Abstract
Objective
To investigate the association between red cell distribution width (RDW) and the RDW to platelet count ratio (RPR) and cardiovascular diseases (CVDs) and to further investigate whether the association involves population differences and dose–response relationships.
Design
Cross-sectional population-based study.
Setting
The National Health and Nutrition Examination Survey (1999–2020).
Participants
A total of 48 283 participants aged 20 years or older (CVD, n=4593; non-CVD, n=43 690) were included in this study.
Primary and secondary outcome measures
The primary outcome was the presence of CVD, while the secondary outcome was the presence of specific CVDs. Multivariable logistic regression analysis was performed to determine the relationship between RDW or the RPR and CVD. Subgroup analyses were performed to test the interactions between demographics variables and their associations with disease prevalence.
Results
A logistic regression model was fully adjusted for potential confounders; the ORs with 95% CIs for CVD across the second to fourth quartiles were 1.03 (0.91 to 1.18), 1.19 (1.04 to 1.37) and 1.49 (1.29 to 1.72) for RDW (p for trend <0.0001) compared with the lowest quartile. The ORs with 95% CIs for CVD across the second to fourth quartiles were 1.04 (0.92 to 1.17), 1.22 (1.05 to 1.42) and 1.64 (1.43 to 1.87) for the RPR compared with the lowest quartile (p for trend <0.0001). The association of RDW with CVD prevalence was more pronounced in females and smokers (all p for interaction <0.05). The association of the RPR with CVD prevalence was more pronounced in the group younger than 60 years (p for interaction=0.022). The restricted cubic spline also suggested a linear association between RDW and CVD and a non-linear association between the RPR and CVD (p for non-linear <0.05).
Conclusion
There are statistical heterogeneities in the association between RWD, RPR distributions and the CVD prevalence, across sex, smoking status and age groups.
Keywords: adult cardiology, coronary heart disease, cardiology, heart failure
Strengths and limitations of this study.
The quality and scale of the National Health and Nutrition Examination Survey database and the rigour of its measures ensure the statistical power and reliability of our results.
The use of a validated survey instrument and standardised data collection methods allows for comparison with other studies.
Interaction tests in subgroup analysis verified the reliability of the results and identified potential interactions in different strata.
This study was limited by its cross-sectional design, and no causal relationships could be determined.
The use of a single database may introduce bias.
Introduction
Studies have shown that the global cardiovascular disease (CVD) prevalence increased from 271 million in 1990 to 523 million in 2019, with deaths increasing from 12.1 million to 18.6 million.1 Despite recent improvements in medications and devices, mortality and morbidity remain high in patients with CVDs. Several indicators and risk factors have been previously identified for traditional CVD assessment, but those that can be used routinely in the clinical setting are relatively limited.2 Many patients with CVD have no traditional risk factors3; however, these patients have the highest number of cardiovascular events.4 A large number of studies have reported the association of new risk factors with CVD.5 In recent years, some studies have shown a correlation between peripheral blood cells and CVD6; however, these studies are mostly limited to small cohorts and have some limitations.7
A complete blood count (CBC) is one of the most commonly used laboratory tests in clinical practice, and automated cell counters are routinely available in many clinical laboratories for determining red cell distribution width (RDW), platelet count and the RDW to platelet count ratio (RPR).8 Recent studies have shown that RDW is elevated to varying degrees in a variety of haematological diseases, acute inflammatory reactions, coronary heart disease (CHD) and congestive heart failure (CHF) and has a role in the diagnosis and prognosis of disease.9 Previous studies have shown the RPR to be useful in the prognosis of pancreatitis, neonatal sepsis,10 hepatitis, hepatic fibrosis11 and burn patients12 as a new predictor of acute myocardial infarction.13
However, previous studies of the association of the RDW and RPR with CVD have some limitations. First, previous studies have had small sample sizes and used different methods to adjust for potential confounders; therefore, the independence of association with identified CVD risk factors cannot be reliably inferred. Second, studies have used inconsistent disease definitions, preventing standardised analysis of CVD subtypes or direct comparisons of associations with the RDW and RPR in multiple conditions. Most importantly, few large cross-sectional studies have explored population differences, and no studies of dose–response relationships have been conducted to explore key inflection points for these metrics.14–16
To address the above limitations, this large cross-sectional study aimed to assess the relationship between RDW and the RPR and CVD.
Materials and methods
Study population
The National Health and Nutrition Examination Survey (NHANES) is an ongoing survey17 conducted by the National Center for Health Statistics of the Centers for Disease Control and Prevention (CDC) to measure the health and nutrition status of the civilian non-institutionalised US population. This survey combines detailed in-person interviews, physical examinations, computer-based questionnaires and laboratory tests to collect a large amount of quantitative and qualitative data.18 More detailed information about the NHANES, sample selection and data collection methodology can be found on the NHANES website. This study was performed according to the Strengthening the Reporting of Observational Studies in Epidemiology guidelines19 for reporting observational studies.
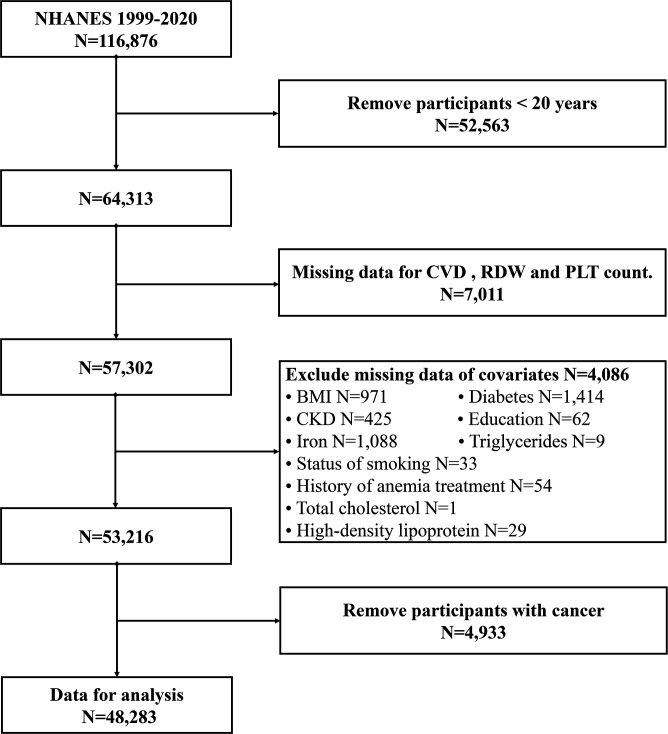
For this study, we used NHANES data collected from 1999 to 2020. The inclusion criterion for the study was subjects aged ≥20 years. Patients with missing data for CVD information, RDW, platelet count and covariates were excluded. In the 1999–2020 cycle of the NHANES, 116 876 participants completed the survey. In the current study, individuals aged <20 years were excluded (N=52 563). Participants without complete CVD, RDW and platelet count information (n=7011) were also excluded. In addition, participants with missing covariate data were excluded from the analysis (N=4086). Participants with cancer were also excluded (N=4933). Ultimately, 48 283 participants participated in our study, as shown in figure 1.
