Abstract
Methadone and buprenorphine have pharmacologic properties that are concerning for a high risk of drug–drug interactions (DDIs). We performed high-throughput screening for clinically relevant DDIs with methadone or buprenorphine by combining pharmacoepidemiologic and pharmacokinetic approaches. We conducted pharmacoepidemiologic screening via a series of self-controlled case series studies (SCCS) in Optum claims data from 2000 to 2019. We included persons 18 years or older who experienced an outcome of interest during target drug treatment. Exposures were all overlapping medications (i.e., the candidate precipitants) during target drug treatment. Outcomes were opioid overdose, non-overdose adverse effects, and cardiac arrest. We used conditional Poisson regression to calculate rate ratios, accounting for multiple comparisons with semi-Bayes shrinkage. We explored the impact of key study design choices in analyses that varied the exposure definitions of the target drugs and the candidate precipitant drugs. Pharmacokinetic screening was conducted by incorporating published data on CYP enzyme metabolism into an equation-based static model. In SCCS analysis, 1,432 events were included from 248,069 new users of methadone or buprenorphine. In the primary analysis, statistically significant DDIs included gabapentinoids with either methadone or buprenorphine; baclofen with methadone; and benzodiazepines with methadone. In sensitivity analysis, additional statistically significant DDIs included methocarbamol, quetiapine, or simvastatin with methadone. Pharmacokinetic screening identified two moderate-to-strong potential DDIs (clonidine and fluconazole with buprenorphine). The combination of clonidine and buprenorphine was also associated with a significantly increased risk of opioid overdose in pharmacoepidemiologic screening. These DDI signals may be the most important targets for future confirmation studies.
Medication-assisted treatment (MAT) with either methadone or buprenorphine is a cornerstone of the management of opioid use disorder.1,2 As access to MAT has expanded,2 the use of methadone and buprenorphine has increased substantially.3 Both drugs also have an increasing role in the management of pain.4,5 Complex comorbid illness and polypharmacy are common in patients treated with methadone and buprenorphine,6,7 which increases the risk for drug–drug interactions (DDIs).
Methadone and buprenorphine have pharmacologic properties that are concerning for a high risk of adverse interactions with many commonly used drugs.8 These properties include metabolism through multiple cytochrome (CYP) P450 enzymes,9,10 transportation by P-glycoprotein (P-gp),11 prolongation of the electrocardiographic QT interval,12-14 and potentially additive or synergistic central nervous system (CNS) depression.15,16 Based on these properties, Micromedex lists over 700 potential DDIs that have been postulated for the two drugs.17 Nevertheless, it is likely that some important DDIs have yet to be identified, whereas many of the currently listed interactions are likely to be clinically irrelevant. Given the potential for serious DDI mediated harm, the paucity of studies8,18,19 characterizing the real-world health effects of DDIs with methadone or buprenorphine is a major concern.
To address this problem, we conducted high-throughput screening for interactions among drug combinations involving methadone or buprenorphine using two distinct, yet complementary methods: (i) pharmacoepidemiologic outcome studies in a national claims database; and (ii) pharmacokinetic simulation. Generation of DDI signals using simulation leverages existing knowledge about drug metabolism as a theoretical basis for predicting high-risk DDIs. Pharmacoepidemiologic screening avoids relying exclusively on existing mechanistic knowledge, which is often incomplete. Integration of these methods broadens the scope of investigation, whereas contrasting the results of the two approaches can enhance inferences on causality. Our objective was to identify and prioritize DDI signals for further investigation.
METHODS
Overview of pharmacoepidemiologic screening
We conducted high-throughput pharmacoepidemiologic screening in a large US commercial health insurance database using the self-controlled case series (SCCS), a rigorous, case-only epidemiologic study design that uses each person as his or her own control (Figure 1).20 SCCS analysis involves three primary steps: (i) identifying individuals who experienced an outcome event of interest during specified windows of time termed “observation periods”; (ii) classifying person-time during observation periods with regard to an exposure(s) of interest; and (iii) comparing the frequency of outcome events that occur during exposed and unexposed person-time. The SCCS design is an attractive approach because exposure contrasts are made within individuals, which inherently controls confounding from both measured and unmeasured factors that do not change over the observation period (e.g., sex, genetics, and chronic diseases). Control of confounding from time-varying factors can be achieved through statistical adjustment.
Figure 1.
Self-controlled case series study design for DDI screening. DDI, drug–drug interactions.
We applied the SCCS method for DDI screening by first defining observation periods of continuous treatment with either methadone or buprenorphine, the target medications. Within these observation periods, we identified all medications that had overlapping exposure with the target medications. Each overlapping medication was then examined for its potential to precipitate an adverse event in SCCS analysis. Separate confounder-adjusted SCCS studies were performed for the outcomes of opioid overdose, non-overdose adverse events, and cardiac arrest, repeated for each target drug+ candidate precipitant drug combination. We used the standard SCCS approach, which includes person-time before and after the occurrence of outcome events.20
Defining observation periods
We queried dispensed prescription claims and Healthcare Common Procedure Coding System (HCPCS) codes from Optum’s de-identified Clinformatics Data Mart Database from May 1, 2000, through June 30, 2019. We identified outpatient episodes of continuous treatment with the target drugs, which served as the observation periods for the SCCS analyses (Section M1 in Supplementary Material). We excluded in-hospital days because in-hospital drug use is poorly documented in claims data. We included HCPCS codes to identify target drug episodes that were used for MAT (Section M2 in Supplementary Material). In defining continuous exposure, we allowed a “grace period” between consecutive dispensed prescription records and at the end of a target drug episode to account for variable adherence. This was calculated as one fifth of the prescription days’ supply (assuming 80% adherence). We used fixed grace periods for exposure episodes that were defined using HCPCS codes because such codes do not have the days’ supply information. The duration of the fixed grace periods was tailored to each target drug based on dosing frequency, duration of effect, and administration route (Section M2 in Supplementary Material).
Eligible observation periods were restricted to episodes of “new use” of the target drugs. New use was defined as initiation of a target drug after a baseline period of at least 183 days of continuous health plan enrollment (allowing a maximum gap of 45 days) that was free from dispensed prescriptions for either target drug that had a prescription fill date that fell within the baseline period. Because SCCS analysis is a case-only approach, we further restricted observation periods to those during which an outcome of interest occurred. We excluded observation periods that occurred in individuals younger than 18 years, those with a dispensed prescription for naltrexone during the 183-day baseline period, and those where both target drugs were initiated on the same day.
