Abstract
Background:
Real world patterns of cannabis use for health concerns are highly variable and rarely overseen by a physician. Pragmatic effectiveness studies with electronic daily diaries that capture person-specific patterns of cannabis use and health symptoms may help clarify risks and benefits.
Methods:
As part of a larger, randomized trial (NCT03224468), adults (N = 181) seeking cannabis for insomnia, pain, or anxiety or depressive symptoms were randomized to obtain a medical cannabis card immediately (MCC) or a waitlist control (WLC) and completed 12-weeks of daily web-based surveys on cannabis use and sleep, pain, and depressive symptoms.
Results:
Completion rates of daily surveys were moderate to high (median completed: 72 out of 90 days). Daily reports of cannabis use were consistent with monthly interview assessments and urinalysis. The MCC group increased cannabis use frequency in the 12 weeks following randomization, while WLC did not. Among the MCC group, self-reported sleep quality was significantly higher on cannabis use days, compared to nonuse days. The MCC group displayed long-term sleep improvements, consistent with increasing cannabis frequency. No improvements were found for pain or depressive symptoms.
Conclusion:
Cannabis use is associated with same day improvements in self-reported sleep quality, but not pain or depressive symptoms, although sleep improvements occurred in the context of increased frequency of cannabis use, raising the risk for cannabis use disorder. Daily web-based assessments of cannabis appear valid and feasible in adults seeking cannabis for health concerns, providing a flexible, complementary method for future real-world effectiveness studies with expanded and objective measures.
Keywords: Daily diary, Cannabis, Longitudinal, Sleep, Pain, Mood, Clinical trial
1. Introduction
Access to cannabis is rapidly increasing in the United States as a growing number of states legalize medical and recreational cannabis (Goodman et al., 2020). Though patients increasingly seek clinician input on the use of cannabis to address chronic mental and physical health challenges, including with sleep, mood, and pain (Lintzeris et al., 2018; Sarris et al., 2020), the efficacy of cannabis to address these concerns is inconclusive (Abrams, 2018; Sarris et al., 2020). Due, in part, to the complex legal status of cannabis use in the United States (federally prohibited but with individual state-level laws Boehnke et al., 2019; Goodman et al., 2020; Mead, 2017; Pacula et al., 2014), many, if not most, individuals using cannabis for health concerns do so without ongoing medical provider supervision (Sexton et al., 2016), lack clear guidelines for dosing (see (MacCallum and Russo, 2018)), and have the flexibility to purchase a wide range of cannabis products (Hazekamp et al., 2013; Cranford et al., 2016) of potentially unclear or mislabeled chemical composition (Gilman et al., 2021; Vandrey et al., 2015). Real-world patterns of medical cannabis use are thus highly variable, patient-specific, and may be influenced by familiarity and/or expectations for use that facilitate navigation of its complex legal status.
Pragmatic clinical trials, that for example randomize individuals to receive access to medical cannabis or to a waitlist control (Gilman et al., 2022), provide an opportunity to characterize the potential therapeutic effectiveness of cannabis under real-world conditions. Experience sampling studies (Csikszentmihalyi and Larson, 2014; Kahneman et al., 2004) that capture patient-specific, in-the moment (ecological momentary assessment) or on the same day (daily diary) patterns of cannabis use (Verdoux et al., 2003) and health symptoms through intensive longitudinal tracking can provide essential, complementary data for these pragmatic studies. However, limited work has used experience sampling designs in studies of cannabis use for health concerns and therefore the feasibility, validity, and utility of this design for clinical research in this area remains largely unknown.
This project was part of a larger pragmatic clinical trial that randomized adults interested in cannabis for health concerns to a medical cannabis card or waitlist control (Gilman et al., 2022). The parent trial (Gilman et al., 2022) demonstrated with conventional lab-based, in-person measures that, relative to the waitlist control group, the medical cannabis card group had increased risk for cannabis use disorder and reported fewer insomnia related symptoms, but had no change in pain severity or anxiety or depressive symptoms. The current project reports exploratory analyses from an intensive longitudinal, electronic daily diary design that was also completed as part of the parent trial. Participants provided daily web-based, self-reports of cannabis use and symptoms of sleep, mood, and pain for the first 90 days of receiving access to a medical cannabis card or placement on the waitlist. The primary aims for this study were to 1) determine the feasibility and validity of intensive longitudinal assessment of cannabis use in adults starting cannabis use for health concerns and 2) examine the association between cannabis use and reported symptoms of sleep, mood, and pain across the multiple timescales afforded by the long-term, daily diary design (e.g., short-term same day effects, long-term change over three months).
2. Method
2.1. Participants
Participants were part of a pragmatic, single-site, single-blinded clinical trial (NCT03224468; (Gilman et al., 2022)) that randomized participants to either obtain a medical cannabis card (MCC) in the community at the time of randomization or to wait 12 weeks before obtaining a medical cannabis card (waitlist control (WLC)). Participants had self-reported symptoms of insomnia, pain, or anxiety and/or depression (“mood”). Participants were generally healthy, with no known, unstable major medical condition, who expressed an interest in obtaining a medical cannabis card but did not yet possess one, reported less than daily current cannabis use, and did not meet criteria for DSM-5 cannabis use disorder (see also Table 1).
Table 1.
Stratification, Demographic, and Baseline Assessments from Analysis Sample.
| MCC | WLC | |||
|---|---|---|---|---|
| N/M (SD) | Range | N/M (SD) | Range | |
| Demographics | ||||
| Participants | 102 | 79 | ||
| Presenting Problem (N Affective | Sleep | Pain) | 44 | 22 | 36 | 37 | 19 | 23 | ||
| N Female | Male | Non-Binary | 68 | 33 | 1 | 50 | 29 | 0 | ||
| Age in years | 38.26 (14.31) | 18–65 | 36.69 (14.56) | 18–65 |
| Education years | 16.71 (2.31) | 11–24 | 16.27 (2.74) | 10–25 |
| Baseline Assessments 1 | ||||
| Cannabis Uses Per-Day | 0.50 (0.63) | 0–2.5 | 0.58 (0.61) | 0–2.5 |
| Athens Insomnia Scale Total2 | 9.71 (4.95) | 0–21 | 9.63 (4.40) | 1–20 |
| HADS-Anxiety Total3 | 7.50 (4.35) | 0–18 | 7.91 (4.34) | 0–20 |
| HADS-Depression Total4 | 5.01 (3.51) | 0–13 | 5.04 (4.05) | 0–16 |
| Brief Pain Inventory Worst Pain5 | 5.12 (2.30) | 0–9 | 5.52 (2.20) | 1 −9 |
Note. Sample size (N), mean (M) and standard deviation (SD) of stratification, demographic, and baseline assessments. No significant differences (p’s > .243) were found between randomized groups (medical cannabis card [MCC], waitlist control [WLC]) on demographic measures or baseline assessments. HADS, Hospital Anxiety and Depression Scale. See document for measure citations.
Baseline assessments are provided for the entire MCC or WLC group (irrespective of presenting problem). See (Gilman et al., 2022) for additional breakdown by presenting problem.
Higher scores indicate more sleep difficulty. 0–7 indicates no insomnia, 8–14 mild insomnia, 15–21 and 22 or more indicate moderate and severe insomnia, respectively.
Higher scores indicate more anxiety, with 0–7 indicating normal, 8–10 indicating borderline anxiety and 11–21 indicating abnormal levels.
