Abstract
Combination therapy with three antiretroviral agents has been integral to successful HIV-1 treatment since 1996. Although the efficacy, adverse effects, and toxicities of contemporary three-drug regimens have improved, even the newest therapies have potential adverse effects. The use of two-drug regimens is one way to reduce lifetime exposure to antiretroviral drugs while maintaining the benefits of viral suppression. Multiple large, randomised trials have shown the virological non-inferiority of certain two-drug regimens versus three-drug comparators, including adverse effect differences that reflect known profiles of the antiretroviral drugs in the respective regimens. Two-drug combinations are now recommended in treatment guidelines and include the first long-acting antiretroviral regimen for the treatment of HIV-1. Recommended two-drug regimens differ in their risks for, and factors associated with, virological failure and emergent resistance. The tolerability, safety, metabolic profiles, and drug interactions of two-drug regimens also vary by the constituent drugs. No current two-drug regimen is recommended for people with chronic hepatitis B virus as none include tenofovir. Two-drug regimens have increased options for individualised care.
Introduction
In the mid-1990s, antiretroviral regimens consisting of three active drugs from at least two different drug classes became the standard of care for people with HIV. These regimens suppressed viral replication and restricted the development of resistance—the main limitations of the initial single and two-drug regimens of the late 1980s and early 1990s. Improvements in antiretroviral drug profiles have led to preferred three-drug regimens with high viral suppression rates and excellent resistance characteristics. The tolerability and safety of recommended antiretroviral drugs have also improved substantially. However, as any drug has potential adverse effects, use of as few drugs as possible, if appropriate, could be beneficial.
Despite the lack of virological efficacy of early two-drug antiretroviral therapy regimens, interest in two-drug antiretroviral therapy persisted as a way of reducing lifetime therapy exposure, specific drug toxicities, and cost.1,2 This interest was increased with the knowledge that some antiretroviral drugs contribute to age-related end-organ and metabolic comorbidities, which is highly relevant with an increasing population of people with HIV older than 50 years.3–5 More than 30 unique antiretroviral drugs have been developed; about half of these are included in current HIV-1 treatment guidelines. Effective and safe regimens that contain only two drugs can and have been established, including the first complete long-acting HIV regimen.
Two-drug antiretroviral therapy
Overview
Although several two-drug regimens have not met expected efficacy or safety standards in pilot or large randomised studies (table 1),6–15 some two-drug regimens have shown virological non-inferiority to standard three-drug therapy, with similar effects on CD4 cell count reconstitution and favourable safety profiles (table 2).16–41 Other studies with extended follow-up (>5 years) have shown long-term efficacy and tolerability of specific two-drug regimens.21,26,29,40,41
Table 1:
Unsuccessful studies of two-drug antiretroviral therapy regimens
| Participants (n) | Experimental regimen | Comparator regimen | Reasons for study failure or poor outcome | |
|---|---|---|---|---|
|
| ||||
| Treatment-naive studies | ||||
| The Kalead Study (2010)6* | 152 | Ritonavir-boosted lopinavir plus tenofovir disoproxil | Ritonavir-boosted lopinavir plus two non-tenofovir disoproxil NRTIs | Efficacy; premature treatment discontinuation due to adverse events |
| ACTG A5262 (2011)7* | 112 | Ritonavir-boosted darunavir plus raltegravir | NA | Efficacy; resistance |
| SPARTAN (2012)8* | 94 | Atazanavir plus raltegravir | Ritonavir-boosted atazanavir plus tenofovir disoproxil and emtricitabine | Resistance; safety and adverse events |
| RADAR (2014)9* | 85 | Ritonavir-boosted darunavir plus raltegravir | Ritonavir-boosted darunavir plus tenofovir disoproxil and emtricitabine | Efficacy; premature treatment discontinuation due to loss to follow up |
| HARNESS (2016)10* | 109 | Ritonavir-boosted atazanavir plus raltegravir | Ritonavir-boosted atazanavir plus tenofovir disoproxil and emtricitabine | Efficacy; resistance; safety and adverse events; premature treatment discontinuation due to poor adherence |
| ROCnRAL (2014)11* | 44 | Raltegravir plus maraviroc | NA | Efficacy; resistance; safety and adverse events |
| MODERN 1 (2016)12† | 797 | Ritonavir-boosted darunavir plus maraviroc | Ritonavir-boosted darunavir plus tenofovir disoproxil and emtricitabine | Efficacy |
| Switch studies | ||||
| MARCH (2016)13† | 395 | Maraviroc plus ritonavir-boosted protease inhibitor or maraviroc plus two NRTIs | Current antiretroviral therapy | Efficacy |
| The COOL Trial (2009)14† | 143 | Tenofovir disoproxil plus efavirenz | Tenofovir disoproxil plus lamivudine and efavirenz | Efficacy; resistance; safety and adverse events |
| The GUSTA Study (2014)15† | 115 | Ritonavir-boosted darunavir plus maraviroc | Standard three-drug antiretroviral therapy | Efficacy |
NA=not available. NRTI=nucleoside reverse transcriptase inhibitor.
Unsuccessful small study or pilot study.
Unsuccessful fully powered, randomised study.
The MIDAS study was a pilot study of 24 participants examining ritonavir-boosted darunavir plus maraviroc, which was successful in terms of virological efficacy and had no grade 4 adverse events or study discontinuations due to adverse events. However, this regimen failed in MODERN, which was prematurely terminated.
Table 2:
Randomised studies of two-drug antiretroviral therapy regimens with non-inferiority to triple therapy
| Study design | Participants (n) | Two-drug or experimental regimen | Control or comparator regimen | Efficacy (%) | Resistance | Safety and adverse events | Comments | |
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Dolutegravir plus lamivudine | ||||||||
| GEMINI 1 and 2 (2018)16,17* | Two identical, phase 3, multicentre, randomised, double-blind, non-inferiority trials | 1433 | Dolutegravir plus lamivudine | Dolutegravir plus tenofovir disoproxil and emtricitabine | Combined; week 48: 90·0% in fixed-dose dolutegravir plus lamivudine group; 93·0% in dolutegravir plus tenofovir disoproxil and emtricitabine group; week 144: 84·3% in fixed-dose dolutegravir plus lamivudine group; 89·4% in dolutegravir plus tenofovir disoproxil and emtricitabine group | Week 48: no emergent resistance-associated mutations; week 144: 12 fixed-dose dolutegravir plus lamivudine participants and nine dolutegravir plus tenofovir disoproxil and emtricitabine participants met virological withdrawal criteria, but still no emergent resistance-associated mutations; one individual in the fixed-dose dolutegravir plus lamivudine group had virological rebound with M184V (week 132) and R263R/K (week 144) emergence | Week 48: more drug adverse events in the dolutegravir plus tenofovir disoproxil and emtricitabine group than in the fixed-dose dolutegravir plus lamivudine group (23·6% vs 17·6%); 2% of participants discontinued the study regimen in each group due to adverse events; week 144: significantly fewer adverse events with fixed-dose dolutegravir plus lamivudine than with dolutegravir plus tenofovir disoproxil and emtricitabine (20% vs 27%; RR 0·76) | Fixed-dose dolutegravir plus lamivudine dual therapy regimen showed non-inferiority to triple therapy with dolutegravir plus tenofovir disoproxil and emtricitabine |
| Boosted protease inhibitor plus lamivudine | ||||||||
| GARDEL (2014)18* | Phase 3, randomised, multicentre, open-label, non-inferiority trial | 373 | Ritonavir-boosted lopinavir plus lamivudine | Ritonavir-boosted lopinavir plus two NRTIs | Week 48: 88·3% in ritonavir-boosted lopinavir plus lamivudine group; 83·7% in ritonavir-boosted lopinavir plus two NRTIs group | No emergent resistance in the ritonavir-boosted lopinavir plus lamivudine group; two cases of resistance in the ritonavir-boosted lopinavir plus two NRTIs group | Adverse event discontinuations were more common in the ritonavir-boosted lopinavir plus two NRTIs group than in the ritonavir-boosted lopinavir plus lamivudine group | Ritonavir-boosted lopinavir plus lamivudine dual therapy regimen showed non-inferiority to triple therapy with ritonavir-boosted lopinavir plus two NRTIs; participants with baseline HIV viral load ≥100 000 copies per mL had similar results in both study groups (87·2% vs 77·9%) |
| Boosted darunavir plus raltegravir | ||||||||
| NEAT 001/ANRS 143 (2014)19* | Phase 3, randomised, multicentre, open-label, non-inferiority trial | 805 | Ritonavir-boosted darunavir plus raltegravir | Ritonavir-boosted darunavir plus tenofovir disoproxil and emtricitabine | Week 96: 82·2% in ritonavir-boosted darunavir plus raltegravir group; 86·2% in ritonavir-boosted darunavir plus tenofovir disoproxil and emtricitabine group | 29·5% of participants in the ritonavir-boosted darunavir plus raltegravir group with virological failure had resistance-associated mutations (NRTI [n=3], protease inhibitor [n=1], INSTI [n=14]) | Discontinuation due to adverse events: 1·5% in the two-drug group vs 2·6% in the standard group (adjusted difference −1·2%, 95% CI −3·1 to 0·7); treatment-restricting adverse events: 3·9 per 100 patient years in the two-drug group vs 4·2 per 100 patient-years in the standard group; rates of serious adverse events: 10·2 per 100 patient-years in the two-drug group vs 8·3 per 100 patient-years in the standard group | Ritonavir-boosted darunavir plus raltegravir dual therapy regimen showed non-inferiority to triple therapy with ritonavir-boosted darunavir plus tenofovir disoproxil and emtricitabine; non-inferiority was not shown in people with HIV viral load >100 000 copies per mL or CD4 cell count <200 at baseline |
| Dolutegravir plus lamivudine | ||||||||
| TANGO (2020)20,21† | Phase 3, multicentre, randomised, open-label, non-inferiority trial | 741 | Dolutegravir plus lamivudine | Current tenofovir alafenamide-based triple antiretroviral therapy | Week 48: 93·2% in fixed-dose dolutegravir plus lamivudine group; 93·0% in tenofovir alafenamide-based triple antiretroviral therapy group; week 144: 85·9% in fixed-dose dolutegravir plus lamivudine group; 81·7% in tenofovir alafenamide-based triple antiretroviral therapy group | No emergence of new resistance-associated mutations seen at week-144 follow-up in fixed-dose dolutegravir plus lamivudine group | Week 48: ≥1 adverse event occurred in 79·9% of the fixed-dose dolutegravir plus lamivudine group and in 78·7% of the tenofovir alafenamide-based triple antiretroviral therapy group; week 144: incidence of ≥1 adverse event similar between groups (fixed-dose dolutegravir plus lamivudine 91·1%; tenofovir alafenamide-based antiretroviral therapy 90·1%) | Fixed-dose dolutegravir plus lamivudine dual therapy regimen showed non-inferiority to continuing tenofovir alafenamide-based triple antiretroviral therapy regimen; no virological failure with fixed-dose dolutegravir plus lamivudine up to week 144 |
