinical study (phase 1b trial) of the ChAdOx1 MERS vaccine, conducted in Saudi Arabia, investigated the reactogenicity and immunogenicity of ChAdOx1 MERS in Middle Eastern adults.84 Positive initial findings led to a current phase 2 study, where a larger number of healthy adults, health-care workers, and occupationally exposed camel handlers will be recruited to explore vaccine safety and immunogenicity to prevent future MERS outbreaks. Limitations of this study were noted to include the small sample size and the open-label, nonrandomized, uncontrolled trial design.
Summary
Although the development of MERS vaccines began with the emergence of MERS-CoV in 2012, vaccine developments have been slow, and most of the vaccine candidates have been evaluated in animal models.85 The low occurrence of human-to-human transmission might be the reason for not prioritizing research in this area. However, the recent MERS outbreak in South Korea, which demonstrated virus emergence in second-generation and third-generation contacts, has reignited public awareness regarding the danger of MERS-CoV.86 As no effective targeted treatment against MERS is currently available, the best solution is to develop a functional MERS vaccine to prevent MERS-CoV infection. More studies are focused on the viral vector-based and subunit vaccines. Even though many promising vaccine candidates have been proposed and reported, as of now, only 3 potential MERS-CoV vaccine candidates have progressed to phase I clinical trials: a DNA vaccine (GLS-5300) and 2 viral vector-based vaccines (MVA-MERS-S and MERS001).85 It is likely that MERS vaccine for human use will not be available in the near future. Considerable efforts should be given to minimize delays in executing clinical trials, such as better understanding and coordination between sponsors, primary investigators, investigators, participants, and stakeholders.
Clinics care points
-
•
Old age (ie, 80 years or older); gender (ie, male); and the presence of comorbid conditions, such as diabetes, chronic lung disease, cardiovascular disease, immunocompromising conditions, chronic kidney disease, and obesity, are risk factors for SARS-CoV2 with poor prognosis and death.
-
•
The preferred method for detection of SARS-COV2 is the RT-PCR.
-
•
The optimal days for RT-PCR testing via nasal or NP swab are days 3 to 4 after symptom onset.
-
•
Asymptomatic testing can be expected to have a significantly high rate of false-negative results.
-
•
Antigen tests can be used at point-of-care because they produce results in 15 to 30 minutes and may be useful as a biomarker to detect contagiousness.
-
•
Vaccination is highly effective against COVID-19–associated hospitalization and death.
-
•Management of nonhospitalized patients included:
-
○Nonpharmacological treatments such as drinking fluids, pulmonary hygiene, and resting in a prone position
-
○Pharmacologic treatments such as ritonavir-boosted nirmatrelvir (Paxlovid) or remdesivir is recommended as the first-line therapy for patients who do not require supplemental oxygen.
-
○Bebtelovimab and molnupiravir is reserved for cases where ritonavir-boosted nirmatrelvir and remdesivir are unavailable or their use is clinically inappropriate.
-
○
-
•Management of hospitalized patients may be subdivided into 3 treatment groups depending on the requirements of oxygen.
-
○Patients who required minimal and conventional oxygen supplementation
-
▪May be treated with remdesivir or combination of remdesivir and dexamethasone. If remdesivir is not available, dexamethasone alone may be used.
-
▪If progressively increasing O2 need, oral baricitinib or IV tocilizumab may be added.
-
▪
-
○Patients who required HFNC or NIV oxygen
-
▪Dexamethasone together with oral baricitinib (or alternatively tofacitinib) or IV tocilizumab (or alternatively sarilumab) are being considered first.
-
▪If baricitinib, tofacitinib, tocilizumab, or sarilumab is not available, dexamethasone alone may be given.
-
▪Remdesivir may be added in certain patients
-
▪
-
○Patients who required MV or ECMO
-
▪Dexamethasone plus oral baricitinib (or alternatively tofacitinib) or IV tocilizumab (or alternatively sarilumab) is given. Dexamethasone alone is considered, if baricitinib, tofacitinib, tocilizumab, or sarilumab is not available.
-
▪
-
○
-
•
The most effective primary prevention of COVID-19 infection is vaccination. COVID-19 vaccination schedule recommendations are published by the CDC and are determined by age, immune status, and the specific vaccine type (see Figs. 8 and 9).
-
•
The MERS-CoV is caused by a single-stranded positive-sense RNA virus.
-
•
This viral infection has been linked to dromedary camels, from which the virus has jumped to humans perhaps during calving season.
