Abstract
BACKGROUND.
The central vein sign (CVS) is a proposed MRI biomarker of multiple sclerosis (MS). The impact of gadolinium-based contrast agent (GBCA) administration on CVS evaluation remains poorly investigated.
OBJECTIVE.
The purpose of this study was to assess the effect of GBCA use on CVS detection and on the diagnostic performance of the CVS for MS using a 3-T FLAIR* sequence.
METHODS.
This study was a secondary analysis of data from the pilot study for the prospective multicenter Central Vein Sign: A Diagnostic Biomarker in Multiple Sclerosis (CAVS-MS), which recruited adults with suspected MS from April 2018 to February 2020. Participants underwent 3-T brain MRI including FLAIR and precontrast and postcontrast echo-planar imaging T2*-weighted acquisitions. Postprocessing was used to generate combined FLAIR and T2*-weighted images (hereafter, FLAIR*). MS diagnoses were established using the 2017 McDonald criteria. Thirty participants (23 women, seven men; mean age, 45 years) were randomly selected from the CAVS-MS pilot study cohort. White matter lesions (WMLs) were marked using FLAIR* images. A single observer, blinded to clinical data and GBCA use, reviewed marked WMLs on FLAIR* images for the presence of the CVS.
RESULTS.
Thirteen of 30 participants had MS. Across participants, on precontrast FLAIR* imaging, 218 CVS-positive and 517 CVS-negative WMLs were identified; on postcontrast FLAIR* imaging, 269 CVS-positive and 459 CVS-negative WMLs were identified. The fraction of WMLs that were CVS-positive on precontrast and postcontrast images was 48% and 58% in participants with MS and 7% and 10% in participants without MS, respectively. The median patient-level CVS-positivity rate on precontrast and postcontrast images was 43% and 67% for participants with MS and 4% and 8% for participants without MS, respectively. In a binomial model adjusting for MS diagnoses, GBCA use was associated with an increased likelihood of at least one CVS-positive WML (odds ratio, 1.6; p < .001). At a 40% CVS-positivity threshold, the sensitivity of the CVS for MS increased from 62% on precontrast images to 92% on postcontrast images (p = .046). Specificity was not significantly different between precontrast (88%) and postcontrast (82%) images (p = .32).
CONCLUSION.
GBCA use increased CVS detection on FLAIR* images, thereby increasing the sensitivity of the CVS for MS diagnoses.
CLINICAL IMPACT.
The postcontrast FLAIR* sequence should be considered for CVS evaluation in future investigational trials and clinical practice.
Keywords: central vein sign, FLAIR*, gadolinium, multiple sclerosis
Prompt diagnosis of multiple sclerosis (MS) is critical, as early treatment initiation is associated with improved clinical outcomes [1]. However, accurately diagnosing MS remains challenging due to the limited specificity of the current diagnostic criteria [2]. In one study, nearly one in five patients with MS were misdiagnosed [3]. Misdiagnosis is problematic due to the costs and risks associated with the use of disease-modifying therapies; in a study of 110 patients misdiagnosed with MS, 70% received disease-modifying therapies, which resulted in unnecessary morbidity in 31% [4].
The identification of white matter lesions (WMLs) on MRI is an important component for the diagnosis of MS. However, many other conditions can be associated with WMLs on MRI that mimic MS, including small-vessel ischemic disease, migraine, and systemic autoimmune diseases [4, 5]. As such, MRI biomarkers with greater specificity for MS are needed. MS lesions are oriented around a central vein, an association that can be visualized using advanced MRI approaches and that is referred to as the central vein sign (CVS) [5, 6]. The CVS may be useful in differentiating WMLs due to MS from those due to mimickers [7–10]. However, further research is needed to standardize MRI evaluation of the CVS and to provide data from large-scale prospective studies before the CVS can be incorporated into clinical practice [5, 11, 12]. Visualization of the CVS has been described with several different T2*-weighted MRI sequences, including FLAIR* [6, 7, 9, 10], susceptibility-weighted imaging (SWI) [13], and quantitative susceptibility mapping (QSM) [14]. FLAIR* imaging combines a 3D echo-planar imaging (EPI) T2*-weighted sequence and 3D FLAIR to facilitate simultaneous WML and CVS visualization [15]. Indeed, available evidence supports optimal CVS detection through the use of a high-resolution isotropic 3D EPI T2*-weighted sequence such as FLAIR* [5].
The current literature is heterogeneous with respect to the impact of gadolinium-based contrast agent (GBCA) administration on CVS detection and diagnostic utility [7, 13, 16–18]. After GBCA administration, veins are more visible on T2*-weighted sequences due to the reduction of the relaxation time of water protons in blood [19]. However, whether this increased visibility translates to improved conspicuity of the CVS in WMLs has not been well investigated. Insight into the impact of GBCA use in this setting is important given both the agent’s additional cost and the potential risk of intracranial gadolinium deposition from repeated contrast-enhanced MRI examinations often performed in patients with MS [20]. The use of GBCA to aid in the detection of the CVS could impact not only initial diagnosis but also treatment planning and modification—for example, by helping to discern, in combination with clinical judgment and other MRI findings, whether WMLs are related to MS or to comorbidities such as small-vessel disease [21]. One recent study found that the use of a GBCA increased the detection of CVS-positive MS lesions when using a 1.5-T SWI sequence [22], but the effect of GBCA use on CVS detection when using FLAIR* or a 3-T system has not been explored to our knowledge.
The goal of the current study was to assess the effect of GBCA use on CVS detection and on the diagnostic performance of the CVS for MS using a FLAIR* sequence at 3 T.
Methods
Participants
This study was a secondary analysis of a subset of patients from the pilot study for the Central Vein Sign: A Diagnostic Biomarker in Multiple Sclerosis (CAVS-MS). The CAVS-MS pilot study was a prospective longitudinal international multicenter observational study conducted by the North American Imaging in MS Cooperative (NAIMS). The study collected preliminary data on the application of the CVS on the FLAIR* sequence on 3-T MRI for evaluation of MS. Institutional review board approval was obtained at all sites, HIPAA compliance was maintained for all research staff, and written informed consent was obtained from all patients. The CAVS-MS pilot study recruited patients who were between 18 and 65 years old and who had been referred to any of 10 MS centers with new clinical or radiologic suspicion for MS between April 2018 and February 2020. Individuals were ineligible for recruitment if they had a contraindication to or intolerance of MRI or GBCA or had received prior treatment with disease-modifying therapy for MS or treatment with systemic corticosteroids in the 4 weeks before potential enrollment.
The CAVS-MS pilot study obtained consent from and enrolled 97 participants. Five enrolled patients were excluded on the basis of an initial quality control assessment (image artifacts, n = 4; no contrast-enhanced acquisition, n = 1) by an MRI physicist (P.S.), resulting in 92 patients in the CAVS-MS pilot study’s final analysis. For the present secondary investigation of the impact of GBCA use, 30 of these 92 participants were randomly selected using a random-number generator. This sample size was estimated to provide 80% power to detect at least a 15% difference in CVS detection based on GBCA use. The 30 participants in the present analysis were recruited from five sites: Cleveland Clinic, University of Toronto (St. Michael’s Hospital), University of Pennsylvania, University of Vermont, and Johns Hopkins University. All participants were included in an earlier report of the primary outcomes from the entire CVS-MS pilot study cohort that did not focus on the impact of GBCA use [23]. Figure 1 provides a flowchart of patient selection.
