Abstract
Background.
Zika virus (ZIKV) can be transmitted sexually but the risk of sexual transmission remains unknown. Most evidence of sexual transmission is from partners of infected travelers returning from areas with ZIKV circulation.
Methods.
We used data from the US national arboviral disease surveillance system on travel-and sexually acquired ZIKV disease cases during 2016–2017 to develop individual-level simulations for estimating risk of male-to-female, male-to-male, and female-to-male sexual transmission of ZIKV via vaginal and/or anal intercourse. We specified parametric distributions to characterize individual-level variability of parameters for ZIKV persistence and sexual behaviors.
Results.
Using ZIKV RNA persistence in semen/vaginal fluids to approximate infectiousness duration, male-to-male transmission had the highest estimated probability (1.3% [95% confidence interval, CI, .4%–6.0%] per anal sex act), followed by male-to-female and female-to-male transmission (0.4% [95% CI, .3%–.6%] per vaginal/anal sex act and 0.1% [95% CI, 0%–.8%] per vaginal sex act, respectively). Models using viral isolation in semen vs RNA detection to approximate infectiousness duration predicted greater risk of sexual transmission.
Conclusions.
While likely insufficient to maintain sustained transmission, the estimated risk of ZIKV transmission through unprotected sex is not trivial and is especially important for pregnant women, as ZIKV infection can cause severe congenital disorders.
Keywords: Zika, sexual transmission, risk estimation, simulation model
While most Zika virus (ZIKV) transmission occurs via Aedes mosquitos, evidence also exists for transmission through sexual contact [1], as well as through intrauterine, perinatal, blood transfusion, and laboratory exposure [2–7]. In addition to these reports, ZIKV RNA has been detected in blood, urine, semen, vaginal or cervical fluids, and saliva, as well as other body fluids [8–10].
Additional evidence suggests the possible importance of sexual transmission, especially by men. Transmission via semen has been observed as long as 32–41 days after symptom onset, and infectious virus has been detected in semen for up to 69 days after onset [11–14]. ZIKV RNA has been detected over even longer time periods. A systemic analysis by Counotte et al reported a median duration of RNA in semen of 40 days with a maximum of 370 days [15]. Detection of ZIKV in cervicovaginal fluids has been less common and mostly of shorter duration. Infectious virus has been found at 3 days and viral RNA up to 6 months after symptom onset [16–18], with Counotte et al reporting a median duration of RNA in the female genital tract of 14 days [15]. Correspondingly, most documented cases of sexual transmission of ZIKV have been from symptomatic men to their female partners, although male-to-male and asymptomatic male-to-female sexual transmission also have been reported [1, 19–23]. In the United States, 1 case of female-to-male sexual transmission with the possible presence of menstrual blood was reported [24]. In addition, a report from France presented the possibility of male-to-female transmission through oral sex in a couple that also had vaginal intercourse without ejaculation [25].
Assessing the risk of ZIKV sexual transmission is challenging as sexual and vector-borne transmission are generally impossible to differentiate in areas with Aedes vector mosquitos and substantial transmission of ZIKV. ZIKV outbreaks have not occurred in areas without vector-borne transmission, and the estimated reproductive number for ZIKV infection by sexual transmission alone is less than 1 [26]. While the estimated contribution of sexual transmission in ZIKV epidemics is low, sexual transmission might contribute to the higher incidence observed in women relative to men [27]. No studies have estimated risk of sexual transmission of ZIKV per sex act. Furthermore, potential differences in the probability of sexual transmission by type of sexual contact have not been described. In this analysis, we used data from the US national arboviral disease surveillance system (ArboNET) from 2016 to 2017 on travel- and sexually acquired ZIKV disease occurring in areas without documented local transmission to develop individual-level simulations for estimating risk of male-to-female, male-to-male, and female-to-male sexual transmission of ZIKV.
METHODS
Cases of ZIKV disease in the United States are reported to ArboNET, a passive surveillance system managed by the Centers for Disease Control and Prevention and state health departments. All cases had clinically compatible illness and laboratory confirmation according to the Council of State and Territorial Epidemiologists (CSTE) case definitions for confirmed and probable ZIKV disease [28]. Travel-associated cases had recent travel to areas with active ZIKV transmission, and cases were designated as sexually acquired when their only known risk factor was sexual contact with a partner with recent travel to an area with active ZIKV transmission [20]. For travel- and sexually acquired cases of ZIKV, individual-level data were available on sex, age group (<18, 18–25, 25–34, 35–44, 45+ years), case status, and month of symptom onset. Frequencies of sexual transmission type (male-to-male, etc.) were available by year of symptom onset.
Modeling Approach
To estimate the risks of sexual transmission, we developed stochastic individual-level simulation models to estimate the number of sex acts among infected travelers that could potentially result in ZIKV transmission. Due to the lack of data on and likely much lower transmission risk of oral sex and insertive anal sex, only vaginal and receptive anal sex acts (ZIKV infected partner inserts penis into anus of uninfected partner) were considered as potentially infectious in the male-to-female transmission model, receptive anal sex acts for male-to-male transmission model, and vaginal sex acts for the female-to-male model. The sequential components of the models are described in Figure 1.
Figure 1.
