Abstract
We sought to assess if COVID-19 infection recovery is associated with increased rates of newly diagnosed erectile dysfunction. Using IBM MarketScan, a commercial claims database, men with prior COVID-19 infection were identified using ICD-10 diagnosis codes. Using this cohort along with an age-matched cohort of men without prior COVID-19 infection, we assessed the incidence of newly diagnosed erectile dysfunction. Covariates were assessed using a multivariable model to determine association of prior COVID-19 infection with newly diagnosed erectile dysfunction. 42,406 men experienced a COVID-19 infection between January 2020 and January 2021 of which 601 (1.42%) developed new onset erectile dysfunction within 6.5 months follow up. On multivariable analysis while controlling for diabetes, cardiovascular disease, smoking, obesity, hypogonadism, thromboembolism, and malignancy, prior COVID-19 infection was associated with increased risk of new onset erectile dysfunction (HR 1.27; 95% CI 1.1–1.5; P = 0.002). Prior to the widespread implementation of the COVID-19 vaccine, the incidence of newly diagnosed erectile dysfunction is higher in men with prior COVID-19 infection compared to age-matched controls. Prior COVID-19 infection was associated with a 27% increased likelihood of developing new-onset erectile dysfunction when compared to those without prior infection.
Subject terms: Erectile dysfunction, Predictive markers, Sexual dysfunction, Preventive medicine, Risk factors
Introduction
As of April 2022, the SARS-CoV-2 virus has led to over 80 million COVID-19 infections and 970,000 deaths in the United States alone [1]. As the pandemic has evolved, the role of endothelial dysfunction during COVID-19 infection and associated long-term sequalae have come into focus [2–4]. Given the well-known association of erectile dysfunction (ED) and cardiovascular disease, many have hypothesized there may be increased risk of ED following COVID-19 [5–11].
Initial reports describing increased risk of ED after COVID-19 have been limited to small cohort studies (<75 COVID-positive men), but have shown reduction of International Index Erectile Function-5 (IIEF-5) scores after COVID-19 infection and increased rates of ED compared to age-matched controls [10, 12, 13]. Evaluation of corporal tissue months after COVID-19 infection revealed persistence of COVID-19 peplomers suggesting a possible pathophysiologic mechanism for ED [7]. An association between COVID-19 infection and ED has been shown in two population-level studies [5, 6]. However, the databases utilized require follow-up to occur in participating institutions in order be captured and as a result, may underestimate the true association [5, 6] IBM MarketScan, an insurance claims database of over 215 million policy holders, is able to capture longitudinal follow up regardless of the location in which follow up occurs.
Given this preliminary evidence, we hypothesize that the incidence of newly diagnosed ED will be higher in men with a history of COVID-19 infection compared to men without prior COVID-19 infection. Thus, we sought to assess the rate of newly diagnosed ED in those with a history of COVID-19 infection compared to those without a history of COVID-19 infection using a large healthcare claims database.
Methods
Data source
After obtaining IRB approval (00123727), we retrospectively queried a large health care claims database, IBM MarketScan, to identify men with a first-time diagnosis of COVID-19 and ED. Data from 2020 is the latest currently available data in IBM MarketScan. Thus, our study period was between January 2020 to January 2021. IBM MarketScan is uniquely able to capture both inpatient, outpatient, and virtual medical visits at any institution given that data is collected based on insurance claims. Available de-identified data includes demographic data, International Classification of Disease (ICD) 9 and 10 diagnosis/procedure codes, Common Procedure Terminology codes, and National Drug Codes. These unique advantages are particularly useful for longitudinal studies and have been leveraged to assess surgical subspecialty outcomes in otolaryngology, neurosurgery, and urology [14–17].
Outcomes
Using ICD 9 and 10 codes, (Supplementary Table 1) we identified men who had a diagnosis of COVID-19 between January 2020 and January 2021 who subsequently were diagnosed with ED for the first time. This cohort was compared to age-matched controls without diagnosis of COVID-19 in the same study period. Inclusion criteria included men >18 years with COVID-19 infection (exposure). (Supplementary Fig. 1) Exclusion criteria included men with prior history of ED and newly diagnosed ED secondary to radiation or genitourinary surgery. (Supplementary Fig. 1). Our primary outcome was newly diagnosed ED. (Supplementary Fig. 1).
