Abstract
Introduction
In young adults, pairing a cognitive task with walking can have different effects on gait and cognitive task performance. In some cases, performance clearly declines whereas in others compensatory mechanisms maintain performance. This study investigates the preliminary finding of behavioral improvement in Go/NoGo response inhibition task performance during walking compared with sitting, which was observed at the piloting stage.
Materials and Methods
Mobile brain/body imaging (MoBI) was used to record electroencephalographic (EEG) activity, 3-dimensional (3D) gait kinematics and behavioral responses in the cognitive task, during sitting or walking on a treadmill.
Results
In a cohort of 26 young adults, 14 participants improved in measures of cognitive task performance while walking compared with sitting. These participants exhibited walking-related EEG amplitude reductions over frontal scalp regions during key stages of inhibitory control (conflict monitoring, control implementation, and pre-motor stages), accompanied by reduced stride-to-stride variability and faster responses to stimuli compared with those who did not improve. In contrast, 12 participants who did not improve exhibited no EEG amplitude differences across physical condition.
Discussion
The neural activity changes associated with performance improvement during dual tasking hold promise as cognitive flexibility markers that can potentially help assess cognitive decline in aging and neurodegeneration.
Keywords: event-related potentials, EEG, response inhibition, gait, error processing
Introduction
Performance of executive functions requires coordination across distributed neural networks for both routine and more complex cognitive processes (Zelazo et al. 1997; Alvarez and Emory 2006; Collette et al. 2006; Barbey et al. 2012). Yet, there are limits to the number and complexity of tasks that can be undertaken at the same time (Marois and Ivanoff 2005). As task demands increase, behavioral performance may begin to deteriorate, indicating a shift towards more effortful cognitive strategies (Andersson et al. 1998; Klingberg 1998). When engaging in 2 tasks simultaneously (termed dual-task performance), this change in cognitive strategy may manifest as concurrent activation of multiple brain regions (D'Esposito et al. 1995; Schubert and Szameitat 2003; Collette et al. 2005). Performing cognitive and motor tasks simultaneously sets the stage for competition for available neural resources leading to performance declines in both modalities. This is referred to as cognitive-motor interference (CMI; Abernethy 1988; Plummer et al. 2013; Leone et al. 2017; McIsaac et al. 2018). Pairing cognitive tasks with walking can elicit dual-task decline in gait performance and task-related behavior, as well as altered patterns of neural activation in older neurotypical adults (Dubost et al. 2006; Hollman et al. 2007; Priest et al. 2008; Herman et al. 2010; Holtzer et al. 2011; Mirelman et al. 2012; Beurskens et al. 2014; Malcolm et al. 2015; Malcolm et al. 2021; Protzak et al. 2021; Protzak and Gramann 2021) and in various patient populations (Cocchini et al. 2004; Lord et al. 2010; Doi et al. 2013; Stegemoller et al. 2014; Maidan et al. 2016; De Sanctis et al. 2020). However, in young adults, the manifestation of decrements in gait and cognitive task performance is not as clear, and as such, the neural activity changes detected in this group reflect interaction but not necessarily interference at a neural resource level. Some studies have reported evidence of no deterioration of response accuracy or increases in gait variability during dual-task (DT) walking in young adults (Beauchet et al. 2005; Hollman et al. 2007; De Sanctis et al. 2014; Mirelman et al. 2014; Larson et al. 2015; Beurskens et al. 2016; Shaw et al. 2018; Reiser et al. 2020; Protzak et al. 2021; Richardson et al. 2022). These studies suggest that young healthy adults adapt their gait and task-related behavior during DT walking and, consistent with this conclusion, report slower reaction times to stimuli (Beurskens et al. 2016; Shaw et al. 2018; Reiser et al. 2020; Protzak et al. 2021), changes in stride length (De Sanctis et al. 2014; Mirelman et al. 2014; Richardson et al. 2022), and reduced gait speed and velocity (Beauchet et al. 2005; Hollman et al. 2007; Mirelman et al. 2014). These findings indicate that young adults adopt a more deliberate approach to both task responses and walking in order to maintain task accuracy, and as such, point to strategy changes that will necessarily involve neural reconfigurations. On the other hand, there are studies that have reported reductions in response accuracy (Plummer et al. 2015; Ladouce et al. 2019) and increases in gait variability (Dubost et al. 2008; Priest et al. 2008; Pizzamiglio et al. 2017; Hoang et al. 2020) during DT walking in young adults. This discrepancy suggests that young adults can adopt different strategies when dual tasking, which might be driven by differences in the activation of compensatory mechanisms in response to the increased task demands. Examining the changes in dual-task-related neural activity can provide a deeper insight into the reallocation of neural resources underlying these different cognitive strategies and their associated compensatory adaptations.
Response inhibition, namely withholding a response to a thought, emotion, or stimulus, is one of the core executive functions and a vital component of everyday living. One often-used approach to studying response inhibition is the Go/NoGo task using a set of visual images as stimuli. The task requires pressing a response button after each novel image is presented (“Go” trial), but withholding the button press in response to the second presentation of a repeated image (“NoGo” trial; Menon et al. 2001; Roche et al. 2004; Rentrop et al. 2008; Smith et al. 2008; Steele et al. 2013; De Sanctis et al. 2014; Malcolm et al. 2015; De Sanctis et al. 2020; Francisco et al. 2020; Wakim et al. 2021). During successful NoGo trials during which the participant properly withholds a response, 2 stimulus-locked event-related potential (ERP) components are typically elicited: the N2 and the P3. The N2 is a negative voltage deflection that peaks ~200–350 ms (Bekker et al. 2005; Kato et al. 2009) post-stimulus-onset and has a frontocentral scalp distribution. This topographical distribution reflects its generation by the anterior cingulate cortex (ACC)—a brain region, which is key for monitoring inhibitory conflict (van Veen and Carter 2002a, 2002b; Bekker et al. 2005; Dias et al. 2006). The P3 is a positive voltage deflection that peaks ~350–600-ms post-stimulus-onset and has a broad distribution, extending from centroparietal to frontal areas (De Sanctis et al. 2014; Guo et al. 2018). During the P3 processing stage, both motor and cognitive components of inhibition are executed (Smith et al. 2008). In parallel to the motor inhibitory component of button press cancellation, higher inhibitory control is putatively exerted by lateral prefrontal areas, specifically the dorsolateral prefrontal cortex (DLPFC), to reduce inhibitory conflict in ensuing trials and hence improve cognitive task performance (Carter and van Veen 2007; Mansouri et al. 2009). During unsuccessful NoGo inhibition trials, an ERP component known as the response-locked event-related negativity (ERN) is elicited. The frontocentral ERN peaks ~50 ms after the erroneous motor response (Falkenstein et al. 2000; O'Connell et al. 2009a, 2009b), and reflects conflict monitoring in error trials and the source has been localized to the ACC (Mathalon et al. 2003; Carter and van Veen 2007; O'Connell et al. 2007).
The effects of walking on the N2 and P3 typically elicited during successful performance of the Go/NoGo response inhibition task has been investigated by previous studies (De Sanctis et al. 2014; Malcolm et al. 2015). De Sanctis et al. (2014) observed amplitude reductions of both the N2 and the P3 ERPs during successful inhibitions while walking, as well as an anteriorization of the P3 distribution suggesting recruitment of frontal cortical circuits. Furthermore, they found no significant differences between sitting and walking in terms of response accuracy and response speed, and no significant changes in stride-to-stride variability when comparing single-task (ST) and DT walking in young adults. In the absence of significant dual-task decrements, these findings were interpreted as a shift to a less automatic (N2 amplitude reduction) and more effortful (P3 frontalization) cognitive strategy during walking.
Combining walking with Go–NoGo response inhibition has been shown to elicit pronounced declines in response accuracy in older healthy adults (Malcolm et al. 2015) as well as in adults with neurological disorders such as multiple sclerosis (De Sanctis et al. 2020) and Parkinson’s disease (Sosnik et al. 2022). In a recent study, St George et al. (2022) found that the interference between gait and concurrent cognitive task performance was higher when the cognitive task required inhibition. Specifically, pairing an inhibition task with walking was linked to enhanced walking speed reduction in young adults, as well as pronounced response accuracy reduction in older adults.
Other studies focused on the correlation between neural activity and several behavioral measures in the context of a Go/NoGo response inhibition task. Falkenstein et al. (1999) found that young healthy individuals who had a high rate of unsuccessful inhibitions exhibited a smaller and later N2 compared to those with a low rate of unsuccessful inhibitions. Roche et al. (2005) showed that highly absentminded, young healthy individuals had larger and earlier N2 and P3 components in successful inhibition trials and larger error-related components in unsuccessful inhibition trials compared with less absentminded individuals of the same age group. Karamacoska et al. (2018) reported smaller P3 amplitudes in young healthy adults with increased response time (RT) variability, who were also found to commit more errors, compared to peers with low RT variability. In each case, different criteria were used to split the cohort into 2 subgroups, depending on the behavioral variable of interest: Certain studies performed a median split (Hester et al. 2004; Roche et al. 2005; Steele et al. 2013; Karamacoska et al. 2018), others leveraged the bimodality of the distribution of the behavioral variable and split the cohort based on the 2 modes (Falkenstein et al. 1999) and studies investigating impulsivity applied the splitting methodology proposed by Pailing and colleagues (Pailing et al. 2002; Ruchsow et al. 2008). In the context of collecting pilot data for the present study, data from 5 young healthy adults were collected. Analysis of these preliminary data showed that 3 of the pilot participants improved response accuracy during walking compared with sitting. Improvement in cognitive task performance with the addition of walking appeared to conflict with the CMI hypothesis. Response accuracy was measured using the d’ score (sensitivity index; Green and Swets 1966; Macmillan and Creelman 2005), since it is a bias-free measure that takes into account both the Go and the NoGo behavior (greater d’ score signifies better response accuracy). These preliminary data were then sequestered (see Supplemental Material). The working hypothesis that young individuals who improve performance during walking would differ in their ability to flexibly allocate neural resources for accomplishing both the motoric and cognitive tasks, and might also differ in the consistency of their gait compared to those who do not improve while walking, was tested in this report. Participants were divided into 2 subgroups based on the walking-minus-sitting d’ difference: (i) participants who exhibited a positive walking-minus-sitting d’ difference (cognitive task performance improved during walking compared with sitting—IMPs) and (ii) participants who exhibited a walking-minus-sitting d’ difference, which was either negative or not significantly different from zero (cognitive task performance did not improve during walking compared with sitting—nIMPs).
The current study examined successful NoGo inhibition trials during the N2 and P3 processing stages and unsuccessful NoGo inhibition trials during the ERN stage for walking-related amplitude changes in neurophysiological activity in the entire young adult cohort, and subsequently in the IMP and nIMP subgroups. Gait variability and response speed were additionally examined for dual-task changes in the same groups, to test whether improvement in response accuracy (IMPs) would be accompanied by trade-offs reflected in other physiological domains; for example whether IMPs would be more accurate but slower in their responses, or they would walk more variably. Identifying potential differences in the way IMPs alter their neural activity in response to dual-task load compared to nIMPs can shed light on the underlying neurocognitive mechanisms that drive their behavioral improvement.
Materials and methods
Participants
Twenty-six-young adults (18–30 years old; age = 22.35 ± 3.27 years; 13 female and 13 male; 23 right-handed and 3 left-handed) participated in the study. All participants provided written informed consent, reported no diagnosed neurological conditions, no recent head injuries, and normal or corrected-to-normal vision. The Institutional Review Board of the University of Rochester approved the experimental procedures (STUDY00001952). All procedures were compliant with the principles laid out in the Declaration of Helsinki for the responsible conduct of research. Participants were paid $15/h for time spent in the lab.
Experimental design
A Go/NoGo response inhibition cognitive task was employed. During each experimental block, images were presented in the central visual field for 67 ms with a fixed stimulus-onset-asynchrony of 1,017 ms. On average, images subtended 20° horizontally by 16° vertically. The task was coded using the Presentation software (version 20.1, Neurobehavioral Systems, Albany, CA, United States). Participants were instructed to press the button of a wireless game controller using their dominant hand as fast and accurately as possible if the presented image was different from the preceding image (“Go” trial). They were instructed to withhold pressing the button if the presented image was the same as the preceding image (“NoGo” trial; Fig. 1). Participants performed blocks of 240 trials in which 209 (87%) were Go trials and 31 (13%) were NoGo trials. NoGo trials were randomly distributed within each block.
Fig. 1.
