Abstract
Different studies have shown that females develop liver diseases at lower levels of alcohol consumption than males. Our aim was to quantify the dose-response relationship between alcohol consumption and the risk of liver cirrhosis by sex and identify the differences between females and males. A systematic review was conducted using PubMed/Medline and Embase to identify longitudinal and case-control studies that analyzed the relationship between the level of alcohol use and liver cirrhosis (LC) incidence, and mortality (ICD-8 and ICD-9 codes 571 and ICD-10 codes K70, K73, K74). Pooled relative risks (RR) were calculated by random effects models. Restricted cubic splines were used to model the dose-response relationship. A total of 24 studies were included in the analysis. There were collectively 2,112,476 females and 924,853 males, and a total of 4,301 and 4,231 cases of LC for females and males, respectively. We identified a non-linear dose-response relationship. Females showed a higher risk for LC compared to males with the same amount of alcohol consumed daily. For instance, drinking 40 g/day showed RRs of 9.35 (95% CI 7.64–11.45) in females and 2.82 (95% CI 2.53–3.14) in males, while drinking 80 g/day presented RRs of 23.32 (95% CI 18.24–29.82) in females and 7.93 (95% CI 7.12–8.83) in males. Additional analyses showed that a higher risk for females was found for morbidity and for mortality. Understanding the influence of sex on the association of alcohol consumption and the risk of LC is needed to develop recommendations and clinical guidelines for prevention and treatment.
Systematic Review Registration:
https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022299680, identifier CRD42022299680.
Keywords: liver cirrhosis, alcohol, systematic review, meta-analysis, epidemiology
Introduction
Globally, the incidence of liver cirrhosis (LC) has shown an increasing trend in females and a stabilized trend in males in recent years (1), with certain areas such as the Eastern Mediterranean Region, African Region and South-East Asia, having an increasing trend of LC in females and males. Other areas had an increasing trend only in males, such as the European Region, and only in females, such as the Region of the Americas. In addition, although death and disability-adjusted life years (DALY) global rates of LC decreased from 1990 to 2019, the number of deaths and DALYs and the proportion of all global deaths due to LC have increased (2), being ranked the 8th most common non-communicable disease cause of death globally in 2019 (1). With alcohol use being a key risk factor in the progression and mortality of LC (3), particularly among females (4, 5), efforts should be made to develop alcohol control policies and prevention measures to address this concerning public health problem.
There is evidence that a dose-response relationship between alcohol consumption and LC exists (6–8) and, at the same level of alcohol consumption, females seem to have a higher risk of developing LC than males (7). By studying how the dose-response relationship varies by cause of LC and by fatal vs non-fatal events in females and males (9), we will have strong evidence to inform policy makers and prevention experts on whether most of their efforts should be directed to the general population level or targeted towards females and males by their levels of alcohol use. For instance, if the risk curves are more linear, a general population approach will yield better results, whereas with more exponential risk curves targeted approaches should be recommended (10). Consequently, there is a need to have global, updated, and precise risk curves on the association of alcohol consumption and LC in females and males from which we can identify the meaningful risk increases for each sex. Accordingly, our aim was to quantify the dose-response relationship on alcohol use and the risk of LC by sex using meta-analysis and meta-regression models, and to identify the differences between females and males in the risks of morbidity and mortality due to LC.
Materials and methods
Systematic review
We conducted a systematic review using PubMed/Medline and Embase databases from their inception to October 12, 2021, applying the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria (see PRISMA checklist (11) in the Supplementary Material 1). An updated search was conducted on January 14, 2022 with no new references discovered. Any combination of key words and MeSH terms relating to alcohol consumption, LC, and observational studies were included (for the complete search strategy, see Supplementary Material 2) and the reference list of relevant articles was reviewed. All references were screened by one author, with independent verification by two additional reviewers. We screened the publications which resulted from a wider review on alcohol consumption and LC (PROSPERO registration number CRD42022299680) in order to identify papers with sex-specific results.
Studies included were articles with a longitudinal or case control design. The exposure variable was the quantity of alcohol use. The outcome was LC morbidity (incidence of LC or decompensated LC) and mortality (ICD-8 and ICD-9 codes 571 and ICD-10 codes K70, K73, K74). Studies were excluded if they were not published as full reports, they used a cross sectional design, or there was not enough data to compute the relative risk related to alcohol use. For this review, we also excluded articles which presented their results with both sexes combined.
Data extraction
Two authors extracted relevant information using a standardized spreadsheet which included the title, first author, year of publication, country, study design, year of study at baseline and follow-up, sex, sample size, cause of LC, socioeconomic status, alcohol consumption categories, time period of alcohol consumption, risk estimates (relative risk (RR), odds ratio (OR) and hazard ratio (HR)) with their corresponding 95% CIs, adjustments, and outcomes.
If the quantity of alcohol use was not presented in grams per day, we converted it based on the size of a standard drink in the country the study was based on. When alcohol was given in ranges, the midpoint was taken. If there was no upper bound for the highest category, 75% of the width from the previous category range was taken and added to the lower bound, and this value was used to represent the highest level of alcohol use.
Quality assessment
We used an adapted version of the Cochrane Risk-of-Bias Tool for Non-Randomized Studies (ROBINS-I) (12) to assess the risk of bias in the studies included in our analysis (for details on the adaptation, see Supplementary Material 3). The evidence was rated based on the Grading of Recommendations Assessment, Development and Evaluation approach (13). Each article was rated by at least two authors and several consensus conferences were held to produce the final ratings. The overall bias for each study was rated with one of the following scores: low, moderate, serious, or critical, with the ranking going from the lowest to the highest risk of bias.
Statistical analysis
We harmonized the reference categories of all the included studies to have lifetime abstainers serve as the reference category. This step was necessary to avoid the “sick quitter bias” which is the bias of including abstainers who quit drinking for health reasons (14) (for similar analyses, see (15)). RRs were pooled with the inverse-variance method using Restricted Maximum Likelihood (REML) random effects model (16). Heterogeneity was quantified using the Cochrane Q-test and the I2 statistic. In order to obtain the dose-response curves, five models were tested (linear, quadratic, restrictive cubic splines, cubic polynomial, and fractional polynomial) (17). The model which best fit our data was selected based on Akaike Information Criterion (AIC) and Bayesian information criterion (BIC) statistics.
We ran meta-regression models, integrating sex, cause of LC, quality score, and outcomes to test their interactions with the amount of alcohol consumed. The differences between the estimates were tested using a Wald-type test. In addition, we conducted a sensitivity analysis excluding the study conducted by Liu and colleges (18), as it had the highest weight in our analysis due to its large sample size. All analysis were conducted using meta (19) and metafor (20) packages in R software version 4.2.1 (21).
Results
A total of 24 studies were included in the analysis (Figure 1); 13 of the studies provided results for females and 19 provided results for males. Table 1 presents the studies’ characteristics. There were collectively 2,112,476 females and 924,853 males, and a total of 4,301 and 4,231 cases of LC for females and males respectively. The majority of studies were cohort studies (67%). In total, 10 studies (42%) provided results for LC morbidity, 11 studies (46%) were mortality studies, and three studies (12%) presented their results with both morbidity and mortality combined. As for the type of LC, 20 studies provided data including all causes of LC, two studies for alcohol-related LC, one study for HCV-induced LC, and one study for alcohol-related LC and HCV-induced LC combined. In the risk-of-bias assessment, 10 studies had a moderate score, six studies had a serious score, and eight studies had a critical score.
FIGURE 1.
