Abstract
Objective
Idiopathic hypersomnia is a debilitating sleep disorder characterized by excessive daytime sleepiness, sleep inertia, and prolonged sleep duration. The patient burden of idiopathic hypersomnia is poorly understood. The Real World Idiopathic Hypersomnia Outcomes Study (ARISE) evaluated symptoms and treatment effectiveness/satisfaction in participants with idiopathic hypersomnia.
Methods
ARISE was a United States–based virtual cross-sectional survey. Participants were adults 21–65 years of age with idiopathic hypersomnia recruited from social media, the Hypersomnia Foundation website, and a patient panel. Self-assessments included the Epworth Sleepiness Scale (ESS), Idiopathic Hypersomnia Severity Scale (IHSS), Treatment Satisfaction Questionnaire for Medication, version II (TSQM-vII), and additional treatment questions. Data were analyzed for all participants and for subgroups with/without long sleep time (LST; ≥11 hours in 24 hours).
Results
Of 75 participants enrolled, most were female (81.3%). The mean (SD) age was 34.1 (10.7) years and 49% had LST. Most participants took off-label prescription medications (89.3%) and/or used other measures (93.3%) to manage their symptoms. The mean (SD) ESS score was 14.5 (3.5) and the mean IHSS score was 35.2 (7.6). Treatment satisfaction was low (mean [SD] TSQM-vII score: overall, 61.9 [21.2]; with LST, 57.9 [21.4]; without LST, 66.7 [20.3]), primarily driven by dissatisfaction with treatment effectiveness. The most common classes of prescription medications used were stimulants (61.3%), wake-promoting agents (28.0%), and antidepressants (18.7%); non-prescription measures used to manage symptoms included caffeine (73.3%), planned naps (34.7%), and individual accommodations (32.0%).
Conclusion
Overall, participants with idiopathic hypersomnia, with or without LST, had substantial symptom burden despite most of the study population taking off-label medications and using nonprescription measures to manage symptoms.
Keywords: sleep disorder, sodium oxybate, wake-promoting agents, stimulants, antidepressants
Video abstract

Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Plain Language Summary
Idiopathic hypersomnia is a disabling neurologic sleep disorder with no known cause. People with this disorder are extremely sleepy during the day and often need to sleep for long amounts of time. They may sleep and nap over 11 hours in a normal day, yet still find it hard to wake up and think clearly. Many prescription treatments (mostly not FDA approved) and changes in habits have been tried to improve the symptoms. However, most patients with idiopathic hypersomnia still cannot function well in many areas of their lives. We used an online survey to better understand how idiopathic hypersomnia and its treatments affect people’s lives. The survey was completed by 75 adults with idiopathic hypersomnia. About half of them slept longer than 11 hours in a normal day. Most of them took a prescription medication and used other measures to help manage their idiopathic hypersomnia. However, most still had a large burden from their symptoms. Overall satisfaction with treatments was low. These findings more clearly define the patient burden of idiopathic hypersomnia. More effective therapies could address this symptom burden.
Introduction
Idiopathic hypersomnia is a debilitating neurologic sleep disorder characterized by excessive daytime sleepiness (EDS; the inability to stay awake and alert during the day, resulting in the irrepressible need to sleep or unplanned lapses into sleep or drowsiness), and by severe sleep inertia (prolonged difficulty waking with frequent reentries into sleep, confusion, and irritability), prolonged nighttime sleep, long and unrefreshing naps, and cognitive impairment.1,2 Idiopathic hypersomnia negatively affects many aspects of daily life. For example, people with idiopathic hypersomnia have a greater risk of driving accidents than healthy controls.3 They experience more anxiety and depression than those without idiopathic hypersomnia,4 and also score lower than the national norms on multiple domains regarding health-related quality of life.5 In addition, symptoms of autonomic dysfunction, such as headaches, temperature dysregulation, fainting, and heart palpitations, are reported by more people with idiopathic hypersomnia than by healthy controls.1,6,7 An early report on idiopathic hypersomnia estimated the prevalence as 2–5 cases per 100,000 persons,8 although a more recent study suggested a diagnosed prevalence of 10.3 cases per 100,000 persons.9 The true prevalence of idiopathic hypersomnia is not known.10 The procedures required for diagnosis are often not covered by insurance in the US,11 which could lead to a lack of diagnoses in people who have the disorder, as well as inaccurate diagnoses of idiopathic hypersomnia in people who may only have insufficient sleep syndrome.1
According to the International Classification of Sleep Disorders, 3rd Edition (ICSD-3), a diagnosis of idiopathic hypersomnia requires demonstration of hypersomnia on 24-hour polysomnography or 7-day wrist actigraphy with a sleep log or hypersomnolence on a multiple sleep latency test, after ruling out of other disorders.1 Either a 24-hour sleep time totaling ≥11 hours or a mean sleep latency of ≤8 minutes must be present for a diagnosis.1 Although the previous edition of the ICSD (version 2) separated idiopathic hypersomnia into 2 distinct disorders based on whether or not an individual had long sleep time (LST; ≥10 hours at nighttime),12 the ICSD-3 stated that evidence was lacking to distinguish idiopathic hypersomnia with LST as a separate disorder.1 Nevertheless, a recent claims analysis reported that at least two-thirds of patients with newly diagnosed idiopathic hypersomnia had the LST phenotype.10 Further, in a real-world study, the proportion of individuals with idiopathic hypersomnia who experienced sleep inertia and brain fog (being unable to think clearly or concentrate at any time throughout the day) was greater in those who slept for cutoffs of at least 10 or 11 hours per day compared with those who slept less.13
There was no approved treatment for idiopathic hypersomnia until August 2021, when the United States (US) Food and Drug Administration (FDA) approved calcium, magnesium, potassium, and sodium oxybates, or low-sodium oxybate (Xywav®), for idiopathic hypersomnia in adults.14–18 Rather, treatments similar to those used to treat narcolepsy typically have been prescribed off-label for idiopathic hypersomnia, including stimulants (eg, amphetamines, methylphenidate) and wake-promoting agents (eg, modafinil, armodafinil).19 The American Academy of Sleep Medicine (AASM) guidelines, published in September 2021, strongly recommend modafinil as a treatment (compared with no treatment) in adults with idiopathic hypersomnia, and conditionally recommend other medications (ie, clarithromycin, methylphenidate, pitolisant, and sodium oxybate).20 However, many patients with idiopathic hypersomnia continue to experience residual symptoms despite off-label pharmacotherapy.13
Information is limited on the burden of symptoms in patients with idiopathic hypersomnia, and little is known about the effectiveness of off-label treatments for this disorder. The Real World Idiopathic Hypersomnia Outcomes Study (ARISE) evaluated the impact of idiopathic hypersomnia on participants’ lives and their perspectives regarding their current treatment. The objectives of ARISE were to assess and characterize the severity of idiopathic hypersomnia symptoms, to evaluate participants’ perceptions regarding satisfaction and effectiveness of their current treatment regimen, and to examine how idiopathic hypersomnia impacts participants’ lives, including their emotional well-being, daily activities, cognition, productivity/employment, and relationships. This paper reports on symptom severity and treatment effectiveness/satisfaction, while the impact of idiopathic hypersomnia on quality of life is reported in a separate publication.