Observation periods were censored upon the earliest of: (i) lapsed treatment with the target drug (allowing for the grace period); (ii) a dispensed prescription for a therapeutic alternative (i.e., buprenorphine in methadone users, or vice versa; or naltrexone in users of either target drug); (iii) a dispensed prescription for a target drug via a route other than oral for methadone or oral/transdermal for buprenorphine; (iv) health plan disenrollment (permitting a 45-day maximum enrollment gap); or (v) the end of the study dataset. Individuals were allowed to contribute multiple observation periods if each period met all eligibility criteria.
Defining exposure to candidate precipitant drugs
We used pharmacy claim dates and days’ supply values to identify episodes of continuous exposure to any orally administered medication prescribed during the observation periods (i.e., the precipitant drugs of interest). To minimize exposure misclassification, we did not allow grace periods. Using the enumerated episodes of precipitant exposure, we classified each day of observation as either precipitant-exposed or precipitant-unexposed (Figure 1).
Outcome definitions
Outcomes were: (i) opioid overdose, defined as outpatient-originating opioid overdose events resulting in emergency department or hospital presentation; (ii) non-overdose opioid adverse drug effects (ADEs), defined as outpatient-originating opioid ADEs resulting in emergency department or hospital presentation that did not involve overdose; and (iii) cardiac arrest, defined as outpatient-originating sudden cardiac arrest or ventricular arrhythmia events that did not involve overdose. Each outcome was operationally defined using validated claims-based algorithms (Section M3 in Supplementary Material).21-23
Statistical analysis
We used Poisson regression models conditioned on the individual to estimate rate ratios and 95% confidence intervals (CIs).20 Separate models were estimated for each target drug-precipitant outcome combination. The unit of analysis was the person-day and the independent variables included a subject identifier, an indicator variable for precipitant exposure on each day, and the time-varying covariates. We do not report rate ratio estimates from models that exhibited statistical instability (Section M4 in Supplementary Material). Models were adjusted for two key time-varying covariates: target drug average daily dose based on the most recent dispensed prescription; and prior occurrence of the outcome of interest. We used semi-Bayes shrinkage to minimize the risk of false-positive results (Section M4 in Supplementary Material).24
We conducted several sensitivity analyses. First, we repeated screening after excluding HCPCS defined episodes (to examine whether results varied when dispensed prescription claims vs. HCPCS code were used defined exposure). Second, we repeated screening after applying a 2-day lag period to all precipitant drugs (to allow for potential delayed onset or offset of precipitant drug effects). Third, we repeated screening analyses after excluding the grace period when defining target drug exposure episodes (to examine the impact of our grace period definition). The University of Pennsylvania’s institutional review board approved this research (#832191).
Pharmacokinetic screening: Base model
Potential pharmacokinetic DDIs were screened using a static pharmacokinetic model.25 The model was used to estimate the area under the concentration–time curve ratios (AUCRs), which compare target drug AUC in the presence of precipitant drug to the target drug AUC in the absence of precipitant drug (Eq. 1):
| (1) |
where fe represents the fraction of the target drug that is excreted unchanged in the urine, [I]unbound is the unbound plasma concentration of the precipitant drug, Ki,j is the inhibition constant of the precipitant drug for the jth inhibited CYP P450 pathway, and fm, CYPj is the fraction of the target drug’s hepatic metabolism through the jth inhibited P450 pathway.
Pharmacokinetic screening: Input parameters
The [I]unbound and Ki,j of the precipitant drugs were collected from DrugBank26 and the published literature. We estimated [I]unbound as Cmax × fu, where Cmax is the maximum concentration, and fu is the fraction of unbound drug in plasma. The pharmacokinetic parameters of the target drugs are shown in Table 1. Target drug fe values were collected from the Biopharmaceutics Drug Disposition Classification System.27 The fraction of hepatic metabolism through CYP pathways (fm) was estimated28 with Eq. 2:
| (2) |
where inhibitionj refers to the percentage of inhibition for the jth enzyme.
Table 1.
Key parameters for methadone and buprenorphine in pharmacokinetic DDI screening
CYP pathways for buprenorphine were inferred from in vitro drug inhibition data.10 Obtaining fm estimates for methadone is complicated by discordance between in vitro data and in vivo studies regarding relative contribution of CYP3A4 vs. CYP2B6.29 In vitro data suggest a prominent role for CYP3A4,9 whereas in vivo studies show at most a minor role for CYP3A4 in the hepatic metabolism of methadone.29 Consequently, fm cannot be extrapolated from in vitro data. We thus developed a method to estimate fm from in vivo DDI studies. We applied a multiple linear regression model to log-transformed AUCR estimates obtained from in vivo DDI studies as summarized in a recent US Food and Drug Administration (FDA) analysis.30 We created a dataset (Table S1) that included the estimated AUCR (log transformed for analysis), the concomitantly administered drug, and indicator variables for the drugs effect on CYP enzymes (coded as one for inhibitors, and negative one for inducers). Assuming that the variance in the reported AUCRs is explained by the contributions of CYP enzyme metabolizing pathways, fm could be estimated by each indicator variable’s contribution to total variance. Consistent with FDA guidelines for evaluating DDIs,31 predicted AUCR values < 1.25 were considered negligible, predicted AUCR values from 1.25 to < 2 were considered weak interactions, predicted AUCR values from 2 to < 5 were considered moderate interactions, and predicted AUCR values of 5 or more were considered strong interactions.