-
•
There is one current rRT-PCR test available under EUA for the detection of MERS. When a suspected case of MERS-CoV presents, an rRT-PCR that detects at least 2 viral genetic targets should be ordered.
-
•
The treatment of MERS-CoV infection has been mostly supportive, which includes the administration of analgesics and antipyretics such as acetaminophen or NSAIDs, as well as hydration and MV support.
-
•
The IVIG has been used and other combination therapies of antivirals together with an immunosuppressant such as cyclosporine, mycophenolic acid, or interferon-alpha have also been investigated.
-
•
Unfortunately, none of these studies has reduced the overall mortality rate in either human or animal models.
-
•
Despite several vaccine candidates under development, there is currently no vaccine licensed specifically for MERS.
Disclosure
The authors have nothing to disclose.
References
- 1.GBD 2017 Influenza Collaborators. Mortality, morbidity, and hospitalisations due to influenza lower respiratory tract infections, 2017: an analysis for the Global Burden of Disease Study 2017. Lancet Respir Med. 2019;7(1):69–89. doi: 10.1016/S2213-2600(18)30496-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abate S.M., Checkol Y.A., Mantefardo B. Global prevalence and determinants of mortality among patients with COVID-19: A systematic review and meta-analysis. Ann Med Surg (Lond) 2021;64:102204. doi: 10.1016/j.amsu.2021.102204. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Disease Burden of Flu. Centers for Disease Control and Prevention website. Available at: https://www.cdc.gov/flu/about/burden/index.html. 2022. Accessed May 25, 2022.
- 4.Prevention CfDCa. Covid Data Tracker. Available at: https://covid.cdc.gov/covid-data-tracker/#prevention-measures-social-impact. Accessed September 6, 2022.
- 5.Prevention CfDCa. Covid Data Tracker. 2022. Available at: https://covid.cdc.gov/covid-data-tracker/#datatracker-home. Accessed July 16, 2022.
- 6.Dorjee K., Kim H., Bonomo E., et al. Prevalence and predictors of death and severe disease in patients hospitalized due to COVID-19: A comprehensive systematic review and meta-analysis of 77 studies and 38,000 patients. PLoS One. 2020;15(12):e0243191. doi: 10.1371/journal.pone.0243191. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hasoksuz M., Kilic S., Sarac F. Coronaviruses and SARS-COV-2. Turk J Med Sci. 2020;50(Si-1):549–556. doi: 10.3906/sag-2004-127. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chams N., Chams S., Badran R., et al. COVID-19: A Multidisciplinary Review. Front Public Health. 2020;8:383. doi: 10.3389/fpubh.2020.00383. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh S.P., Pritam M., Pandey B., et al. Microstructure, pathophysiology, and potential therapeutics of COVID-19: A comprehensive review. J Med Virol. 2021;93(1):275–299. doi: 10.1002/jmv.26254. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ji Y.L., Wu Y., Qiu Z., et al. The Pathogenesis and Treatment of COVID-19: A System Review. Biomed Environ Sci. 2021;34(1):50–60. doi: 10.3967/bes2021.007. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pascarella G., Strumia A., Piliego C., et al. COVID-19 diagnosis and management: a comprehensive review. J Intern Med. 2020;288(2):192–206. doi: 10.1111/joim.13091. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xiao F., Tang M., Zheng X., et al. Evidence for Gastrointestinal Infection of SARS-CoV-2. Gastroenterology. 2020;158(6):1831–1833 e3. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan Y.K., Goh C., Leow A.S.T., et al. COVID-19 and ischemic stroke: a systematic review and meta-summary of the literature. J Thromb Thrombolysis. 2020;50(3):587–595. doi: 10.1007/s11239-020-02228-y. In eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pagali S., Parikh R.S. Severe urticarial rash as the initial symptom of COVID-19 infection. BMJ Case Rep. 2021;14(3):e241793. doi: 10.1136/bcr-2021-241793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mir T, Almas T, Kaur J, et al. Coronavirus disease 2019 (COVID-19): Multisystem review of pathophysiology. Annals of Medicine and Surgery 2021;69:102745. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8381637/pdf/main.pdf. Accessed September 6, 2022. [DOI] [PMC free article] [PubMed]
- 16.Gomez-Mesa J.E., Galindo-Coral S., Montes M.C., et al. Thrombosis and Coagulopathy in COVID-19. Curr Probl Cardiol. 2021;46(3):100742. doi: 10.1016/j.cpcardiol.2020.100742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Prevention CfDCa. Guidance for Antigen Testing for SARS-CoV-2 for Healthcare Providers Testing Individuals in the Community. 2022. Available at: https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antigen-tests-guidelines.html. Accessed June 20, 2022.