| DOLAM (2021)22† | Phase 4, multicentre, randomised, open-label, non-inferiority trial | 265 | Dolutegravir plus lamivudine | Current standard triple antiretroviral therapy | Week 48: 93·1% in dolutegravir plus lamivudine group; 93·3% in standard triple antiretroviral therapy group | No emergence of fixed-dose dolutegravir plus lamivudine resistance-associated mutations | Similar rates of adverse events in both groups; 4 adverse event discontinuations in standard triple antiretroviral therapy group and 3 adverse event discontinuations in fixed-dose dolutegravir plus lamivudine group | Fixed-dose dolutegravir plus lamivudine dual therapy regimen showed non-inferiority to continuing standard triple antiretroviral therapy regimen |
| SALSA (2021)23† | Phase 3, multicentre, randomised, open-label, non-inferiority trial | 493 | Dolutegravir plus lamivudine | Current standard triple antiretroviral therapy regimen | Week 48: 94·3% in dolutegravir plus lamivudine group; 92·7% in standard triple antiretroviral therapy group | No emergence of new resistance-associated mutations observed in either group | 4 participants (2·0%) in dolutegravir plus lamivudine group had drug-related adverse events leading to study withdrawal; 1 participant (<1·0%) had drug-related adverse events leading to study withdrawal in the triple antiretroviral therapy group | Dolutegravir plus lamivudine dual therapy regimen showed non-inferiority to continuing standard triple antiretroviral therapy |
| Dolutegravir plus rilpivirine | ||||||||
| SWORD 1 and 2 (2018)24–26† | Two identical phase 3, multicentre, randomised, open-label, non-inferiority trials | 1024 | Dolutegravir plus rilpivirine | Current standard antiretroviral therapy; at 52 weeks, participants switched to dolutegravir plus rilpivirine (known as the late-switch group) | Week 48: 94·7% in dolutegravir plus rilpivirine group; 94·9% in standard antiretroviral therapy group; week 96: 89·0% in dolutegravir plus rilpivirine group; 93·1% in late-switch group; week 148: 84·2% in dolutegravir plus rilpivirine group; 89·7% in late-switch group | Two virological withdrawals at week 48 in each group; 1 individual in the dolutegravir plus rilpivirine group had an NNRTI resistance-associated mutation; no INSTI resistance | Grade 2–4 adverse events occurred in 6·0% of early-switch switch group participants after 148 weeks of exposure to dolutegravir plus rilpivirine and 3·4% of late-switch group participants after 96 weeks of exposure to dolutegravir plus rilpivirine; discontinuations due to adverse events occurred in 7·8% of early-switch participants up to 148 weeks of exposure and 4·0% of late-switch participants up to 96 weeks of exposure; serious adverse events occurred in 14·0% of early-switch participants after 148 weeks of exposure (0·8% were drug related) and 9·2% of late-switch participants after 96 weeks of exposure (none were drug related) | Dolutegravir plus rilpivirine dual therapy regimen showed non-inferiority to continuing current triple antiretroviral therapy |
| Boosted protease inhibitor plus lamivudine | ||||||||
| OLE (2015)27† | Phase 4, randomised, open-label, non-inferiority trial | 250 | Ritonavir-boosted lopinavir plus lamivudine | Ritonavir-boosted lopinavir plus lamivudine or emtricitabine plus second RTI | Week 48: 87·8% in ritonavir-boosted lopinavir plus lamivudine group; 86·6% in ritonavir-boosted lopinavir plus lamivudine or emtricitabine plus second RTI group | RTI resistance-associated mutations K103N and M184V in 1 participant in the ritonavir-boosted lopinavir plus lamivudine group | Severe adverse events: 6·5% (n=8) of participants in the ritonavir-boosted lopinavir plus lamivudine or emtricitabine plus second RTI group and 3·9% (n=5) in the ritonavir-boosted lopinavir plus lamivudine group; adverse event discontinuation: 3·3% (n=4) in the ritonavir-boosted lopinavir plus lamivudine or emtricitabine plus second RTI group and 0·8% (n=1) in the ritonavir-boosted lopinavir plus lamivudine group | Ritonavir-boosted lopinavir plus lamivudine dual therapy showed non-inferiority to triple therapy regimen of ritonavir-boosted lopinavir plus lamivudine or emtricitabine plus second RTI |
| SALT (2015)28,29† | Phase4, randomised, open label, non-inferiority trial | 286 | Ritonavir-boosted atazanavir plus lamivudine | Ritonavir-boosted atazanavir plus two RTIs | Week 48: 87·8% in ritonavir-boosted atazanavir plus lamivudine group; 86·6% in ritonavir-boosted atazanavir plus two RTIs group (intention to treat); week 96: 74·4% in ritonavir-boosted atazanavir plus lamivudine group; 73·9% in ritonavir-boosted atazanavir plus two RTIs group | 1 participant in the ritonavir-boosted atazanavir plus two RTIs group developed resistance-associated mutations (M184V and L63P) | Similar rates of grade 3–4 adverse events in both groups (70·7% vs 70·2%); treatment discontinuation was less frequent in the ritonavir-boosted atazanavir plus lamivudine group (2·1%, n=3) than in the ritonavir-boosted atazanavir plus two RTIs group (7·0%, n=10) | Ritonavir-boosted atazanavir plus lamivudine dual therapy showed non-inferiority to triple therapy regimen of ritonavir-boosted atazanavir plus two RTIs |
| AtLAS-M (2013)30,31† | Phase 4, multicentre, randomised, open-label, non-inferiority, randomised trial | 266 | Ritonavir-boosted atazanavir plus lamivudine | Ritonavir-boosted atazanavir plus two NRTIs | Week 48: 89·5% in ritonavir-boosted atazanavir plus lamivudine group; 79·7% in ritonavir-boosted atazanavir plus two NRTIs group | No emergence of new resistance-associated mutations observed in either group | Similar proportion of adverse events in both groups | Ritonavir-boosted atazanavir plus lamivudine dual therapy showed non-inferiority and superiority to triple therapy regimen of ritonavir-boosted atazanavir plus two NRTIs |
| DUAL-GESIDA 8014-RIS-EST45 (2017)32† | Phase 4, randomised, multicentre, open-label, non-inferiority trial | 249 | Ritonavir-boosted darunavir plus lamivudine | Ritonavir-boosted darunavir plus tenofovir disoproxil or emtricitabine or ritonavir-boosted darunavir plus abacavir and emtricitabine | Week 48: 88·9% in ritonavir-boosted darunavir plus lamivudine group; 92·7% in ritonavir-boosted darunavir-based triple therapy group | Protease inhibitor resistance-associated mutations L10I, A71T, and L76W in 1 participant (virus remained susceptible to darunavir); no emergent M184V or other emtricitabine resistance-associated mutations | Severe adverse event and adverse event-related discontinuations: 4·8% in ritonavir-boosted darunavir plus lamivudine group; 4·9% in ritonavir-boosted darunavir-based triple therapy group | Dual therapy with ritonavir-boosted darunavir plus lamivudine showed non-inferiority and similar tolerability compared with ritonavir-boosted darunavir-based triple therapy |
| Integrase inhibitor plus boosted protease inhibitor | ||||||||
| DUALIS (2020)33† | Phase 3, randomised, open-label, multicentre, non-inferiority, trial | 292 | Dolutegravir plus boosted darunavir | Continuation of boosted darunavir plus two NRTIs | Week 48: 86·3% in dolutegravir plus boosted darunavir group; 87·9% in boosted darunavir plus two NRTIs triple therapy group | No emergence of new resistance-associated mutations observed | Adverse event discontinuations: 4·6% (n=6) in dolutegravir plus boosted darunavir group; 0·8% (n=1) in boosted darunavir plus two NRTIs triple therapy group | Dolutegravir plus boosted darunavir dual therapy regimen showed non-inferiority to continuing boosted darunavir plus two NRTIs triple therapy |
| Boosted darunavir plus rilpivirine | ||||||||
| PROBE-2 trial (2020)34† | Phase 4, randomised, open-label, non-inferiority trial | 160 | Rilpivirine plus cobicistat-boosted darunavir | Current standard antiretroviral therapy regimen | Week 24: 90·0% in rilpivirine plus cobicistat-boosted darunavir group; 93·8% in standard antiretroviral therapy group | None reported | 8 participants in the rilpivirine plus cobicistat-boosted darunavir group and 3 participants in the standard antiretroviral therapy group discontinued study therapy; 4 patients reported adverse events not leading to treatment discontinuation (1 patient in the rilpivirine plus cobicistat-boosted darunavir group and 3 patients in the standard antiretroviral therapy group); 8 participants discontinued therapy in the rilpivirine plus cobicistat-boosted darunavir group and 3 participants discontinued therapy in the standard antiretroviral therapy group | Rilpivirine plus cobicistat-boosted darunavir dual therapy regimen showed non-inferiority to continuing current antiretroviral therapy |
| Long-acting cabotegravir plus rilpivirine | ||||||||
| FLAIR (2020)35–37† | Phase 3, randomised, open-label, non-inferiority trial | 566 | Cabotegravir plus rilpivirine (long-acting, intramuscular version) | Dolutegravir plus abacavir and lamivudine (oral) | Week48: 93·6% in long-acting cabotegravir plus rilpivirine group; 93·3%, in dolutegravir plus abacavir and lamivudine group; week 96: 9 (3·2%) participants in each group had HIV viral load ≥50 copies per mL | Week 48: three participants in long-acting cabotegravir plus rilpivirine group developed NNRTI and INSTI resistance-associated mutations | 5·0% (n=14) in the long-acting cabotegravir plus rilpivirine group and 1·4% (n=4) in the dolutegravir plus abacavir and lamivudine group had adverse events leading to withdrawal | Long-acting cabotegravir plus rilpivirine dual therapy showed non-inferiority to dolutegravir plus abacavir and lamivudine triple therapy |
| ATLAS (2020)38† | Phase 3, randomised, open-label, non-inferiority trial | 616 | Cabotegravir plus rilpivirine (long-acting, intramuscular version) | Current standard antiretroviral therapy regimen | Week 48: 92·5% in long-acting cabotegravir plus rilpivirine group; 95·5% in standard antiretroviral therapy group | Week 48: 3 participants in the long-acting cabotegravir plus rilpivirine group developed NNRTI resistance-associated mutations | 4·5% (n=14) in the long-acting cabotegravir plus rilpivirine group and 2·0% (n=6) in the standard antiretroviral therapy group had adverse events leading to withdrawal | Long-acting cabotegravir plus rilpivirine dual therapy showed non-inferiority to continuation of standard antiretroviral therapy |
| ATLAS-2M (2021)39–41† | Randomised, multicentre, open-label, phase 3b, non-inferiority trial | 1045 | Cabotegravir plus rilpivirine (long-acting, intramuscular version administered every 8 weeks) | Cabotegravir plus rilpivirine (long-acting, intramuscular version administered every 4 weeks) | Week 96: 90% in every 4 weeks group; 91% in every 8 weeks group | Week 152: 2 participants in every 8 weeks group had virological failure rilpivirine and cabotegravir resistance-associated mutations | Safety profile similar between groups; 80·8% (n=844) participants had an adverse event | Long-acting cabotegravir plus rilpivirine administered every 8 weeks showed non-inferiority to dosing long-acting cabotegravir plus rilpivirine administered every 4 weeks |
A71T=threonine replacing alanine at position 71 in protease. INSTI=integrase strand transfer inhibitor. K103N=asparagine replacing lysine at position 103 in reverse transcriptase. L10I=isoleucine replacing leucine at position 76 in protease. L63P=proline replacing leucine at position 63 on protease. L76W=tryptophan replacing lysine at position 76 in protease. M184V=valine replacing methionine at position 184 in reverse transcriptase. NNRTI=non-nucleoside reverse transcriptase inhibitor. NRTI=nucleoside reverse transcriptase inhibitor. R263R/K=lysine replacing arginine at position 263 in integrase. RR=relative risk. RTI=reverse transcriptase inhibitor.
Study of antiretroviral therapy-naive individuals.
Switch study of virologically suppressed individuals.