Fig. 1—
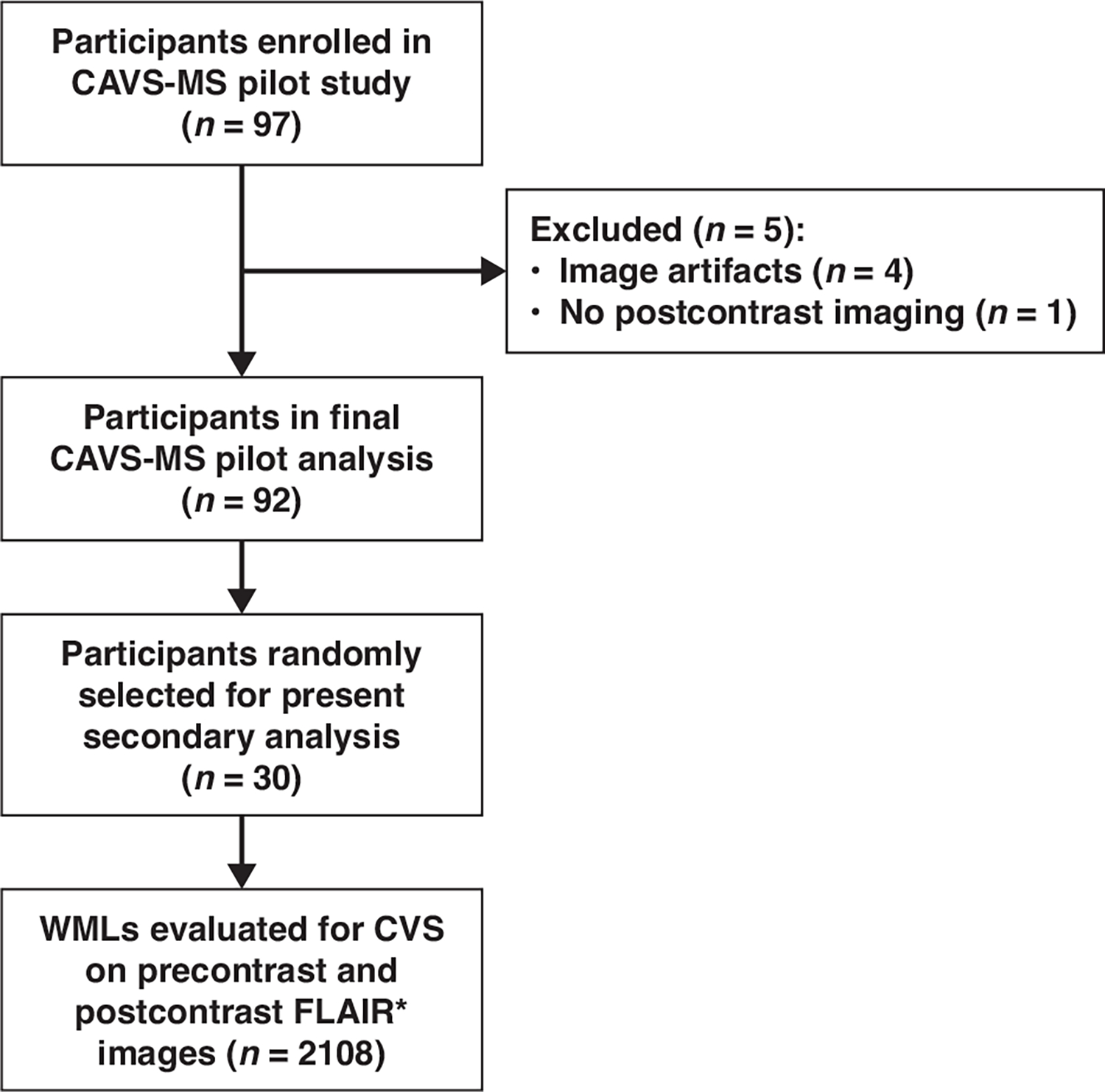
Flowchart shows patient selection. CAVS-MS = Central Vein Sign: A Diagnostic Biomarker in Multiple Sclerosis, CVS = central vein sign, WML = white matter lesion.
Study Design
Participants in the CAVS-MS pilot study underwent a single study visit, which included clinical assessment, neurologic examination, and a single brain MRI examination as described later in the Methods. As part of this visit, neurologists at the local site with subspecialty training in MS classified participants in terms of the presence or absence of an MS diagnosis based on the 2017 McDonald criteria [24]. This determination considered all available clinical data, including MRI findings with the exception of CVS-related findings. In addition to the determination of MS, site neurologists classified brain MRI examinations in terms of dissemination in space and dissemination in time based on the 2017 McDonald criteria [24]. Approximately 12 months after the initial study visit, the neurologists reported participants’ most current diagnosis based on routine care during the interval since the initial study visit, as assessed by chart review.
MRI Acquisitions and Postprocessing
Brain MRI examinations performed during the initial study visit were performed using a 3-T system (two sites: Prisma Fit, Siemens Healthineers; one site: Skyra, Siemens Healthineers; one site: Ingenia Elition X, Philips Healthcare; one site: Achieva dStream, Philips, Healthcare). A manual providing a detailed MRI protocol was provided to all sites. Examinations used a single dose (0.1 mmol/kg) of a macrocyclic GBCA (gadoterate, gadoteridol, or gadobutrol, depending on the site’s routine practice). Examinations included an unenhanced T2*-weighted FLAIR sequence (isotropic resolution, 1.0 mm), a high-resolution T2*-weighted segmented EPI sequence (isotropic resolution, 0.65 mm) performed before and after GBCA administration, and a T1-weighted sequence (isotropic resolution, 1.0 mm) performed before and after GBCA administration (Table 1). All sequences comprised a 3D sagittal acquisition of the entire brain. Images from the MRI examinations were uploaded to an online platform (QMENTA, QMENTA) and accessibly by all CAVS-MS pilot study investigators.
TABLE 1:
Brain MRI Protocol Used at All Participating Sites
| Sequence | Isotropic Resolution (mm) | TR/TE | Acquisition Time | Other Parameters |
|---|---|---|---|---|
|
| ||||
| Precontrast T2-weighted FLAIR | 1.0 | 4800/352 | 7 min 9 s | TI = 1800 ms |
| Pre- and postcontrast T2*-weighted segmented EPI | 0.65 | 64/35 | 5 min 48 s | Flip angle = 10°, EPI factor = 15 |
| Pre- and postcontrast T1-weighted imaging | 1.0 | 7.8/3.0 | 3 min 16 s | Flip angle = 18° |
Note—All sequences comprised a 3D sagittal acquisition of entire brain. TI = inversion time, EPI = echo-planar imaging.
FLAIR* images were generated using three automated postprocessing steps [15]: coregistration between FLAIR and T2*-weighted sequences, interpolation of the registered FLAIR images to match the spatial resolution of the T2*-weighted images, and multiplication of the coregistered interpolated FLAIR images by the T2*-weighted images. This postprocessing was performed using the online platform and required approximately 30 minutes to generate each FLAIR* image set.
White Matter Lesion–Level and Patient-Level Analyses
A 4th-year medical student (L.D.), blinded to MS diagnosis and other clinical information, reviewed the single FLAIR acquisition for all participants to identify and mark the location of all WMLs. This assessment was performed using open-source software (ITK-SNAP) [25]. The WML markings were transferred to precontrast and postcontrast FLAIR* images to allow assessment of corresponding WMLs on both image sets.
One month later, the same investigator (L.D.) manually reviewed the precontrast and postcontrast FLAIR* image sets in all 30 participants (total, 60 FLAIR* image sets reviewed). The 60 image sets were evaluated in random order; the investigator (L.D.) was blinded to MS diagnosis, other clinical information, and GBCA administration (i.e., whether an image set was obtained precontrast or postcontrast). Postcontrast T1-weighted images were not reviewed. The images included a mask depicting the previously marked WMLs. The rater categorized the location of each marked WML as juxtacortical (supratentorial, touched or included cortex), periventricular (supratentorial, touched lateral ventricles), subcortical or deep white matter (supratentorial, touched neither cortex nor ventricles), or infratentorial. The rater also categorized each WML as follows: CVS-positive if the WML clearly showed a central vein on at least two of three orthogonal planes, consistent with NAIMS criteria [5]; CVS-negative if no central vein could be identified in the WML; CVS-indeterminate if a central vein was visualized on only one plane, precluding classification as CVS-positive or CVS-negative; or excluded from CVS evaluation if meeting an NAIMS exclusion criterion [5]. The reasons for excluding CVS from evaluation were recorded.
For each MRI examination, the CVS-positivity rate was calculated as the percentage of CVS-positive WMLs among CVS-positive and CVS-negative WMLs. Examinations were then classified as CVS-positive at the patient level if the CVS-positivity rate reached various thresholds.
A neuroimmunology fellow (M.A.) independently evaluated all 60 FLAIR* image sets in the 30 patients. These evaluations were used for assessing interreader agreement; the first reader’s interpretations were used for all further analyses.
Before the start of the interpretations, all readers underwent training using a standardized dataset of annotated images that contained both CVS-positive and CVS-negative lesions curated from a prior study [26].