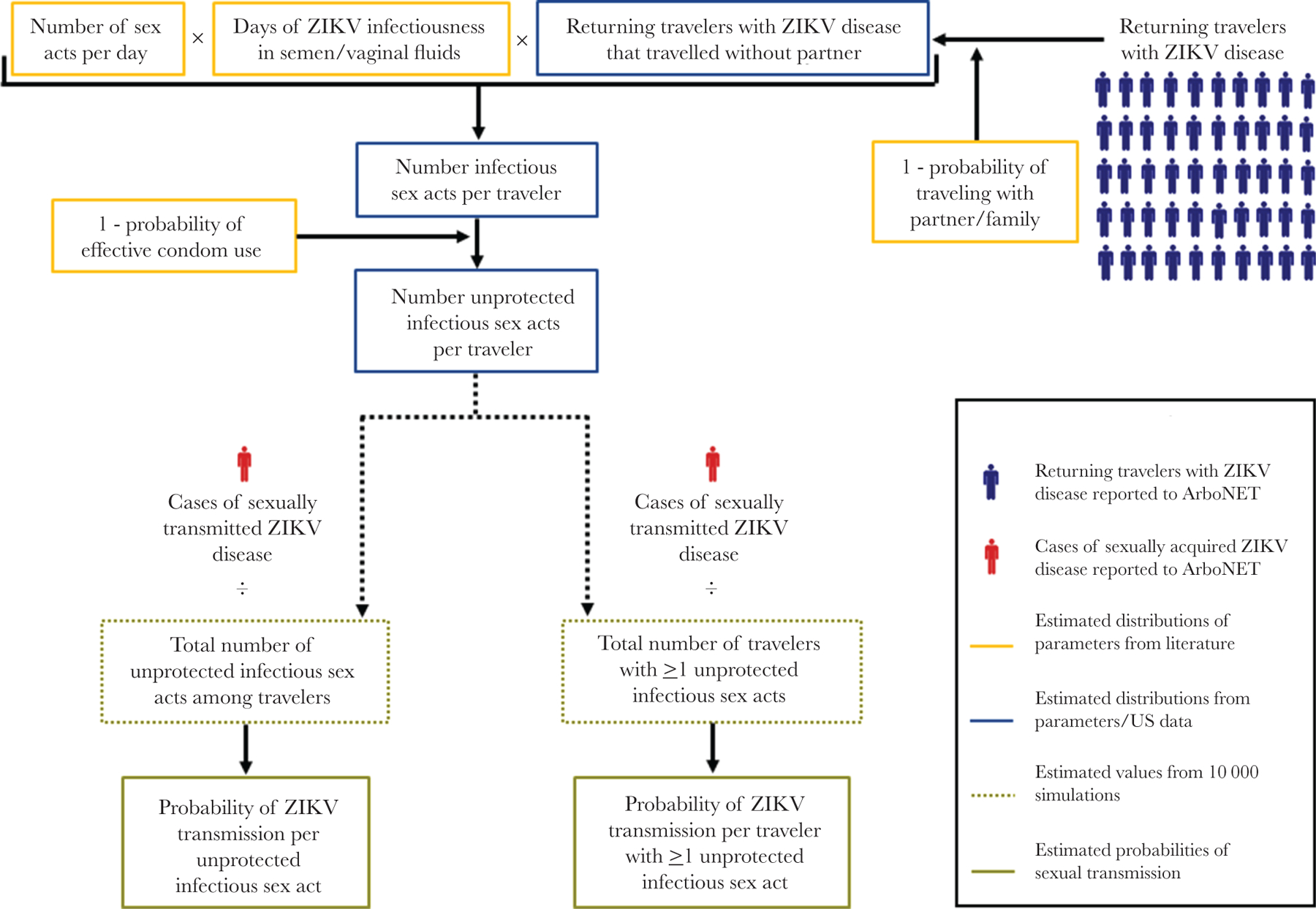
Schematic illustration of Zika virus (ZIKV) sexual transmission probability modeling approach.
Using the number of potentially infectious sex acts per traveler generated from parameter estimates, we performed 10 000 simulations for each of the 3 sexual transmission types to generate these values for each reported male or female traveler with ZIKV disease reported to ArboNET. For each simulation, we summed the total number of unprotected infectious sex acts (NUnprotectedInfectiousSexActs) among travelers who traveled without their partner and the total number of individuals that had at least 1 unprotected infectious sex act (NTravelersWithUprotectedInfectiousSexAct).
Where IOneOrMoreUnprotectedInfectiousSexActs = 1 if nInfectiousSexActs > 0 and IOneOrMoreUnprotectedInfectiousSexActs = 0 if nInfectiousSexActs = 0. To calculate the probabilities of sexual transmission of ZIKV per infected individual who had at least 1 unprotected sex act with an infected traveler, we divided the number of reported sexually acquired cases by the respective denominators.
From the simulations, we computed asymptotic, 95% score confidence intervals (CI) for the probabilities of interest described while accounting for uncertainty in the unknown, true sample sizes, as detailed in the Supplementary Material. Briefly, we assumed that the number of positives given the sample size was binomially distributed with parameters the given sample size and the probability of interest. We then estimated the distribution of the (random) sample size from the simulated data and marginalized against this distribution to compute the distribution for the number of positives, as a function of the probability of interest. This marginal distribution for the number of positives formed the basis for inference, including maximum likelihood point estimation and CI construction.