Statistical analysis
We modeled the likelihood of men with a history of COVID-19 infection to subsequently develop newly diagnosed ED compared to men without a history of COVID-19 infection using a Cox proportional hazards model starting on June 17th, 2020 (Date at which 10% of COVID-19 infections had occurred). The Occurrence of ED was adjusted for common ED risk factors including age, prostate cancer, cardiovascular disease, hypogonadism, obesity, smoking, and diabetes mellitus [11, 18–22]. Bladder cancer, hypertension, hyperlipidemia, spinal cord injury, and geographic region were included as additional cofactors.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Results
Demographics and comorbidities
After applying exclusion criteria, we identified 87,525 men who were continuously enrolled in MarketScan between January 2020 and January 2021 of which 42,406 experienced an insurance-claims documented COVID-19 infection. (Fig. 1) Men with documented COVID-19 infection were more likely to have a prior history of diabetes mellitus (COVID-19 14.7%; Control 11.5%), hyperlipidemia (COVID-19 39.3%; Control 35.5%), hypertension (COVID-19 35.5%; Control 30.6%), and hypogonadism (COVID-19 6.6%; Control 4.9%). (Table 1) Current smoker status was more common in the control group (COVID-19 7.3%; Control 8.5%). (Table 1).
Fig. 1. COVID-19 positive case counts by date.
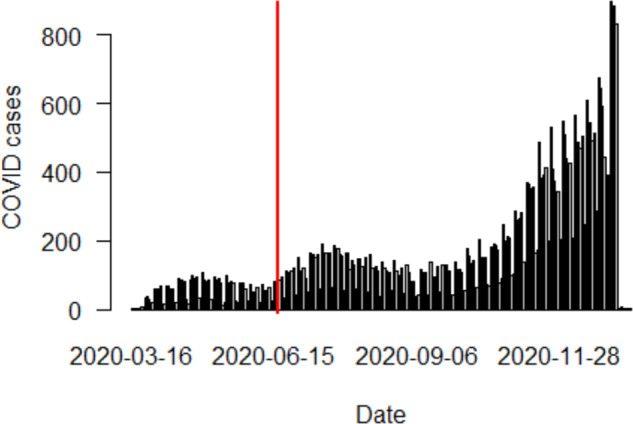
Red line denotes point at which 10% of COVID-19+ cases had occurred (6-17-2020). From this time point until the end of 2020, the incidence of newly diagnosed erectile dysfunction was assessed in men with and without prior COVID-19 infection.
Table 1.
Demographic breakdown of population stratified by prior covid case versus control status.
| Variable | Control | COVID+ | Full Cohort |
|---|---|---|---|
| Total | 45,119 (51.6) | 42,406 (48.5) | 87,525 (100) |
| Age | 41 (32–49) | 41 (32–49) | 41 (32–49) |
| Region | |||
| Northeast | 9025 (20) | 8515 (20.1) | 17,540 (20.0) |
| Midwest | 9955 (22.1) | 8846 (20.9) | 18,801 (21.5) |
| South | 19,338 (42.9) | 19,768 (46.6) | 39,106 (44.7) |
| West | 6664 (14.8) | 5189 (12.2) | 11,853 (13.5) |
| Other | 137 (0.3) | 88 (0.2) | 225 (0.3) |
| Population density (urban) | 39,444 (87.4) | 35,847 (84.5) | 75,291 (86.0) |
| Charlson Comorbidity Index | |||
| 0 | 36,557 (81.0) | 32,240 (76.0) | 68,797 (78.6) |
| 1 | 6356 (14.1) | 7178 (16.9) | 13,534 (15.5) |
| 2 | 1468 (3.3) | 1929 (4.6) | 3397 (3.9) |
| 3+ | 738 (1.6) | 1059 (2.5) | 1797 (2.1) |
| Comorbidities | |||
| Bladder Cancer | 79 (0.2) | 67 (0.2) | 146 (0.2) |
| Prostate Cancer | 316 (0.7) | 349 (0.8) | 665 (0.8) |
| Prostatectomy | 28 (0.1) | 46 (0.1) | 74 (0.1) |
| Diabetes Mellitus | 5196 (11.5) | 6235 (14.7) | 11,431 (13.1) |
| Cardiovascular Disease | 2768 (6.1) | 3372 (8.0) | 6140 (7.0) |
| Hyperlipidemia | 16,030 (35.5) | 16,670 (39.3) | 32,700 (37.4) |
| Hypertension | 13,807 (30.6) | 15,034 (35.5) | 28,841 (33.0) |
| Hypogonadism | 2218 (4.9) | 2796 (6.6) | 5014 (5.7) |
