Abstract
Objectives
To compare the accuracy of the Sequential Organ Failure Assessment (SOFA) and Acute Physiology and Chronic Health Evaluation II (APACHE II) Scores in predicting mortality among intensive care unit (ICU) patients with sepsis in a low-income and middle-income country.
Design
A multicentre, cross-sectional study.
Setting
A total of 15 adult ICUs throughout Vietnam.
Participants
We included all patients aged ≥18 years who were admitted to ICUs for sepsis and who were still in ICUs from 00:00 to 23:59 of the specified study days (ie, 9 January, 3 April, 3 July and 9 October of the year 2019).
Primary and secondary outcome measures
The primary outcome was hospital all-cause mortality (hospital mortality). We also defined the secondary outcome as all-cause deaths in the ICU (ICU mortality).
Results
Of 252 patients, 40.1% died in hospitals, and 33.3% died in ICUs. SOFA Score (areas under the receiver operating characteristic curve (AUROC): 0.688 (95% CI 0.618 to 0.758); cut-off value≥7.5; PAUROC<0.001) and APACHE II Score (AUROC: 0.689 (95% CI 0.622 to 0.756); cut-off value ≥20.5; PAUROC<0.001) both had a poor discriminatory ability for predicting hospital mortality. However, the discriminatory ability for predicting ICU mortality of SOFA (AUROC: 0.713 (95% CI 0.643 to 0.783); cut-off value≥9.5; PAUROC<0.001) was fair and was better than that of APACHE II Score (AUROC: 0.672 (95% CI 0.603 to 0.742); cut-off value≥18.5; PAUROC<0.001). A SOFA Score≥8 (adjusted OR (AOR): 2.717; 95% CI 1.371 to 5.382) and an APACHE II Score≥21 (AOR: 2.668; 95% CI 1.338 to 5.321) were independently associated with an increased risk of hospital mortality. Additionally, a SOFA Score≥10 (AOR: 2.194; 95% CI 1.017 to 4.735) was an independent predictor of ICU mortality, in contrast to an APACHE II Score≥19, for which this role did not.
Conclusions
In this study, SOFA and APACHE II Scores were worthwhile in predicting mortality among ICU patients with sepsis. However, due to better discrimination for predicting ICU mortality, the SOFA Score was preferable to the APACHE II Score in predicting mortality.
Clinical trials registry – India: CTRI/2019/01/016898.
Keywords: Adult intensive & critical care, INTENSIVE & CRITICAL CARE, ACCIDENT & EMERGENCY MEDICINE, BACTERIOLOGY
Strengths and limitations of this study.
An advantage of the present study was data from multi centres, which had little missing data.
Due to the absence of a national registry of intensive care units (ICUs) to allow systematic recruitment of units, we used a snowball method to identify suitable units, which might have led to the selection of centres with a greater interest in sepsis management.
Due to the study’s real-world nature, we did not make a protocol for microbiological investigations. Moreover, we mainly evaluated resources used in ICUs; therefore, the data detailing the point-of-care testing and life-sustaining treatments were not available. Additionally, to improve the feasibility of conducting the study in busy ICUs, we opted not to collect data on antibiotic resistance and appropriateness.
Due to our independent variables (eg, Sequential Organ Failure Assessment Score that was greater than or equal to the cut-off value) that might be associated with primary outcome only measured on ICU admission, the mixed-effects logistic regression model could not be used to predict discrete outcome variables measured at two different times, that is, inside and outside the ICU settings.
Although the sample size was large enough, the CI was slightly wide (±6.03%), which might influence the normal distribution of the sample.
Introduction
Sepsis is a clinical syndrome which has physiological, biological and biochemical abnormalities caused by a dysregulated host response to infection and is a critical global health problem.1 2 Sepsis is the most common cause of in-hospital deaths, with most of the burden in low-income and middle-income countries (LMICs), and extracts a high economic and social cost;3–5 mortality rates remain high at 30%–45% and contribute to as much as 20% of all deaths worldwide.2 4 6 7 There is no reference standard that allows easy, accurate diagnosis and prognosis of sepsis.1 8 Although the 1991 International Consensus Definition Task Force proposed the systemic inflammatory response syndrome criteria to identify patients with a septic host response,9 these criteria do not measure whether the response is injurious, and their utility is limited.1 8
The Acute Physiology and Chronic Health Evaluation II (APACHE II) Score was originally developed for critically ill patients in intensive care units (ICUs).10 It has 12 physiological measures and extra points based on age and the presence of chronic disease.10 The APACHE II Score was shown to have good prognostic value in acutely ill or surgical patients.10 11 However, some limitations of the APACHE II Score are that (1) It is complex and cumbersome to use, (2) It does not differentiate between the sterile and infected necrosis, and finally, (3) It has a poor predictive value at 24 hours.12
In 2016, the Sepsis-3 Task Force proposed that for patients with suspected infection, an increase of 2 points or more in the Sequential Organ Failure Assessment (SOFA) Score could serve as clinical criteria for sepsis,1 and the consensus has not changed since then.13 This approach was justified based on content validity (SOFA reflects the facets of organ dysfunction) and predictive validity (the proposed criteria predict downstream events associated with the condition of interest).14–17 However, the validity of this score was mainly derived from critically ill patients with suspected sepsis by interrogating over a million ICU electronic health record encounters from ICUs in high-income countries (HICs).1 17 18 Moreover, the patients, pathogens and clinical capacity to manage sepsis differ considerably between HIC and LMIC settings.7 Therefore, it’s still unclear whether this score could be applied to different types of infections, locations within the hospital and countries.
Vietnam is an LMIC, ranked fifteenth in the world and third in South-East Asia by population with 96.462 million people.19 Vietnam is also a hot spot for emerging infectious diseases in South-East Asia, including SARS-CoV-1,20 avian influenza A(H5N1)21 22 and the ongoing global COVID-19 outbreaks.23 24 Additionally, severe dengue,25 Streptococcus suis infection,26 malaria27 and increased antibiotic resistance are other major causes of sepsis in ICUs across Vietnam.28 29 Despite its recent economic growth spurt,30 Vietnam is still struggling to provide either enough resources or adequate diagnostic, prognostic and treatment strategies for patients with sepsis in both local and central settings.31 32 In addition, within the healthcare system in Vietnam, central hospitals are responsible for receiving patients who have difficulties being treated in local hospital settings.33 Therefore, the diagnosis, prognosis and initiation of treatment for patients with sepsis are often delayed.
In resource-limited settings, the early identification of infected patients who may go on to develop sepsis or who may be at risk of death from sepsis using accurate scoring systems is a way to decrease sepsis-associated mortality. Therefore, this study aimed to investigate the mortality rate and compare the accuracy of the SOFA Score and the APACHE II Score in predicting mortality in ICU patients with sepsis in Vietnam.
Methods
Source of data
This multicentre observational, cross-sectional, point prevalence study is part of the Management of Severe sepsis in Asia’s Intensive Care unitS (MOSAICS) II Study,34–37 which enrolled patients on 9 January (Winter), 3 April (Spring), 3 July (Summer) and 9 October (Autumn) of the year 2019. All patients received a follow-up till hospital discharge, death in the ICU/hospital or up to 90 days postenrolment, whichever was earliest. In this study, we only used data from Vietnam. A total of 15 adult ICUs (excluding predominantly neurosurgical, coronary, and cardiothoracic ICUs) participated in the MOSAICS II study from 14 hospitals, of which 5 are central, and 9 are provincial, district, or private hospitals throughout Vietnam. Each ICU had one or two representatives who were part of the local study team and the MOSAICS II Study group, as shown in eAppendix 2 of a previously published paper.36 Participation was voluntary and unfunded.
Participants
All patients admitted to participating ICUs on 1 of the 4 days (ie, 9 January, 3 April, 3 July and 9 October, 2019) which represented the different seasons of the year 2019 were screened for eligibility. We included all patients, aged ≥18 years, who were admitted to the ICUs for sepsis, and who were still in the ICUs from 00:00 to 23:59 of the study days. We defined sepsis as infection with a SOFA Score of 2 points or more from baseline (assumed to be 0 for patients without prior organ dysfunction).1
Data collection
We used a standardised classification and case record form (CRF) to collect data on common variables as shown in online supplemental file 1. The data dictionary of the MOSAICS II Study is available as an online supplement of previously published papers.35 36 Data were entered by the representatives of the participating hospitals into the database of the MOSAICS II Study via the password-protected online CRFs. We checked the data for implausible outliers and missing fields and contacted ICU representatives for clarification. We then merged the data sets for the 14 hospitals.
bmjopen-2022-064870supp001.pdf (188.5KB, pdf)
Outcome measures
The primary outcome was hospital all-cause mortality (hospital mortality). We also defined the secondary outcomes as all-cause deaths in the ICU (ICU mortality) and the ICU and hospital lengths of stay (LOS).