RESULTS
Pharmacoepidemiologic screening: Observation period characteristics
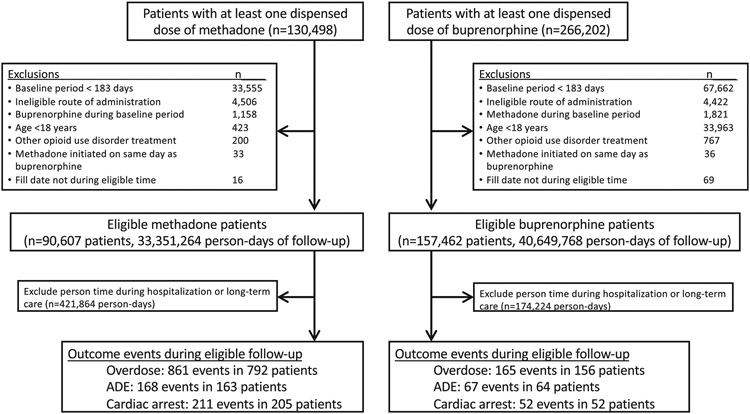
The selection of patients is depicted in Figure 2. We identified 396,700 individuals who had a record of at least one dispensed prescription of a target drug during the study period. From this population, we identified 248,069 individuals who met study criteria, of whom 1,432 had at least one outcome event during an observation period (methadone, n = 1,160; and buprenorphine, n = 272), with the most common event being overdose. Persons experiencing an outcome event had a median age of around 60 years, were predominantly White, and showed a roughly even split of men and women (Table 2). Included patients were drawn from all regions of the United States, with the South Atlantic region being most commonly represented. Opioid use disorder was documented in 16% of methadone patients and 54% of buprenorphine patients.
Figure 2.
Selection of patients.
Table 2.
Observation period characteristics
| Methadone | Buprenorphine | |||||
|---|---|---|---|---|---|---|
| Overdose | Non-overdose adverse drug event |
Cardiac arrest | Overdose | Non-overdose adverse drug event |
Cardiac arrest | |
| Persons | 792 | 163 | 205 | 156 | 64 | 52 |
| Days of observation | ||||||
| Total days | 234,531 | 53,539 | 49,532 | 26,336 | 14,712 | 9,620 |
| Median (IQR) days per person | 123.5 (37.0–387.5) | 167.0 (37.0–448.0) | 96.0 (37.0–299.0) | 87.5 (35.0–221.5) | 128.0 (37.0–282.0) | 124.5 (43.0–198.5) |
| Number of events | 861 | 168 | 211 | 165 | 67 | 52 |
| Demographics | ||||||
| Age, years, median (IQR) | 57.4 (49.6–65.2) | 62.5 (55.5–71.0) | 60.1 (52.8–67.3) | 55.7 (44.3–64.8) | 65.1 (57.3–70.7) | 62.0 (52.8–66.9) |
| Female, n (%) | 430 (54.3) | 97 (59.5) | 89 (43.4) | 73 (46.8) | 39 (60.9) | 18 (34.6) |
| Race, n (%) | ||||||
| White | 565 (71.3) | 121 (74.2) | 138 (67.3) | 111 (71.2) | 49 (76.6) | 37 (71.2) |
| African American | 76 (9.6) | 21 (12.9) | 27 (13.2) | 14 (9.0) | 9 (14.1) | 4 (7.7) |
| Hispanic | 36 (4.5) | 6 (3.7) | 5 (2.4) | 11 (7.1) | 0 (0.0) | 3 (5.8) |
| Asian | 6 (0.8) | 1 (0.6) | 2 (1.0) | 1 (0.6) | 0 (0.0) | 1 (1.9) |
| Unknown | 109 (13.8) | 14 (8.6) | 33 (16.1) | 19 (12.2) | 6 (9.4) | 7 (13.5) |
| Geographic region, n (%) | ||||||
| New England | 21 (2.7) | 9 (5.5) | 4 (2.0) | 4 (2.6) | 3 (4.7) | 3 (5.8) |
| Middle Atlantic | 31 (3.9) | 12 (7.4) | 16 (7.8) | 10 (6.4) | 2 (3.1) | 4 (7.7) |
| East North Central | 83 (10.5) | 31 (19.0) | 32 (15.6) | 17 (10.9) | 7 (10.9) | 5 (9.6) |
| West North Central | 56 (7.1) | 16 (9.8) | 22 (10.7) | 9 (5.8) | 2 (3.1) | 5 (9.6) |
| South Atlantic | 239 (30.2) | 38 (23.3) | 76 (37.1) | 53 (34.0) | 24 (37.5) | 19 (36.5) |
| East South Central | 61 (7.7) | 6 (3.7) | 15 (7.3) | 8 (5.1) | 4 (6.3) | 6 (11.5) |
| West South Central | 72 (9.1) | 9 (5.5) | 9 (4.4) | 22 (14.1) | 6 (9.4) | 2 (3.8) |
| Mountain | 118 (14.9) | 22 (13.5) | 13 (6.3) | 18 (11.5) | 9 (14.1) | 2 (3.8) |
| Pacific | 108 (13.6) | 20 (12.3) | 18 (8.8) | 15 (9.6) | 7 (10.9) | 6 (11.5) |
| Unknown | 3 (0.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| History of opioid use disorder, n (%) | 121 (15.3) | 42 (25.8) | 26 (12.7) | 86 (55.1) | 31 (48.4) | 29 (55.8) |
| Time-varying covariates | ||||||
| Target drug average daily dose (mg), median (IQR) | 40.0 (30.0–80.0) | 40.0 (20.0–80.0) | 40.0 (30.0–80.0) | 16.0 (2.9–20.0) | 16.0 (8.0–24.0) | 16.0 (8.0–24.0) |
| Ever prior occurrence of outcome, n (%) | 79 (9.6) | 8 (4.9) | 3 (1.4) | 26 (16.3) | 5 (7.6) | 1 (1.9) |
IQR, interquartile range.
