Abstract
Cannabis use among pregnant people has increased over the past decade. This is of concern as prenatal cannabis exposure (PCE) is associated with cognitive, motor, and social deficits among offspring. Here, we examined resting-state functional connectivity (rsFC) of the salience network (SN) — a core neurocognitive network that integrates emotional and sensory information — in children with (vs. without) PCE. Using neuroimaging and developmental history data collected from 1210,719 children (M±SD=9.92±0.62 years; 47.9% female) from the Adolescent Brain Cognitive Development (ABCD) study, we assessed the impact of parent-reported PCE (before or after knowledge of pregnancy) on rsFC within and between the SN and five other core neurocognitive networks. We also evaluated whether SN rsFC mediated the association between PCE and child psychopathology. Results showed that PCE before (but not after) knowledge of pregnancy was associated with lower SN-ventral attention network (VAN) rsFC. Further, psychotic-like experiences mediated the association between PCE and SN-VAN rsFC, and reversal of the model was also significant, such that SN-VAN rsFC mediated the association between PCE and psychotic-like symptoms. However, these mediation effects were no longer significant after the inclusion of covariates. Taken together, these findings suggest that developmental alterations in SN-VAN interactions may explain the previously reported association between PCE and elevated risk of child psychopathology.
Keywords: Salience network, gestational marijuana, endocannabinoid, neurocognitive, resting-state, fMRI
Introduction
Rates of cannabis use have dramatically increased in the U.S. over the past several decades. This increase was accompanied by more jurisdictions moving towards decriminalization and legalization, and decreased perceptions of risk of use, particularly among young people (Cohn et al., 2017; Ingstrup et al., 2018; Paul et al., 2021). Indeed, as of November 2021, recreational cannabis use was legal in 18 states; though, it remains illegal at the federal level due to the Supremacy Clause. While there is growing interest and evidence supporting the use of cannabis and cannabinoids for the treatment of various medical conditions (e.g., pediatric epilepsy Whiting et al., 2015), the rising use and potency of cannabis may amplify potential adverse effects on vulnerable populations (e.g., fetuses, children, adolescents).
One population that has shown dramatic recent increases in rates of cannabis use is pregnant women. One study found that past-month cannabis use increased by 106% from 2002 (3.4%) to 2017 (7%) among pregnant women in the U.S. (Volkow et al., 2019). Rates of self-reported cannabis may be as high as 22% among pregnant adolescent women (e.g., ages 18 or less; Young-Wolff et al., 2017). In tandem, one study in Colorado found that nearly 70% of dispensaries recommended that pregnant women use cannabis to help with nausea and morning sickness (Dickson et al., 2018). Indeed, pregnant women often report that they use cannabis to combat anxiety and nausea (for review, see Thompson et al., 2019)
The endocannabinoid (eCB) system plays a crucial role in modulating neural development (Richardson et al., 2016). In particular, it modulates various key functions (e.g., neurogenesis, neuronal migration, progenitor cell proliferation, and synaptogenesis; Harkany et al., 2007), making it foundational for neural development and thus later-life behaviors (e.g., learning, memory). Indeed, the eCB system has been implicated in brain plasticity, but it is already essential as early as fertilization and blastocyst implantation (Silveira et al., 2018). Importantly, research shows that constituents of cannabis, including Δ9-tetrahydrocannabinol (THC), can easily cross the placental barrier and then interfere with fetal neurodevelopment (see review by Thompson et al., 2019)
Recent studies indicate that prenatal cannabis exposure (PCE) may have detrimental effects on offspring, including a heightened risk for premature birth (Corsi et al., 2019), lower birth weight, and impaired cognition (Rodriguez et al., 2019). During the embryonic period, which occurs during gestational weeks 3 through 8, the fetus is particularly vulnerable to teratogens and environmental exposures (Alwan & Chambers, 2015). The embryonic period is also associated with the highest risk for miscarriages (see Thompson et al., 2019 for review). Noteworthy, many individuals do not realize they are pregnant at this early stage (Branum & Ahrens, 2017), so they may not know to cease or reduce their cannabis use. This highlights the importance of increasing awareness about the effects of PCE among women of reproductive age. Although studies to-date are limited, the emerging reports of adverse effects prompted a U.S. Surgeon General advisory in 2019 against the use of cannabis during pregnancy (U.S. Surgeon General’s Advisory: Marijuana Use and the Developing Brain, 2019).
The ongoing NIH Adolescent Brain Cognitive Development (ABCD) study provides an unprecedented opportunity to examine the impact of PCE on neurodevelopmental outcomes in children. The ABCD study began in 2016 and is a large longitudinal study of over 10,000 children, ages 9–10 upon enrollment, from 21 sites across the U.S. (https://abcdstudy.org). Three recently published studies leveraged the ABCD data set to examine associations between PCE and childhood outcomes. These studies linked PCE to childhood sleep disorders, psychosis proneness, and psychopathology (e.g., externalizing and internalizing problems; Ingstrup et al., 2018; Paul et al., 2021). Paul et al. (2021) additionally linked PCE to lower birth weight, lower total intracranial volume, and lower white matter volumes among offspring, suggesting adverse effects on child neurodevelopment.
Neuroimaging studies in adolescent and adult populations with current or previous cannabis use are more common than in individuals with histories of PCE. These studies demonstrate that cannabis use is associated with altered structure and function of core neurocognitive networks (Battistella et al., 2014; Blest-Hopley et al., 2018; Hurd et al., 2019). In particular, several studies suggest functional alterations within and between the salience network (SN) in cannabis-using adolescents and adults (Bhattacharyya et al., 2015; Wijayendran et al., 2018), which is not surprising given that cannabinoid receptors are densely located in core SN regions (Burns et al., 2007). The SN encompasses the anterior insula, the cingulate cortex, and anterior prefrontal cortex and is involved in orienting attention to biologically-relevant stimuli, such as threats or rewards (Chiong et al., 2013; Seeley et al., 2007). Disruptions in the SN are linked to risk of both psychiatric and substance use disorders (Hurd et al., 2019; Zilverstand et al., 2018).
The third trimester of pregnancy is associated with rapid maturation of functional brain networks, including the insula and other SN regions (Tau & Peterson, 2010). Further, developmental studies demonstrate that within and between SN functional connectivity changes dynamically across childhood and adolescence (Marusak et al., 2016), and may be sensitive to variation in eCB levels (Heng et al., 2011). Despite the apparent vulnerability of the SN to developmental insults during the prenatal period, few studies have examined the impact of PCE on the SN. In addition, it is unknown whether variations in SN function and connectivity are also linked to symptoms thought to be associated with psychopathology (e.g., hallucinations, thought disorder, attention problems) that previously have been linked to PCE in the ABCD data set.