Predictor measures
We defined exposure variables as the SOFA and the APACHE II Scores.10 14 All data elements required for calculating the SOFA Score at the time of ICU admission and the APACHE II Score over the first 24 hours of ICU admission were prospectively collected on a CRF and entered into a database via the online CRF for later analysis.
We determined confounding factors as the variables of hospital and ICU characteristics collected on a questionnaire by representatives before patient enrolment, as shown in online supplemental file 2. We also determined confounding factors as variables collected on a CRF by investigators. The CRF contained four sections which is available in online supplemental file 1. The first section focused on baseline characteristics (demographics, documented comorbidities and details of admission). The second section comprised of vital signs on ICU admission, laboratory parameters, site of infection and microbiology. Only microorganisms detected via all cultures, serology, molecular and histological investigations, and deemed to be true pathogens rather than commensals or contaminants were recorded. The third section captured the timing of sepsis bundle elements referencing time zero, determined as follows: (A) Time of triage in the emergency department (ED) for those presenting with sepsis to the ED; (B) Time of clinical documentation of deterioration in the general wards or other non-ED areas for those who developed sepsis after hospital admission; (C) Time of ICU admission for those in which (A) or (B) could not be determined from the clinical documentation. The bundle elements were based on the Surviving Sepsis Campaign’s 2018 update: antibiotics administration, blood cultures, lactate measurement, fluid administration (amount of fluids administered in the first and third hours from time zero) and vasopressor initiation.38 The fourth section concerned life-sustaining treatments provided during the ICU stay.
bmjopen-2022-064870supp002.pdf (108.1KB, pdf)
Sample size
In the present study, hospital mortality served as the primary outcome. We, therefore, used the formula to determine the minimal sample size for estimating a population proportion with a confidence level of 95%, a CI (margin of error) of 6.03% and an assumed population proportion of 61.0%, based on the hospital mortality rate (61.0%) of our cohort reported in a previously published study.39 Therefore, we should have at least 252 patients in our sample. Because of this, our sample size was sufficient and reflected a normal distribution.
where:
z is the z score (z score for a 95% confidence level is 1.96)
ε is the margin of error (ε for a CI of ±6.03% is 0.0603)
is the population proportion ( for a population proportion of 61.0% is 0.61)
n is the sample size
Statistical analyses
We used IBM SPSS Statistics V.22.0 (IBM Corp, Armonk, New York, USA) for data analysis. We report data as numbers (no.) and percentages (%) for categorical variables and medians and IQRs or means and SDs for continuous variables. Comparisons were made between survival and death in the hospital and ICU for each variable, using the χ2 test or Fisher’s exact test for categorical variables and the Mann-Whitney U test, Kruskal-Wallis test, one-way analysis of variance for continuous variables.
Receiver operating characteristic (ROC) curves were plotted and the areas under the ROC curve (AUROC) were calculated to determine the discriminatory ability of the SOFA and APACHE II Scores for deaths in the hospital and ICU. The cut-off value of the SOFA and the APACHE II Scores was determined by the ROC curve analysis and defined as the cut-off point with the maximum value of Youden’s Index (ie, sensitivity+specificity – 1). Based on the cut-off value of the scores, we assigned the patients to two groups: either a score that was less than the cut-off value or a score that was greater than or equal to the cut-off value.
We assessed factors associated with death in the hospital using logistic regression analysis. To reduce the number of predictors and the multicollinearity issue and resolve the overfitting, we used different ways to select variables as follows: (A) We put all variables (including exposure and confounding factors) of hospital and ICU characteristics, baseline characteristics, clinical and laboratory characteristics, and treatments into the univariable logistic regression model; (B) We selected variables if the value of p was <0.05 in the univariable logistic regression analysis between survival and death in the hospital, as well as those that are clinically crucial to put in the multivariable logistic regression model. These variables included university affiliation, training programme in ICU, documented comorbidities (ie, cardiovascular disease, chronic neurological disease), the severity of illness (ie, SOFA and APACHE II Scores that were greater than or equal to the cut-off value), sites of infection (ie, urinary tract, abdominal, skin or cutaneous sites), pathogens detection (ie, no pathogens detected, Gram-negative bacteria), completion of the 1-hour or 3-hour sepsis bundle of care, completion of the initial administration of antibiotics within 1 hour or 3 hours, respiratory support (ie, mechanical ventilation (MV), high-flow nasal oxygen), and additional ICU support (ie, vasopressors/inotropes, renal replacement therapy (RRT), red blood cell transfusion, platelet transfusion, fresh frozen plasma transfusion, surgical source control, and non-surgical source control). Using a stepwise backward elimination method, we started with the full multivariable logistic regression model that included the selected variables. This method then deleted the variables stepwise from the full model until all remaining variables were independently associated with the risk of death in the hospital in the final model. Similarly, we used these methods of variable selection and analysis for assessing factors associated with death in the ICU. We presented the ORs and 95% CIs in the univariable logistic regression model and the adjusted ORs (AORs) and 95% CIs in the multivariable logistic regression model.
For all analyses, significance levels were two-tailed, and we considered p<0.05 as statistically significant.
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Results
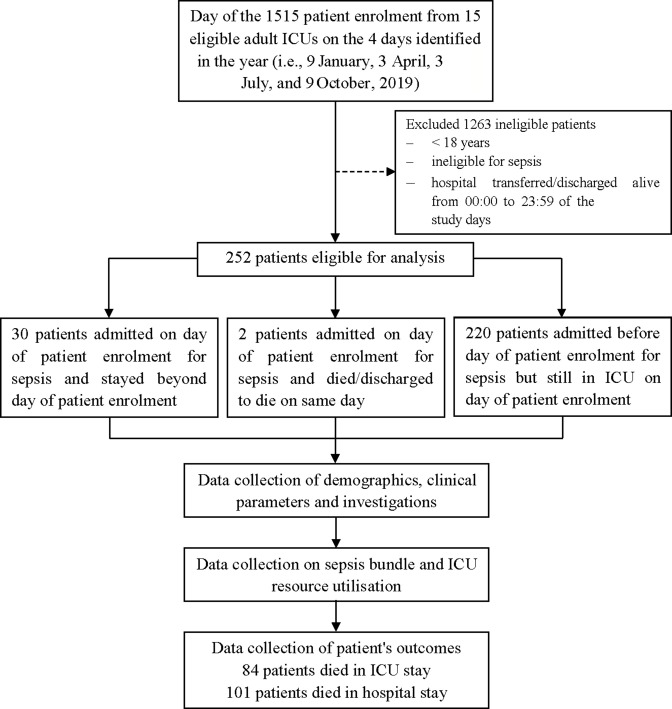
Data on 252 patients with sepsis were submitted to the database of the MOSAICS II Study (figure 1), in which there were little missing data.
Figure 1.
Flow chart of the study design, patient enrolment and follow-up. ICU, intensive care unit; discharged to die, defined as the patients who were in grave condition or dying and were classified with deaths in the ICU at the time of discharge.