Pharmacoepidemiologic screening: SCCS results
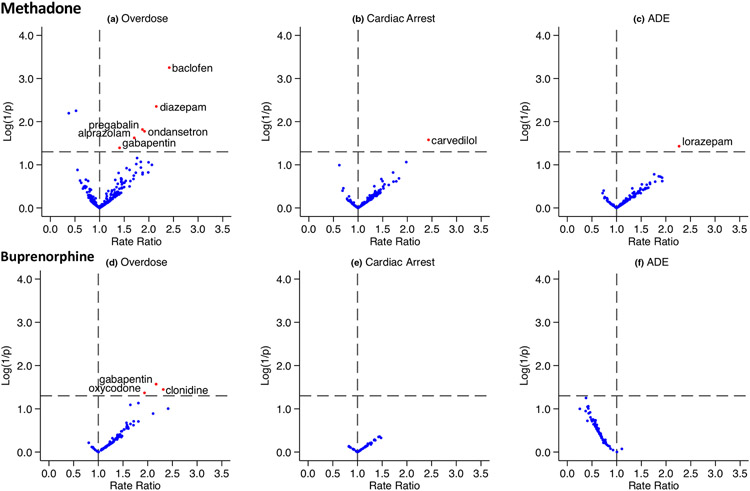
We identified 413 drugs that had overlapping exposure with methadone and 247 drugs that had overlapping exposure with buprenorphine. After restricting to the set of candidate precipitants for which at least 5 persons were co-exposed, we examined 265 methadone-precipitant pairs in 547 SCCS studies; and 116 buprenorphine-precipitant pairs in 229 SCCS studies (the number of studies is greater than the number of drug pairs because each pair could be examined for association with up to 3 outcomes). The semi-Bayes shrunk the adjusted rate ratios from the primary analysis for all target drug-precipitant-outcome combinations are summarized in Figure 3 and Table S2. Across all SCCS studies, 11 of 776 (1.4%) demonstrated statistically significantly elevated adjusted rate ratios after semi-Bayes shrinkage (Table 3).
Figure 3.
Summary of pharmacoepidemiologic screening study results. Each panel depicts a volcano plot, with the x-axis representing the semi-Bayes shrunk adjusted rate ratio for target drug-precipitant pairs, and the y-axis representing the log (1/P value) for the semi-Bayes shrunk the adjusted rate ratio. Data points in the upper right quadrant represent statistically significant elevated rate ratios and are highlighted with red dots. (a) There were 258 candidate precipitants; (b) 147 candidate precipitants; (c) 142 candidate precipitants; (d) 104 candidate precipitants; (e) 54 candidate precipitants; and (f) 71 candidate precipitants. ADE, adverse drug events.
Table 3.
Summary of statistically significant pharmacoepidemiologic screening signals
| Precipitant | Semi-Bayes shrunk adjusted rate ratio | |
|---|---|---|
| Methadone | Buprenorphine | |
| Overdose | ||
| Gabapentin | 1.41 (1.01–1.95) | 2.17 (1.09–4.31) |
| Pregabalin | 1.87 (1.13–3.10) | – |
| Baclofen | 2.42 (1.46–3.99) | – |
| Clonidine | – | 2.32 (1.06–5.07) |
| Diazepam | 2.15 (1.27–3.65) | – |
| Alprazolam | 1.70 (1.07–2.71) | – |
| Oxycodone | – | 1.94 (1.02–3.67) |
| Ondansetron | 1.91 (1.12–3.25) | – |
| Non-overdose adverse drug events | ||
| Lorazepam | 2.27 (1.05, 4.91) | – |
| Cardiac arrest | ||
| Carvedilol | 2.43 (1.11–5.32) | – |
Results of sensitivity analyses are shown in Table S3. Repeating analysis after excluding HCPCS defined target drug episodes produced nearly identical results compared with the primary analysis. Similarly, analysis of lagged precipitant exposure produced consistent results: 10 out of 11 signals identified in the primary analysis remained significant, whereas one signal (alprazolam and methadone overdose) was no longer significant. There were also three new signals in the lagged precipitant analysis: methocarbamol and metoprolol were associated with increased overdose risk in methadone users; and morphine was associated with overdose risk in buprenorphine users. In contrast, the zero-grace period analysis showed greater discrepancy with the primary analysis: 4 out of 11 primary analysis signals remained significant in the zero-grace analysis, and there were 5 new signals: quetiapine, temazepam, simvastatin, and ibuprofen were associated with opioid overdose in methadone users, and acetaminophen was associated with overdose risk in buprenorphine users.
Pharmacokinetic screening
We identified requisite data on precipitant drug pharmacokinetic parameters to screen 43 buprenorphine-precipitant pairs and 91 methadone-precipitant pairs. One drug pair was identified as having potentially weak interaction, one drug pair as having potentially moderate interaction, and one drug pair that had an AUCR >5 was identified as having a potentially strong interaction (Table 4). This latter AUCR value was predicted for concomitant administration of buprenorphine and clonidine, a drug pair that was also associated with a significantly elevated risk of opioid overdose in the SCCS screening studies. The AUCR values of the remaining 131 drug pairs were negligible (Table S2).
Table 4.
Potential DDI signals identified in pharmacokinetic screening
| Precipitant | Predicted AUCR | |
|---|---|---|
| Methadone | Buprenorphine | |
| Clonidine | – | 6.82 (strong) |
| Fluconazole | – | 2.66 (moderate) |
| Metronidazole | – | 1.29 (weak) |
AUCRs were estimated with Eq. 1 described in the methods section, under “Pharmacokinetic screening methods: base model”. Predicted AUCR values < 1.25 were considered negligible, predicted AUCR values from 1.25 to < 2 were considered weak interactions, predicted AUCR values from 2 to < 5 were considered moderate interactions, and predicted AUCR values of 5 or more were considered strong interactions.31
AUCR, area under the concentration–time curve ratios; DDI, drug–drug interaction.
DISCUSSION
We performed high-throughput DDI screening of drugs administered concomitantly with methadone or buprenorphine using two distinct, yet complimentary approaches. First, we conducted pharmacoepidemiologic screening with a series of SCCS studies, varying key study design choices in sensitivity analyses to maximize signal detection. Second, we conducted pharmacokinetic screening with a series of simulations that leveraged available knowledge of target drug metabolism. In the primary pharmacoepidemiologic analysis, we identified several potential DDIs that were associated with increased opioid overdose through potential pharmacodynamic mechanisms: gabapentinoids combined with either methadone or buprenorphine; baclofen combined with methadone; and benzodiazepines combined with methadone. Additional potential pharmacodynamic interactions were identified in sensitivity analyses: methocarbamol, temazepam, and quetiapine combined with methadone; and morphine combined with buprenorphine. In the pharmacokinetic screening analysis, we identified two moderate to strong potential DDIs mediated through CYP-enzyme inhibition (clonidine with buprenorphine; and fluconazole with buprenorphine). The combination of clonidine and buprenorphine was also associated with a significantly increased risk of opioid overdose in pharmacoepidemiologic screening. Given that our objective was signal detection, we view these findings as hypothesis-generating. Of the many hundreds of potential DDIs with methadone and buprenorphine, these interaction signals may be the most important targets for future confirmation studies.
