Abstract
Objectives
Neurokinin 2 receptor (NK2R) agonists may be useful for treating bladder and bowel dysfunction via direct contraction of detrusor and gastrointestinal smooth muscle. The NK2R agonist [Lys5, MeLeu9, Nle10]-NKA(4–10) (LMN-NKA) induces urination and defecation, but also produces the potential side effect of dermal flushing in rats. Although LMN-NKA is a NK2R agonist, it also has affinity for neurokinin 1 receptors (NK1R). Therefore, the goal of this study was to determine the neurokinin receptor (NKR) subtypes responsible for LMN-NKA-induced urination, defecation, and flushing by blocking either NK2Rs or NK1Rs before LMN-NKA administration.
Methods
To accomplish this goal, we developed a simple high-throughput ‘rapid detection voiding assay’ to detect rapid-onset drug-induced urination and defecation in rats. In LMN-NKA dose-response experiments, LMN-NKA (10–100 μg/kg, subcutaneous) was injected and urination, defecation, and flushing were monitored for 30 min. For NKR antagonist experiments, vehicle, the NK2R antagonist GR159897, or the NK1R antagonist CP-99,994 were injected before an acclimation period. Following acclimation, saline or 100 μg/kg LMN-NKA were injected, and behavior was observed for 30 min.
Results
LMN-NKA produced dose-related increases in urination, defecation, and flushing. Blocking NK2Rs reduced urination and blocked defecation, without affecting flushing. Blocking NK1Rs did not change LMN-NKA-induced urination or defecation but reduced LMN-NKA-induced flushing.
Conclusions
Using the rapid detection voiding assay we show that LMN-NKA-induced urination and defecation are mediated by NK2Rs, while flushing is mediated by NK1Rs. Therefore, drugs that are more selective for NK2 vs. NK1Rs should produce rapid-onset urination and defecation without producing the potential side effect of flushing.
Keywords: defecation, neurokinin 1 receptor, neurokinin 2 receptor, rapid detection voiding assay, urination
Introduction
Bladder and bowel dysfunction are common in individuals with diabetes, spinal cord injury (SCI), neurological disease, and the elderly. In many cases, efficient bladder emptying is accomplished by catheterization, which may result in health problems including bleeding, urinary tract infections, urethritis, and pain [1, 2]. Individuals with diabetes, Parkinson’s disease, and multiple sclerosis often struggle with constipation resulting in an incomplete emptying of the bowel [3], [4], [5], and underactive bowel function following SCI often requires time consuming and inconvenient bowel management programs. Although some treatments are available for urinary retention and constipation/underactive bowel, none of the current therapies produce rapid-onset urination and defecation. Therefore, there is a need for a pharmacotherapy to promote rapid “on-demand” bladder and bowel voiding [6, 7].
Tachykinin neurokinin 2 receptor (NK2R) agonists cause contraction of the smooth muscle of the bladder and gastrointestinal tract across species, including humans [8]. Studies using anesthetized animals have shown that the NK2R agonist, [Lys5,MeLeu9,Nle10]-NKA (4–10) (LMN-NKA), produces rapid-onset dose-related increases in bladder and colorectal pressure [9, 10]. In conscious dogs, minipigs, and chronic SCI (cSCI) rats LMN-NKA induces reproducible, rapid-onset urination and defecation [11], [12], [13]. LMN-NKA is ∼74–105-fold more selective for NK2Rs vs. NK1Rs in functional assays measuring calcium mobilization [14]. However, transient hypotension, which is a NK1R mediated effect, has been observed with higher doses of LMN-NKA in anesthetized animal studies [11, 15]. Dermal flushing of the ears and feet was also observed in anesthetized rats and temporally coincides with transient hypotension following similar LMN-NKA doses. Therefore, the goal of the current study was to determine the NKR subtypes that mediate LMN-NKA-induced urination, defecation, and flushing in conscious spinally intact rats.
During investigations of a suitable drug for potential development it is important to have a reliable and rapid method to assess the drug’s desired effects and any adverse side effects. Evaluating rapid-onset drug-induced urination in naive spinally intact rats is challenging compared to dogs, mini-pigs, and chronic SCI rat models, which all have large bladders. Therefore, in the current study, we developed the rapid detection voiding assay to detect rapid, dose-dependent effects of LMN-NKA on urination and defecation concomitant with monitoring flushing and adverse behavior in spinally intact rats. This method displayed the sensitivity to differentiate the NKR subtypes mediating LMN-NKA-induced urination, defecation, and flushing.