Clinical characteristics and outcomes
In our study cohort, 64.3% (162/252) were men and the median age was 65 years (IQR: 52–76.75) (table 1). Among the total patients, the median SOFA Score was 7 (IQR: 4.75–10) at the time of ICU admission, the median APACHE II Score was 18 (IQR: 13–24) over the first 24 hours of ICU admission, and 29.4% (74/252) of patients had septic shock (table 1). Table 1 also shows that the most common documented comorbidities included cardiovascular disease (31.0%; 78/252), diabetes mellitus (26.6%; 67/252) and chronic neurological disease (14.3%; 36/252), the most common sites of infection included respiratory (56.7%; 143/252), abdominal cavity (24.2%; 61/252), urinary tract (14.7%; 37/252), and skin or cutaneous sites (7.5%; 19/252) and Gram-negative bacteria were isolated in 61.9% (156/252) of patients. Table 2 shows that MV was provided for 68.9% (173/251) of patients and RRT for 40.2% (101/251). Overall, 40.1% (101/252) of patients with sepsis died in the hospital, 33.3% (84/252) of whom died in the ICU (figure 1 and table 2). The median hospital and ICU LOS were 16 (IQR: 10–25) and 10 (IQR: 6–18) days, respectively (table 2). The clinical characteristics, severity of illness, sites of infection and microbiology, compliance with sepsis bundle elements, and life-sustaining treatments during ICU stay were compared between patients who survived and patients who died in the hospital and ICU, as shown in tables 1 and 2, and tables S1–S14 (online supplemental file 3).
Table 1.
Baseline characteristics according to hospital survivability of patients with sepsis
| Variables | All cases | Survived | Died | P value* |
| Hospital and ICU characteristics | n=252 | n=151 | n=101 | |
| University affiliation, no. (%) | 99 (39.3) | 46 (30.5) | 53 (52.5) | <0.001 |
| Training programme in ICU, no. (%) | 202 (80.2) | 129 (85.4) | 73 (72.3) | 0.010 |
| Demographics | n=252 | n=151 | n=101 | |
| Age (years), median (IQR) | 65 (52–76.75) | 65 (53–76) | 65 (52–78) | 0.810‡ |
| Sex (male), no. (%) | 162 (64.3) | 93 (61.6) | 69 (68.3) | 0.275 |
| Documented comorbidities | n=252 | n=151 | n=101 | |
| Cardiovascular disease, no. (%) | 78 (31.0) | 41 (27.2) | 37 (36.6) | 0.111 |
| Chronic lung disease, no. (%) | 30 (11.9) | 18 (11.9) | 12 (1.9) | 0.992 |
| Chronic neurological disease, no. (%) | 36 (14.3) | 28 (18.5) | 8 (7.9) | 0.018 |
| Chronic kidney disease, no. (%) | 23 (9.1) | 14 (9.3) | 9 (8.9) | 0.922 |
| Peptic ulcer disease, no. (%) | 9 (3.6) | 5 (3.3) | 4 (4.0) | >0.999† |
| Chronic liver disease, no. (%) | 27 (10.7) | 14 (9.3) | 13 (12.9) | 0.365 |
| Diabetes mellitus, no. (%) | 67 (26.6) | 40 (26.5) | 27 (26.7) | 0.966 |
| Connective tissue disease, no. (%) | 3 (1.2) | 2 (1.3) | 1 (1.0) | >0.999† |
| Immunosuppression, no. (%) | 10 (4.0) | 7 (4.6) | 3 (3.0) | 0.744 |
| Haematological malignancies, no. (%) | 5 (2.0) | 3 (2.0) | 2 (2.0) | >0.999† |
| Solid malignant tumours, no. (%) | 12 (4.8) | 6 (4.0) | 6 (5.9) | 0.551† |
| Vital signs (on admission into ICU) | n=252 | n=151 | n=101 | |
| GCS, median (IQR) | 13 (9–15) | 14 (10–15) | 10 (8–14) | <0.001‡ |
| HR (beats per min), median (IQR) | 110 (95.25–125.75) | 110 (92–125) | 110 (100–129.5) | 0.083‡ |
| Temperature (oC), mean (SD) | 37.79 (1.01) | 37.80 (1.08) | 37.77 (0.91) | 0.871‡ |
| MBP (mmHg), mean(SD) | 75.82 (22.08) | 79.75 (22.88) | 69.93 (19.51) | 0.002‡ |
| RR (breaths per min), median (IQR) | 25 (22–30) | 25 (22–30) | 25 (20–30) | 0.693‡ |
| Blood investigations | n=252 | n=151 | n=101 | |
| Total WBC (x109/L), mean (SD) | 15.73 (9.20) | 15.63 (8.67) | 15.88 (9.98) | 0.914‡ |
| PLT (x109/L), mean (SD) | 185.98 (137.85) | 200.71 (129.67) | 163.95 (147.15) | 0.002‡ |
| Hb (g/dL), mean (SD) | 11.14 (2.59) | 11.36 (2.68) | 10.82 (2.44) | 0.088‡ |
| K+ (mmol/L), mean (SD) | 3.89 (0.79) | 3.90 (0.80) | 3.87 (0.77) | 0.865‡ |
| Na+ (mmol/L), mean (SD) | 136.05 (8.24) | 135.62 (8.81) | 136.69 (7.80) | 0.068‡ |
| Creatinine (µmol/L), mean (SD) | 187.85 (151.92) | 186.15 (171.60) | 190.38 (117.27) | 0.030‡ |
| Bilirubin (µmol/l), mean (SD) | 32.80 (61.49) | 31.74 (72.67) | 34.35 (40.09) | 0.007‡ |
| pH, mean (SD) | 7.37 (0.50) | 7.41 (0.64) | 7.32 (0.14) | 0.004‡ |
| PaO2 (mmHg), mean (SD) | 116.17 (74.28) | 110.23 (56.25) | 124.73 (94.07) | 0.665‡ |
| PaO2/FiO2 ratio, mean (SD) | 262.48 (149.58) | 281.52 (149.39) | 235.26 (146.32) | 0.003‡ |
| Severity of illness scores | n=252 | n=151 | n=101 | |
| SOFA, median (IQR), n=250 | 7 (4.75–10) | 6 (4-9) | 9 (6-12) | <0.001‡ |
| APACHE II, median (IQR) | 18 (13–24) | 15 (12–21) | 22 (16–27) | <0.001‡ |
| Septic shock | 74 (29.4) | 35 (23.2) | 39 (38.6) | 0.008 |
| Site of Infection | n=252 | n=151 | n=101 | |
| Respiratory, no. (%) | 143 (56.7) | 82 (54.3) | 61 (60.4) | 0.339 |
| Urinary tract, no. (%) | 37 (14.7) | 30 (19.9) | 7 (6.9) | 0.004 |
| Abdominal, no. (%) | 61 (24.2) | 34 (22.5) | 27 (26.7) | 0.444 |
| Neurological, no. (%) | 12 (4.8) | 8 (5.3) | 4 (4.0) | 0.767† |
| Bones or joints, no. (%) | 2 (0.8) | 2 (1.3) | 0 (0.0) | 0.518† |
| Skin or cutaneous sites, no. (%) | 19 (7.5) | 7 (4.6) | 12 (11.9) | 0.033 |
| Intravascular catheter, no. (%) | 1 (0.4) | 1 (0.7) | 0 (0.0) | >0.999† |
| Infective endocarditis, no. (%) | 1 (0.4) | 0 (0.0) | 1 (1.0) | 0.401† |
| Primary bacteraemia, no. (%) | 7 (2.8) | 5 (3.3) | 2 (2.0) | 0.705† |
| Systemic, no. (%) | 6 (2.4) | 4 (2.6) | 2 (2.0) | >0.999† |
| Microbiology | n=252 | n=151 | n=101 | |
| No pathogens detected, no. (%) | 67 (26.6) | 47 (31.1) | 20 (19.8) | 0.046 |
| Gram-negative bacteria, no. (%) | 156 (61.9) | 88 (58.3) | 68 (67.3) | 0.147 |
| Gram-positive bacteria, no. (%) | 34 (13.5) | 22 (14.6) | 12 (11.9) | 0.540 |
| Fungi, no. (%) | 7 (2.8) | 4 (2.6) | 3 (3.0) | >0.999† |
| Viruses, no. (%) | 2 (0.8) | 0 (0.0) | 2 (2.0) | 0.160† |
| Other pathogens, no. (%) | 4 (1.6) | 3 (2.0) | 1 (1.0) | 0.651† |
See tables S1–S4 (online supplemental file 3) for additional information.
*Comparison between the patients who survived and died using χ2 test.
†Fisher’s exact test.
‡Mann–Whitney U test.
APACHE II, Acute Physiology and Chronic Health Evaluation II; FiO2, fraction of inspired oxygen; GCS, Glasgow Coma Scale; Hb, haemoglobin; HR, heart rate; ICU, intensive care unit; MBP, mean blood pressure; no, number; PaO2, partial pressure of oxygen in the arterial blood; PLT, platelet count; RR, respiratory rate; SOFA, Sequential Organ Failure Assessment; WBC, white blood cell.
