Abstract
Pain due to chronic conditions is a frequent and insufficiently addressed problem. Current drug options for pain management (either in cases of chronic inflammatory conditions or neuropathy) do not adequately treat pain. Moreover, they are associated with important adverse events in long term use. Luteolin is a flavonoid widely present in the plant kingdom and its sources have been assembled in a comprehensive list of this paper. Luteolin has shown in several research studies a range of pharmacological properties; anti-inflammatory, antioxidant, neuroprotective, and analgesic. In this article, we summarize the effects and potential benefits from introducing luteolin as an adjuvant agent in established protocols for pain management. We review the most indicative in vivo and in vitro evidence of how luteolin can target the molecular pathways involved in pathogenesis of chronic inflammatory and neuropathic pain. The data reviewed strongly support luteolin's promising benefits in pain management and raise the need for further clinical trials that can establish its role in clinical practice.
Keywords: luteolin, inflammatory pain, neuropathic pain, chronic pain, pain managemant
1. Introduction
Pain is defined by The International Association for the Study of Pain (IASP) as: “An unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage” (1).
Acute pain comes from the activation of the nociceptors to noxious stimuli. It works as a normal, warning system that protects the body from potential damage that can be caused by physical, thermal, or chemical threats. It can also result from a damaged (inflamed) tissue. In the latter case, pain is expected to be relieved when resolution of inflammation is achieved and the healing process is completed (2, 3).
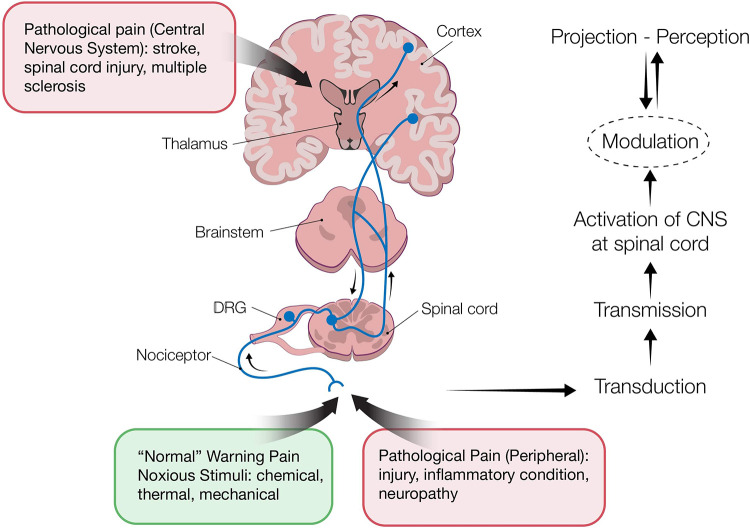
Four processes occur during pain perception: Transduction, transmission, perception and modulation (Figure 1). The ascending pathway represents the signal transmission from peripheral nerves to the brain, whereas modulation can follow an ascending and/or descending pathway. Through modulation, pain impulses can be either enhanced or inhibited. Inhibitory mechanisms have been the basis for various pain control management approaches, e.g., opioids drugs that mimic the endogenous opioid inhibitory system. Neurotransmitters play an important role in the pain transmission and modulation. They can be either produced from peripheral nerves to facilitate pain transmission (e.g., serotonin released at the site of injury or inflammation) or to inhibit pain impulses (e.g., serotonin, GABA and opioids acting in spinal dorsal horn) (4, 5).
Figure 1.
Pain pathways: transduction-transmission-perception-modulation. Transduction refers to activation of peripheral nociceptors (primary afferent neurons) from various stimuli. Cell bodies of nociceptors neurons are located in the dorsal root ganglia (DRG) (innervating the skin, deep tissues, visceral organs) and trigeminal ganglia (innervating the face). Nociceptors express transducers on their distal terminus, which are high-threshold ion channels, such as transient receptor potential ion channels [TRPs, ATP- gated ion channels, and acid-sensing ion channels (ASIC)]. These are responsible for converting the stimuli to action potential (AP). Next is the transmission process during which primary afferent neurons transmit the AP to the spinal cord, via their axons that terminate in the spinal dorsal horn (DH). Perception refers to the projection of pain signals in the brain, during which complex neuronal networks in the brain receive and “translate” from the spinal cord information about duration, location, and intensity of pain. Modulation is the process by which the nervous system either enhances or inhibits pain signals. Alteration of pain impulses occurs due to three endogenous mechanisms. 1. Segmental inhibition during which the inhibitory nerve in the spinal cord can be blocked to transmit noxious stimuli from C-fibers as a result of stimulation of the non-noxious Aβ fibers nociceptors (the “gate theory”). The method of pain management with transcutaneous electrical nerve stimulation is based on this theory. 2. The opioid system consists of opioid receptors in the brain, spinal cord and peripheral nerves that are activated by the binding of endogenous opioids (enkephalins, endorphins, and dynorphins). 3. The descending inhibitory nerve system, projecting via the midbrain, inhibits nociceptive transmission at the spinal cord dorsal horn.
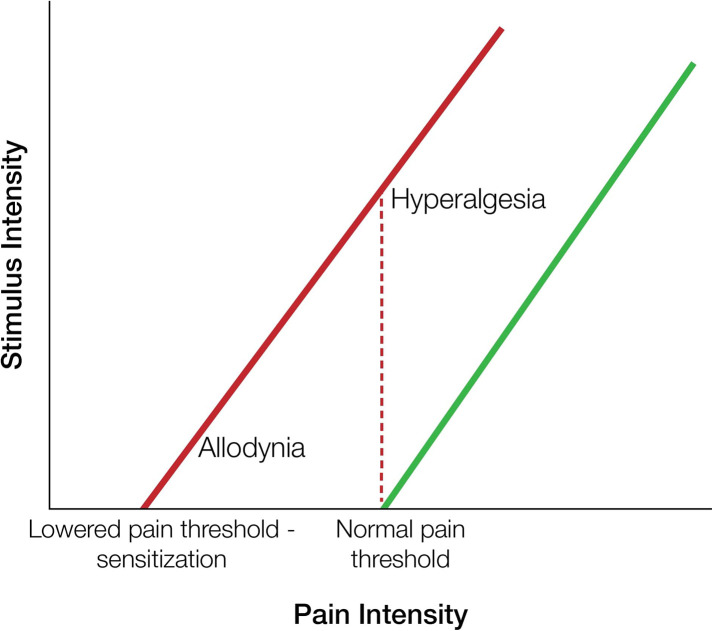
In contrast to acute pain, pain in chronic disorders is a much more complicated state and may involve concurrently different mechanistic types of pain. It can be either nociceptive, neuropathic, or include both components. Nociceptive is defined as the pain caused by damage in non-neuronal tissues, whereas neuropathic pain is the pain resulting from damage in the neuronal system. Prolonged stimulation of the nervous system leads to sensitization, during which the threshold of nociceptors is reduced, and to the phenomenon of hyperalgesia (increased sense of pain from a stimulus that causes pain) or allodynia (pain provoked by a stimulus that normally is not painful) is induced (3) (Figure 2).
Figure 2.
Allodynia and hyperalgesia vs. “normal” pain. Allodynia is the pain perception triggered by stimuli which under normal conditions are not painful. It is caused by a lowered pain threshold of nociceptors. Hyperalgesia is characterized by increased sensitivity to pain intensity triggered by normally painful stimuli.
Experiencing pain due to chronic disorders can have a serious impact on the quality of life of the afflicted individuals. Sleep deprivation, stress, anxiety, and social withdrawal are common consequences which seem to also have a bidirectional relationship in pain perception (6).
Therefore, effective management of pain is crucial in chronic conditions and despite the advances in medical treatments, it remains a challenge in clinical practice. Maximizing effectiveness and minimizing the risks for side effects in long-term use, are the goals in treating pain. However, current drug options do not seem to completely achieve this goal, neither in targeting pain as a symptom of a chronic inflammatory disease, nor as a result of a neuropathic condition. Thus, the need for complementary approaches, which can be both effective and safe, has emerged (7, 8).
In the last decade natural compounds have gained much attention for their potential use in therapeutics. Flavonoids are a group of phytochemicals that is perhaps one of the most studied. They are a group of secondary metabolites of plants, found extensively in fruits, vegetables, and herbs. Due to their wide range of pharmacological activities and safety profile are considered to be promising agents against chronic inflammation and neuropathy (7, 8). Luteolin, a flavone that is abundant in a wide range of medicinal plants and herbs, has been reported to have a range of pharmacological properties. Among these, the anti-inflammatory, antioxidant (9, 10), neuroprotective (11), and analgesic (7) effects are the ones that can be of significant value in pain management.
In this article we will summarize the current knowledge about the activities of luteolin which bring out its potential in managing pain. We will focus on the two “classic” types of pain, chronic inflammatory-nociceptive and neuropathic, and how luteolin could target the mechanisms involved in their pathogenesis. The recently defined as nociplastic pain, referring to central nervous sensitization in the absence of obvious damage (as seen in fibromyalgia, irritable bowel syndrome, and medically unexplained conditions) (12) is not covered in this review, since the mechanisms implicated have not yet been adequately elucidated to support a beneficial role for luteolin.
2. Chemical structure, plant and dietary sources of luteolin
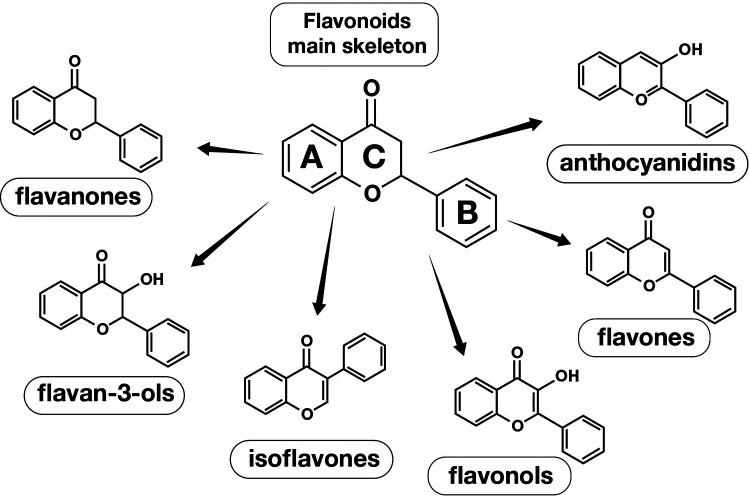
Luteolin belongs to a huge group of substances called flavonoids which are secondary metabolites characterized by a diphenylpropane structure (C6–C3–C6) and which are classified to many groups (Figure 3). They are divided into several subgroups based on structural differences in their ring C involving its oxidation state. The A and B-rings of flavonoids are usually functionalized by OH, OMe, isoprenyl, and glycosyl groups (13).
Figure 3.
Flavonoids main skeleton and chemical structures of flavonoids different groups.
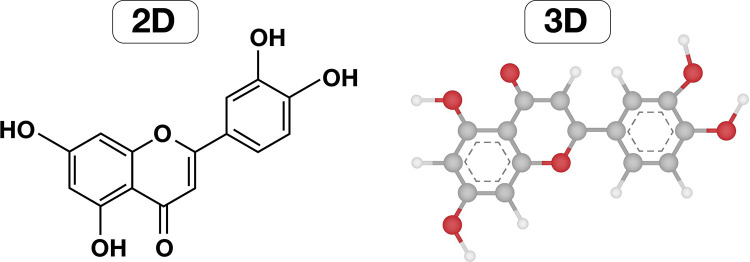
Luteolin is a tetrahydroxyflavone in which the four hydroxyl groups are located in positions 3, 4, 5 and 7 as seen in Figures 4A,B. It is a flavone (3′, 4′, 5, 7-tetra hydroxyl flavone) with a yellow crystalline appearance. Due to its color, the plant Reseda luteola that contains luteolin, has been used as a source of dye from the first millennium B.C. Michel Eugène Chevreul, a France chemist, was the first who isolated luteolin in 1829, but the correct structure was proposed in 1896 by the English chemist Arthur George Perkin (14).
Figure 4.
Chemical structure of luteolin in 2D and 3D.
Luteolin is a substance found in several plant species, including those used in traditional medicine for the treatment of many diseases. It is widely distributed in the plant kingdom and has been studied extensively for its pharmacological properties, such as anti-inflammatory, antioxidant, and neuroprotective. Glycosides of luteolin have been identified in fossils of Ulmaceae species, 36 to 25 million years old. Over 350 plant species have been found to contain luteolin and/or its various glycosidic forms (Supplementary Table S1) (15).
Depending on the country, the daily intake of flavonoids in the human diet differs. The highest intake of flavonoids including polymers is in Ireland with a daily intake of 851 mg/day, while for the same group, (flavonoids including polymers) the lowest daily intake is 225 mg in the Czech Republic. Correspondingly, the lowest intake is in the Flavones with the highest being 10 mg/day in Italy, while the lowest is 2 mg/day in Sweden, Netherlands and the United Kingdom (Table 1).
Table 1.
Daily intake (mg) of flavonoids in adults in the European union (16).
| Countries/Flavonoids | Flavonols | Flavanones | Flavones | Flavonoids (monomeric compounds only) | Flavonoids (including polymers) |
|---|---|---|---|---|---|
| Denmark | 19 | 13 | 3 | 128 | 379 |
| Finland | 17 | 30 | 3 | 134 | 354 |
| Sweden | 18 | 19 | 2 | 110 | 310 |
| Northern Region | 18 ± 1 | 21 ± 5 | 3 | 124 ± 7 | 348 ± 20 |
| Belgium | 19 | 12 | 3 | 97 | 281 |
| Czech Republic | 16 | 10 | 4 | 75 | 225 |
| Germany | 27 | 19 | 3 | 181 | 573 |
| Hungary | 23 | 11 | 4 | 122 | 408 |
| Ireland | 38 | 8 | 3 | 249 | 851 |
| Latvia | 25 | 4 | 9 | 135 | 411 |
| Netherlands | 31 | 18 | 2 | 201 | 643 |
| United Kingdom | 28 | 9 | 2 | 195 | 655 |
| Central Region | 26 ± 2 | 11 ± 2 | 4 ± 1 | 157 ± 21 | 506 ± 75 |
| France | 18 | 10 | 7 | 115 | 352 |
| Italy | 20 | 20 | 10 | 96 | 291 |
| Spain | 15 | 17 | 3 | 75 | 260 |
| Southern Region | 18 ± 1 | 15 ± 3 | 7 ± 2 | 95 ± 11 | 301 ± 27 |
| Europe | 23 ± 2 | 14 ± 2 | 4 ± 1 | 136 ± 14 | 428 ± 49 |
With regards to luteolin, although there are many plant sources (Supplementary Table S1), the daily intake in the European Union in adults ranges from 0 to 2 mg/day (Table 2) (16).
Table 2.