Methods
Subjects
Adult Sprague–Dawley rats (n=27; Charles River, Raleigh, NC) were used in two experimental protocols. For the LMN-NKA dose response experiments n=4 male and n=11 female rats were used. Since no differences were observed in urination or defecation between male and female rats, n=12 female Sprague–Dawley rats were used for the NKR antagonist experiments. Rats were maintained under standard laboratory conditions with ad libitum food and water. Experiments conformed with NIH Guidelines for the Care and Use of Laboratory Animals and were approved by the Integrated Laboratory Systems Animal Care and Use Committee (Animal approved project number 2018-03).
Drug solutions and drug administration
LMN-NKA was dissolved in saline (Sal) at 10 mg/mL (corrected for the active peptide content of 91%) and diluted in Sal to give 0.01–0.1 mg/mL. The NK2R antagonist, GR159897 (Tocris), was solubilized at 50 mM in dimethylsulfoxide (DMSO) and diluted with Sal to a final concentration of 1 mg/mL in 4.8% DMSO. The NK1R antagonist, CP-99,994 (Monomerchem Inc.), was dissolved in Sal at a concentration of 1 mg/mL. Aliquots (1 mL) of each drug or vehicle (Veh) were stored at −20 °C until the day of the experiment. All drugs were given via subcutaneous (SC) injection into the left or right flank, and the site of the injection (left or right flank) alternated daily for the LMN-NKA dose-response experiment. For the NKR antagonist experiment, the first injection was given SC in the right or left flank, and the second injection was administered on the opposite side.
Behavioral monitoring using the rapid detection voiding assay
Rats were habituated to handling for 3 days prior to the initiation of the experiment. During habituation, rats were placed in metabolic cages (Life Science Equipment) 3 days for ∼20 min each day. On test days, rats were placed in the procedure room in their home cage 10–20 min before the experiment began.
All experimental trials were conducted during the light-cycle between 9:00 am and 12:30 pm. The dose-response study was conducted to determine the optimal dose of LMN-NKA to use in the antagonist study. Rats were placed into individual metabolic cages for a 10 min acclimation period. The cylindrical metabolic cages (25.4 cm in diameter and 20.3 cm tall) were placed directly on a grid floor, and a round mirror (25.4 cm in diameter) was placed under the grid floor. The mirror allowed visualization of the ventral surface (urethra and anus) of the animal and was easily slid out from under the cage to collect urine. Urine was drawn up into a 0.5 or 1 mL syringe and the volume and time was recorded immediately after each void. The testing area was illuminated to aid observation of dermal flushing of the ears and paws. After the acclimation period, Sal or LMN-NKA (10, 30, or 100 μg/kg) were administered SC, and behavior was observed for an additional 30 min.
During the NKR antagonist study, rats were administered Veh (Sal or 4.8% DMSO), 1 mg/kg GR159897, or 1 mg/kg CP-99,994 and behavior was monitored during a 20 min acclimation period to provide sufficient time for NKR blockade. Subsequently, Sal or 100 μg/kg LMN-NKA were administered, and behavior was observed for an additional 30 min. A dose of 100 μg/kg LMN-NKA was chosen based on consistent urination, defecation, and dermal flushing during the dose-response study. For both the dose-response study and NKR antagonist study, there were no group differences in behavior during the acclimation period (data not shown), and the data reported for both studies are from the 30 min observation period.
For both studies, rats were dosed using a randomly assigned crossover design. In the dose response study, rats were assigned to 2–3 LMN-NKA dose trials and up to 3 Sal trials (Sal n=15; 10 μg/kg n=10; 30 μg/kg n=11; 100 μg/kg n=10). In the NKR antagonist study, rats were assigned to 1–3 of the LMN-NKA trials and up to 3 Veh + Sal trials (Veh + Sal n=20; Veh + LMN-NKA n=12; CP + LMN-NKA n=10; GR + LMN-NKA n=8). Observers were blinded to the treatment conditions and up to four rats were observed at one time, with the dosing staggered by 6–10 min/rat. Data were entered on score sheets and behavioral measurements included time of each urination event, urination volume, the time of fecal pellet excretion, the number of fecal pellets excreted, consistency of pellets, onset of flushing, and offset of flushing. At the end of the trial, animals were placed back in the home cage, and the fecal pellet weight was recorded. The rapid detection voiding assay can be used to concurrently observe a variety of normal behaviors such as, grooming and locomotion, while also providing initial detection of drug-induced adverse events and behaviors such as, piloerection, lethargy, stereotypy, and abnormal posture/gait.