Table 2.
Treatments and outcomes according to hospital survivability of patients with sepsis
| Variables | All cases | Survived | Died | P value* |
| Completion of the sepsis bundle of care | n=241 | n=146 | n=95 | |
| Completion of the sepsis bundle within 1 hour, no. (%) | 87 (36.1) | 53 (36.3) | 34 (35.8) | 0.936 |
| Completion of the initial administration of antibiotics within 1 hour, no. (%) | 173 (71.8) | 109 (74.7) | 64 (63.4) | 0.219 |
| Completion of the sepsis bundle within 3 hours, no. (%) | 108 (44.8) | 66 (45.2) | 42 (44.2) | 0.879 |
| Completion of the initial administration of antibiotics within 3 hours, no. (%) | 205 (85.1) | 131 (89.7) | 74 (77.9) | 0.012 |
| Life-sustaining treatments | n=251 | n=150 | n=101 | |
| Respiratory support, no. (%) | ||||
| Mechanical ventilation | 173 (68.9) | 82 (54.7) | 91 (90.1) | <0.001 |
| Non-invasive ventilation | 20 (8.0) | 13 (8.7) | 7 (6.9) | 0.618 |
| High-flow nasal oxygen | 38 (15.1) | 29 (19.3) | 9 (8.9) | 0.024 |
| Additional ICU support, no. (%) | ||||
| Vasopressors/inotropes | 163 (64.7) | 82 (54.3) | 81 (80.2) | <0.001 |
| Renal replacement therapy | 101 (40.2) | 43 (28.7) | 58 (57.4) | <0.001 |
| Red blood cell transfusion | 93 (37.1) | 48 (32.0) | 45 (44.6) | 0.043 |
| Platelet transfusion | 50 (19.9) | 20 (13.3) | 30 (29.7) | 0.001 |
| Fresh frozen plasma transfusion | 58 (23.1) | 28 (18.7) | 30 (29.7) | 0.042 |
| Surgical source control | 25 (10.0) | 19 (12.7) | 6 (5.9) | 0.081 |
| Non-surgical source control | 78 (31.1) | 54 (36.0) | 24 (23.8) | 0.040 |
| Outcomes | n=252 | n=151 | n=101 | |
| Patient status, no. (%) | <0.001† | |||
| Alive on current hospital discharge | 150 (59.5) | 150 (99.3) | 0 (0.0) | |
| Alive on discharge from current ICU stay, but died in current hospital stay | 17 (6.7) | 0 (0.0) | 17 (16.8) | |
| Alive on discharge from current ICU stay, but still in current hospital stay after 90 days | 1 (0.4) | 1 (0.7) | 0 (0.0) | |
| Still in current ICU stay after 90 days | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Died in current ICU stay | 84 (33.3) | 0 (0.0) | 84 (83.2) | |
| Length of stay, median days (IQR) | ||||
| Hospital | 16 (10–25) | 17 (11–24.25) | 13 (7–26) | 0.027‡ |
| ICU | 10 (6–18) | 6–1710.5 (6-17) | 10 (5–21) | 0.740‡ |
See tables S5–S7 (online supplemental file 3) for additional information.
*Comparison between the patients who survived and died using χ2 test.
†Fisher’s exact test.
‡Mann–Whitney U test.
ICU, intensive care unit; no, number.
bmjopen-2022-064870supp003.pdf (718.3KB, pdf)
Overall prognostic performance of the severity scoring systems
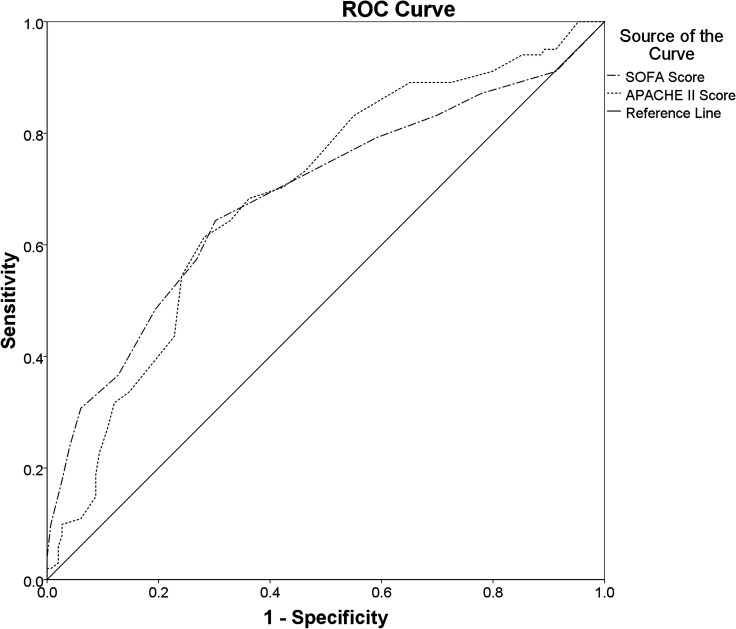
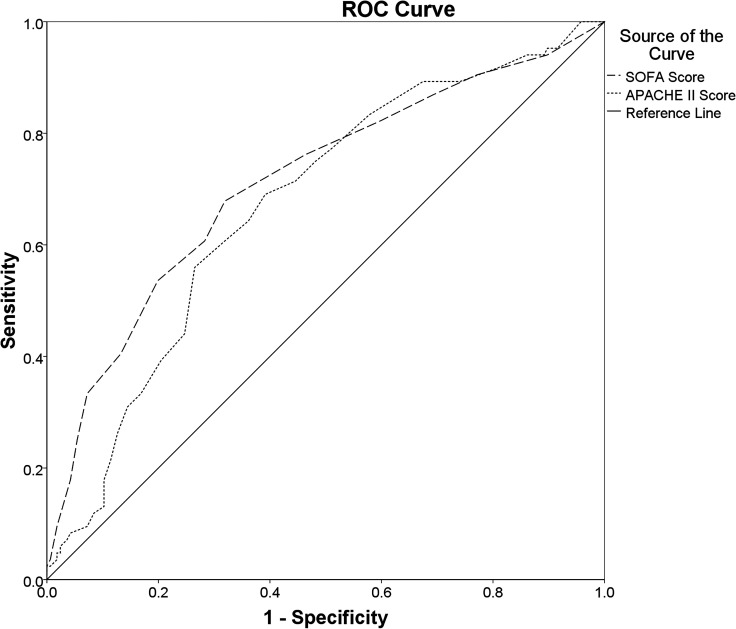
The SOFA Score (AUROC: 0.688 (95% CI 0.618 to 0.758); cut-off value≥7.5; sensitivity: 64.4%; specificity: 69.8%; PAUROC<0.001) and APACHE II Score (AUROC: 0.689 (95% CI 0.622 to 0.756); cut-off value≥20.5; sensitivity: 61.4%; specificity: 71.8%; PAUROC<0.001) both had a poor discriminatory ability for the hospital mortality (figure 2). The discriminatory ability for the ICU mortality of SOFA Score (AUROC: 0.713 (95% CI 0.643 to 0.783); cut-off value≥9.5; sensitivity: 53.6%; specificity: 80.1%; PAUROC<0.001), however, was fair and was better than that of the APACHE II Score (AUROC: 0.672 (95% CI 0.603 to 0.742); cut-off value≥18.5; sensitivity: 69.0%; specificity: 60.8%; PAUROC<0.001) (figure 3).
Figure 2.
Comparisons of the AUROCs: Comparing the overall diagnostic performance of the SOFA Score (AUROC: 0.688 (95% CI 0.618 to 0.758); cut-off value≥7.5; sensitivity: 64.4%; specificity: 69.8%; PAUROC<0.001) and the APACHE II Score (AUROC: 0.689 (95% CI 0.622 to 0.756); cut-off value≥20.5; sensitivity: 61.4%; specificity: 71.8%; PAUROC<0.001) for predicting hospital mortality in ICU patients with sepsis. APACHE II, Acute Physiology and Chronic Health Evaluation II Score; AUROC, areas under the ROC curve; ICU, intensive care unit; ROC, receiver operating characteristics; SOFA, Sequential Organ Failure Assessment.