Higher scores indicate more depression, with 0–7 indicating normal, 8–10 indicating borderline depression and 11–21 indicating abnormal levels.
Scores can range from 0 (no pain) to 10 (pain as bad as you can imagine).
Participants (N = 186; female: n = 122; mean age = 37.2 years, range 18–65-years-old; Table 1) were recruited from local clinical sites and the community within the greater Boston area, and were predominantly of Not Hispanic or Latino, White ethnic/racial backgrounds, with an average of 16.5 years of education. See Gilman et al. (2022) for additional detail on study design, participant characteristics, and randomization. The initial sample for the current project consisted of the 186 participants randomized to MCC or WLC who participated in monthly assessments from the parent trial (Gilman et al., 2022). The final analytic sample (see below) for this study consisted of 181 of these participants. Study procedures were approved by Partners Human Research Committee (protocol number 2015P001600). Participants provided informed consent and were financially compensated for participation. For the daily diary reports, participants were compensated $2 per-survey day and $6 for completing all seven days in a week (possible total of $20 per week).
2.2. Procedures
Following screening to assess basic eligibility, participants were randomized, stratified by sex, age, and the presenting problem for which they were seeking cannabis (self-reported problems with sleep, pain or mood [anxiety or depression]) to either the MCC group, in which they were to obtain a medical cannabis card without delay, or to the WLC group, in which they agreed to wait 12-weeks before obtaining a medical cannabis card. Consistent with the pragmatic clinical trial design, participants were not excluded if they reported secondary symptoms of other presenting problems (Miller and Cano, 2009; Staner, 2010). Specificity and commonality amongst presenting problems was examined throughout the project (see below). Participants randomized to the MCC group were instructed that they could obtain a medical cannabis card without delay to participate in the study, and in detailed subsequent analyses, we determined changes in their cannabis usage via biochemically verified assessments. Participants were randomized 2:1 MCC:WLC to ensure adequate sample size in the MCC group owing to the expected dropout in the MCC group due to financial and logistic challenges inherent in obtaining a medical cannabis card. Participants completed an experimenter administered baseline visit where primary study variables (Gilman et al., 2022) were assessed, and they received instructions on subsequent web-based daily self-reports. For the MCC group, the baseline visit was scheduled to be as close as possible to the receipt date of the medical cannabis card. Following the baseline assessment, participants were prospectively followed for the 12-week, randomized period using 1) in-person or virtual experimenter administered visits (all experimenter administered visits became virtual in March 2020 due to COVID-19 pandemic; see (Gilman et al., 2022)) at 2-, 4-, and 12-weeks following randomization and 2) using daily web-based assessments of self-reported cannabis use, sleep, pain, depression. The current project reports the results of the latter, daily web-based assessments; see the parent trial (Gilman et al., 2022) for the former.
2.3. Measures
2.3.1. Baseline substance use, psychiatric, and medical symptoms
Baseline and monthly cannabis use frequency was assessed at each experimenter administered study visit via an interview with research staff where participants reported their use according to the following options: “Once or more per day”, “5–6 days a week”, “3–4 days a week”, “1–2 days a week”, ”Less than once a week”, “Less than once every two weeks”. Baseline sleep, anxiety, depression, and pain symptoms were assessed via the Athens Insomnia Scale (Soldatos et al., 2000), Hospital Anxiety and Depression Scale (Snaith, 2003), and Brief Pain Inventory (Tan et al., 2004), respectively.
2.3.2. Daily self-reports
Participants provided daily reports on cannabis use and sleep, pain, and depression symptoms via a secure web-based survey application, designed for this study, that was available via smartphone and computer. Participants used their own devices to complete the surveys. Participants were instructed to complete assessments at the same time every day at the end of the day (e.g., end of day schedule: (Trull and Ebner-Priemer, 2020)), but the exact time was flexible to accommodate differences in schedules. Questions were based on the previous 24 hours. For cannabis use, participants first reported whether they had used cannabis (“Did you use Medical Marijuana Today”: “Yes” versus “No”) and if they had used cannabis that day, to report an approximation of the number of cannabis use occasions. Participants also provided daily reports from 1 (low) to 10 (high) using sliders, on pain (“How much pain did you feel today on average”: “(1) No pain”, “(10) Extreme pain”), sleep (“How was your sleep quality last night”: “(1) Very poor”, “(10) Very good”). and depression (“How depressed did you feel today: “(1) Not at all”, “(10) Extremely”). All participants, from both the MCC and WLC group were instructed to complete daily diaries. Participants in the WLC group were not asked to change their normal (minimal; see inclusion criteria) patterns of use and reports of use were not exclusionary.
2.3.3. Urinalysis
At experimenter administered visits, whether in person or virtual (via mail), urine was collected for assessment of cannabinoids and their metabolites. Concentration of THC, CBD, their primary metabolites, and 15 other cannabinoids in urine was assessed via high performance liquid chromatography with tandem mass spectrometry (see Supplemental S1 and (Gilman et al., 2021)).
2.4. Sample size justification
The parent trial (Gilman et al., 2022) aimed to recruit 200 participants and was powered using standard, in-lab monthly assessments to detect a clinically-significant, 30% reduction in health symptoms in the MCC group and a 5% reduction in the WLC group, with sample sizes of approximately 33 participants targeted in both the MCC and WLC groups from the three areas of presenting problems: insomnia, pain, or anxiety and/or depression.
2.5. Analysis
2.5.1. Feasibility and validation of daily cannabis reports
Feasibility and validity analyses used descriptive statistics and linear regression (see below).
2.5.2. Daily diary associations between cannabis use and physical and mental health symptoms
Linear mixed effects models were used to examine associations between cannabis use and sleep, pain, and anxiety and depression symptoms across the 12-week daily diary period. Cannabis use and the effect of cannabis use on symptoms was examined at the within-person, day-level by comparing days with versus days without reported cannabis use. Days with and without use was selected for primary analysis (over a potentially continuous measure of use occasions) to ensure model robustness given the variable number of use days between individuals and that on average, the MCC group reported use on about half the days in the daily diary period (see Fig. 1). Models were specified to account for person-specific (random effects) changes over time in symptoms following randomization, the difference between use days and nonuse days, and their interaction. Between-person (fixed effects) differences in the total number of cannabis use days, age, years of education, and presenting problem (self-reported challenges with sleep, pain, or mood) were included as covariates. See Supplemental S2 for additional detail on model specification.
Fig. 1. Cannabis Daily Diary Completion.