Methods
Participant Selection Criteria
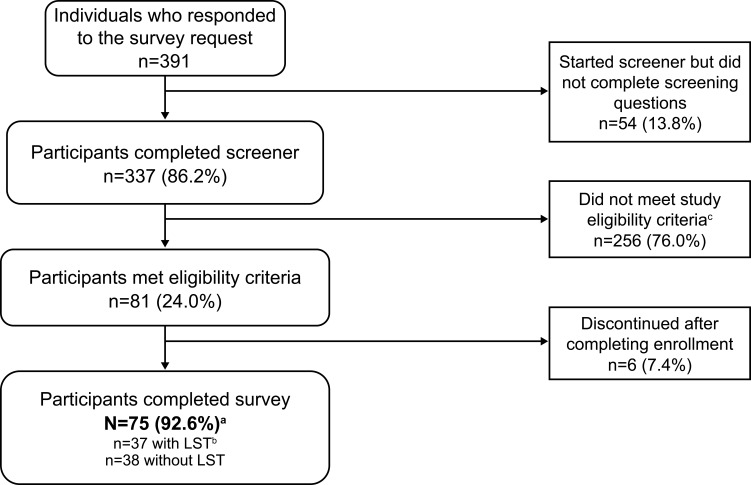
Individuals who were eligible to participate in ARISE were between 21 and 65 years of age and resided in the US. Inclusion criteria were a current diagnosis of idiopathic hypersomnia for at least 6 months, sleeping at least 7 hours in a typical night, no current diagnosis of narcolepsy or obstructive sleep apnea, and no prior episodes of cataplexy. This study used a similar methodology as in a recently published web-based idiopathic hypersomnia registry13 to validate the idiopathic hypersomnia diagnosis. Specifically, during screening, potential participants first were required to select that they have a current idiopathic hypersomnia diagnosis from a list of sleep disorders or related diagnoses. Then, later during screening, they had to select again from a list to confirm that they received a current diagnosis of the condition and that a doctor had informed them that it was the cause of their symptoms. Participants were required to select idiopathic hypersomnia both times to be eligible for inclusion in the study. Individuals who also had a diagnosis of obstructive sleep apnea were excluded in order for the study results to be due to idiopathic hypersomnia alone, given the high amount of symptom overlap with obstructive sleep apnea.1,11 Exclusion criteria were the inability to read or understand English and having no access to a computer or a mobile device to send and receive text messages. Participant recruitment channels included a patient panel, idiopathic hypersomnia–focused social media groups, and the Hypersomnia Foundation (HF) website (https://www.hypersomniafoundation.org/). Participants of this study were a convenience sample of volunteers who responded to the study invitation email. The participant selection process is indicated in Figure 1.
Figure 1.
Participant disposition.
Notes: aHypersomnia Foundation, n=59; inVibe, n=16. bLong sleep was defined as ≥11 hours of sleep in a 24-hour period (self-reported). cMost terminations during screening were because potential participants had obstructive sleep apnea (n=26), narcolepsy (n=12), no idiopathic hypersomnia (n=116), or slept <7 hours a night (n=47).
Abbreviation: LST, long sleep time.
Survey Design
ARISE was a US-based virtual, cross-sectional, internet-based survey using multiple patient-reported outcome measures consisting of validated questionnaires and additional questions related to idiopathic hypersomnia; therefore, all outcomes in this study are based on self-reported information. The survey took approximately 30 minutes to complete, and participants were financially compensated for their participation.
Assessments
Symptom severity was assessed using the Epworth Sleepiness Scale (ESS) and the Idiopathic Hypersomnia Severity Scale (IHSS). The ESS is a validated 8-item scale that is a self-reported measure of daytime sleepiness across a variety of activities.21,22 ESS scores range from 0 to 24, with scores >10 representing pathological sleepiness (eg, as in narcolepsy or idiopathic hypersomnia).21,22 The IHSS is a validated 14-item scale that measures hypersomnolence symptoms, consequences, and responsiveness to treatment in people with idiopathic hypersomnia.23,24 Total IHSS score ranges from 0 to 50, with higher scores indicating greater severity of symptoms. The mean (standard deviation [SD]) IHSS score for controls is 10.49 (6.36). A score of ≥22 has been used as a cutoff to distinguish untreated people with idiopathic hypersomnia from controls, and a score ≥26 has been used to distinguish severe to very severe symptoms from mild to moderate symptoms.23,24 Treatment satisfaction was assessed using the Treatment Satisfaction Questionnaire for Medication, version II (TSQM-vII), which contains 4 components measuring effectiveness, side effects, convenience, and overall satisfaction with treatment.25 The TSQM-vII is assessed on a scale of 0–100, with greater numbers indicating higher satisfaction; a poor appraisal of health was previously found to correspond to mean scores of 64.8 (global satisfaction), 63.3 (effectiveness), 75.8 (side effects), and 83.3 (convenience).26 Additional quantitative questions covered current idiopathic hypersomnia medications (dose taken and time on current medication), other nonprescription medication measures used to manage idiopathic hypersomnia, the most difficult to treat idiopathic hypersomnia symptom, and hours of sleep during a typical day and night.
Compliance and Ethical Approval
All participants signed an electronic informed consent prior to participating in the survey. The study protocol, informed consent forms, and recruitment materials were approved by the WCG institutional review board (tracking number: 20210849) prior to any participants enrolling in the study. This study was conducted in accordance with the Declaration of Helsinki.
Statistical Analyses
Data were collected and processed by inVibe (Costa Mesa, CA). Quality control of data was performed by inVibe, Stratevi (Santa Monica, CA), and ICON plc (Dublin, Ireland). Continuous variables were summarized with descriptive statistics (n, mean, SD, median, first and third quartiles, minimum, and maximum). Frequency counts and percentage of participants within each category were provided for categorical data. Participants were categorized into subgroups of those with or without self-reported LST (≥11 hours of sleep in a 24-hour period).
