Abstract
Immunotherapy is the standard of care for several cancers and the field continues to advance at a rapid pace, with novel combinations leading to indications in an increasing number of disease settings. Durable responses and long-term survival with immunotherapy have been demonstrated in some patients, though lack of initial benefit and recurrence after extended disease control remain major hurdles for the field. Many new combination regimens are in development for patients whose disease progressed on initial immunotherapy. To guide clinical trial design and support analyses of emerging molecular and cellular data surrounding mechanisms of resistance, the Society for Immunotherapy of Cancer (SITC) previously generated consensus clinical definitions for resistance to single-agent anti-PD-1 immune checkpoint inhibitors (ICIs) in three distinct scenarios: primary resistance, secondary resistance, and progression after treatment discontinuation. An unmet need still exists, however, for definitions of resistance to ICI-based combinations, which represent an expanding frontier in the immunotherapy treatment landscape. In 2021, SITC convened a workshop including stakeholders from academia, industry, and government to develop consensus definitions for resistance to ICI-based combination regimens for improved outcome assessment, trial design and drug development. This manuscript reports the minimum drug exposure requirements and time frame for progression that define resistance in both the metastatic setting and the perioperative setting, as well as key caveats and areas for future research with ICI/ICI combinations. Definitions for resistance to ICIs in combination with chemotherapy and targeted therapy will be published in companion volumes to this paper.
Keywords: Clinical Trials as Topic; Guidelines as Topic; Immunotherapy; Tumor Escape; Drug Therapy, Combination
Introduction
Immunotherapy in the form of immune checkpoint inhibitors (ICIs) has transformed the standard of care for a number of solid tumors, offering some patients durable disease control. Despite the dramatic clinical benefit sometimes seen with ICI therapy, resistance remains a major barrier. Many tumors do not initially respond to ICI monotherapy (ie, primary resistance) and recurrence may occur even after periods of extended disease control (ie, secondary resistance).1 2
Previously, the Society for Immunotherapy of Cancer (SITC) developed consensus definitions for clinical phenotypes of resistance to single-agent checkpoint blockade for therapies targeting programmed cell death protein 1 and its ligand (PD-(L)1).3 The definitions identified minimum drug exposure requirements, best response, and requirements for confirmatory scans to define primary resistance, secondary resistance, and disease progression after discontinuation of therapy to support standardized study enrolment criteria and facilitate appropriate comparisons in post-anti-PD-(L)1 clinical trials. The SITC-defined resistance phenotypes for monotherapy have been shown to be associated with distinct clinical outcomes including tumor burden, tumor growth, likelihood to receive further systemic therapy, and post-progression survival. This paper expands on that earlier effort by defining resistance to combinations involving more than one checkpoint inhibitor. Also covered is resistance to ICI combinations in the perioperative setting, an increasingly important concern as immunotherapy demonstrates benefit in the treatment of early-stage disease.
Strategies to overcome primary and secondary immunotherapy resistance are an active and ongoing area of investigation. Combinations involving multiple ICIs, ICIs with chemotherapy, targeted therapies and radiotherapy have been investigated to this aim. Some of these approaches have been already approved by the US Food and Drug Administration (FDA) and by regulatory agencies in other countries in a variety of solid tumor settings. A substantial unmet medical need exists, however, for patients with tumors that progress after ICI-based combinations. Uniform resistance definitions are needed to support drug development by ensuring standardized clinical trial enrollment and stratification criteria.
Recognizing that the clinical definitions of primary resistance, secondary resistance, and resistance that develops after discontinuation of therapy for multidrug approaches may be distinct from those for anti-PD-(L)1 monotherapy, SITC’s Immunotherapy Resistance Committee convened a workshop on immunotherapy combinations. Participants were assigned to working groups to define resistance to one of three broad categories: anti-PD-(L)1 in combination with other ICIs, anti-PD-(L)1 in combination with chemotherapy, and anti-PD-(L)1 in combination with anti-VEGF tyrosine kinase inhibitors (TKIs) or antiangiogenic antibodies. The definitions developed for anti-PD-(L)1 in combination with other ICIs are reported in this paper—definitions for the other classes of combinations are outside the scope of this volume and may be found in companion manuscripts. The definitions reported in this paper are based on the best available evidence and the expert consensus of the SITC Immunotherapy Resistance Committee Immunotherapy Combinations Working Group. As additional evidence becomes available from ongoing trials, understanding of resistance will likely evolve and these definitions may be updated.
Methods
To generate expert consensus definitions on clinical phenotypes of resistance to immunotherapy combinations, SITC convened representatives from academia, industry, and government for a daylong workshop, held virtually in May 2021. Prior to the workshop, attendees completed a survey describing clinical scenarios for resistance to immunotherapy combinations. Discussion of the premeeting survey results in one of three breakout rooms (focused on immunotherapy/immunotherapy combinations, immunotherapy/targeted therapy combinations, and immunotherapy/chemotherapy combinations) led to the definitions reported in this manuscript and its companion volumes. Workshop attendees are listed in online supplemental file 1.
jitc-2022-005921supp001.pdf (556.5KB, pdf)
Disclosures of potential conflicts of interest were made prior to the onset of manuscript development. Recognizing that workshop attendees are among the leading experts on the subject matter under consideration, any identified potential conflicts of interests were managed as outlined in SITC’s disclosure and conflict of interest resolution policies. As noted in these policies, attendees disclosing a real or perceived potential conflict of interest may be permitted to participate in consideration and decision making of a matter related to that conflict, but only if deemed appropriate after discussion and agreement by the participants.
General assumptions on resistance to immunotherapy combinations
The consensus definitions for ICI combinations are intended to inform clinical trial design and drug development in the context of patients with solid tumors being treated with systemic therapy. Consistent with the 2020 SITC definitions for resistance to anti-PD(L)1 monotherapy, these definitions aim to identify patients who would have a ≤5% chance of subsequent clinical benefit if treatment was continued past progression. At this point, the definitions do not encompass recommendations for clinical management, which should be based on the best judgment of the treating physicians for their individual patients.