To address these gaps, this study leverages ABCD data to examine the impact of PCE on within and between network resting-state functional connectivity (rsFC) of the SN and five other core neurocognitive networks (Gordon et al., 2016): (1) the default mode network (DMN), which underlies self-oriented thought and includes the medial prefrontal and poster cingulate cortex; (2) the ventral attention network (VAN), involved in detection of unexpected but behaviorally relevant stimuli and includes the temporoparietal junction and the ventral frontal cortex; (3) the dorsal attention network (DAN), involved in top-down controlled attentional selection and includes the superior parietal lobe, intraparietal sulcus, and ventral premotor cortex; the (4) frontoparietal network (FPN), involved in top-down control of executive functioning and compromised of dorsolateral prefrontal cortex and posterior parietal cortex (Sheffield et al., 2015; Vossel et al., 2014); and (5) the cingulo-opercular network (CON), which is involved in instantiating and maintaining task performance, lies posterior and dorsal to the SN, and includes dorsal anterior insula and anterior medial superior prefrontal cortex (Dosenbach et al., 2006). We also tested for associations among PCE, SN rsFC, and symptoms of child psychopathology, given that prior studies using ABCD data have reported significant effects of PCE on child symptoms associated with psychotic-like experiences, internalizing, externalizing, thought disorder, and problems with attention and social function (Ingstrup et al., 2018; Paul et al., 2021).
Materials and Methods
Participants
The ongoing, large-scale NIH Adolescent Brain Cognitive Development (ABCD) study provides an unprecedented opportunity to examine the impact of PCE on childhood outcomes. Details regarding eligibility criteria for the larger ABCD study are provided elsewhere (Garavan et al., 2018). Data were accessed from the NIMH Data Archive (NDA; Release 3.0). We excluded participants with missing data for (1) prenatal substance exposure history and/or (2) resting-state fMRI data. Parents and/or guardians provided informed consent, youth provided assent, and the local IRB approved all study procedures.
Prenatal cannabis exposure (PCE)
PCE was assessed by parental report of use before and after knowledge of pregnancy. Primary analyses considered exposure to any PCE, as a binary variable (exposed, unexposed). Then, we tested for specificity of effects by examining PCE before vs. after knowledge of pregnancy separately, following Paul et al. (2021).
Demographic variables and covariates
The covariates used in the study included child age, sex, race/ethnicity, household income, maternal education, prenatal exposure to tobacco and alcohol, prematurity. Race/ethnicity was dummy coded into two variables (White, non-White), where the largest racial/ethnic group in the sample (White) served as the reference group. Child sex (female, non-female), prenatal alcohol and tobacco exposure (exposed, unexposed), and prematurity (born prematurely, not born prematurely) were also dummy coded. Annual household income (1 to 5, where 1 = ≤$49,999 and 5 = ≥$200,000) and maternal educational level (0 to 21 y where 0 = never attended school, 21 = doctoral degree) were treated as ordinal variables. Child age (months) was treated as a continuous variable.
Child symptoms of psychopathology
Parents/guardians reported on their child’s symptoms of psychopathology using the Child Behavior Checklist (CBCL; Achenbach & Rescorla, 2001), a well-validated 113-item report of past 6-month problem behaviors. Here, we focused on the following subscales: internalizing, externalizing, thought disorder, attention problems, and social problems. These subscales were selected as scores were previously associated with PCE in the ABCD sample (Ingstrup et al., 2018; Paul et al., 2021). We used age- and sex-normed t-scores for analyses. We also included psychotic-like experiences, as measured by the 21-item child self-report Prodromal Questionnaire-Brief Child Version (Loewy1 et al., 2011). We computed total number of endorsed items (e.g., “do familiar surroundings sometimes seem strange, confusing, threatening, or unreal to you?”), as prior work shows that this total score is associated with psychosis risk factors (Karcher et al., 2018).
Neuroimaging data acquisition, preprocessing, and rsFC analysis
MRI data acquisition and processing has been described previously (Casey et al., 2018). Scanning procedures were harmonized across the 21 sites (Hagler, 2019). Four 5-min resting-state scans were acquired during the imaging session. The sequence is a high spatial and temporal resolution simultaneous multi-slice (SMS)/multiband EPI resting-state sequence. Preprocessing was accomplished using the ABCD Data Analysis and Informatics Core using the standardized ABCD pipeline (Hagler, 2019). Following preprocessing, fMRI time courses were projected onto FreeSurfer’s cortical surface. Using these time courses, within- and between-network connectivity (Pearson correlation) was calculated on the basis of the Gordon parcellation scheme (Gordon et al., 2016) for 12 predefined resting-state networks. Then rsFC values were Fischer Z transformed. Within-network rsFC reflects the average of the correlation over all pairs of regions within a network. Between-network rsFC reflects the average correlation value between all regions of two separate networks. Our analyses focused on six connections: (1) within-network SN connectivity, and between-network SN rsFC with the following five networks: (2) VAN, (3) DMN, (4) FPN, (5) CON, and (6) DAN (see Figure 1).
Figure 1. Core neurocognitive networks of interest.