Daily intake (mg) of individual compounds of flavonoids in adults in the European union (16).
| Country/Flavonoids | Kampherol | Myricetin | Quercetin | Isorhamnetin | Hesperetin | Naringenin | Eriodictyol | Apigenin | Luteolin |
|---|---|---|---|---|---|---|---|---|---|
| Denmark | 4 | 2 | 12 | 1 | 8 | 3 | 1 | 2 | 1 |
| Finland | 3 | 2 | 10 | 2 | 19 | 7 | 3 | 2 | 1 |
| Sweden | 3 | 1 | 12 | 1 | 12 | 5 | 2 | 1 | 1 |
| Northern Region | 4 | 2 | 11 ± 1 | 1 | 13 ± 3 | 5 ± 1 | 2 ± 1 | 2 | 1 |
| Belgium | 3 | 1 | 12 | 2 | 7 | 3 | 1 | 2 | 1 |
| Czech Republic | 4 | 1 | 9 | 3 | 6 | 3 | 1 | 3 | 1 |
| Germany | 6 | 2 | 17 | 2 | 13 | 4 | 1 | 2 | 1 |
| Hungary | 5 | 2 | 14 | 3 | 7 | 3 | 1 | 3 | 1 |
| Ireland | 11 | 4 | 21 | 3 | 5 | 2 | 1 | 3 | 1 |
| Latvia | 4 | 2 | 17 | 2 | 3 | 1 | 0 | 8 | 1 |
| Netherlands | 8 | 3 | 18 | 2 | 12 | 4 | 2 | 1 | 1 |
| United Kingdom | 8 | 3 | 16 | 2 | 6 | 2 | 1 | 1 | 1 |
| Central Region | 6 ± 1 | 2 | 15 ± 1 | 2 | 7 ± 1 | 3 | 1 | 3 ± 1 | 1 |
| France | 3 | 2 | 11 | 2 | 6 | 3 | 1 | 6 | 1 |
| Italy | 4 | 1 | 13 | 3 | 12 | 6 | 2 | 9 | 2 |
| Spain | 2 | 1 | 10 | 2 | 10 | 4 | 2 | 1 | 1 |
| Southern Region | 3 | 1 | 11 ± 1 | 2 | 9 ± 2 | 4 ± 1 | 2 | 5 ± 2 | 1 |
| Europe | 5 ± 1 | 2 | 14 ± 1 | 2 | 9 ± 1 | 4 | 1 | 3 ± 1 | 1 |
3. Luteolin in management of pain in chronic inflammatory conditions
3.1. Chronic inflammation
Pain, along with redness, warmth, swelling are the characteristic symptoms of inflammation. Inflammation is an evolutionary conserved and protective reaction of the body against factors that are threatening its normal functioning and homeostasis. Acute inflammation is a high-grade type of inflammation, triggered either by the presence of pathogenic microorganisms or cellular damages caused by noxious stimuli. Pathogen-associated molecular patterns (PAMPs) are activated in case of infections from pathogens, whereas damage-associated molecular patterns (DAMPs) are activated in case of cellular damage. These molecules are recognized from immune cells such as macrophages and dendritic cells (DC), which in turn are activated to produce inflammatory enzymes, such as cyclooxygenase 2 (COX-2) producing prostaglandin E2 (PGE2), and cytokines, such as tumor necrosis factor-alpha (TNF-α), interleukin 6 (IL-6), and interleukin 1-beta (IL-1β) (17). Recruited neutrophils are further promoting the inflammatory state. All the inflammatory mediators are playing a key role in acute inflammatory pain, through activating the nociceptors (17–19).
After a reasonable period of time and under normal conditions, when threat is eliminated and healing of the tissue has been completed, the resolution of inflammation will be achieved and relative symptoms including pain, will stop. Various factors, including environmental and genetic parameters, can contribute to preserved chronic inflammation, usually activated by DAMPs (sterile type). Persistent, non-resolving inflammation can lead gradually to detrimental effects in tissues and organs' normal structure and functioning. Pain can be one of the symptoms where the underlying pathology is a low-grade, progressive inflammatory state (3, 17, 18). Chronic conditions like osteoarthritis, rheumatoid arthritis, inflammatory bowel disease, are characterized by pathological and persistent pain. This type of pain results from sensitization of nociceptors to the inflammatory mediators' stimuli in the foci of inflammation, causing a shift from their high-threshold to low-threshold activation.
In the absence of cure, inhibition of inflammation is the gold standard approach in management of chronic inflammatory conditions, in order to achieve prolonged remission state, and consequently relief of pain. Non-steroidal anti-inflammatory drugs (NSAIDs), analgesics, corticosteroids, opioids, immunobiological molecules are being used in chronic inflammatory pain. Clinicians' choice is based on pathogenesis of the inflammatory condition itself, as well as on outweighing the effectiveness of the protocols designed and the risks for side effects. However, there is still a need for more effective and safe agents that can complement the current therapies (8).
3.2. Anti-Inflammatory properties of luteolin in chronic inflammatory pain
Luteolin, shows pleiotropic actions in various pathways involved in chronic inflammation, and due to its safe profile can be considered as a promising adjuvant therapeutic choice in inhibition of inflammation and related pain.
Luteolin has shown important effects in regulation of inflammation, in both in vitro and in vivo studies. Its actions are mostly exerted by inhibiting several biochemical pathways and inflammatory mediators, which are involved in several chronic diseases where prolonged inflammation is the common denominator of pathogenesis.
The anti-inflammatory activities of luteolin were comprehensively reviewed in literature in two review papers (9, 10). The reader can refer to these reviews for very detailed information on preclinical evidence about the anti-inflammatory effects of luteolin in various in vitro cell lines and in vivo animal models. Authors described extensively the regulatory effects of luteolin on inflammatory mediators such as cytokines IL-6, IL-1β, TNF-α, enzyme COX-2 and prostaglandins PGEs. Moreover, luteolin can inhibit the increased expression of inducible nitric oxide synthase (iNOS) and metalloproteinases (MMPs) in chronic inflammatory conditions (9, 10).
Nitric oxide (NO) has various roles under normal conditions. It promotes vasodilation, works as a neurotransmitter, and regulator of immune response. However, in chronic inflammation, an inducible form of NO is synthesized by synthase iNOS, leading to nitric oxide overexpression, even 1,000 times more than its physiological production. In this case, nitric oxide acts as an inflammatory mediator (20, 21).
MMPs are a family of metalloproteinases, and their primary action is to degrade extracellular matrix proteins. Although they have been attributed with many physiological roles in various essential functions, such as cell proliferation, tissue repair, wound healing, and others, they are also involved in inflammatory processes. In particular, increased expression of various types of MMPs have been implicated in tissue remodeling and destruction in chronic inflammatory conditions (22, 23).
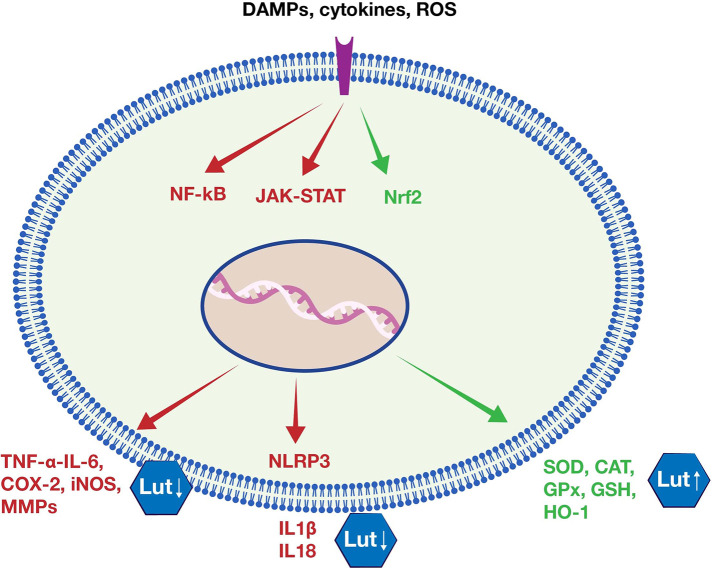
The inflammatory response requires the orchestrated activation of different but interacting signaling pathways. Among of those, nuclear factor kappa B (NF-κΒ), Janus kinase - signal transducer and activator of transcription (JAK-STAT), and inflammasome NOD-like receptor (NLR family) pyrin domain containing 3 (NLRP3) play important roles in expression of pro-inflammatory genes. NF-κB is considered a master transcription factor in both acute and chronic inflammation, involved in expression of pro-inflammatory cytokines, chemokines, adhesion molecules, iNOS, and metalloproteinases (MMPs) (24). JAK-STAT is another signaling pathway whose activation is implicated in auto-immune and inflammatory disorders. It is used as a signal transduction pathway from cytokines to promote the inflammatory response and it relates to NF-κΒ signaling pathway activation (25). NLRP3 is the most studied inflammasome, a multiprotein complex whose oligomerization activated by PAMPs or DAMPs, leads to maturation of the potent immune modulators IL-1β and IL-8 (26). Research studies showed that luteolin can beneficially regulate these pathways during the inflammatory process (10, 27–29), as summarized in Figure 5.
Figure 5.
Luteolin's anti-inflammatory and antioxidant effects. Luteolin inhibits major inflammatory signaling pathways (e.g., NF-κΒ, JAK-STAT, NLRP3), leading consequently to reduced expression of pro-inflammatory mediators (e.g., TNF-α, IL-6, COX-2, iNOS, MMPs, IL1β, IL18). Moreover, luteolin seems able to activate the major antioxidant factor Nrf2, and increase the expression of antioxidant enzymes (e.g., SOD, CAT, GPx, GSH, HO-1).
Based on its multi-targeted anti-inflammatory actions, luteolin appears to be a very promising natural agent for inhibiting abnormal inflammatory responses in chronic inflammatory conditions. In Table 3, we provide a summary of recent in vitro and in vivo studies, reflecting this flavonoid's potential effects on conditions characterized by significant pain during flares of the inflammatory diseases: Rheumatoid Arthritis, Osteoarthritis, and Inflammatory Bowel Disease.
Table 3.
Effects of luteolin on chronic inflammation as shown from in vitro and in vivo studies.
| in vitro/in vivo | Cell line/Animal model | Concentration/Dose and route of adminstration of luteolin | Effect | Refs |
|---|---|---|---|---|
| Rheumatoid Arthritis | ||||
| in vitro | Human synovial sarcoma cell line, SW982 | 1 or 10 μM | Inhibited IL-1b-induced MMPs (MMP-1 and -3), cytokines TNF-a and IL-6 | (27) |
| Rat FLS cells (RSC-364) | luteolin 25 μmol/l in combination with Chlorogenic acid | Inhibited the proliferation of fibroblast-like synoviocytes | (30) | |
| LPS-induced RAW 264.7 macrophages | 0.25–0.5 μM | Inhibited iNOS, IL-6 and TNF-α | (31) | |
| in vivo | Collagen Induced Arthritis (CIA) mice | co-ultramicronized PEA/Palmitoylethanolamide + luteolin 1 mg/kg, intraperitoneally | Reduced neutrophil and mast cell infiltration into the joints, improvements in locomotor activity and pain sensitivity | (32) |
| (FCA)-induced arthritis (AA) in male rats | 10 and 20 mg/kg intragastrically | Reduced, TNF-α, IL-6, IL-1β and IL-17, alleviated synovial hyperplasia, protected from joints destruction | (33) | |
| Osteoarthritis | ||||
| in vitro | rabbits cultured articular chondrocytes | 100 μM | Inhibited IL-1β MMP3 induced expression, and other degradative enzymes | (34) |
| in vivo | knee joint of rats on in vivo IL-1b-stimulated production of MMP-3 from articular cartilage tissues. | 100 μM injection into the right knee joint | Inhibited IL-1b-induced production level of MMP3 | |
| in vitro | interleukin (IL)-1β-stimulated rat chon- drocyte | 25, 50, 100 μΜ | Reduced iNOS, NO, PGE2, COX-2, TNF-α, MMP1, MMP2, MMP3, MMP-8, MMP-9, MMP13, reversed collagen II degradation | (35) |
| in vivo | MIA-induced mice model of OA | 10 mg/kg daily by gavage | Alleviated articular cartilage destruction | |
| Inflammatory Bowel Disease | ||||
| in vitro | Human mast cells - HMC-1 | 50 μM | Inhibited TNF-α, IL-8, IL-6, GM-CSF, and COX-2 | (36) |
| Human colon epithelial cells - HT-29 | 50 and 100 μM | Inhibited IL-8, COX-2, iNOS | (28) | |
| in vivo | DSS-induced colitis mouse model | 8 mg/kg oral administration | Inhibited IL-17 pathway | (37) |
| DSS-induced colitis mouse mode | 50 mg/kg by gastric gavage | Reduced IL-1β IL-6 | (38) | |
Antioxidant properties of luteolin could also contribute to inhibition of chronic inflammation. Oxidative stress and inflammation are closely related. In fact, evidence shows that there is a bidirectional relationship between excessive free radicals' production and inflammation. Chronic inflammatory conditions are characterized by oxidative stress, and vice versa. Excessive production of free radicals is implicated in the pathogenetic triggers of chronic inflammation: It leads gradually to accumulation of damages to fundamental components of cells, such as DNA, lipids and proteins, and induces activation of inflammatory pathways, such as the NF-κΒ (39, 40).
Flavonoids are potent antioxidant agents (41). Luteolin has shown important antioxidant actions, acting directly, via its ability to scavenge free radicals, but also by inducing endogenous antioxidant cell defenses. In various in vitro and in vivo experiments, it was found that luteolin promotes increased production of antioxidant enzymes (9). One of the main pathways involved in this pathway is suggested to be the activation of the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway (Figure 5) (42).
More detailed information on Luteolin's antioxidant capacity will be discussed in the next section about Neuropathic pain, where oxidative stress seems to play a primary role in pathogenesis.
4. Luteolin in management of neuropathic pain
4.1. Neuropathic pain
Neuropathic pain is the type of pain that results from damage or disease of the somatosensory nervous system. Its origin can be either peripheral (e.g., peripheral nerve injury pain, chemotherapy induced, cancer pain, diabetes peripheral neuropathy, alcoholic neuropathy) or central (e.g., spinal cord or brain injury, stroke, neurodegenerative disease). Though the pathogenetic mechanisms have not been fully elucidated, there is growing evidence that nitrosative and oxidative stress, along with neuroinflammation, have crucial roles in neuropathic pain.
Oxidative stress and nitrosative stress represent the loss of redox balance in the cells, due to high levels of reactive oxygen species (ROS) and reactive nitrogen species (RNS) respectively. ROS, produced by nicotinamide adenine dinucleotide phosphate (NAPDH) oxidase (NOX) enzymes, and RNS, produced by iNOS enzymes, are generated constantly during normal metabolic cellular processes. When in low concentrations they have various protective roles, e.g., contributing to a normal immune response, but they are potentially detrimental when highly increased. ROS and RNS can easily react with other molecules, causing important oxidative damages to components which are fundamental for cells' healthy functioning and survival (lipid peroxidation, protein and DNA oxidation) (43–45).
Cells normally have endogenous defenses to maintain concentrations of these reactive species under control (redox balance), including a series of antioxidant enzymes, such as superoxide dismutases (SODs), catalase (CAT), glutathione (GSH), heme oxygenase 1 (HO-1). The transcription factor Nrf2 is considered the governor for the expression of more than 200 antioxidants enzymes. However, this antioxidative ability can be compromised as a result of disease or injury, due to excessive production of ROS and NOS (45, 46).