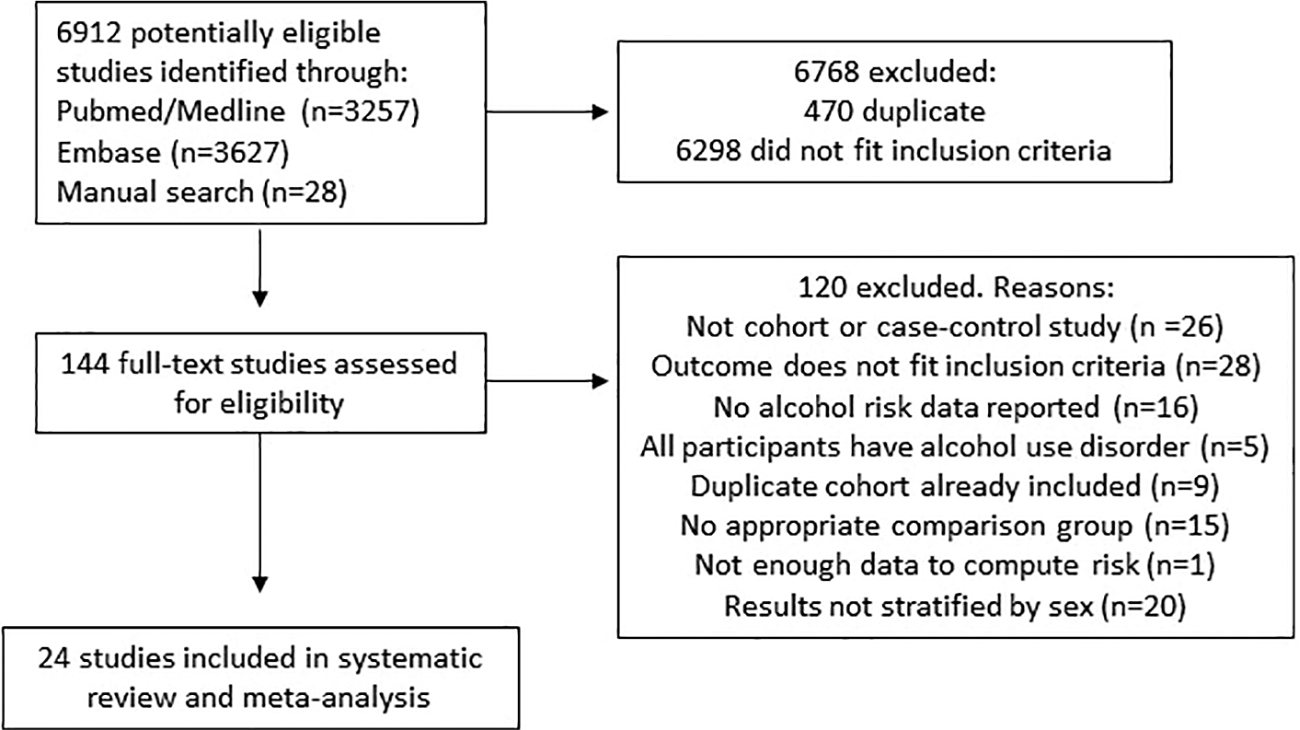
Flow-chart of the study selection process.
TABLE 1.
Characteristics of the included studies.
| Authors | Year | Country | Study design | FU-years | N | Female (%) | Cause LC | Alcohol use in g/day: n | Relative risk* | CI 95% lower | CI 95% upper | Adjusted | Outcome |
Quality score | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Category | P (%) | ||||||||||||||
|
| |||||||||||||||
| Alemy-Carreau et al. (22) | 1996 | France | Case-control | N.A. | 221 | 0 | ALD + HCV | 0 g/d: 24 | Reference | – | – | No | Cirrhosis | 101 (46%) | Critical |
| 100 g/d: 197 | 121.1 | 49.4 | 298.9 | ||||||||||||
| Askgaard et al. (23) | 2015 | Denmark | Cohort | 15 | 55,917 | 52 | ALD | Male | Yes | Cirrhosis and LC mortality | 257 (1%) | Serious | |||
| 0–24 g/d: 15,028 | Reference | – | – | ||||||||||||
| 24–48 g/d: 6,800 | 2.33 | 1.52 | 3.58 | ||||||||||||
| 48–72 g/d: 2,774 | 6.98 | 4.65 | 10.5 | ||||||||||||
| 72–96 g/d: 1,062 | 13.12 | 8.51 | 20.23 | ||||||||||||
| 96–120 g/d: 445 | 29.03 | 18.63 | 45.24 | ||||||||||||
| >120 g/d: 174 | 50 | 30.12 | 82.96 | ||||||||||||
| Female | 85 (0.3%) | ||||||||||||||
| 0–24 g/d: 23,278 | Reference | – | – | ||||||||||||
| 24–48 g/d: 4,242 | 3.49 | 2 | 6.12 | ||||||||||||
| 48–72 g/d: 744 | 16.2 | 9.16 | 28.7 | ||||||||||||
| >72 g/d: 222 | 21.57 | 10.38 | 44.84 | ||||||||||||
| Batey et al. (24) | 1992 | Australia | Case-control | N.A. | 158 | 0 | All cause | ≤40 g/d: 109 | Reference | – | – | No | Cirrhosis | 43 (27%) | Serious |
| 41–80 g/d:23 | 8.8 | 3.2 | 24.3 | ||||||||||||
| >80 g/d: 26 | 21.9 | 7.7 | 62.9 | ||||||||||||
| Becker et al. (25) | 2002 | Denmark | Cohort | 5 | 30,630 | 47 | ALD | Male | Yes | Cirrhosis and LC mortality | 212 (1%) | Moderate | |||
| <1.7 g/d | 7.76 | 3.35 | 18 | ||||||||||||
| 1.7–12 g/d | Reference | – | – | ||||||||||||
| 12–36 g/d | 2.34 | 1.18 | 4.62 | ||||||||||||
| 36–60 g/d | 1.34 | 0.7 | 2.56 | ||||||||||||
| >60 g/d | 2.63 | 1.39 | 5 | ||||||||||||
| Female | 80 (0.6%) | ||||||||||||||
| <1.7 g/d | 1.11 | 0.43 | 2.84 | ||||||||||||
| 1.7–12 g/d | Reference | – | – | ||||||||||||
| 12–36 g/d | 4.48 | 2.21 | 9.08 | ||||||||||||
| 36–60 g/d | 9.08 | 3.6 | 22.77 | ||||||||||||
| >60 g/d | 11.85 | 3.74 | 37.48 | ||||||||||||
| Blackwelder et al. (26) | 1980 | USA | Cohort | 4 | 7888 | 0 | All cause | 0 g/d: 3,747 | 2.11 | 0.25 | 17.49 | No | LC mortality | 16 (0.2%) | Serious |
| 1–10 g/d: 1,316 | Reference | – | – | ||||||||||||
| 11–30 g/d: 1,593 | 1.65 | 0.15 | 18.2 | ||||||||||||
| ≥30 g/d: 1,232 | 7.48 | 0.92 | 60.69 | ||||||||||||
| Boffetta et al. (27) | 1990 | USA | Cohort | 12 | 276,802 | 0 | All cause | 0 g/d: 153,043 | Reference | – | – | Yes | LC mortality | 687 (0.2%) | Moderate |
| 14 g/d: 33,229 | 1.21 | 0.86 | 1.69 | ||||||||||||
| 28 g/d: 23,558 | 3.15 | 2.39 | 4.16 | ||||||||||||
| 42 g/d: 11,257 | 5.39 | 4 | 7.26 | ||||||||||||
| 56 g/d: 7,309 | 8.67 | 6.45 | 11.6 | ||||||||||||
| 70 g/d: 3,368 | 10.6 | 7.36 | 15.3 | ||||||||||||
| ≥84 g/d: 7,698 | 18.1 | 14.1 | 23.2 | ||||||||||||
| Corrao et al. (28) | 1993 | Italy | Case-control | N.A. | 640 | 35 | All cause | Male | Yes | Cirrhosis | 207 (50%) | Serious | |||
| LTA: 28 | Reference | – | – | ||||||||||||
| 25 or 50 g/d: 58 | 1.4 | 0.6 | 3.1 | ||||||||||||
| 75 or 100 g/d: 98 | 1.6 | 0.7 | 3.8 | ||||||||||||
| 125 or 150 g/d: 95 | 2.1 | 0.9 | 4.7 | ||||||||||||
| ≥175 g/d: 135 | 4.9 | 2.2 | 10.9 | ||||||||||||
| Female | 113 (50%) | ||||||||||||||
| LTA: 62 | Reference | – | – | ||||||||||||
| 25 or 50 g/d: 82 | 0.5 | 0.2 | 0.8 | ||||||||||||
| 75 or 100 g/d: 38 | 1.4 | 0.6 | 3.2 | ||||||||||||
| 125 or 150 g/d: 26 | 2.6 | 1 | 6.5 | ||||||||||||
| ≥175 g/d: 18 | 8.7 | 2.3 | 33 | ||||||||||||
| Corrao et al. (29) | 1997 | Italy | Case-control | N.A. | 1,113 | 41 | All cause | Male | Yes | Cirrhosis | 300 (46%) | Serious | |||
| LTA: 48 | Reference | – | – | ||||||||||||
| <50 g/d: 255 | 1.9 | 0.62 | 5.8 | ||||||||||||