This manuscript focuses on ICI combinations consisting of an anti-PD-(L)1 agent administered with another antibody directed at an additional immune checkpoint. Combinations involving non-antibody-based immune-modulating agents such as cytokines or transforming growth factor traps were not included in these definitions. At the time of publication, three ICI combination regimens were approved by the US FDA. The anti-CTLA-4 antibody ipilimumab is given in combination with the anti-PD-1 antibody nivolumab for indications in colorectal cancer with microsatellite instability, advanced renal cell carcinoma (RCC), mesothelioma, melanoma, non-small cell lung cancer (NSCLC) and hepatocellular carcinoma (HCC). Another anti-PD-1 and anti-CTLA-4 combination, durvalumab with tremelimumab, is also FDA approved for advanced HCC and NSCLC. Ipilimumab plus nivolumab is approved as a stand-alone combination, as well as with platinum-based chemotherapy for the treatment of NSCLC. Durvalumab plus tremilimumab is approved in combination with platinum-based chemotherapy in NSCLC as well.4–10 ICI combinations with chemotherapy are discussed in a companion manuscript. The anti-lymphocyte activation gene 3-(LAG-3) ICI relatlimab in combination with nivolumab gained approval in 2022 for the treatment of metastatic melanoma based on the phase III RELATIVITY-047 trial11 and ongoing studies are evaluating its activity in other disease settings. Additional agents targeting checkpoints beyond PD-(L)1, LAG-3, and CTLA-4 are also being evaluated. The anti-T cell immunoreceptor with Ig and ITIM domains (TIGIT) antibody tiragolumab in combination with anti-PD-L1 atezolizumab demonstrated improved ORR and PFS compared with atezolizumab alone for patients with NSCLC.12 However, the subsequent phase III SKYSCRAPER-01 trial failed to meet its coprimary endpoint of progression-free survival, and the overall survival co-primary endpoint was not yet mature at the time of manuscript publication. The resistance definitions offered here are presumed to be agent-agnostic and applicable to approved and investigational ICI combination regimens for the purposes of guiding further development of the same combinations or alternative rational regimens.
Importantly, ICI combinations are administered under distinct dosing regimens. This has implications for determining whether resistance is to one or both agents. Although the mechanism of action of CTLA-4 in antitumor immunity has yet to be elucidated, CTLA-4 is known to suppress effector T cells both by increasing the threshold for activation and attenuating expansion.13 Anti-CTLA-4 agents are thought to support activation and proliferation of CD8+ and CD4+ effector T cells, regardless of TCR specificity, in addition to reducing regulatory T cell-mediated immunosuppression in the draining lymph node. Anti-PD-(L)1 is generally understood to reinvigorate exhausted tumor antigen-specific effector T cells in the tumor microenvironment.14 15 Expression of PD-1 and PD-L1 on other cell types such as myeloid cells and B cells indicates that these populations may also play a role in antitumor immunity.16 17 LAG3 is also expressed on exhausted T cells in tumors and canonically binds to major histocompatibility complex class II. The mechanisms of immune suppression mediated by LAG3 are not known,18 however, anti-LAG3 synergizes with anti-PD-1 to enhance tumor killing by CD8+ T cells in preclinical models. Anti-CTLA-4 and anti-PD-1 act at early and later time points in the cancer-immunity cycle,19 respectively, and combination approaches lead to the expansion of distinct populations of antitumor effector memory T cells compared with monotherapy.20 Combination regimens involving anti-PD-1 and anti-CTLA-4 are also associated with higher incidences of severe toxicity.21
For several indications in disease settings in which the early response elicited by CTLA-4 inhibition in combination with PD-(L)1 blockade can be sustained via anti-PD-(L)1 alone, the anti-CTLA-4 agent is only administered for a limited number of cycles at treatment initiation, which is referred to as ‘induction’ for the purposes of this paper. In other settings, both agents are administered until disease progression or unacceptable toxicity, referred to hereafter as ‘continuous’ dosing. Examples of induction dosing include the FDA-labeled indications for ipilimumab plus nivolumab for metastatic melanoma and RCC among other indications, where an induction phase of a combination of the anti-CTLA-4 agent ipilimumab is administered with the anti-PD-1 agent nivolumab every 3 weeks for 4 doses and is followed by a maintenance phase of the anti-PD-1 agent nivolumab alone of varying durations—the optimal duration remaining unclear and under investigation in some settings. The number of cycles may vary across agents and disease types. For example, the durvalumab plus tremelimumab regimen that demonstrated survival benefit in the treatment of HCC in the phase III HIMALAYA trial consisted of a single priming dose of the anti-CTLA-4 agent tremelimumab.
Settings where dosing is continuous include the ipilimumab plus nivolumab indication for mesothelioma, where the anti-CTLA-4 agent is given every 6 weeks with the anti-PD-1 agent every 3 weeks. Of note, ipilimumab is administered at 1 mg/kg in mesothelioma—a lower dose than the 3 mg/kg used in most cases in advanced melanoma. Frequently, multiple dose levels are evaluated in the early phase trials for combination ICI regimens, and only the regimens with the most favorable efficacy and safety are advanced to registrational studies, leading to inconsistencies in the label dosing schedules across disease settings. As another example, dose reduction of the anti-CTLA-4 component was necessary to reduce adverse events in the phase I CheckMate 012 trial that evaluated ipilimumab in combination with nivolumab for first-line treatment of advanced NSCLC5
Continuous dosing of both agents until disease progression or toxicity is also the case for the combination of the anti-LAG-3 relatlimab in combination with the anti-PD-1 agent nivolumab for the treatment of melanoma, which was FDA approved based on the phase III RELATIVITY-047 trial. The combination on that trial was given as a fixed dose every 4 weeks.
These definitions assume objective assessment of disease burden with Response Evaluation Criteria In Solid Tumors version 1.1 (RECIST v1.1). Of note, for the purposes of these definitions, oligometastatic lesions that are managed with local therapy measures when overall disease control is maintained would not be considered evidence of resistance. As such progressive disease as determined by RECIST v1.1 is not automatically defined as resistance. Progression that defines resistance for the purposes of these definitions is wide-spread progression or appearance of new lesions that are not amenable to local treatment. Although several alternative criteria have been described to account for the atypical patterns of radiographic findings sometimes seen with immunotherapy,22–24 these measures are still not routinely used as endpoints in registration trials. As such, we do not address them here.
Primary resistance
In the 2020 SITC definitions, primary resistance to anti-PD-(L)1 monotherapy was defined to identify the population of patients who would not benefit from prolonged exposure to the drug. The mechanisms of resistance to single-agent and combination ICI therapy may be overlapping or distinct. ICI combinations (ie, adding anti-CTLA-4, anti-LAG-3 or anti-TIGIT to anti-PD-(L)1) may alleviate some tumor-extrinsic (ie, microenvironment-mediated or immune-mediated) mechanisms of primary resistance to monotherapy by enhancing T cell priming and activation in the lymph nodes or relieving exhaustion in the tumor microenvironment.2 25–29 Due to the complexity of conducting continuous molecular assessment of the tumor immune microenvironment, resistance definitions based on clinical parameters are necessary for trial design and drug development. For the purposes of these definitions, primary resistance is defined based on drug exposure and best response.
