Abstract
Background
We evaluated clinical and laboratory findings among patients with nonsevere or severe dengue in Puerto Rico to examine whether clinical manifestations vary by age.
Methods
During 2012–2014, we enrolled patients who arrived at the emergency department with fever or history of fever within 7 days of presentation. Serum samples were tested for dengue virus (DENV) by reverse transcriptase-polymerase chain reaction (RT-PCR) and IgM enzyme-linked immunosorbent assay (ELISA). Severe dengue was defined as severe plasma leakage or shock, severe bleeding, or organ involvement at presentation, during hospitalization, or follow-up.
Results
Of 1089 dengue patients identified, 281 (26%) were severe. Compared to those with nonsevere dengue, patients with severe dengue were more often aged 10–19 years (55% vs 40%, P < .001) and hospitalized (87% vs 30%, P < .001). Severe plasma leakage or shock was more common among children aged 0–9 (59%) or 10–19 years (86%) than adults (49%) (P < .01). Severe bleeding was less common among 10–19 year olds (24%) compared to 0–9 year olds (45%) and adults (52%; P < .01).
Conclusions
Severe plasma leakage was the most common presentation among children, highlighting important differences from adults. Vaccination against dengue could help prevent severe dengue among children in Puerto Rico.
Keywords: dengue, severe, plasma leakage, Puerto Rico, children
We evaluated patients with dengue in Puerto Rico. Most cases were among children. Severe plasma leakage was the most common presentation among children, highlighting important differences from adults. Vaccination could help prevent severe dengue among children in Puerto Rico.
Dengue virus (DENV) and other arboviruses transmitted by Aedes aegypti mosquitoes, including Zika and chikungunya viruses, present an increasing public health challenge in tropical and subtropical regions. There are 4 genetically and antigenically distinct clades, thought of as serotypes (DENV-1–4) [1]. Following an infection with one DENV serotype, the resulting antibodies are initially broadly cross-reactive with other DENV serotypes and provide short-term cross-protection, but over time become serotype specific to provide long-lasting protection against the infecting serotype [2].
Dengue is endemic throughout the tropics and subtropics, with an estimated 3.83 billion people (roughly 53% of the global population) living in areas suitable for DENV transmission, including much of Asia, Africa, and the Americas [3]. Over the past 3 decades, dengue incidence and severity across Latin America has increased substantially [4]. In Puerto Rico, dengue has been endemic since at least the 1960s and epidemics occurred roughly every 3–5 years until chikungunya [5] and Zika virus outbreaks [6] occurred in 2014 and 2015–2016, respectively, after which dengue transmission has remained low. During the last major epidemic in 2012–2013, more than 30 000 suspected dengue cases were reported, approximately 16 000 of which had diagnostic evidence of DENV infection confirmed by reverse transcription polymerase chain reaction (RT-PCR) [7], including 69 fatal cases (Centers for Disease Control and Prevention [CDC], unpublished data, 2019). Reported dengue cases likely underestimate the true burden of dengue in Puerto Rico, as recent estimates suggest that for every reported hospitalized dengue patient there were an additional 5–9 unreported hospitalized cases, and for every reported ambulatory dengue case there were an additional 21–115 unreported ambulatory cases [8].
Typically, <5% of persons with dengue will progress to severe dengue, a more severe and potentially fatal form of the disease. Age, comorbidities, host genetics, and virus strain are risk factors for severe dengue, with heterotypic secondary infections being the most prominent factor associated with severe dengue [2]. The most common form of severe dengue differs from uncomplicated dengue by the presence of increased vascular permeability, leading to plasma leakage into the peritoneum, pleural cavity, or pericardium. If not recognized and treated in a timely manner, the patient may progress to hypovolemic shock, metabolic acidosis, major hemorrhagic manifestations, or death. At present, no effective antiviral treatments are available for dengue. The mainstay for preventing morbidity and mortality is timely and appropriate supportive care, particularly among patients with severe dengue. The case-fatality ratio for severe dengue can be 10% or higher when untreated but can be reduced to less than 1% with appropriate clinical management [9].
The first Food and Drug Administration-approved dengue vaccine, CYD-TDV (chimeric yellow fever-dengue-tetravalent dengue vaccine) manufactured by Sanofi Pasteur, was recently recommended by the Advisory Committee on Immunization Practices (ACIP) for children aged 9–16 years who live in an endemic area in the Unites States and have laboratory evidence of prior DENV infection [10]. Understanding the proportion of dengue and severe dengue in this age group, and whether the severe disease clinical manifestations and complications are different among children compared to adults, is important for clinical management and vaccine prioritization. We utilized data from the Sentinel Enhanced Dengue Surveillance System (SEDSS) to compare clinical and laboratory findings among patients with nonsevere dengue to those with severe dengue and explore differences in manifestations of severe dengue by age.
METHODS
Study Enrollment and Data Collection
SEDSS is a facility-based acute febrile illnesses surveillance system that operates at 2 clinical sites in southern Puerto Rico: Saint Luke’s Episcopal Hospital located in Ponce (SLEH-Ponce), a 425 inpatient bed, tertiary care teaching hospital that has approximately 50 000 annual emergency department (ED) visits; and SLEH-Guayama, a 161 inpatient bed hospital that received approximately 35 000 annual ED visits [11]. We analyzed clinical and laboratory data from patients with diagnostic evidence of dengue enrolled in SEDSS during 2012–2014.
A detailed description of the SEDSS methods have been previously published [11]. In brief, patients who presented to the emergency room at either SEDSS site were eligible for enrollment if fever was present or they reported a history of fever in the last 7 days. The SEDSS protocol was approved by the Ponce Health Sciences University Institutional Review Board and all participants gave informed consent. A form was completed at enrollment to document clinical characteristics and laboratory findings. Hospitalized participants had their clinical course summarized via the hospital admission abstraction form. Blood, urine, nasopharyngeal, and oropharyngeal specimens were collected from all participants for diagnostic testing. Convalescent blood and urine and additional clinical information were collected at the follow-up visit 7–10 days after hospital discharge. Specimens were processed and transported to CDC Dengue Branch in San Juan, Puerto Rico for diagnostic testing.