Data analysis
Data were transcribed into Excel (Microsoft, Redmond, WA) and analyzed using Prism 8 (GraphPad Software Inc., San Diego, CA). Shapiro–Wilk normality tests showed the data were not normally distributed. Therefore, data were analyzed with Kruskal–Wallis tests followed by Dunn’s multiple comparisons tests. Data are presented as median ± interquartile range and a p value<0.05 was considered statistically significant.
Results
LMN-NKA dose-response study
LMN-NKA (10–100 μg/kg) induced dose-dependent urination that was complete by 10 min post-injection. There was a significant increase in voided urine volume and number of urination events 10 min following 30 and 100 μg/kg LMN-NKA (Figure 1A–B). The latency to urinate decreased with increasing concentrations of LMN-NKA (Figure 1C).
Figure 1:
LMN-NKA rapidly induces urination in conscious rats. LMN-NKA (30 and 100 μg/kg) produced a dose-related increase in (A) urine volume (H=17.12, p<0.001) and (B) number of urination events (H=19.18, p<0.0005), and the 100 μg/kg dose significantly reduced (C) the latency to urinate (H=12.34, p<0.01). If no urination occurred, a maximum observation time of 30 min was assigned. Lines above bars indicate statistically significant group differences (p<0.05, Dunn’s multiple comparisons test). Data are expressed as median ± interquartile range (n=10–15/group).
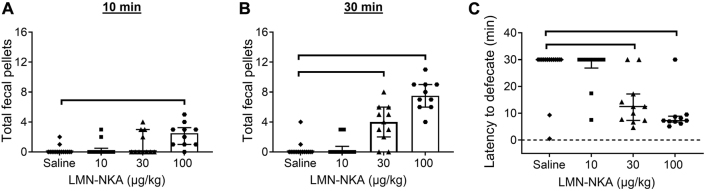
LMN-NKA (30 and 100 μg/kg) induced rapid defecation that continued throughout the 30 min observation period (Figure 2A–B). There was a dose-related decrease in latency to defecate (Figure 2C). The latency to defecate was 4–17 min following 30 μg/kg and approximately 8 min following 100 μg/kg.
Figure 2:
LMN-NKA induces defecation in conscious rats. LMN-NKA (30 μg/kg) increased the total number of fecal pellets at (A) 10 min (H=16.30, p<0.005). Both the 30 and 100 μg/kg doses increased the total number of fecal pellets at (B) 30 min post-injection (H=32.74, p<0.001), and (C) reduced the latency to defecate (H=20.87, p<0.0005). If no defecation occurred, a maximum observation time of 30 min was assigned. Lines above bars indicate statistically significant group differences (p<0.05, Dunn’s multiple comparisons test). Data are expressed as median ± interquartile range (n=10–15/group).
Flushing of the ears and paws occurred 0.5–2 min following LMN-NKA (30 and 100 μg/kg) [p<0.05 compared to saline, Dunn’s multiple comparisons test, n=10–15/group (data not shown)]. Duration of flushing was not recorded. No adverse behavior was observed.
NKR antagonist study
LMN-NKA significantly increased urine volume and the number of urination events, compared to controls (Figure 3). Blocking NK2Rs with GR159897 resulted in urine volumes and urination events similar to Veh + Sal controls (Figure 3). LMN-NKA increased defecation (Figure 4A–B), with a latency of <10 min (Figure 4C). LMN-NKA-induced defecation was blocked by pretreatment with GR159897 (Figure 4). LMN-NKA (100 μg/kg) induced flushing was not inhibited by pretreatment with GR159897 (Figure 5).
Figure 3:
LMN-NKA increases urination in the presence of the NK1R antagonist CP-99,994 but not the NK2R antagonist GR159897. (A−B) at 10 min following LMN-NKA administration the Veh + LMN-NKA and CP + LMN-NKA groups had significantly increased urine volume (H=14.13, p<0.005) and urination events (H=16.06, p<0.005). Lines above bars indicate statistically significant group differences (p<0.05, Dunn’s multiple comparisons test). Data are expressed as median ± interquartile range (n=8–20/group). CP, CP-99,994; GR, GR159897; Sal, saline; Veh, vehicle.