Post Hoc Analysis
At each of the five clinical sites, a single neurologist (2–5 years of posttraining experience) performed a post hoc review of the medical records of participants without MS at 12-month follow-up but who had a CVS-positivity rate of 40% or greater, participants with clinically isolated syndrome at 12-month follow-up, participants with MS who had a CVS-positivity rate of less than 40%, and participants classified as having MS at the initial study visit but not at 12-month follow-up. Observations from this medical record review were summarized qualitatively.
Statistical Analysis
Variables were stratified among various subsets on the basis of MS diagnosis, GBCA use, WML location, and CVS categorization and were compared using a combination of the t test, Fisher exact test, chi-square test, Mann-Whitney U test, and Wilcoxon rank sum test. A binomial model was used to identify associations of MS diagnoses and GBCA use with CVS positivity on a WML level, accounting for multiple WMLs per patient and considering a possible interaction between MS diagnosis and GBCA use. A negative binomial mixed model was used to identify an association of the distribution of WML locations with MS diagnosis or GBCA use, accounting for multiple WMLs per patient with a random intercept and considering a possible interaction between location distribution and MS. The negative binomial model was used due to overdispersion of WML counts. Sensitivity and specificity for MS diagnosis were compared between precontrast and postcontrast FLAIR* images on the basis of patient-level CVS-positivity rate using the McNemar test.
Various sensitivity analyses were performed. In one sensitivity analysis, CVS-indeterminate WMLs were counted as CVS-positive or as CVS-negative. In addition, primary analyses defined patients as having MS at a patient-level CVS-positivity threshold of 40% or greater [8]. Secondary analyses used thresholds of 35% and 50%. Primary analyses also used MS diagnoses from the initial study visit based on 2017 McDonald criteria. Secondary analyses were performed using diagnoses of MS from 12-month follow-up, variably classifying patients with a 12-month follow-up diagnosis of clinically isolated syndrome or radiologically isolated syndrome as having MS or as being excluded from analysis.
Interrater agreement for classifying WMLs as CVS-positive versus CVS-negative and for patient-level CVS-positivity at the 40% threshold were determined for precontrast and postcontrast FLAIR* images using the percentage agreement and Cohen kappa coefficient. This assessment of interrater agreement included only those lesions classified as CVS-positive or CVS-negative by both readers. Kappa coefficients were classified as follows [27]: 0.01–0.20, slight agreement; 0.21–0.40, fair; 0.41–0.60, moderate; 0.61–0.80, substantial; and greater than 0.80, almost perfect.
Results were considered statistically significant at p < .05. All statistical analyses used R (version 4.0.5) or GraphPad Prism (version 9, GraphPad Software) software.
Results
Participants and White Matter Lesions
The characteristics of the 30 randomly selected participants are presented in Table 2 and Table S1 (available in the online supplement). The mean age was 45 ± 12 (SD) years. Twenty-three participants were women, and seven were men. Based on the initial study visit, 13 of 30 participants met 2017 McDonald criteria for MS, and 17 of 30 did not. A total of 11 of 17 participants without MS met dissemination-in-space criteria on MRI, and three of 17 participants without MS met dissemination-in-time criteria on MRI.
TABLE 2:
Comparison of Characteristics of Participants Who Did and Those Who Did Not Meet 2017 McDonald Criteria for MS Diagnosis on Initial Study Visit
| Characteristic | MS (n = 13) | No MSa (n = 17) | p b |
|---|---|---|---|
|
| |||
| Age (y), mean ± SD | 44 ± 12 | 46 ± 12 | .67 |
| Sex | .02 | ||
| Male | 6 (46) | 1 (6) | |
| Female | 7 (54) | 16 (94) | |
| Hypertension | 0 (0) | 7 (41) | .01 |
| Coronary artery disease | 0 (0) | 1 (6) | >.99 |
| Diabetes mellitus | 1 (8) | 3 (18) | .61 |
| Hyperlipidemia | 1 (8) | 4 (24) | .35 |
| Ever use of tobacco | 7 (54) | 4 (24) | .13 |
| Presence of CSF oligoclonal bandsc | 5/7 (71) | 0/5 (0) | .03 |
Note—Unless otherwise indicated, data are numbers of participants with percentages in parentheses. MS = multiple sclerosis.
No diagnosis of MS based on 2017 McDonald criteria during the study.
The p values were calculated using the t test for age and Fisher exact test for categoric variables.
Not assessed in all participants.
A total of 2108 WMLs were identified on FLAIR images in the 30 participants (905 WMLs in participants with MS, 1203 WMLs in participants without MS) and were subsequently evaluated on precontrast and postcontrast FLAIR* images. The distribution of WMLs by location was significantly different between participants with MS and those without MS (p < .001): participants with MS, compared with those without MS, had a lower fraction of WMLs in the subcortical or deep white matter (72% vs 89%, respectively) and higher fractions in periventricular (16% vs 6%) and juxtacortical (10% vs 5%) locations (Table 3).
TABLE 3:
Distribution of WML Locations in Participants With and Those Without MS
| Location | MS (n = 905) | No MSa (n = 1203) |
|---|---|---|
|
| ||
| Supratentorial | ||
| Periventricular (touched lateral ventricles) | 144 (16) | 67 (6) |
| Juxtacortical (touched or included cortex) | 95 (10) | 56 (5) |
| Subcortical or deep white matter (touched neither cortex nor ventricles) | 650 (72) | 1073 (89) |
| Infratentorial | 16 (2) | 7 (1) |
Note—Data are expressed as numbers of lesions with percentages in parentheses. The distribution was significantly different between the two groups (p < .001). WML = white matter lesion, MS = multiple sclerosis.
No diagnosis of MS based on 2017 McDonald criteria during the study.
Effect of GBCA on Central Vein Sign Detection
Based on the NAIMS criteria for CVS evaluation, 1316 of 2108 (62%) WMLs were excluded on precontrast FLAIR* images, and 1315 of 2108 (62%) WMLs were excluded on postcontrast FLAIR* images. The reasons for exclusion of WMLs on postcontrast images included the following: size less than 3 mm in diameter in any plane (1038/1315, 79%), a branching vein (3/1315, 0.2%), a peripheral vein (52/1315, 4.0%), multiple veins (98/1315, 7.5%), confluence (83/1315, 6.3%), and both multiple veins and confluence (38/1315, 2.9%). The fraction of WMLs excluded from CVS evaluation was significantly higher in participants without MS than in those with MS on both precontrast (68% vs 56%, respectively; p < .001) and postcontrast (67% vs 56%; p < .001) images. The median number of WMLs per participant was 57 (range, 7–221). After exclusions, the median number of evaluated WMLs per participant was 16 (range, 3–111) for those without MS and was 25 (range, 6–100) for those with MS.
Table 4 summarizes the distribution of CVS in evaluated WMLs. On precontrast FLAIR* images, a total of 218 CVS-positive and 517 CVS-negative WMLs were identified; on postcontrast FLAIR* images, a total of 269 CVS-positive and 459 CVS-negative WMLs were identified. In patients with MS, 192 of 402 (48%) WMLs were CVS-positive on precontrast FLAIR* images, whereas 229 of 398 (58%) WMLs were CVS-positive on postcontrast FLAIR* images. In patients without MS, 26 of 390 (7%) WMLs were CVS-positive on precontrast FLAIR* images, and 40 of 395 (10%) were CVS-positive on postcontrast FLAIR* images. Figure 2 shows examples of excluded, CVS-positive, CVS-negative, and CVS-indeterminate WMLs. The number of CVS-positive WMLs and CVS-positive rates, stratified by MS diagnosis and WML location, are summarized in Table S2 (available in the online supplement), and the distribution of CVS-positive WMLs across locations, stratified by GBCA use, is summarized in Table S3 (available in the online supplement); GBCA use was not significantly associated with this location distribution (p = .95).