Figure 1.
Flow chart of eligible National Health and Nutrition Examination Survey (NHANES) participants included in this study. BMI, body mass index; CKD, chronic kidney disease; CVD, cardiovascular disease; PLT, platelet; RDW, red cell distribution width.
Assessment of RDW, the RPR and CVD
Blood specimens were collected at NHANES mobile examination centres (MECs). Detailed specimen collection and processing instructions are discussed in the NHANES Laboratory/Medical Technologists Procedures Manual. The RDW and platelet count were included in the CBC and processed via a Beckman Coulter MAXM Instrument, which derives CBC parameters based on the Beckman Coulter method of counting, sizing, automatic diluting and mixing for sample processing. A detailed description of the laboratory methods can be found on the NHANES website. The RPR was defined as the RDW to platelet ratio (ie, RPR=RDW/platelet count).
The primary outcome was the presence of CVD, while the secondary outcomes included CHF, CHD, angina, heart attack and stroke. In this study, we defined total CVD outcomes as any positive self-reported physician diagnosis of CHF, CHD, angina, heart attack or stroke. The medical conditions section contains self-reported data from personal interviews on a wide range of health issues, including CHF, CHD, angina, heart attack and stroke. The participants were asked ‘Has a doctor or other health professional ever told you that you have CHF/CHD/angina/heart attack/stroke?’. CVDs were considered to exist if any of the above questions were answered positively.
Covariates
We considered the following factors as potential covariates in the present study, which have been suggested to be associated with CVD, the RDW and the RPR according to previous studies.20 21
The demographic variables included age, sex, race and education. Education was stratified as <9th grade, 9th to 11th grade, high school graduate, some college or an AA degree, and college graduate or above, as recorded in the original survey. Lifestyle factors, such as smoking status, were obtained by self-reports. Individuals who reported smoking less than 100 cigarettes in their life were classified as never smokers, those who smoked more than 100 cigarettes in their life and had quit smoking were considered former smokers, and those who smoked more than 100 cigarettes in their life and smoked some days or every day were considered current smokers. Body mass index (BMI) was measured at an MEC using standard protocols.
Laboratory parameters included red haemoglobin levels, mean red blood cell volume, serum iron, total cholesterol (TC), high-density lipoprotein cholesterol (HDL) and triglyceride (TG) levels. Serum specimens were processed, stored and shipped to Cooperative Laboratory Services in Ottumwa, Iowa for analysis. Iron concentrations were measured using the DcX800 method. Serum lipid profiles were collected by using a haematology analyser; CBC parameters were measured as detailed in the previous paragraph.
Medical history included diabetes mellitus, hypertension, chronic kidney disease (CKD) and anaemia treatment: diabetes was defined as a diagnosis from a doctor or other health professional, an HbA1c level (%) >6.5, a random blood glucose level (mmol/L) ≥11.1 or the use of medication or insulin for diabetes. CKD was defined as an estimated glomerular filtration rate (eGFR) <60 mL/min/1.73 m2 or an albumin–creatinine ratio of 30 mg/g. eGFR was calculated according to the CKD Epidemiology Collaboration equation.22 Hypertension was defined as a diagnosis by a physician or other health professional and an average blood pressure ≥130/80 mm Hg or the use of medication for hypertension.23
Statistical analysis
Continuous variables are expressed as the weighted mean±SD and were compared using the t-test, whereas categorical variables are expressed as weighted percentages (95% CIs) and were compared using the Rao-Scott χ2 test. Multivariable logistic regression analysis was used to assess the association between RDW and the RPR and CVD and to determine ORs and 95% CIs while adjusting for confounding variables.
We used multivariate logistic generalised linear models to determine the relationship between RDW and the RPR and CVD. Model 1 was adjusted for sex, age and race. Model 2 was adjusted for model 1 plus education, smoking status, BMI, diabetes, hypertension, CKD and history of anaemia treatment. Model 3 was adjusted for model 2 plus haemoglobin, mean blood cell volume, iron, TG, HDL and TC. We tested the variance inflation factors of each covariate using the vif() function in R. The variance inflation factors of each variable were <5, which did not indicate the presence of multicollinearity in the covariates.
Subgroup analysis stratified by age, sex, smoking status, diabetes, CKD and hypertension was also conducted using stratified multivariate regression analysis, covariates that were not analysed in subgroups were included in the model as covariates in the logistic regression. Additionally, we tested the interaction with a likelihood ratio test, and the interaction test clarified the heterogeneity of correlations between subgroups. Moreover, we constructed restricted cubic spline (RCS) to analyse the non-linear relationship. According to Harrell’s suggestion,24 the curve works best when the number of nodes is four, and the key nodes were not known. We used an RCS with four knots at the 5th, 35th, 65th and 95th percentiles to model the non-linear relationship between the log2-transformed RDW and RPR with total and individual CVD.
Weights, created by the CDC, account for the complex survey design of NHANES (including oversampling), survey non-response and poststratification adjustment to match total population counts from the USA. According to the NHANES analysis guidelines, in our study, data from 1998 to 2020 were combined; for all analyses combining data from 1999 to 2000 with data from other survey periods, we constructed combined sample weights using 4-year weights from 1999 to 2002 and 2-year weights from each additional survey period.
All statistical analyses were performed using R’s statistical software25 (V.4.1.3) package (https://www.R-project.org, The R Foundation). All statistical tests were two-tailed, and a p value less than 0.05 was considered statistically significant.
Patient and public involvement
Patients or the public were not involved in the design, or conduct, or reporting, or dissemination plans of our research.
Results
Baseline characteristics of the sample population
The sample in the present study included 48 283 participants, representing 179 161 725 non-institutionalised adults (20 years of age and older) in the USA. As shown in table 1, the mean age of the surveyed population was 45.65±0.17 years. Of the participants, 66.48% (62.76 to 70.21) were non-Hispanic white and 50.47% (48.80 to 52.14) were women. The average RDW was 13.05±0.01 in the total population, and the average RPR was 0.05±0.001. The prevalence of CVD was 7.34% (6.88 to 7.80), while that of CHF, CHD, angina, heart attack and stroke was 1.84% (1.68 to 2.00), 2.97% (2.70 to 3.24), 2.06% (1.85 to 2.26), 2.86% (2.62 to 3.11) and 2.38% (2.19 to 2.57), respectively.
Table 1.