Similar to the definition for primary resistance to anti-PD-(L)1 monotherapy, the task force determined that a minimum drug exposure requirement of a minimum of two full cycles of exposure to both drugs (6–12 weeks, depending on the dosing schedule) would be necessary to define resistance. Rapid responses to combination ipilimumab plus nivolumab were seen in the CheckMate 069 trial, with the majority of patients having tumor reduction on the first scan, which was at 12 weeks. However, 4 out of the 44 responses occurred only after 16 weeks.30 In other disease settings or with other combinations, however, the time to response to therapy may be more prolonged and responses after the 12-week time point are observed. For example, the median time to response to ipilimumab plus nivolumab for advanced renal cell carcinoma in CheckMate 214 was 2.8 months4 and the median time to response to nivolumab plus relatlimab in RELATIVITY-047 was 2.79 months.11 Primary resistance is defined by progressive disease or stable disease lasting less than 6 months, although there was some consideration that a 3-month time frame should be used in some settings (mirroring the mandatory safety follow-up period used on many clinical trials) based on the expected pharmacokinetics and pharmacodynamics of ICIs31 and to avoid delay in switching treatment for aggressive malignancies. Because initial tumor growth followed by response is a possibility with ICI combinations, a confirmatory scan is required after 4 weeks or more after initial evidence of progressive disease to rule out pseudoprogression, provided that the patient is clinically stable. The definition for primary resistance is summarized in table 1.
Table 1.
Drug exposure and best response requirements for primary resistance to ICI combinations
| Resistance phenotype | Drug exposure requirement | Best response | Confirmatory scan for PD requirement | Confirmatory scan time frame |
| Primary resistance | A minimum of 2 cycles of both drugs (6–12 weeks) | PD, SD < 6 months | Yes, if clinically stable | At least 4 weeks after PD, if clinically feasible |
ICIs, immune checkpoint inhibitors; PD, progressive disease; SD, stable disease.
Secondary resistance
Similar to primary resistance, secondary resistance, characterized by initial benefit from an immunotherapy combination followed by progressive disease, may arise via multiple tumor-intrinsic (eg, MHC silencing, T cell exclusion) and tumor-extrinsic (eg, defective T cell priming and activation) mechanisms.2 25 27 28 The mechanisms of secondary resistance to combinations including an anti-PD-(L)1 agent plus an anti-CTLA-4 agent may be distinct as compared with an anti-LAG-3 agent or another investigational agent, given the overlapping but unique roles each checkpoint plays in suppressing antitumor immunity.18 Clinically, the phenotype will manifest as initial benefit, followed by progressive disease regardless of the molecular pathways involved.
Secondary resistance requires initial benefit with therapy. In the absence of widely available biomarker-based approaches to validate immunological tumor control, such a benefit must be established by RECIST v1.1 criteria, with a response that persists for at least 12–24 weeks, depending on the tumor type and the natural history of the disease. As with primary resistance, isolated sites of progression that may be managed with local therapy do not define disease progression for these definitions. The minimum drug exposure time frame requirements and duration of best response to define secondary resistance are summarized in table 2.
Table 2.
Drug exposure and best response requirements for secondary resistance to ICI combinations
| Resistance phenotype | Drug exposure requirement | Best response | Confirmatory scan for PD requirement | Confirmatory scan time frame |
| Secondary resistance* | >6 months | CR, PR, or SD ≥6 months† |
Yes, if clinically stable | At least 4 weeks after PD, if clinically feasible |
*For ICI combinations administered as induction dosing (ie, one agent is dropped after a set number of cycles) secondary resistance is defined for the monotherapy that was continued.
†For aggressive tumors such as mesothelioma and NSCLC, 3 months is required.
CR, complete response; ICI, immune checkpoint inhibitor; NSCLC, non-small cell lung cancer; PD, progressive disease; PR, partial response; SD, stable disease.
A minimum drug exposure of 6 months is necessary to define secondary resistance, with a best response of complete response, partial response, or stable disease, as defined by RECIST v1.1 criteria. Importantly, the resistance definition for ICI combinations depends on the natural history of the tumor type and the dosing of the ICI combination. For aggressive malignancies, a shorter period of disease control may still indicate clinical benefit, and, as such, the consensus of the group was that a shorter duration of best response should be required for the definition for secondary resistance to ICI combinations for patient safety concerns. Therefore, for mesothelioma, NSCLC, and other rapidly growing tumors, secondary resistance requires a 3-month duration of best response.
Under induction dosing, where one ICI is administered by design for only a limited number of initial cycles (typically 4 cycles), progressive disease occurring after 6 months is defined as secondary resistance to the monotherapy for the drug that was continued. If continuous dosing is utilized with a combination ICI regimen, secondary resistance may be determined for the combination.
Resistance in the perioperative setting
Anti-PD-(L)1 agents and other ICIs are increasingly becoming incorporated into the management of early-stage disease, with the intent of reducing the risk of recurrence after curative-intent surgery. The goal of administering ICIs in the neoadjuvant setting is to elicit a robust antitumor immune response while the lesion is in situ, whereas adjuvant immunotherapy is delivered with the aim of immunological control of micrometastatic residual disease. Immunotherapy in the perioperative setting has been demonstrated to improve survival outcomes in many settings, although data are still somewhat sparse on ICI combinations. Emerging evidence indicates that some tumors will require combination immunotherapy approaches in the perioperative setting to obtain maximal benefit.
Definitions of resistance to perioperative immunotherapy are complicated by the difficulty in establishing a bona fide response. Although examination of resection specimens in the neoadjuvant setting is an established technique for determining response to chemotherapies and targeted therapies in many tumor types, the delayed clinical effects and immune memory associated with ICIs may not be apparent at the time of surgery. Moreover, in the postoperative setting, in the absence of easy-to-measure dynamic biomarkers for minimal residual disease after resection, failure of adjuvant immunotherapy cannot be detected until gross regrowth. Regardless of the challenges, ICI-based combinations in the perioperative setting represent an important and rapidly expanding frontier in immunotherapy research. Definitions for immunotherapy resistance in early-stage disease are needed to support drug development with consistent enrollment and stratification criteria across trials.