Successful oral two-drug regimens in phase 3 trials to date include an agent with a high barrier to resistance, specifically dolutegravir (a second-generation integrase strand transfer inhibitor [INSTI]) or a boosted protease inhibitor, combined with an agent blocking HIV-1 reverse transcriptase. Despite their similarities, these two-drug regimens exhibit differences in the risk of resistance emergence during virological rebound, and some regimens have specific weaknesses relative to three-drug comparators (table 2). Like three-drug regimens, the constituent drugs in two-drug regimens determine the safety, tolerability, and metabolic profile of the regimen. The unique strengths and limitations of each two-drug regimen, as well as available clinical trial data, inform their use in different clinical scenarios.
Treatment-naive people with HIV
GARDEL compared ritonavir-boosted lopinavir plus lamivudine with ritonavir-boosted lopinavir plus two nucleoside reverse transcriptase inhibitors (NRTIs) and found similar virological efficacy and a low risk of resistance, even in people with HIV with viral loads of more than 100 000 copies per mL.18 However, this regimen has many issues attributable to ritonavir-boosted lopinavir, including hyperlipidaemia, problems with liver function, gastrointestinal side-effects, drug–drug interactions, and twice-daily dosing. Large, randomised trials have since identified more tolerated and safer two-drug regimens with non-inferior virological efficacy than three-drug therapy. These regimens include dolutegravir plus lamivudine16,17,42 and ritonavir-boosted darunavir plus lamivudine.32 NEAT001/ANRS143 showed the non-inferiority of ritonavir-boosted darunavir plus raltegravir, but subgroup analysis found inferiority to three-drug regimens in participants with baseline CD4 cell counts less than 200 cells per μL, and more virological failures in participants with HIV-1 RNA more than or equal to 100 000 copies per mL at baseline than in participants who had CD4 counts of more than 200 cells per μL or HIV-1 RNA less than 100 000 copies per mL.19 This regimen also requires multiple pills and twice-daily dosing, making it less convenient than other preferred treatments. Data from these studies led to the inclusion of these regimens in many treatment guidelines for initial antiretroviral therapy (table 3).43–46 Of these two-drug regimens, only dolutegravir plus lamivudine is recommended for most treatment-naive people with HIV.43,44,46
Table 3:
Two-drug regimens recommended for treatment of HIV-1 infection
| DHHS guidelines43 | EACS guidelines44 | WHO guidelines45 | IAS guidelines46 | |
|---|---|---|---|---|
|
| ||||
| Recommendations for initial treatment in antiretroviral therapy-naive individuals | ||||
| Recommended regimens | Dolutegravir plus lamivudine (A1); ritonavir-boosted darunavir plus lamivudine (C1); ritonavir-boosted darunavir plus raltegravir (C1) | Dolutegravir plus lamivudine | Not mentioned | Dolutegravir plus lamivudine (A1a) |
| Exclusions for dolutegravir plus lamivudine | Viral load >500 000 copies per mL; HBV co-infection; before results of HIV genotypic resistance or HBV testing | Viral load >500 000 copies per mL; HBV co-infection; PREP failure | NA | Viral load >500 000 copies per mL; HBV co-infection; before results of HIV genotypic resistance or HBV testing; CD4 cell count <200 cells per μL |
| Exclusions for ritonavir-boosted darunavir plus lamivudine | HBV co-infection; before results of HIV genotypic resistance or HBV testing | NA | NA | NA |
| Exclusions for ritonavir-boosted darunavir plus raltegravir | Viral load >100 000 copies per mL; CD4 cell count <200 cells per μL; HBV co-infection; before results of HIV genotypic resistance or HBV testing | NA | NA | NA |
| Recommendations for antiretroviral therapy switch in virologically suppressed individuals | ||||
| Recommended regimens | Dolutegravir plus rilpivirine (A1); dolutegravir plus lamivudine (A1); boosted protease inhibitor plus lamivudine (B1–C1); dolutegravir plus boosted darunavir (C1); long-acting cabotegravir plus rilpivirine (A1) | Dolutegravir plus rilpivirine; dolutegravir plus lamivudine; boosted darunavir plus lamivudine; boosted atazanavir plus lamivudine; boosted darunavir plus rilpivirine;* long-acting cabotegravir plus rilpivirine | Not mentioned | Dolutegravir plus lamivudine (A1a); dolutegravir plus rilpivirine (A1a); ritonavir-boosted darunavir plus lamivudine (A1a); long-acting cabotegravir plus rilpivirine administered every 4 weeks (A1a); long-acting cabotegravir plus rilpivirine administered every 8 weeks (B1b);39,40† |
| Exclusions and limitations of recommendations | HBV co-infection | Reported only as potential options; no historical resistance; absence of chronic HBV co-infection | NA | HBV co-infection |
A1=strong data from randomised controlled trials. A1a=strong panel support from ≥1 randomised controlled trials published in peer-reviewed literature. B1=moderate data from randomised controlled trials. B1b=moderate panel support from ≥1 randomised controlled trials presented in abstract form at peer-reviewed scientific meetings. C1=optional data from randomised controlled trials. DHHS=United States Department of Health and Human Services. EACS=European AIDS Clinical Society. HBV=hepatitis B virus. IAS=International Antiviral Society. NA=not applicable. PREP=pre-exposure prophylaxis.
Use recommended only by small trials.
This rating for long-acting cabotegravir plus rilpivirine administered every 8 weeks could be due to the publication of guidelines before results of the ATLAS-2M trial, which have since become available.
DHHS guidelines were last updated in August, 2021. EACS guidelines were last updated in October, 2021. IAS guidelines were last updated in October, 2020. WHO guidelines were last updated in July, 2021. IAS guidelines do not recommend dolutegravir plus lamivudine for patients with chronic hepatitis B or HIV RNA level above 500 000 copies per mL (and potentially a CD4 cell count below 200 per μL, although this is unclear). IAS guidelines recommend monitoring patients for adherence and virological response. Dolutegravir plus lamivudine is not recommended for patients who are being treated for an active opportunistic infection,such as tuberculosis, toxoplasmosis, and pneumocystis.
ACTG A5353 was a phase 2, single-arm, pilot study of once-daily dolutegravir plus lamivudine in treatment-naive people with HIV, which showed an efficacy of 85% at week 48.47 One participant with poor adherence developed lamivudine-resistance-associated and dolutegravir-resistance-associated mutations. The GEMINI 1 and 2 studies, which enrolled treatment-naive people with HIV without transmitted drug-resistant virus and HIV-1 RNA of 500 000 copies per mL or less, showed non-inferior efficacy of dolutegravir plus lamivudine compared with dolutegravir plus tenofovir disoproxil fumarate and emtricitabine.16,17 Post-hoc analysis in participants with baseline HIV-1 RNA of more than 100 000 copies per mL showed no decline in activity of dolutegravir plus lamivudine in the high viral load strata, although the number of participants in this subgroup was small.48 Some guidelines restrict the recommended use of dolutegravir plus lamivudine to people with HIV with HIV RNA less than 500 000 copies per mL as people with HIV-1 RNA more than 500 000 copies per mL were excluded from the GEMINI studies. Regulatory agencies, including the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA), do not restrict use based on HIV viral load.43,44,46,49,50
Baseline resistance testing documenting susceptibility to lamivudine and dolutegravir, as well as hepatitis B virus (HBV) testing, are recommended with dolutegravir plus lamivudine. Furthermore, some guidelines recommend caution for people with HIV with CD4 cell counts less than 200 cells per μL, as the GEMINI 1 and 2 studies showed numerically lower viral suppression rates in this subgroup than in the control group using FDA snapshot analysis (79% with dolutegravir plus lamivudine vs 93% with three-drug comparator at 48 weeks).17 No difference was seen in the low CD4 cell count subgroup in preplanned treatment-related discontinuation equals failure analysis at the same timepoint.
Although ACTG A5353 highlighted concerns about resistance to dolutegravir and lamivudine during virological failure,47 no treatment-emergent resistance was noted in the larger GEMINI studies until week 144, when one participant developed valine-replacing methionine at position 184 in reverse transcriptase (M184V) and lysine replacing arginine at position 263 in integrase (R263R/K) mutations because of non-adherence.16 This participant developed viraemia of 61 927 copies per mL at week 132 but resuppressed to less than 50 copies per mL 4 weeks later on dolutegravir plus lamivudine, thus not meeting virological withdrawal criteria. Viral rebound occurred again to 135 copies per mL at week 144, leading to study withdrawal. There have been no cases of resistance emergence to dolutegravir or bictegravir plus two NRTIs in clinical trials of treatment-naive individuals to date.
Antiretroviral therapy switch in virologically suppressed people with HIV
Switching to a two-drug regimen is an option for some virologically suppressed people with HIV. Reasons to consider switching include avoiding specific NRTI adverse effects, such as the bone and renal effects of tenofovir disoproxil fumarate or cardiovascular effects associated with abacavir.43,44,46 Even without obvious contraindications to triple therapy, provided the two-drug regimen does not compromise virological outcomes or safety, some clinicians consider reduction in lifetime antiretroviral exposure through two-drug therapy potentially beneficial for patients. Other reasons to switch to a two-drug regimen include payer or insurance restrictions (particularly in the USA), cost, pill size, drug–drug interactions, and drug intolerance. In some people with HIV who could switch to two-drug therapy, maintaining three-drug antiretroviral therapy might be preferable for many reasons, including satisfaction with current antiretroviral therapy, payer or formulary restrictions for two-drug regimens, drug interactions, and personal preference.
Switch studies have been done in virologically suppressed patients (table 2). Recommended two-drug switch options include dolutegravir plus lamivudine, dolutegravir plus rilpivirine, boosted darunavir or atazanavir plus lamivudine (although atazanavir is no longer included in most guidelines), and long-acting cabotegravir plus long-acting rilpivirine (figure).43,44,46 The European AIDS Clinical Society also lists boosted darunavir plus rilpivirine or dolutegravir as possible two-drug regimens for antiretroviral-therapy-experienced people with HIV, noting that their use is only supported by small trials.44 Recommended two-drug regimens preserve the so-called undetectable equals untransmissible rule, a crucial benefit of antiretroviral therapy as HIV transmission does not occur in serodifferent sexual partners when viral load is suppressed to less than 200 copies per mL.51
Figure: Timeline of two-drug antiretroviral therapy studies and guidelines.