General characteristics of included participants (n=48 283) according to the presence or absence of CVD in the National Health and Nutrition Examination Survey 1999–2020
| Characters | Overall (n=48 283) | Non-CVD (n=43 690) | CVD (n=4593) | P value |
| Age, year | 45.65±0.17 | 44.28±0.16 | 62.94±0.29 | <0.0001 |
| Sex | <0.0001 | |||
| Female | 50.47 (48.80 to 52.14) | 50.92 (50.42 to 51.42) | 44.85 (42.76 to 46.94) | |
| Male | 49.53 (47.91 to 51.15) | 49.08 (48.58 to 49.58) | 55.15 (53.06 to 57.24) | |
| Education | <0.0001 | |||
| Less than 9th grade | 5.74 (5.34 to 6.14) | 5.40 (5.00 to 5.80) | 10.01 (8.85 to 11.17) | |
| 9th–11th grade | 10.92 (10.30 to 11.54) | 10.51 (9.94 to 11.08) | 16.09 (14.72 to 17.47) | |
| High school graduate | 24.40 (23.18 to 25.62) | 24.14 (23.34 to 24.95) | 27.66 (25.62 to 29.70) | |
| College degree | 30.99 (29.80 to 32.18) | 31.18 (30.42 to 31.93) | 28.65 (26.91 to 30.38) | |
| College or above | 27.95 (26.33 to 29.57) | 28.77 (27.41 to 30.13) | 17.59 (15.57 to 19.61) | |
| Race | <0.0001 | |||
| Mexican American | 8.78 (7.82 to 9.73) | 9.12 (8.06 to 10.19) | 4.42 (3.54 to 5.30) | |
| Non-Hispanic black | 11.15 (10.24 to 12.05) | 11.07 (10.03 to 12.11) | 12.15 (10.74 to 13.56) | |
| Non-Hispanic white | 66.48 (62.76 to 70.21) | 65.94 (63.98 to 67.89) | 73.35 (71.13 to 75.56) | |
| Other Hispanic | 6.17 (5.37 to 6.97) | 6.34 (5.50 to 7.18) | 4.03 (3.21 to 4.85) | |
| Other race | 7.42 (6.83 to 8.02) | 7.53 (6.90 to 8.17) | 6.05 (5.01 to 7.09) | |
| Smoking status | <0.0001 | |||
| Never | 54.87 (53.09 to 56.65) | 56.06 (55.15 to 56.98) | 39.78 (37.84 to 41.72) | |
| Former | 23.61 (22.51 to 24.71) | 22.49 (21.84 to 23.13) | 37.81 (35.94 to 39.68) | |
| Now | 21.52 (20.52 to 22.52) | 21.45 (20.71 to 22.19) | 22.41 (20.75 to 24.07) | |
| Treatment anaemia | 2.99 (2.77 to 3.21) | 2.68 (2.48 to 2.89) | 6.88 (5.93 to 7.83) | <0.0001 |
| Diabetes | 10.74 (10.27 to 11.21) | 9.07 (8.69 to 9.45) | 31.83 (30.27 to 33.40) | <0.0001 |
| Hypertension | 46.36 (44.58 to 48.13) | 43.64 (42.81 to 44.48) | 80.63 (78.96 to 82.30) | <0.0001 |
| CKD | 12.86 (12.29 to 13.43) | 10.96 (10.54 to 11.39) | 36.81 (35.10 to 38.52) | <0.0001 |
| BMI (kg/m2) | 28.85±0.07 | 28.71±0.07 | 30.59±0.14 | <0.0001 |
| Haemoglobin (g/L) | 14.35±0.02 | 14.37±0.02 | 14.11±0.04 | <0.0001 |
| MCV (fL) | 89.51±0.07 | 89.43±0.07 | 90.49±0.11 | <0.0001 |
| RDW (%) | 13.05±0.01 | 13.01±0.01 | 13.56±0.03 | <0.0001 |
| Platelet count (%) | 254.18±0.64 | 255.65±0.65 | 235.59±1.51 | <0.0001 |
| Iron (µg/dL) | 88.00±0.28 | 88.43±0.28 | 82.56±0.77 | <0.0001 |
| TG (mg/dL) | 148.40±1.02 | 146.81±1.06 | 168.58±2.47 | <0.0001 |
| HDL (mg/dL) | 52.96±0.16 | 53.20±0.16 | 49.84±0.41 | <0.0001 |
| TC (mg/dL) | 195.14±0.36 | 195.87±0.38 | 186.03±1.11 | <0.0001 |
| RPR | 0.05±0.001 | 0.05±0.001 | 0.06±0.001 | <0.0001 |
Values indicate the weighted mean±SD or weighted % (95% CI). The results are weighted based on the survey. P values are weighted.
BMI, body mass index; CKD, chronic kidney disease; CVD, cardiovascular disease; HDL, high-density lipoprotein; MCV, mean corpuscular volume; RDW, red cell volume distribution width; RPR, RDW-to-platelet ratio; TC, total cholesterol; TG, triglyceride.
Among participants with CVD, the mean age was 62.94±0.29 years, of which 55.15% (53.06 to 57.24) were men and 73.35% (71.13 to 75.56) were non-Hispanic white. The RDW and RPR were significantly different between the groups with and without CVD. Participants with CVD tended to be older, have lower education and BMI levels, and have higher rates of hypertension, diabetes, CKD and smoking. In addition, there were significant differences in sex, race and various laboratory indicators in participants with CVD compared with those without CVD (all p<0.0001).
Association between the RDW and RPR and total CVD
RDW levels were positively associated with CVD prevalence in multivariable logistic regression analyses. In the fully adjusted model, the ORs (95% CIs) for CVD prevalence across the RDW quartiles were 1.03 (0.91 to 1.18), 1.19 (1.04 to 1.37) and 1.49 (1.29 to 1.72) (p for trend <0.0001) compared with the first quartile (see table 2). This characteristic of increased ORs for CVD prevalence with increased RDW levels remained in the other two adjusted models (all p for trend <0.0001).
Table 2.
Adjusted ORs for associations between RDW, RPR and total cardiovascular disease
| Cases | N | Model 1 | Model 2 | Model 3 | |
| OR (95% CI) P value |
OR (95% CI) P value |
OR (95% CI) P value |
|||
| RDW (%) | |||||
| Q1 (9.700–12.400) | 678 | 12 761 | Ref. | Ref. | Ref. |
| Q2 (12.400–13.000) | 924 | 12 344 | 1.07 (0.95 to 1.21) | 1.03 (0.90 to 1.16) | 1.03 (0.91 to 1.18) |
| p=0.245 | p=0.690 | p=0.629 | |||
| Q3 (13.000–13.700) | 1181 | 11 414 | 1.33 (1.17 to 1.51) | 1.22 (1.06 to 1.40) | 1.19 (1.04 to 1.37) |
| p<0.0001 | p=0.005 | p=0.013 | |||
| Q4 (13.700–37.800) | 1810 | 11 764 | 1.97 (1.73 to 2.25) | 1.54 (1.35 to 1.76) | 1.49 (1.29 to 1.72) |
| p<0.0001 | p<0.0001 | p<0.001 | |||
| P for trend | <0.0001 | <0.0001 | <0.0001 | ||
| RPR | |||||
| Q1 (0.013–0.045) | 706 | 12 108 | Ref. | Ref. | Ref. |
| Q2 (0.045–0.053) | 909 | 12 681 | 1.01 (0.90 to 1.14) | 1.03 (0.92 to 1.17) | 1.04 (0.92 to 1.17) |
| p=0.887 | p=0.587 | p=0.572 | |||
| Q3 (0.053–0.064) | 1062 | 11 607 | 1.15 (1.00 to 1.33) | 1.25 (1.08 to 1.45) | 1.22 (1.05 to 1.42) |
| p=0.046 | p=0.004 | p=0.009 | |||
| Q4 (0.064–1.650) | 1916 | 11 887 | 1.70 (1.50 to 1.92) | 1.76 (1.55 to 2.00) | 1.64 (1.43 to 1.87) |
| p<0.0001 | p<0.0001 | p<0.0001 | |||
| P for trend | <0.0001 | <0.0001 | <0.0001 | ||
Model 1 adjusted for: sex, age and race. Model 2 was adjusted for model 1 plus education, smoking status, body mass index, diabetes, hypertension, chronic kidney disease and history of anaemia treatment. Model 3 was adjusted for model 2 plus haemoglobin, mean blood cell volume, iron, triglyceride, high-density lipoprotein and total cholesterol. The results are weighted based on the survey.