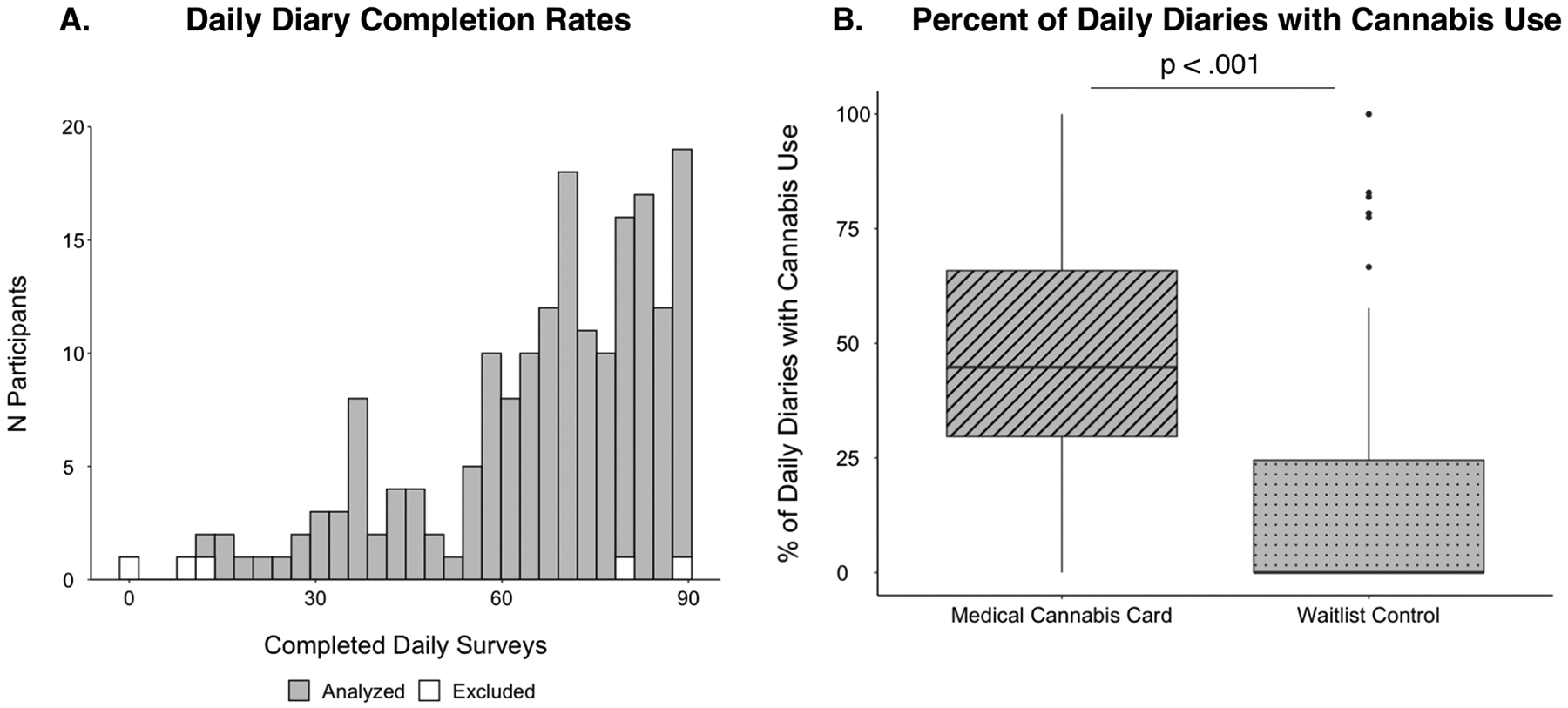
A) Histogram displaying the number of completed diaries for each participant. White bars indicate the five excluded participants; grey bars indicate the 181 participants in the final analytic sample (see Methods). B) Boxplots displaying the percent of completed daily surveys where participants indicated they had used cannabis in the medical cannabis card (MCC) group compared to the waitlist control (WLC) group.
As a central focus of the daily diary design was on two timescales: 1) potential short-term, same-day symptom relief through cannabis use, 2) longer-term changes across the full daily diary period, analyses focused on same-day (i.e., zero lag) associations between cannabis use and health symptoms and changes across the twelve-week period, respectively. For sleep quality, this meant the daily sleep measure (“How was your sleep quality last night”) was shifted forward one day (“lead”) with respect to the cannabis use metric, to model the effect of cannabis use on same-day (for sleep, same-night) symptoms for all outcomes.
Given the protocol instructed the WLC group to wait to seek a medical cannabis card and, consistent with this protocol, their reported and metabolite detected cannabis use was very low, primary statistical analyses linking same day cannabis use to health symptoms were restricted to the MCC group. In addition to the main effects in the MCC group, secondary analyses examined the associations between same day cannabis use and health symptoms in subgroups according to participant’s self-reported presenting problem that was used in stratification for randomization (see Procedure), to align the current work from that of the parent trial. Statistical inference was based on both effect size and significance values (Cumming, 2014).
2.5.3. Probability of cannabis use following randomization
To better understand any potential longer-term changes in health symptoms following randomization, cannabis use frequency (use day versus nonuse day) was modeled as a dependent variable as a function of time since baseline, via generalized linear mixed effects models with a logit link function. Fixed effects again included participant age, years of education, and presenting problem as covariates. Random intercepts and a random slope for days since baseline were included for each participant.
2.5.4. Sensitivity analyses
Sensitivity analyses were performed restricting the analyses to only those MCC participants who had cannabinoid metabolites detected via urinalysis during at least one timepoint of the study period. Although randomization was stratified by biological sex, additional sensitivity analyses were performed while covarying for biological sex, which has been linked to both cannabis use and the examined health symptoms. Unless otherwise stated, the magnitude (effect size) and pattern of significance of primary results were unchanged from those presented in the main document (See Supplemental S3, S4).
3. Results
3.1. Participant inclusion
Owing to delays in receiving a medical cannabis card, two participants from the MCC group completed the majority (> 45 / 90 days) of their daily diary assessments prior to receiving the MCC card and were excluded from primary analyses.
Additionally, to balance result generalizability, which has been widely suggested to be undermined by strict thresholds for participant inclusion on for example the number of completed daily diary assessments (Ji et al., 2018), and model stability, three participants who did not have at least 9 out of 90 (10%) days with completed assessments were excluded from data analysis. Therefore, our final primary analytic sample was 181 participants.
3.2. Daily diary completion rates
Completion rates of daily surveys were high overall and consistent with prior experience sampling studies of cannabis use with shorter study periods (e.g., less than 30 days (Buckner et al., 2012; Goodhines et al., 2019; Verdoux et al., 2003)). Among the full sample (N = 186; no analysis-specific exclusions, see above), the median number of days with completed surveys was 72 out of 90 days, with a mean of 66.21 (SD=20.02) and range of 1–90 (Fig. 1A). Completion rates were nearly identical in the final analytic sample (N = 181; the median number of days with completed surveys was 72 days, range of 12–90, Fig. 1A). Completion rates were comparable across presenting problems and MCC and WLC groups (Supplemental S5) and remained high throughout the entirety of the 90-day study period (see Supplemental S6).
Among the full sample (N = 186), the total number of completed surveys did not significantly differ between MCC and WLC groups, or as a function of presenting problem, and was likewise not related to baseline sleep, pain, depression, or anxiety (p’s > .095). There were significant positive associations between the number of completed surveys and participant age (r = 0.237, p = .001) and years of education (r = 0.175, p = .017). Age and years of education were used as covariates in all subsequent analyses. The pattern of significant associations between completion rates of daily surveys and study variables was unchanged when restricting analyses to only the participants in the primary analytic sample (N = 181).
3.2.1. Medical cannabis card group reports more cannabis use days than waitlist control
Daily diary data confirm randomization to a medical cannabis card (MCC) is associated with more subsequent cannabis use (percentage of daily reports that included cannabis use: MCC: 48.2%, WLC: 15.4%, p < .001)(Fig. 1B). Daily diary data likewise indicated a significant increase in cannabis frequency as a function of time since the baseline visit in the MCC group (p = .007), but not the WLC group (p = .071) (see below).
