Abstract
Background
Systematic reviews showed that systemic postnatal corticosteroids reduce the risk of bronchopulmonary dysplasia (BPD) in preterm infants. However, corticosteroids have also been associated with an increased risk of neurodevelopmental impairment. It is unknown whether these beneficial and adverse effects are modulated by differences in corticosteroid treatment regimens related to type of steroid, timing of treatment initiation, duration, pulse versus continuous delivery, and cumulative dose.
Objectives
To assess the effects of different corticosteroid treatment regimens on mortality, pulmonary morbidity, and neurodevelopmental outcome in very low birth weight infants.
Search methods
We conducted searches in September 2022 of MEDLINE, the Cochrane Library, Embase, and two trial registries, without date, language or publication‐ type limits. Other search methods included checking the reference lists of included studies for randomized controlled trials (RCTs) and quasi‐randomized trials.
Selection criteria
We included RCTs comparing two or more different treatment regimens of systemic postnatal corticosteroids in preterm infants at risk for BPD, as defined by the original trialists. The following comparisons of intervention were eligible: alternative corticosteroid (e.g. hydrocortisone) versus another corticosteroid (e.g. dexamethasone); lower (experimental arm) versus higher dosage (control arm); later (experimental arm) versus earlier (control arm) initiation of therapy; a pulse‐dosage (experimental arm) versus continuous‐dosage regimen (control arm); and individually‐tailored regimens (experimental arm) based on the pulmonary response versus a standardized (predetermined administered to every infant) regimen (control arm). We excluded placebo‐controlled and inhalation corticosteroid studies.
Data collection and analysis
Two authors independently assessed eligibility and risk of bias of trials, and extracted data on study design, participant characteristics and the relevant outcomes. We asked the original investigators to verify if data extraction was correct and, if possible, to provide any missing data. We assessed the following primary outcome: the composite outcome mortality or BPD at 36 weeks' postmenstrual age (PMA). Secondary outcomes were: the components of the composite outcome; in‐hospital morbidities and pulmonary outcomes, and long‐term neurodevelopmental sequelae. We analyzed data using Review Manager 5 and used the GRADE approach to assess the certainty of the evidence.
Main results
We included 16 studies in this review; of these, 15 were included in the quantitative synthesis. Two trials investigated multiple regimens, and were therefore included in more than one comparison. Only RCTs investigating dexamethasone were identified.
Eight studies enrolling a total of 306 participants investigated the cumulative dosage administered; these trials were categorized according to the cumulative dosage investigated, 'low' being < 2 mg/kg, 'moderate' being between 2 and 4 mg/kg, and 'high' > 4 mg/kg; three studies contrasted a high versus a moderate cumulative dose, and five studies a moderate versus a low cumulative dexamethasone dose. We graded the certainty of the evidence low to very low because of the small number of events, and the risk of selection, attrition and reporting bias. Overall analysis of the studies investigating a higher dose versus a lower dosage regimen showed no differences in the outcomes BPD, the composite outcome death or BPD at 36 weeks' PMA, or abnormal neurodevelopmental outcome in survivors assessed. Although there was no evidence of a subgroup difference for the higher versus lower dosage regimens comparisons (Chi2 = 2.91, df = 1 (P = 0.09), I2 = 65.7%), a larger effect was seen in the subgroup analysis of moderate‐dosage regimens versus high‐dosage regimens for the outcome cerebral palsy in survivors. In this subgroup analysis, there was an increased risk of cerebral palsy (RR 6.85, 95% CI 1.29 to 36.36; RD 0.23, 95% CI 0.08 to 0.37; P = 0.02; I² = 0%; NNTH 5, 95% CI 2.6 to 12.7; 2 studies, 74 infants). There was evidence of subgroup differences for higher versus lower dosage regimens comparisons for the combined outcomes death or cerebral palsy, and death and abnormal neurodevelopmental outcomes (Chi2 = 4.25, df = 1 (P = 0.04), I2 = 76.5%; and Chi2 = 7.11, df = 1 (P = 0.008), I2 = 85.9%, respectively). In the subgroup analysis comparing a high dosage regimen of dexamethasone versus a moderate cumulative‐dosage regimen, there was an increased risk of death or cerebral palsy (RR 3.20, 95% CI 1.35 to 7.58; RD 0.25, 95% CI 0.09 to 0.41; P = 0.002; I² = 0%; NNTH 5, 95% CI 2.4 to 13.6; 2 studies, 84 infants; moderate‐certainty evidence), and death or abnormal neurodevelopmental outcome (RR 3.41, 95% CI 1.44 to 8.07; RD 0.28, 95% CI 0.11 to 0.44; P = 0.0009; I² = 0%; NNTH 4, 95% CI 2.2 to 10.4; 2 studies, 84 infants; moderate‐certainty evidence). There were no differences in outcomes between a moderate‐ and a low‐dosage regimen.
Five studies enrolling 797 infants investigated early initiation of dexamethasone therapy versus a moderately early or delayed initiation, and showed no significant differences in the overall analyses for the primary outcomes. The two RCTs investigating a continuous versus a pulse dexamethasone regimen showed an increased risk of the combined outcome death or BPD when using the pulse therapy. Finally, three trials investigating a standard regimen versus a participant‐individualized course of dexamethasone showed no difference in the primary outcome and long‐term neurodevelopmental outcomes.
We assessed the GRADE certainty of evidence for all comparisons discussed above as moderate to very low, because the validity of all comparisons is hampered by unclear or high risk of bias, small samples of randomized infants, heterogeneity in study population and design, non‐protocolized use of ‘rescue’ corticosteroids and lack of long‐term neurodevelopmental data in most studies.
Authors' conclusions
The evidence is very uncertain about the effects of different corticosteroid regimens on the outcomes mortality, pulmonary morbidity, and long term neurodevelopmental impairment. Despite the fact that the studies investigating higher versus lower dosage regimens showed that higher‐dosage regimens may reduce the incidence of death or neurodevelopmental impairment, we cannot conclude what the optimal type, dosage, or timing of initiation is for the prevention of BPD in preterm infants, based on current level of evidence. Further high quality trials would be needed to establish the optimal systemic postnatal corticosteroid dosage regimen.
Keywords: Humans; Infant; Infant, Newborn; Bronchopulmonary Dysplasia; Bronchopulmonary Dysplasia/prevention & control; Cerebral Palsy; Cerebral Palsy/complications; Dexamethasone; Dexamethasone/adverse effects; Glucocorticoids; Glucocorticoids/therapeutic use; Infant, Premature
Plain language summary
Different timing and dosages of corticosteroids to prevent lung injury
Review question
What timing and dosage of corticosteroids (a class of drugs that suppress inflammation) are best for preventing lung injury in babies born very early.
Background
Babies who are born too early have an increased risk of developing lung injury. In medical terms, this is called chronic lung disease (CLD) or bronchopulmonary dysplasia (BPD). Inflammation of the lungs is one of the causes of these lung problems, and for this reason studies have investigated the anti‐inflammatory drugs called corticosteroids. These studies showed that corticosteroid treatment reduced the risk of BPD, but it was also associated with serious side effects on development later in life. To reduce these side effects, doctors have looked for alternative courses of these drugs, such as postponing the start of corticosteroid therapy to a later period in life, lowering the total dose of the drug given, giving the drugs only for some days and then pausing for some time instead of every day, or deciding on the total dose or the length of the course of the drug depending on how the baby is doing instead of using a standard dose for all babies.
What did we do?
We searched electronic databases and found 16 studies investigating two or more different corticosteroid courses in preterm babies. The investigated courses differed in the total dose of the drug that was given, timing of start of the drug, and duration and schedule of therapy.
Main results
We identified 16 studies investigating different timing of initiation and dosages of corticosteroid therapy. The studies comparing a higher‐ versus a lower‐dose course showed no difference in the chance of developing BPD between the two groups, but there are concerns of an increased risk of poor development later in life for infants receiving a lower total dose of the drug. The studies investigating an earlier versus later start of steroids did not show any difference in outcome. Furthermore, courses that gave steroids on some days with pauses in between instead of every day showed a higher chance of BPD compared with everyday treatment. Deciding on the total doses and length of the course depending on how the baby was doing showed no differences compared to using the standard course for all babies.
What are the limitations of the evidence?
We have very limited confidence in the evidence, because most of the studies had limitations in study design. Most studies had a small sample size, and there were considerable differences between the studies that made it hard to compare them. Most of the studies were too short to provide information on the babies' longer‐term development. Therefore, it is not very well known what the best course of therapy is to prevent BPD.
How up to date is this evidence?
This review updates our previous review. The evidence is up to date to September 2022.
Summary of findings
Summary of findings 1. Lower compared to higher cumulative dose dexamethasone regimen for prevention of bronchopulmonary dysplasia in preterm infants.
| Lower compared to higher cumulative dose dexamethasone regimen for prevention of bronchopulmonary dysplasia in preterm infants | ||||||
| Patient or population: preterm infants at risk for bronchopulmonary dysplasia Intervention: lower cumulative dose dexamethasone regimen Comparison: higher cumulative dose dexamethasone regimen | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with higher cumulative dose dexamethasone regimen | Risk with lower | |||||
| Death or bronchopulmonary dysplasia at 36 weeks PMA | Study population | RR 1.03 (0.86 to 1.24) | 268 (7 RCTs) | ⊕⊕⊝⊝ LOWa,b | ||
| 651 per 1000 | 671 per 1000 (560 to 808) | |||||
| Death or cerebral palsy at 1 to 3 years | Study population | RR 1.74 (0.94 to 3.24) | 193 (4 RCTs) | ⊕⊕⊝⊝ LOWa,b | ||
| 308 per 1000 | 535 per 1000 (289 to 997) | |||||
| Death or abnormal neurodevelopmental outcome (various definitions) at 1 to 3 years | Study population | RR 1.86 (0.98 to 3.53) | 100 (3 RCTs) | ⊕⊝⊝⊝ VERY LOWa,b,c | ||
| 172 per 1000 | 319 per 1000 (168 to 606) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; PMA: postmenstrual age; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
a Downgraded one level for risk of bias in some included studies b Downgraded one level for serious imprecision of effect estimates (95% CI around estimate consistent with substantial harm or benefit). c Downgraded one level for serious inconsistency across studies.
Summary of findings 2. Later compared to earlier initiation of dexamethasone therapy for prevention of bronchopulmonary dysplasia in preterm infants.
| Later compared to earlier initiation of dexamethasone therapy for prevention of bronchopulmonary dysplasia in preterm infants | ||||||
| Patient or population: preterm infants at risk for bronchopulmonary dysplasia Intervention: later initiation of dexamethasone therapy Comparison: earlier initiation of dexamethasone therapy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with earlier initiation of dexamethasone therapy | Risk with later | |||||
| Death or bronchopulmonary dysplasia at 36 weeks' PMA | Study population | RR 1.06 (0.87 to 1.29) | 391 (3 RCTs) | ⊕⊕⊝⊝ LOWa,b | ||
| 476 per 1000 | 505 per 1000 (414 to 614) | |||||
| Death or cerebral palsy at 1 to 3 years | Study population | RR 1.12 (0.68 to 1.84) | 86 (1 RCT) | ⊕⊕⊝⊝ LOWa,b | ||
| 412 per 1000 | 461 per 1000 (280 to 758) | |||||
| Death or abnormal neurodevelopmental outcome (various definitions) | Study population | RR 0.87 (0.63 to 1.21) | 167 (2 RCTs) | ⊕⊕⊝⊝ LOWa,b | ||
| 507 per 1000 | 441 per 1000 (319 to 613) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; PMA: postmenstrual age; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
a Downgraded one level for serious study design limitations (unclear methodology or random sequence allocation, lack of blinding of clinicians and outcome assessment (Bloomfield 1998; Merz 1999; Hingre 1992; Halliday 2001a) b Downgraded one level for serious imprecision of effect estimate (95% CI around estimate consistent with substantial harm or benefit and number of events < 300).
Summary of findings 3. Pulse compared to continuous dexamethasone therapy for prevention of bronchopulmonary dysplasia in preterm infants.
| Pulse compared to continuous dexamethasone therapy for prevention of bronchopulmonary dysplasia in preterm infants | ||||||
| Patient or population: preterm infants at risk for bronchopulmonary dysplasia Intervention: pulse dexamethasone therapy Comparison: continuous dexamethasone therapy | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with continuous dexamethasone therapy | Risk with pulse | |||||
| Death or bronchopulmonary dysplasia at 36 weeks' PMA | Study population | RR 1.38 (1.02 to 1.88) | 197 (2 RCTs) | ⊕⊕⊝⊝ LOWa,b | ||
| 390 per 1000 | 538 per 1000 (398 to 733) | |||||
| Death or abnormal neurodevelopmental outcome (various definitions) | Study population | RR 1.23 (0.79 to 1.92) | 76 (1 RCT) | ⊕⊝⊝⊝ VERY LOWa,b,c | ||
| 459 per 1000 | 565 per 1000 (363 to 882) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; PMA: postmenstrual age; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
a Downgraded one level for serious study design limitations (lack of blinding of clinicians and outcome assessment (Bloomfield 1998)) b Downgraded one level for serious imprecision of effect estimate (95% CI around estimate consistent with substantial harm or benefit and number of events < 300). c Downgraded one level for publication bias, since the study by Barkemeyer 2001 was never published as full text.
Summary of findings 4. Individual tailored compared to continuous tapered dexamethasone regimen for prevention of bronchopulmonary dysplasia in preterm infants.
| Individual tailored compared to continuous tapered dexamethasone regimen for prevention of bronchopulmonary dysplasia in preterm infants | ||||||
| Patient or population: preterm infants at risk for bronchopulmonary dysplasia Intervention: individual tailored dexamethasone regimen Comparison: continuous tapered dexamethasone regimen | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with continuous tapered dexamethasone regimen | Risk with individual tailored | |||||
| Death or bronchopulmonary dysplasia at 36 weeks PMA | Study population | RR 1.06 (0.88 to 1.29) | 168 (3 RCTs) | ⊕⊕⊝⊝ LOWa,b | ||
| 639 per 1000 | 677 per 1000 (562 to 824) | |||||
| Death or cerebral palsy at 1 to 3 years | Study population | RR 7.24 (0.95 to 55.26) | 59 (1 RCT) | ⊕⊕⊕⊝ MODERATEb | ||
| 33 per 1000 | 241 per 1000 (32 to 1000) | |||||
| Death or abnormal neurodevelopmental outcome (various definitions) | Study population | RR 1.44 (0.99 to 2.07) | 168 (3 RCTs) | ⊕⊕⊝⊝ LOWa,b | ||
| 313 per 1000 | 451 per 1000 (310 to 648) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; PMA: postmenstrual age; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
a Downgraded one level for serious study design limitations (unclear methodology or random sequence allocation, lack of blinding of clinicians and outcome assessment (Bloomfield 1998; Odd 2004 ) b Downgraded one level for serious imprecision of effect estimate (95% CI around estimate consistent with substatial harm or benefit and number of events < 300).
Background
Description of the condition
The first description of bronchopulmonary dysplasia (BPD) by Northway and colleagues in 1967 was one of severe lung injury in relatively mature preterm infants who were ventilated with high pressures and high concentrations of oxygen before the advent of surfactant therapy (Northway 1967). This so‐called 'classical' BPD is characterized by profound lung parenchymal inflammation, fibrosis, muscle hypertrophy and diffuse airway damage (O'Brodovich 1985). Treatment and survival of the very young has led to a new pattern of lung injury (Coalson 2006; Jobe 1999). This so‐called 'new' BPD is mainly seen in very preterm infants with gestational ages less than 30 weeks. It is characterized by an arrest in lung development with fewer and larger alveoli, and less striking fibrosis and inflammation (Husain 1998). As a result of changes in infant and histological characteristics, the timing at which BPD is diagnosed has shifted from 28 days' postnatal age (PNA) to 36 weeks' postmenstrual age (PMA) (Bancalari 2006). Cohort studies have shown that, compared with 28 days' PNA, diagnosing BPD at 36 weeks' PMA provides a better identification of infants at risk for long‐term pulmonary and neurological sequelae (Ehrenkranz 2005).
BPD, defined as oxygen dependency at 36 weeks' PMA, remains an important complication of preterm birth with a reported incidence ranging from 23% to 73%, depending on the gestational age (Stoll 2010). BPD is characterized by prolonged respiratory support and recurrent respiratory infections during the first years, and compromised lung function lasting into adulthood. Furthermore, BPD is an independent risk factor for neurodevelopmental impairment (Short 2007; Walsh 2005).
BPD is considered a multifactorial disease. Besides genetic susceptibility, intrauterine growth restriction, nutritional deficits, direct mechanical injury caused by artificial ventilation, oxygen toxicity, and pulmonary inflammation has been identified as a key factor in the development of BPD (Carlton 1997; Ferreira 2000; Jobe 2001). Corticosteroids have a strong anti‐inflammatory effect, making them an ideal candidate to attenuate this inflammatory response associated with BPD.
Description of the intervention
Since the 1980s, several randomized controlled trials (RCTs) have investigated the use of corticosteroids, in particular dexamethasone, as a means to reduce the incidence of BPD. Some of these trials started corticosteroid therapy in the first week of life (early), with the aim of preventing progression of the initial acute inflammatory response to BPD (Yeh 1997). Others used corticosteroid therapy in infants who had evolving BPD, starting administration either moderately early (7 to 14 days) or delayed (more than three weeks) after birth (CDTG 1991; Durand 1995).
Current Cochrane Reviews of placebo‐controlled RCTs clearly showed that systemic corticosteroids, mainly dexamethasone, significantly reduced the incidence of BPD and the combined outcome of death or BPD in ventilated preterm infants, independent of the time of postnatal administration (Doyle 2021a; Doyle 2021b). However, at the end of the 1990s the first reports on long‐term neurodevelopmental outcome were published, showing that early postnatal systemic dexamethasone treatment is associated with an increased risk of abnormal neurological development (O'Shea 1999; Yeh 1998).
In response to these reports, the American Academy of Pediatrics, the Canadian Paediatric Society and the European Association of Perinatal Medicine concluded that routine use of systemic dexamethasone in the treatment of evolving BPD can no longer be recommended until further research has established the optimal type, dose and timing of corticosteroid therapy (AAP 2002; Halliday 2001; Watterberg 2010). Following these statements, observational reports have shown a sharp decline in the use of postnatal corticosteroids, a reduction in its cumulative dose, a delay in starting treatment, and a switch to alternative corticosteroids such as hydrocortisone (Kaempf 2003; Shinwell 2003; Walsh 2006).
How the intervention might work
To date, most studies have used a placebo‐controlled design to study the effects of postnatal corticosteroid treatment in preterm infants at risk for BPD. These studies have shown both benefits and harms of corticosteroid treatment. Adjusting the dosage regimen might improve the benefit‐to‐risk ratio of postnatal corticosteroid use. This review identified and analyzed the available randomized trials, using a head‐to‐head comparative design, on five possible treatment regimens.
Alternative corticosteroids The association between systemic dexamethasone treatment and long‐term neurodevelopmental impairment has resulted in the use of alternative anti‐inflammatory corticosteroids, such as hydrocortisone. Animal studies have suggested that, in contrast to dexamethasone, hydrocortisone has no detrimental effect on the brain (Huang 2007). Historical cohort studies have suggested that hydrocortisone treatment is equally effective in reducing death or BPD compared with dexamethasone‐treated infants without increasing the risk of adverse neurological outcome (van der Heide‐Jalving 2003; Karemaker 2006; Lodygensky 2005; Rademaker 2007). Pooled data on placebo‐controlled trials investigating a low hydrocortisone dose initiated at an early treatment onset (< 7 days' PNA) showed reduced rates of mortality, and of the combined outcome of mortality or bronchopulmonary dysplasia, without causing any obvious long‐term harm. However, gastrointestinal perforation was more frequent in the hydrocortisone group (Doyle 2021a). The only large placebo‐controlled randomized trial investigating the use of hydrocortisone after the first week of life in ventilator‐dependent preterm infants showed no improvement in the outcome BPD, or the composite outcome death or BPD (Onland 2019).
Lowering the corticosteroid dose and duration In line with the current opinion of postnatal corticosteroids being 'misguided rockets', clinicians have started to use lower dosage schedules of dexamethasone. The available reviews on placebo‐controlled trials of postnatal corticosteroids stacked information from trials with tremendous heterogeneity in their cumulative dose and duration of therapy (Doyle 2021a; Doyle 2021b). Subgroup analyses using this heterogeneity by dividing the different trials according to the used cumulative dexamethasone dose showed that higher dexamethasone doses reduce the typical risk ratio (RR) for the combined outcome of death or BPD, with the largest treatment effect in trials using a cumulative dose above 4 mg/kg (Onland 2009). No overall effect was found of dosing on the risk of neurodevelopmental sequelae, but in the moderately early treatment studies the risk of death or cerebral palsy (CP) significantly decreased when using a higher cumulative dose (Onland 2009).
Postponing initiation of therapy Besides lowering the cumulative dose, clinicians limited the use of corticosteroids to those infants that do not respond to other supportive therapies and spontaneous improvement over time. As a result, administration of postnatal corticosteroids in those infants is often postponed until the third or fourth week of life. Placebo‐controlled trials administrating dexamethasone after the first week of life differ in their timing of onset. Meta‐analysis dividing the different placebo‐controlled studies according to the timing of initiation used seems to suggest that moderately early administration is more effective in reducing BPD than delayed administration (Onland 2009; Schmidt 2008).
Pulse dose administration To minimize the possible adverse effects associated with continuous corticosteroid use, some have suggested prescribing dexamethasone in a pulse regimen using dexamethasone‐free intervals to minimize the risk of direct toxic effects of dexamethasone, while maintaining the beneficial effects on the lung. One placebo‐controlled trial showed that such a pulse regimen resulted in improved pulmonary outcome without clinically relevant side effects (Brozanski 1995).
Individualized tailored regimen Another approach is to reduce the risk of possible adverse effects of corticosteroids by tailoring the administered cumulative dose to the infant's pulmonary response. For instance, a rapid and clear improvement in respiratory status will allow for a rapid reduction in corticosteroid dose or duration (Bloomfield 1998). To date, there are no placebo‐controlled trials on individualized regime.
Why it is important to do this review
The international neonatal community has discarded the use of early postnatal corticosteroids completely for the reasons stated above. Regarding the use of moderately early or late postnatal systemic corticosteroids, clinicians encounter a dilemma facing those infants at high risk of BPD, since BPD itself is associated with an increased risk of adverse neurological outcome (Ehrenkranz 2005).
It is unknown whether both the beneficial and adverse treatment effects of postnatal corticosteroids can be modulated by the various different dosing regimens described above. Despite all the aforementioned concerns on the long‐term neurodevelopmental sequelae, corticosteroids are still used in approximately 16% of preterm infants (Costeloe 2012). Clinicians remain in doubt as to what the correct drug, cumulative dose, duration and timing of therapy are in terms of the optimal balance between beneficial and adverse effects. Addressing these questions is also important since studies have suggested that restricted use of postnatal corticosteroids resulted in an increased incidence of BPD (Cheong 2013; Shinwell 2007; Yoder 2009).
Objectives
To assess the effects of different corticosteroid treatment regimens on mortality, pulmonary morbidity, and neurodevelopmental outcome in very low birth weight infants.
Methods
Criteria for considering studies for this review
Types of studies
Randomized controlled or quasi‐randomized and cluster‐randomized trials comparing two or more different regimens of systemic corticosteroids in preterm infants at risk for BPD were eligible for this review. Non‐randomized cohort studies were not eligible for this review, given the fact of potential bias of confounding by indication or residual confounding influencing the results of studies with such designs (Fewell 2007; Kyriacou 2016). We excluded studies investigating the effects of one regimen of systemic corticosteroids versus a placebo arm or studies using inhalation corticosteroids. Those studies are included in other Cochrane Reviews.
Types of participants
Eligible participants were preterm infants at risk for BPD, as defined by the original trialists.
Types of interventions
We included trials which randomized infants to treatment with two different regimens of systemic corticosteroids. The following types of intervention were eligible.
An alternative corticosteroid (e.g. hydrocortisone) as the experimental arm versus another type of corticosteroid (e.g. dexamethasone) as the control arm. Any type of corticosteroid in either arm was allowed.
Lower cumulative corticosteroid dosage (experimental arm) versus higher cumulative corticosteroid dosage (control arm). Both arms of the identified trials were categorized according to the cumulative dosage investigated, 'low' being less than 2 mg/kg, 'moderate' being between 2 and 4 mg/kg, and 'high' using a cumulative dosage greater than 4 mg/kg. For inclusion, all comparisons of low‐, moderate‐ or high‐dosage regimens were allowed. Although arbitrary, these cut‐off values were chosen given the results of a systematic review of placebo‐controlled trials (Onland 2009).
Later (experimental arm) versus earlier (control arm) initiation of therapy. We categorized both arms of the identified trials according to the investigated timing of initiation, 'early' being less than 8 days' PNA, 'moderately early' being between 8 and 21 days' PNA, and 'delayed' being greater than 21 days' PNA. Similar to the dosing analyses, all comparisons were allowed. This arbitrary cut‐off point was chosen according to the original Cochrane Reviews on placebo‐controlled trials (Halliday 2003a; Halliday 2003b; Halliday 2003c).
Pulse‐dosage regimen (experimental arm) versus continuous‐dosage regimen (control arm). During pulse therapy, the administration of corticosteroids is interrupted for a period longer than the normal interval between corticosteroid doses. Any period of interruption was allowed.
Individually tailored regimens (experimental arm) based on the pulmonary response defined by the original trialists versus a standardized (predetermined schedule administered to every infant) dosage regimen independent of the pulmonary response (control arm).
Types of outcome measures
In the previous version of this review, two review authors (WO and ADJ) independently extracted the following outcome parameters for each study. In the current update of this review, one review author entered final data into Review Manager (WO) and a second review author checked the data for accuracy (MvdL).
Primary outcomes
Combined outcome of death or BPD at 36 weeks' PMA (BPD defined as the need for respiratory support or oxygen dependency at 36 weeks' PMA).
Secondary outcomes
Mortality at 28 days' PNA, 36 weeks' PMA, hospital discharge and during the first year of life.
BPD (defined by the need for supplemental oxygen) at 28 days' PNA and 36 weeks' PMA.
Failure to extubate at days three and seven after initiating therapy and at the latest reported time point.
Days of mechanical ventilation and supplemental oxygen.
Complications during primary hospitalization: hypertension, defined as more than two standard deviations (SD) according to local protocols; hyperglycemia, defined as greater than 8.3 mmol/L or requiring insulin therapy; rescue treatment with open‐label corticosteroids within or outside the study period; culture‐confirmed and clinically suspected infection; gastrointestinal bleeding or perforation, spontaneous intestinal perforation (SIP); necrotizing enterocolitis (NEC), following Bell's stages; patent ductus arteriosus (PDA), according to trial protocol and requiring therapy; intraventricular hemorrhage (IVH), any and severe grades; periventricular leukomalacia (PVL); cardiac hypertrophy; and retinopathy of prematurity (ROP), any and severe stages.
Long‐term neurodevelopmental sequelae, assessed after at least one year corrected gestational age (CGA) and before a CGA of four years, and at the latest reported time point, including cerebral palsy and Bayley Scales of Infant Development (Mental Development Index, MDI), blindness, and deafness.
Search methods for identification of studies
The Neonatal Group Information Specialist, in consultation with the authors, revised the search strategies for this update to incorporate a more sensitive approach to drug names for interventions (corticosteroids) and bronchopulmonary dysplasia.
Electronic searches
The following databases were searched without language or publication status restrictions. The RCT search was not limited by date; the search for systematic reviews was limited from 2020 forward.
Cochrane Central Register of Controlled Trials, Issue 9, 2022 (Wiley)
Cochrane Database of Systematic Reviews, Issue 9, 2022 (Wiley)
Ovid MEDLINE and Epub Ahead of Print, In‐Process, In‐Data‐Review & Other Non‐Indexed Citations, Daily and Versions 1946 to 9 September 2022
Embase (OVID) 1974 to 12 September 2022
Search strategies are available in Appendix 1; Appendix 2; and Appendix 3. Search strategies used in 2016 are available in Appendix 4.
Searching other resources
Two trial registries were searched without date limits.
US National Library of Medicine's trial registry www.clinicaltrials.gov
World Health Organization International Clinical Trials Registry Platform (ICTRP) trialsearch.who.int/
Search strategies are available in Appendix 5.
We searched the following websites for conference abstracts from 1990 forward.
Pediatric Academic Societies (PAS)
European Society for Pediatric Research
Electronic searches were supplemented by contacting original authors of all studies to confirm details of reported follow‐up studies or to obtain information about long‐term follow‐up where none were reported.
We checked the reference lists of included studies and of systematic reviews related to the topic of this review.
Data collection and analysis
Selection of studies
We used Cochrane’s Screen4Me (S4M) automatic classifier (Noel‐Storr 2020; Noel‐Storr 2021a; Noel‐Storr 2021b; Screen4Me), and S4M’s Known Assessments and RCT Classifier to exclude known non‐RCTs. Two review authors (WO and MvdL) independently screened remaining title/abstracts and full‐texts. At any point in the screening process, disagreements were resolved by discussion. Search results were managed in Endnote and screened using Covidence software (Covidence). We documented reasons for excluding studies after full‐text review, and noted their characteristics. We documented characteristics of ongoing studies and studies awaiting assessment. We collated multiple reports of the same study so that each study, not reference, is the unit of interest in the review. We recorded the search process in sufficient detail to create a study flow diagram (Liberati 2009).
Data extraction and management
In the current update of this review, one review author entered final data into Review Manager (WO) and a second review author checked the data for accuracy (MvdL). Review authors resolved any discrepancies through discussion. In the previous version of this review, two review authors (WO and ADJ) independently extracted the following data for each study, in addition to the predefined outcome measurements, using a predefined data sheet: infant's characteristics (such as birth weight, gestational age, gender); number of participants randomized; treatment with antenatal corticosteroids and postnatal surfactant; type of corticosteroid and regimens (PNA at start, duration of therapy, cumulative dose; dosing interval (fixed or variable); dose adjustments according to infant's characteristics); and the incidence of open‐label (outside the study protocol) use of corticosteroids in both arms of the studies. The original investigators of the included RCTs were asked to confirm whether the data extraction was accurate and, where necessary, to provide additional (unpublished) data.
Assessment of risk of bias in included studies
Two review authors (WO and ADJ) in the previous version, and two reviewers in the current version (WO and MvdL), independently assessed the risk of bias (low, high, or unclear) of all included trials using the Cochrane risk of bias tool in the Cochrane Handbook for Systematic Reviews of Interventions for the following domains (Higgins 2017).
Selection bias
Performance bias
Detection bias
Attrition bias
Reporting bias
Any other bias
Any disagreements were resolved by discussion or by a third assessor. See Appendix 6 for a more detailed description of risk of bias for each domain.
Measures of treatment effect
We conducted data management using the Cochrane statistical package, Review Manager 5 (Review Manager 2020). Where possible, we calculated treatment effect estimates for dichotomous outcomes in all individual trials expressed as the risk ratio (RR) and risk difference (RD), all with a 95% confidence interval (CI). For continuous outcomes reported in individual studies we used the mean values for treatment and control groups, with the SD. If median and range were given in individual studies, and the study authors were not able to provide the mean value and variance from the original data set, we calculated them according to the method described by Hozo 2005. We calculated the number needed to treat for an additional beneficial outcome (NNTB) and number needed to treat for an additional harmful outcome (NNTH) for each different outcome in case of statistical significance (Graphpad 2021).
Unit of analysis issues
If cluster‐randomized trials had been included in the analyses, we would have adjusted their sample sizes using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022).
Dealing with missing data
We asked the study author of each included RCT to confirm whether the data extraction was accurate and, where necessary, to provide additional (unpublished) data.
Assessment of heterogeneity
We assessed heterogeneity between trials by inspecting the forest plots and quantifying the impact of heterogeneity using the I² statistic, using the following categories as defined by the Cochrane Neonatal Review Group.
Less than 25%: no heterogeneity.
25% to 49%: low heterogeneity.
50% to 74%: moderate heterogeneity.
75% or greater: high heterogeneity.
We explored possible causes of statistical heterogeneity using prespecified subgroup analysis (e.g. differences in intervention regimens).
Assessment of reporting biases
We used funnel plots to assess possible reporting or publication biases.
Data synthesis
We performed meta‐analysis of the extracted data using standard Cochrane methods and Review Manager 5 (Review Manager 2020). Treatment effects for dichotomous outcomes were expressed as RR with a 95% CI, RD, and NNTBs or NNTHs in case of significance. We used mean differences (MD) for continuous outcomes. In case of variance of outcome measures (with different SD) measuring the same outcome, we calculated standardized mean differences (SMD) in the meta‐analysis. We used the fixed‐effect model for all meta‐analyses.
Subgroup analysis and investigation of heterogeneity
In case of substantial heterogeneity, we performed subgroup analyses and sensitivity analyses, and, if not appropriate, reconsidered whether an overall summary was meaningful at all. We planned to carry out the following subgroup analyses.
Gestational age using an arbitrary cut‐off point of 26 weeks.
The degree of illness at the start of treatment as defined by mean respiratory index or fractional inspired oxygen, if available, at trial entry.
Ventilated versus non‐ventilated neonates at study entry.
Trials allowing use of open‐label corticosteroids during the study period, by dividing the individual trials according to the percentage of infants treated with open‐label corticosteroids in the experimental arm, using arbitrary cut‐off points of less than 30%, 30% to 50%, and greater than 50% of the included infants; and trials investigating two (or more) of the main comparisons analyzed in both comparisons in subgroups. For example, if a study investigates hydrocortisone at an early initiation versus a dexamethasone regimen at a later treatment onset, this study would be analyzed in both the main comparison type of corticosteroids, as well as the comparison timing of initiation.
Sensitivity analysis
We performed sensitivity analyses when we judged trials to be at high risk of bias, to assess the effect of the bias on the meta‐analysis.
Summary of findings and assessment of the certainty of the evidence
We used the GRADE approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes: the combined outcome of BPD or death at 36 weeks' PMA, as well as the combined outcomes of death or cerebral palsy, and death or abnormal neurodevelopmental outcome.
Two authors independently assessed the quality of the evidence for each of the outcomes above. We considered evidence from randomized controlled trials as high quality but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates and presence of publication bias. We used the GRADEpro GDT Guideline Development Tool to create summary of findings tables to report the quality of the evidence.
The GRADE approach results in an assessment of the quality of a body of evidence in one of four grades.
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Results
Description of studies
Results of the search
Searches identified 8758 references (8225 from databases and trial registries; 533 from conference searching). After removing 2371 duplicates, 6387 records were available for screening. We excluded 6351 references (500 via Screen4Me (Figure 1); 5851 by authors).
1.
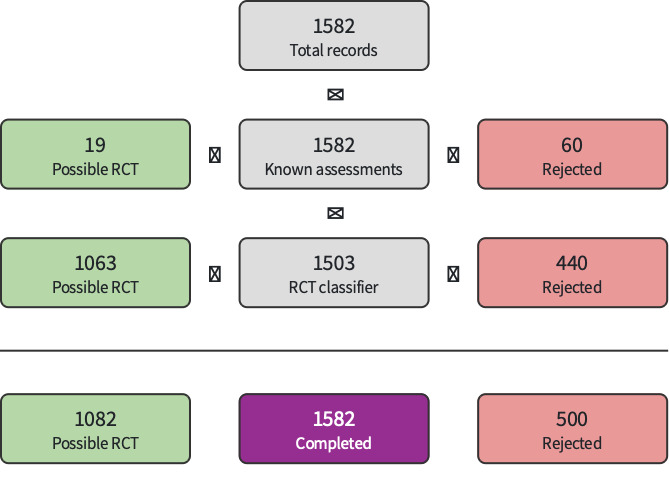
Screen4Me 13 September 2022
We assessed 35 full‐texts and one trial registry record for inclusion. We excluded five studies (five references) with reasons (Characteristics of excluded studies). We placed one study (IRCT20200721048155N1), in Awaiting classification since the current status is that recruitment has been completed but no results have been published yet (Characteristics of studies awaiting classification); and identified one ongoing study (IRCT20201222049802N3).
We included 16 studies (29 references); see Characteristics of included studies. One study, Groneck 1993, could not be included in our quantitative synthesis because outcome data were not presented in the published reports and were not available from the authors of the study. Thus, 15 studies are included in our quantitative synthesis and 16 studies in the qualitative synthesis.
Details of the selection process can be seen in Figure 2.
2.
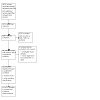
PRISMA flow chart
Included studies
Sixteen trials (29 reports) met inclusion criteria; of these, 15 are included in the quantitative synthesis. The 15 trials randomized a total of 1257 infants. A detailed description of participant characteristics of the individual trials can be found in Table 5. Most trials included preterm infants with similar ranges of gestational age and birth weight, yet there was considerable variation in the use of antenatal corticosteroids and exogenous surfactant. Pulmonary illness, assessed by the amount of supplemental oxygen and the level of mean airway pressure at study entry, differed considerably across the trials. Only three trials reported no late rescue treatment with dexamethasone in both treatment groups. The investigated regimens differed in the used cumulative dose, timing of initiation and duration of therapy.
1. Participant characteristics of individual trials.
| Allocation arm | Participants (N) |
Birthweight (grams) |
Gestational age (weeks) |
Antenatal steroids(%) |
Surfactant(%) | Starting dose (mg/kg/d) | Cumulative dose (mg/kg) | Mean age initiation | Total duration(days) |
Late rescue corticosteroids (%) |
Entry FiO₂ (%) |
Entry MAP (cmH₂0) |
|
| Lower cumulative dosage (experimental arm) versus higher cumulative dosage (control arm) | |||||||||||||
| Cummings 1989 | High | 13 | 818 ± 145 | 26 ± 2 | 38 | 0 | 0.5 | 7.9 | 14 | 42 | 0 | 0.60 ± 0.27 | 10.4 ± 6.0 |
| Moderate | 12 | 810 ± 208 | 26 ± 2 | 25 | 0 | 0.5 | 3.0 | 18 | 0 | 0.51 ± 0.23 | 8.8 ± 2.7 | ||
| DeMartini 1999 | High | 16 | 741 ± 142 | 25.5 ± 1.7 | 62 | 100 | 0.5 | 4.1 | ? | 21 | 0 | 0.61 ± 26.9 | ? |
| Moderate | 14 | 848 ± 224 | 26.4 ± 1.6 | 64 | 100 | 0.5 | 2.7 | 7 | 0 | 0.60 ± 25.2 | |||
| Marr 2019a | High | 30 | 769 ± 149 | 25.2 ± 1.2 | 63 | 97 | 0.5 | 7.96 | 14 ± 4 | 42 | 20 | 0.72 ± 0.13 | 10.3 ± 2.0 |
| Moderate | 29 | 785 ± 167 | 25.2 ± 1.1 | 62 | 86 | 0.5 | 4.04 | 13 ± 3 | 9b | 37 | 0.77 ± 0.16 | 10.4 ± 1.7 | |
| Malloy 2005 | Moderate | 9c | 767 ± 149 | 25.8 ± 0.9 | 75 | 100 | 0.5 | 2.7 | 14.8 ± 6.5 | 7 | 88 | 0.57 ± 0.08 | ? |
| Low | 8 | 773 ± 182 | 26.1 ± 1.8 | 63 | 100 | 0.08 | 0.6 | 16.8 ± 5.7 | 7 | 50 | 0.52 ± 0.16 | ||
| Durand 2002 | Moderate | 23 | 932 ± 182 | 27.1 ± 1.8 | 52 | 87 | 0.5 | 2.4 | 11.5 ± 2.2 | 7 | 22 | 0.43 ± 0.11 | 7.8 ± 2.2 |
| Low | 24 | 858 ± 186 | 26.9 ± 1.6 | 50 | 88 | 0.2 | 1.0 | 11.3 ± 2.7 | 7 | 29 | 0.41 ± 0.10 | 7.0 ± 1.2 | |
| McEvoy 2004 | Moderate | 29 | 839 ± 229 | 26.1 ± 2.0 | 34 | 97 | 0.5 | 2.4 | 10.7 ± 3.7 | 7 | 55 | 0.44 ± 0.13 | 6.8 ± 1.8 |
| Low | 33 | 830 ± 248 | 26.3 ± 1.8 | 48 | 82 | 0.2 | 1.0 | 11.6 ± 4.3 | 7 | 39 | 0.42 ± 0.13 | 7.4 ± 2.2 | |
| Ramanathan 1994 | Moderate | 15 | 850 ± 290 | 27 ± 2 | ? | 67 | 0.4 | 1.9d | 10 to 14 | 7 | 67 | ? | ? |
| Low | 13 | 817 ± 186 | 27 ± 2 | 62 | 0.2 | 1.0d | 7 | 54 | |||||
| Da Silva 2002 | Moderate | 17 | 821 ± 160 | 25.4 ± 0.9 | ? | ? | 0.5 | ? | ? | 7 | ? | ? | ? |
| Low | 21 | 851 ± 465 | 25.7 ± 1.8 | 0.1 | 0.7 | 7 | |||||||
| Later initiation (experimental arm) versus earlier (control arm) | |||||||||||||
| Papile 1998 | ME | 182 | 808 ± 187 | 25.7 ± 1.9 | 29 | 91 | 0.5 | 3.7 | 14 | 14 | 12 | 0.54 ± 0.18 | 8 ± 2 |
| L | 189 | 801 ± 182 | 25.6 ± 1.6 | 27 | 89 | 28 | 16 | 0.54 ± 0.19 | 8 ± 2 | ||||
| Merz 1999 | E | 15 | 980 (710 to 1250) | 27 (25 to 29) | 87 | 87 | 0.5 | 3.1 | 7 | 16 | 0 | 0.3 (0.25 to 0.5) | ? |
| ME | 15 | 938 (680 to 1250) | 27.5 (24 to 29) | 73 | 73 | 14 | 0 | 0.3 (0.25 to 0.55) | |||||
| Halliday 2001a | E | 135 | 1017 ± 290 | 27.4 ± 1.9 | 61 | 95 | 0.5 | 2.7 | 3 | 12 | ? | ? | ? |
| ME | 150 | 1007 ± 283 | 27.1 ± 1.9 | 55 | 92 | 16 | |||||||
| Hingre 1992 | E | 14 | 744 ± 144 | 26 ± 2 | ? | ? | 0.5 | 7.96 | 5 | 42 | ? | ? | ? |
| ME | 21 | 730 ± 135e | 25 ± 2 | 14 | |||||||||
| Pulse dosage regimen (experimental arm) versus continuous dosage regimen (control arm) | |||||||||||||
| Bloomfield 1998 | Pulse/Ef | 39 | 776 ± 25 | 25.8 ± 0.3 | 95 | ? | 0.5 | 5.3 (1.5 to 11.8) | 7 | 34 (11 to 73) | ? | 0.30 ± 0.02 | 8.0 ± 0.3 |
| Cont/ME | 37 | 793 ± 28 | 25.8 ± 0.3 | 73 | 0.5 | 7.1 (4.5 to 7.6) | 14 | 42 (42 to 51) | 0.30 ± 0.01 | 7.8 ± 0.3 | |||
| Barkemeyer 2001 | Pulse | 58 | 816 | 26.1 | 84 | 92 | 0.5 | 4.5 | 7 to 21 | 23 | 41 | ? | ? |
| Cont | 63 | 842 | 26.2 | 78 | 88 | 36 | |||||||
| Individualized tailored (experimental arm) versus standard dosage regimen (control arm) | |||||||||||||
| Odd 2004 | Indiv | 17 | 669 ± 113 | 24 (23 to 27) | ? | ? | 0.5 | 3.8 (2.0 to 5.7) | 12 (7 to 16) | 42 (5 to 73) | ? | 0.40 (0.25 to 1.0) | 9 (7 to 14) |
| Cont | 16 | 720 ± 130 | 24 (23 to 26) | 0.5 | 7.9 | 10 (7 to 23) | 42 | 0.40 (0.21 to 1.0) | 9 (7 to 13) | ||||
a Marr not only in higher versus lower comparison, but also in individual tailored versus standard dosage regimen b 19 of the 29 infants (66%) received one course, 5 infants (17%) 2 courses, and 5 infants (17%) 3 courses of dexamethasone c Including one patient in high dose group who died on the second day of treatment d Estimated cumulative dose based on abstract data e participant characteristics calculated on 16 participants in the moderately early group f Bloomfield not only pulse versus continuous comparison, but also in early versus later initiation and in individualized tailored versus standard dosage regimens comparison
Mean ± standard deviation or median (interquartile range)
Abbreviations: FiO2: fractional inspired oxygen; MAP: mean airway pressure; E: Early initiation (≤ 7 days' PNA); ME: Moderately early initiation (7 to 14 days' PNA); L: Late initiation (> 14 days' PNA); Pulse: Pulse dosage regimen; Cont: Continuous tapered dosage regimen; Indiv: Individual tailored regimen.
The trial by Marr 2019 investigated a high‐dosage tapered regimen of corticosteroids with a duration of 42 days versus a moderate‐dosage regimen of corticosteroids during nine days. Whereas the high‐dosage regimen was only given once, participants on the moderate‐dosage regimen of nine days could receive a second, or even a third course of nine days moderate dose of dexamethasone if entry respiratory criteria were met after completion of the previous course. Based on this design, we used the trial for two comparisons in this review: higher versus lower dosage regimens of corticosteroid treatment, and individualized versus standardized dosing. The trial by Bloomfield 1998 allocated infants to a group receiving a pulse dose of corticosteroids initiated early or a group receiving a continuous tapering dose of corticosteroids started moderately early. In addition, the duration of the pulse dose, but not the continuous tapering dose, was dependent on the pulmonary response of the infant. Based on this design, we used the trial for three comparisons in this review: earlier versus later initiation of corticosteroid treatment, pulse versus continuous dosing, and individualized versus standardized dosing. We included all other studies in one comparison each.
Eight of the 15 original investigators provided the authors with additional data on methodology, intervention, infant characteristics or missing outcome parameters.
Alternative corticosteroids
We did not identify any trials investigating two or more different types of corticosteroids. In fact, all trials included in this review used dexamethasone in both treatment arms.
Lowering the corticosteroid dose and duration
The timing of the eight eligible trials investigating this comparison was moderately early (7 to 21 days). The cumulative dexamethasone doses in the studies ranged from 0.6 to 3.0 mg/kg in the lower‐dosage regimens (experimental arm) to 1.9 to 7.9 mg/kg in the high‐dosage regimens (control arm). Only two dosage comparisons were identified during this review, high (> 4 mg/kg cumulative dose) versus moderate dose (between 2 and 4 mg/kg cumulative dose) and moderate‐ versus low‐ (< 2 mg/kg cumulative dose) dosage regimens. Three trials compared a high dose (control arm) to a moderate dose (Cummings 1989; DeMartini 1999; Marr 2019); and five trials a moderate dose to a low dose (Da Silva 2002; Durand 2002; Malloy 2005; McEvoy 2004; Ramanathan 1994). We analyzed and reported these two comparisons separately.
Postponing initiation of therapy
Five RCTs investigated the effect of timing on the dexamethasone treatment effects in preterm infants (Bloomfield 1998; Halliday 2001a; Hingre 1992; Merz 1999; Papile 1998). Only two comparisons were identified, namely late versus moderately early initiation, and moderately early versus early initiation of corticosteroid therapy. Papile 1998 compared late (> 21 days' PNA (experimental arm)) to moderately early (between 8 and 21 days (control arm)) initiation of treatment. The other four trials contrasted early (≤ 7 days' PNA) to moderately early (experimental arm) initiation of treatment. We analyzed these two comparisons separately. The comparison of moderately early versus early initiation included the trial performed by Halliday 2001a. This RCT used a factorial design with four allocation arms. Two arms administered corticosteroids by inhalation, and we excluded the data of the infants treated with inhalation corticosteroids from this review. The other two arms administered dexamethasone systemically, starting either early or moderately early, and we therefore included these in the analysis.
Pulse dose administration
Two trials compared pulse therapy of dexamethasone (experimental arm) with a continuous tapering dosage regimen (control arm) (Barkemeyer 2001; Bloomfield 1998). Both trials used a pulse dexamethasone therapy (0.5 mg/kg/day) for three consecutive days followed by seven days of no corticosteroid therapy. One trial administered similar cumulative doses of dexamethasone in both allocation arms (Barkemeyer 2001). However, in the other trial the duration of the pulse‐dosage regimen varied, depending on the infant’s pulmonary condition and level of respiratory support (Bloomfield 1998). The continuous tapering dosage regimen in this study, however, was the same for every infant allocated to this arm.
Individualized tailored regimen
Three trials allocated the infants to either an individualized dosage regimen (experimental arm), or a tapering dosage regimen. Two studies initiated the intervention at the same postnatal age (Odd 2004; Marr 2019), whereas the other study initiated the pulse therapy at day seven of life, comparing it to a tapering continuous‐dosage regimen commencing at day 14 of life (Bloomfield 1998).
Excluded studies
We excluded five trials after reading the full text. One had a retrospective study design (DeCastro 2009); one was a placebo controlled trial of early dexamethasone administration (Shipalana 1994); one was a report of a web‐based survey on corticosteroids (Singh 2022); one trial registration was identified without any report of completion of the study or results (Ahrens 2000); and one investigated two different dexamethasone regimens, but in a placebo controlled design (Romagnoli 1999).
Awaiting classification
One study is awaiting classification (IRCT20200721048155N1) (see Characteristics of studies awaiting classification).
Ongoing studies
We identified one trial registration title as an ongoing trial (IRCT20201222049802N3) (see Characteristics of ongoing studies).
Risk of bias in included studies
We deemed the overall risk of bias of the 15 trials to range from unclear to low (Figure 3; Figure 4). Four trials were only published as abstracts, and therefore had insufficient data to allow a proper methodological assessment (Da Silva 2002; DeMartini 1999; Hingre 1992; Ramanathan 1994).
3.