An interesting finding from this study was the signal of interaction between clonidine and buprenorphine in both pharmacoepidemiologic and pharmacokinetic screening, representing a previously unrecognized potential DDI.17 Clonidine is an alpha-2 adrenergic receptor agonist approved for the treatment of hypertension and attention-deficit disorder, and is commonly used off-label for the treatment of opioid withdrawal syndrome.32 In pharmacokinetic screening, clonidine was predicted to increase buprenorphine AUC, based on an in vitro study suggesting that clonidine can inhibit CYP3A4 at high concentrations.33,34 Whether these findings can be extrapolated in vivo is unclear, as doses that are used clinically result in lower serum concentrations compared with those studied in vitro.33,34 The plausibility of an interaction is bolstered by the observation that clonidine was associated with a roughly two-fold increased risk of overdose in SCCS screening. In addition to CYP3A4 inhibition, the association with overdose may be due in part to additive CNS depression, as clonidine has well-characterized sedative effects, particularly at high doses.35 It is also possible that the association is driven at least in part by unmeasured confounding. In particular, because clonidine is used for treatment of opioid withdrawal symptoms, clonidine may be functioning as a surrogate for loss of opioid tolerance or unmeasured opioid abuse in persons experiencing withdrawal.
Our SCCS screening also identified associations between CNS depressants and increased opioid overdose risk in patients taking either methadone or buprenorphine. Additive or synergistic CNS depression is a known risk factor for overdose in opioid users. Numerous studies have shown higher overdose risk when opioids are combined with benzodiazepines,36 and prescribing guidelines recommend against concomitant use of these drug classes.37 Prior studies have also found additive respiratory depression and increased risk of overdose when gabapentinoids are combined with opioids.38,39 The use of gabapentinoids has increased markedly in recent years,40 often in combination with opioids as an “opioid sparing” strategy, although recent evidence has questioned the safety of this approach.41 Similarly, our data suggest that cotreatment with opioids and gabapentinoids might have an unfavorable risk vs. benefit in many patients. Moreover, ours is the first study to find a higher risk of overdose when gabapentin is combined with buprenorphine. Although buprenorphine has a lower intrinsic risk of respiratory depression, owing to its partial agonist mechanism of action,5 our results suggest that preferential use of buprenorphine instead of methadone does not’ fully mitigate the risks of opioid overdose associated with concomitant gabapentin therapy.
An additional notable finding was the association between the use of the skeletal muscle relaxants (SMRs) baclofen (primary analysis) and methocarbamol (lagged precipitants analysis) during methadone exposure and a higher risk of opioid overdose. Baclofen has been associated with opioid overdose risk in other studies. In a large claims-based cohort study of over 15,000,000 opioid users,42 SMR drugs as a class were associated with a higher opioid overdose risk, with the strongest association observed among users of baclofen or carisoprodol. In contrast, methocarbamol was not associated with overdose risk. Similarly, a claims-based screening study of opioid users found that baclofen, but not other SMR drugs (including methocarbamol), was associated with higher opioid overdose risk.43 Taken together, these data support the hypothesis that among drugs in the SMR class, baclofen may pose a uniquely high risk of overdose in opioid users.
Two signals in the methadone screening analysis (carvedilol and cardiac arrest in the primary analysis; and simvastatin and overdose in the zero-grace period analysis) might be explained by effects on P-gp transporters. Both drugs have been identified as inhibitors of P-gp, 44,45 which has an important role in methadone absorption.11,46 Thus, concomitant carvedilol or simvastatin treatment may lead to increased methadone concentrations during the absorption phase after oral administration of methadone, as has been shown with other P-gp inhibitors.46
A novel aspect of our analysis was the approach to estimating methadone fm based on AUCR data from in vivo DDI studies. The rationale for this approach was the well-described discordance between in vitro and in vivo studies of methadone metabolism, which arrive at conflicting conclusions regarding the relative importance of CYP3A4 vs. CYP2B6.9,29 In vitro studies have consistently identified CYP3A4 as the predominant metabolic enzyme.9 However, these studies used much higher methadone concentrations than those observed clinically.29 In contrast, in vivo studies have shown that strong CYP3A4 inhibitors do not increase methadone concentrations, suggesting a minor role of CYP3A4 at therapeutic concentrations.29,30 Such studies have instead identified CYP2B6 activity as the predominant metabolic enzyme,29,30 findings that are in agreement with pharmacogenetic studies that show significantly reduced methadone metabolism in patients who have CYP2B6 loss-of-function alleles.47 Our regression analysis estimated an fm of 74% for CYP2B6 and <5% for CYP3A4, results that align with the current consensus on methadone metabolism.29
Pharmacokinetic screening did not identify any DDI signals for methadone. This is perhaps unsurprising, as there are few strong CYP2B6 inhibitors.48 Of drugs screened in this study, clopidogrel and clotrimazole have ki values that suggest a potential for clinically relevant CYP2B6 inhibition.48 Clotrimazole is also a potent CYP3A4 inhibitor.49 However, both clopidogrel and clotrimazole are highly protein bound and unlikely to achieve sufficient systemic concentrations to mediate important inhibition of hepatic metabolism,48 as reflected by negligible predicted AUCR values (Table S1). Clopidogrel was not associated with adverse health outcomes in pharmacoepidemiologic screening. Clotrimazole (administered as the oral troche) was associated with a higher rate of methadone overdose, but the association was nonsignificant after semi-Bayes adjustment (rate ratio 2.06, 95% CI 0.87–4.89). Clotrimazole oral troches have been associated with moderate changes in exposure to orally administered midazolam,50 most likely mediated through inhibition of intestinal CYP3A4.50 Given that high methadone concentrations are achieved in enterocytes during absorption, it remains possible that clotrimazole troches could alter methadone absorption via inhibition of intestinal CYP3A4 metabolism.