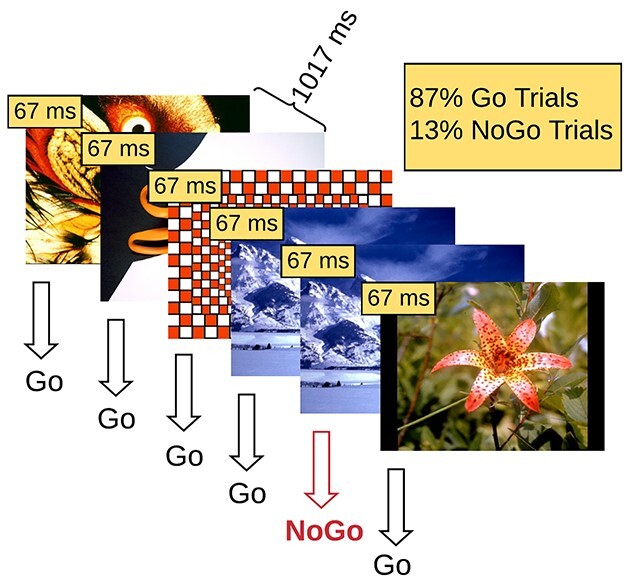
Illustration of the Go/NoGo response inhibition experimental design. Participants are instructed to respond on Go trials and withhold response on NoGo trials.
Four behavioral conditions of the cognitive task were defined: (i) correct rejections, defined as the NoGo trials on which participants correctly withheld their response, (ii) false alarms, defined as the NoGo trials on which participants incorrectly pressed the response button and (iii) hits, defined as the Go trials on which participants correctly pressed the response button, and (iv)misses, defined as the Go trials on which participants failed to press the response button. The behavioral conditions of interest were the ones that required response inhibition (regardless of the behavioral outcome), namely correct rejections and false alarms.
Experimental blocks were performed while the participants were either sitting or walking on a treadmill (Tuff Tread, Conroe, TX, United States), at a distance of 2.25-m approximately from the projection screen on which the images were projected (Barco F35 AS3D, 1,920 × 1,080 pxl). A safety harness was worn while walking to guard against falls (https://youtu.be/HS-5Qk5tvDE). An experimental session consisted of 16 blocks: 1 training block at the beginning, 7 sitting blocks, 7 walking blocks, and 1 ST walking block (walking on the treadmill without a cognitive task). The order of sitting and walking blocks was pseudorandomized; no >3 consecutive walking blocks occurred to prevent exhausting the participants. Participants were allowed to take short breaks between the blocks, each of which lasted 4 min. Most participants took at least 1 break during the experiment. If a break was requested, typically it did not last longer than 10 min. Participants were asked to select a treadmill speed corresponding to brisk walking for them, starting from the recommended speed of 4.8 km/h and increasing or decreasing as necessary. The vast majority of participants (22 out of 26) selected a speed of 4.8 km/h, whereas 4 participants selected lower speeds (3 participants walked at 4.2 km/h and 1 participant at 3.9 km/h). In general, the walking speeds selected corresponded to brisk walking (Piercy et al. 2018).
The pictures used for stimuli were drawn from the International Affective Picture System (IAPS) database (Bradley and Lang 2017). The IAPS database contains pictures of varied emotional valence and semantic content. Positive, neutral, and negative pictures were all used, however analyzing the emotional valence or semantic content of stimuli is beyond the scope of this study.
Electroencephalographic (EEG) data were recorded using a BioSemi Active Two System (BioSemi Inc., Amsterdam, the Netherlands) and a 64-electrode configuration following the International 10–20 system. Neural activity was digitized at 2,048 Hz. Full-body motion capture was recorded using a 16 camera OptiTrack system (Prime 41 cameras), and Motive software (OptiTrack, NaturalPoint, Inc., Corvallis, OR, United States) in a ~37-m2 space. Cameras recorded 41 markers on standard anatomical landmarks along the torso, the head and both arms, hands, legs, and feet at 360 frames per second. Stimulus triggers from Presentation (Neurobehavioral Systems Inc., Berkeley, CA, United States), behavioral responses from the game controller button, motion tracking data, and EEG data were time-synchronized using Lab Streaming Layer (LSL) software (Swartz Center for Computational Neuroscience, University of California, San Diego, CA, United States; available at: https://github.com/sccn/labstreaminglayer). Motion capture data were recorded using custom software written to rebroadcast the data from the Motive software to the LSL lab recorder. EEG data were recorded from available LSL streaming plugins for the BioSemi system. Behavioral event markers were recorded using the built-in LSL functionality in the Presentation software. The long-term test–retest reliability of the mobile brain-body imaging (MoBI) approach has been recently detailed (Malcolm et al. 2019). All behavioral, EEG and motion kinematic data processing and basic analyses were performed using custom MATLAB scripts (MathWorks Inc., Natick, MA, United States) and/or functions from EEGLAB (Delorme and Makeig 2004). Custom code from this study will be made available on GitHub (https://github.com/CNL-R) upon publication.
Cognitive task performance processing & analysis
The exact timing of each button press relative to stimulus-onset, the participant’s RTs, were recorded using the Response Manager functionality of Presentation and stored with precision of 1/10 ms. The Response Manager was set to accept responses only after 183-ms post-stimulus-onset within each experimental trial. Any responses prior to that were considered delayed responses to the previous trial and were ignored. This RT threshold was selected to filter out as many delayed-response trials as possible, without rejecting any valid trials for which the responses were merely fast (Hulsdunker et al. 2019).
Two behavioral conditions of the cognitive task were interrogated in this study, (i) correct rejections and (ii) false alarms. For both correct rejections and false alarms, only trials that were preceded by hits were kept, to ensure that the inhibitory component was present.
Two behavioral measures were calculated: (i) the d’ score (sensitivity index) and (ii) mean RT during (correct) Go trials, namely hits. D’ is a standardized score and it is computed as the difference between the Gaussian standard scores for the false alarm rate (percentage of unsuccessful NoGo trials) and the hit rate (HR; percentage of successful Go trials; Green and Swets 1966; Macmillan and Creelman 2005). D’ can provide a more meaningful measure of inhibitory performance than correct rejection rate (CRR; percentage of successful NoGo trials), since it removes the bias introduced by adopting different response strategies. For example, if accuracy was assessed using CRRs alone, participants who tended to adopt conservative response strategies, thereby exhibiting high CRRs but also high miss rates (percentage of unsuccessful Go trials), would be classified as achieving good inhibitory performance. However, this classification would not be accurate, since these participants would have shifted their response criterion to circumvent part of the inhibitory conflict by “sacrificing” performance in Go trials, and thus they would not actually engage their inhibitory cognitive resources to a degree sufficient for them to be classified as performing well in the inhibition task.
Gait kinematics processing & analysis
Heel markers on each foot were used to track gait kinematics. The 3 dimensions (3D) of movement were defined as follows: X is the dimension of lateral movement (right-and-left relative to the motion of the treadmill belt), Y is the dimension of vertical movement (up-and-down relative to the motion of the treadmill belt), and Z is the dimension of fore-aft movement (parallel to the motion of the treadmill belt). The heel marker motion in 3D is described by the 3 time series of the marker position over time in the X, Y, and Z dimension, respectively. Gait cycle was defined as the time interval between 2 consecutive heel strikes of the same foot. Heel strikes were identified as the local maxima of the Z position waveform over time. To ensure that no “phantom” heel strikes were captured, only peaks with a prominence >0.1 m were kept (“findpeaks” function in MATLAB, “minimum peak prominence” parameter was set to 0.1 m).
Stride-to-stride variability was quantified as the mean Euclidean distance between consecutive 3D gait cycle trajectories, using the dynamic time warping algorithm (DTW; Sakoe and Chiba 1978; Berndt and Clifford 1994). DTW is an algorithm for measuring the similarity between time series, and its efficacy in measuring 3D gait trajectory similarity is well-established (Boulgouris et al. 2004; Engelhard et al. 2016; Switonski et al. 2019). A more complete description of DTW can be found in the Supplementary Material.
Gait cycle trajectories with a kurtosis that exceeded 5 standard deviations of the mean were rejected as outliers. Also, before DTW computation, gait cycle trajectories were resampled to 100 samples. Since DTW essentially calculates the sum of the Euclidean distances between corresponding points of 2 interrogated trajectories, ensuring that all trajectories are resampled to the same length helps avoid bias in the algorithm computations.
The actual measure that was used to quantify each participant’s stride-to-stride variability is the mean across DTW distances occurring from all stride-to-stride comparisons. Right-foot and left-foot stride-to-stride DTW distances were pooled to calculate the mean DTW distance per participant.
EEG activity processing & analysis
EEG signals were first filtered using a zero-phase Chebyshev Type II filter (“filtfilt” function in MATLAB, passband ripple “Apass” = 1 dB, and stopband attenuation “Astop” = 65 dB) (Mazurek et al. 2020), and subsequently down-sampled from 2,048 to 512 Hz. Next, “bad” electrodes were detected based on kurtosis, probability, and spectrum of the recorded data, setting the threshold to 5 standard deviations of the mean, as well as covariance, with the threshold set to ±3 standard deviations of the mean (Mazurek et al. 2020). These “bad” electrodes were removed and interpolated based on neighboring electrodes, using spherical interpolation. All the electrodes were re-referenced offline to a common average reference.
Winkler et al. (2015) have shown that 1–2 Hz highpass filtered EEG data yield the optimal independent component analysis (ICA) decomposition results in terms of signal-to-noise ratio. In order to both achieve a high-quality ICA decomposition and retain as much low-frequency (< 1 Hz) neural activity as possible, after running Infomax ICA (“runica” function in EEGLAB, the number of retained principal components matched the rank of the EEG data) on 1–45 Hz bandpass-filtered data and obtaining the decomposition matrices (weight and sphere matrices), these matrices were transferred and applied to 45-Hz lowpass-filtered data. No highpass filtering was applied, since there is evidence indicating that the best way to avoid introducing artifacts into the ERP waveforms is either to use conservative high-pass filters (≤ 0.1 Hz) or to avoid high-pass filtering altogether (Tanner et al. 2015). ICs were labeled using the ICLabel algorithm (Pion-Tonachini et al. 2019). ICs whose sum of probabilities for the 5 artifactual IC classes (“Muscle,” “Eye,” “Heart,” “Line Noise,” and “Channel Noise”) was higher than 50% were labeled as artifactual and were thus rejected. The remaining ICs were back-projected to the sensor space.
Subsequently, the resulting neural activity was split into temporal epochs. For the correct rejection trials, epochs were locked to the stimulus-onset, beginning 200 ms before and extending until 800 ms after stimulus-onset of the trial. Correct rejection epochs were baseline-corrected relative to the pre-stimulus-onset interval from −100 to 0 ms. For the false alarm trials, epochs were locked to the response onset, beginning 500 ms before and extending until 500 ms after response onset of the trial. False alarm epochs were baselined-corrected relative to the pre-response interval from −400 to −300 ms. Epochs with a maximum voltage greater than ±150 μV or that exceeded 5 standard deviations of the mean in terms of kurtosis and probability were excluded from further analysis. Epochs that deviated from the mean by ±50 dB in the 0–2 Hz frequency window (eye movement detection) and by +25 or −100 dB in the 20–40 Hz frequency window (muscle activity detection) were rejected as well. For the sitting condition, on average 21% of the trials were rejected based on these criteria, whereas for the walking condition the respective percentage was 39%. ERPs were measured by averaging epochs for (2 motor task) × (2 cognitive task) conditions, namely 4 experimental conditions in total. The motor task conditions were (i) sitting and (ii) walking; and the cognitive task conditions were (i) correct rejections and (ii) response-locked false alarms.
Statistical analyses
Cognitive task performance
In the entire young adult cohort, d’ score differences between sitting and walking were assessed using a paired t-test (the walking-minus-sitting d’ score difference was subjected to a Shapiro–Wilk normality test (BenSaïda 2014) and the null hypothesis was not rejected). In addition, mean RTs during Go trials were tested for differences between sitting and walking using a paired t-test (the walking-minus-sitting mean RT difference was subjected to a Shapiro–Wilk normality test and the null hypothesis was not rejected).
Participants were subsequently classified on the basis of whether their d’ score during walking was significantly higher than when they were seated (their cognitive task performance improved (IMP) while walking), or whether they did not improve (nIMP) performance while walking (either because their d’ scores declined or were unchanged). The classification criterion was based on absolute walking-related changes (walking-minus-sitting) in d’ score, since relative changes (walking-minus-sitting divided by sitting), which is the most widely used alternative approach, have been shown to distort the magnitude of the measured effects, have less statistical power and follow highly skewed distributions thus violating the normality assumption (Vickers 2001; Berry and Ayers 2006; Hochman and McCormick 2011). Significant walking-minus-sitting d’ score difference was defined as the difference that lay outside of the 95% confidence interval of the normal distribution that had a mean value of zero and a standard deviation equal to that of the (d’walking–d’sitting) distribution of the entire cohort.
