Abstract
Accumulating data suggest that air pollution increases the risk of internalizing psychopathology, including anxiety and depressive disorders. Moreover, the link between air pollution and poor mental health may relate to neurostructural and neurofunctional changes. We systematically reviewed the MEDLINE database in September 2021 for original articles reporting effects of air pollution on 1) internalizing symptoms and behaviors (anxiety or depression) and 2) frontolimbic brain regions (i.e., hippocampus, amygdala, prefrontal cortex). One hundred and eleven articles on mental health (76% human, 24% animals) and 92 on brain structure and function (11% human, 86% animals) were identified. For literature search 1, the most common pollutants examined were PM2.5 (64.9%), NO2 (37.8%), and PM10 (33.3%). For literature search 2, the most common pollutants examined were PM2.5 (32.6%), O3 (26.1%) and Diesel Exhaust Particles (DEP) (26.1%). The majority of studies (73%) reported higher internalizing symptoms and behaviors with higher air pollution exposure. Air pollution was consistently associated (95% of articles reported significant findings) with neurostructural and neurofunctional effects (e.g., increased inflammation and oxidative stress, changes to neurotransmitters and neuromodulators and their metabolites) within multiple brain regions (24% of articles), or within the hippocampus (66%), PFC (7%), and amygdala (1%). For both literature searches, the most studied exposure time frames were adulthood (48% and 59% for literature searches 1 and 2, respectively) and the prenatal period (26% and 27% for literature searches 1 and 2, respectively). Forty-three percent and 29% of studies assessed more than one exposure window in literature search 1 and 2, respectively. The extant literature suggests that air pollution is associated with increased depressive and anxiety symptoms and behaviors, and alterations in brain regions implicated in risk of psychopathology. However, there are several gaps in the literature, including: limited studies examining the neural consequences of air pollution in humans. Further, a comprehensive developmental approach is needed to examine windows of susceptibility to exposure and track the emergence of psychopathology following air pollution exposure.
Keywords: Air pollution, mental health, frontolimbic, brain, anxiety, depression
1. BACKGROUND/INTRODUCTION
Emerging research links exposure to environmental pollutants, including sources from air pollution, to increased prevalence and/or severity of mental disorders (Braithwaite et al., 2019; Zhao et al., 2018). Understanding the potential role of air pollution in risk of psychiatric disease is a major public health concern given that 99% of the world’s population live in environments that do not meet World Health Organization air quality guidelines (Ambient (Outdoor) Air Pollution Fact Sheet, 2021). Further, in 2019, more than one in ten people globally lived with a mental health disorder (Dattani et al., 2021). Exposure to air pollution is consistently linked to increased risk of internalizing disorders, such as anxiety and depression (Borroni et al., 2022; Trushna et al., 2021). Anxiety and depression are the most common mental disorders across the globe (Dattani et al., 2021) and can increase an individual’s risk of suicide attempts and completion (Soto-Sanz et al., 2019), adversely affect family and social relationships, and are associated with substantial individual and societal economic burden. Indeed, these disorders cost the global economy approximately 1 trillion US dollars each year in lost productivity (The Lancet Global, 2020). Despite the emerging evidence that environmental pollutants play a role in mental health, the biological mechanisms underlying environmental risk of psychiatric disorders (e.g., central nervous system (CNS) disruptions) are unknown.
Atmospheric composition from air pollution is a complex mixture of particulate matter and gases including particulate matter (PM) of varying sizes, nitrogen oxides, ozone (O3), volatile organic compounds (VOCs), polycyclic aromatic hydrocarbons (PAHs) and others (Hahad et al., 2020; Huang et al., 2020; Huang et al., 2017). Anthropogenic sources of air pollution includes both mobile (e.g., motor vehicles) and stationary (e.g., factories, power plants) sources (EPA, 2018). There is substantial regional variability in air pollution levels, with urban areas responsible for nearly 78% of emissions that affect over 50% of the world’s population (Bereitschaft & Debbage, 2013; Liang & Gong, 2020). Additionally, there is substantial spatiotemporal variation in air pollution concentrations, and up to half of the variation is attributed to meteorological conditions (e.g., temperature, humidity, precipitation, wind) (Tai et al., 2010). With climate change concerns on the rise, including increased temperatures and adverse weather events, changes in these meteorological parameters can adversely affect air quality, by changing atmospheric ventilation and dilution, precipitation, and other removal processes (Fiore et al., 2015; Kinney, 2008). Thus, continued research on the health consequences of air pollution is of utmost importance. Air pollution is considered a major environmental health threat and is associated with a range of health outcomes, including adverse birth outcomes, obesity, cancer, and respiratory and cardiovascular disease (see Manisalidis et al. (2020) for a review). Growing evidence indicates that exposure to air pollution can also impact the CNS (Babadjouni et al., 2017; Costa et al., 2020; Kim, Kim, et al., 2020), with studies showing adverse effects on cognitive and behavioral functioning, poor attention, decreased intelligence quotient (IQ), memory, and academic performance (Cipriani et al., 2018; Clifford et al., 2016; Stenson et al., 2021). Recent studies have also identified air pollution as a major risk factor of internalizing psychopathology. For example, a recent meta-analysis found that an increase in ambient PM (PM2.5 and PM10) concentration was strongly associated with increased risk of depression, as well as suicide (Q. Liu et al., 2021). However, the mechanism(s) by which pollutants, such as PM, affect the CNS and contribute to risk of internalizing psychopathology remains unclear.
A growing body of preclinical and human neuroimaging studies indicates that air pollution exposure may increase risk of internalizing psychopathology by altering frontolimbic brain regions, including the hippocampus, amygdala, and prefrontal cortex (PFC) (Ehsanifar, Montazeri, et al., 2021; Salvi et al., 2020; Yao et al., 2015). These regions play a key role in stress responding and emotion regulation and are implicated in the pathophysiology of internalizing disorders (Espinoza Oyarce et al., 2020; Janiri et al., 2020; Kolesar et al., 2019). Preclinical studies suggest that ultrafine particles (UFP) and nanosized particulate matter (nPM) may affect the nervous system directly through crossing the olfactory bulb and blood brain barrier and other air pollutants (PM2.5, PM10, O3, etc.) may indirectly affect the CNS through neuroimmune or neuroinflammatory reactions (Costa et al., 2020; Genc et al., 2012). Indeed, animal studies frequently report an increase in inflammatory and oxidative stress reactions, and changes in neurotransmitter receptor gene expression in frontolimbic brain regions, particularly the hippocampus, amygdala, and PFC following air pollution exposure (Ehsanifar, Montazeri, et al., 2021; Salvi et al., 2020; Yao et al., 2015). Consistent with these findings, human neuroimaging studies show that air pollution exposure is associated with lower frontolimbic gray matter volumes (e.g., PFC, medial temporal regions), and altered microstructure of white matter tracts that connect frontolimbic brain regions (e.g., cingulum bundle) (Herting et al., 2019; Lubczynska et al., 2020). Thus, air pollution exposure may impact the frontolimbic brain regions and pathways associated with stress and emotion regulation, which then may lead to increased risk of internalizing symptomatology.
Several recent systematic reviews have been conducted on the impact of air pollution on mental health (Borroni et al., 2022; Braithwaite et al., 2019; Fan et al., 2020; Q. Liu et al., 2021; Margolis et al., 2022; Trushna et al., 2021; Zeng et al., 2019; Zhao et al., 2018). However, these reviews either focused on one specific air pollutant (e.g., PM) or on specific developmental periods (e.g., adults). Further, only one of these reviews included translational models of internalizing behaviors (e.g., open field test in rodent models); the remainder included only human observational studies. More recently, Margolis et al. (Margolis et al., 2022) published an important review on animal models of prenatal air pollution exposure and internalizing and externalizing behaviors; however, no comparison was made between the animal models and the current human literature. Similarly, while recent systematic reviews have been conducted on air pollution and brain structure and function (Balboni et al., 2022; de Prado Bert et al., 2018; Herting et al., 2019), none have included preclinical studies, which account for most studies on this topic. Furthermore, only one review focused on frontolimbic brain regions, that are highly relevant to internalizing psychopathology (Balboni et al., 2022).
To address these gaps, we performed two systematic reviews to examine the literature, both human and animal studies, on the effects of air pollution on (1) anxiety and depressive symptoms and behaviors, and on (2) frontolimbic brain regions involved in internalizing psychopathology (i.e., PFC, amygdala, and hippocampus). We also explored the impact of exposure timing (e.g., prenatal/early-life, childhood, adolescent, adulthood), timing of outcome assessment, technique of exposure assessment, sex differences and age differences on psychopathology and neural outcomes. We synthesize the results, discuss potential neurobiological mechanisms (e.g., neuroinflammation), and highlight gaps in the literature. We end by discussing directions for future research and the implications of neurobehavioral alterations for the prevention and treatment of internalizing disorders in at-risk individuals, such as urban inhabitants.
2. METHODOLOGY
2.1. Search Strategy
On September 13, 2021, we performed two searches of the MEDLINE database through PubMed to identify publications linking outdoor air pollution with (1) internalizing psychopathology (anxiety or depression), and (2) outcomes in frontolimbic brain regions commonly implicated in the pathophysiology of these disorders. The literature search included both human and nonhuman animal studies, and English-language studies only. Figure 1 outlines the study selection process for searches 1 and 2. Each step of the review was informed by PRISMA guidelines (Page et al., 2021). Additional information on the search strategy and search terms used can be found in the Supplemental Material.
Figure 1.
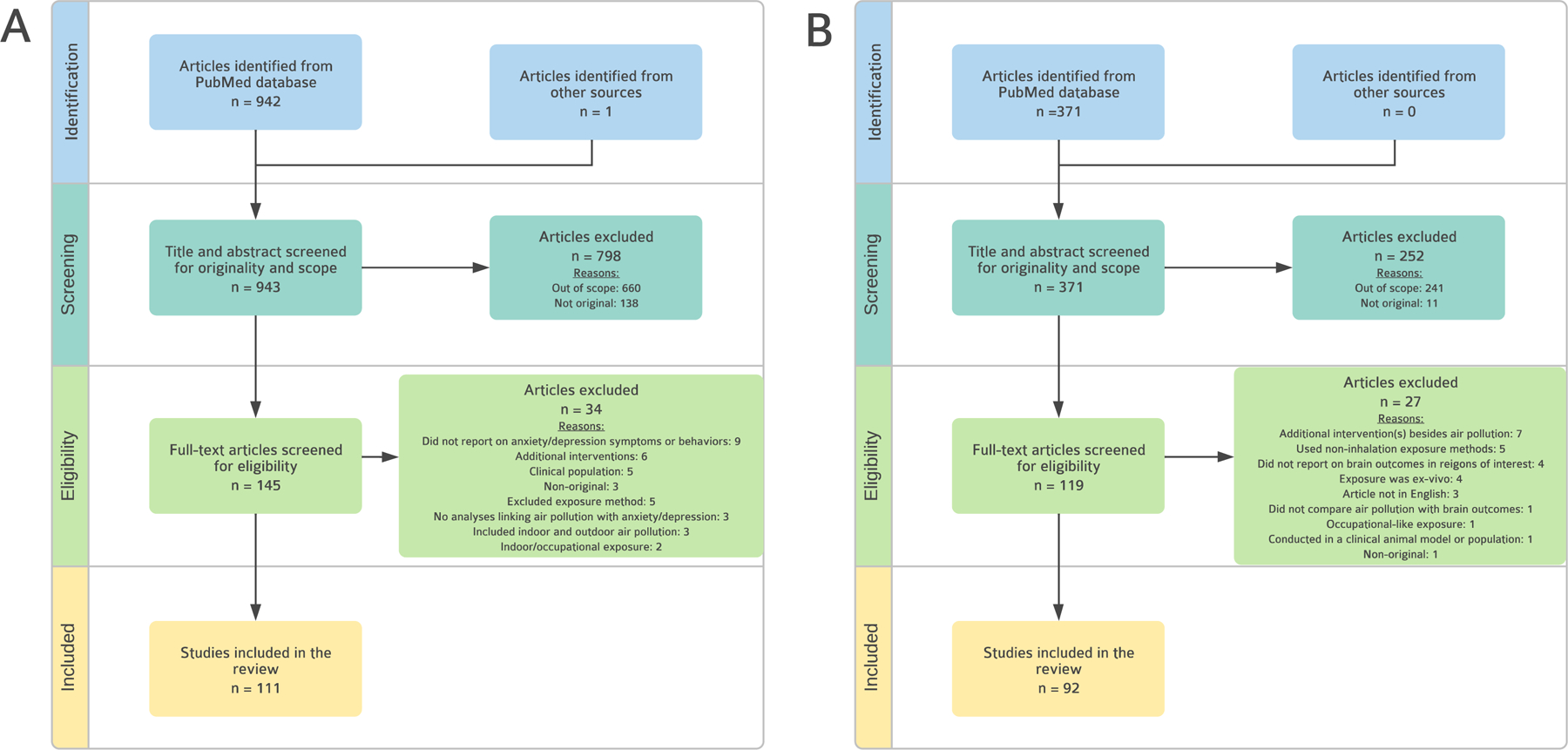
Flowchart for Literature Search 1 and 2.
Panel A describes Literature Search 1: Air Pollution and Internalizing Behaviors and Symptoms. Panel B describes Literature Search 2: Air Pollution and Frontolimbic Brain Regions Each step of the reviews was informed by PRISMA guidelines (Page et al., 2021).
2.2. Study Screening
Titles and abstracts, and then full texts were subsequently screened to determine relevance to the review. Studies were included in both searches if they were a (i) full-length original research article, and (ii) reported on exposure to ambient air pollution, for animals – delivered via inhalation, intranasal, intratracheal, or oropharyngeal aspiration/instillation, and for humans – through fixed ground monitor stations, geospatial estimates, personal air monitoring, or targeted recruitment from highly polluted areas. Included studies examined air pollution exposure during the prenatal, early-life, childhood, adolescent, adult, and late-life periods. Studies were excluded from both searches if they were (i) out of scope, (ii) did not compare air pollution with anxiety/depression or brain outcomes, (iii) focused on indoor or occupational air pollutants (e.g., secondhand smoking, solvent exposure), (iv) conducted in a clinical animal model or population (e.g., Alzheimer’s phenotype), and (v) included additional interventions or exposures (e.g., air pollution + high fat diet). For search 2, we excluded ex-vivo studies and studies that did not report on our brain regions of interest (i.e., hippocampus, amygdala, (pre)frontal cortex). Although ventromedial prefrontal cortex (vmPFC) and adjacent ventral anterior cingulate cortex (vACC) are particularly implicated in emotion regulation and internalizing psychopathology (Hiser & Koenigs, 2018), most animal studies reported on frontal cortex or did not specify the cortical area. To limit our focus to frontal regions, we excluded studies that reported only on cortical regions or did not specify the location to be in the frontal cortex. Full texts were screened by CZ and HM, and uncertainty was discussed by both authors together.
2.3. Study classification
Articles were classified by (1) species (e.g., human, mice, rats), (2) pollutant (e.g., PM2.5, PAHs), (3) exposure window (e.g., prenatal, early-life, adolescence, adulthood, and later life), (4), duration of exposure, (5) sample size, (6) gender or sex, (7) exposure assessment method (e.g., land-use regression, fixed site monitoring), (8) period of behavioral assessment (e.g., prenatal, early-life, adolescence, adulthood, and late-life) and (9) outcome measure, see Tables 1 and 2. For human studies, 0–5 years of age was considered ‘early-life’, 6–9 ‘childhood’, 10–17 ‘adolescence’, 18–64 years was considered ‘adulthood’, and ≥65 years was considered ‘late-life’. For studies in rodents, we followed Semple et al. (2013)’s benchmarks of maturation and vulnerability to injury across species which considers post-natal day (PND) 1–21 to be ‘early-life’, PND 25–35 to be ‘childhood’, PND 35–49 as ‘adolescence’, and PND 60+ as ‘adulthood’. To determine when rodents were considered senescent or the equivalent of human “late-life”, we followed the Jackson Laboratory’s established protocol of 18–24 months of age, when senescent changes in biomarkers can be detected (Flurkey et al., 2007; Life Span as a Biomarker, 2022). Thus, animal studies in which assessments were conducted during PND 540+ were considered “late-life”. We also provide a brief overview of methodologically rigorous studies within each category.
Table 1.
Final Included Articles from Literature Search 1: Air Pollution and Internalizing Symptoms and Behaviors
| First author & Year | Species | Pollutant | Exposure Window | Duration of Exposure | N | Gender or sex | Exposure Assessment Method | Period of Behavioral Assessment | Internalizing outcome assessed | Internalizing increased or decreased |
|---|---|---|---|---|---|---|---|---|---|---|
| Emik and Plata (1969) | Mice (C57 BL) | Multiple Pollutants | Adulthood | Continuously for 8 weeks | 24 | Male | Whole Body Inhalation | Late-life | Depression | Increased |
| Campbell et al. (1970) | Mice (C57BL) | Peroxyacetyl nitrate (PAN) | Adulthood | 224 days, 7–8 months | 9–12/group | Male | Whole Body Inhalation | Adulthood | Depression | Increased |
| Tepper et al. (1982) | Rats (Long-Evans) | Ozone | Adulthood | 6 hours | 16 | Male | Whole Body Inhalation | Adulthood | Depression | Increased |
| Evans et al. (1988) | Human | Ozone | Adulthood | Not reported | 1,002 | Both | Conducted during a heavy and light polluted seasons | Adulthood | Both | Mixed |
| Musi et al. (1994) | Mice (CD-1) | Ozone | Not reported | 13 days | 10/group | Both | Whole Body Inhalation | Not reported | Both | Increased |
| Szyszkowicz (2007) | Human | Multiple Pollutants | Early-life through Late-life | 0–2 days | 15,556 | Both | Used measurements from fixed ground monitoring stations | Early-life through late-life | Both | Mixed |
| Szyszkowicz et al. (2009) | Human | Multiple Pollutants | Early-life through Late-life | 0–2 days | 27,047 | Both | Used measurements from fixed ground monitoring stations | Early-life through late-life | Depression | Increased |
| Szyszkowicz et al. (2010) | Human | Multiple Pollutants | Early-life through Late-life | 0–2 days | 9,358 | Both | Used measurements from fixed ground monitoring stations | Early-life through late-life | Depression | Increased |
| Fonken et al. (2011) | Mice (C57BL/6) | PM2.5 | From Childhood through Adulthood | 10 months | Not reported | Male | Whole Body Inhalation | Adulthood | Both | Increased |
| Perera et al. (2011) | Human | PAH | Prenatal, Childhood | Biomarkers used, not specified | 215 | Both | Biological markers | Childhood | Both | Increased |
| (Bowler et al., 2012) | Human | Manganese | Adulthood | Estimated off of years of residence, mean was 36.1 + 15.8 years. | 190, 100 exposed, 90 controls | Both | Dispersion modeling | Adulthood | Anxiety | Increased |
| Lim et al. (2012) | Human | Multiple Pollutants | Adulthood to Late-life | 0–28 days | 537 | Both | Used measurements from fixed ground monitoring stations | Adulthood and Late-life | Depression | Increased |
| Perera et al. (2012) | Human | PAH | Prenatal | 3 months | 253 | Both | Personal air monitoring | Childhood | Both | Increased |
| Davis et al. (2013) | Mice (C57BL/6J) | nPM | Prenatal | 10 weeks | 5–10/group | Both | Whole Body Inhalation | Adulthood | Both | Mixed |
| Peiffer et al. (2013) | Rats (Wistar Han) | PAH | Adulthood | 14 days | 18/group | Male | Nose-only inhalation | Adulthood | Anxiety | Decreased |
| Y. Wang et al. (2014) | Human | Multiple Pollutants | Late-life | 1–14 days, 1 year | 765 | Both | Combination | Late-life | Depression | No effects observed |
| Power et al. (2015) | Human | Multiple Pollutants | Adulthood and Late-life | 1, 3, 6, months, 1 year, 15 years | 71,271 | Female | General additive mixed models | Adulthood & Late-life | Anxiety | Increased |
| Kim et al. (2016) | Human | PM2.5 | Adolescence, Adulthood, Late-life | 1 year, 3 years | 27,270 | Both | Used measurements from fixed ground monitoring stations | Adolescence, Adulthood, Late-life | Depression | Increased |
| Margolis et al. (2016) | Human | PAH | Prenatal | 3–4 months | 462 | Both | Biological markers | Early-life, childhood, adolescence | Both | Increased |
| Miller et al. (2016) | Mice (BALB/cByj) | PAH | Prenatal | 3 weeks | 14–18/group | Both | Whole Body Inhalation | Adulthood | Anxiety | Mixed |
| Szyszkowicz et al. (2016) | Human | Multiple Pollutants | Early-life through Late-life | 0–8 days | 118,602 | Both | Used measurements from fixed ground monitoring stations | Early-life through late-life | Depression | Increased |
| Yokota, Oshio and Takeda (2016) | Mice (ICR) | DEP | Prenatal | 5 days | 30/group | Male | Intratracheal Administration | Adulthood | Anxiety | Increased |
| Zijlema et al. (2016) | Human | Multiple Pollutants | Adulthood and Late-life | 3 years | 70,928 | Both | Land use regression models | Adulthood & Late-life | Depression | No effects observed |
| S. Chen et al. (2017) | Human | PM2.5 | Adolescence, Adulthood | 1 week | 102 | Both | Conducted during a heavy and light polluted week | Adolescence and Adulthood | Both | Mixed |
| Kim and Kim (2017) | Human | PM10 | Adolescence through Late-life | 9 years | 23,139 | Both | Used measurements from fixed ground monitoring stations | Adulthood & Late-life | Depression | No effects observed |
| Kioumourtzoglou et al. (2017) | Human | Multiple Pollutants | Adulthood and Late-life | 1, 2, 5 years | 41,844 | Female | Generalized additive models | Adulthood & Late-life | Depression | Increased |
| Lin et al. (2017) | Human | Multiple Pollutants | Adulthood | 0–15 days | 1,931 | Female | Used measurements from fixed ground monitoring stations | Adulthood | Both | Increased |
| Pun et al. (2017) | Human | PM2.5 | Adulthood and Late-life | 7, 30, 180 days, 1, 4 years | Wave 1 – 3,005, Wave 2 – 3,377 | Both | Generalized additive mixed models | Adulthood & Late-life | Both | Increased |
| Salvi et al. (2017) | Rats (Sprague-Dawley) | DEP | Adulthood | 2 weeks | 13/group | Male | Whole Body Inhalation | Adulthood | Both | Increased |
| Vert et al. (2017) | Human | Multiple Pollutants | Adulthood and Late-life | 4–5 years | 958 | Both | Land use regression models | Adulthood & Late-life | Both | Increased |
| Zhang et al. (2017) | Human | Multiple Pollutants | Adulthood | 1 day | 23,259 | Both | Used measurements from fixed ground monitoring stations | Adulthood | Depression | Increased |
| Kulas et al. (2018) | Mice (FVB) | PM2.5 | Prenatal | 3 weeks | 10/group | Male | Whole Body Inhalation | Adulthood | Anxiety | No effects observed |
| Liu et al. (2018) | Mice (C57BL/6) | PM2.5 | Adulthood | 4, 8, 12 weeks | 12/group | Male | Whole Body Inhalation | Adulthood | Depression | Increased |
| Sheffield et al. (2018) | Human | PM2.5 | Prenatal | 9 months | 557 | Female | General additive mixed models | Adulthood | Both | Increased |