3.2.2. Validating cannabis daily diary with field-standard interview assessments and urinalysis
To validate cannabis use data collected via web-based, daily diaries, we compared the MCC group’s (n = 101 [1 participant did not have 1 month follow-up data]) cannabis use frequency from this method (percentage of daily surveys reporting cannabis use) with 1) cannabis frequency identified in a field-standard, in person interview-based assessment (see Methods) querying the first month of the daily diary period and 2) the presence of cannabinoid metabolites in urine after the first month of the daily diary period (i.e., urinalysis from experimenter-administered, two-week or one-month visits). The MCC group (and not the WLC group) was used in this analysis because the protocol instructed the WLC group to wait to seek a medical cannabis card and, consistent with this protocol, their cannabis use was very low (See (Gilman et al., 2021)). The first month of data (and not subsequent assessment periods) were used in these analyses given the potential lingering effects of cannabinoid metabolites in urine following use, and to avoid “carry--over” effects where qualitatively positive urinalysis results from the first month might influence subsequent assessment periods, even if there was no use (cf., (Schuster et al., 2020)). Qualitative results (detected versus not detected) were used given the high degree of individual variability in metabolite detection from urinalysis that is dependent on person-specific and methodological factors ((Gilman et al., 2021; Goodwin et al., 2008) see Supplemental S1 for additional discussion).
Cannabis use frequency collected via daily diaries (percentage of daily surveys with cannabis use) over the first month of the study was moderately to strongly associated with retrospective past month cannabis use frequency (number of days used) determined via structured interview questions with study staff at the experimenter-administered, four-week visit (standardized coefficient: β = 0.685, p < .001, R2=.468) (Fig. 2A). Cannabis use frequency collected via daily diaries (per-participant percentage of collected diaries with reported cannabis use) also disambiguated those in the MCC group with cannabinoid metabolites in their urine compared to those without detectable cannabinoids in their urine at either the two week or one month experimenter administered (p < .001) (Fig. 2B) as well as specifically those with THC metabolites (p < .001).
Fig. 2. Cannabis Daily Diary Validation.
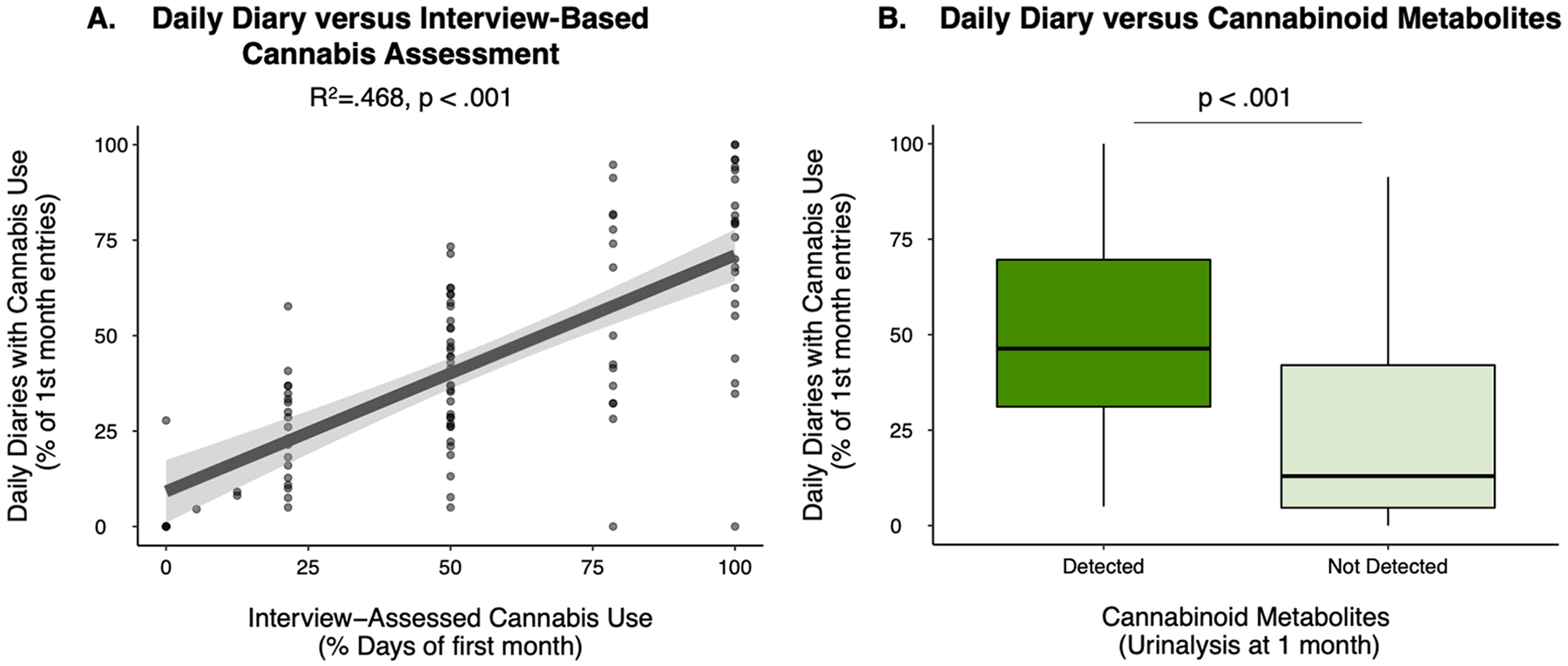
A) Association between per-participant past month cannabis use frequency assessed via daily diary (y-axis) and via experimenter interview (x-axis) among those participants in the medical cannabis card (MCC, n = 101) group. Model covaries for participant age and years of education. B) Per-participant past month cannabis use frequency derived via daily diary for those participants in the MCC with (n = 75) and without (n = 26) cannabinoid metabolites detected in their urine.
3.2.3. Same day associations between cannabis use and health symptoms in the MCC group
Among the full MCC group (N = 102), better sleep quality was reported for the night following cannabis use days compared to nonuse days (difference in standard deviation units: Δz =0.115, p < .001) (Fig. 3A). As our primary analyses utilized within-person day-level cannabis use and health symptom information and covaried for between-person associations between cannabis frequency and sleep (as well as salient demographic variables: see Methods), this result is consistent with cannabis use being associated with small, but significant same day improvements in self-reported sleep quality. The effect of same-day cannabis use on sleep quality significantly varied (omnibus test statistic for interaction from mixed model: χ2 = 7.32, p = .026) as a function of participants’ presenting problem at baseline (sleep, pain or mood), with post-hoc testing demonstrating significant, same day improvements of sleep on cannabis use days, compared to nonuse days for participants who entered the study based on self-reported problems with sleep (Δz =0.178, p = .007) and mood (Δz =0.178, p < .001) but not pain (Δz =0.022, p = .623)(Fig. 3A). The effect sizes and statistical significance of associations between cannabis use days vs. nonuse days with sleep quality did not change when restricting the MCC sample to only those with cannabinoid metabolites detected via urinalysis (Supplemental S2). Cannabis use days and nonuse days did not statistically differ in self-reported pain (Δz =0.023, p = .428)(Fig. 3B), the interaction between presenting problem (self-reported challenges sleep, pain, or mood) and cannabis use vs. nonuse days was not significant with respect to pain symptoms (χ2 = 2.44, p = .295), and there was not a significant difference in use days compared to nonuse days specifically in participants with a presenting problem of pain (Δz =0.074, p = .109). There was a very small, but statistically significant difference between cannabis use days and nonuse days in the full MCC sample (Δz = −0.058, p = .026), suggestive of use days being associated with slightly lower depressive symptoms compared to nonuse days; Fig. 3C). This effect however was not significant in supplemental analyses (S2) that restricted the sample to those with cannabinoid metabolites detected via urinalysis (Δz = −0.038, p = .197). The interaction between presenting problem and use vs. nonuse days was not significant with respect to depressive symptoms in the full MCC sample (χ2 = 4.32, p = .115) or in those with cannabinoid metabolites detected via urinalysis (χ2 = 2.37, p = .306), where the difference between use vs. nonuse days for depressive symptoms was also not significantly different specifically in the group with mood as a primary presenting problem (Δz =0.078, p = .073).