Assumptions and Parameter Estimates
All cases of ZIKV disease were symptomatic and therefore represented a subset of all infections that may have occurred. For the purpose of this study, we assumed that travel-associated mosquito-acquired and sexually acquired infections were equally likely to have been symptomatic and reported such that both types of cases represent a similar proportion of the true total number of each type of infection. We assumed that the probability of sexual transmission of ZIKV was likely to be low and short lived, such that on average most infected travelers would sexually transmit ZIKV to fewer than 1 person. We also assumed that the infectious period in travelers began after their return to the United States, sexual behavior during the infectious period was consistent with estimates from nationally representative samples, and ZIKV sexual transmission occurred via vaginal and receptive anal sex acts. Model assumptions are listed in Table 1 and parameters are described in Table 2.
Table 1.
Model Assumptions for Zika Virus (ZIKV) Sexual Transmission Risk Models
| Assumption |
|---|
| Travel and sexually acquired ZIKV infections were equally likely to have been symptomatic and reported to ArboNet. |
| An infected traveler would transmit ZIKV to fewer than 1 person, on average, even if they had multiple sexual partners. |
| The infectious period for sexual transmission of ZIKV in travelers began after their return to the United States. |
| Duration of ZIKV RNA or ZIKV isolation detection in semen approximate the infectious period duration in men; duration of ZIKV RNA in vaginal fluids approximates infectious period duration in women. |
| Frequency of sexual contact and condom use were consistent with representative sample estimates and sexual transmission was equally likely to occur throughout the infectious period. |
| Male-to-female transmission occurred through vaginal or anal sex, female-to-male transmission occurred via vaginal sex, and male-to-male transmission occurred via receptive anal sex. |
Table 2.
Data Source, Distribution, and Estimates of Parameters for ZIKV Sexual Transmission Risk Models
| Parameter | Variable | Data Source | Distribution | Estimated Median (2.5%-97.5% Range) | Model |
|---|---|---|---|---|---|
| Proportion of individuals that travel internationally with partner | P TravelingWithPartner | US Dept of Commerce [29, 30] | Uniform (0.23–0.37) | 0.3 (0.23–0.37) | All |
| Proportion of males who are MSM | P msm | Meta-analysis of US population-based surveys [31] | Uniform (0.025–0.035) | 0.03 (0.025–0.035) | Male-to-male |
| ZIKV persistence, days | |||||
| By RNA detection in semen | t Da ysInfectiousZIKV | Prospective studies [32, 33] | Log normal (42, 0.65)/d | 42 (12–150) | Male-to-female, male-to-male |
| By RNA detection in vaginal fluid | Systematic review, prospective study [15, 32] | Log normal (1, 0.95)/d | 1 (0–7) | Female-to-male | |
| By virus isolation in semen | Prospective studies [32, 33] | Log normal (10, 0.65)/d | 10 (3–36) | Male-to-female, male-to-male | |
| Sex acts per day | |||||
| Vaginal and anal acts among MSW | n SexActsPerDay | NSFG, NSSHB [34–36] | Log normal (2.5, 1.2)/28 | 0.09 (0.01–0.94) | Male-to-female |
| Vaginal acts among women | NSFG [34] | Log normal (2.9, 1.15)/28 | 0.10 (0.01–1.07) | Female-to-male | |
| Insertive anal acts among MSM | Secondary analyses of GSS, NHSLS, HIVNET VPS, NHBS [37, 38] | Log normal (75/365, 0.5) | 0.20 (0.08–0.56) | Male-to-male | |
| Proportion of sex acts with condom use | |||||
| Vaginal and anal acts among MSW | P CondomUse | NSSHB [39] | Beta (3.4, 12) | 0.21 (0.06–0.45) | Male-to-female |
| Vaginal acts among women | NSSHB [39] | Beta (2.6, 11) | 0.18 (0.04–0.43) | Female-to-male | |
| Insertive anal acts among MSM | NHBSS [40] | Beta (13, 10) | 0.57 (0.35–0.75) | Male-to-male | |
| Proportion of condom use that effectively prevents ZIKV transmission | |||||
| Sex acts among MSW | P CondomEffectivity | Meta-analysis of prospective and case-control/cross-sectional studies [41] | Beta (28.2, 12.7) | 0.69 (0.54–0.82) | Male-to-female |
| Sex acts among women | Meta-analysis of EXPLORE and VAX 004 trials [42] | Beta (6.2, 4.9) | 0.56 (0.27–0.82) | Female-to-male | |
| Sex acts among MSM | Meta-analysis of prospective and case-control/cross-sectional studies [41] | Beta (56.2, 21.8) | 0.72 (0.62–0.81) | Male-to-male |
Abbreviations: GSS, General Social Survey; HIVNET VPS, HIVNET Vaccine Preparedness Study; MSM, men who have sex with men; MSW, men who have sex with women; NHBS, National HIV Behavioral Surveillance; NHSLS, National Health and Social Life Survey; NSFG, National Survey of Family Growth; NSSHB, National Survey of Sexual Health and Behavior; ZIKV, Zika virus.