| 50–100 g/d: 132 | 9.1 | 2.94 | 28.18 | ||||||||||||
| ≥100 g/d: 220 | 31.4 | 10.3 | 95.76 | ||||||||||||
| Female | 162 (35%) | ||||||||||||||
| LTA: 104 | Reference | – | – | ||||||||||||
| <50 g/d: 306 | 2.09 | 0.69 | 6.3 | ||||||||||||
| 50–100 g/d: 35 | 7.5 | 3.46 | 16.25 | ||||||||||||
| ≥100 g/d: 13 | 20.36 | 6.3 | 65.79 | ||||||||||||
| Fuchs et al. (30) | 1995 | USA | Cohort | 12 | 85,709 | 100 | All cause | 0 g/d: 25535 | 4.76 | 1.66 | 39.29 | Yes | LC mortality | 52 (0.06%) | Moderate |
| 0.1–1.4 g/d: 11304 | Reference | – | – | ||||||||||||
| 1.5–4.9 g/d: 18460 | 3.29 | 1.14 | 9.43 | ||||||||||||
| 5–14.9 g/d: 17783 | 6.05 | 2.57 | 14.33 | ||||||||||||
| 15–29.9 g/d: 8106 | 8.86 | 3.62 | 21.86 | ||||||||||||
| ≥30 g/d: 4521 | 12.14 | 5.05 | 29.1 | ||||||||||||
| Garfinkel et al. (31) | 1988 | USA | Cohort | 12 | 581,321 | 100 | All cause | LTA: 467,382 | Reference | – | – | No | LC mortality | 589 (0.01%) | Critical |
| 14 g/d: 20,000 | 2.46 | 1.87 | 3.25 | ||||||||||||
| 28 g/d:13,000 | 7.4 | 5.9 | 9.27 | ||||||||||||
| 49 g/d: 10,000 | 14.21 | 10.77 | 18.75 | ||||||||||||
| 77 g/d: 12,000 | 16.7 | 12.1 | 23.04 | ||||||||||||
| ≥84 g/d: 2,000 | 28.29 | 20.21 | 39.59 | ||||||||||||
| Gordon et al. (32) | 1984 | USA | Cohort | 22 | 4747 | 56 | All cause | Male | No | LC mortality | 14 (0.7%) | Critical | |||
| 0 g/d: 402 | Reference | – | – | ||||||||||||
| 0.7–20 g/d: 1107 | 1.45 | 0.16 | 12.96 | ||||||||||||
| 21–41 g/d: 344 | 3.51 | 0.37 | 33.55 | ||||||||||||
| 41–61 g/d: 131 | 9.21 | 0.97 | 87.75 | ||||||||||||
| 62–82 g/d: 50 | 8.04 | 0.51 | 126.55 | ||||||||||||
| 83–248 g/d: 72 | 11.17 | 1.03 | 121.55 | ||||||||||||
| Female | |||||||||||||||
| 0–7-20 g/d: 2434 | Reference | – | – | ||||||||||||
| 21–61 g/d: 191 | 1.59 | 0.2 | 12.67 | ||||||||||||
| 62 g/d-248 g/d: 16 | 19 | 2.52 | 143.34 | ||||||||||||
| Gordon et al. (33) | 1987 | USA | Cohort | 28 | 1,762 | 0 | All cause | 0 g/d: 585 | Reference | – | – | No | LC mortality | 15 (0.9%) | Critical |
| 0.7–20 g/d: 842 | 0.52 | 0.12 | 2.32 | ||||||||||||
| 21–41 g/d: 175 | 2.51 | 0.57 | 11.1 | ||||||||||||
| 41–61 g//d: 100 | 2.93 | 0.54 | 15.76 | ||||||||||||
| > 62 g/d: 60 | 7.31 | 1.68 | 31.91 | ||||||||||||
| Im et al. (34) | 2021 | China | Cohort | 10.1 | 218,341 | 59 | All cause | Male | Yes | Cirrhosis | 1210 (1%) | Moderate | |||
| 1 g/d: 117,072 | Reference | – | – | ||||||||||||
| <20 g/d: 24,171 | 1.29 | 1.06 | 1.57 | ||||||||||||
| 20–40 g/d: 18,182 | 2.47 | 2.1 | 2.91 | ||||||||||||
| 40–60 g/d: 12,318 | 3.13 | 2.59 | 3.79 | ||||||||||||
| ≥ 60 g/d: 12,318 | 8.15 | 7.04 | 9.43 | ||||||||||||
| Female | 946 (3%) | ||||||||||||||
| 1 g/d: 28,396 | Reference | – | – | ||||||||||||
| 16 g/d: 5,896 | 1.09 | 0.71 | 1.68 | ||||||||||||
| Khan et al. (35) | 2000 | Japan | Case-control | N.A. | 106 | 0 | HCV | 0 g/d: 40 | Reference | – | – | No | Cirrhosis | 54 (51%) | Critical |
| <80 g/d: 42 | 6 | 2.29 | 15.69 | ||||||||||||
| >80 g/d: 24 | 6 | 1.98 | 18.21 | ||||||||||||
| Klatsky et al. (36) | 2003 | USA | Cohort | 20 | 128,934 | 56 | All cause | Male | No | LC mortality | 146 (0.3%) | Moderate | |||
| LTA: 4,125 | Reference | – | – | ||||||||||||
| <0.5 g/d: 8,105 | 0.7 | 0.32 | 1.53 | ||||||||||||
| <14 g/d: 21,264 | 0.5 | 0.25 | 1.02 | ||||||||||||
| 14–28 g/d: 13,512 | 1.3 | 0.68 | 2.48 | ||||||||||||
| 42–70 g/d: 5,905 | 3.3 | 1.7 | 6.4 | ||||||||||||
| ≥84 g/d: 1,535 | 8.3 | 3.97 | 17.34 | ||||||||||||
| Female | 86 (0.1%) | ||||||||||||||
| LTA: 11,373 | Reference | – | – | ||||||||||||
| <0.5 g/d: 19,312 | 1.2 | 0.42 | 3.42 | ||||||||||||
| <14 g/d: 26,631 | 2.5 | 1 | 6.26 | ||||||||||||
| 14–28 g/d: 9,896 | 4.7 | 2.02 | 10.95 | ||||||||||||
| 42–70 g/d: 2,523 | 14.2 | 5.94 | 33.96 | ||||||||||||
| ≥84 g/d: 469 | 15.2 | 5.81 | 39.76 | ||||||||||||
| Kono et al. (37) | 1986 | Japan | Cohort | 18 | 5,135 | 0 | All cause | LTA: 1,074 | Reference | – | – | Yes | LC mortality | 43 (0.8%) | Moderate |