Resistance in the adjuvant setting
Adjuvant therapy is typically administered for a fixed duration after definitive therapy, typically surgery or chemoradiation. The duration may vary by disease histology and indication, though these definitions assume that the patients received surgery and adjuvant therapy as planned and adequate drug exposure was achieved. While the minimum drug exposure requirements to elicit antitumor immunity are still not known, and durable responses have been observed with single doses or reduced doses in some settings, the consensus of this manuscript development group was that two cycles of treatment is necessary to define resistance. Although no ICI combinations were approved in the adjuvant setting at the time of manuscript writing, multiple dual immunotherapy regimens are being evaluated postresection in ongoing trials.
For ICI combinations in the adjuvant setting, the consensus was that resistance should be defined by the time interval elapsed after discontinuation of the combination. Primary resistance is defined as recurrence on therapy or within a window where the drugs and their effects on cytotoxic immune cells are assumed to still be active. Recurrence after the time period at which an ICI would no longer be expected to be present31 32 may not confer resistance. While the pharmacokinetics and pharmacodynamics of ICIs are complex, with prolonged receptor occupancy and durable effects on immune cells possible even after clearance, the group was in agreement that five half-lives was a reasonable time threshold to consider that the majority of the agent is cleared. As such, the consensus of the group was that recurrence ≤12 weeks after the last dose of therapy should be deemed primary resistance and recurrence that occurs 12 weeks after the last dose may not be determined to be resistance, as summarized in table 3. Rechallenge studies will be needed to validate the time points that define resistance after adjuvant therapy discontinuation, such as the results that have been reported in the metastatic setting in melanoma.33 34
Table 3.
Resistance definitions for the adjuvant setting
| Resistance phenotype | Time from adjuvant therapy discontinuation |
| Primary resistance in the adjuvant setting | ≤12 weeks or recurrence on therapy |
| Undeterminable | >12 weeks |
As with primary resistance in the advanced disease setting, the dosing schedule has implications for the resistance type. If one drug only was administered during a maintenance period or discontinued due to toxicity, then recurrence after adjuvant therapy is resistance to the monotherapy. If both agents are administered continuously, progression within 12 weeks after the last dose is considered resistance to the combination of both agents.
Confirmatory biopsies to confirm relapse of the same malignancy is recommended for the definitions of resistance in the adjuvant setting, especially if the recurrence occurs more than 6 months after the primary resection. Even in cases where regrowth is considered highly likely to originate from the primary resected lesion and in a location distinct from common sites of relapse, biopsies are important to collect in order to understand mechanisms of resistance. At academic centers, this can be assessed if the patient is enrolled in a biomarker protocol.
Resistance in the neoadjuvant setting
At the time of manuscript preparation, somewhat sparse data were available on ICI combinations in the neoadjuvant setting. Case reports have described pathological complete responses with preoperative single-agent checkpoint blockade.35–37 Neoadjuvant nivolumab in combination with ipilimumab has been shown to result in the expansion of more tumor-infiltrating T cell clones compared with the same regimen in the adjuvant setting.38 Single-agent pembrolizumab given in the neoadjuvant setting and continued in the adjuvant setting after surgery was associated with a 42% reduced risk of recurrence compared with adjuvant therapy alone in patients with high-risk melanoma in the SWOG 1801 trial.39 Neoadjuvant anti-LAG3 in combination with anti-PD-1 continued into the adjuvant setting was associated with a 57% pathological complete response rate and a 70% overall pathological response rate among 30 patients with high-risk melanoma treated in a phase II trial.40 Pathological response was observed in 20/20 (100%, 95% CI 86 to 100%) mismatch repair deficient rectal tumors, with 19 major pathological responses (defined as ≤10% residual viable tumor) and 12 pathological complete responses in NICHE-2.41 In the phase II OpACIN-neo trial, all of the patients with macroscopic stage III melanoma who achieved pathological response after neoadjuvant treatment with nivolumab in combination with ipilimumab (n=7 out of 9) remained relapse-free at 4 years, and tumor mutation burden as well as interferon gamma gene expression signatures were identified as predictive biomarkers.42 The PRADO trial, which was an expansion of OpACIN-neo, reported a 72% pathological response rate in 99 patients, and the 24-month relapse-free survival and distant metastasis-free survival rates were 93% and 98% in patients with a major pathological response in the resection specimen (<10% viable tumor).43 These studies are exciting and lay the groundwork for larger trials to establish neoadjuvant ICI combinations in the treatment paradigm for early-stage disease.
Despite the remarkable rate of pathological response, which often exceed the clinical and radiographic responses, pathological responses to immunotherapy have not yet been validated as a reliable surrogate for overall survival.44 Furthermore, neoadjuvant therapy is typically given for a limited number of cycles before resection. Given the sometimes prolonged time to first response with ICI therapy, the short course of drug exposure during typical neoadjuvant treatment may be inadequate to assess for an adequate response to the drug combination. Further research on blood-based and tumor-based biomarkers of response and resistance to neoadjuvant therapy is needed. Circulating tumor DNA (ctDNA) has been demonstrated to associate with a survival benefit in clinical trials with ICI therapy in the adjuvant setting in lung cancer and in urothelial cancer,45 46 and big data approaches for analysis of resection specimens using multiplex immunofluorescence is capable of identifying lymphocyte infiltration phenotypes associated with long-term benefit with neoadjuvant checkpoint blockade.47 As technologies mature and more data becomes available, concrete definitions for resistance may be developed.
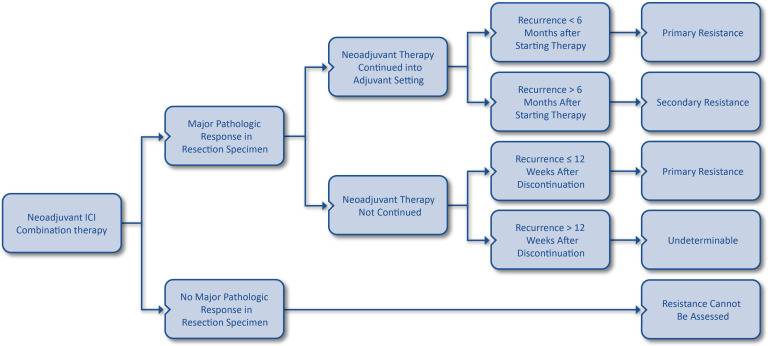
Importantly, however, sensitivity may be defined in the neoadjuvant setting if a major pathological response is observed in the resection specimen, providing more compelling evidence of activity than by radiologic response alone. If a major pathological response is observed in the resection specimen, provided neoadjuvant immunotherapy is administered and the treatment is continued after surgery, then the definitions for primary and secondary resistance to the combination may be applied if tumor regrowth is seen following combination therapy. In cases in which postoperative therapy is not administered after neoadjuvant therapy, yet major pathological response was achieved, primary and secondary resistance definitions in the adjuvant setting may be used. Secondary resistance would be considered to have occurred if a patient experiences a major pathological response and has relapse of disease within 12 weeks of administration of combination ICIs. If no major pathological response is observed in the resection specimen after neoadjuvant immunotherapy, then the resistance phenotype cannot be determined. Evaluation of resistance based on response in the resection specimen is summarized in figure 1.