Results
Study Population
The survey was conducted from March 11 to April 5, 2021. Of the 391 individuals who responded to the survey invitation, 256 did not meet the eligibility criteria (including 116 who did not have idiopathic hypersomnia) and were excluded from participation (Figure 1). Seventy-five participants completed the survey. The mean (SD) age was 34.1 (10.7) years and the majority of participants (81.3%) were female (Table 1). Most participants had been diagnosed with idiopathic hypersomnia 2 or more years previously. The mean (SD) self-reported 24-hour sleep duration was 11.6 (3.4) hours. Nearly half of all participants reported having a psychiatric comorbidity, most commonly anxiety. Thirty-seven participants (49.3%) reported having LST. Participants with LST had a mean (SD) self-reported 24-hour sleep duration of 14.3 (2.7) hours and those without LST slept 8.9 (1.1) hours.
Table 1.
Demographics and Patient Characteristics
| All Participants N=75 |
Participants With LST n=37a |
Participants Without LST n=38a |
|
|---|---|---|---|
| Age, years, mean (SD) | 34.1 (10.7) | 33.7 (10.7) | 34.4 (10.9) |
| Female, n (%) | 61 (81.3) | 27 (73.0) | 34 (89.5) |
| Time since idiopathic hypersomnia diagnosis, years, n (%) | |||
| <2 | 12 (16.0) | 5 (13.5) | 7 (18.4) |
| 2–4 | 34 (45.3) | 20 (54.1) | 14 (36.8) |
| 5–9 | 18 (24.0) | 8 (21.6) | 10 (26.3) |
| ≥10 | 11 (14.7) | 4 (10.8) | 7 (18.4) |
| Patient-reported sleep duration, mean (SD) | |||
| Approximate hours of sleep in a typical 24-hour period | 11.6 (3.4) | 14.3 (2.7) | 8.9 (1.1) |
| Approximate hours of sleep in a typical night | 8.9 (1.6) | 9.8 (1.7) | 8.0 (0.8) |
| Approximate hours of sleep in a typical day | 2.7 (2.8) | 4.5 (2.9) | 0.9 (1.0) |
| Current self-reported comorbidity (≥5%), n (%)b | |||
| Any psychiatric disorderc | 33 (44.0) | 16 (43.2) | 17 (44.7) |
| Anxiety | 26 (34.7) | 13 (35.1) | 13 (34.2) |
| Major depressive disorder | 9 (12.0) | 2 (5.4) | 7 (18.4) |
| Bipolar disorder or any psychotic disorder | 6 (8.0) | 5 (13.5) | 1 (2.6) |
| Hypothyroidism | 6 (8.0) | 4 (10.8) | 2 (5.3) |
| PTSD | 5 (6.7) | 3 (8.1) | 2 (5.3) |
| Restless legs syndrome | 5 (6.7) | 3 (8.1) | 2 (5.3) |
| Other disorder that may be associated with excessive daytime sleepiness or fatigued | 5 (6.7) | 1 (2.7) | 4 (10.5) |
| Anxiety and PTSDe | 4 (5.3) | 3 (8.1) | 1 (2.6) |
Notes: aLong sleep was defined as ≥11 hours of sleep in a 24-hour period (self-reported). bA participant could have had ≥1 comorbidity. cIncludes anxiety, major depressive disorder, bipolar disorder, or any psychotic disorder. dIncludes 1 participant each who had depression; mild depression, possible attention-deficit disorder (but could be sleep disorder); persistent depressive disorder; small fiber neuropathy; and inflammatory bowel disease. eIncludes participants who had both separate diagnoses.
Abbreviations: LST, long sleep time; PTSD, post-traumatic stress disorder; SD, standard deviation.
Symptoms of Idiopathic Hypersomnia
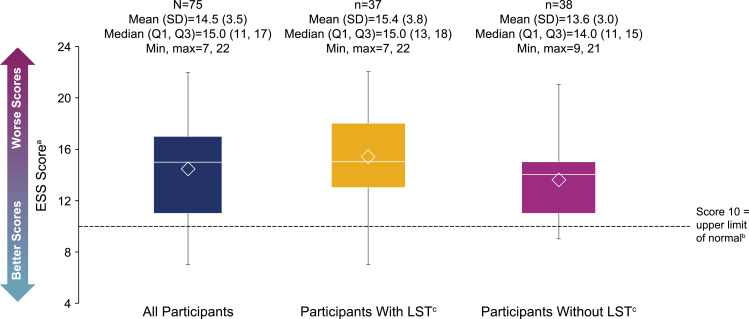
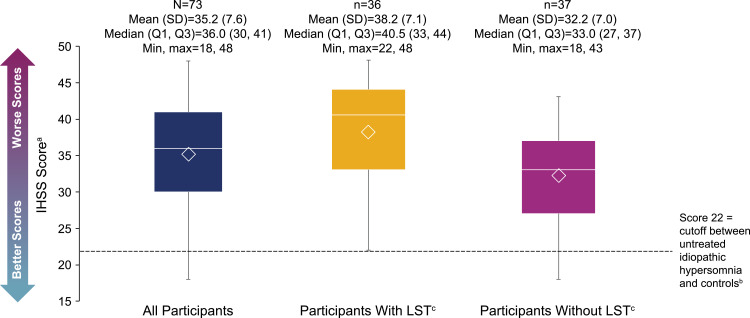
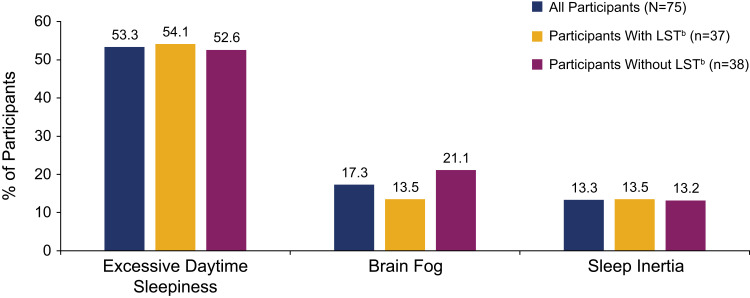
The mean (SD) ESS score was 14.5 (3.5). For participants with and without LST, these scores were 15.4 (3.8) and 13.6 (3.0), respectively (Figure 2). Most participants (88.0%) scored >10, indicating pathological sleepiness; this was the case for participants with LST (89.2%) or without LST (86.8%). The mean (SD) IHSS score was in the severe range24 at 35.2 (7.6) for all participants, 38.2 (7.1) for participants with LST, and 32.2 (7.0) for those without LST (Figure 3). All participants with LST, and 91.9% of participants without LST, had IHSS scores ≥22 (comparable to untreated patients with idiopathic hypersomnia23). The most difficult to treat symptoms reported by participants overall were EDS (53.3%), brain fog (17.3%), and sleep inertia (13.3%) (Figure 4).
