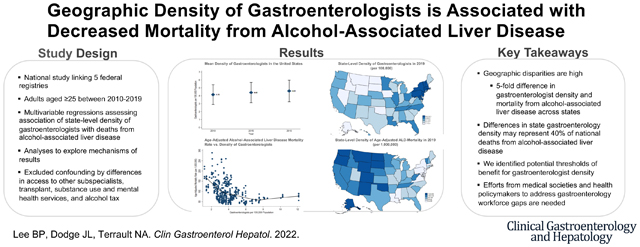
Abstract
BACKGROUND&AIMS
Alcohol-associated liver disease (ALD) is the leading cause of liver-related mortality, and has been increasing. To inform public health efforts to addressing the growing incidence of ALD, we assessed the association of geographic density of gastroenterologists with ALD-related mortality.
METHODS
National data was obtained for adults aged≥25, with state-level demographics and 2010–2019 mortality estimates by linking federally-maintained registries (WONDER, NSSATS, BRFSS, HRSA, US Census Bureau). Multivariable linear regression was used to assess the association of state-level geographic density of gastroenterologists with ALD-related mortality, adjusting for age, sex, race/ethnicity, and other potential confounders.
RESULTS
Among 50 states and the District of Columbia, the national mean geographic density of gastroenterologists was 4.6 per 100,000 population and annual ALD-related mortality rate was 85.6 per 1,000,000 population. There was greater than 5-fold differences in geographic density of gastroenterologists and ALD-related mortality across states. In multivariable analysis, the geographic density of gastroenterologists was significantly associated with lower ALD-related mortality (9.0 [95%CI 1.3–16.7] fewer ALD-related deaths per 1,000,000 population for each additional gastroenterologist per 100,000 population). The association appeared to peak at a threshold of ≥7.5 gastroenterologists per 100,000 population. We estimated that differences in geographic density of gastroenterologists across states may potentially represent 40% of national ALD-related mortality. Exploratory analyses to assess for confounding by generalized subspecialty care, transplant access, alcohol taxation, and substance use or mental health services did not affect primary results.
CONCLUSIONS
State-level geographic density of gastroenterologists is associated with lower ALD-related mortality. These results may inform medical societies and health policymakers to address anticipated workforce gaps to address the growing epidemic of ALD.
Keywords: workforce, hepatology, disparities, inequities
Graphical Abstract
Alcohol-associated liver disease (ALD) accounts for half of global liver-related deaths.1 Liverrelated mortality in recent years has increased faster than each of the leading 10 causes of death (e.g. cardiovascular disease, cancer, etc.) in the United States, increasing 65% from 2009 to 2016.2 Mortality due to ALD has experienced the highest increase compared to other causes of liver disease, particularly among young adults, women, and racial minorities.2 Assessment of factors associated with mitigating ALD-related mortality is thus critical to inform interventions to address the growing contribution of ALD to public health inequities and disease burden.
Prior Veteran Affairs (VA) studies have shown that patients with an ICD-10 code of liver disease who had completed a gastroenterology visit at any time after diagnosis,3 and patients with hepatocellular carcinoma who saw a hepatologist within 30 days of diagnosis4 had lower mortality rates. These findings have not been assessed beyond a VA cohort or among patients with ALD, which is important to inform generalizability and national initiatives regarding subspecialty workforce to address the rising incidence of ALD-related mortality. The VA patient population is unique and does not necessarily reflect the fastest growing populations of ALD (i.e. young adults, women, and racial minorities), and there is significant variation in the distribution of gastroenterologists for the general US population,5 which may not be as pronounced within VA attachment areas.
The purpose of this study was to inform these knowledge gaps and to assess the association of state-level geographic density of gastroenterologists with ALD-related mortality in the entire US population.
METHODS
Primary Outcome
The primary outcome was the annual state-level mortality from ALD (ICD-10 K70) per 1,000,000 adults. Deaths based on death certificate data were obtained from the Center for Disease Control and Prevention’s Wide-ranging Online Data for Epidemiologic Research (CDC WONDER) (Supplemental Methods). We extracted age-adjusted mortality rates by state of residence and year of death from 2010–2019. The study period was determined to reflect the significant rise in ALD-related mortality and most updated follow-up at the time of this analysis. Mortality rates were standardized to the 2000 US standard population using the direct method in 10-year age groups (25–34, 35–44, 45–54, 55–64, 65–74, 75–84, ≥85).
Primary Exposure
The primary exposure was the number of gastroenterologists per 100,000 population obtained from the Health Resources and Services Administration, in years 2010, 2015, and 2019. The Health Resources and Services Administration is maintained by the United States Department of Health and Human Services, to provide annual Area Health Resources Files (data.hrsa.gov) with accurate data regarding supply, demand, distribution, and education of health workers.
Time-Varying Co-Variates
State-level characteristics which we a priori hypothesized could be associated with mortality were obtained from national registries. We modeled these characteristics as time-varying co-variates by year. For years with unavailable co-variate data, these were linked at the closest prior year of available data. From the US Census Bureau, we obtained the proportion of residents that were female, non-Hispanic Black, Hispanic, and the median household income for the overall population. From the Behavioral Risk Factor Surveillance System, we obtained the proportion of adults with diabetes, obesity, reporting any binge drinking in the past 30 days. From the Health Resources and Services Administration, we obtained the number of primary care physicians per 100,000 population, number of transplant surgeons per 100,000 population, the number of mental health providers per 100,000 population, and the proportion of residents living in rural areas. From the National Survey of Substance Abuse Treatment Services, we obtained the geographic density of substance use treatment centers treating alcohol use disorder per 100,000 population. The full variable list with years of data availability is detailed in Supplemental Table 1.
This study is a population-based, national observational cohort capturing all reported deaths via death certificate data in the CDC WONDER database in the United States from 2010–2019.
Data are expected to be 100% complete for all-cause mortality.