Figure 4:
LMN-NKA induced defecation is NK2R dependent. (A−B) Veh + LMN-NKA and CP + LMN-NKA significantly increased defecation at 10 min (H=25.69, p<0.0001) and 30 min post-injection (H=34.97, p<0.0001), and blocking NK2Rs completely blocked this effect (GR + LMN-NKA group). (C) The Veh + LMN-NKA and CP + LMN-NKA groups show reduced latency to defecate (H=33.72, p<0.0001). If no defecation occurred, a maximum observation time of 30 min was assigned. (D) The Veh + LMN-NKA and CP + LMN-NKA groups produced significantly greater total fecal pellet weights (H=38.10, p<0.0001), compared to the Veh + Sal and GR + LMN-NKA groups. Lines above bars indicate statistically significant group differences (p<0.05, Dunn’s multiple comparisons test). Data are expressed as median ± interquartile range (n=8–20/group). CP, CP-99,994; GR, GR159897; Veh, vehicle; Sal, saline.
Figure 5:
Blocking NK1Rs with CP-99,994 reduces LMN-NKA induced dermal flushing. (A) The Veh + LMN-NKA and GR + LMN-NKA groups displayed a rapid-onset of flushing (H=31.78, p<0.0001) and this effect was inhibited by pretreatment with the NK1R antagonist CP-99,994 (CP + LMN-NKA group). A value of 30 min indicates no flushing was observed. (B) The Veh + LMN-NKA and GR + LMN-NKA groups display significantly longer duration of flushing than the CP + LMN-NKA group (H=36.12, p<0.0001). Lines above bars indicate statistically significant group differences (p<0.05, Dunn’s multiple comparisons test). Data are expressed as median ± interquartile range (n=5–12/group). CP, CP-99,994; GR, GR159897; Sal, saline; Veh, vehicle.
Pretreatment with the NK1R antagonist, CP-99,994, had no effect on LMN-NKA-induced urination (Figure 3) or defecation (Figure 4). However, NK1R antagonism inhibited LMN-NKA-induced flushing, delaying the onset of flushing and reducing flushing duration (Figure 5).
Discussion
In the current study, we examined the NKR subtypes mediating LMN-NKA-induced urination, defecation, and flushing in rats. To measure rapid-onset drug-induced urination and defecation using spinally intact rats we developed the rapid detection voiding assay. This assay has adequate sensitivity to detect rapid onset, dose-related increases in urination and defecation, as well as changes in behavior and adverse events that are important to identify during drug screening (see methods). Using the rapid detection voiding assay we demonstrated that LMN-NKA-induced urination and defecation are inhibited by pretreatment with the NK2R antagonist GR159897 and LMN-NKA-induced flushing is mediated by NK1Rs receptors.
Administration of 30 and 100 μg/kg LMN-NKA rapidly induced urination and the number of urination events (Figure 1A–B). These results are particularly promising since no measures were taken to ensure there was urine present in the bladder at the time of drug administration. Based on results from the dose-response study, the 100 μg/kg dose of LMN-NKA was used to test the effects of NK1 or NK2R blockade since this dose produced consistent urination, defecation, and flushing. In the antagonist study, LMN-NKA-induced urination was inhibited after NK2 but not NK1R blockade (Figure 3A–B). These findings are consistent with previous studies that show blocking NK2Rs prevents LMN-NKA mediated increases in bladder pressure in anesthetized minipigs, dogs, and acute SCI rats [9, 10, 12]. Overall, drug-induced urination was more variable than drug-induced defecation in spinally intact rats. This is likely due to variability in the amount of urine present in the bladder at the onset of the experiment, coupled with the small size of the bladder in spinally intact rats. We did not see a sex difference in drug-induced urination and defection in the dose-response experiments. Therefore, only female rats were used in the antagonist study, which could have increased the variability since female rats have smaller bladder capacities than males [16]. Furthermore, handling and drug injections are stressful for rats, which can result in urination. Therefore, sufficient habituation of rats to the rapid detection voiding assay is critical to optimize the urination signal to noise ratio.