RDW, red cell distribution width; RPR, red cell distribution width–platelet ratio.
In all three logistic regression models (table 2), RPR levels were also positively associated with CVD prevalence (all p for trend <0.0001). Participants with RPR levels in higher quartiles had higher odds of CVD than those in the lowest reference quartile, with ORs (95% CI) of 1.04 (0.92 to 1.17), 1.22 (1.05 to 1.42) and 1.64 (1.43 to 1.87), respectively, for the fully adjusted model.
Association between RDW and individual CVDs
Our data show that high RDW levels are positively associated with high CVD prevalence (table 3). In fully adjusted logistic regression model 3, the third and fourth RDW quartiles were associated with higher CHF and heart attack prevalence compared with the lowest RDW quartile (p for trend <0.0001); the ORs (95% CIs) for CHF were 1.51 (1.11 to 2.04) and 2.82 (2.08 to 3.82) and ORs (95% CIs) for heart attack were 1.30 (1.04 to 1.63) and 1.58 (1.27 to 1.97), respectively. In the analysis of CHD and stroke, the highest RDW quartile was shown to be associated with higher disease prevalence (p for trend <0.05), with ORs (95% CI) of 1.49 (1.20 to 1.86) and 1.33 (1.06 to 1.68) for CHD and stroke, respectively. There was no correlation between RDW levels and angina (p for trend >0.05).
Table 3.
Adjusted ORs for associations between RDW, RPR and individual cardiovascular diseases
| N | Congestive heart failure | Coronary heart disease | Angina | Heart attack | Stroke | ||||||
| Case | OR (95% CI) | Case | OR (95% CI) | Case | OR (95% CI) | Case | OR (95% CI) | Case | OR (95% CI) | ||
| P value | P value | P value | P value | P value | |||||||
| RDW (%) | |||||||||||
| Q1 (9.700–12.400) | 12 761 | 125 | Ref. | 252 | Ref. | 207 | Ref. | 252 | Ref. | 220 | Ref. |
| Q2 (12.400–13.000) | 12 344 | 212 | 1.25 (0.92 to 1.69) | 348 | 1.04 (0.83 to 1.30) | 256 | 0.93 (0.73 to 1.18) | 372 | 1.17 (0.95 to 1.43) | 314 | 1.10 (0.89 to 1.36) |
| p=0.150 | p=0.732 | p=0.522 | p=0.133 | p=0.396 | |||||||
| Q3 (13.000–13.700) | 11 414 | 294 | 1.51 (1.11 to 2.04) | 450 | 1.18 (0.93 to 1.48) | 274 | 0.91 (0.70 to 1.18) | 472 | 1.30 (1.04 to 1.63) | 425 | 1.25 (1.00 to 1.56) |
| p=0.009 | p=0.165 | p=0.470 | p=0.023 | p=0.050 | |||||||
| Q4 (13.700–37.800) | 11 764 | 669 | 2.82 (2.08 to 3.82) | 650 | 1.49 (1.20 to 1.86) | 414 | 1.17 (0.91 to 1.52) | 700 | 1.58 (1.27 to 1.97) | 658 | 1.33 (1.06 to 1.68) |
| p<0.0001 | p<0.001 | p=0.214 | p<0.0001 | p=0.015 | |||||||
| P for trend | <0.0001 | <0.001 | 0.144 | <0.001 | 0.007 | ||||||
| RPR | |||||||||||
| Q1 (0.013–0.045) | 12 108 | 149 | Ref. | 194 | Ref. | 186 | Ref. | 236 | Ref. | 275 | Ref. |
| Q2 (0.045–0.053) | 12 681 | 241 | 1.49 (1.12 to 1.97) | 266 | 0.93 (0.72 to 1.21) | 241 | 0.97 (0.77 to 1.23) | 320 | 1.03 (0.80 to 1.33) | 349 | 1.12 (0.91 to 1.38) |
| p=0.006 | p=0.599 | p=0.811 | p=0.804 | p=0.265 | |||||||
| Q3 (0.053–0.064) | 11 607 | 286 | 1.70 (1.25 to 2.31) | 388 | 1.34 (1.06 to 1.70) | 261 | 1.08 (0.85 to 1.36) | 431 | 1.42 (1.13 to 1.77) | 364 | 1.13 (0.89 to 1.44) |
| p<0.001 | p=0.016 | p=0.522 | p=0.003 | p=0.305 | |||||||
| Q4 (0.064–1.650) | 11 887 | 624 | 2.25 (1.75 to 2.89) | 852 | 1.93 (1.54 to 2.44) | 463 | 1.38 (1.09 to 1.76) | 809 | 1.67 (1.35 to 2.08) | 629 | 1.37 (1.11 to 1.70) |
| p<0.0001 | p<0.0001 | p=0.009 | p<0.0001 | p=0.004 | |||||||
| P for trend | <0.0001 | <0.0001 | 0.002 | <0.001 | 0.004 | ||||||
Analyses were adjusted for: sex, age, race, education, smoking status, body mass index, diabetes, hypertension, chronic kidney disease, history of anaemia treatment, haemoglobin, mean blood cell volume, iron, triglyceride, high-density lipoprotein and total cholesterol. The results are weighted based on the survey.
RDW, red cell distribution width; RPR, red cell distribution width–platelet ratio.
Association between RPR and individual CVDs
Our data show similar trends in the relationship between RPR and individual CVD prevalence (table 3). In model 3, the odds of CHF were higher (p for trend <0.0001) for all participants in the second, third and fourth RPR quartiles, with ORs (95% CI) of 1.49 (1.12 to 1.97), 1.70 (1.25 to 2.31) and 2.25 (1.75 to 2.89), respectively. In the CHD and heart attack populations, participants in the third and fourth RPR quartiles had higher odds of having CHD or heart attack compared with the first RPR reference quartile (p for trend <0.0001), with ORs (95% CIs) of 1.34 (1.06 to 1.70) and 1.93 (1.54 to 2.44), respectively, for CHD. The ORs (95% CIs) for heart attack were 1.42 (1.13 to 1.77) and 1.67 (1.35 to 2.08), respectively. Analysis of the risk of angina and stroke prevalence showed that participants with RPR levels in the fourth quartile were more likely to have angina and stroke (p for trend <0.01), with ORs (95% CIs) of 1.38 (1.09 to 1.76) and 1.37 (1.11 to 1.70), respectively.