Figure 3.
Comparisons of the AUROCs: Comparing the overall diagnostic performance of the SOFA Score (AUROC: 0.713 (95% CI 0.643 to 0.783); cut-off value≥9.5; sensitivity: 53.6%; specificity: 80.1%; PAUROC<0.001) and the APACHE II Score (AUROC: 0.672 (95% CI 0.603 to 0.742); cut-off value≥18.5; sensitivity: 69.0%; specificity: 60.8%; PAUROC<0.001) for predicting ICU mortality in ICU patients with sepsis. APACHE II, Acute Physiology and Chronic Health Evaluation II; AUROC, areas under the ROC curve; ICU, intensive care unit; ROC, receiver operating characteristics; SOFA, Sequential Organ Failure Assessment.
Risk factors for mortality
In the multivariable analysis, a SOFA Score of 8 and above (AOR: 2.717; 95% CI 1.371 to 5.382) and an APACHE II Score of 21 and above (AOR: 2.668; 95% CI 1.338 to 5.321) were independently associated with an increased risk of hospital mortality (table 3). Additionally, a SOFA Score of 10 and above (AOR: 2.801; 95% CI 1.332 to 5.891) was independently associated with an increased risk of ICU mortality, in contrast to an APACHE II Score of 19 and above, for which this independent association was not observed (table 4). Other factors were significantly or independently associated with the risk of hospital and ICU mortalities, as shown in tables 3 and 4, and tables S15–S18) (online supplemental file 3).
Table 3.
Factors relating to hospital mortality in patients with sepsis
| Factors | Univariable logistic regression analyses* | Multivariable logistic regression analyses† | ||||||
| OR | 95% CI for OR | P value | AOR | 95% CI for AOR | P value | |||
| Lower | Upper | Lower | Upper | |||||
| Hospital and ICU characteristics | ||||||||
| University affiliation | 2.520 | 1.495 | 4.248 | 0.001 | NA | NA | NA | NA |
| Training programme in ICU | 0.445 | 0.237 | 0.833 | 0.011 | 0.392 | 0.162 | 0.949 | 0.038 |
| Documented comorbidities | ||||||||
| Cardiovascular disease | 1.551 | 0.903 | 2.664 | 0.112 | 2.181 | 1.019 | 4.664 | 0.044 |
| Chronic neurological disease | 0.378 | 0.165 | 0.867 | 0.022 | 0.179 | 0.058 | 0.546 | 0.003 |
| Severity of illness scores | ||||||||
| SOFA Score≥8 | 4.173 | 2.440 | 7.137 | <0.001 | 2.717 | 1.371 | 5.382 | 0.004 |
| APACHE II Score≥21 | 4.126 | 2.414 | 7.051 | <0.001 | 2.668 | 1.338 | 5.321 | 0.005 |
| Site of infection | ||||||||
| Urinary tract | 0.300 | 0.126 | 0.714 | 0.006 | 0.312 | 0.105 | 0.932 | 0.037 |
| Abdominal | 1.256 | 0.701 | 2.249 | 0.444 | NA | NA | NA | NA |
| Skin or cutaneous sites | 2.774 | 1.053 | 7.309 | 0.039 | NA | NA | NA | NA |
| Microbiology | ||||||||
| No pathogens detected | 0.546 | 0.300 | 0.994 | 0.048 | NA | NA | NA | NA |
| Gram-negative bacteria | 1.475 | 0.871 | 2.498 | 0.148 | NA | NA | NA | NA |
| Completion of sepsis bundle elements | ||||||||
| Completion of the sepsis bundle within 1 hour | 0.978 | 0.571 | 1.675 | 0.936 | NA | NA | NA | NA |
| Completion of the administration of antibiotics within 1 hour | 0.701 | 0.397 | 1.237 | 0.220 | NA | NA | NA | NA |
| Completion of the sepsis bundle within 3 hours | 0.961 | 0.571 | 1.615 | 0.879 | NA | NA | NA | NA |
| Completion of the administration of antibiotics within 3 hours | 0.403 | 0.196 | 0.830 | 0.014 | 0.381 | 0.151 | 0.965 | 0.042 |
| Life-sustaining treatments during ICU stay | ||||||||
| Respiratory support | ||||||||
| Mechanical ventilation | 7.546 | 3.645 | 15.625 | <0.001 | 4.391 | 1.912 | 10.085 | <0.001 |
| High-flow nasal oxygen | 0.408 | 0.184 | 0.904 | 0.027 | NA | NA | NA | NA |
| Additional ICU support | ||||||||
| Vasopressors/inotropes | 3.408 | 1.899 | 6.116 | <0.001 | NA | NA | NA | NA |
| Renal replacement therapy | 3.356 | 1.976 | 5.702 | <0.001 | NA | NA | NA | NA |
| Red blood cell transfusion | 1.708 | 1.014 | 2.876 | 0.044 | NA | NA | NA | NA |
| Platelet transfusion | 2.746 | 1.455 | 5.185 | 0.002 | NA | NA | NA | NA |
| Fresh frozen plasma transfusion | 1.841 | 1.018 | 3.329 | 0.043 | NA | NA | NA | NA |
| Surgical source control | 0.435 | 0.168 | 1.132 | 0.088 | NA | NA | NA | NA |
| Non-surgical source control | 0.554 | 0.314 | 0.977 | 0.041 | NA | NA | NA | NA |
| Constant | 0.230 | 0.007 | ||||||
See tables S15 and S16 (online supplemental file 3) for additional information.
*Each variable of hospital and ICU characteristics, baseline characteristics, clinical and laboratory characteristics, and treatments was analysed in the univariable logistic regression model and was considered in the multivariable logistic regression model if the value of p was<0.05 in univariable logistic regression analysis between survival and death in the hospital, as well as clinically crucial factors.
†All selected variables were included in the multivariable logistic regression model with the stepwise backward elimination method. Variables, then, were deleted stepwise from the full model until all remaining variables were independently associated with death in the hospital.
AOR, adjusted OR; APACHE II, Acute Physiology and Chronic Health Evaluation II; ICU, intensive care unit; NA, not available; SOFA, Sequential Organ Failure Assessment.
Table 4.