Because it is unfeasible to specify an optimal study design for all drug combinations examined in the context of high throughput screening, we conducted sensitivity analyses in which we varied key study design choices. Notably, there were discrepancies in signal detection across the analyses, particularly for the zero-grace period analysis. Although the reasons for discrepancies among the analyses are unclear, some observations can be made. First, rate ratios that changed from “significant” to “nonsignificant” (or vice versa) across analyses tended to be associations that were near the threshold for significance in all analyses. For example, gabapentin and clonidine were significantly associated with overdose in buprenorphine users in both the primary and lagged precipitant analyses, but just missed significance in the zero-grace period analysis (P = 0.074 and 0.067, respectively). Second, it might be expected that the zero-grace period analysis demonstrates greater discrepancy with the primary analysis compared with other analyses, as it represents a significant design change, resulting in a shorter average duration of target drug exposure episodes (because episodes are truncated at the beginning of a grace period); a reduced number of outcome events included (because some events occurred after a grace period, and are thus dropped from analysis); and varying prevalence of precipitant exposure, as (like outcome events) some precipitant exposure occurs only after grace periods. Finally, it is possible that discrepancies between the zero-grace period analysis and the primary analysis are due in part to effects on misclassification bias.
Our study has several limitations. First, although the SCCS design inherently controls confounding from both measured and unmeasured factors that do not change over time, there remains a risk of unmeasured confounding from time-varying factors. To minimize such bias, we controlled for two key factors (object drug dose and history of the outcome). However, given the high-throughput nature of the studies, it was not feasible to control for all plausible time-varying factors. We thus consider our findings hypothesis-generating only. Second, we defined drug exposure based on dispensed prescription claims, which may not reflect actual drug ingestion. Third, our findings may not be generalizable beyond a commercially insured, ambulatory care population. Fourth, we only included outcome events that led to an emergency department visit or hospitalization, which could underestimate event rates. However, given the high specificity of our claims algorithms, biases in the rate ratio estimates should be minimal. Fifth, because the observation periods of the SCCS studies were restricted to person-time exposed to the target drugs, we are unable to distinguish drug interactions from inherent effects of the overlapping medications. Sixth, although we attempted to minimize the risk of false-positive findings using semi-Bayes shrinkage, it remains possible that some of our findings are due to chance. Seventh, the number of events was quite limited for the ADE and cardiac arrest outcomes, providing power to detect only very strong associations. Eighth, overdose events occurring during target drug exposure could lead to discontinuation of the target drugs (particularly methadone), which would violate an assumption of the SCCS study design.20 However, recent studies suggest the magnitude of this bias, if present, is generally small.51 Eighth, our pharmacokinetic simulation model only considered the effects of competitive hepatic CYP-enzyme inhibition. It is possible that this approach missed interactions mediated through other mechanisms. Ninth, our model for estimating methadone fm from in vivo DDI studies assumes the same magnitude of effect for all inducers and inhibitors included in the analysis, which is likely an oversimplification. However, we chose this parsimonious approach because there is insufficient evidence in the literature to accurately estimate the relative potency of induction and/or inhibition effects of the included antiretrovirals. Further, our results assume that our methadone fm estimates are generalizable to other inhibitors. Violation of either one of these assumptions could produce both false-positive signals (overestimation of the fm due to one or more enzymes) and false-negative results (underestimation of the fm due to one or more enzymes).
CONCLUSION
Identifying clinically relevant DDIs among users of methadone and buprenorphine is a major public health priority. By combining pharmacoepidemiologic and pharmacokinetic screening approaches, we have generated an evidence-based list of DDI signals that may be the most important targets for future validation studies.
Supplementary Material
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
Methadone and buprenorphine have properties that are concerning for a high risk of drug–drug interactions (DDIs), including extensive cytochrome P450 metabolism, prolongation of the electrocardiographic QT interval, and potentially synergistic effects on central nervous system depression. Despite this, few studies have examined the impact of DDIs on adverse health outcomes during treatment with these drugs.
WHAT QUESTION DID THIS STUDY ADDRESS?
We aimed to identify clinically relevant DDIs through high-throughput screening of drugs administered concomitantly with methadone or buprenorphine by combining pharmacoepidemiologic and pharmacokinetic screening.
WHAT DOES THIS STUDY ADD TO OUR KNOWLEDGE?
We screened 776 drug–drug outcome associations in pharmacoepidemiologic screening, 11 of which identified a significant DDI, including gabapentinoids with either methadone or buprenorphine; baclofen with methadone; and benzodiazepines with methadone. Pharmacokinetic screening identified two moderate-to-strong potential DDIs (clonidine and fluconazole with buprenorphine). Clonidine with buprenorphine was also associated with significantly increased opioid overdose risk in pharmacoepidemiologic screening.
HOW MIGHT THIS CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE?
These DDI signals may be the most important targets for future confirmation studies.
ACKNOWLEDGMENTS
The authors thank Ms. Min Du and Ms. Qing Liu for their computer programming support.
FUNDING
This work was funded by National Institutes of Health (K08DK124658, R01AG025152, R01DA048001, R01AG060975, and R01DA042299); and the University of Pennsylvania University Research Foundation. Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Footnotes
CONFLICT OF INTEREST
Dr. Leonard is an Executive Committee Member of and Dr. Hennessy directs the University of Pennsylvania’s Center for Pharmacoepidemiology Research and Training, which has received funds from Pfizer and Sanofi to support pharmacoepidemiology education. Dr. Hennessy has received grants from Pfizer, and Johnson and Johnson during the conduct of the study, consulted for Novo Nordisk, Arbor Pharmaceuticals, the Medullary Thyroid Cancer Consortium (Novo Nordisk, AstraZeneca, GlaxoSmithKline, and Eli Lilly), Biogen, Intercept Pharmaceuticals, Provention Bio, Bluebird Bio, and Amylyx Pharmaceuticals, and is a special government employee of the FDA. Dr. Leonard recently received honoraria from the American College of Clinical Pharmacy Foundation, the University of Florida, and the University of Massachusetts and is a Special Government Employee of the FDA. The opinions expressed in this manuscript are those of the authors and should not be interpreted as the position of the FDA. Dr. Leonard consults for the Reagan-Udall Foundation. Dr. Leonard’s spouse is employed by Merck; neither he nor she own stock in the company. Dr. Bilker serves on multiple data safety monitoring boards for Genentech. Dr. Woody has consulted with the Drug Enforcement Administration, the Bureau of Professional and Occupational Affairs of the State of Pennsylvania, and BioCorRx. Dr. Pham receives support from Acadia Pharmaceuticals Inc., unrelated to this project. All other authors declared no competing interests for this work.