Nerve cells are more vulnerable to nitro-oxidative damage, because they have weaker antioxidant defenses mechanisms and higher lipid content, compared to other types of cells. Therefore, nitro-oxidative stress in neurons can be considered as a primary cause of neuropathic pain. It can lead to mitochondrial dysfunction - further increasing ROS levels - and induce pro-inflammatory cytokines production. Moreover, ROS have been involved in the enhancement of excitatory signaling of nociceptive nervous cells. They can induce excitatory N-methyl-D-aspartate (NMDA) receptors, inhibiting glutamatergic regulation, and contribute to reduction of GABA-ergic inhibitory signaling. In addition, ROS seem to contribute to activation of the ion channels TRP, which are highly expressed in nociceptors neurons and have an important role in transduction of pain (44, 47).
Apart from damage caused directly by ROS and NOS, neuroinflammation seems to be a key process in induction and maintenance of neuropathic pain. Neuroinflammation is the type of inflammation triggered by damage of neuronal tissue in the peripheral or central nervous system. When the peripheral nerve is damaged, resident immune cells (e.g., mast cells and macrophages), along with immune cells recruited from blood circulation (e.g., neutrophils and T-cells) release inflammatory mediators, such as cytokines IL-1b and TNF-a, prostaglandins, and ROS. Moreover, microglia and astrocytes in the spinal dorsal horn are activated, which in turn contribute to the release of inflammatory mediators in the central nervous system and enhancement of excitability. This cascade of inflammatory events leads to peripheral and central sensitization (44, 48).
Both inflammation and nitro-oxidative stress shall not be considered as independent processes in neuropathic pain, as they rather interact in a bidirectional relationship.
Excessive ROS and RNS induce neuronal inflammatory response and vice versa. For example, the transcriptional factor NF-κΒ is one of the most characteristic examples of an inflammatory biochemical pathway that can be activated by ROS/RNS leading to increased expression of inflammatory mediators, but also itself can result to increased nitro-oxidative species production (44, 48, 49).
Pharmacological options currently available for neuropathic pain target the pain as a symptom, rather than inhibit the causes of its pathogenesis. First line drugs include tricyclic antidepressants, serotonin-norepinephrine reuptake inhibitors, gabapentin and pregabalin. Second-line therapy includes tramadol, capsaicin patches, and lidocaine patches. Last line of choice are strong opioids. Due to their inadequate efficacy and important side effects in long-term use of these drugs, effective management of neuropathic pain seems to be quite difficult. An outcome of 30%–50% pain reduction is considered meaningful (50, 51), with a significant number of patients, even >50% in relative surveys, reporting insufficient management of their painful symptoms (52, 53).
Direct antioxidants (e.g., Vitamins C, E, coenzyme Q) have been tried but clinical outcomes failed to support their use (α-Lipoic acid seems however to have therapeutic efficacy in case of diabetic neuropathy). It is worth noting that apart from their unfavorable pharmacokinetic and pharmacodynamic profile, another possible reason for the failure of direct antioxidants is that they may interfere with the physiological (and protective) functions of ROS (44, 45).
Newer and safer approaches that could target selectively multiple pathways may be more effective, protecting from nitro-oxidative damage and at the same time inhibiting neuroinflammation.
4.2. Neuroprotective and analgesic properties of luteolin in neuropathic pain
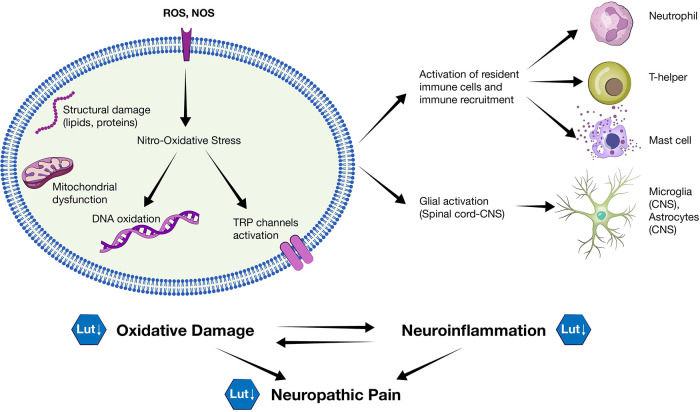
Luteolin is a very promising agent against neuropathic pain, due to its effects on two molecular mechanisms involved in neuropathy pathogenesis: oxidative stress and inflammation. Its potent antioxidant abilities, its actions against neuroinflammation, along with its analgesic effects, justify luteolin as a possible complementary therapeutic compound in neuropathy (Figure 6).
Figure 6.
Oxidative damage and neuroinflammation in neuropathy. An illustration of the pathogenetic mechanisms implicated in neuropathic pain. ROS and NOS can induce nitro-oxidative damage in the vulnerable neuronal cells and activation of TRP channels which are involved in neuropathic pain transduction. Neuroinflammation - induced from neuronal cell damage - involves the activation of resident immune cells and glial activation. Oxidative damage and neuroinflammation, which are considered to be reciprocal processes, can be inhibited by luteolin.
Luteolin's antioxidant properties are well established. It works as a direct free radical scavenger, due to the presence of the hydroxyl groups in the B ring that act as an electron-donating system. In addition, it has been shown that luteolin can induce the enhancement of endogenous antioxidant capacity. It can lead to increased expression of antioxidant enzymes, such as SOD, CAT, glutathione peroxidase (GPx), GSH, HO-1, NADPH quinone dehydrogenase 1 (NQO1). This particular action is of very special interest in the case of neuropathic pain (9, 42, 54–56). As mentioned previously, direct antioxidants may not be as effective as expected, and current research has turned its focus on agents that induce endogenous antioxidant mechanisms, rather than just neutralize directly the free radicals. Luteolin seems to achieve this action by activating the major antioxidant transcription factor Nrf2. Moreover, luteolin has been extensively researched for its neuroprotective effects. It appears that it can cross the blood-brain barrier and exert important antioxidant and anti-inflammatory effects in the brain (57–59).
In various preclinical studies, it has been shown that luteolin has a pleiotropic function against mechanisms involved in neurodegenerative diseases, such as Alzheimer's, Parkinson's, and Multiple Sclerosis. It can prevent the activation of microglial and astrocytes, the upregulation of inflammatory mediators, such as cytokines and iNOS, and can also reduce oxidative stress. Again, the major signaling pathways on which Luteolin acts, inducing this anti-inflammatory and antioxidant profile, include the inhibition of NF-κΒ and induction of Nrf2 respectively, among others (11, 60, 61).
Even though the neuroprotective role of luteolin in the central nervous system is well established from in vitro and in vivo studies, its benefits on peripheral neuroinflammation still lack evidence. However, as one would notice from the present review, the pathways involved in inflammatory response and oxidative damage are universal, independently of the tissue damaged. Therefore, we can suspect that luteolin will most likely act beneficially also against damages of the peripheral nervous system. In fact, the limited but important in vivo studies about luteolin's effect on neuropathic pain are very encouraging (Table 4).
Table 4.
In vivo studies of luteolin's analgesic effects in neuropathic models.
| Animal model | Dose and route of administration of luteolin | Effect | Refs |
|---|---|---|---|
| male Sprague–Dawley rats | intrathecally administered luteolin 1.5 mg - intraperitoneally administered luteolin 50 mg/kg | Attenuated mechanical and cold hyperalgesia | (62) |
| diabetic rats - neuropathy induced by intraperitoneal injection of streptozotocin in rats | intraperitoneally 50 mg/kg, 100 mg/kg and 200 mg/kg | improved the impaired nerve functions, activated Nrf2, increased activities of antioxidant enzymes SOD, GST, GPx and CAT, and reduced ROS production, attenuated hyperalgesia and allodynia in a dose dependent manner | (63) |
| sciatic nerve ligated mice luteolin | intraperitoneally 5 mg/kg and 10 mg/kg body weight | reduced hyperalgesia in acute and chronic phase of formalin test | (64) |
| Lewis's lung cancer induced bone pain in mice | intraperitoneally 10 mg/kg and 50 mg/kg | ameliorated pain in the pain behavior test in a dose-dependent manner, inhibited activation of glial cells in spinal dorsal root and NLRP3 inflammasome | (65) |
Luteolin could attenuate in a dose dependent manner, the mechanical and cold hyperalgesia in an animal model of neuropathic pain (62). The researchers also investigated the mechanism of its antinociceptive action. Antihyperalgesic effects of luteolin (intrathecally administered luteolin 1.5 mg or intraperitoneally administered luteolin 50 mg/kg) were inhibited by intrathecal pretreatment with the γ-aminobutyric acid A (GABAA) receptor antagonist bicuculline and μ-opioid receptor antagonist naloxone, but not by intrathecal pretreatment with either the benzodiazepine receptor antagonist flumazenil or glycine receptor antagonist strychnine.
In a diabetic neuropathy model, luteolin was able to improve the impaired nerve functions (63). Intraperitoneally daily treatment with luteolin (50 mg/kg, 100 mg/kg and 200 mg/kg) induced activation of Nrf2, increased activities of antioxidant enzymes SOD, GST, GPx and CAT, and reduced ROS production. Nerve function, as measured by increase of motor and sensory conduction velocities were also improved with luteolin treatment. Moreover, mechanical withdrawal threshold, cold, and heat withdrawal latencies were increased, indicating that luteolin can attenuate hyperalgesia and allodynia. All results were induced in a dose dependent manner, with doses of 100 mg/kg and 200 mg/kg of luteolin treatment leading to a significantly better outcome than dose of 50 mg/kg.
Administration of luteolin dose (5 mg/kg and 10 mg/kg body weight) significantly reduced neuropathic pain in another animal model. Interestingly, co-administration of luteolin and morphine (1 mg/kg), potentiated morphine analgesic effects (64).
Intraperitoneally treatment with luteolin ameliorated Lewis's lung cancer (LLC)-induced bone pain in mice in a dose-dependent manner (65) (50 mg/kg significantly improvement in pain behavior, than 1 mg/kg and 10 mg/kg). Bone cancer pain (BCP) is a severe complication in cancer patients with both inflammatory and neuropathic components. Intrathecal injection of luteolin 0.1 mg also reduced BCP in this study. Further analysis about the mechanism of action, showed that luteolin inhibited important components of neuroinflammation, such as activation of glial cells in spinal dorsal root and NLRP3 inflammasome.
5. Discussion
Pain management, especially in chronic conditions, remains a major challenge for clinicians. In case of a chronic inflammatory disorder, the goal of treatment is to inhibit the inflammatory process, prolong, and increase frequency of remission stages, and consequently reduce pain symptoms. In neuropathic pain, management is mainly symptomatic, targeting the pain as a symptom rather than the pathogenetic mechanisms involved. In either case, we are looking mostly at management rather than therapeutic approaches. Moreover, the drugs used are associated with important side effects in long term use, which raises the need for more efficient and safer options (8).
In this paper, we reviewed the potential beneficial effects of a flavone, luteolin, in pain management. Luteolin has potent anti-inflammatory actions, inhibiting important mediators of inflammation, that are also involved in pain, such as cytokines (e.g., TNF-a, IL-1, IL-6, IL-8) and enzymes (COX-2, iNOS). Luteolin also exhibits strong antioxidant activity, acting as a scavenger of free radicals, but also inducing expression of endogenous antioxidant enzymes (e.g., SOD, CAT, GPx, GSH) (9, 10). Since inflammatory process and nitro-oxidative damage are key mechanisms in both chronic inflammation and neuropathy, luteolin shows favorable effects in management of these conditions.
Moreover, the antinociceptive effects of flavonoids, including luteolin and its derivatives, have been observed in experiments even from earlier decades (7, 66–68). The mechanism of luteolin's effect on pain modulation process still remains unclear. There have been indications from in vitro and in vivo studies about its possible activity in GABA and opioid receptors. As mentioned previously in this review, analgesic effects of luteolin in a rat neuropathic model were inhibited by pretreatment with the γ-aminobutyric acid (GABAA) receptor antagonist bicuculline and μ-opioid receptor antagonist naloxone, but not by pretreatment with either the benzodiazepine receptor antagonist flumazenil or glycine receptor antagonist strychnine (62). The proposed mechanism that luteolin may act on GABAA receptor in a bicuculline-sensitive and flumazenil-insensitive manner has been further supported (69, 70). However, further studies are required to provide strong evidence on the involvement of luteolin effect on the GABAergic system and its activity profile at GABAA receptor subtypes. It will also be interesting to investigate any possible activity of luteolin on pain transmission, e.g., via modulation of ion channels activity, such as TRP, since other flavonoids have shown such effects (71).
However, from research studies to actual establishment luteolin as a therapeutic agent there are crucial parameters that need to be examined and fully clarified.
Bioavailability is one of these important factors. This measurement identifies the proportion of the substance that is available in the systemic circulation, taking into account the intestinal absorption and first-pass metabolism. Flavonoids, in general, are well known for their low oral bioavailability, because of their low water solubility and their rapid, and extensive first-pass metabolism. However, this does not necessarily reflect a low bioactivity. There is an evidence-based theory trying to explain the paradoxical phenomenon for polyphenols’ high bioactivity, in contrast to their low bioavailability. According to this theory, polyphenols, including flavonoids, could also exert their beneficial effects through their metabolites. In addition, prolonged bioavailability due to flavonoids' enterohepatic recycling, can contribute to their bioactivity (72, 73).
Luteolin, after its efficient absorption in intestinal epithelium, is extensively metabolized via glucuronidation, sulphation, or methylation. Research data (in vivo and in humans) have shown that after oral administration, luteolin presents in systemic circulation mainly in the form of luteolin conjugates, with more abundant being the luteolin glucuronides and luteolin sulfates (74, 75). Apart from the known bioactivity of free luteolin, its metabolites seem to be also biologically active, with evidence on their anti-inflammatory activity (76, 77). Another interesting process that was identified in a study, is the hydrolysis of luteolin monoglucuronide to luteolin at the site of inflammation. β-glucuronidase released from neutrophils as a result of the inflammation, was able to transform the luteolin glucuronide conjugates to free luteolin (78). Moreover, there are indications for the enterohepatic recirculation of luteolin, as studies have shown a second peak of their concentration and prolonged presence of luteolin and its metabolites in plasma (74, 77). From these data we can conclude that despite luteolin's low bioavailability (e.g., ranging from 4,1% at dose of 50 mg/kg (79), to 17,5% at dose 200 mg/kg (77) in rats), luteolin metabolites and recycling can contribute to luteolin's bioactivity.
In any case, a variety of methods have been tried successfully to enhance water solubility, bioavailability, and efficacy of luteolin. For example, complexes of luteolin with cyclodextrin (physicochemical data) (80) and phospholipids (physicochemical and in vivo evidence) (81, 82) have shown promising results. Moreover, nanoencapsulation of luteolin in liposomes (83, 84), micelles (85) and nanoparticles (86) as well as utilization of a microemulsion system (87) were able to improve in vivo luteolin's bioavailability and efficacy. Undoubtedly, there is a need for clinical studies proving these effects in humans.
