Abstract
Introduction
During the processing of fresh plants, prolonged exposure to the air can cause rapid oxidative changes, and this is further accelerated if they have large surface areas. These changes can ultimately lead to losses in valuable ingredients and deterioration of the final product. Consequently, in the food, cosmetics, and pharmaceutical industries, oxidation and the use of antioxidant conservatives are major considerations during processing and production. However, similar considerations are not currently made for herbal medicines. The differences in the UV-Vis spectra of several commercial herbal mother tinctures were investigated here to determine if the oxidation process occurred and should thus be considered during their production.
Methods
The impact of air exposure on comminuted fresh Melissa officinalis and on the quality of the resulting mother tincture was evaluated using UV-Vis spectrophotometric analysis, antioxidant tests (potassium permanganate and FOLIN-Ciocalteu), and high-performance thin-layer chromatography.
Results
A time-dependent decrease in phenolic compounds, UV absorbance, and antioxidant capacity of the Melissa officinalis mother tincture were observed. Specifically, the antioxidant capacity of ground Melissa officinalis in the resulting herbal mother tincture was reduced by 40.47% and 55.52% after 5 and 30 min of air exposure, respectively.
Conclusions
The results indicate that the Melissa officinalis mother tincture is affected if its comminuted starting material is exposed to air during the manufacturing process and that this should be considered when producing fresh herbal medicine plant products in the future.
Keywords: Herbal mother tinctures, Oxidation, Polyphenol oxydase, UV-Vis spectrophotometry, Antioxidant assay
Zusammenfassung
Einleitung
Bei der Verarbeitung von Frischpflanzen kann es bei längerem Kontakt mit der Luft schnell zu oxidativen Veränderungen kommen, die durch große Oberflächen beschleunigt werden. Diese Veränderungen können letztlich zu Verlusten an wertvollen Inhaltsstoffen und zur Beeinträchtigung des Endprodukts führen. In Verarbeitungs- und Herstellungsprozessen der Lebensmittel-, Kosmetik- und Pharmaindustrie sind Oxidation und der Einsatz von Antioxidationsmitteln daher ein allgegenwärtiges Thema. Im Bereich der pflanzlichen Arzneimittel werden ähnliche Überlegungen derzeit jedoch nicht angestellt. In dieser Arbeit wurde anhand UV-Vis-Spektren von verschiedenen kommerziell erhältichen pflanzlichen Urtinkturen untersucht, ob Anzeichen stattgefundener Oxidationsprozesse festzustellen sind und ob dies daher bei der Herstellung berücksichtigt werden sollte.
Methoden
Die Auswirkungen der Luftexposition auf frisches zerkleinertes Pflanzenmaterial von Melissa officinalis und auf die Qualität der daraus resultierenden Urtinkturen wurden mit Hilfe von UV-Vis-Spektralphotometrie, Antioxidationstests (Kaliumpermanganat und FOLIN-Ciocalteu) und Hochleistungs-Dünnschichtchromatographie (HPTLC) untersucht.
Ergebnisse
Es wurde eine zeitabhängige Abnahme der phenolischen Verbindungen, der UV-Absorption und der antioxidativen Kapazität der Melissa officinalis Urtinktur beobachtet. Insbesondere war die antioxidative Kapazität der Melissa officinalis Urtinktur durch 5 bzw. 30 Minuten Luftexposition des frischen gemahlenen Ausgangsmaterials um 40.47% bzw. 55.52% reduziert.
Schlussfolgerungen
Die Ergebnisse deuten darauf hin, dass die Melissa officinalis Urtinktur beeinträchtigt wird, wenn das zerkleinerte Frischpflanzenmaterial während des Herstellungsprozesses der Luft ausgesetzt wird, und dass dies bei der Herstellung von Arzneimitteln aus Frischpflanzen in Betracht gezogen werden sollte. © 2022 The Author(s).
Schlüsselwörter: Pflanzliche Urtinkturen, Oxidation, Polyphenoloxidase, UV-Vis Spektrophotometrie, Antioxidations-Assay
Introduction
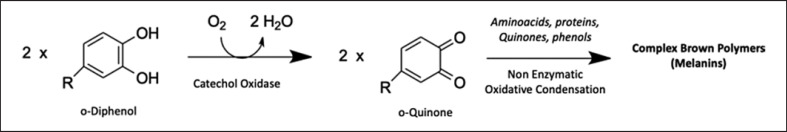
Herbal mother tinctures (HMTs) are homeopathic drug preparations, which have to be prepared in European countries through the maceration of fresh (or sometimes dried or otherwise prepared) herbal drugs according to the methods described in the European Pharmacopeia (PhEur), monograph 2,371: METHODS OF PREPARATION OF HOMOEOPATHIC STOCKS AND POTENTISATION. This monograph is in accordance with the equivalent document in the “Homöopathisches Arzneibuch (HAB)” in Germany [1, 2]. The amount of the hydro-alcoholic solvent that should be added to each herbal material is determined using the loss value after the plant material is dried, in accordance with the corresponding monograph in PhEur or HAB, in this case the HAB-Monograph “Melissa officinalis” [2]. In the industrial production of HMTs, pharmaceutical companies follow the monographs of the pharmacopoeia in their manufacturing process. These regulations leave some room for maneuvering, and consequently, there are several variables that can have a large influence on the quality of the finished product. Preharvest factors such as soil composition, varying climatic conditions, cultivar, and harvesting time may influence the active ingredient content but are difficult to control [3]. However, variables during the subsequent manufacturing process, such as storage conditions and time until processing, as well as processing techniques, can be better monitored. This is because ongoing metabolic processes, exposure to light, heat, and air, or volatilization, may have a major influence on the quality of the finished product and could result in low-quality products from even the highest quality starting materials [3]. When fresh material is used for extraction, the process should be carried out as soon as possible after collection to avoid possible deterioration [4]. The grinding process is a key step in plant processing. Reducing the size of the starting material allows the hydro-alcoholic solvent to access the chemicals more easily, improving the extraction rate [3, 4]. Furthermore, various metabolic processes are also slowed as the enzymes are denatured by the alcohol. However, excessive grinding can degrade the plant material through mechanical heating and oxidation due to air exposure [4]. It is commonly accepted that an oxidation reaction involving phenolic