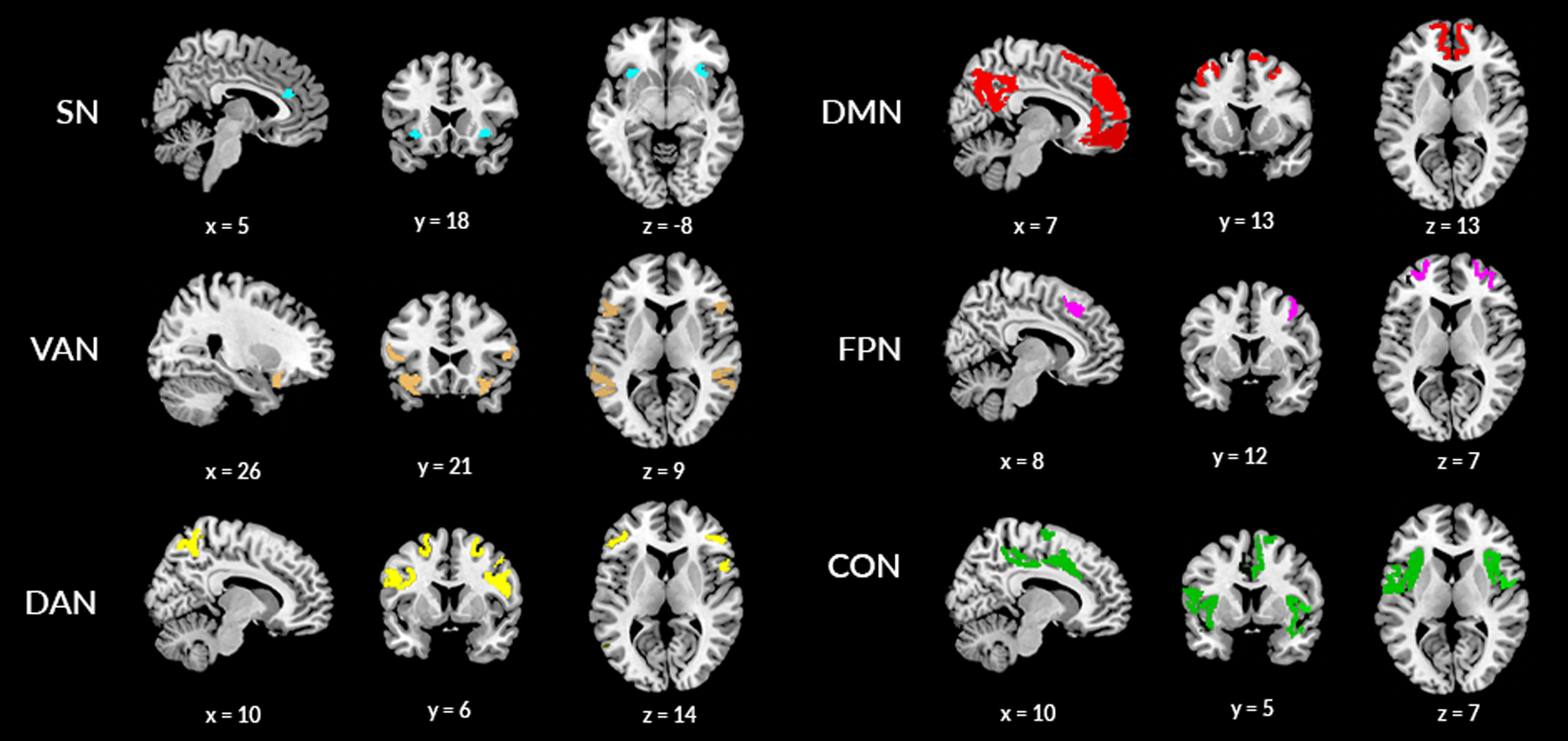
SN, salience network; DAN, dorsal attention network; DMN, default mode network; FPN, frontoparietal network; VAN, ventral attention network.Xyz indicates Montreal Neurological Institute (MNI) coordinates.
Statistical Analyses
First, we tested for effects of PCE on sociodemographic and developmental history variables using Pearson bivariate correlation (e.g., age, sex, race, income, maternal education, prematurity, tobacco and alcohol exposure). These variables were evaluated based on a prior report by Paul et al. (2021) linking PCE to child behavioral outcomes in the ABCD sample. We also assessed the effects of these sociodemographic variables on SN connectivity.
Our main analyses examined the impact of PCE on rsFC of each of the six rsFC connections of interest using linear mixed effects models. Site and family were added as random effects to the models, with family nested within site. PCE was added as the predictor in each model, following Paul et al. (2021). RsFC was entered as the outcome variable. Our primary analysis considered any PCE exposure. Then, to decompose significant effects of PCE, we performed separate models for PCE before and PCE after knowledge of pregnancy. Benjamini-Hochberg false discovery rate (FDR) correction was applied to control for multiple comparisons. Regression analyses were repeated with the inclusion of covariates. Covariates were added in a stepwise fashion. First, we controlled for sociodemographic and developmental history variables. These variables were selected based on prior literature or their potential roles as confounders of the relationship between PCE and rsFC (cf. Dennis et al., 2009). Then, we repeated analyses additionally controlling for prematurity and prenatal alcohol and tobacco exposure, to evaluate whether results remained significant after adjusting for these factors. This stepwise approach was based on a recent study suggesting best practices for control of covariates in the ABCD data set (Dick et al., 2021). Sex x PCE interactions were explored. Analyses were performed in R Studio 2022.07.1 Build 554 with a p value of 0.05 (two-tailed).
Given that Paul et al. (2021) observed PCE-related effects on child psychopathology in the ABCD sample (internalizing, externalizing, thought disorder, attention problems, social problems, and psychotic-like experiences), we additionally tested whether network connections that showed significant associations with PCE were also associated with symptomology. First, we confirmed that PCE before and/or after knowledge of pregnancy was associated with these symptoms. Then, we performed regressions to test whether these symptom dimensions were also associated with rsFC values that were associated with PCE. For rsFC values showing significant associations with both PCE and symptomology, we performed exploratory mediation using the SPSS PROCESS macro (version 3.4) (Hayes, 2012). The PROCESS macro uses a bootstrapping approach (5,000 resamples), and significant mediation is indicated by 95% confidence intervals for the indirect effect that do not include zero.
Results
Of the 10,719 children in our sample, 4% and 1.2% of parents/guardians reported using cannabis before and after knowledge of pregnancy, respectively. PCE before and after knowledge of pregnancy were both associated with female child sex (r’s > 0.02, p < 0.05), prenatal tobacco (r’s > 0.15, p < 0.001) and alcohol exposure (r’s > 0.05, p < 0.001), lower maternal education (r’s < −0.06, p < 0.001), and non-white race (r’s > 0.03, p < 0.01). PCE before (but not after) knowledge of pregnancy was associated with lower annual household income (r = −0.03, p = 0.002; see Table 1). PCE before and after knowledge of pregnancy was associated with later reports of finding out that they were pregnant (p < 0.05).
Table 1.
Participant demographics overall and by prenatal cannabis exposure (PCE).