| Spinal Cord Injury | 51 (0.1) | 58 (0.1) | 109 (0.1) |
| Body Mass Index (BMI) 25–30 | 4176 (9.3) | 3757 (8.9) | 7933 (9.1) |
| Body Mass Index (BMI) 30–40 | 4874 (10.8) | 5516 (13.0) | 10,390 (11.9) |
| Body Mass Index (BMI) 40+ | 1357 (3.0) | 1903 (4.5) | 3260 (3.7) |
| Smoking | 3818 (8.5) | 3082 (7.3) | 6900 (7.9) |
| Deep Vein Thrombosis | 427 (1.0) | 445 (1.1) | 872 (1.0) |
| Pulmonary Embolism | 241 (0.5) | 269 (0.6) | 510 (0.6) |
Continuous variables presented as median (with inter-quartile range). Categorical variables presented as number (with percentage of group). Percentages in ‘Total’ row denote percentage of full cohort. Percentages in other rows denote percentage of group.
Outcomes
Between January 2020 and January 2021, 1111 new diagnoses of ED were identified in men without prior history of ED. (Table 2) Of the 1111 men with newly diagnosed ED, 601 (54.1%) occurred in men with a prior COVID-19 infection compared to 510 (45.9%) in the control group. (Table 2) Newly diagnosed ED occurred in 1.4% and 1.1% of the COVID-19 and control cohorts, respectively.
Table 2.
Newly diagnosed erectile dysfunction events in those without prior COVID diagnosis (Control) versus men with prior history of COVID + test.
| Variable | Control | COVID+ | Full cohort |
|---|---|---|---|
| Outcome | |||
| Erectile Dysfunction | 510 (1.1) | 601 (1.4) | 1111 (1.2) |
Cohort percentages in parentheses.
On multivariable logistic regression, prior COVID-19 infection was independently associated with newly diagnosed ED (Hazard Ratio, 1.27; 95% CI [1.1–1.5]; P = 0.002). Several additional covariates were also associated with ED including: diabetes (HR 1.34), 1-year increase in age (HR 1.05), hypogonadism (HR 2.04), BMI 25–30 (HR 1.27), and BMI 30–40 (HR 1.34). (Table 3) When controlling for the previously mentioned covariates, the Kaplan Meier graph of our Cox regression analysis highlights a lower erectile dysfunction disease-free survival associated with men with prior history of COVID-19 compared to those without a history of infection (Fig. 2).
Table 3.
Model effects from Cox-Proportional Hazards Model.
| HR | 95% CI | P -Value | |
|---|---|---|---|
| COVID+ | 1.27 | 1.09–1.47 | 0.002 |
| Age | 1.05 | 1.04–1.07 | 0.000 |
| Bladder Cancer | 1.25 | 0.4–3.93 | 0.697 |
| Prostate Cancer | 1.24 | 0.74–2.10 | 0.409 |
| Diabetes Mellitus | 1.34 | 1.16–1.56 | 0.000 |
| Cardiovascular Disease | 0.90 | 0.73–1.11 | 0.339 |
| Hypogonadism | 2.04 | 1.71–2.43 | 0.000 |
| BMI 25–30 | 1.27 | 1.06–1.53 | 0.011 |
| BMI 30–40 | 1.34 | 1.15–1.60 | 0.000 |
| BMI 40+ | 1.08 | 0.81–1.43 | 0.604 |
| Smoking | 1.12 | 0.92–1.38 | 0.268 |
| Deep Vein Thrombosis | 1.16 | 0.69–1.95 | 0.565 |
| Pulmonary Embolism | 0.63 | 0.27–1.46 | 0.284 |
P-values of 0 indicate < 0.001. Effect of COVID and comorbidities relative to patients without that comorbidity. Effect of age represents increase of one year. HR Hazard Ratio, 95% CI 95% Confidence Interval, BMI Body Mass Index. Bolded P-values denoted for statistical significance.