Dengue Diagnostic Testing
Serum specimens collected ≤6 days postonset (DPO) were tested by DENV-serotype–specific real-time, reverse transcriptase-polymerase chain reaction assay (rRT-PCR) [12]. Specimens collected ≥ 4 DPO were tested by IgM antibody capture enzyme-linked immunosorbent assay (MAC-ELISA) anti-DENV IgM Capture [13]. Serostatus was determined by testing RT-PCR–positive specimens collected <7 DPO by anti-DENV IgG ELISA [14].
Definitions
Participants were patients who were offered and accepted enrollment in SEDSS. Dengue cases were participants that tested positive for dengue by rRT-PCR or anti-DENV IgM ELISA. Primary DENV infection was a dengue case for which anti-DENV IgG antibody was not detected, and those with secondary DENV infection had anti-DENV IgG antibody in specimens collected ≤5 DPO. Dengue clinical case classifications followed the 2009 World Health Organization (WHO) guidelines [15]. Dengue warning signs were defined by abdominal pain or tenderness; persistent vomiting (ie, ≥3 episodes of emesis in <24 hours); pleural effusion or ascites; mucosal bleeding defined as bleeding from gums or nose, vaginal bleeding, or hematuria; lethargy or restlessness; hepatomegaly (ie, liver enlargement >2 cm); or hemoconcentration.
Dengue cases were classified as severe dengue if at enrollment or follow-up 7–10 days DPO they developed severe dengue manifestations, defined as (1) severe plasma leakage or shock; (2) severe bleeding; or (3) severe organ impairment (definitions provided in Box 1 and Supplementary Table 1).
Box 1. Definition of Severe Dengue.
| Severe plasma leakage or shock | Severe dengue defined as (1) severe plasma leakage or shock; OR (2) severe bleeding; OR (3) severe organ impairment | |
| Shock OR respiratory distress AND plasma leakage | ||
| Shock, any of 3 criteria: 1. Shock listed in medical assessment at any time 2. Use of vasopressors, inotropics, or vasodilators; use of albumin 3. Pulse pressure <20 mmHg OR hypotension OR drop in systolic blood pressure >40 mmHg AND any 2 of the following: Elevated heart rate Capillary refill > 2 seg Mottled skin Thready, weak pulse Pale and/or cold skin Blue skin or lips Cyanotic limbs Cold limbs |
Respiratory distress and plasma leakage, both criteria: 1. Respiratory compromise: any of the following Tachypnea Respiratory distress Accessory muscles use Supplemental oxygen Intubation 2. Plasma leakage: any of the following Pleural effusion Pericardial effusion Ascites Free fluid in abdomen Hematocrit change >20% during illness Hematocrit value >20% above baseline for age and sex at any time Albumin low for age |
|
| Severe bleeding | Any of the following: Blood transfusion Hematochezia Melena Hematemesis |
|
| Severe organ involvement | Any of the following: AST or ALT ≥ 1000 U Intubation/mechanical ventilation PT INR ≥ 1.5 Encephalopathy Myocarditis Acute hepatitis Glasgow score <11 Aseptic meningitis Acute paralysis |
|
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; INR, international normalized ratio; PT, prothrombin time.
Data Analysis
We compared the distribution of demographic variables, clinical signs and symptoms, and laboratory findings between dengue and severe dengue cases using R statistical package. We used the Mann-Whitney-Wilcoxon test to compare medians and χ2 test to estimate the difference in proportions. Separate multivariate logistic regression models explored the association of laboratory findings and clinical symptoms with severe dengue, adjusted by age and DPO. Interaction terms between variables significantly associated with severe dengue in bivariate analyses and age were evaluated. Separate adjusted odds ratios (OR) by age category are presented. Because anti-DENV IgM antibody may remain detectable for several months and potentially result in false-positive cases due to prior rather than acute infection, we conducted a sensitivity analysis that only included dengue cases confirmed by RT-PCR. Findings and significant associations were unchanged when IgM ELISA cases were excluded.
RESULTS
During 2012–2014, a total of 1089 dengue cases were enrolled in SEDSS, of which 720 (66%) tested positive by RT-PCR and 369 (34%) by anti-DENV IgM ELISA only. A total of 281 (26%) cases had severe dengue and 808 (74%) did not (Table 1). Median age among severe dengue cases was 16 years compared to 15 years among nonsevere cases (P = .04); 55% of severe dengue cases and 40% of nonsevere cases were aged 10–19 years (P < .001). Nealy half (45%) of severe dengue cases occurred among 9–16 year olds, the age group for whom the CYD-TDV dengue vaccine is currently recommended. Severe dengue cases presented for care significantly later in the clinical course than nonsevere cases (mean DPO, 4 [range, 0–7] vs 3 [0–7], respectively, P < .001). A total of 483 (44%) dengue cases were hospitalized, including 87% (245/281) of severe dengue cases and 30% (238/808) of nonsevere cases. Of the 36 severe dengue cases not hospitalized at the study sites, 2 were transferred to another facility, 30 had severe bleeding, and 4 had severe plasma leakage or shock. There was no difference in age or sex between hospitalized and nonhospitalized severe cases. Of the 238 nonsevere cases that were hospitalized, 32% had a comorbidity and 81% had at least 1 warning sign.
Table 1.