Fig. 3. Differences in Health Symptoms between Cannabis Use Days and Nonuse Days in the Medical Cannabis Card Group.
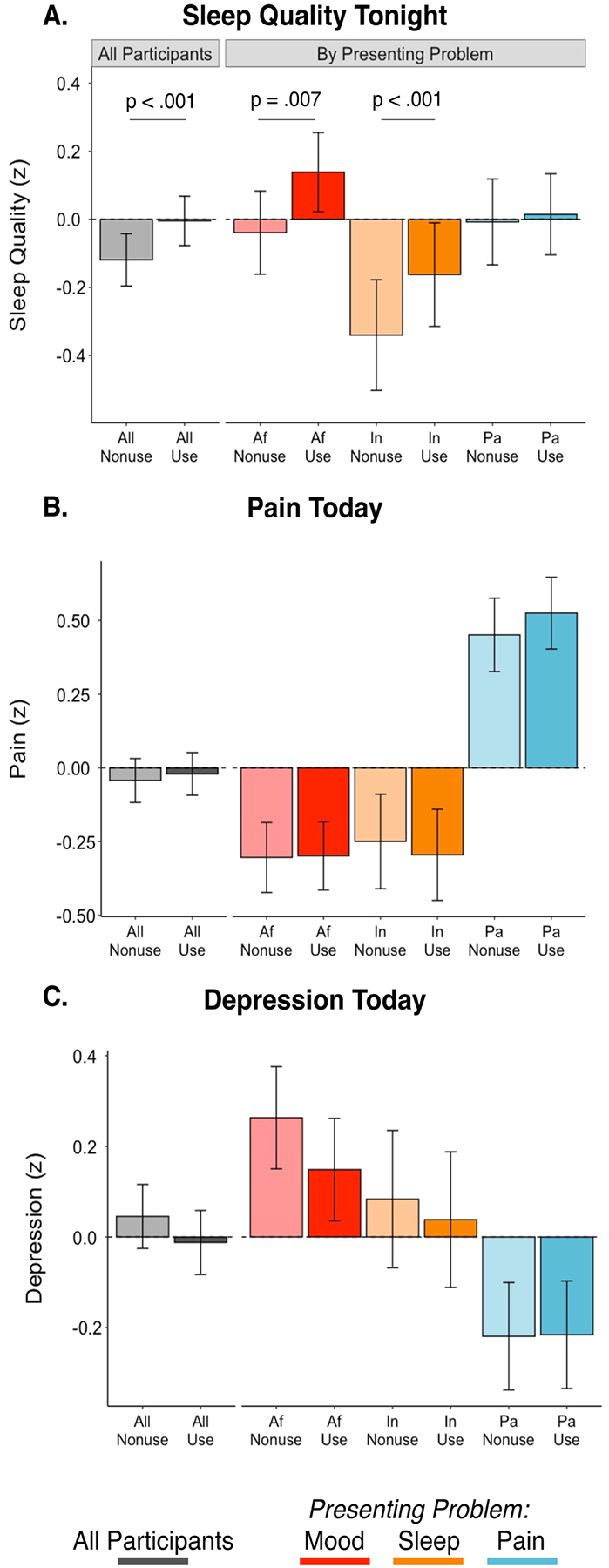
Differences in self-reported same night sleep quality (A), same day pain (B), and same day depression symptoms (C) in cannabis use days (darker colors) and nonuse days (lighter colors) in the medical cannabis card (MCC) group. Values shown for all MCC participants (N = 102; grey) and those whose presenting problem was mood (depression or anxiety symptoms, Af: n = 44, red), challenges with sleep (In: n = 22, orange), or pain (Pa: n = 36, blue). Data are z scored (standard deviation units) to support effect size interpretation. Displayed values are estimated marginal means and their standard errors from linear mixed effects models fit in primary analyses (covariates include per-participant estimates of age, years of education, and the proportion of daily diary days that included cannabis use [i.e., between-person effect]); see Methods for more detail on parameterization).
3.2.4. Linking daily and long-term sleep self-reported sleep quality changes associated with new cannabis use
Across the daily diary period (90 days), the MCC group displayed significant aggregate increases in self-reported sleep quality following randomization (per-day association (z units): β = 0.002, p = .007; total sleep change (z units): .195), while the WLC group did not (per-day standardized association (z units): β = −0.0003, p = .652; total sleep change (z units): .030) (Fig. 4A). The difference between MCC and WLC groups was significant (group by time interaction: χ2 = 4.70, p = .030). This effect was driven by those in the MCC group with a presenting problem of insomnia, whose sleep quality significantly increased over time (per-day association (z units): β = 0.006, p < .001; total sleep change (z units): .675) and was significantly different (χ2 = 11.51, p = .003) than MCC participants with a presenting problem of pain or mood (Fig. 4A). The pattern was mirrored in the insomnia group being the only MCC subgroup with a significant increase (per-day odds ratio: 1.02, p < .001) in the frequency of cannabis use days following randomization (Fig. 4B). This suggested that long-term improvements in sleep-quality were likely driven by an increase in cannabis use frequency, rather than for example, the lasting effect of a single cannabis use.
Fig. 4. Long-term Changes in Self-Reported Sleep and Cannabis Use.