TABLE 4:
Comparison of CVS Classification Between Precontrast and Postcontrast FLAIR* Sequences, Stratified by MS Diagnosis
| MS (n = 905) | No MSa (n = 1203) | |||
|---|---|---|---|---|
|
|
||||
| CVS Classification | Precontrast | Postcontrast | Precontrast | Postcontrast |
|
| ||||
| Excluded WMLs | 503 (56) | 507 (56) | 813 (68) | 808 (67) |
| Evaluated WMLs | 402 (44) | 398 (44) | 390 (32) | 395 (33) |
| CVS-positive | 192 (48) | 229 (58) | 26 (7) | 40 (10) |
| CVS-negative | 162 (40) | 127 (32) | 355 (91) | 332 (84) |
| CVS-indeterminate | 48 (12) | 42 (11) | 9 (2) | 23 (6) |
Note—Data are expressed as numbers of white matter lesions (WMLs) with percentages in parentheses. Percentages for CVS-positive, CVS-negative, and CVS-indeterminate WMLs were calculated among evaluated WMLs. CVS = central vein sign, MS = multiple sclerosis.
No diagnosis of MS based on 2017 McDonald criteria during the study.
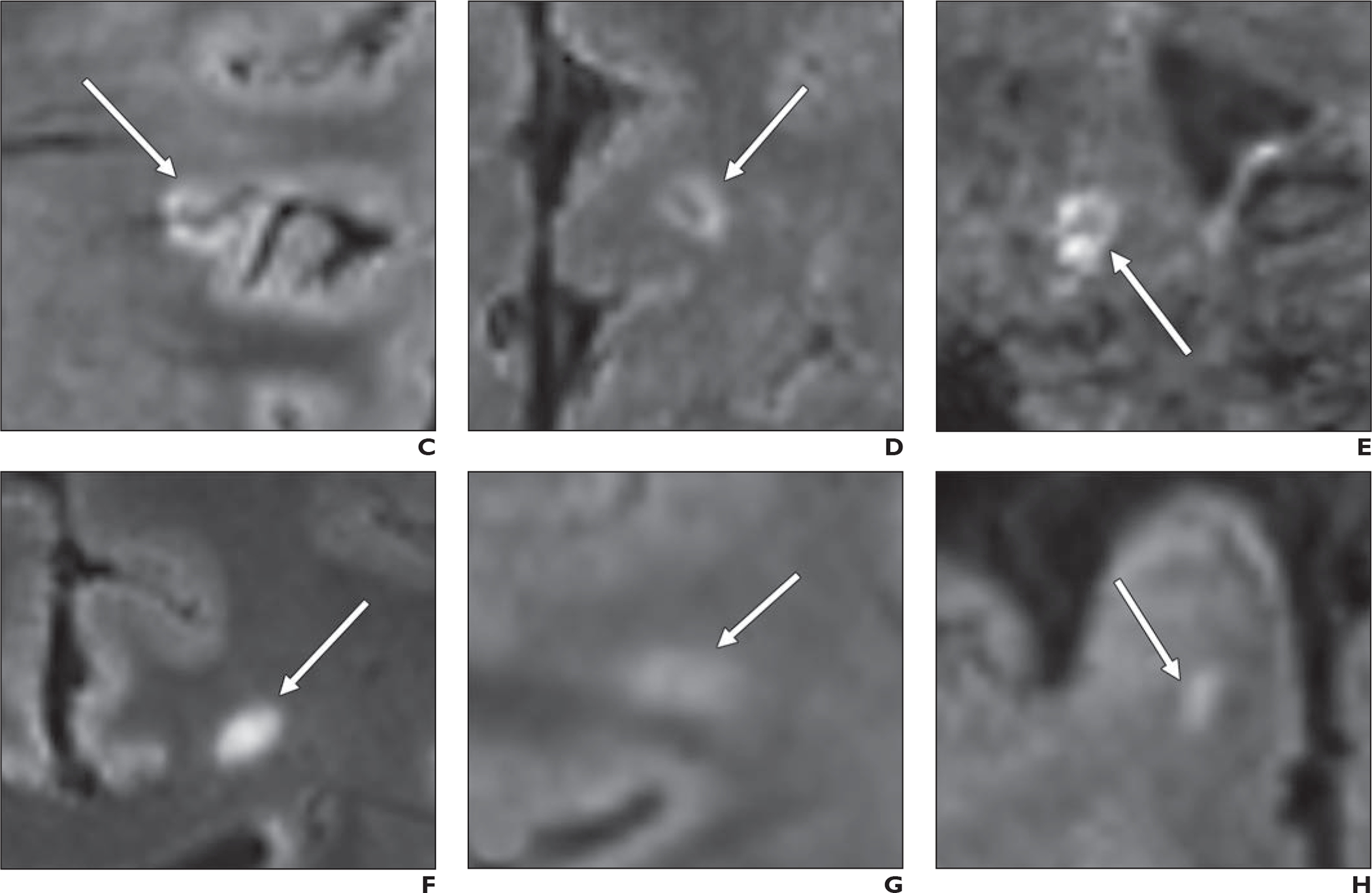
Fig. 2—
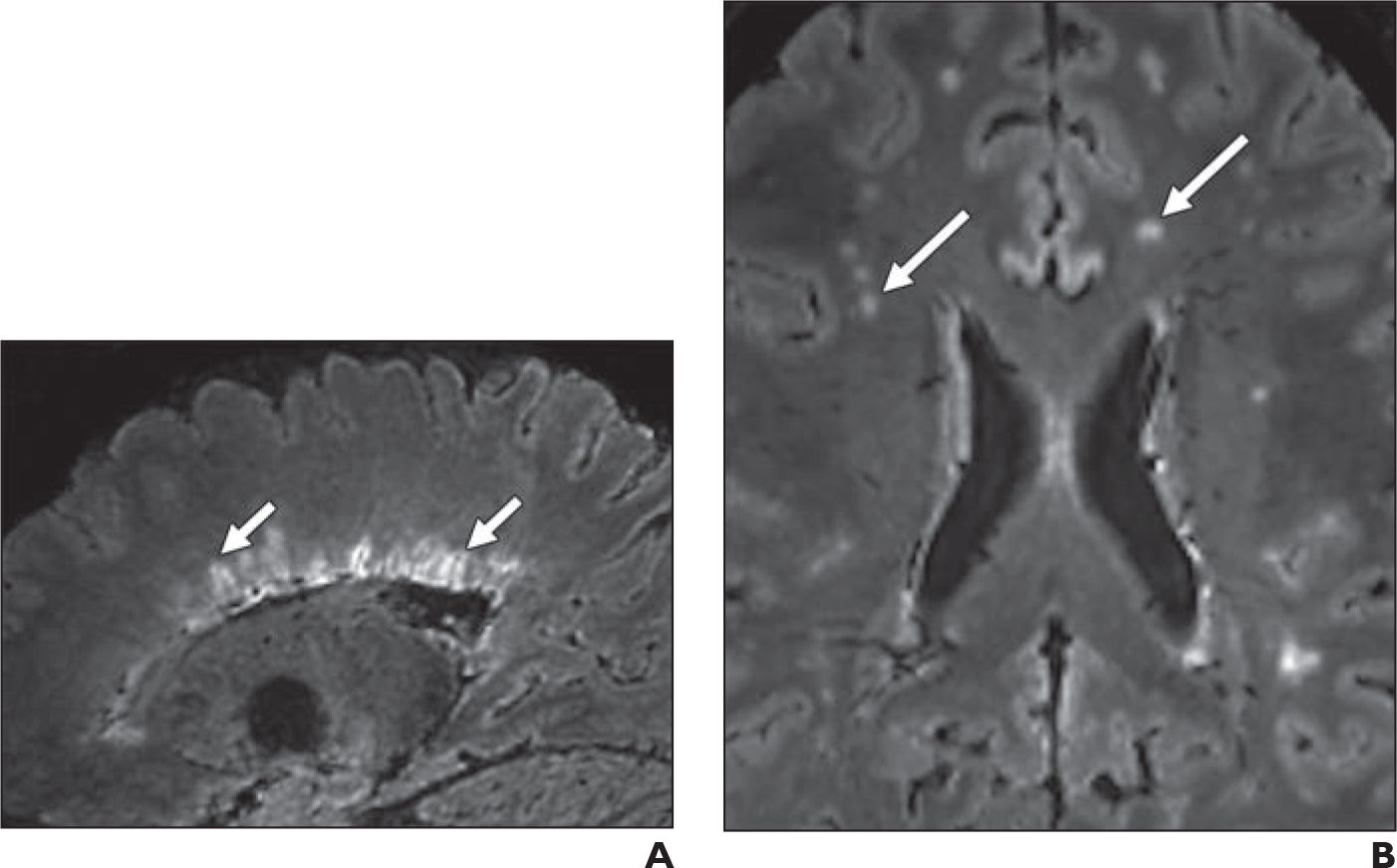
Representative FLAIR* MR images of study participants. MS = multiple sclerosis, CVS = central vein sign, WML = white matter lesion.