Dose–response relationship between the RDW and RPR and CVD
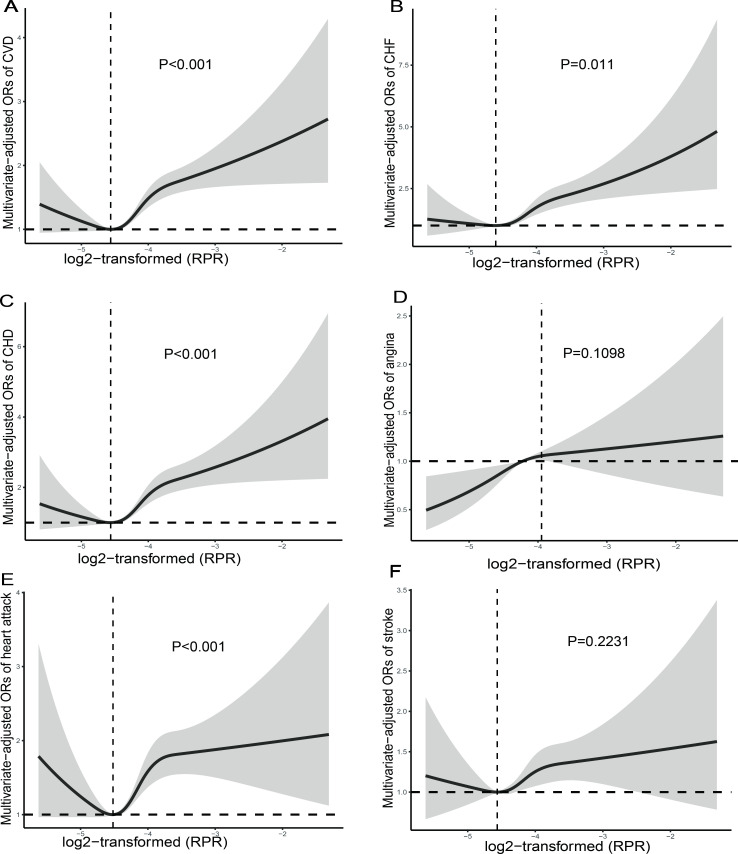
We used an RCS model with four knots (at the 5th, 35th, 65th and 95th percentiles) to simulate the relationship between the RDW and RPR and total CVD risk. RCS analysis of RDW using natural logarithm transformation suggested (figure 2) a linear relationship between the RDW and total CVD risk (p for non-linearity=0.1667) and a non-linear association between the RPR and total CVD risk (p for non-linearity <0.001), with an inflection point occurring at a log2-transformed RPR of −4.562 (figure 3).
Figure 2.
Restricted cubic spline plot of the association between RDW and CVD (A), CHF (B), CHD (C), angina (D), heart attack (E), stroke (F). RDW was log2-transformed. Adjustments were made according to gender, age, race, education, smoking status, body mass index, diabetes, hypertension, chronic kidney disease, history of anaemia treatment, haemoglobin, mean blood cell volume, iron, triglyceride, high-density lipoprotein and total cholesterol. The results are weighted based on the survey. P indicates the results of a test for non-linearity. CHD, coronary heart disease; CHF, congestive heart failure; CVD, cardiovascular disease; RDW, red cell distribution width.
Figure 3.
Restricted cubic spline plot of the association between RPR and CVD (A), CHF (B), CHD (C), angina (D), heart attack (E), stroke (F). RPR was log2-transformed. Adjustments were made according to gender, age, race, education, smoking status, body mass index, diabetes, hypertension, chronic kidney disease, history of anaemia treatment, haemoglobin, mean blood cell volume, iron, triglyceride, high-density lipoprotein and total cholesterol. P indicates the results of a test for non-linearity. CHD, coronary heart disease; CHF, congestive heart failure; CVD, cardiovascular disease; RPR, red cell distribution width-to-platelet ratio.
The RCS of log2-transformed RDW levels suggests a non-linear association between RDW levels and CHF and CHD (p for non-linearity <0.05). The log2-transformed RDW with CHD and angina shows a saturation effect, which occurs at the log2-transformed RDW=3.8354. The RCS plot showed a non-linear association between log2-transformed RPR levels and CHF, CHD and heart attack (p for non-linearity <0.05), and an inflection point occurring at a log2-transformed RPR of −3.9833.
Subgroup analyses
Considering the effect of different characteristics on CVD risk (table 4), we performed subgroup analysis to verify whether the effect of RDW and RPR levels on the risk of CVD was constant. Significant interaction was found between RDW and total CVD risk stratified by sex and smoking status (interaction p values of 0.026 and 0.023, respectively); a significant interaction in association between total CVD prevalence age, RPR quartile were found (interaction p=0.022). The interaction suggests that, unlike in males, in females, the prevalence of CVD increases when the RDW is classified in a quartile higher than the first quartile; similarly, the prevalence of CVD increases in the smoking population when the RDW is classified in a quartile higher than the second quartile compared with the non-smoking population. The OR between RPR upper quartiles and RPR first quartile are significantly different in two age groups. In the remaining subgroup analyses, the interaction effect was not significant (p for interaction >0.05), indicating consistent results across subcomponent layers and reliable findings.
Table 4.