| Shin et al. (2018) | Human | Multiple Pollutants | Adulthood | 1 year | 124,205 | Both | Used measurements from fixed ground monitoring stations | Adulthood | Depression | Increased |
| Umezawa et al. (2018) | Mice (NMRI) | PM2.5 | Prenatal | 14 days | 10/group | Both | Whole Body Inhalation | Adulthood | Anxiety | Decreased |
| F. Wang, H. Liu, et al. (2018) | Human | Multiple Pollutants | Early-life through late-life | 0–7 days | 19,646 | Both | Used measurements from fixed ground monitoring stations | Early-life through late-life | Depression | Increased |
| R. Wang et al. (2018) | Human | PM2.5 | Adulthood | 1 year | 20,861 | Both | Kriging model | Adulthood | Depression | Increased |
| Wang and Yang (2018) | Human | Multiple Pollutants | Adulthood and Late-life | 2 years | 11,634 | Both | Used measurements from fixed ground monitoring stations | Adulthood & Late-life | Depression | Increased |
| Woodward et al. (2018) | Rats (Sprague-Dawley) | nPM | Prenatal through Adulthood | 28 weeks | 15–17 | Male | Whole Body Inhalation | Adulthood | Both | Mixed |
| Zhang et al. (2018) | Mice (SPF Kunming) | PM2.5 | Prenatal | 7 days | 6/group | Both | Intratracheal Instillation | Adolescence | Both | Increased |
| Zock et al. (2018) | Human | Multiple Pollutants | Early-life through Late-life | 1 year | 4,450 | Both | Land use regression models | Early-life through late-life | Both | Mixed |
| Brokamp, Strawn, et al. (2019) | Human | PM2.5 | Adolescence | 0–3 days | 6,812 | Both | Combination | Adolescence | Both | Increased |
| Brunst et al. (2019) | Human | TRAP | Prenatal/Early-life through Adolescence | 1 year, 12 years | 145 | Both | Land use regression models | Adolescence | Anxiety | Increased |
| Chu et al. (2019) | Rats (Sprague-Dawley) and Mice (Wild Type) | PM2.5 | Rats - Adulthood, Mice - Adolescence through Adulthood | Rats - 12 weeks, Mice - 9 weeks | 8/group | Male | Whole Body Inhalation | Adulthood | Both | Increased |
| Generaal, Hoogendijk, et al. (2019) | Human | PM2.5 | Adulthood | 1 year | 32,487 | Both | Land use regression models | Adulthood | Depression | Increased |
| Generaal, Timmermans, et al. (2019) | Human | PM2.5 | Adulthood | 1 year | 2,980 | Both | Land use regression models | Adulthood | Both | Mixed |
| Ehsanifar, Jafari, et al. (2019) | Mice (NMRI) | DEP | Adulthood | 2, 5, 7 hrs | 12/group | Male | Whole Body Inhalation | Adulthood | Anxiety | Increased |
| Ehsanifar, Tameh, et al. (2019) | Mice (NMRI) | DEP | Adulthood | 12 weeks | 12/treatment/time, 48 total | Male | Whole Body Inhalation | Adulthood | Both | Increased |
| Fan et al. (2019) | Human | PM2.5 | Adolescence | 3 years | 21,780 | Both | Land use regression models | Adolescence | Depression | Increased |
| Jorcano et al. (2019) | Human | Multiple Pollutants | Prenatal through Adolescence | 1 year, 7 years | 13,182 | Both | Land use regression models | Childhood, adolescence | Both | No effects observed |
| Khan et al. (2019) | Human | Multiple Pollutants | For the U.S.: Early-life through Late-life. For Denmark - Early-life through Childhood | U.S. - Not reported, Denmark - 10 years | For U.S.: 151,104,811. For Denmark: 1,435,074 | Both | Combination | For the U.S.: early-life through late-life, for Denmark: not reported | Depression | Increased |
| Lee et al. (2019) | Human | PM10 | Early-life through Late-life | 0–5 days | 30,704 | Both | Used measurements from fixed ground monitoring stations | Early-life through late-life | Depression | Increased |
| Liu et al. (2019) | Rats (Sprague-Dawley) | PM2.5 | Early-life | 12 days | 20/group | Both | Intranasal instillation | Childhood, Adulthood | Both | Increased |
| Morris-Schaffer et al. (2019) | Mice (C57BL/6J) | UFP | Early-life | 6 days | Not reported | Both | Whole Body Inhalation | Not reported | Anxiety | No effects observed |
| Motesaddi Zarandi et al. (2019) | Rats (Wistar) | PM2.5 | Adolescence through Adulthood | 3 months, 6 months | 96, 32/group | Both | Whole Body Inhalation | Adulthood | Depression | No effects observed |
| Petkus et al. (2019) | Human | PM2.5 | Late-life | 3 years | 1,989 | Female | Bayesian Maximum Entropy | Late-life | Depression | Increased |
| Pun et al. (2019) | Human | Multiple Pollutants | Late-life | 1 year | 4118 | Both | Distance to major roadway | Late-life | Both | Increased |
| Qiu et al. (2019) | Human | Multiple Pollutants | Early-life through Late-life | 0–7 days | 10,947 | Both | Used measurements from fixed ground monitoring stations | Early-life through late-life | Depression | Increased |
| Roberts et al. (2019) | Human | Multiple Pollutants | Adolescence | 1 year | 284 | Both | KCLurban model - kernel modeling approach | Adolescence and Adulthood | Both | Mixed |
| Wang et al. (2019) | Human | PM2.5 | Adulthood | 1 year | 20,861 | Both | Used measurements from fixed ground monitoring stations | Adulthood | Depression | Increased |
| Yolton et al. (2019) | Human | TRAP | Prenatal/Early-life through Adolescence | 1 year, 12 years | 344 | Both | Land use regression models | Adolescence | Both | Increased |
| Yue et al. (2020) | Human | Multiple Pollutants | Early-life through Late-life | 0–7 days | 16,601 | Both | Used measurements from fixed ground monitoring stations | Early-life through late-life | Anxiety | Increased |
| Zhang et al. (2019) | Human | Multiple Pollutants | Adulthood | 1, 5 years | 123,045 | Both | Land use regression models | Adulthood | Depression | Increased |
| Zhao et al. (2019) | Human | Multiple Pollutants | Adolescence | 0–7 days, 1 year | 2827 | Both | Combination | Adolescence | Depression | No effects observed |
| Altug et al. (2020) | Human | Multiple Pollutants | Late-life | Not reported | 821 | Female | Land use regression models | Late-life | Depression | Increased |
| Diaz et al. (2020) | Human | Multiple Pollutants | Not reported | 0–8 days | 1,461 | Both | Used measurements from fixed ground monitoring stations | Not reported | Both | No effects observed |
| H. Gu et al. (2020) | Human | PM2.5 | Adulthood | 1 year | 14,772 | Both | Satellite-based measurements | Adulthood | Both | Increased |
| X. Gu et al. (2020) | Human | Multiple Pollutants | Early-life through Late-life | 0–7 days | 111,620 | Both | Used measurements from fixed ground monitoring stations | Early-life through Late-life | Depression | Increased |
| Haghani, Johnson, Safi, et al. (2020) | Mice (C57BL/6NJ) | nPM | Prenatal | 3 weeks | 5–16/group | Both | Whole Body Inhalation | Adulthood | Depression | Increased |
| Haghani, Johnson, Woodward, et al. (2020) | Mice (C57BL/6J) | nPM | Prenatal | 3 weeks | Not reported | Both | Whole Body Inhalation | Adulthood | Depression | Increased |
| Kim, Cho, et al. (2020) | Human | Multiple Pollutants | Adulthood and Late-life | 5 years | 2,729 | Both | Kriging model | Adulthood & Late-life | Depression | Increased |
| Li and Zhou (2020) | Human | Multiple Pollutants | Adulthood | 1 year | 11,908 | Both | Used measurements from fixed ground monitoring stations | Adulthood | Depression | Increased |
| Lu et al. (2020) | Human | Multiple Pollutants | Early-life, Childhood, Adolescence, Adulthood, Late-life | 0–5 days | 111,842 | Both | Used measurements from fixed ground monitoring stations | Early-life, childhood, adolescence, adulthood, late-life | Both | Increased |
| McGuinn et al. (2020) | Human | PM2.5 | Prenatal | Up to 39 weeks | 539 | Both | Combination | Childhood | Both | No effects observed |
| Nephew et al. (2020) | Rats (Sprague-Dawley) | PM2.5 | Prenatal through Early-life | 29 days | 18/group | Male | Whole Body Inhalation | Childhood, Adolescence | Anxiety | Increased |
| Niedzwiecki et al. (2020) | Human | PM2.5 | Adulthood | Up to 39 weeks | 509 | Female | Land use regression models | Adulthood | Both | Mixed |
| Nishimura et al. (2020) | Human | Multiple Pollutants | Not reported | 1 month | Not reported | Both | Used measurements from fixed ground monitoring stations | Not reported | Depression | No effects observed |
| Petkus et al. (2020) | Human | PM2.5 | Late-life | 3 years | 2,202 | Female | Bayesian Maximum Entropy | Late-life | Depression | No effects observed |
| Pinault et al. (2020) | Human | Multiple Pollutants | Adulthood and Late-life | 1 year | 84,800 | Both | Chemical transport | Adulthood & Late-life | Both | Increased |
| Roe et al. (2020) | Human | PM2.5 | Adulthood, Late-life | 15–20 mins | 11 | Both | Personal air monitoring | Adulthood & Late-life | Both | Mixed |
| Shi et al. (2020) | Human | Multiple Pollutants | Adulthood and Late-life | 2 weeks | 1,811 | Both | Used measurements from fixed ground monitoring stations | Adulthood & Late-life | Both | Increased |
| Tsai et al. (2020) | Human | Ozone | Not reported | 0–2 days | 80,813 | Not reported | Used measurements from fixed ground monitoring stations | Not reported | Depression | Increased |
| Wang et al. (2020) | Human | PM2.5 | Adulthood and Late-life | 1 year | 24,623 | Both | Chemical transport | Adulthood and Late-life | Depression | Increased |
| Wei et al. (2020) | Human | Multiple Pollutants | Adolescence, Adulthood, Late-life | 0–7 days | 16,225 | Both | Used measurements from fixed ground monitoring stations | Adolescence, Adulthood, Late-life | Depression | Increased |
| Zhao et al. (2020) | Human | Multiple Pollutants | Childhood, Adolescence, Adulthood, Late-life | 10 years | 1,126,014 | Both | Used measurements from fixed ground monitoring stations | Adolescence, Adulthood, Late-life | Both | Increased |
| Zu et al. (2020) | Human | Multiple Pollutants | Not reported | Not reported | 4,721 | Both | Used measurements from fixed ground monitoring stations | Adulthood | Depression | Increased |
| Ahlers and Weiss (2021) | Human | PM2.5 | Adulthood | 3 months, 9 months | 50 | Female | Used measurements from fixed ground monitoring stations | Adulthood | Depression | Increased |
| Allaouat et al. (2021) | Human | PM2.5 | Adulthood, Late-life | 4 years | 5,895 | Both | Dispersion modeling | Adulthood, Late-life | Depression | No effects observed |
| Chen et al. (2021) | Human | PM2.5 | Not reported | 1 year | 1,782 | Not reported | Used measurements from fixed ground monitoring stations | Not reported | Anxiety | Increased |
| Dores et al. (2021) | Human | Multiple Pollutants | Adolescence, Adulthood | 1–3 years | 55,650 | Both | Chemical transport | Adolescence and Adulthood | Depression | No effects observed |
| Ehsanifar, Jafari, et al. (2021) | Mice (NMRI) | DEP | Adulthood | 12 weeks | 12/group | Male | Whole Body Inhalation | Adulthood | Anxiety | Increased |
| Jeong et al. (2021) | Mice (C57BL/6nCrlOri) | DEP | Adulthood | 7 days | 8/group | Male | Intratracheal Instillation | Adulthood | Anxiety | Increased |
| Joo et al. (2021) | Human | PM2.5 | Adolescence, Adulthood | 1 year | 1,484 | Both | Used measurements from fixed ground monitoring stations | Adolescence, Adulthood | Both | Increased |
| Kanner et al. (2021) | Human | Multiple Pollutants | Adolescence through Adulthood | 15 months | 11,173 | Female | Chemical transport | Adolescence and Adulthood | Depression | Increased |
| Lamichhane et al. (2021) | Human | Multiple Pollutants | Adulthood | 3 months | 1,481 | Female | Land use regression models | Adulthood | Both | Increased |
| Latham et al. (2021) | Human | Multiple Pollutants | Childhood | 1 year | 2,066 | Both | Chemical transport model | Adulthood | Depression | No effects observed |
| Muhsin et al. (2022) | Human | Multiple Pollutants | Adulthood, Late-life | 0–7 days | 81,548 | Both | Used measurements from fixed ground monitoring stations | Adulthood, late-life | Both | Increased |
| Nguyen et al. (2021) | Human | Multiple Pollutants | Early-life through Late-life | 0–29 days | 1,997,992 | Both | Used measurements from fixed ground monitoring stations | Early-life through late-life | Depression | Increased |
| Pelgrims et al. (2021) | Human | Multiple Pollutants | Adulthood | 1 year | 1,325 | Both | Dispersion modeling | Adulthood | Both | Mixed |
| Petkus, Wang, et al. (2021) | Human | Multiple Pollutants | Late-life | 3 years | 6,118 | Female | Kriging model | Late-life | Both | Increased |
| Petkus, Younan, et al. (2021) | Human | Multiple Pollutants | Late-life | 3 years | 1,583 | Female | Kriging model | Late-life | Depression | Increased |
| Rasnick et al. (2021) | Human | PM2.5 | Early-life through Early-adolescence | 12 years | 263 | Both | Land use random forest model | Adolescence | Both | Increased |
| Reuben et al. (2021) | Human | Multiple Pollutants | Adolescence, Adulthood | 1 year | 2,039 | Both | combination | Adolescence and Adulthood | Both | Increased |
| Roberts and Helbich (2021) | Human | PM2.5 | Adulthood | 1 year | 393 | Both | Land use regression models | Adulthood | Depression | No effects observed |
| Tsai et al. (2021) | Human | Multiple Pollutants | Not reported | 0–3 days | 80,813 | Not reported | Used measurements from fixed ground monitoring stations | Not reported | Depression | Increased |
| Wen et al. (2021) | Mice (C57BL/6) | Multiple Pollutants | Prenatal | 3 weeks | 8/group | Both | Intratracheal Instillation | Adolescence | Anxiety | Increased |
| Xue et al. (2021) | Human | PM2.5 | Adulthood and Late-life | 1 year | 15,954 | Both | Chemical transport model | Adulthood & Late-life | Depression | Increased |
| Yao et al. (2022) | Human | PM2.5 | Adulthood and Late-life | 1 month - 2 years | 15,105 | Both | Used measurements from fixed ground monitoring stations | Adulthood & Late-life | Depression | Increased |
| Zhou, An, et al. (2021) | Human | Multiple Pollutants | Not reported | 0–5 days | 92,387 | Both | Used measurements from fixed ground monitoring stations | Not reported | Depression | Increased |
| Zhou, Fan, et al. (2021) | Human | Multiple Pollutants | Not reported | 0–3 days | 23,773 | Both | Used measurements from fixed ground monitoring stations | Not reported | Anxiety | Increased |
0 days refers to pollution estimates that occurred on the same day as the outcome
Table 2.
Final Included Articles from Literature Search 2: Air Pollution and Frontolimbic Brain Regions
| First author & year | Species | Pollutant | Exposure Window | Duration of Exposure | N | Gender or sex | Exposure Assessment Method | Period of Behavioral Assessment | Brain regions assessed | Brain outcome assessed |
|---|---|---|---|---|---|---|---|---|---|---|
| Avila-Costa et al. (1999) | Rats (Wistar) | Ozone | Not reported | 4 hours | 24 | Male | Whole Body Inhalation | Not reported | Hippocampus | Dendritic spine length or neurite changes |
| Avila-Costa et al. (2001) | Rats (Wistar) | Ozone | Not reported | 4 hours | 24 | Male | Whole Body Inhalation | Not reported | Prefrontal Cortex | Dendritic spine length or neurite changes |
| Dorado-Martínez et al. (2001) | Rats (Wistar) | Ozone | Not reported | 4 hours | 136 | Male | Whole Body Inhalation | Not reported | Hippocampus, Frontal Cortex | Lipid peroxidation |
| Niño-Cabrera (2002) | Rats (Wistar) | Ozone | Late-life | 4 hours | 7 (3 controls) | Male | Whole Body Inhalation | Late--ife | Hippocampus CA1 and Prefrontal Cortex | Necrotic processes, myelin alterations, altered astrocytes |
| Calderon-Garciduenas et al. (2003) | Dogs | Multiple pollutants | Lifetime | Dogs - 1 year, Humans - 2–10 years | 40 (14 controls) | Both | Whole Body Inhalation | Adulthood | Hippocampus, Frontal Cortex | Altered DNA, amyloid, immune reactions, inflammatory reactions, altered astrocytes |
| Calderon-Garciduenas et al. (2004) | Humans | Multiple pollutants | Lifetime | 34–83 years | 19 (9 low pollution, 10 high pollution) | Both | Lived in polluted city versus unpolluted city | Adulthood & Late-life | Hippocampus, Frontal Cortex | Altered DNA, amyloid, inflammatory reactions |
| Santucci et al. (2006) | Mice (CD-1) | Ozone | Prenatal | 47 days | 8 (4 females, 4 males) | Both | Whole Body Inhalation | Adulthood | Hippocampus | Neurotrophins |
| Calderon-Garciduenas et al. (2008) | Humans, Dogs | Multiple pollutants | Lifetime | Humans - 9.2 + 2.3 years, Dogs - 12–19 months | 73 children (55 high polluted, 18 low polluted), 12 dogs (7 high polluted, 5 low polluted) | Both | Lived in polluted city versus unpolluted city | Childhood | Subcortical prefrontal white matter | White matter lesions, inflammatory reactions |
| Gerlofs-Nijland et al. (2010) | Rats (Fischer F344/DUCRL) | Multiple pollutants | Adulthood | 4 weeks | 15/group | Male | Combination of whole body inhalation and nose-only inhalation | Adulthood | Hippocampus | No effects observed (inflammatory reactions, immune reactions) |
| Rivas-Arancibia et al. (2010) | Rats (Wistar) | Ozone | Not reported | 15–90 days | 110 (22 in each group) | Male | Whole Body Inhalation | Not reported | Hippocampus | Microglia, altered neurogenesis, altered cell proliferation, lipid peroxidation, altered astrocytes, |
| Suzuki et al. (2010) | Mice (ICR) | DEP | Prenatal | 2 weeks | 272 (114 exposed, 161 control) | Male | Whole Body Inhalation | Childhood | Prefrontal Cortex, Hippocampus | Neurotransmitter or neuromodulator metabolites and receptors |
| Calderon-Garciduenas et al. (2011) | Humans | Multiple pollutants | Lifetime | 7.1 + 0.69 years | 30 (10 low polluted, 20 high polluted) | Both | Lived in polluted city versus unpolluted city | Childhood | Prefrontal white matter, temporal white matter, hippocampus, amygdala | Brain volumes, white matter lesions |
| Fonken et al. (2011) | Mice (C57BL/6) | PM2.5 | Childhood through Adulthood | 10 months | Not reported | Male | Whole Body Inhalation | Adulthood | Hippocampus | Dendritic spine length or neurite changes, inflammatory reactions |
| Gackiere et al. (2011) | Rats (Wistar) | Ozone | Adolescence | Up to 120 hours | Not reported | Male | Whole Body Inhalation | Adolescence | Amygdala | Activated neurons |
| Morgan et al. (2011) | Mice (C57BL/6J) and Rats (F344) | nPM | Adulthood | 10 weeks | Not reported | Male | Whole Body Inhalation | Adulthood | Hippocampus | Microglia, neurotransmitter or neuromodulator metabolites and receptors, dendritic spine length or neurite changes, altered astrocytes, inflammatory reactions |
| Bos et al. (2012) | Mice (C57BL6) | PM2.5 | Adolescence | 5 days | 20 | Male | Whole Body Inhalation | Adulthood | Hippocampus | Inflammatory reactions, immune reactions |
| Hallberg et al. (2012) | Rats (Wistar Han) and Mice (C57BL/6) | DEP | Not reported | 6 hours | 5/group | Both | Whole Body Inhalation | Not reported | Hippocampus | No effects observed (inflammatory reactions) |
| Davis et al. (2013) | Mice (C57BL/6J) | nPM | Prenatal | 10 weeks | 4/group | Both | Whole Body Inhalation | Early-life | Hippocampus | Necrotic processes |
| Guerra et al. (2013) | Rats (Sprague-Dawley) | Multiple pollutants | Adolescence through Adulthood | 8 weeks | Not reported | Male | Whole Body Inhalation | Adulthood | Frontal cortex, Hippocampus | Mitochondrial changes, misfolded proteins, inflammatory reactions |
| Rodriguez-Martinez et al. (2013) | Rats (Wistar) | Ozone | Not reported | Up to 60 days | 180 (36/group) | Male | Whole Body Inhalation | Not reported | Hippocampus | Oxidative stress markers, swollen and damaged cells, mitochondrial changes |
| Win-Shwe et al. (2013) | Mice (BALB/c) | Multiple pollutants | Adulthood | 1 single intranasal instillation dose of 50 ul | 24, 8/group | Male | Intranasal instillation | Adulthood | Hippocampus | Inflammatory reactions |
| Gomez-Crisostomo et al. (2014) | Rats (Wistar) | Ozone | Not reported | Up to 90 days | 72 | Male | Whole Body Inhalation | Not reported | Hippocampus | Necrotic processes, altered cell proliferation, oxidative stress markers |
| Kinawy et al. (2014) | Rats (Wister) | DEP | Not reported | Single session - 30 mins, Chronic session - 8 weeks | 90, 30/group | Male | Whole Body Inhalation | Not reported | Hippocampus | Neurotransmitter or neuromodulator metabolites and receptors |
| F. Wang et al. (2014) | Mice (Kun Ming) | VOCs | Childhood through Adulthood | 3 months | 60 | Male | Whole Body Inhalation | Adulthood | Hippocampus | Decreased number of neurons, altered cell proliferation, oxidative stress markers, lipid peroxidation, neurotransmitter or neuromodulator metabolites and receptors |
| Win-Shwe et al. (2014) | Mice (BALB/c) | DEP | Childhood through Adulthood | 1–3 months | 6/group | Both | Whole Body Inhalation | Adulthood | Hippocampus | Neurotransmitter or neuromodulator metabolites and receptors |
| Calderon-Garciduenas et al. (2015) | Humans | Multiple pollutants | Lifetime | Children - 12.45 + 3.4 years. Adults - 37.5 + 6.77 | 57 polluted children and 9 control children. 48 polluted adults, and 7 control adults. | Both | Lived in polluted city versus unpolluted city | Childhood and Adolescence, Adulthood | Hippocampus | MR Spectroscopy |
| Heidari Nejad et al. (2015) | Mice (BALB/c) | DEP | Adulthood | 8 days | 12/group | Both | Whole Body Inhalation | Adulthood | Hippocampus | Altered astrocytes, blood brain barrier integrity |
| Hallberg et al. (2015) | Rats (Wistar Han) | DEP | Not reported | Up to 24 months | 10, 5/group | Both | Not reported | Not reported | Hippocampus | No effects observed (oxidative stress makers) |
