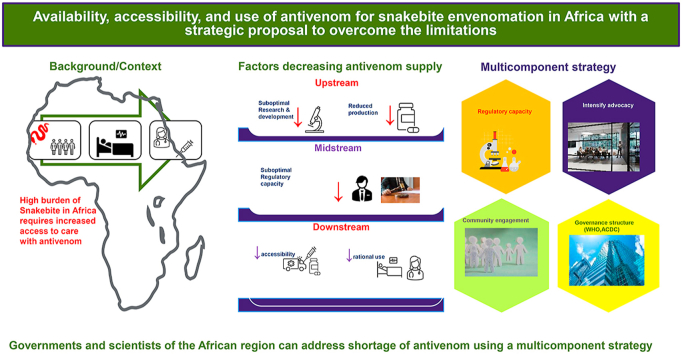
Abstract
Africa remains one of the regions with the highest incident and burden of snakebite. The goal of the World Health Organization to halve the global burden of snakebite by 2030 can only be achieved if sub-optimal access to antivenoms in the most affected regions is addressed. We identified upstream, midstream, and downstream factors along the antivenom value chain that prevent access to antivenoms in the African region. We identified windows of opportunities that could be utilized to ensure availability, accessibility, and affordability for snakebite endemic populations in Africa. These include implementation of multicomponent strategies such as intensified advocacy, community engagement, healthcare worker trainings, and leveraging the institutional and governance structure provided by African governments to address the challenges identified.
Keywords: Antivenoms, Snakebite envenoming, Burden, Accessibility, Affordability, Africa
Graphical abstract
Highlights
-
•
Windows of opportunities to improve availability and accessibility of antivenoms in Africa.
-
•
Reiterating need to implement multicomponent strategies to address antivenom shortage.
-
•
New perspective on leveraging governance structure of African governments to address antivenom access and utilization.
1. Background
The chance of getting bitten by a snake, remote as it may seem, is real for majority of the population in the tropics. The continuous encroachment by humans of the ecosystem hitherto occupied by venomous snakes makes this more realistic. All inhabitants of affected countries, especially rural poor populations, live with the reality of being among the over 5.4 million bitten yearly by snakes with half as many developing envenomation that results in nearly 140,000 deaths (Gutierrez et al., 2017; WHO, 2021; Harrison et al., 2009). Chippaux estimated a million bites per year in Africa as far back as 1998 with more recent works suggesting much higher numbers (Chippaux, 1998; Habib et al., 2015a). In addition, for every death from snakebite, there are three victims surviving with long-term physical or psychological sequelae such as amputation, blindness, physical disabilities, anxiety, depression, and posttraumatic stress disorder (Muhammed et al., 2017; Habib et al., 2020).
Antivenoms, i.e., preparations of immunoglobulins or immunoglobulin fragments purified from the plasma of animals immunized with venoms, constitute the mainstay in the therapy of these envenomations (Gutierrez et al., 2017). Thus, identifying and addressing challenges along the whole snake antivenom value chain remain a priority for reducing the burden of this neglected tropical disease. Following intense and persistent advocacy, the WHO declared snakebite envenomation as a category A neglected tropical disease (NTD) in 2017, and by 2019 set the goal of halving the global burden of snakebite by 2030 (Minghui et al., 2019). Finding and addressing factors that militate against achieving this target is an important determinant of success. While there are no appropriate placebo-controlled Randomized Controlled Trials determining efficacy, there is evidence antivenoms are effective in reducing mortality from snakebite (Hamza et al., 2021; Habib and Warrell, 2013; Iliyasu et al., 2015). Thus, access to safe and effective antivenom remains a rate limiting step to effective control of snake envenomation (Gutierrez et al., 2017; Minghui et al., 2019). By extension, it contributes towards achieving the United Nations’ (UN) Sustainable Development Goal (SDG) 3.8 - “access to safe, effective, quality and affordable essential medicines and vaccines for all” (UN General Assembly, 2017). For populations affected with endemic snakebite, availability of antivenoms contributes towards achieving Universal Health Coverage (UHC).
Despite recognition of the effectiveness of antivenoms against mortality, as with other vaccines and biologicals, there is the need to democratize the capacity/technology for development, funding, regulations, and other demand and supply factors affecting availability and effective utilization. The WHO and other key stakeholders should utilize the lessons learnt from polio eradication initiative as well as control of other vaccine preventable diseases to ensure the political will required for snakebite control is in place.
Even though the WHO has included snake antivenom in the first ever published list of essential medicines, the lack of access to these medicines in resource-limited settings should be addressed. These challenges and their effects have been detailed in earlier works by Potet et al. classified into upstream, midstream, and downstream factors, and the multicomponent strategy expounded by Gutierrez et al. a concise summary of these concepts is presented in Table 1 (Gutiérrez et al., 2014; Potet et al., 2021; Pedrique et al., 2013; Wirtz et al., 2016). Effective strategies to enhance access to medicines in resource-limited settings need to be tailored to the specific product characteristics, the clinical context and the target populations vis a vis their socio-cultural and political peculiarities (Potet et al., 2021).