Subgroup analysis of the association between RDW, RPR and prevalence of total CVD among US population in the National Health and Nutrition Examination Survey 1999–2020
| RDW | RPR | |||||||||||
| Q1 | Q2 | Q3 | Q4 | Pa | Pb | Q1 | Q2 | Q3 | Q4 | Pa | Pb | |
| Age | 0.347 | 0.022 | ||||||||||
| <60 years | Ref. | 1.12 (0.91 to 1.38) p=0.296 | 1.52 (1.23 to 1.88) p<0.001 | 1.84 (1.48 to 2.29) p<0.0001 | <0.0001 | Ref. | 1.32 (1.09 to 1.59) p=0.004 | 1.65 (1.33 to 2.05) p<0.0001 | 2.00 (1.65 to 2.43) p<0.0001 | <0.0001 | ||
| ≥60 years | Ref. | 1.08 (0.92 to 1.26) p=0.360 | 1.20 (1.01 to 1.42) p=0.040 | 1.66 (1.40 to 1.96) p<0.0001 | <0.0001 | Ref. | 0.91 (0.76 to 1.10) p=0.336 | 1.13 (0.93 to 1.37) p=0.210 | 1.74 (1.46 to 2.09) p<0.0001 | <0.0001 | ||
| Sex | 0.026 | 0.213 | ||||||||||
| Male | Ref. | 0.88 (0.74 to 1.04) p=0.143 | 1.04 (0.85 to 1.26) p=0.729 | 1.48 (1.19 to 1.85) p<0.001 | <0.0001 | Ref. | 1.07 (0.87 to 1.33) p=0.506 | 1.37 (1.09 to 1.73) p=0.007 | 1.68 (1.37 to 2.08) p<0.0001 | <0.0001 | ||
| Female | Ref. | 1.26 (1.03 to 1.54) p=0.026 | 1.42 (1.17 to 1.71) p<0.001 | 1.54 (1.27 to 1.88) p<0.0001 | <0.0001 | Ref. | 1.04 (0.87 to 1.24) p=0.661 | 1.12 (0.92 to 1.35) p=0.254 | 1.67 (1.39 to 1.99) p<0.0001 | <0.0001 | ||
| Smoking status | 0.023 | 0.292 | ||||||||||
| Never | Ref. | 0.90 (0.74 to 1.10) p=0.314 | 1.23 (0.99 to 1.52) p=0.056 | 1.37 (1.08 to 1.73) p=0.009 | 0.001 | Ref. | 0.91 (0.72 to 1.14) p=0.400 | 1.05 (0.85 to 1.30) p=0.634 | 1.59 (1.32 to 1.91) p<0.0001 | <0.0001 | ||
| Former | Ref. | 1.20 (0.93 to 1.54) p=0.161 | 1.03 (0.82 to 1.29) p=0.791 | 1.35 (1.02 to 1.79) p=0.035 | 0.063 | Ref. | 1.02 (0.80 to 1.29) p=0.874 | 1.25 (0.98 to 1.60) p=0.072 | 1.58 (1.26 to 1.97) p<0.0001 | <0.0001 | ||
| Now | Ref. | 1.06 (0.75 to 1.49) p=0.753 | 1.41 (1.01 to 1.95) p=0.041 | 2.01 (1.44 to 2.82) p<0.0001 | <0.0001 | Ref. | 1.28 (0.96 to 1.70) p=0.087 | 1.52 (1.12 to 2.06) p=0.008 | 1.77 (1.32 to 2.39) p<0.001 | <0.001 | ||
| DM | 0.414 | 0.839 | ||||||||||
| Yes | Ref. | 1.07 (0.78 to 1.45) p=0.687 | 1.33 (1.02 to 1.72) p=0.036 | 1.87 (1.43 to 2.45) p<0.0001 | <0.0001 | Ref. | 1.12 (0.82 to 1.52) p=0.474 | 1.38 (1.02 to 1.86) p=0.037 | 1.98 (1.48 to 2.65) p<0.0001 | <0.0001 | ||
| No | Ref. | 1.03 (0.89 to 1.19) p=0.708 | 1.16 (0.99 to 1.35) p=0.065 | 1.36 (1.15 to 1.62) p<0.001 | <0.001 | Ref. | 1.01 (0.87 to 1.16) p=0.928 | 1.17 (0.99 to 1.39) p=0.069 | 1.52 (1.32 to 1.75) p<0.0001 | <0.0001 | ||
| CKD | 0.187 | 0.078 | ||||||||||
| Yes | Ref. | 1.04 (0.84 to 1.29) p=0.709 | 1.04 (0.83 to 1.31) p=0.707 | 1.54 (1.25 to 1.89) p<0.0001 | <0.0001 | Ref. | 1.17 (0.94 to 1.46) p=0.162 | 1.16 (0.93 to 1.46) p=0.189 | 1.96 (1.57 to 2.45) p<0.0001 | <0.0001 | ||
| No | Ref. | 1.03 (0.88 to 1.21) p=0.730 | 1.25 (1.06 to 1.48) p=0.010 | 1.45 (1.20 to 1.76) p<0.001 | <0.0001 | Ref. | 0.99 (0.84 to 1.16) p=0.865 | 1.25 (1.05 to 1.48) p=0.011 | 1.50 (1.27 to 1.77) p<0.0001 | <0.0001 | ||
| Hypertension | 0.083 | 0.640 | ||||||||||
| Yes | Ref. | 1.03 (0.89 to 1.20) p=0.655 | 1.10 (0.94 to 1.29) p=0.245 | 1.44 (1.22 to 1.69) p<0.0001 | <0.0001 | Ref. | 1.10 (0.96 to 1.26) p=0.185 | 1.28 (1.09 to 1.51) p=0.003 | 1.71 (1.47 to 1.99) p<0.0001 | <0.0001 | ||
| No | Ref. | 1.01 (0.72 to 1.40) p=0.976 | 1.49 (1.10 to 2.02) p=0.010 | 1.60 (1.16 to 2.21) p=0.005 | <0.001 | Ref. | 0.84 (0.59 to 1.18) p=0.316 | 1.03 (0.73 to 1.47) p=0.851 | 1.39 (1.02 to 1.88) p=0.036 | 0.004 | ||
Analyses were adjusted for: sex, age, race, education, smoking status, body mass index, diabetes, hypertension, chronic kidney disease, and history of anaemia treatment, haemoglobin, mean blood cell volume, iron, triglyceride, high-density lipoprotein and total cholesterol. The results are weighted based on the survey.
CKD, chronic kidney disease; DM, diabetes mellitus; Pa, p for trend; Pb, p for interaction; RDW, red cell distribution width; RPR, RDW to platelet ratio.
Discussion
Our cross-sectional study showed that high RDW and RPR levels were associated with a high prevalence of total CVD and individual CVD, and this association remained after adjusting for multiple covariates, including demographic variables, traditional risk factors, lipids and blood counts. The fourth quantile RDW and RPR across all subgroup analysis indicates a significant higher CVD prevalence compared with first quartile, there was an interaction between elevated RDW levels and increased CVD prevalence in women and in the smoking population, as well as between elevated RPR levels and increased CVD prevalence in younger age groups (age <60 years).
The present study extends previous work on this topic.21 26 27 In 2009, Perlstein et al,21 with the aid of the NHANES III database, examined RDW and CVD mortality and all-cause mortality and found that a high RDW level was associated with all causes of death and that this relationship was not specific to CVD. In a meta-analysis by Hou et al27 examining the prognostic impact of RDW on CVD risk, which included 28 studies and 102 689 participants, the combined HR associated with all-cause mortality was 1.12 (95% CI 1.09 to 1.15) for every 1% increase in the RDW level, and that associated with major adverse cardiac events was 1.12 (95% CI 1.09 to 1.15) for every 1% increase in the RDW level (95% CI 1.08 to 1.17).
Our study has some innovations based on previous research. The results of our subgroup analysis suggest that women, smokers and young populations are particular groups of interest in this study area. In females and in the smoking population, the RDW showed an interaction effect.28 The association between high RDW levels and CVD in female patients has also been previously reported.29 The authors attribute this to women seeking more medical advice, but Lassale et al’s6 study showed the opposite, with high RDW levels in men indicating a higher risk of CVD; therefore, this direction requires more research in the future. In addition, Borné et al’s30 study showed a significant interaction between RDW and smoking on the incidence of coronary events; however, a high RDW level was also correlated with CVD in both ex-smokers and non-smokers, so it is unlikely that the association between RDW levels and CVD can be explained exclusively by smoking. A possible explanation is that smoking itself is a high risk factor for CVD, in addition to the fact that smoking can cause elevated RDW levels,31 which is indirectly associated with a high prevalence of CVD. In the younger population (aged <60 years), there is an interaction between high RPR levels and CVD; however, there are few studies examining RPR levels and CVD, and more studies are needed to investigate the relationship in the future. In future population-based CVD screening, if examiners find elevated RDW levels in women or smokers or elevated RPR levels in younger populations, increased attention should be given to these individuals.