Figure 2.
ESS scores. The bottom and top edges of the box indicate Q1 and Q3, the line inside the box is the median, and the marker inside the box is the mean. The whiskers extending from the box indicate the minimum and maximum values.
Notes: aThe range of possible scores is 0–24. bESS scores of >10 suggest pathologic sleepiness.22 cLong sleep was defined as ≥11 hours of sleep in a 24-hour period (self-reported).
Abbreviations: ESS, Epworth Sleepiness Scale; LST, long sleep time; Max, maximum; Min, minimum; Q1, first quartile; Q3, third quartile; SD, standard deviation.
Figure 3.
IHSS scores. The bottom and top edges of the box indicate Q1 and Q3, the line inside the box is the median, and the marker inside the box is the mean. The whiskers extending from the box indicate the minimum and maximum values.
Notes: aThe range of possible scores is 0–50. bIHSS scores of ≥22 are a cutoff separating untreated patients with idiopathic hypersomnia from controls.23 cLong sleep was defined as ≥11 hours of sleep in a 24-hour period (self-reported).
Abbreviations: IHSS, Idiopathic Hypersomnia Severity Scale; LST, long sleep time; Max, maximum; Min, minimum; Q1, first quartile; Q3, third quartile; SD, standard deviation.
Figure 4.
Most difficult to treat symptom of idiopathic hypersomniaa.
Notes: aOther most difficult to treat symptoms were long sleep (n=8), intentional nap (n=1), falling asleep during the day without meaning to (n=1), difficulty remembering things (n=1), and automatic behaviors (n=1). bLong sleep was defined as ≥11 hours of sleep in a 24-hour period (self-reported).
Abbreviation: LST, long sleep time.
Treatment Characteristics and Symptom Management
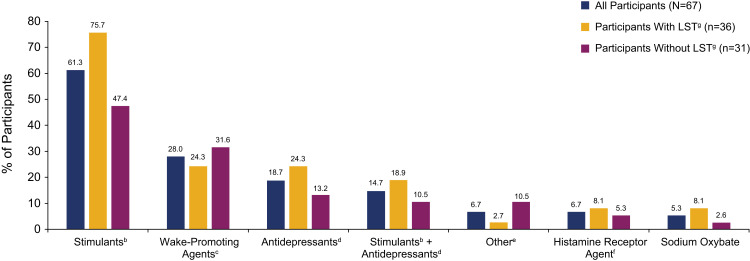
Most participants took off-label prescription medications (89.3%) and/or used other measures (93.3%) to manage idiopathic hypersomnia symptoms (Table 2). All but 1 participant with LST, and 81.6% of participants without LST, took prescription medications. Stimulants, wake-promoting agents, and antidepressants were the most common medications, taken by 61.3%, 28.0%, and 18.7% of participants, respectively (Figure 5). Stimulants and antidepressants were taken, respectively, by 75.7% and 24.3% of participants with LST, and 47.4% and 13.2% of participants without LST. Wake-promoting agents were taken by 24.3% and 31.6% of participants with and without LST, respectively. Most participants had been taking medication for idiopathic hypersomnia for ≥1 year (data not shown).
Table 2.
Off-Label Prescription Medications and Other Measures Taken to Manage Idiopathic Hypersomnia Symptoms
| All Participants N=75 | Participants With LST n=37a |
Participants Without LST n=38a |
|
|---|---|---|---|
| Off-label medications | |||
| Participants, n (%) | 67 (89.3) | 36 (97.3) | 31 (81.6) |
| Number of medication classes taken, mean (SD)b | 1.5 (0.9) | 1.6 (1.0) | 1.4 (0.7) |
| Min, Max | 1, 4 | 1, 4 | 1, 3 |
| Other measures used | |||
| Participants, n (%) | 70 (93.3) | 35 (94.6) | 35 (92.1) |
| Number of other measures used, mean (SD)c | 2.0 (1.1) | 2.2 (1.2) | 1.7 (1.0) |
| Min, Max | 0, 4 | 0, 4 | 0, 4 |
Notes: aLong sleep was defined as ≥11 hours of sleep in a 24-hour period (self-reported). bIncludes participants taking any medication. cIncludes all participants.
Abbreviations: LST, long sleep time; Max, maximum; Min, minimum; SD, standard deviation.
Figure 5.
Types of off-label prescription medications taken by participants to treat idiopathic hypersomniaa.
Notes: aA participant could have taken ≥1 medication; the 8 participants in the full study population who reported no medication use are not included in this analysis. bIncludes amphetamine and methylphenidate. cIncludes modafinil and armodafinil. dIncludes selective serotonin reuptake inhibitors, serotonin and norepinephrine reuptake inhibitors, tricyclic antidepressants, and monoamine oxidase inhibitors. eIncludes bupropion HCl, bupropion XL, flumazenil, and levothyroxine. fPitolisant. gLong sleep was defined as ≥11 hours of sleep in a 24-hour period (self-reported).
Abbreviation: LST, long sleep time.