| Hernandez-Zimbron and Rivas-Arancibia (2015) | Rats (Wistar) | Ozone | Not reported | Up to 90 days | 72, 12/group | Male | Whole Body Inhalation | Not reported | Hippocampus | Amyloid, mitochondrial changes |
| Kodavanti et al. (2015) | Rats (Long-Evans) | VOCs | Adulthood | Acute - 6 hours, Subchronic - 13 weeks | Not reported | Male | Whole Body Inhalation | Adulthood | Frontal Cortex, Hippocampus | Oxidative stress markers |
| Peterson et al. (2015) | Humans | PAH | Prenatal, Childhood | Prenatal - 48 hours, Postnatal 5 years | 40 | Both | Combination of personal air monitoring, and urinary metabolites | Childhood | Frontal lobe white matter, Temporal lobe white matter, dorsal prefrontal white matter | Brain volumes |
| Yao et al. (2015) | Rats (Wistar) | SO2 | Not reported | 90 days | 20/group | Male | Whole Body Inhalation | Adulthood | Hippocampus | Neurotransmitter or neuromodulator metabolites and receptors, inflammatory reactions, memory related kinases and genes |
| Calderon-Garciduenas et al. (2016) | Humans and Dogs | Multiple pollutants | Lifetime | Dogs - 3.11 + 0.67 years, Humans - 12.67 + 4.9 years | 9 high polluted dogs, 6 control dogs | Both | Lived in polluted city versus unpolluted city | Not reported | Frontal white and gray matter in dogs. Prefrontal white and gray matter in children | Cerebrovascular changes |
| Cole et al. (2016) | Mice (C57BL/6) | DEP | Adulthood | 6 hours | 3–6/group | Both | Whole Body Inhalation | Adulthood | Hippocampus | Inflammatory reactions, microglia, lipid peroxidation |
| Hernandez-Zimbron and Rivas-Arancibia (2016) | Rats (Wistar) | Ozone | Not reported | Up to 90 days | 72 (12/group) | Male | Whole Body Inhalation | Not reported | Hippocampus | Endoplasmic reticulum changes, amyloid |
| Rodriguez-Martinez et al. (2016) | Rats (Wistar) | Ozone | Not reported | Up to 90 days | 108 | Male | Whole Body Inhalation | Not reported | Hippocampus | Endoplasmic reticulum changes, necrotic processes |
| Yokota, Oshio, Moriya, et al. (2016) | Mice (ICR) | DEP | Prenatal | 2 weeks | 15/group | Male | Whole Body Inhalation | Adulthood | Prefrontal cortex, amygdala | Neurotransmitter or neuromodulator metabolites and receptors |
| Chao et al. (2017) | Rats (Sprague-Dawley) | PM2.5 | Prenatal | Up to 25 mg instillation intratracheal test | 12 | Not reported | Intratracheal instillation | Not reported | Hippocampus | Memory related kinases and genes, endoplasmic reticulum changes, altered cell proliferation |
| J. C. Chen et al. (2017) | Humans | Multiple pollutants | Adulthood, Late-life | 9 years | 1403 | Female | Bayesian maximum entropy | Late-life | Hippocampus, Frontal and Temporal Gray and White Matter | Brain volumes |
| Cheng et al. (2017) | Rats (Sprague-Dawley) | PM2.5 | Adulthood | 28 days | 20, 10/group | Male | Whole Body Inhalation | Adulthood | Hippocampus | Dendritic spine length or neurite changes |
| Ku et al. (2017) | Mice (C57BL/6) | PM2.5 | Adulthood | 4 weeks | Not reported | Male | Oropharyngeal aspiration | Adulthood | Hippocampus | Amyloid, neurotransmitter or neuromodulator metabolites and receptors, synaptic changes, altered cell proliferation |
| Nway et al. (2017) | Mice (C3H/HeN) | DEP | Prenatal and Early-life | 5 days | 8/group | Both | Whole Body Inhalation | Early-life | Hippocampus | Neurotransmitter or neuromodulator metabolites and receptors, inflammatory reactions, microglia |
| Rivas-Arancibia et al. (2017) | Rats (Wistar) | Ozone | Not reported | Up to 90 days | 72, 12/group | Male | Whole Body Inhalation | Not reported | Hippocampus | Amyloid |
| Woodward, Levine, et al. (2017) | Mice (C57BL/6J) | nPM | Adulthood | 10 weeks | Not reported | Female | Whole Body Inhalation | Adulthood | Hippocampus | Inflammatory reactions, immune reactions |
| Woodward, Pakbin, et al. (2017) | Mice (C67BL/6J) | nPM | Adulthood through Late-life | 10 weeks | 9/group | Female | Whole Body Inhalation | Adulthood and Late-life | Hippocampus | Microglia, dendritic spine length or neurite changes, neurotransmitter or neuromodulator metabolites and receptors, inflammatory reactions, myelin alterations |
| Yang et al. (2017) | Rats (Wistar) | Multiple pollutants | Not reported | 10 days | 6/group | Male | Intratracheal instillation | Not reported | Hippocampus | Inflammatory reactions, amyloid |
| Andrade-Oliva et al. (2018) | Rats (Sprague Dawley) | PM2.5 | Adulthood | Acute - 3 days, Subchronic - 8 weeks | Not reported | Male | Whole Body Inhalation | Not reported | Prefrontal Cortex | No effects observed (Neurotransmitter or neuromodulator metabolites and receptors, altered astrocytes |
| Jia et al. (2018) | Mice (C57BL/6J) | PM2.5 | Adulthood | 20 weeks | 10/group | Male | Whole Body Inhalation | Adulthood | Hippocampus | Dendritic spine length or neurite changes, inflammatory reactions, glucocorticoid receptors, |
| Kim et al. (2018) | Mice (BALB/c) | DEP | Adolescence through Adulthood | 4 weeks, 8 weeks | 32, 8/group | Female | Whole Body Inhalation | Adulthood | Prefrontal Cortex, Temporal Cortex | Synaptic changes, neurotrophins, oxidative stress markers |
| Li et al. (2018) | Rats (Sprague-Dawley) | PM2.5 | Early-life | 2 weeks | Not reported | Male | Intranasal instillation | Early-life | Hippocampus, Prefrontal Cortex | Inflammatory reactions, autism genes expression, altered astrocytes, microglia |
| Liu et al. (2018) | Mice (C57BL/6) | Multiple pollutants | Adulthood through Late-life | Up to 12 weeks | Not reported | Male | Whole Body Inhalation | Not reported | Hippocampus | Neurotrophins, necrotic processes, dendritic spine length or neurite changes, inflammatory reactions |
| Ning et al. (2018) | Mice (C57BL/6) | PM2.5 | Childhood through Adolescence | 4 weeks | Not reported | Not reported | Oropharyngeal aspiration | Not reported | Hippocampus | Energy metabolites, cholesterol metabolites, arachidonic acid metabolites, inositol phosphate metabolites, aspartic acid metabolites |
| Shih et al. (2018) | Rats (Sprague Dawley) | TRAP | Adulthood | 3 months, 6 months | 9/group | Male | Whole Body Inhalation | Adulthood | Hippocampus | Dendritic spine length or neurite changes, inflammatory reactions, brain volumes, arachidonic acid metabolites, necrotic processes |
| Valand et al. (2018) | Rats (Fisher344) | DEP | Not reported | 28 days | 7/group | Male | Whole Body Inhalation | Not reported | Hippocampus, Frontal Cortex | Genes involved in bronchial smooth muscle cells, genes associated with neuronal development, alterations in neuronal migration, swollen and damaged cells, synaptic changes, immune reactions, oxidative stress markers, dendritic spine length or neurite changes, inflammatory reactions |
| F. Wang, Z. Fangfang, et al. (2018) | Mice (Kunming) | VOCs | Childhood | 10 days | 10/group | Male | Whole Body Inhalation | Adolescence | Hippocampus | Dendritic spine length or neurite changes, neurotransmitter or neuromodulator metabolites and receptors, oxidative stress markers |
| Woodward et al. (2018) | Rats (Sprague-Dawley) | TRAP | Prenatal through Adulthood | 28 weeks | Not reported | Male | Whole Body Inhalation | Adulthood | Hippocampus | Cerebral microbleeds, altered neurogenesis, blood brain barrier integrity, microglia |
| Zheng et al. (2018) | Mice (Kunming) | PM2.5 | Prenatal | 7 days | 6/group | Not reported | Tracheal drip | Early-life | Hippocampus | Activated neurons, altered neurogenesis, mitochondrial changes, synaptic changes, immune reactions, altered cell proliferation, necrotic processes, dendritic spine length or neurite changes, inflammatory reactions |
| Bai et al. (2019) | Rats (Sprague Dawley) | TRAP | Adulthood | 3 months, 6 months | Not reported | Male | Whole Body Inhalation | Adulthood | Hippocampus | Microglia |
| Bello-Medina et al. (2019) | Rats (Wistar) | Ozone | Not reported | Up to 90 days | 80, 10/group | Male | Whole Body Inhalation | Not reported | Hippocampus | Dendritic spine length or neurite changes |
| Brunst et al. (2019) | Humans | TRAP | Early-life, Childhood, Cumulative | 1 year, 12 years | 145 | Both | Land use regression models | Adolescence | Anterior Cingulate Cortex (ACC) | MR Spectroscopy |
| Chu et al. (2019) | Rats (Sprague-Dawley) and Mice (WT and Nrfs−/− (KO)) | PM2.5 | Rats - Adulthood, Mice - Adolescence through Adulthood | Rats - 12 weeks, Mice - 9 weeks | 24, 8/group | Male | Whole Body Inhalation | Adulthood | Prefrontal Cortex | Dendritic spine length or neurite changes, inflammatory reactions, heavy metal deposits, neurotrophins, altered astrocytes, necrotic processes, oxidative stress markers, neurotransmitter or neuromodulator metabolites and receptors |
| Custodio et al. (2019) | Rats (Wistar) | Ozone | Prenatal | 20 days | 18 exposed, 16 controls | Both | Whole Body Inhalation | Adulthood | Prefrontal Cortex, Hippocampus | Neurotransmitter or neuromodulator metabolites and receptors, altered cell proliferation |
| Ehsanifar, Jafari, et al. (2019) | Mice (NMRI) | DEP | Prenatal | 3 weeks | 10/group | Male | Whole Body Inhalation | Adulthood | Hippocampus | Dendritic spine length or neurite changes, inflammatory reactions, neurotransmitter or neuromodulator metabolites and receptors |
| Ehsanifar, Tameh, et al. (2019) | Mice (NMRI) | DEP | Adulthood | 12 weeks | 48, 12/group | Male | Whole Body Inhalation | Adulthood | Hippocampus | Oxidative stress markers |
| Hedges et al. (2019) | Humans | Multiple pollutants | Adulthood and Late-life | 1 year | 18,278 | Both | Land use regression models | Adulthood & Late-life | Hippocampus | Brain volumes |
| Kim et al. (2019) | Mice (BALB/c) | DEP | Adolescence into Adulthood | 4 weeks, 8 weeks | 32, 8/group | Female | Whole Body Inhalation | Adulthood | Prefrontal Cortex, Temporal Cortex | Immune reactions, synaptic changes, necrotic processes, neurotransmitter or neuromodulator metabolites and receptors, inflammatory reactions, neuronal plasticity measures, genes associated with neuronal development |
| Li et al. (2019) | Mice (C57BL/6J) | PM2.5 | Adulthood | Acute - 24 hours, Chronic - up to 4.5 months | Acute - 10, Chronic - 6 | Male | Whole Body Inhalation | Adulthood | Hippocampus | Altered DNA |
| Liu et al. (2019) | Rats (Sprague Dawley) | PM2.5 | Early-life | 12 days | 8/group | Both | Intranasal instillation | Childhood or Adulthood | Hippocampus | Neurotrophins, synaptic changes |
| Armstrong et al. (2020) | Mice (C57/BL/6) | DEP | Adulthood and Late-life | 50 days | 16/group | Male | Whole Body Inhalation | Adulthood, Late-life | Hippocampus | Cerebrovascular changes, altered cell proliferation, amyloid, oxidative stress markers |
| Calderon-Garciduenas et al. (2020) | Humans and Mice (C57BL/6J) | Multiple pollutants | Humans - not reported, adulthood for animals | Humans - 29.8 years, Mice - 7 months | Humans (5 controls, 9 exposed). Mice - 4/group | Humans - both. Mice - Female | Humans - Lived in polluted city versus unpolluted city, Mice - whole body inhalation | Humans- Adulthood Mice – Adulthood | Humans - Frontal white matter, Mice - Frontal Cortex | Tau-related pathology, heavy metal deposits, altered DNA |
| Cho et al. (2020) | Humans | Multiple pollutants | Adulthood and Late-life | 1 year, 5 years | 957 | Both | Kriging model | Adulthood, Late-life | Frontal Cortex, Temporal Cortex, Hippocampus, Amygdala | Brain volumes |
| Cole et al. (2020) | Mice (C57BL/6J) | DEP | Prenatal through Early-life | 3 weeks | Not reported | Both | Whole Body Inhalation | Early-life, Adulthood | Hippocampus | Altered neurogenesis |
| Di Domenico et al. (2020) | Mice (Balb/C) | PM2.5 | Prenatal, Childhood through Adulthood | 85 days | Not reported | Male | Whole Body Inhalation | Adulthood | Hippocampus | Neurotrophins, altered astrocytes, microglia |
| Gale et al. (2020) | Humans | Multiple pollutants | Adulthood and Late-life | 1 year, 4 years | 18,288 | Both | Land use regression models | Adulthood & Late-life | Prefrontal Cortex | Brain volumes |
| Greve et al. (2020) | Rats (Wistar Kyoto) | DEP | Adulthood | 1 month | 7–8/group | Male | Whole Body Inhalation | Adulthood | Hippocampus, Frontal Cortex | Microglia, inflammatory reactions |
| Haghani, Johnson, Woodward, et al. (2020) | Mice (C57BL/6J) | nPM | Prenatal | 3 weeks | Not reported | Both | Whole Body Inhalation | Adulthood | Hippocampus | Neurotransmitter or neuromodulator metabolites and receptors, inflammatory reactions, altered neurogenesis, altered cell proliferation, immune reactions |
| Hajipour et al. (2020) | Rats (Wistar) | Dusty PM | Not reported | 4 weeks | 88, 22/group | Male | Whole Body Inhalation | Not reported | Hippocampus | Synaptic changes |
| Li et al. (2020) | Mice (C57BL/6) | DEP | Adulthood | 14 days | 10/group | Male | Intranasal instillation | Adulthood | Hippocampus | Inflammatory reactions, mitochondrial changes, immune reactions, microglia |
| Liu et al. (2020) | Mice (C57BL/6) | Multiple pollutants | Adulthood | Up to 12 weeks | 72, 12/group | Male | Whole Body Inhalation | Adulthood | Hippocampus | Necrotic processes, dendritic spine length or neurite changes, inflammatory reactions, heavy metal deposits |
| Milani et al. (2020) | Mice (BALB/cOlaHsd) | DEP | Adolescence into Adulthood | Acute - single instillation, Subacute - 3 instillations | 6/group | Male | Intratracheal instillation | Adulthood | Hippocampus | Amyloid, oxidative stress markers, inflammatory reactions |
| Nephew et al. (2020) | Rats (Sprague-Dawley) | TRAP | Prenatal through Early-life | 6 weeks | 6/group | Male | Whole Body Inhalation | Early-life | Hippocampus, Anterior Cingulate | Diffusion tensor imaging |
| Park et al. (2020) | Mice (C57Bl6/J) | UFP | Adulthood | 3 weeks | 10/group | Male | Whole Body Inhalation | Adulthood | Hippocampus | Amyloid, oxidative stress markers, inflammatory reactions, |
| Patten et al. (2020) | Rats (Sprague Dawley) | TRAP | Prenatal to adulthood | 11 days | 15/group | Both | Whole Body Inhalation | Adulthood | Hippocampus | Necrotic processes, microglia, inflammatory reactions, alterations in neuronal migration, genes associated with neuronal development, altered neurogenesis, brain volumes, altered astrocytes |
| Zhou et al. (2020) | Mice (ICR) | PM2.5 | Prenatal, Early-life | 8 days | Not reported | Both | Intratracheal instillation | Childhood | Hippocampus | Altered DNA, neurotrophins |
| Ehsanifar, Jafari, et al. (2021) | Mice (NMRI) | DEP | Adulthood | 12 weeks | 48, 12/group | Male | Whole Body Inhalation | Adulthood | Hippocampus | Lipid peroxidation, neurotransmitter or neuromodulator metabolites and receptors, dendritic spine length or neurite changes, inflammatory reactions |
| Ehsanifar, Montazeri, et al. (2021) | Mice (NMRI) | DEP | Adulthood | 14 weeks | 10/group | Both | Whole Body Inhalation | Adulthood | Hippocampus | Oxidative stress markers, neurotransmitter or neuromodulator metabolites and receptors, dendritic spine length or neurite changes, inflammatory reactions, lipid peroxidation |
| Kodavanti et al. (2021) | Rats (Brown Norway) | Ozone | Adulthood, Late-life | 13 weeks | Not reported | Male | Whole Body Inhalation | Adulthood & Late-life | Frontal cortex, Hippocampus | Oxidative stress markers, mitochondrial changes |
| F. Liu et al. (2021) | Mice (ICR) | Multiple pollutants | Adulthood | 4 weeks | 6/group | Male | Intranasal instillation | Adulthood | Hippocampus, Frontal Cortex | Necrotic processes, oxidative stress markers, dendritic spine length or neurite changes, CA2+ signaling pathway-related genes, decreased number of neurons, heavy metal deposits, altered neurogenesis, neurotrophins, synaptic changes |
| Lubczynska et al. (2021) | Humans | Multiple pollutants | Prenatal through childhood | Prenatal - 9 months, Childhood - 9–12 years | 3133 | Both | Land use regression models | Childhood | Hippocampus, Amygdala | Brain volumes |
| Sahu et al. (2021) | Mice (C57BL/6;C3H) | PM2.5 | Adulthood | 3 months | Not reported | Male | Whole Body Inhalation | Adulthood | Hippocampus | No effects observed (altered astrocytes, microglia, inflammatory reactions, amyloid) |
| Wen et al. (2021) | Mice (C57BL/6) | Multiple pollutants | Prenatal | 3 months | 6/group | Both | Intratracheal instillation | Adulthood | Hippocampus | Thyroid hormone signaling pathway genes |
| Zhang et al. (2021) | Mice (SPF ICR) | PM2.5 | Prenatal | Not reported | Not reported | Both | Endotracheal nebulization | Early-life | Hippocampus | Inflammatory reactions, immune reactions |
3. RESULTS
3.1. Literature search 1: Effects of air pollution on internalizing psychopathology (i.e., anxiety and depression)
From an initial search that yielded 943 articles, 145 articles were eligible for full-text screening based on title and abstract review. Of these studies, 34 were excluded, leaving 111 articles for the review. See Figure 1 for a depiction of the review process for literature search 1. Much of the research on air pollution and internalizing symptom and behaviors included in the final systematic review was conducted within the past 3 years (see Figure 2, Panel A).
Figure 2.
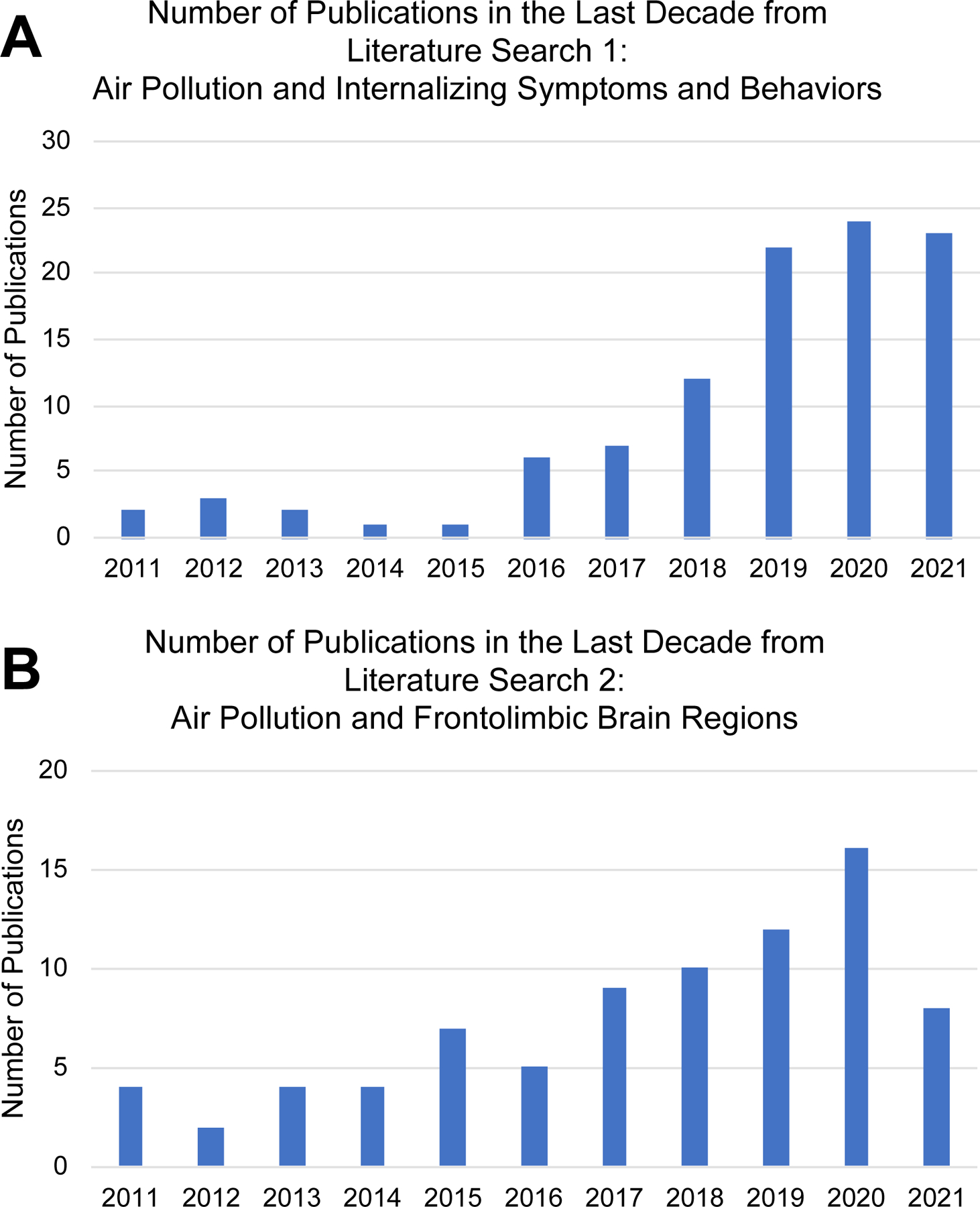
Publications by Year for Literature Searches 1 (A) and 2 (B).
Of the 111 articles reviewed, 81 (73%) reported increased internalizing symptoms or behaviors with air pollution exposure, 16 articles (14%) did not observe any significant effects of air pollution on internalization, 12 articles (11%) reported mixed effects (non-significant for depression, but significant for anxiety, or vice-versa), and only 2 articles (2%) reported associations between air pollution exposure and decreased internalizing symptoms and behaviors. Of the internalizing symptoms and behaviors assessed in this review, 47% assessed depression alone, 38% assessed both anxiety and depression, and 15% assessed anxiety alone. Eighty-four studies (76%) reported findings in humans and 27 (24%) in animal models. Most studies examined more than one pollutant (46%). Across all studies, the most examined pollutants were PM2.5 (64.9%), NO2 (37.8%), and PM10 (33.3%). See Table 3 for all pollutants examined by studies within literature search 1. Eighty articles (72%) utilized air pollution estimates (e.g., land-use regression, chemical transport models). Of those, 61 (76%) controlled for meteorological conditions (e.g., temperature, wind, humidity), while 19 (24%) did not. Thirty-one articles (28%) did not utilize specific air pollutant estimates (e.g., animal studies using direct exposures, human studies using indirect estimates such as nearest roadway, etc.). Forty-three percent of studies examined exposures that spanned more than one developmental window and 8% of studies did not specify the exposure window. Adulthood (48%) was the most commonly examined exposure window across studies, followed by prenatal (26%), late-life (13%), adolescence (7%), early-life (4%), and childhood (2%). The results for literature search 1 are graphically represented in Figure 3.
Table 3.
Pollutants Examined in Literature Searches 1 and 2
| Type of Pollutant | Percentage of Articles for Literature Search 1 | Percentage of Articles for Literature Search 2 |
|---|---|---|
| Benzo[a]pyrene (B[a]P) | 0.0 | 1.1 |
| Black Carbon (BC) | 1.8 | 0.0 |
| CO | 14.4 | 0.0 |
| Diesel Exhaust Particles (DEP) | 5.4 | 26.1 |
| Distance from major roadway | 0.9 | 0.0 |
| Elemental carbon (EC) | 0.9 | 0.0 |
| Heavy Metals (Pb, Mn, Zn, etc.) | 0.9 | 3.3 |
| Methane (CH4) | 0.9 | 0.0 |
| NO | 3.6 | 1.1 |
| NO2 | 37.8 | 2.2 |
| Non-methane hydrocarbons (NMHC) | 0.9 | 0.0 |
| NOx | 7.2 | 2.2 |
| Nanoscale Particulate Matter or Ultrafine Particulates (nPM/UFP) | 5.4 | 8.7 |
| O2 | 0.9 | 0.0 |
| Organic Carbon (OC) | 0.9 | 1.1 |
| O3 | 27.0 | 26.1 |
| Polycyclic Aromatic Hydrocarbons (PAHs) | 5.4 | 2.2 |
| Peroxyacetyle nitrate (PAN) | 0.9 | 0.0 |
| PM | 0.9 | 8.7 |
| PM10 | 33.3 | 6.5 |
| PM2.5 | 64.9 | 32.6 |
| PM2.5absorbance | 3.6 | 1.1 |
| PM2.5–10 | 0.9 | 2.2 |
| PMcoarse | 1.8 | 1.1 |
| SO2 | 21.6 | 2.2 |
| SO4 | 1.8 | 0.0 |
| Total Hydrocarbons | 1.8 | 0.0 |
| Total Suspended Particles (TSP) | 0.9 | 0.0 |
| Traffic-Related Air Pollution (TRAP) | 1.8 | 6.5 |
| Volatile Organic Compounds (VOCs) | 0.0 | 3.3 |
Figure 3.
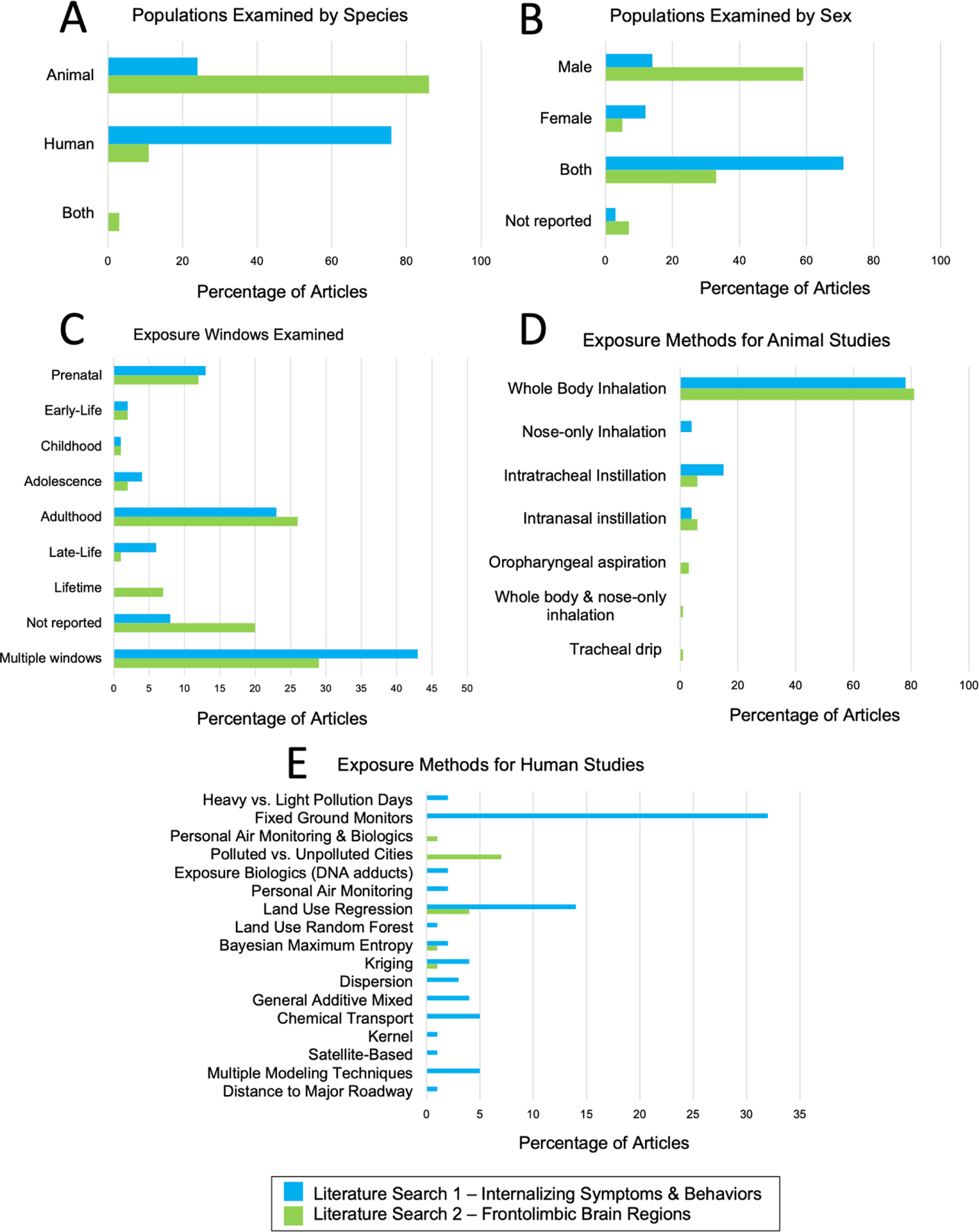
Summary of Study Types for Literature Searches 1 (blue) and 2 (green). Panel A – Populations Examined by Species, Panel B – Populations Examined by Sex, Panel C – Exposure Windows Examined Panel D – Exposure Methods for Animal Studies, Panel E – Exposure Methods for Human Studies
3.1.1. Literature search 1 – Animal studies
Of the 27 nonhuman animal model studies, 70% were conducted in mice, 26% in rats, and 1 article (4%) used both a mouse and rat model. The majority of studies (59%) were conducted in males only and 41% included both male and female animals. The exposure methods used were whole body inhalation (78% of articles), intratracheal instillation (15%), intranasal instillation (4%), and nose-only inhalation (4%). The most common behavioral assessments in animal studies included the elevated plus maze (37%), open field test (37%), forced swim test (33%), and running wheel – voluntary activity (15%); see Table 4 for description of all behavioral assessments.