Table 1.
Factors that limit availability and accessibility of antivenoms from design and manufacture to point of care (adapted from Potet et al. (2021) and Gutiérrez et al. (2014)).
| Factor | Challenges | Implications | Solution |
|---|---|---|---|
| Upstream factors | |||
| 1. Adequacy of manufacturing | I. Most manufacturers primarily aim to address local needs (some like the Serum Institute of India produce for external use) II. Poor interest from large multinational pharmaceutical producers |
I. Inadequate number of manufacturers II. Inadequate doses to address need III. Lack of species-specific antivenom in some countries IV. Vulnerability of endemic countries depending on foreign supply |
I. Increase number of local manufacturing hubs II. Increase capacity of existing manufacturers to address need III. Create facilities for collection of venoms from local snake species IV. R&D to address species-specific envenomation V. Higher level interventions (WHO, Africa CDC, others) to address commercial interest of manufacturers and advocate for corporate social responsibility |
| 2. Quality of antivenoms | I. Poor adherence to good manufacturing practice (GMP) II. Lack of regulatory capacity to register/license quality antivenoms III. Use of substandard products |
I. Flooding of market with substandard antivenoms II. Poor outcomes from use of substandard antivenoms |
I. Advocacy for and improving capacity for GMP II. Improve regulatory capacity for registration and licensing |
| 3. R&D for new antivenoms | I. Poor capacity of some endemic countries to manufacture or purchase new antivenoms II. Limited local R&D capacity for antivenom development |
Lack of effective antivenoms to address envenoming from local species in some instances | I. Advocacy for local ownership of the efforts to address antivenom production to address locally relevant species II. Strengthening local R & D capacity in universities and private and public institutions |
| Mid-stream factors | |||
| 1. Regulatory capacity for evaluation, validation, and registration | Lack of capacity to evaluate and register antivenoms | I. Flooding of the market with products that haven't been evaluated thoroughly II. Procurement, distribution, and widespread use of substandard antivenoms |
I. Strengthen capacity for assessment, validation, and registration of antivenoms II. Foster partnerships between regulatory agencies and university research groups to strengthen local regulatory capacity |
| 2. Cost and financing | I. Lack of cost-effective approach to procurement, distribution, and utilization of antivenoms II. Antivenoms not prioritized among the essential drug list by countries healthcare financing models |
I. High cost of antivenoms II. Resort to substandard, cheap, and harmful antivenoms III. Increased use of unorthodox, harmful practices |
I. Develop regional stockpiles that will enable more competitive pricing II. Develop regional stockpiles that will improve the cost-effectiveness of antivenoms of proven quality |
| Downstream factors | |||
| 1. Availability and accessibilty | I. Effective antivenoms often unavailable in countries they are needed II. Poor distribution of antivenoms to some remote areas where they are needed |
I. Use of ineffective, substandard antivenoms II. Lack of access to effective antivenoms in remote areas when needed III. Resort to unorthodox and harmful practices |
Develop national and regional mechanisms of antivenom acquisition and distribution based on sound epidemiological information and effective deployment strategies |
| 2. Rational use | Lack of capacity of frontline health workers to determine the need for antivenoms and use the correct antivenom | I. Wastage of antivenoms II. Poor utilization of antivenoms III. Stock outs |
I. Train healthcare workers on clinical assessment of envenomations and utilization of antivenom II. Provide off site support using a hub-and-spoke model III. Build capacity for use inventory for managing stock |
| 3. Community perception and misconception | Use of unorthodox, potentially harmful interventions | Suboptimal utilization of antivenoms | I. Improve risk communication and community engagement II. Promote effective communication channels with traditional healers on the correct management of envenomations |
Regulatory capacity for quality control and African readiness
One of the major challenges for development of biomedicals is the capacity for regulation and quality control. Manufacturers need good regulatory capacity for approval of their products to ensure acceptability. Even though most countries in Africa have regulatory agencies, the capacity to regulate, evaluate and establish safety and efficacy of the products remain sub-optimal. This has led to flooding of the market with substandard products, leaving little space for quality antivenoms, with misconceptions on management of snakebites further complicating an already bad situation (Potet et al., 2021). Based on these challenges, earlier works on challenges of availability and accessibility of antivenom recommended multifaceted interventions including but not limited to development of regional hubs for development, evaluation, and regulation of antivenoms alongside other recommendations by the WHO Strategy for Prevention and Control of Snakebite Envenoming (WHO, 2019). To address this problem, the WHO established an independent assessment of antivenoms to support procurement agencies and public health officials decide which antivenoms are likely to be safe and efficacious, that its quality, safety and efficacy are acceptable, and that, when used to treat snake bite in the countries in which it is marketed, its benefits will outweigh any foreseeable risks. The process involves joint desk review assessment, as well as WHO laboratory