Risk of bias graph: review authors' judgments about each risk of bias item presented as percentages across all included studies.
4.

Risk of bias summary: review authors' judgments about each risk of bias item for each included study.
Allocation
Five trials described the random sequence generation insufficiently, whereas four trials did not mention the method of allocation concealment. Therefore, only nine trials described both these items properly, and we judged them as having low risk of selection bias.
Blinding
Six trials did not attempt to blind the intervention; thus caregivers, parents and outcome assessors were not blinded. We judged these trials as being at high risk for performance and detection bias. In two trials no information on blinding was available, making it impossible to assess bias (Hingre 1992; Ramanathan 1994).
Incomplete outcome data
We judged the RCTs to be at low risk of attrition bias, because all but two trials reported data on 'loss to follow‐up' or participant selection, or both. Malloy 2005 excluded one infant who died during the study course, and for this reason was assessed as being at high risk of attrition bias. However, this infant was included in the current analyses. Hingre 1992 excluded five deceased infants in the late treatment group from the analysis, but these infants were included in the current analysis for mortality.
Selective reporting
None of the included trials published a study protocol. Four included studies were published only as abstracts (Da Silva 2002; DeMartini 1999; Hingre 1992; Ramanathan 1994); therefore, this item could not be assessed. All studies reported sufficiently on the predefined outcome parameters.
Other potential sources of bias
We judged most trials as having an unclear risk for other potential sources of bias, because the authors did not state in the manuscript if and how the studies were funded (Bloomfield 1998; Cummings 1989; Da Silva 2002; DeMartini 1999; Durand 2002; Marr 2019; Merz 1999; Odd 2004; Ramanathan 1994). Malloy 2005 was terminated prematurely; and in Halliday 2001a, a large proportion of the infants randomized to delayed selective treatment either died or did not fulfill the entry criteria. We judged the other trials to be at low risk.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Comparison 1. Lower (experimental arm) versus higher (control arm) cumulative dosage regimens of dexamethasone
Primary outcome
Combined outcome of death or BPD at 36' weeks PMA
Meta‐analysis suggests that a lower compared with a higher cumulative corticosteroid dosage may not affect the risk of death or BPD at 36' weeks PMA (RR 1.03, 95% CI 0.86 to 1.24; P = 0.73; I²= 7%; 7 trials, 268 participants; Analysis 1.1). There was no evidence of a subgroup effect by 'moderate versus high cumulative dose regimen' (Chi2 = 1.09, df = 1 (P = 0.30), I2 = 8%). We graded the certainty of the evidence low using GRADE methods because of the small number of events, and the risk of selection, attrition and reporting bias (Table 1). We were unable to assess potential publication bias in a funnel plot as fewer than 10 eligible RCTs reported this outcome. Furthermore, the planned subgroup or sensitivity analyses by gestational age, severity of illness, mode of ventilation, and use of open‐label corticosteroids were not possible because of paucity of available data. Meta‐analysis including only those trials without high risk of bias in any domains suggests that a lower compared with a higher cumulative corticosteroid dosage may not affect the risk of death or BPD at 36' weeks PMA (RR 1.09, 95% CI 0.93 to 1.29; I² = 41%; 4 trials, 142 participants).
1.1. Analysis.

Comparison 1: Lower versus higher cumulative dose dexamethasone regimen, Outcome 1: Death or bronchopulmonary dysplasia at 36 weeks PMA
Secondary outcomes
Mortality at 28 days' PNA, at 36 weeks' PMA and at hospital discharge.
No data were retrieved on mortality at 28 days' PNA. Meta‐analysis suggests that a lower compared with a higher cumulative corticosteroid dosage may not affect the risk of the outcome of death at 36 weeks' PMA, and at hospital discharge (RR 0.68, 95% CI 0.29 to 1.60; P = 0.38; I² = 0%; 7 trials, 268 participants; subgroup differences Chi2 = 1.03, df = 1 (P = 0.31), I2 = 2.9%; Analysis 1.2; and RR 0.93, 95% CI 0.48 to 1.81; P = 0.84; I² = 0%; 7 trials, 268 participants; subgroup differences Chi2 = 2.14, df = 1 (P = 0.14), I2 = 53.3%; Analysis 1.3, respectively). Subgroups for moderate‐ versus high‐ and low‐ versus moderate‐dosage regimens also showed no evidence of a difference.
1.2. Analysis.