Drug-induced defecation using the rapid detection voiding assay was highly reproducible. Both 30 and 100 μg/kg LMN-NKA significantly increased defecation and decreased the latency to defecate (Figure 2). In the NKR antagonist study, LMN-NKA-induced defecation was blocked by the NK2R antagonist GR159897 (Figure 4). In contrast, blocking NK1Rs with CP-99,994 had no effect on LMN-NKA-induced defecation. These results extend previous findings that showed blocking NK2Rs prevents LMN-NKA-mediated increases in colorectal activity in anesthetized minipigs, dogs, and acute SCI rats [9, 10, 12], and that LMN-NKA produced rapid-onset defecation in conscious dogs that was not altered by the NK1R antagonist CP-99,994 [11]. Furthermore, in humans the endogenous NK2R agonist, neurokinin A (NKA), increases gastrointestinal motility, which can be reduced by the NK2R antagonist MEN 1142 (Nepadutant) [17]. Therefore, examining the effectiveness of NK2R agonists to induce gastrointestinal activity and defecation in rats shows translational promise for human underactive bowel disorders.
The current results suggest that LMN-NKA-induced flushing in rats is mediated by NK1Rs. Blocking NK1Rs with CP-99,994 significantly reduced the onset and duration of flushing (Figure 5). In humans, flushing is a potential adverse event that is associated with sweating, skin irritation, itching, warm sensation, and can result in patient discomfort [18]. Dermal vasodilation is a well-recognized effect of NK1R agonists in humans [19]. Specifically, substance p, the endogenous ligand for NK1Rs, has been shown to produce vasodilation and dermal flushing in humans [20, 21], presumably by activation of NK1Rs located on cutaneous blood vessels [22]. The endogenous NK2R agonist, NKA, is also a potent agonist at the septide-sensitive binding site of NK1Rs and binds to NK1Rs with subnanomolar affinity [23]. Intravenous infusion of NKA in humans has been shown to produce flushing that was not blocked by the NK2R antagonist MEN 1142 [17], which is similar to the current findings with LMN-NKA-mediated flushing in rats. Unfortunately, that study did not test whether an NK1R antagonist could prevent NKA-induced flushing in humans. Taken together, the data from humans and the current data in rats suggest that dermal flushing produced by the NKR agonists LMN-NKA, NKA, and substance p are all mediated by NK1Rs and not NK2Rs.
Rapid detection voiding assay
There are several factors to consider when using the rapid detection voiding assay to test drug efficacy in spinally intact rats. First, this assay is best suited to study rapid-onset urination and/or defecation (within 5–30 min), while concurrently providing an immediate assessment of potential adverse side effects. If long term measurement of voiding behavior is desired, metabolism cages placed above an analytical balance with a computer interface are the standard method for accurate long-term urination measurement [24]. While this method provides accurate and long-term measurements of voiding it does not typically assess real time adverse events. Furthermore, methods used to examine defecation in rats are typically focused on evaluating gastrointestinal motility over a relatively long timeframe.
Habituation of rats to handling, injection procedures, metabolism cages, and the testing facility each day, at the same time each day, for a minimum of 3 occasions are important to prevent high levels of baseline urination and defecation. The amount of habituation required varies depending on the strain, age, and model being used. For example, in our experience the Sprague Dawley and Fischer Brown Norway strains require less habituation than Fischer 344 rats, which require habituation for 5 or more days. In addition, young adult rats (2–6 months) require less habituation than older rats used for aging studies (15–20 months), that require ≥6 days of habituation. Moreover, results can be optimized by performing the experiment ∼2 h into the dark cycle (lights off) and illuminating the room with red light so the experimenter can observe behavior. Experiments performed during the dark cycle take advantage of the highest level of rat activity, which is highest near the beginning of the dark cycle [25]. Currently, we have successfully used the rapid detection voiding assay to detect rapid drug-induced voiding and/or defecation following SC, intramuscular, and intrarectal drug administrations, but other routes of administration should be easily adaptable for use with the assay. Furthermore, rodent experiments using neuromodulation to induce urination and/or defecation would be well suited for the rapid detection voiding assay as well. One of the limitations of using the rapid detection voiding assay with spinally intact rats is that the bladder volumes at the onset of the experiment are unknown, which contributes to variability in the urination results. But overall, the rapid detection voiding assay provides a simple and effective high-throughput method for evaluating rapid-onset drug-induced urination and defecation in rats.
In conclusion, using the rapid detection voiding assay we demonstrate that LMN-NKA produces rapid-onset urination and defecation via NK2R activation in conscious rats. We also show that LMN-NKA-induced dermal flushing is mediated by NK1Rs. Therefore, drugs that are more selective for NK2Rs vs. NK1Rs may be useful for inducing on-demand urination and defecation in individuals with underactive bladder and bowel function while limiting unwanted side effects.