Factors relating to intensive care unit mortality in patients with sepsis
| Factors | Univariable logistic regression analyses* | Multivariable logistic regression analyses† | ||||||
| OR | 95% CI for OR | P value | AOR | 95% CI for AOR | P value | |||
| Lower | Upper | Lower | Upper | |||||
| Hospital and ICU characteristics | ||||||||
| University affiliation | 2.260 | 1.322 | 3.862 | 0.003 | 2.562 | 1.164 | 5.639 | 0.019 |
| Intensivist to patient ratio | ||||||||
| 1 intensivist : 5 or fewer patients | Reference | 0.082 | NA | NA | ||||
| 1 intensivist : 6 to 8 patients | 0.553 | 0.298 | 1.025 | 0.060 | NA | NA | NA | NA |
| 1 intensivist : 12 or more patients | 1.750 | 0.540 | 5.668 | 0.351 | NA | NA | NA | NA |
| Training programme in ICU | 0.458 | 0.243 | 0.861 | 0.015 | 0.267 | 0.100 | 0.713 | 0.008 |
| Documented comorbidities | ||||||||
| Cardiovascular disease | 1.506 | 0.863 | 2.627 | 0.150 | 2.047 | 0.954 | 4.391 | 0.066 |
| Chronic neurological disease | 0.526 | 0.229 | 1.212 | 0.131 | 4.630 | 1.130 | 18.970 | 0.033 |
| Solid malignant tumours | 2.077 | 0.649 | 6.648 | 0.218 | NA | NA | NA | NA |
| Severity of illness scores | ||||||||
| SOFA Score≥10 | 4.650 | 2.620 | 8.254 | <0.001 | 2.801 | 1.332 | 5.891 | 0.007 |
| APACHE II Score≥19 | 3.535 | 1.025 | 6.171 | <0.001 | NA | NA | NA | NA |
| Site of infection | ||||||||
| Urinary tract | 0.340 | 0.136 | 0.851 | 0.021 | 0.276 | 0.087 | 0.878 | 0.029 |
| Abdominal | 1.416 | 0.779 | 2.575 | 0.254 | NA | NA | NA | NA |
| Skin or cutaneous sites | 2.387 | 0.931 | 6.123 | 0.070 | 3.074 | 0.982 | 9.629 | 0.054 |
| Microbiology | ||||||||
| No pathogens detected | 0.599 | 0.320 | 1.121 | 0.109 | NA | NA | NA | NA |
| Gram-negative bacteria | 1.258 | 0.729 | 2.171 | 0.409 | NA | NA | NA | NA |
| Completion of sepsis bundle elements | ||||||||
| Completion of the sepsis bundle within 1 hour | 0.931 | 0.532 | 1.630 | 0.802 | NA | NA | NA | NA |
| Completion of the administration of antibiotics within 1 hour | 0.671 | 0.374 | 1.202 | 0.180 | NA | NA | NA | NA |
| Completion of the sepsis bundle within 3 hours | 0.938 | 0.546 | 1.609 | 0.815 | NA | NA | NA | NA |
| Completion of the administration of antibiotics within 3 hours | 0.434 | 0.211 | 0.889 | 0.023 | 0.344 | 0.122 | 0.970 | 0.044 |
| Life-sustaining treatments during ICU stay | ||||||||
| Respiratory support | ||||||||
| Mechanical ventilation | 6.856 | 3.109 | 15.116 | <0.001 | 3.086 | 1.180 | 8.072 | 0.022 |
| High-flow nasal oxygen | 0.257 | 0.096 | 0.685 | 0.007 | NA | NA | NA | NA |
| Additional ICU support | ||||||||
| Vasopressors/inotropes | 2.956 | 1.600 | 5.460 | 0.001 | NA | NA | NA | NA |
| Renal replacement therapy | 4.239 | 2.432 | 7.388 | <0.001 | 3.433 | 1.669 | 7.058 | 0.001 |
| Red blood cell transfusion | 1.682 | 0.983 | 2.879 | 0.058 | NA | NA | NA | NA |
| Platelet transfusion | 2.966 | 1.571 | 5.597 | 0.001 | NA | NA | NA | NA |
| Fresh frozen plasma transfusion | 1.891 | 1.036 | 3.453 | 0.038 | NA | NA | NA | NA |
| Surgical source control | 0.599 | 0.230 | 1.562 | 0.295 | NA | NA | NA | NA |
| Non-surgical source control | 0.535 | 0.293 | 0.977 | 0.042 | 0.385 | 0.175 | 0.842 | 0.017 |
| Constant | 0.182 | 0.004 | ||||||
See tables S17 and S18 (online supplemental file 3) for additional information.
*Each variable of hospital and ICU characteristics, baseline characteristics, clinical and laboratory characteristics, and treatments was analysed in the univariable logistic regression model and was considered in the multivariable logistic regression model if the value of p was <0.05 in univariable logistic regression analysis between survival and death in the ICU, as well as clinically crucial factors.
†All selected variables were included in the multivariable logistic regression model with the stepwise backward elimination method. Variables, then, were deleted stepwise from the full model until all remaining variables were independently associated with death in the ICU.
AOR, adjusted OR; APACHE II, Acute Physiology and Chronic Health Evaluation II; ICU, intensive care unit; NA, not available; SOFA, Sequential Organ Failure Assessment.
Discussion
Of 252 patients with sepsis included in our analysis, two-fifths (40.1%) died in the hospital, and about a third (33.3%) died during the ICU stay (figure 1 and table 2). The SOFA and APACHE II Scores had a poor discriminatory ability for predicting hospital mortality (figure 2). However, the overall performance of the SOFA Score for predicting ICU mortality was fair and was better than that of the APACHE II Score (figure 3). A SOFA Score of 8 and above and an APACHE II Score of 21 and above were independently associated with an increased risk of hospital mortality (table 3). Additionally, a SOFA score of 10 and above was an independent predictor of ICU mortality, in contrast to an APACHE II score of 19 and above, for which this role did not appear (table 4).
In our study, the hospital mortality rate was lower than that of the MOSAICS I Study (44.5%; 572/1285),40 as well as the rates previously reported from LMICs in South-East Asia, including Indonesia (68.3%; 41/60),41 Thailand (42%; 263/627)42 and Vietnam (61.0%; 75/123).39 These findings may be because the diagnosis and treatment of sepsis have significantly changed over the previous 10 years to increase patient survival in sepsis and septic shock.1 8 13 36 38 43 44 However, our study showed rates for ICU and hospital mortality that were higher than rates reported in the international Extended Study on Prevalence of Infection in Intensive Care III (EPIC III) (28% (99/352) and 31.1% (110/352) in LMICs, 26.4% (821/3114) and 32.7% (1019/3114) in upper-middle-income countries (UMICs), and 21.3% (950/4470) and 28.5% (1275/4470) in HICs).45 These variations might be because EPIC III included ICU-acquired infection rather than only sepsis.45 Despite the distinct inclusion criteria, our median SOFA Score at the time of ICU admission was comparable to that of EPIC III (7 points (IQR: 4–11) in LMICs/UMICs/HICs).45 However, patients in our study received invasive organ support treatments (ie, MV and RRT) during ICU stays more frequently than those in EPIC III (54.4% (4377/8045) and 15.7% (1253/8045)).45 Previous studies showed that MV was a crucial predictor of mortality at any point throughout the ICU stay.4 35 Additionally, the utilisation of RRT at any time during the ICU stay was also associated with a higher fatality rate.4 35 46–48 Furthermore, Acinetobacter baumannii (17.9%, 45/252; table S4, online supplemental file 3), one of the most harmful pathogens, was more frequently isolated from patients in the present study than in those from the HIC cohort (4.4%; 137/3113) of the EPIC III Study.45 The previous studies showed that A. baumannii infection was often due to a lack of strict infection control bundles49 and associated with an increased risk of death.50 51 The fact that our proportions for ICU and hospital mortality were higher than those reported in EPIC III suggested that patients, pathogens and clinical capacity to manage sepsis vary significantly between regions, particularly between HIC and LMIC settings.
In this study, we found a poor ability of both SOFA and APACHE II Scores to predict hospital mortality (figure 2). However, with the SOFA Score, the discrimination for predicting ICU mortality was fair, and it was better than those of the APACHE II Score (figure 3). The APACHE scoring system is among the most widely used, of which there are four versions (APACHE I through IV Scores). Although APACHE IV Score is the most up-to-date version, some centres still use older versions including APACHE II Score. In the present study, despite having a poor discriminatory ability for predicting hospital and ICU mortalities, an APACHE II Score of 21 and above was independently associated with an increased risk of deaths in hospitals (table 3). However, in contrast to a SOFA Score of 10 and above, an APACHE II Score of 19 and above was not an independent predictor of ICU mortality (table 4). Previous studies revealed that the APACHE II Score had a good prognostic value in acutely ill or surgical patients10 11 but did not differentiate between sterile and infected necrotising pancreatitis and had a poor predictive value for the severity of acute pancreatitis at 24 hours.12
In contrast, the SOFA Score was proposed for patients with a suspected infection that an increase of 2 points or more could serve as clinical criteria for sepsis.1 In ICU patients with suspected infection, discrimination of the SOFA Score was fair for predicting hospital mortality, with an AUROC value of 0.74 (95% CI, 0.73 to 0.76; PAUROC<0.001), reported in the previously published studies.1 17 However, our study showed that the discriminatory ability of the SOFA Score was poor for predicting hospital mortality (figure 2). This difference might be due to our SOFA Score only calculated on ICU admission, in contrast to the SOFA Score in the previously published study that was calculated for the time window from 48 hours before to 24 hours after the onset of an infection, as well as on each calendar day.17 This difference also might be because the burden and causes of sepsis and its management differ considerably between HIC and LMIC settings,7 35 37 which might make the accuracy of critical illness severity scoring systems vary widely in the different countries, particularly between HICs and LMICs. However, our study revealed that the SOFA Score had a fair discriminatory ability for predicting ICU mortality (figure 3). Moreover, a SOFA Score of 8 and above and a score of 10 and above were independently associated with an increased risk of deaths in hospitals and ICUs, respectively (tables 3 and 4). Overall, this study shows that both SOFA and APACHE II Scores were worthwhile in predicting hospital and ICU mortalities in ICU patients with sepsis. However, because of having better discrimination for predicting ICU mortality, the SOFA Score was preferable to the APACHE II Score in predicting mortality.