Table 4.
Internalizing Outcomes Assessed (Literature Search 1)
| Internalizing Outcomes | N (%) |
|---|---|
| Animal Studies | |
| Elevated Plus Maze | 10 (37) |
| Open Field | 10 (37) |
| Forced Swim | 9 (33) |
| Running Wheel - Voluntary Activity | 4 (15) |
| Tail Suspension Test | 4 (15) |
| Light Dark Box | 3 (11) |
| Marble Burying Test | 2 (7) |
| Elevated Zero Maze | 1 (4) |
| Hole Board Test | 1 (4) |
| Novelty Suppression Feed Test | 1 (4) |
| Sucrose Preference Test | 1 (4) |
| Cricket predation test | 1 (4) |
| Grooming behaviors | 1 (4) |
| Human Studies | |
| Center for Epidemiologic Studies Depression Scale (CES-D) | 18 (21) |
| Emergency Department Visits | 8 (10) |
| Hospital admissions for depression | 8 (10) |
| Patient Health Questionnaire-9 (PHQ9) | 6 (7) |
| Geriatric Depression Scale (GDS) | 5 (6) |
| SCL-90 | 5 (6) |
| CBCL Anxious/Depressed | 4 (5) |
| Hospital Anxiety and Depression Scale (HADS) | 4 (5) |
| Behavior Assessment System for Children - 2 (BASC2) | 3 (4) |
| Doctor Diagnosis of Major Depressive Disorder | 3 (4) |
| Use of anti-depressant medication | 3 (4) |
| 28-item Inventory of Depression Symptomatology (IDS) | 2 (2) |
| Children’s Depression Inventory (CDI) | 2 (2) |
| Diagnostic Interview Schedule | 2 (2) |
| Edinburg Postnatal Depression Scale (EPDS) | 2 (2) |
| Hospital admissions for anxiety | 2 (2) |
| Insurance claims for depression using ICD-9, ICD-10 codes | 2 (2) |
| Kessler Psychological Distress Scale (K6) | 2 (2) |
| Presence or absence of depressiveness (such as a feeling of sadness or hopelessness lasting more than 2 consecutive weeks) | 2 (2) |
| Self-reported history of depression disorders | 2 (2) |
| Semi-structured Composite International Diagnostic Interview (SCID) | 2 (2) |
| Spence Children’s Anxiety Scale (SCAS) | 2 (2) |
| Suicide attempts | 2 (2) |
| Medical Symptoms Questionnaire (MSQ) | 1 (1) |
| Crown Crisp Phobic Anxiety Scale | 1 (1) |
| Deficient Emotional Self-Regulation (DESR) | 1 (1) |
| Mini International Neuropsychiatric Interview (MINI) | 1 (1) |
| “Have you ever felt sadness or despair in the last two consecutive weeks in the recent year”? | 1 (1) |
| Use of anxiety medication | 1 (1) |
| Self-reported history of anxiety disorders | 1 (1) |
| Hedonic Unhappiness | 1 (1) |
| ICPC codes for Depression or Anxiety | 1 (1) |
| Depression Screener for Teenagers (DesTeen) | 1 (1) |
| Beck Anxiety Inventory (BAI) | 1 (1) |
| Multidimensional Anxiety Scale Children (MASC) | 1 (1) |
| 40-item four dimensional symptom questionnaire (4DSQ) | 1 (1) |
| Beck Depression Inventory (BDI) | 1 (1) |
| Strength and Difficulties Questionnaire (SDQ) | 1 (1) |
| Insurance claims for anxiety using ICD-9, ICD-10 codes | 1 (1) |
| Cause of death was suicide (ICD-10 codes) | 1 (1) |
| Frequency of depressed emotions in recent months | 1 (1) |
| K10-distress | 1 (1) |
| University of Wales Institute of Science and Technology (UWIST) Mood Adjective Check List (MACL) | 1 (1) |
| Generalized Anxiety Disorder (GAD-7) scale | 1 (1) |
| General Health Questionnaire (GHQ-12) | 1 (1) |
| Composite International Diagnostic Interview (CIDI) | 1 (1) |
| Structured clinical interview (DSM-IV) | 1 (1) |
| “Has a doctor diagnosed or treated you for depression during the last year (12 months)?” | 1 (1) |
| State-Trait Anxiety Inventory (STAI) | 1 (1) |
| Outpatient anxiety visits | 1 (1) |
| Outpatient depression visit | 1 (1) |
| SF-36 | 1 (1) |
| Search words related to anxiety | 1 (1) |
A recent example of one of the animal studies included in literature search 1 examined whether exposure to air pollutants was associated with increased anxiety-like behaviors (Ehsanifar, Jafari, et al., 2021). Ehsanifar, Jafari and colleagues exposed male NMRI mice to 300–350 μg/m3 nanoscale diesel exhaust particles (DEPs) via whole-body inhalation for 2, 5, and 7 hours. Anxiety-like behavior was measured using the elevated plus maze. The results showed that exposed mice (2, 5, and 7 hours) demonstrated a significantly decreased ability to enter the open arms and a shorter elapsed time as compared to control mice, both indicators of increased anxiety-like behaviors.
3.1.2. Literature search 1 – Human studies
Of the 84 human studies, most (81%) included both men and women in their study design, 15% of studies assessed women only, and 3% did not report or did not specify. For the exposure methods utilized, 42% used measurements from fixed ground monitoring stations, 18% used land-use regression models, 7% used a combination of modeling techniques (e.g., ground monitoring measurements and land-use regression models), 6% used chemical transport models, 5% used general additive mixed models, 5% kriging models, and 4% dispersion models. Other assessment methods included collecting measurements during both heavy and light pollution time points, biological markers of exposure (e.g., DNA adducts), land use random forest models, personal air monitoring, kernel models, distance to major roadway, Bayesian maximum entropy models, and satellite-based measurements (each making up less than 2% of articles). Internalizing outcomes varied widely, and the most common measures included the Center for Epidemiological Studies Depression Scale (CES-D) (21% of articles), emergency department visits (10%), hospital admissions for depression (10%), and the Patient Health Questionaire-9 (PHQ-9) (7%); see Table 4 for description of all outcomes assessed. Most of the observational studies were conducted in the United States (29%), China (27%), and South Korea (11%). See Figure 4, Panel A for a map of locations of the human observational studies included in literature search 1.
Figure 4.
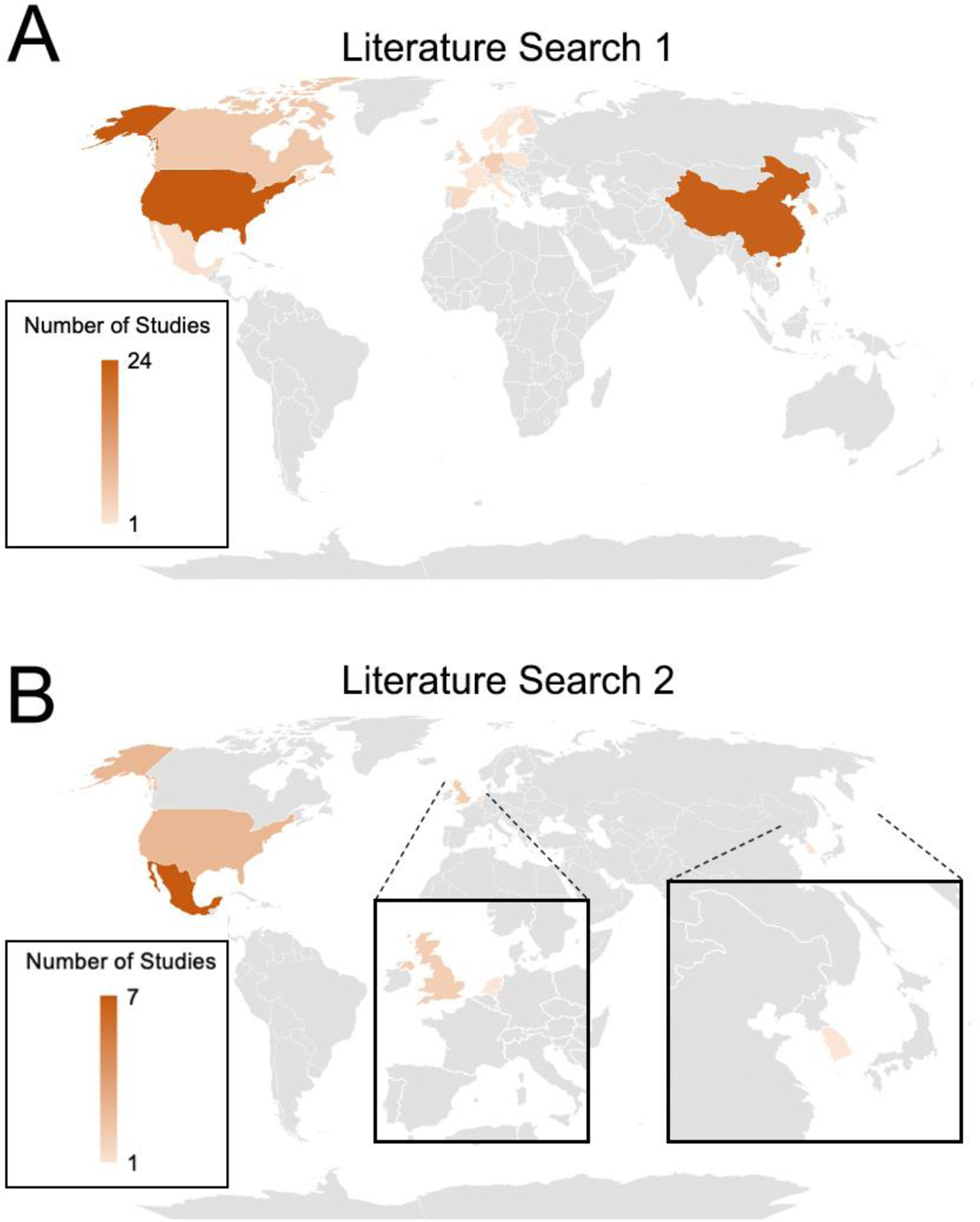
Map of Locations of Human Observations Studies for each Literature Search. Panel A – Literature Search 1 – Internalizing Symptoms & Behaviors, Panel B – Literature Search 2 – Frontolimbic Brain Regions
A recent example of one of the human studies included in literature search 1 examined whether air pollution exposure during childhood and adolescence was associated with increased depression and anxiety symptoms at age 18 (Reuben et al., 2021). Reuben and colleagues estimated childhood (past year at age 10) and late-adolescence (past year at age 18) air pollution based on participant’s residential address using a combination of the U.S. Environmental Protect Agency’s Community Multiscale Air Quality Modeling System and atmospheric dispersion model. Anxiety and depression symptoms were measured using a structured interview designed to assess internalizing-spectrum disorder symptoms from DSM-IV symptoms of Depression and Generalized Anxiety Disorder. Covariates included in the analyses were sex, family socioeconomic status, family psychiatric history, participant history of emotional and behavioral problems in early childhood, and tobacco smoking up to 18 years of age. Importantly, Reuben and colleagues also controlled for several disadvantageous neighborhood characteristics such as deprivation, dilapidation, disconnection, and dangerousness. The results showed that increased NOx exposure during childhood and late adolescence was associated with increased internalizing symptoms at age 18 and adjusting for the neighborhood characteristics did not change the results.
3.1.3. Literature search 1 – Sex-specific effects
Sixteen articles (12 human, 4 animal) were identified that reported on sex or gender specific effects in the impact of air pollution exposure on internalizing outcomes. The results from these studies were mixed. In the human studies, 6 studies reported findings in which women were more susceptible to the effects of air pollution than men (H. Gu et al., 2020; Szyszkowicz, 2007; Szyszkowicz et al., 2016; F. Wang, H. Liu, et al., 2018; Wei et al., 2020; Yue et al., 2020), 3 studies reported that men were more susceptible than women (Kim, Cho, et al., 2020; Pun et al., 2019; Shin et al., 2018), and 3 studies had mixed findings in which effects were observed for both sexes but had differential outcomes (e.g., differential lag times, different pollutants) (Lu et al., 2020; Zhang et al., 2017). In the animal studies, 3 studies reported that males were more susceptible to internalizing behaviors following air pollution exposure than females (Davis et al., 2013; Haghani, Johnson, Safi, et al., 2020; Haghani, Johnson, Woodward, et al., 2020), while 1 study reported that females were more susceptible than males (Miller et al., 2016).
3.1.4. Literature search 1 – Age effects
Twelve articles (11 human, 1 animal) were identified that reported age effects in the impact of air pollution exposure on internalizing outcomes. The results from these studies were mixed. For example, in the human studies, following recent air pollution exposure (up to 3 years before outcome assessed), five studies found that older adults (≥65) were more vulnerable to internalizing symptoms compared to younger age groups (<65 yrstd) (Kim et al., 2016; Pun et al., 2019; Szyszkowicz et al., 2009; F. Wang, H. Liu, et al., 2018; Wei et al., 2020), 2 found that those who were middle-aged (35–64 years) were more vulnerable to developing internalizing symptoms following recent air pollution exposure, compared to those <35 as well as those >64 years of age (Muhsin et al., 2022; Xue et al., 2021), and one found effects on internalizing symptoms for differing pollutants for middle-aged versus older adults following recent air pollution exposure (e.g., middle adults were sensitive to PM2.5, PM10, NO2, and SO2, while older adults were sensitive to PM2.5, NO2, and O3) (Lu et al., 2020). Two studies found that children and adolescents were more vulnerable to internalizing symptoms following air pollution exposure (Nguyen et al., 2021; Rasnick et al., 2021). Nguyen et al. (2021) found that children and adolescents (0–18 years) were more vulnerable to internalizing symptoms following recent (up to 7 days prior to outcome assessed) air pollution exposure than individuals ages 19–34 years. Rasnick et al. (2021) investigated sensitive exposure windows for 12-year-old adolescents. They found that the most sensitive time for air pollution exposure on anxiety, was in early childhood, between four years and four months and five years and eleven months, compared to all other timepoints from birth through 12 years. Interestingly, two studies (1 human, 1 animal) found that internalizing symptoms and behaviors developed later (into adolescence and adulthood) following prenatal and early-life exposure to air pollution, suggesting that these effects may be delayed (Liu et al., 2019; Margolis et al., 2016).
3.2. Literature search 2: Effects of air pollution on frontolimbic brain regions
From an initial search that yielded 371 articles, 119 articles were eligible for full-text screening. Of these studies, 27 were excluded, leaving 92 articles for the review. See Figure 1 for a depiction of the review process for literature search 2. Much of the research (49%) on air pollution and frontolimbic brain regions included in the final systematic review was conducted within the past 4 years (see Figure 2, Panel B).
Of the 92 articles reviewed, 87 (95%) reported significant effects of air pollution on frontolimbic brain regions. Seventy-nine articles (86%) were conducted in animal models (mice, rat, dogs), and ten articles (11%) assessed humans only. A small number (~3%) of studies included both human and animal models. A large portion of studies examined more than one pollutant (22%). Across all studies, the most examined pollutants were PM2,5 (32.6%), O3 (26.1%), and Diesel Exhaust Particles (DEP) (26.1%). See Table 3 for all pollutants examined by studies within literature search 2. Of the brain regions assessed in this review, 66% of articles assessed the hippocampus alone, 7% PFC, and 1% each the amygdala, anterior cingulate cortex, and frontal cortex. Twenty-two articles (24%) assessed more than one of our brain regions of interest. The measured neurobiological outcomes varied; the most common measured outcomes included inflammatory reactions (39% of articles) and neurite changes (21%), followed by changes to neurotransmitter metabolites or receptors (20%) and oxidative stress markers (17%); see Table 5 for description of all outcomes assessed.
Table 5.
Neurobiological outcomes assessed (Literature Search 2)
| Neurobiological Outcomes Assessed | N (%) of Articles |
|---|---|
| Inflammatory Reactions (COX-2, NF-KB, Cytokines, T-lymphocytes, HO-1, Nrf-2, TLR4, PGE2, MMP9) | 36 (39.1) |
| Dendritic Spine Length or Neurite Changes | 19 (20.7) |
| Neurotransmitters or neuromodulator metabolites and receptors (Dopamine, serotonin, noradrenaline, glutamate, NMDA, ARC mRNA, GAD67) | 18 (19.6) |
| Oxidative stress markers (protein carbonyl, Mn-SOD, GPx, SDH, ROS, FoxO 3a, CAT, SOD, GSH, T-AOC, MDA, NQO1, UBIQ-RD) | 16 (17.4) |
| Microglia (Iba1) | 13 (14.1) |
| Necrotic Processes (apoptosis, JNK1, Caspase 3, TUNEL, LC3ii/I, Caspase-8,9, Bax, Bcl-2, MMP14) | 12 (13.0) |
| Amyloid (APP, Abeta42, ADAM10, BACE1) | 11 (12.0) |
| Altered Astrocytes (GFAP, S100beta) | 11 (12.0) |
| Immune Reactions (iNOS, MyD88, NFKB1, ADAMTS1, p65, NLRP3) | 10 (10.9) |
| Altered cell proliferation (p53, karyopycnosis, karyolysis, cyclin D, FoxO 1a, Lin28, Kbtbd8, mir-574–5p, ACE1) | 9 (9.8) |
| Brain volumes | 9 (9.8) |
| Neurotrophins (NGF, BDNF, CREB, p-CREB) | 8 (8.7) |
| Synaptic changes (EPSCs, PSD-95, fEPSP, PNNs, PV-positive interneurons, VAMP2, GAP43, SYP, VGLUT1, VGLUT2, VGAT) | 8 (8.7) |
| Altered neurogenesis (doublecortin, Neu-N, EdU, Sox2, Trb2) | 7 (7.6) |
| Lipid Peroxidation (MDA, TBA-RS) | 6 (6.5) |
| Mitochondrial Changes (SOD-2, MitoSox fluorescence, cytochrome c, Presenilin 1 and 2, JC-, Ndufa1, Ndufa2, Atp5h, total aconitase activity) | 6 (6.5) |
| Altered DNA (global methylation, Dnmt1, H3K9me2/me3, γ-H2A.X) | 5 (5.4) |
| Heavy metal deposits (Cr, Co, Ti, Li, Be, Al, Ni, Se, Cd, Ba, Pb) or Nanoparticle Deposits | 4 (4.3) |
| Endoplasmic Retiuclum changes (Syx5, Ildr2. Caspase-12) | 3 (3.3) |
| Genes associated with neuronal development (AUTS2, neurocan, IGf1) | 3 (3.3) |
| Activated Neurons (c-Fos) | 2 (2.2) |
| Alterations in neuronal migration (Dcx) | 2 (2.2) |
| Arachidonic Acid Metabolites (methyl arachidonic acid, linoleicacid, 8-isoprostane) | 2 (2.2) |
| Blood Brain Barrier Integrity (plasma-derived IgG, ZO-1) | 2 (2.2) |
| Cerebrovascular changes (Ang II-AT1) | 2 (2.2) |
| Decreased number of neurons | 2 (2.2) |
| Memory related kinases, genes (PKA, PKC, CaMKIIalpha, ADAM11) | 2 (2.2) |
| Myelin Alterations (MBP) | 2 (2.2) |
| Spectroscopy (MRS) | 2 (2.2) |
| Swollen and damaged cells (vaculoes, neuropil) | 2 (2.2) |
| White Matter Lesions | 2 (2.2) |
| Aspartic Acid Metabolites (aspartic acid, asparagine, homoserine) | 1 (1.1) |
| Autism (ASD) Genes expression - Shank3 | 1 (1.1) |
| Ca2+ signaling pathway-related genes | 1 (1.1) |
| Cerebral Microbleeds (Iron deposits, hemosiderin) | 1 (1.1) |
| Cholesterol metabolites (desmosterol, lanosterol, campesterol) | 1 (1.1) |
| Diffusion Tensor Imaging (DTI) | 1 (1.1) |
| Energy metabolites (citric acid, succinic acid, malic acid, maltose, and creatinine) | 1 (1.1) |
| Genes involved in bronchial smooth muscle cells (ADRB2) | 1 (1.1) |
| Glucocorticoid receptors | 1 (1.1) |
| Inositol phosphate metabolites (myto-inositol-1-phosphate, methyl-phosphate, myo-inositol) | 1 (1.1) |
| Misfolded proteins (BiP) | 1 (1.1) |
| Neuronal Plasticity measures (tenascin c) | 1 (1.1) |
| Tau-related pathology (AT8, Tau5, tau protein phosphorylation) | 1 (1.1) |
| Thyroid hormone signaling pathway genes (Prkca, Med12l, Ep300, Slc16a10) | 1 (1.1) |
Of the 33 studies (32 preclinical, 1 postmortem human study) evaluating inflammatory reactions, all but one reported significant increase in inflammation following air pollution exposure. Inflammatory reactions were most studied in the hippocampus (73% or studies), followed by PFC (6%), or in multiple frontolimbic brain regions (21% of studies). Of the 19 studies (all preclinical), that evaluated neurite changes (e.g., dendritic spine lengths, neuronal degeneration), all studies reported significant findings including decreases in the hippocampus (79% of studies), PFC (11%), or in multiple frontolimbic brain regions (11%), following air pollution exposure. While 17 studies (all preclinical) investigated changes to neurotransmitter or neuromodulator systems, the directionality of results were mixed (i.e., increases vs. decreases); however, 100% of studies reported significant effects air pollution exposure. Studies on neurotransmitters were commonly focused on the glutamatergic system only (50% of studies), and half of the studies investigated multiple systems, e.g., dopaminergic, serotonin, gamma-aminobutyric acid (GABA). Changes to neurotransmitter systems were reported primarily in the hippocampus (71% of studies), PFC (6%), and in multiple frontolimbic brain areas (18%). Fifteen studies (all preclinical) reported significant increases in oxidative stress markers (e.g., MnSOD, GSH, MDA) following exposure to air pollution. Studies reporting increases in oxidative stress makers focused primarily on the hippocampus only (60% of studies), 7% PFC only, and 33% in multiple frontolimbic brain regions. Seven articles (8%) utilized air pollution estimates. Of those seven articles, only one controlled for meteorological conditions. Eighty-five articles (92%) did not utilize estimates (e.g., animal studies). Adulthood (59%) was the most frequently examined exposure window across studies, followed by prenatal (27%), early-life (5%), adolescence (5%), childhood (2%), and late-life (2%). The results for literature search 2 are graphically represented in Figure 3.
3.2.1. Literature search 2 – Animal studies
Of the nonhuman animal model studies, 51% were in mice, 44% in rats, 4% in both mice and rats within the same study, and 1% in dogs. Most studies (66%) were conducted in males only, 6% in females only, 24% included both male and female animals, and 3% did not specify. The exposure methods used were whole body inhalation (81% of articles), intratracheal instillation (6%), intranasal instillation (6%), oropharyngeal aspiration (3%), combination whole body inhalation and nose-only inhalation (1%), tracheal drip (1%), endotracheal nebulization (1%), and one study did not specify.
A recent example of one of the animal studies included in literature search 2 examined the developmental neurotoxicity of traffic-related air pollution (TRAP) exposure (Patten et al., 2020). Sprague-Dawley rats were exposed to TRAP 24 hours/day via whole-body inhalation, from gestational day (GD) 14 to PND 47–51. Both male and female rats were included in this study. Animals were sacrificed 2–4 days after final exposure. Exposed rats had increased levels of microglia and astrocyte activity within the hippocampus, compared to control rats. Regarding cellular neuroinflammatory responses, exposed female rats have significantly higher levels of an anti-inflammatory cytokine (IL-10) and more mature neurons in the hippocampus compared to control female rats. Additionally, exposed male rats had increased neurogenesis, cell proliferation, and expression of a growth factor implicated in autism spectrum disorder (Igf1) in the hippocampus, compared to control rats.
3.2.2. Literature search 2 – Human studies
Most studies (92%) included both men and women, and 1 study (7%) included women only. Recruitment from high-polluted vs. low-polluted areas was used as the exposure method for four of the ten studies. Three of the human studies used land-use regression models to estimate exposures, one used a Bayesian maximum entropy (BME) model, one used a universal kriging model, and one used a combination of personal air monitoring and urinary metabolites. Most observational studies were conducted in Mexico (50%), followed by the United States (22%), the United Kingdom (14%), the Netherlands (7%) and South Korea (7%), See Figure 4, Panel B for a map of locations of the human observational studies included in literature search 2. Of the 13 studies that included humans, 3 conducted brain assessments post-mortem and 10 utilized in vivo neuroimaging approaches. Of the studies that were conducted post-mortem, outcomes examined included altered DNA expression and damage, amyloid and tau-related pathologies, cerebrovascular changes, inflammatory reactions, and heavy metal deposits, within hippocampal and frontal cortex tissues. Of the studies that utilized neuroimaging, two studies examined alterations in neurochemistry though magnetic resonance spectroscopy (MRS), two studies examined white matter hyperintensities or lesions using T2 or FLAIR MRI scans, and the remaining 6 studies examined brain volumes and morphology using structural T1 MRI scans.