secondary metabolites can occur when plant cell compartmentation is damaged, as this enables polyphenol oxidase (PPO; stored in plastids) to contact the phenolic substances (initially stored in the plant vacuole) [5]. These enzymes are also known as tyrosinases, which are a group of multi-copper glycoproteins, such as catechol oxidases, capable of catalyzing the oxidation of phenolic compounds in the presence of molecular oxygen as a co-substrate (reaction shown in Fig. 1) [5]. The resulting oxidized products (o-quinone) are highly reactive species that undergo further spontaneous non-enzymatic reactions with nucleophilic substances such as phenols, amino acids, or proteins to produce a complex mixture of brown products known as melanin [5, 6]. The melanin polymer complexes formed because of cellular damage can reduce the nutritional value of the plant tissue. It is for this reason that in the food, cosmetic, and pharmaceutical industries, oxidation is a major concern and why many of these products are stabilized with antioxidants [7]. The relationship between the degradation reactions of phenolic compounds and the deterioration of the quality of the final product has been well studied in areas such as food processing [7, 8, 9] but not widely investigated or considered in the production of herbal medicines. Phenolic acids and flavonoids are highly reactive with PPO and could suffer a major loss during the cutting and grinding process [7]. Phenolic compounds have been shown to possess a variety of beneficial pharmacological effects, such as antioxidant, anti-inflammatory, anti-allergic, anti-thrombotic, and anti-mutagenic, participating largely in the biological activity of herbal medicines [10]. Therefore, the degradation of phenolic compounds could also be a concern, not only for the quality but also the efficacy of herbal medicines, especially those prepared from fresh plants. Routine quality control methods for the market release of HMTs, including density, dry residue, and thin-layer chromatography, are not sufficient for assessment, thus making mindful production more important [11, 12].
Fig. 1.
Suggested reaction of ortho-diphenol oxidation in ortho-quinone catalyzed by catechol oxidase (CO) in the presence of molecular oxygen and subsequent transformation in melanins.
Due to its high content of phenolic compounds [13] and its sensitivity to browning in the transformation process [14, 15], Melissa officinalis was chosen as a model plant to conduct the experiments described herein. Phytochemical investigations showed the presence of several phenolic compounds known to exhibit important antioxidant activity such as the caffeic acid derivatives rosmarinic acid, lithospermic acid, and chlorogenic acid [16].
This study started after the comparison of different HMTs available on the market through a UV-Vis spectrophotometric analysis that revealed interesting differences between the analyzed batches. The impact of oxidation during the production of herbal medicinal products was suspected to be the main reason for the observed differences. To further investigate this, we have characterized the influence of air exposure on the oxidation of fresh M. officinalis using simple analytical methods. In addition, the results obtained were integrated to develop a suitable and simple analytical method to identify and describe the oxidation status of a finished HMT.
Materials and Methods
Chemicals
Potassium permanganate (≥99%) and FOLIN & Ciocalteu's phenol reagent (2 M, with respect to acid) were purchased from Sigma-Aldrich (Darmstadt, Germany). Sodium carbonate anhydrous (reagent grade), potassium hydroxide (extra pure), potassium dihydrogen phosphate (≥99.5%), and dipotassium hydrogen phosphate (≥99.5%) were purchased from Scharlau (Barcelona, Spain). Ethyl acetate (≥99.5%), ethyl methyl ketone (≥99.5%), formic acid (≥98%), ethanol (≥99.5%), methanol (≥99.5%), diphenylboryloxyethylamine, polyethylene glycol, rutoside, chlorogenic acid, and caffeic acid (analytical grade) were purchased from Roth (Karlsruhe, Germany). Distilled water was prepared using a Millipore Q water purification system (Millipore, Billerica, MA, USA). Liquid nitrogen was purchased from Pangas (Dagmersellen, Switzerland), and mushroom tyrosinase (>1,000 Ui) was obtained from Sigma-Aldrich (Darmstadt, Germany).
Herbal Material
The fresh aerial parts of Melissa officinalis used for the preparation of the extract during the air exposure test were obtained from Ekkharthof (Lengwil, Switzerland) and harvested before flowering.
Melissa officinalis HMTs
Samples of Melissa officinalis HMTs were obtained from Ceres Heilmittel AG (Kesswil, Switzerland) and from four different manufacturers by purchasing their products directly from a local pharmacy. All the HMTs were prepared according to the PhEur method 3a and were stored hermetically under appropriate climatic conditions until use. The different tinctures were conventionally named with a code consisting of a letter “C” for tinctures from Ceres Heilmittel AG and “(n)MT” preceded by a number (n) for mother tinctures obtained from local pharmacies. The letter is followed by two numbers indicating the year of production (e.g., “C18,” “1MT18,” “2MT18,” and “3MT18” indicate mother tinctures produced in 2018 by Ceres and by other manufacturers; each of the digits 1–3 denotes a different manufacturer).
Standard PhEur Methods for HMT Control (Dry Residue, Relative Density, and Organoleptics)
The dry residue of each HMT sample was determined using the Moisture Analyzer DBS 60-3 (Kern). First, 2 g of undiluted HMT was placed in DBS 60-3 and evaporated at 105°C. The remaining weight was recorded and expressed as the percentage of drying loss. The relative density of each HMT was evaluated by submitting 2 mL for analysis using the densitometer DMA 4100 M (Anton Paar). The organoleptic identity checks (taste, smell, and color) were carried out in accordance with the GHP, as follows: 2 mL of HMT was placed on a glass disc (6 cm diameter; 0.5 cm height) and the smell and color were recorded subjectively by the operator. A 2-mL glass pipette was used to collect the HMTs, and few drops were administered on the tongue as the taste control.