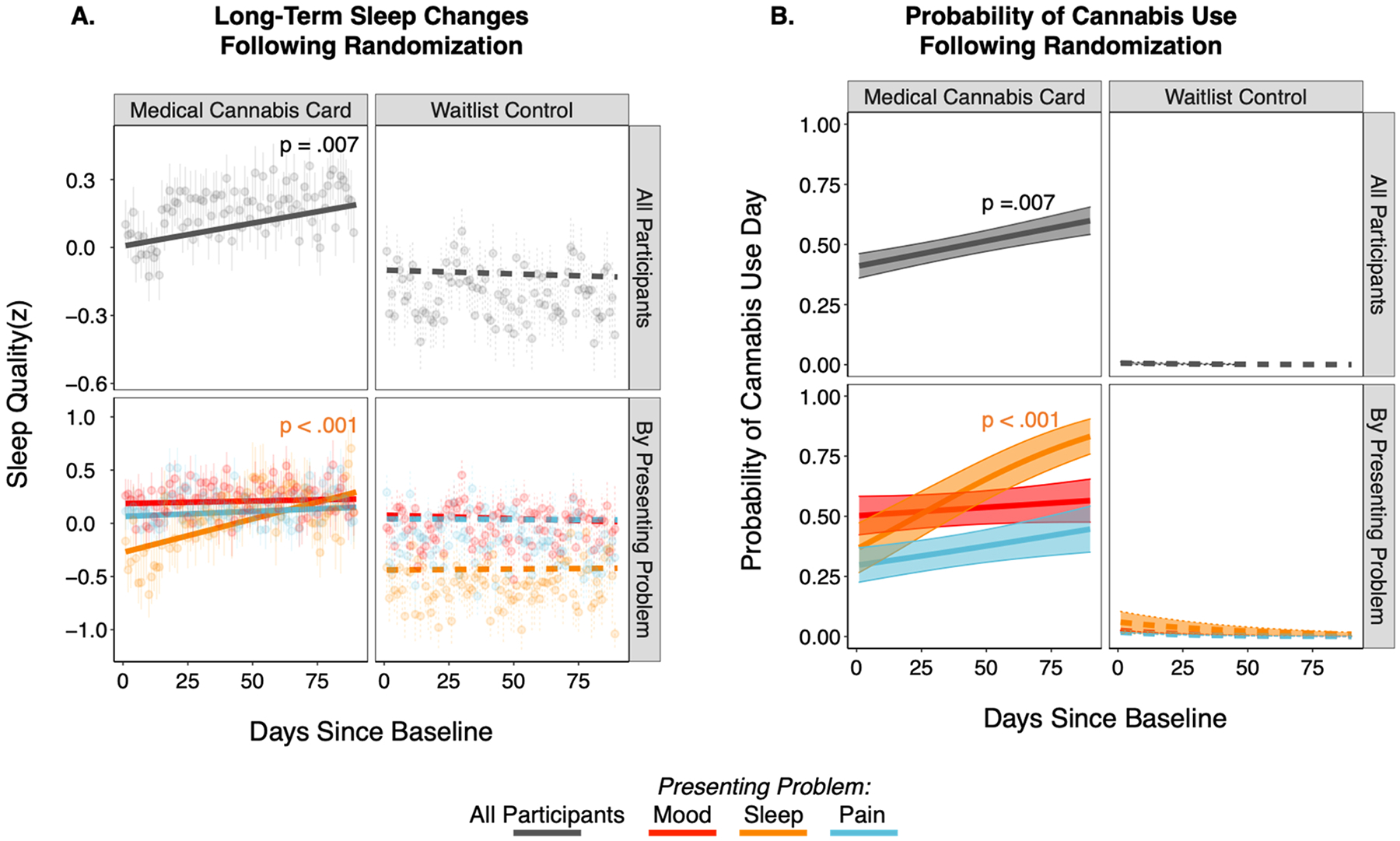
A) Sleep quality changes across the 90-day daily diary period for medical cannabis card (MCC, n = 102; left) and waitlist control (WLC, n = 79; right) groups shown for all participants in each group (top row) and separately by presenting problem (bottom row). Note, fits and data are averaged across use days and nonuse days. Data points are cross-participant means and standard errors of raw data; model fit lines are fixed effects that account for primary model covariates (per-participant age, years of education, and proportion of daily diary days that included cannabis use; see Methods for more detail on parameterization). B) Probability of a cannabis use day across the 90-day daily diary period for the MCC (left) and WLC (right) groups for all participants (top row) and by presenting problem (bottom row).
4. Discussion
4.1. Feasibility and validity of daily diary design in studies of cannabis use for health concerns
Completion rates of daily surveys were high across our long-term (90 days) daily diary design (80% of surveys on average) and approximately equivalent to other, shorter experience sampling studies of cannabis use (e.g., >1 month 60–90% completion rates (Buckner et al., 2012; Goodhines et al., 2019; Verdoux et al., 2003)). Using per-participant and group-level analysis procedures, we also found that completion rates remained within this range throughout the study period (Supplemental S6).
The current work also provides validation for the daily diary, experience sampling method for cannabis use metrics, as cannabis use frequency derived from daily surveys moderately-to-well aligned with field-standard, interview-based cannabis assessment as well as cannabinoid metabolite detection from urinalysis. This alignment while robust, however was not perfect and future daily diary, experience sampling studies of cannabis will benefit (as we have here) from concurrent interview-based and urinalysis cannabis assessments. Larger, well-powered multisite validity studies may be particularly useful towards addressing observed individual differences in cannabis use and health symptoms.
Web-based assessments of cannabis use are well-suited to current, highly variable real-world patterns of cannabis use for health concerns. Such daily diary assessments of cannabis use for health concerns may capture both real-world patterns of cannabis use in future pragmatic clinical trials and complement formal efficacy trials with in-laboratory measures to monitor study adherence and collect additional day-to-day or moment-to-moment use patterns. Web-based daily diary cannabis assessments will also be useful in future research that integrates photography or video to better remotely document cannabis product labels and ultimately estimate dosage.
4.2. Cannabis use is associated with same day improvements in sleep symptoms
Using the daily diary design, we demonstrate that cannabis use is associated with an improvement in same night sleep quality. The observed improvement in sleep quality on cannabis use days, compared to nonuse days, with respect to effect size, was small-to-moderate considering current effect size benchmarks (Gignac and Szodorai, 2016) and is considered to be practically (Funder and Ozer, 2019) and likely clinically (Rutledge and Loh, 2004) meaningful. This is notable as such small-to-moderate improvement is observed on the level of single days (see (Gabriel et al., 2019)). Same day sleep improvements among adults starting cannabis for health concerns is consistent with prior work examining recreational use among a community sample of college students during a shorter monitoring period (14 days)(Goodhines et al., 2019). These same day, within-person effects of cannabis-related sleep improvements are further consistent with a meta-analysis of sleep outcomes from clinical trials of therapeutic cannabinoids (Abrams, 2018). Supporting clinical research, basic science highlights a key role of the endogenous cannabinoid system in sleep (see (Prospéro-García et al., 2016),(Babson et al., 2017) for review). However, a recent small, but well controlled trial (Walsh et al., 2021), demonstrated that a cannabinoid formulation, relative to placebo, improved insomnia and sleep-related actigraphy outcomes, but not polysomnography measurements.
In the context of emerging research, expanded investigation of cannabinoids as a treatment for sleep challenges is likely warranted. The success of the current daily diary design suggests such research may benefit from flexible, ecologically valid designs together with expanded and more objective sleep assessments (e.g., actigraphy watches) to complement lab-based sleep studies. Larger, well powered multisite studies and consideration for broader health policy and cannabis regulation are likewise necessary.
4.3. Cannabis use is not associated with same day improvements in pain or mood symptoms
We did not find same day improvements in pain or depressive symptoms, which is consistent with our prior work looking at 12-week changes in health symptoms from in-person monthly assessments (i.e., non-daily diary) in adults randomized to receive a medical cannabis card (Gilman et al., 2021). Among health concerns, chronic pain is one of the most common complaints leading to an interest in cannabis use (Reinarman et al., 2011) and while some preclinical models suggest cannabinoids may regulate pain (Woodhams et al., 2015), clinical results have been inconclusive (Haroutounian et al., 2021; Mücke et al., 2018; National Academies of Sciences, Medicine, 2017). The current project did not find substantial evidence of cannabis improving same day pain symptoms. Given the relative success of the daily diary method in the current project and established intraindividual variability in chronic pain symptoms (Mun et al., 2019) (O’Brien et al., 2011), however, future work may use experience sampling designs to further explore same day associations in refined samples with expanded self-report capturing more comprehensive pain assessments.