The data from this study suggest that high RDW and RPR levels are associated with an increased incidence of CVD. In the RCS analysis, we found the saturation effect occurred at a log2-transformed RDW of 3.8354. Before the point, the prevalence of CHD and angina increased rapidly with increasing RDW levels, but after the point, this prevalence levelling off. A U-shaped correlation was shown between RPR levels and CVD prevalence, with the prevalence of CVD decreasing with increasing RPR levels and an inflection point at a log2-transformed RPR of −4.562, where the prevalence of CVD was lowest, followed by a gradual increase in CVD prevalence. Few previous studies have evaluated the correlation between RPR levels and CVD prevalence,16 and most have shown that the risk of various inflammation-related diseases gradually increases with increasing RPR levels; however, due to the limited sample size, whether this correlation was linear was not discussed. The present study has made progress on the basis of these studies.11
CVDs are a group of systemic, progressive, inflammatory diseases.32 The half-life of red blood cells is higher than that of bilirubin and albumin; therefore, the RDW represents a more stable index.33 One possible inference is that inflammation, increased adrenergic and neuroendocrine system activity, and activation of the renin angiotensin system lead to an increase in RDW levels.34 Under acute inflammatory conditions, oxidative stress is also effective in increasing RDW levels through damage to the erythrocyte membrane and the release of immature erythrocytes from the bone marrow into the peripheral blood.7 One inference against the relevance of the RPR to CVD is that thrombosis and inflammation play an important role in the pathophysiology of CVD, and reduced platelet counts are associated with an increased extent and degree of coronary stenosis, mean platelet volume, and platelet aggregability.35 Activated platelets attach to the vessel wall at coronary plaque rupture sites, triggering arterial thrombosis and leading to ischaemia or infarction. Platelets release inflammatory markers, such as soluble CD40 ligand and β-globulin, and directly stimulate inflammatory cells, which leads to further release of inflammatory markers.36 As a new marker and a more powerful predictor of significant fibrosis, the RPR reflects the severity of inflammation. The RPR is used in clinical practice along with concomitant assessments, and it may be a useful and important marker for predicting mortality in patients with certain chronic diseases.37
The strengths of this study include the representative sample of a large general population from the NHANES, the exploration of non-linear relationships, and covariate adjustment. However, our study has several limitations. A single measurement of RDW and platelet levels in the blood may not be representative of long-term changes. Second, this was a cross-sectional study, which limits our ability to make causal interpretations. In addition, the inability to include inflammatory indicators such as C reactive protein levels in the covariates due to inconsistencies in measurement methods may bias the results.
Conclusions
Elevated RDW and RPR levels were positively correlated with increased CVD prevalence, and RPR levels were non-linearly correlated with CVD prevalence. The interaction suggested that elevated RDW levels in women and smokers and elevated RPR levels in those aged <60 years imply high CVD prevalence. However, the RDW/RPR mechanisms associated with CVD need to be further investigated.
Supplementary Material
Acknowledgments
We thank all the staff of the National Health and Nutrition Examination Survey (NHANES) programme for their efforts in data collection.
Footnotes
AA, KK and AA contributed equally.
Contributors: AAiniwaer, KK and AAbulizi contributed to the conception and design, acquisition, analysis, interpretation of the data, and drafting of the manuscript or critical revision for important intellectual content. WQH, RR, HM and MY collected and organised data. XM and Y-TM contributed to the conception and design and reviewing of the manuscript or critical revision for important intellectual content. XM is responsible for the overall content as the guarantor. All authors approved the final version, and agree to be accountable for all aspects of the work.
Funding: This research was supported by Grant 2019D04017 from the Construction of Key Laboratories in the Xinjiang Uygur Autonomous Region.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available in a public, open access repository. The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found online (https://wwwn.cdc.gov/nchs/nhanes/).
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
This study involves human participants and was reviewed and approved by the NCHS Research Ethics Review Committee. The patients/participants provided their written informed consent to participate in this study (protocols numbers: NHANES 2019-2020:Protocol #2018-01, NHANES 2017-2018;Protocol #2018-01, NHANES 2011-2016:Protocol #2011-17, NHANES 2005-2010:Protocol #2005-06, NHANES 1999-2004:Protocol #98-12). Participants gave informed consent to participate in the study before taking part.
References
- 1.Roth GA, Mensah GA, Johnson CO, et al. Global burden of cardiovascular diseases and risk factors, 1990–2019. Journal of the American College of Cardiology 2020;76:2982–3021. 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McClellan M, Brown N, Califf RM, et al. Call to action: urgent challenges in cardiovascular disease: a presidential advisory from the American heart association. Circulation 2019;139:e44–54. 10.1161/CIR.0000000000000652 [DOI] [PubMed] [Google Scholar]
- 3.Avis SR, Vernon ST, Hagström E, et al. Coronary artery disease in the absence of traditional risk factors: a call for action. Eur Heart J 2021;42:3822–4. 10.1093/eurheartj/ehab474 [DOI] [PubMed] [Google Scholar]
- 4.Wang TJ. Assessing the role of circulating, genetic, and imaging biomarkers in cardiovascular risk prediction. Circulation 2011;123:551–65. 10.1161/CIRCULATIONAHA.109.912568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saba PS, Parodi G, Ganau A. From risk factors to clinical disease. J Am Coll Cardiol 2021;77:1436–8. 10.1016/j.jacc.2021.01.040 [DOI] [PubMed] [Google Scholar]
- 6.Lassale C, Curtis A, Abete I, et al. Elements of the complete blood count associated with cardiovascular disease incidence: findings from the EPIC-NL cohort study. Sci Rep 2018;8:3290. 10.1038/s41598-018-21661-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danese E, Lippi G, Montagnana M. Red blood cell distribution width and cardiovascular diseases. J Thorac Dis 2015;7:E402–11. 10.3978/j.issn.2072-1439.2015.10.04 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arkew M, Gemechu K, Haile K, et al. Red blood cell distribution width as novel biomarker in cardiovascular diseases: a literature review. J Blood Med 2022;13:413–24. 10.2147/JBM.S367660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li N, Zhou H, Tang Q. Red blood cell distribution width: a novel predictive indicator for cardiovascular and cerebrovascular diseases. Dis Markers 2017;2017:7089493. 10.1155/2017/7089493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song S-Y, Hua C, Dornbors D, et al. Baseline red blood cell distribution width as a predictor of stroke occurrence and outcome: a comprehensive meta-analysis of 31 studies. Front Neurol 2019;10:1237. 10.3389/fneur.2019.01237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cetinkaya E, Senol K, Saylam B, et al. Red cell distribution width to platelet ratio: new and promising prognostic marker in acute pancreatitis. World J Gastroenterol 2014;20:14450–4. 10.3748/wjg.v20.i39.14450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiu L, Chen C, Li S-J, et al. Prognostic values of red blood cell distribution width, platelet count, and red cell distribution width-to-platelet ratio for severe burn injury. Sci Rep 2017;7:13720. 10.1038/s41598-017-13151-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taefi A, Huang CC, Kolli K, et al. Red cell distribution width to platelet ratio, a useful indicator of liver fibrosis in chronic hepatitis patients. Hepatol Int 2015;9:454–60. 10.1007/s12072-015-9638-9 [DOI] [PubMed] [Google Scholar]
- 14.Su C, Liao L-Z, Song Y, et al. The role of red blood cell distribution width in mortality and cardiovascular risk among patients with coronary artery diseases: a systematic review and meta-analysis. J Thorac Dis 2014;6:1429–40. 10.3978/j.issn.2072-1439.2014.09.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tkaczyszyn M, Comín-Colet J, Voors AA, et al. Iron deficiency and red cell indices in patients with heart failure: iron deficiency and red cell indices in heart failure. Eur J Heart Fail 2018;20:114–22. 10.1002/ejhf.820 [DOI] [PubMed] [Google Scholar]
- 16.Celık T, Balta S, Demır M, et al. Predictive value of admission red cell distribution width-platelet ratio for no-reflow phenomenon in acute ST segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Cardiol J 2016;23:84–92. 10.5603/CJ.a2015.0070 [DOI] [PubMed] [Google Scholar]
- 17.Johnson CL, Paulose-Ram R, Ogden CL, et al. National health and nutrition examination survey: analytic guidelines, 1999-2010. Vital Health Stat 2 2013:1–24. [PubMed] [Google Scholar]
- 18.Data from: centers for disease control and prevention, national center for health statistics. national health and nutrition examination survey. Available: www.cdc.gov/nchs/nhanes/about_nhanes.html [Accessed 1 Aug 2022].