High-Performance Thin-Layer Chromatography
Following the internal quality control routine method “A2,” 10 μL of undiluted HMT was used for each sample, and 5 μL of a methanolic solution containing 1 mg/mL each of rutoside, chlorogenic acid, and caffeic acid was used as the reference. Samples and references were applied to 10-mm bands, which were 8 mm in height on 200 × 100 mm high-performance thin-layer chromatography silica gel 60 W F254 plates (Merck, Darmstadt). Plates were developed until 65 mm in height in a pre-saturated glass chamber (Camag). The mobile phase consisted of ethyl acetate, ethyl methyl ketone, 98% formic acid, and water (50:30:10:10 vol/vol). Post-chromatographic chemical detection was carried out by spraying a solution of diphenylboryloxyethylamine dissolved in methanol (10 g/L) followed by a solution of polyethylene glycol 400 diluted in ethanol (50 mL/L) using an automatic derivatizer (Camag). The plate was placed on a TLC visualizer (Camag), observed, and recorded at 254 and 365 nm.
Extract Preparation (Air Exposure Test)
The fresh aerial parts of M. officinalis (leaves and stems) were processed immediately after harvesting by adapting the 3a method described in the PhEur. The plants were chopped into approximately 5-cm pieces with a knife and cryoground manually in a mortar for 2.5 min covered with liquid nitrogen. Three different extracts were prepared using this procedure. A reference “oxidation-free” extract (CRYO) was prepared by adding the extraction solvent to the ground material immediately after grinding. Oxidized extracts were prepared analogously to the reference extract, adding the extractant to the ground material only after 5 min (CRYO_5) and 30 min (CRYO_30) at room temperature (22 ± 2°C). The air exposure of the ground material in the waiting time was carried out by transferring the cryoground material to a new mortar at room temperature, leaving it in contact with the air and manually stirring it with a spatula for 5 min and 30 min, respectively. For each extract, 15 g of ground material was macerated in ethanol 86% (m/m) while protected from light under an airtight seal. The amount of ethanol 86% (m/m) to be added was calculated using equation (1), with m being the mass of the fresh herbal material and T being the percentage loss after drying the starting material.
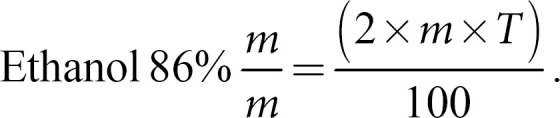
The three macerated samples were shaken daily. After 15 days at room temperature, the macerated samples were centrifuged at 4,000 rpm for 20 min (Heraeus Megafuge), and the upper phase was recovered and filtered through a 12–25-μm filter (Black Ribbon) into a 20-mL volumetric flask. In order to visually assess the impact of oxidation due to exposure of ground Melissa officinalis to air, the “oxidation-free” ground material (CRYO) and the material exposed for 30 min (CRYO_30) were photographed and the images were subsequently compared to each other.
UV-Visible Spectrophotometry
Spectroscopic analysis was conducted using a Specord 200 plus spectrophotometer (Analytik Jena) and 3-mL quartz cuvette. Absorption spectra of 1:250 aqueous dilutions of the HMTs were recorded in the range of 200–500 nm. Additionally, to evaluate the impact of oxidation in the air exposure test, two additional dilutions were prepared diluting 1 mL of the 1:250 CRYO sample with distilled water until it reached the same maximum absorbance (at 325 nm) as the CRYO_5 and CRYO_30 sample dilution. Measurements were performed using AspectUV software. The raw data were exported and processed in Microsoft Excel.
Tyrosinase Oxidation Assay
In a 3-mL quartz cuvette, 2 mL of 50 mM phosphate buffer solution (pH 6.8) was mixed with 0.25 mL of the HMT (diluted 1:25 v/v with the previous buffer) and 0.25 mL of the tyrosinase solution (8.3 U/mL). Repeated scans (recorded every minute) of the UV-Vis absorption spectra over the 200–500 nm range were recorded over 60 min. The reaction sample was measured against a blank containing all the same reagents except for a 60% m/m ethanol dilution (1:25 v/v) used instead of the HMT dilution. The first spectrum was recorded immediately after the addition of the tyrosinase solution.
Potassium Permanganate (KMnO4) Antioxidant Assay
Antioxidant activity, expressed as the gallic acid equivalent (GAE), was measured by adapting the potassium permanganate radical scavenging assay described by Amponsah et al. [17]. A potassium dihydrogen orthophosphate (KH2PO4) buffer solution was prepared by dissolving 8.7 g of KH2PO4 in 400 mL distilled water and adjusting with 1 M potassium hydroxide (KOH) to pH 9. To conduct the test, 3 mL of KMnO4 (80 mg/L), obtained by diluting KMnO4 with the buffer solution to maintain an alkaline environment (pH 9), was added to 1 mL of a 1:250 vol/vol aqueous dilution of the HMT sample. After 30 min at 22°C ± 2°C, in the absence of light, absorbance was measured against the buffer solution at 525 nm. A sample containing 1 mL of distilled water + 3 mL of the KMnO4 solution was used as a blank. Distilled water was used to prepare 1:250 dilutions of HMT to avoid ethanol interference reacting with the KMnO4. The scavenging effect was expressed as the GAE using a calibration curve (r2 = 0.995; y = 1.3955x + 1.7649) with gallic acid in a range of 0–48 μg/mL. Results were blank resp. solvent corrected and normalized on a dry residue. Each test was conducted in triplicate, and the results were expressed as the mean GAE per dry residue ± standard deviation (mg/mL).