Demographic Characteristics and Clinical Laboratory Findings Among Patients With Dengue and Severe Dengue, Sentinel Enhanced Dengue Surveillance System, Puerto Rico, 2012–2014
| Characteristic | Total, No. (%) (n = 1089) | Severe, No. (%) (n = 281) | Nonsevere, No. (%) (n = 808) | P Value,a Severe vs Nonsevere |
|---|---|---|---|---|
| Sex | ||||
| Male | 579 (53.2) | 158 (56.2) | 421 (52.1) | .26 |
| Female | 510 (46.8) | 123 (43.8) | 387 (47.9) | .26 |
| Age, y, median (range) | 15.3 (0–97.1) | 16.1 (0.2–82.8) | 14.9 (0–97.1) | .04 |
| 0–9 | 274 (25.2) | 49 (17.4) | 225 (27.8) | .001 |
| 10–19 | 478 (43.9) | 155 (55.2) | 323 (40.0) | .001 |
| 20–29 | 119 (10.9) | 25 (8.9) | 94 (11.6) | .25 |
| 30–39 | 59 (5.4) | 15 (5.3) | 44 (5.4) | 1.0 |
| 40–49 | 47 (4.3) | 9 (3.2) | 38 (4.7) | .37 |
| 50+ | 112 (10.3) | 28 (10.0) | 84 (10.4) | .93 |
| Year of recruitment | ||||
| 2012 | 418 (42.8) | 115 (48.5) | 303 (40.9) | .05 |
| 2013 | 455 (46.6) | 108 (45.6) | 347 (46.9) | .78 |
| 2014 | 104 (10.6) | 14 (5.9) | 90 (12.2) | .01 |
| Days after illness onset, median (range) | 3 (0–7.0) | 4 (0–7.0) | 3 (0–7.0) | <.001 |
| <3 | 417 (38.3) | 63 (22.4) | 354 (43.8) | <.001 |
| 3–5 | 592 (54.4) | 191 (68.0) | 401 (49.6) | <.001 |
| 6–7 | 80 (7.3) | 27 (9.6) | 53 (6.6) | .12 |
| Disposition | ||||
| Admitted | 483 (44.4) | 245 (87.2) | 238 (29.5) | <.001 |
| Died | 1 (0.1) | 0 (0) | 1 (0.1) | 1.0 |
| Sent home | 602 (55.3) | 34 (12.1) | 568 (70.3) | <.001 |
| Transferred | 3 (0.3) | 2 (0.7) | 1 (0.1) | .34 |
| Duration of hospitalization among those hospitalized | n = 483 | n = 245 | n = 238 | |
| Duration of hospitalization in days, median (range) | 3.0 (0–14.0) | 3.5 (0–14.0) | 3.0 (1.0–10.0) | <.001 |
| Secondary DENV infection | n = 719 | n = 183 | n = 536 | |
| No | 153 (21.3) | 24 (13.1) | 129 (24.1) | |
| Yes | 566 (78.7) | 159 (86.9) | 407 (75.9) | .003 |
| Infecting DENV | n = 720 | n = 176 | n = 544 | |
| DENV-1 | 679 (94.3) | 161 (91.5) | 518 (95.2) | .09 |
| DENV-2 | 3 (0.4) | 0 (0) | 3 (0.6) | .75 |
| DENV-4 | 38 (5.3) | 15 (8.5) | 23 (4.2) | .04 |
| Laboratory findings | ||||
| Highest AST U/L, median (range) | 104 (10–8111) | 153 (13–8111) | 86 (10–909) | <.001 |
| AST <50 U/L | 129 (22.8) | 49 (19.4) | 80 (25.4) | .11 |
| AST 51–500 U/L | 388 (68.4) | 164 (65.1) | 224 (71.1) | .15 |
| AST 501–1000 U/L | 38 (6.7) | 27 (10.7) | 11 (3.5) | .001 |
| AST >1000 U/L | 12 (2.1) | 12 (4.8) | 0 (0) | <.001 |
| Lowest ALT U/L, median (range) | 87 (160–7548) | 136 (20–7548) | 68 (16–968) | <.001 |
| ALT <50 U/L | 161 (28.3) | 49 (19.4) | 112 (35.4) | <.001 |
| ALT 51–500 U/L | 364 (64.0) | 170 (67.2) | 194 (61.4) | .18 |
| ALT 501–1000 U/L | 39 (6.9) | 29 (11.5) | 10 (3.2) | <.001 |
| ALT >1000 U/L | 5 (0.9) | 5 (2.0) | 0 (0) | .0396 |
| Lowest WBC/microL, median (range) | 3400 (800–24 000) | 2900 (800–19 000) | 3700 (900–24 000) | <.001 |
| WBC ≤4000 cells/microL | 661 (62.2) | 212 (76.5) | 449 (57.2) | <.001 |
| WBC 4001–10 000 cells/microL | 358 (33.7) | 60 (21.7) | 298 (38) | <.001 |
| WBC >10 000 cells/microL | 43 (4.0) | 5 (1.8) | 38 (4.8) | .04 |
| Lowest PLT/microL, median (range) | 11 4000 (2000–511 000) | 62 000 (2000–457 000) | 134 000 (3000–511 000) | <.001 |
| PLT < 50 000/microL | 158 (14.9) | 87 (31.3) | 71 (9.1) | <.001 |
| PLT 50 000–100 000/microL | 294 (27.7) | 119 (42.8) | 175 (22.3) | <.001 |
| PLT >100 000/microL | 609 (57.4) | 72 (25.9) | 537 (68.6) | <.001 |
| Thrombocytopenia <100 000 platelets/microL | 452 (42.6) | 206 (74.1) | 246 (31.4) | <.001 |
| Highest hematocrit %, median (range) | 41.2 (28.5–57.4) | 41.3 (28.9–55.5) | 41.0 (28.5–57.4) | .95 |
| Hemoconcentration | 60 (5.6) | 30 (10.8) | 30 (3.8) | <.001 |
| Low albumin g/dL | 309 (65.5) | 208 (85.6) | 101 (44.1) | <.001 |
| Lowest albumin g/dL, median (range) | 3.2 (1.9–5.0) | 3.1 (1.9–4.1) | 3.4 (2.0–5.0) | <.001 |
Data are No. (%) except where indicated.
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; DENV, dengue virus; PLT, platelets; WBC, white blood cell; U/L, units per litre; microL, microlitre; dL, decilitre; g, grams.
Calculated with the Mann-Whitney-Wilcoxon test to compare medians and χ2 test to estimate the difference in proportions.