A recent example of one of the human studies included in literature search 2 examined whether prenatal or childhood exposure to air pollution was associated with changes in brain morphology in pre-adolescence (Lubczynska et al., 2021). Lubczyńska and colleagues estimated air pollution exposure based on participants’ residential addresses using well-validated land-use regression models. The specific pollutants examined included PM10, PM2.5, absorbance of PM2.5 fraction, composition of PM2.5 consisting of polycyclic aromatic hydrocarbons (PAH), benzo[a]pyrene, organic carbon, copper, iron, potassium, silicon (Si), zinc, and oxidative potential of PM2.5 (OP). Brain morphology was measured using structural magnetic resonance imaging (MRI) on a 3T scanner. Regional gray matter volumes of subcortical brain regions, including the hippocampus and amygdala, were computed. Covariates included in the analyses were parental education, household income, country of birth, parental age, maternal smoking and alcohol consumption during pregnancy, parity, marital status, parental psychological distress, pre-pregnancy BMI, maternal IQ at child’s age 6, child sex, and child current age. Results showed that prenatal exposure to PAH was associated with smaller hippocampal volumes, and higher prenatal exposure to Si was associated with larger amygdala volume. Higher exposure to OP during childhood was associated with smaller hippocampal volumes. No associations were observed between childhood exposures and amygdala volumes.
3.2.3. Literature search 2 – Sex-specific effects
Nine articles (3 human, 6 animal) were identified that reported on sex- or gender- specific effects in the impact of air pollution exposure on frontolimbic brain outcomes. The results from these studies were mixed. For example, in the animal studies, 4 studies reported that the effects on frontolimbic brain regions following air pollution exposure were stronger in males as compared to females (Cole et al., 2016; Ehsanifar, Montazeri, et al., 2021; Haghani, Johnson, Woodward, et al., 2020; Nway et al., 2017), while 2 studies reported mixed findings (e.g., the effects of air pollution on certain brain outcomes were stronger in males and others were stronger in females) (Custodio et al., 2019; Patten et al., 2020). In the human studies, two studies reported that females were more susceptible to the effects of air pollution on frontolimbic brain regions than males (Hedges et al., 2019; Peterson et al., 2015), and one study reported that males were more susceptible than females (Cho et al., 2020).
3.2.4. Literature search 2 – Age effects
Three articles (all animal) were identified that reported on age effects. The results from these studies were mixed. One study found differential effects in both young and aged rats following exposure to air pollution in adulthood and late-life (e.g., both young and aged exposed rats demonstrated oxidative damage in frontolimbic brain regions, however only the young exposed rats had effects observed in the frontal cortex) (Kodavanti et al., 2021). One study found that effects of air pollution exposure (i.e., altered cell proliferation, increased oxidative stress, cerebrovascular changes, amyloid deposits) on frontolimbic brain regions were more pronounced in exposed aged mice as compared to exposed young mice (Armstrong et al., 2020). In contrast, one study reported that effects of exposure (i.e., myelin alterations, changes to microglia, dendritic spine length or neurite changes, neurotransmitter or neuromodulator changes, increase inflammation) on frontolimbic brain regions were diminished in older mice and did not exacerbate effects associated with normal aging; young mice, in contrast, displayed significant effects on frontolimbic brain regions following exposure (Woodward, Pakbin, et al., 2017).
4. DISCUSSION
This paper is the first, to the best of our knowledge, to systematically review the literature on the effects of air pollution on (1) both internalizing symptoms and behaviors in humans and animal models, and (2) the impact on underlying frontolimbic brain regions. An overall conceptual model of neurobehavioral mechanisms by which exposure to air pollution increases risk of internalizing symptoms and behavior is provided in Figure 5. Here, we summarize results of our systematic reviews, discuss gaps in the literature, and identify future directions for research.
Figure 5.
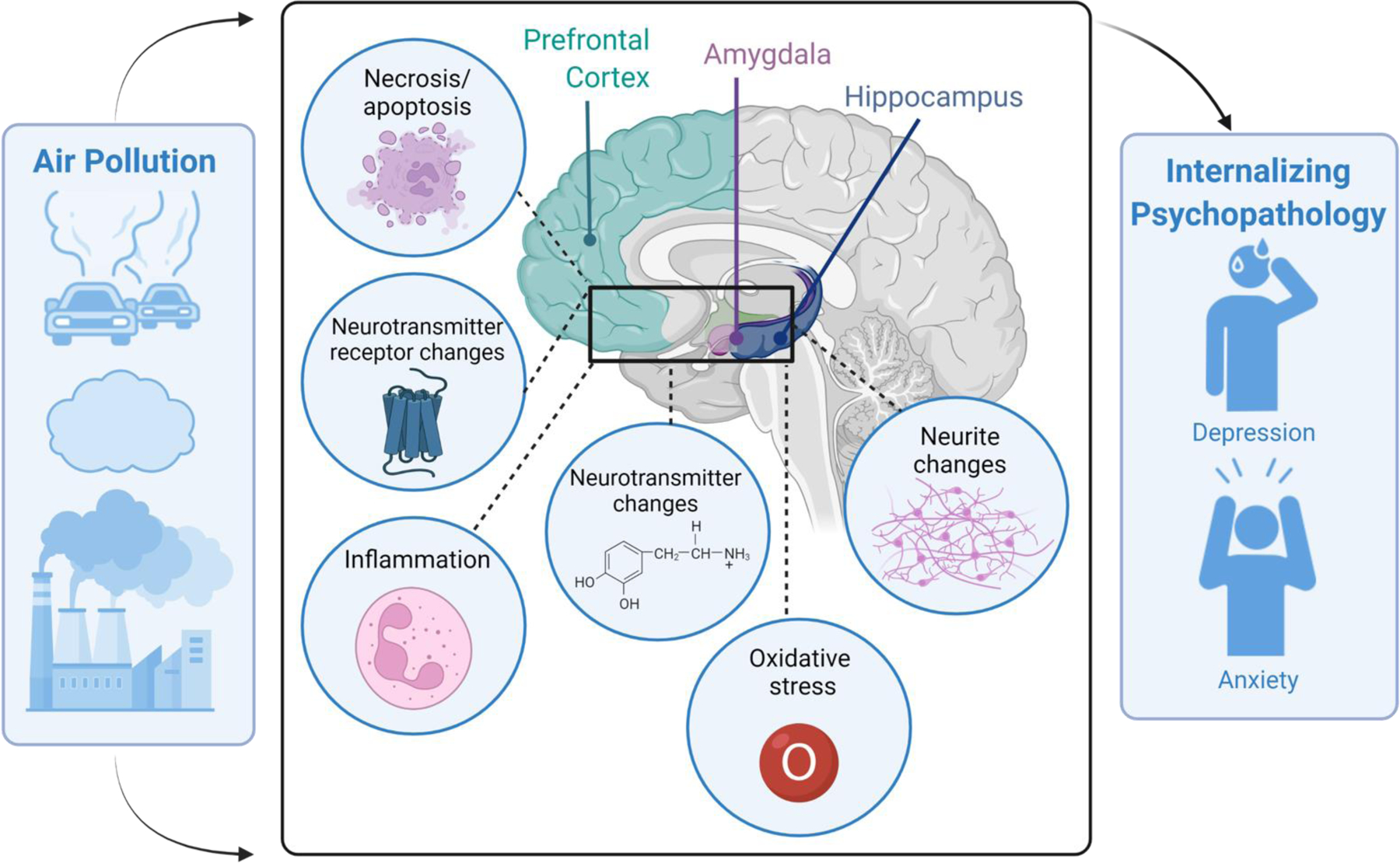
A conceptual model of neurobehavioral mechanisms by which air pollution exposure increases risk of internalizing psychopathology through structural and functional changes in frontolimbic brain regions. This figure was created with BioRender.com.
4.1. Summary of systematic reviews
In summary, our first systematic review on air pollution and internalizing symptoms and behaviors revealed that air pollution exposure is consistently associated with increased anxiety and depression across different exposure windows and in both human and animal models. We found that most research focused specifically on depression outcomes, while only 15% of articles focused on anxiety alone. Most studies examined multiple pollutants, while studies that focused on one pollutant primarily assessed PM2.5 and DEPs — two pollutants that have been shown to directly affect the CNS (Ehsanifar, Tameh, et al., 2019; Ferreira et al., 2022; Hartz et al., 2008; Kang et al., 2021). Most articles included in literature review 1 examined exposures that spanned multiple developmental windows, with less attention given to exposure windows specifically within childhood and adolescence, a period of dramatic neurodevelopment (Arain et al., 2013). Within the human studies, most obtained air pollution estimates from fixed ground monitoring stations, rather than using modeling techniques that incorporate land-use and meteorological variables.
Our second systematic review, which focused on air pollution exposure and frontolimbic brain regions, revealed that air pollution exposure is associated with several neurobiological changes, predominantly increased inflammation, neuronal degeneration, and oxidative stress. The hippocampus was the most commonly assessed brain region with less attention given to the PFC and the amygdala. In contrast to the first systematic review on internalizing symptoms and behaviors, most studies included in this review on frontolimbic brain regions were conducted predominantly in animal models, with only 10 articles that assessed humans. Of note, the majority of the animal model studies (66%) focused exclusively on males, which is a significant gap given that internalizing disorders are more prevalent among females than males (Dattani et al., 2021). Similar to the first systematic review, the majority of studies examined multiple pollutants, with studies that focused on one pollutant primarily assessing PM2.5 and DEPs. Additionally, most articles examined exposures that spanned more than one developmental window, with childhood and adolescence less studied.
Both systematic reviews identified several studies that reported on sex effects following air pollution exposure; however, the results from these studies were mixed, and thus no conclusion can be drawn. Sex effects were reported in both the neurotoxic effects on frontolimbic brain regions as well in the internalizing symptoms and behaviors observed following air pollution exposure. Future studies should take a more targeted approach at investigating the differential sex effects and their potential mechanisms (e.g., menstrual cycle fluctuations, changes in estrogen/testosterone), and how these changes may influence vulnerability.
Additionally, both systematic reviews identified studies that reported on age effects following air pollution exposure. While findings were mostly mixed, a large portion of studies suggested that children and adolescents and older adults were more vulnerable to both the neurotoxic effects on frontolimbic brain regions and internalizing symptoms and behaviors following air pollution exposure, as compared to young and middle adults. While these studies indicated increased vulnerability for these specific age groups, the exposure windows within these studies were extremely variable (i.e., recent exposures versus prenatal or early-life) and thus it is difficult to conclude at what point in the life cycle individuals are associated with enhanced vulnerability to the negative effects of air pollution exposure. For example, most studies examined recent exposures, which for children would impact their developing brains, but for older adults would impact fully developed or aging brains. Thus, future studies are needed that address all developmental windows or exposures and subsequent timepoints in which assessments occur (i.e., in childhood or decades later). In fact, accelerated longitudinal study designs would be most equipped to help further elucidate age effects, including delayed effects, following air pollution exposure.
4.2. Gaps in the literature
For both literature search 1 and 2 the most assessed exposure window was adulthood followed by the prenatal period. Early-life, childhood, and adolescence exposure windows were rarely assessed (accounting for 1–4% of studies in each search). This is especially concerning when evaluating neurobehavioral outcomes as the brain continues to develop until young adulthood and may therefore be particularly sensitive to neurotoxic effects of air pollution during development (Arain et al., 2013). While prenatal exposure may have a substantial impact on development, less is known about post-natal exposures and how those may affect brain neural circuity, which undergoes dramatic development and refinement throughout adolescence (Gogtay et al., 2004). For example, the neural circuity underlying PFC-hippocampal interactions, a system that is implicated in emotion regulation and internalizing psychopathology, develops throughout adolescence (Calabro et al., 2020). Thus, more preclinical, and clinical studies specifically assessing frontolimbic or internalizing outcomes in childhood and adolescence are sorely needed.
Within literature search 2, a majority of studies focused on the hippocampus, when examining effects of air pollution exposure on frontolimbic brain regions. While the hippocampus is an essential part of the frontolimbic neural circuity, the PFC and amygdala — and interactions within these structures — are also important for internalizing psychopathology. For example, meta-analyses on brain function and structure within anxiety disorders have consistently revealed hyperactivation in the amygdala and hypoactivation and decreased volume within the PFC relative to healthy controls (Etkin & Wager, 2007; Janiri et al., 2020; Kolesar et al., 2019). Meta-analyses on brain function and structure within depressive disorders have revealed hyperactivation within the PFC relative to healthy controls (Espinoza Oyarce et al., 2020; Miller et al., 2015; Wang et al., 2012). Studies that have correlated these neural differences to internalizing symptoms have found that greater resting state functional connectivity between the amygdala and PFC is associated with increased rumination and worry (Feurer et al., 2021). Thus, future studies should investigate changes associated with air pollution in both the PFC and amygdala in addition to the hippocampus, and interactions between these regions.
Additionally, within literature search 2, only 10 studies were conducted in humans, three of which were post-mortem assessments. While the animal literature has extensively shown that air pollution can induce a multitude of changes to frontolimbic brain regions, including inflammatory and oxidative stress reactions, the replication of these studies within humans is lacking. The 7 neuroimaging studies examining the effects of air pollution primarily assessed brain volumetrics through T1 MRI, white matter hyperintensities or lesions via T2 or FLAIR MRI, and alterations in neurochemistry assessed by MR spectroscopy imaging. While the majority of studies assessed total brain volumes (e.g., total volume of the frontal lobe), future studies should incorporate region-of-interest analyses to identify regional changes in the hippocampus, PFC, and amygdala, and their associations to internalizing symptoms and behaviors. No studies included in this review assessed functional MRI outcomes (either resting-state or task-based). While structural imaging is useful for detecting brain damage and abnormalities, functional imaging can often detect changes that precede structural changes or subtle changes in cerebral blood flow or activation) as opposed to brain atrophy (Gore, 2003; Grajski et al., 2019). Additionally, functional imaging can identify changes in activity that occur during specific behavioral tasks, allowing for the potential identification of neurobehavioral mechanisms linking air pollution exposure and internalizing psychopathology. Thus, future studies are needed to examine the effect of air pollution on neuroimaging outcomes, specifically functional neuroimaging techniques, within humans.
Finally, only 13 articles were identified in both literature search 1 and literature search 2 that examined internalizing symptomology and frontolimbic brain regions within the same study. To elucidate the neurobehavioral mechanisms underlying the associations between air pollution exposure and mental health, additional studies, both preclinical and clinical, are needed that assess these outcomes within the same study design, and to evaluate these associations through a mediation design (see conceptual model in Figure 5). For example, does air pollution affect internalizing symptomology through changes in frontolimbic brain regions? This question could be more readily answered in studies that analyze both brain and behavioral outcomes.
4.3. Directions for future research
While the epidemiological evidence associating air pollution exposure with increased internalizing symptoms and behaviors continues to be replicated in different populations and with differing exposure windows, less is known concerning the neurobiological underpinnings. Preclinical studies have shown that air pollution affects frontolimbic brain regions involved in internalizing symptoms and behaviors in a multitude of ways, however human neuroimaging studies have been less prevalent and have focused more on global measures, rather than region-of-interest-based approaches. In fact, literature search 1, on internalizing symptoms and behaviors, was disproportionally composed of human studies (76%), whereas literature search 2, on neurobiological effects on frontolimbic brain regions, was composed primarily of animal models (86%). Thus, future human neuroimaging studies are sorely needed and should target their investigations on emotion-regulation brain regions, such as the hippocampus, amygdala, and PFC, and should incorporate emotion-based functional tasks into their studies.
One of the most prevalent gaps in the literature is the lack of knowledge surrounding windows of susceptibility of air pollution exposure and subsequent mental health and brain outcomes. A developmental approach to these associations is warranted, as many mental health disorders develop during adolescence in concordance with the development of emotion-regulation neural circuits (Calabro et al., 2020; Kessler et al., 2005). Preclinical studies that clearly state the age of the animal at exposure and during behavioral and brain assessments are also critical for elucidating critical periods of exposure. Further, while only two studies were identified that reported delayed internalizing symptoms and behaviors following air pollution exposure in youth (Liu et al., 2019; Margolis et al., 2016), more longitudinal human neuroimaging studies are critical to forming the trajectory of air pollution-based changes in mental health and frontolimbic structure and function. In fact, the Adolescent Brain Cognitive Development (ABCD) study (https://abcdstudy.org/), the largest long-term study of brain development and child health in the United States, has begun to look at the effects of air pollution (Burnor et al., 2021; Cserbik et al., 2020). As the ABCD study continues with yearly follow-ups, the effects of air pollution on brain development as well as neuropsychiatric outcomes will be explored.
Additionally, only three studies were identified in our literature searches that used a pre- and post-exposure study design that allows for individuals to serve as their own control, thus reducing between-subject variability. For example, Chen and colleagues examined mood symptoms in the same participants once during a week that had low levels of air pollution and again during a week that had extremely high levels of air pollution, and found that depression symptoms were significantly higher on the day with extreme air pollution levels(S. Chen et al., 2017). Additionally, Roe and colleagues measured mood symptoms immediately following two different walking routes, one with high air pollution exposure (e.g., near highway) and one with low air pollution exposure (e.g., more greenspace). They found that hedonic depression symptoms decreased significantly following the low air pollution exposure route, while no significant change in symptoms was observed after the high air pollution route (Roe et al., 2020). Further, Brokamp and colleagues used a time-stratified case-crossover study to examine the associations between PM2.5 exposure and psychiatry pediatric emergency department visits. This design that allows each participant to serve as their own control (Brokamp, Strawn, et al., 2019). While these studies are rare and often difficult to conduct, future studies may benefit from designs that incorporate participants as their own controls so that the specific effects of air pollution exposure —independent of the effects of potential confounders (e.g., interindividual variability) — can continue to be identified.
Future research should also incorporate more advanced modeling of air pollution estimates or the use of personal air monitors. In both literature searches, most human studies relied on measurements from fixed ground monitoring stations, often using a single monitor or several monitors averaged while weighted by distance. Monitoring is prohibitively expensive (most counties in the US do not have a regulatory air pollution monitor) and cannot fully capture the complex spatiotemporal variations in air pollution. Alternatives are to utilize personal sampling, whereby individuals use personal monitors to better capture variability in air pollution due to time-behavior patterns, or to use exposure assessment models, which use spatiotemporal features (e.g., meteorological data, satellite-based measures, land characteristics, and measures of air pollution sources like vehicles and industrial activity) to predict air pollution concentrations in locations and times that measurements were unavailable. Additionally, these two exposure techniques (i.e., fixed ground modeling and personal monitoring) are utilized to answer different scientific questions. For example, fixed air monitoring can help examine the effects of ambient air pollution concentrations at the neighborhood level, while personal monitoring can examine the effects of an individual’s personal concentration of air pollutants. While these two types of exposures (i.e., community and personal) may synergistically contribute to health effects, they are studied using different methods. For a more detailed discussion on the advantages and disadvantages of model-based and personal sample strategies, see Brokamp, Brandt, et al. (2019).
Further, most human studies examine the average air pollution estimates across time periods, and do not necessarily evaluate the cumulative air pollution an individual may be exposed to, which contrasts with preclinical/animal studies. This discrepancy has led to calls by the Office of Research and Development of the United States Environmental Protection Agency for further research into cumulative impacts, as the “single pollutant/single exposure” paradigm is not well suited to the reality that individuals are exposed to several pollutants over time (EPA, 2022). Lastly, future studies should investigate independent contributions of indoor and outdoor air pollution to internalizing symptoms and behaviors and changes in frontolimbic brain regions.
4.4. Limitations and conclusions
The systematic review performed in this paper is not without limitations. First, potentially eligible studies may have been missed. To minimize this risk, a wide search was performed on one of the best tools for biomedical electronic research, MEDLINE. While this review focused on a comprehensive report on all air pollutants, future reviews may take a pollutant-specific approach to examine if there are differential effects of different types of pollutants on both internalizing symptoms and behaviors and frontolimbic brain regions. Further, while our review on internalizing symptoms and behaviors following air pollution exposure encompassed many definitions and measurements of anxiety and depression, the observed pattern (i.e., increased symptoms post-exposure) appears to be consistent across measurement types. Next, we focused our search on frontolimbic brain regions, given their key role in emotion regulation and internalizing psychopathology (Espinoza Oyarce et al., 2020; Janiri et al., 2020; Kolesar et al., 2019). However, future studies should consider other brain regions and white matter pathways that may be involved in emotional regulation and their susceptibility to air pollutant exposures.
In conclusion, air pollution exposure is associated with increased internalizing symptoms and behaviors as well as structural and functional changes to frontolimbic brain regions across the lifespan. Further investigation with improvements in design and reporting would fill the following key gaps in literature: First, more assessments of the brain and behavioral effects of air pollution are needed during childhood and adolescence, and longitudinal evaluations would be a welcome addition. Next, more human neuroimaging assessments are needed to replicate or compare the effects of air pollution on frontolimbic brain regions that have been reported in the nonhuman animal literature. Lastly, more comprehensive studies are needed that examine both internalizing symptoms and frontolimbic brain outcomes within the same study design, which will allow for mediation analyses to be explored. The identification of the neurobiological mechanisms underlying the associations between air pollution exposure and increased mental health issues is imperative. This research would identify biological targets for intervention to stem the pathophysiology of internalizing disorders. While air pollution exposure may not be decreased as quickly and effectively as needed, additional research will aid in the development of appropriate interventions that will mediate air pollution’s negative effects on the brain and subsequent mental health.