SUPPORTING INFORMATION
Supplementary information accompanies this paper on the Clinical Pharmacology & Therapeutics website (www.cpt-journal.com).
References
- 1.The ASAM National Practice Guideline for the Treatment of Opioid Use Disorder – 2020. Focused Update <https://www.asam.org/Quality-Science/quality/2020-national-practice-guideline>. Accessed March 13, 2021.
- 2.Substance Abuse and Mental Health Services Administration (US); Office of the Surgeon General (US). Facing addiction in America: The surgeon general’s report on alcohol, drugs, and health. Washington (DC): US Department of Health and Human Services; <https://www.ncbi.nlm.nih.gov/books/NBK424857/> (2016). Accessed May 22, 2021. [PubMed] [Google Scholar]
- 3.Alderks CE Trends in the use of methadone, buprenorphine, and extended-release naltrexone at substance abuse treatment facilities: 2003–2015 (update). In The CBHSQ Report 2013–2017 (Substance Abuse and Mental Health Services Administration (US), Rockville, MD, 2017). [PubMed] [Google Scholar]
- 4.Hanna V & Senderovich H Methadone in pain management: a systematic review. J. Pain 22, 233–245 (2021). [DOI] [PubMed] [Google Scholar]
- 5.Hale M, Garofoli M & Raffa RB Benefit-risk analysis of buprenorphine for pain management. J. Pain Res 14, 1359–1369 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.West NA & Dart RC Prescription opioid exposures and adverse outcomes among older adults. Pharmacoepidemiol. Drug Saf 25, 539–544 (2016). [DOI] [PubMed] [Google Scholar]
- 7.Mark TL, Dilonardo J, Vandivort R & Miller K Psychiatric and medical comorbidities, associated pain, and health care utilization of patients prescribed buprenorphine. J. Subst. Abuse Treat 44, 481–487 (2013). [DOI] [PubMed] [Google Scholar]
- 8.McCance-Katz EF, Sullivan LE & Nallani S Drug interactions of clinical importance among the opioids, methadone and buprenorphine, and other frequently prescribed medications: a review. Am. J. Addict 19, 4–16 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang Y, Fang WB, Lin SN & Moody DE Stereo-selective metabolism of methadone by human liver microsomes and cDNA-expressed cytochrome P450s: a reconciliation. Basic Clin. Pharmacol. Toxicol 108, 55–62 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Picard N, Cresteil T, Djebli N & Marquet P In vitro metabolism study of buprenorphine: evidence for new metabolic pathways. Drug Metab. Dispos 33, 689–695 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Hassan HE, Myers AL, Coop A & Eddington ND Differential involvement of P-glycoprotein (ABCB1) in permeability, tissue distribution, and antinociceptive activity of methadone, buprenorphine, and diprenorphine: in vitro and in vivo evaluation. J. Pharm. Sci 98, 4928–4940 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris SC, Morganroth J, Ripa SR, Thorn MD & Colucci S Effects of buprenorphine on QT intervals in healthy subjects: results of 2 randomized positive- and placebo-controlled trials. Postgrad. Med 129, 69–80 (2017). [DOI] [PubMed] [Google Scholar]
- 13.Westermeyer J, Adabag S, Anand V, Thuras P, Yoon G & Batres-y-Carr T Methadone maintenance dose/weight ratio, long QTc, and EKG screening. Am. J. Addict 25, 499–507 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Kao DP, Haigney MCP, Mehler PS & Krantz MJ Arrhythmia associated with buprenorphine and methadone reported to the Food and Drug Administration. Addiction 110, 1468–1475 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mégarbane B, Hreiche R, Pirnay S, Marie N & Baud FJ Does high-dose buprenorphine cause respiratory depression? Possible mechanisms and therapeutic consequences. Toxicol. Rev 25, 79–85 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Chou R, Weimer MB & Dana T Methadone overdose and cardiac arrhythmia potential: findings from a review of the evidence for an American pain society and college on problems of drug dependence clinical practice guideline. J. Pain 15, 338–365 (2014). [DOI] [PubMed] [Google Scholar]
- 17.IBM Corporation; IBM Watson Health; Truven Health Analytics. Micromedex® (electronic version) <http://www.micromedexsolutions.com/micromedex2/librarian> (2018). Accessed May 21, 2021. [Google Scholar]
- 18.Nielsen S, Dietze P, Lee N, Dunlop A & Taylor D Concurrent buprenorphine and benzodiazepines use and self- reported opioid toxicity in opioid substitution treatment. Addiction 102, 616–622 (2007). [DOI] [PubMed] [Google Scholar]
- 19.Abrahamsson T, Berge J, Öjehagen A & Håkansson A Benzodiazepine, z-drug and pregabalin prescriptions and mortality among patients in opioid maintenance treatment—a nation-wide register-based open cohort study. Drug Alcohol Depend. 174, 58–64 (2017). [DOI] [PubMed] [Google Scholar]
- 20.Whitaker HJ, Farrington CP, Spiessens B & Musonda P Tutorial in biostatistics: the self-controlled case series method. Stat. Med 25, 1768–1797 (2006). [DOI] [PubMed] [Google Scholar]
- 21.Green CA et al. Identifying and classifying opioid-related overdoses: a validation study. Pharmacoepidemiol. Drug Saf 28, 1127–1137 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Green CA, Perrin NA, Janoff SL, Campbell CI, Chilcoat HD & Coplan PM Assessing the accuracy of opioid overdose and poisoning codes in diagnostic information from electronic health records, claims data, and death records. Pharmacoepidemiol. Drug Saf 26, 509–517 (2017). [DOI] [PubMed] [Google Scholar]
- 23.Hennessy S et al. Validation of diagnostic codes for outpatient-originating sudden cardiac death and ventricular arrhythmia in Medicaid and Medicare claims data. Pharmacoepidemiol. Drug Saf 19, 555–562 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steenland K, Bray I, Greenland S & Boffetta P Empirical Bayes adjustments for multiple results in hypothesis-generating or surveillance studies. Cancer Epidemiol. Biomarkers Prev 9, 895–903 (2000). [PubMed] [Google Scholar]