A, Sagittal image in 32-year-old participant with MS shows periventricular CVS-positive WML (arrows).
B, Axial image in 41-year-old participant without MS shows subcortical and deep white matter CVS-negative WMLs (arrows).
Representative FLAIR* MR images of study participants. MS = multiple sclerosis, CVS = central vein sign, WML = white matter lesion.
C, Coronal image in 38-year-old participant with MS shows juxtacortical CVS-positive WML (arrow).
D, Axial image in 36-year-old participant with MS shows subcortical CVS-positive WML (arrow).
E, Sagittal image in 38-year-old participant with MS shows infratentorial CVS-positive WML (arrow).
F, Axial image in 42-year-old participant without MS shows subcortical CVS-negative WML (arrow).
G, Axial image in 37-year-old participant with MS shows subcortical CVS-indeterminate WML (arrow).
H, Coronal image in 57-year-old participant without MS shows subcortical WML (arrow) that was excluded owing to size of 2.2 mm.
The median patient-level CVS-positivity rate was higher for participants with MS than for those without MS for both precontrast (43% [IQR, 25–74] vs 4% [IQR, 0–24], respectively) and postcontrast (67% [IQR, 56–81] vs 8% [IQR, 2–27]) FLAIR* images (Fig. 3). In a binomial model adjusting for the effect of GBCA, the likelihood of having at least one CVS-positive lesion was significantly higher for participants with than for those without MS (odds ratio [OR], 15.5 [95% CI, 11.6–21.0], p < .001). No significant interaction was identified between MS diagnosis and GBCA use (p = .99). Among participants with MS, the median patient-level CVS-positivity rate was higher for postcontrast (67% [IQR, 56–81]) than precontrast (43% [IQR, 25–74]) FLAIR* images (p < .001). In a binomial model adjusting for the effect of MS diagnoses, the frequency of having at least one CVS-positive WML was significantly higher for postcontrast than precontrast FLAIR* images (OR, 1.6 [95% CI, 1.2–2.0], p < .001). Based on the binomial model, in participants with MS, GBCA use increased the probability of having at least one CVS-positive WML from 0.54 to 0.65 (Table S4, available in the online supplement). Finally, GBCA use was associated with a mean of 2.8 additional CVS-positive WMLs per participant with MS and 0.8 additional CVS-positive WML per participant without MS. Figure 4 shows examples of WMLs classified as CVS-negative on precontrast FLAIR* images but as CVS-positive on postcontrast FLAIR* images.
Fig. 3—
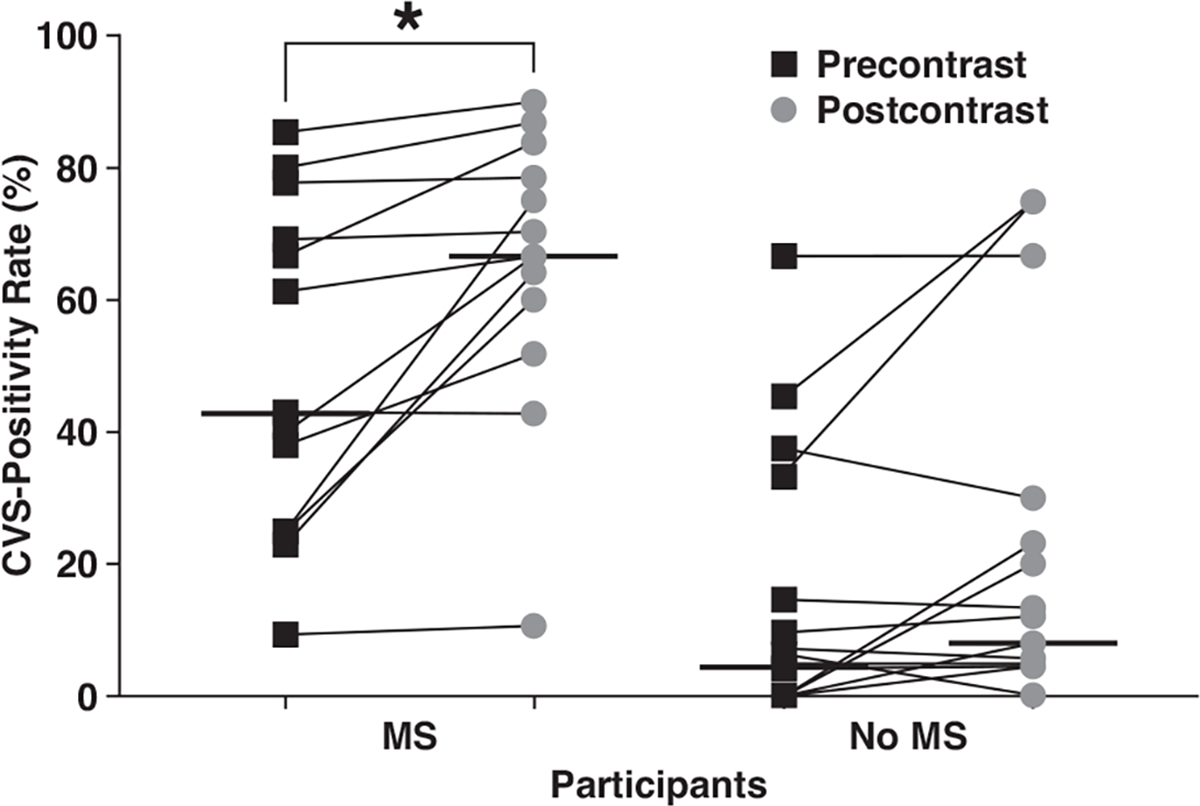
Graph shows patient-by-patient comparison of central vein sign (CVS)-positivity rate between precontrast (black boxes) and postcontrast (gray circles) FLAIR* images. Participants with multiple sclerosis (MS) diagnosis and participants with no MS diagnosis based on 2017 McDonald criteria during study are shown. Thin diagonal lines connect precontrast and postcontrast values for individual participants, and thick horizontal lines denote median percentage across participants for precontrast and postcontrast images. Asterisk indicates statistically significant difference (p < .001).