Statistical Analysis
State-level characteristics for the most recent year (2019) were summarized as means (standard deviations), weighted by the state population. The geographic density of gastroenterologist was mapped by state for the year 2019 (Stata grmap) and plotted as weighted means with standard deviations by year (years 2010, 2015, and 2019 when data were collected) using box and whisker plots. Linear regression was used to model annual state-level age-adjusted mortality rates (AAMR) as the dependent variable to evaluate the association between mortality and geographic density of gastroenterologists. To address clustering by state, Huber-White sandwich estimators were used, which are robust to heteroscedasticity and autocorrelation. Multivariable linear regression models were adjusted for state-level characteristics including the proportion of the state population in each 10-year age group (25–34, 35–44, 45–54, 55–64, 65–74, 75–84, ≥85), proportion female, proportion non-Hispanic Black race and Hispanic ethnicity, proportion with binge drinking in the last 30 days, median household income, proportion with obesity, proportion of rural residents, geographic density of primary care physicians, gastroenterologists, and substance use treatment centers treating alcohol use disorder, and year of death. Fully adjusted mortality rates and 95% confidence intervals were estimated from the multivariable model for given densities of gastroenterologists.
All models were weighted to the state population. The relationship between AAMR for ALD and geographic density of gastroenterologists was visualized using locally weighted regression (Stata lowess). Based on the resulting plot, gastroenterologist density was modeled as a piecewise linear function with a knot at 7.5 gastroenterologists per 100,000 to test for a change in slope at this point.
Exploratory Analyses
We performed several exploratory analyses to assess the robustness and explore potential reasons for our results:
To assess whether our results could be explained by access to liver transplantation, we repeated the multivariable analysis adding the state-level geographic density of transplant surgeons per 100,000 population to the model.
To assess whether our results could be explained by access to mental health specialists, we repeated the multivariable analysis adding the state-level geographic density of substance use, behavioral disorder, and mental health counselors per 100,000 population.
To assess whether gastroenterologist density may be a surrogate for access to subspecialty services and specialized healthcare resources rather than gastroenterology-specific services, we repeated the multivariable analysis substituting gastroenterologist density for state-level geographic density of ophthalmologists, and separately, dermatologists per 100,000 population.
To evaluate if our results were specific to ALD, we repeated the analysis using all cirrhosis and liver cancer-related mortality (ALD, non-ALD, hepatocellular carcinoma, using ICD-10 codes previously used by Tapper et al2: K70.3, K74.5, K74.6).
To evaluate if our results could be related to increased access to direct anti-viral therapy (DAA) for hepatitis C, given the frequency of co-morbid hepatitis C among ALD, we repeated the multivariable analysis restricted to 2010–2013 (pre-DAA) vs. 2014–2019 (post-DAA) eras.
To evaluate if our results could be confounded by differences in state-level alcohol taxation, we repeated the multivariable analysis adjusting for the weighted state tax per drink for all beverage types, as previously calculated by prior investigators.6 This analysis was restricted to the 32 states and Washington DC who did not have wholesale or retail monopolies on beverage types. The remaining 18 states have wholesale or retail monopolies on beverage types, in which tax data on wine and distilled spirits are generally not available.
P-values are from two-sided hypothesis tests where values less than 0.05 indicate statistical significance for the primary outcome. P-values for exploratory analyses are not presented due to the exploratory nature of the analyses, and confidence intervals are instead provided. Data analysis was conducted using SAS v9.4 (Cary, NC), Stata/MP 16.1 (College Station, TX).
The sample size was fixed and utilized the entirety of each state’s population, rather than a sample, therefore estimates reflect the whole population and sample size/power were not calculated as the study size has full national case ascertainment. Confidence intervals are provided to guide interpretation of results.
RESULTS
State-level Characteristics
The study included 50 states and the District of Columbia. The national mean geographic density of gastroenterologists was 4.6 per 100,000 population—2019 characteristics are summarized in Table 1. Between 2010–2019, the geographic density of gastroenterologists in the United States was fairly stable (Figure 1). There was variability in the state-level geographic density of gastroenterologists (lowest: Alaska [1.8 per 100,000 population]; highest: District of Columbia [10.1 per 100,000 population]) (Figure 2a).
Table 1.
State-Level Characteristics in 2019 *
| Characteristic | N=51 |
|---|---|
| Gastroenterologists per 100,000 Residents | 4.6±1.4 |
| Women, % | 51.6±0.7 |
| Non-Hispanic Black, % | 12.0±7.7 |
| Non-Hispanic White, % | 64.3±14.9 |
| Hispanic, % | 15.5±11.7 |
| Asian, % | 6.1±5.2 |
| Native American/Alaska Native, % | 0.7±1.3 |
| Lives in Rural County, % | 37.9±23.4 |
| In Poverty, % | 12.3±2.0 |
| Household Income | $67,072±10,347 |
| Primary Care Physicians per 100,000 Residents | 30.4±7.3 |
| Hospital Beds per 1,000 Residents | 2.8±0.6 |
| Substance Use Treatment Centers Offering Treatment for Alcohol Use Disorder per 100,000 Residents | 5.2±2.0 |
| Transplant Surgeons per 100,000 Residents | 0.08±0.05 |
| Ophthalmologists per 100,000 Residents | 5.8±1.4 |
| Dermatologists per 100,000 Residents | 4.0±1.1 |
| Underlying Medical Conditions | |
| Diabetes, % | 11.0±1.5 |
| Obese, % | 31.3±3.9 |
| Current Smoking, % | 15.3±3.4 |
| Any Alcohol in Past 30 Days, % | 53.0±5.4 |
| Binge Alcohol in Past 30 Days, % | 16.7±2.1 |
| Self-Reported Good or Better Health, % | 81.4±2.5 |
| Ever Myocardial Infarction or Coronary Heart Disease, % | 6.3±1.2 |
| Ever Stroke, % | 3.4±0.7 |
| Asthma, % | 9.0±1.3 |
Means (standard deviations) and percentages are weighted by the state population aged ≥25 years. Percentages represent the mean percentage of the state-level population with the given characteristic. ± denotes standard deviation.
Figure 1. Mean Geographic Density of Gastroenterologists in the United States in 2010, 2015 and 2019.