Comparison 1: Lower versus higher cumulative dose dexamethasone regimen, Outcome 2: Mortality at 36 weeks' PMA
1.3. Analysis.

Comparison 1: Lower versus higher cumulative dose dexamethasone regimen, Outcome 3: Mortality at hospital discharge
BPD at 28 days' PNA and 36 weeks' PMA
No data were retrieved on the outcome of BPD at 28 days' PNA. Meta‐analysis suggests that a lower compared with a higher cumulative corticosteroid dosage may not affect the risk of the outcome BPD at 36 weeks' PMA (RR 1.12, 95% CI 0.91 to 1.37; P = 0.28; I² = 31%; 7 trials, 268 participants; subgroup differences Chi2 = 0.00, df = 1 (P = 0.99), I2 = 0%; Analysis 1.4). Subgroups moderate versus high and low versus moderate dosage regimen also showed no evidence of a difference.
1.4. Analysis.

Comparison 1: Lower versus higher cumulative dose dexamethasone regimen, Outcome 4: Bronchopulmonary dysplasia at 36 weeks' PMA
Failure to extubate
Meta‐analysis suggests that a lower compared with a higher cumulative corticosteroid dosage may not affect the outcome of 'failure to extubate day three after initiation therapy' (RR 1.12, 95% CI 0.98 to 1.29; P = 0.09; I² = 0%; 5 trials, 209 participants; subgroup differences Chi2 = 0.04, df = 1 (P = 0.85), I2 = 0%; Analysis 1.5). However, compared to the infants allocated to the higher dosage regimens, the infants allocated to the lower‐dose regimen had a higher incidence of failing extubation at day seven after initiation of therapy (RR 1.32, 95% CI 1.08 to 1.60; P = 0.006; I²= 5%; NNTH 6, 95% CI 3 to 17; 5 trials, 210 participants; Analysis 1.6). Although there was no evidence of a subgroup difference between the high versus moderate dosage regimen compared with the moderate versus low dosage regimen (Chi2 = 1.03, df = 1 (P = 0.31), I2 = 3.2%), a larger effect was found in the higher dosage regimen subgroup (RR 1.49, 95% CI 1.10 to 2.02; P = 0.01; I²=54%; NNTH 4, 95% CI 2 to 12; 2 trials, 58 participants; Analysis 1.6.1). For the subgroup low versus moderate dosage regimen there was no evidence of a difference in failure to extubate seven days after initiation of therapy.
1.5. Analysis.

Comparison 1: Lower versus higher cumulative dose dexamethasone regimen, Outcome 5: Failure to extubate 3 days after initiation
1.6. Analysis.

Comparison 1: Lower versus higher cumulative dose dexamethasone regimen, Outcome 6: Failure to extubate 7 days after initiation
Days of mechanical ventilation and supplemental oxygen
Meta‐analysis suggests that a lower compared with a higher cumulative corticosteroid dosage may not affect the duration of mechanical ventilation (MD 4.50, 95% CI ‐0.68 to 9.67; P = 0.09; I² = 68%; 6 trials, 218 participants; Analysis 1.7). Although there was no evidence of a subgroup difference (Chi2 = 1.40, df = 1 (P = 0.24), I2 = 28.7%) in the high‐dosage regimen compared to the moderate‐dosage regimen, duration of mechanical ventilation was shorter (MD 8.09, 95% CI 0.21 to 15.96; P = 0.04; I² = 85%; 3 trials; 112 participants; Analysis 1.7.1). No evidence of a difference was seen in the outcome 'days of supplemental oxygen' (MD 0.30, 95% CI ‐20.14 to 20.74; P = 0.98; I² = 0%; 2 trials, 80 participants; subgroup differences Chi2 = 0.16, df = 1 (P = 0.69), I2 = 0%; Analysis 1.8).
1.7. Analysis.

Comparison 1: Lower versus higher cumulative dose dexamethasone regimen, Outcome 7: Days of mechanical ventilation
1.8. Analysis.

Comparison 1: Lower versus higher cumulative dose dexamethasone regimen, Outcome 8: Days on supplemental oxygen
Complications during primary hospitalization
Compared to the infants allocated to the higher‐dosage regimen, the infants allocated to the lower‐corticosteroid regimen showed a lower incidence of the short‐term adverse effect of hypertension (RR 0.31, 95% CI 0.12 to 0.77; P = 0.01; I² = 0%; NNTB 10, 95% CI 5.9 to 32; 6 trials, 240 participants; Analysis 1.9). Although there was no evidence of a subgroup difference between the high versus moderate dosage regimen and the moderate versus low dosage regimen (Chi2 = 0.01, df =1 (P = 0.91), I2 = 0%), a smaller confidence interval was found in the lower dosage regimen subgroup (RR 0.31, 95% CI 0.11 to 0.87; P = 0.03; I² = 0%; NNTH 7, 95% CI 4 to 30; 3 trials, 126 participants; Analysis 1.9.2).
1.9. Analysis.

Comparison 1: Lower versus higher cumulative dose dexamethasone regimen, Outcome 9: Hypertension
For hyperglycemia a similar result was found for higher versus lower dosage regimen (RR 0.60, 95% CI 0.37 to 0.97; P = 0.04; I² = 13%; NNTB 10, 95% CI 4.8 to 113; 6 trials, 240 participants; Analysis 1.10). Although there was no evidence of a subgroup difference between the high versus moderate dosage regimen compared with the moderate versus low dosage regimen (Chi2 = 1.93, df =1 (P = 0.17), I2 = 48.1%), a larger effect was seen in the low versus moderate dosage regimen (RR 0.40, 95% CI 0.17 to 0.93; P = 0.03; I² = 0%; NNTH 7, 95% CI 4 to 41; 3 trials, 126 participants; Analysis 1.10.2). No differences were seen between the moderate and high dosage comparison.
1.10. Analysis.

Comparison 1: Lower versus higher cumulative dose dexamethasone regimen, Outcome 10: Hyperglycemia
There was no evidence of a difference between the different dosage regimens for the following outcomes:
incidence of late ‘rescue’ therapy with open‐label corticosteroids (RR 0.93, 95% CI 0.68 to 1.28; P = 0.66; I² = 10%; 7 trials, 268 participants; subgroup differences Chi2 = 2.50, df = 1 (P = 0.11), I2 = 59.9%; Analysis 1.11);
culture confirmed infection (RR 0.96, 95% CI 0.67 to 1.39; P = 0.72; I² = 0%; 7 trials, 289 participants; subgroup differences Chi2 = 1.31, df = 1 (P = 0.25), I2 = 23.7%; Analysis 1.12);
clinical suspected infection (RR 1.03, 95% CI 0.62 to 1.70; P = 0.44; I² = 0%; 3 trials, 131 participants; subgroup differences Chi2 = 0.52, df = 1 (P = 0.47), I2 = 0%; Analysis 1.13);
gastrointestinal hemorrhage (no events in either allocation arm; 3 trials, 101 participants), gastrointestinal perforation (RR 0.92, 95% CI 0.13 to 6.28; P = 0.96; I² = 0%; 4 trials, 185 participants; subgroup differences not applicable; Analysis 1.14);
NEC (RR 0.53, 95% CI 0.18 to 1.56; P = 0.57; I² = 0%; 4 trials, 198 participants; subgroup differences Chi2 = 1.05, df = 1 (P = 0.31), I2 = 4.9%; Analysis 1.15);
severe IVH (RR 1.68, 95% CI 0.65 to 4.37; P = 0.63; I² = 0%; 3 trials, 101 participants; subgroup differences Chi2 = 0.00, df = 1 (P = 1.00), I2 = 0%; Analysis 1.16);
PVL (RR 0.93, 95% CI 0.20 to 4.39; P = 0.92; I² = 0%; 2 trials, 121 participants; subgroup differences Chi2 = 0.01, df = 1 (P = 0.92), I2 = 0%; Analysis 1.17); or
severe ROP (RR 0.64, 95% CI 0.32 to 1.28; P = 0.83; I² = 0%; 5 trials, 176 participants; subgroup differences Chi2 = 0.02, df = 1 (P = 0.89), I2 = 0%; Analysis 1.18)
1.11. Analysis.

Comparison 1: Lower versus higher cumulative dose dexamethasone regimen, Outcome 11: Open‐label corticosteroids
1.12. Analysis.

Comparison 1: Lower versus higher cumulative dose dexamethasone regimen, Outcome 12: Culture confirmed infection
1.13. Analysis.

Comparison 1: Lower versus higher cumulative dose dexamethasone regimen, Outcome 13: Clinical suspected infection
1.14. Analysis.

Comparison 1: Lower versus higher cumulative dose dexamethasone regimen, Outcome 14: Gastrointestinal perforation
1.15. Analysis.

Comparison 1: Lower versus higher cumulative dose dexamethasone regimen, Outcome 15: Necrotizing enterocolitis
1.16. Analysis.

Comparison 1: Lower versus higher cumulative dose dexamethasone regimen, Outcome 16: Intraventricular hemorrhage (> grade II)
1.17. Analysis.

Comparison 1: Lower versus higher cumulative dose dexamethasone regimen, Outcome 17: Periventricular leukomalacia (PVL)
1.18. Analysis.

Comparison 1: Lower versus higher cumulative dose dexamethasone regimen, Outcome 18: Severe retinopathy of prematurity
No data were retrieved on the outcomes PDA and cardiac hypertrophy.
Long‐term neurodevelopmental sequelae
Four trials reported the long‐term neurodevelopmental outcomes of cerebral palsy, visual impairment or the Bayley MDI in survivors, including 66% to 100% of their randomized infants. Malloy 2005 performed long‐term neurodevelopmental assessment, but used the modified Gesell Developmental Appraisal, which was deemed not to be comparable with the Bayley MDI reported in the other studies. Analysis showed a difference in the incidence of cerebral palsy. Compared to the infants allocated to the higher‐dosage regimens, the infants allocated to the lower‐dose regimen had a higher incidence of cerebral palsy assessed between one and three years of age (RR 2.64, 95% CI 1.02 to 6.83; P = 0.04; I² = 3%; NNTH 9, 95% CI 4.5 to 87; 4 trials, 149 participants; Analysis 1.19). Although there was no evidence of a subgroup difference for the higher versus lower dosage regimens comparisons (Chi2 = 2.91, df = 1 (P = 0.09), I2 = 65.7%), a larger effect was seen in the subgroup analysis of moderate‐dosage regimens versus high‐dosage regimens (RR 6.85, 95% CI 1.29 to 36.36; P = 0.02; I² = 0%; NNTH 5, 95% CI 2.6 to 12.7; 2 trials, 74 participants; Analysis 1.19.1).
1.19. Analysis.

Comparison 1: Lower versus higher cumulative dose dexamethasone regimen, Outcome 19: Cerebral palsy in survivors assessed at 1‐3 years
Meta‐analysis suggests that a lower compared with a higher cumulative corticosteroid dosage may not affect the composite outcome of death or cerebral palsy of all trials (Analysis 1.20). However, there was evidence of a subgroup difference for higher versus lower dosage regimens (Chi2 = 4.25, df = 1 (P = 0.04), I2 = 76.5%). A larger effect was seen in the group of infants allocated to the high dosage regimen comparing a moderate dosage regimen (RR 3.20, 95% 1.35 to 7.58; P = 0.008; I² = 25%; NNTH 5, 95% CI 2.6 to 13.1; 2 trials, 84 participants; Analysis 1.20.1).
1.20. Analysis.

Comparison 1: Lower versus higher cumulative dose dexamethasone regimen, Outcome 20: Death or cerebral palsy at 1‐3 years
There were no differences in the number of infants with Bayley MDI less than ‐2 SD in the higher versus lower dosage comparison. However, there was evidence of a subgroup difference for higher versus lower dosage regimens (Chi2 = 4.26, df = 1 (P = 0.04), I2 = 76.5%). In participants treated with a moderate‐dosage regimen compared to a high‐dosage regimen, an increased risk was found on the Bayley MDI < ‐2 SD (RR 3.99, 95% CI 1.06 to 15.08; P = 0.04; I² = 0%; NNTB 6, 95% CI 3 to 27; 2 trials, 72 participants; Analysis 1.21.1).
1.21. Analysis.

Comparison 1: Lower versus higher cumulative dose dexamethasone regimen, Outcome 21: Bayley's MDI < 2 SD in survivors assessed
Meta‐analysis suggests that a lower compared with a higher cumulative corticosteroid dosage may not affect the outcome visual impairment (RR 0.66, 95% CI 0.17 to 2.66; P = 0.69; I² = 0%; 5 trials, 166 participants; subgroup differences Chi2 = 1.47, df = 1 (P = 0.22), I2 = 32.1%; Analysis 1.22).
1.22. Analysis.

Comparison 1: Lower versus higher cumulative dose dexamethasone regimen, Outcome 22: Severe blindness in survivors assessed
Three studies reported on the incidence of abnormal neurodevelopmental outcome as defined by the trialists. The meta‐analyses of low‐ versus moderate‐dosage regimens did not reveal any differences. However, there was evidence of a subgroup difference for higher versus lower dosage regimens (Chi2 = 6.39, df = 1 (P = 0.01), I2 = 84.3%). Compared to the infants allocated to a high‐dosage regimen, a higher incidence of abnormal neurodevelopmental outcome was seen in the group of infants allocated to the moderate‐dosage regimen (RR 7.60, 95% CI 1.45 to 39.78; P = 0.02; I² = 0%; NNTH 4, 95% CI 2.4 to 9.8; 2 trials, 74 participants; Analysis 1.23.1). Meta‐analysis suggests that a lower compared with a higher cumulative corticosteroid dosage may not affect the composite outcome of abnormal neurodevelopmental outcome or death assessed between one and three years of age (RR 1.86, 95% CI 0.98 to 3.53; P = 0.06; I² = 73%; 3 trials, 100 participants; Analysis 1.24). However, there was evidence of a subgroup difference for higher versus lower dosage regimens (Chi2 = 7.12, df = 1 (P = 0.008), I2 = 85.9%). The composite outcome of abnormal neurodevelopmental outcome or death showed the same benefits in favor of the high‐dosage group (RR 3.41, 95% CI 1.44 to 8.07; P = 0.005; I² = 41%; NNTH 4, 95% CI 2.2 to 10.4; 2 trials, 84 participants; moderate certainty; Analysis 1.24.1).
1.23. Analysis.

Comparison 1: Lower versus higher cumulative dose dexamethasone regimen, Outcome 23: Abnormal neurodevelopmental outcome in survivors assessed (various definitions) at 1‐3 years
1.24. Analysis.

Comparison 1: Lower versus higher cumulative dose dexamethasone regimen, Outcome 24: Death or abnormal neurodevelopmental outcome (various definitions) at 1‐3 years
We graded the certainty of the evidence for the composite outcomes death or CP, and death or abnormal neurodevelopmental outcome as very low because of the inconsistency across studies, the small number of events, and the risk of performance, detection and attrition bias (Table 1). Cummings 1989 and Marr 2019) also reported the neurodevelopmental outcome at age seven years and 15 years, respectively. No meta‐analysis was possible given the single study results for each time of assessment, but these individual studies showed similar results in the reduced risk of neurodevelopmental impairment in the group of infants allocated to high dosage regimen, compared to the infants treated with moderate dosage regimens.
Comparison 2. Later (experimental arm) versus earlier (control arm) initiation of dexamethasone
Primary outcome
Combined outcome of death or BPD at 36 weeks' PMA
Meta‐analysis suggests that a later compared with an earlier cumulative corticosteroid dosage may not affect the combined outcome of death or BPD at 36 weeks' PMA (RR 1.06, 95% CI 0.87 to 1.29; P = 0.57; I² = 17%; 3 trials, 391 participants; subgroup differences not applicable; Analysis 2.1). We graded the certainty of the evidence as low because of the small number of events, and the risk of performance and detection bias in all three trials and unclear selection bias in one trial (Table 2). We were unable to assess potential publication bias in a funnel plot as fewer than 10 eligible RCTs reported this outcome. We were unable to undertake the planned subgroup or sensitivity analyses by gestational age, severity of illness, mode of ventilation, and use of open‐label corticosteroids, because of paucity of available data. We were not able to perform a meta‐analysis including only those trials without high risk of bias in any domains, because all were judged to have a high risk of bias.
2.1. Analysis.

Comparison 2: Later versus earlier initiation of dexamethasone therapy, Outcome 1: Death or bronchopulmonary dysplasia at 36 weeks' PMA
Secondary outcomes
Mortality at 28 days' PNA, 36 weeks' PMA and at hospital discharge
Meta‐analysis suggests that a later compared with an earlier cumulative corticosteroid dosage may not affect mortality at 28 days' PNA (RR 1.01, 95% CI 0.69 to 1.47; P = 0.97; I² = 59%; 4 trials, 762 participants; subgroup differences Chi2 = 4.42, df = 1 (P = 0.04), I2 = 77.4%; Analysis 2.2), 36 weeks' PMA (RR 0.93, 95% CI 0.68 to 1.28; P = 0.66; I² = 54%; 4 trials, 762 participants; subgroup differences Chi2 = 3.98, df = 1 (P = 0.05), I2 = 74.9%; Analysis 2.3) and mortality at hospital discharge (RR 1.00, 95% CI 0.75 to 1.33; P = 0.30; I² = 18%; 5 trials, 797 participants; subgroup differences Chi2 = 2.67, df = 1 (P = 0.10), I2 = 62.5%; Analysis 2.4).
2.2. Analysis.

Comparison 2: Later versus earlier initiation of dexamethasone therapy, Outcome 2: Mortality at 28 days' PNA
2.3. Analysis.

Comparison 2: Later versus earlier initiation of dexamethasone therapy, Outcome 3: Mortality at 36 weeks' PMA
2.4. Analysis.

Comparison 2: Later versus earlier initiation of dexamethasone therapy, Outcome 4: Mortality at hospital discharge
BPD at 28 days' PNA and 36 weeks' PMA
Compared to the infants who were allocated to earlier initiation, the infants allocated to later initiation had a higher incidence of the outcome BPD at 28 days' PNA (RR 1.12, 95% CI 1.02 to 1.23; P = 0.02; I² = 49%; NNTH 14, 95% CI 7 to 90; 4 trials, 762 participants; Analysis 2.5). Although there was no evidence of a subgroup difference between the earlier versus later initiation regimens (Chi2 = 0.33, df =1 (P = 0.56), I2 = 0%), a larger effect was found in the late versus moderate early initiation regimen subgroup. Compared to the infants who were allocated to moderately early initiation, the infants allocated to delayed initiation had a higher incidence of the outcome BPD at 28 days' PNA (RR 1.15, 95% CI 1.05 to 1.26; P = 0.004; I² = not applicable; NNTH 9, 95% CI 5 to 26; 1 trial, 371 participants; Analysis 2.5.1). Later versus earlier initiation of corticosteroids showed no effect on the outcome BPD at 36 weeks' PMA. Although there was no evidence of a subgroup difference between the earlier versus later initiation regimens (Chi2 = 3.10, df =1 (P = 0.08), I2 = 67.7%), a larger effect was found in the moderate early versus early initiation regimen subgroup. Compared to the infants allocated in the early administration, the infants who were allocated in the moderately early group had a higher incidence of BPD at 36 weeks' PMA (RR 1.38, 95% CI 1.01 to 1.90; P = 0.05; I² = 17%; NNTH 11, 95% CI 6 to 333; 3 trials, 391 participants; Analysis 2.6.2).
2.5. Analysis.

Comparison 2: Later versus earlier initiation of dexamethasone therapy, Outcome 5: Bronchopulmonary dysplasia at 28 days' PNA
2.6. Analysis.

Comparison 2: Later versus earlier initiation of dexamethasone therapy, Outcome 6: Bronchopulmonary dysplasia at 36 weeks' PMA
Failure to extubate
Compared to late initiation, moderately early initiation resulted in a reduction in the number of infants failing extubation at day three and day seven in the only trial reporting this outcome (RR 1.10, 95% CI 1.05 to 1.15; P < 0.0001; I² = not applicable; 1 trial, 371 participants; subgroup differences not applicable; Analysis 2.7; RR 1.22, 95% CI 1.14 to 1.32; P < 0.00001; I² = not applicable; 1 trial, 378 participants; subgroup differences not applicable; Analysis 2.8).
2.7. Analysis.

Comparison 2: Later versus earlier initiation of dexamethasone therapy, Outcome 7: Failure to extubate 3 days after initiation
2.8. Analysis.

Comparison 2: Later versus earlier initiation of dexamethasone therapy, Outcome 8: Failure to extubate 7 days after initiation
Days of mechanical ventilation and supplemental oxygen
The trials publishing data on the duration of mechanical ventilation showed a difference between early administration and moderately early administration (MD 12.71, 95% CI 4.44 to 20.99; P = 0.003; I² = 0%; 2 trials, 60 participants; subgroup differences not applicable; Analysis 2.9), and the only trial reporting the outcome supplemental days of oxygen showed that compared to moderately early initiation, early initiation of dexamethasone results in a reduction in days on supplemental oxygen (RR 29.00, 95% CI 7.10 to 50.90; P = 0.009; I² = not applicable; 1 trial, 30 participants; subgroup differences not applicable; Analysis 2.10).
2.9. Analysis.

Comparison 2: Later versus earlier initiation of dexamethasone therapy, Outcome 9: Days of mechanical ventilation
2.10. Analysis.

Comparison 2: Later versus earlier initiation of dexamethasone therapy, Outcome 10: Days of supplemental oxygen
Complications during primary hospitalization
Meta‐analysis suggests that a later compared with an earlier cumulative corticosteroid dosage may not affect the incidence of hypertension (RR 0.99, 95% CI 0.67 to 1.47; P = 0.98; I² = 34%; 4 trials, 762 participants; subgroup differences Chi2 = 1.52, df = 1 (P = 0.22), I2 = 34.2%; Analysis 2.11).
2.11. Analysis.

Comparison 2: Later versus earlier initiation of dexamethasone therapy, Outcome 11: Hypertension
Compared to the infants allocated to the earlier administration arm, the infants allocated to the later initiation arm had a lower incidence of hyperglycemia (RR 0.66, 95% CI 0.53 to 0.82; P = 0.0001; I² = 21%; NNTB 8, 95% CI 5.0 to 15.7; 4 trials, 726 participants; Analysis 2.12). No subgroup differences were seen (Chi2 = 0.00, df = 1 (P = 1.00), I2 = 0%). These effects were seen both in the subgroup late versus moderately early, and moderately early versus early initiation of therapy (RR 0.66, 95% CI 0.46 to 0.95; P = 0.03; I² = not applicable; NNTB 10, 95% CI 5 to 73; 1 trial, 371 participants; Analysis 2.12.1 and RR 0.66, 95% CI 0.51 to 0.85; P = 0.002; I² = 47%; NNTB 6, 95% CI 4 to 15; 3 trials, 355 participants; Analysis 2.12.2, respectively).
2.12. Analysis.

Comparison 2: Later versus earlier initiation of dexamethasone therapy, Outcome 12: Hyperglycemia
The incidence of ‘rescue’ therapy with open‐label corticosteroids was higher in the group of infants allocated to late initiation (RR 1.71, 95% CI 1.04 to 2.81; P = 0.03; NNTH 25, 95% CI 12.50 to 462.4; I² = 66%; 3 trials, 732 participants; subgroup differences Chi2 = 2.67, df = 1 (P = 0.10), I2 = 62.6%; Analysis 2.13). Overall, no evidence of a difference was seen in the outcome culture‐proven infection between later and earlier initiation of therapy (Analysis 2.14). However, there was some evidence of a subgroup difference for earlier versus later initiation of therapy in both outcomes (Chi2 = 6.39, df = 1 (P = 0.01), I2 = 84.3%). Compared to the infants allocated to the moderately early dexamethasone initiation, the infants allocated to late initiation showed a lower incidence of the outcomes of culture‐proven infection (RR 0.67, 95% CI 0.54 to 0.84; P = 0.0005; I² = not applicable; NNTH 6, 95% CI 3.50 to 12.00; 1 trial, 175 participants; Analysis 2.14.1). No data were reported on the outcome clinically suspected infection.
2.13. Analysis.

Comparison 2: Later versus earlier initiation of dexamethasone therapy, Outcome 13: Open‐label corticosteroids
2.14. Analysis.

Comparison 2: Later versus earlier initiation of dexamethasone therapy, Outcome 14: Culture confirmed infection
Furthermore, no evidence of a difference was seen in the outcome gastrointestinal hemorrhage between later and earlier initiation of therapy (Analysis 2.15). Although no difference was found between the subgroups (Chi2 = 0.57, df = 1 (P = 0.45), I2 = 0%), a higher incidence of gastrointestinal hemorrhage was found in the subgroup comparing late versus moderate early initiation of therapy (RR 0.60, 95% CI 0.38 to 0.95; P = 0.04; I² = 0%; NNTH 12, 95% CI 6.0 to 98.5; 4 trials, 762 participants; Analysis 2.15.1). Meta‐analysis suggests that a later compared with an earlier cumulative corticosteroid dosage may not affect for the outcomes gastrointestinal perforation (RR 0.75, 95% CI 0.23 to 2.40; P = 0.63; I² = not applicable; 2 trials, 315 participants; subgroup differences not applicable; Analysis 2.16), and NEC (RR 1.44, 95% CI 0.82 to 2.55; P = 0.21; I² = 17%; 4 trials, 725 participants; subgroup differences Chi2 = 0.16, df = 1 (P = 0.69), I2 = 0%; Analysis 2.17).
2.15. Analysis.

Comparison 2: Later versus earlier initiation of dexamethasone therapy, Outcome 15: Gastrointestinal hemorrhage
2.16. Analysis.

Comparison 2: Later versus earlier initiation of dexamethasone therapy, Outcome 16: Gastrointestinal perforation
2.17. Analysis.

Comparison 2: Later versus earlier initiation of dexamethasone therapy, Outcome 17: Necrotizing enterocolitis
Compared to the infants allocated to the early initiation group, the infants allocated to the moderately early initiation arm had an increased risk of a PDA requiring therapy (RR 1.74, 95% CI 1.32 to 2.29; P < 0.0001; I² = not applicable; NNTH 5, 95% CI 2.80 to 7.60; 1 trial, 285 participants; Analysis 2.18). Meta‐analysis suggests that a later compared with an earlier cumulative corticosteroid dosage may not affect the outcomes IVH (RR 2.37, 95% CI 0.49 to 11.48; P = 0.28; I² = not applicable; 1 trial, 76 participants; subgroup differences not applicable; Analysis 2.19); ROP any grade (RR 0.80, 95% CI 0.52 to 1.23; P = 0.31; I² = 0%; 2 trials, 324 participants; subgroup differences not applicable; Analysis 2.20); or severe ROP (RR 1.50, 95% CI 0.63 to 3.53; P = 0.58; I² = 0%; 3 trials, 391 participants; subgroup differences not applicable; Analysis 2.21). No data were reported on the outcome PVL.
2.18. Analysis.