| Variable | Total (N = 10,719) | PCE before knowledge of pregnancy (n = 434) | PCE after knowledge of pregnancy (n = 130) | No PCE (n = 10,271) |
|---|---|---|---|---|
| Child age, mean (SD), y | 9.92 (0.62) | 9.88 (0.63) | 9.83 (0.60) | 9.92 (0.62) |
| Female sex, no. (%) | 5,133 (47.9%) | 230 (53%) | 78 (60%) | 4,897 (47.7%) |
| Other | 1032 (10%) | 45 (10%) | 14 (11%) | 986 (10%) |
| ≥$200,000 | 1,168 (11%) | 17 (4%) | 1 (1%) | 1151 (11%) |
| Weeks found out pregnant, m (SD) | 6.93 (6.78) | 7.84 (6.33) | 8.21 (7.29) | 6.85 (6.75) |
| Prenatal alcohol exposure before knowledge of pregnancy, no. (%) | 2,324 (22%) | 216 (50%) | 54 (42%) | 2106 (21%) |
| Prenatal alcohol exposure after knowledge of pregnancy, no. (%) | 234 (2%) | 28 (7%) | 16 (12%) | 205 (2%) |
| Prenatal tobacco exposure before knowledge of pregnancy, no. (%) | 1,240 (12%) | 247 (57%%) | 71 (55%) | 985 (10%) |
| Prenatal tobacco exposure after knowledge of pregnancy, no. (%) | 218 (3.9%) | 90 (21%) | 44 (34%) | 332 (3%) |
Missing data: 589 parents/guardians did not report on prenatal alcohol exposure before knowledge of pregnancy; 55 for prenatal alcohol after knowledge of pregnancy; 131 for prenatal tobacco exposure before knowledge of pregnancy; 66 for prenatal tobacco exposure after knowledge of pregnancy; 449 missing for income. PCE, prenatal cannabis exposure. Note that analyses were performed for any PCE, and then PCE before and after knowledge of pregnancy, separately, rather than three mutually-exclusive groups.
Sociodemographic and developmental history variables were also associated with rsFC outcomes. In particular, non-white race was negatively associated with SN-VAN, SN-CON, SN-DMN, and SN-SN rsFC, and positively associated with SN-DAN rsFC (p’s < 0.02). Female child sex was associated with lower SN-DAN and SN-FPN rsFC (p’s < 0.001), age was negatively associated with SN-VAN and SA-DMN (p’s < 0.002), and maternal education was positively associated with SN-VAN rsFC (p = 0.0008).
PCE and rsFC without covariates
PCE was associated with significantly lower SN-VAN rsFC (β = −0.017, t = 3.043, SE = 0.005, p = 0.002; see Figure 2). This effect passed FDR correction and was specific to PCE before (β = −0.019, t = 3.53, SE = 0.004, p = 0.0004) but not after (p = 0.2) knowledge of pregnancy. No other network connections reached significance levels.
Figure 2. Prenatal cannabis exposure (PCE) before knowledge of pregnancy is associated with lower rsFC between the SN and VAN.
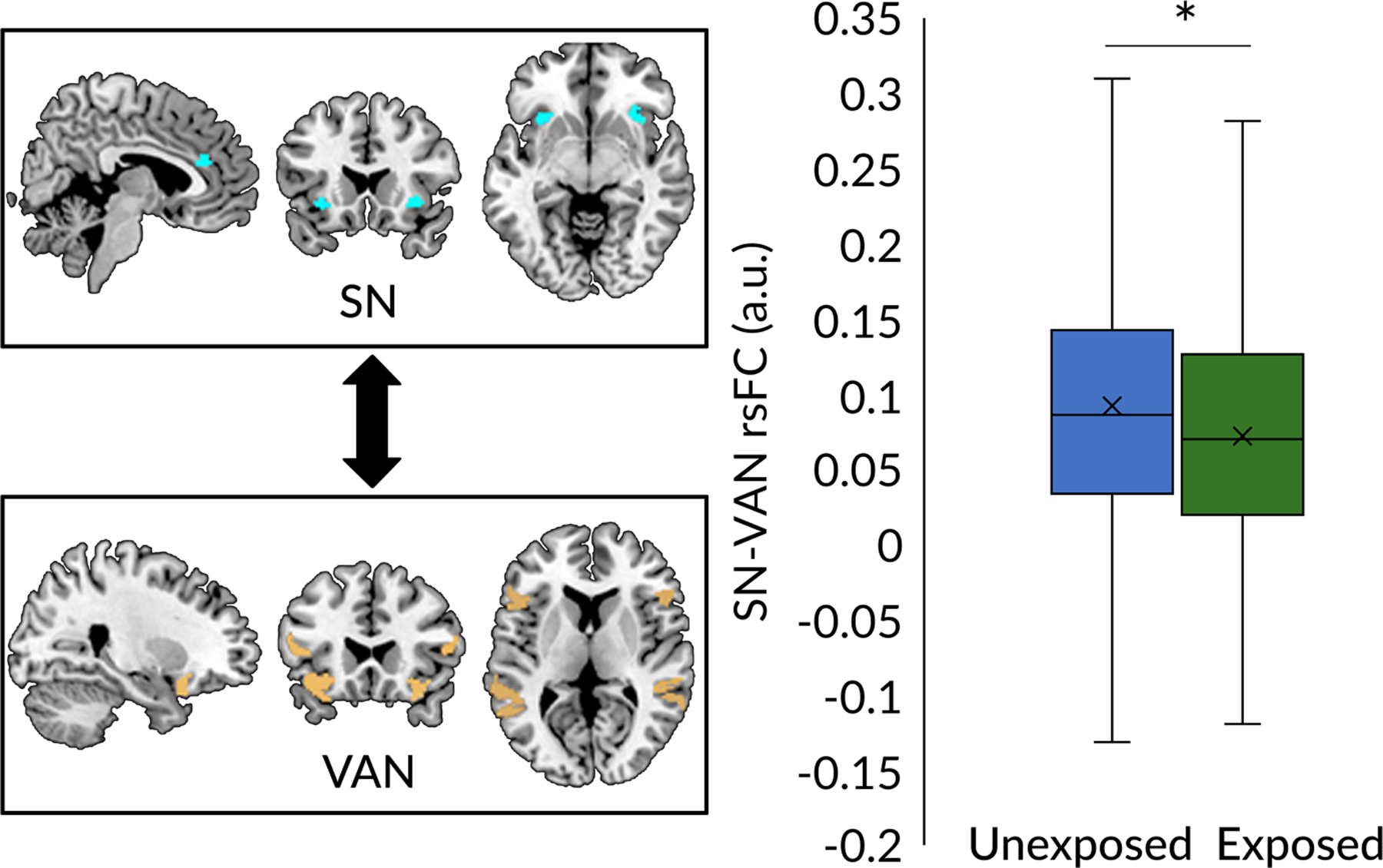
Box plots indicate median and first and third quartiles. The sample mean is indicated with an “X”. SN, salience network; PCE, prenatal cannabis exposure; VAN, ventral attention network; rsFC, resting-state functional connectivity. *p < 0.05.
PCE and rsFC with covariates
When controlling for sociodemographic variables (child age, sex, race, income, and maternal education), the effect of PCE before knowledge of pregnancy on SN-VAN rsFC remained significant (β = −0.018, t = 2.279, SE = 0.008, p = 0.02). This effect was observed after controlling for prenatal alcohol and tobacco exposure, prematurity (β = −0.018, t = 2.088, SE = 0.008, p = 0.037), and after FDR correction. PCE after knowledge of pregnancy remained a non-significant predictor of SN-VAN rsFC after adjusting for covariates (p = 0.2). There were no sex x PCE interactions on SN-VAN rsFC (p’s > 0.05).