The present study’s data from many centres, which contained few missing data points, was a benefit (tables S19, online supplemental file 3). The following are some drawbacks of the current study, though: first, since there isn't a national registry of ICUs to enable systematic recruitment of units, we used the snowball method to find suitable units, which may have resulted in the selection of centres with a greater interest in managing sepsis; as a result, our data are subject to selection bias and might not accurately reflect intensive care in all of Vietnam; second, we did not create a protocol for microbiological investigations due to the study’s real-world aspect. The data on point-of-care tests (such as lactate clearance) and life-sustaining therapies (such as fluid balance, steroid administration, and modalities of RRT and MV) were also missing since we primarily evaluated resources used in ICUs. Additionally, we decided not to gather information on antibiotic resistance and appropriateness to increase the practicality of performing the study in busy ICUs; third, the mixed-effects logistic regression model could not be used to predict the discrete outcome variables measured at two different times, that is, inside and outside the ICU settings, due to our independent variables (eg, SOFA Score that was greater than or equal to the cut-off value), which might be associated with the primary outcome only measured on ICU admission; finally, even though the sample size was sufficient, the CI was a little bit broad (6.03%), which may have an impact on the sample’s normal distribution. Therefore, more studies with bigger sample sizes may be required to strengthen the findings.
Conclusions
Our cohort was a selected population of patients with sepsis admitted to the ICUs in Vietnam with a high mortality rate. The SOFA and APACHE II Scores were worthwhile in predicting mortality among ICU patients with sepsis. However, due to better discrimination for predicting ICU mortality, the SOFA Score was preferable to the APACHE II Score in predicting mortality.
Supplementary Material
Acknowledgments
The authors thank all ED and ICU staff of participating hospitals for their support with this study. The authors also thank the staff of the Faculty of Public Health at Thai Binh University of Medicine and Pharmacy for their support and statistical advice. The authors also thank Miss Phuong Thi Tran from the Center for Emergency Medicine of Bach Mai Hospital, Hanoi, Vietnam, and Miss Mai Phuong Nguyen from St Paul American School, Hanoi, Vietnam, for their support with this study.
Footnotes
Twitter: @drchinh
MHN and DTP contributed equally.
Contributors: SND contributed to the conception, design of the work, acquisition, and interpretation of data for the work, and revised the draft critically for important intellectual content; CQL contributed to the conception, design of the work, acquisition, analysis, and interpretation of data for the work, and wrote the first draft of the work; CXD and TAN contributed to the design of the work, acquisition, interpretation of data for the work, and revised the draft critically for important intellectual content; DTP and MHN contributed to the design of the work, analysis, and interpretation of data for the work; NTN, DQH, QTAH, CVB, TDV, HNB, HTN, HBH, TTPL, LTBN, PTD, TDN, VHL and GTTP contributed to the acquisition and interpretation of data for the work; GTHB, TVB, TTNP, CVN and ADN contributed to the interpretation of data for the work; JP and AL contributed to the design of the work, interpretation of data for the work, and revised the draft critically for important intellectual content. All authors reviewed and edited the work and approved its final version. CQL is responsible for the overall content as the guarantor.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants. The Scientific and Ethics Committees of Bach Mai Hospital approved this study (approval number: 2919/QĐ–BM; project code: BM-2017-883-89). The authors also obtained permission from the heads of institutions and departments of all participating hospitals and their respective institutional review boards wherever available. The study was conducted according to the principles of the Declaration of Helsinki. In this non-intervention study, all collected information has received verbal informed consent from patients or, when unavailable, from family members at the ICUs, and witnessed by the on-duty medical staff. Written informed consent, however, was waived by the Bach Mai Hospital Scientific and Ethics Committees since it was not feasible to undergo such a methodical process of collection when the subject was comprised of an urgent situation in which a patient or a family member’s condition was severe or life-threatening. Public notification of the study was made by public posting. All data analyses were based upon data sets kept in password-protected systems, and all final presented data have been made anonymous. Participants gave informed consent to participate in the study before taking part.
References
- 1. Singer M, Deutschman CS, Seymour CW, et al. The third International consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016;315:801–10. 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rudd KE, Johnson SC, Agesa KM, et al. Global, regional, and national sepsis incidence and mortality, 1990-2017: analysis for the global burden of disease study. Lancet 2020;395:200–11.:S0140-6736(19)32989-7. 10.1016/S0140-6736(19)32989-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Liu V, Escobar GJ, Greene JD, et al. Hospital deaths in patients with sepsis from 2 independent cohorts. JAMA 2014;312:90–2. 10.1001/jama.2014.5804 [DOI] [PubMed] [Google Scholar]
- 4. Sakr Y, Jaschinski U, Wittebole X, et al. Sepsis in intensive care unit patients: worldwide data from the intensive care over nations audit. Open Forum Infect Dis 2018;5:ofy313. 10.1093/ofid/ofy313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Torio CM, Moore BJ. National inpatient hospital costs: the most expensive conditions by payer, 2013: statistical brief #204. In: Knutson D, ed. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD): Agency for Healthcare Research and Quality (US), 2006. [PubMed] [Google Scholar]
- 6. Bauer M, Gerlach H, Vogelmann T, et al. Mortality in sepsis and septic shock in Europe, North America and Australia between 2009 and 2019- results from a systematic review and meta-analysis. Crit Care 2020;24:239. 10.1186/s13054-020-02950-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Schultz MJ, Dunser MW, Dondorp AM, et al. Current challenges in the management of sepsis in ICUs in resource-poor settings and suggestions for the future. Intensive Care Med 2017;43:612–24. 10.1007/s00134-017-4750-z [DOI] [PubMed] [Google Scholar]
- 8. Levy MM, Fink MP, Marshall JC, et al. 2001 sccm/esicm/accp/ats/sis international sepsis definitions conference. Intensive Care Med 2003;29:530–8. 10.1007/s00134-003-1662-x [DOI] [PubMed] [Google Scholar]
- 9. Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest 1992;101:1644–55. 10.1378/chest.101.6.1644 [DOI] [PubMed] [Google Scholar]
- 10. Knaus WA, Draper EA, Wagner DP, et al. Apache II: a severity of disease classification system. Crit Care Med 1985;13:818–29. 10.1097/00003246-198510000-00009 [DOI] [PubMed] [Google Scholar]
- 11. Capuzzo M, Valpondi V, Sgarbi A, et al. Validation of severity scoring systems saps II and APACHE II in a single-center population. Intensive Care Med 2000;26:1779–85. 10.1007/s001340000715 [DOI] [PubMed] [Google Scholar]
- 12. Banks PA, Freeman ML, Practice Parameters Committee of the American College of Gastroenterology . Practice guidelines in acute pancreatitis. Am J Gastroenterol 2006;101:2379–400. 10.1111/j.1572-0241.2006.00856.x [DOI] [PubMed] [Google Scholar]
- 13. Evans L, Rhodes A, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med 2021;47:1181–247. 10.1007/s00134-021-06506-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vincent J-L, Moreno R, Takala J, et al. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. Intensive Care Med 1996;22:707–10. 10.1007/BF01709751 [DOI] [PubMed] [Google Scholar]
- 15. Vincent J-L, de Mendonca A, Cantraine F, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units. Critical Care Medicine 1998;26:1793–800. 10.1097/00003246-199811000-00016 [DOI] [PubMed] [Google Scholar]
- 16. Ferreira FL, Bota DP, Bross A, et al. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA 2001;286:1754–8. 10.1001/jama.286.14.1754 [DOI] [PubMed] [Google Scholar]