FOLIN-Ciocalteu Total Polyphenol Content
The total polyphenol content was calculated by adapting the FOLIN-Ciocalteu assay described by Singleton et al. [18]. First, 2.5 mL of FOLIN reagent (2 M), diluted at 1:10 vol/vol with distilled water, was added to 0.5 mL of 1:100 vol/vol aqueous dilutions of HMT samples. After a 3-min incubation period, 2 mL of 7.5% m/vol sodium carbonate (Na2CO3) aqueous solution was added to each sample. After 60 min at 22°C ± 2°C, in the absence of light, absorbance was measured at 750 nm. A linear calibration curve (r2 = 0.995; y = 0.0103 × −0.1562) was prepared using gallic acid at 0–160 μg/mL. The test was conducted in triplicate, and the results were blank corrected and expressed as the mean GAE per dry residue ± standard deviation (mg/mL).
Statistical Analysis
Significant differences in the HMTs analyzed using the KMnO4 scavenging activity and FOLIN-Ciocalteu total polyphenol content tests were highlighted using one-way analysis of variance, followed by a Tukey HSD pairwise test using R programming for statistical analysis. These tests are often used to compare the significant differences (p value ≤ 0.05) between multiple data groups. The respective quantitative results were expressed as the mean ± standard deviation of at least three replicates for each experiment and were calculated using Microsoft Office Excel.
Results
Standard PhEur Methods for HMT Control (Dry Residue, Relative Density)
The dry residue percentage of the analyzed HMTs, representing the total amount of extracted substances (Table 1), was 2.50–1.03 for the air exposure tests and 2.68–1.56 for the commercial batches. All investigated HMTs were in accordance with the specifications of the HAB-Monograph “Melissa officinalis,” except for the dry residue value of the cryoground extract exposed to air for 30 min (CRYO_30).
Table 1.
Results of standard GHP analysis on the tested mother tinctures
| Analyzed samples | Dry residue (≥1.2%), % | Rel density (0.900–0.920) | Color (G, GB) | Smell (C) | Taste (C) |
|---|---|---|---|---|---|
| CRYO | 2.50 | 0.907 | Conform | Conform | Conform |
| CRYO_5 | 2.06 | 0.902 | Conform | Conform | Conform |
| CRYO_30 | 1.03 | 0.901 | Conform | Conform | Conform |
| C19 | 2.68 | 0.910 | Conform | Conform | Conform |
| C18 | 2.05 | 0.907 | Conform | Conform | Conform |
| 1MT18 | 1.56 | 0.904 | Conform | Conform | Conform |
| 2MT18 | 2.11 | 0.904 | Conform | Conform | Conform |
| 3MT18 | 1.79 | 0.907 | Conform | Conform | Conform |
| C17 | 2.14 | 0.907 | Conform | Conform | Conform |
| C16 | 2.48 | 0.908 | Conform | Conform | Conform |
| 4MT16 | 2.3 | 0.913 | Conform | Conform | Conform |
Rel density, relative density; G, green; GB, green-brown; C, characteristic.
High-Performance Thin-Layer Chromatography
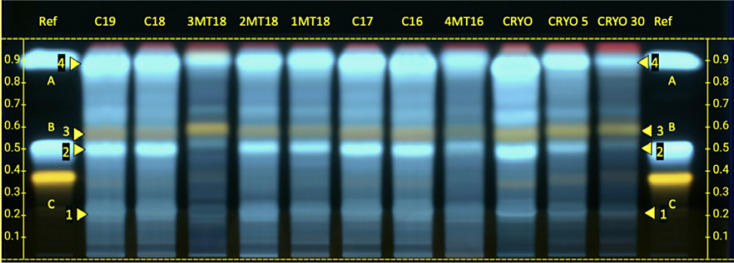
The extract prepared in the air exposure test and the commercially available HMTs were analyzed together on the same plate (Fig. 2). All the tinctures complied with the internal monograph (the required zones are indicated by numbers 1–4). A decrease in signal intensity in the area between caffeic acid (A) and chlorogenic acid (B) that was proportional to the air exposure time was observed in the test mother tinctures CRYO, CRYO_5, and CRYO_30 comparisons. The intensity of the band fluorescence varies between different commercially available mother tinctures, and the tinctures manufactured by Ceres Heilmittel AG generally had higher band intensity.
Fig. 2.
HPTLC plate of Melissa officinalis mother tinctures displayed under 365 nm after application of detection reagent. HPTLC, high-performance thin-layer chromatography.
UV-Visible Spectrophotometry
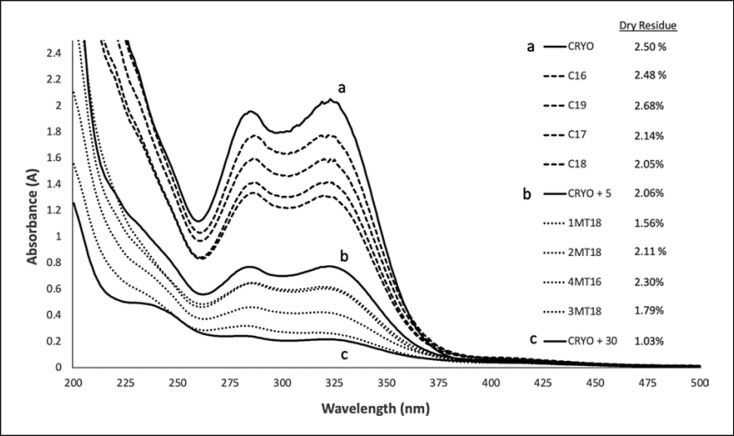
The UV-Vis absorption spectra of the M. officinalis samples analyzed from 200 to 500 nm typically showed a minimum absorption at 262 ± 2 nm and two absorption maxima between 285–287 nm and 318–325 nm, whereby the absorption and ratio between the curve minima and maxima varied within samples (Fig. 3). The spectra of the commercially available mother tinctures showed similar patterns with different intensities and grades of curve flattening (described by the ratio between maximum and minimum). The mother tinctures from Ceres Heilmittel AG, even those produced somewhat earlier (like C16, produced in 2016), generally showed a higher absorbance than the comparative commercial samples (produced in 2018), and they also had a less pronounced flattening (Fig. 3).