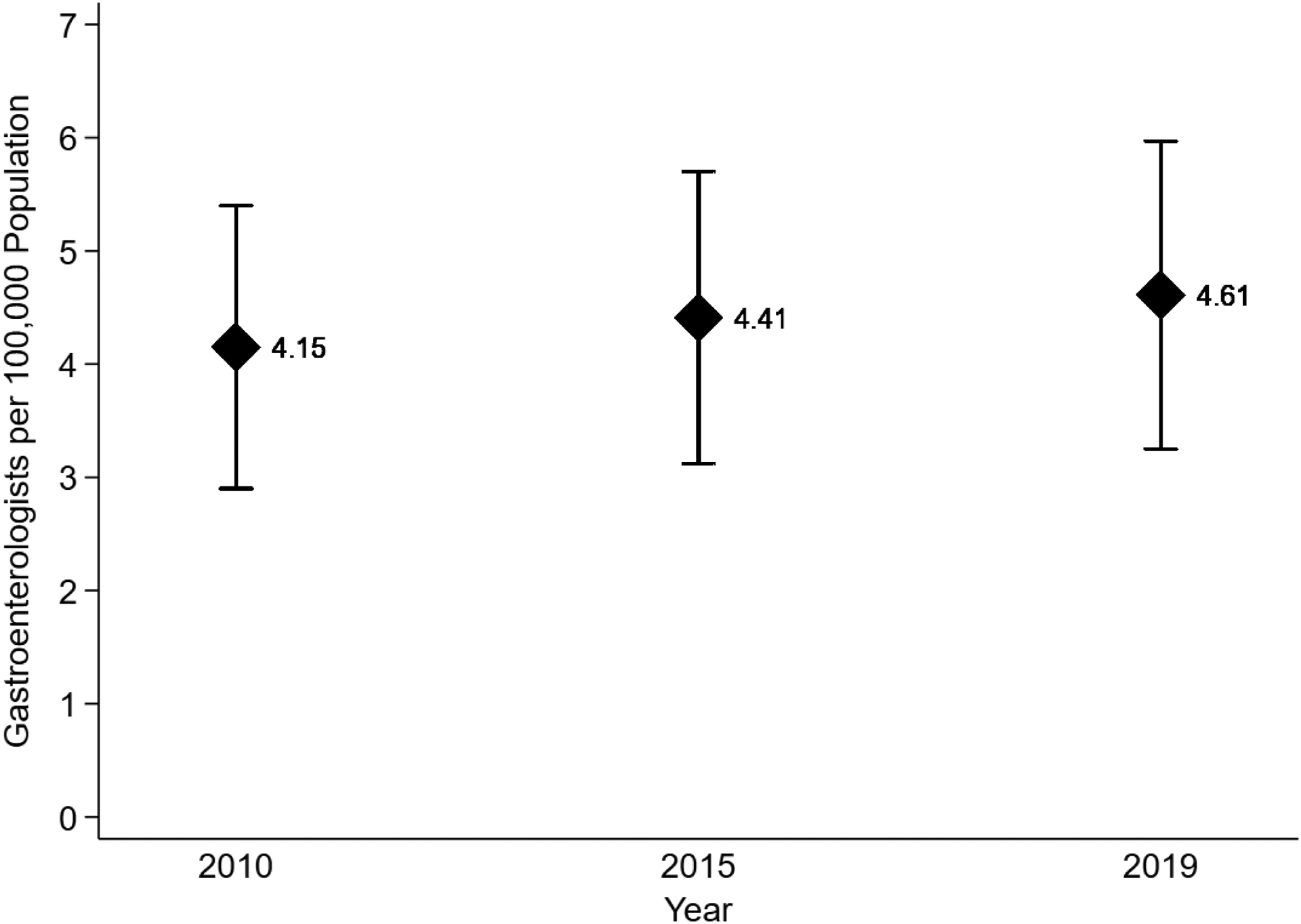
This figure represents the weighted mean density of gastroenterologists (diamond) with standard deviation (error bars) in the United States in 2010, 2015 and 2019.
Figure 2A. State-Level Geographic Density of Gastroenterologists in 2019.
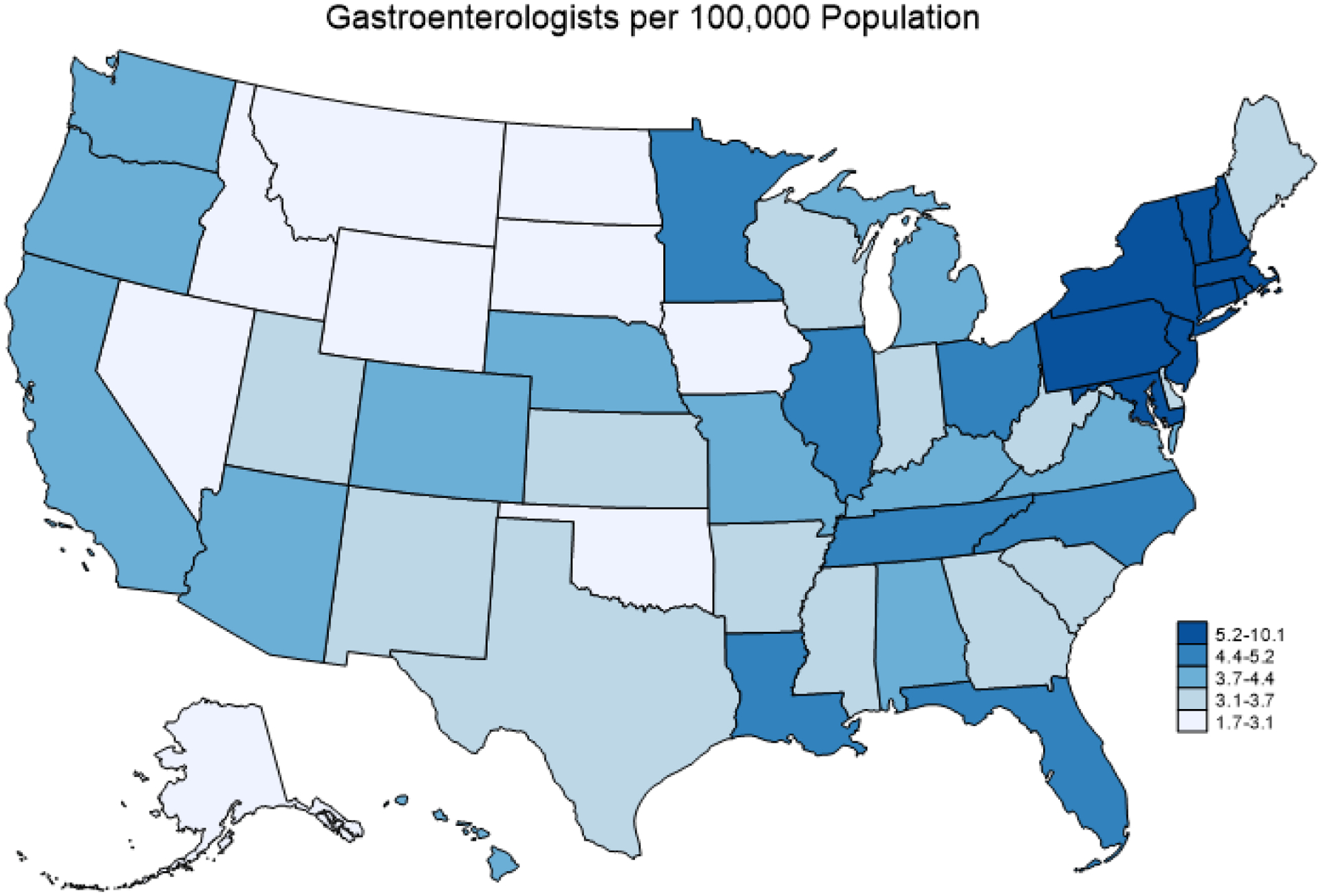
This map represents the geographic density of gastroenterologists by state in 2019, with darker shades representing higher geographic density. There was variability in the state-level geographic density of gastroenterologists (lowest: Alaska [1.8 per 100,000 population]; highest: District of Columbia [10.1 per 100,000 population]).