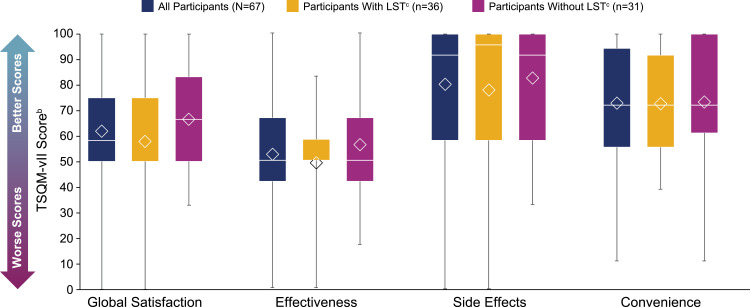
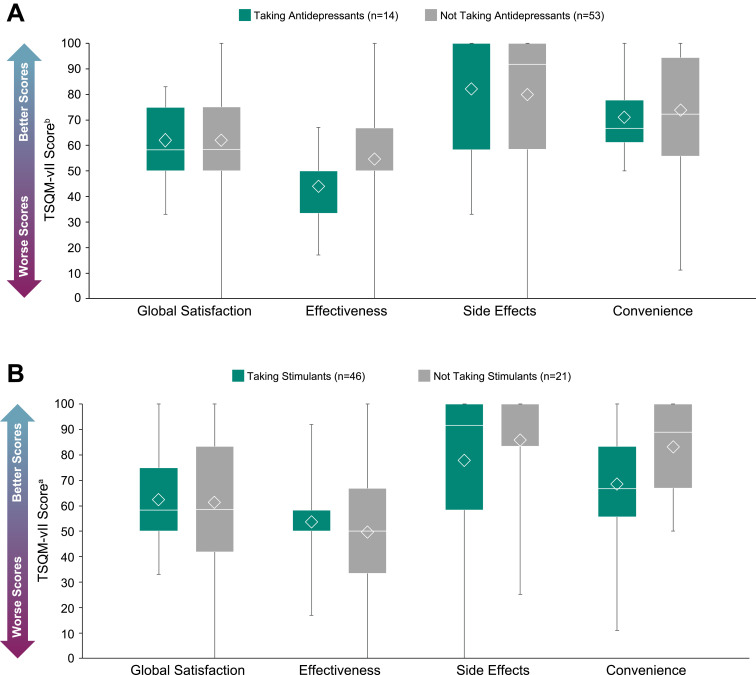
The mean (SD) global satisfaction score on the TSQM-vII was 61.9 (21.2) for participants taking prescription medications. For participants with and without LST, these scores were 57.9 (21.4) and 66.7 (20.3), respectively (Figure 6). Treatment effectiveness was scored the lowest out of all TSQM-vII components (mean [SD], 52.4 [18.3]). The mean (SD) treatment effectiveness scores for participants with and without LST were 49.1 (16.6) and 56.2 (19.7), respectively (Figure 6). TSQM-vII scores of participants who did or did not take antidepressants or stimulants are shown in Figure 7. The mean (SD) treatment effectiveness score for participants taking antidepressants (n=14) was 44.0 (12.8), and for those not taking antidepressants (n=53), this was 54.6 (19.0). The mean (SD) convenience rating for participants taking stimulants (n=46) was 68.6 (20.4), and for those not taking stimulants (n=21), this was 83.1 (18.9).
Figure 6.
TSQM-vII scoresa. The bottom and top edges of the box indicate Q1 and Q3, the line inside the box is the median, and the marker inside the box is the mean. The whiskers extending from the box indicate the minimum and maximum values.
Notes: aThe 8 participants in the full study population who reported no medication use are not included in this analysis. bScale of 0–100, with greater numbers indicating higher satisfaction; a poor appraisal of health was previously found to correspond to mean scores of 64.8 (global satisfaction), 63.3 (effectiveness), 75.8 (side effects), and 83.3 (convenience).26 cLong sleep was defined as ≥11 hours of sleep in a 24-hour period (self-reported).
Abbreviations: LST, long sleep time; Max, maximum; Min, minimum; Q1, first quartile; Q3, third quartile; SD, standard deviation; TSQM-vII, Treatment Satisfaction Questionnaire for Medication, version II.
Figure 7.
TSQM-vII scores in participantsa taking antidepressants (A) or stimulants (B) The bottom and top edges of the box indicate Q1 and Q3, the line inside the box is the median, and the marker inside the box is the mean. The whiskers extending from the box indicate the minimum and maximum values.
Notes:aThe 8 participants in the full study population who reported no medication use are not included in this analysis. bScale of 0–100, with greater numbers indicating higher satisfaction; a poor appraisal of health was previously found to correspond to mean scores of 64.8 (global satisfaction), 63.3 (effectiveness), 75.8 (side effects), and 83.3 (convenience).26
Abbreviations: Max, maximum; Min, minimum; Q1, first quartile; Q3, third quartile; SD, standard deviation; TSQM-vII, Treatment Satisfaction Questionnaire for Medication, version II.
In addition to prescription medications, 93.3% of participants used other measures to manage their idiopathic hypersomnia symptoms (Table 2); 62.7% of participants used multiple measures. The most common other measures used by participants overall were caffeine, planned naps, and individual accommodations (eg, additional time on testing and assignments, or delayed morning start time; Table 3).
Table 3.
Other Measures Used to Manage Idiopathic Hypersomnia Symptomsa
| Other Measures Used, n (%) | All Participants N=75 | Participants With LST n=37e | Participants Without LST n=38e |
|---|---|---|---|
| Caffeine | 55 (73.3) | 27 (73.0) | 28 (73.7) |
| Planned naps | 26 (34.7) | 17 (45.9) | 9 (23.7) |
| Individual accommodationsb | 24 (32.0) | 12 (32.4) | 12 (31.6) |
| Dietary changesc | 12 (16.0) | 8 (21.6) | 4 (10.5) |
| Cognitive behavioral therapy | 10 (13.3) | 7 (18.9) | 3 (7.9) |
| Melatonin | 11 (14.7) | 6 (16.2) | 5 (13.2) |
| None | 5 (6.7) | 2 (5.4) | 3 (7.9) |
| Otherd | 7 (9.3) | 2 (5.4) | 5 (13.2) |
Notes: aA participant could have used ≥1 other measure. bFor example, additional time on testing and assignments, delayed morning start time, excused absences related to medication holidays or prolonged sleep durations. cFor example, maintain a low-carbohydrate diet. dIncludes the following items: afternoon and evening naps whenever feasible; cardio exercise; consistent sleep schedule and regular physical exercise; exercise; I usually do not start work before 10:00 am; I wake up to take medication in order to actually get out of bed at a certain time; using a night and morning routine to prepare my body for sleep and wakefulness and keeping a steady sleep schedule. eLong sleep was defined as ≥11 hours of sleep in a 24-hour period (self-reported).
Abbreviation: LST, long sleep time.