Of 719 dengue cases that could be classified as primary or secondary DENV infections, 87% (159/183) of severe dengue cases had secondary DENV infection compared to 76% (407/536) of nonsevere cases (P = .003). Among 720 dengue cases for which the infecting DENV was identified, most (94%) were infected with DENV-1, followed by DENV-4 (5%) and DENV-2 (<1%). Among cases infected with DENV-1, 24% (161/679) were severe, compared to 40% (23/38) of DENV-4 cases (P = .04). Almost all severe cases infected with DENV-4 (92%) or DENV-1 (84%) were secondary infections. Severe dengue cases compared to nonsevere dengue cases more frequently had leukopenia (77% and 57%, respectively, P < .001), thrombocytopenia (74% and 31%, respectively, P < .001), low albumin (86% and 44%, P < .001), and hemoconcentration (11% vs 4%, P < .001). Similar differences were observed when limiting analysis to laboratory tests conducted at presentation (Supplementary Table 2). After adjusting for age and DPO, leukopenia (aOR, 1.4; 95% confidence interval [CI], 1.0–2.1; P < .001), thrombocytopenia (aOR, 5.3; 95% CI, 3.8–7.4) and secondary DENV infection (aOR, 2.0; 95% CI, 1.2–3.3) were significantly more prevalent among patients with severe dengue. These associations varied by age, with few significant interactions (Supplementary Table 3). Low albumin and thrombocytopenia had a stronger association with severe dengue among cases aged 10–19 years. Secondary DENV infection was only associated with severe dengue among children and not among adults, as most adults had secondary infections.
Many signs and symptoms were more prevalent among severe dengue cases. Those that persisted after adjusting for age and DPO were: jaundice, rash, petechia/purpura, facial or neck erythema, pruritic skin, red or swollen joints, diarrhea, and irritability. Warning signs were significantly more common among severe dengue cases, and the association remained significant after adjusting by age and DPO for mucosal bleeding (18% vs 11%, P = .01), nervousness/anxiety (51% vs 34%, P < .001), abdominal pain (78% vs 58%, P < .001), persistent vomiting (41% vs 24%, P < .001), and signs of fluid accumulation (9% vs 0.6%, P < .001). Interactions between age and selected clinical findings showed difference in the association of abdominal pain and severe dengue by age (Supplementary Table 3), with a stronger association among cases aged 10–19 years.
Characteristics of Severe Dengue Cases
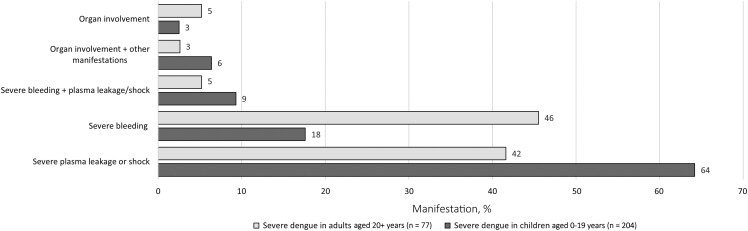
Among all severe dengue cases, the most common manifestation of severe dengue was severe plasma leakage/shock (71%) followed by severe bleeding (35%). Most severe bleeding was gastrointestinal and less frequently pulmonary (Table 2). Organ involvement (9%) was an uncommon manifestation of severe dengue. Few cases had multiple manifestations of severe dengue, including 8% with severe bleeding and severe plasma leakage/shock, and 5% with organ involvement and another manifestation (Figure 1).
Table 2.
Signs and Symptoms Associated With Severe Dengue Among Laboratory-Positive Dengue Patients, Sentinel Enhanced Dengue Surveillance System, Puerto Rico, 2012–2014
| Characteristic | Total, No. (%) (n = 1089) | Severe, No. (%) (n = 281) | Nonsevere, No. (%) (n = 808) | P aValue |
|---|---|---|---|---|
| Comorbidities | ||||
| Any chronic medical condition | 339 (31.1) | 81 (28.8) | 258 (31.9) | .37 |
| Asthma | 203 (18.6) | 50 (17.8) | 153 (18.9) | .7380 |
| Coronary heart disease | 37 (3.4) | 12 (4.3) | 25 (3.1) | .4554 |
| Diabetes | 70 (6.4) | 14 (5.0) | 56 (6.9) | .3144 |
| High blood pressure | 87 (8.0) | 19 (6.8) | 68 (8.4) | .4513 |
| High cholesterol | 44 (4.0) | 11 (3.9) | 33 (4.1) | 1.0000 |
| Cancer | 16 (1.5) | 3 (1.1) | 13 (1.6) | .7175 |
| Thyroid | 43 (3.9) | 8 (2.8) | 35 (4.3) | .3560 |
| Sickle cell disease | 7 (0.6) | 2 (0.7) | 5 (0.6) | 1.0000 |
| Other | 23 (2.1) | 7 (2.5) | 16 (2.0) | .7854 |
| Signs and symptoms | ||||
| Eye pain | 614 (57.2) | 165 (58.7) | 449 (56.6) | .59 |
| Headache | 915 (84.0) | 245 (87.2) | 670 (82.9) | .11 |