Mortality from Alcohol-Associated Liver Disease
The national mean annual ALD-related mortality rate was 85.6 per 1,000,000 population during the study period. There was variability among states by ALD-related mortality (lowest: New Jersey, Maryland, Hawaii [52 per 1,000,000 population]; highest: Wyoming [289 per 1,000,000 population]) (Figure 2b). In multivariable analysis, the geographic density of gastroenterologists was significantly associated with a lower ALD-related mortality (9.0 [95%CI 1.3–16.7] fewer ALD-related deaths per 1,000,000 population for each additional gastroenterologist per 100,000 population)—full multivariable model is outlined in Table 2. The rate of ALD-related mortality as associated with geographic density of gastroenterologists plateaued at approximately 7.5 gastroenterologists per 100,000 population (Figure 3). Using multivariable piecewise linear regression, we confirmed this plateau-effect, finding that the association of geographic density of gastroenterologists with ALD-related mortality was decreasing in a relatively linear relationship between 0–7.5 gastroenterologists per 100,000 population (10.7 [95%CI 3.4–18.1] fewer ALD-related deaths per 1,000,000 population for each additional gastroenterologist per 100,000 population) and flat with ≥7.5 gastroenterologists per 100,000 population (+9.8 [95%CI −4.7 to +24.3] ALD-related deaths per 1,000,000 population for each additional gastroenterologist per 100,000 population); the difference in slope between these two segments was statistically significant (p=0.006). We estimated the fully-adjusted ALD-related mortality to be 91.1 (95%CI 84.4–97.8) per 1,000,000 population for a state with 3.7 (lowest quartile) gastroenterologists per 100,000 population, as compared to 56.8 (95%CI 32.6–81.2) ALD-related deaths per 1,000,000 for a state with 7.5 gastroenterologists per 100,000 population.
Figure 2B. State-Level Age-Adjusted Alcohol-Associated Liver Disease Mortality in 2019.
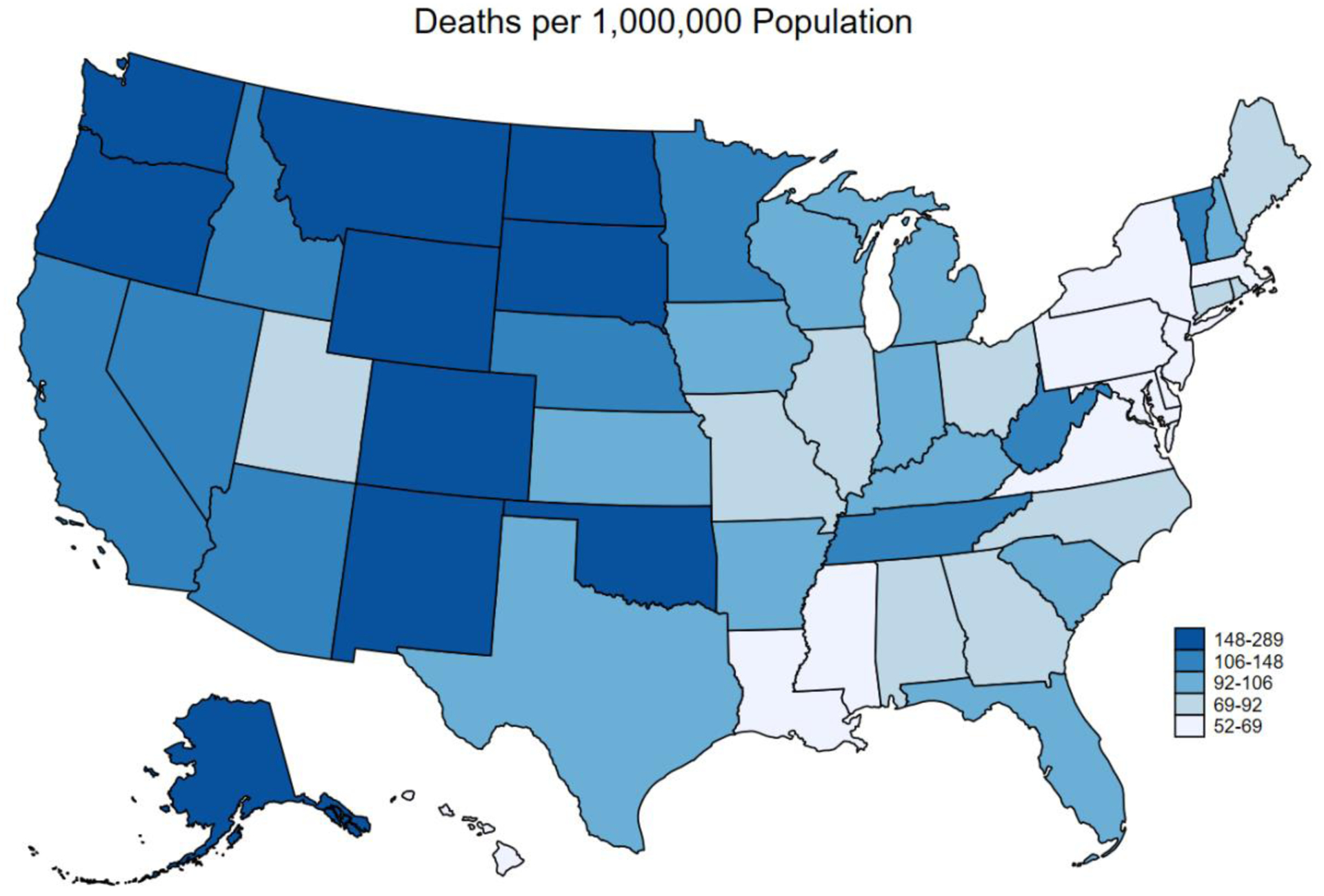
This map represents the age-adjusted alcohol-associated liver disease mortality by state in 2019, with darker shades representing higher mortality. There was variability in the state-level age-adjusted alcohol-associated liver disease mortality (lowest: New Jersey, Maryland, Hawaii [52 per 1,000,000 population]; highest: Wyoming [289 per 1,000,000 population]).