Discussion
The ARISE study was conducted to provide real-world data on symptom severity, symptom management, and treatment satisfaction in participants with idiopathic hypersomnia. The demographic characteristics of the ARISE study population were generally consistent with other recent, larger studies of people with idiopathic hypersomnia (eg, mean age [years] in the mid-30s and mostly female).13,27 Approximately half of participants in ARISE had LST; this proportion is similar to what was observed in a registry study but higher than that seen in a randomized clinical trial.13,27
Most ARISE participants had ESS scores in the pathological range. The mean ESS score from the ARISE population (14.5; 89.3% treated with medications) was similar to that of patients with idiopathic hypersomnia at baseline in a randomized, controlled study (14.0–15.0).28 The ESS scores in ARISE were slightly higher than those from 2 observational studies. One study found mean ESS scores of 13.5 in an idiopathic hypersomnia population (medications included modafinil [25.6%], methylphenidate [16.3%], and antidepressants [16.3%]).29 The other study found a mean ESS score of 12.1 in an idiopathic hypersomnia population (medications were not reported for this group alone, but 79.6% of a combined idiopathic hypersomnia and narcolepsy group took modafinil).30 All ARISE participants had an IHSS score that was above the mean for controls without idiopathic hypersomnia (10.5), and most had a score in the “severe” range (≥26), established in prior studies.23 The mean IHSS score in ARISE participants (35.2) was slightly higher than that of a Phase 3 study population at baseline (32.1; 57% treated with medications),27 and those of treated or untreated patients with idiopathic hypersomnia in an IHSS validation study (26.1 and 31.0, respectively).24
The present findings demonstrate a substantial burden of idiopathic hypersomnia symptoms that is characterized not just by EDS (as assessed with the ESS), but also sleep inertia, cognitive difficulties, and unrefreshing sleep (as assessed with the IHSS). This burden was observed despite most patients being treated with the off-label medications that were available at the time of the study and also using other nonprescription measures to manage their idiopathic hypersomnia symptoms. This broad symptom burden was also reflected in a larger real-world study from the Hypersomnia Foundation (HF) in which brain fog, difficulty remembering things, and difficulty waking up and functioning with normal alertness were reported by approximately 72% to 83% of 468 participants with idiopathic hypersomnia, nearly all of whom also reported EDS.13 Even with treatment, more than half of those participants still experienced EDS (64.1%), trouble waking up and functioning with normal alertness (61.1%), and brain fog (54.0%). Consistent with this, ARISE participants identified EDS, brain fog, and sleep inertia as the symptoms that were the most difficult to treat. These symptoms of idiopathic hypersomnia have previously been demonstrated to have a negative impact on quality of life and functioning.5,30,31 This presents a challenge in treatment, as therapies that are effective at reducing EDS may not be sufficient in managing the burden of other symptoms in people with idiopathic hypersomnia.
Most ARISE participants (89.3%) reported taking off-label prescription medications for idiopathic hypersomnia symptoms, most commonly stimulants, followed by wake-promoting agents. These results are consistent with those from the HF registry study,13 implying that no better approved pharmacotherapy options had appeared in the interim between these 2 studies. Approximately 11% of participants in the present study were not currently taking any medications for idiopathic hypersomnia, in contrast to approximately 18% in the HF study.13 In that study, the majority of untreated participants had previously tried 1 or more medications without success.13 A retrospective study found that 42% of patients had tried at least 2 medications for idiopathic hypersomnia.32 However, neither of these studies directly assessed treatment satisfaction. In ARISE, treatment satisfaction was evaluated with the TSQM-vII, which demonstrated that participants with idiopathic hypersomnia had low global satisfaction with the treatments available to them at the time. ARISE participants reported a mean global satisfaction score of 61.9, which is lower than that reported by people in a TSQM validation study who rated their health as “poor” (64.8) or their illness as “severe” (67.3).26 Additional TSQM-vII results from ARISE indicated that the primary shortcoming of treatments was their effectiveness; tolerability and convenience appeared to have relatively minor roles in contributing to the poor global satisfaction. This is consistent with a previous survey of people with idiopathic hypersomnia, in which they rated medication effectiveness as 5.4 on a 10-point scale (with 1 being “not effective”).31
In addition to off-label prescription medications, more than 90% of participants in the present study also adopted measures other than prescription medications to manage their symptoms. The most common of these were caffeine, planned naps, and individual accommodations, such as sleeping longer and having a delayed morning start time. This is similar to another survey study, in which most people with idiopathic hypersomnia reported using caffeine (82.2%), scheduled nighttime sleep (75.2%), and daytime naps (81.4%) for symptom management.31 However, these symptom management options do not appear to be as effective as they are in people with narcolepsy; for example, intentional napping during the day is typically unrefreshing for people with idiopathic hypersomnia.1,19,31,33
Collectively, these results underscore that there was an unmet need for therapies with better efficacy. At the time of this study, there were no approved treatments for idiopathic hypersomnia. However, after the study completed, the US FDA approved the first treatment for adults with idiopathic hypersomnia (low-sodium oxybate).
Although the ARISE study was not designed to evaluate group differences between participants with or without LST, several other studies have reported on LST phenotype differences. In the HF study, a greater proportion of participants with LST reported having trouble waking and maintaining alertness (88.3%), experiencing brain fog (86.9%), and needing to use more than 1 alarm to wake up (77.5%), compared with those without LST (69.3%, 78.1%, and 61.7%, respectively).13 Two studies reported no difference in ESS scores based on LST phenotype.4,29 However, participants with LST (defined as sleeping >10 hours at night or >11 hours in a 24-hour period) had significantly worse scores on the Fatigue Severity Scale and the Sleep Inertia Questionnaire compared with participants without LST.29
The ARISE study has limitations. This study was entirely internet based, and as such, all data were self-reported. The most important variables potentially affected by this were the idiopathic hypersomnia diagnosis and the LST groupings. To increase confidence in the validity of the self-reported diagnosis, participants were asked to answer this question twice during screening, with different wording (a methodology used by a previous real-world study).13 The LST groupings were based on the participants’ self-reported assessments of hours slept, and may be less accurate than data measured in a sleep clinic. It is possible that the results would be different if objective measures of sleep duration had been used to group participants into the LST and non-LST categories. As ARISE was a real-world study, participants were not randomized to their treatments, and no causal attribution of reported differences according to treatment should be inferred. Individuals who participated in the study may have been motivated to do so because they were less happy with their symptom management, whereas those who had better symptom management may have not felt the need to participate. For participants who reported comorbidities, information on whether those comorbidities were sufficiently managed with treatments was not collected. Because the aim of the ARISE study was to gain real-world data on the experience of people with idiopathic hypersomnia, no healthy controls or individuals with other sleep disorders (eg, narcolepsy) were included. The sample size in ARISE (N=75) is smaller compared with another real-world study of people with idiopathic hypersomnia (N=468).13 Finally, the study population was entirely within the US; thus, some findings may not be applicable in other countries.
Conclusions
People with idiopathic hypersomnia in the ARISE study demonstrated a significant burden of disease, which warrants effective therapy. Many participants reported this high disease burden despite differences in symptom management with off-label medications and other means, and regardless of LST phenotype. The findings from this real-world study provide valuable insight into the unique characteristics and unmet treatment needs of people with idiopathic hypersomnia. With the US FDA approval of the first treatment indicated for adults with idiopathic hypersomnia, a more complete understanding of the symptom burden of idiopathic hypersomnia is important to accurately assess therapeutic impact. Additional studies should be conducted to further quantify these unmet needs in people with idiopathic hypersomnia. Available therapies with demonstrated efficacy over the symptoms of idiopathic hypersomnia may help to reduce this patient burden.