Fig. 3.
Spectrophotometric UV-visible analysis of commercial Melissa mother tinctures compared with the extracts prepared in the air exposure test. The letter a, b, and c correspond to the sample CRYO, CRYO_5, and CRYO_30, respectively. Name of extracts in the legends are ordered in descendent intensity of maximum absorption (between 318 and 323 nm).
The mother tinctures whose starting materials were either not exposed to air (a) or exposed to air in a controlled manner (b and c) showed a time-dependent change in their absorption patterns. As the time that the crushed fresh plant was in the air increased, the UV absorption decreased, as well as the ratio between the maximum and minimum, showing an increasing flattening of the absorbance shape. The dry residue was also massively reduced, even if the identical starting material and identical ratios of plant material and extractant were used, as in the preparations for the test mother tinctures (a, b, and c) (Fig. 3; Table 1).
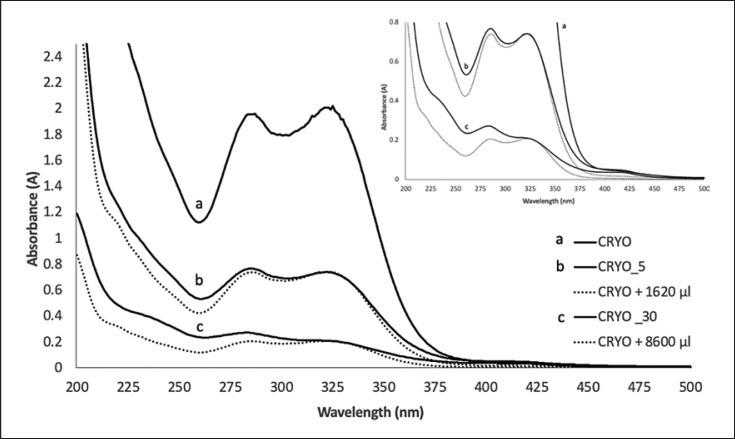
As the UV absorption could be dependent on the substance content and this varies within the samples (as can be seen from the loss on drying), the “oxidation-free” reference tincture (CRYO) was diluted to such an extent that it achieved the same absorption maximum as the CRYO_5 and CRYO_30 (Fig. 4). Thereby, the exposure of the starting material did not only lead to a decrease in UV intensity but also to a flattening of the curve pattern of the resulting mother tincture. This flattening of the absorption curve can be described or detected by a relative increase in absorption intensity in the region of the minimum (262 ± 2 nm) and a relative decrease in the absorption maximum in the range of 318–325 nm (Fig. 4).
Fig. 4.
Spectrophotometric UV-visible analysis of “oxidation-free” reference extract (CRYO) (a) diluted with distilled water (CRYO + 1,620 μL and CRYO + 8,600 µL) until it reached the same maximum of (b) cryoground + 5 min air exposed (CRYO + 5) and (c) cryoground + 30 min air exposed (CRYO + 30). Name of extracts in the legends are ordered in descendent intensity of maximum absorbance (between 318 and 323 nm).
Tyrosinase Oxidation Assay
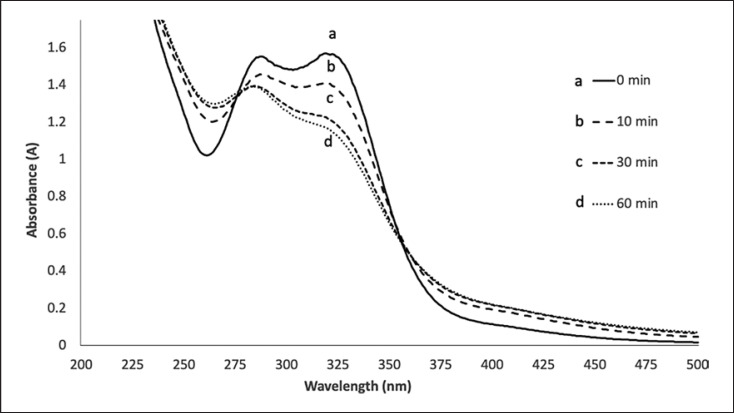
Incubation of tyrosinase with C16 showed a time-dependent flattening of the typical absorbance shape of the mother tincture (Fig. 5). This change in shape appears to be proportional to the reaction time and appears to occur rapidly (within the first 30 min). After the addition of the tyrosinase solution, the maximum absorbance at 319 nm showed a decrease, while the minimum at 261 nm showed an increase. The HMT C16 was chosen as the sample for the assay due to its higher absorbance and higher maximum/minimum ratio between the commercial batches (Fig. 3).
Fig. 5.
Oxidation of C16 Melissa officinalis mother tincture by tyrosinase (8.3 U/mL) in 50 mM phosphate buffer. Spectra recorded a immediately after enzyme addition; b after 10 min; c after 30 min; and d after 60 min.