Table 2.
Univariable and Multivariable Analysis for Alcohol-Associated Liver Disease Mortality
| Univariable | Multivariable | |||
|---|---|---|---|---|
| State-Level Characteristic | Coefficienta (95% CI) | P | Coefficienta (95% CI) | P |
| Gastroenterologists per 100,000 population | −10.6 (−15.3 to −5.8) | <0.001 | −9.0 (−1.7 to −1.3) | 0.02 |
| Primary Care Physicians per 100,000 population | +1.8 (+0.7 to +2.9) | 0.002 | +1.2 (+0.03 to +2.0) | 0.01 |
| Substance Use Treatment | ||||
| >10 | +19.3 (−8.0 to +46.7) | 0.16 | −1.3 (−18.3 to +15.6) | 0.87 |
| Binge Drinking in last 30 daysb | −0.04 (−2.5 to +2.4) | 0.97 | −1.2 (−3.5 to +1.0) | 0.28 |
| Femaleb | −34.7 (−45.9 to −23.5) | <0.001 | −0.5 (−17.8 to +16.8) | 0.95 |
| Non-Hispanic Blackb | −2.6 (−3.8 to −1.5) | <0.001 | −1.0 (−2.1 to +0.07) | 0.07 |
| Hispanicb | +1.4 (+0.2 to +2.6) | 0.02 | +1.4 (+0.5 to +2.3) | 0.003 |
| Income (per $10,000) | +4.2 (−7.9 to +16.2) | 0.49 | −3.2 (−12.1 to +5.7) | 0.47 |
| Obesityb | −2.1 (−5.5 to +1.4) | 0.24 | −3.7 (−6.1 to −1.2) | 0.004 |
| Diabetesb | −2.9 (−7.6 to +1.8) | 0.22 | +2.8 (−2.4 to +8.0) | 0.28 |
| Living in Rural Areab | −0.3 (−0.8 to +0.3) | 0.30 | −0.2 (−0.6 to +0.2) | 0.32 |
| ≥85 | −5.6 (−43.1 to +32.0) | 0.77 | −19.4 (−59.4 to +20.6) | 0.34 |
| 2019 | +25.9 (+20.8 to +30.9) | <0.001 | −24.8 (−74.2 to +24.7) | 0.32 |
Coefficients, 95% confidence intervals (CI), and p-values in this table are derived from linear regression. Coefficients reflect the mean difference in age-adjusted mortality rates for alcohol-associated liver disease per 1,000,000 population.
Per % increase in state-level proportion
Figure 3. Age-Adjusted Alcohol-Associated Liver Disease Mortality Rate vs. Geographic Density of Gastroenterologists.
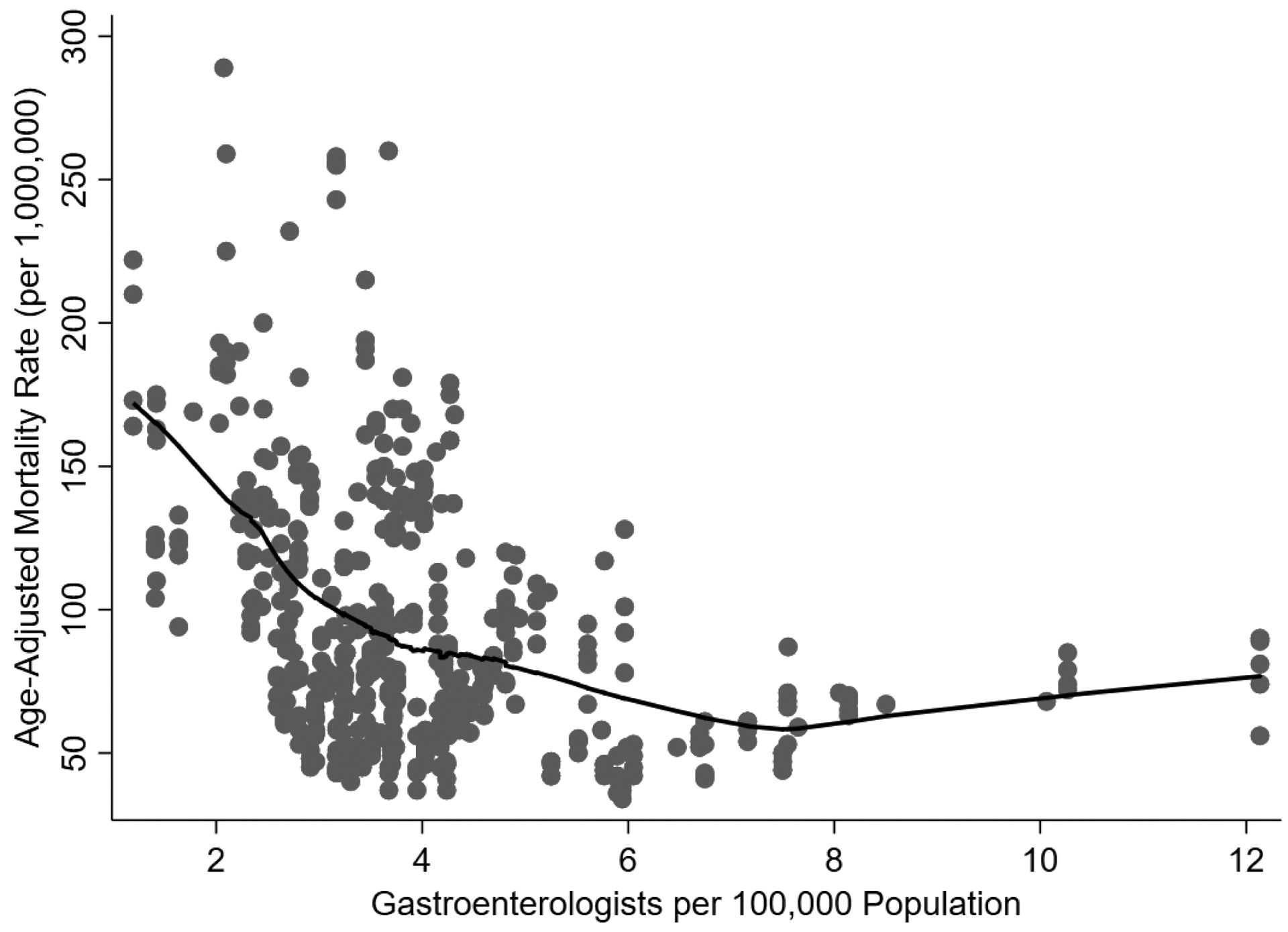
This figure represents the age-adjusted mortality rate for alcohol-associated liver disease (y-axis) as compared to the geographic density of gastroenterologists (x-axis). Each dot represents a unique combination of state and year. The black line represents the fitted smooth curve using LOESS regression. The rate of ALD-related mortality as associated with geographic density of gastroenterologists plateaued at approximately 7.5 gastroenterologists per 100,000 population.
Exploratory Analyses
In the exploratory analysis including transplant surgeon density, the association of state-level geographic density of gastroenterologists with ALD-related mortality was similar to the primary results: 8.4 (95%CI 0.04–16.9) fewer ALD-related deaths per 1,000,000 population for each additional gastroenterologist per 100,000 population (Supplemental Table 2). In the exploratory analysis including state-level geographic density of substance use, behavioral disorder, and mental health counselors, the association of state-level geographic density of gastroenterologists with ALD-related mortality was similar to the primary results: 9.5 (95%CI 1.3–17.8) fewer ALD-related deaths per 1,000,000 population for each additional gastroenterologist per 100,000 population (Supplemental Table 