Comparison 2: Later versus earlier initiation of dexamethasone therapy, Outcome 18: Patent ductus arteriosus requiring therapy
2.19. Analysis.

Comparison 2: Later versus earlier initiation of dexamethasone therapy, Outcome 19: Intraventricular hemorrhage (> grade II)
2.20. Analysis.

Comparison 2: Later versus earlier initiation of dexamethasone therapy, Outcome 20: Retinopathy of prematurity (any)
2.21. Analysis.

Comparison 2: Later versus earlier initiation of dexamethasone therapy, Outcome 21: Severe retinopathy of prematurity
Long‐term neurodevelopmental sequelae
Two trials investigating moderately early versus early initiation of dexamethasone reported long‐term neurodevelopmental outcomes using various definitions. No data were reported on the Mental Developmental Index of the Bayley Scales of Infant Development in these trials. Meta‐analysis showed no evidence of a difference in the incidence of cerebral palsy in survivors assessed between both allocation arms (RR 1.95, 95% CI 0.43 to 8.86; P = 0.39; I² = not applicable; 1 trial, 61 participants; subgroup differences not applicable; Analysis 2.22), and the composite outcome death or cerebral palsy (RR 1.12, 95% CI 0.68 to 1.84; P = 0.65; I² = not applicable; 1 trial, 86 participants; subgroup differences not applicable; Analysis 2.23). None of the infants had severe blindness, regardless of the allocation group (1 trial, 61 participants). Analyses showed no evidence of a difference in the incidence of abnormal neurodevelopmental outcome in survivors assessed between both allocation arms (RR 1.06, 95% CI 0.66 to 1.69; P = 0.82; I² = 0%; 2 trials, 155 participants; subgroup differences not applicable; Analysis 2.24), or the composite outcome of death or long‐term neurodevelopmental outcome (RR 0.87, 95% CI 0.63 to 1.21; P = 0.42; I² = 0%; 2 trials, 167 participants; subgroup differences not applicable; Analysis 2.25). The certainty of the evidence was low because of the small number of events, and the risk of performance and detection bias and unclear selection bias (Table 2).
2.22. Analysis.

Comparison 2: Later versus earlier initiation of dexamethasone therapy, Outcome 22: Cerebral palsy in survivors assessed
2.23. Analysis.

Comparison 2: Later versus earlier initiation of dexamethasone therapy, Outcome 23: Death or cerebral palsy
2.24. Analysis.

Comparison 2: Later versus earlier initiation of dexamethasone therapy, Outcome 24: Abnormal neurodevelopmental outcome in survivors assessed (various definitions)
2.25. Analysis.

Comparison 2: Later versus earlier initiation of dexamethasone therapy, Outcome 25: Death or abnormal neurodevelopmental outcome (various definitions)
Comparison 3. Pulse therapy (experimental arm) versus continuous tapered (control arm) dosage regimens of dexamethasone
Primary outcome
Combined outcome death or BPD at 36 weeks' PMA
Compared to the infants allocated to the continuous tapered dosage regimen, the infants allocated to pulse therapy showed an increase in the incidence of the combined outcome of death or BPD at 36 weeks' PMA (RR 1.38, 95% CI 1.02 to 1.88; P = 0.04; I² = not applicable; NNTH 7, 95% CI 4 to 155; 1 trial, 197 participants; Analysis 3.1). We graded the certainty of the evidence as low because of the small number of events, and the risk of performance and detection bias in one trial (Table 3). We were unable to assess potential publication bias in a funnel plot as fewer than 10 eligible RCTs reported this outcome. Furthermore, the planned subgroup or sensitivity analyses by gestational age, severity of illness, mode of ventilation, and use of open‐label corticosteroids were not possible because of paucity of available data. Meta‐analysis including the only trial without high risk of bias in any domains suggests that a pulse compared with a continuous tapered corticosteroid regimen may not affect the risk of death or BPD at 36' weeks PMA (RR 1.42, 95% CI 0.99 to 2.05; I² not applicable; 1 trials, 121 participants).
3.1. Analysis.

Comparison 3: Pulse versus continuous dexamethasone therapy, Outcome 1: Death or bronchopulmonary dysplasia at 36 weeks PMA
Secondary outcomes
Mortality at 28 days, 36 weeks' PMA and at hospital discharge
Meta‐analysis suggests that a pulse compared with a continuous tapered corticosteroid regimen may not affect the outcome of mortality at any time point (RR 2.85, 95% CI 0.31 to 26.15; P = 0.36; I² = not applicable; 1 trial, 76 participants; Analysis 3.2; RR 2.04, 95% CI 0.72 to 5.78; P = 0.18; I² = 0%; 2 trials, 197 participants; Analysis 3.3; RR 2.04, 95% CI 0.72 to 5.78; P = 0.18; I² = 0%; 2 trials, 197 participants; Analysis 3.4, respectively).
3.2. Analysis.

Comparison 3: Pulse versus continuous dexamethasone therapy, Outcome 2: Mortality at 28 days PNA
3.3. Analysis.

Comparison 3: Pulse versus continuous dexamethasone therapy, Outcome 3: Mortality at 36 weeks PMA
3.4. Analysis.

Comparison 3: Pulse versus continuous dexamethasone therapy, Outcome 4: Mortality at hospital discharge
BPD at 28 days' PNA and at 36 weeks' PMA
Meta‐analysis suggests that a pulse compared with a continuous tapered corticosteroid regimen may not affect the outcomes of BPD at 28 days' PNA or 36 weeks' PMA (RR 1.40, 95% CI 0.92 to 2.13; P = 0.12; I² = not applicable; 1 trial, 76 participants; Analysis 3.5; RR 1.29, 95% CI 0.90 to 1.83; P = 0.16; I² = 0%; 2 trials, 197 participants; Analysis 3.6, respectively).
3.5. Analysis.

Comparison 3: Pulse versus continuous dexamethasone therapy, Outcome 5: Bronchopulmonary dysplasia at 28 days PNA
3.6. Analysis.

Comparison 3: Pulse versus continuous dexamethasone therapy, Outcome 6: Bronchopulmonary dysplasia at 36 weeks PMA
Failure to extubate
No data could be retrieved on the outcomes of failure to extubate.
Days of mechanical ventilation and supplemental oxygen
No data could be retrieved on the days of mechanical ventilation or supplemental oxygen.
Complications during primary hospitalization
No evidence of a difference between the two allocation arms was found for the outcomes hypertension (RR 0.50, 95% CI 0.20 to 1.23; P = 0.13; I² = not applicable; 2 trials, 197 participants; Analysis 3.7), or hyperglycemia (RR 1.08, 95% CI 0.71 to 1.65; P = 0.73; I² = 31%; 2 trials, 160 participants; Analysis 3.8). The use of open‐label corticosteroids was similar in both groups in the trial providing this information (RR 0.97, 95% CI 0.64 to 1.47; P = 0.87; I² = not applicable; 2 trials, 197 participants; Analysis 3.9). No evidence of a difference was seen for the following outcomes:
3.7. Analysis.

Comparison 3: Pulse versus continuous dexamethasone therapy, Outcome 7: Hypertension
3.8. Analysis.

Comparison 3: Pulse versus continuous dexamethasone therapy, Outcome 8: Hyperglycemia
3.9. Analysis.

Comparison 3: Pulse versus continuous dexamethasone therapy, Outcome 9: Open‐label corticosteroids
culture‐proven infection (RR 1.32, 95% CI 0.87 to 2.01; P = 0.19; I² = not applicable; 1 trial, 121 participants; Analysis 3.10);
clinically suspected infection (RR 1.21, 95% CI 0.70 to 2.10; P = 0.49; I² = not applicable; 1 trial, 121 participants; Analysis 3.11);
gastrointestinal hemorrhage (RR 0.65, 95% CI 0.25 to 1.68; P = 0.38; I² = not applicable; 2 trials, 197 participants; Analysis 3.12);
NEC (RR 0.78, 95% CI 0.33 to 1.83; P = 0.57; I² = 60%; 2 trials, 160 participants; Analysis 3.13);
IVH above grade II (RR 2.37, 95% CI 0.49 to 11.48; P = 0.28; I² = not applicable; 1 trial, 76 participants; Analysis 3.14);
ROP (RR 0.53, 95% CI 0.05 to 5.34; P = 0.59; I² = not applicable; 1 trial, 39 participants; Analysis 3.15); and
severe ROP (RR 0.24, 95% CI 0.05 to 1.07; P = 0.06; I² = not applicable; 1 trial, 121 participants; Analysis 3.16).
3.10. Analysis.

Comparison 3: Pulse versus continuous dexamethasone therapy, Outcome 10: Culture confirmed infection
3.11. Analysis.

Comparison 3: Pulse versus continuous dexamethasone therapy, Outcome 11: Clinical suspected infection
3.12. Analysis.

Comparison 3: Pulse versus continuous dexamethasone therapy, Outcome 12: Gastrointestinal hemorrhage
3.13. Analysis.

Comparison 3: Pulse versus continuous dexamethasone therapy, Outcome 13: Necrotizing enterocolitis
3.14. Analysis.

Comparison 3: Pulse versus continuous dexamethasone therapy, Outcome 14: Intraventricular hemorrhage (> grade II)
3.15. Analysis.

Comparison 3: Pulse versus continuous dexamethasone therapy, Outcome 15: Retinopathy of prematurity (any)
3.16. Analysis.

Comparison 3: Pulse versus continuous dexamethasone therapy, Outcome 16: Severe retinopathy of prematurity
Long‐term neurodevelopmental sequelae
Follow‐up was only performed in one trial. No data were reported on Bayley Scales of Infant Development or cerebral palsy outcomes in this trial. No evidence of a difference was found for the outcome abnormal neurodevelopmental outcome alone (RR 0.88, 95% CI 0.54 to 1.44; P = 0.62; I² = not applicable; 1 trial, 64 participants; Analysis 3.17), or combined with death (RR 1.23, 95% CI 0.79 to 1.92; P = 0.37; I² = not applicable; 1 trial, 76 participants; Analysis 3.18). We graded the certainty of the evidence as very low because of the small number of events, and the risk of performance and detection bias in one trial and potential publication bias of one trial (Table 3).
3.17. Analysis.

Comparison 3: Pulse versus continuous dexamethasone therapy, Outcome 17: Abnormal neurodevelopmental outcome in survivors assessed (various definitions)
3.18. Analysis.

Comparison 3: Pulse versus continuous dexamethasone therapy, Outcome 18: Death or abnormal neurodevelopmental outcome (various definitions)
Comparison 4. Individual tailored (experimental arm) versus continuous tapered (control arm) dosage regimens of dexamethasone
Primary outcome
Combined outcome death or BPD at 36 weeks' PMA
Meta‐analysis suggests that an individual tailored regimen compared with a continuous tapered corticosteroid regimen may not affect the outcome of combined death or BPD at 36 weeks' PMA (RR 1.06, 95% CI 0.88 to 1.29; P = 0.54; I² = 10%; 3 trials, 168 participants; Analysis 4.1). We graded the certainty of the evidence as low because of the small number of events, and the risk of performance and detection bias (Table 4). We were unable to assess potential publication bias in a funnel plot as fewer than 10 eligible RCTs reported this outcome. We were unable to undertake the planned subgroup or sensitivity analyses by gestational age, severity of illness, mode of ventilation, use of open‐label corticosteroids, because of paucity of available data. Meta‐analysis including the only trial without high risk of bias in any domains suggests that an individual tailored regimen compared with a continuous tapered corticosteroid regimen may not affect the risk of death or BPD at 36' weeks PMA (RR 0.96, 95% CI 0.82 to 1.12; I² = not applicable; 1 trial, 59 participants).
4.1. Analysis.

Comparison 4: Individual tailored versus continuous tapered dexamethasone regimen, Outcome 1: Death or bronchopulmonary dysplasia at 36 weeks PMA
Secondary outcomes
Mortality at 28 days' PNA, 36 weeks' PMA and at hospital discharge
Meta‐analysis suggests that an individual tailored regimen compared with a continuous tapered corticosteroid regimen may not affect mortality at 28 days' PNA and 36 weeks' PMA (RR 2.83, 95% CI 0.60 to 13.32; P = 0.19; I² = 0%; 2 trials, 109 participants; Analysis 4.2; RR 1.54, 95% CI 0.63 to 3.79; P = 0.35; I² = 0%; 3 trials, 168 participants; Analysis 4.3; RR 1.84, 95% CI 0.77 to 4.37; P = 0.17; I² = 0%; 3 trials, 168 participants; Analysis 4.4, respectively).
4.2. Analysis.

Comparison 4: Individual tailored versus continuous tapered dexamethasone regimen, Outcome 2: Mortality at 28 days PNA
4.3. Analysis.

Comparison 4: Individual tailored versus continuous tapered dexamethasone regimen, Outcome 3: Mortality at 36 weeks PMA
4.4. Analysis.

Comparison 4: Individual tailored versus continuous tapered dexamethasone regimen, Outcome 4: Mortality at hospital discharge
BPD at 28 days' PNA and 36 weeks' PMA
Meta‐analysis suggests that an individual tailored compared with a continuous tapered corticosteroid regimen may not affect the outcome BPD at 28 days' PNA or 36 weeks' PMA (RR 1.15, 95% CI 0.88 to 1.50; P = 0.30; I² = 81%; 2 trials, 109 participants; Analysis 4.5; RR 0.99, 95% CI 0.79 to 1.25; P = 0.96; I² = 0%; 3 trials, 168 participants; Analysis 4.6, respectively).
4.5. Analysis.

Comparison 4: Individual tailored versus continuous tapered dexamethasone regimen, Outcome 5: Bronchopulmonary dysplasia at 28 days PNA
4.6. Analysis.

Comparison 4: Individual tailored versus continuous tapered dexamethasone regimen, Outcome 6: Bronchopulmonary dysplasia at 36 weeks PMA
Failure to extubate
Meta‐analysis suggests that an individual tailored compared with a continuous tapered corticosteroid regimen may not affect the outcomes of failure to extubate three days after initiation of therapy (RR 1.16, 95% CI 0.95 to 1.43; P = 0.15; I² = not applicable; 1 trial, 59 participants; Analysis 4.7). Compared to the infants allocated to the continuous tapered regimen, the infants who were allocated to the individual tailored dosage regimen had an increased risk of failure to extubate seven days after initiation of therapy (RR 1.72, 95% CI 1.17 to 2.54, NNTH 3, 95% CI 2 to 7; P = 0.006; I² = not applicable; 1 trial, 59 participants; Analysis 4.8).
4.7. Analysis.

Comparison 4: Individual tailored versus continuous tapered dexamethasone regimen, Outcome 7: Failure to extubate 3 days after initiation
4.8. Analysis.

Comparison 4: Individual tailored versus continuous tapered dexamethasone regimen, Outcome 8: Failure to extubate 7 days after initiation
Days of mechanical ventilation and supplemental oxygen
Compared to the infants allocated to the continuous tapered regimen, the infants who were allocated to the individual tailored dosage regimen had a decreased duration of mechanical ventilation (MD 9.26, 95% CI 4.32 to 14.21; I2 = 69%; 2 studies, 90 participants; Analysis 4.9). Meta‐analysis suggests that an individual tailored regimen compared with a continuous tapered corticosteroid regimen may not affect the days of supplemental oxygen (MD 8.00, 95% CI ‐34.64 to 50.64; P = 0.71; I² = not applicable; 1 trial, 52 participants; Analysis 4.10).
4.9. Analysis.

Comparison 4: Individual tailored versus continuous tapered dexamethasone regimen, Outcome 9: Days of mechanical ventilation
4.10. Analysis.

Comparison 4: Individual tailored versus continuous tapered dexamethasone regimen, Outcome 10: Days on supplemental oxygen
Complications during primary hospitalization
Meta‐analysis suggests that an individual tailored compared with a continuous tapered corticosteroid regimen may not affect the following outcomes:
hypertension (RR 0.34, 95% CI 0.01 to 8.13; P = 0.51; I² = not applicable; 2 trials, 135 participants; Analysis 4.11);
hyperglycemia (RR 0.66, 95% CI 0.26 to 1.66; P = 0.37; I² = not applicable; 2 trials, 98 participants; Analysis 4.12);
open‐label corticosteroids (RR 1.72, 95% CI 0.72 to 4.13; P = 0.22; I² = not applicable; 2 trials, 135 participants; Analysis 4.13);
culture‐proven infection (RR 1.27, 95% CI 0.58 to 2.76; P = 0.55; I² = not applicable; 2 trials, 92 participants; Analysis 4.14);
clinically suspected infection (no events in either allocation arms; 1 trial, 59 participants), gastrointestinal hemorrhage and perforation (no events in either allocation arms; 2 trials, 135 participants; no events in either allocation arms; 1 trial, 59 participants, respectively);
NEC (RR 1.75, 95% CI 0.48 to 6.35; P = 0.39; I² = not applicable; 2 trials, 98 participants; Analysis 4.15);
IVH above grade II (RR 2.00, 95% CI 0.78 to 5.18; P = 0.15; I² = 0%; 3 trials, 168 participants; Analysis 4.16);
PVL (RR 1.03, 95% CI 0.07 to 15.77; P = 0.98; I² = not applicable; 1 trial, 59 participants; Analysis 4.17); and
ROP (RR 0.83, 95% CI 0.24 to 2.92; P = 0.77; I² = 0%; 2 trials, 98 participants; Analysis 4.18).
4.11. Analysis.

Comparison 4: Individual tailored versus continuous tapered dexamethasone regimen, Outcome 11: Hypertension
4.12. Analysis.

Comparison 4: Individual tailored versus continuous tapered dexamethasone regimen, Outcome 12: Hyperglycemia
4.13. Analysis.

Comparison 4: Individual tailored versus continuous tapered dexamethasone regimen, Outcome 13: Open‐label corticosteroids
4.14. Analysis.

Comparison 4: Individual tailored versus continuous tapered dexamethasone regimen, Outcome 14: Culture confirmed infection
4.15. Analysis.

Comparison 4: Individual tailored versus continuous tapered dexamethasone regimen, Outcome 15: Necrotizing enterocolitis
4.16. Analysis.

Comparison 4: Individual tailored versus continuous tapered dexamethasone regimen, Outcome 16: Intraventricular hemorrhage (> grade II)
4.17. Analysis.

Comparison 4: Individual tailored versus continuous tapered dexamethasone regimen, Outcome 17: Periventricular leucomalacia
4.18. Analysis.

Comparison 4: Individual tailored versus continuous tapered dexamethasone regimen, Outcome 18: Retinopathy of prematurity (any)
Long‐term neurodevelopmental sequelae
The included trials reporting in this comparison did not show any evidence of difference in the following outcomes:
abnormal neurodevelopmental outcome, defined as either the presence of severe cerebral palsy alone (RR 4.62, 95% CI 0.55 to 38.74; P = 0.16; I² = not applicable; 1 trial, 56 participants; Analysis 4.19), or in combination with death (RR 7.24, 95% CI 0.95 to 55.26; P = 0.06; I² = not applicable; 1 trial, 59 participants; Analysis 4.20);
a Bayley mental score greater than 2 SD below the mean (RR 2.69, 95% CI 0.57 to 12.69; P = 0.21; I² = not applicable; 1 trial, 54 participants; Analysis 4.21);
bilateral blindness (RR 3.44, 95% CI 0.15 to 81.09; P = 0.44; I² = not applicable; 1 trial, 56 participants; Analysis 4.22); or
abnormal neurodevelopmental outcome in survivors alone (RR 1.09, 95% CI 0.71 to 1.70; P = 0.69; I² = 39%; 3 trials, 143 participants; Analysis 4.23), or in combination with death (RR 1.44, 95% CI 0.99 to 2.07; P = 0.05; I² = 52%; 3 trials, 168 participants; Analysis 4.24)
4.19. Analysis.

Comparison 4: Individual tailored versus continuous tapered dexamethasone regimen, Outcome 19: Cerebral palsy in survivors assessed at 1‐3 years
4.20. Analysis.

Comparison 4: Individual tailored versus continuous tapered dexamethasone regimen, Outcome 20: Death or cerebral palsy at 1‐3 years
4.21. Analysis.

Comparison 4: Individual tailored versus continuous tapered dexamethasone regimen, Outcome 21: Bayley's MDI < ‐2 SD
4.22. Analysis.

Comparison 4: Individual tailored versus continuous tapered dexamethasone regimen, Outcome 22: Severe blindness
4.23. Analysis.

Comparison 4: Individual tailored versus continuous tapered dexamethasone regimen, Outcome 23: Abnormal neurodevelopmental outcome in survivors assessed (various definitions)
4.24. Analysis.