Based on the criteria for reporting sexually acquired ZIKV disease cases to ArboNET, ZIKV disease cases in sexual partners who travelled together would not be classified as sexual transmission as both partners would have also experienced risk of vector-borne ZIKV transmission. In 2016 and 2017, an estimated 23%–24% of overseas travelers from the United States were accompanied by their partner and an additional 13% were accompanied by family (which might also reflect travel with a partner) [29, 30]. We therefore assumed that 23%–37% of travelers traveled with their partner and characterized this probability with a uniform distribution:
An infected traveler either traveled alone (TravelAlone = 1) or did not (TravelAlone = 0), so for each individual traveler, we sampled from a Bernoulli distribution where the probability of traveling alone is 1 minus the probability of traveling with a partner:
For the male-to-male transmission model, we used a Bernoulli distribution to approximate the proportion of male travelers that had sex with male partners (pMSM) using national population estimates the proportion of men who engaged in same-sex behavior in the past year [31]:
We estimated the infectious period (tDaysInfectiousZIKV) for each male traveler with 1 of 2 different indicators of ZIKV infectiousness in semen: (1) the detection of live ZIKV by viral isolation and (2) the detection of ZIKV RNA. Due to limited available data on viral isolation in vaginal fluids, the infectious period in female travelers was estimated using ZIKV RNA persistence data only. For each indicator, we used a log-normal distribution to characterize individual-level variability based on the median (m) and log standard deviation (σ). We used a median of 42 days for ZIKV RNA in semen, a median of 10 days for viral culture in semen, and a log standard deviation of 0.65 for both distributions [32, 33]. For ZIKV RNA in vaginal fluids, we used a median of 1 day and a log standard deviation of 0.95 [15, 32]:
To estimate the average number of sex acts per day (nSexActsPerDay), we used published data from the National Survey of Family Growth for median vaginal acts among men and women [34]. To estimate the average number of anal sex acts, we considered published estimates of opposite sex engagement in anal sex from the National Survey of Sexual Health and Behavior [35, 36] and secondary analyses of men who have sex with men (MSM) data from the HIVNET Vaccine Preparedness Study, General Social Survey, and National Health and Social Life Survey [37, 38]. We approximated the distributions for average sex acts per day with a LogNormal distribution, using median (m) vaginal and anal acts per 28 days (d) for opposite sex partners and median anal sex acts per 365 days for MSM. We assumed that half of the estimated average anal sex acts per day among MSM were insertive (pInsertiveAnalAct) where an infected traveler might transmit ZIKV through semen.
The individual-level number of potentially infectious vaginal and/or anal sex acts among travelers who traveled without their partner was sampled from a Poisson distribution, where the proportion of male travelers that had sex with male partners (pMSM) and the proportion of anal sex acts among infected male travelers with male partners that were insertive (pInsertive Act) were only applied to the male-to-male model.
For the probability of condom use during vaginal and anal sexual intercourse (pCondomUse) we used published data from the National Survey of Sexual Health and Behavior and estimates from the National HIV Behavioral Surveillance System, and applied a Beta distribution [39, 40]. We also used a Beta distribution to estimate effectivity of condom use in preventing transmission using estimates from National HIV studies for opposite sex couples and MSM [41, 42]. Then, we estimated the probability of effective condom use by multiplying condom use and condom effectivity probabilities:
The number of unprotected vaginal and/or anal sex acts per traveler (nUnprotectedInfectiousSexActs) assumed a binomial distribution of the individual-level number of sex acts and the probability of no or ineffective condom use (1−pEffectiveCondomUse):
Sensitivity Analyses
To evaluate the sensitivity of transmission probability estimates to plausible changes in parameters, we conducted sensitivity analyses for the male-to-female model using ZIKV RNA persistence in semen to approximate duration of infectiousness. We varied parameter inputs according to their upper and lower 95% CIs from the data source or, for the proportion of individuals who traveled with a partner, to the upper and lower distribution boundaries. Transmission probabilities per unprotected infectious sex act and per traveler with ≥1 unprotected infectious sex act were recalculated for each parameter variation.
Computing Environment
All distribution approximations and simulations were performed in R 4.0.4 [43]. The full code is available from the corresponding author.
RESULTS
During 2016–2017, 3446 female cases and 1888 male cases of travel-acquired ZIKV disease and 49 female cases and 3 male cases of sexually acquired ZIKV disease were reported to ArboNET. All sexually acquired female cases had suspected male-to-female transmission; 2 of the sexually acquired male cases had suspected male-to-male transmission and 1 had suspected female-to-male transmission. Other parameters for the model are detailed in Table 2; the results of the simulations to estimate unprotected infectious sex acts per traveler and among all travelers as well the number of travelers with ≥1 unprotected infectious sex act are reported for each model in Supplementary Table 1.
The probability of male-to-female transmission per male traveler with ≥1 unprotected infectious vaginal or anal sex act varied from 4.2% (95% CI, 3.2%–5.6%) when based on ZIKV RNA detection to 6.4% (95% CI, 4.9%–8.5%) when based on ZIKV isolation (Table 3). The probability of male-to-female transmission per unprotected vaginal or anal sex act was 0.4% (95% CI, .3%–.6%) based on ZIKV RNA detection and 1.8% (95% CI, 1.4%–2.5%) when based on ZIKV isolation.
Table 3.
Estimated ZIKV Transmission Probabilities per Sex Act and Traveler by Transmission Type and Persistence Estimate
| Estimated Transmission | Probability, % | 95% CI, % |
|---|---|---|
| Male-to-female based on ZIKV RNA detection in semen | ||
| Per unprotected vaginal or anal sex act | 0.4 | .3–.6 |
| Per male traveler with ≥1 unprotected infectious vaginal sex act | 4.2 | 3.2–5.6 |
| Male-to-female based on ZIKV isolation in semen | ||
| Per unprotected vaginal sex act | 1.8 | 1.4–2.5 |
| Per male traveler with ≥1 unprotected infectious vaginal sex act | 6.4 | 4.9–8.5 |
| Male-to-male based on ZIKV RNA detection in semen | ||
| Per unprotected insertive anal sex act | 1.3 | .4–6.0 |
| Per male traveler with ≥1 unprotected infectious anal sex act | 5.6 | 1.5–20.3 |
| Male-to-male based on ZIKV isolation in semen | ||
| Per unprotected insertive anal sex act | 5.6 | 1.5–25.6 |
| Per male traveler with ≥1 unprotected infectious anal sex act | 9.9 | 2.7–36.1 |
| Female-to-male based on ZIKV RNA detection in vaginal fluids | ||
| Per unprotected vaginal sex act | 0.1 | .0–.8 |
| Per female traveler with ≥1 unprotected infectious vaginal sex act | 0.2 | .0–1.3 |
Abbreviation: CI, confidence interval; ZIKV, Zika virus.