Fig. 2. Kaplan Meier Curve.
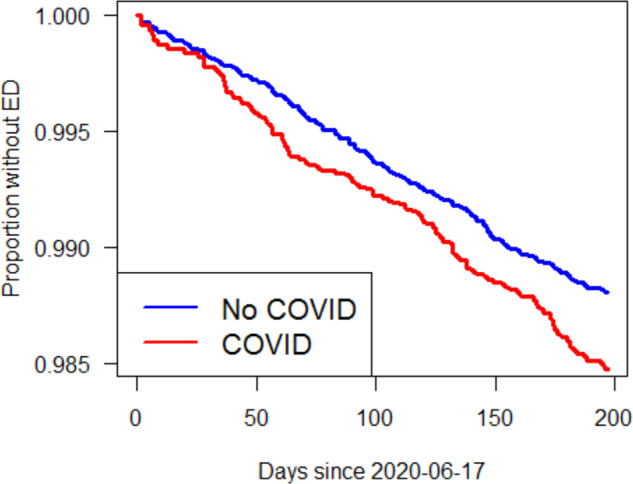
Kaplan Meier curve representing proportion without ED among those with and without prior COVID-19 infection.
Discussion
This study showed a significant association of prior COVID-19 infection with a new diagnosis of ED when evaluated on a population level and when controlled for common risk factors for ED (age, diabetes, prostate cancer, hypogonadism, BMI, and smoking status). COVID-19 infection was associated with a 27% increased risk of ED, roughly comparable to a new diagnosis of diabetes.
The link between ED and COVID-19
Although the overall body of literature linking COVID-19 infection to ED is limited, there has been increasing evidence of this association over the past 18 months. The role of endothelial cell dysfunction during COVID-19 infection and its subsequent impact on organ systems has been well documented in the literature [23–25]. This led many to hypothesize that COVID-19 infection may influence subsequent risk of ED. However, the initial studies evaluating this association were limited by small sample size and short follow-up.
In March 2021, Sansone et al. reported increased self-reported ED men with recent COVID-19 infection compared to age-matched controls [10]. Between April and May 2020, 25 men with a recent COVID-19 infection were assessed for ED using an online survey and were compared to 75 age-matched controls. The authors reported increased risk of ED in the COVID-19 cohort (28%) compared to controls (9.33%). In this study, COVID-19 infection was independently associated with ED (OR 5.66) [10].
In July 2021, a potential pathophysiologic basis was described by Kresch et al. who confirmed the presence of COVID-19 peplomers in corporal tissue of two patients undergoing inflatable penile prosthesis surgery for newly reported erectile dysfunction after COVID-19 infection [7]. The authors concluded that COVID-19 infection-related endothelial dysfunction may increase risk of ED [7].
In August 2021, Nassau et al. reviewed potential mechanisms for COVID-19-related ED highlighting the increased susceptibility of men for developing COVID-19 infection-related morbidity and mortality [9]. The authors further described the role of ACE2 and TMPRSS2 which are required for SARS-CoV-2 uptake and are ubiquitous within endothelial cells. These findings further reinforced the potential association of COVID-19 infection with vasculogenic ED [9].
In November 2021, Hu et al. assessed the risk of new erectile dysfunction in 67 men following COVID-19 infection 6 months prior [12]. At 3 and 6 months following infection, patients were evaluated with symptom checklist 90 and IIEF-5. Compared to controls, men with prior COVID-19 infection had increased risk of a first-time ED diagnosis (44.8% versus 17.1%) [12].