Comparison 4: Individual tailored versus continuous tapered dexamethasone regimen, Outcome 24: Death or abnormal neurodevelopmental outcome (various definitions)
The certainty of the evidence was moderate for the outcome death or cerebral palsy and low for the outcome death or abnormal neurodevelopmental outcome because of the small number of events, and the risk of performance and detection bias (Table 4).
Discussion
It has been proven in RCTs that corticosteroids reduce the combined outcome of death or BPD at 36 weeks' PMA. However, concerns have arisen about negative long‐term neurodevelopmental effects of this therapy. Despite the firm recommendations of several pediatric societies to stop using postnatal systemic dexamethasone outside the realm of randomized clinical trials, clinicians are still using dexamethasone to treat ventilator‐dependent preterm infants. Therefore, attempts to identify the optimal corticosteroid treatment regimen remain clinically relevant and important. Questions that need to be answered are: 1) what is the optimal time to start corticosteroid treatment; 2) what is optimal cumulative dose; 3) what is the optimal duration of therapy; 4) what is the optimal corticosteroid to use? This systematic review summarizes all published studies that have investigated the comparative effects of different corticosteroid treatment regimens head‐to‐head on the incidence of the combined outcome of death or BPD and the risk of adverse effects on neurodevelopment.
Summary of main results
This review examines four different types of postnatal corticosteroid regimens. The first intervention, investigated in eight RCTs (n = 306) compares a lower versus a higher dose of dexamethasone. The absolute dexamethasone dose used to contrast a higher versus a lower dose varied considerably between the included trials. This heterogeneity in dose contrast precluded a pooled analysis of all available trials. For this reason, we divided the studies into a high‐range contrast subgroup, comparing a high cumulative dose (> 4 mg/kg) to a moderate dose (2 to 4 mg/kg) and a low‐range contrast subgroup, comparing a moderate to a low cumulative dose (< 2 mg/kg). We would like to emphasize that the terms 'high', 'moderate', and 'low' should be interpreted from a relative perspective, because compared to the physiological levels of corticosteroids all reported doses are supraphysiological (i.e. 'high'). The analyses showed no evidence of outcome differences when contrasting a low to a moderate dexamethasone dose, except the analyses of the outcomes hypertension and hyperglycemia. However, these adverse effects seen in the group of infants treated with a moderate dosage regimen might be outweighed by beneficial effect on long term outcomes. The analyses also showed no evidence of a difference in the outcome BPD at 36 weeks' PMA, and the combined outcome of death or BPD at 36 weeks' PMA when contrasting a moderate to a high dexamethasone dose. However, compared to a moderate dose, a high dexamethasone dose reduced the risk of failure to extubate, prolonged duration of mechanical ventilation, cerebral palsy or abnormal neurodevelopmental outcome assessed between one and three years of age, and the composite outcome death or cerebral palsy or abnormal neurodevelopmental outcome.
In contrast with a previous meta‐analysis assessing the impact of (different) cumulative dexamethasone doses used in placebo‐controlled trials (Onland 2009), no evidence of a difference was found for the primary outcome death or BPD at 36 weeks' PMA in the meta‐analyses of studies investigating a higher versus lower dosage regimen. This lack of evidence of a difference changed compared with the previous review published 2017 (Onland 2017). However, the current review was able to include the full publication of the trial conducted by Marr 2019. No differences were found in that trial comparing a moderate versus high dosage regimen of dexamethasone, hence the effect estimate changed from borderline significant (RR 1.35, 95% CI 1.00 to 1.82) in the previous version of this review towards no significant difference in the current version (Analysis 1.1). We can only speculate on the possible explanations for this change in findings compared to the previous version of this review (Onland 2017). First, the a priori risk of BPD might have been different between the different studies and comparisons. One of the studies in the high‐range contrast comparison was performed in the pre‐surfactant era (Cummings 1989), and another study in this comparison included infants with a quite low birth weight and gestational age with considerable supplemental oxygen and mean airway pressure at trial entry (Marr 2019). Both factors are known BPD risk factors and might explain the statistical heterogeneity found in these analyses. Second, the use of additional ('rescue') dexamethasone treatment outside the study protocol by infants in both allocation arms was only observed in the studies comparing a low to moderate cumulative dose and the Marr 2019 study. This could well have resulted in an underestimation of the true treatment effect in these trials (Onland 2010). Finally, these results may also suggest that a relatively low cumulative dexamethasone dose as used in the low‐range contrast comparison is, in a pharmacodynamic sense, not sufficient to change the rate of BPD and hence any contrast in this dosing range will not result in a group difference in BPD.
In contrast with the analyses of the primary outcome showing no difference, the short term pulmonary outcomes, namely duration of mechanical ventilation and failure to extubate seven days after initiation of therapy in the studies investigating a moderate versus a high dosage regimen did show a difference between groups in favor of the high‐dosage regimens. Furthermore, this review suggests that the benefit of high‐dose dexamethasone on these short‐term pulmonary outcomes is not outweighed by an increased risk of neurodevelopmental impairment. It even suggests that, compared to a moderate cumulative dose, neurodevelopment might be improved in the infants treated with a high dose, although this finding should be interpreted cautiously for the following reasons. First, the low a priori chance of adverse neurodevelopmental outcomes in combination with the relatively small number of included infants in this review might not be sufficient to detect small but clinically relevant treatment effects on these outcomes. Second, the number of infants lost to follow‐up was more than 10% in two of the three studies, which might have biased the results, since children with cerebral palsy are especially difficult to follow up. A possible benefit of high‐dose dexamethasone on neurodevelopmental outcome might be mediated by the reduced duration of mechanical ventilation. This outcome is associated with an increased risk of neurodevelopmental impairment and may, in the high‐risk infant, override a possible direct toxic effect of dexamethasone on the brain (Doyle 2014; Vliegenthart 2017; Vliegenthart 2019; Walsh 2005). In line with the aforementioned results of these meta‐analyses and reasoning, a recent network analysis including 62 RCTs evaluating 14 different inhaled and systemic administered corticosteroid regimens showed that moderately early initiation of a systemic moderate‐dosage regimen of dexamethasone might be the most appropriate treatment for the prevention of BPD and mortality (Ramaswamy 2021). However, more high‐quality evidence is needed to support or refute this hypothesis.
The second intervention, contrasting a later versus earlier initiation of therapy, showed conflicting results. The subgroup analyses comparing trials that started corticosteroids within the first week to trials starting after the first week of life showed a reduction of ventilation days and supplemental oxygen, as well as a decreased risk of BPD when treatment was initiated earlier. This beneficial effect of early treatment did not come at the expense of an increased risk of adverse neurodevelopmental outcome, as reported in the meta‐analysis of placebo‐controlled trials starting dexamethasone in the first week of life (Doyle 2021a). However, it is important to emphasize that only three studies performed a head‐to‐head comparison of moderately early versus early dexamethasone treatment reporting these outcomes, and included a small number of participants.
Analyses of primary comparisons including trials investigating late‐initiated dexamethasone versus initiation in the moderately early period revealed no benefits on long‐term pulmonary outcomes. Although postponing the start of dexamethasone treatment did reduce the risk of hypertension and culture‐proven sepsis, data on long‐term neurodevelopmental outcomes were not reported. These results are in contrast with the meta‐analyses of the placebo‐controlled trials, showing a lower number needed to treat for an additional beneficial outcome (NNTB) for reducing BPD when starting treatment moderately early compared to delayed administration (Onland 2009; Schmidt 2008).
The third intervention summarized in this review involved studies exploring the effect of a pulse‐dosing regimen on both the beneficial and adverse effects of dexamethasone treatment. These analyses showed that a pulse‐dosing regimen increased the risk of the combined outcome death or BPD compared with a continuous‐dosing regimen. Although speculative, it might be that the ongoing inflammatory response causing the development of BPD will not be suppressed by a pulse therapy regimen, which incorporated a seven‐day treatment pause.
Finally, tailoring the dexamethasone dose to the individual pulmonary response of the infant seems a logical approach, since there is a wide spectrum of lung damage in preterm infants. More inflamed and damaged lungs could theoretically benefit from a higher cumulative corticosteroid dose. To date, only three trials including a small number of infants have investigated this contrast, with no difference in the primary or secondary outcomes.
Overall completeness and applicability of evidence
We were not able to perform funnel plot analyses of the primary outcomes to identify potential publication bias, because less then 10 RCTs were identified per comparison. Therefore, we cannot rule out that other small RCTs were performed, but not published. Several studies were only published as abstracts, limiting methodological assessment and data on the primary and secondary outcomes. Another major problem that this review uncovered is that even when full text was published, not every trial reported on our stated primary and secondary outcomes. Specifically, few studies reported on neurodevelopmental outcome parameters, and those that did used various definitions or assessed neurodevelopment at different points in time. Although we pooled the data as if they were statistically homogeneous, this clinical heterogeneity might compromise the validity of the results of our meta‐analysis. It remains unclear how this influences the conclusions of this review. We could not perform the previously mentioned subgroup analyses, i.e. according to gestational age and respiratory status at trial entry, due to lack of data or heterogeneity between the trials on these clinical characteristics.
Quality of the evidence
Except for the trials that were only published as abstracts, and for which assessment of potential biases was not possible, we deemed the risk of bias in the trials as unclear to very low and we believe that bias has no large influence on the results. However, the overall quality of the evidence provided by the meta‐analyses using the GRADE approach for each outcome was moderate to very low due to several severe study limitations, such as risk of bias; potential publication bias; and imprecision of effect estimates. First, as mentioned earlier, the overall sample size in these analyses was small, resulting in inadequate power to detect small but clinically relevant differences in some of the important outcome parameters. Second, although most studies contrasted two dosing regimens of dexamethasone, there was considerable diversity in the study designs, like the cumulative dexamethasone dose that was used, the starting dose and the duration of therapy. It remains unclear whether and how these differences affect the observed treatment effect in the different interventions. Third, the use of late 'rescue' corticosteroids outside the study protocol was considerable in the majority of the trials; this may have confounded (contaminated) the true dexamethasone treatment effect.
Potential biases in the review process
The 2021 search did not include independent searches of EMBASE, ClinicalTrials.gov, or the World Health Organization’s International Clinical Trials Registry Platform (ICTRP). Although records from these sources are included in Cochrane CENTRAL, their omission may have reduced sensitivity of the search. Subsequent updates of this review will include these sources to minimize potential bias.
Agreements and disagreements with other studies or reviews
The first systematic review investigating the effect of different dosage regimens on the outcome BPD was published in 2008 (Onland 2008). The conclusion on the pulmonary outcome remains unchanged. However, that review did not include the abstract of Marr 2011, relating to the now‐published Marr 2019 study. Including the data from that study into the 2017 version of this review (Onland 2017) changed the cumulative effect estimate of long‐term neurodevelopmental outcome, showing a significantly reduced risk when administrating a higher‐dosage regimen. Since the Onland 2017 version of this review, the full publication of the study by Marr became available (Marr 2019), and the effect estimate of the outcome death or BPD showed no difference comparing a high‐ versus moderate‐dosage regimen of dexamethasone (Analysis 1.1). However, although the evidence is very uncertain, the meta‐analyses showing the long‐term neurodevelopmental outcomes suggest a beneficial effect in favor of higher dosage regimens including this trial (Analysis 1.20; Analysis 1.24), compared to a moderate‐dosage regimen of dexamethasone. Therefore, the results of the current review do not support the recommendation of international guidelines proclaiming that steroids should be dosed as short and low as possible (AAP 2002; Watterberg 2010).
Authors' conclusions
Implications for practice.
The present review includes all randomized studies to date that have investigated different corticosteroid treatment regimens head‐to‐head; all studies used the corticosteroid dexamethasone. Despite the fact that some studies reported a modulating effect of a specific treatment regimen in favor of higher dosage on the incidence of neurodevelopmental impairment, we cannot draw conclusions on the optimal type, dosage, or timing of initiation for the prevention of bronchopulmonary dysplasia(BPD) in preterm infants, based on current evidence. The evidence is very uncertain about the effects of different corticosteroid regimens on the outcomes mortality, pulmonary morbidity, and long term neurodevelopmental impairment. Furthermore, the results of this review do not justify a change in the recommendation published in international guidelines of corticosteroid use to not use these drugs outside the realm of a well‐designed clinical trial. This review demonstrated that a well‐designed large randomized controlled trial (RCT) is needed to establish the optimal systemic postnatal corticosteroid dosage regimen.
Implications for research.
In light of the ongoing use of dexamethasone in the clinical setting, we feel that an RCT on dexamethasone dose and timing is justified and, in fact, urgently needed. A large multicenter study with a factorial design should compare a higher cumulative dexamethasone dose with a lower dose, as well as timing of initiation. Although the current evidence prevents firm recommendations, the present review suggests the trial should compare dexamethasone doses in the higher ranges. Obviously, the trial should be adequately powered to detect small but clinically relevant treatment effects and interaction between dose and timing of initiation. It should include ventilated preterm infants with a high risk for BPD based on the known determinants in the development of BPD. The time window to initiate dexamethasone treatment between seven days and 14 days after birth should be compared with initiation after that time period, as suggested by the recently published network analysis (Ramaswamy 2021). We recommend that data on the following primary outcome parameters be collected in any future comparative study: BPD at 36 weeks' postmenstrual age (PMA), mortality at 36 weeks' PMA and at discharge, and neurodevelopmental outcome using predefined definitions, assessed with standardized, validated, reliable diagnostic and functional outcome measurement instruments at standardized time points. In addition, short‐term benefits (time of extubation, ventilation time, discharge home on oxygen, length of hospital stay) and adverse effects (hypertension, sepsis, hyperglycemia, and the need for tracheostomy) should be collected as secondary outcomes. Various threats to the internal validity of the trial should be recognized and contained, such as potential dilution of treatment effect due to the use of 'rescue' corticosteroids outside the study protocol, or crossing over between trial arms. In any event, additional treatments should be adequately reported in order to assess the possibility of confounding.
What's new
| Date | Event | Description |
|---|---|---|
| 21 February 2024 | Amended | In the main text, the direction of effect was mistakenly reversed and did not match the associated Forest Plots. This has been corrected for Failure to extubate, Analyses 2.7 and 2.8. |
History
Protocol first published: Issue 1, 2014 Review first published: Issue 1, 2017
| Date | Event | Description |
|---|---|---|
| 13 March 2023 | New citation required and conclusions have changed | We identified two additional studies for inclusion; one of these studies had sufficient data to incorporate in the quantitative synthesis (Marr 2019); the other informs the qualitative synthesis (Groneck 1993). In contrast to the previous version of this review, meta‐analyses of studies investigating a moderate dosage regimen dexamethasone versus a high dosage regimen showed no differences in the outcome death or bronchopulmonary dysplasia, but a reduction in adverse neurodevelopmental outcomes in favor of higher dosage regimens. |
| 13 March 2023 | New search has been performed | Revised search strategy run without date limits. |
Notes
Part of this systematic review on one of the comparisons has been published before (Onland 2008).
Acknowledgements
We would like to thank Michelle Fiander, Information Specialist, for writing search strategies, running searches, writing search methods, writing results of search, and running the Screen4Me process.
We would like to thank the Cochrane Neonatal editorial base: Michelle Fiander, Jane Cracknell, and Fiona Russell, Managing Editors; William McGuire and Roger Soll, Co‐ordinating Editors. We also thank Colleen Ovelman, former Managing Editor, and Yoland Brosseau, former Information Specialist, for support on previous versions of this review.
We would like to thank Dr A De Jaegere for her participation of the previous version of this review (Onland 2017).
The authors thank Dr JK Muraskas, Loyola University Medical Center, Dr M Durand, Los Angeles County‐University of Southern California Medical Center, Dr C McEvoy, Oregon Health Sciences University, Dr CA Malloy, Children’s Memorial Hospital, Northwestern University's Feinberg School of Medicine, Dr BM Barkemeyer, LSU Health Sciences Center, New Orleans, Dr JJ Cummings, Brody School of Medicine, East Carolina University, and Dr BL Marr, Crouse Hospital, Syracuse NY for providing us with additional data and thoughtful review of the draft.
We thank the following peer reviewers:
Danielle EY Ehret, MD, MPH Associate Professor of Pediatrics Asfaw Yemiru Green and Gold Professor of Global Health University of Vermont, Larner College of Medicine, USA.
Dr Vibhuti Shah Staff Neonatologist, Department of Paediatrics, Mount Sinai Hospital Professor, Department of Paediatrics and Institute of Health Policy, Management and Evaluation, University of Toronto, Canada.
Appendices
Appendix 1. Cochrane Library search strategy
| Cochrane Library:, Issue 9, 2022 (Wiley) | ||
| Date run: 13 September 2022 23:24:19 | ||
| ID | Terms | Hits |
| #1 | MeSH descriptor: [Adrenal Cortex Hormones] explode all trees | 15283 |
| #2 | MeSH descriptor: [Steroids] explode all trees | 62755 |
| #3 | MeSH descriptor: [Glucocorticoids] explode all trees | 4807 |
| #4 | (adrenal cortex hormone* or dexamethasone or betamethasone or hydrocortisone or steroid or steroids or corticosteroid* or prednisolone or methylprednisolone or glucocorticoid*):ti,ab,kw | 73523 |
| #5 | (Beclomethasone or Betamethasone or Betamethasone Valerate or Budesonide or Clobetasol or Desoximetasone or Diflucortolone or Flumethasone or Fluocinolone Acetonide or Fluocinonide or Fluocortolone or Fluorometholone or Fluprednisolone or Flurandrenolone or Fluticasone‐Salmeterol or Melengestrol Acetate or Methylprednisolone or Paramethasone or Prednisolone or Prednisone or (Tobramycin adj2 Dexamethasone) or Triamcinolone):ti,ab,kw | 34663 |
| #6 | (Androstane* or (Bile Acids and Salts) or Cardanolide* or Cholane* or Cholestan*? or Cyclosteroid* or Estrane* or Gonane* or Homosteroid* or Hydroxysteroid* or Ketosteroid* or Norsteroid* or Pregnane* or Sapogenin* or Secosteroid*):ti,ab,kw | 1345 |
| #7 | ("17‐Ketosteroid*" or Androstenedione or Androsterone or Dehydroepiandrosterone or Estrone or Etiocholanolone or Hydroxycorticosteroid* or "11‐Hydroxycorticosteroid*" or "17‐Hydroxycorticosteroid*" or Desoxycorticosterone or Pregnenolone):ti,ab,kw | 2676 |
| #8 | #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 | 120371 |
| #9 | MeSH descriptor: [Respiratory Distress Syndrome, Newborn] explode all trees | 1797 |
| #10 | MeSH descriptor: [Transient Tachypnea of the Newborn] explode all trees | 47 |
| #11 | MeSH descriptor: [Hyaline Membrane Disease] explode all trees | 100 |
| #12 | hyaline membrane disease*:ti,ab,kw OR "transient tachypne*":ti,ab,kw | 187 |
| #13 | #9 OR #10 OR #11 OR #12 | 1878 |
| #14 | MeSH descriptor: [Bronchopulmonary Dysplasia] explode all trees | 580 |
| #15 | (bronchopulmonar* or bronchio* or bronchia* or pulmonar* or lung or lungs):ti,ab,kw | 118881 |
| #16 | (bpd OR cld):ti,ab,kw | 2263 |
| #17 | #15 OR #16 | 120115 |
| #18 | MeSH descriptor: [Oxygen Inhalation Therapy] this term only | 1332 |
| #19 | MeSH descriptor: [Ventilator‐Induced Lung Injury] this term only | 56 |
| #20 | MeSH descriptor: [Respiration, Artificial] explode all trees | 6986 |
| #21 | MeSH descriptor: [Respiratory Distress Syndrome] this term only | 1533 |
| #22 | (ventilator* or ventilation or ventilating):ti,ab,kw | 36437 |
| #23 | (artificial NEAR/2 respiration):ti,ab,kw | 3997 |
| #24 | (respiratory NEAR/2 distress*):ti,ab,kw | 7860 |
| #25 | (oxygen NEAR/2 Therap*):ti,ab,kw | 5858 |
| #26 | #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 | 45735 |
| #27 | MeSH descriptor: [Infant, Newborn] explode all trees | 17651 |
| #28 | (infant or infants or infant? or infantile or infancy or newborn* or new born or new borns or newly born or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or pre term or premies or low birth weight or low birthweight or VLBW or LBW or ELBW or NICU):ti,ab,kw | 110862 |
| #29 | #27 OR #28 | 110862 |
| #30 | #8 AND #13 | 332 |
| #31 | #8 AND (#17 OR #26) AND #29 | 2159 |
| #32 | #30 OR #31 [protocols and systematic reviews, 2020‐] | 21 |
| #33 | #30 OR #31 [Trials; all years] | 2020 |
| #34 | Total | 2041 |
Appendix 2. Medline search strategy
| Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process, In‐Data‐Review & Other Non‐Indexed Citations, Daily and Versions 1946 to 9 September, 2022 | ||
| # | Searches | Results |
| 1 | exp Adrenal Cortex Hormones/ | 418034 |
| 2 | exp Steroids/ | 905349 |
| 3 | exp Glucocorticoids/ | 203989 |
| 4 | (adrenal cortex hormone* or dexamethasone or betamethasone or hydrocortisone or steroid or steroids or corticosteroid* or prednisolone or methylprednisolone or glucocorticoid*).mp. | 615216 |
| 5 | (Beclomethasone or Betamethasone or Betamethasone Valerate or Budesonide or Clobetasol or Desoximetasone or Diflucortolone or Flumethasone or Fluocinolone Acetonide or Fluocinonide or Fluocortolone or Fluorometholone or Fluprednisolone or Flurandrenolone or Fluticasone‐Salmeterol or Melengestrol Acetate or Methylprednisolone or Paramethasone or Prednisolone or Prednisone or (Tobramycin adj2 Dexamethasone) or Triamcinolone).ti,ab,kw,kf. [Glucocorticoids ] | 98977 |
| 6 | (Androstane? or (Bile Acids and Salts) or Cardanolide? or Cholane? or Cholestane? or Cyclosteroid? or Estrane? or Gonane? or Homosteroid? or Hydroxysteroid? or Ketosteroid? or Norsteroid? or Pregnane? or Sapogenin? or Secosteroid?).ti,ab,kw,kf. [Steroids] | 27222 |
| 7 | (17‐Ketosteroid? or Androstenedione or Androsterone or Dehydroepiandrosterone or Estrone or Etiocholanolone or Hydroxycorticosteroid? or 11‐Hydroxycorticosteroid? or 17‐Hydroxycorticosteroid? or Desoxycorticosterone or Pregnenolone).ti,ab,kw,kf. [Adrenal Cortex Hormones] | 39824 |
| 8 | or/1‐7 [Interventions including drug terms] | 1236655 |
| 9 | Bronchopulmonary Dysplasia/ | 5696 |
| 10 | (bronchopulmonar* or bronchio* or bronchia* or pulmonar* or lung or lungs).ti,ab,kw,kf. | 1249423 |
| 11 | (BPD or CLD).ti,ab,kw,kf. | 15518 |
| 12 | or/9‐11 [BPD] | 1260102 |
| 13 | respiratory distress syndrome, newborn/ or hyaline membrane disease/ or "transient tachypnea of the newborn"/ | 15817 |
| 14 | (hyaline membrane disease? or Transient Tachypne*).ti,ab,kw,kf. | 2408 |
| 15 | or/13‐14 [BPD similar conditions specific to newborns; combine only with intervention terms] | 16533 |
| 16 | (BPD or CLD).ti,ab,kw,kf. | 15518 |
| 17 | Oxygen Inhalation Therapy/ or Ventilator‐Induced Lung Injury/ or Respiration, Artificial/ or respiratory distress syndrome/ | 88385 |
| 18 | (ventilator? or ventilation or ventilating).ti,ab,kw,kf. | 177115 |
| 19 | (artificial adj2 respiration).ti,ab,kw,kf. | 3122 |
| 20 | (respiratory adj2 distress*).ti,ab,kw,kf. | 51232 |
| 21 | (oxygen adj2 therap*).ti,ab,kw,kf. | 15556 |
| 22 | or/17‐21 [Additional terms related to BPD] | 261027 |
| 23 | exp infant, newborn/ | 659347 |
| 24 | (infant or infants or infant? or infantile or infancy or newborn* or new born or new borns or newly born or neonat* or baby* or babies or premature or prematures or prematurity or preterm or preterms or pre term or premies or low birth weight or low birthweight or VLBW or LBW or ELBW or NICU).ti,ab,kw,kf. | 984103 |
| 25 | or/23‐24 [Filter: Neonatal Population 2021] | 1266473 |
| 26 | (randomized controlled trial or controlled clinical trial).pt. | 666756 |
| 27 | (randomized or randomised or randomly).ti,ab,kw,kf. | 1048987 |
| 28 | placebo.ab. | 231535 |
| 29 | (trial or groups).ab. | 2857311 |
| 30 | drug therapy.fs. | 2527015 |
| 31 | ((single or doubl* or tripl* or treb*) and (blind* or mask*)).ti,ab,kw,kf. | 216824 |
| 32 | Double‐Blind Method/ | 172977 |
| 33 | exp Animals/ not humans/ | 5043875 |
| 34 | (or/26‐32) not 33 [RCT filter] | 4811651 |
| 35 | systematic review.pt. | 206453 |
| 36 | (systematic adj2 review).ti. | 198382 |
| 37 | meta analysis/ | 167078 |
| 38 | (meta‐analysis or metaanalysis).ti,ab,kw. | 214611 |
| 39 | (cochrane or systematic review?).jw. | 19501 |
| 40 | overview of reviews.ti. | 105 |
| 41 | or/35‐40 [SR filter] | 375070 |
| 42 | 8 and 12 and 25 [Corticosteroids AND BPD AND Neonate] | 5511 |
| 43 | 8 and 15 [Corticosteroids and Infant‐specific BPD type conditions] | 1963 |
| 44 | 8 and 22 and 25 [Corticosteroids AND BPD‐related terms AND Neonate] | 3752 |
| 45 | or/42‐44 [Results before filters] | 7665 |
| 46 | 45 and 34 [RCT Results] | 3345 |
| 47 | 45 and 41 and 202*.yr. [SR results 2020‐] | 56 |
| 48 | or/46‐47 [Medline results 2022] | 3361 |
Appendix 3. Embase search strategy
| Embase 1974 to 12 September 2022 | ||
| # | Searches | Results |
| 1 | exp corticosteroid/ or exp glucocorticoid/ [no specific heading for adrenal cortex hormone] | 1046269 |
| 2 | exp steroid/ | 1689551 |
| 3 | dexamethasone/ or betamethasone/ or hydrocortisone/ or prednisolone/ or methylprednisolone/ | 508271 |
| 4 | (adrenal cortex hormone* or dexamethasone or betamethasone or hydrocortisone or steroid or steroids or corticosteroid* or prednisolone or methylprednisolone or glucocorticoid*).mp. | 1206131 |
| 5 | (Beclomethasone or Betamethasone or Betamethasone Valerate or Budesonide or Clobetasol or Desoximetasone or Diflucortolone or Flumethasone or Fluocinolone Acetonide or Fluocinonide or Fluocortolone or Fluorometholone or Fluprednisolone or Flurandrenolone or Fluticasone‐Salmeterol or Melengestrol Acetate or Methylprednisolone or Paramethasone or Prednisolone or Prednisone or (Tobramycin adj2 Dexamethasone) or Triamcinolone).ti,ab,kw,kf. [Glucocorticoids ] | 153995 |
| 6 | (Androstane? or (Bile Acids and Salts) or Cardanolide? or Cholane? or Cholestane? or Cyclosteroid? or Estrane? or Gonane? or Homosteroid? or Hydroxysteroid? or Ketosteroid? or Norsteroid? or Pregnane? or Sapogenin? or Secosteroid?).ti,ab,kw,kf. [Steroids] | 26290 |
| 7 | (17‐Ketosteroid? or Androstenedione or Androsterone or Dehydroepiandrosterone or Estrone or Etiocholanolone or Hydroxycorticosteroid? or 11‐Hydroxycorticosteroid? or 17‐Hydroxycorticosteroid? or Desoxycorticosterone or Pregnenolone).ti,ab,kw,kf. [Adrenal Cortex Hormones] | 38205 |
| 8 | or/1‐7 [Interventions including drug terms] | 1843331 |
| 9 | lung dysplasia/ | 14316 |
| 10 | (bronchopulmonar* or bronchio* or bronchia* or pulmonar* or lung or lungs).ti,ab,kw,kf. | 1678563 |
| 11 | (BPD or CLD).ti,ab,kw,kf. | 23364 |
| 12 | or/9‐11 [BPD] | 1696549 |
| 13 | hyaline membrane disease/ or neonatal respiratory distress syndrome/ or "transient tachypnea of the newborn"/ | 12624 |
| 14 | (hyaline membrane disease? or transient tachypne*).ti,ab,kw,kf. | 2614 |
| 15 | or/13‐14 [BPD similar conditions specific to newborns; combine only with intervention terms] | 13512 |
| 16 | oxygen therapy/ or ventilator induced lung injury/ or artificial ventilation/ or respiratory distress syndrome/ | 212066 |
| 17 | (ventilator? or ventilation or ventilating).ti,ab,kw,kf. | 262559 |
| 18 | (artificial adj2 respiration).ti,ab,kw,kf. | 2381 |
| 19 | (respiratory adj2 distress*).ti,ab,kw,kf. | 74540 |
| 20 | (oxygen adj2 therap*).ti,ab,kw,kf. | 20817 |
| 21 | or/16‐20 [BPD synonyms any population] | 417511 |
| 22 | newborn/ or prematurity/ or newborn intensive care/ or newborn care/ or gestational age/ | 756156 |
| 23 | (babe or babes or baby* or babies or gestational age? or infant? or infantile or infancy or low birth weight or low birthweight or neonat* or neo‐nat* or newborn* or new born? or newly born or premature or pre‐mature or pre‐matures or prematures or prematurity or pre‐maturity or preterm or preterms or pre term? or preemie or preemies or premies or premie or VLBW or VLBWI or VLBW‐I or VLBWs or LBW or LBWI or LBWs or ELBW or ELBWI or ELBWs or NICU or NICUs).ti,ab,kw,kf. | 1191466 |
| 24 | or/22‐23 [Filter: Neonatal Population 03‐2022‐OVID EMBASE] | 1451323 |
| 25 | Randomized controlled trial/ or Controlled clinical study/ | 917765 |
| 26 | random$.ti,ab,kw. | 1837854 |
| 27 | Randomization/ | 94976 |
| 28 | placebo.ti,ab,kw. | 346737 |
| 29 | ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab,kw. | 260451 |
| 30 | double blind procedure/ | 198581 |
| 31 | (controlled adj7 (study or design or trial)).ti,ab,kw. | 417936 |
| 32 | parallel group$1.ti,ab. | 30008 |
| 33 | (crossover or cross over).ti,ab. | 118070 |