- 25.Guest EJ, Rowland-Yeo K, Rostami-Hodjegan A, Tucker GT, Houston JB & Galetin A Assessment of algorithms for predicting drug–drug interactions via inhibition mechanisms: comparison of dynamic and static models. Br. J. Clin. Pharmacol 71, 72–87 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wishart DS et al. DrugBank 5.0: a major update to the DrugBank database for 2018. Nucleic Acids Res. 46, D1074–D1082 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benet LZ, Broccatelli F & Oprea TI BDDCS applied to over 900 drugs. AAPS J. 13, 519–547 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hua L et al. The cancer drug fraction of metabolism database. CPT Pharmacometrics Syst. Pharmacol 8, 511–519 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kharasch ED Current concepts in methadone metabolism and transport. Clin. Pharmacol. Drug Dev 6, 125–134 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Younis IR, Lakota EA, Volpe DA, Patel V, Xu Y & Sahajwalla CG drug–drug interaction studies of methadone and antiviral drugs: lessons learned. J. Clin. Pharmacol 59, 1035–1043 (2019). [DOI] [PubMed] [Google Scholar]
- 31.US Food and Drug Administration. Guidance for industry: drug interaction studies—study design, data analysis, implications for dosing, and labeling recommendations (Rockville, Center for Drug Evaluation and Research (CDER), 2012) <https://www.federalregister.gov/documents/2012/02/21/2012-3958/draft-guidance-for-industry-on-drug-interaction-studies-study-design-data-analysis-implications-for>. Accessed March 22, 2021. [Google Scholar]
- 32.Srivastava AB, Mariani JJ & Levin FR New directions in the treatment of opioid withdrawal. Lancet 395, 1938–1948 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tanaka E, Nakamura T, Inomata S & Honda K Effects of premedication medicines on the formation of the CYP3A4-dependent metabolite of ropivacaine, 2′, 6’-Pipecoloxylidide, on human liver microsomes in vitro. Basic Clin. Pharmacol. Toxicol 98, 181–183 (2006). [DOI] [PubMed] [Google Scholar]
- 34.Vasseur B et al. Comparison of the systemic and local pharmacokinetics of clonidine mucoadhesive buccal tablets with reference clonidine Oral tablets in healthy volunteers: an open-label randomised cross-over trial. Adv. Ther 34, 2022–2032 (2017). [DOI] [PubMed] [Google Scholar]
- 35.Maze M & Tranquilli W Alpha 2 adrenoceptor agonists: defining the role in clinical anaesthesia. Anesthesiology 74, 581–605 (1991). [PubMed] [Google Scholar]
- 36.Sun EC, Dixit A, Humphreys K, Darnall BD, Baker LC & Mackey S Association between concurrent use of prescription opioids and benzodiazepines and overdose: retrospective analysis. BMJ 356, j760 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dowell D, Haegerich TM & Chou R CDC guideline for prescribing opioids for chronic pain—United States, 2016. MMWR Recomm. Rep 65, 1–49 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Gomes T, Juurlink DN, Antoniou T, Mamdani MM, Paterson JM & van den Brink W Gabapentin, opioids, and the risk of opioid-related death: a population-based nested case–control study. PLoS Med. 14, e1002396 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gomes T et al. Pregabalin and the risk for opioid-related death: a nested case–control study. Ann. Intern. Med 69, 732–734 (2018). [DOI] [PubMed] [Google Scholar]
- 40.Johansen M Gabapentinoid use in the United States 2002 through 2015. JAMA Intern. Med 178, 292–294 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bykov K, Bateman BT, Franklin JM, Vine SM & Patorno E Association of Gabapentinoids with the risk of opioid-related adverse events in surgical patients in the United States. JAMA Netw. Open 3, e2031647 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Y et al. Risk of opioid overdose associated with concomitant use of opioids and skeletal muscle relaxants: a population-based cohort study. Clin. Pharmacol. Ther 108, 81–89 (2020). [DOI] [PubMed] [Google Scholar]
- 43.Khan NF, Bykov K, Glynn RJ, Barnett ML & Gagne JJ Coprescription of opioids with other medications and risk of opioid overdose. Clin. Pharmacol. Ther 110, 1011–1017 (2021). [DOI] [PubMed] [Google Scholar]
- 44.Chen C et al. Differential interaction of 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors with ABCB1, ABCC2, and OATP1B1. Drug Metab. Dispos 33, 537–546 (2005). [DOI] [PubMed] [Google Scholar]
- 45.Aiba T, Ishida K, Yoshinaga M, Okuno M & Hashimoto Y Pharmacokinetic characterization of transcellular transport and drug interaction of digoxin in Caco-2 cell monolayers. Biol. Pharm. Bull 28, 114–119 (2005). [DOI] [PubMed] [Google Scholar]
- 46.Kharasch ED, Hoffer C & Whittington D The effect of quinidine, used as a probe for the involvement of P-glycoprotein, on the intestinal absorption and pharmacodynamics of methadone. Br. J. Clin. Pharmacol 57, 600–610 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kharasch ED, Regina KJ, Blood J & Friedel C Methadone pharmacogenetics: CYP2B6 polymorphisms determine plasma concentrations, clearance, and metabolism. Anesthesiology 123, 1142–1153 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walsky RL, Astuccio AV & Obach RS Evaluation of 227 drugs for in vitro inhibition of cytochrome P450 2B6. J. Clin. Pharmacol 46, 1426–1438 (2006). [DOI] [PubMed] [Google Scholar]
- 49.Zhang W, Ramamoorthy Y, Kilicarslan T, Nolte H, Tyndale RF & Sellers EM Inhibition of cytochromes P450 by antifungal imidazole derivatives. Drug Metab. Dispos 30, 314–318 (2002). [DOI] [PubMed] [Google Scholar]
- 50.Shord SS et al. Effects of oral clotrimazole troches on the pharmacokinetics of oral and intravenous midazolam. Br. J. Clin. Pharmacol 69, 160–166 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bykov K, Franklin JM, Li H & Gagne JJ Comparison of self-controlled designs for evaluating outcomes of drug–drug interactions: simulation study. Epidemiology 30, 861–866 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.