Supplementary Material
ACKNOWLEDGEMENTS
HM is supported by the National Institute of Mental Health (K01MH119241) and Eunice Kennedy Shriver National Institute of Child Health and Human Development (R21HD105882). HM, CZ, and YH acknowledge funding support from the Center for Urban Responses to Environmental Stressors (CURES) (P30ES020957). PR and CB are supported by the National Institute of Environmental Health Sciences (R01ES031621). JRS is supported by the National Institute of Child Health and Human Development (R01HD099775, R01HD09875) and the Yung Family Foundation. YH acknowledges funding support from the Faculty Competition for Postdoctoral Fellows of the Office of Vice President for Research at Wayne State University. The grant providers had no influence on study design, the collection, analyses and interpretation of data, report writing nor decision of submission for publication
REFERENCES
- Ahlers NE, & Weiss SJ (2021). Exposure to particulate matter, prenatal depressive symptoms and HPA axis dysregulation. Heliyon, 7(6), e07166. 10.1016/j.heliyon.2021.e07166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allaouat S, Yli-Tuomi T, Tiittanen P, Turunen AW, Siponen T, Kukkonen J, Kangas L, Kauhaniemi M, Aarnio M, Ngandu T, & Lanki T (2021). Long-term exposure to ambient fine particulate matter originating from traffic and residential wood combustion and the prevalence of depression. J Epidemiol Community Health, 75(11), 1111–1116. 10.1136/jech-2021-216772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altug H, Fuks KB, Huls A, Mayer AK, Tham R, Krutmann J, & Schikowski T (2020). Air pollution is associated with depressive symptoms in elderly women with cognitive impairment. Environ Int, 136, 105448. 10.1016/j.envint.2019.105448 [DOI] [PubMed] [Google Scholar]
- Ambient (Outdoor) Air Pollution Fact Sheet. (2021). https://www.who.int/news-room/fact-sheets/detail/ambient-(outdoor)-air-quality-and-health
- Andrade-Oliva MD, Aztatzi-Aguilar OG, Garcia-Sierra F, De Vizcaya-Ruiz A, & Arias-Montano JA (2018). Effect of in vivo exposure to ambient fine particles (PM2.5) on the density of dopamine D2-like receptors and dopamine-induced [(35)S]-GTPgammaS binding in rat prefrontal cortex and striatum membranes. Environ Toxicol Pharmacol, 60, 58–65. 10.1016/j.etap.2018.04.001 [DOI] [PubMed] [Google Scholar]
- Arain M, Haque M, Johal L, Mathur P, Nel W, Rais A, Sandhu R, & Sharma S (2013). Maturation of the adolescent brain. Neuropsychiatr Dis Treat, 9, 449–461. 10.2147/NDT.S39776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong TD, Suwannasual U, Kennedy CL, Thasma A, Schneider LJ, Phillippi D, & Lund AK (2020). Exposure to Traffic-Generated Pollutants Exacerbates the Expression of Factors Associated with the Pathophysiology of Alzheimer’s Disease in Aged C57BL/6 Wild-Type Mice. J Alzheimers Dis, 78(4), 1453–1471. 10.3233/JAD-200929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avila-Costa MR, Colín-Barenque L, Fortoul TI, Machado-Salas P, Espinosa-Villanueva J, Rugerio-Vargas C, Borgonio G, Dorado C, & Rivas-Arancibia S (2001). Motor impairments in an oxidative stress model and its correlation with cytological changes on rat striatum and prefrontal cortex. The International journal of neuroscience, 108(3–4), 193–200. [DOI] [PubMed] [Google Scholar]
- Avila-Costa MR, Colín-Barenque L, Fortoul TI, Machado-Salas P, Espinosa-Villanueva J, Rugerio-Vargas C, & Rivas-Arancibia S (1999). Memory deterioration in an oxidative stress model and its correlation with cytological changes on rat hippocampus CA1. Neuroscience letter, 270(2), 107–109. [DOI] [PubMed] [Google Scholar]
- Babadjouni RM, Hodis DM, Radwanski R, Durazo R, Patel A, Liu Q, & Mack WJ (2017). Clinical effects of air pollution on the central nervous system; a review. J Clin Neurosci, 43, 16–24. 10.1016/j.jocn.2017.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai KJ, Chuang KJ, Chen CL, Jhan MK, Hsiao TC, Cheng TJ, Chang LT, Chang TY, & Chuang HC (2019). Microglial activation and inflammation caused by traffic-related particulate matter. Chem Biol Interact, 311, 108762. 10.1016/j.cbi.2019.108762 [DOI] [PubMed] [Google Scholar]
- Balboni E, Filippini T, Crous-Bou M, Guxens M, Erickson LD, & Vinceti M (2022). The association between air pollutants and hippocampal volume from magnetic resonance imaging: A systematic review and meta-analysis. Environ Res, 204(Pt A), 111976. 10.1016/j.envres.2021.111976 [DOI] [PubMed] [Google Scholar]
- Bello-Medina PC, Prado-Alcala RA, & Rivas-Arancibia S (2019). Effect of Ozone Exposure on Dendritic Spines of CA1 Pyramidal Neurons of the Dorsal Hippocampus and on Object-place Recognition Memory in Rats. Neuroscience, 402, 1–10. 10.1016/j.neuroscience.2019.01.018 [DOI] [PubMed] [Google Scholar]
- Bereitschaft B, & Debbage K (2013). Urban Form, Air Pollution, and CO2 Emissions in Large U.S. Metropolitan Areas. The Professional Geographer, 64(4), 612–635. [Google Scholar]
- Borroni E, Pesatori AC, Bollati V, Buoli M, & Carugno M (2022). Air pollution exposure and depression: A comprehensive updated systematic review and meta-analysis. Environ Pollut, 292(Pt A), 118245. 10.1016/j.envpol.2021.118245 [DOI] [PubMed] [Google Scholar]
- Bos I, De Boever P, Emmerechts J, Buekers J, Vanoirbeek J, Meeusen R, Van Poppel M, Nemery B, Nawrot T, & Panis LI (2012). Changed gene expression in brains of mice exposed to traffic in a highway tunnel. Inhal Toxicol, 24(10), 676–686. 10.3109/08958378.2012.714004 [DOI] [PubMed] [Google Scholar]
- Bowler RM, Harris M, Gocheva V, Wilson K, Kim Y, Davis SI, Bollweg G, Lobdell DT, Ngo L, & Roels HA (2012). Anxiety affecting parkinsonian outcome and motor efficiency in adults of an Ohio community with environmental airborne manganese exposure. Int J Hyg Environ Health, 215(3), 393–405. 10.1016/j.ijheh.2011.10.005 [DOI] [PubMed] [Google Scholar]
- Braithwaite I, Zhang S, Kirkbride JB, Osborn DPJ, & Hayes JF (2019). Air Pollution (Particulate Matter) Exposure and Associations with Depression, Anxiety, Bipolar, Psychosis and Suicide Risk: A Systematic Review and Meta-Analysis. Environ Health Perspect, 127(12), 126002. 10.1289/EHP4595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brokamp C, Brandt EB, & Ryan PH (2019). Assessing exposure to outdoor air pollution for epidemiological studies: Model-based and personal sampling strategies. J Allergy Clin Immunol, 143(6), 2002–2006. 10.1016/j.jaci.2019.04.019 [DOI] [PubMed] [Google Scholar]
- Brokamp C, Strawn JR, Beck AF, & Ryan P (2019). Pediatric Psychiatric Emergency Department Utilization and Fine Particulate Matter: A Case-Crossover Study. Environ Health Perspect, 127(9), 97006. 10.1289/EHP4815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunst KJ, Ryan PH, Altaye M, Yolton K, Maloney T, Beckwith T, LeMasters G, & Cecil KM (2019). Myo-inositol mediates the effects of traffic-related air pollution on generalized anxiety symptoms at age 12years. Environ Res, 175, 71–78. 10.1016/j.envres.2019.05.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnor E, Cserbik D, Cotter DL, Palmer CE, Ahmadi H, Eckel SP, Berhane K, McConnell R, Chen JC, Schwartz J, Jackson R, & Herting MM (2021). Association of Outdoor Ambient Fine Particulate Matter With Intracellular White Matter Microstructural Properties Among Children. JAMA Netw Open, 4(12), e2138300. 10.1001/jamanetworkopen.2021.38300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabro FJ, Murty VP, Jalbrzikowski M, Tervo-Clemmens B, & Luna B (2020). Development of Hippocampal-Prefrontal Cortex Interactions through Adolescence. Cereb Cortex, 30(3), 1548–1558. 10.1093/cercor/bhz186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Engle R, Mora-Tiscareno A, Styner M, Gomez-Garza G, Zhu H, Jewells V, Torres-Jardon R, Romero L, Monroy-Acosta ME, Bryant C, Gonzalez-Gonzalez LO, Medina-Cortina H, & D’Angiulli A (2011). Exposure to severe urban air pollution influences cognitive outcomes, brain volume and systemic inflammation in clinically healthy children. Brain Cogn, 77(3), 345–355. 10.1016/j.bandc.2011.09.006 [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Herrera-Soto A, Jury N, Maher BA, Gonzalez-Maciel A, Reynoso-Robles R, Ruiz-Rudolph P, van Zundert B, & Varela-Nallar L (2020). Reduced repressive epigenetic marks, increased DNA damage and Alzheimer’s disease hallmarks in the brain of humans and mice exposed to particulate urban air pollution. Environ Res, 183, 109226. 10.1016/j.envres.2020.109226 [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Maronpot RR, Torres-Jardon R, Henriquez-Roldan C, Schoonhoven R, Acuna-Ayala H, Villarreal-Calderon A, Nakamura J, Fernando R, Reed W, Azzarelli B, & Swenberg JA (2003). DNA damage in nasal and brain tissues of canines exposed to air pollutants is associated with evidence of chronic brain inflammation and neurodegeneration. Toxicol Pathol, 31(5), 524–538. 10.1080/01926230390226645 [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Mora-Tiscareno A, Melo-Sanchez G, Rodriguez-Diaz J, Torres-Jardon R, Styner M, Mukherjee PS, Lin W, & Jewells V (2015). A Critical Proton MR Spectroscopy Marker of Alzheimer’s Disease Early Neurodegenerative Change: Low Hippocampal NAA/Cr Ratio Impacts APOE varepsilon4 Mexico City Children and Their Parents. J Alzheimers Dis, 48(4), 1065–1075. 10.3233/JAD-150415 [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Mora-Tiscareno A, Ontiveros E, Gomez-Garza G, Barragan-Mejia G, Broadway J, Chapman S, Valencia-Salazar G, Jewells V, Maronpot RR, Henriquez-Roldan C, Perez-Guille B, Torres-Jardon R, Herrit L, Brooks D, Osnaya-Brizuela N, Monroy ME, Gonzalez-Maciel A, Reynoso-Robles R, … Engle RW (2008). Air pollution, cognitive deficits and brain abnormalities: a pilot study with children and dogs. Brain Cogn, 68(2), 117–127. 10.1016/j.bandc.2008.04.008 [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Reed W, Maronpot RR, Henriquez-Roldan C, Delgado-Chavez R, Calderon-Garciduenas A, Dragustinovis I, Franco-Lira M, Aragon-Flores M, Solt AC, Altenburg M, Torres-Jardon R, & Swenberg JA (2004). Brain inflammation and Alzheimer’s-like pathology in individuals exposed to severe air pollution. Toxicol Pathol, 32(6), 650–658. 10.1080/01926230490520232 [DOI] [PubMed] [Google Scholar]
- Calderon-Garciduenas L, Reynoso-Robles R, Vargas-Martinez J, Gomez-Maqueo-Chew A, Perez-Guille B, Mukherjee PS, Torres-Jardon R, Perry G, & Gonzalez-Maciel A (2016). Prefrontal white matter pathology in air pollution exposed Mexico City young urbanites and their potential impact on neurovascular unit dysfunction and the development of Alzheimer’s disease. Environ Res, 146, 404–417. 10.1016/j.envres.2015.12.031 [DOI] [PubMed] [Google Scholar]
- Campbell KI, Emik LO, Clarke GL, & Plata RL (1970). Inhalation Toxicity of Peroxyacetyl Nitrate. Archives of Environmental Health, 20, 22–27. [DOI] [PubMed] [Google Scholar]
- Chao MW, Yang CH, Lin PT, Yang YH, Chuang YC, Chung MC, & Tseng CY (2017). Exposure to PM2.5 causes genetic changes in fetal rat cerebral cortex and hippocampus. Environ Toxicol, 32(4), 1412–1425. 10.1002/tox.22335 [DOI] [PubMed] [Google Scholar]
- Chen B, Ma W, Pan Y, Guo W, & Chen Y (2021). PM2.5 exposure and anxiety in China: evidence from the prefectures. BMC Public Health, 21(1), 429. 10.1186/s12889-021-10471-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JC, Wang X, Serre M, Cen S, Franklin M, & Espeland M (2017). Particulate Air Pollutants, Brain Structure, and Neurocognitive Disorders in Older Women. Research report (Health Effects Institute), 193, 1–65. [PMC free article] [PubMed] [Google Scholar]
- Chen S, Kong J, Yu F, & Peng K (2017). Psychopathological Symptoms under Smog: The Role of Emotion Regulation. Front Psychol, 8, 2274. 10.3389/fpsyg.2017.02274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L, Lau WKW, Fung TKH, Lau BWM, Chau BKH, Liang Y, Wang Z, So KF, Wang T, Chan CCH, & Lee TMC (2017). PM2.5 Exposure Suppresses Dendritic Maturation in Subgranular Zone in Aged Rats. Neurotox Res, 32(1), 50–57. 10.1007/s12640-017-9710-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho J, Noh Y, Kim SY, Sohn J, Noh J, Kim W, Cho SK, Seo H, Seo G, Lee SK, Seo S, Koh SB, Oh SS, Kim HJ, Seo SW, Shin DS, Kim N, Kim HH, Lee JI, & Kim C (2020). Long-Term Ambient Air Pollution Exposures and Brain Imaging Markers in Korean Adults: The Environmental Pollution-Induced Neurological EFfects (EPINEF) Study. Environ Health Perspect, 128(11), 117006. 10.1289/EHP7133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C, Zhang H, Cui S, Han B, Zhou L, Zhang N, Su X, Niu Y, Chen W, Chen R, Zhang R, & Zheng Y (2019). Ambient PM2.5 caused depressive-like responses through Nrf2/NLRP3 signaling pathway modulating inflammation. J Hazard Mater, 369, 180–190. 10.1016/j.jhazmat.2019.02.026 [DOI] [PubMed] [Google Scholar]
- Cipriani G, Danti S, Carlesi C, & Borin G (2018). Danger in the Air: Air Pollution and Cognitive Dysfunction. Am J Alzheimers Dis Other Demen, 33(6), 333–341. 10.1177/1533317518777859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford A, Lang L, Chen R, Anstey KJ, & Seaton A (2016). Exposure to air pollution and cognitive functioning across the life course--A systematic literature review. Environ Res, 147, 383–398. 10.1016/j.envres.2016.01.018 [DOI] [PubMed] [Google Scholar]
- Cole TB, Chang YC, Dao K, Daza R, Hevner R, & Costa LG (2020). Developmental exposure to diesel exhaust upregulates transcription factor expression, decreases hippocampal neurogenesis, and alters cortical lamina organization: relevance to neurodevelopmental disorders. journal of neurodevelopmental disorders, 12(1), 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole TB, Coburn J, Dao K, Roque P, Chang YC, Kalia V, Guilarte TR, Dziedzic J, & Costa LG (2016). Sex and genetic differences in the effects of acute diesel exhaust exposure on inflammation and oxidative stress in mouse brain. Toxicology, 374, 1–9. 10.1016/j.tox.2016.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, Cole TB, Dao K, Chang YC, Coburn J, & Garrick JM (2020). Effects of air pollution on the nervous system and its possible role in neurodevelopmental and neurodegenerative disorders. Pharmacol Ther, 210, 107523. 10.1016/j.pharmthera.2020.107523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cserbik D, Chen JC, McConnell R, Berhane K, Sowell ER, Schwartz J, Hackman DA, Kan E, Fan CC, & Herting MM (2020). Fine particulate matter exposure during childhood relates to hemispheric-specific differences in brain structure. Environ Int, 143, 105933. 10.1016/j.envint.2020.105933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Custodio V, Rubio C, & Paz C (2019). Prenatal Ozone Exposure Induces Memory Deficiencies in Newborns Rats. Front Mol Neurosci, 12, 244. 10.3389/fnmol.2019.00244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dattani S, Ritchie H, & Roser M (2021). Mental Health. Published online at OurWorldInData.org. https://ourworldindata.org/mental-health
- Davis DA, Bortolato M, Godar SC, Sander TK, Iwata N, Pakbin P, Shih JC, Berhane K, McConnell R, Sioutas C, Finch CE, & Morgan TE (2013). Prenatal exposure to urban air nanoparticles in mice causes altered neuronal differentiation and depression-like responses. PLoS One, 8(5), e64128. 10.1371/journal.pone.0064128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Prado Bert P, Mercader EMH, Pujol J, Sunyer J, & Mortamais M (2018). The Effects of Air Pollution on the Brain: a Review of Studies Interfacing Environmental Epidemiology and Neuroimaging. Curr Environ Health Rep, 5(3), 351–364. 10.1007/s40572-018-0209-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Domenico M, Benevenuto SGM, Tomasini PP, Yariwake VY, de Oliveira Alves N, Rahmeier FL, da Cruz Fernandes M, Moura DJ, Nascimento Saldiva PH, & Veras MM (2020). Concentrated ambient fine particulate matter (PM2.5) exposure induce brain damage in pre and postnatal exposed mice. Neurotoxicology, 79, 127–141. 10.1016/j.neuro.2020.05.004 [DOI] [PubMed] [Google Scholar]
- Diaz J, Lopez-Bueno JA, Lopez-Ossorio JJ, Gonzalez JL, Sanchez F, & Linares C (2020). Short-term effects of traffic noise on suicides and emergency hospital admissions due to anxiety and depression in Madrid (Spain). Sci Total Environ, 710, 136315. 10.1016/j.scitotenv.2019.136315 [DOI] [PubMed] [Google Scholar]
- Dorado-Martínez C, Paredes-Carbajal C, Mascher D, Borgonio-Pérez G, & Rivas-Arancibia S (2001). Effects of different ozone doses on memory, motor activity and lipi peroxidation levels, in rats. The International journal of neuroscience, 108(3–4), 149–161. [DOI] [PubMed] [Google Scholar]
- Dores AK, Fick GH, MacMaster FP, Williams JVA, Bulloch AGM, & Patten SB (2021). Outdoor Air Pollution and Depression in Canada: A Population-Based Cross-Sectional Study from 2011 to 2016. Int J Environ Res Public Health, 18(5). 10.3390/ijerph18052450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehsanifar M, Jafari AJ, Montazeri Z, Kalantari RR, Gholami M, & Ashtarinezhad A (2021). Learning and memory disorders related to hippocampal inflammation following exposure to air pollution. J Environ Health Sci Eng, 19(1), 261–272. 10.1007/s40201-020-00600-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehsanifar M, Jafari AJ, Nikzad H, Zavareh MS, Atlasi MA, Mohammadi H, & Tameh AA (2019). Prenatal exposure to diesel exhaust particles causes anxiety, spatial memory disorders with alters expression of hippocampal pro-inflammatory cytokines and NMDA receptor subunits in adult male mice offspring. Ecotoxicol Environ Saf, 176, 34–41. 10.1016/j.ecoenv.2019.03.090 [DOI] [PubMed] [Google Scholar]
- Ehsanifar M, Montazeri Z, Taheri MA, Rafati M, Behjati M, & Karimian M (2021). Hippocampal inflammation and oxidative stress following exposure to diesel exhaust nanoparticles in male and female mice. Neurochem Int, 145, 104989. 10.1016/j.neuint.2021.104989 [DOI] [PubMed] [Google Scholar]
- Ehsanifar M, Tameh AA, Farzadkia M, Kalantari RR, Zavareh MS, Nikzaad H, & Jafari AJ (2019). Exposure to nanoscale diesel exhaust particles: Oxidative stress, neuroinflammation, anxiety and depression on adult male mice. Ecotoxicology and Environmental Safety, 168, 338–347. [DOI] [PubMed] [Google Scholar]
- Emik LO, & Plata RL (1969). Depression of Running Activity in Mice by Exposure to Polluted Air. Archives of Environmental Health, 18, 574–579. [DOI] [PubMed] [Google Scholar]
- EPA. (2018). Pollutants and Sources. United States Enviornmental Protection Agency. Retrieved 02/15/2022 from https://www3.epa.gov/airtoxics/pollsour.html [Google Scholar]
- EPA, U. (2022). Cumulative Impacts Research: Recommendations for EPA’s Office of Research and Development (EPA/600/R-22/014a). https://www.epa.gov/system/files/documents/2022-09/Cumulative%20Impacts%20Research%20Final%20Report_FINAL-EPA%20600-R-22-014a.pdf
- Espinoza Oyarce DA, Shaw ME, Alateeq K, & Cherbuin N (2020). Volumetric brain differences in clinical depression in association with anxiety: a systematic review with meta-analysis. J Psychiatry Neurosci, 45(6), 406–429. 10.1503/jpn.190156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etkin A, & Wager TD (2007). Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry, 164(10), 1476–1488. 10.1176/appi.ajp.2007.07030504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW, Colome SD, & Shearer DF (1988). Psychological Reactions to Air Pollution. Environmental Research, 45, 1–15. [DOI] [PubMed] [Google Scholar]
- Fan B, Wang T, Wang W, Zhang S, Gong M, Li W, Lu C, & Guo L (2019). Long-term exposure to ambient fine particulate pollution, sleep disturbance and their interaction effects on suicide attempts among Chinese adolescents. J Affect Disord, 258, 89–95. 10.1016/j.jad.2019.08.004 [DOI] [PubMed] [Google Scholar]
- Fan SJ, Heinrich J, Bloom MS, Zhao TY, Shi TX, Feng WR, Sun Y, Shen JC, Yang ZC, Yang BY, & Dong GH (2020). Ambient air pollution and depression: A systematic review wtih meta-analysis up to 2019. The Science of the total environment. [DOI] [PubMed] [Google Scholar]
- Ferreira APS, Ramos JMO, Gamaro GD, Gioda A, Gioda CR, & Souza ICC (2022). Experimental rodent models exposed to fine particulate matter (PM2.5) highlighting the injuries in the central nervous system: A systematic review. Atmospheric Pollution Research, 13(5). [Google Scholar]
- Feurer C, Jimmy J, Chang F, Langenecker SA, Phan KL, Ajilore O, & Klumpp H (2021). Resting state functional connectivity correlates of rumination and worry in internalizing psychopathologies. Depress Anxiety, 38(5), 488–497. 10.1002/da.23142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore AM, Naik V, & Leibensperger EM (2015). Air quality and climate connections. J Air Waste Manag Assoc, 65(6), 645–685. 10.1080/10962247.2015.1040526 [DOI] [PubMed] [Google Scholar]
- Flurkey K, Currer JM, & Harrison DE (2007). Mouse models in aging research. Faculty research 2000–2009, 1685. https://mouseion.jax.org/stfb2000_2009/1685 [Google Scholar]
- Fonken LK, Xu X, Weil ZM, Chen G, Sun Q, Rajagopalan S, & Nelson RJ (2011). Air pollution impairs cognition, provokes depressive-like behaviors and alters hippocampal cytokine expression and morphology. Mol Psychiatry, 16(10), 987–995, 973. 10.1038/mp.2011.76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gackiere F, Saliba L, Baude A, Bosler O, & Strube C (2011). Ozone inhalation activates stress-responsive regions of the CNS. J Neurochem, 117(6), 961–972. 10.1111/j.1471-4159.2011.07267.x [DOI] [PubMed] [Google Scholar]
- Gale SD, Erickson LD, Anderson JE, Brown BL, & Hedges DW (2020). Association between exposure to air pollution and prefrontal cortical volume in adults: A cross-sectional study from the UK biobank. Environ Res, 185, 109365. 10.1016/j.envres.2020.109365 [DOI] [PubMed] [Google Scholar]
- Genc S, Zadeoglulari Z, Fuss SH, & Genc K (2012). The adverse effects of air pollution on the nervous system. J Toxicol, 2012, 782462. 10.1155/2012/782462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Generaal E, Hoogendijk EO, Stam M, Henke CE, Rutters F, Oosterman M, Huisman M, Kramer SE, Elders PJM, Timmermans EJ, Lakerveld J, Koomen E, Ten Have M, de Graaf R, Snijder MB, Stronks K, Willemsen G, Boomsma DI, Smit JH, & Penninx B (2019). Neighbourhood characteristics and prevalence and severity of depression: pooled analysis of eight Dutch cohort studies. Br J Psychiatry, 215(2), 468–475. 10.1192/bjp.2019.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Generaal E, Timmermans EJ, Dekkers JEC, Smit JH, & Penninx B (2019). Not urbanization level but socioeconomic, physical and social neighbourhood characteristics are associated with presence and severity of depressive and anxiety disorders. Psychol Med, 49(1), 149–161. 10.1017/S0033291718000612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlofs-Nijland ME, van Berlo D, Cassee FR, Schins RP, Wang K, & Campbell A (2010). Effect of prolonged exposure to diesel engine exhaust on proinflammatory markers in different regions of the rat brain. Part Fibre Toxicol, 7, 12. 10.1186/1743-8977-7-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, & Thompson PM (2004). Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A, 101(21), 8174–8179. 10.1073/pnas.0402680101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Crisostomo NP, Rodriguez Martinez E, & Rivas-Arancibia S (2014). Oxidative stress activates the transcription factors FoxO 1a and FoxO 3a in the hippocampus of rats exposed to low doses of ozone. Oxid Med Cell Longev, 2014, 805764. 10.1155/2014/805764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore JC (2003). Principles and practice of functional MRI of the human brain. Journal of Clinical Investigation, 112(1), 4–9. 10.1172/jci200319010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grajski KA, Bressler SL, & Alzheimer’s Disease Neuroimaging I (2019). Differential medial temporal lobe and default-mode network functional connectivity and morphometric changes in Alzheimer’s disease. Neuroimage Clin, 23, 101860. 10.1016/j.nicl.2019.101860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greve HJ, Mumaw CL, Messenger EJ, Kodavanti P, Royland JL, Kodavanti UP, & Block ML (2020). Diesel exhaust impairs TREM2 to dysregulate neuroinflammation. Journal of neuroinflammation, 17(1), 351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu H, Yan W, Elahi E, & Cao Y (2020). Air pollution risks human mental health: an implication of two-stages least squares estimation of interaction effects. Environ Sci Pollut Res Int, 27(2), 2036–2043. 10.1007/s11356-019-06612-x [DOI] [PubMed] [Google Scholar]
- Gu X, Guo T, Si Y, Wang J, Zhang W, Deng F, Chen L, Wei C, Lin S, Guo X, & Wu S (2020). Association Between Ambient Air Pollution and Daily Hospital Admissions for Depression in 75 Chinese Cities. Am J Psychiatry, 177(8), 735–743. 10.1176/appi.ajp.2020.19070748 [DOI] [PubMed] [Google Scholar]