To date, only two studies have assessed the association of COVID-19 and ED on a population level. In February 2022, Chu et al. compared 230,517 men with prior COVID-19 infection to 232,645 men without documented COVID-19 infection using the TriNETx database between January 2020 and November 2021 [5]. The authors found an increased association of ED following COVID-19 infection, OR 1.20 (1.004–1.248; P = 0.04) [5]. A similar association was shown by Katz et al. using a database of participating the University of Florida Healthcare System centers [6]. The authors identified 3098 COVID-19-recovered men between January 2020 and June 2021 with this cohort being 3.68 times more likely to report ED compared to controls [6]. Although, electronic health record (EHR) databases such as TriNetX have the distinct advantage of including both insured and uninsured patients, there are many limitations of longitudinal data in these databases, since patients may often get care outside of the health care system that is used for the study.
Why our data is unique
EHR-based databases such as TriNetX, and Informatics for Integrating Biology, and the Bedside Database collate medical records from participating healthcare institutions medical records. The Informatics for Integrating Biology and the Bedside Database, utilized by Katz et al. included participating University of Florida Healthcare Centers [6]. While the TriNetX Database is made up of 63 participating institutions in 8 countries with 93% of patients being from the United States [26]. However, a significant limitation of these databases is that it requires patients to seek longitudinal medical care at the institutions represented in the database. For example, a patient is admitted to a tertiary center for a COVID-19 infection early in the pandemic, recovers, and then subsequently presents to their local primary care provider to report new onset ED. Both the primary care provider and the tertiary center must be members of the same EHR database to adequately capture this longitudinal data which likely underestimates the effect of COVID-19 infection. This limitation is a unique strength of the IBM MarketScan database.
The IBM MarketScan commercial claims database includes over 3 billion medical encounters made by 215 million policy holders, spouses, and dependents. Most importantly, medical visits and associated diagnoses are captured longitudinally as long as insurance is billed, thus, patients do not have to receive all of their care in a participating EHR database institution to be captured. In the previously discussed example, a patient presenting to a tertiary hospital for a COVID-19 infection early in the pandemic who subsequently received follow-up care at a primary care physician’s office would be accurately captured as long as insurance was billed for each encounter. This unique characteristic allows increased accuracy for longitudinally collected data and may explain why our cohort had an increased rate of newly diagnosed ED following COVID-19 infection (1.42%) compared to Chu et al. (0.48%) [5]. It should be emphasized, that this higher capture rate using MarketScan occurred with shorter follow-up of 6.5 months compared to Chu et al. (exact follow-up not reported; estimated Jan 2020 to time of manuscript acceptance Aug 2021) [5].
Despite a maximum follow-up time of 6.5 months, our findings showed a 27% increased risk of first-time ED compared to controls over the same timeframe. (Table 3) The results of our study are likely biased towards less of an association of COVID-19 and ED, since there were likely some patients in the control arm with COVID-19 that were never captured in claims data. If everyone that had COVID-19 was captured the association may be higher between COVID-19 and erectile dysfunction. However, it should be acknowledged that this selection bias occurs at baseline in the population given that patients may have cleared a COVID-19 infection without symptoms and thus without a positive test. In fact, the COVID-19 positive test rate in our study mirrored the positive test rate seen in the US through 2020. (Fig. 1 & Supplementary Fig. 2)
As the most recent available data in MarketScan is January 2021, our data only represents the first 9 months of the pandemic and limits our ability for long-term follow-up. However, our dataset removes vaccine administration, over-the-counter home testing, and variant strains as potential confounders. The first COVID-19 vaccine administered in the US was on December 14th, 2020, with only 2.8 million Americans receiving the first dose of the two-shot series by the conclusion of 2020 (<1% of US population) [27]. Likewise, Federal Drug Administration approval of the first over-the-counter COVID-19 antigen test did not occur until December 15th, 2020 [28]. Lastly, variant strains of SARS-COV-2 are associated with varying degrees of transmissibility and pathogenicity. Initial SARS-COV-2 variants Alpha (B.1.1.7) and Beta (B.1.351) were first documented in the United Kingdom and South Africa, respectively [29]. However, both strains were not labeled variants of concern by the World Health Organization until December 18th, 2020 [29]. Thus, our findings may be more representative of the association of COVID-19 infection prior to widespread vaccine uptake, home testing, and variant strain transmission.