3). In the exploratory analyses assessing for potential confounding by generalized subspecialty effect, the state-level geographic density of ophthalmologists (−3.2 [95%CI −9.3 to +2.9]) (Supplemental Table 4) and state-level geographic density of dermatologists (−0.2 [95%CI −6.3 to +5.9]) (Supplemental Table 5) were not associated with ALD-related mortality. In the exploratory analysis assessing all cirrhosis and liver cancer-related mortality as the outcome, the geographic density of gastroenterologists was not significantly associated: −0.2 (95%CI −7.4 to +7.0) cirrhosis and liver-cancer related deaths per 1,000,000 population for each additional gastroenterologist per 100,000 population (Supplemental Table 6); when obesity and diabetes were removed from the model, the association was less attenuated: −4.9 (95%CI −14.1 to +4.3) cirrhosis and liver-cancer related deaths per 1,000,000 population for each gastroenterologist per 100,000 population (Supplemental Table 7); when obesity, diabetes, and income were removed from the model, the association was almost the same as the primary results: −7.3 (95%CI −15.3 to +0.7) cirrhosis and liver-cancer related deaths per 1,000,000 population for each gastroenterologist per 100,000 population (Supplemental Table 8). In the exploratory analysis assessing pre-DAA vs. post-DAA eras, the associations of geographic density of gastroenterologists with ALD-related mortality were similar (pre-DAA: −8.0 [95%CI −17.3 to +1.2]; post-DAA: −9.5 [95%CI −17.7 to −1.2] ALD-related deaths per 1,000,000 population for each additional gastroenterologist per 100,000 population (Supplemental Tables 9–10). In the exploratory analysis adjusting for state-level alcohol taxation, the association of geographic density of gastroenterologists with ALD-related mortality was similar (−7.6 [95%CI −15.6 to +4.7] ALD-related deaths per 1,000,000 population for each additional gastroenterologist per 100,000 population), and the weighted state-level tax per drink was not associated (+2.4 [95%CI −11.5 to +16.2] per dollar tax increase) with ALD-related mortality (Supplemental Table 11).
DISCUSSION
In this national cohort study, we show that the state-level geographic density of gastroenterologists is associated with lower ALD-related mortality. Remarkably, the absolute differential between the lowest quartile (3.7 gastroenterologists per 100,000 population) and the plateau (7.5 gastroenterologists per 100,000 population) of effect was almost 35 ALD-related deaths per 1,000,000 population, representing 40% of the national ALD-related mortality. These findings build on prior VA studies3,4 emphasizing the importance of an adequate gastroenterology workforce and access to subspecialty care to address increasing rates of ALD-related mortality throughout the United States. These findings also provide some quantification regarding a potential target ALD-threshold density of gastroenterologists (7.5 gastroenterologists per 100,000 population) and anticipated effects to ALD-related mortality should this threshold be achieved.