To increase the effectiveness of luteolin against inflammatory and neuropathic pain, an interesting approach is to investigate a possible synergistic action in combination with other flavonoids. Quercetin is one of the most studied flavonoids, including in vitro, in vivo, and human studies. Like luteolin, quercetin shows potent antioxidant (88), anti-inflammatory (89), and neuroprotective (90) actions. Clinical trials have provided promising evidence on quercetin's beneficial role in alleviating chronic inflammation (91). A randomized, double-blind, placebo-controlled study investigated quercetin effects (500 mg/day) in 50 women with rheumatoid arthritis. Inflammation, stiffness and pain significantly reduced (92). Moreover, extensive preclinical research supports quercetin's protective actions against neuroinflammation and neuropathy. In addition, quercetin exerts analgesic effects (93). Examples of other flavonoids, well known for their anti-inflammatory, antioxidant, and analgesic properties are apigenin, rutin, naringenin, genistein (8, 94).
Even though there is already strong evidence on the anti-inflammatory, antioxidant, and neuroprotective effects of luteolin from preclinical studies, results from clinical trials are only scarce. However, beneficial outcomes of these studies pave the way for further investigation of luteolin's effects on patients suffering from chronic inflammatory conditions and/or neuropathies.
A nutritional supplement containing a mixture of flavonoids, luteolin (100 mg), quercetin (70 mg), and rutin (30 mg), has shown to improve clinical outcome in children with autism spectrum disorder (ASD). Treatment with 2 capsules/20 kg of body weight for at least 4 weeks led to significant improvement in gastrointestinal and allergy symptoms in about 75% of the children, eye contact and attention in 50%, social interaction in 25%, and resumption of speech in about 10% (95). These results were followed by another clinical study, during which treatment with 1 capsule/10 kg of body weight for 26 weeks, resulted in significant benefit in ASD children both in adaptive functioning and behavioral difficulties (assessed by the Vineland Adaptive Behavior Scales, and Aberrant Behavior Checklist respectively) (96). Researchers collected and analyzed a series of serum inflammatory markers from the children with ASD who participated in the latter study, before and after treatment with luteolin supplementation. Control samples were gathered from healthy children unrelated to the ASD subjects. The analysis showed that cytokines TNF-α and IL-6 were elevated in ASD patients compared to controls, and treatment with the formula containing luteolin led to a significant decrease of these inflammatory indices. The decrease was observed in the children with ASD whose behavior improved the most after supplementation (97). It is worth noticing that this is the first study that provided clinical evidence about luteolin's effects on inflammatory markers in relation to a beneficial clinical outcome.
Another interesting observation is the potential synergistic and beneficial effect of luteolin with Palmitoylethanolamide (PEA). Preclinical and clinical studies have reported an anti-inflammatory and analgesic action of PEA, a naturally occurring lipid mediator molecule (98). A two-arms study was performed to investigate the neuroprotective effects of a co-ultramicronized composite containing PEA and luteolin (co-ultraPEALut). In the first arm, rats subjected to middle cerebral artery occlusion and treated with co-ultraPEALut showed reduced edema, improved neurobehavioral functions, and reduced neuroinflammation after treatment. In the second arm, researchers examined the effects of a pharmaceutical co-ultraPEALut preparation treatment for 60 days on a cohort of 250 stroke patients undergoing neurorehabilitation. At study end, indices of neurological status, cognitive abilities, degree of spasticity, pain, and independence in daily living showed significant gains (99). Despite this being an observational rather than a controlled clinical trial, it adds clinical evidence on luteolin's beneficial effects.
In efforts to manage pain in chronic conditions we shall also consider that anxiety and depression are frequent comorbidities. In fact, there is evidence that a bidirectional relationship exists between them. Increased pain perception may result because of a chronic condition, and vice versa. A chronic painful condition increases the risk for psychopathological disorders (6, 100, 101). Flavonoids, including luteolin, have shown anxiolytic actions (102, 103). Characteristic example is the case of Chamomilla matricaria, a herb used traditionally for centuries in anxiety and depression. Its anxiolytic effects are attributed to its content in the flavonoid apigenin (104, 105).
Apart from its bioavailability and efficacy, safety is also of utmost importance when considering any compound for therapeutic purposes. Flavonoids are generally considered quite safe compounds even in doses largely exceeding daily dietary intake (106). Oral administration of luteolin has shown LD50 values even above 5,000 mg/kg (10, 106). In the clinical trials mentioned previously, safety of oral supplementation with luteolin was also recorded (95–97, 99). However, there is still a need for further toxicological studies to fully determine luteolin safety profile.
This review summarized the key properties of luteolin englighting its potential in management of pain in chronic conditions.
-
•
Luteolin appears to be an excellent candidate for alleviating pain in chronic inflammatory conditions (e.g., rheumatoid arthritis, osteoarthritis, inflammatory bowel disease), inhibiting major inflammatory mediators involved in manifestation of pain as a symptom of the disease.
-
•
Based on its strong anti-inflammatory and antioxidant properties shown in preclinical studies, luteolin can inhibit the major components of pain pathogenesis in neuropathy, namely oxidative stress and neuroinflammation, that lead to nerve damage and chronic pain.
-
•
Moreover, as we described previously, there is evidence that it can also show analgesic effect via interaction with GABAA receptors.
-
•
Luteolin appears (from preclinical and clinical data) to have a very good safety profile, making it even more appealing for clinical implementation.
However there are limitations before it can be further developed into an analgesic drug for pain management. Additional studies in chronic inflammatory and neuropathic disease models should be conducted to expand knowledge about luteolin's efficacy, methods enhancing its bioavailability, mechanism of its direct analgesic action, and to ascertain its safety. Clinical trials specifically using luteolin as a complementary analgesic strategy in chronic inflammatory and neuropathic conditions are necessary to establish an effective and safe dose for patients.
In conclusion, luteolin is considered a very promising, safe, and effective natural compound in the management of pain, both in chronic inflammatory conditions and neuropathy. Further studies, especially clinical trials, are necessary to establish its role as an adjuvant agent in therapeutic protocols.
Funding Statement
Article processing fees were funded by “Whisper P.C.”.
Author contributions
Conceptualization, writing, review, editing, FN and AT. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpain.2023.1114428/full#supplementary-material.
References
- 1.https://www.iasp-pain.org/publications/iasp-news/iasp-announces-revised-definition-of-pain/?ItemNumber=10475 IASP Announces Revised Definition of Pain - International Association for the Study of Pain (IASP). Available at: (Cited October 11, 2022).
- 2.Johnson MI. The landscape of chronic pain: broader perspectives. Medicina (Lithuania). (2019) 55(5):182–201. 10.3390/medicina55050182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, et al. Chronic inflammation in the etiology of disease across the life span. Nat Med. (2019) 25(12):1822–32. 10.1038/s41591-019-0675-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ellison DL. Physiology of pain. Crit Care Nurs Clin North Am. (2017) 29(4):397–406. 10.1016/j.cnc.2017.08.001 [DOI] [PubMed] [Google Scholar]
- 5.Lee GI, Neumeister MW. Pain: pathways and physiology. Clin Plast Surg. (2020) 47(2):173–80. 10.1016/j.cps.2019.11.001 [DOI] [PubMed] [Google Scholar]
- 6.Cohen SP, Vase L, Hooten WM. Chronic pain: an update on burden, best practices, and new advances. Lancet. (2021) 397(10289):2082–97. 10.1016/S0140-6736(21)00393-7 [DOI] [PubMed] [Google Scholar]
- 7.Xiao X, Wang X, Gui X, Chen L, Huang B. Natural flavonoids as promising analgesic candidates: a systematic review. Chem Biodivers. (2016) 13(11):1427–40. 10.1002/cbdv.201600060 [DOI] [PubMed] [Google Scholar]
- 8.Ferraz CR, Carvalho TT, Manchope MF, Artero NA, Rasquel-Oliveira FS, Fattori V, et al. Therapeutic potential of flavonoids in pain and inflammation: mechanisms of action, pre-clinical and clinical data, and pharmaceutical development. Molecules. (2020) 25(3):762. 10.3390/molecules25030762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seelinger G, Merfort I, Schempp CM. Anti-oxidant, anti-inflammatory and anti-allergic activities of luteolin. Planta Med. (2008) 74(14):1667–77. 10.1055/s-0028-1088314 [DOI] [PubMed] [Google Scholar]
- 10.Aziz N, Kim MY, Cho JY. Anti-inflammatory effects of luteolin: a review of in vitro, in vivo, and in silico studies. J Ethnopharmacol. (2018) 225:342–58. 10.1016/j.jep.2018.05.019 [DOI] [PubMed] [Google Scholar]
- 11.Nabavi SF, Braidy N, Gortzi O, Sobarzo-Sanchez E, Daglia M, Skalicka-Woźniak K, et al. Luteolin as an anti-inflammatory and neuroprotective agent: a brief review. Brain Res Bull. (2015) 119:1–11. 10.1016/j.brainresbull.2015.09.002 [DOI] [PubMed] [Google Scholar]
- 12.Fitzcharles MA, Cohen SP, Clauw DJ, Littlejohn G, Usui C, Häuser W. Nociplastic pain: towards an understanding of prevalent pain conditions. Lancet. (2021) 397(10289):2098–110. 10.1016/S0140-6736(21)00392-5 [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Liu M, Cui W, Yang J, Yang B. Total synthesis of luteolin. J Chem Res. (2014) 38(1):60–1. 10.3184/174751914X13867643876192 [DOI] [Google Scholar]
- 14.Mu N, Ugli G, Teshaboy PA. Some flavonoids in the yarrow (Achillea Millefolium L.) plant and their effects on human health. AJSHR. (2021) 2(5):116–20. Available at: https://grnjournals.us/index.php/AJSHR [Google Scholar]
- 15.López-Lázaro M. Distribution and biological activities of the flavonoid luteolin. Med Chem (Los Angeles). (2009) 9(1):31–59. 10.2174/138955709787001712 [DOI] [PubMed] [Google Scholar]
- 16.Vogiatzoglou A, Mulligan AA, Lentjes MAH, Luben RN, Spencer JPE, Schroeter H, et al. Flavonoid intake in European adults (18 to 64 years). PLoS One. (2015) 10(5):e0128132. 10.1371/journal.pone.0128132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Netea MG, Balkwill F, Chonchol M, Cominelli F, Donath MY, Giamarellos-Bourboulis EJ, et al. A guiding map for inflammation. Nat Immunol. (2017) 18(8):826–31. 10.1038/ni.3790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kidd BL, Urban LA. Mechanisms of infammatory pain. Br J Anaesth. (2001) 87(1):3–11. 10.1093/bja/87.1.3 [DOI] [PubMed] [Google Scholar]
- 19.Zhang JM, An J. Cytokines, inflammation, and pain. Int Anesthesiol Clin. (2007) 45(2):27–37. 10.1097/AIA.0b013e318034194e [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma JN, Al-Omran A, Parvathy SS. Role of nitric oxide in inflammatory diseases. Inflammopharmacology. (2007) 15(6):252–9. 10.1007/s10787-007-0013-x [DOI] [PubMed] [Google Scholar]
- 21.Xue Q, Yan Y, Zhang R, Xiong H. Regulation of iNOS on immune cells and its role in diseases. Int J Mol Sci. (2018) 19(12):3805. 10.3390/ijms19123805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fingleton B. Matrix metalloproteinases as regulators of inflammatory processes. Biochim Biophys Acta Mol Cell Res. (2017) 1864(11):2036–42. 10.1016/j.bbamcr.2017.05.010 [DOI] [PubMed] [Google Scholar]
- 23.Serra R. Matrix metalloproteinases in health and disease. Biomolecules. (2020) 10(8):1–3. 10.3390/biom10081138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther. (2017) 2:17023. 10.1038/sigtrans.2017.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Banerjee S, Biehl A, Gadina M, Hasni S, Schwartz DM. JAK–STAT signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs. (2017) 77(5):521–46. 10.1007/s40265-017-0701-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozaki E, Campbell M, Doyle SL. Targeting the NLRP3 inflammasome in chronic inflammatory diseases: current perspectives. J Inflamm Res. (2015) 8:15–27. 10.2147/JIR.S51250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi EM, Lee YS. Luteolin suppresses IL-1β-induced cytokines and MMPs production via p38 MAPK, JNK, NF-kappaB and AP-1 activation in human synovial sarcoma cell line, SW982. Food Chem Toxicol. (2010) 48(10):2607–11. 10.1016/j.fct.2010.06.029 [DOI] [PubMed] [Google Scholar]