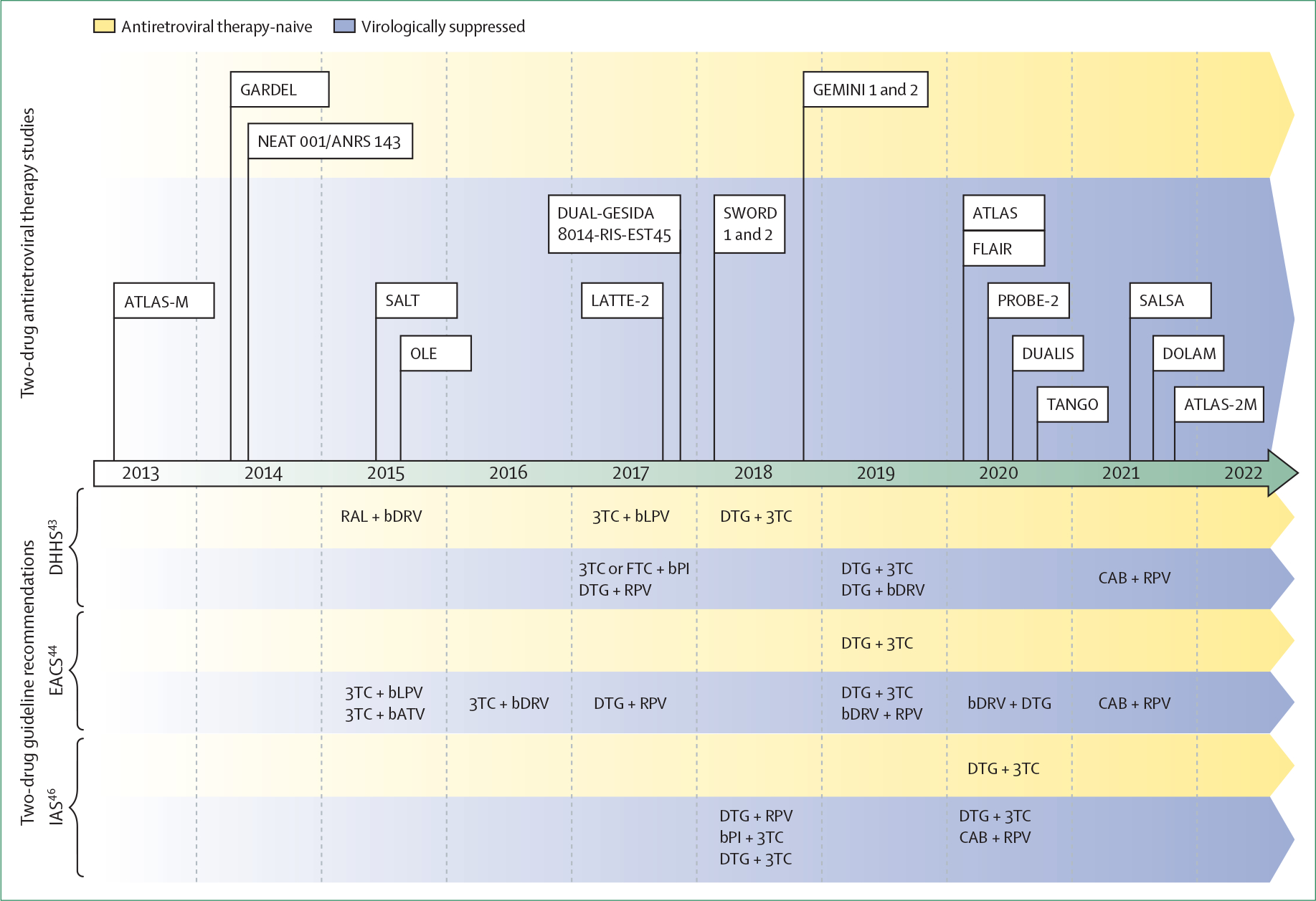
Two-drug regimens evaluated in each study: ATLAS-M: ritonavir-boosted atazanavir plus lamivudine;30,31 GARDEL: ritonavir-boosted lopinavir plus lamivudine;18 NEAT 001/ANRS 143: ritonavir-boosted darunavir plus raltegravir;19 SALT: ritonavir-boosted atazanavir plus lamivudine;28,29 OLE: ritonavir-boosted lopinavir plus lamivudine;27 LATTE-2: long-acting cabotegravir plus long-acting rilpivirine;53 DUAL-GESIDA 8014-RIS-EST45: ritonavir-boosted darunavir plus lamivudine;32 SWORD 1 and 2: dolutegravir plus rilpivirine;24–26 GEMINI 1 and 2: dolutegravir plus lamivudine;16,17 ATLAS: long-acting cabotegravir plus long-acting rilpivirine;38 FLAIR: long-acting cabotegravir plus long-acting rilpivirine;35,37 PROBE-2: cobicistat-boosted darunavir plus rilpivirine;34 DUALIS: dolutegravir plus ritonavir-boosted darunavir;33 TANGO: dolutegravir plus lamivudine;20,21 SALSA: dolutegravir plus lamivudine;23 DOLAM: dolutegravir plus lamivudine;22 ATLAS-2M: long-acting cabotegravir plus long-acting rilpivirine.39–41 3TC=lamivudine. bATV=boosted atazanavir. bDRV=boosted darunavir. bLPV=boosted lopinavir. bPI=boosted protease inhibitor. CAB=cabotegravir. DHHS=Department of Health and Human Services. DTG=dolutegravir. EACS=European AIDS Clinical Society. FTC=emtricitabine. IAS=International Antiviral Society. RAL=raltegravir. RPV=rilpivirine.
Among oral two-drug regimens, dolutegravir plus lamivudine has advantages, including an absence of the food and calorie requirements of dolutegravir plus rilpivirine and the metabolic and drug interaction concerns with boosted darunavir. Numerous studies have shown the efficacy and safety of dolutegravir plus lamivudine as a switch strategy for people with HIV with no history of unsuccessful treatment or resistance. In the largest trials, no participants had virological failure, including up to week 144 in TANGO23 and week 48 in SALSA.21 Furthermore, no participants in the dolutegravir plus lamivudine groups in ASPIRE, TANGO, DOLAM, or SALSA developed resistance-associated mutations to INSTIs or reverse transcriptase inhibitors.
Dolutegravir plus rilpivirine showed non-inferiority to continuation of three-drug antiretroviral therapy in the SWORD 1 and 2 trials.24–26 Two individuals had virological failure at week 48 in the dolutegravir plus rilpivirine group, one of whom developed non-nucleoside reverse transcriptase inhibitor (NNRTI) resistance (101K/E mixture).24 Although virological failure remained uncommon (1%) at follow-up, up to week 148, emergence of NNRTI-resistance-associated mutations occurred in more than half (6 or 11) of participants with virological failure.26 No INSTI-resistance-associated mutations occurred up to week 148. Boosted darunavir plus dolutegravir and boosted protease inhibitors plus lamivudine have also shown virological efficacy, but pill burden, adverse metabolic and gastrointestinal effects, and drug interactions restrict the use of these regimens.
Studies have also shown the safety and efficacy of boosted darunavir plus rilpivirine and raltegravir plus a boosted protease inhibitor as potential two-drug switch regimens. However, they are not widely adopted because of scarce data, complex dosing, risk of resistance, and few additional advantages over alternative regimens. Other regimens that have been investigated in small studies but have issues and are not recommended include raltegravir plus etravirine, nevirapine plus lamivudine, boosted lopinavir plus raltegravir, and dolutegravir plus emtricitabine.
Long-acting injectable regimens
The LATTE trial showed non-inferiority of oral cabotegravir plus rilpivirine, allowing for further study of long-acting injectable cabotegravir plus rilpivirine.52 Long-acting cabotegravir plus rilpivirine was approved as the first long-acting HIV-1 treatment regimen and is licensed by both the FDA and EMA for maintenance of viral suppression in people with HIV with no history of virological failure and no known or suspected resistance to its components. This regimen is administered every 4 or 8 weeks as a depot preparation, although only the 8-week dosing is available in Europe and many other countries. The phase 2b LATTE-2 study showed the safety and efficacy of long-acting cabotegravir plus rilpivirine administered every 4 weeks or every 8 weeks compared with oral therapy.53
The phase 3 registrational studies underpinning regulatory approval include cabotegravir plus rilpivirine administered every 4 weeks compared with an oral standard of care comparator in two treatment populations: the FLAIR study for treatment-naive people with HIV and the ATLAS study for virologically suppressed people with HIV.38,54 ATLAS-2M evaluated cabotegravir plus rilpivirine administered every 4 weeks versus administered every 8 weeks in virally suppressed people with HIV.39–41 The injectable cabotegravir plus rilpivirine regimen has been shown to have similar efficacy and safety to an oral comparator in phase 2 and 3 trials of virally suppressed adults without HBV and no previous INSTI-resistance-associated or NNRTI-resistance-associated mutations (except asparagine replacing lysine at position 103 in reverse transcriptase [K103N]).35,39,40 Durability of suppression has been established up to 124 weeks in FLAIR and up to 152 weeks in ATLAS-2M.37,41
In the phase 3 trial programme, virological failure occurred in approximately 1% of participants taking cabotegravir plus rilpivirine and occurred most commonly within the first 24 weeks of administration, with more occurring in recipients of 2-month dosing than in recipients of 1-month dosing.37 Participants with virological failure tended to have NNRTI and INSTI resistance, causing the failure.35 Presence of rilpivirine-resistance-associated mutations at baseline, HIV-1 genotype A1/A6, BMI more than 30 kg/m2, and low week-8 rilpivirine trough concentration were associated with virological failure in multivariate analysis. The presence of two or more of these factors was linked to increased risk of virological failure.55,56 Notably, week-152 results from ATLAS-2M showed 2·1% of participants had virological failure in the 8-week administration group and 0·4% had it in the 4-week administration group.
Participants frequently reported injection site reactions, which were predominantly mild and rarely resulted in discontinuation.38,39,54 Participants also substantially preferred the long-acting regimen to the daily oral therapy, and many preferred 8-week dosing to 4-week-dosing.41 Importantly, people with HIV enrolled in these studies knowing that they might receive otherwise unavailable long-acting therapy. Therefore, in clinical practice, these preference data are probably most applicable to people with HIV who express interest in long-acting therapy or stopping daily therapy.
An important challenge when implementing long-acting cabotegravir plus rilpivirine is the need to carefully choose candidates to minimise the risk of virological failure with resistance. Each clinic will also need to establish an efficient workflow for refrigerated medication storage and administration of injections by trained staff using scheduling and reminder procedures that maximise adherence. To overcome these challenges, clinics will need to use strategies that prioritise patient preference and clinical success and adapt them depending on locally available human resources and clinic capacity.
Two-drug antiretroviral therapy in individuals with known or increased risk of resistance
Regulatory agencies and expert guidelines recommend against the use of dolutegravir plus rilpivirine or long-acting cabotegravir plus rilpivirine in people with HIV who have known or suspected resistance to any component of these regimens.43,44,46,50 Several studies have shown archived rilpivirine mutations increase the risk of virological failure with both dolutegravir plus rilpivirine and long-acting cabotegravir plus rilpivirine.26,56 Furthermore, the increased risk of resistance emergence during virological failure of these regimens, particularly with the limitation of subsequent treatment options among individuals who have long-acting cabotegravir plus rilpivirine failure, should be included when considering these regimens.
Although regulatory agencies and expert guidelines also recommend against treatment with dolutegravir plus lamivudine in people with HIV with known or suspected resistance to any components of this regimen, there is ongoing research investigating the effect historical resistance to lamivudine might have on the efficacy of this regimen. In TANGO, proviral DNA genotyping detected M184V/I in four of 322 participants in the dolutegravir plus lamivudine group at baseline. However, none of these participants developed virological failure. In the ART-PRO trial, none of the 21 participants with historical M184V/I mutations, which were not detected in proviral DNA genotyping with population sequencing, had virological failure after 96 weeks of follow up.57
A 2021 cohort study of 695 virologically suppressed participants with an available genotype, including 105 with past M184V/I mutations, who were switched to dolutegravir plus lamivudine found no difference in the probability of virological failure regardless of history of previous M184V/I mutation after a median follow-up length of 1·2 years.58 These results should be interpreted with caution until further research can be done. Large studies are needed to completely understand the effect previous resistance to lamivudine will have on the activity of dolutegravir plus lamivudine for maintenance of virological suppression. We do not currently recommend this strategy in clinical practice.
Two-drug antiretroviral therapy in low-income and middle-income countries
Although many guidelines in Europe and North America have incorporated two-drug regimens, WHO has not incorporated them.45 Concerns regarding HBV co-infection, no data in pregnant people, and the high frequency of tuberculosis co-infection in some regions (requiring dolutegravir to be taken twice a day) were some of the reasons for not including the two-drug regimen in the WHO guidelines. Furthermore, some low-income and middle-income countries have a high prevalence of NRTI and NNRTI resistance in both antiretroviral-therapy naive and experienced people with HIV, highlighting the need for HIV resistance testing. Although the use of two-drug regimens, including long-acting cabotegravir plus rilpivirine, is one way to reduce toxicities of long-term NRTI use in resource-constrained settings, implementation of this strategy will depend on local infrastructure, including capacity to test for HBV, do HIV resistance testing, and maintain cold-chain storage for long-acting rilpivirine.