The estimated risk for sexual transmission of ZIKV was greatest and had the most uncertainty for male-to-male transmission with probabilities of 5.6% (95% CI, 1.5%–20.3%) and 9.9% (95% CI, 2.7%–36.1%) per male traveler with ≥1 unprotected infectious insertive anal sex act, based on ZIKV RNA detection and ZIKV isolation, respectively. The probability of male-to-male transmission per sex act was 1.3% (95% CI, .4%–6.0%) based on ZIKV RNA detection and 5.6% (95% CI, 1.5%–25.6%) when based on ZIKV isolation.
Estimates were lowest for female-to-male transmission. The probability of transmission for female-to-male per female traveler with ≥1 unprotected infectious vaginal sex act was 0.2% (95% CI, 0%–1.3%) based on ZIKV RNA detection, and the transmission probability per unprotected vaginal sex act was 0.1% (95% CI, 0%–.8%).
In the sensitivity analyses performed for male-to-female transmission, the lowest and highest estimates for the probability of ZIKV transmission per male traveler with ≥1 unprotected infectious sex act were 3.8% and 4.7%, with both estimates generated from varying the proportion of individuals that travel with their partner across its distribution boundaries (Table 4). The probability of transmission per unprotected infectious vaginal and anal sex acts varied from 0.37% to 0.53%, with both estimates generated from varying the infectious period across the 95% CI boundaries for ZIKV RNA persistence in semen. Variations in condom use and effectiveness had the smallest effects on both transmission probability estimates.
Table 4.
Sensitivity Analyses for Male-to-Female Sexual Transmission Probability Estimates Using ZIKV RNA Persistence to Approximate Duration of Infectiousness
| Parameter | Low and High Parameter Estimatesa | % Transmission Probability per Unprotected Infectious Sex Act (95% CI)b | % Transmission Probability per Male Traveler With ≥1 Unprotected Infectious Sex Act (95% CI)b |
|---|---|---|---|
| Proportion of individuals that travel in-ternationally with partner/family | 0.23, 0.37 | Low: 0.40 (.29–.54) | Low: 3.8 (2.9–5.1) |
| High: 0.49 (.36–.66) | High: 4.7 (3.6–6.2) | ||
| ZIKV persistence, d | |||
| By RNA detection in semen | 35, 50 | Low: 0.53 (.39–.71) | Low: 4.4 (3.3–5.8) |
| High: 0.37 (.27–.50) | High: 4.1 (3.1–5.4) | ||
| Sex acts per 28 d | |||
| Vaginal and anal acts among MSW | 2.1, 2.8 | Low: 0.39 (.28–.53) | Low: 4.1 (3.1–5.4) |
| High: 0.51 (.38–.70) | High: 4.4 (3.3–5.7) | ||
| Proportion of sex acts with condom use | |||
| Vaginal and anal acts among MSW | 0.196, 0.235 | Low: 0.42 (.31–.58) | Low: 4.2 (3.2–5.5) |
| High: 0.44 (.3–60) | High: 4.2 (3.2–5.6) | ||
| Proportion of condom use that effectively prevents ZIKV transmission | |||
| Sex acts among MSW | 0.52, 0.80 | Low: 0.41 (.30–.56) | Low: 4.2 (3.2–5.5) |
| High: 0.44 (.32–.60) | High: 4.2 (3.2–5.7) |
Abbreviations: CI, confidence interval; MSW, men who have sex with women; ZIKV, Zika virus.
Based on high and low distribution boundaries from proportion of individuals that travel with a partner and central tendency 95% CI from data sources for all other parameters.
Probabilities are calculated using the low estimate and high estimate for each parameter.
DISCUSSION
Comprehensive studies of ZIKV sexual transmission are complicated by the primary role of mosquito-borne transmission and an inability to differentiate vector-borne and sexual transmission. However, the large scale of the 2016–2017 pandemic led to many travel-associated infections and a unique opportunity to assess sexual transmission risk among cases detected in travelers and their subsequent sex partners. The majority of reported sexually acquired cases (n = 49) were due to male-to-female transmission. Our analysis estimated a probability of male-to-female transmission per unprotected sex act of 0.4% based on RNA detection and an even greater risk for male-to-male transmission of 1.3% per sex act. This latter estimate had the highest uncertainty due to the low number of observed cases (n = 2) and relatively lower number of expected male-to-male sex acts among infected travelers. Risk was estimated to be lowest for female-tomale, with a transmission probability of 0.1% per sex act.
The difference between estimates made using ZIKV RNA persistence vs ZIKV isolation as proxies for infectiousness and results of the sensitivity analysis indicate that the duration of the infectious period is a key source of uncertainty, particularly for estimating transmission probability per unprotected sex act. Approximating this period using RNA positivity resulted in lower risk estimates as the extended infectious period increases the number of potentially infectious sex acts while the number of reported cases remains the same. In contrast, presence of infectious virus is more challenging to measure, resulting in fewer available data and additional limits to sensitivity. Therefore, the infectious period approximated from the infectious virus data may underestimate the true infectious period and, therefore, overestimate risk as a shorter infectious period would mean that there are fewer infectious sex acts.