- 28.Nunes C, Almeida L, Barbosa RM, Laranjinha J. Luteolin suppresses the JAK/STAT pathway in a cellular model of intestinal inflammation. Food Funct. (2017) 8(1):387–96. 10.1039/C6FO01529H [DOI] [PubMed] [Google Scholar]
- 29.Zhang BC, Li Z, Xu W, Xiang CH, Ma YF. Luteolin alleviates NLRP3 inflammasome activation and directs macrophage polarization in lipopolysaccharide-stimulated RAW264.7 cells. Am J Transl Res. (2018) 10(1):265–73. Available at: www.ajtr.org PMID: ; PMCID: [PMC free article] [PubMed] [Google Scholar]
- 30.Lou L, Liu Y, Zhou J, Wei Y, Deng J, Dong B, et al. Chlorogenic acid and luteolin synergistically inhibit the proliferation of interleukin-1 β -induced fibroblast-like synoviocytes through regulating the activation of NF- B and JAK/STAT-signaling pathways. Immunopharmacol Immunotoxicol. (2015) 37(6):499–507. 10.3109/08923973.2015.1095763 [DOI] [PubMed] [Google Scholar]
- 31.Sun YW, Bao Y, Yu H, Chen QJ, Lu F, Zhai S, et al. Anti-rheumatoid arthritis effects of flavonoids from Daphne genkwa. Int Immunopharmacol. (2020) 83:106384. 10.1016/j.intimp.2020.106384 [DOI] [PubMed] [Google Scholar]
- 32.Impellizzeri D, Esposito E, di Paola R, Ahmad A, Campolo M, Peli A, et al. http://arthritis-research.com/content/15/6/R192 Palmitoylethanolamide and luteolin ameliorate development of arthritis caused by injection of collagen type II in mice (2013). Available at: [DOI] [PMC free article] [PubMed]
- 33.Shi F, Zhou D, Ji Z, Xu Z, Yang H. Anti-arthritic activity of luteolin in Freund's Complete adjuvant-induced arthritis in rats by suppressing P2X4 pathway. Chem Biol Interact. (2015) 226:82–7. 10.1016/j.cbi.2014.10.031 [DOI] [PubMed] [Google Scholar]
- 34.Kang BJ, Ryu J, Lee CJ, Hwang SC. Luteolin inhibits the activity, secretion and gene expression of MMP-3 in cultured articular chondrocytes and production of MMP-3 in the rat knee. Biomol Ther (Seoul). (2014) 22(3):239–45. 10.4062/biomolther.2014.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fei J, Liang B, Jiang C, Ni H, Wang L. Luteolin inhibits IL-1β-induced inflammation in rat chondrocytes and attenuates osteoarthritis progression in a rat model. Biomedicine and Pharmacotherapy. (2019) 109:1586–92. 10.1016/j.biopha.2018.09.161 [DOI] [PubMed] [Google Scholar]
- 36.Kang OH, Choi JG, Lee JH, Kwon DY. Luteolin isolated from the flowers of Lonicera japonica suppresses inflammatory mediator release by blocking NF-κB and MAPKs activation pathways in HMC-1 cells. Molecules. (2010) 15(1):385–98. 10.3390/molecules15010385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin TJ, Yin SY, Hsiao PW, Yang NS, Wang IJ. Transcriptomic analysis reveals a controlling mechanism for NLRP3 and IL-17A in dextran sulfate sodium (DSS)-induced colitis. Sci Rep. (2018) 8(1): 14927. 10.1038/s41598-018-33204-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li P, Wang Y, Luo J, Zeng Q, Wang M, Bai M, et al. Downregulation of OCTN2 by cytokines plays an important role in the progression of inflammatory bowel disease. Biochem Pharmacol. (2020) 178:114115. 10.1016/j.bcp.2020.114115 [DOI] [PubMed] [Google Scholar]
- 39.Khansari N, Shakiba Y, Mahmoudi M. Chronic inflammation and oxidative stress as a Major cause of age- related diseases and cancer. Recent Pat Inflamm Allergy Drug Discov. (2009) 3(1):73–80. 10.2174/187221309787158371 [DOI] [PubMed] [Google Scholar]
- 40.Lingappan K. NF-κB in oxidative stress. Curr Opin Toxicol. (2018) 7:81–6. 10.1016/j.cotox.2017.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hussain T, Tan B, Yin Y, Blachier F, Tossou MCB, Rahu N. Oxidative stress and inflammation: what polyphenols can do for us? Oxid Med Cell Longev. (2016) 2016:7432797. 10.1155/2016/7432797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kitakaze T, Makiyama A, Yamashita Y, Ashida H. Low dose of luteolin activates Nrf2 in the liver of mice at start of the active phase but not that of the inactive phase. PLoS One. (2020) 15(4):e0231403. 10.1371/journal.pone.0231403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Costigan M, Scholz J, Woolf CJ. Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci. (2009) 32:1–32. 10.1146/annurev.neuro.051508.135531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Teixeira-Santos L, Albino-Teixeira A, Pinho D. Neuroinflammation, oxidative stress and their interplay in neuropathic pain: focus on specialized pro-resolving mediators and NADPH oxidase inhibitors as potential therapeutic strategies. Pharmacol Res. (2020) 162:105280. 10.1016/j.phrs.2020.105280 [DOI] [PubMed] [Google Scholar]
- 45.Grace PM, Gaudet AD, Staikopoulos V, Maier SF, Hutchinson MR, Salvemini D, et al. Nitroxidative signaling mechanisms in pathological pain. Trends Neurosci. (2016) 39(12):862–79. 10.1016/j.tins.2016.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saha S, Buttari B, Panieri E, Profumo E, Saso L. An overview of Nrf2 signaling pathway and its role in inflammation. Molecules. (2020) 25(22):5474. 10.3390/molecules25225474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Carrasco C, Naziroglu M, Rodríguez AB, Pariente JA. Neuropathic pain: delving into the oxidative origin and the possible implication of transient receptor potential channels. Front Physiol. (2018) 9:95. 10.3389/fphys.2018.00095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ellis A, Bennett DLH. Neuroinflammation and the generation of neuropathic pain. Br J Anaesth. (2013) 111(1):26–37. 10.1093/bja/aet128 [DOI] [PubMed] [Google Scholar]
- 49.Morgan MJ, Liu ZG. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. (2011) 21(1):103–15. 10.1038/cr.2010.178. Available at: https://www.nature.com/articles/cr2010178 (Cited October 11, 2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cohen K, Shinkazh N, Frank J, Israel I, Fellner C. Pharmacological treatment of diabetic peripheral neuropathy. Pharmacy and Therapeutics. (2015) 40(6):375–88. PMID: ; PMCID: [PMC free article] [PubMed] [Google Scholar]
- 51.Finnerup NB, Attal N, Haroutounian S, McNicol E, Baron R, Dworkin RH, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. (2015) 14(2):162–73. 10.1016/S1474-4422(14)70251-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. (2006) 10(4):287. 10.1016/j.ejpain.2005.06.009 [DOI] [PubMed] [Google Scholar]
- 53.Holbrook T, Obradovic M, Liedgens H. Treatment satisfaction and its association with health outcomes in patients with neuropathic pain. Value Health. (2013) 16(7):A388. 10.1016/j.jval.2013.08.377 [DOI] [Google Scholar]
- 54.Park CM, Park JY, Noh KH, Shin JH, Song YS. Taraxacum officinale weber extracts inhibit LPS-induced oxidative stress and nitric oxide production via the NF-κB modulation in RAW 264.7 cells. J Ethnopharmacol. (2011) 133(2):834–42. 10.1016/j.jep.2010.11.015 [DOI] [PubMed] [Google Scholar]
- 55.Crascì L, Cardile V, Longhitano G, Nanfitò F, Panico A. Anti-degenerative effect of apigenin, luteolin and quercetin on human keratinocyte and chondrocyte cultures: sAR evaluation. Drug Res. (2018) 68(3):132–8. 10.1055/s-0043-120662 [DOI] [PubMed] [Google Scholar]
- 56.Rajput SA, Shaukat A, Wu K, Rajput IR, Baloch DM, Akhtar RW, et al. Luteolin alleviates aflatoxinb1-induced apoptosis and oxidative stress in the liver of mice through activation of nrf2 signaling pathway. Antioxidants. (2021) 10(8):1268. 10.3390/antiox10081268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang YC, Gan FF, Shelar SB, Ng KY, Chew EH. Antioxidant and Nrf2 inducing activities of luteolin, a flavonoid constituent in Ixeris sonchifolia Hance, provide neuroprotective effects against ischemia-induced cellular injury. Food Chem Toxicol. (2013) 59:272–80. 10.1016/j.fct.2013.05.058 [DOI] [PubMed] [Google Scholar]
- 58.Nazari QA, Kume T, Takada-Takatori Y, Izumi Y, Akaike A. Protective effect of luteolin on an oxidative-stress model induced by microinjection of sodium nitroprusside in mice. J Pharmacol Sci. (2013) 122(2):109–17. 10.1254/jphs.13019FP [DOI] [PubMed] [Google Scholar]
- 59.Yao ZH, Yao XL, Zhang Y, Zhang SF, Hu JC. Luteolin could improve cognitive dysfunction by inhibiting neuroinflammation. Neurochem Res. (2018) 43(4):806–20. 10.1007/s11064-018-2482-2 [DOI] [PubMed] [Google Scholar]
- 60.Daily JW, Kang S, Park S. Protection against Alzheimer's Disease by luteolin: role of brain glucose regulation, anti-inflammatory activity, and the gut microbiota-liver-brain axis. BioFactors. (2021) 47(2):218–31. 10.1002/biof.1703 [DOI] [PubMed] [Google Scholar]
- 61.Siddique YH. Role of luteolin in overcoming Parkinson's Disease. BioFactors. (2021) 47(2):198–206. 10.1002/biof.1706 [DOI] [PubMed] [Google Scholar]
- 62.Hara K, Haranishi Y, Terada T, Takahashi Y, Nakamura M, Sata T. Effects of intrathecal and intracerebroventricular administration of luteolin in a rat neuropathic pain model. Pharmacol Biochem Behav. (2014) 125:78–84. 10.1016/j.pbb.2014.08.011 [DOI] [PubMed] [Google Scholar]
- 63.Li M, Li Q, Zhao Q, Zhang J, Lin J. Luteolin improves the impaired nerve functions in diabetic neuropathy: behavioral and biochemical evidences. Int J Clin Exp Pathol. (2015) 8(9):10112–20. PMID: ; PMCID: [PMC free article] [PubMed] [Google Scholar]
- 64.Hashemzaei M, Abdollahzadeh M, Iranshahi M, Golmakani E, Rezaee R, Tabrizian K. Effects of luteolin and luteolin-morphine co-administration on acute and chronic pain and sciatic nerve ligated-induced neuropathy in mice. J Complement Integr Med. (2017) 14(1):20160066. 10.1515/jcim-2016-0066 [DOI] [PubMed] [Google Scholar]
- 65.Zhou YS, Cui Y, Zheng JX, Quan YQ, Wu SX, Xu H, et al. Luteolin relieves lung cancer-induced bone pain by inhibiting NLRP3 inflammasomes and glial activation in the spinal dorsal horn in mice. Phytomedicine. (2022) 96:153910. 10.1016/j.phymed.2021.153910 [DOI] [PubMed] [Google Scholar]
- 66.Block LC, Santos ARS, Maria De Souza M, Scheidt C, Yunes RA, Santos A, et al. Chemical and pharmacological examination of antinociceptive constituents of Wedelia paludosa. J Ethnopharmacol. (1998) 61:85–9. 10.1016/S0378-8741(98)00019-1 [DOI] [PubMed] [Google Scholar]
- 67.Ramesh M, Rao YN, Rao AVNA, Prabhakar MC, Rao CS, Muralidhar N, et al. Antinociceptive and anti-inflammatory activity of a flavonoid isolated from Caralluma attenuata. J Ethnopharmacol. (1998) 62:63–6. 10.1016/S0378-8741(98)00048-8 [DOI] [PubMed] [Google Scholar]
- 68.Backhouse N, Rosales L, Apablaza C, Goïty L, Erazo S, Negrete R, et al. Analgesic, anti-inflammatory and antioxidant properties of buddleja globosa, buddlejaceae. J Ethnopharmacol. (2008) 116(2):263–9. 10.1016/j.jep.2007.11.025 [DOI] [PubMed] [Google Scholar]
- 69.Raines T, Jones P, Moe N, Duncan R, McCall S, Ceremuga TE. Investigation of the anxiolytic effects of luteolin, a lemon balm flavonoid in the male sprague–dawley rat. AANA J. (2009) 77:33–6. PMID: [PubMed] [Google Scholar]
- 70.de la Peña JB, Kim CA, Lee HL, Yoon SY, Kim HJ, Hong EY, et al. Luteolin mediates the antidepressant-like effects of Cirsium japonicum in mice, possibly through modulation of the GABAA receptor. Arch Pharm Res. (2014) 37(2):263–9. 10.1007/s12272-013-0229-9 [DOI] [PubMed] [Google Scholar]
- 71.Abbas MA. Modulation of TRPV1 channel function by natural products in the treatment of pain. Chem Biol Interact. (2020) 330:109178. 10.1016/j.cbi.2020.109178 [DOI] [PubMed] [Google Scholar]
- 72.Zeng M, Sun R, Basu S, Ma Y, Ge S, Yin T, et al. Disposition of flavonoids via recycling: direct biliary excretion of enterically or extrahepatically derived flavonoid glucuronides. Mol Nutr Food Res. (2016) 60(5):1006–19. 10.1002/mnfr.201500692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Luca SV, Macovei I, Bujor A, Miron A, Skalicka-Woźniak K, Aprotosoaie AC, et al. Bioactivity of dietary polyphenols: the role of metabolites. Crit Rev Food Sci Nutr. (2020) 60(4):626–59. 10.1080/10408398.2018.1546669 [DOI] [PubMed] [Google Scholar]
- 74.Deng C, Gao C, Tian X, Chao B, Wang F, Zhang Y, et al. Pharmacokinetics, tissue distribution and excretion of luteolin and its major metabolites in rats: metabolites predominate in blood, tissues and are mainly excreted via bile. J Funct Foods. (2017) 35:332–40. 10.1016/j.jff.2017.05.056 [DOI] [Google Scholar]