Unique populations and contexts
Rapid antiretroviral therapy (antiretroviral therapy initiation before the availability of some or all baseline test results, such as HIV-1 genotype, HBV serologies, viral load, and renal function) might improve viral suppression, retention in care, and clinical outcomes.59 Although most controlled trials of this strategy were done in resource-constrained settings, it is now widely used in countries where the infrastructure exists, in part because people with HIV generally prefer starting treatment rather than delaying it. STAT was a single-arm study that investigated rapid antiretroviral therapy initiation with dolutegravir plus lamivudine.60 Participants whose results later showed HBV infection or transmitted baseline lamivudine resistance had tenofovir alafenamide or tenofovir disoproxil fumarate added within 2 weeks. Results were favourable in the as-treated population, with no virological failures due to resistance, even in individuals with baseline HIV RNA more than 500 000 copies per mL. Despite STAT suggesting that rapid antiretroviral therapy initiation with dolutegravir plus lamivudine was feasible, this study had a small sample size and short follow-up. Guidelines currently recommend standard three-drug regimens for rapid antiretroviral therapy initiation.43–46
Current recommendations for HIV and HBV co-infection include regimens containing tenofovir alafenamide or tenofovir disoproxil fumarate plus lamivudine and emtricitabine. If tenofovir cannot be used, people with HIV and HBV co-infection should be treated with entecavir in addition to a fully suppressive antiretroviral therapy regimen. Two-drug regimens are not recommended for people with HIV and HBV co-infection as no currently approved regimen contains tenofovir. HBV infection has occurred during treatment with two-drug regimens; therefore, testing for HBV before initiating two-drug regimens is recommended. Ideally, clinicians should ensure that people with HIV being considered for two-drug regimens who are at risk of HBV infection are vaccinated and have documented immunity to HBV.
There are no published data on the efficacy and safety of two-drug antiretroviral therapy regimens in pregnancy to date. People who become pregnant while receiving two-drug regimens should be enrolled in appropriate antiretroviral pregnancy registries. Currently, clinicians should discuss treatment guidelines with the patient and explain that two-drug regimens are not currently recommended when pregnant.43–46,61
The randomised clinical trials that led to the inclusion of oral two-drug regimens in antiretroviral therapy guidelines were done in adult populations. Therefore, these regimens are not recommended in paediatric populations. In April, 2022, the FDA approved long-acting cabotegravir plus rilpivirine for maintenance of virological suppression in adolescents aged 12 years or older weighing at least 35 kg with no previous treatment failure and no known or suspected resistance to either component drug on the basis of the week 16 interim analysis of the ongoing MOCHA study (NCT03497676).62 The ongoing PENTA-17 SMILE trial of boosted darunavir plus an INSTI in children aged 6–18 years showed non-inferior virological suppression at week 48.63
The most efficacious option for treating active tuberculosis is a rifampin-containing regimen. In low-income and middle-income countries, rifabutin—an alternative to rifampin with fewer drug interactions—is often unavailable. As dolutegravir is partly metabolised by the cytochrome P450–3A system, an enzyme system strongly induced by rifampin, dolutegravir should be administered twice daily if co-administered with rifampin. Therefore, the option to use single tablet, once-daily dolutegravir plus lamivudine as a complete regimen is not available when treating an individual with tuberculosis. Furthermore, rifampin might decrease protease inhibitor concentrations, either decrease or increase ritonavir concentrations, and increase the risk of hepatotoxicity, thus contraindicating protease inhibitor-based two-drug antiretroviral therapy when cotreating tuberculosis.
The management of individuals with virological failure is challenging as there is often a history of suboptimal adherence or resistance. Although there are data supporting use of some two-drug regimens, such as dolutegravir plus ritonavir-boosted darunavir, in this context,64 a full discussion is outside the scope of this Review.
No two-drug regimen is currently recommended for initial antiretroviral therapy in people with HIV who have not responded to HIV pre-exposure prophylaxis, including individuals who were taking long-acting cabotegravir as pre-exposure prophylaxis.
Two-drug therapy and metabolic outcomes
Non-AIDS comorbidities in people with HIV are affected by traditional risk factors (eg, obesity, tobacco use, age, and family history or genetics), chronic inflammation, immune activation from HIV, and, in some cases, the effects of long-term antiretroviral therapy.65 Risk reduction involves maintaining viral suppression and managing modifiable risk factors, including optimising antiretroviral therapy regimens to minimise potential metabolic complications. Like three-drug regimens, the metabolic profile of two-drug regimens is determined by their constituent agents. Many two-drug antiretroviral therapy trials have examined surrogate markers of metabolic outcomes, including blood and urine markers of renal function, lipid metabolism, glucose or insulin resistance, radiological studies assessing bone density, and weight or BMI changes (appendix pp 1–3).
Tenofovir disoproxil fumarate is known to cause elevations in serum creatinine and decreased bone mineral density, and studies examining two-drug regimens that do not contain tenofovir disoproxil fumarate versus tenofovir-disoproxil-fumarate-containing three-drug regimens have consistently shown benefits in markers of bone and renal health.17,23,26 Tenofovir alafenamide, however, has favourable bone and renal profiles; in TANGO there was no difference in renal or bone biomarkers in the dolutegravir plus lamivudine versus tenofovir-alafenamide-containing triple-drug antiretroviral therapy groups.20,21 Some antiretroviral drugs have distinct effects on lipid metabolism, as reflected in serum lipid profiles. The beneficial effects of tenofovir-disoproxil-fumarate-containing three-drug therapy on lipids was shown in multiple studies compared with two-drug therapy.17,32,31,36 Conversely, TANGO showed an overall improvement in lipids with switching to dolutegravir plus lamivudine compared with continuing tenofovir-alafenamide-containing three-drug therapy, potentially affected by three-drug regimens that included boosted protease inhibitors.20,21,66,67 Abacavir has been independently linked to an increased risk of cardiovascular disease.68 Therefore, starting or switching to a regimen that does not contain abacavir, such as currently approved two-drug regimens, could be beneficial in people with increased risk of cardiovascular disease.
Some antiretroviral drugs also influence bodyweight; INSTIs and tenofovir alafenamide are associated with weight gain and tenofovir disoproxil fumarate is associated with weight suppression.69–72 The differential effect of tenofovir disoproxil fumarate on weight was seen in the GEMINI 1 and 2 studies, with participants receiving dolutegravir plus lamivudine having 1·3 kg more weight gain, on average, than those receiving tenofovir-disoproxil-fumarate-containing three-drug regimens (3·7 kg vs 2·4 kg at week 144).16,72 Switching from tenofovir-disoproxil-fumarate-containing three-drug regimens to dolutegravir plus lamivudine or tenofovir-alafenamide-containing three-drug therapy has also been consistently associated with weight gain.22,23,73 TANGO found no differences in weight gain, BMI, blood glucose, or insulin resistance between participants randomised to dolutegravir plus lamivudine compared with continuation of tenofovir-alafenamide-containing three-drug antiretroviral therapy.20,21 Currently, no evidence suggests starting or switching to preferred two-drug regimens will favourably influence antiretroviral-therapy-associated weight changes. The PASO-DOBLE study (NCT04884139) will compare switching from a suppressive regimen to dolutegravir plus lamivudine or bictegravir plus lamivudine and tenofovir alafenamide to assess this potential effect.
Two-drug therapy and other important features of antiretroviral therapy
An important benefit of viral suppression is improving atypical immune activation and inflammation compared with untreated HIV. Atypical immune activation and inflammation during antiretroviral therapy have been associated with poor clinical outcomes.74 Substudies of pivotal trials have compared biomarkers of residual inflammation or immune activation in people with HIV receiving two-drug versus three-drug antiretroviral therapy regimens and found mixed effects of unclear clinical significance.23,75,76 Sometimes, changes in biomarkers were similar between two-drug and three-drug regimens during short follow-up.24,25,,77–81 Focused studies with a longer timeframe will improve our understanding of the inflammatory profiles of the different two-drug regimens compared with three-drug regimens and the potential clinical consequences.
HIV might continue to replicate in anatomical sanctuaries, including the CNS, lymphoid tissue, and genital tissue, where antiretroviral drug concentrations and viral dynamics might differ from the systemic compartment. It is important to know whether two-drug antiretroviral therapy regimens adequately reach and act in these compartments as suboptimal antiretroviral therapy penetration could lead to persistent viral replication, select for resistance, and increase HIV transmission risk.82,83
Multiple small studies have examined the clinical effects or penetration of particular two-drug antiretroviral therapy in the CNS. In a substudy of DOLAM, all participants who switched to dolutegravir plus lamivudine maintained both plasma and cerebrospinal fluid (CSF) HIV viral suppression up to week 48, and there was no increase in CSF markers of inflammation or neuronal injury.84 Another study examined 78 people with HIV on three-drug therapy and 19 people with HIV on two-drug therapy and found no difference in rates of undetectable CSF HIV-1 RNA or CSF escape between the regimens.85 Another study examined drug concentrations and viral suppression in ten people with HIV after switching to a two-drug regimen of boosted atazanavir plus lamivudine and found that all but one participant with suboptimal antiretroviral therapy adherence maintained plasma and CSF HIV-1 RNA less than 40 copies 12 weeks after switching.86 A neurocognitive substudy within the SALT trial showed stable neurocognitive function up to week 96 in both the two-drug and three-drug groups.87
A substudy of 18 participants from the ANRS 167 LAMIDOL study who switched from dolutegravir-based triple-therapy to dolutegravir plus lamivudine found seminal plasma HIV concentrations to be unchanged after 24 weeks of follow-up.88 These results were consistent with the results of a study that examined genital HIV-1 shedding with dolutegravir plus lamivudine in both treatment-naive and switch participants compared with three-drug regimens and found no differences in genital shedding between groups.89
HIV replication continues at low rates in many people with HIV on suppressive antiretroviral therapy, but whether this affects inflammation, risk of virological failure, or emergence of resistance-associated mutations or not is unknown. Scarce data suggest that specific two-drug regimens have similar rates of low HIV replication to comparator three-drug regimens. Specifically, dolutegravir plus lamivudine was similar to three-drug therapy in incidence of viral blips and HIV RNA less than 40 copies per mL with target not detected.48,90 A subanalysis of the ATLAS-M study found similar HIV DNA concentrations with ritonavir-boosted atazanavir plus lamivudine versus three-drug therapy.91
Cost
The cost of antiretroviral therapy to individuals varies between and within countries due to differences in pricing and payment schemes; the cost profile for some two-drug regimens might be more favourable than for three-drug regimens in countries like the USA if the overall costs reflect the relatively low cost of generic lamivudine.92 A cost-effectiveness and budget-effect study using data from the GEMINI studies estimated that initial treatment with dolutegravir plus lamivudine would be cost-saving compared with three-drug regimens containing dolutegravir, bictegravir, or boosted darunavir.93 Cost projections using TANGO data also showed significant savings from switching to dolutegravir plus lamivudine compared with continuing tenofovir-alafenamide-containing three-drug therapy. Studies using data from DOLAMA94 found cost benefits with dolutegravir plus lamivudine. In Taiwan, dolutegravir plus rilpivirine was found to have cost advantages, but not when compared with efavirenz, emtricitabine, and tenofovir disoproxil fumarate.95 Budget-effect analysis in ATLAS-M found simplification from ritonavir-boosted atazanavir plus two NRTIs to ritonavir-boosted atazanavir plus lamivudine at a national level would lead to substantial reduction in medical costs for the Italian national health system.96,97
Potential cost advantages of two-drug therapy are not always replicated. For example, more active pharmaceutical ingredient (by total weight) is needed to manufacture dolutegravir plus lamivudine compared with bictegravir, emtricitabine, and tenofovir alafenamide. Therefore, a potential generic dolutegravir plus lamivudine treatment is possibly more expensive to deliver on a mass scale to resource-constrained settings than a potential generic bictegravir, emtricitabine, and tenofovir alafenamide treatment.