Depressive symptoms are also associated with increased cannabis use in epidemiological samples (Degenhardt et al., 2003; Onaemo et al., 2020) and are frequently cited as a reason to pursue medical cannabis (Reinarman et al., 2011). The current project however did not find substantive improvement in self-reported depressive symptoms, which is largely consistent with existing evidence (Abrams, 2018). The significant interest in use of cannabis for depressive symptoms, together with the lack of improvement in depression symptoms among those using cannabis, is concerning given that those with affective disorders (e.g., depression, anxiety disorders) are likely at significantly increased risk for developing a cannabis use disorder (Gilman et al., 2022; Onaemo et al., 2020). It is therefore important for future cannabis treatment research to carefully assess symptoms of cannabis use disorder during treatment and evaluate for exacerbation in depressive and other psychiatric symptoms.
Owing to the noted variability, within and between individuals, for pain (May et al., 2018) and mood (Colombo et al., 2019) symptoms, as well as the current success of remote assessment, experience sampling techniques can provide an important complement to future efficacy studies assessing these health concerns. The current project emphasized a long monitoring period (90 days) but collected a single assessment per-day (daily diary design). Future work may nevertheless be supported by more direct, in-the-moment and event contingent designs that track health symptoms and cannabis use multiple times throughout the day and are more proximal to specific cannabis use occasions. Hybrid approaches that balance participant burden to assess symptoms across a long, clinically relevant time scale with such fine-grained temporal information may be particularly useful.
4.4. Limitations
Despite the strengths of the current research, there are limitations worth noting. First, the current project relied on exploratory analyses of daily self-reported single-item assessments of sleep, pain, and mood symptoms that were designed to minimize participant burden and maximize completion over the very long study period (90 daily diary days). It is, however, essential for future work to consider replicating these results with larger samples and objective measures with validated, clinician-blinded ratings. Another potential limitation is the likelihood of a high degree of variability in cannabis route of administration, dose, and potency among participants using cannabis for health concerns. In part, the current, pragmatic study of cannabis use for health concerns was designed to test for effectiveness given these variable, real-world conditions. As use patterns aligned with urinalysis and interview-based cannabis assessments, the daily diary responses appear valid in capturing broad use and frequency patterns. However, as participants purchased their own cannabis products, and our study lacked daily information on per-participant exact dosing, route of administration, and potency, clear conclusions regarding pharmacological effects of cannabis are not discernable from this project. An additional limitation of the current work concerns the generalizability of the current sample to other larger and more diverse samples. Future work with expanded recruitment efforts and larger, more diverse samples should seek to replicate and refine the current results.
5. Conclusion
This project establishes feasibility and validity of integrating a daily diary design into a pragmatic clinical trial of cannabis use for health concerns. Within this design, exploratory analyses support same-day improvements in sleep, but not pain or mood symptoms. Future work with expanded and objective measures of cannabis use and health symptoms can build upon the ecologically valid longitudinal design.
Supplementary Material
Acknowledgements
Funding/Support:
This work was funded by the National Institutes of Health R01DA042043; PI: JMG
Role of Funder/Sponsor:
The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Disclosures
AEE has served as a consultant to Charles River Analytics (NIDA SBIR grant) and Karuna Pharmaceuticals (Chair Data Monitoring Board). BTC has equity holdings in Abbot Laboratories, Gilead Sciences Inc., Medtronic PLC, Pfizer Inc., Thermo Fisher Scientific, Varian Medical Systems Inc., and Waters Corporation. GNP has equity holdings in Pfizer Inc. Other authors report no disclosures.
Footnotes
Conflict of Interest
No conflict Declared.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.drugalcdep.2022.109760.
Data Sharing Statement:
All data, code, and materials used in the analyses can be provided by Brenden Tervo-Clemmens, Jodi Gilman, and Massachusetts General Hospital pending scientific review and a completed data use agreement/material transfer agreement. Requests for all materials should be submitted to Brenden Tervo-Clemmens and Jodi Gilman.
References
- Abrams DI, 2018. The therapeutic effects of Cannabis and cannabinoids: an update from the National Academies of Sciences, Engineering and Medicine report. Eur. J. Intern. Med 49, 7–11. [DOI] [PubMed] [Google Scholar]
- Babson KA, Sottile J, Morabito D, 2017. Cannabis, cannabinoids, and sleep: a review of the literature. Curr. Psychiatry Rep 19, 1–12. [DOI] [PubMed] [Google Scholar]
- Boehnke KF, Gangopadhyay S, Clauw DJ, Haffajee RL, 2019. Qualifying conditions of medical cannabis license holders in the United States. Health Aff. 38, 295–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner JD, Crosby RD, Silgado J, Wonderlich SA, Schmidt NB, 2012. Immediate antecedents of marijuana use: an analysis from ecological momentary assessment. J. Behav. Ther. Exp. Psychiatry 43, 647–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo D, Fernández-Álvarez J, Patanè A, Semonella M, Kwiatkowska M, García-Palacios A, Cipresso P, Riva G, Botella C, 2019. Current state and future directions of technology-based ecological momentary assessment and intervention for major depressive disorder: a systematic review. J. Clin. Med 8, 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranford JA, Bohnert KM, Perron BE, Bourque C, Ilgen M, 2016. Prevalence and correlates of “Vaping” as a route of cannabis administration in medical cannabis patients. Drug Alcohol Depend. 169, 41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csikszentmihalyi M, Larson R, 2014. Validity and reliability of the experience-sampling method. In: Flow and the Foundations of Positive Psychology. Springer, pp. 35–54. [DOI] [PubMed] [Google Scholar]
- Cumming G, 2014. The new statistics: Why and how. Psychol. Sci 25, 7–29. [DOI] [PubMed] [Google Scholar]
- Degenhardt L, Hall W, Lynskey M, 2003. Exploring the association between cannabis use and depression. Addiction 98, 1493–1504. [DOI] [PubMed] [Google Scholar]
- Funder DC, Ozer DJ, 2019. Evaluating effect size in psychological research: sense and nonsense. Adv. Methods Pract. Psychol. Sci 2, 156–168. [Google Scholar]
- Gabriel AS, Podsakoff NP, Beal DJ, Scott BA, Sonnentag S, Trougakos JP, Butts MM, 2019. Experience sampling methods: a discussion of critical trends and considerations for scholarly advancement. Organ. Res. Methods 22, 969–1006. [Google Scholar]
- Gignac GE, Szodorai ET, 2016. Effect size guidelines for individual differences researchers. Personal. Individ. Differ 102, 74–78. [Google Scholar]