In the context of the split-group analysis, IMPs and nIMPs were compared in terms of average d’ score, i.e. 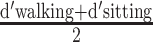 , using an independent samples t-test (the average d’ scores of IMPs and nIMPs were subjected to a Shapiro–Wilk normality test and the null hypothesis was not rejected for either group). In addition, mean RTs during Go trials were subjected to a 2 (Group: IMPs and nIMPs) × 2 (Motor Load: sitting and walking) analysis of variance (ANOVA) to test for response speed differences between IMPs and nIMPs, as well as for differences in how response speed was modulated by the addition of walking in these groups.
, using an independent samples t-test (the average d’ scores of IMPs and nIMPs were subjected to a Shapiro–Wilk normality test and the null hypothesis was not rejected for either group). In addition, mean RTs during Go trials were subjected to a 2 (Group: IMPs and nIMPs) × 2 (Motor Load: sitting and walking) analysis of variance (ANOVA) to test for response speed differences between IMPs and nIMPs, as well as for differences in how response speed was modulated by the addition of walking in these groups.
Gait kinematics
In the entire young adult cohort, mean DTW distance differences between ST walking and DT walking were assessed using a Wilcoxon signed rank test (the DT-minus-ST mean DTW distance difference was subjected to a Shapiro–Wilk normality test and the null hypothesis was rejected). One participant did not have ST walking recordings and was therefore excluded from this analysis.
In the context of the split-group analysis, mean DTW distance was subjected to a 2 (Group: IMPs and nIMPs) × 2 (cognitive load: ST walking and DT walking) ANOVA to test for stride-to-stride variability differences between IMPs and nIMPs, as well as for differences in how the addition of cognitive task performance modulated stride-to-stride variability in these groups. The participant who was excluded from the group-level analysis belonged to the IMP subgroup.
EEG activity
The EEG statistical analyses were performed using the FieldTrip toolbox (Oostenveld et al. 2011; http://fieldtriptoolbox.org). To compare ERP waveforms between sitting and walking, paired t-tests and cluster-based permutation tests were used (Pernet et al. 2015). As stated in the Introduction, significant differences between sitting and walking were hypothesized to be found in N2 ([200, 350] ms (Bekker et al. 2005; Kato et al. 2009)) and P3 ([350, 600] ms (De Sanctis et al. 2014; Guo et al. 2018)) latencies and topographies during correct rejection trials, and in ERN ([−50, 100] ms (Falkenstein et al. 2000; O'Connell et al. 2009b)) latencies and topographies during response-locked false alarm trials. Despite having formulated specific hypotheses about the latency and the topographies of the effects, the full set of 64 electrodes and all the epoch timepoints were included in the analyses, to explore potential effects that might have been overlooked by previous studies. By using this approach, both the hypothesis-driven and the exploratory component of this study are satisfied at once.
First, the mean walking-minus-sitting difference ERP waveform was obtained for each electrode and for each subject by subtracting the within-subject mean sitting ERP waveform from the corresponding mean walking ERP waveform. Next, 1-sample t-tests were performed on the mean difference ERP waveforms coming from all subjects, at each electrode and timepoint. To correct for multiple comparisons, cluster-based permutation tests were performed, using the Monte Carlo method (5,000 permutations, significance level of the permutation tests 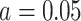 , probabilities corrected for performing 2-sided tests) and the weighted cluster mass statistic (Hayasaka and Nichols 2004; cluster significance level
, probabilities corrected for performing 2-sided tests) and the weighted cluster mass statistic (Hayasaka and Nichols 2004; cluster significance level 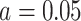 , parametric cluster threshold). This procedure was performed separately for each one of the interrogated behavioral conditions of the cognitive task (correct rejection, response-locked false alarm), first for the entire cohort and, subsequently, for the IMP and nIMP subgroups. The results of the point-wise t-tests from all 64 electrodes and all timepoints were displayed as an intensity plot to efficiently summarize and facilitate the identification of the onset and general topographical distribution of walking-related changes in ERP activity. The x, y, and z axes, respectively, represent time, electrode location, and the t-statistic (indicated by a color value) at each electrode-timepoint pair.
, parametric cluster threshold). This procedure was performed separately for each one of the interrogated behavioral conditions of the cognitive task (correct rejection, response-locked false alarm), first for the entire cohort and, subsequently, for the IMP and nIMP subgroups. The results of the point-wise t-tests from all 64 electrodes and all timepoints were displayed as an intensity plot to efficiently summarize and facilitate the identification of the onset and general topographical distribution of walking-related changes in ERP activity. The x, y, and z axes, respectively, represent time, electrode location, and the t-statistic (indicated by a color value) at each electrode-timepoint pair.
Multiple comparisons correction
The 2-stage step-up false discovery rate (FDR) method of Benjamini, Krieger, and Yekutieli (Benjamini et al. 2006) was applied on the combined set of all the P-values occurring from all the cognitive task performance, gait kinematic and EEG activity statistical tests, both from the group-level (entire cohort) and the split-group (IMP/nIMP) analyses. The calculations were conducted in MATLAB (Groppe 2010). For each paired-sample or independent-sample test, one P-value was obtained. For each 2 × 2 (Group × Motor Load) ANOVA, 3 P-values were obtained: 2 for the main effects and 1 for the interaction effect. Within each comparison conducted as part of the EEG activity statistical analysis, cluster-based statistics revealed no, 1, or >1 clusters of significant neural effects, each of which had their own cluster P-value. For FDR calculation purposes, the minimum cluster P-value within each comparison was added to the abovementioned combined set. In total, this combined set comprised of 16 P-values. The critical P-value yielded by FDR procedure was 0.0492 (false discovery rate = 5%). Therefore, all tests whose uncorrected p-values were found to be less than or equal to 0.0492 were significant after FDR correction.
Results
Group-level analysis
Cognitive task performance
In previous studies, CRR and HR have been used to assess cognitive task performance in a Go/NoGo response inhibition task (De Sanctis et al. 2014). However, CRR and HR in isolation can be impacted by changes in the participant’s response criterion. The sensitivity index (d’) measures the discriminability between the Go and the NoGo conditions and is independent of the response criterion. Higher d’ scores indicate an increased ability to properly detect and respond to both Go and NoGo stimuli.
In the current cohort, d’ scores during sitting and walking were calculated and d’ differences between sitting and walking were examined using a paired t-test. Overall, d’ scores were higher during walking compared to sitting (d’sitting = 2.27 ± 1.20, d’walking = 2.50 ± 1.07; t25 = 2.85, P = 0.0087, Cohen’s d = 0.56) indicating better performance when participants were walking on the treadmill (Fig. 2A). This observation appears to be inconsistent with the hypothesis that there will be interference between motor and cognitive tasks (CMI) during dual-task conditions (Leone et al. 2017).
Fig. 2.
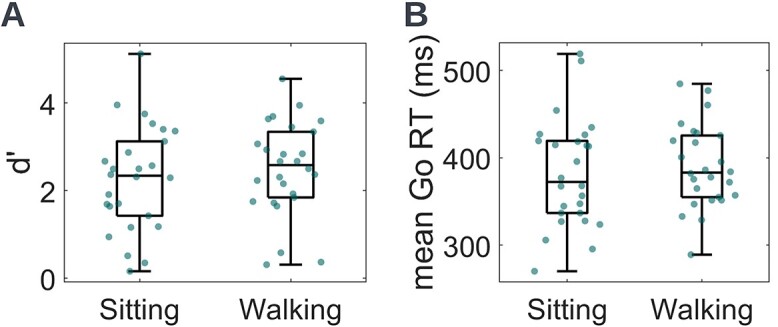
Sitting and walking A) d’ scores and B) mean Go RTs of the entire young adult cohort. Dots represent individual participants. The central mark of each box indicates the median, and the bottom and top edges indicate the 25th and 75th percentiles, respectively. The whiskers extend to the most extreme data points not considered outliers. There were no outliers here. D′ scores during walking were higher compared to sitting, indicating better cognitive task performance during walking in young adults. No significant differences were found in mean go RTs between sitting and walking.
Mean Go RT differences between sitting and walking were assessed using a paired t-test. No significant differences were found between sitting and walking mean Go RTs (mean RT Go sitting = 382 ± 62 ms, mean RT Go walking = 390 ± 47 ms; t25 = 1.36, P = 0.1862, Cohen’s d = 0.27; Fig. 2B).
Based on these data, young adults responded more accurately to task-related stimuli during walking than while sitting. This behavioral improvement was not accompanied by response speed costs (i.e. no speed-accuracy trade-off was observed).
Gait kinematic activity
Walking on a treadmill imposes a fixed walking speed, and as a result stride time variability may underestimate the impact of cognitive tasks on gait. To evaluate gait kinematics across the entire gait cycle (stance and swing phases) and compare 3D trajectories of consecutive strides, a DTW approach was used (details in Methods).
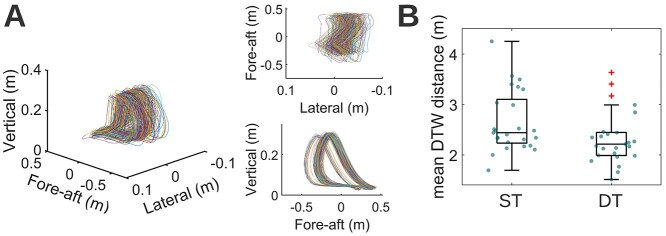
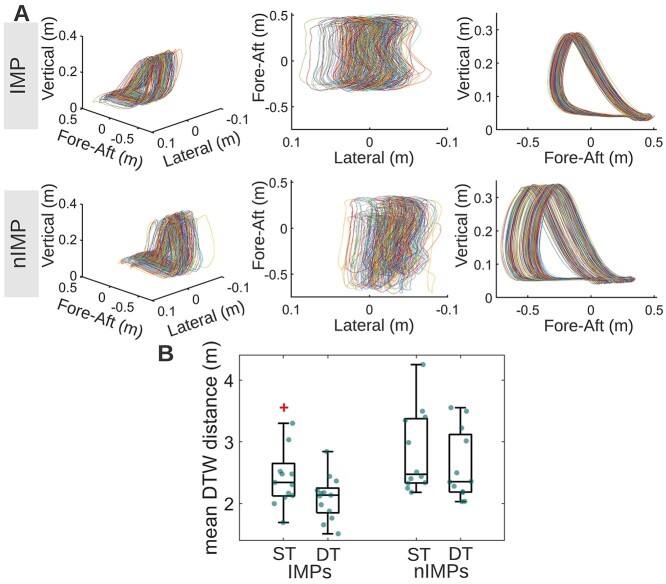
Using DTW, the variability from one stride to the next was quantified as DTW distance and, subsequently, the mean DTW distance of all stride-to-stride comparisons was extracted per participant (Fig. 3A). Mean DTW distance was calculated during both ST and DT walking. One participant did not have ST walking recordings and was therefore excluded from this analysis, resulting in a set of 25 participants. Mean DTW distance differences between ST and DT walking were assessed using a Wilcoxon signed rank test. Mean DTW distances were greater during ST compared to DT walking (mean DTW distance ST = 2.44 ± 0.61 m, mean DTW distance DT = 2.21 ± 0.52 m; z = 4.02, P = 0.0001, Cohen’s r = 0.57). As illustrated, walking variability “decreased” when combined with the response inhibition task compared to walking in isolation (Fig. 3B).
Fig. 3.
A) 3D representations of trajectories of a series of strides. Lateral is the dimension of movement right-and-left relative to the motion of the treadmill belt. Vertical is the dimension movement up-and-down relative to the motion of the treadmill belt. Fore-aft is the dimension of movement parallel to the motion of the treadmill belt. Perspective (left), top (upper right), and right (lower right) views are provided. Using DTW, the variability from one stride to the next was quantified as DTW distance (see Methods) and the mean DTW distance of all stride-to-stride comparisons was extracted per participant. B) Mean DTW distance distribution during ST walking and DT walking; mean DTW distances of individual participants are represented as dots scattered on the boxes. Mean DTW distance was smaller during DT compared to ST walking. Red “+” symbols indicate outliers.