Acknowledgments
The authors thank the individuals who participated in this study. This study was supported by Jazz Pharmaceuticals. Under the direction of the authors, Emily C. Bruggeman, PhD of Peloton Advantage, LLC, an OPEN Health company, provided medical writing and editorial support for this manuscript, which was funded by Jazz Pharmaceuticals. Some of the findings from this study have been presented in posters at Psych Congress (2021), the 74th Annual Meeting of the American Academy of Neurology (2022), and the 36th Annual Meeting of the Associated Professional Sleep Societies (2022). An abstract was also published in Neurology. 2022;98 (18 Supplement).
Abbreviations
AASM, American Academy of Sleep Medicine; ARISE, Real World Idiopathic Hypersomnia Outcomes Study; EDS, excessive daytime sleepiness; ESS, Epworth Sleepiness Scale; FDA, Food and Drug Administration; HF, Hypersomnia Foundation; ICSD-3, International Classification of Sleep Disorders, 3rd Edition; IHSS, Idiopathic Hypersomnia Severity Scale; LST, long sleep time; TSQM-vII, Treatment Satisfaction Questionnaire for Medication, version II; US, United States.
Data Sharing Statement
All relevant data are provided within the article and supplemental material. Jazz has established a process to review requests from qualified external researchers for data from Jazz-sponsored clinical trials in a responsible manner that includes protecting patient privacy, assurance of data security and integrity, and furthering scientific and medical innovation. Additional details on Jazz Pharmaceuticals data sharing criteria and process for requesting access can be found at: https://www.jazzpharma.com/science/clinical-trial-data-sharing/.
Author Contributions
All authors have substantially revised or critically reviewed the article; have agreed on the journal to which the article will be submitted; reviewed and agreed on all versions of the article before submission, during revision, the final version accepted for publication, and any significant changes introduced at the proofing stage; and agreed to take responsibility and be accountable for the contents of the article. LD Schneider: Conceptualization of the study (equal); Methodology of the study (equal); Writing of the article – review and editing (equal). J Stevens: Conceptualization of the study (equal); Methodology of the study (equal); Project administration of the study (lead); Supervision of the study (lead); Writing of the article – review and editing (equal). AM Husain: Conceptualization of the study (equal); Methodology of the study (equal); Writing of the article – review and editing (equal). D Ito: Conceptualization of the study (equal); Methodology of the study (equal); Writing of the article – review and editing (equal). DS Fuller: Data curation of the study (lead); Formal analysis of the study results (lead); Methodology of the study (equal); Visualization of the study results (equal); Writing of the article – review and editing (equal). PC Zee: Writing of the article – review and editing (equal). W Macfadden: Conceptualization of the study (supporting); Methodology of the study (equal); Supervision of the study (supporting); Writing of the article – review and editing (equal).
Disclosure
LD Schneider is a compensated member of advisory boards and speakers bureaus for Jazz Pharmaceuticals, Eisai, and Harmony Biosciences. He is an employee of Alphabet, Inc. J Stevens, W Macfadden, and DS Fuller are full-time employees of Jazz Pharmaceuticals who, in the course of this employment, have received stock options exercisable for, and other stock awards of, ordinary shares of Jazz Pharmaceuticals, plc. AM Husain has received consultancy fees and/or research funding from Jazz Pharmaceuticals, UCB, BlackThorn, Sage, Eisai, Marinus, Pipeline Pharm, and Neurelis, as well as royalties from Springer, Demos Medical, and Wolters Kluwer, and holds an editorship role with Wolters Kluwer. He is a Data and Safety Monitoring Board (DSMB) member for Merck, Eisai and UCB Pharma. D Ito is an employee of Stratevi, a consulting firm that received research funding from Jazz Pharmaceuticals to conduct this study. PC Zee serves on scientific advisory boards for Jazz, Eisai, and Harmony Biosciences and, as a consultant for CVS Caremark, owns stock in Teva. The authors report no other conflicts of interest in this work.
References
- 1.American Academy of Sleep Medicine. Idiopathic hypersomnia. In: International Classification of Sleep Disorders. 3rd ed. Darien, IL: American Academy of Sleep Medicine; 2014. [Google Scholar]
- 2.Trotti LM. Idiopathic hypersomnia. Sleep Med Clin. 2017;12(3):331–344. doi: 10.1016/j.jsmc.2017.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pizza F, Jaussent I, Lopez R, et al. Car crashes and central disorders of hypersomnolence: a French study. PLoS One. 2015;10(6):e0129386. doi: 10.1371/journal.pone.0129386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vernet C, Arnulf I. Idiopathic hypersomnia with and without long sleep time: a controlled series of 75 patients. Sleep. 2009;32(6):753–759. doi: 10.1093/sleep/32.6.753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ozaki A, Inoue Y, Nakajima T, et al. Health-related quality of life among drug-naive patients with narcolepsy with cataplexy, narcolepsy without cataplexy, and idiopathic hypersomnia without long sleep time. J Clin Sleep Med. 2008;4(6):572–578. doi: 10.5664/jcsm.27352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vernet C, Leu-Semenescu S, Buzare MA, Arnulf I. Subjective symptoms in idiopathic hypersomnia: beyond excessive sleepiness. J Sleep Res. 2010;19(4):525–534. doi: 10.1111/j.1365-2869.2010.00824.x [DOI] [PubMed] [Google Scholar]
- 7.Miglis MG, Schneider L, Kim P, Cheung J, Trotti LM. Frequency and severity of autonomic symptoms in idiopathic hypersomnia. J Clin Sleep Med. 2020;16(5):749–756. doi: 10.5664/jcsm.8344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bassetti C, Aldrich MS. Idiopathic hypersomnia. A series of 42 patients. Brain. 1997;120(Pt 8):1423–1435. doi: 10.1093/brain/120.8.1423 [DOI] [PubMed] [Google Scholar]
- 9.Acquavella J, Mehra R, Bron M, Suomi JMH, Hess GP. Prevalence of narcolepsy, other sleep disorders, and diagnostic tests from 2013–2016: insured patients actively seeking care. J Clin Sleep Med. 2020;16(8):1255–1263. doi: 10.5664/jcsm.8482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saad R, Ben-Joseph R, Prince P, Stack C, Bujanover S, Taylor B. Utilization of diagnostic sleep testing prior to idiopathic hypersomnia diagnosis among US adults: a real-world claims analysis [poster 499]. Presented at: Annual Meeting of the Associated Professional Sleep Societies; June 10–13; 2021. [Google Scholar]
- 11.Dauvilliers Y, Bogan RK, Arnulf I, Scammell TE, St Louis EK, Thorpy MJ. Clinical considerations for the diagnosis of idiopathic hypersomnia. Sleep Med Rev. 2022;66:101709. doi: 10.1016/j.smrv.2022.101709 [DOI] [PubMed] [Google Scholar]
- 12.American Academy of Sleep Medicine. Idiopathic hypersomnia with long sleep time. In: International Classification of Sleep Disorders: Diagnostic and Coding Manual. 2nd ed. Westchester, IL: American Academy of Sleep Medicine; 2005:98–103. [Google Scholar]
- 13.Trotti LM, Ong JC, Plante DT, Friederich MC, King R, Bliwise DL. Disease symptomatology and response to treatment in people with idiopathic hypersomnia: initial data from the Hypersomnia Foundation registry. Sleep Med. 2020;75:343–349. doi: 10.1016/j.sleep.2020.08.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jazz Pharmaceuticals, Inc. Xywav® (Calcium, Magnesium, Potassium, and Sodium Oxybates) Oral Solution, CIII [Prescribing Information]. Palo Alto, CA: Jazz Pharmaceuticals, Inc.; 2022. [Google Scholar]
- 15.Jazz Pharmaceuticals announces U.S. FDA approval of Xywav® (calcium, magnesium, potassium, and sodium oxybates) oral solution for idiopathic hypersomnia in adults [press release]; 2021. Available from: http://investor.jazzpharma.com/news-releases/news-release-details/jazz-pharmaceuticals-announces-us-fda-approval-xywavr-calcium. Accessed October 4, 2021.