Figure 1.
Schematic for evaluation of resistance to immune checkpoint inhibitor (ICI) combinations based on presence of pathological response and continuation of therapy in the neoadjuvant setting. Note that the definition for primary resistance in the neoadjuvant setting requires both drugs to have been received. Major pathological response should be evaluated based on remaining viable tumor thresholds specific to individual disease settings.
Resistance after halting therapy
Although the optimal duration of ICI therapy has not been established, multiple scenarios may lead to a patient discontinuing treatment before evidence of relapse or progression, including toxicities, trial design, socioeconomic reasons, patient preference, or other factors. Unlike conventional chemotherapy and targeted therapy, however, the effects of immunotherapy can often persist after discontinuation of treatment.48 49 Indeed, delayed toxicity is also a possibility.50 The definition for resistance after discontinuation depends on the reason for halting therapy. Importantly, a major distinction exists between stopping treatment due to the achievement of maximal clinical benefit or trial design and cessation of therapy due to adverse events or financial and/or social reasons. Importantly, tumor regrowth or recurrence after therapy is discontinued due to toxicity does not define resistance. Additionally, prolonged benefit even after discontinuation due to toxicity has been observed in some cases, for example, with the use of anti-CTLA-4 plus anti-PD-1 therapy for the treatment of advanced melanoma.8 Even for patients who discontinue the anti-CTLA-4 portion of their treatment as a result of toxicity, therapeutic benefit has often been observed. Therefore, the timing of progression after discontinuation for toxicity should be used in the same way to define resistance as when progression occurs after discontinuation due to completion of planned therapy.
When therapy is discontinued for reasons other than toxicity and adequate drug exposure (ie, 2 cycles or 6–12 weeks) has been achieved, the definition of resistance depends on timing of progression after discontinuation of therapy. These definitions are concordant with the timeline for resistance in the adjuvant setting. If a patient had adequate drug exposure (ie, 2 cycles or 6–12 weeks) and tumor regrowth occurs ≤12 weeks after the last dose is given, the tumor has primary resistance. For checkpoint inhibitors administered on a continuous schedule (double checkpoint inhibitors), progressive disease ≤12 weeks after the last dose of both agents should be deemed resistance to the full combination. For induction regimens (ie, one drug was administered for a fixed number of cycles and then monotherapy was given as maintenance) progressive disease ≤12 weeks after the last dose should define resistance to the monotherapy. If complete or partial response was achieved and progressive disease occurs >12 weeks after discontinuation, the resistance phenotype must be assessed based on rechallenge. Confirmatory scans are not required to establish resistance if regrowth occurs 12 weeks or longer after therapy is halted. Resistance scenarios after discontinuation of therapy are summarized in table 4.
Table 4.
Resistance scenarios after halting therapy in the metastatic disease setting
| Administration schedule | Timing of tumor regrowth | Resistance phenotype |
| Continuous | ≤12 weeks after the last dose | Primary resistance to the combination |
| >12 weeks after the last dose | Secondary resistance to the combination | |
| Induction | ≤12 weeks after the last dose | Primary resistance to the drug that was continued |
| >12 weeks after the last dose | Must be assessed based on rechallenge |
These definitions assume that adequate drug exposure (ie, 12 weeks or 2–4 cycles) was provided.
Future directions
Through the development of the resistance definitions in this manuscript, several important areas for future research were identified. First, prospective trials are needed to establish validity of these definitions. Additionally, data should be collected on outcomes after rechallenge and stratified by time interval. Moreover, it will be important to understand the implications of the different types of resistance as defined in this manuscript, both retrospectively and prospectively, by assessing overall survival rates from the time of disease progression and stratifying the analyses by primary and secondary resistance scenarios. Future studies evaluating later-line treatments in the post-ICI setting should enroll by resistance phenotype to identify effective salvage therapies, such as the pilot trial demonstrating that fecal microbiota transplant provided clinical benefit in 6 out of 15 patients with primary resistance to anti-PD-1.51
The identification and validation of biomarkers of response and resistance to immunotherapy is an ongoing priority for the field. Trials evaluating immunotherapy combination should be designed with integrated biomarker collection and analyses so that future research efforts can define resistance with greater certainty. In particular, samples to measuring ctDNA should be collected at baseline and on-treatment. Measurement of ctDNA has demonstrated prognostic utility to identify patients with lung or urothelial cancer who benefit from atezolizumab.52 53 Further studies are needed to determine if dynamics of this blood-based biomarker may be used as a surrogate for response and recurrence. Longitudinal studies with dynamic ctDNA assessment, imaging, and liquid biomarker measurements should be performed to identify signatures of primary and secondary resistance at different time points during and after cessation of therapy. Other factors that have been identified that are associated with response to ICI such as PD-L1, TMB, and other markers of intratumoral inflammation should also be collected and analyzed for correlations with resistance phenotypes. Big data approaches47 for evaluating resection specimens and tumor biopsies should also be encouraged to continue identifying the cellular populations associated with non-response to ICIs within the tumor microenvironment.
Conclusion
ICI combinations are becoming the standard of care for several solid tumors. The pace of drug development shows no signs of abating, and combination immunotherapy is demonstrating clinical benefit even in some tumors that typically do not respond to single-agent PD-(L)1 blockade. Basket trials such as the SWOG 1609 DART study54 that evaluate a single intervention in a population of patients with multiple diseases with common unifying risk factors are expanding the landscape by evaluating dual CTLA-4/PD-(L)1 blockade in rare tumors. Ongoing studies are advancing novel ICI targets such as LAG-3,18 TIM-3,55 and TIGIT56 from preclinical models through late-stage trials. As ever-increasing numbers of patients are treated with ICI combinations, clear and clinically relevant definitions for resistance are clearly essential for future drug development. Additionally—although clinical management is beyond the scope of this manuscript—clinical definitions of resistance are important in order to avoid treatment beyond futility and unnecessary toxicity as well as to guide and allow for rechallenge with a given immunotherapy regimen if resistance definitions have not been met.