Next steps
This initial data, showing an increased association of new-onset ED following COVID-19 recovery warrants further evaluation. First, our findings should be confirmed in claims databases with longer follow up as it becomes available. This data along with population data in the setting of increasing vaccination should be evaluated to determine if vaccination on a population level reduces the risk of new-onset ED. Until data is available on the impact of asymptomatic infection and infection in the setting of prior vaccination, our data should reinforce the findings from Katz and Chu et al. [5, 6] and be used to inform patients of the short-term risks associated with COVID-19 infection.
Limitations
Our study is not without limitations that are inherent to the retrospective analysis of an insurance claim database. First, race and socioeconomic status were not available and could impact our findings. Available data is based on medical diagnoses associated with an insurance claim. Thus, COVID-19 home tests or outpatient tests in which an insurance claim is not submitted are not captured. As a result, it is likely that our capture rate of COVID-19 infection is underestimated. Likewise, MarketScan data is only available through the end of 2020 which represents the early stage of the COVID-19 pandemic and limited the follow-up window to observe our outcome.
Additionally, individual medical records (paper charts or electronic medical records) are not available in this database which has several implications. First, we are unable to specifically assess the morbidity associated with each COVID-19 infection. As a result, we intentionally did not stratify COVID-19 cohort by diagnosis location (inpatient versus outpatient) as MarketScan lacks the granular details that would allow us to delineate an admission that was primarily related to a COVID-19 infection versus admission for a different diagnosis with an associated finding of COVID-19 positivity. It should be acknowledged that there is likely a step wise increase in risk of ED following COVID-19 infection depending on location of diagnosis (outpatient versus inpatient) and intensive care utilization. However, it is important to note that these limitations would bias our findings to a lower association between ED and COVID-19 and there is likely a stronger association than our study results show.
Conclusion
In this cohort of >87,500 unvaccinated men with continuous follow-up through the first year of the COVID-19 pandemic, COVID-19 infection was associated with a 27% increased likelihood of subsequent ED when controlled for diabetes, smoking status, cardiovascular disease, and obesity. Future studies should focus on the morbidity of COVID-19 infections, vaccination status, and subsequent ED risk given the high prevalence of COVID-19 infections and the ubiquity of vaccines.
Supplementary information
Acknowledgements
The computational resources used were partially funded by the NIH, Share Instrumentation Grant 1S10OD021644-011A1. No financial assistance was received in support of this work.
Author contributions
KJH, RM, JJH, NP, RD, BJM, JBM, and JMH each contributed to the study design, manuscript drafting, approval of the final version, and agree to be accountable for the accuracy of the work.
Funding
The University of Utah receives educational grants from Boston Scientific and Coloplast. Both are companies that produce prosthetics which is related to, but not the subject of this manuscript.
Data availability
The data that support the findings of this study are available from IBM MarketScan but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of IBM MarketScan.
Competing interests
JMH receives fellowship/research grants from Boston Scientific, Acerus, Coloplast, and Endo. JMH is a consultant for Turtle Health, Maximus. JMH has leadership positions in FirmTech, StreamDx, and Inherent Bioscience. JBM is a consultant for Cooper Medical. The University of Utah Division of Urology receives fellowship educational grants from Boston Scientific and Coloplast. KJH, RM, JJH, NP, RD, and BJM have no disclosures to report.
Ethical approval
International review board approval (IRB 00123727) was obtained prior to data access and analysis per standard protocol.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41443-023-00687-4.
References
- 1.Prevention CfDCa. COVID Data Tracker Atlanta, GA: US Department of Health and Human Services; 2022. Available from: https://covid.cdc.gov/covid-data-tracker.