evaluation to confirm essential product features like proof of preclinical efficacy, supplemented by on-site inspection by regulatory, herpetological and medical experts from the relevant African countries, complemented by international experts. Consequently, only antivenoms with favourable risk‒benefit ratio will be entered into a list on the WHO website. Furthermore, the WHO's Global Benchmarking Tool (GBT) for objective evaluation of regulatory systems provide both an evaluation and quality improvement platform with four defined Maturity Levels (MLs), for National Regulatory Agencies (NRAs). A minimum of ML3 is needed for NRAs to be provided approval and oversight to enable them to apply for WHO Pre-Qualification (PQ). This PQ in turn is required before any manufacturer could export vaccines to most self-financing countries and the UNICEF. In this regard, efforts by the WHO to publish “target product profile” (TPP) for antivenoms intended for use in Sub-Saharan Africa is a welcomed development a process that is ongoing now. The TPP describes the characteristics needed to produce good antivenoms for Africa thereby supporting the efforts towards designing and producing new antivenoms that really respond to needs of the African countries. In addition, the ongoing efforts by the Africa CDC to develop capacity for manufacture of vaccine could be leveraged upon to address the antivenom need. The Partnerships for African Vaccine Manufacturing (PAVM) Framework for Action is another window of opportunity to address regulatory requirements for antivenom production in Africa. Program 3 of the PAVM viz: ‘Strengthening National Regulatory Agencies and Regional Centers of Regulatory Excellence to build vaccine regulatory excellences’ specifically outlined the requirements and steps needed by the continent to achieve them. Moreover, there is another Africa Union specialized agency, the African Medicines Agency (AMA), that aims to enhance the capacity of African Union Member States and Regional Economic Communities (RECs) to regulate medical products to improve access to quality, safe and efficacious products in the African continent. It will carry out regulatory oversight of medicinal products through convening of pooled scientific expertise, coordinating data collection and information sharing among countries and RECs, leverage on existing Regional Hubs and Regional Centers of Excellence to jointly assess and inspect medicines and vaccines for priority diseases in Africa and strengthen networking for optimal use of the limited resources. This agency, among other efforts, will also perform market surveillance to regulate and reduce substandard and falsified medical products in African Countries. The existing and ongoing efforts of this agency can be leveraged in the regulation of antivenoms to ensure its safety and efficacy in the continent.
1.1. Estimates of burden and budgetary need
Any effort on addressing the antivenom need should factor the essential needs in terms of the burden of snakebite, the predominant species, institutional capacity requirement, logistics for distribution and cold chain maintenance, human resource, training, and supportive supervision. There is abundant information in the literature on the burden of snakebite, projected antivenom need, and estimated cost to meet the need (Chippaux, 2011; Halilu et al., 2019).
However, it is necessary to improve the information on the actual burden of snakebites in different African countries, so as to have a more robust platform on which to build strategies for improving antivenom accessibility and availability. To make progress, a dedicated group should take the advocacy and lobby further to ensure that deliberate steps are taken to ensure that the key stakeholders are engaged and prevailed upon to make the political and financial commitment to translate the data for action to tangible deliverables.
One of its strategic approaches of the African Union Continental Framework for the control and elimination of NTDs is to increase political engagement to increase domestic resources for research and development of NTDs in African countries. This is also highlighted in the fourth pillar of the Africa CDC's call for a New Public Health Order that advocates for increased domestic investment in Health. So, to make progress, a dedicated group should take the advocacy and lobby further to ensure that deliberate steps are taken to ensure that the key stakeholders are engaged and prevailed upon to make the political and financial commitment to translate the data for action to tangible deliverables.
1.2. Facilities producing antivenom in Africa
It is important to review the antivenoms intended for use in Africa, locally produced or by international manufacturers that distribute their products in Africa. This information should include, among other elements, the types of antivenoms they produce, what percentage of venomous snakes are covered, what is the volume of production and whether the production rate and capacity is enough to address the needs. Moreover, it is necessary to have a detailed understanding on the preclinical efficacy of these antivenoms towards the medically most relevant snake venoms in various African countries, especially in those where these antivenoms are distributed.
Table 2 summarised the facilities producing antivenoms used in Africa, country of production, and venoms against which antivenom is active according to product insert. It is striking that only one manufacturer of antivenoms intended for use in sub-Saharan Africa is actually located in sub-Saharan Africa: this is SAVP, a public manufacturer based in South Africa.
Table 2.
Summary of antivenom available for use in Africa, country of production, the type of active substance and the species covered.