| Jaundice/icteric sclera | 57 (5.3) | 35 (12.5) | 22 (2.8) | <.001 |
| Red conjunctiva | 614 (57.2) | 165 (58.7) | 449 (56.6) | .59 |
| Skin rash | 613 (56.9) | 191 (68.0) | 422 (53.0) | <.001 |
| Petechia/purpura | 383 (35.4) | 137 (48.8) | 246 (30.7) | <.001 |
| Facial and/or neck erythema | 637 (59.3) | 190 (68.3) | 447 (56.2) | <.001 |
| Pruritic skin | 412 (38.3) | 132 (47.1) | 280 (35.2) | <.001 |
| Chills | 867 (80.2) | 245 (87.5) | 622 (77.7) | <.001 |
| Muscle/bone/back pain | 833 (77.5) | 237 (84.6) | 596 (75.0) | .001 |
| Joint pain | 667 (62.5) | 194 (69.3) | 473 (60.0) | .007 |
| Red/swollen joints | 177 (16.7) | 61 (22.0) | 116 (14.8) | .008 |
| Cough | 475 (44.1) | 136 (48.7) | 339 (42.5) | .08 |
| Rhinorrhea | 356 (33.1) | 105 (37.6) | 251 (31.5) | .07 |
| Sore throat | 410 (38.3) | 118 (42.1) | 292 (37.0) | .14 |
| Anorexia | 869 (80.8) | 250 (89.6) | 619 (77.7) | <.001 |
| Nausea | 744 (69.5) | 222 (79.9) | 522 (65.9) | <.001 |
| Diarrhea | 449 (41.9) | 148 (53.2) | 301 (37.9) | <.001 |
| Dizziness | 674 (62.5) | 205 (73.2) | 469 (58.8) | <.001 |
| Tiredness, lethargy | 953 (88.1) | 266 (95.3) | 687 (85.6) | <.001 |
| Irritability | 357 (33.2) | 127 (45.4) | 230 (28.9) | <.001 |
| Seizure | 19 (1.8) | 9 (3.2) | 10 (1.2) | .06 |
| Warning signs | ||||
| Mucosal bleeding | 140 (12.9) | 51 (18.1) | 89 (11.1) | .01 |
| Nose/gums | 100 (9.2) | 36 (12.8) | 64 (8.0) | .02 |
| Unusual vaginal | 26 (8.8) | 10 (11.0) | 16 (7.9) | .52 |
| Macroscopic blood in urine | 26 (2.4) | 13 (4.7) | 13 (1.6) | .009 |
| Abdominal pain | 679 (63.1) | 218 (77.9) | 461 (57.9) | <.001 |
| Vomiting (3 or more episodes in day) | 304 (28.1) | 115 (40.9) | 189 (23.6) | <.001 |
| Nervousness, anxiety | 410 (38.3) | 142 (51.1) | 268 (33.8) | <.001 |
| Hemoconcentration | 60 (5.6) | 30 (10.8) | 30 (3.8) | <.001 |
| Fluid accumulation | ||||
| Ascites | 2 (0.8) | 2 (1.3) | 0 (0) | .68 |
| Pleural effusion | 23 (4.0) | 21 (8.4) | 2 (0.6) | <.001 |
| Any fluid accumulation | 24 (4.2) | 22 (8.8) | 2 (0.6) | <.001 |
| Signs of respiratory distress | ||||
| Tachypnea | 491 (46.3) | 209 (75.5) | 282 (36.0) | <.001 |
| Signs of shock | ||||
| Any sign of poor circulation | 716 (65.7) | 219 (77.9) | 497 (61.5) | <.001 |
| At least 2 signs of poor circulation | 210 (19.3) | 69 (24.6) | 141 (17.5) | .0120 |
| Narrow pulse pressure | 21 (2.0) | 9 (3.2) | 12 (1.5) | .14 |
| Hypotension | 71 (15.0) | 47 (19.4) | 24 (10.4) | .009 |
| Drop systolic blood pressure | 15 (3.4) | 10 (5.3) | 5 (2.0) | .11 |
| Use of vasopressors | 4 (0.8) | 4 (1.7) | 0 (0) | .14 |
| Diagnoses of shock | 1 (0.1) | 1 (0.4) | 0 (0) | .58 |
| Severe dengue classification | ||||
| Severe bleeding | 99 (9.1) | 99 (35.2) | 0 (0) | |
| Vomit with blood | 28 (2.6) | 28 (10.0) | 0 (0) | |
| Feces with blood | 17 (1.6) | 17 (6.0) | 0 (0) | |
| Black tarry stools | 49 (4.5) | 49 (17.4) | 0 (0) | |
| Pulmonary hemorrhage | 2 (0.4) | 2 (0.9) | 0 (0) | |
| Any blood product received | 17 (3.6) | 17 (7.0) | 0 (0) | |
| Severe plasma leakage + shock | 200 (18.4) | 200 (71.2) | 0 (0) | |
| Severe plasma leakage | 187 (17.2) | 187 (66.5) | 0 (0) | |
| Fluid accumulation plus respiratory distress | 16 (2.7) | 16 (6.3) | 0 (0) | |
| Shock | 22 (2.0) | 22 (7.8) | 0 (0) | |
| Severe organ involvement | 24 (4.0) | 24 (9.3) | 0 (0) |
Calculated with the Mann-Whitney-Wilcoxon test to compare medians and χ2 test to estimate the difference in proportions.
Figure 1.
Combination of manifestations of severe dengue, Sentinel Enhanced Dengue Surveillance System, Puerto Rico, 2012–2014.
Frequency of severe bleeding was significantly higher among adults compared to 10–19 year olds (52% vs 24%; OR, 0.3; 95% CI, .2–.5) (Table 3). Frequency of organ involvement was similar across age groups (9%–13%). Compared to adults (49%), severe plasma leakage/shock was significantly more common among cases aged 10–19 years (86%; OR, 6.4; 95% CI, 3.5–12.1) or 0–9 years (59%; OR, 1.7; 95% CI, .8–3.6) (Table 3). This association remained significant after adjusting for serostatus.
Table 3.