Fig. 4—
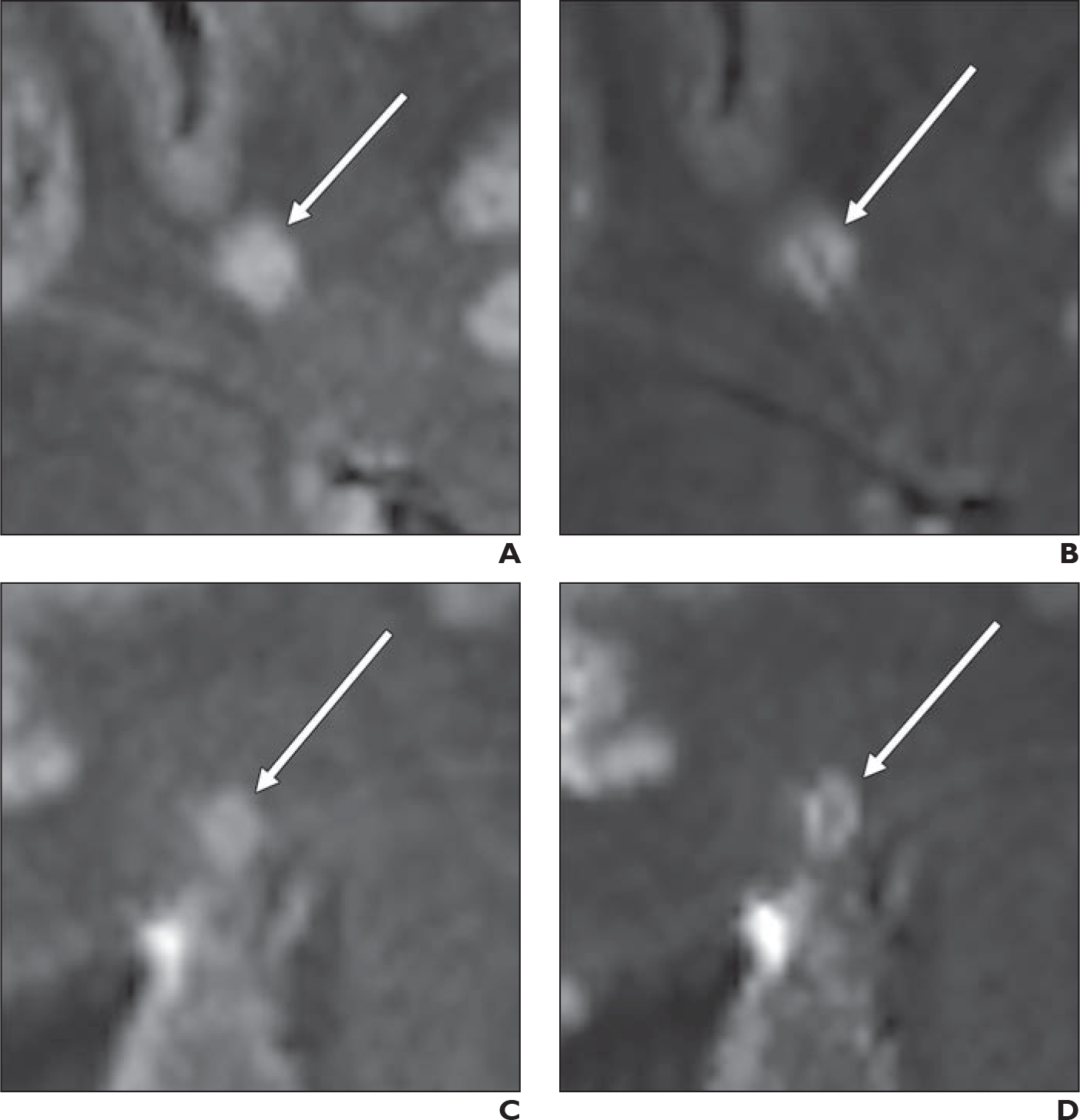
Comparison of precontrast and postcontrast FLAIR* images in terms of central vein sign (CVS) visualization in white matter lesions (WMLs). In this example, patient is 43-year-old participant with multiple sclerosis.
A and B, WML (arrow) was classified as CVS-negative on precontrast image (A) but as CVS-positive on postcontrast image (B).
C and D, WML (arrow) was classified as CVS-negative on precontrast image (C) but as CVS-positive on postcontrast image (D).
A sensitivity analysis in which CVS-indeterminate WMLs were classified as CVS-positive showed similar findings. Participants with MS had a higher median CVS-positivity rate on postcontrast (71% [IQR, 63–85]) than precontrast (50% [IQR, 40–76]) FLAIR* images. After adjusting for MS diagnosis, the likelihood of having at least one CVS-positive WML was significantly higher for postcontrast than precontrast FLAIR* images (OR = 1.6 [95% CI, 1.2–2.0], p < .001). In participants with MS, a mean of an additional 2.4 CVS-positive WMLs on postcontrast than precontrast FLAIR* images. Likewise, when CVS-indeterminate lesions were classified as CVS-negative, participants with MS had a higher median CVS-positivity rate on postcontrast (60% [IQR, 43–73]) than precontrast (40% [IQR, 21–60]) FLAIR* images; the likelihood of having at least one CVS-positive WML was higher for postcontrast than precontrast FLAIR* images (adjusted OR = 1.5 [95% CI, 1.2–1.9], p = .001); and, in participants with MS, a mean of an additional 2.8 CVS-positive WMLs were identified on postcontrast than precontrast FLAIR* images.
Effect of GBCA on Diagnostic Performance of Central Vein Sign for Multiple Sclerosis
Table 5 summarizes the sensitivity and specificity of CVS for diagnosing MS on the basis of various criteria. At a 40% patient-level CVS-positivity threshold, the sensitivity of CVS for MS increased from 62% on precontrast FLAIR* images to 92% on postcontrast FLAIR* images (p = .046). Specificity of CVS for MS was not significantly different between precontrast FLAIR* (88%) and postcontrast FLAIR* (82%) images (p = .32). At the 40% threshold, five of 13 (38%) participants with MS were incorrectly classified as not having MS on precontrast FLAIR* images compared with one of 13 (8%) participants on postcontrast FLAIR* images (i.e., four additional participants were correctly classified by postcontrast FLAIR* images).
TABLE 5:
Comparison of Precontrast and Postcontrast Images in Terms of Sensitivity and Specificity of CVS for Diagnosis of MS, Based on Varying Criteria
| Sensitivity | Specificity | |||
|---|---|---|---|---|
|
|
||||
| Outcome | Valuea | p b | Valuea | p b |
|
| ||||
| Patient-level CVS-positivity rate (35% threshold as positive for MS) | .08 | >.99 | ||
| Precontrast | 69 (9/13) | 82 (14/17) | ||
| Postcontrast | 92 (12/13) | 82 (14/17) | ||
| Patient-level CVS-positivity rate (40% threshold as positive for MS) | .046 | .32 | ||
| Precontrast | 62 (8/13) | 88 (15/17) | ||
| Postcontrast | 92 (12/13) | 82 (14/17) | ||
| Patient-level CVS-positivity rate (50% threshold as positive for MS) | .03 | .16 | ||
| Precontrast | 46 (6/13) | 94 (16/17) | ||
| Postcontrast | 85 (11/13) | 82 (14/17) | ||
| Patient-level CVS-positivity rate (40% threshold as positive for MS) with, based on 12-mo follow-up diagnoses, participants with RIS or CIS classified as positive for MS | .046 | .32 | ||
| Precontrast | 47 (8/17) | 85 (11/13) | ||
| Postcontrast | 71 (12/17) | 77 (10/13) | ||
| Patient-level CVS-positivity rate (40% threshold as positive for MS) with, based on 12-mo follow-up diagnoses, participants with RIS or CIS excluded | .046 | .32 | ||
| Precontrast | 58 (7/12) | 85 (11/13) | ||
| Postcontrast | 92 (11/12) | 77 (10/13) | ||
Note—CVS = central vein sign, MS = multiple sclerosis, RIS = radiologically isolated syndrome, CIS = clinically isolated syndrome.
Data represent percentage, with numerator and denominator in parentheses.
The p values were obtained using the McNemar test.
Thresholds of 35% and 50% yielded similar results (Table 5), for example yielding an additional three and five participants, respectively, being correctly classified on postcontrast FLAIR* images. Sensitivity of CVS for MS was significantly higher for precontrast FLAIR* (46%) than postcontrast FLAIR* (85%) images at the 50% threshold (p = .03) but was not significantly different between precontrast FLAIR* (69%) and postcontrast FLAIR* (92%) images at the 35% threshold (p = .08). Specificity was not significantly different between precontrast and postcontrast FLAIR* images for either threshold (all p > .05).
Twelve-Month Follow-Up
None of the 17 patients without an MS diagnosis at the initial study visit had an established MS diagnosis at 12-month follow-up. Clinical diagnoses at 12-month follow-up in these 17 patients were as follows: migraine (n = 3, with additional post-concussion syndrome in two patients), small-vessel ischemic disease (n = 3, with additional transient ischemic attack in one patient), clinically isolated syndrome (n = 3), nonspecific white matter changes (n = 2), radiologically isolated syndrome (n = 2), mixed connective tissue disease (n = 1), paresthesias (n = 1), and traumatic cervical myelopathy (n = 1); the remaining one participant did not have an established diagnosis at 12-month follow-up. Of the 13 participants who met 2017 McDonald criteria for MS at the initial study visit, 12 had a diagnosis of MS at 12-month follow-up, and one participant was later diagnosed with postinfectious encephalomyelitis. Tables S5 and S6 (available in the online supplement) provide additional participant-level information.
Figures S1 and S2 (available in the online supplement) show the impact of GBCA use on CVS detection based on 12-month follow-up diagnoses when classifying participants with final diagnoses of clinically isolated syndrome or radiologically isolated syndrome as having MS or as being excluded, respectively. Table 5 shows the effect of GBCA use on the sensitivity and specificity of CVS for MS based on 12-month follow-up diagnoses when classifying participants with final diagnoses of clinically isolated syndrome or radiologically isolated syndrome as having MS or as being excluded. These analyses showed similar results in terms of GBCA use significantly increasing the likelihood of having at least one CVS-positive WML and of being associated with a significant increase in the sensitivity of CVS for MS without a significant change in specificity.