Investigational two-drug regimens
Two-drug regimens, including subcutaneous lenacapavir (the first-in-class capsid inhibitor) dosed every 6 months, showed promising virological efficacy in treatment-naive people with HIV. However, resistance emerged in a few individuals with poor adherence and virological failures.98 Studies involving long-acting lenacapavir were stopped in December, 2021, due to FDA concerns about the compatibility of lenacapavir with borosilicate vials, but began again in May, 2022, with new data on the compatibility of lenacapavir with an alternative vial made from aluminosilicate glass. In June, 2022, the EMA recommended granting a marketing authorisation for lenacapavir for the treatment of people with HIV with multidrug-resistant HIV-1.99 Development of two-drug regimens containing islatravir was placed on clinical hold due to an occurrence of lymphopenia in some participants. However, in September, 2022, Merck announced a new phase 3 clinical trial programme for daily doravirine plus a low dose of islatravir, and planned resumption of the phase 2 trial of weekly lenacapavir plus islatravir at a reduced dose.100 Finally, a proof-of-concept study (ACTG A5357) is investigating long-acting cabotegravir plus VRC07–523 leucine and serine, a long-acting, broadly neutralising HIV-1 antibody (NCT03739996).
Conclusion
Some two-drug antiretroviral therapy regimens, including the first approved long-acting injectable regimen, increase treatment options for the individualised care of people with HIV. By reducing cumulative exposure to antiretroviral drugs, effective two-drug regimens might reduce long-term toxicities and costs, depending on the constituent drugs and the setting. The use of any two-drug regimen should be informed by the characteristics of that regimen, exceptions to its recommended use, and risks and predictors of virological failure and resistance emergence. Treatment guidelines do not recommend two-drug therapy in people with chronic HBV, pregnancy, active tuberculosis, or resistance to any of the component antiretroviral drugs. The high burden of chronic HBV and restricted infrastructure in some settings pose challenges to the widespread use of two-drug antiretroviral therapy regimens as a preferred global public health approach. However, multiple two-drug regimens have been shown to be effective for HIV treatment with low rates of virological failure, good tolerability, and high durability. These regimens are now included in guidelines as standard antiretroviral therapy options for treatment-naive and treatment-experienced people with HIV.
Supplementary Material
Search strategy and selection criteria.
We searched the literature using medical subject headings to find original articles using the search terms: “two drug”, “two-drug”, “2 drug”, “2-drug”, “dual therapy”, “dual treatment”, “two drug therapy”, “two drug regimen”, “two-drug therapy”, “two-drug regimen”, “HIV”, “HIV Infections”, and “Human Immunodeficiency Virus” in PubMed, Embase, Web of Knowledge, and Google Scholar. Abstracts were screened and reviewed for relevance. This search was restricted to articles published in English. The database searches were done between Oct 29 and Nov 15, 2021, and on April 4, 2022.
Footnotes
Declaration of interests
SGK has served on advisory boards for Gilead, ViiV, and Theratechnologies. JRA has received advisory fees, speaker fees, and grant support from ViiV, Janssen, Gilead, Merck, Aelix, and Thera. PC has worked on advisory boards at ViiV and Merck and received research and speaker funds from ViiV, CanSino, and Janssen and has received honoraria as a data safety and monitoring board member for Moderna. CO has received payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Gilead, ViiV, Janssen, GlaxoSmithKline, AstraZeneca, and Merck. ESD has received research support from and is a consultant for Gilead, Merck, GlaxoSmithKline, and ViiV. PES receives research support from Gilead, GlaxoSmithKline, and ViiV. He serves as a scientific advisory board member and consultant for Gilead, GlaxoSmithKline, ViiV, Merck, and Janssen; and has editorial positions with UpToDate, Medscape, New England Journal of Medicine Journal Watch, and Open Forum Infectious Diseases. BOT has served as a consultant and received honoraria from GlaxoSmithKline, ViiV, Gilead, Merck, and Johnson & Johnson. KMG received a training grant from the Agency For Health Care Research and Quality (T32HS026122).
Contributor Information
Kevin M Gibas, Division of Infectious Diseases, Vanderbilt University Medical Center, Vanderbilt University, Nashville, TN, USA.
Sean G Kelly, Division of Infectious Diseases, Vanderbilt University Medical Center, Vanderbilt University, Nashville, TN, USA.
Jose R Arribas, Infectious Diseases Unit, La Paz University Hospital, Hospital La Paz Institute for Health Research, Madrid, Spain; School of Medicine, Universidad Autónoma de Madrid, Madrid, Spain; Centro de Investigación Biomédica en Red de Enfermedades Infecciosas, Madrid, Spain.
Pedro Cahn, Fundación Huésped, Buenos Aires, Argentina.
Chloe Orkin, Department of Immunobiology, Queen Mary University of London, London, UK.
Eric S Daar, The Lundquist Institute, Harbor University of California, Los Angeles, Torrence, CA, USA.
Paul E Sax, Division of Infectious Diseases, Brigham and Women’s Hospital, Boston, MA, USA; Harvard Medical School, Harvard University, Boston, MA, USA.
Babafemi O Taiwo, Division of Infectious Diseases, Feinberg School of Medicine, Northwestern University, Chicago, IL, USA.
References
- 1.Moreno S, Perno CF, Mallon PW, et al. Two-drug vs. three-drug combinations for HIV-1: do we have enough data to make the switch? HIV Med 2019; 20 (suppl 4): 2–12. [DOI] [PubMed] [Google Scholar]
- 2.Colasanti J, Marconi VC, Taiwo B. Antiretroviral reduction: is it time to rethink the unthinkable? AIDS 2014; 28: 943–47. [DOI] [PubMed] [Google Scholar]
- 3.Work Group for HIV and Aging Consensus Project. Summary report from the Human Immunodeficiency Virus and Aging Consensus Project: treatment strategies for clinicians managing older individuals with the human immunodeficiency virus. J Am Geriatr Soc 2012; 60: 974–79. [DOI] [PubMed] [Google Scholar]
- 4.Overton ET. Metabolic complications of HIV infection and its therapies. Top Antivir Med 2014; 22: 651–54. [PMC free article] [PubMed] [Google Scholar]
- 5.Althoff KN, Stewart CN, Humes E, et al. The shifting age distribution of people with HIV using antiretroviral therapy in the United States. AIDS 2022; 36: 459–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pinola L, Lazzarin A, Antinori A, et al. Lopinavir/ritonavir plus tenofovir dual therapy versus lopinavir/ritonavir-based triple therapy in HIV-infected antiretroviral naive subjects: the Kalead Study. Journal of Antivirals and Antiretrovirals 2010; 4: 56–62. [Google Scholar]
- 7.Taiwo B, Zheng L, Gallien S, et al. Efficacy of a nucleoside-sparing regimen of darunavir/ritonavir plus raltegravir in treatment-naive HIV-1-infected patients (ACTG A5262). AIDS 2011; 25: 2113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kozal MJ, Lupo S, DeJesus E, et al. A nucleoside- and ritonavir-sparing regimen containing atazanavir plus raltegravir in antiretroviral treatment-naïve HIV-infected patients: SPARTAN study results. HIV Clin Trials 2012; 13: 119–30. [DOI] [PubMed] [Google Scholar]
- 9.Bedimo RJ, Drechsler H, Jain M, et al. The RADAR study: week 48 safety and efficacy of RAltegravir combined with boosted DARunavir compared to tenofovir/emtricitabine combined with boosted darunavir in antiretroviral-naive patients. Impact on bone health. PLoS One 2014; 9: e106221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Lunzen J, Pozniak A, Gatell JM, et al. Brief report: switch to ritonavir-boosted atazanavir plus raltegravir in virologically suppressed patients with HIV-1 infection: a randomized pilot study. J Acquir Immune Defic Syndr 2016; 71: 538–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katlama C, Assoumou L, Valantin MA, et al. Maraviroc plus raltegravir failed to maintain virological suppression in HIV-infected patients with lipohypertrophy: results from the ROCnRAL ANRS 157 study. J Antimicrob Chemother 2014; 69: 1648–52. [DOI] [PubMed] [Google Scholar]
- 12.Stellbrink HJ, Le Fevre E, Carr A, et al. Once-daily maraviroc versus tenofovir/emtricitabine each combined with darunavir/ritonavir for initial HIV-1 treatment. AIDS 2016; 30: 1229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pett SL, Amin J, Horban A, et al. Maraviroc, as a switch option, in HIV-1-infected individuals with stable, well-controlled HIV replication and R5-tropic virus on their first nucleoside/nucleotide reverse transcriptase inhibitor plus ritonavir-boosted protease inhibitor regimen: week 48 results of the randomized, multicenter MARCH study. Clin Infect Dis 2016; 63: 122–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Girard PM, Cabié A, Michelet C, et al. A randomized trial of two-drug versus three-drug tenofovir-containing maintenance regimens in virologically controlled HIV-1 patients. J Antimicrob Chemother 2009; 64: 126–34. [DOI] [PubMed] [Google Scholar]
- 15.Gagliardini R, Rossetti B, Bianco C, et al. Safety and therapeutic efficacy of the switch to maraviroc plus darunavir/ritonavir in HIV/HCV coinfected patients: initial results from GUSTA study. J Int AIDS Soc 2014; 17 (suppl 3): 19818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cahn P, Sierra Madero J, Arribas JR, et al. Three-year durable efficacy of dolutegravir plus lamivudine in antiretroviral therapy—naive adults with HIV-1 infection. AIDS 2022; 36: 39–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cahn P, Madero JS, Arribas JR, et al. Dolutegravir plus lamivudine versus dolutegravir plus tenofovir disoproxil fumarate and emtricitabine in antiretroviral-naive adults with HIV-1 infection (GEMINI-1 and GEMINI-2): week 48 results from two multicentre, double-blind, randomised, non-inferiority, phase 3 trials. Lancet 2019; 393: 143–55. [DOI] [PubMed] [Google Scholar]
- 18.Cahn P, Andrade-Villanueva J, Arribas JR, et al. Dual therapy with lopinavir and ritonavir plus lamivudine versus triple therapy with lopinavir and ritonavir plus two nucleoside reverse transcriptase inhibitors in antiretroviral-therapy-naive adults with HIV-1 infection: 48 week results of the randomised, open label, non-inferiority GARDEL trial. Lancet Infect Dis 2014; 14: 572–80. [DOI] [PubMed] [Google Scholar]
- 19.Raffi F, Babiker AG, Richert L, et al. Ritonavir-boosted darunavir combined with raltegravir or tenofovir-emtricitabine in antiretroviral-naive adults infected with HIV-1: 96 week results from the NEAT001/ANRS143 randomised non-inferiority trial. Lancet 2014; 384: 1942–51. [DOI] [PubMed] [Google Scholar]
- 20.van Wyk J, Ajana F, Bisshop F, et al. Efficacy and safety of switching to dolutegravir/lamivudine fixed-dose 2-drug regimen vs continuing a tenofovir alafenamide-based 3- or 4-drug regimen for maintenance of virologic suppression in adults living with human immunodeficiency virus type 1: phase 3, randomized, noninferiority TANGO study. Clin Infect Dis 2020; 71: 1920–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osiyemi O, De Wit S, Ajana F, et al. Efficacy and safety of switching to dolutegravir/lamivudine (DTG/3TC) versus continuing a tenofovir alafenamide-based 3- or 4-drug regimen for maintenance of virologic suppression in adults living with HIV-1: results through week 144 from the phase 3, non-inferiority TANGO randomized trial. Clin Infect Dis 2022; published online Jan 25. 10.1093/cid/ciac036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rojas J, de Lazzari E, Negredo E, et al. Efficacy and safety of switching to dolutegravir plus lamivudine versus continuing triple antiretroviral therapy in virologically suppressed adults with HIV at 48 weeks (DOLAM): a randomised non-inferiority trial. Lancet HIV 2021; 8: e463–73. [DOI] [PubMed] [Google Scholar]
- 23.Llibre J, Brites C, Cheng CY, et al. Switching to the 2-drug regimen of dolutegravir/lamivudine (DTG/3TC) fixed-dose combination (FDC) is non-inferior to continuing a 3-drug regimen through 24 weeks in a randomized clinical trial (SALSA). 2021. https://www.natap.org/2021/IAS/IAS_20.htm (accessed Jan 30, 2022).