Acknowledgments
We thank Integrated Laboratory Systems for their collaboration. We also thank Dr. Nadia Rupniak for a thorough and thoughtful review of the manuscript.
Footnotes
Research funding: This study was funded by the NIDDK of the National Institutes of Health under award number R44DK112437.
Author contributions: JBC performed experiments, collected and analyzed the data, wrote the manuscript. RP performed experiments, collected data, edited manuscript. LM conceived the study, edited manuscript, acquired funding.
Competing interests: Jason B. Cook, Raymond Piatt, and Lesley Marson are employed by Dignify Therapeutics LLC. Lesley Marson has equity ownership in Dignify Therapeutics LLC.
Informed consent: Not applicable.
Ethical approval: Experiments conformed with NIH Guidelines for the Care and Use of Laboratory Animals and were approved by the Integrated Laboratory Systems Animal Care and Use Committee (Animal approved project number 2018-03).
References
- 1.Singh R, Rohilla RK, Sangwan K, Siwach R, Magu NK, Sangwan SS. Bladder management methods and urological complications in spinal cord injury patients. Indian J Orthop. 2011;45:141–7. doi: 10.4103/0019-5413.77134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newman DK, Willson MM. Review of intermittent catheterization and current best practices. Urol Nurs. 2011;31:12–28. doi: 10.7257/1053-816x.2012.31.1.12. quiz 29. [DOI] [PubMed] [Google Scholar]
- 3.Dibley L, Coggrave M, McClurg D, Woodward S, Norton C. It’s just horrible”: a qualitative study of patients’ and carers’ experiences of bowel dysfunction in multiple sclerosis. J Neurol. 2017;264:1354–61. doi: 10.1007/s00415-017-8527-7. [DOI] [PubMed] [Google Scholar]
- 4.Stocchi F, Torti M. Constipation in Parkinson’s disease. Int Rev Neurobiol. 2017;134:811–26. doi: 10.1016/bs.irn.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 5.Bytzer P, Talley NJ, Leemon M, Young LJ, Jones MP, Horowitz M. Prevalence of gastrointestinal symptoms associated with diabetes mellitus: a population-based survey of 15,000 adults. Arch Intern Med. 2001;161:1989–96. doi: 10.1001/archinte.161.16.1989. [DOI] [PubMed] [Google Scholar]
- 6.van Koeveringe GA, Vahabi B, Andersson KE, Kirschner-Herrmans R, Oelke M. Detrusor underactivity: a plea for new approaches to a common bladder dysfunction. Neurourol Urodyn. 2011;30:723–8. doi: 10.1002/nau.21097. [DOI] [PubMed] [Google Scholar]
- 7.Awad RA. Neurogenic bowel dysfunction in patients with spinal cord injury, myelomeningocele, multiple sclerosis and Parkinson’s disease. World J Gastroenterol. 2011;17:5035–48. doi: 10.3748/wjg.v17.i46.5035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warner FJ, Miller RC, Burcher E. Human tachykinin NK2 receptor: a comparative study of the colon and urinary bladder. Clin Exp Pharmacol Physiol. 2003;30:632–9. doi: 10.1046/j.1440-1681.2003.03887.x. [DOI] [PubMed] [Google Scholar]
- 9.Rupniak NMJ, Katofiasc M, Marson L, Thor KB. NK2 and NK1 receptor-mediated effects of NKA and analogs on colon, bladder, and arterial pressure in anesthetized dogs. Naunyn-Schmiedeberg’s Arch Pharmacol. 2018;391:299–308. doi: 10.1007/s00210-017-1458-0. [DOI] [PubMed] [Google Scholar]
- 10.Kullmann FA, Katofiasc M, Thor KB, Marson L. Pharmacodynamic evaluation of Lys(5), MeLeu(9), Nle(10)-NKA(4-10) prokinetic effects on bladder and colon activity in acute spinal cord transected and spinally intact rats. Naunyn-Schmiedeberg’s Arch Pharmacol. 2017;390:163–73. doi: 10.1007/s00210-016-1317-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rupniak NMJ, Katofiasc M, Walz A, Thor KB, Burgard EC. [Lys(5), MeLeu(9), Nle(10)]-NKA(4-10) elicits NK2 receptor-mediated micturition and defecation, and NK1 receptor-mediated emesis and hypotension, in conscious dogs. J Pharmacol Exp Therapeut. 2018;366:136–44. doi: 10.1124/jpet.118.248765. [DOI] [PubMed] [Google Scholar]