| Name of Facility and Country | Brand Name | African country used | IgG Type | Species venom active against (according to product insert) | Status of WHO assessment |
|---|---|---|---|---|---|
| Polyspecific | |||||
| Bharat Serums and Vaccines, India | ASNA antivenom C (ASNA-C) | Sub-Saharan Africa | F(ab’)2— equine | Bitis arietans, B. gabonica, B. nasicornis, Dendroaspis. angusticeps, D. jamesoni, D. polylepis, E. carinatus, Naja haje, N. melanoleuca, N. nigricollis, N. nivea | No risk-benefit assessment |
| Bharat Serums and Vaccines, India | ASNA antivenom D (ASNA-D) | Sub-Saharan Africa | F(ab’)2— equine |
B. arietans, B. gabonica, B. nasicornis, D. angusticeps, D. jamesoni, D. polylepis, E. ocellatus, N. haje, N. melanoleuca, N. nigricollis, N. nivea |
No risk-benefit assessment |
| MicroPharm Ltd, United Kingdom | Fav-Afrique (FAV-A) | Sub-Saharan Africa | F(ab’)2— equine |
B. arietans, B. gabonica, D. jamesoni, D. polylepis, D. viridis, Echis leucogaster, E. ocellatus, N. haje, N. melanoleuca, N. nigricollis |
No risk-benefit assessment |
| MicroPharm Ltd, United Kingdom | Favirept | North Africa | F(ab)’2 | B. arietans, Cerastes cerastes, D. deserti, E. leucogaster, M. deserti, N. haje, N. nigricollis | No risk-benefit assessment |
| Instituto Clodomiro Picado, University of Costa Rica, Costa Rica | EchiTabPlus (ET-Plus) | Nigeria; Burkina Faso, Central African Republic, South Sudan | Intact IgG —equine |
B. arietans, B. gabonica, B. rhinoceros, B. nasicornis, E. ocellatus, E. leucogaster, E. pyramidum, E. coloratus, N. nigricollis, N. ashei, N. mossambica, N. katiensis | Undergoing risk-benefit assessment |
| Inosan Biopharma, S.A., Spain |
Inoserp Pan-Africa (Inoserp-P) | Benin; Burkina Faso; Gabon; Ghana; Guinea; Cote D'Ivoire; Kenya; Mali; Senegal; Tanzania; Togo; Uganda; Cameroon |
F(ab’)2— equine | B. arietans, B. gabonic, B. nasicornis, B. rhinoceros, D. angusticeps, D. jamesoni, D. polylepis, D. viridis, E. leucogaster, E. ocellatus, E. pyramidum, N. haje, N. katiensis, N. melanoleuca, N. nigricollis, N. nivea, N. pallida | Terminated risk-benefit assessment |
| Institut Pasteur d'Algerie, Algeria | IPAVIP Antiviperin Sera | Algeria | IgG2a | C. cerastes, Macrovipera lebetina | No risk-benefit assessment |
| Egyptian Organisation for Biological Products and Vaccines (VACSERA), Egypt | Polyvalent Anti-Snake Serum | Egypt | F(ab’)2 | C. cerastes, N. haje; N. nubiae | No risk-benefit assessment |
| Egyptian Organisation for Biological Products and Vaccines (VACSERA), Egypt | Polyvalent Anti-Vipers Serum | Egypt | F(ab’)2 | C. cerastes, E. coloratus, E. pyramidum | No risk-benefit assessment |
| Instituto Bioclon/Silanes, Mexico | Antivipmyn-Africa (Antivip-A) | Sub-Saharan Africa | F(ab’)2— equine |
B. arietans, E. ocellatus, E. pyramidum, E. romani |
Positive risk-benefit assessment |
| Premium Serums and Vaccines, India | Snake Venom Antiserum -PanAfrica (Premium-A) | Sub-Saharan Africa | F(ab’)2— Equine |
B. arietans, B. gabonica, B. nasicornis, B. rhinoceros, D. angusticeps, D. jamesoni, D. polylepis, D. viridis, E. carinatus, E. leucogaster, E. ocellatus, N. nigricollis, N. haje, N. melanoleuca |
Undergoing risk-benefit assessment |
| Premium Serums and Vaccines, India | Snake Venom Antiserum - Central Africa (Premium-CA) |
Sub-Saharan Africa | F(ab’)2— Equine |
B. rhinoceros, E. carinatus, D. polylepis, Daboia russelli | No risk-benefit assessment |
| Premium Serums and Vaccines, India | Combipack of Snake Venom Antiserum (African- Ten) | Sub-Saharan Africa | F(ab’)2— Equine |
B. rhinoceros, E. carinatus, Daboia russelli, D. polylepis | No risk-benefit assessment |
| South African Vaccine Producers (SAVP), South Africa | SAIMR Polyvalent Antivenom | South Africa | F(ab’)2— equine | B. arietans, B. gabonica, D. angusticeps, D. jamesoni, D. polylepis, Hemachatus haemachatus, N. annulifera, N. melanoleuca, N. mossambica, N. nivea | Undergoing risk-benefit assessment |
| VINS Bioproducts, India | Snake Venom Antiserum African - 10 (Afriven 10) | Kenya; Cote D'Ivoire (Ivory Coast); Nigeria; Ghana; Zambia; North Africa |
F(ab’)2— equine | B. arietans, B. gabonica, D. jamesoni, D. polylepis, D. viridis, E. leucogaster, E. ocellatus, N. haje, N. melanoleuca, N. nigricollis | Undergoing risk-benefit assessment |
| VINS Bioproducts, India | Menaven, Snake Venom Antitoxin | North Africa | F(ab)’2— equine | C. cerastes; N. haje; N. nigricollis | No risk-benefit assessment |
| VINS Bioproducts, India | Snake Venom Antiserum Echiven Plus | Sub-Saharan Africa | F(ab)’2— equine | N. nigricollis, N. haje, D. polylepis, B. arietans, E. ocellatus | No risk-benefit assessment |
| VINS Bioproducts, India | Anti-Snake Venom Serum Central Africa (VINS-CA) |
F(ab)’2— equine | B. gabonica, E. carinatus, Daboia russelli, D. polylepis | No risk-benefit assessment | |
| Biological E Limited, India | Anti Snake Venom Serum Central Africa - 6 | Sub-Saharan Africa | F(ab’)2— equine | B. arietans; C. cerastes; D. deserti; E. leucogaster; N. haje; N. nigricollis | No risk-benefit assessment |