The American Gastroenterology Association (AGA) highlighted in “Looking at the Future of Gastroenterology” that the gastroenterologist workforce has not increased in conjunction with the increasing US population and burden of digestive diseases. In parallel, the American Associated for the Study of Liver Diseases (AASLD) Workforce Study Group highlighted a “critical need for more information”5 regarding workforce needs, and commissioned a recent modeling study7 predicting a critical shortage in gastroenterologists and hepatologists based on predicted future demand for liver disease care. Our results provide timely data to support these issues, and highlight the variability in gastroenterologist density and ALD-related mortality across states. We found that there was a greater than 5-fold difference both in geographic density of gastroenterologists and ALD-related mortality across states. In general, Mid-Atlantic states had the highest geographic density of gastroenterologists and lowest ALD-related mortality, whereas Mountain states had the lowest geographic density of gastroenterologists and highest ALD-related mortality. Recent studies show that medical providers are concentrated in urban (vs. rural) areas and in proximity to medical schools,8,9 but the geographic maldistribution of gastroenterologists/hepatologists remains understudied. Our study should encourage further research to better understand geographic inequities in gastroenterology/hepatology care, and interventions to ameliorate such inequities. Incentives to facilitate gastroenterology/hepatology training and increasing reimbursement for healthcare workers with low densities of gastroenterologists and high rates of ALD-related mortality are potential ideas to address the growing epidemic of ALD in the United States.
We performed a number of exploratory analyses to assess the mechanisms that might explain our results. First, neither the geographic density of ophthalmologists nor the density of dermatologists were associated with ALD-related mortality in our multivariable models, suggesting that the association between geographic density of gastroenterologists with ALD-related mortality is independent of any potential increased geographic density in generalized subspecialty care. Second, the addition of transplant surgeon density or substance use, behavioral health, and mental health counselors did not significantly alter our primary results, which seems to suggest that the association between state-level of gastroenterologists and ALD-related mortality is independent of any potential increased geographic density in transplant access and mental health counselors. These analyses strengthen the hypothesis that our findings are related to services provided by gastroenterologists, including deeper disease-specific fund of knowledge, greater adherence to best practices guidelines-based care, and provision of preventative liver-specific care such as variceal and liver cancer screening. Indeed, a recent study10 showed that gastroenterology/hepatology consultation was associated with improved quality metric performance, lower 30-day hospital readmissions, and mortality as compared to primary care. Our results add to growing evidence regarding the value in subspecialty care provided by gastroenterologists/hepatologists to address growing rates of liver-related mortality.
Next, the association of gastroenterologist density with all cirrhosis and liver-cancer mortality was examined. There was minimal association: −0.2 (95%CI −7.4 to +7.0) cirrhosis and liver-cancer related deaths per 1,000,000 population for each additional gastroenterologist per 100,000 population. We hypothesize that this is in part due to competing risks: while the most common cause of mortality in ALD is liver-disease, patients with non-ALD are more likely to die from cardiovascular or non-liver related malignancy causes, which would not be captured in this analysis. For example, a patient with non-alcoholic fatty liver disease who dies from a myocardial infarction will not be captured in WONDER as mortality caused by cirrhosis. Indeed, when obesity and diabetes are removed from the multivariable model with all cirrhosis and liver-cancer mortality as the outcome, the attenuation is less pronounced. How gastroenterology/hepatology care affects mortality from non-liver related causes in patients with liver disease was outside the scope of this study, and should be assessed in prospective studies with individual-level data. A second hypothesis can be drawn from prior data11 showing that among patients with cirrhosis, those with alcohol-associated cirrhosis have greater morbidity, hospitalization, and re-hospitalization rates. Thus, ALD (vs. non-ALD) may be associated with more acute decompensations and hospitalizations where interfacing with gastroenterology/hepatology care (i.e. in the hospital) has a greater influence on survival. Also, given that ALD-mortality is more acute and less centered on preventative care, the influence of income and access to outpatient care may be less pronounced. Indeed, when income, obesity and diabetes are removed from the model, the point estimate association is almost the same as ALD-related mortality: −7.3 (95% −15.3 to +0.7) cirrhosis and liver-cancer deaths per 1,000,000 population for each additional gastroenterologist per 100,000 population. These findings highlight that ALD outcomes and interventions to address ALD may be quite different from non-ALD etiologies of cirrhosis.
We found that geographic density of treatment centers for alcohol use disorder and density of substance use, behavioral disorder, and mental health counselors were not associated with ALD-related mortality. To our knowledge, these associations have not been previously assessed and was an unexpected negative result. However, these variables are not fully representative of resources for treatment of harmful alcohol use (e.g. does not include group therapies such as Alcoholics Anonymous) and conversely may include services that go beyond treatment for alcohol use disorder (e.g. illicit substances), and should thus be interpreted with caution. This finding should nonetheless encourage future research using more granular data to better understand the dynamics between the presence of resources to treat alcohol use disorder and ALD-related mortality. Indeed, there are cognitive and pharmacologic therapies with robust data to decrease heavy drinking and promote abstinence among alcohol use disorder, which would be anticipated to reduce ALD-related mortality.12 However, we hypothesize that the presence of such therapies does not necessarily translate to patients receiving such resources, and future research should examine whether specific barriers can be intervened upon to maximize the impact of resources available for individuals with harmful alcohol use. Given that mental health and transplant-related variables were not associated with ALD-related mortality, we hypothesize that gastroenterology/hepatology-related interventions that may be influencing ALD-related mortality include corticosteroid therapy for alcohol-associated hepatitis, treatment and surveillance for hepatocellular carcinoma, treatment and surveillance for esophageal and gastric varices, and management of hepatic encephalopathy and ascites.
Non-Hispanic Black race was associated with a modest trend (coef=−1.0, p=0.07) towards lower ALD mortality—while non-Hispanic Blacks more frequently have harmful alcohol use and greater fibrosis and risk of mortality at ALD diagnosis, they have lower overall prevalence of ALD (vs. non-Hispanic Whites and Hispanics).2,13,14 We thus hypothesize that this association is related to the fact that states with higher proportions of non-Hispanics Blacks conversely have higher proportions of other races with higher prevalence of ALD.