- 75.Taheri Y, Sharifi-Rad J, Antika G, Yilmaz YB, Tumer TB, Abuhamdah S, et al. Paving luteolin therapeutic potentialities and agro-food-pharma applications: emphasis on in vivo pharmacological effects and bioavailability traits. Oxid Med Cell Longev. (2021) 2021:1987588. 10.1155/2021/1987588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kure A, Nakagawa K, Kondo M, Kato S, Kimura F, Watanabe A, et al. Metabolic fate of luteolin in rats: its relationship to anti-inflammatory effect. J Agric Food Chem. (2016) 64(21):4246–54. 10.1021/acs.jafc.6b00964 [DOI] [PubMed] [Google Scholar]
- 77.Hayasaka N, Shimizu N, Komoda T, Mohri S, Tsushida T, Eitsuka T, et al. Absorption and metabolism of luteolin in rats and humans in relation to in vitro anti-inflammatory effects. J Agric Food Chem. (2018) 66(43):11320–9. 10.1021/acs.jafc.8b03273 [DOI] [PubMed] [Google Scholar]
- 78.Shimoi K, Saka N, Kaji K, Nozawa R, Kinae N. Metabolic fate of luteolin and its functional activity at focal site. BioFactors. (2000) 12:181–6. 10.1002/biof.5520120129 [DOI] [PubMed] [Google Scholar]
- 79.Sarawek S, Derendorf H, Butterweck V. Pharmacokinetics of luteolin and metabolites in rats. Nat Prod Commun. (2008) 3(12):128–32. 10.1177/1934578X0800301218 [DOI] [Google Scholar]
- 80.Liu B, Li W, Zhao J, Liu Y, Zhu X, Liang G. Physicochemical characterisation of the supramolecular structure of luteolin/cyclodextrin inclusion complex. Food Chem. (2013) 141(2):900–6. 10.1016/j.foodchem.2013.03.097 [DOI] [PubMed] [Google Scholar]
- 81.Xu K, Liu B, Ma Y, Du J, Li G, Gao H, et al. Physicochemical properties and antioxidant activities of luteolin-phospholipid complex. Molecules. (2009) 14(9):3486–93. 10.3390/molecules14093486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Khan J, Saraf S, Saraf S. Preparation and evaluation of luteolin-phospholipid complex as an effective drug delivery tool against GalN/LPS induced liver damage. Pharm Dev Technol. (2016) 21:475–86. 10.3109/10837450.2015.1022786 [DOI] [PubMed] [Google Scholar]
- 83.Li J, Cheng X, Chen Y, He W, Ni L, Xiong P, et al. Vitamin E TPGS modified liposomes enhance cellular uptake and targeted delivery of luteolin: an in vivo/in vitro evaluation. Int J Pharm. (2016) 512(1):262–72. 10.1016/j.ijpharm.2016.08.037 [DOI] [PubMed] [Google Scholar]
- 84.Wu G, Li J, Yue J, Zhang S, Yunusi K. Liposome encapsulated luteolin showed enhanced antitumor efficacy to colorectal carcinoma. Mol Med Rep. (2018) 17(2):2456–64. 10.3892/mmr.2017.8185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zheng S, Cheng Y, Teng Y, Liu X, Yu T, Wang Y, et al. Application of luteolin nanomicelles anti-glioma effect with improvement in vitro and in vivo. Oncotarget. (2017) 8(37):61146–62. 10.18632/oncotarget.18019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Majumdar D, Jung KH, Zhang H, Nannapaneni S, Wang X, Amin AR, et al. Luteolin nanoparticle in chemoprevention: in vitro and in vivo anticancer activity. Cancer Prev Res (Phila). (2014) 7(1):65–73. 10.1158/1940-6207.CAPR-13-0230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Miyashita A, Ito J, Parida IS, Naoki Syoji N, Fujii T, Takahashi H, et al. Improving water dispersibility and bioavailability of luteolin using microemulsion system. Sci Rep. (2022) 12:11949. 10.1038/s41598-022-16220-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Xu D, Hu MJ, Wang YQ, Cui YL. Antioxidant activities of quercetin and its complexes for medicinal application. Molecules. (2019) 24(6):1123. 10.3390/molecules24061123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li Y, Yao J, Han C, Yang J, Chaudhry MT, Wang S, et al. Quercetin, inflammation and immunity. Nutrients. (2016) 8(3):167. PMID: ; PMCID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Costa LG, Garrick JM, Roquè PJ, Pellacani C. Mechanisms of neuroprotection by quercetin: counteracting oxidative stress and more. Oxid Med Cell Longev. (2016) 2016:2986796. 10.1155/2016/2986796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ulusoy HG, Sanlier N. A minireview of quercetin: from its metabolism to possible mechanisms of its biological activities. Crit Rev Food Sci Nutr. (2020) 60(19):3290–303. 10.1080/10408398.2019.1683810 [DOI] [PubMed] [Google Scholar]
- 92.Javadi F, Ahmadzadeh A, Eghtesadi S, Aryaeian N, Zabihiyeganeh M, Rahimi Foroushani A, et al. The effect of quercetin on inflammatory factors and clinical symptoms in women with rheumatoid arthritis: a double-blind, randomized controlled trial. J Am Coll Nutr. (2017) 36(1):9–15. 10.1080/07315724.2016.1140093 [DOI] [PubMed] [Google Scholar]
- 93.Carullo G, Cappello AR, Frattaruolo L, Badolato M, Armentano B, Aiello F. Quercetin and derivatives: useful tools in inflammation and pain management. Future Med Chem. (2017) 9(1):79–93. 10.4155/fmc-2016-0186 [DOI] [PubMed] [Google Scholar]
- 94.Uddin MS, al Mamun A, Rahman MA, Kabir MT, Alkahtani S, Alanazi IS, et al. Exploring the promise of flavonoids to combat neuropathic pain: from molecular mechanisms to therapeutic implications. Front Neurosci. (2020) 14:1–18. 10.3389/fnins.2020.00478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Theoharides RC, Asadp S, Panagiotidou S. A case series of a luteolin formulation (NeuroProtek®) in children with autism spectrum disorders. Int J Immunopathol Pharmacol. (2012) 25(2):317–23. 10.1177/039463201202500201 [DOI] [PubMed] [Google Scholar]
- 96.Taliou A, Zintzaras E, Lykouras L, Francis K. An open-label pilot study of a formulation containing the anti-inflammatory flavonoid luteolin and its effects on behavior in children with autism spectrum disorders. Clin Ther. (2013) 35(5):592–602. 10.1016/j.clinthera.2013.04.006 [DOI] [PubMed] [Google Scholar]
- 97.Tsilioni I, Taliou A, Francis K, Theoharides TC. Children with autism spectrum disorders, who improved with a luteolin-containing dietary formulation, show reduced serum levels of TNF and IL-6. Transl Psychiatry. (2015) 5(9):e647. 10.1038/tp.2015.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cordaro M, Cuzzocrea S, Crupi R. An update of palmitoylethanolamide and luteolin effects in preclinical and clinical studies of neuroinflammatory events. Antioxidants. (2020) 9(3):216. 10.3390/antiox9030216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Caltagirone C, Cisari C, Schievano C, di Paola R, Cordaro M, Bruschetta G, et al. Co-ultramicronized palmitoylethanolamide/luteolin in the treatment of cerebral ischemia: from rodent to man. Transl Stroke Res. (2016) 7(1):54–69. 10.1007/s12975-015-0440-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Woo A, Mb W, Frca BS. Depression and anxiety in pain. Rev Pain. (2010) 4(1):8–12. 10.1177/204946371000400103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gayman MD, Brown RL, Cui M. Depressive symptoms and bodily pain: the role of physical disability and social stress. Stress Health. (2011) 27(1):52–63. 10.1002/smi.1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jäger AK, Saaby L. Flavonoids and the CNS. Molecules. (2011) 16:1471–85. 10.3390/molecules16021471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gadotti VM, Zamponi GW. Anxiolytic effects of the flavonoid luteolin in a mouse model of acute colitis. Mol Brain. (2019) 12(1):114. 10.1186/s13041-019-0539-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Salehi B, Venditti A, Sharifi-Rad M, Kręgiel D, Sharifi-Rad J, Durazzo A, et al. The therapeutic potential of apigenin. Int J Mol Sci. (2019) 20(6):1305. MDPI AG. 10.3390/ijms20061305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mihyaoui AE, Esteves Da Silva JCG, Charfi S, Castillo MEC, Lamarti A, Arnao MB. Chamomile (Matricaria chamomilla L.): a review of ethnomedicinal use, phytochemistry and pharmacological uses. Life. (2022) 12(4):479. 10.3390/life12040479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Alzaabi MM, Hamdy R, Ashmawy NS, Hamoda AM, Alkhayat F, Khademi NN, et al. Flavonoids are promising safe therapy against COVID-19. Phytochem Rev. (2022) 21(1):291–312. 10.1007/s11101-021-09759-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vaya J, Mahmood S. Flavonoid content in leaf extracts of the fig (Ficus carica L.), carob (Ceratonia siliqua L.) and pistachio (Pistacia lentiscus L.). BioFactors. (2006) 28:169–75. 10.1002/biof.5520280303 [DOI] [PubMed] [Google Scholar]
- 108.Seeram NP, Zhang Y, Henning SM, Lee R, Niu Y, Lin G, et al. Pistachio skin phenolics are destroyed by bleaching resulting in reduced antioxidative capacities. J Agric Food Chem. (2006) 54(19):7036–40. 10.1021/jf0614948 [DOI] [PubMed] [Google Scholar]
- 109.Manzoor M, Ahmad N, Manzoor A, Kalsoom A. Food based phytochemical luteolin their derivatives, sources and medicinal benefits. International Journal of Agricultural ad Life Sciences. (2017) 3(2):195–207. 10.22573/spg.ijals.017.s12200084 [DOI] [Google Scholar]
- 110.Park JC, Park JG, Kim HJ, Hur JM, Lee JH, Sung NJ, et al. Effects of extract from Angelica keiskei and its component, cynaroside, on the hepatic bromobenzene-metabolizing enzyme system in rats. Phytother Res. (2002) 16(Suppl. 1):S24–7. 10.1002/ptr.783 [DOI] [PubMed] [Google Scholar]
- 111.Dall’Acqua S, Giorgetti M, Cervellati R, Innocenti G. Deoxypodophyllotoxin content and antioxidant activity of aerial parts of Anthriscus sylvestris hoffm. Zeitschrift fur Naturforschung - Section C Journal of Biosciences. (2006) 61(9–10):658–62. 10.1515/znc-2006-9-1008 [DOI] [PubMed] [Google Scholar]
- 112.Lin LZ, Lu S, Harnly JM. Detection and quantification of glycosylated flavonoid malonates in celery, Chinese celery, and celery seed by LC-DAD-ESI/MS. J Agric Food Chem. (2007) 55(4):1321–6. 10.1021/jf0624796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pistelli L, Noccioli C, Giachi I, Dimitrova B, Gevrenova R, Morelli I, et al. Lupane-triterpenes from Bupleurum flavum. Nat Prod Res. (2005) 19(8):783–8. 10.1080/14786410500045119 [DOI] [PubMed] [Google Scholar]
- 114.Dall’Acqua S, Innocenti G. Antioxidant compounds from Chaerophyllum hirsutum extracts. Fitoterapia. (2004) 75(6):592–5. 10.1016/j.fitote.2004.05.007 [DOI] [PubMed] [Google Scholar]
- 115.Shalaby NMM, Maghraby AS, El-Hagrassy AM. Effect of Daucus carota var. boissieri extracts on immune response of schistosoma mansoni infected mice. Folia Microbiol. (1999) 44(4):441–8. 10.1007/BF02903720. Available at: http://w’aw.biolned,cas.cz/tabu/folia/ [DOI] [PubMed] [Google Scholar]
- 116.El-Sayed NH, Ammar NM, Al-Okbi SY, El-Kassem ALT, Mabry TJ. Antioxidant activity and two new flavonoids from Washingtonia filifera. Nat Prod Res. (2006) 20(1):57–61. 10.1080/1478641500059276 [DOI] [PubMed] [Google Scholar]
- 117.Lee D, Cuendet M, Schunke Vigo J, Graham JG, Cabieses F, Fong HHS, et al. A novel cyclooxygenase-inhibitory stilbenolignan from the seeds of Aiphanes aculeata. Org Lett. (2001) 3(14):2169–70. 10.1021/ol015985j [DOI] [PubMed] [Google Scholar]
- 118.Ramesh M, Rao YN, Rao AVNA, Prabhakar MC, Rao CS, Muralidhar N, et al. Antinociceptive and anti-inflammatory activity of a flavonoid isolated from Caralluma attenuata. J Ethnopharmacol. (1998) 62(1):63–6. 10.1016/S0378-8741(98)00048-8 [DOI] [PubMed] [Google Scholar]
- 119.Bader A, Braca A, de Tommasi N, Morelli I. Further constituents from Caralluma negevensis. Phytochemistry. (2003) 62(8):1277–81. 10.1016/S0031-9422(02)00678-7 [DOI] [PubMed] [Google Scholar]
- 120.Shabana M, Gonaid M, Salama MM, Abdel-Sattar E. Phenylalkylamine alkaloids from Stapelia hirsuta L. Nat Prod Res. (2006) 20(8):710–4. 10.1080/14786410500137718 [DOI] [PubMed] [Google Scholar]
- 121.Keyhanian S, Stahl-Biskup E. Phenolic constituents in dried flowers of Aloe vera (Aloe barbadensis) and their in vitro antioxidative capacity. Planta Med. (2007) 73(6):599–602. 10.1055/s-2007-967202 [DOI] [PubMed] [Google Scholar]
- 122.Iwpshina T, Matsumoto S, Ozawa K, Akuzawa K. Flavone glycosides from asplenium normale. Phytochemistry. (1990) 29(11):3543–6. 10.1016/0031-9422(90)85272-H [DOI] [PubMed] [Google Scholar]
- 123.Glasl S, Mucaji P, Werner I, Presser A, Jurenitsch J. Sesquiterpenes and flavonoid aglycones from a Hungarian taxon of the Achillea millefolium group. Z Naturforsch. (2002) 57:976–82. 10.1515/znc-2002-11-1203. Available at: www.znaturforsch.com [DOI] [PubMed] [Google Scholar]
- 124.Kasaj D, Krenn L, Prinzb S, Hüfnerb A, Haslingerb E, Shan Yuc S, et al. Flavon-and flavonolglycosides from A chillea pannonica scheele. Z Naturforsch. (2001) 56:521–5. 10.1515/znc-2001-7-808. Available at: www.znaturforsch.com [DOI] [PubMed] [Google Scholar]
- 125.Merfort I, Wendisch D. New flavonoid glycosides from Arnicae flos DAB 9. Planta Med. (1992) 58(4):355–7. 10.1055/s-2006-961484 [DOI] [PubMed] [Google Scholar]