After adjusting for covariates, there was a significant sex x PCE interaction (β = 0.02, t = 2.2, SE = 0.01, p = 0.027) for SN-FPN rsFC. This effect was also observed after controlling for prenatal alcohol and tobacco exposure, and prematurity (β = 0.025, t = 2.344, SE = 0.01, p = 0.019). This effect was specific to PCE before (β = 0.024, t = 2.3, SE = 0.01, p = 0.024) but not after (p = 0.736) knowledge of pregnancy. When split by child sex, the effect of PCE was driven by lower SN-FPN rsFC among males (β = −0.017, t = 2.031, SE = 0.008, p = 0.043) but not females (p = 0.29). With the addition of prenatal alcohol and tobacco exposure, as well as prematurity to the model, there was also a significant negative effect of PCE on SN-FPN rsFC (β = −0.017, t = 2.193, SE = 0.008, p = 0.028). However, the effects of PCE or sex x PCE interaction on SN-FPN rs-FC did not pass FDR correction. PCE was not associated with any other network connections after adjusting for covariates (p’s > 0.05).
Symptoms of psychopathology and rsFC without covariates
First, we confirmed the previously reported (Paul et al., 2021) association between PCE and symptoms of psychopathology (i.e., internalizing, externalizing, attention problems, social problems, thought disorder, and psychotic-like experiences; p’s < 0.001) in this sample. Next, we explored whether SN-VAN rsFC was additionally associated with symptoms of psychopathology. Greater psychotic-like experiences were associated with lower SN-VAN rsFC (β = −0.01, p = −0.028, R2 = 0.010). No other symptoms were associated with SN-VAN rsFC. Given that both PCE before knowledge of pregnancy and psychotic-like experiences were associated with SN-SN rsFC, we performed exploratory mediation analyses. First, we tested whether SN-SN rsFC mediated the association between PCE before knowledge of pregnancy and psychotic-like experiences. The indirect effect was not significant. Then we tested whether SN-VAN mediated the same association. The indirect effect was significant (effect = 0.018, boot SE = 0.0084, bootstrap lower level confidence interval [LLCI] = 0.0084, bootstrap upper level confidence interval [ULCI] = 0.0371), suggesting that SN-VAN rsFC mediated the association between PCE and psychotic-like experiences (R2 = 0.027; Figure 3a). This effect was specific to PCE before knowledge of pregnancy and was not significant for PCE after knowledge of pregnancy (boot LLCI = −0.0031, boot ULCI = 0.04). Reversal of the model was also significant, such that psychotic-like experiences mediated the association between PCE and SN-VAN rsFC (effect = −0.0006, boot SE = 0.0002, boot LLCI = −0.0012, boot ULCI = −0.000146; Figure 3b).
Figure 3: Mediation analyses.

A: Psychotic-like experiences mediate the association between PCE before knowledge of pregnancy and SN-VAN rsFC. B: Reversal of the model was also significant, such that SN-VAN rsFC mediates the association between PCE before knowledge of pregnancy and psychotic-like symptoms. c = total effect; c’ = direct effect. *p < 0.05, **p < 0.01, ***p < 0.001.
Symptoms of psychopathology and rsFC with covariates
The effects of psychotic-like experiences on SN-VAN rsFC were non-significant after adjusting for sociodemographic variables, prenatal alcohol, cannabis, and tobacco exposure, and prematurity. No other symptoms were associated with SN-VAN rsFC after adjusting for covariates.
Discussion
This study leveraged a large sample of over 10,000 youth from the ABCD cohort to examine the neurodevelopmental consequences of PCE on within and between functional connectivity of the SN in children, and the association of these changes with behavioral symptoms. Our results suggest that altered neurodevelopment of the SN may explain previously reported associations between PCE and child psychopathology (Paul et al., 2021). Specifically, we found that PCE before (but not after) knowledge of pregnancy was associated with lower between-network rsFC of the SN with the VAN, and these effects remained significant folloing the inclusion of covariates. Further, psychotic-like experiences mediated the association between PCE and SN-VAN rsFC, and reversal of the model was also significant, such that SN-VAN rsFC mediated the association between PCE and psychotic-like symptoms. However, these mediation effects were no longer significant after the inclusion of covariates. Given the critical role of the SN and VAN in modulating attentional control, our results suggest that alterations in core neurocognitive networks involved in attention may contribute to elevated risk of attention-related psychopathology following PCE.
Relative to unexposed children, we observed lower SN-VAN rsFC in children with PCE, which could indicate reduced coherence between networks implicated in attentional control. Indeed, both the SN and VAN are involved in detecting and orienting attention to salient or unexpected, biologically relevant stimuli (Chiong et al., 2013; Seeley et al., 2007; Vossel et al., 2014). Disruptions to the SN and/or VAN are linked to increased risk of psychiatric and substance use disorders, particularly psychotic disorders (Hurd et al., 2019; Sylvester et al., 2013; Zilverstand et al., 2018). Therefore, lower connectivity between these core attentional networks may contribute to increased risk of psychopathology following PCE. In line with this hypothesis, we observed initial evidence that psychotic-like experiences mediated the association between PCE and SN-VAN rsFC. These findings underscore that symptomatology might affect functioning of attention-related neural networks. However, reversal of the model was also significant, suggesting that disruptions in attentional networks may also affect symptomatology. Importantly, these effects were specific to PCE before (but not after) knowledge of pregnancy, which suggests that cannabis may have greater effects on functional network organization early during gestation (e.g., during the embryonic period). Although the mediation effects were no longer significant after adjusting for potential confounders, future analyses incorporating longitudinal waves of the ABCD data set may be valuable for uncovering the role of neural network alterations in the emergence of psychopathology.
The notion that PCE exerts lasting effects on neurodevelopment early during gestation, in particular, is in line with prior studies on the role of the eCB system during development. ECBs, such as anandamide (AEA), are shown to be present and functional early during neural development. Indeed, AEA is a partial agonist for the orphan g-protein coupled receptor GPR55 (as reviewed by Sharir & Abood, 2010) that is involved in regulation of axon growth and refinement during development (Cherif et al., 2015). Therefore, administration of exogenous cannabinoids, such as THC and/or cannabidiol, may disrupt the developing eCB system and impact postnatal outcomes. Indeed, a recent preclinical study in a cannabis inhalation model demonstrated that up to 30% of THC in circulating maternal blood reaches the fetal brain (Baglot et al., 2022). In sum, our findings add to the growing body of evidence suggesting that PCE, particularly during early gestation, can have detrimental effects on offspring neurodevelopment.