- 17. Seymour CW, Liu VX, Iwashyna TJ, et al. Assessment of clinical criteria for sepsis: for the third International consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016;315:762–74. 10.1001/jama.2016.0288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shankar-Hari M, Phillips GS, Levy ML, et al. Developing a new definition and assessing new clinical criteria for septic shock: for the third International consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016;315:775–87. 10.1001/jama.2016.0289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. World . World development indicators. Washington, D.C., United States: The World Bank Group, 2019. Available: https://databank.worldbank.org/data/download/POP.pdf [Google Scholar]
- 20. WHO . Viet nam SARS-free. 2003. in: weekly epidemiological record [internet]. 2003: 145–6. Available: https://www.who.int/wer/2003/en/wer7818.pdf
- 21. South East Asia Infectious Disease Clinical Research Network . Effect of double dose oseltamivir on clinical and virological outcomes in children and adults admitted to hospital with severe influenza: double blind randomised controlled trial. BMJ 2013;346(may30 2):f3039. 10.1136/bmj.f3039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. World Health Organization . Cumulative number of confirmed human cases for avian influenza A(H5N1) reported to WHO, 2003-2021, 15 april 2021. Geneva, Switzerland: The World Health Organization; 2021. Available: www.who.int/publications/m/item/cumulative-number-of-confirmed-human-cases-for-avian-influenza-a(h5n1)-reported-to-who-2003-2021-15-april-2021 [Google Scholar]
- 23. World Health Organization . COVID-19 situation reports in viet nam geneva. Switzerland: The World Health Organization; 2021. Available: www.who.int/vietnam/emergencies/coronavirus-disease-(covid-19)-in-viet-nam/covid-19-situation-reports-in-viet-nam/ [Google Scholar]
- 24. Do TV, Manabe T, Vu GV, et al. Clinical characteristics and mortality risk among critically ill patients with COVID-19 owing to the B.1.617.2 (delta) variant in Vietnam: a retrospective observational study. PLoS One 2023;18:e0279713. 10.1371/journal.pone.0279713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Anders KL, Nguyet NM, Chau NVV, et al. Epidemiological factors associated with dengue shock syndrome and mortality in hospitalized dengue patients in Ho Chi Minh City, Vietnam. Am J Trop Med Hyg 2011;84:127–34. 10.4269/ajtmh.2011.10-0476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mai NTH, Hoa NT, Nga TVT, et al. Streptococcus suis meningitis in adults in Vietnam. Clin Infect Dis 2008;46:659–67. 10.1086/527385 [DOI] [PubMed] [Google Scholar]
- 27. Maude RJ, Ngo TD, Tran DT, et al. Risk factors for malaria in high incidence areas of Viet Nam: a case-control study. Malar J 2021;20:373. 10.1186/s12936-021-03908-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nguyen KV, Thi Do NT, Chandna A, et al. Antibiotic use and resistance in emerging economies: a situation analysis for Viet Nam. BMC Public Health 2013;13:1158. 10.1186/1471-2458-13-1158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Phu VD, Wertheim HFL, Larsson M, et al. Burden of hospital acquired infections and antimicrobial use in Vietnamese adult intensive care units. PLoS One 2016;11:e0147544. 10.1371/journal.pone.0147544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. The World . The world bank in vietnam. Washington: The World Bank; 2020. Available: www.worldbank.org/en/country/vietnam/overview [Google Scholar]
- 31. Dat VQ, Long NT, Giang KB, et al. Healthcare infrastructure capacity to respond to severe acute respiratory infection (SARI) and sepsis in Vietnam: a low-middle income country. J Crit Care 2017;42:109–15.:S0883-9441(17)30405-7. 10.1016/j.jcrc.2017.07.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chinh LQ, Manabe T, Son DN, et al. Clinical epidemiology and mortality on patients with acute respiratory distress syndrome (ARDS) in Vietnam. PLoS One 2019;14:e0221114. 10.1371/journal.pone.0221114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Takashima K, Wada K, Tra TT, et al. A review of Vietnam’s healthcare reform through the direction of healthcare activities (Doha). Environ Health Prev Med 2017;22:74. 10.1186/s12199-017-0682-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Asian critical care clinical trials group. In: Management of Sepsis in Asia’s Intensive Care unitS II (MOSAICS II) Study Singapore: Society of Intensive Care Medicine (Singapore). 2019. Available: https://sicm.org.sg/article/YDNc8 [Google Scholar]
- 35. Do SN, Luong CQ, Pham DT, et al. Factors relating to mortality in septic patients in Vietnamese intensive care units from a subgroup analysis of mosaics II study. Sci Rep 2021;11:18924. 10.1038/s41598-021-98165-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li A, Ling L, Qin H, et al. Epidemiology, management, and outcomes of sepsis in ICUs among countries of differing national wealth across Asia. Am J Respir Crit Care Med 2022;206:1107–16. 10.1164/rccm.202112-2743OC [DOI] [PubMed] [Google Scholar]
- 37. Do SN, Luong CQ, Nguyen MH, et al. Predictive validity of the quick sequential organ failure assessment (qsofa) score for the mortality in patients with sepsis in Vietnamese intensive care units. PLoS One 2022;17:e0275739. 10.1371/journal.pone.0275739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Levy MM, Evans LE, Rhodes A. The surviving sepsis campaign bundle: 2018 update. Intensive Care Med 2018;44:925–8. 10.1007/s00134-018-5085-0 [DOI] [PubMed] [Google Scholar]
- 39. Thao PTN, Tra TT, Son NT, et al. Reduction in the IL-6 level at 24 H after admission to the intensive care unit is a survival predictor for Vietnamese patients with sepsis and septic shock: a prospective study. BMC Emerg Med 2018;18:39. 10.1186/s12873-018-0191-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Phua J, Koh Y, Du B, et al. Management of severe sepsis in patients admitted to Asian intensive care units: prospective cohort study. BMJ 2011;342:d3245. 10.1136/bmj.d3245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dewi RS, Radji M, Andalusia R. Evaluation of antibiotic use among sepsis patients in an intensive care unit: a cross-sectional study at a referral hospital in Indonesia. Sultan Qaboos Univ Med J 2018;18:e367–73. 10.18295/squmj.2018.18.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rudd KE, Hantrakun V, Somayaji R, et al. Early management of sepsis in medical patients in rural Thailand: a single-center prospective observational study. J Intensive Care 2019;7:55. 10.1186/s40560-019-0407-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rhodes A, Evans LE, Alhazzani W, et al. Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med 2017;45:486–552. 10.1097/CCM.0000000000002255 [DOI] [PubMed] [Google Scholar]
- 44. Phua J, Lim C-M, Faruq MO, et al. The story of critical care in Asia: a narrative review. J Intensive Care 2021;9:60. 10.1186/s40560-021-00574-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vincent J-L, Sakr Y, Singer M, et al. Prevalence and outcomes of infection among patients in intensive care units in 2017. JAMA 2020;323:1478–87. 10.1001/jama.2020.2717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sharma S, Kelly YP, Palevsky PM, et al. Intensity of renal replacement therapy and duration of mechanical ventilation: secondary analysis of the acute renal failure trial network study. Chest 2020;158:1473–81.:S0012-3692(20)31606-8. 10.1016/j.chest.2020.05.542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Elseviers MM, Lins RL, Van der Niepen P, et al. Renal replacement therapy is an independent risk factor for mortality in critically ill patients with acute kidney injury. Crit Care 2010;14:R221. 10.1186/cc9355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Barbar SD, Clere-Jehl R, Bourredjem A, et al. Timing of renal-replacement therapy in patients with acute kidney injury and sepsis. N Engl J Med 2018;379:1431–42. 10.1056/NEJMoa1803213 [DOI] [PubMed] [Google Scholar]
- 49. Aboshakwa AM, Lalla U, Irusen EM, et al. Acinetobacter baumannii infection in a medical intensive care unit: the impact of strict infection control. Afr J Thorac Crit Care Med 2019;25. 10.7196/AJTCCM.2019.v25i1.239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. John AO, Paul H, Vijayakumar S, et al. Mortality from Acinetobacter infections as compared to other infections among critically ill patients in South India: a prospective cohort study. Indian J Med Microbiol 2020;38:24–31. 10.4103/ijmm.IJMM_19_492 [DOI] [PubMed] [Google Scholar]
- 51. Shargian-Alon L, Gafter-Gvili A, Ben-Zvi H, et al. Risk factors for mortality due to Acinetobacter baumannii bacteremia in patients with hematological malignancies-a retrospective study. Leuk Lymphoma 2019;60:2787–92. 10.1080/10428194.2019.1599113 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-064870supp001.pdf (188.5KB, pdf)
bmjopen-2022-064870supp002.pdf (108.1KB, pdf)
bmjopen-2022-064870supp003.pdf (718.3KB, pdf)
Data Availability Statement
Data are available upon reasonable request. All data relevant to the study are included in the article or uploaded as supplementary information.