- 126.Kim NM, Kim J, Chung HY, Choi JS. Isolation of luteolin 7-O-rutinoside and esculetin with potential antioxidant activity from the aerial parts of Artemisia Montana. Arch Pharm Res. (2000) 23(3):237–9. 10.1007/BF02976451 [DOI] [PubMed] [Google Scholar]
- 127.Michal W, Monika T, Michal T, Jan G, Iwona W. Antioxidant activity of extracts and flavonoids from bidens tripartita. Acta Poloniae Pharmaceutica ñ Drug Research. (2007) 63(5):441–7. [PubMed] [Google Scholar]
- 128.Jeong DM, Jung HA, Choi JS. Comparative antioxidant activity and HPLC profiles of some selected Korean thistles. Arch Pharm Res. (2008) 31(1):28–33. 10.1007/s12272-008-1116-7 [DOI] [PubMed] [Google Scholar]
- 129.Tundis R, Statti G, Menichini F, Delle Monache F. Arctiin and onopordopicrin from Carduus micropterus ssp. Perspinosus. Fitoterapia. (2000) 71(5):600–1. 10.1016/S0367-326X(00)00203-3 [DOI] [PubMed] [Google Scholar]
- 130.Lee JY, Chang EJ, Kim HJ, Park H, Choi SW. Antioxidative flavonoids from leaves of Carthamus tinctorius. Arch Pharm Res. (2002) 25(3):313–9. 10.1007/BF02976632. Available at: http://apr.psk.or.kr [DOI] [PubMed] [Google Scholar]
- 131.Diaa Y, August WF. Constituents of the EgyptianGentaurea scoparia; III. PhenolicConstituents of the aerial parts. Planta Med. (1995) 61:570–3. 10.1055/s-2006-959378 [DOI] [PubMed] [Google Scholar]
- 132.Fonseca FN, Tavares MFM, Horváth C. Capillary electrochromatography of selected phenolic compounds of Chamomilla recutita. J Chromatogr A. (2007) 1154(1–2):390–9. 10.1016/j.chroma.2007.03.106 [DOI] [PubMed] [Google Scholar]
- 133.Suksamrarn A, Chotipong A, Suavansri T, Boongird S, Tirnsuksai P, Vimuttipong S, et al. Antimycobacterial activity and cytotoxicity of flavonoids from the flowers of Chromolaena odorata. Arch Pharm Res. (2004) 27(5):507–11. 10.1007/BF02980123. Available at: http://apr.psk.or.kr [DOI] [PubMed] [Google Scholar]
- 134.Nguyen M, Awale S, Tezuka Y, Tran Q, Watanabe H, Kadota S. Xanthine oxidase inhibitory activity of Vietnamese medicinal plants. Biol Pharm Bull. (2004) 27(9):1414–21. 10.1248/bpb.27.1414 [DOI] [PubMed] [Google Scholar]
- 135.Ganzera M, Pöcher A, Stuppner H, Ganzera M. Differentiation of Cirsium japonicum and C. setosum by TLC and HPLC-MS. Phytochem Anal. (2005) 16:205–9. 10.1002/pca.846. Available at: www.interscience.wiley.com [DOI] [PubMed] [Google Scholar]
- 136.Nazaruk J, Gudej J. Flavonoid compounds from the flowers of cirsium rivulare (JACQ.) all. Acta Pol Pharm. (2003) 60(1):87–9. PMID: [PubMed] [Google Scholar]
- 137.Kong LD, Abliz Z, Zhou CX, Li LJ, Cheng CHK, Tan RX. Glycosides and xanthine oxidase inhibitors from Conyza bonariensis. Phytochemistry. (2001) 58(4):645–51. 10.1016/S0031-9422(01)00176-5. Available at: www.elsevier.com/locate/phytochem [DOI] [PubMed] [Google Scholar]
- 138.Nazaruk J. Flavonoid aglycones and phytosterols from the Erigeron acris L. Herb. Acta Pol Pharm. (2006) 63(4):317–9. PMID: [PubMed] [Google Scholar]
- 139.Schauss AG, Wu X, Prior RL, Ou B, Patel D, Huang D, et al. Phytochemical and nutrient composition of the freeze-dried amazonian palm berry, Euterpe oleraceae mart. (acai). J Agric Food Chem. (2006) 54(22):8598–603. 10.1021/jf060976g [DOI] [PubMed] [Google Scholar]
- 140.Hsu HF, Houng JY, Chang CL, Wu CC, Chang FR, Wu YC. Antioxidant activity, cytotoxicity, and DNA information of Glossogyne tenuifolia. J Agric Food Chem. (2005) 53(15):6117–25. 10.1021/jf050463u [DOI] [PubMed] [Google Scholar]
- 141.Süzgeç S, Meriçli AH, Houghton PJ, Çubukçu B. Flavonoids of Helichrysum compactum and their antioxidant and antibacterial activity. Fitoterapia. (2005) 76(2):269–72. 10.1016/j.fitote.2004.12.006 [DOI] [PubMed] [Google Scholar]
- 142.Park EJ, Kim Y, Kim J. Acylated flavonol glycosides from the flower of Inula britannica. J Nat Prod. (2000) 63(1):34–6. 10.1021/np990271r [DOI] [PubMed] [Google Scholar]
- 143.Zhang Y, Chen J, Ma XM, Shi YP. Simultaneous determination of flavonoids in Ixeridium gracile by micellar electrokinetic chromatography. J Pharm Biomed Anal. (2007) 45(5):742–6. 10.1016/j.jpba.2007.08.014 [DOI] [PubMed] [Google Scholar]
- 144.Ma JY, Wang ZT, Xu LS, Xu GJ. A sesquiterpene lactone glucoside from Ixeris denticulata f. pinnatipartita. Phytochemistry. (1999) 50:113–5. 10.1016/S0031-9422(98)00452-X [DOI] [PubMed] [Google Scholar]
- 145.Hou CC, Lin SJ, Cheng JT, Hsu FL. Antidiabetic dimeric guianolides and a lignan glycoside from Lactuca indica. J Nat Prod. (2003) 66(5):625–9. 10.1021/np0205349 [DOI] [PubMed] [Google Scholar]
- 146.DuPont MS, Mondin Z, Williamson G, Price KR. Effect of variety, processing, and storage on the flavonoid glycoside content and composition of lettuce endive. J Agric Food Chem. (2000) 48(9):3957–64. 10.1021/jf0002387 [DOI] [PubMed] [Google Scholar]
- 147.Kim DK. Antioxidative components from the aerial parts of Lactuca scariola L. Arch Pharm Res. (2001) 24(5):427–30. 10.1007/BF02975189 [DOI] [PubMed] [Google Scholar]
- 148.Schwaiger S, Seger C, Wiesbauer B, Schneider P, Ellmerer EP, Sturm S, et al. Development of an HPLC-PAD-MS assay for the identification and quantification of major phenolic edelweiss (Leontopodium alpium cass.) constituents. Phytochem Anal. (2006) 17(5):291–8. 10.1002/pca.917 [DOI] [PubMed] [Google Scholar]
- 149.Grael CFF, Albuquerque S, Lopes JLC. Chemical constituents of Lychnophora pohlii and trypanocidal activity of crude plant extracts and of isolated compounds. Fitoterapia. (2005) 76(1):73–82. 10.1016/j.fitote.2004.10.013 [DOI] [PubMed] [Google Scholar]
- 150.Gongora L, Manez S, Giner R, Recio C, Gray A, Rios J. Phenolic glycosides from Phagnalon rupestre. Phytochemistry. (2002) 59(8):857–60. 10.1016/S0031-9422(02)00011-0. Available at: www.elsevier.com/locate/phytochem [DOI] [PubMed] [Google Scholar]
- 151.Souleles C, Laskaris G. Flavonoids from Picnomon acarna. Planta Med. (1988) 54(1):47–8. 10.1055/s-2006-962332 [DOI] [PubMed] [Google Scholar]
- 152.Cottiglia F, Casu L, Bonsignore L, Casu M, Floris C, Sosa S, et al. Topical anti-inflammatory activity of flavonoids and a new Xanthone from Santolina insularis. Z Naturforsch. (2005) 60:63–6. 10.1515/znc-2005-1-212. Available at: http://www.znaturforsch.com [DOI] [PubMed] [Google Scholar]
- 153.Seo CS, Kim JH, Huang DS, Shin HK. Simultaneous determination of seven compounds in samsoeum by HPLC-PDA. J Orient Med Prescription. (2010) 18(1):95–103. PMID: ; PMCID: 24082332 [Google Scholar]
- 154.Báthori M, Zupkó I, Hunyadi A, Gácsné-Baitz E, Dinya Z, Forgó P. Monitoring the antioxidant activity of extracts originated from various Serratula species and isolation of flavonoids from Serratula coronata. Fitoterapia. (2004) 75(2):162–7. 10.1016/j.fitote.2003.12.009 [DOI] [PubMed] [Google Scholar]
- 155.Ghanta S, Banerjee A, Poddar A, Chattopadhyay S. Oxidative DNA damage preventive activity and antioxidant potential of Stevia rebaudiana (bertoni) bertoni, a natural sweetener. J Agric Food Chem. (2007) 55(26):10962–7. 10.1021/jf071892q [DOI] [PubMed] [Google Scholar]
- 156.Williams CA, Goldstone F, Greenham J. Flavonoids, cinnamic acids and coumarins from the different tissues and medicinal preparations of taraxacum officinale. Phytochemistry. (1996) 42(1):121–7. 10.1016/0031-9422(95)00865-9 [DOI] [PubMed] [Google Scholar]
- 157.Block LC, Santos ARS, Maria De Souza M, Scheidt C, Yunes RA, Santos A, et al. Chemical and pharmacological examination of antinociceptive constituents of Wedelia paludosa. J Ethnopharmacol. (1998) 61(1):85–9. 10.1016/S0378-8741(98)00019-1 [DOI] [PubMed] [Google Scholar]
- 158.Ooi LSM, Wang H, He Z, Ooi VEC. Antiviral activities of purified compounds from Youngia japonica (L.) DC (Asteraceae, Compositae). J Ethnopharmacol. (2006) 106(2):187–91. 10.1016/j.jep.2005.12.028 [DOI] [PubMed] [Google Scholar]
- 159.Sharaf M, El-Ansari MA, Saleh NAM. New flavonoids from Äicennia marina. Fitoterapia. (2000) 71(3):274–7. 10.1016/S0367-326X(99)00169-0 [DOI] [PubMed] [Google Scholar]
- 160.Ueda Y, Oku H, Linuma M, Ishiguro K. Effects on blood pressure decrease in response to PAF of Impatiens textori MIQ. Biol Pharm Bull. (2003) 26(10):1505–7. 10.1248/bpb.26.1505 [DOI] [PubMed] [Google Scholar]
- 161.Liang H-R, Vuorela P, Vuorela H, Hiltunen R. Isolation and immunomodulatory effect of flavonol glycosides from Epimedium hunanense. Planta Med. (1997) 63(4):316–9. 10.1055/s-2006-957690 [DOI] [PubMed] [Google Scholar]
- 162.Chen CC, Huang YL, Sun CM, Shen CC, Ko FN, Teng CM. New prenylflavones from the leaves of Epimedium sagittatum. J Nat Prod. (1996) 59(4):412–4. 10.1021/np9601925 [DOI] [PubMed] [Google Scholar]
- 163.Gormann R, Kaloga M, Ferreira D, Marais JPJ, Kolodziej H. Newbouldiosides A-C, phenylethanoid glycosides from the stem bark of Newbouldia laevis. Phytochemistry. (2006) 67(8):805–11. 10.1016/j.phytochem.2006.01.016 [DOI] [PubMed] [Google Scholar]
- 164.Han HY, Shan S, Zhang X, Wang NL, Lu XP, Yao XS. Down-regulation of prostate specific antigen in LNCaP cells by flavonoids from the pollen of Brassica napus L. Phytomedicine. (2007) 14(5):338–43. 10.1016/j.phymed.2006.09.005 [DOI] [PubMed] [Google Scholar]
- 165.Bringmann G, Ochse M, Zotz G, Peters K, Peters EM, Brun R, et al. 6-Hydroxyluteolin-7-O-(1′′-α-rhamnoside) From Vriesea sanguinolenta cogn. And marchal (Bromeliaceae). Phytochemistry. (2000) 53(8):965–9. 10.1016/S0031-9422(99)00550-6. Available at: www.elsevier.com/locate/phytochem [DOI] [PubMed] [Google Scholar]
- 166.Backhouse N, Rosales L, Apablaza C, Goïty L, Erazo S, Negrete R, et al. Analgesic, anti-inflammatory and antioxidant properties of buddleja globosa, Buddlejaceae. J Ethnopharmacol. (2008) 116(2):263–9. 10.1016/j.jep.2007.11.025 [DOI] [PubMed] [Google Scholar]
- 167.Georges K, Jayaprakasam B, Dalavoy SS, Nair MG. Pest-managing activities of plant extracts and anthraquinones from Cassia nigricans from Burkina Faso. Bioresour Technol. (2008) 99(6):2037–45. 10.1016/j.biortech.2007.02.049 [DOI] [PubMed] [Google Scholar]
- 168.Tshikalange TE, Meyer JJM, Hussein AA. Antimicrobial activity, toxicity and the isolation of a bioactive compound from plants used to treat sexually transmitted diseases. J Ethnopharmacol. (2005) 96(3):515–9. 10.1016/j.jep.2004.09.057 [DOI] [PubMed] [Google Scholar]
- 169.Ingkaninan K, Ijzerman AP, Verpoorte R. Luteolin, a compound with adenosine A1 receptor-binding activity, and chromone and dihydronaphthalenone constituents from Senna siamea. J Nat Prod. (2000) 63(3):315–7. 10.1021/np9904152 [DOI] [PubMed] [Google Scholar]
- 170.Sudjaroen Y, Haubner R, Würtele G, Hull WE, Erben G, Spiegelhalder B, et al. Isolation and structure elucidation of phenolic antioxidants from tamarind (Tamarindus indica L.) seeds and pericarp. Food Chem Toxicol. (2005) 43(11):1673–82. 10.1016/j.fct.2005.05.013 [DOI] [PubMed] [Google Scholar]
- 171.Zhou Y, Wang Y, Wang R, Guo F, Yan C. Two-dimensional liquid chromatography coupled with mass spectrometry for the analysis of Lobelia chinensis lour. Using an ESI/APCI multimode ion source. J Sep Sci. (2008) 31(13):2388–94. 10.1002/jssc.200700685 [DOI] [PubMed] [Google Scholar]
- 172.Vanhoenacker G, van Rompaey P, de Keukeleire D, Sandra P. Chemotaxonomic features associated with flavonoids of cannabinoid-free cannabis (Cannabis sativa subsp. sativa L.) in relation to hops (Humulus lupulus L.). Nat Prod Lett. (2002) 16(1):57–63. 10.1080/1057563029001/4863 [DOI] [PubMed] [Google Scholar]
- 173.Li Y-Q, Yang S-L, Li H-R, Xu L-Z. Two new alkaloids from Capparis himalayensis. Chem Pharm Bull. (2008) 56(2):189–91. 10.1248/cpb.56.189 [DOI] [PubMed] [Google Scholar]
- 174.Sousa A, Ferreira ICFR, Calhelha R, Andrade PB, Valentão P, Seabra R, et al. Phenolics and antimicrobial activity of traditional stoned table olives “alcaparra.”. Bioorg Med Chem. (2006) 14(24):8533–8. 10.1016/j.bmc.2006.08.027 [DOI] [PubMed] [Google Scholar]
- 175.Yao X, Jiang G, Chen G. ORIGINAL RESEARCH determination of active constituents in Lonicera confusa DC. By capillary electrophoresis with amperometric detection. Biomed Chromatogr. (2006) 20:1192–9. 10.1002/bmc.684. Available at: www.interscience.wiley.com [DOI] [PubMed] [Google Scholar]
- 176.Elbandy M, Miyamoto T, Lacaille-Dubois MA. Sulfated lupane triterpene derivatives and a flavone C-glycoside from Gypsophila repens. Chem Pharm Bull. (2007) 808(5):808–11. 10.1248/cpb.55.808 [DOI] [PubMed] [Google Scholar]
- 177.Li HY, Koike K, Ohmoto T. Triterpenoid saponins from dzanthus chinenszs. Phytochemistry. (1994) 35(3):751–6. 10.1016/S0031-9422(00)90599-5 [DOI] [PubMed] [Google Scholar]
- 178.Andrade-Cetto A, Wiedenfeld H. Hypoglycemic effect of Cecropia obtusifolia on streptozotocin diabetic rats. J Ethnopharmacol. (2001) 78:145–9. 10.1016/S0378-8741(01)00335-X. Available at: www.elsevier.com/locate/jethpharm [DOI] [PubMed] [Google Scholar]