| <27 g/d: 1,034 | 0.3 | 0.1 | 1.1 | ||||||||||||
| >27 g/d: 925 | 1.8 | 0.8 | 4 | ||||||||||||
| Liu et al. (18) | 2009 | UK | Cohort | 6 | 1,290,413 | 100 | All cause | 0 g/d: 305,652 | 1.41 | 1.23 | 1.61 | Yes | Cirrhosis and LC mortality | 2105 (0.2%) | Moderate |
| 1–2 g/d:372,065 | Reference | – | – | ||||||||||||
| 3–7 g/d:294,353 | 1.24 | 1.08 | 1.44 | ||||||||||||
| 8–16 g/d: 241,307 | 1.84 | 1.6 | 2.11 | ||||||||||||
| ≥17 g/d: 67,360 | 4.32 | 3.71 | 5.03 | ||||||||||||
| Norton et al. (38) | 1987 | Australia | Case-control | N.A. | 135 | 100 | All cause | <40 g/d: 102 | Reference | – | – | No | Cirrhosis | 36 (27%) | Critical |
| >40 g/d: 33 | 784 | 84.52 | 7272.06 | ||||||||||||
| Pequignot et al. (39) | 1978 | France | Case-control | N.A. | 962 | 0 | All cause | 0–20 g/d: 188 | Reference | – | – | Yes | Cirrhosis | 184 (19%) | Serious |
| 21–40 g/d: 222 | 3.1 | 0.84 | 11.42 | ||||||||||||
| 41–60 g/d: 180 | 6.2 | 1.76 | 21.83 | ||||||||||||
| 61–80 g/d: 132 | 13.8 | 4.06 | 46.92 | ||||||||||||
| 81–100 g/d: 88 | 29.6 | 8.71 | 100.55 | ||||||||||||
| 101–120 g/d: 54 | 41 | 11.61 | 144.83 | ||||||||||||
| 121–140 g/d: 38 | 124.3 | 33.1 | 466.75 | ||||||||||||
| >141 g/d: 60 | 659.3 | 159.57 | 2723.98 | ||||||||||||
| Schult et al. (40) | 2017 | Sweden | Cohort | 33 | 1,462 | 100 | All cause | 0 g/d | Reference | – | – | No | Cirrhosis | 11 (0.8%) | Critical |
| 10 g/d | 2.16 | 1.57 | 2.99 | ||||||||||||
| 30 g/d | 10.08 | 3.87 | 26.73 | ||||||||||||
| Stroffolini et al. (41) | 2010 | Italy | Case-control | N.A. | 397 | 34 | All cause | Male | No | Cirrhosis | 111 (14%) | Critical | |||
| 12–24 g/d: 215 | Reference | – | – | ||||||||||||
| 36 g/d: 56 | 1.2 | 0.5 | 2.9 | ||||||||||||
| ≥36 g/d: 239 | 4.3 | 2.5 | 7.3 | ||||||||||||
| Female | 26 (3%) | ||||||||||||||
| 12–24 g/d: 204 | Reference | – | – | ||||||||||||
| 36 g/d: 18 | 0.8 | 0.1 | 6.5 | ||||||||||||
| ≥36 g/d: 37 | 5.7 | 2.3 | 14.5 | ||||||||||||
| Yang et al. (42) | 2012 | China | Cohort | 15 | 218,189 | 0 | ALD | 0 g/d: 145,323 | Reference | – | – | Yes | LC mortality | 418 (0.2%) | Moderate |
| <20 g/d: 14,208 | 1.12 | 0.76 | 1.64 | ||||||||||||
| 20–40 g/d: 19,391 | 0.84 | 0.59 | 1.21 | ||||||||||||
| 40–60 g/d: 18,681 | 1.16 | 0.85 | 1.56 | ||||||||||||
| 60–100 g/d: 10,870 | 1.22 | 0.83 | 1.79 | ||||||||||||
| ≥100 g/d: 9,716 | 1.74 | 1.22 | 2.48 | ||||||||||||
| Yi et al. (43) | 2016 | Korea | Cohort | 6 | 107,735 | 0 | ALD | <1.3 g/d: 34,435 | Reference | – | – | Yes | LC mortality | 178 (0.2%) | Moderate |
| 1.3–8 g/d: 31,341 | 1.32 | 0.81 | 2.18 | ||||||||||||
| 9–17 g/d: 15,162 | 2.45 | 1.48 | 4.07 | ||||||||||||
| 18–35 g/d: 15,764 | 2.77 | 1.71 | 4.5 | ||||||||||||
| ≥36 g/d: 11,033 | 4.03 | 2.51 | 6.46 | ||||||||||||
| Yuan et al. (44) | 1997 | China | Cohort | 9 | 18,244 | 0 | ALD | LTA: 10,471 | Reference | – | – | Yes | LC mortality | 35 (0.2%) | Moderate |
| 1.7–48 g/d: 6,189 | 0.46 | 0.17 | 1.22 | ||||||||||||
| ≥50 g/d: 1,201 | 2.99 | 1.12 | 7.94 | ||||||||||||
FU, follow-up; N, sample size; LTA, lifetime abstainers; CI, confidence intervals; P, percent in sample; N.A., not applicable; ALD, alcohol-related liver disease; HCV, hepatitis C virus; LC, liver cirrhosis.
Risk-relation estimates were based on either odds ratios, relative risks, or hazard ratios.
A non-linear dose-response curve was identified for both females and males. The restrictive cubic spline model (17) was the model that best fit our data (for reasoning on the selection of the model see Supplementary Material 4). For the same amount of alcohol use, females presented a higher relative risk of LC than males (Figure 2). For both curves, the risk of LC increased with the average number of grams of alcohol consumed daily. Table 2 presents the RRs and the risk ratios of females vs males. The risk ratio was not constant, first increasing in value and then decreasing as the level of alcohol consumption became higher.
FIGURE 2.