Post Hoc Analysis
Three patients without MS had a CVS-positivity rate on postcontrast FLAIR* images that was greater than 40% (participants 4, 8, and 12; Table S5). Participant 8 had no CSF oligoclonal bands, and participants 4 and 8 had cervical and thoracic spinal cord imaging that did not show any WMLs. These three participants had 12-month follow-up diagnoses of radiologically isolated syndrome, migraines with postconcussive syndrome, and nonspecific white matter changes, respectively.
Of the three participants with clinically isolated syndrome at 12-month follow-up (participants 7, 9, and 17; Table S5), none had a precontrast or postcontrast CVS-positivity rate higher than 40%. One participant with MS had a CVS-positivity rate on postcontrast FLAIR* images that was less than 40% (participant 24, 11%; Table S6). This participant had a typical sensory presentation of MS but also had other factors potentially associated with WML development (age of 63 years and smoking use). The one participant who was classified as having MS at the initial study visit but who did not have an MS diagnosis at the 12-month follow-up (participant 19, diagnosis of postinfectious encephalomyelitis at 12-month follow-up; Table S6) had a CVS-positivity rate of 43% on both precontrast and postcontrast FLAIR* images.
Interobserver Agreement
The interreader agreement for classifying WMLs as CVS-positive versus CVS-negative was 88% agreement (κ = 0.71, indicating substantial agreement) on precontrast FLAIR* images and 87% agreement (κ = 0.73, indicating substantial agreement) on postcontrast FLAIR* images. The interreader agreement for patient-level CVS-positivity at the 40% threshold showed 77% agreement (κ = 0.35, fair agreement) for precontrast FLAIR* images and 80% agreement (κ = 0.60, moderate agreement) for postcontrast FLAIR* images.
Discussion
This multicenter study evaluated the impact of GBCA use on CVS detection, assessed by NAIMS criteria, and on performance of the CVS for diagnosing MS, when evaluated by a 3-T FLAIR* sequence. GBCA use increased CVS detection independent of MS diagnoses, across various sensitivity analyses. For example, GBCA use was associated with a 1.6-times greater odds of detecting at least one CVS-positive WML in all participants and a mean of 2.8 additional CVS-positive WMLs per participant with MS. GBCA use also improved the performance of the CVS for diagnosing MS based on the 2017 McDonald criteria. For example, at a 40% CVS-positivity threshold, GBCA use increased the sensitivity of CVS for diagnosing MS from 62% to 92% without significantly impacting specificity. When GBCA was used, four additional participants were correctly classified by the CVS as having MS. These findings support the use of GBCA for optimizing the role of the CVS on 3-T FLAIR* images as a diagnostic biomarker in MS evaluation.
Given concerns about long-term intracranial gadolinium accumulation [20], as well as increased costs, GBCA should be used prudently, and insight into the effect of GBCA will help guide protocol decisions in patients with MS. The role of GBCA in improving CVS detection, as observed in the current study, could be helpful for establishing MS diagnoses when using abbreviated CVS counting methods in individuals with a low volume of WMLs [26]. In this context, the additional detection of a few CVS-positive WMLs could markedly impact patient-level CVS-positivity rates. Accordingly, the observed effect of GBCA use may be particularly impactful early in the MS disease course when, typically, fewer WMLs are present. Nonetheless, in patients with established MS, assessment of newly developed WMLs for CVS positivity could also be used to guide disease management [28].
This study had limitations. First, the type of GBCA was not recorded for each examination, precluding assessment of the effect of different GBCA relaxivities on CVS conspicuity. Second, MRI examinations did not include SWI, which is less sensitive for CVS than the T2*-weighted EPI sequence evaluated in this study [13]. A recent study showed increased visibility of the CVS on SWI after GBCA administration [29], and a subsequent study of 19 patients with MS found that the CVS-positivity rate for SWI increased from 54% on precontrast images to 86% on postcontrast images [22], larger than the increase in CVS positivity for FLAIR* images observed by the current study. The MRI examinations also did not include QSM. A recent study reported visualization of a central vein in 31% of MS lesions on postcontrast QSM [14], which is substantially lower than the frequency of the CVS for postcontrast FLAIR* images in the current study. Third, patient-level analyses were performed using CVS-positivity rates, which may not be representative of patient-level CVS assessments based on machine learning algorithms or simplified clinical tools (e.g., select three WMLs) that rely on counting CVS-positive WMLs rather than determining percentages [26]. Such methods may be less intensive for interpreting radiologists; in addition, percentages may be less reliable when the denominator is small. Fourth, no participant without an initial MS diagnosis had a change in diagnosis to MS at 12-month follow-up. Longer follow-up might have allowed definitive diagnoses in participants with clinically isolated syndrome or radiologically isolated syndrome. Fifth, we did not differentiate between WMLs that were enhancing or nonenhancing on T1-weighted images. In a previous study, the CVS was found to be less visible in enhancing WMLs [28]. Sixth, 62% of all WMLs were excluded from evaluation by NAIMS criteria, of which 79% were excluded due to small size. Seventh, all MRI examinations were performed at 3 T. A recent meta-analysis showed a lower fraction of CVS in WMLs evaluated at 1.5 T (58%) than at 3 T (74%) or 7 T (82%) [30]. Given the decreased CVS detection at 1.5 T, GBCA use may have a larger impact on CVS detection at the lower field strength. Finally, the sample size was small. A prospective multicenter study to evaluate the CVS as a diagnostic biomarker in MS is currently underway (NCT04495556) and has a target recruitment of 400 participants.
In conclusion, in patients with suspicion for MS, CVS detection was significantly higher for postcontrast than precontrast 3-T FLAIR* images; GBCA use resulted in a larger mean number of CVS-positive WMLs per participant and in a larger fraction of participants having at least one CVS-positive WML. In addition, GBCA use significantly increased the sensitivity of CVS for MS without affecting specificity, thereby correctly classifying a larger number of participants in terms of MS diagnoses. The postcontrast FLAIR* sequence should be considered for CVS evaluation in future investigational trials and clinical practice.
Supplementary Material
HIGHLIGHTS.
Key Finding
In a model accounting for MS diagnoses, GBCA use was associated with increased likelihood of at least one CVS-positive WML on FLAIR* images (OR = 1.6, p < .001). CVS had higher sensitivity for MS on postcontrast than precontrast FLAIR* images (92% vs 62%, p = .046) without a difference in specificity (82% vs 88%, p = .32).
Importance
GBCA use improves visualization of central veins in patients being evaluated for MS. Postcontrast FLAIR* images should be considered for future trials and clinical practice.
Acknowledgments
P. Rodrigues is an employee of and owns options in QMENTA, Inc. C. Azevedo is on the scientific advisory boards of Genentech, EMD Serono, Alexion Pharmaceuticals, and Sanofi Genzyme. B. A. C. Cree is a consultant to Alexion Pharmaceuticals, Atara Biotherapeutics, Biogen, EMD Serono, Novartis, Sanofi, and TG Therapeutics. L. Freeman is on the advisory boards of Genentech, Novartis, and Celgene; is a consultant to EMD Serono, Celgene, and Biogen; and receives program sponsorship from Biogen and EMD Serono. R. G. Henry is a consultant to Novartis, Sanofi Genzyme, Genentech/Roche, Celgene, Atara Biotherapeutics, and MedDay. E. E. Longbrake is a consultant to Genentech, Genzyme, Alexion Pharmaceuticals, Biogen, EMD Serono, Celegene/Bristol Myers Squibb. K. Nakamura receives a licensing fee from Biogen and research support from the U.S. Department of Defense, NIH, Patient-Centered Outcomes Research Institute (PCORI), Genzyme, and Biogen. J. Oh receives research support from Biogen Idec, Roche, and EMD Serono and is a consultant to EMD Serono, Sanofi Genzyme, Biogen Idec, Roche, Celgene, and Novartis. D. Pelletier is a consultant to EMD Serono, Sanofi Genzyme, Roche, and Novartis. R. D. Samudralwar is on the advisory boards of Biogen, EMD Serono, and Sanofi Genzyme and is a consultant to EMD Serono and Biogen. E. S. Sotirchos is on the scientific advisory boards of Viela Bio and Genentech and receives speaker honoraria from Viela Bio. N. L. Sicotte receives research support from NIH, the National Multiple Sclerosis Society, PCORI, Race to Erase MS, and Biogen Idec. A. J. Solomon is a consultant to EMD Serono, Biogen, Alexion Pharmaceuticals, and Celgene; is a nonpromotional speaker for EMD Serono; receives research funding from Biogen; and is contracted for research by Biogen, Novartis, Actelion, and Genentech/Roche. D. Ontaneda receives research support from NIH, National Multiple Sclerosis Society, PCORI, Race to Erase MS, Genentech, Genzyme, and Novartis and is a consultant to Biogen Idec, Genentech/Roche, Genzyme, Novartis, and Merck. P. Sati receives research support from NIH and the National Multiple Sclerosis Society. The remaining authors declare that there are no other disclosures relevant to the subject matter of this article.