We found initial associations among PCE, SN rsFC, and symptoms related to psychosis proneness, particularly psychotic-like experiences; however, these findings were no longer significant after adjusting for covariates. Several recent studies have reported increased vulnerability to psychosis symptoms in children exposed to cannabis in utero (Bolhuis et al., 2018; Paul et al., 2021), although these initial reports rely on cross-sectional data and parent retrospective report of cannabis use during pregnancy. Nonetheless, disruptions to the SN and/or VAN are consistently linked to psychosis. Indeed, a voxel-wise meta-analysis of 56 rsFC data sets demonstrated a consistent pattern of reduced connectivity within and between the SN and VAN in patients with schizophrenia relative to healthy controls (Dong et al., 2018). Functioning of the SN, particularly the insula — one of the core SN nodes — is frequently characterized as aberrant in neurobiological models of schizophrenia (Damaraju et al., 2014). Specifically, the saliency or “dysconnectivity” hypothesis of schizophrenia suggests that aberrant functioning within and between this network and other core neurocognitive networks underlies difficulties in differentiating inner experiences from external stimuli (Damaraju et al., 2014; Dong et al., 2018; Palaniyappan & Liddle, 2012). The core function of the SN is to detect salient stimuli and appropriately engage neurocognitive networks involved in attentional control, internal processing, or executive functioning. Therefore, our results suggest the intriguing possibility that altered neurodevelopment of SN-VAN connections may be an intermediate phenotype leading to elevated risk of psychosis in children with PCE. This hypothesis should be explored in future research.
Our results should be interpreted in the context of limitations. First, mediation analyses relied on cross-sectional data and should therefore be considered preliminary. Future longitudinal studies, including in the ABCD data set, are needed to confirm these associations. Second, in the ABCD data set, PCE was measured via parent self-report, which is known to under-estimate use (Young-Wolff et al., 2017). However, the use of a large diverse cohort increased sensitivity to detect effects. Future studies including toxicology tests may reveal stronger associations than what is reported here. Similarly, the timing of prenatal substance exposures was estimated using parent retrospective report of exposure before vs. after knowledge of pregnancy, which is not a precise measure of fetal age. On average, women who reported cannabis use before knowledge of pregnancy were at almost 8 weeks when they found out they were pregnant. Therefore, on average, our findings may reflect the impact of PCE during the embryonic period, and also encompasses a period of time in which the eCB system is thought to be present and functional (at around 5 weeks). However, there is substantial variation in when individuals reported finding out that they were pregnant; therefore, studies in animal models may better identify periods of greatest in utero vulnerability. Finally, our analysis focused a priori on core neurocognitive networks, specifically the SN, given its critical role in cognitive and emotional neurodevelopment. Future studies are needed to explore whole-brain functional organization, and effects of PCE on organization of other networks (e.g., sensory, motor).
To the best of our knowledge, this is the first study to examine the impact of PCE on functional organization of large-scale neurocognitive networks in children. Our results demonstrate that PCE is associated with reduced functional interactions between the SN and VAN — networks critical for modulating salience processing and attentional control. This study also extends recent reports of elevated risk of symptoms of psychopathology in children with PCE, particularly psychotic-like experiences. Indeed, here we find initial evidence that disruptions in SN-VAN rsFC may serve as an intermediate phenotype linking PCE to elevated risk of psychosis in offspring. These findings illustrate the need for future studies examining the impact of PCE on the development of large-scale neural networks and subsequent risk of psychopathology in offspring.
Significance Statement.
Cannabis use among pregnant people has increased over the past decade. This is of concern as prenatal cannabis exposure is associated with cognitive, motor, and social deficits among children. However, the neural mechanisms underlying these associations are unclear. This study leveraged the Adolescent Brain Cognitive Development study to examine the impact of prenatal cannabis exposure on organization of large-scale core neurocognitive networks in over 10,000 children. We also linked changes in functional neural networks to symptoms thought to be associated with psychopathology (e.g., hallucinations, thought disorder, attention problems) that previously have been linked to prenatal cannabis exposure.
Support or grant information:
Dr. Marusak is supported by National Institute of Mental Health grant K01MH119241 and Eunice Kennedy Shriver National Institute of Child Health and Human Development grant R21HD105882. Mr. Faraj was supported by a Wayne State University School of Medicine Research Fellowship (MSRF). Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive DevelopmentSM (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children age 9–10 and follow them over 10 years into early adulthood. The ABCD Study® is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041048, U01DA050989, U01DA051016, U01DA041022, U01DA051018, U01DA051037, U01DA050987, U01DA041174, U01DA041106, U01DA041117, U01DA041028, U01DA041134, U01DA050988, U01DA051039, U01DA041156, U01DA041025, U01DA041120, U01DA051038, U01DA041148, U01DA041093, U01DA041089, U24DA041123, U24DA041147
Footnotes
Conflict of Interest and other Ethics Statements: The authors declare no conflict of interest.
Data availability statement:
These data were derived from data available to authorized users on the NIMH Data Archive System at https://nda.nih.gov/abcd.