A major obstacle to evaluating resistance definitions identified during the discussion was a lack of an immunotherapy comparator arm in many randomized trials. Designing immunotherapy trials with maximum likelihood of positive results and useful data is a priority for SITC,57 with several ongoing efforts at guidance documents on optimal endpoints, rationale, and analyses for phase III studies. The resistance definitions described herein will further help inform trial design to ensure appropriate comparisons within and across studies.
The definitions set forth in this paper were based on the expert consensus and clinical experience of attendees at the SITC Immunotherapy Resistance Committee Combinations Resistance Workshop, with the intent of identifying patients with a 95% chance of not responding if therapy was continued, or resumed in the case of induction therapy. The workshop attendees recognized that limited data were available to inform the definitions of resistance in some scenarios (eg, neoadjuvant therapy) and, as such, a second major outcome of the meeting was a call for data sharing to validate the differences in survival outcomes depending on the resistance scenario. Additionally, prospective data should be collected on outcomes after rechallenge, as described in the Future directions section. Finally, although this paper strictly defined resistance phenotypes based on clinical presentations, mechanistic studies to understand the biology of non-responsiveness to immunotherapy are also essential. In addition to supporting drug development, the authors hope that these consensus definitions will also facilitate the important translational and reverse translational research on immunotherapy resistance by biologically comparing samples from similar resistance scenarios.
Acknowledgments
The authors thank Adnan Jaigirdar, MD, Marc Theoret, MD, and Peter Bross, MD of the US Food and Drug Administration for contributing to the discussion at the SITC Immunotherapy Combinations Resistance Workshop and for providing critical review of manuscript drafts. The authors acknowledge SITC staff for their contributions including Sam Million-Weaver, PhD for medical writing; Claire Griffiths, MD, MPH; for editorial support; and Peter J. Intile, PhD for project management and assistance. Additionally, the authors wish to thank SITC for supporting the manuscript development.
Footnotes
Twitter: @HTawbi_MD
Contributors: HK and ES served as chairs of the manuscript development group. HK, RJS, and HT are chairs of the SITC Immunotherapy Resistance committee. All other authors participated on surveys, provided input during discussions at the SITC Immunotherapy Combinations Resistance Workshop, and contributed to the writing, critical review, and editing during the manuscript development and are thus listed alphabetically by last name.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: HK—consulting fees: Iovance, Immunocore, Celldex, Array Biopharma, Merck, Elevate Bio, Instil Bio, Bristol Myers Squibb, Clinigen, Shionogi, Chemocentryx, Calithera, Signatero. JCB—salary and employment: AstraZeneca; Ownership interest less than 5%: AstraZeneca. JFG—consulting fees: Bristol-Myers Squibb, Genentech/Roche, Takeda, Loxo/Lilly, Blueprint, Oncorus, Regeneron, Gilead, Mirati, Moderna, AstraZeneca, Pfizer, Novartis, Merck, iTeos, Karyopharm, Jazz Pharmaceuticals, Silverback Therapeutics, and GlydeBio; Contracted research: Novartis, Genentech/Roche, Takeda, Bristol-Myers Squibb, Tesaro, Moderna, Blueprint, Jounce, Array Biopharma, Merck, Adaptimmune, Novartis, and Alexo; Ownership interest less than 5%: Ironwood Pharmaceuticals; Spousal employment: Ironwood Pharmaceuticals. MH—consulting fees: Bristol Myers Squibb, CRISPR Therapeutics, Exelixis, Nektar Therapeutics, Janssen; Spousal employment: Arvinas. OH—consulting fees: Aduro, Akeso, Amgen, Beigene, Bioatla, Bristol Myers Squibb, Genentech/Roche, GSK, Immunocore, Idera, Incyte, Iovance, Instil Bio, Janssen, Merck, Nextcure, Novartis, Pfizer, Sanofi Regeneron, SeaGen, Tempus, Zelluna; Fees for non-CME services: Bristol Myers Squibb, Novartis, Pfizer, Sanofi Regeneron; Contracted research: Arcus, Aduro, Akeso, Amgen, Bioatla, Bristol Myers Squibb, Cytomx, Exelixis, Genentech/Roche, GSK, Immunocore, Idera, Incyte, Iovance, Merck, Moderna, Merck Serono, NextCure, Novartis, Pfizer, Rubius, Sanofi Regeneron, SeaGen, Torque, Zelluna. RAM—salary and employment: Bristol Myers Squibb; Ownership interest less than 5%: Bristol Myers Squibb. RZ—IP rights: Memorial Sloan Kettering, Weill Cornell Medical College; Consulting fees: Leap Therapeutics, iTEOS. TL—Salary and employment: Coherus Biosciences; IP rights: AstraZeneca, Parker Institute for Cancer Immunotherapy, Celldex, EntreMed; Consulting fees: TRex Bio, Grey Wolf Therapeutics, Exosis, LisCure Biosciences, BiOne Cure, Inovio, 1440 Foundation; Ownership interest less than 5%: AstraZeneca, Coherus Biosciences. RJS—consulting fees: Asana Biosciences, AstraZeneca, Bristol Myers Squibb, Eisai, Iovance, Merck, Novartis, OncoSec, Pfizer, Replimune; Contracted research: Merck, Amgen. HT—consulting fees: Genentech/Roche, Bristol Myers Squibb, Novartis, Merck, Pfizer, Eisai, Karyopharm, Boxer Capital; Contracted research: Genentech/Roche, Bristol Myers Squibb, Novartis, Merck, GSK. ES—nothing to disclose. SITC Staff: SMW, CG, PJI—nothing to disclose.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1.Zhao X, Subramanian S. Intrinsic resistance of solid tumors to immune checkpoint blockade therapy. Cancer Res 2017;77:817–22. 10.1158/0008-5472.CAN-16-2379 [DOI] [PubMed] [Google Scholar]
- 2.Sharma P, Hu-Lieskovan S, Wargo JA, et al. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 2017;168:707–23. 10.1016/j.cell.2017.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kluger HM, Tawbi HA, Ascierto ML, et al. Defining tumor resistance to PD-1 pathway blockade: recommendations from the first meeting of the SITC immunotherapy resistance taskforce. J Immunother Cancer 2020;8:e000398. 10.1136/jitc-2019-000398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med 2018;378:1277–90. 10.1056/NEJMoa1712126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med 2019;381:2020–31. 10.1056/NEJMoa1910231 [DOI] [PubMed] [Google Scholar]