EEG activity
Correct rejections
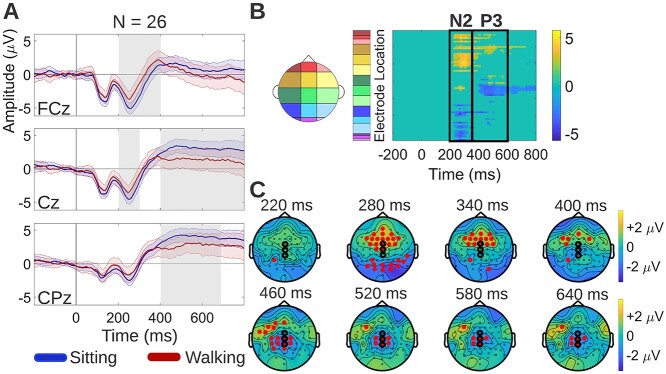
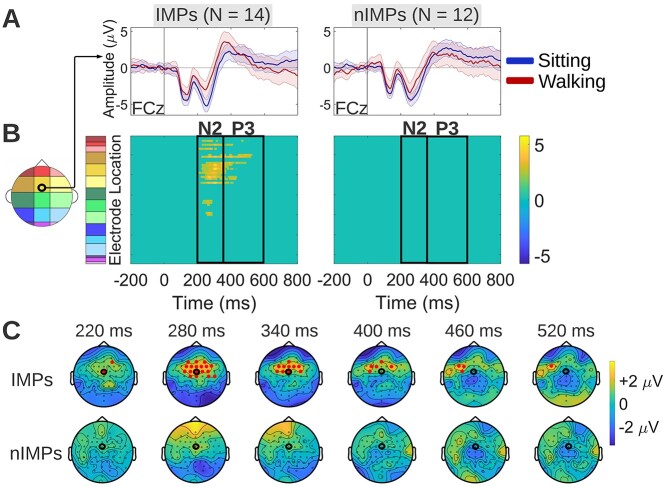
Cluster-based permutation tests were used to examine neural activity differences between sitting and walking during correct rejection trials. First, 3 midline electrode locations—a frontocentral midline electrode (FCz), a central midline electrode (Cz), and a centroparietal midline electrode (CPz)—were plotted and inspected for differences (Fig. 4A, latency intervals of significant differences are highlighted in gray). The selection of these electrodes was based on previous studies showing that the N2 amplitude is maximal over frontocentral midline scalp and the P3 is maximal over centroparietal midline scalp (Smith et al. 2008; Barry and De Blasio 2013). Reduced ERP amplitudes during walking were found at FCz and Cz during the N2 latency interval, and at the Cz and CPz during the P3 latency interval (Fig. 4A). The cluster-based permutation approach also allowed for exploring the existence of walking-related effects on ERPs in the entire electrode set and at all the epoch timepoints. The effects that this approach revealed were ERP amplitude reductions during walking (i) over frontal and frontocentral scalp (yellow in the Fig. 4B statistical clusterplot) and over parietal and occipital scalp (blue) during the N2 latency interval, (ii) over left prefrontal (yellow) and over central and centroparietal scalp (blue) during the P3 latency interval, and (iii) over central and centroparietal scalp during latencies beyond the P3 latency interval, until the end of the epoch (blue). The topographical distribution of the average (walking-minus-sitting) neural activity difference during correct rejection trials is shown for selected timepoints at which this difference was found to be significant (Fig. 4C, red dots on the maps show electrodes that exhibit significant differences).
Fig. 4.
Neural activity differences between sitting and walking during correct rejection trials. A) Grand average sitting and walking ERP waveforms, at 3 midline electrode locations: frontocentral midline (FCz), central midline (Cz), and centroparietal midline (CPz) electrode. The shaded regions around the ERP waveforms indicate the standard error of the mean (SEM) across participants. The latency interval of significant differences in each electrode is highlighted in gray. B) Spatiotemporal walking-minus-sitting ERP differences using cluster-based permutation tests. The statistical clusterplot shows the t-values for the electrode-timepoint pairs at which significant ERP differences between sitting and walking were found. Positive t-values (yellow) indicate that walking ERP amplitude was greater than sitting ERP amplitude. Significant differences were found (i) over frontal and frontocentral scalp (yellow) and over parietal and occipital scalp (blue) during the N2 latency interval, (ii) over left prefrontal (yellow) and over central and centroparietal scalp (blue) during the P3 latency interval, and (iii) over central and centroparietal scalp during latencies beyond the P3 latency interval, until the end of the epoch (blue). The black rectangles indicate latencies corresponding to the N2 and P3. C) Topographical maps showing the average (walking-minus-sitting) neural activity difference for selected timepoints at which this difference was found to be significant. The electrodes exhibiting significant differences are depicted as red dots on the maps. The electrodes to which the ERP waveforms of panel A correspond are circled in black (vertical order matched).
Response-locked false alarms
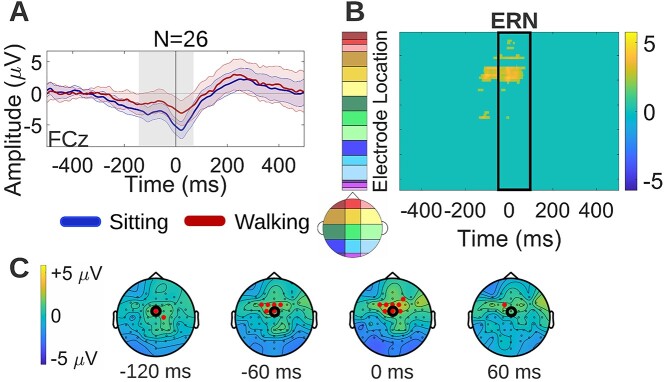
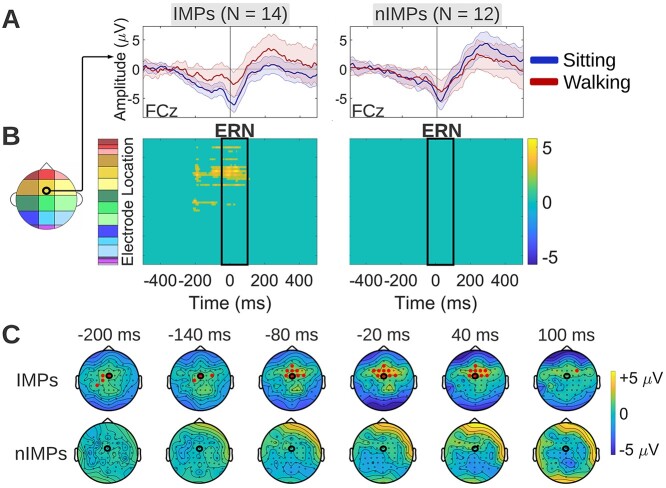
Cluster-based permutation tests were used to examine neural activity differences between sitting and walking during response-locked false alarm trials. First, the FCz electrode was plotted and inspected for differences (Fig. 5A), since the ERN has been shown to have maximal amplitude over frontocentral midline scalp (Riesel et al. 2013; Nguyen et al. 2016). Reduced ERP amplitudes during walking were found at FCz during the ERN latency interval. By exploring the entire electrode set and all the epoch timepoints, the effects that the cluster-based permutation approach revealed were frontocentral walking-related amplitude reductions during ERN latencies, as well as during latencies preceding the ERN ([−130, −50 ms] approximately, corresponding to the yellow points outside of the black rectangle in Fig. 5B statistical clusterplot). The latency and topography of these earlier, pre-ERN differences indicate reduction in pre-motor neural activity reflected by the premovement positivity (PMP) ERP (Deecke et al. 1969; Bortoletto et al. 2006). The topographical distribution of the average (walking-minus-sitting) neural activity difference during response-locked false alarm trials is shown for selected timepoints at which this difference was found to be significant (Fig. 5C, red dots on the maps show electrodes that exhibit significant differences).
Fig. 5.
Neural activity differences between sitting and walking during response-locked false alarm trials (modeled per Fig. 4). A) Grand average sitting and walking ERP waveforms during response-locked false alarm trials, at a frontocentral midline electrode (FCz). B) Spatiotemporal walking-minus-sitting ERP differences using cluster-based permutation tests. Significant differences, shown in yellow in the statistical clusterplot, were found over frontocentral scalp during the ERN latency interval, and over similar frontocentral scalp during pre-ERN latencies. The latter indicated reduction in the pre-motor positivity (PMP). C) Topographical maps showing the average (walking-minus-sitting) neural activity difference for selected timepoints at which this difference was found to be significant. The electrode to which the ERP waveforms of panel a correspond is circled in black.
Split-group differences based on cognitive task performance
Cognitive task performance
As shown above, mean d’ scores for the entire cohort improved when participants were walking on the treadmill. This is an indication of cognitive-motor enhancement, rather than the more typically expected CMI often observed in dual-task paradigms. In other words, the response inhibition task appears to have gotten easier when coupled with walking in this young adult cohort. Several questions arise from this observation: Does each individual improve? Are there neural patterns that differ based on behavioral improvement versus nonimprovement while walking? Are there differences in gait variability for those that improve compared to those that do not? These questions are addressed below in split-group analyses that compare those who improved their performance while walking (IMPs) and those who did not (nIMPs).
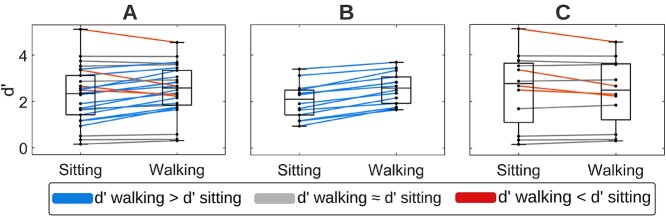
The (d’walking–d’sitting) difference was calculated for each participant and its significance was subsequently tested by determining whether it lay outside of the 95% confidence interval of the normal distribution having a mean value of zero and a standard deviation equal to that of the (d’walking–d’sitting) distribution of the entire cohort. If d’walking > d’sitting, namely the participant improved significantly during walking compared to sitting, they were classified into the IMP subgroup. If d’walking ≤ d’sitting, namely the participant did not improve significantly during walking compared with sitting, they were classified into the nIMP subgroup. In total, 14 participants were classified into the IMP subgroup and 12 participants into the nIMP subgroup (8 had d’walking ≈ d’sitting, 4 had d’walking < d’sitting), as shown in Fig. 6A.
Fig. 6.
Sitting and walking d’ scores of A) the entire young adult cohort, B) participants who improved during walking (IMPs), and C) participants who did not improve during walking (nIMPs). Each line corresponds to one participant.
In the subsequent analyses, RTs, ERPs, and gait kinematic variability were contrasted between IMPs (Fig. 6B) and nIMPs (Fig. 6C).
No significant differences in average d’ scores were found between IMPs and nIMPs, as the independent samples t-test indicated (average d’ IMPs = 2.30 ± 0.70, average d’ nIMPs = 2.48 ± 1.49; t24 = 0.39, P = 0.7020, Cohen’s d = 0.15).
The 2 × 2 ANOVA assessing the effects of Group (IMPs vs nIMPs) and Motor Load (sitting vs walking) on mean RTs revealed a significant main effect of Group (F1,24 = 4.86, P = 0.0373, η2 = 0.16). This indicated that IMPs (mean RT sitting = 360 ± 54 ms, mean RT walking = 372 ± 41 ms) were overall faster than nIMPs (mean RT sitting = 407 ± 64 ms, mean RT walking = 411 ± 47 ms) to respond to image presentation. No significant effects of Motor Load (F1,24 = 1.65, P = 0.2108, η2 < 0.01) or Group/Motor Load interaction was found (F1,24 = 0.55, P = 0.4649, η2 < 0.01).
Gait kinematic activity
The effects of Group (IMPs vs nIMPs) and Cognitive Load (ST vs DT walking) on mean DTW distance were assessed by means of a 2 × 2 ANOVA. This ANOVA revealed a significant main effect of Group (F1,23 = 4.71, P = 0.0406, η2 = 0.14) indicating that IMPs (mean DTW distance ST = 2.46 ± 0.54 m, mean DTW distance DT = 2.08 ± 0.36 m) walked less variably than nIMPs (mean DTW distance ST = 2.82 ± 0.67 m, mean DTW distance DT = 2.59 ± 0.57 m; Fig. 7B). The significant main effect of Cognitive Load (F1,23 = 13.48, P = 0.0013, η2 = 0.07) that occurred is expected, since the same effect was tested using the Wilcoxon signed rank test as part of the group-level analysis. Within-group post-hoc t-tests showed that mean DTW distance decreased significantly during DT walking in both the IMP (t12 = 2.59, P = 0.0235, Cohen’s d = 0.72) and the nIMP subgroups (t11 = 3.35, P = 0.0064, Cohen’s d = 0.97). No significant Group/Cognitive Load interaction was found (F1,23 = 0.80, P = 0.3811, η2 < 0.01; Fig. 7B). Of note, the 1 participant excluded from the corresponding group-level analysis was an IMP, thus resulting in a set of 13 IMPs and 12 nIMPs entered into this analysis.
Fig. 7.
A) 3D representations of trajectories of a series of strides for an IMP (top) and a nIMP (bottom). For both the IMP and the nIMP, perspective (left), top (middle), and right (right) views are provided in the respective panels. B) Mean DTW distance distribution during ST walking and DT walking, for IMPs and nIMPs. Mean DTW distance was smaller in IMPs compared to nIMPs.
Figure 7A shows 3D representations of trajectories of a series of strides for an IMP (top) and a nIMP (bottom), to give an example of what a lower-variability series of strides (IMP) looks like compared to a higher-variability series of strides (nIMP).