- 18.Dinnes J., Deeks J.J., Berhane S., et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst Rev. 2021;3(3):Cd013705. doi: 10.1002/14651858.CD013705.pub2. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Teymouri M., Mollazadeh S., Mortazavi H., et al. Recent advances and challenges of RT-PCR tests for the diagnosis of COVID-19. Pathol Res Pract. 2021;221:153443. doi: 10.1016/j.prp.2021.153443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopera T.J., Alzate-Angel J.C., Diaz F.J., et al. The Usefulness of Antigen Testing in Predicting Contagiousness in COVID-19. Microbiol Spectr. 2022;10(2):e01962-21. doi: 10.1128/spectrum.01962-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prevention CfDCa. Interim Guidelines for COVID-19 Antibody Testing. 2022. Available at: https://www.cdc.gov/coronavirus/2019-ncov/lab/resources/antibody-tests-guidelines.html. Accessed July 21, 2022.
- 22.Mekonnen D., Mengist H.M., Derbie A., et al. Diagnostic accuracy of serological tests and kinetics of severe acute respiratory syndrome coronavirus 2 antibody: A systematic review and meta-analysis. Rev Med Virol. 2021;31(3):e2181. doi: 10.1002/rmv.2181. [DOI] [PubMed] [Google Scholar]
- 23.Peeling R.W., Heymann D.L., Teo Y.-Y., et al. Diagnostics for COVID-19: moving from pandemic response to control. Lancet. 2022;399(10326):757–768. doi: 10.1016/S0140-6736(21)02346-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guan W.J.N.Z., Hu Y., Liang W.H., et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fu L., Wang B., Yuan T., et al. Clinical characteristics of coronavirus disease 2019 (COVID-19) in China: A systematic review and meta-analysis. J Infect. 2020;80(6):656–665. doi: 10.1016/j.jinf.2020.03.041. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kovacs A., Palasti P., Vereb D., et al. The sensitivity and specificity of chest CT in the diagnosis of COVID-19. Eur Radiol. 2021;31(5):2819–2824. doi: 10.1007/s00330-020-07347-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Radiology ACo. ACR Recommendations for the use of Chest Radiography and Computed Tomography (CT) for Suspected COVID-19 Infection. 2020. Available at: https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection. Accessed July 26, 2022.
- 28.Jacobi A.C., BernheimA E.C. Portable chest X-ray in coronavirus disease-19 (COVID-19): a pictorial review. Clin Imag. 2020;(64):35–42. doi: 10.1016/j.clinimag.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Panel. C-TG. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. . 2021 Available at: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/. Accessed July 27, 2022. [PubMed]
- 30.Yek C., Warner S., Wiltz J.L., et al. Risk Factors for Severe COVID-19 Outcomes Among Persons Aged ≥18 Years Who Completed a Primary COVID-19 Vaccination Series — 465 Health Care Facilities, United States, December 2020–October 2021. MMWR Morb Mortal Wkly Rep. 2022;71:19–25. doi: 10.15585/mmwr.mm7101a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Panel C-TG. Covid-19 Treatment Guidelines Clinical Management Summary. 2022 Available at: https://www.covid19treatmentguidelines.nih.gov/management/clinical-management/clinical-management-summary/. Accessed July 26, 2022.
- 32.Panel C-TG. Covid-19 Treatment Guidelines Therapeutic Management of Nonhospitalized Adults With COVID-19. 2022 Available at: https://www.covid19treatmentguidelines.nih.gov/management/clinical-management/nonhospitalized-adults--therapeutic-management/. Accessed July 26, 2022.
- 33.NIH. Antiviral Agents, Including Antibody Products. NIH. Available at: https://www.covid19treatmentguidelines.nih.gov/therapies/antivirals-including-antibody-products/summary-recommendations/. Accessed March 6, 2022.
- 34.Inc. P. Paxlovid (nirmatrelvir/ritonavir) (fact sheet). Available at: https://www.fda.gov/media/155050/download#:∼:text=The%20dosage%20for%20PAXLOVID%20is,each%20active%20ingredient%20within%20PAXLOVID. Accessed June 28, 2022.