| 34 | ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. | 387870 |
| 35 | (open adj label).ti,ab. | 100078 |
| 36 | (quasirandom* or quasi‐random* or randomi* or randomly).ti,ab,kw,kf. | 1498581 |
| 37 | (control* adj2 (group? or random*)).ti,ab,kw,kf. | 1220582 |
| 38 | or/25‐37 [ Terms based on Cochrane Central strategy‐ How Central is Created] | 3140009 |
| 39 | (exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/) and (human/ or normal human/ or human cell/) | 24087606 |
| 40 | exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ | 31065873 |
| 41 | 40 not 39 [Animal Exclusion‐https://community‐cochrane‐org.ezproxy.uvm.edu/sites/default/files/uploads/inline‐files/Embase%20animal%20filter.pdf] | 6978267 |
| 42 | 38 not 41 [Filter: RCT‐EMBASE] | 2700062 |
| 43 | meta‐analysis/ or "systematic review"/ or "meta analysis (topic)"/ [EMTREE] | 527291 |
| 44 | ((systematic* adj3 (review* or overview*)) or (methodologic* adj3 (review* or overview*))).ti,ab,kw. | 344257 |
| 45 | ((integrative adj3 (review* or overview*)) or (collaborative adj3 (review* or overview*)) or (pool* adj3 analy*)).ti,ab,kw. | 50245 |
| 46 | (data synthes* or data extraction* or data abstraction*).ti,ab,kw. | 44779 |
| 47 | (hand search* or handsearch*).ti,ab,kw. | 12965 |
| 48 | (mantel haenszel or peto or der simonian or dersimonian or fixed effect* or latin square*).ti,ab,kw. | 43604 |
| 49 | (meta analy* or metanaly* or meta regression* or metaregression*).ti,ab,kw. | 315252 |
| 50 | (medline or cochrane or pubmed or medlars or embase or cinahl).ab. | 379847 |
| 51 | (cochrane or systematic review?).jn,jx. | 30609 |
| 52 | (overview adj2 reviews).ti. | 120 |
| 53 | or/43‐52 [SR Filter: EMBASE based on CADTH filter: https://searchfilters.cadth.ca] | 809956 |
| 54 | 8 and 12 and 24 [Corticosteroids AND BPD AND Neonate] | 11819 |
| 55 | 8 and 15 [Corticosteroids and Infant‐specific BPD type conditions] | 2399 |
| 56 | 8 and 21 and 24 [Corticosteroids AND BPD‐related terms AND Neonate] | 9830 |
| 57 | or/54‐56 [Results before filters] | 17432 |
| 58 | 42 and 57 [RCT Results] | 2698 |
| 59 | 53 and 57 and 202*.yr. [SR results 2020‐] | 180 |
| 60 | or/58‐59 [Embase Results 2022] | 2811 |
Appendix 4. Pre‐2022 search strategies
We conducted a comprehensive search including: Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 2) in the Cochrane Library (searched 21 March 2016); MEDLINE via PubMed (1966 to 21 March 2016); Embase (1980 to 21 March 2016); CINAHL (1982 to 21 March 2016) using the MeSH terms and text words: ('adrenal cortex hormones' OR 'dexamethasone' OR 'betamethasone' OR 'hydrocortisone' OR 'prednisolone' OR 'methylprednisolone' OR 'steroids' OR 'corticosteroids' OR 'glucocorticoids'), and Limits:
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh])) Embase: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial) CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial) Cochrane Library: (infant or newborn or neonate or neonatal or premature or preterm or very low birth weight or low birth weight or VLBW or LBW)
Appendix 5. Trial registry search strategies
| Date | Site | Terms | Results |
| 13 September 2022 | clinicaltrials.gov | Other terms: corticosteroid OR glucocorticoid OR dexamethasone or betamethasone or hydrocortisone or steroid AND Condition: Bronchopulmonary Dysplasia AND Child (Birth to 17 years) | 27 |
| 13 September 2022 | clinicaltrials.gov | Other terms: Beclomethasone or Betamethasone or Budesonide or Clobetasol or Desoximetasone or Diflucortolone or Flumethasone or Fluocinolone Acetonide or Fluocinonide or Fluocortolone or Fluorometholone or Fluprednisolone or Flurandrenol AND Condition: Bronchopulmonary Dysplasia AND Child (Birth‐17) | 0 |
| 13 September 2022 | ICTRP | bronchopulmonary dysplasia AND corticosteroid [Main search page] AND trials in children | 3 |
| 13 September 2022 | ICTRP | bronchopulmonary dysplasia AND glucocorticoid [Main search page] AND trials in children | 1 |
| 13 September 2022 | ICTRP | bronchopulmonary dysplasia AND dexamethasone AND trials in children | 6 |
| 13 September 2022 | ICTRP | bronchopulmonary dysplasia AND betamethasone AND trials in children | 0 |
| 13 September 2022 | ICTRP | bronchopulmonary dysplasia AND hydrocortisone AND trials in children | 5 |
| 42 |
Appendix 6. Risk of bias tool
The following issues were evaluated and entered into the risk of bias table.
Adequate sequence generation? For each included study, we categorized the risk of selection bias as:
low risk ‐ adequate (any truly random process, e.g. random number table; computer random number generator;
high risk ‐ inadequate (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk ‐ no or unclear information provided.
Allocation concealment? For each included study, we categorized the risk of bias regarding allocation concealment as
low risk ‐ adequate (e.g. telephone or central randomizations; consecutively numbered sealed opaque envelopes);
high risk ‐ inadequate (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk ‐ no or unclear information provided.
-
Blinding?
-
Performance bias? For each included study, we categorized the methods used to blind study personnel from knowledge of which intervention a participant received (as our study population consists of neonates, they are all blinded to the study intervention).
low risk ‐ adequate for personnel (a placebo that could not be distinguished from the active drug was used in the control group);
high risk ‐ inadequate ‐ personnel aware of group assignment;
unclear risk ‐ no or unclear information provide.
-
Detection bias? For each included study, we categorized the methods used to blind outcome assessors from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or classes of outcomes. We categorized the methods used with regards to detection bias as:
low risk ‐ adequate; follow‐up was performed with assessors blinded to group assignment;
high risk ‐ inadequate; assessors at follow‐up were aware of group assignment;
unclear risk ‐ no or unclear information provided.
-
Incomplete data addressed (attrition bias)? For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomized participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information is reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorized the methods with respect to the risk attrition bias as:
low risk ‐ adequate (< 10% missing data);
high risk ‐ inadequate (>10% missing data);
unclear risk ‐ no or unclear information provided.
-
Free of selective reporting (reporting bias)? For each included study, we investigated the risk of selective outcome reporting bias and what we found. We assessed the methods as:
low risk ‐ adequate (where it is clear that all of the study's prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk ‐ inadequate (where not all the study's prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest were reported incompletely and so cannot be used; study failed to include results of a key outcome that would have been expected to have been reported);
unclear risk ‐ no or unclear information provided (the study protocol was not available).
-
Free of other bias? For each included study, we described any important concerns we had about other possible sources of bias (e.g. whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as:
low risk ‐ no concerns of other bias raised;
high risk ‐ concerns raised about multiple looks at the data with the results made known to the investigators, difference in number of patients enrolled in abstract and final publications of the paper;
unclear ‐ concerns raised about potential sources of bias that could not be verified by contacting the authors.
Overall risk of bias
Explicit judgments were made about whether studies are at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2022). The magnitude and direction of the bias was assessed and the possible impact on the findings. The impact of the level of bias was explored through undertaking sensitivity analyses ‐ see Sensitivity analysis. If necessary, the original investigators were asked to provide additional information.
Data and analyses
Comparison 1. Lower versus higher cumulative dose dexamethasone regimen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Death or bronchopulmonary dysplasia at 36 weeks PMA | 7 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.86, 1.24] |
| 1.1.1 Moderate versus high cumulative dose regimen | 3 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.95, 1.30] |
| 1.1.2 Low versus moderate cumulative dose regimen | 4 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.50, 1.40] |
| 1.2 Mortality at 36 weeks' PMA | 7 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.29, 1.60] |
| 1.2.1 Moderate versus high cumulative dose regimen | 3 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.34, 3.41] |
| 1.2.2 Low versus moderate cumulative dose regimen | 4 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.11, 1.63] |
| 1.3 Mortality at hospital discharge | 7 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.48, 1.81] |
| 1.3.1 Moderate versus high cumulative dose regimen | 3 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.62, 2.99] |
| 1.3.2 Low versus moderate cumulative dose regimen | 4 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.11, 1.63] |
| 1.4 Bronchopulmonary dysplasia at 36 weeks' PMA | 7 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.91, 1.37] |
| 1.4.1 Moderate versus high cumulative dose regimen | 3 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.93, 1.35] |
| 1.4.2 Low versus moderate cumulative dose regimen | 4 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.65, 1.95] |
| 1.5 Failure to extubate 3 days after initiation | 5 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.98, 1.29] |
| 1.5.1 Moderate versus high cumulative dose regimen | 2 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.93, 1.32] |
| 1.5.2 Low versus moderate dose regimen | 3 | 125 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.93, 1.39] |
| 1.6 Failure to extubate 7 days after initiation | 5 | 210 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [1.08, 1.60] |
| 1.6.1 Moderate versus high cumulative dose regimen | 2 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [1.10, 2.02] |
| 1.6.2 Low versus moderate cumulative dose regimen | 3 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.94, 1.56] |
| 1.7 Days of mechanical ventilation | 6 | 218 | Mean Difference (IV, Fixed, 95% CI) | 4.50 [‐0.68, 9.67] |
| 1.7.1 Moderate versus high cumulative dose regimen | 3 | 112 | Mean Difference (IV, Fixed, 95% CI) | 8.09 [0.21, 15.96] |
| 1.7.2 Low versus moderate cumulative dose regimen | 3 | 106 | Mean Difference (IV, Fixed, 95% CI) | 1.77 [‐5.09, 8.64] |
| 1.8 Days on supplemental oxygen | 2 | 80 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐20.14, 20.74] |
| 1.8.1 Moderate versus high cumulative dose regimen | 1 | 52 | Mean Difference (IV, Fixed, 95% CI) | 8.00 [‐34.64, 50.64] |
| 1.8.2 Low versus moderate cumulative dose regimen | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | ‐2.00 [‐25.29, 21.29] |
| 1.9 Hypertension | 6 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.12, 0.77] |
| 1.9.1 Moderate versus high cumulative dose regimen | 3 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.27 [0.03, 2.34] |
| 1.9.2 Low versus moderate cumulative dose regimen | 3 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.31 [0.11, 0.87] |
| 1.10 Hyperglycemia | 6 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.37, 0.97] |
| 1.10.1 Moderate versus high cumulative dose regimen | 3 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.47, 1.46] |
| 1.10.2 Low versus moderate cumulative dose regimen | 3 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.17, 0.93] |
| 1.11 Open‐label corticosteroids | 7 | 268 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.68, 1.28] |
| 1.11.1 Moderate versus high cumulative dose regimen | 3 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [0.72, 4.13] |
| 1.11.2 Low versus moderate cumulative dose regimen | 4 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.57, 1.14] |
| 1.12 Culture confirmed infection | 7 | 289 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.67, 1.39] |
| 1.12.1 Moderate versus high cumulative dose regimen | 3 | 114 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.71, 2.01] |
| 1.12.2 Low versus moderate cumulative dose regimen | 4 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.46, 1.32] |
| 1.13 Clinical suspected infection | 3 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.62, 1.70] |
| 1.13.1 Moderate versus high cumulative dose regimen | 2 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.71, 2.09] |
| 1.13.2 Low versus moderate cumulative dose regimen | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.32, 2.08] |
| 1.14 Gastrointestinal perforation | 4 | 185 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.13, 6.28] |
| 1.14.1 Moderate versus high cumulative dose regimens | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.14.2 Low versus moderate cumulative dose regimen | 3 | 126 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.13, 6.28] |
| 1.15 Necrotizing enterocolitis | 4 | 198 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.18, 1.56] |
| 1.15.1 Moderate versus high cumulative dose regimen | 2 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.23, 3.19] |
| 1.15.2 Low versus moderate cumulative dose regimen | 2 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.03, 1.97] |
| 1.16 Intraventricular hemorrhage (> grade II) | 3 | 101 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [0.65, 4.37] |
| 1.16.1 Moderate versus high cumulative dose regimen | 2 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [0.49, 5.72] |
| 1.16.2 Low versus moderate cumulative dose regimen | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [0.37, 7.67] |
| 1.17 Periventricular leukomalacia (PVL) | 2 | 121 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.20, 4.39] |
| 1.17.1 Moderate versus high cumulative dose regimens | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.07, 15.77] |
| 1.17.2 Low versus moderate cumulative dose regimen | 1 | 62 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.13, 5.85] |
| 1.18 Severe retinopathy of prematurity | 5 | 176 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.32, 1.28] |
| 1.18.1 Moderate versus high cumulative dose regimen | 2 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.19, 1.92] |
| 1.18.2 Low versus moderate cumulative dose regimen | 3 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.28, 1.57] |
| 1.19 Cerebral palsy in survivors assessed at 1‐3 years | 4 | 149 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.64 [1.02, 6.83] |
| 1.19.1 Moderate versus high cumulative dose regimen | 2 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.85 [1.29, 36.36] |
| 1.19.2 Low versus moderate cumulative dose regimen | 2 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.29, 4.00] |
| 1.20 Death or cerebral palsy at 1‐3 years | 4 | 193 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.74 [0.94, 3.24] |
| 1.20.1 Moderate versus high cumulative dose regimen | 2 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.20 [1.35, 7.58] |
| 1.20.2 Low versus moderate dose regimen | 2 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.28, 2.18] |
| 1.21 Bayley's MDI < 2 SD in survivors assessed | 4 | 147 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.71, 2.92] |
| 1.21.1 Moderate versus high cumulative dose regimen | 2 | 72 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.99 [1.06, 15.08] |
| 1.21.2 Low versus moderate cumulative dose regimen | 2 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.29, 1.83] |
| 1.22 Severe blindness in survivors assessed | 5 | 166 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.17, 2.66] |
| 1.22.1 Moderate versus high cumulative dose regimen | 2 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.44 [0.15, 81.09] |
| 1.22.2 Low versus moderate cumulative dose regimen | 3 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.06, 2.19] |
| 1.23 Abnormal neurodevelopmental outcome in survivors assessed (various definitions) at 1‐3 years | 3 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.22 [0.86, 5.76] |
| 1.23.1 Moderate versus high cumulative dose regimen | 2 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.60 [1.45, 39.78] |
| 1.23.2 Low versus moderate cumulative dose regimen | 1 | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.30 [0.05, 1.97] |
| 1.24 Death or abnormal neurodevelopmental outcome (various definitions) at 1‐3 years | 3 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.86 [0.98, 3.53] |
| 1.24.1 Moderate versus high cumulative dose regimen | 2 | 84 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.41 [1.44, 8.07] |
| 1.24.2 Low versus moderate cumulative dose regimen | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.43 [0.12, 1.51] |
Comparison 2. Later versus earlier initiation of dexamethasone therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Death or bronchopulmonary dysplasia at 36 weeks' PMA | 3 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.87, 1.29] |
| 2.1.1 Moderate early versus early initiation | 3 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.87, 1.29] |
| 2.2 Mortality at 28 days' PNA | 4 | 762 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.69, 1.47] |
| 2.2.1 Late versus moderate early initiation | 1 | 371 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.20 [0.93, 5.23] |
| 2.2.2 Moderate early versus early initiation | 3 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.51, 1.20] |
| 2.3 Mortality at 36 weeks' PMA | 4 | 762 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.68, 1.28] |
| 2.3.1 Late versus moderate early initiation | 1 | 371 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.83, 2.62] |
| 2.3.2 Moderate early versus early initiation | 3 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.49, 1.07] |
| 2.4 Mortality at hospital discharge | 5 | 797 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.75, 1.33] |
| 2.4.1 Late versus moderate early initiation | 1 | 371 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.83, 2.62] |
| 2.4.2 Moderate early versus early initiation | 4 | 426 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.61, 1.18] |
| 2.5 Bronchopulmonary dysplasia at 28 days' PNA | 4 | 762 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [1.02, 1.23] |
| 2.5.1 Late versus moderate early initiation | 1 | 371 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [1.05, 1.26] |
| 2.5.2 Moderate early versus early initiation | 3 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.91, 1.29] |
| 2.6 Bronchopulmonary dysplasia at 36 weeks' PMA | 4 | 762 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.97, 1.28] |
| 2.6.1 Late versus moderate early initiation | 1 | 371 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.88, 1.17] |
| 2.6.2 Moderate early versus early initiation | 3 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.01, 1.90] |
| 2.7 Failure to extubate 3 days after initiation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.7.1 Late versus moderate early initiation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.8 Failure to extubate 7 days after initiation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.8.1 Late versus moderate early initiation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.9 Days of mechanical ventilation | 2 | 60 | Mean Difference (IV, Fixed, 95% CI) | 12.71 [4.44, 20.99] |
| 2.9.1 Moderate early versus early initiation | 2 | 60 | Mean Difference (IV, Fixed, 95% CI) | 12.71 [4.44, 20.99] |
| 2.10 Days of supplemental oxygen | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.10.1 Moderate early versus early initiation | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.11 Hypertension | 4 | 762 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.67, 1.47] |
| 2.11.1 Late versus moderate early initiation | 1 | 371 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.72, 2.36] |
| 2.11.2 Moderate early versus early initiation | 3 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.47, 1.34] |
| 2.12 Hyperglycemia | 4 | 726 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.53, 0.82] |
| 2.12.1 Late versus moderate early initiation | 1 | 371 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.46, 0.95] |
| 2.12.2 Moderate early versus early initiation | 3 | 355 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.51, 0.85] |
| 2.13 Open‐label corticosteroids | 3 | 732 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [1.04, 2.81] |
| 2.13.1 Late versus moderate early initiation | 1 | 371 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.82, 2.31] |
| 2.13.2 Moderate early versus early initiation | 2 | 361 | Risk Ratio (M‐H, Fixed, 95% CI) | 15.31 [0.89, 262.78] |
| 2.14 Culture confirmed infection | 3 | 732 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.68, 0.98] |
| 2.14.1 Late versus moderate early initiation | 1 | 371 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.54, 0.84] |
| 2.14.2 Moderate early versus early initiation | 2 | 361 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.83, 1.63] |
| 2.15 Gastrointestinal hemorrhage | 4 | 762 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.45, 0.97] |
| 2.15.1 Late versus moderate early initiation | 1 | 371 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.38, 0.95] |
| 2.15.2 Moderate early versus early initiation | 3 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.41, 1.71] |
| 2.16 Gastrointestinal perforation | 2 | 315 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.23, 2.40] |
| 2.16.1 Moderate early versus early initiation | 2 | 315 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.23, 2.40] |
| 2.17 Necrotizing enterocolitis | 4 | 725 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.82, 2.55] |
| 2.17.1 Late versus moderate early initiation | 1 | 371 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.59, 5.07] |
| 2.17.2 Moderate early versus early initiation | 3 | 354 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.68, 2.61] |
| 2.18 Patent ductus arteriosus requiring therapy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.18.1 Moderate early versus early initiation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.19 Intraventricular hemorrhage (> grade II) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.19.1 Moderate early versus early initiation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.20 Retinopathy of prematurity (any) | 2 | 324 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.52, 1.23] |
| 2.20.1 Moderate early versus early initiation | 2 | 324 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.52, 1.23] |
| 2.21 Severe retinopathy of prematurity | 3 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.63, 3.53] |
| 2.21.1 Moderate early versus early initiation | 3 | 391 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.63, 3.53] |
| 2.22 Cerebral palsy in survivors assessed | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.22.1 Moderate early versus early initiation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.23 Death or cerebral palsy | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.23.1 Moderate early versus early initiation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.24 Abnormal neurodevelopmental outcome in survivors assessed (various definitions) | 2 | 155 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.66, 1.69] |
| 2.24.1 Moderate early versus early initiation | 2 | 155 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.66, 1.69] |
| 2.25 Death or abnormal neurodevelopmental outcome (various definitions) | 2 | 167 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.63, 1.21] |
| 2.25.1 Moderate early versus early | 2 | 167 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.63, 1.21] |
Comparison 3. Pulse versus continuous dexamethasone therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Death or bronchopulmonary dysplasia at 36 weeks PMA | 2 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.02, 1.88] |
| 3.2 Mortality at 28 days PNA | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.3 Mortality at 36 weeks PMA | 2 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.72, 5.78] |
| 3.4 Mortality at hospital discharge | 2 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.72, 5.78] |
| 3.5 Bronchopulmonary dysplasia at 28 days PNA | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.6 Bronchopulmonary dysplasia at 36 weeks PMA | 2 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.90, 1.83] |
| 3.7 Hypertension | 2 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.20, 1.23] |
| 3.8 Hyperglycemia | 2 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.71, 1.65] |
| 3.9 Open‐label corticosteroids | 2 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.64, 1.47] |
| 3.10 Culture confirmed infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.11 Clinical suspected infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.12 Gastrointestinal hemorrhage | 2 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.25, 1.68] |
| 3.13 Necrotizing enterocolitis | 2 | 160 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.33, 1.83] |
| 3.14 Intraventricular hemorrhage (> grade II) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.15 Retinopathy of prematurity (any) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.16 Severe retinopathy of prematurity | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.17 Abnormal neurodevelopmental outcome in survivors assessed (various definitions) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.18 Death or abnormal neurodevelopmental outcome (various definitions) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 4. Individual tailored versus continuous tapered dexamethasone regimen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Death or bronchopulmonary dysplasia at 36 weeks PMA | 3 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.88, 1.29] |
| 4.2 Mortality at 28 days PNA | 2 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.83 [0.60, 13.32] |
| 4.3 Mortality at 36 weeks PMA | 3 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.63, 3.79] |
| 4.4 Mortality at hospital discharge | 3 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.84 [0.77, 4.37] |
| 4.5 Bronchopulmonary dysplasia at 28 days PNA | 2 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.88, 1.50] |
| 4.6 Bronchopulmonary dysplasia at 36 weeks PMA | 3 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.79, 1.25] |
| 4.7 Failure to extubate 3 days after initiation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.8 Failure to extubate 7 days after initiation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.9 Days of mechanical ventilation | 2 | 90 | Mean Difference (IV, Fixed, 95% CI) | 9.26 [4.32, 14.21] |
| 4.10 Days on supplemental oxygen | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.11 Hypertension | 2 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.01, 8.13] |
| 4.12 Hyperglycemia | 2 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.26, 1.66] |
| 4.13 Open‐label corticosteroids | 2 | 135 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [0.72, 4.13] |
| 4.14 Culture confirmed infection | 2 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.58, 2.76] |
| 4.15 Necrotizing enterocolitis | 2 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.48, 6.35] |
| 4.16 Intraventricular hemorrhage (> grade II) | 3 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.78, 5.18] |
| 4.17 Periventricular leucomalacia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.18 Retinopathy of prematurity (any) | 2 | 98 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.24, 2.92] |
| 4.19 Cerebral palsy in survivors assessed at 1‐3 years | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.20 Death or cerebral palsy at 1‐3 years | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.21 Bayley's MDI < ‐2 SD | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.22 Severe blindness | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4.23 Abnormal neurodevelopmental outcome in survivors assessed (various definitions) | 3 | 143 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.71, 1.70] |
| 4.24 Death or abnormal neurodevelopmental outcome (various definitions) | 3 | 168 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.44 [0.99, 2.07] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barkemeyer 2001.
| Study characteristics | ||
| Methods | Randomized controlled trial investigating a pulse‐dosage versus continuous‐dosage regimen. | |
| Participants | Infants were eligible for enrollment with birth weight < 1500 grams, a history of respiratory distress syndrome, and ventilator dependence at 7 to 21 days of life. Infants were excluded if significant anomalies of cardiac or respiratory systems, or clinically significant patent ductus arteriosus at time of enrollment. |
|
| Interventions | The infants were randomly assigned to 1 of 2 regimens.
All administrations were in 2 divided doses. |
|
| Outcomes | Primary endpoint of the study was survival of 36 weeks' PMA without the need for supplemental oxygen. Secondary endpoints included survival, days of mechanical ventilation, days of supplemental oxygen, and length of hospital stay. Potential side effects were evaluated included hyperglycemia, hypertension, infection, left ventricular hypertrophy, necrotizing enterocolitis, gastritis, abnormal head ultrasound, retinopathy of prematurity, growth delay, and leucocytosis. No long‐term neurodevelopmental outcomes were assessed (personal communication). | |
| Notes | Funding: Partial funding by a grant from the APS‐SPR Multicenter Clinical Trials Program. Declarations of interest: not reported. Trial was only published as abstract, but the original author provided unpublished manuscript with additional data on secondary outcomes. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer random number generator. |
| Allocation concealment (selection bias) | Low risk | Centralized random number generator program. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Only the pharmacists at the participating centers were aware of the randomization assignments, caregivers and parents were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Attending physicians were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis with no missing data. |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes were mentioned in the manuscript. |
| Other bias | Low risk | No concerns of other biases. |
Bloomfield 1998.
| Study characteristics | ||
| Methods | Randomized controlled trial comparing a pulse course against high‐dosage regimen dexamethasone. | |
| Participants | Infants with a birth weight ≤ 1250 grams, and ventilated at ≥ 15 cycles/min at 7 days of age. Infants with major congenital malformations or who were ventilated for surgical reasons were excluded. |
|
| Interventions | The infants were randomly assigned to 1 of 2 regimens.
The initial dosage administration of 0.5 mg/kg/day was in 2 divided doses. |
|
| Outcomes | The primary outcome was linear growth, measured as weight gain, crown‐heel length, and head circumference. Secondary outcomes were hypertension, hyperglycemia requiring insulin therapy, necrotizing enterocolitis, retinopathy of prematurity, proven infections, myocardial hypertrophy, supplemental oxygen at 28 days' PNA and 36 weeks' PMA, BPD at 28 days' PNA and 36 weeks' PMA. In addition, a Synacthen test was performed 1 week after discontinuation of the dexamethasone. The long‐term follow‐up manuscript reported on neurodevelopmental outcome with an extended inclusion rate. Infants were classified into 1 of 4 outcome categories defined and modified from Kitchen 1987. |