| Biological E Limited, India | Anti Snake Venom Serum Pan Africa - 10 | Ghana | F(ab’)2— equine | B. arietans, B. gabonica, D. jamesoni, D. polylepis, D. viridis, E. leucogaster, E. ocellatus, N. haje, N. melanoleuca, N. nigricollis | No risk-benefit assessment |
| Monospecific | |||||
| MicroPharm Ltd, United Kingdom | EchiTAbG | Nigeria | Intact IgG —ovine |
E. coloratus, E. ocellatus, E. pyramidum | Positive risk-benefit assessment |
| Institut Pasteur de Tunis, Tunisia | Gamma-Vip | Tunisia | F(ab)’2 | C. cerastes, M. lebetina | No risk-benefit assessment |
| South African Vaccine Producers (SAVP), South Africa | SAIMR Echis | South Africa | F(ab)’2— equine | E. carinatus, E. ocellatus, E. coloratus, E. pyramidum, C. cerastes | No risk-benefit assessment |
| South African Vaccine Producers (SAVP), South Africa | SAIMR-Boomslang (SAIMR-Boom) | South Africa | F(ab)’2— equine | Dispholidus typus | No risk-benefit assessment |
| VINS Bioproducts, India | Snake venom antiserum Echis ocellatus (VINS-Echis) | Sub-Saharan Africa | F(ab)’2— equine | E. ocellatus | No risk-benefit assessment |
| VINS Bioproducts, India | Snake Venom Antiserum (Echiven) | Sub-Saharan Africa | F(ab)’2— equine | E. ocellatus | No risk-benefit assessment |
| Biological E Limited, India | Anti Snake Venom Serum Monovalent Echis ocellatus | Sub-Saharan Africa | F(ab’)2— equine | E. ocellatus | No risk-benefit assessment |
1.3. Facilitating sustainable local production
The two public health institutions with mandate for public health response in the African region provided clear guidance and commitment to sustainable development of vaccines and biologicals. The WHO in its strategy rightly placed countries at the centre of all efforts to address snake envenomation (WHO, 2019). In addition, the Africa CDC also made it clear in its new public health order that was derived from lessons learnt from COVID-19 and focuses on five key elements: local production of therapeutics, vaccines and diagnostics; respectful, action oriented partnerships; increased domestic financing; a strong public-health workforce and strengthened public-health institutions (Nkengasong and Tessema, 2020; Nkengasong et al., 2017; CDC, 2022). All the five pillars will be key to any efforts to ensure acquisition or production, equitable distribution, and rational and effective use of antivenoms in the region. Through the leadership of the continent's public health institutions, Africa should be able to use existing knowledge of geographical distribution of snakes in the region and available expertise, to strategically station antivenom production structures that will address antivenom needs of the continent. In the short-term, the power of collective bargaining should be used to procure and distribute antivenoms from existing producers with home transfer of technical expertise and technology will be agreed as part of the massive procurement agreement. An example of where this concept has been successfully implemented is the Africa Medical Supplies Platform, a joint initiative between the African Union, Africa CDC, Afreximbank, and UNECA with the support of several partners, which allowed countries to benefit from preferential access and prices to COVID-19 vaccines and other consumables through pooled procurement. This platform can be leveraged to improve access to diagnostics and treatment such as antivenom.
The lessons learned from the pandemic and structures already established therefrom could be utilized for this purpose. The recent realization that less than one percent of vaccines used in Africa are produced within the continent and the impact on this on effective response to pandemics and other health emergencies led to development of the PAVM (African Union, 2022). This initiative aims to increase local production of vaccines to 60% by the year 2040. For this, the continent should consider up-front investment in infrastructure, materials, and technical capacity. Similar efforts should be promoted in the field of antivenoms.
To achieve the above milestones, the following sequence of steps should be taken by the continent:
-
1.
A committee of experts, advocates, and vaccine champions should be formed to come up with a strategy aimed at bringing the who and who in public health of the continent. They could meet online for their deliberations and have a few strategic physical meetings funded by the WHO and/or Africa CDC. The outcome of their deliberations will include mapping of infrastructure, regulatory capacity, the identification of medically relevant snake species, the burden of snake envenomation in the various sub-regions or countries, and available infrastructure, manpower, and funding. This effort could work synergistically to facilitate realization of the $4.29 m and $15.58 m budgeted/proposed for 2019–2020 and 2021–2024 in the WHO strategy (WHO, 2019).