- 35.Veklury (remdesivir) (package insert). Foster City, CA: Gilead Sciences, Inc. Available at: https://www.gilead.com/-/media/files/pdfs/medicines/covid-19/veklury/veklury_pi.pdf. Revised June, 2022. Accessed June 20, 2022.
- 36.Merck and Co. Lagevrio (molnupiravir) (fact sheet). U.S. Food and Drug Administration website. Available at: https://www.fda.gov/media/155054/download. Accessed June 20, 2022.
- 37.Eli Lilly and Company. Bebtelovimab (fact sheet). U.S. Food and Drug Administration website. Available at: https://www.fda.gov/media/156152/download. Accessed June 28, 2022.
- 38.LP AP. EVUSHELD (tixagevimab co-packaged with cilgavimab) (Fact sheet). US Food and Drug Administration website. 2022 Available at: https://www.fda.gov/media/154701/download. Accessed August 10, 2022.
- 39.Prevention CfDCa. COVID-19 Treatments and Medications. CDC. Available at: https://www.cdc.gov/coronavirus/2019-ncov/your-health/treatments-for-severe-illness.html. Accessed March 6, 2022.
- 40.Sanofi & Regeneron Pharmaceuticals I. KEVZARA (sarilumab) (Fact sheet). Sanofi & Regeneron Pharmaceuticals, Inc. 2018 Available at: https://products.sanofi.us/Kevzara/Kevzara.pdf. Accessed August 24, 2022.
- 41.Health NIo. COVID-19 Treatment Guidelines: Interleukin-6 Inhibitors. 2021 https://www.covid19treatmentguidelines.nih.gov/therapies/immunomodulators/interleukin-6-inhibitors/. Accessed August 24, 2022.
- 42.Genentech, Inc. Actemra (tocilizumab). U.S. Food and Drug Administration website. Available at: https://www.fda.gov/media/150321/download. Accessed June 28, 2022.
- 43.Eli Lilly and Company. Baricitinib (fact sheet). U.S. Food and Drug Administration website. Available at: https://www.fda.gov/media/143823/download. Accessed June 28, 2022.
- 44.Health NIo. COVID-19 Treatment Guidelines: Kinase Inhibitors: Janus Kinase Inhibitors and Bruton’s Tyrosine Kinase Inhibitors. 2022 Available at: https://www.covid19treatmentguidelines.nih.gov/therapies/immunomodulators/kinase-inhibitors/. Accessed August 24, 2022.
- 45.Health NIo. COVID-19 Treatment Guidelines: Corticosteroids. 2022 Available at: https://www.covid19treatmentguidelines.nih.gov/therapies/immunomodulators/corticosteroids/. Accessed August 24, 2022.
- 46.Panel. C-TG. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. National Institutes of Health. Therapeutic Management of Hospitalized Adults With COVID-19. 2022 Available at: https://www.covid19treatmentguidelines.nih.gov/management/clinical-management/hospitalized-adults--therapeutic-management/. Accessed July 27, 2022. [PubMed]
- 47.NIH. COVID-19 Treatment Guidelines. 2022 Available at: https://www.covid19treatmentguidelines.nih.gov/tables/management-of-hospitalized-adults-summary/. Accessed March 8, 2022.
- 48.Karampitsakos T., Papaioannou O., Tsiri P., et al. Tocilizumab versus baricitinib in hospitalized patients with severe COVID-19: an open label, randomized controlled trial. Clin Microbiol Infect. 2023;29(3):372–378. doi: 10.1016/j.cmi.2022.10.015. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Panel. C-TG. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Special Populations. National Institutes of Health. 2022 Available at: https://www.covid19treatmentguidelines.nih.gov/special-populations/. Accessed August 3, 2022. [PubMed]
- 50.Panel. C-TG. Coronavirus Disease 2019 (COVID-19) Treatment Guidelines. Antithrombotic Therapy in Patients With COVID-19. National Institutes of Health. 2022 Available at: https://www.covid19treatmentguidelines.nih.gov/therapies/antithrombotic-therapy/. Accessed August 3, 2022. [PubMed]
- 51.NIH. COVID-19 Treatment Guidelines. 2022 Available at: https://www.covid19treatmentguidelines.nih.gov/tables/management-of-nonhospitalized-adults-summary/. Accessed March 7, 2022.