EEG activity
Correct rejections
To compare the spatiotemporal effects of walking on neurophysiology between IMPs and nIMPs during correct rejection trials, cluster-based permutation tests were performed separately for each group to examine differences between the sitting and the walking correct rejection ERP waveform. During correct rejection trials, IMPs exhibited reduced walking ERP amplitudes over frontocentral scalp during the N2 latency interval and over left prefrontal scalp during the P3 latency interval, whereas nIMPs exhibited no detectable ERP differences between sitting and walking (Fig. 8B). These walking-related effects in IMPs were represented by the yellow cluster in the Fig. 8B statistical clusterplot. By comparing these walking-related effects to the corresponding group-level effects shown in the Fig. 4B statistical clusterplot, it can be observed that the yellow cluster was almost identical between IMPs and the entire cohort, indicating that IMPs maintain only the frontal/frontocentral portion of walking-related effects that were found in the overall combined cohort. Sitting and walking ERPs of IMPs and nIMPs during correct rejection trials are depicted at FCz, since this electrode belongs to the frontocentral cluster of significant effects (Fig. 8A). The topographical maps of Fig. 8C show the scalp distribution of the average (walking-minus-sitting) neural activity difference in IMPs and nIMPs during correct rejection trials, for selected timepoints at which this difference was found to be significant in IMPs.
Fig. 8.
Walking-minus-sitting ERP differences in IMPs and nIMPs during correct rejection trials. A) Grand average sitting and walking ERP waveforms of IMPs (left column) and IMPs (right column), at a frontocentral midline electrode (FCz) that exhibits significant differences between the 2 motor load conditions for IMPs. The latency interval of significant differences is highlighted in gray. B) Spatiotemporal walking-minus-sitting ERP differences in IMPs (left column) and IMPs (right column), using cluster-based permutation tests. Such differences were found only in IMPs, over frontocentral scalp during the N2 latency interval and left prefrontal scalp during the P3 latency interval (yellow cluster). C) Topographical maps showing the average (walking-minus-sitting) neural activity difference for selected timepoints at which this difference was found to be significant in IMPs. Maps are illustrated both for IMPs and nIMPs, for comparison purposes. The electrode to which the ERP waveforms of panel A correspond is circled in black.
Response-locked false alarms
To compare the spatiotemporal effects of walking on neurophysiology between IMPs and nIMPs during response-locked false alarm trials, cluster-based permutation tests were performed separately for each group to examine differences between the sitting and walking response-locked false alarm ERP waveform. During response-locked false alarm trials, IMPs exhibited reduced walking-related ERP amplitudes over frontal/frontocentral scalp during the ERN latency interval and over central/frontocentral scalp during pre-ERN latencies (Fig. 9B). Pre-ERN amplitude reductions, which were not part of our initial hypothesis, were also encountered in the group-level analysis (Fig. 5B) where they were interpreted as reduction in the PMP. Here, even though differential effects started earlier than in the combined group (−210 vs −130 ms, approximately), they are still thought to reflect PMP amplitude reduction during walking based on their latency and topography, only more pronounced compared to the entire cohort. No detectable ERP differences between sitting and walking were found in nIMPs (Fig. 9B). The walking-related effects in IMPs are represented by the yellow cluster in the Fig. 9B statistical clusterplot. By comparing these walking-related effects to the corresponding group-level effects shown in the Fig. 5B statistical clusterplot, it can be observed that the yellow cluster manifested in IMPs was qualitatively similar to the corresponding cluster of the entire cohort, with the difference that the IMP cluster had an earlier onset (more pronounced PMP reduction during walking in IMPs) and it spread over more electrodes during the ERN latency interval (more pronounced ERN reduction during walking in IMPs). This indicates that IMPs in general maintain the walking-related effects that were found in the overall combined cohort. Sitting and walking ERPs of IMPs and nIMPs during response-locked false alarm trials are depicted at FCz, since this electrode belongs to the frontocentral cluster of significant effects (Fig. 9A). The topographical maps of Fig. 9C show the scalp distribution of the average (walking-minus-sitting) neural activity difference in IMPs and nIMPs during response-locked false alarm trials, for selected timepoints at which this difference was found to be significant in IMPs.
Fig. 9.
Walking-minus-sitting ERP differences in IMPs and nIMPs during the response-locked false alarm trials (modeled per Fig. 8). A) Grand average sitting and walking ERP waveforms of IMPs (left column) and IMPs (right column), at a frontocentral midline electrode (FCz) that exhibits significant differences between the 2 motor load conditions for IMPs. The latency interval of significant differences is highlighted in gray. B) Spatiotemporal walking-minus-sitting ERP differences in IMPs (left column) and IMPs (right column), using cluster-based permutation tests. Such differences were found only in IMPs, over frontocentral scalp during the ERN latency interval and over central/frontocentral scalp during latencies preceding the ERN (yellow cluster). The latter indicated reduction in the PMP. C) Topographical maps showing the average (walking-minus-sitting) neural activity difference for selected timepoints at which this difference was found to be significant in IMPs. Maps are illustrated both for IMPs and nIMPs, for comparison purposes. The electrode to which the ERP waveforms of panel A correspond is circled in black.
Discussion
CMI (Abernethy 1988) predicts that walking will have a detrimental effect on cognitive task performance and/or on gait kinematics due to competition in neural resource allocation between the 2 concurrent task components. However, in the current cohort, walking actually improved performance in more than half of the participants (14 out of 26). The remaining 12 participants showed either no change in performance (n = 8) while walking, or had the expected decline (n = 4). Those who improved (IMPs) responded more quickly to Go stimuli and had significantly reduced stride-to-stride variability compared with those who did not improve (nIMPs). Behavioral improvement effects while walking were accompanied by ERP changes during both the correct rejection trials and the response-locked false alarm trials. No dual-task-related ERP differences were present in participants who did not improve performance while walking. The findings above suggest that IMPs may adjust their cognitive strategy in response to the increased task demands, and this adjustment is reflected in the ERP amplitude reductions during key processing stages of inhibitory control.
Reduction in stride-to-stride variability during DT walking compared to ST walking was found both in the cohort overall and in the IMP and nIMP subgroups, suggesting that this effect may characterize young healthy adults in general, independent of cognitive task performance. A number of studies have reported findings similar to this, especially in young adults (Grabiner and Troy 2005; Lovden et al. 2008; Decker et al. 2016; Wrightson et al. 2016; Hamacher et al. 2019a, 2019b; Richardson et al. 2022). One possibility to account for this pattern is that shifting attention solely to motor control of walking, a largely automated motor pattern (Clark 2015), increases susceptibility to endogenous or exogenous noise, which, in turn, can compromise walking performance (Beilock et al. 2002; Beilock and Gray 2012; Hamacher et al. 2019b). In contrast, with the addition of a cognitive load, attention shifts away from walking-related motor control, enhancing automaticity and improving the consistency of the generated walking patterns (Lovden et al. 2008; Wrightson et al. 2016). Indeed, a recent paper demonstrated that the addition of cognitive load, via performance of a Go/NoGo task, reduced the impact of perturbations in ongoing optic flow inputs on both gait parameters and EEG outcomes while participants were engaged in treadmill walking (Malcolm et al. 2018).
During correct rejection trials, ERP amplitude reductions during walking were found (i) over frontal, frontocentral, parietal, and occipital scalp regions during the N2 latency interval, (ii) over left prefrontal, central, and centroparietal scalp regions during the P3 latency interval, and (iii) over central and centroparietal scalp regions during latencies beyond the P3 latency interval, until the end of the epoch (Fig. 4). From these effects, the walking-related amplitude reductions of the frontocentral N2 and the centroparietal P3 are consistent with previous literature (De Sanctis et al. 2014). However, the left prefrontal amplitude reductions during the P3 latency interval, along with the late central/centroparietal amplitude reductions during post-P3 latencies, have not been reported by previous studies (Fig. 4). The latter central/centroparietal effect is thought to stem from differences in the EEG processing pipelines; neural activity was not highpass-filtered here, allowing more low-frequency content to be retained. IMPs maintained the anterior (frontal/frontocentral) portion of effects found in the combined cohort, namely they exhibited ERP amplitude reductions during walking over frontal/frontocentral scalp during the N2 latency interval and in left prefrontal scalp during the P3 latency interval (Fig. 8). In contrast, nIMPs exhibited no detectable walking-related effects during correct rejection trials (Fig. 8). Focusing on the effects found in IMPs, the N2 is thought to reflect the conflict between the 2 competing response tendencies (the “Go” and the “NoGo”) and its generation has been traced to the ACC, a region that plays a key role in conflict monitoring (van Veen and Carter 2002a, 2002b; Dias et al. 2003; Botvinick et al. 2004; Dias et al. 2006). The neural activity differences between sitting and walking in IMPs during these latencies had a frontal/frontocentral topographical distribution, which aligns with ACC engagement (Fig. 8). During the P3 latencies, a topographical shift of the walking-minus-sitting ERP differences towards left lateral prefrontal scalp regions was observed (Fig. 8). This finding too can be interpreted using the conflict monitoring hypothesis, which predicts that, once conflict between active representations is detected by the ACC, a conflict-related signal is relayed to lateral prefrontal resources, and more specifically the DLPFC, where inhibitory control is implemented. In that way, the top-down attentional resources hosted by the DLPFC are activated towards implementing behavioral adjustments, to reduce conflict in ensuing trials (Carter and van Veen 2007). Neuroimaging studies have shown that that increased left DLPFC activation in particular is associated with lower activation levels in conflict-related brain regions in subsequent trials of a Go/NoGo response inhibition task, thus emphasizing the central role that the left DLPFC plays in exerting top-down cognitive control (Fassbender et al. 2009). Taken together, our neurophysiological findings during correct rejection trials suggest that successful inhibition in IMPs during walking is driven by modulation of the conflict monitoring (N2 stage) and the subsequent control implementation (P3 stage) neural processes compared to sitting, with such effects being absent in nIMPs.
During response-locked false alarm trials, ERP amplitude reductions during walking were found over frontocentral scalp regions during the ERN latency interval, as well as during earlier latencies preceding the ERN, which were interpreted as PMP ERP effects (Fig. 5). IMPs exhibited a pronounced version of the walking-related ERP effects found in the combined cohort, maintaining the general topography and latency of the effects, whereas nIMPs exhibited no detectable effects during the response-locked false alarm trials (Fig. 9). Focusing on the effects found in IMPs, the ERN is thought to reflect the conflict between the erroneous motor response and corrective processes, a few milliseconds following the motor response (it peaks roughly 50-ms post-response, in accord with the results shown in Fig. 9). The source of the ERN has been localized to the ACC (Mathalon et al. 2003; Carter and van Veen 2007; O'Connell et al. 2009a), same as the N2, consistent with the frontocentral topographical distribution of the walking-minus-sitting ERP differences demonstrated in Fig. 9 during this latency interval. The main difference compared to the N2 is that here the ACC is activated and thus conflict is detected after the response instead of before (Van Veen and Carter 2002b; Carter and van Veen 2007). Therefore, the timing of the activation of the ACC, which functions as a conflict monitor, plays a critical role in determining the behavioral outcome. Regarding the earlier pre-ERN differences, the [−210, −50] ms latency interval during which they were observed has been associated with pre-motor processes reflected by the PMP (Deecke et al. 1969; Bortoletto et al. 2006). The PMP has been proposed to reflect the “go-ahead” signal generated by the supplementary motor area (SMA) and pre-SMA to allow movement execution, consistent with the central/frontocentral topography of the walking-minus-sitting ERP differences detected during these latencies (Fig. 9). Summarizing the findings during response-locked false alarm trials, IMPs seem to manage an unsuccessful inhibition differently during walking compared to sitting, and this difference was pinpointed to modulation of the pre-motor neural processes preceding the erroneous motor response (PMP stage), as well as of the conflict monitoring neural processes immediately following the erroneous response (ERN stage). No such modulation was evident in nIMPs.
The CMI hypothesis proposes that cognitive task-related and gait kinematic performance decrements stem from underlying competition in the allocation of neural resources between the cognitive and the motor task. The IMP phenotype of improvement in all interrogated domains clearly contradicts the CMI hypothesis, suggesting that neural resource competition is likely absent in this subgroup, or that other factors are at play. Given that IMPs were found to alter neural activity during walking compared with sitting, whereas nIMPs did not, these IMP-specific walking-related neural activity changes might hold the key to understanding how IMPs manage to flexibly recalibrate the underlying neural processes in order to avoid inter-task neural resource competition. It is proposed that these neural activity changes manifested by IMPs in response to motoric load increase hold promise as neural markers of cognitive flexibility.