-
2.
A compendium of existing global manufacturers producing and supplying the continent should be collated, analysed, and prioritized with a view to engage and secure their commitment through the proposed collective bargaining. Earlier efforts at documentation should be followed up and optimized (Potet et al., 2021).
-
3.
The result from above should be used to brief the African Union Heads of State and Government and WHO continent leadership with the aim of advocating for and securing commitment for the strategy
-
4.
A revolving fund for antivenom procurement, stockpiling, and distribution should be establish for the African region same way similar structures were formed for Nigeria and the West African sub-region (Dada et al., 2022). This will serve as a stop gap measure to address the immediate needs for vaccine while more sustainable structures are being formed. The WHO planned launch of the pilot phase of African antivenom stockpile at the beginning of 2023 could utilize the lessons learned from the revolving fund for outbreak response that was successful in the health security space.
-
5.
Advocate for hub-and-spoke approach to snakebite management by identifying and strategically training healthcare workers that could provide consultations and mentorship to harder to reach populations (Hamza et al., 2020). The use of drones could serve remote locations requiring immediate access to antivenoms. This is being used for distribution of life-saving commodities like blood and vaccines, among others (Sham et al., 2022; Sing et al., 2019; Nwannekanma, 2022)
-
6.
Use the universal health coverage debate to ensure antivenoms are part of essential drugs list within countries. The cost of antivenom should be part of the basic services covered by health insurance in endemic countries.
-
7.
Strategically commence work towards antivenom production based on the WHO Roadmap for regional public–private partnerships for antivenom production in LMICs.
-
8.
Advocate for counterpart funding from individual nation states or collectively through the continental pool with Africa CDC using the potential for good return on investment based on computation that evaluate under which conditions the cost of establishing the antivenom production plant would compare favourably with the cost of importation of antivenom needs for the continent. Given the economy of scales of the burden of snakebite in Africa, a business case could be made in addition to lives saved and other opportunity costs related to the burden of snake envenomation in the region could be a strong advocacy tool (Habib et al., 2015b)
-
9.
Engage the private sector to contribute to these aims by utilizing their social corporate responsibility and incentives like tax breaks.
-
10.
Maintain advocacy and monitoring and evaluation based on evidence-based project management tools to maintain the whatever momentum gained from above steps
1.4. Improving demand, correct, and rational use of antivenoms
This goal has been captured by the one of the five pillars of the new public health order by Africa CDC: a strong public-health workforce and strengthened public-health institutions (Nkengasong and Tessema, 2020). For endemic regions, training healthcare workers on effective management of snakebite is as important as training for infection prevention and control, antimicrobial stewardship, and surveillance and response to outbreaks. There should be advocacy for utilization of training opportunities for other public health priorities to capture snakebite management. A hub-and-spoke model could be used to provide supportive supervision and on-the-job training from more specialized centers to more rural, under-staffed settings where most of the burden is. The use of telemedicine to support this could be explored. This will provide confidence, access, and increased use of antivenoms on one hand, and ensure more rational use and reduced wastage on the other hand. Furthermore, sensitising the public on the need for prompt reporting to established healthcare facilities for access to efficacious antivenoms could create demand and enhance the utilization of antivenoms.
2. Advocacy for research and development, funding, and equitable distribution
2.1. Procurement
As mentioned above, local production of therapeutics, vaccines and diagnostics through trusted partnerships are in line with the first two pillars of Africa CDC's new public health order (Nkengasong and Tessema, 2020; African Union, 2022). Given that this is a long-term approach, in the interim, the WHO and Africa CDC should provide leadership for procurement and equitable distribution of vaccines the same way COVID19 vaccines, and IPC consumables were procured and distributed. This should be through rigorous estimation of the volume of antivenom needed, solid epidemiological information, and anticipated rational antivenom use.
2.2. Quantity and quality of antivenoms
As part of the mapping of resources and technical capacity earlier mentioned, Africa should identify hubs with regulatory capacity to be responsible for quality assurance of antivenoms. They should serve as quality control hub for procurement and/or production of antivenoms for the continent. The demonstration of preclinical and clinical efficacy of antivenoms distributed in African countries, either produced locally or elsewhere, is of paramount relevance to attain this goal.
2.3. Logistics, supply chain, and viability
The African continent has vast experience with logistic and supply chain maintenance with managing the AIDS pandemic through the U.S. President's Emergency Plan for AIDS Relief (PEPFAR) and Global Fund as well as Polio eradication initiatives through the GAVI initiatives among others. This should be utilized to support the logistics for equitable distribution of antivenoms. As mentioned earlier, newer technologies like the use of drones could address viability and supply to hard-to-reach populations. The ongoing efforts by the Africa CDC to acquire and distribute COVID-19 vaccine through the Saving Lives and Livelihood Programme as well as other initiatives should be leveraged upon to address other public health priorities like snake envenomation.