Higher transmission probabilities among MSM and from male-to-female compared with female-to-male were expected based on what has been reported for other sexually transmitted diseases [44]. These findings are also supported by data from animal studies. In a mouse model, sexual transmission was observed from males to females but not from females to males [45]. High levels of ZIKV replication in the testes in mice and sustained detection of viral RNA and of virus in testicular and epididymal epithelia in mice is consistent with the longer duration of detection seen in men compared to women [45, 46]. Rectal mucosa is also susceptible to ZIKV infection [46], and likely an important route of transmission based on our results.
The probability of sexual transmission of ZIKV in our study is higher than what has been reported for HIV (4–138 per 10 000 exposures) [47], but lower than that for other sexually transmitted infections. For example, Chlamydia transmission probability per partnership has been estimated at 55% (interquartile range [IQR], 49%–63%) [48], while the Chlamydia transmission probability per sex act is estimated at 10% (IQR, 6%–17%). Transmission probabilities are even higher for gonorrhea, estimated at 46% (95% CI, 30%–62%) for male-to-female transmission per sex act and 23% (95% CI, 16%–32%) for female-to-male transmission per sex act [44].
Only 2 studies have used mathematical models to estimate the reproductive number for ZIKV sexual transmission. Gao et al used a deterministic model with surveillance data from Brazil, Colombia, and El Salvador, and derived a reproductive number of 0.136 (95% CI, .009–.521) [49]. Towers et al using a deterministic model parametrized to data from Colombia, concluded the reproductive number was “likely below one” [50]. The 2 studies calculated the proportion of ZIKV infections resulting from sexual transmission as 3.04% (95% CI, .12%–45.73%) [49] and 23% (95% CI, 1%–47%) [50]. Neither study provided information about the transmission probability per sex act. In addition, surveillance data on which these studies based their results did not distinguish between vectortransmitted ZIKV and sexually transmitted ZIKV infections.
Our study had some limitations. The transmission probabilities are based on symptomatic infections, which may have resulted in biased estimates. However, we assumed that this reliance would equally affect the travel-acquired ZIKV cases that could potentially transmit ZIKV sexually and the sexually acquired ZIKV cases. As our data on cases are limited to symptomatic, reported cases, it may be biased by differences in care-seeking by sex, detection, reporting of travel or sexual transmission cases, or other factors. Sexual transmissions among partners who travelled together may have been misclassified as mosquito-acquired infections during travel. For the male-to-female model, expected anal sex acts were relatively low and combined with vaginal sex acts to obtain a single per-act transmission probability. However, risk estimates from the other 2 models suggest anal sex has a higher probability of ZIKV transmission than vaginal sex. Finally, estimates for male-to-male and female-to-male transmission were based on only 2 and 1 reported cases, respectively.
We also made assumptions on types and consistency of sexual behaviors and the infectious period duration that may have biased ZIKV sexual transmission risk estimates. Due to limited evidence of ZIKV transmission through oral sex in the absence of other types of sexual contact, we did not account for it in our risk estimates, potentially leading to overestimation of per-act transmission risk. Additionally, we estimated the ZIKV transmission probability for receptive anal sex, not considering scenarios where infection may have occurred during insertive anal sex. As data on timing of ZIKV diagnosis and symptom duration and severity among infected travelers were unavailable, we assumed sexual behaviors were consistent with national representative sample estimates throughout the infectious period. This may have resulted in underestimation of transmission risk if illness and/or ZIKV diagnosis decreased sex act frequency and/or increased condom use, reducing the number of potentially infectious sex acts. Finally, uncertainty regarding duration of infectiousness in genital fluids greatly impacts risk estimates, and our assumption that the entire infectious period occurred after return from travel may also have led to underestimation of transmission risk.
Although sexual transmission is not enough to sustain ZIKV transmission due primarily to duration of infectiousness [49, 50], our study documented that the risk among discordant partners engaging in unprotected sex is not trivial. The risk of sexual transmission of ZIKV is particularly relevant for women who are pregnant or planning a pregnancy, as ZIKV infection can cause severe congenital disorders. Promoting condom use and other strategies to prevent sexual transmission for residents and travelers visiting and returning from areas with ongoing ZIKV transmission is essential.