One explanation for not observing CMI effects in IMPs is that the employed task might not have been sufficiently taxing on their cognitive resources. Walking is a relatively automatic motor task (Clark 2015), so it may have sufficed to recruit certain cortical (e.g. sensorimotor) or non-cortical (e.g. subcortical, brainstem, and spinal) neural networks to produce this automatic walking pattern (Miyai et al. 2001; Wagner et al. 2012; Seeber et al. 2014; Tattersall et al. 2014; Takakusaki 2017) without interfering with the neural resources used by the response inhibition task. Also, the generally good performance of the IMP subgroup on the Go/NoGo response inhibition task suggests that this is not an especially difficult task for them. It will be interesting to observe in future work whether increasing the difficulty of the motor and/or the cognitive task will prompt IMPs to adopt a more effortful cognitive strategy, and as such, make individuals of this group manifest effects consistent with CMI. However, IMPs did not just maintain performance; they actually achieved better performance when walking. Findings such as this, although unanticipated based on CMI, have been reported and interpreted before. There is evidence that moderate exercise like walking can enhance sustained attention and facilitate cognitive task performance (Tomporowski 2003; Davranche and Audiffren 2004; Lambourne and Tomporowski 2010). This facilitatory effect has been explained using neurotransmitter models, proposing that moderate exercise induces an increase in catecholamine levels, which, in turn, boosts the signal-to-noise ratio during processing by prefrontal attentional systems (Mesulam 1990; McMorris 2009). This hypothesis aligns with the present findings, since the observed modulation in lateral prefrontal neural activity in IMPs coexisted with reduced conflict-related ERP amplitudes (N2 in correct rejections and ERN in response-locked false alarms) during walking, as shown in Figs. 8 and 9. In addition, attenuated N2 and ERN amplitudes have been found under conditions of increased expectancy of the “NoGo” trials, when reliance on proactive control is thought to be dominant (Nieuwenhuis et al. 2003; Gajewski et al. 2008; O'Connell et al. 2009b; Smith et al. 2010). Therefore, this reduction in inhibitory conflict that IMPs manifest during walking might stem from a more active and/or efficient engagement of top-down attentional resources, which presumably induces a shift to a more proactive cognitive strategy and hence promotes better anticipation of the rare “NoGo” events when walking.
Treadmill walking, the approach used here, is likely to play a role in reducing competition between the cognitive and the motor components of the task. When paired with a cognitive task, treadmill walking has been shown to interfere minimally with gait stability as well as elicit improvement in cognitive task performance in healthy adults under certain conditions, in contrast to overground walking, which has been associated with more pronounced CMI effects (Wrightson and Smeeton 2017; Penati et al. 2020; Wrightson et al. 2020). Even though it is more predictable and spatially restricted, which probably is the root cause of the above differences in observed effects between walking modalities, treadmill walking can offer consistency of motor loading throughout the experimental procedure. This feature can be especially useful for designs that are meant to be translated to older or clinical populations, since it provides more standardized measurements of dual-task-related effects on gait and cognitive parameters.
A limitation of this study is that nIMPs were more variable in terms of d’ scores than IMPs, both for sitting and walking. Based on Fig. 6, although IMPs all lie within a range of medium d’ performance, nIMPs were scattered over the full d’ range, encompassing low, medium, and high performers. As part of future research, we aim to collect more nIMP datasets in order to compare IMPs and nIMPs having d’ scores within similar ranges. Despite this limitation, the neural signatures of improvement that this study yielded hold significant potential as cognitive flexibility markers, which could potentially be translated to older neurotypical or patient populations to assess and quantify age-related or neurodegeneration-related cognitive decline, respectively. As a first step in this direction, we aim to expand the methodology employed here to older neurotypical adults to test its efficacy in distinguishing “super-agers” from older adults that exhibit normal or aggravated age-related cognitive decline (De Sanctis et al. 2009). Older adults have been found to manifest response accuracy decline and absence of dual-task-related neural activity recalibration when adding walking to inhibition task performance (Malcolm et al. 2015), similar to the dual-task-related signatures of nIMPs. The present study showed that, even within the same age group, different behavioral phenotypes can coexist, each one correlating with different neural activity patterns. When subjecting older adults to concurrent walking and inhibitory performance, even though the dominant phenotype is expected to be the one described by Malcolm and colleagues (Malcolm et al. 2015), it is also expected that there will be few older adults who will exhibit dual-task-related signatures more consistent with those of IMPs. If such older individuals indeed exist and can be identified, responding more flexibly to dual-task load would suggest that their cognitive resources presumably remain relatively unaffected by aging processes. As neural circuitry gets further compromised by degenerative processes, behavior is anticipated to converge to a phenotype of enhanced dual-task-related costs.
In conclusion, this study examined differences in neural activity, stride-to-stride variability and response speed between (i) young adults who improved in terms of response accuracy during walking compared to sitting (IMPs) and (ii) young adults who did not improve (nIMPs) under the same conditions. This split of the young adult cohort was motivated by findings at the piloting stage of the study, where 3 out of 5 young adults performed better during walking compared to sitting, conflicting with the CMI hypothesis. During correct rejection trials, ERP amplitude reductions were found during walking in IMPs, specifically over frontocentral scalp regions during N2 latencies and over left prefrontal scalp regions during P3 latencies. Also, during response-locked false alarm trials, IMPs exhibited reduced ERP amplitudes while walking over frontocentral scalp regions, during ERN and pre-ERN latencies. No detectable differences were found in the neural activity of nIMPs between sitting and walking, neither during correct rejection trials nor during response-locked false alarm trials. The present findings indicate that IMPs can flexibly modulate frontal brain activity during walking during key stages of inhibitory control (conflict monitoring for N2 and ERN, control implementation for P3, and pre-motor for pre-ERN), something that nIMPs do not seem to do. Combining these neurophysiological findings with findings of faster responses and less stride-to-stride variability in IMPs, these neural activity differences were interpreted as neural signatures of behavioral improvement during DT walking. Future research can test the potential of these neural signatures as markers for assessing cognitive flexibility in populations where it tends to get compromised, for example older adults who either age normally or have been diagnosed with neurodegenerative diseases, such as Parkinson’s or Alzheimer’s disease.
Supplementary Material
Acknowledgments
We would like to thank each of the participants that enrolled in the study. Special thanks to George Kassis, Emma Mantel, Nicholas Abraham, Soma Mizobuchi, and Suzan Hoffman for their help with this study.
Contributor Information
Eleni Patelaki, The Frederick J. and Marion A. Schindler Cognitive Neurophysiology Laboratory, The Del Monte Institute for Neuroscience, Department of Neuroscience, University of Rochester School of Medicine and Dentistry, 601 Elmwood Avenue, Rochester, NY 14642, United States; Department of Biomedical Engineering, University of Rochester, 201 Robert B. Goergen Hall Rochester, NY 14627, United States.
John J Foxe, The Frederick J. and Marion A. Schindler Cognitive Neurophysiology Laboratory, The Del Monte Institute for Neuroscience, Department of Neuroscience, University of Rochester School of Medicine and Dentistry, 601 Elmwood Avenue, Rochester, NY 14642, United States.
Kevin A Mazurek, The Frederick J. and Marion A. Schindler Cognitive Neurophysiology Laboratory, The Del Monte Institute for Neuroscience, Department of Neuroscience, University of Rochester School of Medicine and Dentistry, 601 Elmwood Avenue, Rochester, NY 14642, United States; Department of Physiology and Biomedical Engineering, Mayo Clinic, Joseph Building 4-184W, 200 First Street SW, Rochester, MN 55905, United States; Well Living Lab, Well Living Lab, Inc., 221 First Avenue SW, Rochester, MN 55902, United States.
Edward G Freedman, The Frederick J. and Marion A. Schindler Cognitive Neurophysiology Laboratory, The Del Monte Institute for Neuroscience, Department of Neuroscience, University of Rochester School of Medicine and Dentistry, 601 Elmwood Avenue, Rochester, NY 14642, United States.
Funding
This work was partially supported by the University of Rochester’s Del Monte Institute for Neuroscience pilot grant program, funded through the Roberta K. Courtman Trust (EGF). EP was partially supported by the Gerondelis Foundation Graduate Scholarship. KAM was supported by the University of Rochester Clinical and Translational Science Institute (CTSI) Career Development Program (KL2), National Institutes of Health (NIH) (grant no. 5KL2TR001999). Recordings were conducted at the Translational Neuroimaging and Neurophysiology Core of the University of Rochester Intellectual and Developmental Disabilities Research Center (UR-IDDRC), which is supported by a center grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant no. P50 HD103536 to JJF). The content is solely the responsibility of the authors and does not necessarily represent the official views of any of the above funders.
Conflict of interest statement: None declared.
References
- Abernethy B. Dual-task methodology and motor skills research: some applications and methodological constraints. J Hum Mov Stud. 1988:14:101–132. [Google Scholar]
- Alvarez JA, Emory E. Executive function and the frontal lobes: a meta-analytic review. Neuropsychol Rev. 2006:16(1):17–42. [DOI] [PubMed] [Google Scholar]
- Andersson G, Yardley L, Luxon L. A dual-task study of interference between mental activity and control of balance. Am J Otol. 1998:19(5):632–637. [PubMed] [Google Scholar]
- Barbey AK, Colom R, Solomon J, Krueger F, Forbes C, Grafman J. An integrative architecture for general intelligence and executive function revealed by lesion mapping. Brain. 2012:135(Pt 4):1154–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry RJ, De Blasio FM. Sequential processing in the equiprobable auditory Go/NoGo task: a temporal PCA study. Int J Psychophysiol. 2013:89(1):123–127. [DOI] [PubMed] [Google Scholar]
- Beauchet O, Dubost V, Herrmann FR, Kressig RW. Stride-to-stride variability while backward counting among healthy young adults. J Neuroeng Rehabil. 2005:2:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilock SL, Gray R. From attentional control to attentional spillover: a skill-level investigation of attention, movement, and performance outcomes. Hum Mov Sci. 2012:31(6):1473–1499. [DOI] [PubMed] [Google Scholar]
- Beilock SL, Carr TH, MacMahon C, Starkes JL. When paying attention becomes counterproductive: impact of divided versus skill-focused attention on novice and experienced performance of sensorimotor skills. J Exp Psychol Appl. 2002:8(1):6–16. [DOI] [PubMed] [Google Scholar]
- Bekker EM, Kenemans JL, Verbaten MN. Source analysis of the N2 in a cued Go/NoGo task. Brain Res Cogn Brain Res. 2005:22(2):221–231. [DOI] [PubMed] [Google Scholar]
- BenSaïda SA. Shapiro-Wilk and Shapiro-Francia normality tests. MATLAB Central File Exchange. 2014: (https://www.mathworks.com/matlabcentral/fileexchange/13964-shapiro-wilk-and-shapiro-francia-normality-tests) (last accessed 7 June 2020). [Google Scholar]
- Benjamini Y, Krieger AM, Yekutieli D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika. 2006:93(3):491–507. [Google Scholar]
- Berndt DJ, Clifford J. Using dynamic time warping to find patterns in time series. In: Proceedings of the 3rd international conference on knowledge discovery and data mining. Seattle, WA: AAAI Press; 1994. pp. 359–370 [Google Scholar]
- Berry DA, Ayers GD. Symmetrized percent change for treatment comparisons. Am Stat. 2006:60(1):27–31. [Google Scholar]
- Beurskens R, Helmich I, Rein R, Bock O. Age-related changes in prefrontal activity during walking in dual-task situations: a fNIRS study. Int J Psychophysiol. 2014:92(3):122–128. [DOI] [PubMed] [Google Scholar]