- Gilman JM, Schmitt WA, Wheeler G, Schuster RM, Klawitter J, Sempio C, Evins AE, 2021. Variation in cannabinoid metabolites present in the urine of adults using medical cannabis products in Massachusetts. JAMA Netw. Open 4 e215490–e215490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman JM, Schuster RM, Potter KW, Schmitt W, Wheeler G, Pachas GN, Hickey S, Cooke ME, Dechert A, Plummer R, 2022. Effect of medical marijuana card ownership on pain, insomnia, and affective disorder symptoms in adults: a randomized clinical trial. JAMA Netw. Open 5 e222106–e222106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodhines PA, Gellis LA, Ansell EB, Park A, 2019. Cannabis and alcohol use for sleep aid: a daily diary investigation. Health Psychol. 38, 1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman S, Wadsworth E, Leos-Toro C, Hammond D, team ICPS, 2020. Prevalence and forms of cannabis use in legal vs. illegal recreational cannabis markets. Int. J. Drug Policy 76, 102658. [DOI] [PubMed] [Google Scholar]
- Goodwin RS, Darwin WD, Chiang CN, Shih M, Li S-H, Huestis MA, 2008. Urinary elimination of 11-nor-9-carboxy-Δ9-tetrahydrocannnabinol in cannabis users during continuously monitored abstinence. J. Anal. Toxicol 32, 562–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haroutounian S, Arendt-Nielsen L, Belton J, Blyth FM, Degenhardt L, Di Forti M, Eccleston C, Finn DP, Finnerup NB, Fisher E, 2021. IASP Presidential Task Force on Cannabis and Cannabinoid Analgesia: research agenda on the use of cannabinoids, cannabis, and cannabis-based medicines for pain management. Pain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazekamp A, Ware MA, Muller-Vahl KR, Abrams D, Grotenhermen F, 2013. The medicinal use of cannabis and cannabinoids—an international cross-sectional survey on administration forms. J. Psychoact. Drugs 45, 199–210. [DOI] [PubMed] [Google Scholar]
- Ji L, Chow S-M, Schermerhorn AC, Jacobson NC, Cummings EM, 2018. Handling missing data in the modeling of intensive longitudinal data. Struct. Equ. Model.: A Multidiscip. J 25, 715–736. 10.1080/10705511.2017.1417046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahneman D, Krueger AB, Schkade DA, Schwarz N, Stone AA, 2004. A survey method for characterizing daily life experience: The day reconstruction method. Science 306, 1776–1780. [DOI] [PubMed] [Google Scholar]
- Lintzeris N, Driels J, Elias N, Arnold JC, McGregor IS, Allsop DJ, 2018. Medicinal cannabis in Australia, 2016: the cannabis as medicine survey (CAMS-16). Med. J. Aust 209, 211–216. [DOI] [PubMed] [Google Scholar]
- MacCallum CA, Russo EB, 2018. Practical considerations in medical cannabis administration and dosing. Eur. J. Intern. Med 49, 12–19. [DOI] [PubMed] [Google Scholar]
- May M, Junghaenel DU, Ono M, Stone AA, Schneider S, 2018. Ecological momentary assessment methodology in chronic pain research: a systematic review. J. Pain 19, 699–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead A, 2017. The legal status of cannabis (marijuana) and cannabidiol (CBD) under US law. Epilepsy Behav. 70, 288–291. [DOI] [PubMed] [Google Scholar]
- Miller LR, Cano A, 2009. Comorbid chronic pain and depression: who is at risk? J. Pain 10, 619–627. [DOI] [PubMed] [Google Scholar]
- Mücke M, Phillips T, Radbruch L, Petzke F, Häuser W, 2018. Cannabis-based medicines for chronic neuropathic pain in adults. Cochrane Database Syst. Rev [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mun CJ, Suk HW, Davis MC, Karoly P, Finan P, Tennen H, Jensen MP, 2019. Investigating intraindividual pain variability: methods, applications, issues, and directions. Pain 160, 2415–2429. [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, Medicine, 2017. The health effects of cannabis and cannabinoids: the current state of evidence and recommendations for research. [PubMed]
- O’Brien EM, Waxenberg LB, Atchison JW, Gremillion HA, Staud RM, McCrae CS, Robinson ME, 2011. Intraindividual variability in daily sleep and pain ratings among chronic pain patients: bidirectional association and the role of negative mood. Clin. J. Pain 27, 425–433. [DOI] [PubMed] [Google Scholar]
- Onaemo VN, Fawehinmi TO, D’Arcy C, 2020. Comorbid cannabis use disorder with major depression and generalized anxiety disorder: a systematic review and meta-analyses of nationally representative epidemiological surveys. J. Affect. Disord [DOI] [PubMed] [Google Scholar]
- Pacula RL, Boustead AE, Hunt P, 2014. Words can be deceiving: a review of variation among legally effective medical marijuana laws in the United States. J. Drug Policy Anal 7, 1–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prospéro-García O, Amancio-Belmont O, Meĺendez ALB, Ruiz-Contreras AE, Méndez-Díaz M, 2016. Endocannabinoids and sleep. Neurosci. Biobehav. Rev 71, 671–679. [DOI] [PubMed] [Google Scholar]
- Reinarman C, Nunberg H, Lanthier F, Heddleston T, 2011. Who are medical marijuana patients? Population characteristics from nine California assessment clinics. J. Psychoact. Drugs 43, 128–135. [DOI] [PubMed] [Google Scholar]
- Rutledge T, Loh C, 2004. Effect sizes and statistical testing in the determination of clinical significance in behavioral medicine research. Ann. Behav. Med 27, 138–145. [DOI] [PubMed] [Google Scholar]
- Sarris J, Sinclair J, Karamacoska D, Davidson M, Firth J, 2020. Medicinal cannabis for psychiatric disorders: a clinically-focused systematic review. BMC Psychiatry 20, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster RM, Potter K, Vandrey R, Hareli M, Gilman J, Schoenfeld D, Evins AE, 2020. Urinary 11-nor-9-carboxy-tetrahydrocannabinol elimination in adolescent and young adult cannabis users during one month of sustained and biochemically-verified abstinence. J. Psychopharmacol 34, 197–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sexton M, Cuttler C, Finnell JS, Mischley LK, 2016. A cross-sectional survey of medical cannabis users: patterns of use and perceived efficacy. Cannabis Cannabinoid Res. 1, 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaith RP, 2003. The hospital anxiety and depression scale. Health Qual. life Outcomes 1, 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldatos CR, Dikeos DG, Paparrigopoulos TJ, 2000. Athens Insomnia Scale: validation of an instrument based on ICD-10 criteria. J. Psychosom. Res 48, 555–560. [DOI] [PubMed] [Google Scholar]
- Staner L, 2010. Comorbidity of insomnia and depression. Sleep. Med. Rev 14, 35–46. [DOI] [PubMed] [Google Scholar]
- Tan G, Jensen MP, Thornby JI, Shanti BF, 2004. Validation of the Brief Pain Inventory for chronic nonmalignant pain. The. J. Pain 5, 133–137. [DOI] [PubMed] [Google Scholar]
- Trull TJ, Ebner-Priemer UW, 2020. Ambulatory assessment in psychopathology research: a review of recommended reporting guidelines and current practices. J. Abnorm. Psychol 129, 56. [DOI] [PubMed] [Google Scholar]
- Vandrey R, Raber JC, Raber ME, Douglass B, Miller C, Bonn-Miller MO, 2015. Cannabinoid dose and label accuracy in edible medical cannabis products. Jama 313, 2491–2493. [DOI] [PubMed] [Google Scholar]
- Verdoux H, Gindre C, Sorbara F, Tournier M, SWENDSEN JD, 2003. Effects of cannabis and psychosis vulnerability in daily life: an experience sampling test study. Psychol. Med 33, 23. [DOI] [PubMed] [Google Scholar]
- Walsh JH, Maddison KJ, Rankin T, Murray K, McArdle N, Ree MJ, Hillman DR, Eastwood PR, 2021. Treating insomnia symptoms with medicinal cannabis: a randomized, crossover trial of the efficacy of a cannabinoid medicine compared with placebo. Sleep. 44, zsab1 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhams SG, Sagar DR, Burston JJ, Chapman V, 2015. The role of the endocannabinoid system in pain. Pain. Control 119–143. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data, code, and materials used in the analyses can be provided by Brenden Tervo-Clemmens, Jodi Gilman, and Massachusetts General Hospital pending scientific review and a completed data use agreement/material transfer agreement. Requests for all materials should be submitted to Brenden Tervo-Clemmens and Jodi Gilman.