- 6.Yau T, Kang Y-K, Kim T-Y, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the checkmate 040 randomized clinical trial. JAMA Oncol 2020;6:e204564. 10.1001/jamaoncol.2020.4564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baas P, Scherpereel A, Nowak AK, et al. First-line nivolumab plus ipilimumab in unresectable malignant pleural mesothelioma (checkmate 743): a multicentre, randomised, open-label, phase 3 trial. Lancet 2021;397:375–86. 10.1016/S0140-6736(20)32714-8 [DOI] [PubMed] [Google Scholar]
- 8.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Five-year survival with combined nivolumab and ipilimumab in advanced melanoma. N Engl J Med 2019;381:1535–46. 10.1056/NEJMoa1910836 [DOI] [PubMed] [Google Scholar]
- 9.Abou-Alfa GK, Chan SL, Kudo M, et al. Phase 3 randomized, open-label, multicenter study of tremelimumab (T) and durvalumab (D) as first-line therapy in patients (PTS) with unresectable hepatocellular carcinoma (uhcc): Himalaya. JCO 2022;40:379. 10.1200/JCO.2022.40.4_suppl.379 [DOI] [Google Scholar]
- 10.Johnson ML, Cho BC, Luft A, et al. Durvalumab with or without tremelimumab in combination with chemotherapy as first-line therapy for metastatic non-small-cell lung cancer: the phase III Poseidon study. J Clin Oncol 2022:JCO2200975. 10.1200/JCO.22.00975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tawbi HA, Schadendorf D, Lipson EJ, et al. Relatlimab and nivolumab versus nivolumab in untreated advanced melanoma. N Engl J Med 2022;386:24–34. 10.1056/NEJMoa2109970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez-Abreu D, Johnson ML, Hussein MA, et al. Primary analysis of a randomized, double-blind, phase II study of the anti-TIGIT antibody tiragolumab (tira) plus atezolizumab (atezo) versus placebo plus atezo as first-line (1L) treatment in patients with PD-L1-selected NSCLC (CITYSCAPE). JCO 2020;38:9503. 10.1200/JCO.2020.38.15_suppl.9503 [DOI] [Google Scholar]
- 13.Korman AJ, Peggs KS, Allison JP. Checkpoint blockade in cancer immunotherapy. Adv Immunol 2006;90:297–339. 10.1016/S0065-2776(06)90008-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buchbinder EI, Desai A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am J Clin Oncol 2016;39:98–106. 10.1097/COC.0000000000000239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Das R, Verma R, Sznol M, et al. Combination therapy with anti-CTLA-4 and anti-PD-1 leads to distinct immunologic changes in vivo. J Immunol 2015;194:950–9. 10.4049/jimmunol.1401686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strauss L, Mahmoud MAA, Weaver JD, et al. Targeted deletion of PD-1 in myeloid cells induces antitumor immunity. Sci Immunol 2020;5:43. 10.1126/sciimmunol.aay1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang X, Wang G, Wang Z, et al. PD-1-expressing B cells suppress CD4+ and CD8+ T cells via PD-1/PD-L1-dependent pathway. Molecular Immunology 2019;109:20–6. 10.1016/j.molimm.2019.02.009 [DOI] [PubMed] [Google Scholar]
- 18.Maruhashi T, Sugiura D, Okazaki I-M, et al. LAG-3: from molecular functions to clinical applications. J Immunother Cancer 2020;8:e001014. 10.1136/jitc-2020-001014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen DS, Mellman I. Oncology meets immunology: the cancer-immunity cycle. Immunity 2013;39:1–10. 10.1016/j.immuni.2013.07.012 [DOI] [PubMed] [Google Scholar]
- 20.Gide TN, Quek C, Menzies AM, et al. Distinct immune cell populations define response to anti-PD-1 monotherapy and anti-PD-1/anti-CTLA-4 combined therapy. Cancer Cell 2019;35:238–55. 10.1016/j.ccell.2019.01.003 [DOI] [PubMed] [Google Scholar]
- 21.Arnaud-Coffin P, Maillet D, Gan HK, et al. A systematic review of adverse events in randomized trials assessing immune checkpoint inhibitors. Int J Cancer 2019;145:639–48. 10.1002/ijc.32132 [DOI] [PubMed] [Google Scholar]
- 22.Wolchok JD, Hoos A, O’Day S, et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 2009;15:7412–20. 10.1158/1078-0432.CCR-09-1624 [DOI] [PubMed] [Google Scholar]
- 23.Hodi FS, Hwu W-J, Kefford R, et al. Evaluation of immune-related response criteria and RECIST v1.1 in patients with advanced melanoma treated with pembrolizumab. J Clin Oncol 2016;34:1510–7. 10.1200/JCO.2015.64.0391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Seymour L, Bogaerts J, Perrone A, et al. IRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 2017;18:e143–52. 10.1016/S1470-2045(17)30074-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zaretsky JM, Garcia-Diaz A, Shin DS, et al. Mutations associated with acquired resistance to PD-1 blockade in melanoma. N Engl J Med 2016;375:819–29. 10.1056/NEJMoa1604958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koyama S, Akbay EA, Li YY, et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat Commun 2016;7:10501. 10.1038/ncomms10501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ascierto ML, Makohon-Moore A, Lipson EJ, et al. Transcriptional mechanisms of resistance to anti-PD-1 therapy. Clin Cancer Res 2017;23:3168–80. 10.1158/1078-0432.CCR-17-0270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paulson KG, Voillet V, McAfee MS, et al. Acquired cancer resistance to combination immunotherapy from transcriptional loss of class I HLA. Nat Commun 2018;9:3868. 10.1038/s41467-018-06300-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O’Donnell JS, Teng MWL, Smyth MJ. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat Rev Clin Oncol 2019;16:151–67. 10.1038/s41571-018-0142-8 [DOI] [PubMed] [Google Scholar]
- 30.Postow MA, Chesney J, Pavlick AC, et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015;372:2006–17. 10.1056/NEJMoa1414428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Centanni M, Moes DJAR, Trocóniz IF, et al. Clinical pharmacokinetics and pharmacodynamics of immune checkpoint inhibitors. Clin Pharmacokinet 2019;58:835–57. 10.1007/s40262-019-00748-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brahmer JR, Drake CG, Wollner I, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol 2010;28:3167–75. 10.1200/JCO.2009.26.7609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lipson EJ. Re-orienting the immune system: durable tumor regression and successful re-induction therapy using anti-PD1 antibodies. Oncoimmunology 2013;2:e23661. 10.4161/onci.23661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reschke R, Ziemer M. Rechallenge with checkpoint inhibitors in metastatic melanoma. J Dtsch Dermatol Ges 2020;18:429–36. 10.1111/ddg.14091 [DOI] [PubMed] [Google Scholar]