Supported by the Race to Erase MS, the North American Imaging in MS Cooperative, the NINDS Intramural Research Program, and the NIH Medical Research Scholars Program (R01NS112274).
Footnotes
Provenance and review: Not solicited; externally peer reviewed.
An electronic supplement is available online at doi.org/10.2214/AJR.22.27731.
Based on presentations at the 2020 Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) virtual annual meeting and the 2021 ACTRIMS virtual annual meeting.
References
- 1.Kavaliunas A, Manouchehrinia A, Stawiarz L, et al. Importance of early treatment initiation in the clinical course of multiple sclerosis. Mult Scler 2017; 23:1233–1240 [DOI] [PubMed] [Google Scholar]
- 2.Schwenkenbecher P, Wurster U, Konen FF, et al. Impact of the McDonald criteria 2017 on early diagnosis of relapsing-remitting multiple sclerosis. Front Neurol 2019; 10:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaisey M, Solomon AJ, Luu M, Giesser BS, Sicotte NL. Incidence of multiple sclerosis misdiagnosis in referrals to two academic centers. Mult Scler Relat Disord 2019; 30:51–56 [DOI] [PubMed] [Google Scholar]
- 4.Solomon AJ, Bourdette DN, Cross AH, et al. The contemporary spectrum of multiple sclerosis misdiagnosis: a multicenter study. Neurology 2016; 87:1393–1399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sati P, Oh J, Constable RT, et al. ; NAIMS Cooperative. The central vein sign and its clinical evaluation for the diagnosis of multiple sclerosis: a consensus statement from the North American Imaging in Multiple Sclerosis Cooperative. Nat Rev Neurol 2016; 12:714–722 [DOI] [PubMed] [Google Scholar]
- 6.Kilsdonk ID, Lopez-Soriano A, Kuijer JPA, et al. Morphological features of MS lesions on FLAIR* at 7 T and their relation to patient characteristics. J Neurol 2014; 261:1356–1364 [DOI] [PubMed] [Google Scholar]
- 7.Maggi P, Absinta M, Grammatico M, et al. Central vein sign differentiates multiple sclerosis from central nervous system inflammatory vasculopathies. Ann Neurol 2018; 83:283–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tallantyre EC, Dixon JE, Donaldson I, et al. Ultra-high-field imaging distinguishes MS lesions from asymptomatic white matter lesions. Neurology 2011; 76:534–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kilsdonk ID, Wattjes MP, Lopez-Soriano A, et al. Improved differentiation between MS and vascular brain lesions using FLAIR* at 7 Tesla. Eur Radiol 2014; 24:841–849 [DOI] [PubMed] [Google Scholar]
- 10.Mistry N, Abdel-Fahim R, Samaraweera A, et al. Imaging central veins in brain lesions with 3-T T2*-weighted magnetic resonance imaging differentiates multiple sclerosis from microangiopathic brain lesions. Mult Scler 2016; 22:1289–1296 [DOI] [PubMed] [Google Scholar]
- 11.Rovira À, Wattjes MP, Tintoré M, et al. ; MAGNIMS Study Group. Evidence-based guidelines: MAGNIMS consensus guidelines on the use of MRI in multiple sclerosis: clinical implementation in the diagnostic process. Nat Rev Neurol 2015; 11:471–482 [DOI] [PubMed] [Google Scholar]
- 12.Traboulsee A, Simon JH, Stone L, et al. Revised recommendations of the Consortium of MS Centers Task Force for a standardized MRI protocol and clinical guidelines for the diagnosis and follow-up of multiple sclerosis. AJNR 2016; 37:394–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samaraweera APR, Clarke MA, Whitehead A, et al. The central vein sign in multiple sclerosis lesions is present irrespective of the T2* sequence at 3 T. J Neuroimaging 2017; 27:114–121 [DOI] [PubMed] [Google Scholar]
- 14.Zhang S, Nguyen TD, Hurtado Rúa SM, et al. Quantitative susceptibility mapping of time-dependent susceptibility changes in multiple sclerosis lesions. AJNR 2019; 40:987–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sati P, George IC, Shea CD, Gaitán MI, Reich DS. FLAIR*: a combined MR contrast technique for visualizing white matter lesions and parenchymal veins. Radiology 2012; 265:926–932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campion T, Smith RJP, Altmann DR, et al. FLAIR* to visualize veins in white matter lesions: a new tool for the diagnosis of multiple sclerosis? Eur Radiol 2017; 27:4257–4263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Solomon AJ, Schindler MK, Howard DB, et al. “Central vessel sign” on 3T FLAIR* MRI for the differentiation of multiple sclerosis from migraine. Ann Clin Transl Neurol 2015; 3:82–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Clarke MA, Samaraweera AP, Falah Y, et al. Single test to arrive at multiple sclerosis (STAR-MS) diagnosis: a prospective pilot study assessing the accuracy of the central vein sign in predicting multiple sclerosis in cases of diagnostic uncertainty. Mult Scler 2020; 26:433–441 [DOI] [PubMed] [Google Scholar]
- 19.Sati P, Thomasson DM, Li N, et al. Rapid, high-resolution, whole-brain, susceptibility-based MRI of multiple sclerosis. Mult Scler 2014; 20:1464–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.U.S. FDA website. FDA drug safety communication: FDA warns that gadolinium-based contrast agents (GBCAs) are retained in the body—requires new class warnings. www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-warns-gadolinium-based-contrast-agents-gbcas-are-retained-body. Published December 19, 2017. Updated May 16, 2018. Accessed February 28, 2022
- 21.Guisset F, Lolli V, Bugli C, et al. The central vein sign in multiple sclerosis patients with vascular comorbidities. Mult Scler 2021; 27:1057–1065 [DOI] [PubMed] [Google Scholar]
- 22.Sparacia G, Agnello F, Iaia A, Banco A, Galia M, Midiri M. Multiple sclerosis: prevalence of the ‘central vein’ sign in white matter lesions on gadolinium-enhanced susceptibility-weighted images. Neuroradiol J 2021; 34:470–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Daboul L, O’Donnell C, Moreno-Dominguez D, et al. A multicenter evaluation of the diagnostic performance of the central vein sign using simplified algorithms. Mult Scler J 2021; 27:(suppl)19 [Google Scholar]
- 24.Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17:162–173 [DOI] [PubMed] [Google Scholar]
- 25.Yushkevich PA, Piven J, Hazlett HC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 2006; 31:1116–1128 [DOI] [PubMed] [Google Scholar]
- 26.Solomon AJ, Watts R, Ontaneda D, Absinta M, Sati P, Reich DS. Diagnostic performance of central vein sign for multiple sclerosis with a simplified three-lesion algorithm. Mult Scler 2018; 24:750–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977; 33:159–174 [PubMed] [Google Scholar]
- 28.Al-Louzi O, Letchuman V, Manukyan S, et al. Central vein sign profile of newly developing lesions in multiple sclerosis: a 3-year longitudinal study. Neurol Neuroimmunol Neuroinflamm 2022; 9:e1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Maggi P, Mazzoni LN, Moretti M, Grammatico M, Chiti S, Massacesi L. SWI enhances vein detection using gadolinium in multiple sclerosis. Acta Radiol Open 2015; 4:2047981614560938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Castellaro M, Tamanti A, Pisani AI, Pizzini FB, Crescenzo F, Calabrese M. The use of the central vein sign in the diagnosis of multiple sclerosis: a systematic review and meta-analysis. Diagnostics (Basel) 2020; 10:1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