Potassium Permanganate − Antioxidant Assay
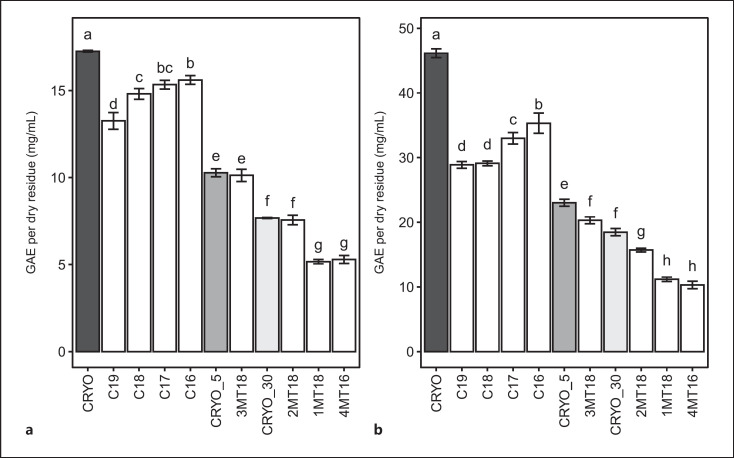
In line with the general pattern of absorbance observed in the UV-Vis spectra (Fig. 3), the results of the KMnO4 assay (Fig. 6a; Table 2) showed a statistically significant decrease in scavenging activity that was in relation to the time of exposure to air in the extracts prepared during the air exposure oxidation test. Results were normalized to the % dry residue values and indicated as GAE per dry residue. Mother tinctures manufactured by Ceres Heilmittel AG showed a higher scavenging activity (expressed as GAE per dry residue) when compared to the other commercially available mother tinctures.
Fig. 6.
Comparison of scavenging effect (potassium permanganate method (a)) and total polyphenol content (FOLIN-Ciocalteu method (b)) expressed as gallic acid equivalent (GAE) per dry residue (mg/mL) in the analyzed Melissa officinalis (L.) mother tinctures. Bar plots not sharing any letter indicate samples which are significantly different (p ≤ 0.05).
Table 2.
Means of the potassium permanganate and FOLIN-Ciocalteu assays, expressed as gallic acid equivalent (GAE) per dry residue ± standard deviation (mg/mL) for each analyzed sample
| Sample | GAE per dry residue, mg/mL |
|
|---|---|---|
| potassium permanganate | FOLIN-Ciocalteu | |
| CRYO | 17.26±0.05a | 46.14±0.68a |
| CRYO_5 | 10.27±0.23b | 23.02±0.54b |
| CRYO_30 | 7.68±0.03c | 18.47±0.57c |
| C19 | 13.26±0.48d | 28.88±0.52d |
| C18 | 14.81±0.30e | 29.11±0.37d |
| C17 | 15.34±0.25ef | 32.97±0.89e |
| C16 | 15.60±0.25f | 35.32±1.56f |
| 1MT18 | 5.17±0.13g | 11.18±0.34h |
| 2MT18 | 7.56±0.28c | 15.71±0.29g |
| 3MT18 | 10.13±0.35b | 20.30±0.54c |
| 4MT16 | 5.29±0.23g | 10.30±0.58h |
| HSD | 0.78 | 2.07 |
HSD, Tukey's honestly significant difference at 5% level of significance.
Within a columnn means without a common superscript differ (p < 0.05).
Within a columnn means without a common superscript differ (p < 0.05).
Within a columnn means without a common superscript differ (p < 0.05).
Within a columnn means without a common superscript differ (p < 0.05).
Within a columnn means without a common superscript differ (p < 0.05).
Within a columnn means without a common superscript differ (p < 0.05).
Within a columnn means without a common superscript differ (p < 0.05).
Within a columnn means without a common superscript differ (p < 0.05).
FOLIN-Ciocalteu Total Polyphenol Content
The results obtained for the total polyphenol content of the HMTs using the FOLIN reagent (Fig. 6b; Table 2) showed a similar relative trend to the results obtained in the potassium permanganate (KMnO4) antioxidant assay.
Discussion
The first experiments performed on the commercial HMTs showed that their UV-Vis absorbance shape was related to the presence of hydroxycinnamic acid derivatives, such as chlorogenic acid [16], lithospermic acid [13], or other similar characteristic substances of M. officinalis (L.) [16]. When the commercial batches were compared, a clear difference was identified in the absorbance intensity and curve shape flattening, which is, respectively, higher and less pronounced in the Ceres HMTs. Similar findings regarding the absorption shape flattening were previously identified in the literature, when the absorbance of phenolic compounds such as chlorogenic acid [19, 20] and compounds with a similar chemical structure such as trans-resveratrol [21] were oxidized by tyrosinase. The hypothesis for oxidation impact was tested by performing an air exposure test. By using a cryogenic grinding procedure, liquid nitrogen was able to guarantee an inert environment as it displaces oxygen and also reduces enzymatic processes to a minimum due to its low temperature [14, 22]. Subsequently, detectable differences in the mother tinctures produced from this material resulted exclusively due to the different waiting times in the air until the addition of alcohol. All other parameters, including and most importantly the quality of the fresh starting material, were identical. Exposing freshly chopped M. officinalis leaves to the air for 5 and 30 min led to (1) a decrease in UV absorbance and (2) a flattening of the curve shape (Fig. 3, 4).
In general, the decrease in UV absorbance should be directly proportional to a decrease in substance content; however, when we compared the dry residues from all the analyzed HMTs and their absorbance values, this trend was not always maintained (e.g., when 4MT18 is compared with CRYO_5). This suggested that the dry residue was not the only factor playing a role in the observed differences. The KMnO4 assay and FOLIN test showed a time-dependent decrease in antioxidant activity and total polyphenol content when the ground M. officinalis material was oxidized by exposure to the air for 5 and 30 min. This decrease is not dependent on the dry residue since the GAE was normalized, indicating that the reduction in antioxidant potential is not only due to the dry residue but also to a certain degree of oxidation. Moreover, the extracts from CRYO_5 and CRYO_30 showed clear increases in flattening, and, as shown in Figure 5, this shape change seems to be directly related to an oxidation process involving phenolic compounds and tyrosinase. This hypothesis is also supported by the experiment of diluting the CRYO sample to the same maximum observed in CRYO_5 and CRYO_30 (shown in Fig. 4). Two overlapping spectra should be expected if the total amount of substances was the only factor playing a role in the intensity of their absorbance. Here, however, a clear flattening (characterized by a higher minimum) is observed in CRYO_5 and CRYO_30, indicating a possible impact of oxidation.