- 12.Rupniak NMJ, Katofiasc MA, Marson L, Ricca DJ, Thor KB, Burgard EC. Prokinetic effects of the neurokinin NK2 receptor agonist [Lys(5), MeLeu(9), Nle(10)]-NKA(4-10) on bladder and colorectal activity in minipigs. Neuropeptides. 2019;77:101956. doi: 10.1016/j.npep.2019.101956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marson L, Piatt RK, Katofiasc MA, Bobbitt C, Thor KB. Chronic, twice-daily dosing of an NK2 receptor agonist [Lys(5), MeLeu(9), Nle(10)]-NKA(4-10), produces consistent drug-induced micturition and defecation in chronic spinal rats. J Neurotrauma. 2019;37:868–76. doi: 10.1089/neu.2019.6676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rupniak NMJ, Perdona E, Griffante C, Cavallini P, Sava A, Ricca DJ, et al. Affinity, potency, efficacy, and selectivity of neurokinin a analogs at human recombinant NK2 and NK1 receptors. PLoS One. 2018;13:e0205894. doi: 10.1371/journal.pone.0205894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marson L, Thor KB, Katofiasc M, Burgard EC, Rupniak NMJ. Prokinetic effects of neurokinin-2 receptor agonists on the bladder and rectum of rats with acute spinal cord transection. Eur J Pharmacol. 2018;819:261–9. doi: 10.1016/j.ejphar.2017.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cruz Y, Downie JW. Sexually dimorphic micturition in rats: relationship of perineal muscle activity to voiding pattern. Am J Physiol Regul Integr Comp Physiol. 2005;289:R1307–18. doi: 10.1152/ajpregu.00088.2005. [DOI] [PubMed] [Google Scholar]
- 17.Lordal M, Navalesi G, Theodorsson E, Maggi CA, Hellstrom PM. A novel tachykinin NK2 receptor antagonist prevents motility-stimulating effects of neurokinin a in small intestine. Br J Pharmacol. 2001;134:215–23. doi: 10.1038/sj.bjp.0704217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kamanna VS, Ganji SH, Kashyap ML. The mechanism and mitigation of niacin-induced flushing. Int J Clin Pract. 2009;63:1369–77. doi: 10.1111/j.1742-1241.2009.02099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newby DE, Sciberras DG, Ferro CJ, Gertz BJ, Sommerville D, Majumdar A, et al. Substance p-induced vasodilatation is mediated by the neurokinin type 1 receptor but does not contribute to basal vascular tone in man. Br J Clin Pharmacol. 1999;48:336–44. doi: 10.1046/j.1365-2125.1999.00017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hannah-Shmouni F, Stratakis CA, Koch CA. Flushing in (neuro) endocrinology. Rev Endocr Metab Disord. 2016;17:373–80. doi: 10.1007/s11154-016-9394-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muckadell OBSD, Aggestrup S, Stentoft P. Flushing and plasma substance p concentration during infusion of synthetic substance p in normal man. Scand J Gastroenterol. 1986;21:498–502. doi: 10.3109/00365528609015169. [DOI] [PubMed] [Google Scholar]
- 22.Wong BJ, Minson CT. Neurokinin-1 receptor desensitization attenuates cutaneous active vasodilatation in humans. J Physiol. 2006;577:1043–51. doi: 10.1113/jphysiol.2006.112508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hastrup H, Schwartz TW. Septide and neurokinin a are high-affinity ligands on the NK-1 receptor: evidence from homologous versus heterologous binding analysis. FEBS Lett. 1996;399:264–6. doi: 10.1016/s0014-5793(96)01337-3. [DOI] [PubMed] [Google Scholar]
- 24.Gumbel JH, Yang CB, Hubscher CH. Timeline of changes in biomarkers associated with spinal cord injury-induced polyuria. Neurotrauma Rep. 2021;2:462–75. doi: 10.1089/neur.2021.0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Evans HL. Rats’ activity: influence of light-dark cycle, food presentation and deprivation. Physiol Behav. 1971;7:455–9. doi: 10.1016/0031-9384(71)90094-1. [DOI] [PubMed] [Google Scholar]