Difference in Severe Dengue Manifestations and Selected Laboratory Parameters by Age, Sentinel Enhanced Dengue Surveillance System, Puerto Rico, 2012–2014
| Criteria for severe dengue | Total No. Severe Dengue Cases in Each Category (n = 281) | No. (%, Row) | P Valuea | Odds Ratioa (95% CI) |
|---|---|---|---|---|
| Severe bleeding | 281 | 99 (35.2) | ||
| 0–9 y | 49 | 22 (44.9) | .44 | 0.8 (.4–1.5) |
| 10–19 y | 155 | 37 (23.9) | .0000 | 0.3 (.2–.5) |
| 20+ y | 77 | 40 (51.9) | Reference | |
| Severe plasma leakage or shock | 281 | 200 (71.2) | ||
| 0–9 y | 49 | 29 (59.2) | .1380 | 1.7 (.8–3.6) |
| 10–19 y | 155 | 133 (85.8) | <.001 | 6.4 (3.5–12.1) |
| 20+ y | 77 | 38 (49.4) | Reference | |
| Severe organ involvement | 257 | 24 (9.3) | ||
| 0–9 y | 39 | 5 (12.8) | .54 | 1.5 (.4–5.3) |
| 10–19 y | 152 | 13 (8.6) | .95 | 1.0 (.4–2.9) |
| 20+ y | 66 | 6 (9.1) | Reference | |
| Select laboratory values | ||||
| Secondary DENV infection | 183 | 159 (86.9) | ||
| 0–9 y | 30 | 24 (80.0) | .27 | 0.5 (.1–1.8) |
| 10–19 y | 114 | 100 (87.7) | .68 | 0.8 (.2–2.4) |
| 20+ y | 39 | 35 (89.7) | Reference | |
| Leukopenia (WBC ≤4000 cells/microL) | 277 | 212 (76.5) | ||
| 0–9 y | 47 | 29 (61.7) | .80 | 0.9 (.4–1.9) |
| 10–19 y | 155 | 135 (87.1) | <.001 | 3.8 (1.9–7.4) |
| 20+ y | 75 | 48 (64.0) | Reference | |
| Thrombocytopenia (<100 000 platelets/microL) | 278 | 206 (74.1) | ||
| 0–9 y | 47 | 21 (44.7) | .02 | 0.4 (.2–.9) |
| 10–19 y | 155 | 135 (87.1) | .001 | 3.1 (1.6–6.2) |
| 20+ y | 76 | 50 (65.8) | Reference | |
| Low albumin (g/dL) | 243 | 208 (85.6) | ||
| 0–9 y | 37 | 28 (75.7) | .53 | 1.4 (.5–3.6) |
| 10–19 y | 147 | 139 (94.6) | <.001 | 7.5 (3.1–19.5) |
| 20+ y | 59 | 41 (69.5) | Reference | |
| High AST or ALT (>500 U/L) | 252 | 41 (16.3) | ||
| 0–9 y | 38 | 4 (10.5) | .73 | 0.8 (.2–2.8) |
| 10–19 y | 152 | 29 (19.1) | .36 | 1.5 (.7–3.7) |
| 20+ y | 62 | 8 (12.9) | Reference | |
Abbreviations: ALT, alanine transaminase; AST, aspartate transaminase; CI, confidence interval; DENV, dengue virus; WBC, white blood cell; U/L, units per litre; microL, microlitre; dL, decilitre; g, grams.
Odds ratios adjusted by days postonset.
Most severe dengue cases had leukopenia (84%). Thrombocytopenia was common among those aged 10–19 years (87%), but less frequent among those aged <10 years (45%) or >19 years (66%) (Table 3). The association between thrombocytopenia and age remained significant after adjusting by serostatus. Severe bleeding was more common among those with platelet counts greater than 100 000/microL, as it occurred with a frequency of 35%, 24%, and 51% among those with a platelet count of <50 000/microL, 50 000–100 000/microL, and >100 000 platelets/microL, respectively. In contrast, severe plasma leakage was more common among those with the lowest platelet count, and was 78%, 82%, and 49% among those with a platelet count of <50 000/microL, 50 000–100 000/microL, and >100 000 platelets/microL, respectively (P < .001).
Most dengue and severe dengue cases had evidence of past DENV infection. Among 719 with IgG testing available, there were 24 severe dengue cases with primary DENV infections, 2 were among infants, 18 among ages 1–19 years, and 4 cases among adults. Of the 24 severe primary DENV cases, 12 had severe plasma leakage alone; 6 had severe bleeding alone; 2 had severe plasma leakage and severe bleeding; 1 had severe plasma leakage, severe bleeding, and shock; 1 had severe bleeding, plasma leakage, and organ involvement; 1 had organ involvement and plasma leakage; and 1 had shock alone.
DISCUSSION
We report the results of an emergency department- and hospital-based study conducted during the most recent dengue epidemic in Puerto Rico. In this study, more than two-thirds of cases of dengue and severe dengue were among patients aged 0–19 years. Most severe dengue cases and one-third of nonsevere cases were hospitalized, underscoring the burden to the health care system during dengue outbreaks. Most dengue patients in this study were experiencing secondary DENV, and as expected secondary DENV infection was more common among those with severe dengue as documented in other studies [16]. This study shows important differences in severe dengue manifestation among children versus adults: while severe plasma leakage was the most common manifestation for severe dengue among children, severe bleeding was more common among adults.
CYD-TDV is the only dengue vaccine approved and recommended for use in the United States, in children aged 9–16 years who live in endemic areas and have diagnostic evidence of prior DENV infection. Although CYD-TDV is not likely to reduce DENV transmission at the population level, it is highly efficacious in reducing the risk of disease, hospitalizations, and severe dengue among seropositive children [10]. As an estimated 250 000 children live in Puerto Rico and half are estimated to be seropositive [10], routine vaccination among this age group can be an important tool to combat dengue and reduce hospital burden during outbreaks. Nonetheless, as CYD-TDV is only recommended for children aged 9–16 years, and important morbidity occurs among younger and older individuals [10], alternative or additional approaches to reduce the burden of dengue will be needed. Furthermore, this vaccine can increase the risk of severe dengue if administered to seronegative children. Therefore, prevaccination screening with highly specific tests must be implemented as part of a vaccination program, which complicates logistics. Vaccines developed by Takeda (TAK-003) and NIH/Merck (TV003/TV005) have completed or are in phase 3 clinical trials [17, 18], may not require prevaccination screening, and could be used for a broader age range, thus having potentially a larger impact on herd immunity if they are recommended for use by ACIP.
In this study, secondary infection was associated with severe dengue among pediatric patients, and most nonsevere dengue cases were also experiencing secondary infection. Multiple epidemiologic studies have shown that the risk of severe disease is higher during a secondary DENV infection than during a primary infection [16]. Nearly 1 of every 10 severe dengue cases resulted from primary DENV infections, similar to previous reports [16]. The severe dengue cases among primary DENV infections mainly occurred among younger participants, two-thirds of whom presented with plasma leakage and one-third with severe bleeding. Although antibody-dependent enhancement can lead to higher viremia and subsequently severe dengue, it is not the only explanation for the manifestations of severe dengue. Occurrence of severe dengue in primary infections could result from a higher inoculum, viral strain, serotype, or host factors [19–21].