- 35.Kaseb AO, Vence L, Blando J, et al. Immunologic correlates of pathologic complete response to preoperative immunotherapy in hepatocellular carcinoma. Cancer Immunol Res 2019;7:1390–5. 10.1158/2326-6066.CIR-18-0605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tucker MD, Beckermann KE, Gordetsky JB, et al. Complete pathologic responses with immunotherapy in metastatic renal cell carcinoma: case reports. Front Oncol 2020;10:609235. 10.3389/fonc.2020.609235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tang Y, Li Y, Zhang L, et al. Pathologic complete response to preoperative immunotherapy in a lung adenocarcinoma patient with bone metastasis: a case report. Thorac Cancer 2020;11:1094–8. 10.1111/1759-7714.13361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blank CU, Rozeman EA, Fanchi LF, et al. Neoadjuvant versus adjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma. Nat Med 2018;24:1655–61. 10.1038/s41591-018-0198-0 [DOI] [PubMed] [Google Scholar]
- 39.Patel S, Othus M, Prieto V, et al. LBA6 neoadjvuant versus adjuvant pembrolizumab for resected stage III-IV melanoma (SWOG S1801). Annals of Oncology 2022;33:S1408. 10.1016/j.annonc.2022.08.039 [DOI] [Google Scholar]
- 40.Amaria RN, Postow M, Burton EM, et al. Neoadjuvant relatlimab and nivolumab in resectable melanoma. Nature 2022;611:155–60. 10.1038/s41586-022-05368-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chalabi M, Fanchi LF, Dijkstra KK, et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat Med 2020;26:566–76. 10.1038/s41591-020-0805-8 [DOI] [PubMed] [Google Scholar]
- 42.Rozeman EA, Menzies AM, van Akkooi ACJ, et al. Identification of the optimal combination dosing schedule of neoadjuvant ipilimumab plus nivolumab in macroscopic stage III melanoma (opacin-neo): a multicentre, phase 2, randomised, controlled trial. Lancet Oncol 2019;20:948–60. 10.1016/S1470-2045(19)30151-2 [DOI] [PubMed] [Google Scholar]
- 43.Reijers ILM, Menzies AM, van Akkooi ACJ, et al. Personalized response-directed surgery and adjuvant therapy after neoadjuvant ipilimumab and nivolumab in high-risk stage III melanoma: the PRADO trial. Nat Med 2022;28:1178–88. 10.1038/s41591-022-01851-x [DOI] [PubMed] [Google Scholar]
- 44.Menzies AM, Amaria RN, Rozeman EA, et al. Pathological response and survival with neoadjuvant therapy in melanoma: a pooled analysis from the international neoadjuvant melanoma consortium (INMC). Nat Med 2021;27:301–9. 10.1038/s41591-020-01188-3 [DOI] [PubMed] [Google Scholar]
- 45.Hussain MHA, Powles T, Albers P, et al. IMvigor010: primary analysis from a phase III randomized study of adjuvant atezolizumab (atezo) versus observation (obs) in high-risk muscle-invasive urothelial carcinoma (MIUC). JCO 2020;38:5000. 10.1200/JCO.2020.38.15_suppl.5000 [DOI] [Google Scholar]
- 46.Soh J, Hamada A, Fujino T, et al. Perioperative therapy for non-small cell lung cancer with immune checkpoint inhibitors. Cancers (Basel) 2021;13:4035. 10.3390/cancers13164035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berry S, Giraldo NA, Green BF, et al. Analysis of multispectral imaging with the astropath platform informs efficacy of PD-1 blockade. Science 2021;372:eaba2609. 10.1126/science.aba2609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Robert C, Marabelle A, Herrscher H, et al. Immunotherapy discontinuation-how, and when? Data from melanoma as a paradigm. Nat Rev Clin Oncol 2020;17:707–15. 10.1038/s41571-020-0399-6 [DOI] [PubMed] [Google Scholar]
- 49.Marron TU, Ryan AE, Reddy SM, et al. Considerations for treatment duration in responders to immune checkpoint inhibitors. J Immunother Cancer 2021;9:e001901. 10.1136/jitc-2020-001901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Couey MA, Bell RB, Patel AA, et al. Delayed immune-related events (dire) after discontinuation of immunotherapy: diagnostic hazard of autoimmunity at a distance. J Immunother Cancer 2019;7:165. 10.1186/s40425-019-0645-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Davar D, Dzutsev AK, McCulloch JA, et al. Fecal microbiota transplant overcomes resistance to anti-PD-1 therapy in melanoma patients. Science 2021;371:595–602. 10.1126/science.abf3363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Herbreteau G, Langlais A, Greillier L, et al. Circulating tumor DNA as a prognostic determinant in small cell lung cancer patients receiving atezolizumab. J Clin Med 2020;9:12. 10.3390/jcm9123861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Powles T, Assaf ZJ, Davarpanah N, et al. Ctdna guiding adjuvant immunotherapy in urothelial carcinoma. Nature 2021;595:432–7. 10.1038/s41586-021-03642-9 [DOI] [PubMed] [Google Scholar]
- 54.Patel SP, Othus M, Chae YK, et al. A phase II basket trial of dual anti-CTLA-4 and anti-PD-1 blockade in rare tumors (DART SWOG 1609) in patients with nonpancreatic neuroendocrine tumors. Clin Cancer Res 2020;26:2290–6. 10.1158/1078-0432.CCR-19-3356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Acharya N, Sabatos-Peyton C, Anderson AC. Tim-3 finds its place in the cancer immunotherapy landscape. J Immunother Cancer 2020;8:e000911. 10.1136/jitc-2020-000911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chauvin J-M, Zarour HM. Tigit in cancer immunotherapy. J Immunother Cancer 2020;8:e000957. 10.1136/jitc-2020-000957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Atkins MB, Abu-Sbeih H, Ascierto PA, et al. Maximizing the value of phase III trials in immuno-oncology: A checklist from the society for immunotherapy of cancer (SITC). J Immunother Cancer 2022;10:e005413. 10.1136/jitc-2022-005413 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
jitc-2022-005921supp001.pdf (556.5KB, pdf)