- 19.von Elm E, Altman DG, Egger M, et al. The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007;370:1453–7. 10.1016/S0140-6736(07)61602-X [DOI] [PubMed] [Google Scholar]
- 20.Leong DP, Joseph PG, McKee M, et al. Reducing the global burden of cardiovascular disease, part 2: prevention and treatment of cardiovascular disease. Circ Res 2017;121:695–710. 10.1161/CIRCRESAHA.117.311849 [DOI] [PubMed] [Google Scholar]
- 21.Perlstein TS, Weuve J, Pfeffer MA, et al. Red blood cell distribution width and mortality risk in a community-based prospective cohort. Arch Intern Med 2009;169:588–94. 10.1001/archinternmed.2009.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rovin BH, Adler SG, Barratt J. KDIGO 2021 clinical practice guideline for the management of glomerular diseases. Kidney Int 2021;100:S1–276. 10.1016/j.kint.2021.05.021 [DOI] [PubMed] [Google Scholar]
- 23.Whelton PK, Carey RM, Aronow WS, et al. 2017 acc/aha/aapa/abc/acpm/ags/apha/ash/aspc/nma/pcna guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: executive summary: a report of the american college of cardiology/american heart association task force on clinical practice guidelines. Hypertension 2018;71:1269–324. 10.1161/HYP.0000000000000066 [DOI] [PubMed] [Google Scholar]
- 24.Harrell FE. Regression modeling strategies. In: Regression modeling strategies: with applications to linear models, logistic regression, and survival analysis. New York, NY: Springer, 2001. 10.1007/978-1-4757-3462-1 [DOI] [Google Scholar]
- 25.Lumley TS. Complex surveys. Hoboken, NJ, USA: Wiley Publishing, 2010. 10.1002/9780470580066 [DOI] [Google Scholar]
- 26.Uyarel H, Isik T, Ayhan E, et al. Red cell distrubition width (RDW): a novel risk factor for cardiovascular disease. Int J Cardiol 2012;154:351–2. 10.1016/j.ijcard.2011.10.126 [DOI] [PubMed] [Google Scholar]
- 27.Hou H, Sun T, Li C, et al. An overall and dose-response meta-analysis of red blood cell distribution width and CVD outcomes. Sci Rep 2017;7:43420. 10.1038/srep43420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonati LH, Gregson J, Dobson J, et al. Restenosis and risk of stroke after stenting or endarterectomy for symptomatic carotid stenosis in the international carotid stenting study (ICSS): secondary analysis of a randomised trial. Lancet Neurol 2018;17:587–96. 10.1016/S1474-4422(18)30195-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arbel Y, Weitzman D, Raz R, et al. Red blood cell distribution width and the risk of cardiovascular morbidity and all-cause mortality. A population-based study. Thromb Haemost 2014;111:300–7. 10.1160/TH13-07-0567 [DOI] [PubMed] [Google Scholar]
- 30.Borné Y, Smith JG, Melander O, et al. Red cell distribution width in relation to incidence of coronary events and case fatality rates: a population-based cohort study. Heart 2014;100:1119–24. 10.1136/heartjnl-2013-305028 [DOI] [PubMed] [Google Scholar]
- 31.Jayasuriya NA, Kjaergaard AD, Pedersen KM, et al. Smoking, blood cells and myeloproliferative neoplasms: meta-analysis and Mendelian randomization of 2·3 million people. Br J Haematol 2020;189:323–34. 10.1111/bjh.16321 [DOI] [PubMed] [Google Scholar]
- 32.Parizadeh SM, Jafarzadeh-Esfehani R, Bahreyni A, et al. The diagnostic and prognostic value of red cell distribution width in cardiovascular disease; current status and prospective. Biofactors 2019;45:507–16. 10.1002/biof.1518 [DOI] [PubMed] [Google Scholar]
- 33.Sun X, Chen W, Sun Z, et al. Impact of red blood cell distribution width on long-term mortality in patients with ST-elevation myocardial infarction. Cardiology 2014;128:343–8. 10.1159/000359994 [DOI] [PubMed] [Google Scholar]
- 34.Haybar H, Pezeshki SMS, Saki N. Evaluation of complete blood count parameters in cardiovascular diseases: an early indicator of prognosis? Exp Mol Pathol 2019;110:104267. 10.1016/j.yexmp.2019.104267 [DOI] [PubMed] [Google Scholar]
- 35.Gregg D, Goldschmidt-Clermont PJ. Platelets and cardiovascular disease. Circulation 2003;108:13. 10.1161/01.CIR.0000086897.15588.4B [DOI] [PubMed] [Google Scholar]
- 36.Khodadi E. Platelet function in cardiovascular disease: activation of molecules and activation by molecules. Cardiovasc Toxicol 2020;20:1–10. 10.1007/s12012-019-09555-4 [DOI] [PubMed] [Google Scholar]
- 37.Takeuchi H, Abe M, Takumi Y, et al. Elevated red cell distribution width to platelet count ratio predicts poor prognosis in patients with breast cancer. Sci Rep 2019;9:3033. 10.1038/s41598-019-40024-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available in a public, open access repository. The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found online (https://wwwn.cdc.gov/nchs/nhanes/).