- 52.West E. China and India approve nasal COVID vaccines — are they a game changer? Nature 2022;609 (News) Available at: https://www.nature.com/articles/d41586-022-02851-0.pdf. Accessed August 3, 2022. [DOI] [PubMed]
- 53.Sano K, Bhavsar D, Singh G, et al. SARS-CoV-2 vaccination induces mucosal antibody responses in previously infected individuals. Nat Commun 2022;13(1):5135. [DOI] [PMC free article] [PubMed]
- 54.Prevention CfDCa. Overview of COVID-19 Vaccines. 2022 Available at: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/overview-COVID-19-vaccines.html?s_cid=11758:different%20covid%20vaccines:sem.ga:p:RG:GM:gen:PTN:FY22. Accessed August 24, 2022.
- 55.Andrews N., Al E., Author Affiliations N.A., et al. Covid-19 vaccine effectiveness against the Omicron (B.1.1.529) variant. N Engl J Med. 2022;386(16):1532–1546. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Prevention CfDCa. COVID Data Tracker. Atlanta, GA: US Department of Health and Human Services, CDC; 2022. Available at: https://covid.cdc.gov/covid-data-tracker. Accessed September 6, 2022.
- 57.Borobia A.M., Carcas A.J., Perez-Olmeda M., et al. Immunogenicity and reactogenicity of BNT162b2 booster in ChAdOx1-S-primed participants (CombiVacS): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet. 2021;398(10295):121–130. doi: 10.1016/S0140-6736(21)01420-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Benning L, Tollner M, Hidmark A, et al. Heterologous ChAdOx1 nCoV-19/BNT162b2 prime-boost vaccination induces strong humoral responses among health care workers. Vaccines 2021;9(8):857. Available at: https://mdpi-res.com/d_attachment/vaccines/vaccines-09-00857/article_deploy/vaccines-09-00857.pdf?version=1628080404. [DOI] [PMC free article] [PubMed]
- 59.Rashedi R., Samieefar N., Masoumi N., et al. COVID-19 vaccines mix-and-match: The concept, the efficacy and the doubts. J Med Virol. 2022;94(4):1294–1299. doi: 10.1002/jmv.27463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schmidt T, Klemis V, Schub D, et al. Immunogenicity and reactogenicity of heterologous ChAdOx1 nCoV-19/mRNA vaccination. Nat med 2021;27(9):1530-1535. Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8440177/pdf/41591_2021_Article_1464.pdf. [DOI] [PMC free article] [PubMed]
- 61.Prevention CfDCa. At-A-Glance COVID-19 Vaccination Schedules. Available at: https://www.cdc.gov/vaccines/covid-19/downloads/COVID-19-vacc-schedule-at-a-glance-508.pdf. Accessed August 31, 2022.
- 62.Prevention CfDCa. Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Approved or Authorized in the United States. 2023 Available at: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html. Accessed March 8, 2022.
- 63.Prevention CfDCa. Interim Clinical Considerations for Use of COVID-19 Vaccines Currently Approved or Authorized in the United States. 2022 Available at: https://www.cdc.gov/vaccines/covid-19/clinical-considerations/interim-considerations-us.html. Accessed March 8, 2022.
- 64.Administration UFD. COVID-19 Vaccines. Available at: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/covid-19-vaccines. Accessed August 31, 2022.
- 65.Novavax I. NOVAVAX COVID-19 VACCINE, ADJUVANTED (Fact Sheet). 2022 Available at: https://www.fda.gov/media/159897/download. Accessed September 6, 2022.
- 66.Astrazeneca I. COVID-19 Vaccine (ChAdOx1-S (recombinant)) (Package insert). 2021. Available at: https://www.fda.gov.ph/wp-content/uploads/2021/04/Package-insert-v3.0.pdf. Accessed August 31, 2022.
- 67.Yan Y., Chang L., Wang L. Laboratory testing of SARS-CoV, MERS-CoV, and SARS-CoV-2 (2019-nCoV): Current status, challenges, and countermeasures. Rev Med Virol. 2020;30(3):e2106. doi: 10.1002/rmv.2106. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Prevention CfDCa. Middle East Respiratory Syndrome (MERS). 2019 Available at: https://www.cdc.gov/coronavirus/mers/about/index.html. Accessed April 20, 2022.