- Guerra R, Vera-Aguilar E, Uribe-Ramirez M, Gookin G, Camacho J, Osornio-Vargas AR, Mugica-Alvarez V, Angulo-Olais R, Campbell A, Froines J, Kleinman TM, & De Vizcaya-Ruiz A (2013). Exposure to inhaled particulate matter activates early markers of oxidative stress, inflammation and unfolded protein response in rat striatum. Toxicol Lett, 222(2), 146–154. 10.1016/j.toxlet.2013.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghani A, Johnson R, Safi N, Zhang H, Thorwald M, Mousavi A, Woodward NC, Shirmohammadi F, Coussa V, Wise JP Jr., Forman HJ, Sioutas C, Allayee H, Morgan TE, & Finch CE (2020). Toxicity of urban air pollution particulate matter in developing and adult mouse brain: Comparison of total and filter-eluted nanoparticles. Environ Int, 136, 105510. 10.1016/j.envint.2020.105510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haghani A, Johnson RG, Woodward NC, Feinberg JI, Lewis K, Ladd-Acosta C, Safi N, Jaffe AE, Sioutas C, Allayee H, Campbell DB, Volk HE, Finch CE, & Morgan TE (2020). Adult mouse hippocampal transcriptome changes associated with long-term behavioral and metabolic effects of gestational air pollution toxicity. Transl Psychiatry, 10(1), 218. 10.1038/s41398-020-00907-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahad O, Lelieveld J, Birklein F, Lieb K, Daiber A, & Munzel T (2020). Ambient Air Pollution Increases the Risk of Cerebrovascular and Neuropsychiatric Disorders through Induction of Inflammation and Oxidative Stress. Int J Mol Sci, 21(12). 10.3390/ijms21124306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajipour S, Farbood Y, Gharib-Naseri MK, Goudarzi G, Rashno M, Maleki H, Bakhtiari N, Nesari A, Khoshnam SE, Dianat M, Sarkaki B, & Sarkaki A (2020). Exposure to ambient dusty particulate matter impairs spatial memory and hippocampal LTP by increasing brain inflammation and oxidative stress in rats. Life Sci, 242, 117210. 10.1016/j.lfs.2019.117210 [DOI] [PubMed] [Google Scholar]
- Hallberg LM, Ward JB, & Committee HHR (2012). Part 3. Assessment of genotoxicity and oxidative stress after exposure to diesel exhaust from U.S. 2007-compliant diesel engines: report on 1- and 3-month exposure in the ACES bioassay. Research report (Health Effects Institute), 166, 163–184. [PubMed] [Google Scholar]
- Hallberg LM, Ward JB, Hernandez C, Ameredes BT, Wickliffe JK, & Committee HHR (2015). Part 3. Assessment of genotoxicity and oxidative damage in rats after chronic exposure to new-technology diesel exhaust in the ACES bioassay. Research report (Health Effects Institute), 184, 87–171. [PubMed] [Google Scholar]
- Hartz AM, Bauer B, Block ML, Hong JS, & Miller DS (2008). Diesel exhaust particles induce oxidative stress, proinflammatory signaling, and P-glycoprotein up-regulation at the blood-brain barrier. FASEB journal: offical publication of the Federation of American Societies for Experiemental Biology, 22(8), 2723–2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges DW, Erickson LD, Kunzelman J, Brown BL, & Gale SD (2019). Association between exposure to air pollution and hippocampal volume in adults in the UK Biobank. Neurotoxicology, 74, 108–120. 10.1016/j.neuro.2019.06.005 [DOI] [PubMed] [Google Scholar]
- Heidari Nejad S, Takechi R, Mullins BJ, Giles C, Larcombe AN, Bertolatti D, Rumchev K, Dhaliwal S, & Mamo J (2015). The effect of diesel exhaust exposure on blood-brain barrier integrity and function in a murine model. J Appl Toxicol, 35(1), 41–47. 10.1002/jat.2985 [DOI] [PubMed] [Google Scholar]
- Hernandez-Zimbron LF, & Rivas-Arancibia S (2015). Oxidative stress caused by ozone exposure induces beta-amyloid 1–42 overproduction and mitochondrial accumulation by activating the amyloidogenic pathway. Neuroscience, 304, 340–348. 10.1016/j.neuroscience.2015.07.011 [DOI] [PubMed] [Google Scholar]
- Hernandez-Zimbron LF, & Rivas-Arancibia S (2016). Syntaxin 5 Overexpression and beta-Amyloid 1–42 Accumulation in Endoplasmic Reticulum of Hippocampal Cells in Rat Brain Induced by Ozone Exposure. Biomed Res Int, 2016, 2125643. 10.1155/2016/2125643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herting MM, Younan D, Campbell CE, & Chen JC (2019). Outdoor Air Pollution and Brain Structure and Function From Across Childhood to Young Adulthood: A Methodological Review of Brain MRI Studies. Front Public Health, 7, 332. 10.3389/fpubh.2019.00332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiser J, & Koenigs M (2018). The Multifaceted Role of the Ventromedial Prefrontal Cortex in Emotion, Decision Making, Social Cognition, and Psychopathology. Biol Psychiatry, 83(8), 638–647. 10.1016/j.biopsych.2017.10.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Unger N, Harper K, & Heyes C (2020). Global Climate and Human Health Effects of the Gasoline and Diesel Vehicle Fleets. Geohealth, 4(3), e2019GH000240. 10.1029/2019GH000240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Wu S, Kramer LJ, Helmig D, & Honrath RE (2017). Surface ozone and its precursors at Summit, Greenland: comparison between observations and model simulations. Atmospheric Chemistry and Physics, 17(23), 14661–14674. 10.5194/acp-17-14661-2017 [DOI] [Google Scholar]
- Janiri D, Moser DA, Doucet GE, Luber MJ, Rasgon A, Lee WH, Murrough JW, Sani G, Eickhoff SB, & Frangou S (2020). Shared Neural Phenotypes for Mood and Anxiety Disorders: A Meta-analysis of 226 Task-Related Functional Imaging Studies. JAMA Psychiatry, 77(2), 172–179. 10.1001/jamapsychiatry.2019.3351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong S, Lee JH, Ha JH, Kim J, Kim I, & Bae S (2021). An Exploratory Study of the Relationships Between Diesel Engine Exhaust Particle Inhalation, Pulmonary Inflammation and Anxious Behavior. Int J Environ Res Public Health, 18(3). 10.3390/ijerph18031166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z, Wei Y, Li X, Yang L, Liu H, Guo C, Zhang L, Li N, Guo S, Qian Y, & Li Z (2018). Exposure to Ambient Air Particles Increases the Risk of Mental Disorder: Findings from a Natural Experiment in Beijing. International journal of environmental research and public health, 15(1), 160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo YS, Kim J, Lee J, & Chung IJ (2021). Understanding the link between exposure to fine particulate matter and internalizing problem behaviors among children in South Korea: Indirect effects through maternal depression and child abuse. Health Place, 68, 102531. 10.1016/j.healthplace.2021.102531 [DOI] [PubMed] [Google Scholar]
- Jorcano A, Lubczynska MJ, Pierotti L, Altug H, Ballester F, Cesaroni G, El Marroun H, Fernandez-Somoano A, Freire C, Hanke W, Hoek G, Ibarluzea J, Iniguez C, Jansen PW, Lepeule J, Markevych I, Polanska K, Porta D, Schikowski T, … Guxens M (2019). Prenatal and postnatal exposure to air pollution and emotional and aggressive symptoms in children from 8 European birth cohorts. Environ Int, 131, 104927. 10.1016/j.envint.2019.104927 [DOI] [PubMed] [Google Scholar]
- Kang YJ, Tan HY, Lee CY, & Cho H (2021). An Air Particulate Pollutant Induces Neuroinflammation and Neurodegeneration in Human Brain Models. Adv Sci (Weinh), 8(21), e2101251. 10.1002/advs.202101251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner J, Pollack AZ, Ranasinghe S, Stevens DR, Nobles C, Rohn MCH, Sherman S, & Mendola P (2021). Chronic exposure to air pollution and risk of mental health disorders complicating pregnancy. Environ Res, 196, 110937. 10.1016/j.envres.2021.110937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, & Walters EE (2005). Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry, 62(6), 593–602. [DOI] [PubMed] [Google Scholar]
- Khan A, Plana-Ripoll O, Antonsen S, Brandt J, Geels C, Landecker H, Sullivan PF, Pedersen CB, & Rzhetsky A (2019). Environmental pollution is associated with increased risk of psychiatric disorders in the US and Denmark. PLoS Biol, 17(8), e3000353. 10.1371/journal.pbio.3000353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Cho J, Isehunwa O, Noh J, Noh Y, Oh SS, Koh SB, & Kim C (2020). Marriage as a social tie in the relation of depressive symptoms attributable to air pollution exposure among the elderly. J Affect Disord, 272, 125–131. 10.1016/j.jad.2020.04.059 [DOI] [PubMed] [Google Scholar]
- Kim H, Kim WH, Kim YY, & Park HY (2020). Air Pollution and Central Nervous System Disease: A Review of the Impact of Fine Particulate Matter on Neurological Disorders. Front Public Health, 8, 575330. 10.3389/fpubh.2020.575330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, & Kim H (2017). Demographic and Environmental Factors Associated with Mental Health: A Cross-Sectional Study. Int J Environ Res Public Health, 14(4). 10.3390/ijerph14040431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KN, Lim YH, Bae HJ, Kim M, Jung K, & Hong YC (2016). Long-Term Fine Particulate Matter Exposure and Major Depressive Disorder in a Community-Based Urban Cohort. Environ Health Perspect, 124(10), 1547–1553. 10.1289/EHP192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Kim JK, Park SH, Kim BG, Jang AS, Oh SH, Lee JH, Suh MW, & Park MK (2018). Effects of inhaled particulate matter on the central nervous system in mice. Neurotoxicology, 67, 169–177. 10.1016/j.neuro.2018.06.001 [DOI] [PubMed] [Google Scholar]
- Kim SY, Lee DH, Park S, Kim BG, Jang AS, Oh SH, Lee JH, Suh MW, & Park MK (2019). Neuronal and perineuronal changes of cerebral cortex after exposure to inhaled particulate matter. Sci Rep, 9(1), 19421. 10.1038/s41598-019-55956-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinawy AA, Ezzat AR, & Al-Suwaigh BR (2014). Inhalation of air polluted with gasoline vapours alters the levels of amino acid neurotransmitters in the cerebral cortex, hippocampus, and hypothalamus of the rat. Exp Toxicol Pathol, 66(5–6), 219–224. 10.1016/j.etp.2014.02.001 [DOI] [PubMed] [Google Scholar]
- Kinney PL (2008). Climate change, air quality, and human health. Am J Prev Med, 35(5), 459–467. 10.1016/j.amepre.2008.08.025 [DOI] [PubMed] [Google Scholar]
- Kioumourtzoglou MA, Power MC, Hart JE, Okereke OI, Coull BA, Laden F, & Weisskopf MG (2017). The Association Between Air Pollution and Onset of Depression Among Middle-Aged and Older Women. Am J Epidemiol, 185(9), 801–809. 10.1093/aje/kww163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodavanti PR, Royland JE, Moore-Smith DA, Besas J, Richards JE, Beasley TE, Evansky P, & Bushnell PJ (2015). Acute and subchronic toxicity of inhaled toluene in male Long-Evans rats: Oxidative stress markers in brain. Neurotoxicology, 51, 10–19. 10.1016/j.neuro.2015.09.001 [DOI] [PubMed] [Google Scholar]
- Kodavanti PRS, Valdez M, Richards JE, Agina-Obu DI, Phillips PM, Jarema KA, & Kodavanti UP (2021). Ozone-induced changes in oxidative stress parameters in brain regions of adult, middle-age, and senescent Brown Norway rats. Toxicol Appl Pharmacol, 410, 115351. 10.1016/j.taap.2020.115351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolesar TA, Bilevicius E, Wilson AD, & Kornelsen J (2019). Systematic review and meta-analyses of neural structural and functional differences in generalized anxiety disorder and healthy controls using magnetic resonance imaging. Neuroimage Clin, 24, 102016. 10.1016/j.nicl.2019.102016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku T, Li B, Gao R, Zhang Y, Yan W, Ji X, Li G, & Sang N (2017). NF-kappaB-regulated microRNA-574–5p underlies synaptic and cognitive impairment in response to atmospheric PM2.5 aspiration. Part Fibre Toxicol, 14(1), 34. 10.1186/s12989-017-0215-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulas JA, Hettwer JV, Sohrabi M, Melvin JE, Manocha GD, Puig KL, Gorr MW, Tanwar V, McDonald MP, Wold LE, & Combs CK (2018). In utero exposure to fine particulate matter results in an altered neuroimmune phenotype in adult mice. Environ Pollut, 241, 279–288. 10.1016/j.envpol.2018.05.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamichhane DK, Jung DY, Shin YJ, Lee KS, Lee SY, Ahn K, Kim KW, Shin YH, Suh DI, Hong SJ, & Kim HC (2021). Association of ambient air pollution with depressive and anxiety symptoms in pregnant women: A prospective cohort study. Int J Hyg Environ Health, 237, 113823. 10.1016/j.ijheh.2021.113823 [DOI] [PubMed] [Google Scholar]
- Latham RM, Kieling C, Arseneault L, Botter-Maio Rocha T, Beddows A, Beevers SD, Danese A, De Oliveira K, Kohrt BA, Moffitt TE, Mondelli V, Newbury JB, Reuben A, & Fisher HL (2021). Childhood exposure to ambient air pollution and predicting individual risk of depression onset in UK adolescents. J Psychiatr Res, 138, 60–67. 10.1016/j.jpsychires.2021.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Jung J, Myung W, Baek JH, Kang JM, Kim DK, & Kim H (2019). Association between dust storm occurrence and risk of suicide: Case-crossover analysis of the Korean national death database. Environ Int, 133(Pt A), 105146. 10.1016/j.envint.2019.105146 [DOI] [PubMed] [Google Scholar]
- Li F, & Zhou T (2020). Effects of objective and subjective environmental pollution on well-being in urban China: A structural equation model approach. Social science & medicine, 249. [DOI] [PubMed] [Google Scholar]
- Li K, Li L, Cui B, Gai Z, Li Q, Wang S, Yan J, Lin B, Tian L, Liu H, Liu X, & Xi Z (2018). Early Postnatal Exposure to Airborne Fine Particulate Matter Induces Autism-like Phenotypes in Male Rats. Toxicological sciences : an official journal of the Society of Toxicology, 162(1), 189–199. [DOI] [PubMed] [Google Scholar]
- Li X, Zhang Y, Li B, Yang H, Cui J, Li X, Zhang X, Sun H, Meng Q, Wu S, Li S, Wang J, Aschner M, & Chen R (2020). Activation of NLRP3 in microglia exacerbates diesel exhaust particles-induced impairment in learning and memory in mice. Environ Int, 136, 105487. 10.1016/j.envint.2020.105487 [DOI] [PubMed] [Google Scholar]
- Li Z, Li N, Guo C, Li X, Qian Y, Wu J, Yang Y, & Wei Y (2019). Genomic DNA methylation signatures in different tissues after ambient air particulate matter exposure. Ecotoxicol Environ Saf, 179, 175–181. 10.1016/j.ecoenv.2019.04.049 [DOI] [PubMed] [Google Scholar]
- Liang L, & Gong P (2020). Urban and air pollution: a multi-city study of long-term effects of urban landscape patterns on air quality trends. Sci Rep, 10(1), 18618. 10.1038/s41598-020-74524-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Life Span as a Biomarker. (2022). Jackson Laboratory. Retrieved 02-03-2022 from https://www.jax.org/research-and-faculty/research-labs/the-harrison-lab/gerontology/life-span-as-a-biomarker [Google Scholar]
- Lim YH, Kim H, Kim JH, Bae S, Park HY, & Hong YC (2012). Air pollution and symptoms of depression in elderly adults. Environ Health Perspect, 120(7), 1023–1028. 10.1289/ehp.1104100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Zhou L, Xu J, Luo Z, Kan H, Zhang J, Yan C, & Zhang J (2017). The impacts of air pollution on maternal stress during pregnancy. Sci Rep, 7, 40956. 10.1038/srep40956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Wang Z, Wei Y, Liu R, Jiang C, Gong C, Liu Y, & Yan B (2021). The leading role of absorbed lead in PM2.5-induced hippocampal neuronal apoptosis and synaptic damage. Journal of hazardous materials, 416. [DOI] [PubMed] [Google Scholar]
- Liu J, Yang C, Yang J, Song X, Han W, Xie M, Cheng L, Xie L, Chen H, & Jiang L (2019). Effects of early postnatal exposure to fine particulate matter on emotional and cognitive development and structural synaptic plasticity in immature and mature rats. Brain Behav, 9(12), e01453. 10.1002/brb3.1453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Wang W, Gu X, Deng F, Wang X, Lin H, Guo X, & Wu S (2021). Association between particulate matter air pollution and risk of depression and suicide: a systematic review and meta-analysis. Environ Sci Pollut Res Int, 28(8), 9029–9049. 10.1007/s11356-021-12357-3 [DOI] [PubMed] [Google Scholar]
- Liu X, Qian X, Xing J, Wang J, Sun Y, Wang Q, & Li H (2018). Particulate Matter Triggers Depressive-Like Response Associated With Modulation of Inflammatory Cytokine Homeostasis and Brain-Derived Neurotrophic Factor Signaling Pathway in Mice. Toxicol Sci, 164(1), 278–288. 10.1093/toxsci/kfy086 [DOI] [PubMed] [Google Scholar]
- Liu X, Wang J, Zhou M, Dai Q, Wang Q, Li H, & Qian X (2020). Particulate matter exposure disturbs inflammatory cytokine homeostasis associated with changes in trace metal levels in mouse organs. Sci Total Environ, 727, 138377. 10.1016/j.scitotenv.2020.138377 [DOI] [PubMed] [Google Scholar]
- Lu P, Zhang Y, Xia G, Zhang W, Xu R, Wang C, Guo Y, & Li S (2020). Attributable risks associated with hospital outpatient visits for mental disorders due to air pollution: A multi-city study in China. Environ Int, 143, 105906. 10.1016/j.envint.2020.105906 [DOI] [PubMed] [Google Scholar]
- Lubczynska MJ, Muetzel RL, El Marroun H, Basagana X, Strak M, Denault W, Jaddoe VWV, Hillegers M, Vernooij MW, Hoek G, White T, Brunekreef B, Tiemeier H, & Guxens M (2020). Exposure to Air Pollution during Pregnancy and Childhood, and White Matter Microstructure in Preadolescents. Environ Health Perspect, 128(2), 27005. 10.1289/EHP4709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubczynska MJ, Muetzel RL, El Marroun H, Hoek G, Kooter IM, Thomson EM, Hillegers M, Vernooij MW, White T, Tiemeier H, & Guxens M (2021). Air pollution exposure during pregnancy and childhood and brain morphology in preadolescents. Environ Res, 198, 110446. 10.1016/j.envres.2020.110446 [DOI] [PubMed] [Google Scholar]
- Manisalidis I, Stavropoulou E, Stavropoulos A, & Bezirtzoglou E (2020). Environmental and Health Impacts of Air Pollution: A Review. Front Public Health, 8, 14. 10.3389/fpubh.2020.00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis AE, Herbstman JB, Davis KS, Thomas VK, Tang D, Wang Y, Wang S, Perera FP, Peterson BS, & Rauh VA (2016). Longitudinal effects of prenatal exposure to air pollutants on self-regulatory capacities and social competence. J Child Psychol Psychiatry, 57(7), 851–860. 10.1111/jcpp.12548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis AE, Liu R, Conceicao VA, Ramphal B, Pagliaccio D, DeSerisy ML, Koe E, Selmanovic E, Raudales A, Emanet N, Quinn AE, Beebe B, Pearson BL, Herbstman JB, Rauh VA, Fifer WP, Fox NA, & Champagne FA (2022). Convergent neural correlates of prenatal exposure to air pollution and behavioral phenotypes of risk for internalizing and externalizing problems: Potential biological and cognitive pathways. Neurosci Biobehav Rev, 104645. 10.1016/j.neubiorev.2022.104645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuinn LA, Bellinger DC, Colicino E, Coull BA, Just AC, Kloog I, Osorio-Valencia E, Schnaas L, Wright RJ, Tellez-Rojo MM, Wright RO, & Horton MK (2020). Prenatal PM2.5 exposure and behavioral development in children from Mexico City. Neurotoxicology, 81, 109–115. 10.1016/j.neuro.2020.09.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milani C, Farina F, Botto L, Massimino L, Lonati E, Donzelli E, Ballarini E, Crippa L, Marmiroli P, Bulbarelli A, & Palestini P (2020). Systemic Exposure to Air Pollution Induces Oxidative Stress and Inflammation in Mouse Brain, Contributing to Neurodegeneration Onset. Int J Mol Sci, 21(10). 10.3390/ijms21103699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CH, Hamilton JP, Sacchet MD, & Gotlib IH (2015). Meta-analysis of Functional Neuroimaging of Major Depressive Disorder in Youth. JAMA Psychiatry, 72(10), 1045–1053. 10.1001/jamapsychiatry.2015.1376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RL, Yan Z, Maher C, Zhang H, Gudsnuk K, McDonald J, & Champagne FA (2016). Impact of prenatal polycyclic aromatic hydrocarbon exposure on behavior, cortical gene expression and DNA methylation of the Bdnf gene. Neuroepigenetics, 5, 11–18. 10.1016/j.nepig.2016.02.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan TE, Davis DA, Iwata N, Tanner JA, Snyder D, Ning Z, Kam W, Hsu YT, Winkler JW, Chen JC, Petasis NA, Baudry M, Sioutas C, & Finch CE (2011). Glutamatergic neurons in rodent models respond to nanoscale particulate urban air pollutants in vivo and in vitro. Environ Health Perspect, 119(7), 1003–1009. 10.1289/ehp.1002973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris-Schaffer K, Merrill A, Jew K, Wong C, Conrad K, Harvey K, Marvin E, Sobolewski M, Oberdorster G, Elder A, & Cory-Slechta DA (2019). Effects of neonatal inhalation exposure to ultrafine carbon particles on pathology and behavioral outcomes in C57BL/6J mice. Part Fibre Toxicol, 16(1), 10. 10.1186/s12989-019-0293-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motesaddi Zarandi S, Shahsavani A, Khodagholi F, & Fakhri Y (2019). Alzheimer and depressive cognitive-like behaviors in male and female rats: A new method for exposure to ambient air pollution. MethodsX, 6, 690–703. 10.1016/j.mex.2019.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhsin HA, Steingrimsson S, Oudin A, Astrom DO, & Carlsen HK (2022). Air pollution and increased number of psychiatric emergency room visits: A case-crossover study for identifying susceptible groups. Environ Res, 204(Pt A), 112001. 10.1016/j.envres.2021.112001 [DOI] [PubMed] [Google Scholar]
- Musi B, Dell’Omo G, Ricceri L, Santucci D, Laviola G, Bignami G, & Alleva E (1994). Effects of Acute and Continuous Ozone (O3) Exposure on Activity/Exploration and Social Behavior of CD-1 Mice. Neurotoxicology, 15(4), 827–836. [PubMed] [Google Scholar]
- Nephew BC, Nemeth A, Hudda N, Beamer G, Mann P, Petitto J, Cali R, Febo M, Kulkarni P, Poirier G, King J, Durant JL, & Brugge D (2020). Traffic-related particulate matter affects behavior, inflammation, and neural integrity in a developmental rodent model. Environ Res, 183, 109242. 10.1016/j.envres.2020.109242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen AM, Malig BJ, & Basu R (2021). The association between ozone and fine particles and mental health-related emergency department visits in California, 2005–2013. PLoS One, 16(4), e0249675. 10.1371/journal.pone.0249675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedzwiecki MM, Rosa MJ, Solano-Gonzalez M, Kloog I, Just AC, Martinez-Medina S, Schnaas L, Tamayo-Ortiz M, Wright RO, Tellez-Rojo MM, & Wright RJ (2020). Particulate air pollution exposure during pregnancy and postpartum depression symptoms in women in Mexico City. Environ Int, 134, 105325. 10.1016/j.envint.2019.105325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ning X, Li B, Ku T, Guo L, Li G, & Sang N (2018). Comprehensive hippocampal metabolite responses to PM2.5 in young mice. Ecotoxicol Environ Saf, 165, 36–43. 10.1016/j.ecoenv.2018.08.080 [DOI] [PubMed] [Google Scholar]
- Niño-Cabrera HG (2002). Differences between hippocampus and cerebral cortex in aged rats in an oxidative stress model. International Journal of Neuroscience, 112, 373–381. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Tsai IJ, Yamauchi H, Nakatani E, Fukushima M, & Hsu CY (2020). Association of Geomagnetic Disturbances and Suicide Attempts in Taiwan, 1997–2013: A Cross-Sectional Study. Int J Environ Res Public Health, 17(4). 10.3390/ijerph17041154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nway NC, Fujitani Y, Hirano S, Mar O, & Win-Shwe TT (2017). Role of TLR4 in olfactory-based spatial learning activity of neonatal mice after developmental exposure to diesel exhaust origin secondary organic aerosol. Neurotoxicology, 63, 155–165. 10.1016/j.neuro.2017.10.001 [DOI] [PubMed] [Google Scholar]
- Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hrobjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, … Moher D (2021). The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ, 372, n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SJ, Lee J, Lee S, Lim S, Noh J, Cho SY, Ha J, Kim H, Kim C, Park S, Lee DY, & Kim E (2020). Exposure of ultrafine particulate matter causes glutathione redox imbalance in the hippocampus: A neurometabolic susceptibility to Alzheimer’s pathology. Sci Total Environ, 718, 137267. 10.1016/j.scitotenv.2020.137267 [DOI] [PubMed] [Google Scholar]