- 2.Mesquida J, Caballer A, Cortese L, Vila C, Karadeniz U, Pagliazzi M, et al. Peripheral microcirculatory alterations are associated with the severity of acute respiratory distress syndrome in COVID-19 patients admitted to intermediate respiratory and intensive care units. Crit Care. 2021;25:381. doi: 10.1186/s13054-021-03803-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nappi F, Avtaar Singh SS. Endothelial dysfunction in SARS-CoV-2 infection. Biomedicines. 2022;10:654. [DOI] [PMC free article] [PubMed]
- 4.Oikonomou E, Souvaliotis N, Lampsas S, Siasos G, Poulakou G, Theofilis P, et al. Endothelial dysfunction in acute and long-standing COVID-19: a prospective cohort study. Vascul Pharmacol. 2022;144:106975. doi: 10.1016/j.vph.2022.106975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu KY, Nackeeran S, Horodyski L, Masterson TA, Ramasamy R. COVID-19 infection is associated with new onset erectile dysfunction: insights from a National Registry. Sex Med. 2022;10:100478. doi: 10.1016/j.esxm.2021.100478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katz J, Yue S, Xue W, Gao H. Increased odds ratio for erectile dysfunction in COVID-19 patients. J Endocrinol Invest. 2022;45:859–64. doi: 10.1007/s40618-021-01717-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kresch E, Achua J, Saltzman R, Khodamoradi K, Arora H, Ibrahim E, et al. COVID-19 endothelial dysfunction can cause erectile dysfunction: histopathological, immunohistochemical, and ultrastructural study of the human penis. World J Mens Health. 2021;39:466–9. doi: 10.5534/wjmh.210055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mostafaei H, Mori K, Hajebrahimi S, Abufaraj M, Karakiewicz PI, Shariat SF. Association of erectile dysfunction and cardiovascular disease: an umbrella review of systematic reviews and meta-analyses. BJU Int. 2021;128:3–11. doi: 10.1111/bju.15313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nassau DE, Best JC, Kresch E, Gonzalez DC, Khodamoradi K, Ramasamy R. Impact of the SARS-CoV-2 virus on male reproductive health. BJU Int. 2022;129:143–50. doi: 10.1111/bju.15573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sansone A, Mollaioli D, Ciocca G, Colonnello E, Limoncin E, Balercia G, et al. “Mask up to keep it up”: Preliminary evidence of the association between erectile dysfunction and COVID-19. Andrology. 2021;9:1053–9. doi: 10.1111/andr.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao B, Hong Z, Wei Y, Yu D, Xu J, Zhang W. Erectile dysfunction predicts cardiovascular events as an independent risk factor: a systematic review and meta-analysis. J Sex Med. 2019;16:1005–17. doi: 10.1016/j.jsxm.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 12.Hu B, Ruan Y, Liu K, Wei X, Wu Y, Feng H, et al. A mid-to-long term comprehensive evaluation of psychological distress and erectile function in COVID-19 recovered patients. J Sex Med. 2021;18:1863–71. doi: 10.1016/j.jsxm.2021.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karkin K, Alma E. Erectile dysfunction and testosterone levels prior to COVID-19 disease: What is the relationship? Arch Ital Urol Androl. 2021;93:460–4. doi: 10.4081/aiua.2021.4.460. [DOI] [PubMed] [Google Scholar]
- 14.Dai JC, Ahn JS, Holt SK, May PC, Sorensen MD, Harper JD. National imaging trends after percutaneous nephrolithotomy. J Urol. 2018;200:147–53. doi: 10.1016/j.juro.2018.01.078. [DOI] [PubMed] [Google Scholar]
- 15.Hebert KJ, Matta R, Horns JJ, Paudel N, Das R, Kohler TS, et al. Risk of postoperative thromboembolism in men undergoing urological prosthetic surgery: an assessment of 21,413 men. J Urol. 2022;208:878–85. doi: 10.1097/JU.0000000000002801. [DOI] [PubMed] [Google Scholar]
- 16.Idowu OA, Boyajian HH, Ramos E, Shi LL, Lee MJ. Trend of spine surgeries in the outpatient hospital setting versus ambulatory surgical center. Spine. 2017;42:E1429–36. doi: 10.1097/BRS.0000000000002180. [DOI] [PubMed] [Google Scholar]