References:
- Achenbach T., & Rescorla L. (2001). Manual for the ASEBA School-Age Forms & Profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families. [Google Scholar]
- Alwan S, & Chambers CD (2015). Identifying Human Teratogens : An Update. 39–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglot SL, VanRyzin JW, Marquardt AE, Aukema RJ, Petrie GN, Hume C, Reinl EL, Bieber JB, McLaughlin RJ, McCarthy MM, & Hill MN (2022). Maternal-fetal transmission of delta-9-tetrahydrocannabinol (THC) and its metabolites following inhalation and injection exposure during pregnancy in rats. Journal of Neuroscience Research, 100(3), 713–730. 10.1002/jnr.24992 [DOI] [PubMed] [Google Scholar]
- Battistella G, Fornari E, Annoni JM, Chtioui H, Dao K, Fabritius M, Favrat B, Mall JF, Maeder P, & Giroud C (2014). Long-term effects of cannabis on brain structure. Neuropsychopharmacology, 39(9), 2041–2048. 10.1038/npp.2014.67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Falkenberg I, Martin-Santos R, Atakan Z, Crippa JA, Giampietro V, Brammer M, & McGuire P (2015). Cannabinoid Modulation of Functional Connectivity within Regions Processing Attentional Salience. Neuropsychopharmacology, 40(6), 1343–1352. 10.1038/npp.2014.258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blest-Hopley G, Giampietro V, & Bhattacharyya S (2018). Residual effects of cannabis use in adolescent and adult brains — A meta-analysis of fMRI studies. Neuroscience and Biobehavioral Reviews, 88(March), 26–41. 10.1016/j.neubiorev.2018.03.008 [DOI] [PubMed] [Google Scholar]
- Bolhuis K, Kushner SA, Yalniz S, Hillegers MHJ, Jaddoe VWV, Tiemeier H, & El Marroun H (2018). Maternal and paternal cannabis use during pregnancy and the risk of psychotic-like experiences in the offspring. Schizophrenia Research, 202(2018), 322–327. 10.1016/j.schres.2018.06.067 [DOI] [PubMed] [Google Scholar]
- Branum AM, & Ahrens KA (2017). Trends in Timing of Pregnancy Awareness Among US Women. Maternal and Child Health Journal, 21(4), 715–726. 10.1007/s10995-016-2155-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns HD, Van Laere K, Sanabria-Bohórquez S, Hamill TG, Bormans G, Eng WS, Gibson R, Ryan C, Connolly B, Patel S, Krause S, Vanko A, Van Hecken A, Dupont P, De Lepeleire I, Rothenberg P, Stoch SA, Cote J, Hagmann WK, … Hargreaves RJ (2007). [18F]MK-9470, a positron emission tomography (PET) tracer for in vivo human PET brain imaging of the cannabinoid-1 receptor. Proceedings of the National Academy of Sciences of the United States of America. 10.1073/pnas.0703472104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Cannonier T, Conley MI, Cohen AO, Barch DM, Heitzeg MM, Soules ME, Teslovich T, Dellarco DV, Garavan H, Orr CA, Wager TD, Banich MT, Speer NK, Sutherland MT, Riedel MC, Dick AS, Bjork JM, Thomas KM, … Dale AM (2018). The Adolescent Brain Cognitive Development (ABCD) study: Imaging acquisition across 21 sites. In Developmental Cognitive Neuroscience. 10.1016/j.dcn.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherif H, Argaw A, Cécyre B, Bouchard A, Gagnon J, Javadi P, Desgent S, Mackie K, & Bouchard JF (2015). Role of GPR55 during axon growth and target innervation. ENeuro, 2(5). 10.1523/ENEURO.0011-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiong W, Wilson SM, D’Esposito M, Kayser AS, Grossman SN, Poorzand P, Seeley WW, Miller BL, & Rankin KP (2013). The salience network causally influences default mode network activity during moral reasoning. Brain, 136(6), 1929–1941. 10.1093/brain/awt066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn AM, Johnson AL, Rose SW, Rath JM, & Villanti AC (2017). Support for Marijuana Legalization and Predictors of Intentions to Use Marijuana More Often in Response to Legalization Among U.S. Young Adults. Substance Use and Misuse, 52(2), 203–213. 10.1080/10826084.2016.1223688 [DOI] [PubMed] [Google Scholar]
- Corsi DJ, Walsh L, Weiss D, Hsu H, El-Chaar D, Hawken S, Fell DB, & Walker M (2019). Association between Self-reported Prenatal Cannabis Use and Maternal, Perinatal, and Neonatal Outcomes. JAMA - Journal of the American Medical Association, 322(2), 145–152. 10.1001/jama.2019.8734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damaraju E, Allen EA, Belger A, Ford JM, McEwen S, Mathalon DH, Mueller BA, Pearlson GD, Potkin SG, Preda A, Turner JA, Vaidya JG, Van Erp TG, & Calhoun VD (2014). Dynamic functional connectivity analysis reveals transient states of dysconnectivity in schizophrenia. NeuroImage: Clinical, 5(July), 298–308. 10.1016/j.nicl.2014.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, & Fletcher JMJM (2009). Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. In Journal of the International Neuropsychological Society. 10.1017/S1355617709090481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick AS, Lopez DA, Watts AL, Heeringa S, Reuter C, Bartsch H, Fan CC, Kennedy DN, Palmer C, Marshall A, Haist F, Hawes S, Nichols TE, Barch DM, Jernigan TL, Garavan H, Grant S, Pariyadath V, Hoffman E, … Thompson WK (2021). Meaningful associations in the adolescent brain cognitive development study. In NeuroImage. 10.1016/j.neuroimage.2021.118262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickson B, Mansfield C, Guiahi M, Allshouse AA, Borgelt LM, Sheeder J, Silver RM, & Metz TD (2018). Recommendations from cannabis dispensaries about first-trimester cannabis use. Obstetrics and Gynecology, 131(6), 1031–1038. 10.1097/AOG.0000000000002619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong D, Wang Y, Chang X, Luo C, & Yao D (2018). Dysfunction of Large-Scale Brain Networks in Schizophrenia: A Meta-analysis of Resting-State Functional Connectivity. Schizophrenia Bulletin, 44(1), 168–181. 10.1093/schbul/sbx034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NUF, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, & Bradley L (2006). A Core System for the Implementation of Task Sets. Neuron, 50(5), 799–812. https://doi.org/doi: 10.1016/j.neuron.2006.04.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garavan H, Bartsch H, Conway K, Decastro A, Goldstein RZ, Heeringa S, Jernigan T, Potter A, Thompson W, & Zahs D (2018). Recruiting the ABCD sample: Design considerations and procedures. Developmental Cognitive Neuroscience. 10.1016/j.dcn.2018.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon EM, Laumann TO, Adeyemo B, Huckins JF, Kelley WM, & Petersen SE (2016). Generation and Evaluation of a Cortical Area Parcellation from Resting-State Correlations. Cerebral Cortex. 10.1093/cercor/bhu239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagler. (2019). Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. Neuroimage, 202. https://doi.org/doi: 10.1016/j.neuroimage.2019.116091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkany T, Guzmán M, Galve-Roperh I, Berghuis P, Devi LA, & Mackie K (2007). The emerging functions of endocannabinoid signaling during CNS development. In Trends in Pharmacological Sciences. 10.1016/j.tips.2006.12.004 [DOI] [PubMed] [Google Scholar]