Supplementary Material
Footnotes
Presented in part: 68th Annual Meeting of the American Society of Tropical Medicine and Hygiene, 14–20 November 2019, National Harbor, MD.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Foy BD, Kobylinski KC, Chilson Foy JL, et al. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg Infect Dis 2011; 17:880–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brooks JT, Friedman A, Kachur RE, LaFlam M, Peters PJ, Jamieson DJ. Update: interim guidance for prevention of sexual transmission of Zika virus—United States, July 2016. MMWR Morb Mortal Wkly Rep 2016; 65:745–7. [DOI] [PubMed] [Google Scholar]
- 3.Perez S, Tato R, Cabrera JJ, et al. Confirmed case of Zika virus congenital infection, Spain, March 2016. Euro Surveill 2016; 21:30261. [DOI] [PubMed] [Google Scholar]
- 4.Besnard M, Lastere S, Teissier A, Cao-Lormeau V, Musso D. Evidence of perinatal transmission of Zika virus, French Polynesia, December 2013 and February 2014. Euro Surveill 2014; 19:20751. [PubMed] [Google Scholar]
- 5.Musso D, Nhan T, Robin E, et al. Potential for Zika virus transmission through blood transfusion demonstrated during an outbreak in French Polynesia, November 2013 to February 2014. Euro Surveill 2014; 19:20761. [DOI] [PubMed] [Google Scholar]
- 6.Petersen EE, Meaney-Delman D, Neblett-Fanfair R, et al. Update: interim guidance for preconception counseling and prevention of sexual transmission of Zika virus for persons with possible Zika virus exposure—United States, September 2016. MMWR Morb Mortal Wkly Rep 2016; 65:1077–81. [DOI] [PubMed] [Google Scholar]
- 7.Gregory CJ, Oduyebo T, Brault AC, et al. Modes of transmission of Zika virus. J Infect Dis 2017; 216:875–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mead PS, Hills SL, Brooks JT. Zika virus as a sexually transmitted pathogen. Curr Opin Infect Dis 2018; 31:39–44. [DOI] [PubMed] [Google Scholar]
- 9.Grischott F, Puhan M, Hatz C, Schlagenhauf P. Non-vector-borne transmission of Zika virus: a systematic review. Travel Med Infect Dis 2016; 14:313–30. [DOI] [PubMed] [Google Scholar]
- 10.Roze B, Najioullah F, Signate A, et al. Zika virus detection in cerebrospinal fluid from two patients with encephalopathy, Martinique, February 2016. Euro Surveill 2016; 21:30205. [DOI] [PubMed] [Google Scholar]
- 11.Turmel JM, Abgueguen P, Hubert B, et al. Late sexual transmission of Zika virus related to persistence in the semen. Lancet 2016; 387:2501. [DOI] [PubMed] [Google Scholar]
- 12.Arsuaga M, Bujalance SG, Díaz-Menéndez M, Vázquez A, Arribas JR. Probable sexual transmission of Zika virus from a vasectomised man. Lancet Infect Dis 2016; 16:1107. [DOI] [PubMed] [Google Scholar]
- 13.Barzon L, Pacenti M, Franchin E, et al. Infection dynamics in a traveller with persistent shedding of Zika virus RNA in semen for six months after returning from Haiti to Italy, January 2016. Euro Surveill 2016; 21:30316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nicastri E, Castilletti C, Liuzzi G, Iannetta M, Capobianchi MR, Ippolito G. Persistent detection of Zika virus RNA in semen for six months after symptom onset in a traveller returning from Haiti to Italy, February 2016. Euro Surveill 2016; 21:30314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Counotte MJ, Kim CR, Wang J, et al. Sexual transmission of Zika virus and other flaviviruses: A living systematic review. PLoS Med 2018; 15:e1002611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Penot P, Brichler S, Guilleminot J, et al. Infectious Zika virus in vaginal secretions from an HIV-infected woman, France, August 2016. Euro Surveill 2017; 22:30444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nicastri E, Castilletti C, Balestra P, Galgani S, Ippolito G. Zika virus infection in the central nervous system and female genital tract. Emerg Infect Dis 2016; 22:2228–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Reyes Y, Bowman NM, Becker-Dreps S, et al. Prolonged shedding of Zika virus RNA in vaginal secretions, Nicaragua. Emerg Infect Dis 2019; 25:808–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Venturi G, Zammarchi L, Fortuna C, et al. An autochthonous case of Zika due to possible sexual transmission, Florence, Italy, 2014. Euro Surveill 2016; 21:30148. [DOI] [PubMed] [Google Scholar]
- 20.Hills SL, Russell K, Hennessey M, et al. Transmission of Zika virus through sexual contact with travelers to areas of ongoing transmission—continental United States, 2016. MMWR Morb Mortal Wkly Rep 2016; 65:215–6. [DOI] [PubMed] [Google Scholar]
- 21.Freour T, Mirallie S, Hubert B, et al. Sexual transmission of Zika virus in an entirely asymptomatic couple returning from a Zika epidemic area, France, April 2016. Euro Surveill 2016; 21:30254. [DOI] [PubMed] [Google Scholar]
- 22.Brooks RB, Carlos MP, Myers RA, et al. Likely sexual transmission of Zika virus from a man with no symptoms of infection—Maryland, 2016. MMWR Morb Mortal Wkly Rep 2016; 65:915–6. [DOI] [PubMed] [Google Scholar]
- 23.Deckard DT, Chung WM, Brooks JT, et al. Male-to-male sexual transmission of Zika virus—Texas, January 2016. MMWR Morb Mortal Wkly Rep 2016; 65:372–4. [DOI] [PubMed] [Google Scholar]
- 24.Davidson A, Slavinski S, Komoto K, Rakeman J, Weiss D. Suspected female-to-male sexual transmission of Zika virus—New York City, 2016. MMWR Morb Mortal Wkly Rep 2016; 65:716–7. [DOI] [PubMed] [Google Scholar]
- 25.D’Ortenzio E, Matheron S, Yazdanpanah Y, et al. Evidence of sexual transmission of Zika virus. N Engl J Med 2016; 374:2195–8. [DOI] [PubMed] [Google Scholar]
- 26.Yakob L, Kucharski A, Hue S, Edmunds WJ. Low risk of a sexually-transmitted Zika virus outbreak. Lancet Infect Dis 2016; 16:1100–2. [DOI] [PubMed] [Google Scholar]
- 27.Coelho FC, Durovni B, Saraceni V, et al. Higher incidence of Zika in adult women than adult men in Rio de Janeiro suggests a significant contribution of sexual transmission from men to women. Int J Infect Dis 2016; 51:128–32. [DOI] [PubMed] [Google Scholar]
- 28.Centers for Disease Control and Prevention. Zika virus disease and Zika virus infection 2016 case definition, approved June 2016. https://ndc.services.cdc.gov/case-definitions/zika-virus-disease-and-zika-virus-infection-2016-06-01/. Accessed 1 August 2020.