The rate of hospitalization in this study was high, similar to what has been reported in Asia but lower than in other parts of Latin America [22]. This may be influenced by recruitment for our study being based at major referral hospitals with a bias towards more severe cases and hospitalizations. This burden of dengue on the health care system has significant economic impact. The global average estimated cost per dengue case is US$70.10 (95% uncertainty interval, $66.66–$74.63) for cases admitted to hospital, and $51.16 ($49.80–$53.71) for an ambulatory case [23]. In contrast, the cost of hospitalizing dengue patients in Puerto Rico (US$2132; 95% CI, 1705–2558) is substantially higher than the global average (US$70.10) [24, 25]. Similarly, the disability-adjusted life year (DALY) losses due to dengue in Puerto Rico are on the same order of magnitude of those due to malaria, tuberculosis, or hepatitis elsewhere in Latin America and the Caribbean [26]. Hence, implementation of dengue vaccination in Puerto Rico, even if only among 9–16 year olds initially, could have economic impact.
The most common presentation of severe dengue was severe plasma leakage followed by severe bleeding. Our findings support the generally accepted view that severe vascular leakage is more common in children [27, 28]. Age is known to influence intrinsic vascular permeability, with children demonstrating a lower threshold for leakage than adults [27]. Shock was uncommon and present in less than one-tenth of severe dengue patients. Contrary to previous reports suggesting that hemorrhage in children is rare and associated with shock [20, 27], we observed hemorrhage in one-third of severe dengue cases among children aged <10 years. Co-occurrence of severe plasma leakage/shock and hemorrhage was uncommon. While thrombocytopenia was highly prevalent among severe dengue cases, it was not associated with hemorrhage in this study but was associated with severe plasma leakage, as reported elsewhere [29].
Whereas strengths of this study include utilization of a relatively large sample size and identification of dengue patients among all age groups, there were several limitations. We did not have results on serostatus and serotype among all participants. DENV-1 predominated during the study and findings may not be generalizable to other serotypes. Most participants with plasma leakage were classified as severe because of low albumin, which can be caused by other chronic comorbidities such as liver injury; however, most patients with plasma leakage were children among whom comorbidities were uncommon. Dengue cases were defined as either IgM positive or PCR positive, which may have led to some misclassification. However, a sensitivity analyses including only PCR-positive cases found similar results. Also, clinical findings were not available for dengue cases transferred or admitted to other facilities, hence the frequency of some manifestations and patient outcomes may have been underestimated. Last, definition of severe dengue was based on WHO criteria and included signs of gastrointestinal bleeding. Gastrointestinal bleeding without shock, hemodynamic instability, or need for blood transfusion may not indicate clinically severe dengue [11], in particular if it was self-reported, as in this study. Hence, some cases of severe dengue may have been misclassified. This helps explain why 34 cases of severe dengue were not hospitalized; self-reported data on severe bleeding could have been ruled out by clinicians after exploring further, resulting in the patient being discharged.
This study is one of the larger studies describing severe dengue in the current literature and contributes to the understanding of the prevalence of the different manifestations of severe dengue. Because most severe dengue cases were among children aged 10–19 years, the currently approved dengue vaccine for children and adolescents aged 9–16 years could help reduce disease and hospitalizations among this group. Clinicians should be aware of the different manifestations of severe dengue in children versus adults and carefully evaluate pediatric patients for plasma leakage, which may be harder to identify than severe hemorrhage. This may help clinicians to initiate supportive management rapidly and judiciously.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Supplementary Material
Contributor Information
Gabriela Paz-Bailey, Centers for Disease Control and Prevention, San Juan, Puerto Rico.
Liliana Sánchez-González, Centers for Disease Control and Prevention, San Juan, Puerto Rico.
Brenda Torres-Velasquez, Centers for Disease Control and Prevention, San Juan, Puerto Rico.
Emma S Jones, Centers for Disease Control and Prevention, Fort Collins, Colorado, USA.
Janice Perez-Padilla, Centers for Disease Control and Prevention, San Juan, Puerto Rico.
Tyler M Sharp, Centers for Disease Control and Prevention, San Juan, Puerto Rico; US Public Health Service, Rockville, Maryland, USA.
Olga Lorenzi, Centers for Disease Control and Prevention, San Juan, Puerto Rico.
Mark Delorey, Centers for Disease Control and Prevention, Fort Collins, Colorado, USA.
Jorge L Munoz-Jordan, Centers for Disease Control and Prevention, San Juan, Puerto Rico.
Kay M Tomashek, Centers for Disease Control and Prevention, San Juan, Puerto Rico; US Public Health Service, Rockville, Maryland, USA; National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Stephen H Waterman, Centers for Disease Control and Prevention, San Juan, Puerto Rico; US Public Health Service, Rockville, Maryland, USA.
Luisa I Alvarado, Ponce Health Sciences University/Ponce Research Institute, Ponce, Puerto Rico.
Vanessa Rivera-Amill, Ponce Health Sciences University/Ponce Research Institute, Ponce, Puerto Rico.
Notes
Acknowledgment. We thank the contribution of all study participants.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Financial support. This work was funded by the Centers for Disease Control and Prevention (grant numbers U01CK000437 and U01CK000580).
References
- 1. Katzelnick LC, Fonville JM, Gromowski GD, et al. . Dengue viruses cluster antigenically but not as discrete serotypes. Science 2015; 349:1338–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wilder-Smith A, Ooi EE, Horstick O, Wills B. Dengue. Lancet 2019; 393:350–63. [DOI] [PubMed] [Google Scholar]
- 3. Messina JP, Brady OJ, Golding N, et al. . The current and future global distribution and population at risk of dengue. Nat Microbiol 2019; 4:1508–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pan American Health Organization . PLISA Health Information Platform for the Americas. Dengue. https://www3.paho.org/data/index.php/en/mnu-topics/indicadores-dengue-en.html. Accessed 24 May2021.