3. Policy implications
The ultimate solution for lack of quality and affordable antivenoms in Africa, and of the effective clinical management of these envenomnations, is a multi-pronged approach of having locally produced antivenom driven by African governments and institutions, complemented by strategic acquisition of safe and effective antivenoms manufactured abroad, and supervised by rigorous regulatory policies (Gutiérrez, 2018). This should be driven by the primary objective of reducing morbidity and mortality from snake envenomation.
3.1. Future directions
The ambitious goal of the WHO to halve the global burden of snakebite by 2030 provide a window of opportunity to intensify advocacy to address the multicomponent challenges that militate against availability and accessibility of safe and effective antivenoms. The case for locally produced antivenoms, driven by African governments have been made repeatedly by the scientists. There is the need to focus on leveraging the institutional and governance structure provided by African government through the Africa CDC under the African Union to bring all stakeholders and ensure synergy of efforts towards ensuring long-term and sustainable flow of antivenoms. These will include the upstream, mid-stream, and downstream factors as earlier highlighted and advocated for by other workers. In parallel, training programs for physicians, nurses and other health care workers should be promoted, together with community engagement efforts aimed at improving prevention and early management of envenomations.
Credit author statement
‘Mahmood Dalhat: design of the study, collection of information, preparation of the first draft, editing of the first draft, revision of the subsequent and final drafts. Julien Potet: critical review of first draft, provision of guidance, revision and validation of the final draft. Abdulaziz Mohammed, Nafiisah Chotun, and Hanna Amanuel Tesfahunei: critical review of the draft and providing context and guidance on African Union governance, revision and validation of the final draft. Abdulrazaq Habib: design of the study, provision of guidance, critical review of first draft, revision and validation of the final draft.’
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We remain indebted to the senior colleagues that dedicated their lives building a case for making snake envenomation a global health priority. We reserved special thanks to José María Gutiérrez for making active contributions towards preparation and improvement in the quality of the manuscript.
Handling Editor: Denise Tambourgi
Data availability
No data was used for the research described in the article.
References
- African Union. Partnerships for African Vaccine Manufacturing (PAVM) Framework for Action. 2022;2022(Version 1):1–99.
- CDC A. Call to action: Africa’s new public health order. 2022. https://africacdc.org/wp-content/uploads/2022/09/Call-to-Action-NPHO-Final-CTA-20-Sep-Edited.pdf Available from:
- Chippaux J. Snake-bites: appraisal of the global situation [Internet] 1998. ncbi.nlm.nih.gov/pmc/articles/PMC2305789/ Available from. [PMC free article] [PubMed]
- Chippaux J.P. Estimate of the burden of snakebites in sub-Saharan Africa: a meta-analytic approach. Toxicon. 2011;57(4):586–599. doi: 10.1016/j.toxicon.2010.12.022. Mar 15. [DOI] [PubMed] [Google Scholar]
- Dada A.O., Lee C.T., Elisha A., Oyebanji O., Danjuma J.S., Sagir K., et al. Impact of a newly established revolving outbreak Investigation fund on timeliness of response to public health emergencies in Nigeria. 2022. https://www.liebertpub.com/doi/10.1089/hs.2021.0126?url_ver=Z39.88-2003&rfr_id=ori:rid:crossref.org&rfr_dat=cr_pub 0pubmed 20(2). Available from: [DOI] [PubMed]
- Gutiérrez J. Global availability of antivenoms: The relevance of public manufacturing laboratories. Toxins (Basel) 2018;11(1):5. doi: 10.3390/toxins11010005. http://www.mdpi.com/2072-6651/11/1/5 Dec 24 [cited 2022 Oct 6] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez J.M., Burnouf T., Harrison R.A., Calvete J.J., Kuch U., Warrell A. 2014. A Multicomponent Strategy to Improve the Availability of Antivenom for Treating Snakebite Envenoming; pp. 526–532. October 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez J.M., Calvete J.J., Habib A.G., Harrison R.A., Williams D.J., Warrell D.A. Snakebite envenoming. Nat. Rev. Dis. Prim. 2017;3(17063) doi: 10.1038/nrdp.2017.63. [DOI] [PubMed] [Google Scholar]
- Habib A.G., Warrell D.A. Antivenom therapy of carpet viper (Echis ocellatus) envenoming: effectiveness and strategies for delivery in West Africa. Toxicon. 2013;69:82–89. doi: 10.1016/j.toxicon.2013.01.002. Jul 1. [DOI] [PubMed] [Google Scholar]