- 24.Llibre JM, Hung CC, Brinson C, et al. Efficacy, safety, and tolerability of dolutegravir-rilpivirine for the maintenance of virological suppression in adults with HIV-1: phase 3, randomised, non-inferiority SWORD-1 and SWORD-2 studies. Lancet 2018; 391: 839–49. [DOI] [PubMed] [Google Scholar]
- 25.Aboud M, Orkin C, Podzamczer D, et al. Efficacy and safety of dolutegravir-rilpivirine for maintenance of virological suppression in adults with HIV-1: 100-week data from the randomised, open-label, phase 3 SWORD-1 and SWORD-2 studies. Lancet HIV 2019; 6: e576–87. [DOI] [PubMed] [Google Scholar]
- 26.van Wyk J, Orkin C, Rubio R, et al. Brief report: durable suppression and low rate of virologic failure 3 years after switch to dolutegravir plus rilpivirine 2-drug regimen: 148-week results from the SWORD-1 and SWORD-2 randomized clinical trials. J Acquir Immune Defic Syndr 2020; 85: 325–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arribas JR, Girard PM, Landman R, et al. Dual treatment with lopinavir-ritonavir plus lamivudine versus triple treatment with lopinavir-ritonavir plus lamivudine or emtricitabine and a second nucleos(t)ide reverse transcriptase inhibitor for maintenance of HIV-1 viral suppression (OLE): a randomised, open-label, noninferiority trial. Lancet Infect Dis 2015; 15: 785–92. [DOI] [PubMed] [Google Scholar]
- 28.Perez-Molina JA, Rubio R, Rivero A, et al. Dual treatment with atazanavir-ritonavir plus lamivudine versus triple treatment with atazanavir-ritonavir plus two nucleos(t)ides in virologically stable patients with HIV-1 (SALT): 48 week results from a randomised, open-label, non-inferiority trial. Lancet Infect Dis 2015; 15: 775–84. [DOI] [PubMed] [Google Scholar]
- 29.Perez-Molina JA, Rubio R, Rivero A, et al. Simplification to dual therapy (atazanavir/ritonavir plus lamivudine) versus standard triple therapy [atazanavir/ritonavir plus two nucleos(t)ides] in virologically stable patients on antiretroviral therapy: 96 week results from an open-label, non-inferiority, randomized clinical trial (SALT study). J Antimicrob Chemother 2017; 72: 246–53. [DOI] [PubMed] [Google Scholar]
- 30.Di Giambenedetto S, Fabbiani M, Colafigli M, et al. Safety and feasibility of treatment simplification to atazanavir/ritonavir plus lamivudine in HIV-infected patients on stable treatment with two nucleos(t)ide reverse transcriptase inhibitors plus atazanavir/ritonavir with virological suppression (Atazanavir and Lamivudine for treatment Simplification, AtLaS pilot study). J Antimicrob Chemother 2013; 68: 1364–72. [DOI] [PubMed] [Google Scholar]
- 31.Di Giambenedetto S, Fabbiani M, Quiros Roldan E, et al. Treatment simplification to atazanavir/ritonavir plus lamivudine versus maintenance of atazanavir/ritonavir plus two NRTIs in virologically suppressed HIV-1-infected patients: 48 week results from a randomized trial (ATLAS-M). J Antimicrob Chemother 2017; 72: 1163–71. [DOI] [PubMed] [Google Scholar]
- 32.Pulido F, Ribera E, Lagarde M, et al. Dual therapy with darunavir and ritonavir plus lamivudine vs triple therapy with darunavir and ritonavir plus tenofovir disoproxil fumarate and emtricitabine or abacavir and lamivudine for maintenance of human immunodeficiency virus type 1 viral suppression: randomized, open-label, noninferiority DUAL-GESIDA 8014-RIS-EST45 trial. Clin Infect Dis 2017; 65: 2112–18. [DOI] [PubMed] [Google Scholar]
- 33.Spinner CD, Kümmerle T, Schneider J, et al. Efficacy and safety of switching to dolutegravir with boosted darunavir in virologically suppressed adults with HIV-1: a randomized, open-label, multicenter, phase 3, noninferiority trial: the DUALIS study. Open Forum Infect Dis 2020; 7: ofaa356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maggiolo F, Gianotti N, Comi L, et al. Rilpivirine plus cobicistat-boosted darunavir as a two-drug switch regimen in HIV-infected, virologically suppressed subjects on steady standard three-drug therapy: a randomized, controlled, non-inferiority trial (PROBE 2). J Antimicrob Chemother 2020; 75: 1332–37. [DOI] [PubMed] [Google Scholar]
- 35.Orkin C, Oka S, Philibert P, et al. Long-acting cabotegravir plus rilpivirine for treatment in adults with HIV-1 infection: 96-week results of the randomised, open-label, phase 3 FLAIR study. Lancet HIV 2021; 8: e185–96. [DOI] [PubMed] [Google Scholar]
- 36.Bernardino JI, Mocroft A, Mallon PW, et al. Bone mineral density and inflammatory and bone biomarkers after darunavir-ritonavir combined with either raltegravir or tenofovir-emtricitabine in antiretroviral-naive adults with HIV-1: a substudy of the NEAT001/ANRS143 randomised trial. Lancet HIV 2015; 2: e464–73. [DOI] [PubMed] [Google Scholar]
- 37.Orkin C, Bernal Morell E, Tan DHS, et al. Initiation of long-acting cabotegravir plus rilpivirine as direct-to-injection or with an oral lead-in in adults with HIV-1 infection: week 124 results of the open-label phase 3 FLAIR study. Lancet HIV 2021; 8: e668–78. [DOI] [PubMed] [Google Scholar]
- 38.Swindells S, Andrade-Villanueva JF, Richmond GJ, et al. Long-acting cabotegravir and rilpivirine for maintenance of HIV-1 suppression. N Engl J Med 2020; 382: 1112–23. [DOI] [PubMed] [Google Scholar]
- 39.Overton ET, Richmond G, Rizzardini G, et al. Long-acting cabotegravir and rilpivirine dosed every 2 months in adults with HIV-1 infection (ATLAS-2M), 48-week results: a randomised, multicentre, open-label, phase 3b, non-inferiority study. Lancet 2021; 396: 1994–2005. [DOI] [PubMed] [Google Scholar]
- 40.Jaeger H, Overton ET, Richmond G, et al. Long-acting cabotegravir and rilpivirine dosed every 2 months in adults with HIV-1 infection (ATLAS-2M), 96-week results: a randomised, multicentre, open-label, phase 3b, non-inferiority study. Lancet HIV 2021; 8: e679–89. [DOI] [PubMed] [Google Scholar]
- 41.Overton ET, Richmond G, Rizzardini G, et al. Long-acting cabotegravir plus rilpivirine every 2 months: ATLAS-2M week 152 results. CROI Conference; Feb 12–16, 2022 (abstr 479). [Google Scholar]
- 42.Gillman J, Janulis P, Gulick R, et al. Comparable viral decay with initial dolutegravir plus lamivudine versus dolutegravir-based triple therapy. J Antimicrob Chemother 2019; 74: 2365–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clinical Info. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. 2022. https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-arv/whats-new-guidelines (accessed Jan 30, 2022).
- 44.European AIDS Clinical Society. Initial regimens: ART-naive adult. 2022. https://eacs.sanfordguide.com/art/initial-regimens-arv-naive-adults (accessed Jan 30, 2022).
- 45.WHO. Consolidated guidelines on HIV prevention, testing, treatment, service delivery and monitoring: recommendations for a public health approach. https://www.who.int/publications-detail-redirect/9789240031593 (accessed Feb 3, 2022). [PubMed]
- 46.Saag MS, Gandhi RT, Hoy JF, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2020 recommendations of the International Antiviral Society-USA panel. JAMA 2020; 324: 1651–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Taiwo BO, Zheng L, Stefanescu A, et al. ACTG A5353: a pilot study of dolutegravir plus lamivudine for initial treatment of human immunodeficiency virus-1 (HIV-1)-infected participants with HIV-1 RNA <500000 copies/mL. Clin Infect Dis 2018; 66: 1689–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eron J, Hung CC, Baril JG, et al. Brief report: virologic response by baseline viral load with dolutegravir plus lamivudine vs dolutegravir plus tenofovir disoproxil fumarate/emtricitabine: pooled analysis. J Acquir Immune Defic Syndr 2020; 84: 60–65. [DOI] [PubMed] [Google Scholar]
- 49.No authors listed. Dovato package insert. https://gskpro.com/content/dam/global/hcpportal/en_US/Prescribing_Information/Dovato/pdf/DOVATO-PI-PIL.PDF (accessed March 11, 2022).
- 50.European Medicines Agency. Dovato. 2019. https://www.ema.europa.eu/en/medicines/human/EPAR/dovato (accessed Feb 3, 2022).
- 51.Madeddu G, De Vito A, Cozzi-Lepri A, et al. Time spent with HIV-RNA ≤ 200 copies/ml in a cohort of people with HIV during the U=U era. AIDS 2021; 35: 1103–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Margolis DA, Brinson CC, Smith GHR, et al. Cabotegravir plus rilpivirine, once a day, after induction with cabotegravir plus nucleoside reverse transcriptase inhibitors in antiretroviral-naive adults with HIV-1 infection (LATTE): a randomised, phase 2b, dose-ranging trial. Lancet Infect Dis 2015; 15: 1145–55. [DOI] [PubMed] [Google Scholar]
- 53.Margolis DA, Gonzalez-Garcia J, Stellbrink HJ, et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet 2017; 390: 1499–510. [DOI] [PubMed] [Google Scholar]
- 54.Orkin C, Arasteh K, Górgolas Hernández-Mora M, et al. Long-acting cabotegravir and rilpivirine after oral induction for HIV-1 infection. N Engl J Med 2020; 382: 1124–35. [DOI] [PubMed] [Google Scholar]
- 55.Smith GHR, Henry WK, Podzamczer D, et al. Efficacy, safety, and durability of long-acting cabotegravir and rilpivirine in adults with human immunodeficiency virus type 1 infection: 5-year results from the LATTE-2 Study. Open Forum Infect Dis 2021; 8: ofab439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cutrell AG, Schapiro JM, Perno CF, et al. Exploring predictors of HIV-1 virologic failure to long-acting cabotegravir and rilpivirine: a multivariable analysis. AIDS 2021; 35: 1333–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rial-Crestelo D, de Miguel R, Montejano R, et al. Long-term efficacy of dolutegravir plus lamivudine for maintenance of HIV viral suppression in adults with and without historical resistance to lamivudine: week 96 results of ART-PRO pilot study. J Antimicrob Chemother 2021; 76: 738–42. [DOI] [PubMed] [Google Scholar]
- 58.Hocqueloux L, Allavena C, Sécher S, et al. Archived mutation M184V does not increase virologic failure during maintenance therapy with dolutegravir plus lamivudine in the French DAT’AIDS cohort. 2021. https://eacs2021.abstractserver.com/program/#/details/presentations/382 (accessed Feb 3, 2022).