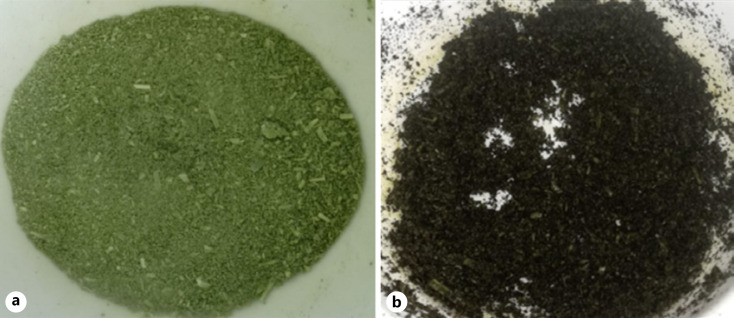
Exposure to air also causes an evident browning of the plant material before extraction (Fig. 7) which could indicate the oxidation of phenolic compounds and the subsequent formation of melanin complexes (reaction shown in Fig. 1), which are characterized by this brown color. One possible hypothesis to explain the decrease in the dry residue is the formation of these complex polymers, which are known to be insoluble in hydro-alcoholic solvents [6, 23]. If these molecules cannot be extracted, a decrease in the dry residue should be expected. However, additional experiments should be performed to verify the presence of melanin in the extracted material.
Fig. 7.
Browning of Melissa officinalis ground leaves in the air exposure test. The figure shows the cryoground material used to prepare the “oxidation-free” reference extract (CRYO) (a) compared with the cryoground material exposed to air for 30 min used for the oxidized extract (CRYO_30) (b).
Although the differences observed between HMTs could also be attributed to the selection of different raw plant materials, an impact due to oxidation during air exposure is highly likely since M. officinalis is a very sensitive plant, evidenced by the fact that if the ground material is exposed to the air for only 5 min, this can lead to considerable loss of antioxidant activity. This strongly suggests that not only the starting material but also the manufacturing process could determine the quality and variability of the finished product. The oxidation hypothesis is supported by the results of the tyrosinase enzyme assay and by the normalized antioxidant potential. Both of these experiments were shown to correlate with the UV-Vis analysis in terms of shape flattening and their intensity, respectively.
The analysis of different commercially available M. officinalis mother tinctures showed a large amount of heterogeneity, whereby it could be shown that the tinctures of Ceres Heilmittel AG performed very well in the experiments described. In principle, the Ceres tinctures seem to have somewhat higher substance contents and were less oxidized. At this stage, however, it can only be stated that there are clear differences in this respect. A conclusive statement on the cause of these differences cannot be made since there are too many different variables and unknown parameters.
In the manufacturing of HMTs at Ceres Heilmittel AG, the comminution process of the starting material is carried out gently by hand. The structural characteristics of the plant material and, therefore, most of the plant cells are largely preserved until the extraction agent is added, as the sections are not cut too small. The extractant required for the extraction was added and mixed at low speed in the patented mortar grinder. Therefore, manufacturing takes place with as little air circulation as possible. This workflow would be contrasted with comminution in an industrial blender, which could process the plant material into an oxidation-sensitive pulp in a short time. With the addition of alcohol, the oxidation-sensitive phase ends as the oxidation via the PPO is inhibited [24] and the exposure to air is drastically reduced, regardless of the previous comminution method.
Conclusion
Freshly ground M. officinalis (L.) leaves are very sensitive to oxidation during exposure to air, which could profoundly affect the herbal medicinal products prepared from them. As shown in this work, this oxidation process occurs rapidly and seems to be of certain relevance in commonly applied production processes.
The air exposure experiment showed that oxidation seems to be reflected mainly in a flattening of the curve, a decrease in antioxidant potential (KMnO4 and FOLIN assay), and a decrease in absorbance intensity. In addition, there seems to be a dependence between the results observed in the UV-Vis analysis and the results of the antioxidant assays, tyrosinase assays, and high-performance thin-layer chromatography. These results may suggest that the observation of the intensity and shape of the absorption curve by UV spectroscopy could be used as an efficient and convenient stand-alone method by which to rapidly assess the oxidation status of an HMT.
This work aims to raise awareness of this issue and is intended to highlight that the oxidation of fresh plant material during the preparation of HMTs should be considered. In the future, further studies could be undertaken to improve the current work by including other plants in the analyses performed and by identifying substances formed in the oxidation process.
Statement of Ethics
An ethics statement was not required for this study type; no human or animal subjects or materials were used.
Conflict of Interest Statement
D.B., S.H., and M.P. are employees of Ceres Heilmittel AG. C.K. is the CEO of Ceres Heilmittel AG. R.K. is the founder of Ceres Heilmittel AG.
Funding Sources
The research project was funded by Ceres Heilmittel AG.
Author Contributions
Roger Kalbermatten provided the theoretical and observational foundation upon which this research project was built. Roger Kalbermatten, Christoph Kalbermatten, Samuel Hasler, and Didier Barmaverain devised the project and the main conceptual ideas; Didier Barmaverain performed the experiments and analyzed and elaborated the data; Samuel Hasler participated in the results interpretation with important feedbacks; and Didier Barmaverain performed original draft preparation in consultation with Samuel Hasler. All authors provided critical feedback and helped shape the research, analysis, and the manuscript.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, C.K., upon reasonable request.
Funding Statement
The research project was funded by Ceres Heilmittel AG.
References
- 1.EDQM. European Pharmacopeia Edition 10.8. Monograph 2371. Methods of preparation of homoeopathic stocks and potentisation. 2022.