- Hayes AF (2012). PROCESS: A versatile computational tool for observed variable mediation, moderation, and conditional process modeling. In White paper (pp. 1–39). https://doi.org/978-1-60918-230-4 [Google Scholar]
- Heng L, Beverley JA, Steiner H, & Tseng KY (2011). Differential developmental trajectories for CB1 cannabinoid receptor expression in limbic/associative and sensorimotor cortical areas. Synapse. 10.1002/syn.20844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd YL, Manzoni OJ, Pletnikov MV, Lee FS, Bhattacharyya S, & Melis M (2019). Cannabis and the developing brain: Insights into its long-lasting effects. Journal of Neuroscience, 39(42), 8250–8258. 10.1523/JNEUROSCI.1165-19.2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingstrup KG, Liu X, Gasse C, Debost JCP, & Munk-Olsen T (2018). Prescription drug use in pregnancy and variations according to prior psychiatric history. Pharmacoepidemiology and Drug Safety, 27(1), 105–113. 10.1002/pds.4355 [DOI] [PubMed] [Google Scholar]
- Karcher NR, Barch DM, Avenevoli S, Savill M, Huber RS, Simon TJ, Leckliter IN, Sher KJ, & Loewy RL (2018). Assessment of the Prodromal Questionnaire–Brief Child Version for Measurement of Self-reported Psychoticlike Experiences in Childhood. 75(8), 853–861. https://doi.org/doi: 10.1001/jamapsychiatry.2018.1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewy1 RL, Pearson R, Vinogradov S, Bearden CE, & Cannon D. T (2011). Psychosis Risk Screening with the Prodromal Questionnaire – Brief version (PQ-B). Schizophr Res, 129(1), 42–46. https://doi.org/doi: 10.1016/j.schres.2011.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusak HA, Calhoun VD, Brown S, Crespo LM, Sala-Hamrick K, Gotlib IH, & Thomason ME (2016). Dynamic functional connectivity of neurocognitive networks in children. Human Brain Mapping. 10.1002/hbm.23346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palaniyappan L, & Liddle PF (2012). Does the salience network play a cardinal role in psychosis? An emerging hypothesis of insular dysfunction. Journal of Psychiatry and Neuroscience, 37(1), 17–27. 10.1503/jpn.100176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul SE, Hatoum AS, Fine JD, Johnson EC, Hansen I, Karcher NR, Moreau AL, Bondy E, Qu Y, Carter EB, Rogers CE, Agrawal A, Barch DM, & Bogdan R (2021). Associations between Prenatal Cannabis Exposure and Childhood Outcomes: Results from the ABCD Study. JAMA Psychiatry, 78(1), 64–76. 10.1001/jamapsychiatry.2020.2902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson KA, Hester AK, & McLemore GL (2016). Prenatal cannabis exposure - The “first hit” to the endocannabinoid system. Neurotoxicology and Teratology, 58(2016), 5–14. 10.1016/j.ntt.2016.08.003 [DOI] [PubMed] [Google Scholar]
- Rodriguez CE, Sheeder J, Allshouse AA, Scott S, Wymore E, Hopfer C, Hermesch A, & Metz TD (2019). Marijuana use in young mothers and adverse pregnancy outcomes: a retrospective cohort study. BJOG: An International Journal of Obstetrics and Gynaecology, 126(12), 1491–1497. 10.1111/1471-0528.15885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, & Greicius MD (2007). Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience, 27(9), 2349–2356. 10.1523/JNEUROSCI.5587-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharir H, & Abood ME (2010). Pharmacological characterization of GPR55, a putative cannabinoid receptor. Pharmacology and Therapeutics, 126(3), 301–313. 10.1016/j.pharmthera.2010.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield JM, Repovs G, Harms MP, Carter CS, Gold JM A. W. M 3rd, Ragland JD, Silverstein SM, Godwin D, & Barch DM (2015). Fronto-parietal and cingulo-opercular network integrity and cognition in health and schizophrenia. Neuropsychologia, 73, 82–93. 10.1016/j.neuropsychologia.2015.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira MM, Arnold JC, Laviolette SR, Hillard CJ, Celorrio M, Aymerich MS, Adams WK, Zilverstand A, Huang AS, Alia-Klein N, Goldstein RZ, Dick AS, Lopez DA, Watts AL, Heeringa S, Reuter C, Bartsch H, Fan CC, Kennedy DN, … Bhattacharyya S (2018). Cannabis and the developing brain: Insights into its long-lasting effects. Neuropsychopharmacology, 39(3), 1–18. 10.1111/j.1365-2826.2008.01670.x [DOI] [Google Scholar]
- Sylvester CM, Barch DM, Corbetta M, Power JD, Schlaggar BL, & Luby JL (2013). Resting State Functional Connectivity of the Ventral Attention Network in Children With a History of Depression or Anxiety. J Am Acad Child Adolesc Psychiatry, 52(12). 10.1016/j.jaac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tau GZ, & Peterson BS (2010). Normal development of brain circuits. Neuropsychopharmacology, 35(1), 147–168. 10.1038/npp.2009.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R, Dejong K, Lo J, Resident O, Health O, Resident O, Health O, & Health O (2019). Marijuana Use in Pregnancy: A Review. Obstet Gynecol Surv, 74(7), 415–428. https://doi.org/doi: 10.1097/OGX.0000000000000685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Surgeon General’s Advisory: Marijuana Use and the Developing Brain. (n.d.).
- Volkow N, Han B, Compton W, & et al. (2019). Self-reported Medical and Nonmedical Cannabis Use Among Pregnant Women in the United States. JAMA - Journal of the American Medical Association, 322(2), 167–169. https://doi.org/doi: 10.1001/jama.2019.7982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vossel S, Geng JJ, & Fink GR (2014). Dorsal and ventral attention systems: Distinct neural circuits but collaborative roles. Neuroscientist. 10.1177/1073858413494269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting PF, Wolff RF, Deshpande S, Di Nisio M, Duffy S, Hernandez AV, Keurentjes JC, Lang S, Misso K, Ryder S, Schmidlkofer S, Westwood M, & Kleijnen J (2015). Cannabinoids for medical use: A systematic review and meta-analysis. In JAMA - Journal of the American Medical Association. 10.1001/jama.2015.6358 [DOI] [PubMed] [Google Scholar]
- Wijayendran SB, O’neill A, & Bhattacharyya S (2018). The effects of cannabis use on salience attribution: A systematic review. Acta Neuropsychiatrica, 30(1), 43–57. 10.1017/neu.2016.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young-Wolff KC, Tucker LY, Alexeeff S, Armstrong MA, Conway A, Weisner C, & Goler N (2017). Trends in self-reported and biochemically tested Marijuana use among pregnant females in California from 2009–2016. In JAMA - Journal of the American Medical Association. 10.1001/jama.2017.17225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilverstand A, Huang AS, Alia-Klein K, & Goldstein1 RZ (2018). Neuroimaging Impaired Response Inhibition and Salience Attribution in Human Drug Addiction. A systematic review. Physiology & Behavior, 98(5), 886–903. 10.1016/j.neuron.2018.03.048.Neuroimaging [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
These data were derived from data available to authorized users on the NIMH Data Archive System at https://nda.nih.gov/abcd.