- 16.Szarfman A, Kuchenberg T, Soreth J, Lajmanovich S. Declaring the sodium content of drug products. N Engl J Med. 1995;333(19):1291. doi: 10.1056/NEJM199511093331917 [DOI] [PubMed] [Google Scholar]
- 17.US Food and Drug Administration, Center for Drug Evaluation and Research. Clinical review for Binosto, NDA 202344; 2012.. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2012/202344Orig1s000MedR.pdf. Accessed February 28, 2023
- 18.US Food and Drug Administration, Center for Drug Evaluation and Research. Quantitative labeling of sodium, potassium, and phosphorus for human over-the-counter and prescription drug products [guidance for industry]; 2022. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/quantitative-labeling-sodium-potassium-and-phosphorus-human-over-counter-and-prescription-drug. Accessed October 11, 2022
- 19.Billiard M, Sonka K. Idiopathic hypersomnia. Sleep Med Rev. 2016;29:23–33. doi: 10.1016/j.smrv.2015.08.007 [DOI] [PubMed] [Google Scholar]
- 20.Maski K, Trotti LM, Kotagal S, et al. Treatment of central disorders of hypersomnolence: an American Academy of Sleep Medicine clinical practice guideline. J Clin Sleep Med. 2021;17(9):1881–1893. doi: 10.5664/jcsm.9328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johns M, Hocking B. Daytime sleepiness and sleep habits of Australian workers. Sleep. 1997;20(10):844–849. doi: 10.1093/sleep/20.10.844 [DOI] [PubMed] [Google Scholar]
- 22.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540 [DOI] [PubMed] [Google Scholar]
- 23.Dauvilliers Y, Evangelista E, Barateau L, et al. Measurement of symptoms in idiopathic hypersomnia: the Idiopathic Hypersomnia Severity Scale. Neurology. 2019;92(15):e1754–e1762. doi: 10.1212/WNL.0000000000007264 [DOI] [PubMed] [Google Scholar]
- 24.Rassu AL, Evangelista E, Barateau L, et al. Idiopathic Hypersomnia Severity Scale to better quantify symptoms severity and their consequences in idiopathic hypersomnia. J Clin Sleep Med. 2022;18(2):617–629. doi: 10.5664/jcsm.9682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Atkinson MJ, Kumar R, Cappelleri JC, Hass SL. Hierarchical construct validity of the treatment satisfaction questionnaire for medication (TSQM version II) among outpatient pharmacy consumers. Value Health. 2005;8(suppl 1):S9–S24. doi: 10.1111/j.1524-4733.2005.00066.x [DOI] [PubMed] [Google Scholar]
- 26.Atkinson MJ, Sinha A, Hass SL, et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes. 2004;2:12. doi: 10.1186/1477-7525-2-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dauvilliers Y, Arnulf I, Foldvary-Schaefer N, et al. Safety and efficacy of lower-sodium oxybate in adults with idiopathic hypersomnia: a phase 3, placebo-controlled, double-blind, randomised withdrawal study. Lancet Neurol. 2022;21(1):53–65. doi: 10.1016/S1474-4422(21)00368-9 [DOI] [PubMed] [Google Scholar]
- 28.Mayer G, Benes H, Young P, Bitterlich M, Rodenbeck A. Modafinil in the treatment of idiopathic hypersomnia without long sleep time—a randomized, double-blind, placebo-controlled study. J Sleep Res. 2015;24(1):74–81. doi: 10.1111/jsr.12201 [DOI] [PubMed] [Google Scholar]
- 29.Nevsimalova S, Susta M, Prihodova I, Maurovich Horvat E, Milata M, Sonka K. Idiopathic hypersomnia: a homogeneous or heterogeneous disease? Sleep Med. 2021;80:86–91. doi: 10.1016/j.sleep.2021.01.031 [DOI] [PubMed] [Google Scholar]
- 30.Dauvilliers Y, Paquereau J, Bastuji H, Drouot X, Weil JS, Viot-Blanc V. Psychological health in central hypersomnias: the French Harmony study. J Neurol Neurosurg Psychiatry. 2009;80(6):636–641. doi: 10.1136/jnnp.2008.161588 [DOI] [PubMed] [Google Scholar]
- 31.Neikrug AB, Crawford MR, Ong JC. Behavioral sleep medicine services for hypersomnia disorders: a survey study. Behav Sleep Med. 2017;15(2):158–171. doi: 10.1080/15402002.2015.1120201 [DOI] [PubMed] [Google Scholar]
- 32.Ali M, Auger RR, Slocumb NL, Morgenthaler TI. Idiopathic hypersomnia: clinical features and response to treatment. J Clin Sleep Med. 2009;5(6):562–568. doi: 10.5664/jcsm.27658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schinkelshoek MS, Fronczek R, Lammers GJ. Update on the treatment of idiopathic hypersomnia. Curr Sleep Med Rep. 2019;5(4):207–214. doi: 10.1007/s40675-019-00158-7 [DOI] [Google Scholar]