- 59.Ford N, Migone C, Calmy A, et al. Benefits and risks of rapid initiation of antiretroviral therapy. AIDS 2018; 32: 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rolle CP, Berhe M, Singh T, et al. Dolutegravir/lamivudine as a first-line regimen in a test-and-treat setting for newly diagnosed people living with HIV. AIDS 2021; 35: 1957–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Clinical Info. Recommendations for the use of antiretroviral drugs during pregnancy and interventions to reduce perinatal HIV transmission in the United States. 2022. https://clinicalinfo.hiv.gov/en/guidelines/perinatal/whats-new-guidelines (accessed July 1, 2022).
- 62.Bolton Moore C, Capparelli E, Calabrese K, et al. Safety and PK of long-acting cabotegravir and rilpivirine in adolescents. CROI Conference; Feb 12–16, 2022 (abstr 738). [Google Scholar]
- 63.Compagnucci A, Chan M, Saïdi Y, et al. Once-daily integrase inhibitor (INSTI) with boosted darunavir is non-inferior to standard of care in virologically suppressed children, week 48 results of the SMILE PENTA-17 TRIAL. 2021. https://theprogramme.ias2021.org/Abstract/Abstract/1079 (accessed July 18, 2022). [DOI] [PMC free article] [PubMed]
- 64.Armenia D, Bouba Y, Gagliardini R, et al. Virological response and resistance profile in highly treatment-experienced HIV-1-infected patients switching to dolutegravir plus boosted darunavir in clinical practice. HIV Med 2021; 22: 519–25. [DOI] [PubMed] [Google Scholar]
- 65.Overton ET. Metabolic complications of HIV infection and its therapies. Top Antivir Med 2014; 22: 651–54. [PMC free article] [PubMed] [Google Scholar]
- 66.Quercia R, Roberts J, Martin-Carpenter L, Zala C. Comparative changes of lipid levels in treatment-naive, HIV-1-infected adults treated with dolutegravir vs efavirenz, raltegravir, and ritonavir-boosted darunavir-based regimens over 48 weeks. Clin Drug Investig 2015; 35: 211–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crane HM, Grunfeld C, Willig JH, et al. Impact of NRTIs on lipid levels among a large HIV-infected cohort initiating antiretroviral therapy in clinical care. AIDS 2011; 25: 185–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dorjee K, Choden T, Baxi SM, Steinmaus C, Reingold AL. Risk of cardiovascular disease associated with exposure to abacavir among individuals with HIV: a systematic review and meta-analyses of results from 17 epidemiologic studies. Int J Antimicrob Agents 2018; 52: 541–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sax PE, Erlandson KM, Lake JE, et al. Weight gain following initiation of antiretroviral therapy: risk factors in randomized comparative clinical trials. Clin Infect Dis 2020; 71: 1379–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shah S, Pilkington V, Hill A. Is tenofovir disoproxil fumarate associated with weight loss? AIDS 2021; 35 (suppl 2): S189–95. [DOI] [PubMed] [Google Scholar]
- 71.Lake JE, Trevillyan J. Impact of integrase inhibitors and tenofovir alafenamide on weight gain in people with HIV. Curr Opin HIV AIDS 2021; 16: 148–51. [DOI] [PubMed] [Google Scholar]
- 72.Erlandson KM, Carter CC, Melbourne K, et al. Weight change following antiretroviral therapy switch in people with viral suppression: pooled data from randomized clinical trials. Clin Infect Dis 2021; 73: 1440–51. [DOI] [PubMed] [Google Scholar]
- 73.Mallon PW, Brunet L, Hsu RK, et al. Weight gain before and after switch from TDF to TAF in a US cohort study. J Int AIDS Soc 2021; 24: e25702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity 2013; 39: 633–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bernardino JI, Mocroft A, Mallon PW, et al. Bone mineral density and inflammatory and bone biomarkers after darunavir-ritonavir combined with either raltegravir or tenofovir-emtricitabine in antiretroviral-naive adults with HIV-1: a substudy of the NEAT001/ANRS143 randomised trial. Lancet HIV 2015; 2: e464–73. [DOI] [PubMed] [Google Scholar]
- 76.Belmonti S, Lombardi F, Quiros-Roldan E, et al. Systemic inflammation markers after simplification to atazanavir/ritonavir plus lamivudine in virologically suppressed HIV-1-infected patients: ATLAS-M substudy. J Antimicrob Chemother 2018; 73: 1949–54. [DOI] [PubMed] [Google Scholar]
- 77.Katlama C, Assoumou L, Valantin MA, et al. Dual therapy combining raltegravir with etravirine maintains a high level of viral suppression over 96 weeks in long-term experienced HIV-infected individuals over 45 years on a PI-based regimen: results from the Phase II ANRS 163 ETRAL study. J Antimicrob Chemother 2019; 74: 2742–51. [DOI] [PubMed] [Google Scholar]
- 78.Tan DHS, Rolon MJ, Figueroa MI, et al. Inflammatory biomarker levels over 48 weeks with dual vs triple lopinavir/ritonavir-based therapy: substudy of a randomized trial. PLoS One 2019; 14: e0221653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hernandez B, Kahl L, Matthews J, et al. Bone, renal, and inflammatory biomarkers up to week 100 post switch to DTG plus RPV: the SWORD-1 and SWORD-2 studies. HIV & Hepatitis Nordic Conference; Sept 26–28, 2018 (abstr P9). [Google Scholar]
- 80.Kahl LP. Changes in inflammatory biomarkers in SWORD-1 and SWORD-2 studies—Authors’ reply. Lancet HIV 2020; 7: e158–59. [DOI] [PubMed] [Google Scholar]
- 81.Llibre JM, Cahn PE, Lo J, et al. Changes in inflammatory and atherogenesis biomarkers with the 2-drug regimen dolutegravir plus lamivudine in antiretroviral therapy-experienced, virologically suppressed people with HIV-1: a systematic literature review. Open Forum Infect Dis 2022; 9: ofac068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Peng X, Isnard S, Lin J, Fombuena B, Royston L, Routy JP. HIV compartmentalization in male genital tract: relevance for viral eradication. Infect Dis Immun. 2021; 1: 86–92. [Google Scholar]
- 83.Wong JK, Yukl SA. Tissue reservoirs of HIV. Curr Opin HIV AIDS 2016; 11: 362–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tiraboschi JM, Rojas J, Zetterberg H, et al. No changes in human immunodeficiency virus (HIV) suppression and inflammatory markers in cerebrospinal fluid in patients randomly switched to dolutegravir plus lamivudine (Spanish HIV/AIDS Research Network, PreEC/RIS 62). J Infect Dis 2021; 223: 1928–33. [DOI] [PubMed] [Google Scholar]
- 85.Trunfio M, Rugge W, Mighetto L, et al. Dual antiretroviral therapies are effective and safe regimens in the central nervous system of neurologically symptomatic people living with HIV. AIDS 2020; 34: 1899–906. [DOI] [PubMed] [Google Scholar]
- 86.Imaz A, Niubó J, Amara A, et al. Cerebrospinal fluid drug concentrations and viral suppression in HIV-1-infected patients receiving ritonavir-boosted atazanavir plus lamivudine dual antiretroviral therapy (Spanish HIV/AIDS Research Network, PreEC/RIS 39). J Neurovirol 2018; 24: 391–97. [DOI] [PubMed] [Google Scholar]
- 87.Pérez-Valero I, Pasquau J, Rubio R, et al. Neurocognitive safety after 96 weeks on dual therapy with atazanavir/ritonavir plus lamivudine: results of the neurocognitive substudy of the SALT randomized clinical trial. J Antimicrob Chemother 2018; 73: 2444–51. [DOI] [PubMed] [Google Scholar]
- 88.Charpentier C, Peytavin G, Raffi F, et al. Pharmacovirological analyses of blood and male genital compartment in patients receiving dolutegravir plus lamivudine dual therapy as a switch strategy (ANRS 167 LAMIDOL trial). J Antimicrob Chemother 2020; 75: 1611–17. [DOI] [PubMed] [Google Scholar]
- 89.Gianella S, Marconi VC, Berzins B, et al. Genital HIV-1 shedding with dolutegravir (DTG) plus lamivudine (3TC) dual therapy. J Acquir Immune Defic Syndr 2018; 79: e112–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Underwood M, Urbaityte R, Wang R, et al. DTG+3TC in GEMINI-1&−2: HIV-1 replication at <50 c/mL and VL blips through 144 weeks. 2021. https://theprogramme.ias2021.org/Abstract/Abstract/1447 (accessed July 14, 2022).
- 91.Lombardi F, Belmonti S, Quiros-Roldan E, et al. Evolution of blood-associated HIV-1 DNA levels after 48 weeks of switching to atazanavir/ritonavir plus lamivudine dual therapy versus continuing triple therapy in the randomized AtLaS-M trial. J Antimicrob Chemother 2017; 72: 2055–59. [DOI] [PubMed] [Google Scholar]
- 92.Girouard MP, Sax PE, Parker RA, et al. The cost-effectiveness and budget impact of 2-drug dolutegravir-lamivudine regimens for the treatment of HIV infection in the United States. Clin Infect Dis 2016; 62: 784–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Butler K, Anderson SJ, Hayward O, et al. Cost-effectiveness and budget impact of dolutegravir/lamivudine for treatment of human immunodeficiency virus (HIV-1) infection in the United States. J Manag Care Spec Pharm 2021; 27: 891–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hidalgo-Tenorio C, Cortés LL, Gutiérrez A, et al. DOLAMA study: effectiveness, safety and pharmacoeconomic analysis of dual therapy with dolutegravir and lamivudine in virologically suppressed HIV-1 patients. Medicine (Baltimore) 2019; 98: e16813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Anderson SJ, Hsu CY, Ou HT, Ko NY, Yang CT, Lopes S. Cost-effectiveness of Juluca for human immunodeficiency virus infection treatment in virologically suppressed adults in Taiwan. Value Health Reg Issues 2021; 24: 216–23. [DOI] [PubMed] [Google Scholar]
- 96.Restelli U, Fabbiani M, Di Giambenedetto S, Nappi C, Croce D. Budget impact analysis of the simplification to atazanavir plus ritonavir plus lamivudine dual therapy of HIV-positive patients receiving atazanavir-based triple therapies in Italy starting from data of the Atlas-M trial. Clinicoecon Outcomes Res 2017; 9: 173–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Restelli U, Fabbiani M, Di Giambenedetto S, Nappi C, Croce D. Update of the budget impact analysis of the simplification to atazanavir plus ritonavir plus lamivudine dual therapy of HIV-positive patients receiving atazanavir-based triple therapies in Italy starting from data of the Atlas-M trial. Clinicoecon Outcomes Res 2017; 9: 569–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gupta S, Sims J, Brinson C, et al. Lenacapavir as part of a combination regimen in treatment naive PWH: week 54 results. CROI Conference; Feb 12–16, 2022 (abstr 138). [Google Scholar]
- 99.Sunlenca EMA. Pending EC decision. 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/sunlenca
- 100.Merck. Merck to initiate new phase 3 clinical program with lower dose of daily oral islatravir in combination with doravirine for treatment of people with HIV-1 infection. 2022. https://www.merck.com/news/merck-to-initiate-new-phase-3-clinical-program-with-lower-dose-of-daily-oral-islatravir-in-combination-with-doravirine-for-treatment-of-people-with-hiv-1-infection/ (accessed Oct 9, 2022).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