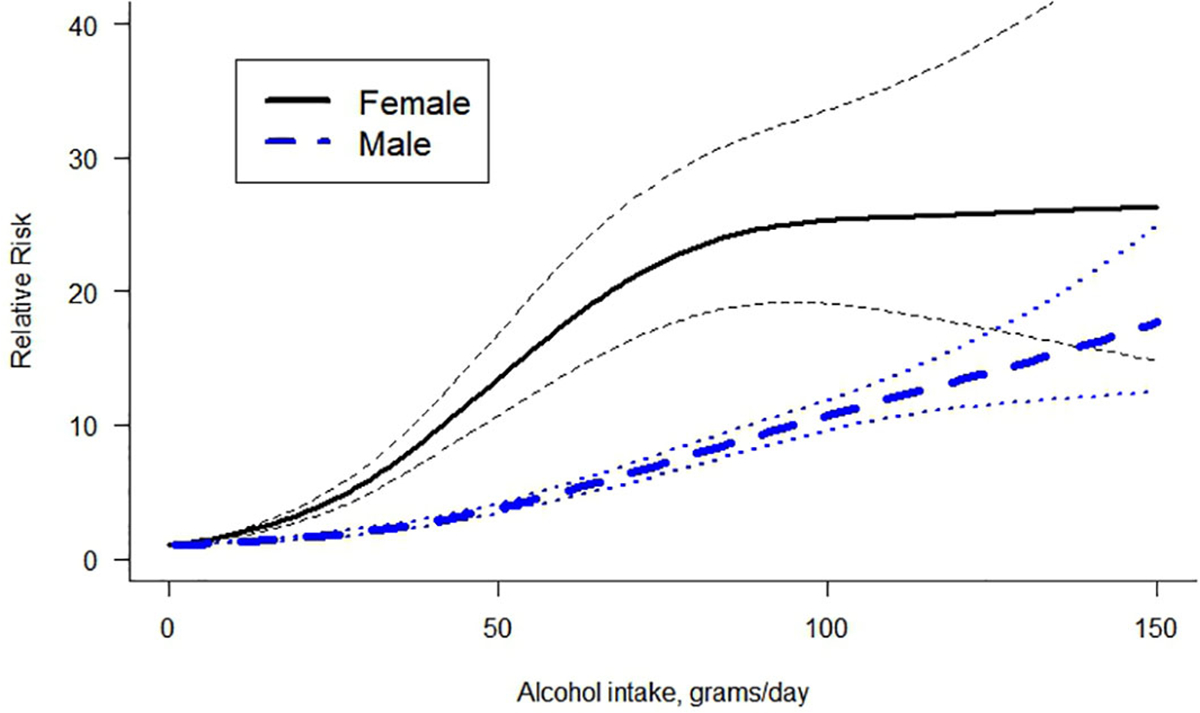
Dose-response curve between alcohol intake in grams per day and the risk of liver cirrhosis by sex.
TABLE 2.
Relative risk and risk ratio by sex for some levels of alcohol use.
| Grams of pure alcohol per day | Female RR (95% CI) | Male RR (95% CI) | Risk Ratio |
|---|---|---|---|
|
| |||
| 20 | 3.34 (2.81 – 3.97) | 1.60 (1.46 – 1.77) | 2.09 |
| 40 | 9.35 (7.64 – 11.45) | 2.82 (2.53 – 3.14) | 3.32 |
| 60 | 17.54 (13.80 – 22.91) | 5.09 (4.85 – 5.65) | 3.45 |
| 80 | 23.32 (18.24 – 29.82) | 7.93 (7.12 – 8.83) | 2.94 |
| 100 | 25.33 (19.09 – 33.60) | 10.76 (9.69 – 11.94) | 2.35 |
| 120 | 25.77 (17.62 – 37.68) | 13.40 (11.30 – 15.88) | 1.92 |
We identified several statistically significant differences in the overall meta-regression model (for full model results and estimates, see Supplementary Material 5). We obtained a significantly higher LC risk for females than for males (beta=−0.0319, se=0.00407; estimate for the interaction between sex and dose of alcohol consumption with female as reference). In addition, mortality studies presented a significantly higher risk compared to morbidity studies. Based on the quality scores obtained in the risk assessment, studies with serious and critical scores presented a higher risk compared to studies with moderate scores. Finally, we identified a significant steeper slope in HCV-related studies compared to all-cause LC and a steeper slope in all-cause LC compared to alcohol-related LC, this latter not being statistically significant.
In addition, we identified a higher risk for morbidity outcomes in females compared to males and a significantly higher risk for mortality due to LC in females compared to males (Figure 3, for full model results, see Supplementary Material 6). We also tested the interaction of morbidity and mortality stratified by sex (for full model results, see Supplementary Material 7). In females, we did not identify statistically significant differences between morbidity studies and mortality studies. For males, mortality studies presented a significantly higher risk compared to morbidity studies.
FIGURE 3.
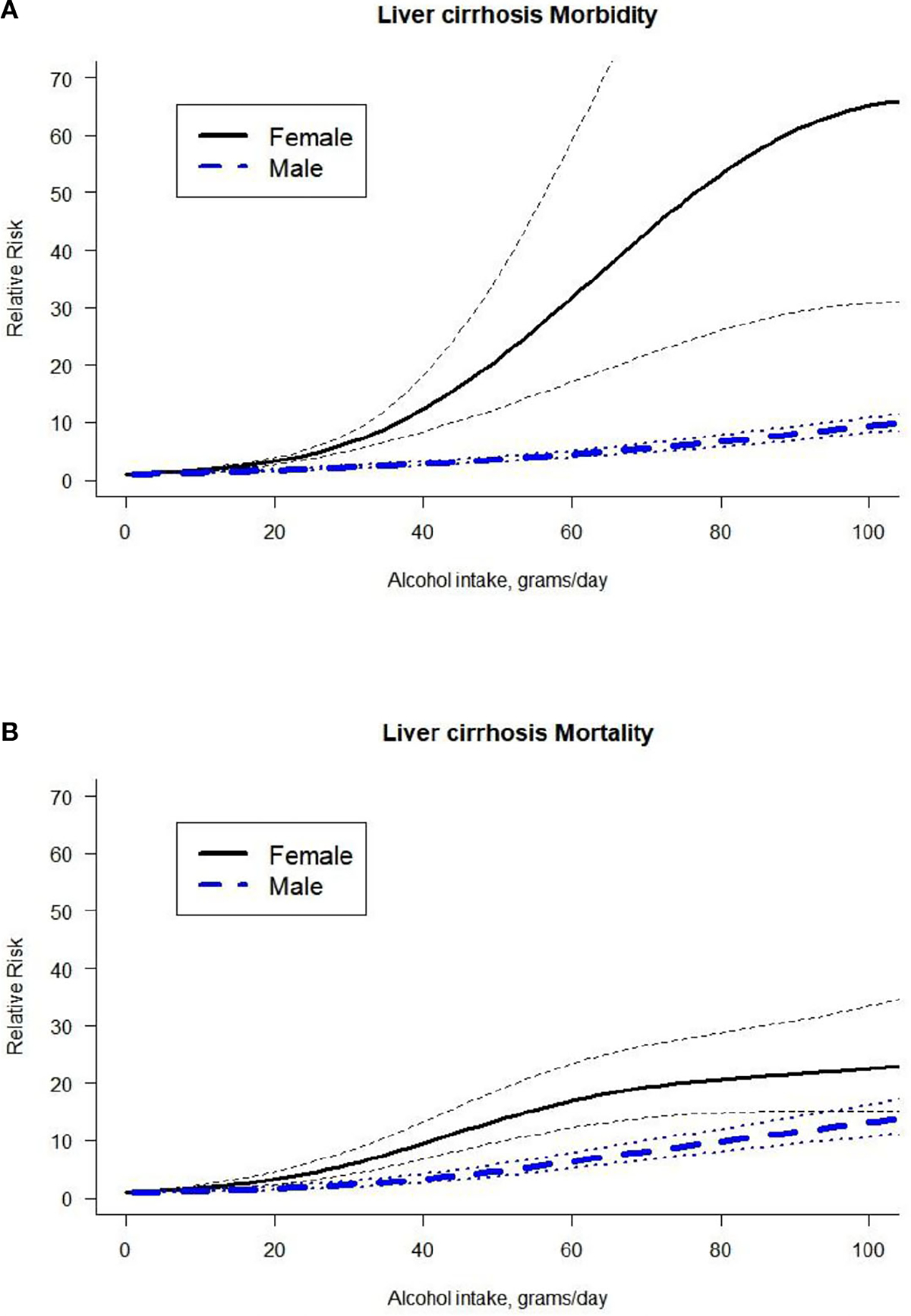
Dose-response curve between alcohol intake in grams per day and the risk of liver cirrhosis by outcome (A. Liver cirrhosis morbidity, B. Liver cirrhosis mortality) and by sex.