- 69.de Wit E., van Doremalen N., Falzarano D., et al. SARS and MERS: recent insights into emerging coronaviruses. Nat Rev Microbiol. 2016;14(8):523–534. doi: 10.1038/nrmicro.2016.81. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dudas G., Carvalho L.M., Rambaut A., et al. MERS-CoV spillover at the camel-human interface. Elife. 2018:7. doi: 10.7554/eLife.31257. In eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bleibtreu A., Bertine M., Bertin C., et al. Focus on Middle East respiratory syndrome coronavirus (MERS-CoV) Med Mal Infect. 2020;50(3):243–251. doi: 10.1016/j.medmal.2019.10.004. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Organization WH. MERS Situation Update July 2022. World Health Organization Regional Office for the Eastern Mediterranean. 2022. Available at: https://applications.emro.who.int/docs/WHOEMCSR546E-eng.pdf?ua=1. Accessed September 6, 2022.
- 73.Heesoo Joo P., Brian A., Maskery PhD., et al. Economic Impact of the 2015 MERS Outbreak on the Republic of Korea's Tourism-Related Industries. Health Security. 2019;17(2):100–108. doi: 10.1089/hs.2018.0115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Widagdo W., Sooksawasdi Na Ayudhya S., Hundie G.B., et al. Host Determinants of MERS-CoV Transmission and Pathogenesis. Viruses. 2019;11(3) doi: 10.3390/v11030280. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bosch B.J., Raj V.S., Haagmans B.L. Spiking the MERS-coronavirus receptor. Cell Res. 2013;23(9):1069–1070. doi: 10.1038/cr.2013.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Al Mutair A., Ambani Z. Narrative review of Middle East respiratory syndrome coronavirus (MERS-CoV) infection: updates and implications for practice. J Int Med Res. 2020;48(1) doi: 10.1177/0300060519858030. 300060519858030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nam H.S., Park J.W., Ki M., et al. High fatality rates and associated factors in two hospital outbreaks of MERS in Daejeon, the Republic of Korea. Int J Infect Dis. 2017;58:37–42. doi: 10.1016/j.ijid.2017.02.008. In eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Seys L.J.M., Widagdo W., Verhamme F.M., et al. DPP4, the Middle East Respiratory Syndrome Coronavirus Receptor, is Upregulated in Lungs of Smokers and Chronic Obstructive Pulmonary Disease Patients. Clin Infect Dis. 2017;66(1):45–53. doi: 10.1093/cid/cix741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chong Z.X., Liew W.P.P., Ong H.K., et al. Current diagnostic approaches to detect two important betacoronaviruses: Middle East respiratory syndrome coronavirus (MERS-CoV) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Pathol Res Pract. 2021;225:153565. doi: 10.1016/j.prp.2021.153565. (In eng) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shirato K., Nao N., Matsuyama S., et al. Ultra-Rapid Real-Time RT-PCR Method for Detecting Middle East Respiratory Syndrome Coronavirus Using a Mobile PCR Device, PCR1100. Japn J Infect Dis. 2020;73(3):181–186. doi: 10.7883/yoken.JJID.2019.400. [DOI] [PubMed] [Google Scholar]
- 81.Kim S.Y., Park S.J., Cho S.Y., et al. Viral RNA in Blood as Indicator of Severe Outcome in Middle East Respiratory Syndrome Coronavirus Infection. Emerg Infect Dis. 2016;22(10):1813–1816. doi: 10.3201/eid2210.160218. In eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rabaan A.A., Al-Ahmed S.H., Sah R., et al. MERS-CoV: epidemiology, molecular dynamics, therapeutics, and future challenges. Ann Clin Microbiol Antimicrob. 2021;20(1):8. doi: 10.1186/s12941-020-00414-7. In eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mo Y., Fisher D. A review of treatment modalities for Middle East Respiratory Syndrome. J Antimicrob Chemother. 2016;71(12):3340–3350. doi: 10.1093/jac/dkw338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bosaeed M, Balkhy HH, Almaziad S, et al. Safety and immunogenicity of ChAdOx1 MERS vaccine candidate in healthy Middle Eastern adults (MERS002): an open-label, non-randomised, dose-escalation, phase 1b trial. Lancet Microbe 2022;3(1):e11-e20. (Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8565931/pdf/main.pdf). [DOI] [PMC free article] [PubMed]
- 85.Yong CY, Ong HK, Yeap SK, et al. Recent advances in the vaccine development against Middle East respiratory syndrome-coronavirus. Front microb 2019;10:1781. (Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6688523/pdf/fmicb-10-01781.pdf). [DOI] [PMC free article] [PubMed]
- 86.Organization WH. MERS Outbreak in the Republic of Korea, 2015. Available at: https://www.who.int/westernpacific/emergencies/2015-mers-outbreak. Accessed September 6, 2022.