- Beurskens R, Steinberg F, Antoniewicz F, Wolff W, Granacher U. Neural correlates of dual-task walking: effects of cognitive versus motor interference in young adults. Neural Plast. 2016:2016:8032180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortoletto M, Sarlo M, Poli S, Stegagno L. Pre-motion positivity during self-paced movements of finger and mouth. Neuroreport. 2006:17(9):883–886. [DOI] [PubMed] [Google Scholar]
- Botvinick MM, Cohen JD, Carter CS. Conflict monitoring and anterior cingulate cortex: an update. Trends Cogn Sci. 2004:8(12):539–546. [DOI] [PubMed] [Google Scholar]
- Boulgouris NV, Plataniotis KN, Hatzinakos D. Gait recognition using dynamic time warping. In: IEEE 6th workshop on multimedia signal processing. Vol. 2004; 2004. pp. 263–266 [Google Scholar]
- Bradley MM, Lang PJ. International affective picture system. In: Zeigler-Hill V, Shackelford TK, editors. Encyclopedia of personality and individual differences. Cham: Springer International Publishing; 2017. pp. 1–4 [Google Scholar]
- Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cogn Affect Behav Neurosci. 2007:7(4):367–379. [DOI] [PubMed] [Google Scholar]
- Clark DJ. Automaticity of walking: functional significance, mechanisms, measurement and rehabilitation strategies. Front Hum Neurosci. 2015:9:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cocchini G, Della Sala S, Logie RH, Pagani R, Sacco L, Spinnler H. Dual task effects of walking when talking in Alzheimer's disease. Rev Neurol (Paris). 2004:160(1):74–80. [PubMed] [Google Scholar]
- Collette F, Olivier L, Van der Linden M, Laureys S, Delfiore G, Luxen A, Salmon E. Involvement of both prefrontal and inferior parietal cortex in dual-task performance. Brain Res Cogn Brain Res. 2005:24(2):237–251. [DOI] [PubMed] [Google Scholar]
- Collette F, Hogge M, Salmon E, Van der Linden M. Exploration of the neural substrates of executive functioning by functional neuroimaging. Neuroscience. 2006:139(1):209–221. [DOI] [PubMed] [Google Scholar]
- Davranche K, Audiffren M. Facilitating effects of exercise on information processing. J Sports Sci. 2004:22(5):419–428. [DOI] [PubMed] [Google Scholar]
- De Sanctis P, Gomez-Ramirez M, Sehatpour P, Wylie GR, Foxe JJ. Preserved executive function in high-performing elderly is driven by large-scale recruitment of prefrontal cortical mechanisms. Hum Brain Mapp. 2009:30(12):4198–4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sanctis P, Butler JS, Malcolm BR, Foxe JJ. Recalibration of inhibitory control systems during walking-related dual-task interference: a mobile brain-body imaging (MOBI) study. NeuroImage. 2014:94:55–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Sanctis P, Malcolm BR, Mabie PC, Francisco AA, Mowrey WB, Joshi S, Molholm S, Foxe JJ. Mobile brain/body imaging of cognitive-motor impairment in multiple sclerosis: deriving EEG-based neuro-markers during a dual-task walking study. Clin Neurophysiol. 2020:131(5):1119–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker LM, Cignetti F, Hunt N, Potter JF, Stergiou N, Studenski SA. Effects of aging on the relationship between cognitive demand and step variability during dual-task walking. Age (Dordr). 2016:38(4):363–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deecke L, Scheid P, Kornhuber HH. Distribution of readiness potential, pre-motion positivity, and motor potential of the human cerebral cortex preceding voluntary finger movements. Exp Brain Res. 1969:7(2):158–168. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004:134(1):9–21. [DOI] [PubMed] [Google Scholar]
- D'Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S, Grossman M. The neural basis of the central executive system of working memory. Nature. 1995:378(6554):279–281. [DOI] [PubMed] [Google Scholar]
- Dias EC, Foxe JJ, Javitt DC. Changing plans: a high density electrical mapping study of cortical control. Cereb Cortex. 2003:13(7):701–715. [DOI] [PubMed] [Google Scholar]
- Dias EC, McGinnis T, Smiley JF, Foxe JJ, Schroeder CE, Javitt DC. Changing plans: neural correlates of executive control in monkey and human frontal cortex. Exp Brain Res. 2006:174(2):279–291. [DOI] [PubMed] [Google Scholar]
- Doi T, Makizako H, Shimada H, Park H, Tsutsumimoto K, Uemura K, Suzuki T. Brain activation during dual-task walking and executive function among older adults with mild cognitive impairment: a fNIRS study. Aging Clin Exp Res. 2013:25(5):539-44. [DOI] [PubMed] [Google Scholar]
- Dubost V, Kressig RW, Gonthier R, Herrmann FR, Aminian K, Najafi B, Beauchet O. Relationships between dual-task related changes in stride velocity and stride time variability in healthy older adults. Hum Mov Sci. 2006:25(3):372–382. [DOI] [PubMed] [Google Scholar]
- Dubost V, Annweiler C, Aminian K, Najafi B, Herrmann FR, Beauchet O. Stride-to-stride variability while enumerating animal names among healthy young adults: result of stride velocity or effect of attention-demanding task? Gait Posture. 2008:27(1):138–143. [DOI] [PubMed] [Google Scholar]
- Engelhard MM, Dandu SR, Patek SD, Lach JC, Goldman MD. Quantifying six-minute walk induced gait deterioration with inertial sensors in multiple sclerosis subjects. Gait Posture. 2016:49:340–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Hohnsbein J. ERP components in Go/Nogo tasks and their relation to inhibition. Acta Psychol. 1999:101(2–3):267–291. [DOI] [PubMed] [Google Scholar]
- Falkenstein M, Hoormann J, Christ S, Hohnsbein J. ERP components on reaction errors and their functional significance: a tutorial. Biol Psychol. 2000:51(2–3):87–107. [DOI] [PubMed] [Google Scholar]
- Fassbender C, Hester R, Murphy K, Foxe JJ, Foxe DM, Garavan H. Prefrontal and midline interactions mediating behavioural control. Eur J Neurosci. 2009:29(1):181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francisco AA, Horsthuis DJ, Popiel M, Foxe JJ, Molholm S. Atypical response inhibition and error processing in 22q11.2 deletion syndrome and schizophrenia: towards neuromarkers of disease progression and risk. Neuroimage Clin. 2020:27:102351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gajewski PD, Stoerig P, Falkenstein M. ERP--correlates of response selection in a response conflict paradigm. Brain Res. 2008:1189:127–134. [DOI] [PubMed] [Google Scholar]
- Grabiner MD, Troy KL. Attention demanding tasks during treadmill walking reduce step width variability in young adults. J Neuroeng Rehabil. 2005:2:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DM, Swets JA. Signal detection theory and psychophysics. Oxford, England: John Wiley; 1966 [Google Scholar]
- Groppe DM. Two-stage Benjamini, Krieger, & Yekutieli FDR procedure. MATLAB Central File Exchange; 2010. (https://www.mathworks.com/matlabcentral/fileexchange/27423-two-stage-benjamini-krieger-yekutieli-fdr-procedure) (last accessed 9 December 2020). [Google Scholar]
- Guo Z, Chen R, Liu X, Zhao G, Zheng Y, Gong M, Zhang J. The impairing effects of mental fatigue on response inhibition: an ERP study. PLoS One. 2018:13(6):e0198206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamacher D, Hamacher D, Müller R, Schega L, Zech A. The effect of a cognitive dual task on the control of minimum toe clearance while walking. Mot Control. 2019a:23(3):344–353. [DOI] [PubMed] [Google Scholar]
- Hamacher D, Koch M, Lowe S, Zech A. Less noise during dual-task walking in healthy young adults: an analysis of different gait variability components. Exp Brain Res. 2019b:237(12):3185–3193. [DOI] [PubMed] [Google Scholar]
- Hayasaka S, Nichols TE. Combining voxel intensity and cluster extent with permutation test framework. NeuroImage. 2004:23(1):54–63. [DOI] [PubMed] [Google Scholar]
- Herman T, Mirelman A, Giladi N, Schweiger A, Hausdorff JM. Executive control deficits as a prodrome to falls in healthy older adults: a prospective study linking thinking, walking, and falling. J Gerontol A Biol Sci Med Sci. 2010:65(10):1086–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester R, Fassbender C, Garavan H. Individual differences in error processing: a review and reanalysis of three event-related fMRI studies using the GO/NOGO task. Cereb Cortex. 2004:14(9):986–994. [DOI] [PubMed] [Google Scholar]
- Hoang I, Ranchet M, Derollepot R, Moreau F, Paire-Ficout L. Measuring the cognitive workload during dual-task walking in young adults: a combination of neurophysiological and subjective measures. Front Hum Neurosci. 2020:14:592532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochman M, McCormick D. Endpoint selection and relative (versus absolute) risk reporting in published medication trials. J Gen Intern Med. 2011:26(11):1246–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollman JH, Kovash FM, Kubik JJ, Linbo RA. Age-related differences in spatiotemporal markers of gait stability during dual task walking. Gait Posture. 2007:26(1):113–119. [DOI] [PubMed] [Google Scholar]
- Holtzer R, Mahoney JR, Izzetoglu M, Izzetoglu K, Onaral B, Verghese J. fNIRS study of walking and walking while talking in young and old individuals. J Gerontol A Biol Sci Med Sci. 2011:66(8):879–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulsdunker T, Ostermann M, Mierau A. The speed of neural visual motion perception and processing determines the visuomotor reaction time of young elite table tennis athletes. Front Behav Neurosci. 2019:13:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karamacoska D, Barry RJ, Steiner GZ. Electrophysiological underpinnings of response variability in the Go/NoGo task. Int J Psychophysiol. 2018:134:159–167. [DOI] [PubMed] [Google Scholar]
- Kato Y, Endo H, Kizuka T. Mental fatigue and impaired response processes: event-related brain potentials in a Go/NoGo task. Int J Psychophysiol. 2009:72(2):204–211. [DOI] [PubMed] [Google Scholar]
- Klingberg T. Concurrent performance of two working memory tasks: potential mechanisms of interference. Cereb Cortex. 1998:8(7):593–601. [DOI] [PubMed] [Google Scholar]
- Ladouce S, Donaldson DI, Dudchenko PA, Ietswaart M. Mobile EEG identifies the re-allocation of attention during real-world activity. Sci Rep. 2019:9(1):15851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambourne K, Tomporowski P. The effect of exercise-induced arousal on cognitive task performance: a meta-regression analysis. Brain Res. 2010:1341:12–24. [DOI] [PubMed] [Google Scholar]
- Larson MJ, LeCheminant JD, Carbine K, Hill KR, Christenson E, Masterson T, LeCheminant R. Slow walking on a treadmill desk does not negatively affect executive abilities: an examination of cognitive control, conflict adaptation, response inhibition, and post-error slowing. Front Psychol. 2015:6:723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone C, Feys P, Moumdjian L, D'Amico E, Zappia M, Patti F. Cognitive-motor dual-task interference: a systematic review of neural correlates. Neurosci Biobehav Rev. 2017:75:348–360. [DOI] [PubMed] [Google Scholar]
- Lord S, Rochester L, Hetherington V, Allcock LM, Burn D. Executive dysfunction and attention contribute to gait interference in ‘off' state Parkinson's disease. Gait Posture. 2010:31(2):169–174. [DOI] [PubMed] [Google Scholar]
- Lovden M, Schaefer S, Pohlmeyer AE, Lindenberger U. Walking variability and working-memory load in aging: a dual-process account relating cognitive control to motor control performance. J Gerontol B Psychol Sci Soc Sci. 2008:63(3):P121–P128. [DOI] [PubMed] [Google Scholar]
- Macmillan NA, Creelman CD. Detection theory: a user's guide. 2nd ed. Mahwah, NJ, US: Lawrence Erlbaum Associates Publishers; 2005 [Google Scholar]
- Maidan I, Nieuwhof F, Bernad-Elazari H, Reelick MF, Bloem BR, Giladi N, Deutsch JE, Hausdorff JM, Claassen JA, Mirelman A. The role of the frontal lobe in complex walking among patients with Parkinson's disease and healthy older adults: an fNIRS study. Neurorehabil Neural Repair. 2016:30(10):963–971. [DOI] [PubMed] [Google Scholar]
- Malcolm BR, Foxe JJ, Butler JS, De Sanctis P. The aging brain shows less flexible reallocation of cognitive resources during dual-task walking: a mobile brain/body imaging (MoBI) study. NeuroImage. 2015:117:230–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm BR, Foxe JJ, Butler JS, Molholm S, De Sanctis P. Cognitive load reduces the effects of optic flow on gait and electrocortical dynamics during treadmill walking. J Neurophysiol. 2018:120(5):2246–2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm BR, Foxe JJ, Butler JS, Mowrey WB, Molholm S, De Sanctis P. Long-term test-retest reliability of event-related potential (ERP) recordings during treadmill walking using the mobile brain/body imaging (MoBI) approach. Brain Res. 2019:1716:62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malcolm BR, Foxe JJ, Joshi S, Verghese J, Mahoney JR, Molholm S, De Sanctis P. Aging-related changes in cortical mechanisms supporting postural control during base of support and optic flow manipulations. Eur J Neurosci. 2021:54(12):8139–8157. [DOI] [PubMed] [Google Scholar]