- 29.US Department of Commerce. International Trade Administration. 2016 Profile of U.S. resident travelers visiting overseas destinations (outbound). https://www.trade.gov/survey-international-air-travelers-siat. Accessed 30 March 2019.
- 30.US Department of Commerce. International Trade Administration. 2017 Profile of U.S. resident travelers visiting overseas destinations (outbound). https://www.trade.gov/survey-international-air-travelers-siat. Accessed 30 March 2019.
- 31.Purcell DW, Johnson CH, Lansky A, et al. Estimating the population size of men who have sex with men in the United States to obtain HIV and syphilis rates. Open AIDS J 2012; 6:98–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Paz-Bailey G, Rosenberg ES, Doyle K, et al. Persistence of Zika virus in body fluids–final report. N Engl J Med 2018; 379:1234–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mead PS, Duggal NK, Hook SA, et al. Zika virus shedding in semen of symptomatic infected men. N Engl J Med 2018; 378:1377–85. [DOI] [PubMed] [Google Scholar]
- 34.Leichliter JS, Chesson HW, Sternberg M, Aral SO. The concentration of sexual behaviours in the USA: a closer examination of subpopulations. Sex Transm Infect 2010; 86:iii45–51. [DOI] [PubMed] [Google Scholar]
- 35.Herbenick D, Reece M, Schick V, Sanders SA, Dodge B, Fortenberry JD. Sexual behavior in the United States: results from a national probability sample of men and women ages 14–94. J Sex Med 2010; 7:255–65. [DOI] [PubMed] [Google Scholar]
- 36.Reece M, Herbenick D, Schick V, Sanders SA, Dodge B, Fortenberry JD. Sexual behaviors, relationships, and perceived health among adult men in the United States: results from a national probability sample. J Sex Med 2010; 7:291–304. [DOI] [PubMed] [Google Scholar]
- 37.Sullivan PS, Salazar L, Buchbinder S, Sanchez TH. Estimating the proportion of HIV transmissions from main sex partners among men who have sex with men in five US cities. AIDS 2009; 23:1153–62. [DOI] [PubMed] [Google Scholar]
- 38.Wall KM, Stephenson R, Sullivan PS. Frequency of sexual activity with most recent male partner among young, Internet-using men who have sex with men in the United States. J Homosex 2013; 60:1520–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reece M, Herbenick D, Schick V, Sanders SA, Dodge B, Fortenberry JD. Condom use rates in a national probability sample of males and females ages 14 to 94 in the United States. J Sex Med 2010; 7:266–76. [DOI] [PubMed] [Google Scholar]
- 40.Sanchez T, Finlayson T, Drake A, et al. Human immunodeficiency virus (HIV) risk, prevention, and testing behaviors- -United States, National HIV Behavioral Surveillance System: men who have sex with men, November 2003-April 2005. MMWR Surveill Summ 2006; 55:1–16. [PubMed] [Google Scholar]
- 41.Giannou FK, Tsiara CG, Nikolopoulos GK, et al. Condom effectiveness in reducing heterosexual HIV transmission: a systematic review and meta-analysis of studies on HIV serodiscordant couples. Expert Rev Pharmacoecon Outcomes Res 2016; 16:489–99. [DOI] [PubMed] [Google Scholar]
- 42.Smith DK, Herbst JH, Zhang X, Rose CE. Condom effectiveness for HIV prevention by consistency of use among men who have sex with men in the United States. J Acquir Immune Defic Syndr 2015; 68:337–44. [DOI] [PubMed] [Google Scholar]
- 43.R Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2021. [Google Scholar]
- 44.Johnson LF, Alkema L, Dorrington RE. A Bayesian approach to uncertainty analysis of sexually transmitted infection models. Sex Transm Infect 2010; 86:169–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duggal NK, Ritter JM, Pestorius SE, et al. Frequent Zika virus sexual transmission and prolonged viral RNA shedding in an immunodeficient mouse model. Cell Rep 2017; 18:1751–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Haddow AD, Nalca A, Rossi FD, et al. High infection rates for adult macaques after intravaginal or intrarectal inoculation with Zika virus. Emerg Infect Dis 2017; 23:1274–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Patel P, Borkowf CB, Brooks JT, Lasry A, Lansky A, Mermin J. Estimating per-act HIV transmission risk: a systematic review. AIDS 2014; 28:1509–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Althaus CL, Heijne JC, Low N. Towards more robust estimates of the transmissibility of Chlamydia trachomatis. Sex Transm Dis 2012; 39:402–4. [DOI] [PubMed] [Google Scholar]
- 49.Gao D, Lou Y, He D, et al. Prevention and control of Zika as a mosquito-borne and sexually transmitted disease: a mathematical modeling analysis. Sci Rep 2016; 6:28070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Towers S, Brauer F, Castillo-Chavez C, Falconar AKI, Mubayi A, Romero-Vivas CME. Estimate of the reproduction number of the 2015 Zika virus outbreak in Barranquilla, Colombia, and estimation of the relative role of sexual transmission. Epidemics 2016; 17:50–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