- 5. Sharp TM, Ryff KR, Alvarado L, et al. . Surveillance for chikungunya and dengue during the first year of chikungunya virus circulation in Puerto Rico. J Infect Dis 2016; 214:S475–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sharp TM, Quandelacy TM, Adams LE, et al. . Epidemiologic and spatiotemporal trends of Zika virus disease during the 2016 epidemic in Puerto Rico. PLoS Negl Trop Dis 2020; 14:e0008532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sharp TM, Ryff KR, Santiago GA, Margolis HS, Waterman SH. Lessons learned from dengue surveillance and research, Puerto Rico, 1899–2013. Emerg Infect Dis 2019; 25:1522–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shankar MB, Rodriguez-Acosta RL, Sharp TM, Tomashek KM, Margolis HS, Meltzer MI. Estimating dengue under-reporting in Puerto Rico using a multiplier model. PLoS Negl Trop Dis 2018; 12:e0006650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kabra SK, Verma IC, Arora NK, Jain Y, Kalra V. Dengue haemorrhagic fever in children in Delhi. Bull World Health Organ 1992; 70:105–8. [PMC free article] [PubMed] [Google Scholar]
- 10. Paz-Bailey G, Adams L, Wong JM, et al. . Dengue vaccine: recommendations of the advisory committee on immunization practices, United States, 2021. MMWR Recomm Rep 2021; 70:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tomashek KM, Lorenzi OD, Andujar-Perez DA, et al. . Clinical and epidemiologic characteristics of dengue and other etiologic agents among patients with acute febrile illness, Puerto Rico, 2012–2015. PLoS Negl Trop Dis 2017; 11:e0005859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Santiago GA, Vergne E, Quiles Y, et al. . Analytical and clinical performance of the CDC real time RT-PCR assay for detection and typing of dengue virus. PLoS Negl Trop Dis 2013; 7:e2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martin DA, Muth DA, Brown T, Johnson AJ, Karabatsos N, Roehrig JT. Standardization of immunoglobulin M capture enzyme-linked immunosorbent assays for routine diagnosis of arboviral infections. J Clin Microbiol 2000; 38:1823–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miagostovich MP, Nogueira RM, dos Santos FB, Schatzmayr HG, Araujo ES, Vorndam V. Evaluation of an IgG enzyme-linked immunosorbent assay for dengue diagnosis. J Clin Virol 1999; 14:183–9. [DOI] [PubMed] [Google Scholar]
- 15. World Health Organization . Dengue: guidelines for diagnosis, treatment, prevention and control - new edition. France: World Health Organization, 2009. [PubMed] [Google Scholar]
- 16. Sangkaew S, Ming D, Boonyasiri A, et al. . Risk predictors of progression to severe disease during the febrile phase of dengue: a systematic review and meta-analysis. Lancet Infect Dis 2021; 21:1014–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Biswal S, Borja-Tabora C, Martinez Vargas L, et al. . Efficacy of a tetravalent dengue vaccine in healthy children aged 4-16 years: a randomised, placebo-controlled, phase 3 trial. Lancet 2020; 395:1423–33. [DOI] [PubMed] [Google Scholar]
- 18. Whitehead SS. Development of TV003/TV005, a single dose, highly immunogenic live attenuated dengue vaccine; what makes this vaccine different from the Sanofi-Pasteur CYD vaccine? Expert Rev Vaccines 2016; 15:509–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vaughn DW, Green S, Kalayanarooj S, et al. . Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J Infect Dis 2000; 181:2–9. [DOI] [PubMed] [Google Scholar]
- 20. Gubler DJ. Dengue and dengue hemorrhagic fever. Clin Microbiol Rev 1998; 11:480–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kumar Y, Liang C, Bo Z, Rajapakse JC, Ooi EE, Tannenbaum SR. Serum proteome and cytokine analysis in a longitudinal cohort of adults with primary dengue infection reveals predictive markers of DHF. PLoS Negl Trop Dis 2012; 6:e1887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Harris E, Videa E, Perez L, et al. . Clinical, epidemiologic, and virologic features of dengue in the 1998 epidemic in Nicaragua. Am J Trop Med Hyg 2000; 63:5–11. [DOI] [PubMed] [Google Scholar]
- 23. Stanaway JD, Shepard DS, Undurraga EA, et al. . The global burden of dengue: an analysis from the Global Burden of Disease Study 2013. Lancet Infect Dis 2016; 16:712–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Von Allmen SD, Lopez-Correa RH, Woodall JP, Morens DM, Chiriboga J, Casta-Velez A. Epidemic dengue fever in Puerto Rico, 1977: a cost analysis. Am J Trop Med Hyg 1979; 28:1040–4. [DOI] [PubMed] [Google Scholar]
- 25. Espana G, Leidner AJ, Waterman SH, Perkins TA. Cost-effectiveness of dengue vaccination in Puerto Rico. PLoS Negl Trop Dis 2021; 15:e0009606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Meltzer MI, Rigau-Perez JG, Clark GG, Reiter P, Gubler DJ. Using disability-adjusted life years to assess the economic impact of dengue in Puerto Rico: 1984–1994. Am J Trop Med Hyg 1998; 59:265–71. [DOI] [PubMed] [Google Scholar]
- 27. Trung DT, Thao le TT, Dung NM, et al. . Clinical features of dengue in a large Vietnamese cohort: intrinsically lower platelet counts and greater risk for bleeding in adults than children. PLoS Negl Trop Dis 2012; 6:e1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Carlos CC, Oishi K, Cinco MT, et al. . Comparison of clinical features and hematologic abnormalities between dengue fever and dengue hemorrhagic fever among children in the Philippines. Am J Trop Med Hyg 2005; 73:435–40. [PubMed] [Google Scholar]
- 29. Wills B, Tran VN, Nguyen TH, et al. . Hemostatic changes in Vietnamese children with mild dengue correlate with the severity of vascular leakage rather than bleeding. Am J Trop Med Hyg 2009; 81:638–44. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.