In our sensitivity analysis, we excluded the study by Liu and colleges (18) which provided four estimate points with the corresponding weights: 14%, 14%, 15%, 13% in the restrictive cubic splines model in the overall dose-response relationship for females. When we ran the analysis with the study excluded, the shape of the curve attenuated but there were still statistically significant differences compared to males (see Supplementary Material 8) and there were no changes in the significance and direction of the regression weights for the other variables studied.
Discussion
Differences in the risk of LC conditional on alcohol use between females and males have been identified, showing that, for females, the same level of alcohol consumed presents a higher risk of LC than for males as well as a higher mortality risk of LC, irrespective of the cause of LC. Our study is the most comprehensive and presents the most up to date estimates currently available. These results are consistent with previous reviews published (6, 7), which identified an increasing dose-response relationship in females. Our review identified six additional studies for females and 13 additional studies for males compared to the most recent review (7) mainly due to the inclusion of additional biological pathways of LC. In addition, it was not restricted to studies of all causes of LC combined, and thus we had the necessary power to study the impact of other variables. Importantly, the dose-response curves for females accelerate quickly, characterized by a non-linear curve with a more exponential relationship before flattening out.
The basis for this difference may partly be explained by differences in alcohol pharmacokinetics and pharmacodynamics between females and males (45, 46). Females generally have a relatively lower total water content and are generally smaller than males, resulting in higher blood alcohol concentrations for the same amount of ingested alcohol. Frezza et al. (47), confirmed this theory and identified that females also present a lower rate of alcohol dehydrogenase activity in the gastric mucosa, consequently producing a higher bioavailability of alcohol when lower doses of alcohol are consumed and a longer time period during which these levels are elevated. Thus, the activity of gastric metabolism is decreased in dependent alcohol use and sex differences are less apparent in heavy drinkers (48). In addition, estrogens are involved in alcohol-related liver damage by increasing the susceptibility of Kupffer cells to endotoxins (49). When activated, Kupffer cells release cytokines and produce hepatic inflammation, playing a role in hepatotoxicity after alcohol exposure (50). In contrast, when estrogen is blocked in animal models, the alcohol-related injury is attenuated (51). Therefore, Kupffer cells are an additional key element in the sex differences in liver injury caused by alcohol use.
The gender-gap that historically existed in the level of alcohol consumption between men and women is narrowing, with women increasing their consumption, including a notable increase in high-risk drinking (52). This phenomenon is a concerning public health problem which can consequently lead to adverse health effects. Additionally, gender-related differences in alcohol use regarding drinking patterns and problematic drinking (53), have been decreasing as the newest generations are being exposed to a social, economic and cultural homogenization (54). Knowing the unique risk that alcohol has for women, increases in their consumption are concerning and efforts should be directed to build awareness for this issue. More specifically for LC, prevention campaigns should take into consideration that females incur more than twofold risks of LC compared to males at all levels of alcohol consumption, with the excess risk of females being highest at 60 grams of pure alcohol per day.
In addition, given the quick acceleration of risk curves in females, targeted approaches directed to females in the healthcare system seem promising. This is especially true with the apparent gender-gap in today’s clinical practice, since women are not routinely asked about their alcohol use (4, 5). In general, alcohol assessment during healthcare visits is key to the early identification of harmful alcohol use as well as for quickly conducting brief interventions or referrals for specialty treatment. Brief interventions and alcohol use disorder treatment have been shown to be effective and relatively cost effective in decreasing consumption (55, 56), and thus in reducing the risk for LC. One study reported that men have a higher rate of accessing healthcare prior to being diagnosed with alcohol-related LC than women, but they are less likely to receive interventions or referrals than women (57). Nevertheless, women report having less social support for treatment engagement when dealing with alcohol use disorders (58). To formulate effective and accessible prevention and treatment options, these factors should be taken into account.
There are some limitations to our study that should be considered. First, we performed a meta-analysis based on an underlying literature search. Therefore, the evidence is limited to the research available and there is a longstanding and consistent history of poor measurement of alcohol use in epidemiological studies due to social desirability bias (underreporting) resulting from survey data. Also, there was a lack of data regarding the causes of LC, which leads us to be cautious when interpreting the differences identified between underlying causes of LC. Nevertheless, the same differences were identified in a wider analysis that included studies with both sexes combined. In addition, females accounted for more than twice the subjects and cases than males, and the study conducted by Liu and colleagues (18) represented more than half of the weight in our analysis. This large prospective study had a relatively high-quality methodology and, although the dose-response curve attenuated when we excluded this study from our analysis, we did not find differences in the direction of the curves or in beta-coefficients for other variables. As for the interpretation of the risk of bias score, although more than half of our studies received a serious or critical score, this was mainly because they did not include a time-variant confounding variable, which left us without a single article with a low bias score, and many articles with scores of serious and critical bias. Finally, alcohol use is often measured by self-report, which may lead to bias, although it has been shown to be valid overall (59).
By using a systematic and standardized methodology we were able to quantify the dose-response relationships in females and males and identify the differences in both curves and according to the outcomes. The higher risk observed in females, both in the progression of and mortality due to LC, calls for action. In primary care, unless alcohol use is screened for systematically, womeńs drinking tends to be overlooked (60). Early identification of excessive alcohol use should be a regular practice in health-care facilities to properly diagnose and treat at-risk individuals and prevent further progression in individuals with lower levels of consumption (61).
Supplementary Material
Acknowledgments
We would like to thank Ms. Astrid Otto for referencing and copyediting the text.