- 2.Homöpathisches Arzneibuch Amtliche Ausgabe, ISBN 978-3-7692-7807-1 2022.
- 3.Silva Junior JOC, Ribeiro Costa RM, Martins F, Ramos Barbos WL. Processing and quality control of herbal drugs and their derivatives. Quality Control of Herbal Medicines and Related Areas. 2011 Nov 4;Vol. 11:p. 195–221. [Google Scholar]
- 4.WHO guidelines on good herbal processing practices for herbal medicines WHO Technical Report Series No. 1010. 2018;107 [cited 2022 Jan 7]. Available from: https://www.gmp-compliance.org/files/guidemgr/TRS1010annex1.pdf. [Google Scholar]
- 5.Boeckx T, Winters AL, Webb KJ, Kingston-Smith AH. Polyphenol oxidase in leaves; is there any significance to the chloroplastic localization? J Exp Bot. 2015 Mar;66((12)):3571–3579. doi: 10.1093/jxb/erv141. [DOI] [PubMed] [Google Scholar]
- 6.Glagoleva AY, Shoeva OY, Khlestkina EK. Melanin pigment in plants current knowledge and future perspectives. Front Plant Sci. 2020 Jun 23;11:770. doi: 10.3389/fpls.2020.00770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheynier V. Polyphenols in foods are more complex than often thought. Am J Clin Nutr. 2005 Jan 1;81((Suppl l)):223S–229S. doi: 10.1093/ajcn/81.1.223S. [DOI] [PubMed] [Google Scholar]
- 8.Tiwari U, Cummins E. Factors influencing levels of phytochemicals in selected fruit and vegetables during pre- and post-harvest food processing operations. Food Res Int. 2013 Mar;50((2)):497–506. [Google Scholar]
- 9.Lingnert H. Influence of food processing on lipid oxidation and flavor stability lipid oxidation in food. In Angelo SAJ editor. ACS Symposium series. Vol. 500:p. 292–301. [Google Scholar]
- 10.Hano C, Tungmunnithum D. Plant polyphenols more than just simple natural antioxidants oxidative stress, aging and age-related diseases. Medicines. 2020 May 9;7(5):26. doi: 10.3390/medicines7050026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biber A, Franck-Karl G, Waimer F, Riegert U, Wiget R. Analytical characterisation of homoeopathic mother tinctures. Pharmeur Sci Notes. 2009 Mar;2009((1)):1–4. [PubMed] [Google Scholar]
- 12.Scheepmake M, Gower N. The quality of selected South African and international homoeopathic mother tinctures. Afr J Trad Complement Altern Med. 2011 Jul 15;8((Suppl 5)):46–52. doi: 10.4314/ajtcam.v8i5S.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ozarowski M, Mikolajczak PL, Piasecka A, Kachlicki P, Kujawski R, Bogacz A, et al. Influence of the Melissa officinalis leaf extract on long-term memory in scopolamine animal model with assessment of mechanism of action. Evid Based Complement Alternat Med. 2016;2016:9729818. doi: 10.1155/2016/9729818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doğan S, Ayyildiz Y, Doğan M, Alan ü, Diken ME. Characterisation of polyphenol oxidase from Melissa officinalis L.subsp.officinalis (lemon balm) Czech J Food Sci. 2013 Apr 18;31((No. 2)):156–165. [Google Scholar]
- 15.Mirahmadi SF, Norouzi R, Ghorbani Nohooji M. The influence of drying treatments on the essential oil content and composition of Melissa officinalis L. Compared with the fresh sample. J Med Plants. 2017;16((61)):68–78. [Google Scholar]
- 16.Shakeri A, Sahebkar A, Javadi B. Melissa officinalis L. a review of its traditional uses, phytochemistry and pharmacology. J Ethnopharmacol. 2016 Jul;188:204–228. doi: 10.1016/j.jep.2016.05.010. [DOI] [PubMed] [Google Scholar]
- 17.Amponsah IK, Orman E, Mensah AY, Sarpong FM, Armah FA, Sarpong LM. Development and validation of a radical scavenging antioxidant assay using potassium permanganate. J Sci Innov Res. 2016;5((2)):36–42. [Google Scholar]
- 18.Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999;299:152–178. [Google Scholar]
- 19.Tono T, Fujita S, Kawasaki H, Li Z-F. Assay of chlorogenic acid oxidase by difference spectra spectrophotometry. Agric Biol Chem. 1987;51((10)):2843–2844. [Google Scholar]
- 20.Kuijpers TFM, Narváez-Cuenca CE, Vincken JP, Verloop AJW, van Berkel WJH, Gruppen H. Inhibition of enzymatic browning of chlorogenic acid by sulfur-containing compounds. J Agric Food Chem. 2012 Apr 4;60((13)):3507–3514. doi: 10.1021/jf205290w. [DOI] [PubMed] [Google Scholar]
- 21.Gonzálvez AG, González Ureña Á, Lewis RJ, van der Zwan G. Spectroscopy and kinetics of tyrosinase catalyzed trans -resveratrol oxidation. J Phys Chem B. 2012 Mar 1;116((8)):2553–2560. doi: 10.1021/jp209753q. [DOI] [PubMed] [Google Scholar]
- 22.Junghare H, Hamjade M, Patil CK, Girase SB, Lele MM. A review on cryogenic grinding. Int J Curr Eng Technol. 2017 Mar;5((7)):420–423. [Google Scholar]
- 23.Harki E, Talou T, Dargent R. Purification characterisation and analysis of melanin extracted from Tuber melanosporum Vitt. Food Chem. 1997;58((1–2)):69–73. [Google Scholar]
- 24.Valero E, Varon R, Garcia-Carmona F. Inhibition of grape polyphenol oxidase by several natural aliphatic alcohols. J Agric Food Chem. 1990 Apr;38((4)):1097–1100. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, C.K., upon reasonable request.