|
| Notes | Funding: no statement provided. Declarations of interest: not reported. Original authors provided additional data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By computer randomization. |
| Allocation concealment (selection bias) | Low risk | By computer randomization, no additional details. Randomizaton was balanced in blocks of 6 and stratified by sex and birth weight. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis. 1 infant was found to have a birth weight of > 1250 grams. 3 infants were lost to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes were mentioned in the manuscript. |
| Other bias | Unclear risk | No concerns of other biases,. |
Cummings 1989.
| Study characteristics | ||
| Methods | Single center, randomized, double‐blind, placebo‐controlled study investigating a moderate dosage versus a high dosage of dexamethasone. | |
| Participants | Preterm infants with a birth weight ≤ 1250 grams, a gestational age of ≤ 30 weeks, and a postnatal age of more than 14 days. All infants were ventilated with a rate of at least 15 cycles per minute and received more than 30% oxygen. Attempts to wean these settings failed over a period of at least 72 hours. Infants with a symptomatic PDA, renal failure or sepsis at entry were excluded. |
|
| Interventions | The included infants were randomly assigned to 1 of 3 dosage regimens.
Medication was given intravenously and divided into 2 dosages per day. Each infant received the same volume of medication by using different concentrations of dexamethasone. Infants in the low‐dosage regimen group received additional saline injections to complete the 42‐day course. The placebo group was excluded for the purpose of this review. No treatment with corticosteroids outside the protocol was allowed. |
|
| Outcomes | The primary outcomes were mortality, duration of mechanical ventilation and duration of oxygen dependence. Secondary outcomes were the duration of hospitalizations, ROP, bloody gastric aspirates, number of transfusions, and occurrence of clinically suspected sepsis, hypertension, hyperglycemia and hypertriglyceridemia. Growth and neurodevelopment (abnormal neurological outcome and the Bayley Scales of Infant Development) were assessed at 6 and 15 months of age corrected for prematurity. Normal neurodevelopmental outcome was defined as having Bayley Mental and Psychomotor Indexes of more than 84 and normal neurological findings (not specified). Further follow‐up studies were done at 4 years and 15 years. Neurological exams and the cognitive function using the McCarthy Scales of Children's Abilities were assessed at the age of 4, whereas at 15 years neurological examination, IQ and the need for specialized education was assessed. |
|
| Notes | Funding: none stated. Declarations of interest: not reported. The original investigator provided additional data on duration of mechanical ventilation, failure to extubate on day 7 and the total number of patients with a Bayley MDI < 2 SD. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sequential assignment by random number table. |
| Allocation concealment (selection bias) | Low risk | Performed by a pharmacist unaware of the clinical status of the infant. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Individual daily doses were drawn from a specific vial designated for that treatment day, ensuring the same volume of study medication every day. Infants in the moderate‐dosage regimen received placebo saline for the remaining 24 days. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All members of the medical team, including the investigators, remained blinded to group assignment throughout the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized infants were evaluated and no missing data. |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes were mentioned in the manuscript. |
| Other bias | Unclear risk | No concerns of other biases. |
Da Silva 2002.
| Study characteristics | ||
| Methods | Single center double‐blind randomized trial on moderate‐ versus low‐dosage regimen of dexamethasone. | |
| Participants | Extremely low birth weight infants (≤ 1500 grams), initial starting administration between 7 and 21 days. | |
| Interventions | The included infants were randomly assigned to 1 of 2 dosage regimens.
|
|
| Outcomes | Primary outcomes were growth parameters (weight, length and head circumference) at 36 weeks' corrected gestational age. Secondary outcomes were documented sepsis and long‐term growth parameters at 9 months of corrected age (actual numbers not provided). | |
| Notes | Funding: no statement. Declarations of interest: not reported. Trial was only published as an abstract and original authors could not provide any additional data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described in abstract. |
| Allocation concealment (selection bias) | Unclear risk | Not described in abstract. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Stated in the abstract as being double blinded, actual procedure not described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Stated in the abstract as being double blinded, actual procedure not described. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unknown. |
| Selective reporting (reporting bias) | Unclear risk | Unknown. |
| Other bias | Unclear risk | Unknown. |
DeMartini 1999.
| Study characteristics | ||
| Methods | Single center randomized controlled trial. | |
| Participants | Intubated preterm infants | |
| Interventions | The infants were randomly assigned to 1 of 2 dosage regimens.
All medication was given divided into 2 dosages per day. No patients were treated with any corticosteroids outside the study protocol. |
|
| Outcomes | The primary outcomes were mortality, duration of mechanical ventilation and duration of oxygen dependence. Secondary outcomes were the occurrence of clinically suspected sepsis, NEC, hypertension, hyperglycemia and hypertriglyceridemia. No long‐term follow‐up was performed. |
|
| Notes | Funding: no statement. Declarations of interest: not reported. Only published as abstract. The original investigator provided data on the incidence of BPD, defined as oxygen dependence at 36 weeks' PMA, combined with mortality at 36 weeks. No long‐term follow‐up performed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information. |
| Allocation concealment (selection bias) | Low risk | By personal communication, no information on the methods. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | By personal communication. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | By personal communication. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not specified in the abstract. |
| Selective reporting (reporting bias) | Unclear risk | Unknown due to abstract form. |
| Other bias | Unclear risk | Unknown due to abstract form. |
Durand 2002.
| Study characteristics | ||
| Methods | Single center randomized controlled trial. | |
| Participants | Infants were included when having a birth weight between 501 and 1500 grams, a gestational age between 24 weeks and 32 weeks, postnatal age between 7 and 14 days and at entry on ventilation support with a rate of 15 cycles per minute or more, and 30% supplemental oxygen or more to maintain a pulse oxymeter oxygen saturation of 90% or higher, despite weaning trials. Infants were excluded from the randomization if they had multiple congenital anomalies or chromosomal abnormalities, systemic hypertension, congenital heart disease, IVH grade IV, renal failure or sepsis at entry. |
|
| Interventions | The included infants were randomly assigned to 1 of 2 dosage regimens.
All medication was given divided into 2 dosages per day. Administration of open‐label dexamethasone was allowed after the study period at the discretion of the attending neonatologist. |
|
| Outcomes | The primary outcomes were the dynamic respiratory mechanics, measured before and on days 2, 5 and 7 of dexamethasone therapy. Secondary outcomes were ventilator settings, occurrence of CLD, defined as dependence on oxygen supplementation at 36 weeks' PMA, survival without CLD, duration of mechanical ventilation, duration of hospitalizations, hyperglycemia, hypertension, ROP, NEC, spontaneous GI perforation, sepsis and pulmonary air leaks. |
|
| Notes | Funding: no statement. Declarations of interest: not reported. Data of the long‐term follow‐up were retrieved from the original investigator. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blind drawing of random cards. |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | An outside investigator blinded to the group assignment evaluated the dynamic pulmonary mechanics and graphics. However, assessment of clinical diagnosis was not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of the 59 infants eligible, 7 parents were unavailable and 5 parents refused. 1 included participant had a few doses of dexamethasone withheld because of suspected infection. |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes were mentioned in the manuscript. |
| Other bias | Unclear risk | No concerns of other biases. |
Groneck 1993.
| Study characteristics | ||
| Methods | Single center non‐blinded randomized controlled trial investigating moderately early versus late administration of systemic dexamethasone | |
| Participants | Inclusion criteria were as follows: 1. Infants were ventilator dependent at postnatal age of 10 days; 2. Had either a fraction of inspired oxygen ≥ 0.3 and/or peak inspiratory pressure ≥ 16 cmH2O; and 3. No radiological evidence of pneumonia and no clinical or laboratory signs of local or systemic infection. | |
| Interventions | Eligible infants meeting the inclusion criteria were randomly assigned to treatment with dexamethasone on day 10 of life or day 16 of life. Dexamethasone was given intravenously every 12 hours in divided doses during 28 days. The dosage tapered schedule was 0.5 mg/kg/day for 3 days, 0.3 mg/kg/day for 3 days, a 10% decrease every 3 days until 0.1 mg/kg/day until the dose of 0.1 mg was reached on day 24, which was given every second day until day 28. | |
| Outcomes | The primary objective of this study was to investigate the effects of dexamethasone on inflammatory indicators in tracheobronchial aspirate fluid such as leukotriene B4, interleukin‐1, elastase, and albumin. | |
| Notes | Funding: Supported by a grant of the Deutsche Forschungsgemeinschaft (Sp 239/3‐1). Declarations of interest: not reported. No clinical data were reported on the outcomes of the participants. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information about sequence generation in manuscript. |
| Allocation concealment (selection bias) | Unclear risk | No methods of randomization described in the manuscript. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding of the personnel for treatment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No blinding of outcome assessment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No clinical outcomes reported |
| Selective reporting (reporting bias) | Unclear risk | No clinical outcomes reported |
| Other bias | Low risk | No signs of other bias. |
Halliday 2001a.
| Study characteristics | ||
| Methods | Multicenter partly double‐blinded randomized controlled trial with a factorial design investigating early versus late administration of inhaled and systemic dexamethasone. | |
| Participants | Intubated infants < 30 weeks' gestational age, a postnatal age < 72 hours and with an inspired oxygen concentration > 30%. Infants with a gestational age between 30 and 31 weeks could be included if needing inspired oxygen > 50%. Infants with lethal congenital anomalies, severe IVH > III, and proven infections were excluded. When strong suspicion of infection, hypertension or hyperglycemia, inclusion was postponed until resolved. |
|
| Interventions | Eligible infants were randomized in 1 of 4 arms, of which 2 contained inhaled corticosteroids. These infants were excluded from this review. The remaining infants were randomized into 1 of 2 arms.
All medication was given divided into 2 dosages per day. |
|
| Outcomes | Primary outcome was death or oxygen dependency at 36 weeks' PMA. Secondary outcomes were death or major cerebral abnormality, death or oxygen dependency at 28 days and expected date of delivery, duration of > 40% oxygen, duration of any oxygen, duration of mechanical ventilation, and duration of hospital stay. Furthermore, complications such as pneumothorax, necrotizing enterocolitis, hypertension, hyperglycemia, sepsis, pneumonia, persistent ductus arteriosus requiring therapy, pulmonary hemorrhage, seizures, recurrent apnea, retinopathy of prematurity, gastric hemorrhage, gastrointestinal perforation were reported. The follow‐up manuscript reported on neurodevelopmental outcome at 7 years of age, including level of disability, cerebral palsy, cognitive ability using the British Ability Scales (BAS 2nd edition), behavioral difficulties using the Strengths and Difficulties Questionnaire (SDQ), competencies using the Child Behavior Checklist for Children, growth, and respiratory symptoms. Impairment was defined as BAS cluster score < 10th percentile, weight or height < 2nd percentile, head circumference < 2nd or > 98th percentile, seizures, borderline SDQ total difficulties score (14 to 16), strabismus, or nystagmus. |
|
| Notes | Funding: The study was supported by a grant from Action Research, United Kingdom, Trudell Medical London, Ontario, Canada and Astra Draco, Lund, Sweden. Declarations of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not mentioned. |
| Allocation concealment (selection bias) | Low risk | Supervising clinician telephoned the randomization center in Belfast. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Of the 47 participating NICUs, 11 conducted a double‐blinded study. In the remaining centers the design was open because some clinicians wanted to prescribe broad spectrum antibiotics or H2 blockers, or both. In the 11 double‐blinded centers intravenous saline was given. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | See above. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analyses were on intention‐to‐treat analyses. 5 infants allocated to early treatment were not treated within 5 days, whereas 10 infants allocated to the moderately early period were treated before the 10th day. 2 infants were given the wrong drug. |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes were mentioned in the manuscript. |
| Other bias | Unclear risk | A large proportion of the total included infants randomized to delayed selective treatment either died or did not fulfill the entry criteria. |
Hingre 1992.
| Study characteristics | ||
| Methods | Single center randomized placebo controlled trial | |
| Participants | 35 infants born between April 1989 and April 1991 with a birth weight < 1000 gram, ventilator dependent(IMV > 15) and oxygen (FiO2> 0.30) on the 4th day of life. | |
| Interventions | All infants received dexamethasone intravenously 0.5 mg/kg/day for 3 days, followed by a tapering schedule for 39 days. Infants were randomized to an early group (Day 5) or a late group (Day 14) of life. | |
| Outcomes | Outcomes reported were mortality, mechanical ventilation, supplemental oxygen, length of hospital stay, and neurologic abnormalities at 6 months adjusted age (not specified). | |
| Notes | Funding: No statement. Declarations of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information in the abstract |
| Allocation concealment (selection bias) | Unclear risk | No information in the abstract |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Although stated as a placebo‐controlled trial, no information was given on how the groups were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Although stated as a placebo‐controlled trial, no information was given on how the groups were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Five infants randomized in the late group died and were excluded in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | No information in the abstract |
| Other bias | Unclear risk | No statement in abstract on funding of the study |
Malloy 2005.
| Study characteristics | ||
| Methods | Single center, randomized double‐blinded controlled trial | |
| Participants | 17 infants of birth weight < 1500 grams and gestational age of 34 weeks, randomized before the 28th day. | |
| Interventions | The included infants were randomly assigned to 1 of 2 dosage regimens.
|
|
| Outcomes | Clinical outcomes on the already included patients were mortality on discharge, duration of mechanical ventilation and oxygen dependence, survival without CLD, retreatment with dexamethasone, and number of days on oxygen supplementation, number of hospital days, IVH, NEC, gastrointestinal perforation, ROP requiring laser photocoagulation, hypertension, and hyperglycemia. Long‐term follow‐up was performed through 3 years of age and neurodevelopmental status was assessed by using the modified Gesell Developmental Appraisal. |
|
| Notes | Funding: no statement. Declarations of interest: not reported. Additional data on failure to extubate on day 3, days on mechanical ventilation and blindness or poor vision were retrieved from the original investigator. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By personal communication, method not specified. |
| Allocation concealment (selection bias) | Low risk | By personal communication, method not specified. Infants were stratified into 3 groups according to birth weight. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Only study pharmacist, with no clinical involvement, was aware of doses administered. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 1 infant in the high‐dose group died on the 2nd day, whereas an infant in the low‐dose died at 4 months of age (1 month after hospital discharge). These infants were included in the analyses of the review. 2 infants in the moderate allocation group were withdrawn from the study on the 6th day of study medication. |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes were mentioned in the manuscript. |
| Other bias | Unclear risk | This study was terminated prematurely due to the 2002 statement from the American Academy of Pediatrics and the Canadian Paediatric Society. |
Marr 2019.
| Study characteristics | ||
| Methods | Single center randomized controlled trial | |
| Participants | Infants were eligible for study if they were born at a gestation between 24 and 27 weeks and were between 10 and 21 days after birth. Further inclusion criteria were radiographic findings consistent with the diagnosis of evolving BPD with ventilator support with sustained (≥ 18 hours) FiO2 ≥ 60% and mean airway pressure ≥ 8 cm H2O. Infants were excluded in case of a birthweight or head circumference < 10th percentile for gestational age, chromosomal anomalies and congenital heart disease, grade IV intracranial hemorrhage, a low 5‐minute Apgar score < 3, or a history of seizures or base deficit of > 15. Infants with sepsis or significant patent ductus arteriosus became study eligible if these issues were treated before the end of the enrollment window. | |
| Interventions | The included infants were randomly assigned to 1 of 2 dosage regimens.
|
|
| Outcomes | Primary outcome was intact survival at 7 years of age, defined as survival without severe neurologic, cognitive, or academic handicap (normal neurologic examination, IQ > 70, and receiving education in a regular classroom without an individualized education program). Secondary outcomes were duration of mechanical ventilation, supplemental oxygen requirement at 36 weeks of corrected age (BPD), feeding tolerance, transfusion exposure, sepsis, length of initial hospitalization, rates of re‐hospitalization, and growth at 7 years of age. | |
| Notes | Funding: no statement. Declarations of interest: no conflict of interest declared. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Infants were assigned randomly by computerized allocation sequence. |
| Allocation concealment (selection bias) | Low risk | An individual not involved with the study generated the random allocation sequence. Access to this sequence and all protocol assignments was limited to 2 study pharmacists. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | All investigators and care givers remained blinded to treatment group. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All developmental testing was carried out by examiners blinded to infant treatment group. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All randomized infants were evaluated and no missing data |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes were mentioned in the manuscript. |
| Other bias | Unclear risk | No concerns of other biases. |
McEvoy 2004.
| Study characteristics | ||
| Methods | Single center randomized controlled trial | |
| Participants | Infants were included when between 7 and 21 days of postnatal age, with a birth weight of > 501 grams and < 1500 grams, a gestational age of > 24 weeks and < 32 weeks. The infants were dependent on ventilation support with 15 cycles per minute or more and oxygen levels of 30% or more at entry. Infants with multiple congenital anomalies, systemic hypertension, congenital heart disease, IVH grade IV, renal failure, and sepsis at entry were excluded. |
|
| Interventions | The included infants were randomly assigned to 1 of 2 dosage regimens.
All medication was given divided into 2 dosages per day. The use of open‐label dexamethasone therapy was discouraged, but could be administered at the discretion of the attending neonatologist. |
|
| Outcomes | The primary outcomes were the functional residual capacity and passive respiratory compliance before and during the 7‐day therapy. Secondary outcome measurements were the ventilator settings, the duration of mechanical ventilation, the duration of hospitalizations, CLD (defined as oxygen dependence at 36 weeks' PMA), survival without CLD, PDA, hyperglycemia, hypertension, IVH, periventricular leukomalacia, ROP, NEC, spontaneous GI perforation, sepsis, pulmonary air leaks. At 1 year of corrected age the infants were assessed for early neurodevelopmental follow‐up (cerebral palsy and Bayley Scales of Infant Development) by a developmental pediatrician, a pediatric neurologist and specialized personnel. Cerebral palsy was defined as non‐progressive motor impairment characterized by abnormal muscle tone and decreased range/control of movements. Severe cognitive delay was defined as lower than 70 on the mental developmental index (MDI) score. |
|
| Notes | Funding: American Lung Association. Declarations of interest: not reported. Additional data on duration of mechanical ventilation, failure to extubate on day 3 and 7, were retrieved from the original investigator. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Group assignment was done by the pharmacy using a randomization table. |
| Allocation concealment (selection bias) | Low risk | Investigators and clinical staff was unaware of treatment allocation, because a staff pharmacist was in charge of randomization and study drug preparation. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Although method not specified in manuscripts. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Although method not specified in manuscripts. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | In 3 patients of the high‐dose group, 1 dose of dexamethasone was withheld due to blood in the gastric tube or hypertension. For 1 patient of the low dose group, a dose was inadvertently not given. 66% of the survivors were assessed for follow‐up. No statement on the influence on the neurodevelopmental outcome. |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes were mentioned in the manuscript. |
| Other bias | Low risk | No concerns of other biases. |
Merz 1999.
| Study characteristics | ||
| Methods | Single center randomized controlled study investigating moderately early versus late administration of dexamethasone. | |
| Participants | Infants with birth weight ≤ 1250 grams, gestational age between 24 and 30 weeks, ventilator dependent at 7 days of age with rate ≥ 15 cycles/min and oxygen requirement 25%. Infants with sepsis, multiple or severe congenital anomalies or evidence of hypertension were excluded. |
|
| Interventions | The included infants were randomly assigned to 1 of 2 regimens.
Both arms received a starting dose of 0.5 mg/kg/day for 3 days, followed by 0.3 days for 3 days, followed by 0.1 mg/kg/day, and followed by this dose alternatively every 2nd day until day 16. All medication was given divided into 2 dosages per day. |
|
| Outcomes | The primary outcome was the time of extubation. Secondary outcomes were duration of supplemental oxygen, the incidence of BPD at 28 days' PNA and pulmonary function tests. Side effects were collected including sepsis, hypertension, hyperglycemia, and adrenal suppression. | |
| Notes | Funding: no statement. Declarations of interest: not reported. Original investigator was not able to provide additional data. No long‐term follow‐up was performed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomization list was provided by the department of medical statistics. |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes with information on timing of initiation were drawn after informed consent by opening the envelope with the lowest number. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No masked intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No masked intervention. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In addition to predefined outcomes, data on necrotizing enterocolitis and gastrointestinal perforation were collected. |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes were mentioned in the manuscript. |
| Other bias | Unclear risk | No concerns of other biases. |
Odd 2004.
| Study characteristics | ||
| Methods | Single center randomized controlled trial investigating a continuous dosage regimen versus an individualized course tailored to the infants' respiratory status. | |
| Participants | Infants ≤ 1250 grams, ventilated between postnatal age of 7 days and 28 days for which dexamethasone was indicated. Infants with congenital anomalies and surgical problems were excluded. |
|
| Interventions | The included infants were randomly assigned to 1 of 2 regimens.
|
|
| Outcomes | The primary outcome was linear growth, measured by knemometry, weight, crown‐heel length, and head circumference. Secondary outcomes were hypertension, myocardial hypertrophy, respiratory status (mode, peak inspiratory pressure, and end expiratory pressure and FiO₂ at enrolment, study days 14, 42, 28 days' postnatal age and 36 weeks' corrected gestational age, hyperglycemia requiring insulin therapy, renal and cranial ultrasounds, proven and suspected infections. In addition a Synacthen test was performed 1 week after discontinuation of the dexamethasone. The long‐term neurodevelopmental outcome were assessed at 9 and 18 months using the Bayley Scales of Infant Development II. Infants were classified into 1 of 4 outcome categories defined and modified from Kitchen 1987. |
|
| Notes | Funding: no statement. Declarations of interest: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | By computer generated random numbers. |
| Allocation concealment (selection bias) | Low risk | Stratified by sex and birth weight. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Parents and personnel were aware of the allocation of the patient. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Clinical outcome assessment was not blinded, although the primary outcome was (knemometry), as well as ultrasounds performed by staff unaware of treatment allocation. The developmental psychologist was also unaware of the treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In 1 infant in the individual group, the dexamethasone treatment was stopped on day 10. Intention‐to‐treat analyses were performed. |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes were mentioned in the manuscript. |
| Other bias | Unclear risk | No concerns of other biases. |
Papile 1998.
| Study characteristics | ||
| Methods | Multicenter double‐blinded randomized controlled trial investigating dexamethasone therapy initiated moderately early versus late. | |
| Participants | Ventilator‐dependent infants with birth weight 501 to 1500 grams, at a postnatal age between 13 and 15 days, with a respiratory index of ≥ 2.4. Infants who received glucocorticoid therapy after birth, had proven or suspected sepsis, or congenital anomaly of cardiovascular, pulmonary, or central nervous system were excluded. |
|
| Interventions | The included infants were randomly assigned to 1 of 2 regimens.
Both dexamethasone regimens started with 0.5 mg/kg/day (divided in 2 doses) for 5 days, followed by 0.15 mg/kg, 0.07 mg/kg, and 0.03 mg/kg for 3 days each. |
|
| Outcomes | Primary outcome was the number of days from randomization to ventilator independence. Secondary outcomes were death before hospital discharge, duration of assisted ventilation, supplemental oxygen, and hospital stay, BPD at 36 weeks, hyperglycemia, hypertension, changes in weight and head circumference, proven sepsis, necrotizing enterocolitis, and gastric hemorrhage. | |
| Notes | Funding: National Institute of Child Health and Human Development and by the General Clinical Research Center grants. Dexamethasone was provided by Merck Sharp & Dohme. Declarations of interest: not reported. No long‐term follow‐up was performed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An order form was sent to each center's pharmacy, where the infants were randomly assigned to 1 of 2 treatment groups. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | To blind clinical staff, different volumes of placebo were prepared to match the various doses of dexamethasone. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 infants did not receive any of the assigned treatments. Of the 173 infants in the late dexamethasone group who were alive on treatment day 14, 31 did not meet the criteria for starting dexamethasone treatment. Results were analyzed on intention‐to‐treat method. |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes were mentioned in the manuscript. |
| Other bias | Low risk | Funded by National Institute of Child Health and Human Development and by the General Clinical Research Center grants. Dexamethasone was provided by Merck Sharp & Dohme. |
Ramanathan 1994.
| Study characteristics | ||
| Methods | Single center randomized controlled trial | |
| Participants | 28 infants of birth weight between 520 and 1440 grams and gestational age of 27 weeks. | |
| Interventions | The included infants were randomly assigned at 10 to 14 days of age to 1 of 2 dosage regimens.
|
|
| Outcomes | Clinical outcomes were mortality on discharge, duration of mechanical ventilation and oxygen dependence, survival without CLD, retreatment with dexamethasone, ROP > stage II, sepsis and hyperglycemia. | |
| Notes | Funding: no statement. Declarations of interest: not reported. Trial only in abstract form. No long‐term follow‐up was reported and no additional data were retrieved from the original authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information in the abstract. |
| Allocation concealment (selection bias) | Unclear risk | No information in the abstract. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information in the abstract. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information in the abstract. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information in the abstract. |
| Selective reporting (reporting bias) | Unclear risk | No information in the abstract. |
| Other bias | Unclear risk | No information in the abstract. |
BPD = bronchopulmonary dysplasia
CLD = chronic lung disease
FiO2 = fractional inspired oxygen
GI = gastrointestinal