Funding
Research reported in this publication was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under Award Numbers R01AA028009 and R01AA028224. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of interest
Author AL reported grants from Swiss National Science Foundation (SNSF) during the conduct of the study.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fgstr.2022.1005729/full#supplementary-material
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
References
- 1.Global Burden of Disease Collaborative Network. Global burden of disease study 2019 (GBD 2019) results [Internet] (2020). Seattle, United States: Institute for Health Metrics and Evaluation (IHME. Available at: https://vizhub.healthdata.org/gbd-results/ (Accessed July 17, 2022). [Google Scholar]
- 2.GBD 2017 Cirrhosis Collaborators. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: A systematic analysis for the global burden of disease study 2017. Lancet Gastroenterol Hepatol (2020) 5(3):245–66. doi: 10.1016/S2468-1253(19)30349-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shield K, Manthey J, Rylett M, Probst C, Wettlaufer A, Parry CDH, et al. National, regional, and global burdens of disease from 2000 to 2016 attributable to alcohol use: A comparative risk assessment study. Lancet Public Heal (2020) 5(1): e51–61. doi: 10.1016/S2468-2667(19)30231-2 [DOI] [PubMed] [Google Scholar]
- 4.Kezer CA, Simonetto DA, Shah VH. Sex differences in alcohol consumption and alcohol-associated liver disease. Mayo. Clin Proc (2021) 96(4):1006–16. doi: 10.1016/j.mayocp.2020.08.020 [DOI] [PubMed] [Google Scholar]
- 5.McCaul ME, Roach D, Hasin DS, Weisner C, Chang G, Sinha R. Alcohol and women: A brief overview. Alcohol Clin Exp Res. 43(5):774–9. doi: 10.1111/acer.13985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rehm J, Taylor B, Mohapatra S, Irving H, Baliunas D, Patra J, et al. Alcohol as a risk factor for liver cirrhosis: A systematic review and meta-analysis. Drug Alcohol Rev (2010) 29(4):437–45. doi: 10.1111/j.1465-3362.2009.00153.x [DOI] [PubMed] [Google Scholar]
- 7.Roerecke M, Vafaei A, Hasan OSM, Chrystoja BR, Cruz M, Lee R, et al. Alcohol consumption and risk of liver cirrhosis: A systematic review and meta-analysis. Am J Gastroenterol (2019) 114(10):1574–86. doi: 10.14309/ajg.0000000000000340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Corrao G, Bagnardi V, Zambon A, Arico S. Exploring the dose-response relationship between alcohol consumption and the risk of several alcohol-related conditions: A meta-analysis. Addiction. (1999) 94(10):1551–73. doi: 10.1046/j.1360-0443.1999.9410155111.x [DOI] [PubMed] [Google Scholar]
- 9.Rehm J, Rovira P, Llamosas-Falcón L, Shield KD. Dose-response relationships between levels of alcohol use and risks of mortality or disease, for all people, by age, sex, and specific risk factors. Nutrients (2021) 13(8):2652. doi: 10.3390/nu13082652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Skog OJ. The prevention paradox revisited. Addiction. (1999) 94(5):751–7. doi: 10.1046/j.1360-0443.1999.94575113.x [DOI] [PubMed] [Google Scholar]
- 11.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (2021) 372:n71. doi: 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ (2016) 355:i4919. doi: 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. rating the quality of evidence. J Clin Epidemiol. (2011) 64(4):401–6. doi: 10.1016/j.jclinepi.2010.07.015 [DOI] [PubMed] [Google Scholar]
- 14.Shaper AG, Wannamethee G, Walker M. Alcohol and mortality in British men: explaining the U-shaped curve. Lancet (1988) 2(8623):1267–73. doi: 10.1016/s0140-6736(88)92890-5 [DOI] [PubMed] [Google Scholar]
- 15.Llamosas-Falcón L, Shield KD, Gelovany M, Hasan OSM, Manthey J, Monteiro M, et al. Impact of alcohol on the progression of HCV-related liver disease: A systematic review and meta-analysis. J Hepatol (2021) 75(3):536–46. doi: 10.1016/j.jhep.2021.04.018 [DOI] [PubMed] [Google Scholar]
- 16.Corbeil RR, Searle SR. Restricted maximum likelihood (REML) estimation of variance components in the mixed model. Technometrics (1976) 18(1):31–8. doi: 10.2307/1267913 [DOI] [Google Scholar]
- 17.Harrell FE. Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. New York: Springer-Verlag; (2010). [Google Scholar]
- 18.Liu B, Balkwill A, Roddam A, Brown A, Beral V. Separate and joint effects of alcohol and smoking on the risks of cirrhosis and gallbladder disease in middle-aged women. Am J Epidemiol. (2009) 169(2):153–60. doi: 10.1093/aje/kwn280 [DOI] [PubMed] [Google Scholar]
- 19.Harrer M, Cuijpers P, Furukawa TA, Ebert DD. Doing meta-analysis with r: A hands-on guide [Internet]. 1st ed. Boca Raton, FL and London: Chapman & Hall/CRC Press; (2021). Available at: https://www.routledge.com/Doing-Meta-Analysis-with-R-A-Hands-On-Guide/Harrer-Cuijpers-Furukawa-Ebert/p/book/9780367610074. [Google Scholar]
- 20.Viechtbauer W Conducting meta-analyses in r with the metafor package. J Stat Softw. (2010) 36:1–48. doi: 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- 21.R Core Team. A language and environment for statistical computing. In: R foundation for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; (2022). Available at: https://www.r-project.org/. [Google Scholar]
- 22.Alemy-Carreau M, Durbec JP, Giordanella J, Rousseau S, Blanc G, Monges D, et al. Lack of interaction between hepatitis c virus and alcohol in the pathogenesis of cirrhosis. A. Stat Study. J Hepatol (1996) 25(5):627–32. doi: 10.1016/S0168-8278(96)80230-3 [DOI] [PubMed] [Google Scholar]
- 23.Askgaard G, Grønbæk M, Kjær MS, Tjønneland A, Tolstrup JS. Alcohol drinking pattern and risk of alcoholic liver cirrhosis: A prospective cohort study. J Hepatol (2015) 62(5):1061–7. doi: 10.1016/j.jhep.2014.12.005 [DOI] [PubMed] [Google Scholar]
- 24.Batey RG, Burns T, Benson JR, Byth K. Alcohol consumption and the risk of cirrhosis. Med J Aust (1992) 156(6):8–11. doi: 10.5694/j.1326-5377.1992.tb139846.x [DOI] [PubMed] [Google Scholar]
- 25.Becker U, Gronbak M, Johansen D, Sorensen TIA. Lower risk for alcohol-induced cirrhosis in wine drinkers. Hepatology. (2002) 35(4):868–75. doi: 10.1053/jhep.2002.32101 [DOI] [PubMed] [Google Scholar]
- 26.Blackwelder WC, Yano K, Rhoads GG, Kagan A, Gordon T, Palesch Y. Alcohol and mortality: The Honolulu heart study. Am J Med (1980) 68(2):164–9. doi: 10.1016/0002-9343(80)90350-2 [DOI] [PubMed] [Google Scholar]
- 27.Boffetta P, Garfinkel L. Alcohol drinking and mortality among men enrolled in an American cancer society prospective study. Epidemiology. (1990) 1(5):342–8. doi: 10.1097/00001648-199009000-00003 [DOI] [PubMed] [Google Scholar]
- 28.Corrao G, Aricò S, Lepore AR, Valenti M, Torchio P, Galatola G, et al. Amount and duration of alcohol intake as risk factors of symptomatic liver cirrhosis: A case-control study. J Clin Epidemiol. (1993) 46(7):601–7. doi: 10.1016/0895-4356(93)90032-V [DOI] [PubMed] [Google Scholar]
- 29.Corrao G, Aricò S, Zambon A, Torchio P, Di Orio F. Female sex and the risk of liver cirrhosis. Scand J Gastroenterol (1997) 32(11):1174–80. doi: 10.3109/00365529709002999 [DOI] [PubMed] [Google Scholar]
- 30.Fuchs CS, Stampfer MJ, Colditz GA, Giovannucci EL, Manson JE, Kawachi I, et al. Alcohol consumption and mortality among women. N. Engl J Med (1995) 332(19):1245–50. doi: 10.1056/NEJM199505113321901 [DOI] [PubMed] [Google Scholar]
- 31.Garfinkel L, Boffetta P, Stellman SD. Alcohol and breast cancer: A cohort study. Prev Med (Baltim). (1988) 17(6):686–93. doi: 10.1016/0091-7435(88)90086-2 [DOI] [PubMed] [Google Scholar]