- Mansouri FA, Tanaka K, Buckley MJ. Conflict-induced behavioural adjustment: a clue to the executive functions of the prefrontal cortex. Nat Rev Neurosci. 2009:10(2):141–152. [DOI] [PubMed] [Google Scholar]
- Marois R, Ivanoff J. Capacity limits of information processing in the brain. Trends Cogn Sci. 2005:9(6):296–305. [DOI] [PubMed] [Google Scholar]
- Mathalon DH, Whitfield SL, Ford JM. Anatomy of an error: ERP and fMRI. Biol Psychol. 2003:64(1–2):119–141. [DOI] [PubMed] [Google Scholar]
- Mazurek KA, Richardson D, Abraham N, Foxe JJ, Freedman EG. Utilizing high-density electroencephalography and motion capture technology to characterize sensorimotor integration while performing complex actions. IEEE Trans Neural Syst Rehabil Eng. 2020:28(1):287–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIsaac TL, Fritz NE, Quinn L, Muratori LM. Cognitive-motor interference in neurodegenerative disease: a narrative review and implications for clinical management. Front Psychol. 2018:9:2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMorris T. Exercise and cognitive function: a neuroendocrinological explanation. Exercise and Cognitive Function. 2009:41–68. [Google Scholar]
- Menon V, Adleman NE, White CD, Glover GH, Reiss AL. Error-related brain activation during a go/NoGo response inhibition task. Hum Brain Mapp. 2001:12(3):131–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol. 1990:28(5):597–613. [DOI] [PubMed] [Google Scholar]
- Mirelman A, Herman T, Brozgol M, Dorfman M, Sprecher E, Schweiger A, Giladi N, Hausdorff JM. Executive function and falls in older adults: new findings from a five-year prospective study link fall risk to cognition. PLoS One. 2012:7(6):e40297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirelman A, Maidan I, Bernad-Elazari H, Nieuwhof F, Reelick M, Giladi N, Hausdorff JM. Increased frontal brain activation during walking while dual tasking: an fNIRS study in healthy young adults. J Neuroeng Rehabil. 2014:11:85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyai I, Tanabe HC, Sase I, Eda H, Oda I, Konishi I, Tsunazawa Y, Suzuki T, Yanagida T, Kubota K. Cortical mapping of gait in humans: a near-infrared spectroscopic topography study. NeuroImage. 2001:14(5):1186–1192. [DOI] [PubMed] [Google Scholar]
- Nguyen AT, Moyle JJ, Fox AM. N2 and P3 modulation during partial inhibition in a modified go/nogo task. Int J Psychophysiol. 2016:107:63–71. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, van den Wildenberg W, Ridderinkhof KR. Electrophysiological correlates of anterior cingulate function in a go/no-go task: effects of response conflict and trial type frequency. Cogn Affect Behav Neurosci. 2003:3(1):17–26. [DOI] [PubMed] [Google Scholar]
- O'Connell RG, Dockree PM, Bellgrove MA, Kelly SP, Hester R, Garavan H, Robertson IH, Foxe JJ. The role of cingulate cortex in the detection of errors with and without awareness: a high-density electrical mapping study. Eur J Neurosci. 2007:25(8):2571–2579. [DOI] [PubMed] [Google Scholar]
- O'Connell RG, Bellgrove MA, Dockree PM, Lau A, Hester R, Garavan H, Fitzgerald M, Foxe JJ, Robertson IH. The neural correlates of deficient error awareness in attention-deficit hyperactivity disorder (ADHD). Neuropsychologia. 2009a:47(4):1149–1159. [DOI] [PubMed] [Google Scholar]
- O'Connell RG, Dockree PM, Bellgrove MA, Turin A, Ward S, Foxe JJ, Robertson IH. Two types of action error: electrophysiological evidence for separable inhibitory and sustained attention neural mechanisms producing error on go/no-go tasks. J Cogn Neurosci. 2009b:21(1):93–104. [DOI] [PubMed] [Google Scholar]
- Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci. 2011:2011:156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pailing PE, Segalowitz SJ, Dywan J, Davies PL. Error negativity and response control. Psychophysiology. 2002:39(2):198–206. [DOI] [PubMed] [Google Scholar]
- Penati R, Schieppati M, Nardone A. Cognitive performance during gait is worsened by overground but enhanced by treadmill walking. Gait Posture. 2020:76:182–187. [DOI] [PubMed] [Google Scholar]
- Pernet CR, Latinus M, Nichols TE, Rousselet GA. Cluster-based computational methods for mass univariate analyses of event-related brain potentials/fields: a simulation study. J Neurosci Methods. 2015:250:85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piercy KL, Troiano RP, Ballard RM, Carlson SA, Fulton JE, Galuska DA, George SM, Olson RD. The physical activity guidelines for Americans. JAMA. 2018:320(19):2020–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pion-Tonachini L, Kreutz-Delgado K, Makeig S. ICLabel: an automated electroencephalographic independent component classifier, dataset, and website. NeuroImage. 2019:198:181–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzamiglio S, Naeem U, Abdalla H, Turner DL. Neural correlates of single- and dual-task walking in the real world. Front Hum Neurosci. 2017:11:460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer P, Eskes G, Wallace S, Giuffrida C, Fraas M, Campbell G, Clifton KL, Skidmore ER, American Congress of Rehabilitation Medicine Stroke Networking Group Cognition Task F . Cognitive-motor interference during functional mobility after stroke: state of the science and implications for future research. Arch Phys Med Rehabil. 2013:94(12):2565–2574 e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer P, Apple S, Dowd C, Keith E. Texting and walking: effect of environmental setting and task prioritization on dual-task interference in healthy young adults. Gait Posture. 2015:41(1):46–51. [DOI] [PubMed] [Google Scholar]
- Priest AW, Salamon KB, Hollman JH. Age-related differences in dual task walking: a cross sectional study. J Neuroeng Rehabil. 2008:5:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protzak J, Gramann K. EEG beta-modulations reflect age-specific motor resource allocation during dual-task walking. Sci Rep. 2021:11(1):16110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protzak J, Wiczorek R, Gramann K. Peripheral visual perception during natural overground dual-task walking in older and younger adults. Neurobiol Aging. 2021:98:146–159. [DOI] [PubMed] [Google Scholar]
- Reiser JE, Wascher E, Rinkenauer G, Arnau S. Cognitive-motor interference in the wild: assessing the effects of movement complexity on task switching using mobile EEG. Eur J Neurosci. 2020. [DOI] [PubMed] [Google Scholar]
- Rentrop M, Backenstrass M, Jaentsch B, Kaiser S, Roth A, Unger J, Weisbrod M, Renneberg B. Response inhibition in borderline personality disorder: performance in a Go/Nogo task. Psychopathology. 2008:41(1):50–57. [DOI] [PubMed] [Google Scholar]
- Richardson DP, Foxe JJ, Mazurek KA, Abraham N, Freedman EG. Neural markers of proactive and reactive cognitive control are altered during walking: a mobile brain-body imaging (MoBI) study. NeuroImage. 2022:247:118853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesel A, Weinberg A, Endrass T, Meyer A, Hajcak G. The ERN is the ERN is the ERN? Convergent validity of error-related brain activity across different tasks. Biol Psychol. 2013:93(3):377–385. [DOI] [PubMed] [Google Scholar]
- Roche RA, Dockree PM, Garavan H, Foxe JJ, Robertson IH, O'Mara SM. EEG alpha power changes reflect response inhibition deficits after traumatic brain injury (TBI) in humans. Neurosci Lett. 2004:362(1):1–5. [DOI] [PubMed] [Google Scholar]
- Roche RA, Garavan H, Foxe JJ, O'Mara SM. Individual differences discriminate event-related potentials but not performance during response inhibition. Exp Brain Res. 2005:160(1):60–70. [DOI] [PubMed] [Google Scholar]
- Ruchsow M, Groen G, Kiefer M, Hermle L, Spitzer M, Falkenstein M. Impulsiveness and ERP components in a Go/Nogo task. J Neural Transm (Vienna). 2008:115(6):909–915. [DOI] [PubMed] [Google Scholar]
- Sakoe H, Chiba S. Dynamic programming algorithm optimization for spoken word recognition. IEEE Trans Acoust Speech Signal Process. 1978:26:159–165. [Google Scholar]
- Schubert T, Szameitat AJ. Functional neuroanatomy of interference in overlapping dual tasks: an fMRI study. Brain Res Cogn Brain Res. 2003:17(3):733–746. [DOI] [PubMed] [Google Scholar]
- Seeber M, Scherer R, Wagner J, Solis-Escalante T, Muller-Putz GR. EEG beta suppression and low gamma modulation are different elements of human upright walking. Front Hum Neurosci. 2014:8:485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw EP, Rietschel JC, Hendershot BD, Pruziner AL, Miller MW, Hatfield BD, Gentili RJ. Measurement of attentional reserve and mental effort for cognitive workload assessment under various task demands during dual-task walking. Biol Psychol. 2018:134:39–51. [DOI] [PubMed] [Google Scholar]
- Smith JL, Johnstone SJ, Barry RJ. Movement-related potentials in the Go/NoGo task: the P3 reflects both cognitive and motor inhibition. Clin Neurophysiol. 2008:119(3):704–714. [DOI] [PubMed] [Google Scholar]
- Smith JL, Smith EA, Provost AL, Heathcote A. Sequence effects support the conflict theory of N2 and P3 in the Go/NoGo task. Int J Psychophysiol. 2010:75(3):217–226. [DOI] [PubMed] [Google Scholar]
- Sosnik R, Danziger-Schragenheim S, Possti D, Fahoum F, Giladi N, Hausdorff JM, Mirelman A, Maidan I. Impaired inhibitory control during walking in Parkinson’s disease patients: an EEG study. J Parkinsons Dis. 2022:12:243–256. [DOI] [PubMed] [Google Scholar]
- St George RJ, Jayakody O, Healey R, Breslin M, Hinder MR, Callisaya ML. Cognitive inhibition tasks interfere with dual-task walking and increase prefrontal cortical activity more than working memory tasks in young and older adultsi. Gait & Posture. 2022:95:186–191. [DOI] [PubMed] [Google Scholar]
- Steele VR, Aharoni E, Munro GE, Calhoun VD, Nyalakanti P, Stevens MC, Pearlson G, Kiehl KA. A large scale (N=102) functional neuroimaging study of response inhibition in a Go/NoGo task. Behav Brain Res. 2013:256:529–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegemoller EL, Wilson JP, Hazamy A, Shelley MC, Okun MS, Altmann LJ, Hass CJ. Associations between cognitive and gait performance during single- and dual-task walking in people with Parkinson disease. Phys Ther. 2014:94(6):757–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Switonski A, Josinski H, Wojciechowski K. Dynamic time warping in classification and selection of motion capture data. Multidim Syst Sign Process. 2019:30(3):1437–1468. [Google Scholar]
- Takakusaki K. Functional neuroanatomy for posture and gait control. J Mov Disord. 2017:10(1):1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner D, Morgan-Short K, Luck SJ. How inappropriate high-pass filters can produce artifactual effects and incorrect conclusions in ERP studies of language and cognition. Psychophysiology. 2015:52(8):997–1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall TL, Stratton PG, Coyne TJ, Cook R, Silberstein P, Silburn PA, Windels F, Sah P. Imagined gait modulates neuronal network dynamics in the human pedunculopontine nucleus. Nat Neurosci. 2014:17(3):449–454. [DOI] [PubMed] [Google Scholar]
- Tomporowski PD. Effects of acute bouts of exercise on cognition. Acta Psychol. 2003:112(3):297–324. [DOI] [PubMed] [Google Scholar]
- Van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulate cortex. J Cogn Neurosci. 2002a:14(4):593–602. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiol Behav. 2002b:77(4–5):477–482. [DOI] [PubMed] [Google Scholar]
- Vickers AJ. The use of percentage change from baseline as an outcome in a controlled trial is statistically inefficient: a simulation study. BMC Med Res Methodol. 2001:1:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner J, Solis-Escalante T, Grieshofer P, Neuper C, Muller-Putz G, Scherer R. Level of participation in robotic-assisted treadmill walking modulates midline sensorimotor EEG rhythms in able-bodied subjects. NeuroImage. 2012:63(3):1203–1211. [DOI] [PubMed] [Google Scholar]
- Wakim KM, Freedman EG, Molloy CJ, Vieyto N, Cao Z, Foxe JJ. Assessing combinatorial effects of HIV infection and former cocaine dependence on cognitive control processes: a high-density electrical mapping study of response inhibition. Neuropharmacology. 2021:195:108636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler I, Debener S, Muller KR, Tangermann M. On the influence of high-pass filtering on ICA-based artifact reduction in EEG-ERP. Annu Int Conf IEEE Eng Med Biol Soc. 2015:2015:4101–4105. [DOI] [PubMed] [Google Scholar]
- Wrightson JG, Smeeton NJ. Walking modality, but not task difficulty, influences the control of dual-task walking. Gait Posture. 2017:58:136–138. [DOI] [PubMed] [Google Scholar]
- Wrightson JG, Ross EZ, Smeeton NJ. The effect of cognitive-task type and walking speed on dual-task gait in healthy adults. Mot Control. 2016:20(1):109–121. [DOI] [PubMed] [Google Scholar]
- Wrightson JG, Schafer L, Smeeton NJ. Dual-task prioritization during overground and treadmill walking in healthy adults. Gait Posture. 2020:75:109–114. [DOI] [PubMed] [Google Scholar]
- Zelazo P, Carter A, Reznick J, Frye D. Early development of executive function: a problem-solving framework. Rev Gen Psychol. 1997:1:198–226. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.