IVH = intraventricular hemorrhage
NEC = necrotizing enterocolitis
NICU = neonatal intensive care unit
PMA = postmenstrual age
PNA = postnatal age
ROP = retinopathy of prematurity
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahrens 2000 | Trial registration only, no results or report available |
| DeCastro 2009 | Retrospective cohort study, no randomized controlled trial |
| Romagnoli 1999 | Two separate placebo controlled studies |
| Shipalana 1994 | Placebo controlled study |
| Singh 2022 | Ineligible study design, web‐based survey |
Characteristics of studies awaiting classification [ordered by study ID]
IRCT20200721048155N1.
| Methods | Single center randomized controlled study, single blinded |
| Participants | This study will be conducted on 30 preterm neonates with gestational age of 37 weeks or lower hospitalized in the NICU ward of Ghaem hospital due to respiratory distress syndrome who cannot be disconnected from ventilator any longer than 14 days. |
| Interventions | Both groups receive the same treatment but the intervention groups, in addition to intravenous dexamethasone, receives hydrocortisone. Patient are unaware of groupings. Intravenous dexamethasone 24 hours before until 48 hours after extubation with the dose of 0.5 mg/kg /day will be administered. In addition, hydrocortisone will be used for 5 days where in the first 3 days, it is administered twice daily with the dose of 0.5 mg/kg and in the next 2 days, once daily with the dose of 0.5 mg/kg. |
| Outcomes | Status of need for mechanical ventilation; duration of connection to the ventilation device |
| Notes | Current status: recruitment completed, no publication identified yet |
Characteristics of ongoing studies [ordered by study ID]
IRCT20201222049802N3.
| Study name | Comparative study of the effect of dexamethasone and injectable hydrocortisone in reducing the need for oxygen in preterm infants |
| Methods | Double‐blind randomized controlled trial |
| Participants | Fetal age less than 33 weeks, the risk of bronchopulmonary dysplasia more than 60% according to the National Institute of Child Health and Human Development (NICHD) definition, parental consent, having received antenatal corticosteroid |
| Interventions | Dexamethasone group: Infants of the dexamethasone group will receive dexamethasone injection from day 14 of birth (0.2 mg per kg body weight for the first 3 days and 0.1 mg per kg body weight for the next 4 days). Hydrocortisone group: Infants of the hydrocortisone group will receive hydrocortisone injection from the 14th day of birth (1 mg per kg of body weight for 7 days). |
| Outcomes | Oxygen saturation. Time point: day 28 after birth. Method of measurement: pulse oximeter. |
| Starting date | 2021‐09‐22 |
| Contact information | Asghar Marzban Address: Ayatollah Mousavi Hospital, Gavazang Road, Above Shahid Sabouti Boulevard, Zanjan, Iran. 4513956183 Telephone: +98 24 3342 0651 Email: Drmarzban@zums.ac.ir Affiliation: Zanjan University of Medical Sciences |
| Notes |
Differences between protocol and review
In this update, we included Marr 2019, a conference abstract placed in Awaiting Assessment in the previous version of this review (Onland 2017). Full data for Marr 2019 allowed us to assess risk of bias and include the study in this update.
In this update, we included an additional comparison, individually tailored regimen, for Bloomfield 1998.
In this update, we assessed the quality of evidence for the main comparisons at the outcome level using the GRADE approach.
Contributions of authors
WO has full access to all of the data in the study and will take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: WO, AvK. Acquisition of data: WO, MvdL Analysis and interpretation of data: WO, MvdL, MO, AvK. Drafting of the manuscript: WO. Critical revision of the manuscript for important intellectual content: WO, MvdL, MO, AvK. Statistical analysis: WO, MvdL. Study supervision: MO, AvK.
Sources of support
Internal sources
No sources of support provided
External sources
-
Vermont Oxford Network, USA
Cochrane Neonatal Reviews are produced with support from Vermont Oxford Network, a worldwide collaboration of health professionals dedicated to providing evidence‐based care of the highest quality for newborn infants and their families
Declarations of interest
WO: No financial disclosure to be declared. WO is co‐author of the Dutch national guideline on prevention and treatment of bronchopulmonary dysplasia, no other potential conflicts of interest are known.
MvdL is project manager for an overview of corticosteroids systematic reviews for bronchopulmonary dysplasia in preterm infants, but receives no financial or other support. MvdL is leading author of the Dutch national guideline on prevention and treatment of bronchopulmonary dysplasia, no other potential conflicts of interest known.
MO: No financial disclosure to be declared. No potential conflicts of interest known.
AvK: No financial disclosure to be declared. AvK is co‐author of the Dutch national guideline on prevention and treatment of bronchopulmonary dysplasia, no potential conflicts of interest known.
Edited (no change to conclusions)
References
References to studies included in this review
Barkemeyer 2001 {published data only}
- Barkemeyer BM, Davey A, Cummings JJ, Pappagallo M, Durand M, Stevens D, et al. Pulse vs. continuous dexamethasone therapy for neonatal chronic lung disease (CLD) in very low birthweight (VLBW) infants. Pediatric Research 2001;47(4):276A. [Google Scholar]
Bloomfield 1998 {published data only}
- Armstrong D, Bloomfield FH, Penrice J, Dezoete A, Knight D, Harding JE. A randomized trial of two courses of dexamethasone for preterm babies at risk of chronic lung disease: follow up at 18 months. Early Human Development 2000;60(1):51-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong DL, Penrice J, Bloomfield FH, Knight DB, Dezoete JA, Harding JE. Follow up of a randomised trial of two different courses of dexamethasone for preterm babies at risk of chronic lung disease. Archives of Disease in Childhood. Fetal and Neonatal Edition 2002;86(2):F102-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield FH, Knight DB, Harding JE. Side effects of 2 different dexamethasone courses for preterm infants at risk of chronic lung disease: a randomized trial. Journal of Pediatrics 1998;133(3):395-400. [PMID: ] [DOI] [PubMed] [Google Scholar]
Cummings 1989 {published data only}
- Cummings J, Slagle T, D'Eugenio D, Gross S. Controlled trial of dexamethasone (DEX) to prevent bronchopulmonary dysplasia (BPD) in ventilator-dependent preterm infants. In: Pediatric Research. Vol. 23. 1988:405A.
- Cummings JJ, D'Eugenio DB, Gross SJ. A controlled trial of dexamethasone in preterm infants at high risk for bronchopulmonary dysplasia. New England Journal of Medicine 1989;320(23):1505-10. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Gross SJ, Anbar RD, Mettelman BB. Follow-up at 15 years of preterm infants from a controlled trial of moderately early dexamethasone for the prevention of chronic lung disease. Pediatrics 2005;115(3):681-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Gross SJ, Cummings JJ. Four year follow-up of a controlled trial of dexamethasone (DEX) in ventilator dependent preterm infants. Pediatric Research 1994;35(4):204A. [Google Scholar]
Da Silva 2002 {published data only}
- da Silva OP, Kumaran VS, Knoppert DC. Randomized controlled trial comparing two regimens of dexamethasone in the neonate with chronic lung disease. In: Pediatric Research. Vol. 53. 2002:369A.
DeMartini 1999 {published data only}
- DeMartini TJ, Muraskas JK. Pulse versus tapered dosing dexamethasone for evolving bronchopulmonary dysplasia (BPD). Pediatric Research 1999;45(4):300A. [Google Scholar]
Durand 2002 {published data only}
- Durand M, Mendoza ME, Tantivit P, Kugelman A, McEvoy C. A randomized trial of moderately early low-dose dexamethasone therapy in very low birth weight infants: dynamic pulmonary mechanics, oxygenation, and ventilation. Pediatrics 2002;109(2):262-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Groneck 1993 {published data only}
- Groneck P, Oppermann M, Speer CP. Levels of complement anaphylatoxin C5a in pulmonary effluent fluid of infants at risk for chronic lung disease and effects of dexamethasone treatment. Pediatric Research 1993;34(5):586-90. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Groneck P, Reuss D, Gotze-Speer B, Speer CP. Effects of dexamethasone on chemotactic activity and inflammatory mediators in tracheobronchial aspirates of preterm infants at risk for chronic lung disease. Journal of Pediatrics 1993;122(6):938-44. [PMID: ] [DOI] [PubMed] [Google Scholar]
Halliday 2001a {published data only}
- Halliday HL, Patterson CC, Halahakoon CW. A multicenter, randomized open study of early corticosteroid treatment (OSECT) in preterm infants with respiratory illness: comparison of early and late treatment and of dexamethasone and inhaled budesonide. Pediatrics 2001;107(2):232-40. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Wilson TT, Waters L, Patterson CC, McCusker CG, Rooney NM, Marlow N, et al. Neurodevelopmental and respiratory follow-up results at 7 years for children from the United Kingdom and Ireland enrolled in a randomized trial of early and late postnatal corticosteroid treatment, systemic and inhaled (the Open Study of Early Corticosteroid Treatment). Pediatrics 2006;117(6):2196-205. [PMID: ] [DOI] [PubMed] [Google Scholar]
Hingre 1992 {published data only}
- Hingre RV, Richman RA, Gross S. Comparative efficacy of early (day 4) versus late (day 14) dexanethasone (DEX) therapy in ventilator. Pediatric Research 1992;31:310A. [Google Scholar]
Malloy 2005 {published data only}
- Malloy CA, Hilal K, Weiss MG, Rizvi Z, Muraskas JK. A prospective, randomized, double-masked trial comparing low dose to conventional dose dexamethasone in neonatal chronic lung disease. Internet Journal of Pediatrics and Neonatology 2005;5(1):10473.
- Malloy CA, Hilal K, Weiss MG, Rizvi Z, Muraskas JK. Randomized controlled trial comparing standard vs. lower dose dexamethasone therapy in neonates with chronic lung disease. In: E-PAS. 2003:2776.
- Malloy CA, Hilal K, Weiss MG, Rizvi Z, Muraskas JK. Randomized trial: standard vs lower dose dexamethasone in neonatal chronic lung disease. Journal of Perinatology 2003;23(7):602. [Google Scholar]
Marr 2019 {published and unpublished data}
- Marr BL, Bode MM, Gross SJ. Trial of 42 day vs. 9 day courses of dexamethasone (DEX) for the treatment of evolving bronchopulmonary dysplasia (BPD) in extremely preterm (EP) infants. In: E-PAS20111660.6. 2011. [DOI] [PubMed]
- Marr BL, Mettelman BB, Bode MM, Gross SJ. Randomized trial of 42-day compared with 9-day courses of dexamethasone for the treatment of evolving bronchopulmonary dysplasia in extremely preterm Infants. Journal of Pediatrics 2019;211:20-26.e1. [PMID: ] [DOI] [PubMed] [Google Scholar]
McEvoy 2004 {published data only}
- McEvoy C, Bowling S, Williamson K, McGaw P, Durand M. Randomized, double-blinded trial of low-dose dexamethasone: II. Functional residual capacity and pulmonary outcome in very low birth weight infants at risk for bronchopulmonary dysplasia. Pediatric Pulmonology 2004;38(1):55-63. [PMID: ] [DOI] [PubMed] [Google Scholar]
Merz 1999 {published data only}
- Merz U, Peschgens T, Kusenbach G, Hornchen H. Early versus late dexamethasone treatment in preterm infants at risk for chronic lung disease: a randomized pilot study. European Journal of Pediatrics 1999;158(4):318-22. [PMID: ] [DOI] [PubMed] [Google Scholar]
Odd 2004 {published data only}
- Cranefield DJ, Odd DE, Harding JE, Teele RL. High incidence of nephrocalcinosis in extremely preterm infants treated with dexamethasone. Pediatric Radiology 2004;34(2):138-42. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Odd DE, Armstrong DL, Teele RL, Kuschel CA, Harding JE. A randomised trial of two dexamethasone regimens to prevent chronic lung disease of prematurity. In: Perinatal Society of Australia and New Zealand. 2003:P46. [DOI] [PubMed]
- Odd DE, Armstrong DL, Teele RL, Kuschel CA, Harding JE. A randomized trial of two dexamethasone regimens to reduce side-effects in infants treated for chronic lung disease of prematurity. Journal of Paediatrics and Child Health 2004;40(5-6):282-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Papile 1998 {published data only}
- NCT00011362. Dexamethasone therapy in VLBW Infants at risk of CLD (Dexamethasone). clinicaltrials.gov/ct2/show/NCT00011362 (first received 19 February 2001).
- Papile L, Stoll B, Donovan E, Tyson J, Bauer C, Wright L, et al. Dexamethasone therapy in infants at risk for chronic lung disease (CLD): a multi-center, randomized, double-masked trial. Pediatric Research 1996;39(4):236A. [Google Scholar]
- Papile LA, Tyson JE, Stoll BJ, Wright LL, Donovan EF, Bauer CR, et al. A multicenter trial of two dexamethasone regimens in ventilator-dependent premature infants. New England Journal of Medicine 1998;338(16):1112-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
- Stoll BJ, Temprosa M, Tyson JE, Papile LA, Wright LL, Bauer CR, et al. Dexamethasone therapy increases infection in very low birth weight infants. Pediatrics 1999;104(5):e63. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ramanathan 1994 {published data only}
- Ramanathan R, Siassi B, Sardesai S, deLemos RA. Comparison of two dosage regimens of dexamethasone for early treatment of chronic lung disease in very low birth weight (VLBW). Pediatric Research 1994;34:250A. [Google Scholar]
References to studies excluded from this review
Ahrens 2000 {published data only}
- NCT00004785. Phase III randomized, double-blind study of dexamethasone vs dexamethasone/methylprednisolone vs placebo for bronchopulmonary dysplasia. clinicaltrials.gov/ct2/show/study/NCT00004785 (first received 25 February 2000).
DeCastro 2009 {published data only}
- DeCastro M, El-Khoury N, Parton L, Ballabh P, LaGamma EF. Postnatal betamethasone vs dexamethasone in premature infants with bronchopulmonary dysplasia: a pilot study. Journal of Perinatology 2009;29(4):297-304. [PMID: ] [DOI] [PubMed] [Google Scholar]
Romagnoli 1999 {published data only}
- Romagnoli C, Zecca E, Vento G, Maggio L, Papacci P, Tortorolo G. Effect on growth of two different dexamethasone courses for preterm infants at risk of chronic lung disease. A randomized trial. Pharmacology 1999;59(5):266-74. [PMID: ] [DOI] [PubMed] [Google Scholar]
Shipalana 1994 {published data only}
- Shipalana N, Cooper PA, Strahlendorff C. Early postnatal steroids for non-ventilated infants weighing less than 1000 g at birth - a randomised trial. Pediatric Reviews and Communications 1994;8(1):29-33. [Google Scholar]
Singh 2022 {published data only}
- Singh N, Gautham KS. Pattern of postnatal steroid use for bronchopulmonary dysplasia in extremely preterm infants. Journal of Perinatology 2022;42(9):1258-9. [DOI: 10.1038/s41372-022-01353-1] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
IRCT20200721048155N1 {unpublished data only}
- IRCT20200721048155N1. Investigation of the effect of low-dose hydrocortisone on neonatal respiratory status under mechanical ventilation. rct.ir/trial/49795 (first received 1 August 2001).
References to ongoing studies
IRCT20201222049802N3 {unpublished data only}
- IRCT20201222049802N3. Comparative study of the effect of dexamethasone and injectable hydrocortisone in reducing the need of oxygen in preterm infants. en.irct.ir/trial/61257 25-01-2022.
Additional references
AAP 2002
- Committee on Fetus and Newborn. Postnatal corticosteroids to treat or prevent chronic lung disease in preterm infants. Pediatrics 2002;109(2):330-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Bancalari 2006
- Bancalari E, Claure N. Definitions and diagnostic criteria for bronchopulmonary dysplasia. Seminars in Perinatology 2006;30(4):164-70. [PMID: ] [DOI] [PubMed] [Google Scholar]
Brozanski 1995
- Brozanski BS, Jones JG, Gilmour CH, Balsan MJ, Vazquez RL, Israel BA, et al. Effect of pulse dexamethasone therapy on the incidence and severity of chronic lung disease in the very low birth weight infant. Journal of Pediatrics 1995;126(5 Pt 1):769-76. [PMID: ] [DOI] [PubMed] [Google Scholar]
Carlton 1997
- Carlton DP, Albertine KH, Cho SC, Lont M, Bland RD. Role of neutrophils in lung vascular injury and edema after premature birth in lambs. Journal of Applied Physiology 1997;83(4):1307-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
CDTG 1991
- Collaborative Dexamethasone Trial Group. Dexamethasone therapy in neonatal chronic lung disease: an international placebo-controlled trial. Pediatrics 1991;88(3):421-7. [PMID: ] [PubMed] [Google Scholar]
Cheong 2013
- Cheong JL, Anderson P, Roberts G, Duff J, Doyle LW. Postnatal corticosteroids and neurodevelopmental outcomes in extremely low birthweight or extremely preterm infants: 15-year experience in Victoria, Australia. Archives of Disease in Childhood. Fetal and Neonatal Edition 2013;98(1):F32-6. [PMID: ] [DOI] [PubMed] [Google Scholar]
Coalson 2006
- Coalson JJ. Pathology of bronchopulmonary dysplasia. Seminars in Perinatology 2006;30(4):179-84. [PMID: ] [DOI] [PubMed] [Google Scholar]
Costeloe 2012
- Costeloe KL, Hennessy EM, Haider S, Stacey F, Marlow N, Draper ES. Short term outcomes after extreme preterm birth in England: comparison of two birth cohorts in 1995 and 2006 (the EPICure studies). BMJ 2012;345:e7976. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence [Computer program]
- Covidence. Version accessed 24 October 2022. Melbourne, Australia: Veritas Health Innovation. Available at covidence.org.
Doyle 2014
- Doyle LW, Halliday HL, Ehrenkranz RA, Davis PG, Sinclair JC. An update on the impact of postnatal systemic corticosteroids on mortality and cerebral palsy in preterm infants: effect modification by risk of bronchopulmonary dysplasia. Journal of Pediatrics 2014;165(6):1258-60. [PMID: ] [DOI] [PubMed] [Google Scholar]
Doyle 2021a
- Doyle LW, Cheong JL, Hay S, Manley BJ, Halliday HL, Soll R. Early (< 7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database of Systematic Reviews 2021, Issue 10. Art. No: CD001146. [DOI: 10.1002/14651858.CD001146.pub6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Doyle 2021b
- Doyle LW, Cheong JL, Hay S, Manley BJ, Halliday HL. Late (≥ 7 days) systemic postnatal corticosteroids for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database of Systematic Reviews 2021, Issue 11. Art. No: CD001145. [DOI: 10.1002/14651858.CD001145] [DOI] [PMC free article] [PubMed] [Google Scholar]
Durand 1995
- Durand M, Sardesai S, McEvoy C. Effects of early dexamethasone therapy on pulmonary mechanics and chronic lung disease in very low birth weight infants: a randomized, controlled trial. Pediatrics 1995;95(4):584-90. [PMID: ] [PubMed] [Google Scholar]
Ehrenkranz 2005
- Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, et al. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics 2005;116(6):1353-60. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ferreira 2000
- Ferreira PJ, Bunch TJ, Albertine KH, Carlton DP. Circulating neutrophil concentration and respiratory distress in premature infants. Journal of Pediatrics 2000;136(4):466-72. [PMID: ] [DOI] [PubMed] [Google Scholar]
Fewell 2007
- Fewell Z, Davey Smith G, Sterne JA. The impact of residual and unmeasured confounding in epidemiologic studies: a simulation study. American Journal of Epidemiology 2007;166(6):646-55. [PMID: ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 24 October 2022. Hamilton (ON): McMaster University (developed by Evidence Prime). Available at gradepro.org.
Graphpad 2021 [Computer program]
- GraphPad Software. Version assessed in October 2022. San Diego, CA 92108. Available at www.graphpad.com.
Halliday 2001
- Halliday HL. Guidelines on neonatal steroids. Prenatal Neonatal Medicine 2001;6:371-3. [Google Scholar]
Halliday 2003a
- Halliday HL, Ehrenkranz RA, Doyle LW. Early postnatal (<96 hours) corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database of Systematic Reviews 2003, Issue 1. Art. No: CD001146. [DOI: 10.1002/14651858.CD001146] [PMID: ] [DOI] [PubMed] [Google Scholar]
Halliday 2003b
- Halliday HL, Ehrenkranz RA, Doyle LW. Moderately early (7-14 days) postnatal corticosteroids for preventing chronic lung disease in preterm infants. Cochrane Database of Systematic Reviews 2003, Issue 1. Art. No: CD001144. [DOI: 10.1002/14651858.CD001144] [PMID: ] [DOI] [PubMed] [Google Scholar]
Halliday 2003c
- Halliday HL, Ehrenkranz RA, Doyle LW. Delayed (>3 weeks) postnatal corticosteroids for chronic lung disease in preterm infants. Cochrane Database of Systematic Reviews 2003, Issue 1. Art. No: CD001145. [DOI: 10.1002/14651858.CD001145] [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2017
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Churchill R, Chandler J, Cumpston MS, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.2.0 (updated June 2017). Cochrane, 2017. Available from training.cochrane.org/handbook/archive/v5.2.
Higgins 2022
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6.3 (updated February 2022). Cochrane 2022. Available from www.training.cochrane.org/handbook.
Hozo 2005
- Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Medical Research Methodology 2005;5:13. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2007
- Huang CC, Lin HR, Liang YC, Hsu KS. Effects of neonatal corticosteroid treatment on hippocampal synaptic function. Pediatric Research 2007;62(3):267-70. [PMID: ] [DOI] [PubMed] [Google Scholar]
Husain 1998
- Husain AN, Siddiqui NH, Stocker JT. Pathology of arrested acinar development in post surfactant bronchopulmonary dysplasia. Human Pathology 1998;29(7):710-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jobe 1999
- Jobe AJ. The new BPD: an arrest of lung development. Pediatric Research 1999;46(6):641-3. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jobe 2001
- Jobe AH, Bancalari E. Bronchopulmonary dysplasia. American Journal of Respiratory and Critical Care Medicine 2001;163(7):1723-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kaempf 2003
- Kaempf JW, Campbell B, Sklar RS, Arduza C, Gallegos R, Zabari M, et al. Implementing potentially better practices to improve neonatal outcomes after reducing postnatal dexamethasone use in infants born between 501 and 1250 grams. Pediatrics 2003;111(4 Pt 2):e534-41. [PMID: ] [PubMed] [Google Scholar]
Karemaker 2006
- Karemaker R, Heijnen CJ, Veen S, Baerts W, Samsom J, Visser GH, et al. Differences in behavioral outcome and motor development at school age after neonatal treatment for chronic lung disease with dexamethasone versus hydrocortisone. Pediatric Research 2006;60(6):745-50. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kitchen 1987
- Kitchen WH, Ford GW, Rickards AL, Lissenden JV, Ryan MM. Children of birth weight less than 1000 g: changing outcome between ages 2 and 5 years. Journal of Pediatrics 1987;110(2):283-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Kyriacou 2016
- Kyriacou DN, Lewis RJ. Confounding by indication in clinical research. Journal of the American Medical Association 2016;316(17):1818-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Liberati 2009
- Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Medicine 2009;6(7):e1000100. [DOI: 10.1136/bmj.b2700] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lodygensky 2005
- Lodygensky GA, Rademaker K, Zimine S, Gex-Fabry M, Lieftink AF, Lazeyras F, et al. Structural and functional brain development after hydrocortisone treatment for neonatal chronic lung disease. Pediatrics 2005;116(1):1-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Marr 2011
Noel‐Storr 2020
- Noel-Storr AH, Dooley G, Wisniewski S, Glanville J, Thomas J, Cox S, et al. Cochrane Centralised Search Service showed high sensitivity identifying randomized controlled trials: a retrospective analysis. Journal of Clinical Epidemiology 2020;127:142-50. [PMID: ] [DOI] [PubMed] [Google Scholar]
Noel‐Storr 2021a
- Noel-Storr A, Dooley G, Affengruber L, Gartlehner G. Citation screening using crowdsourcing and machine learning produced accurate results: evaluation of Cochrane's modified Screen4Me service. Journal of Clinical Epidemiology 2021;130:23-31. [PMID: ] [DOI] [PubMed] [Google Scholar]
Noel‐Storr 2021b
- Noel-Storr A, Dooley G, Elliott J, Steele E, Shemilt I, Mavergames C, et al. An evaluation of Cochrane Crowd found that crowdsourcing produced accurate results in identifying randomized trials. Journal of Clinical Epidemiology 2021;133:130-9. [DOI] [PubMed] [Google Scholar]
Northway 1967
- Northway WH Jr, Rosan RC, Porter DY. Pulmonary disease following respiratory therapy of hyaline-membrane disease. Bronchopulmonary dysplasia. New England Journal of Medicine 1967;276(7):357-68. [PMID: ] [DOI] [PubMed] [Google Scholar]
O'Brodovich 1985
- O'Brodovich HM, Mellins RB. Bronchopulmonary dysplasia. Unresolved neonatal acute lung injury. American Review of Respiratory Disease 1985;132(3):694-709. [PMID: ] [DOI] [PubMed] [Google Scholar]
O'Shea 1999
- O'Shea TM, Kothadia JM, Klinepeter KL, Goldstein DJ, Jackson BG, Weaver RG 3rd, et al. Randomized placebo-controlled trial of a 42-day tapering course of dexamethasone to reduce the duration of ventilator dependency in very low birth weight infants: outcome of study participants at 1-year adjusted age. Pediatrics 1999;104(1 Pt 1):15-21. [PMID: ] [DOI] [PubMed] [Google Scholar]
Onland 2009
- Onland W, Offringa M, De Jaegere AP, Kaam AH. Finding the optimal postnatal dexamethasone regimen for preterm infants at risk of bronchopulmonary dysplasia: a systematic review of placebo-controlled trials. Pediatrics 2009;123(1):367-77. [PMID: ] [DOI] [PubMed] [Google Scholar]
Onland 2010
- Onland W, Kaam AH, De Jaegere AP, Offringa M. Open-label glucocorticoids modulate dexamethasone trial results in preterm infants. Pediatrics 2010;126(4):e954-64. [PMID: ] [DOI] [PubMed] [Google Scholar]
Onland 2019
- Onland W, Cools F, Kroon A, Rademaker K, Merkus MP, Dijk PH, et al. Effect of hydrocortisone therapy initiated 7 to 14 days after birth on mortality or bronchopulmonary dysplasia among very preterm infants receiving mechanical ventilation: a randomized clinical trial. Journal of the American Medical Association 2019;321(4):354-63. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rademaker 2007
- Rademaker KJ, Uiterwaal CS, Groenendaal F, Venema MM, Bel F, Beek FJ, et al. Neonatal hydrocortisone treatment: neurodevelopmental outcome and MRI at school age in preterm-born children. Journal of Pediatrics 2007;150(4):351-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ramaswamy 2021
- Ramaswamy VV, Bandyopadhyay T, Nanda D, Bandiya P, Ahmed J, Garg A, et al. Assessment of postnatal corticosteroids for the prevention of bronchopulmonary dysplasia in preterm neonates: a systematic review and network meta-analysis. JAMA pediatrics 2021;175(6):e206826. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: The Cochrane Collaboration, 2020.
Schmidt 2008
- Schmidt B, Roberts R, Millar D, Kirpalani H. Evidence-based neonatal drug therapy for prevention of bronchopulmonary dysplasia in very-low-birth-weight infants. Neonatology 2008;93(4):284-7. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s). Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach (updated October 2013). GRADE Working Group, 2013. Available from gdt.guidelinedevelopment.org/app/handbook/handbook.html.
Screen4Me
- Screen4Me. Screen4Me: Frequently asked questions [web site]. Available at community.cochrane.org/sites/default/files/uploads/S4M_Users_FAQs.pdf (accessed September 2021).
Shinwell 2003
- Shinwell ES, Karplus M, Bader D, Dollberg S, Gur I, Weintraub Z, et al. Neonatologists are using much less dexamethasone. Archives of Disease in Childhood. Fetal and Neonatal Edition 2003;88(5):F432-3. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shinwell 2007
- Shinwell ES, Lerner-Geva L, Lusky A, Reichman B. Less postnatal steroids, more bronchopulmonary dysplasia: a population-based study in very low birth weight infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2007;92(1):F30-3. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Short 2007
- Short EJ, Kirchner HL, Asaad GR, Fulton SE, Lewis BA, Klein N, et al. Developmental sequelae in preterm infants having a diagnosis of bronchopulmonary dysplasia: analysis using a severity-based classification system. Archives of Pediatrics and Adolescent Medicine 2007;161(11):1082-7. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stoll 2010
- Stoll BJ, Hansen NI, Bell EF, Shankaran S, Laptook AR, Walsh MC, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics 2010;126(3):443-56. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
van der Heide‐Jalving 2003
- Heide-Jalving M, Kamphuis PJ, Laan MJ, Bakker JM, Wiegant VM, Heijnen CJ, et al. Short- and long-term effects of neonatal glucocorticoid therapy: is hydrocortisone an alternative to dexamethasone? Acta Paediatrica 2003;92(7):827-35. [PMID: ] [DOI] [PubMed] [Google Scholar]
Vliegenthart 2017
- Vliegenthart RJ, Onland W, Wassenaer-Leemhuis AG, De Jaegere AP, Aarnoudse-Moens CS, Kaam AH. Restricted ventilation associated with reduced neurodevelopmental impairment in preterm infants. Neonatology 2017;112(2):172-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vliegenthart 2019
- Vliegenthart RJ, Kaam AH, Aarnoudse-Moens CS, Wassenaer AG, Onland W. Duration of mechanical ventilation and neurodevelopment in preterm infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2019;104(6):F631-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Walsh 2005
- Walsh MC, Morris BH, Wrage LA, Vohr BR, Poole WK, Tyson JE, et al. Extremely low birthweight neonates with protracted ventilation: mortality and 18-month neurodevelopmental outcomes. Journal of Pediatrics 2005;146(6):798-804. [PMID: ] [DOI] [PubMed] [Google Scholar]
Walsh 2006
- Walsh MC, Yao Q, Horbar JD, Carpenter JH, Lee SK, Ohlsson A. Changes in the use of postnatal steroids for bronchopulmonary dysplasia in 3 large neonatal networks. Pediatrics 2006;118(5):e1328-35. [PMID: ] [DOI] [PubMed] [Google Scholar]
Watterberg 2010
- Watterberg KL. Policy statement - postnatal corticosteroids to prevent or treat bronchopulmonary dysplasia. Pediatrics 2010;126(4):800-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Yeh 1997
- Yeh TF, Lin YJ, Hsieh WS, Lin HC, Lin CH, Chen JY, et al. Early postnatal dexamethasone therapy for the prevention of chronic lung disease in preterm infants with respiratory distress syndrome: a multicenter clinical trial. Pediatrics 1997;100(4):E3. [PMID: ] [DOI] [PubMed] [Google Scholar]
Yeh 1998
- Yeh TF, Lin YJ, Huang CC, Chen YJ, Lin CH, Lin HC, et al. Early dexamethasone therapy in preterm infants: a follow-up study. Pediatrics 1998;101(5):E7. [DOI] [PubMed] [Google Scholar]
Yoder 2009
- Yoder BA, Harrison M, Clark RH. Time-related changes in steroid use and bronchopulmonary dysplasia in preterm infants. Pediatrics 2009;124(2):673-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Onland 2014
- Onland W, De Jaegere AP, Offringa M, Kaam A. Systemic corticosteroid regimens for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database of Systematic Reviews 2014, Issue 1. Art. No: CD010941. [DOI: 10.1002/14651858.CD010941] [DOI] [PMC free article] [PubMed] [Google Scholar]
Onland 2008
- Onland W, De Jaegere AP, Offringa M, Kaam AH. Effects of higher versus lower dexamethasone doses on pulmonary and neurodevelopmental sequelae in preterm infants at risk for chronic lung disease: a meta-analysis. Pediatrics 2008;122(1):92-101. [PMID: ] [DOI] [PubMed] [Google Scholar]
Onland 2017
- Onland W, De Jaegere AP, Offringa M, Kaam A. Systemic corticosteroid regimens for prevention of bronchopulmonary dysplasia in preterm infants. Cochrane Database of Systematic Reviews 2017, Issue 1. Art. No: CD010941. [DOI: 10.1002/14651858.CD010941.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