- Habib A.G., Kuznik A., Hamza M., Abdullahi M.I., Chedi B.A., Chippaux J.P., et al. Snakebite is under appreciated: appraisal of burden from west Africa. PLoS Neglected Trop. Dis. 2015;9(9):4–11. doi: 10.1371/journal.pntd.0004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib A.G., Lamorde M., Dalhat M.M., Habib Z.G., Kuznik A. Cost-effectiveness of antivenoms for snakebite envenoming in Nigeria. PLoS Neglected Trop. Dis. 2015;9(1) doi: 10.1371/journal.pntd.0003381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habib Z.G., Salihu A.S., Hamza M., Yakasai A.M., Iliyasu G., Yola I.M., et al. Posttraumatic stress disorder and psycho-social impairment following snakebite in Northeastern Nigeria. Int. J. Psychiatr. Med. 2020;56(2):97–115. doi: 10.1177/0091217420913400. Mar 26. [DOI] [PubMed] [Google Scholar]
- Halilu S., Iliyasu G., Hamza M., Chippaux J.P., Kuznik A., Habib A.G. Snakebite burden in Sub-Saharan Africa: estimates from 41 countries. Toxicon. 2019;159:1–4. doi: 10.1016/j.toxicon.2018.12.002. Mar 1. [DOI] [PubMed] [Google Scholar]
- Hamza M., Kuznik A., Id J.C. Challenges and prospects of snake antivenom supply in sub-Saharan Africa. 2020. 811(Table 1):1–10. Available from. [DOI] [PMC free article] [PubMed]
- Hamza M., Knudsen C., Gnanathasan C.A., Monteiro W., Lewin M.R., Laustsen A.H., et al. Clinical management of snakebite envenoming: Future perspectives. Toxicon X. 2021;11 doi: 10.1016/j.toxcx.2021.100079. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison R.A., Hargreaves A., Wagstaff S.C., Faragher B., Lalloo D.G. 2009. Snake Envenoming: A Disease of Poverty. 3(12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliyasu G., Tiamiyu A.B., Daiyab F.M., Tambuwal S.H., Habib Z.G., Habib A.G. Effect of distance and delay in access to care on outcome of snakebite in rural north-eastern Nigeria. Rural Remote Health. 2015;15(4):76–81. https://search.informit.org/doi/10.3316/informit.223800636408692 Dec 1. [PubMed] [Google Scholar]
- Minghui R., Malecela M.N., Cooke E., Abela-ridder B. Comment WHO ’ s snakebite envenoming strategy for prevention and control. Lancet Glob. Heal. [Internet] 2019;7(7) doi: 10.1016/S2214-109X(19)30225-6. Available from: [DOI] [PubMed] [Google Scholar]
- Muhammed A., Dalhat M.M., Joseph B.O., Ahmed A., Nguku P., Poggensee G., et al. Predictors of depression among patients receiving treatment for snakebite in general hospital, kaltungo, gombe state, Nigeria: August 2015. Int. J. Ment. Health Syst. 2017;11(1):26. doi: 10.1186/s13033-017-0132-8. Dec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkengasong J.N., Tessema S.K. Africa needs a new public health order to tackle infectious disease threats. Cell. 2020;183(2):296–300. doi: 10.1016/j.cell.2020.09.041. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nkengasong J., Djoudalbaye B., Maiyegun O. Comment A new public health order for Africa ’ s health security. Lancet Glob. Heal. 2017;5(11) doi: 10.1016/S2214-109X(17)30363-7. Available from: [DOI] [PubMed] [Google Scholar]
- Nwannekanma B. Zipline. Kaduna begin first drone medical deliveries in Nigeria. Guardian. 2022 https://guardian.ng/news/zipline-kaduna-begin-first-drone-medical-deliveries-in-nigeria/ Available from: [Google Scholar]
- Pedrique B., Strub-wourgaft N., Some C., Olliaro P., Trouiller P., Ford N., et al. The drug and vaccine landscape for neglected diseases (2000 – 11): a systematic assessment. Lancet. 2013;1:371–379. doi: 10.1016/S2214-109X(13)70078-0. December. [DOI] [PubMed] [Google Scholar]
- Potet J., Beran D., Ray N., Alcoba G., Garba A., Iliyasu G., et al. Access to antivenoms in the developing world: A multidisciplinary analysis. Toxicon X. 2021;12 doi: 10.1016/j.toxcx.2021.100086. https://undocs.org/A/RES/71/313 Available from: (Accessed) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sham R., Siau C.S., Tan S., Kiu D.C., Sabhi H., Thew H.Z., et al. Drone usage for medicine and vaccine delivery during the COVID-19 pandemic: attitude of health care workers in rural medical centres. Drones. 2022;6(109):1–10. [Google Scholar]
- Sing M., Hii Y., Courtney P., Royall P.G. An evaluation of the delivery of medicines using drones. Drones. 2019;3(52):1–20. https://www.mdpi.com/2504-446X/3/3/52 Available from: [Google Scholar]
- UN General Assembly . 2017. Work of the Statistical Commission pertaining to the 2030 Agenda for Sustainable Development; p. 11371. July. [Google Scholar]
- WHO . 2019. Snakebite Envenoming: a Strategy for Prevention and Control.https://www.who.int/publications/i/item/9789241515641 Geneva, Available from: [DOI] [PubMed] [Google Scholar]
- WHO . 2021. Snakebite envenoming.https://www.who.int/news-room/fact-sheets/detail/snakebite-envenoming Fact sheets. [Google Scholar]
- Wirtz V.J., Hogerzeil H.V., Gray A.L., Bigdeli M., Joncheere CP De, Ewen M.A., et al. Essential medicines for universal health coverage. Lancet. 2016;(389):403–476. doi: 10.1016/%0AS0140-6736(16)31599-9. Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.