- Patten KT, Gonzalez EA, Valenzuela A, Berg E, Wallis C, Garbow JR, Silverman JL, Bein KJ, Wexler AS, & Lein PJ (2020). Effects of early life exposure to traffic-related air pollution on brain development in juvenile Sprague-Dawley rats. Transl Psychiatry, 10(1), 166. 10.1038/s41398-020-0845-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peiffer J, Cosnier F, Grova N, Nunge H, Salquebre G, Decret MJ, Cossec B, Rychen G, Appenzeller BM, & Schroeder H (2013). Neurobehavioral toxicity of a repeated exposure (14 days) to the airborne polycyclic aromatic hydrocarbon fluorene in adult Wistar male rats. PLoS One, 8(8), e71413. 10.1371/journal.pone.0071413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelgrims I, Devleesschauwer B, Guyot M, Keune H, Nawrot TS, Remmen R, Saenen ND, Trabelsi S, Thomas I, Aerts R, & De Clercq EM (2021). Association between urban environment and mental health in Brussels, Belgium. BMC Public Health, 21(1), 635. 10.1186/s12889-021-10557-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Tang D, Wang S, Vishnevetsky J, Zhang B, Diaz D, Camann D, & Rauh V (2012). Prenatal polycyclic aromatic hydrocarbon (PAH) exposure and child behavior at age 6–7 years. Environ Health Perspect, 120(6), 921–926. 10.1289/ehp.1104315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Wang S, Vishnevetsky J, Zhang B, Cole KJ, Tang D, Rauh V, & Phillips DH (2011). Polycyclic aromatic hydrocarbons-aromatic DNA adducts in cord blood and behavior scores in New York city children. Environ Health Perspect, 119(8), 1176–1181. 10.1289/ehp.1002705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson BS, Rauh VA, Bansal R, Hao X, Toth Z, Nati G, Walsh K, Miller RL, Arias F, Semanek D, & Perera F (2015). Effects of prenatal exposure to air pollutants (polycyclic aromatic hydrocarbons) on the development of brain white matter, cognition, and behavior in later childhood. JAMA Psychiatry, 72(6), 531–540. 10.1001/jamapsychiatry.2015.57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkus AJ, Wang X, Beavers DP, Chui HC, Espeland MA, Gatz M, Gruenewald T, Kaufman JD, Manson JE, Resnick SM, Stewart JD, Wellenius GA, Whitsel EA, Widaman K, Younan D, & Chen JC (2021). Outdoor air pollution exposure and inter-relation of global cognitive performance and emotional distress in older women. Environ Pollut, 271, 116282. 10.1016/j.envpol.2020.116282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkus AJ, Younan D, Wang X, Beavers DP, Espeland MA, Gatz M, Gruenewald TL, Kaufman JD, Chui HC, Manson JE, Resnick SM, Wellenius GA, Whitsel EA, Widaman K, & Chen JC (2021). Air Pollution and the Dynamic Association Between Depressive Symptoms and Memory in Oldest-Old Women. J Am Geriatr Soc, 69(2), 474–484. 10.1111/jgs.16889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkus AJ, Younan D, Wang X, Serre M, Vizuete W, Resnick S, Espeland MA, Gatz M, Chui H, Manson JE, & Chen JC (2019). Particulate Air Pollutants and Trajectories of Depressive Symptoms in Older Women. Am J Geriatr Psychiatry, 27(10), 1083–1096. 10.1016/j.jagp.2019.04.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkus AJ, Younan D, Widaman K, Gatz M, Manson JE, Wang X, Serre M, Vizuete W, Chui H, Espeland MA, Resnick S, & Chen JC (2020). Exposure to fine particulate matter and temporal dynamics of episodic memory and depressive symptoms in older women. Environ Int, 135, 105196. 10.1016/j.envint.2019.105196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinault L, Thomson EM, Christidis T, Colman I, Tjepkema M, van Donkelaar A, Martin RV, Hystad P, Shin H, Crouse DL, & Burnett RT (2020). The association betwee ambient air pollution concentrations and psychological distress. Health reports, 31(7), 3–11. [DOI] [PubMed] [Google Scholar]
- Power MC, Kioumourtzoglou MA, Hart JE, Okereke OI, Laden F, & Weisskopf MG (2015). The relation between past exposure to fine particulate air pollution and prevalent anxiety: observational cohort study. BMJ, 350, h1111. 10.1136/bmj.h1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pun VC, Manjourides J, & Suh H (2017). Association of Ambient Air Pollution with Depressive and Anxiety Symptoms in Older Adults: Results from the NSHAP Study. . Environmental health persepctives, 125(3), 342–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pun VC, Manjourides J, & Suh HH (2019). Close proximity to roadway and urbanicity associated with mental ill-health in older adults. Sci Total Environ, 658, 854–860. 10.1016/j.scitotenv.2018.12.221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu H, Zhu X, Wang L, Pan J, Pu X, Zeng X, Zhang L, Peng Z, & Zhou L (2019). Attributable risk of hospital admissions for overall and specific mental disorders due to particulate matter pollution: A time-series study in Chengdu, China. Environmental Research, 170, 230–237. [DOI] [PubMed] [Google Scholar]
- Rasnick E, Ryan PH, Bailer AJ, Fisher T, Parsons PJ, Yolton K, Newman NC, Lanphear BP, & Brokamp C (2021). Identifying sensitive windows of airborne lead exposure associated with behavioral outcomes at age 12. Environ Epidemiol, 5(2), e144. 10.1097/EE9.0000000000000144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben A, Arseneault L, Beddows A, Beevers SD, Moffitt TE, Ambler A, Latham RM, Newbury JB, Odgers CL, Schaefer JD, & Fisher HL (2021). Association of Air Pollution Exposure in Childhood and Adolescence With Psychopathology at the Transition to Adulthood. JAMA Netw Open, 4(4), e217508. 10.1001/jamanetworkopen.2021.7508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas-Arancibia S, Guevara-Guzman R, Lopez-Vidal Y, Rodriguez-Martinez E, Zanardo-Gomes M, Angoa-Perez M, & Raisman-Vozari R (2010). Oxidative stress caused by ozone exposure induces loss of brain repair in the hippocampus of adult rats. Toxicol Sci, 113(1), 187–197. 10.1093/toxsci/kfp252 [DOI] [PubMed] [Google Scholar]
- Rivas-Arancibia S, Rodriguez-Martinez E, Badillo-Ramirez I, Lopez-Gonzalez U, & Saniger JM (2017). Structural Changes of Amyloid Beta in Hippocampus of Rats Exposed to Ozone: A Raman Spectroscopy Study. Front Mol Neurosci, 10, 137. 10.3389/fnmol.2017.00137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts H, & Helbich M (2021). Multiple environmental exposures along daily mobility paths and depressive symptoms: A smartphone-based tracking study. Environ Int, 156, 106635. 10.1016/j.envint.2021.106635 [DOI] [PubMed] [Google Scholar]
- Roberts S, Arseneault L, Barratt B, Beevers S, Danese A, Odgers CL, Moffitt TE, Reuben A, Kelly FJ, & Fisher HL (2019). Exploration of NO2 and PM2.5 air pollution and mental health problems using high-resolution data in London-based children from a UK longitudinal cohort study. Psychiatry Res, 272, 8–17. 10.1016/j.psychres.2018.12.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Martinez E, Martinez F, Espinosa-Garcia MT, Maldonado P, & Rivas-Arancibia S (2013). Mitochondrial dysfunction in the hippocampus of rats caused by chronic oxidative stress. Neuroscience, 252, 384–395. 10.1016/j.neuroscience.2013.08.018 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Martinez E, Nava-Ruiz C, Escamilla-Chimal E, Borgonio-Perez G, & Rivas-Arancibia S (2016). The Effect of Chronic Ozone Exposure on the Activation of Endoplasmic Reticulum Stress and Apoptosis in Rat Hippocampus. Front Aging Neurosci, 8, 245. 10.3389/fnagi.2016.00245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe J, Mondschein A, Neale C, Barnes L, Boukhechba M, & Lopez S (2020). The Urban Built Environment, Walking and Mental Health Outcomes Among Older Adults: A Pilot Study. Front Public Health, 8, 575946. 10.3389/fpubh.2020.575946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu B, Mackos AR, Floden AM, Wold LE, & Combs CK (2021). Particulate Matter Exposure Exacerbates Amyloid-beta Plaque Deposition and Gliosis in APP/PS1 Mice. J Alzheimers Dis, 80(2), 761–774. 10.3233/JAD-200919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi A, Liu H, & Salim S (2020). Involvement of oxidative stress and mitochondrial mechanisms in air pollution-related neurobiological impairments. Neurobiol Stress, 12, 100205. 10.1016/j.ynstr.2019.100205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi A, Patki G, Liu H, & Salim S (2017). Psychological Impact of Vehicle Exhaust Exposure: Insights from an Animal Model. Scientific Reports, 7(1), 8306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santucci D, Sorace A, Francia N, Aloe L, & Alleva E (2006). Prolonged prenatal exposure to low-level ozone affects aggressive behaviour as well as NGF and BDNF levels in the central nervous system of CD-1 mice. Behav Brain Res, 166(1), 124–130. 10.1016/j.bbr.2005.07.032 [DOI] [PubMed] [Google Scholar]
- Semple BD, Blomgren K, Gimlin K, Ferriero DM, & Noble-Haeusslein LJ (2013). Brain development in rodents and humans: Identifying benchmarks of maturation and vulnerability to injury across species. Prog Neurobiol, 106-107, 1–16. 10.1016/j.pneurobio.2013.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield PE, Speranza R, Chiu YM, Hsu HL, Curtin PC, Renzetti S, Pajak A, Coull B, Schwartz J, Kloog I, & Wright RJ (2018). Association between particulate air pollution exposure during pregnancy and postpartum maternal psychological functioning. PLoS One, 13(4), e0195267. 10.1371/journal.pone.0195267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W, Li T, Zhang Y, Sun Q, Chen C, Wang J, Fang J, Zhao F, Du P, & Shi X (2020). Depression and Anxiety Associated with Exposure to Fine Particulate Matter Constituents: A Cross-Sectional Study in North China. Environ Sci Technol, 54(24), 16006–16016. 10.1021/acs.est.0c05331 [DOI] [PubMed] [Google Scholar]
- Shih CH, Chen JK, Kuo LW, Cho KH, Hsiao TC, Lin ZW, Lin YS, Kang JH, Lo YC, Chuang KJ, Cheng TJ, & Chuang HC (2018). Chronic pulmonary exposure to traffic-related fine particulate matter causes brain impairment in adult rats. Part Fibre Toxicol, 15(1), 44. 10.1186/s12989-018-0281-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J, Park JY, & Choi J (2018). Long-term exposure to ambient air pollutants and mental health status: A nationwide population-based cross-sectional study. PLoS One, 13(4), e0195607. 10.1371/journal.pone.0195607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto-Sanz V, Castellvi P, Piqueras JA, Rodriguez-Marin J, Rodriguez-Jimenez T, Miranda-Mendizabal A, Pares-Badell O, Almenara J, Alonso I, Blasco MJ, Cebria A, Gabilondo A, Gili M, Lagares C, Roca M, & Alonso J (2019). Internalizing and externalizing symptoms and suicidal behaviour in young people: a systematic review and meta-analysis of longitudinal studies. Acta Psychiatr Scand, 140(1), 5–19. 10.1111/acps.13036 [DOI] [PubMed] [Google Scholar]
- Stenson C, Wheeler AJ, Carver A, Donaire-Gonzalez D, Alvarado-Molina M, Nieuwenhuijsen M, & Tham R (2021). The impact of Traffic-Related air pollution on child and adolescent academic Performance: A systematic review. Environ Int, 155, 106696. 10.1016/j.envint.2021.106696 [DOI] [PubMed] [Google Scholar]
- Suzuki T, Oshio S, Iwata M, Saburi H, Odagiri T, Udagawa T, Sugawara I, Umezawa M, & Takeda K (2010). In utero exposure to a low concentration of diesel exhaust affects spontaneous locomotor activity and monoaminergic system in male mice. Part Fibre Toxicol, 7, 7. 10.1186/1743-8977-7-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyszkowicz M (2007). Air pollution and emergency department visits for depression in Edmonton, Canada. Int J Occup Med Environ Health, 20(3), 241–245. 10.2478/v10001-007-0024-2 [DOI] [PubMed] [Google Scholar]
- Szyszkowicz M, Kousha T, Kingsbury M, & Colman I (2016). Air Pollution and Emergency Department Visits for Depression: A Multicity Case-Crossover Study. Environ Health Insights, 10, 155–161. 10.4137/EHI.S40493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyszkowicz M, Rowe BH, & Colman I (2009). Air pollution and daily emergency department visits for depression. Int J Occup Med Environ Health, 22(4), 355–362. 10.2478/v10001-009-0031-6 [DOI] [PubMed] [Google Scholar]
- Szyszkowicz M, Willey JB, Grafstein E, Rowe BH, & Colman I (2010). Air pollution and emergency department visits for suicide attempts in vancouver, Canada. Environ Health Insights, 4, 79–86. 10.4137/EHI.S5662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai APK, Mickley LJ, & Jacob DJ (2010). Correlations between fine particulate matter (PM2.5) and meteorological variables in the United States: Implications for the sensitivity of PM2.5 to climate change. Atmospheric Environment, 44(32), 3976–3984. [Google Scholar]
- Tepper JL, Weiss B, & Cox C (1982). Microanalysis of Ozone Depression of Motor Activity. Toxicology and Applied Pharmacology, 64, 317–326. [DOI] [PubMed] [Google Scholar]
- The Lancet Global, H. (2020). Mental health matters. The Lancet Global Health, 8(11). 10.1016/s2214-109x(20)30432-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trushna T, Dhiman V, Raj D, & Tiwari RR (2021). Effects of ambient air pollution on psychological stress and anxiety disorder: a systematic review and meta-analysis of epidemiological evidence. Reviews on environmental health, 36(4), 501–521. [DOI] [PubMed] [Google Scholar]
- Tsai SS, Chiu YW, Weng YH, & Yang CY (2020). Association between ozone air pollution levels and hospitalizations for depression in Taipei: a time-stratified case-crossover study. Journal of toxicology and environmental health, Part A, 83(17–18). [DOI] [PubMed] [Google Scholar]
- Tsai SS, Chiu YW, Weng YH, & Yang CY (2021). Relationship between fine particulate air pollution and hospital admissions for depression: a case-crossover study in Taipei. J Toxicol Environ Health A, 84(17), 702–709. 10.1080/15287394.2021.1932652 [DOI] [PubMed] [Google Scholar]
- Umezawa M, Onoda A, Korshunova I, Jensen ACO, Koponen IK, Jensen KA, Khodosevich K, Vogel U, & Hougaard KS (2018). Maternal inhalation of carbon black nanoparticles induces neurodevelopmental changes in mouse offspring. Part Fibre Toxicol, 15(1), 36. 10.1186/s12989-018-0272-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valand R, Magnusson P, Dziendzikowska K, Gajewska M, Wilczak J, Oczkowski M, Kamola D, Królikowski T, Kruszewski M, Lankoff A, Mruk R, Marcus Eide D, Sapierzyński R, Gromadzka-Ostrowska J, Duale N, Øvrevik J, & Myhre O (2018). Gene expression changes in rat brain regions after 7- and 28 days inhalation exposure to exhaust emissions from 1st and 2nd generation biodiesel fuels - The FuelHealth project. Inhalation toxicology, 30(7–8), 299–312. [DOI] [PubMed] [Google Scholar]
- Vert C, Sanchez-Benavides G, Martinez D, Gotsens X, Gramunt N, Cirach M, Molinuevo JL, Sunyer J, Nieuwenhuijsen MJ, Crous-Bou M, & Gascon M (2017). Effect of long-term exposure to air pollution on anxiety and depression in adults: A cross-sectional study. Int J Hyg Environ Health, 220(6), 1074–1080. 10.1016/j.ijheh.2017.06.009 [DOI] [PubMed] [Google Scholar]
- Wang F, Fangfang Z, Guo X, Chen W, Yao W, Liu H, Lyu C, Zhang Y, & Fan C (2018). Effects of volatile organic compounds and carbon monoxide mixtures on learning and memory, oxidative stress, and monoamine neurotransmitters in the brains of mice. Toxicol Ind Health, 34(3), 178–187. 10.1177/0748233717747504 [DOI] [PubMed] [Google Scholar]
- Wang F, Li C, Liu W, & Jin Y (2014). Potential mechanisms of neurobehavioral disturbances in mice caused by sub-chronic exposure to low-dose VOCs. Inhal Toxicol, 26(4), 250–258. 10.3109/08958378.2014.882447 [DOI] [PubMed] [Google Scholar]
- Wang F, Liu H, Li H, Liu J, Guo X, Yuan J, Hu Y, Wang J, & Lu L (2018). Ambient concentrations of particulate matter and hospitalization for depression in 26 Chinese cities: A case-crossover study. Environ Int, 114, 115–122. 10.1016/j.envint.2018.02.012 [DOI] [PubMed] [Google Scholar]
- Wang L, Hermens DF, Hickie IB, & Lagopoulos J (2012). A systematic review of resting-state functional-MRI studies in major depression. J Affect Disord, 142(1–3), 6–12. 10.1016/j.jad.2012.04.013 [DOI] [PubMed] [Google Scholar]
- Wang Q, & Yang Z (2018). Does chronic disease influence susceptibility to the effects of air pollution on depressive symptoms in China? Int J Ment Health Syst, 12, 33. 10.1186/s13033-018-0212-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Liu Y, Xue D, Yao Y, Liu P, & Helbich M (2019). Cross-sectional associations between long-term exposure to particulate matter and depression in China: The mediating effects of sunlight, physical activity, and neighborly reciprocity. J Affect Disord, 249, 8–14. 10.1016/j.jad.2019.02.007 [DOI] [PubMed] [Google Scholar]
- Wang R, Xue D, Liu Y, Liu P, & Chen H (2018). The Relationship between Air Pollution and Depression in China: Is Neighbourhood Social Capital Protective? Int J Environ Res Public Health, 15(6). 10.3390/ijerph15061160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Yang B, Liu P, Zhang J, Liu Y, Yao Y, & Lu Y (2020). The longitudinal relationship between exposure to air pollution and depression in older adults. Int J Geriatr Psychiatry, 35(6), 610–616. 10.1002/gps.5277 [DOI] [PubMed] [Google Scholar]
- Wang Y, Eliot MN, Koutrakis P, Gryparis A, Schwartz JD, Coull BA, Mittleman MA, Milberg WP, Lipsitz LA, & Wellenius GA (2014). Ambient air pollution and depressive symptoms in older adults: results from the MOBILIZE Boston study. Environ Health Perspect, 122(6), 553–558. 10.1289/ehp.1205909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F, Wu M, Qian S, Li D, Jin M, Wang J, Shui L, Lin H, Tang M, & Chen K (2020). Association between short-term exposure to ambient air pollution and hospital visits for depression in China. Sci Total Environ, 724, 138207. 10.1016/j.scitotenv.2020.138207 [DOI] [PubMed] [Google Scholar]
- Wen Y, Ding X, Guan Q, Hu W, Wang B, Hu Q, Bigambo FM, Zhou Z, Wang X, & Xia Y (2021). Effects of exposure to urban particulate matter SRM 1648a during pregnancy on the neurobehavioral development of offspring mice. Ecotoxicol Environ Saf, 215, 112142. 10.1016/j.ecoenv.2021.112142 [DOI] [PubMed] [Google Scholar]
- Win-Shwe TT, Fujitani Y, Kyi-Tha-Thu C, Furuyama A, Michikawa T, Tsukahara S, Nitta H, & Hirano S (2014). Effects of diesel engine exhaust origin secondary organic aerosols on novel object recognition ability and maternal behavior in BALB/c mice. Int J Environ Res Public Health, 11(11), 11286–11307. 10.3390/ijerph111111286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Win-Shwe TT, Fujitani Y, Sone H, Furuyama A, Nitta H, & Hirano S (2013). Effect of acute single intranasal instillation of secondary organic aerosol on neurological and immunological biomarkers in the brain and lung of BALB/c mice. The Journal of Toxicological Sciences, 38(1), 71–82. [DOI] [PubMed] [Google Scholar]
- Woodward NC, Haghani A, Johnson RG, Hsu TM, Saffari A, Sioutas C, Kanoski SE, Finch CE, & Morgan TE (2018). Prenatal and early life exposure to air pollution induced hippocampal vascular leakage and impaired neurogenesis in association with behavioral deficits. Translational Psychiatry, 8(1), 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward NC, Levine MC, Haghani A, Shirmohammadi F, Saffari A, Sioutas C, Morgan TE, & Finch CE (2017). Toll-like receptor 4 in glial inflammatory responses to air pollution in vitro and in vivo. J Neuroinflammation, 14(1), 84. 10.1186/s12974-017-0858-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward NC, Pakbin P, Saffari A, Shirmohammadi F, Haghani A, Sioutas C, Cacciottolo M, Morgan TE, & Finch CE (2017). Traffic-related air pollution impact on mouse brain accelerates myelin and neuritic aging changes with specificity for CA1 neurons. Neurobiol Aging, 53, 48–58. 10.1016/j.neurobiolaging.2017.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue T, Guan T, Zheng Y, Geng G, Zhang Q, Yao Y, & Zhu T (2021). Long-term PM2.5 exposure and depressive symptoms in China: A quasi-experimental study. Lancet Reg Health West Pac, 6, 100079. 10.1016/j.lanwpc.2020.100079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Chen Y, Zhang Y, Li R, & Dong C (2017). The role of pro-/anti-inflammation imbalance in Abeta42 accumulation of rat brain co-exposed to fine particle matter and sulfur dioxide. Toxicol Mech Methods, 27(8), 568–574. 10.1080/15376516.2017.1337256 [DOI] [PubMed] [Google Scholar]
- Yao G, Yue H, Yun Y, & Sang N (2015). Chronic SO2 inhalation above environmental standard impairs neuronal behavior and represses glutamate receptor gene expression and memory-related kinase activation via neuroinflammation in rats. Environ Res, 137, 85–93. 10.1016/j.envres.2014.11.012 [DOI] [PubMed] [Google Scholar]
- Yao Y, Lu T, Liu Y, Qin Q, Jiang J, & Xiang H (2022). Association of depressive symptoms with ambient PM2.5 in middle-aged and elderly Chinese adults: A cross-sectional study from the China health and Retirement Longitudinal Study wave 4. Environ Res, 203, 111889. 10.1016/j.envres.2021.111889 [DOI] [PubMed] [Google Scholar]
- Yokota S, Oshio S, Moriya N, & Takeda K (2016). Social Isolation-Induced Territorial Aggression in Male Offspring Is Enhanced by Exposure to Diesel Exhaust during Pregnancy. PLoS One, 11(2), e0149737. 10.1371/journal.pone.0149737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota S, Oshio S, & Takeda K (2016). In utero exposure to diesel exhaust particles induced anxiogenic effects on male offspring via chronic activation of serotonergic neuron in dorsal raphe nucleus. The Journal of Toxicological Sciences, 41(5), 583–593. [DOI] [PubMed] [Google Scholar]
- Yolton K, Khoury JC, Burkle J, LeMasters G, Cecil K, & Ryan P (2019). lifetime exposure to traffic-related air pollution and symptoms of depression and anxiety at age 12 years. Environ Res, 173, 199–206. 10.1016/j.envres.2019.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue JL, Liu H, Li H, Liu JJ, Hu YH, Wang J, Lu L, & Wang F (2020). Association between ambient particulate matter and hospitalization for anxiety in China: A multicity case-crossover study. Int J Hyg Environ Health, 223(1), 171–178. 10.1016/j.ijheh.2019.09.006 [DOI] [PubMed] [Google Scholar]
- Zeng Y, Lin R, Liu L, Liu Y, & Li Y (2019). Ambient air pollution exposure and risk of depression: A systematic review and meta-analysis of observational studies. Psychiatry Res, 276, 69–78. 10.1016/j.psychres.2019.04.019 [DOI] [PubMed] [Google Scholar]
- Zhang T, Sun L, Wang T, Liu C, Zhang H, Zhang C, & Yu L (2021). Gestational exposure to PM2.5 leads to cognitive dysfunction in mice offspring via promoting HMGB1-NLRP3 axis mediated hippocampal inflammation. Ecotoxicology and Environmental Safety, 223. [DOI] [PubMed] [Google Scholar]
- Zhang T, Zheng X, Wang X, Zhao H, Wang T, Zhang H, Li W, Shen H, & Yu L (2018). Maternal Exposure to PM2.5 during Pregnancy Induced Impaired Development of Cerebral Cortex in Mice Offspring. International Journal of Molecular Sciences, 19(1), 257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang X, & Chen X (2017). Happiness in the Air: How Does a Dirty Sky Affect Mental Health and Subjective Well-being? J Environ Econ Manage, 85, 81–94. 10.1016/j.jeem.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zhao D, Hong YS, Chang Y, Ryu S, Kang D, Monteiro J, Shin HC, Guallar E, & Cho J (2019). Long-Term Particulate Matter Exposure and Onset of Depression in Middle-Aged Men and Women. Environ Health Perspect, 127(7), 77001. 10.1289/EHP4094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, Markevych I, Romanos M, Nowak D, & Heinrich J (2018). Ambient ozone exposure and mental health: A systematic review of epidemiological studies. Environ Res, 165, 459–472. 10.1016/j.envres.2018.04.015 [DOI] [PubMed] [Google Scholar]
- Zhao T, Markevych I, Standl M, Schulte-Korne G, Schikowski T, Berdel D, Koletzko S, Bauer CP, von Berg A, Nowak D, & Heinrich J (2019). Ambient ozone exposure and depressive symptoms in adolescents: Results of the GINIplus and LISA birth cohorts. Environ Res, 170, 73–81. 10.1016/j.envres.2018.12.014 [DOI] [PubMed] [Google Scholar]
- Zhao T, Tesch F, Markevych I, Baumbach C, Janssen C, Schmitt J, Romanos M, Nowak D, & Heinrich J (2020). Depression and anxiety with exposure to ozone and particulate matter: An epidemiological claims data analysis. Int J Hyg Environ Health, 228, 113562. 10.1016/j.ijheh.2020.113562 [DOI] [PubMed] [Google Scholar]
- Zheng X, Wang X, Wang T, Zhang H, Wu H, Zhang C, Yu L, & Guan Y (2018). Gestational Exposure to Particulate Matter 2.5 (PM2.5) Leads to Spatial Memory Dysfunction and Neurodevelopmental Impairment in Hippocampus of Mice Offspring. Front Neurosci, 12, 1000. 10.3389/fnins.2018.01000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Zhang M, Liu W, Li Y, Qin Y, & Xu Y (2020). Transgenerational transmission of neurodevelopmental disorders induced by maternal exposure to PM2.5. Chemosphere, 255, 126920. 10.1016/j.chemosphere.2020.126920 [DOI] [PubMed] [Google Scholar]
- Zhou YM, An SJ, Tang EJ, Xu C, Cao Y, Liu XL, Yao CY, Xiao H, Zhang Q, Liu F, Li YF, Ji AL, & Cai TJ (2021). Association between short-term ambient air pollution exposure and depression outpatient visits in cold seasons: a time-series analysis in northwestern China. J Toxicol Environ Health A, 84(9), 389–398. 10.1080/15287394.2021.1880507 [DOI] [PubMed] [Google Scholar]
- Zhou YM, Fan YN, Yao CY, Xu C, Liu XL, Li X, Xie WJ, Chen Z, Jia XY, Xia TT, Li YF, Ji AL, & Cai TJ (2021). Association between short-term ambient air pollution and outpatient visits of anxiety: A hospital-based study in northwestern China. Environ Res, 197, 111071. 10.1016/j.envres.2021.111071 [DOI] [PubMed] [Google Scholar]
- Zijlema WL, Wolf K, Emeny R, Ladwig KH, Peters A, Kongsgard H, Hveem K, Kvaloy K, Yli-Tuomi T, Partonen T, Lanki T, Eeftens M, de Hoogh K, Brunekreef B, BioShaRe, Stolk RP, & Rosmalen JG (2016). The association of air pollution and depressed mood in 70,928 individuals from four European cohorts. Int J Hyg Environ Health, 219(2), 212–219. 10.1016/j.ijheh.2015.11.006 [DOI] [PubMed] [Google Scholar]
- Zock JP, Verheij R, Helbich M, Volker B, Spreeuwenberg P, Strak M, Janssen NAH, Dijst M, & Groenewegen P (2018). The impact of social capital, land use, air pollution and noise on individual morbidity in Dutch neighbourhoods. Environ Int, 121(Pt 1), 453–460. 10.1016/j.envint.2018.09.008 [DOI] [PubMed] [Google Scholar]
- Zu D, Zhai K, Qiu Y, Pei P, Zhu X, & Han D (2020). The Impacts of Air Pollution on Mental Health: Evidence from the Chinese University Students. Int J Environ Res Public Health, 17(18). 10.3390/ijerph17186734 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