Our study had limitations. First, not all gastroenterologists actively practice hepatology, our study does not consider advanced-care providers (e.g. nurse practitioners), and there is not a hepatology-specific variable in federally-maintained databases. However, a recent AASLD-commissioned study7 shows that gastroenterologists who practice 50% or more hepatology comprise approximately 80% of the hepatology workforce, with the remaining 20% comprising hepatologists and advanced-care practitioners. Likewise, the contribution of gastroenterologists not practicing hepatology would likely bias our results towards the null, suggesting robustness of our primary findings. Nevertheless, given the growing contribution of liver disease to national all-cause morbidity and mortality rates, our study highlights that implementation of more liver-specific variables, including geographic density of hepatologists, within federally-maintained databases should be strongly encouraged. Second, the data are observational and aggregate, so causal inference is limited and residual confounding based on more granular (e.g. county- or individual-level) characteristics is possible. The geographic density of gastroenterologists, which is higher in urban centers, could be a surrogate for other factors related to ALD-related mortality, including greater access to medical care in general, but we would note that the association with ALD-related mortality was not observed with geographic density of ophthalmologists, dermatologists, primary care physicians, and our analyses did adjust for the proportion of rural residents. Future studies using more granular datasets should focus on issues related to access, patient demographics, and clinical characteristics affecting outcomes to maximize the impact of subspecialty care in patients with ALD. Third, as the primary outcome (ALD-related death) is derived from physician-completed death certificates, we cannot exclude the possibility that access to gastroenterology care is associated with the accuracy of ALD-related mortality classifications on death certificates. Finally, our primary outcome is based on death certificate data which relies on the primary cause of death, so misclassification is possible.
In conclusion, this study provides a national view regarding the state-level association of geographic density of gastroenterologists with ALD-related mortality. These findings add to data suggesting the critical need for an increased gastroenterology/hepatology workforce to address the growing public health burden of ALD.
Supplementary Material
WHAT YOU NEED TO KNOW.
Background
Alcohol-associated liver disease accounts for the majority of liver-related deaths and is rising in incidence. Research to inform interventions to address this public health crisis is critical.
Findings
This national study found that higher geographic density of gastroenterologists was associated with fewer alcohol-associated liver disease deaths. Differences in gastroenterologist density could represent 40% of national alcohol-associated liver disease deaths.
Implications for Patient Care
Increasing the number of gastroenterologists in geographic areas where workforce gaps exist may help to address the growing epidemic of alcohol-associated liver disease.
Acknowledgements:
Supported in part by the USC Research Center for Liver Diseases (P30DK048522).
Abbreviations
- AAMR
age-adjusted mortality rate
- ALD
alcohol-associated liver disease
- BRFSS
Behavioral Risk Factor Surveillance System
- HRSA
Health Resources and Services Administration
- NSSATS
National Survey of Substance Abuse Treatment Services
- US
United States
- VA
Veterans Affairs
- WONDER
Center for Disease Control and Prevention’s Wide-ranging Online Data for Epidemiologic Research
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have no potential conflicts of interest to disclose.
Contributor Information
Brian P. Lee, Division of Gastroenterology and Liver Diseases, University of Southern California, Los Angeles, CA, USA.
Jennifer L. Dodge, Division of Research Medicine and Preventive Medicine, University of Southern California, Los Angeles, CA, USA.
Norah A. Terrault, Division of Gastroenterology and Liver Diseases, University of Southern California, Los Angeles, CA, USA.
References
- 1.Singal AK, Bataller R, Ahn J, Kamath PS, Shah VH. ACG Clinical Guideline: Alcoholic Liver Disease. Am J Gastroenterol 2018;113:175–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tapper EB, Parikh ND. Mortality due to cirrhosis and liver cancer in the United States, 1999–2016: observational study. BMJ 2018;362:k2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mellinger JL, Moser S, Welsh DE, et al. Access to Subspecialty Care And Survival Among Patients With Liver Disease. Am J Gastroenterol 2016;111:838–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Serper M, Taddei TH, Mehta R, et al. Association of Provider Specialty and Multidisciplinary Care With Hepatocellular Carcinoma Treatment and Mortality. Gastroenterology 2017;152:1954–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russo MW, Koteish AA, Fuchs M, Reddy KG, Fix OK. Workforce in hepatology: Update and a critical need for more information. Hepatology 2017;65:336–40. [DOI] [PubMed] [Google Scholar]
- 6.Blanchette JG, Chaloupka FJ, Naimi TS. The Composition and Magnitude of Alcohol Taxes in States: Do They Cover Alcohol-Related Costs? J Stud Alcohol Drugs 2019;80:408–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Russo MW, Fix OK, Koteish AA, et al. Modeling the Hepatology Workforce in the United States: A Predicted Critical Shortage. Hepatology 2020;72:1444–54. [DOI] [PubMed] [Google Scholar]
- 8.Naylor KB, Tootoo J, Yakusheva O, Shipman SA, Bynum JPW, Davis MA. Geographic variation in spatial accessibility of U.S. healthcare providers. PLoS One 2019;14:e0215016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Machado SR, Jayawardana S, Mossialos E, Vaduganathan M. Physician Density by Specialty Type in Urban and Rural Counties in the US, 2010 to 2017. JAMA Netw Open 2021;4:e2033994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tapper EB, Hao S, Lin M, et al. The Quality and Outcomes of Care Provided to Patients with Cirrhosis by Advanced Practice Providers. Hepatology 2020;71:225–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mellinger JL, Shedden K, Winder GS, et al. The high burden of alcoholic cirrhosis in privately insured persons in the United States. Hepatology 2018;68:872–82. [DOI] [PubMed] [Google Scholar]
- 12.Rogal S, Youk A, Zhang H, et al. Impact of Alcohol Use Disorder Treatment on Clinical Outcomes Among Patients With Cirrhosis. Hepatology 2020;71:2080–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anstee QM, Seth D, Day CP. Genetic Factors That Affect Risk of Alcoholic and Nonalcoholic Fatty Liver Disease. Gastroenterology 2016;150:1728–44 e7. [DOI] [PubMed] [Google Scholar]
- 14.Grant BF, Chou SP, Saha TD, et al. Prevalence of 12-Month Alcohol Use, High-Risk Drinking, and DSM-IV Alcohol Use Disorder in the United States, 2001–2002 to 2012–2013: Results From the National Epidemiologic Survey on Alcohol and Related Conditions. JAMA Psychiatry 2017;74:911–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