- 32.Gordon T, Kannel WB. Drinking and mortality. the framinghan study. Am J Epidemiol. (1984) 120(1):97–107. doi: 10.1093/oxfordjournals.aje.a113879 [DOI] [PubMed] [Google Scholar]
- 33.Gordon T, Doyle JT. Drinking and mortality: The Albany study. Am J Epidemiol. (1987) 125(2):263–70. doi: 10.1093/oxfordjournals.aje.a114525 [DOI] [PubMed] [Google Scholar]
- 34.Im PK, Millwood IY, Kartsonaki C, Guo Y, Chen Y, Turnbull I, et al. Alcohol drinking and risks of liver cancer and non-neoplastic chronic liver diseases in China: A 10-year prospective study of 0.5 million adults. BMC Med (2021) 19(1):1–13. doi: 10.1186/s12916-021-02079-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khan KN, Yatsuhashi H. Effect of alcohol consumption on the progression of hepatitis c virus infection and risk of hepatocellular carcinoma in Japanese patients. Alcohol Alcohol (2000) 35(3):286–95. doi: 10.1093/alcalc/35.3.286 [DOI] [PubMed] [Google Scholar]
- 36.Klatsky AL, Friedman GD, Armstrong MA, Kipp H. Wine, liquor, beer, and mortality. Am J Epidemiol. (2003) 158(6):585–95. doi: 10.1093/aje/kwg184 [DOI] [PubMed] [Google Scholar]
- 37.Kono S, Ikeda M, Tokudome S, Nishizumi M, Kuratsune M. Alcohol and mortality: A cohort study of male Japanese physicians. Int J Epidemiol. (1986) 15(4):527–32. doi: 10.1093/ije/15.4.527 [DOI] [PubMed] [Google Scholar]
- 38.Norton R, Bates R, Dwyer T, Macmahon S. Alcohol consumption and the risk of alcohol related cirrhosis in women. Br Med J (1987) 295(6590):80–2. doi: 10.1136/bmj.295.6590.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pequignot G, Tuyns AJ, Berta JL. Ascitic cirrhosis in relation to alcohol consumption. Int J Epidemiol. (1978) 7(2):113–20. doi: 10.1093/ije/7.2.113 [DOI] [PubMed] [Google Scholar]
- 40.Schult A, Mehlig K, Björkelund C, Wallerstedt S, Kaczynski J. Waist-to-hip ratio but not body mass index predicts liver cirrhosis in women. Scand J Gastroenterol (2018) 53(2):212–7. doi: 10.1080/00365521.2017.1420219 [DOI] [PubMed] [Google Scholar]
- 41.Stroffolini T, Cotticelli G, Medda E, Niosi M, Del Vecchio-Blanco C, Addolorato G, et al. Interaction of alcohol intake and cofactors on the risk of cirrhosis. Liver. Int (2010) 30(6):867–70. doi: 10.1111/j.1478-3231.2010.02261.x [DOI] [PubMed] [Google Scholar]
- 42.Yang L, Zhou M, Sherliker P, Cai Y, Peto R, Wang L, et al. Alcohol drinking and overall and cause-specific mortality in China: Nationally representative prospective study of 220 000 men with 15 years of follow-up. Int J Epidemiol. (2012) 41(4):1101–13. doi: 10.1093/ije/dys075 [DOI] [PubMed] [Google Scholar]
- 43.Yi SW, Hong JS, Yi JJ, Ohrr H. Impact of alcohol consumption and body mass index on mortality from nonneoplastic liver diseases, upper aerodigestive tract cancers, and alcohol use disorders in Korean older middle-aged men: Prospective cohort study. Medicine (2016) 95(39):e4876. doi: 10.1097/MD.0000000000004876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yuan JM, Ross RK, Gao YT, Henderson BE, Yu MC. Follow up study of moderate alcohol intake and mortality among middle aged men in shanghai, China. BMJ (1997) 314(7073):18–23. doi: 10.1136/bmj.314.7073.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ward RJ, Coutelle C. Women and alcohol susceptibility: Could differences in alcohol metabolism predispose women to alcohol-related diseases? Arch Womens. Ment Health (2003) 6(4):231–8. doi: 10.1007/s00737-003-0015-7 [DOI] [PubMed] [Google Scholar]
- 46.Thomasson HR. Gender differences in alcohol metabolism. Physiological responses to ethanol. Recent Dev Alcohol an. Off. Publ. Am Med Soc Alcohol Res Soc Alcohol Natl Counc. Alcohol (1995) 12:163–79. doi: 10.1007/0-306-47138-8_9 [DOI] [PubMed] [Google Scholar]
- 47.Frezza M, di Padova C, Pozzato G, Terpin M, Baraona E, Lieber CS. High blood alcohol levels in women. the role of decreased gastric alcohol dehydrogenase activity and first-pass metabolism. N. Engl J Med (1990) 322(2):95–9. doi: 10.1056/NEJM199001113220205 [DOI] [PubMed] [Google Scholar]
- 48.DiPadova C, Worner TM, Julkunen RJ, Lieber CS. Effects of fasting and chronic alcohol consumption on the first-pass metabolism of ethanol. Gastroenterology (1987) 92(5 Pt 1):1169–73. doi: 10.1016/s0016-5085(87)91073-0 [DOI] [PubMed] [Google Scholar]
- 49.Ikejima K, Enomoto N, Iimuro Y, Ikejima A, Fang D, Xu J, et al. Estrogen increases sensitivity of hepatic kupffer cells to endotoxin. Am J Physiol (1998) 274 (4):G669–76. doi: 10.1152/ajpgi.1998.274.4.G669 [DOI] [PubMed] [Google Scholar]
- 50.Thurman RG. Sex-related liver injury due to alcohol involves activation of kupffer cells by endotoxin. Can J Gastroenterol (2000) 14 Suppl D:129D–35D. doi: 10.1155/2000/735262 [DOI] [PubMed] [Google Scholar]
- 51.Järveläinen HA, Lukkari TA, Heinaro S, Sippel H, Lindros KO. The antiestrogen toremifene protects against alcoholic liver injury in female rats. J Hepatol (2001) 35(1):46–52. doi: 10.1016/s0168-8278(01)00050-2 [DOI] [PubMed] [Google Scholar]
- 52.Grant BF, Chou SP, Saha TD, Pickering RP, Kerridge BT, Ruan WJ, et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IV alcohol use disorder in the united states, 2001–2002 to 2012–2013: Results from the national epidemiologic survey on alcohol and related conditions. JAMA Psychiatry (2017) 74(9):911–23. doi: 10.1001/jamapsychiatry.2017.2161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.White AM. Gender differences in the epidemiology of alcohol use and related harms in the united states. Alcohol Res (2020) 40(2):1. doi: 10.35946/arcr.v40.2.01 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Erol A, Karpyak VM. Sex and gender-related differences in alcohol use and its consequences: Contemporary knowledge and future research considerations. Drug Alcohol Depend. (2015) 156:1–13. doi: 10.1016/j.drugalcdep.2015.08.023 [DOI] [PubMed] [Google Scholar]
- 55.Kaner EF, Beyer FR, Muirhead C, Campbell F, Pienaar ED, Bertholet N, et al. Effectiveness of brief alcohol interventions in primary care populations. Cochrane Database Syst Rev (2018) 2(2):CD004148. doi: 10.1002/14651858.CD004148.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Carvalho AF, Heilig M, Perez A, Probst C, Rehm J. Alcohol use disorders. Lancet (London. England). (2019) 394(10200):781–92. doi: 10.1016/S0140-6736(19)31775-1 [DOI] [PubMed] [Google Scholar]
- 57.Otete HE, Orton E, West J, Fleming KM. Sex and age differences in the early identification and treatment of alcohol use: A population-based study of patients with alcoholic cirrhosis. Addiction. (2015) 110(12):1932–40. doi: 10.1111/add.13081 [DOI] [PubMed] [Google Scholar]
- 58.Maxwell AM, Harrison K, Rawls E, Zilverstand A. Gender differences in the psychosocial determinants underlying the onset and maintenance of alcohol use disorder. Front Neurosci (2022) 16:808776. doi: 10.3389/fnins.2022.808776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Midanik LT. Validity of self-reported alcohol use: A literature review and assessment. Br J Addict. (1988) 83(9):1019–30. doi: 10.1111/j.1360-0443.1988.tb00526.x [DOI] [PubMed] [Google Scholar]
- 60.Hettema J, Cockrell S, Russo J, Corder-Mabe J, Yowell-Many A, Chisholm C, et al. Missed opportunities: Screening and brief intervention for risky alcohol use in women’s health settings. J Womens. Health (Larchmt). (2015) 24(8):648–54. doi: 10.1089/jwh.2014.4961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Greaves L, Poole N, Brabete AC. Sex, gender, and alcohol use: Implications for women and low-risk drinking guidelines. Int J Environ Res Public Health (2022) 19(8): 4523. doi: 10.3390/ijerph19084523 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.