- 17.Wright LN, Moghalu OI, Das R, Horns J, Campbell A, Hotaling J, et al. Erectile dysfunction and treatment: an analysis of associated chronic health conditions. Urology. 2021;157:148–54. doi: 10.1016/j.urology.2021.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Hebert KJ, Findlay BL, Yang DY, Houlihan MD, Bole R, Avant RA, et al. Incidence of venous thromboembolism and safety of perioperative subcutaneous heparin during inflatable penile prosthesis surgery. Urology. 2021;157:155–60. doi: 10.1016/j.urology.2021.08.002. [DOI] [PubMed] [Google Scholar]
- 19.Isidori AM, Buvat J, Corona G, Goldstein I, Jannini EA, Lenzi A, et al. A critical analysis of the role of testosterone in erectile function: from pathophysiology to treatment-a systematic review. Eur Urol. 2014;65:99–112. doi: 10.1016/j.eururo.2013.08.048. [DOI] [PubMed] [Google Scholar]
- 20.Kouidrat Y, Pizzol D, Cosco T, Thompson T, Carnaghi M, Bertoldo A, et al. High prevalence of erectile dysfunction in diabetes: a systematic review and meta-analysis of 145 studies. Diabet Med. 2017;34:1185–92. doi: 10.1111/dme.13403. [DOI] [PubMed] [Google Scholar]
- 21.Pizzol D, Smith L, Fontana L, Caruso MG, Bertoldo A, Demurtas J, et al. Associations between body mass index, waist circumference and erectile dysfunction: a systematic review and META-analysis. Rev Endocr Metab Disord. 2020;21:657–66. doi: 10.1007/s11154-020-09541-0. [DOI] [PubMed] [Google Scholar]
- 22.Sivaratnam L, Selimin DS, Abd Ghani SR, Nawi HM, Nawi AM. Behavior-related erectile dysfunction: a systematic review and meta-analysis. J Sex Med. 2021;18:121–43. doi: 10.1016/j.jsxm.2020.09.009. [DOI] [PubMed] [Google Scholar]
- 23.Evans PC, Rainger GE, Mason JC, Guzik TJ, Osto E, Stamataki Z, et al. Endothelial dysfunction in COVID-19: a position paper of the ESC Working Group for Atherosclerosis and Vascular Biology, and the ESC Council of Basic Cardiovascular Science. Cardiovasc Res. 2020;116:2177–84. doi: 10.1093/cvr/cvaa230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perico L, Benigni A, Casiraghi F, Ng LFP, Renia L, Remuzzi G. Immunity, endothelial injury and complement-induced coagulopathy in COVID-19. Nat Rev Nephrol. 2021;17:46–64. doi: 10.1038/s41581-020-00357-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang J, Tecson KM, McCullough PA. Endothelial dysfunction contributes to COVID-19-associated vascular inflammation and coagulopathy. Rev Cardiovasc Med. 2020;21:315–9. doi: 10.31083/j.rcm.2020.03.126. [DOI] [PubMed] [Google Scholar]
- 26.Taquet M, Geddes JR, Husain M, Luciano S, Harrison PJ. 6-month neurological and psychiatric outcomes in 236,379 survivors of COVID-19: a retrospective cohort study using electronic health records. Lancet Psychiatry. 2021;8:416–27. doi: 10.1016/S2215-0366(21)00084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rebecca Spalding COD. U.S. vaccinations in 2020 fall short of target of 20 million people. Reuters. 2020 [cited 2022 Jun 2]. Available from: https://www.reuters.com/article/us-health-coronavirus-usa-vaccinations/u-s-vaccinations-in-2020-fall-far-short-of-target-of-20-million-people-idUSKBN29512W.
- 28.Coronavirus (COVID-19) Update: FDA authorizes antigen test as first over-the-counter fully at-home diagnostic test for COVID-19. FDA.gov 2020 [cited 2022 Jun. 2]. Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-antigen-test-first-over-counter-fully-home-diagnostic.
- 29.WHO. Tracking SAR-COV-2 variants. 2022 [updated May 25, 2022]. Available from: https://www.who.int/activities/tracking-SARS-CoV-2-variants.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from IBM MarketScan but restrictions apply to the availability of these data, which were used under license for the current study, and so are not publicly available. Data are however available from the authors upon reasonable request and with permission of IBM MarketScan.