- 179.Kandil FE, Grace MH. Polyphenols from Cornulaca monacantha. Phytochemistry. (2001) 58(4):611–3. 10.1016/S0031-9422(01)00265-5. Available at: www.elsevier.com/locate/phytochem [DOI] [PubMed] [Google Scholar]
- 180.Rocha L, Marston A, Potterat O, Auxiliadora M, Kaplan C, Stoeckli-Evans H, et al. Antibacterial phloroglucinols and flavonoids from hypericum brasiliense. Phytochemistry. (1995) 40(5):1447–52. 10.1016/0031-9422(95)00507-4 [DOI] [PubMed] [Google Scholar]
- 181.Jurgenliemk G, Nahrstedt A. Phenolic compounds from Hypericum perforatum. Planta Med. (2002) 68:88–91. 10.1055/s-2002-20053 [DOI] [PubMed] [Google Scholar]
- 182.Sfltlupinar N, Husek A, Potèilová H, Dvofáková S, Hanus V, Sedmerat P, et al. Alkaloids and phenolics of coichicum cilicicum. Planta Med. (1988) 53(3):243–5. 10.1055/s-2006-962417 [DOI] [PubMed] [Google Scholar]
- 183.Pettit GR, Hoard MS, Doubek DL, Schmidt JM, Pettit RK, Tackett LP, et al. L. Antineoplastic agents 338. The cancer cell growth inhibitory. Constituents of Terminalia arjuna (Combretaceae). J Ethnopharmacol. (1996) 53(2):57–63. 10.1016/S0378-8741(96)01421-3 [DOI] [PubMed] [Google Scholar]
- 184.Saleem A, Husheem M, Härkönen BP, Pihlaja K. Inhibition of cancer cell growth by crude extract and the phenolics of Terminalia chebula retz. Fruit. J Ethnopharmacol. (2002) 81:327–36. 10.1016/S0378-8741(02)00099-5. Available at: www.elsevier.com/locate/jethpharm [DOI] [PubMed] [Google Scholar]
- 185.Marzouk M, El-Toumy S, Moharram F, Shalaby N, Ehmed A. Pharmacologically active ellagitannins from Terminalia myriocarpa. Planta Med. (2002) 68:523–7. 10.1055/s-2002-32549 [DOI] [PubMed] [Google Scholar]
- 186.Du Q, Xu Y, Li L, Zhao Y, Jerz G, Winterhalter P. Antioxidant constituents in the fruits of Luffa cylindrica (L.) roem. J Agric Food Chem. (2006) 54(12):4186–90. 10.1021/jf0604790 [DOI] [PubMed] [Google Scholar]
- 187.Siciliano T, de Tommasi N, Morelli I, Braca A. Study of flavonoids of Sechium edule (jacq) swartz (Cucurbitaceae) different edible organs by liquid chromatography photodiode array mass spectrometry. J Agric Food Chem. (2004) 52(21):6510–5. 10.1021/jf040214q [DOI] [PubMed] [Google Scholar]
- 188.Chu Q, Tian X, Lin M, Ye J. Electromigration profiles of Cynomorium songaricum based on capillary electrophoresis with amperometric detection. J Agric Food Chem. (2006) 54(21):7979–83. 10.1021/jf061574b [DOI] [PubMed] [Google Scholar]
- 189.Basile A, Sorbo S, Joséantonio Lópezlópez-Saéz J, Castaldo Cobianchi R. Effects of seven pure flavonoids from mosses on germination and growth of Tortula muralis HEDW. (bryophyta) and Raphanus sativus L. (magnoliophyta). Phytochemistry. (2003) 62(7):1145–51. 10.1016/S0031-9422(02)00659-3. Available at: www.elsevier.com/locate/phytochem [DOI] [PubMed] [Google Scholar]
- 190.Oh H, Kim DH, Cho JH, Kim YC. Hepatoprotective and free radical scavenging activities of phenolic petrosins and flavonoids isolated from Equisetum arvense. J Ethnopharmacol. (2004) 95(2–3):421–4. 10.1016/j.jep.2004.08.015 [DOI] [PubMed] [Google Scholar]
- 191.Hawas UW. Antioxidant activity of brocchlin carboxylic acid and its methyl ester from Chrozophora brocchiana. Nat Prod Res. (2007) 21(7):632–40. 10.1080/14786410701371124 [DOI] [PubMed] [Google Scholar]
- 192.Otsuka H, Hirata E, Shinzato T, Takeda Y. Glochiflavanosides A-D: flavanol glucosides from the leaves of Glochidion zeylanicum (GAERTN) A. JUSS. Chem Pharm Bull. (2001) 49(7):921–3. 10.1248/cpb.49.921 [DOI] [PubMed] [Google Scholar]
- 193.El-Desouky SK, Ryu SY, Kim YK. A new cytotoxic acylated apigenin glucoside from Phyllanthus emblica L. Nat Prod Res. (2008) 22(1):91–5. 10.1080/14786410701590236 [DOI] [PubMed] [Google Scholar]
- 194.Fu Y, Zu Y, Liu W, Efferth T, Zhang N, Liu X, et al. Optimization of luteolin separation from pigeonpea [Cajanus cajan (L.) millsp.] leaves by macroporous resins. J Chromatogr A. (2006) 1137(2):145–52. 10.1016/j.chroma.2006.08.067 [DOI] [PubMed] [Google Scholar]
- 195.Mun’im A, Negishi O, Ozawa T. Antioxidative compounds from crotalari sessiliflora. Biosci Biotechnol Biochem. (2003) 67(2):410–4. 10.1271/bbb.67.410 [DOI] [PubMed] [Google Scholar]
- 196.Barreiros ALBS, David JP, de Queiroz LP, David JM. A-type proanthocyanidin antioxidant from Dioclea lasiophylla. Phytochemistry. (2000) 55(7):805–8. 10.1016/S0031-9422(00)00297-1. Available at: www.elsevier.com/locate/phytochem [DOI] [PubMed] [Google Scholar]
- 197.Erdem Y, Koichiro T, Yoshihisa T, Kazuyoshi K. Isolation and characterization of free radical scavenging flavonoid glycosides from the flowers of Spartium junceum by activity-guided fractionation. J Ethnopharmacol. (2000) 73:471–8. 10.1016/S0378-8741(00)00327-5. Available at: www.elsevier.com/locate/jethpharm [DOI] [PubMed] [Google Scholar]
- 198.Mosaddik A, Forster PI, Waterman PG. Three new 3-benzylbenzofuran-2-one derivatives from Homalium brachybotrys (Flacourtiaceae/Salicaceae s. l.). Nat Prod Res. (2007) 21(13):1191–8. 10.1080/14786410601130679 [DOI] [PubMed] [Google Scholar]
- 199.Reddy SV, Tiwari AK, Kumar US, Rao RJ, Rao JM. Free radical scavenging, enzyme inhibitory constituents from antidiabetic ayurvedic medicinal plant Hydnocarpus wightiana blume. Phytother Res. (2005) 19(4):277–81. 10.1002/ptr.1491 [DOI] [PubMed] [Google Scholar]
- 200.Orhan DD, Aslan M, Aktay G, Ergun E, Yesilada E, Ergun F. Evaluation of hepatoprotective effect of Gentiana olivieri herbs on subacute administration and isolation of active principle. Life Sci. (2003) 72(20):2273–83. 10.1016/S0024-3205(03)00117-6 [DOI] [PubMed] [Google Scholar]
- 201.Wu QX, Li Y, Shi YP. Antioxidant phenolic glucosides from Gentiana piasezkii. J Asian Nat Prod Res. (2006) 8(5):391–6. 10.1080/10286020500172368 [DOI] [PubMed] [Google Scholar]
- 202.Lacaille-Dubois MA, Galle K, Wagner H. Secoiridoids and xanthones from Gentianella nitida. Planta Med. (1996) 62(4):365–7. 10.1055/s-2006-957908 [DOI] [PubMed] [Google Scholar]
- 203.Markham KR, Bloor SJ, Nicholson R, Rivera R, Shemluck M, Kevan PG, et al. Black flower coloration in wild Lisianthius nigrescens: its chemistry and ecological consequences. Z Naturforsch. (2004) 59:625–30. 10.1515/znc-2004-9-1003. Available at: http://www.znaturforsch.com [DOI] [PubMed] [Google Scholar]
- 204.Greenham J, Vassiliades DD, Harborne rey B, Williams CA, Eagles J, Grayer RJ, et al. A distinctive favonoid chemistry for the anomalous genus Biebersteinia. Phytochemistry. (2001) 56(1):87–91. 10.1016/S0031-9422(00)00355-1. Available at: www.elsevier.com/locate/phytochem [DOI] [PubMed] [Google Scholar]
- 205.Erhard D, Pohnert G, Gross EM. Chemical defense in Elodea nuttallii reduces feeding and growth of aquatic herbivorous lepidoptera. J Chem Ecol. (2007) 33(8):1646–61. 10.1007/s10886-007-9307-0 [DOI] [PubMed] [Google Scholar]
- 206.Abdalla MF, Saleh Nabiel AM, Garb S, Abu-Eyta AM, El-Said H. Flavone glycosides of salua triloba. Phytochemistry. (1983) 22(9):2057–60. 10.1016/0031-9422(83)80044-2 [DOI] [Google Scholar]
- 207.Yamauchi H, Kakuda R, Yaoita Y, Machida K, Kikuchi M. Two new glycosides from the whole plants of Glechoma hederacea L. Chem Pharm Bull. (2007) 55(2):346–7. 10.1248/cpb.55.346 [DOI] [PubMed] [Google Scholar]
- 208.Woo ER, Piao MS. Antioxidative constituents from Lycopus lucidus. Arch Pharm Res. (2004) 27(2):173–6. 10.1007/BF02980102 [DOI] [PubMed] [Google Scholar]
- 209.Epidmnidij S, Coli E, Pneumoniae K, Wlgarij P, Aenrginosa P. Antibacterial activity studies of flavonoids from salvla palaestzna. J Nat Prod. (1983) 46(6):874–5. 10.1021/np50030a007 [DOI] [PubMed] [Google Scholar]
- 210.Ulubelen A, Topcu G, Eris C, Sonmez U, Kartal M, Kurucu S, et al. Terpenoids from salvia sclarea. Phytochemistry. (1994) 36(4):971–4. 10.1016/S0031-9422(00)90474-6 [DOI] [PubMed] [Google Scholar]
- 211.Nikolova M, Asenov A. Surface flavonoid aglycones in newly studied plant species. Nat Prod Res. (2006) 20(1):103–6. 10.1080/14786410500046463 [DOI] [PubMed] [Google Scholar]
- 212.Jung MJ, Kang SS, Jung HA, Kim J, Choi JS. Isolation of flavonoids and a cerebroside from the stem bark of albizzia julibrissin. Arch Pharm Res. (2004) 27(6):593–9. 10.1007/BF02980155 [DOI] [PubMed] [Google Scholar]
- 213.Tewtrakul S, Miyashiro H, Nakamura N, Hattori M, Kawahata T, Otake T, et al. HIV-1 integrase inhibitory substances from Coleus parvifolius. Phytother Res. (2003) 17(3):232–9. 10.1002/ptr.1111 [DOI] [PubMed] [Google Scholar]
- 214.Saeidnia S, Gohari AR, Ito M, Kiuchi F, Honda G. Bioactive constituents from Dracocephalum subcapitatum (O. Kuntze) Lipsky. Z Naturforsch. (2005) 60:22–4. 10.1515/znc-2005-1-204. Available at: http://www.znaturforsch.com [DOI] [PubMed] [Google Scholar]
- 215.Wang FM, Yao TW, Zeng S, Zeng S. Analysis of luteolin in Elsholtzia blanda benth. By RP-HPLC. Pharmazie. (2005) 60(9):648–9. PMID: [PubMed] [Google Scholar]
- 216.Liu AL, Liu B, Qin HL, Lee SMY, Wang YT, Du GH. Anti-influenza virus activities of flavonoids from the medicinal plant Elsholtzia rugulosa. Planta Med. (2008) 74(8):847–51. 10.1055/s-2008-1074558 [DOI] [PubMed] [Google Scholar]
- 217.Luo M, Lu H, Ma H, Zhao L, Liu X, Jiang S. Separation and determination of flavonoids in lamiophlomis rotata by capillary electrophoresis using borate as electrolyte. J Pharm Biomed Anal. (2007) 44(4):881–6. 10.1016/j.jpba.2007.04.010 [DOI] [PubMed] [Google Scholar]
- 218.Gabriel C, Kokkalou E. A new acetylated glucoside of luteolin and two flavone glucosides from Lavandula stoechas ssp. stoechas. Pharmazie. (2003) 58(6):426–7. PMID: [PubMed] [Google Scholar]
- 219.Parejo I, Caprai E, Bastida J, Viladomat F, Jáuregui O, Codina C. Investigation of Lepechinia graveolens for its antioxidant activity and phenolic composition. J Ethnopharmacol. (2004) 94(1):175–84. 10.1016/j.jep.2004.05.017 [DOI] [PubMed] [Google Scholar]
- 220.Gumbinger H G, Winterhoff H, Wylde R, Sosa A. On the influence of the sugar moiety on the antigonadotropic activity of luteoline glycosides. Planta Med. (1992) 58(1):49–50. 10.1055/s-2006-961388 [DOI] [PubMed] [Google Scholar]
- 221.Bucar F, Kartnig T. Flavone glucuronides of Lycopus virginicus. Planta Med. (1995) 61(4):378–80. 10.1055/s-2006-958111 [DOI] [PubMed] [Google Scholar]
- 222.Modnicki D, Tokar M, Klimek B. Flavonoids and phenolic acids of Nepeta cataria L. Var. citriodora (becker) balb. (Lamiaceae). Acta Pol Pharm. (2007) 64(3):247–52. PMID: [PubMed] [Google Scholar]
- 223.Vieira RF, Grayer RJ, Paton A, Simon JE, Simon JE. Genetic diversity of Ocimum gratissimum L. Based on volatile oil constituents, flavonoids and RAPD markers. Biochem Syst Ecol. (2001) 29(3):287–304. 10.1016/S0305-1978(00)00062-4 [DOI] [PubMed] [Google Scholar]
- 224.Koukoulitsa C, Karioti A, Bergonzi MC, Pescitelli G, di Bari L, Skaltsa H. Polar constituents from the aerial parts of Origanum vulgare L. Ssp. hirtum growing wild in Greece. J Agric Food Chem. (2006) 54(15):5388–92. 10.1021/jf061477i [DOI] [PubMed] [Google Scholar]
- 225.Yoshida K, Kameda K, Kondot T. Diglucuronoflavones from purple leaves of perilla ocimoides. Phytochemistry. (1993) 33(4):917–9. 10.1016/0031-9422(93)85304-A [DOI] [PubMed] [Google Scholar]
- 226.Kamel MS, Mohamed KM, Hassanean HA, Ohtani K, Kasai R, Yamasaki K. Iridoid and megastigmane glycosides from Phlomis aurea. Phytochemistry. (2000) 55(4):353–7. 10.1016/S0031-9422(00)00331-9. Available at: www.elsevier.com/locate/phytochem [DOI] [PubMed] [Google Scholar]
- 227.Kirmizibekmez H, Çalis I, Perozzo R, Brun R, Dönmez AA, Linden A, et al. Inhibiting activities of the secondary metabolites of Phlomis brunneogaleata against parasitic protozoa and plasmodial enoyl-ACP reductase, a crucial enzyme in fatty acid biosynthesis. Planta Med. (2004) 70(8):711–7. 10.1055/s-2004-827200 [DOI] [PubMed] [Google Scholar]
- 228.Çaliş I, Kirmizibekmez H. Glycosides from Phlomis lunariifolia. Phytochemistry. (2004) 65(18):2619–25. 10.1016/j.phytochem.2004.04.038 [DOI] [PubMed] [Google Scholar]
- 229.Snchezde Rojas VR, Somoza B, ViIlar AM. Isolation of vasodilatory active flavonoids from the traditional remedy Satureja obovata. Planta Med. (1995) 62:272–4. 10.1055/s-2006-957876 [DOI] [PubMed] [Google Scholar]
- 230.Yoshitera O, Shotaro T, Hiroshi H. Schizonodiol, schizonol, and schizonepetosides D and E, monoterpenoids of schizonepeta tenuifolia spikes. Planta Med. (1989) 55:179–80. 10.1055/s-2006-961918 [DOI] [PubMed] [Google Scholar]
- 231.Sato Y, Suzaki S, Nishikawa T, Kihara M, Shibata H, Higuti T. Phytochemical flavones isolated from Scutellaria barbata and antibacterial activity against methicillin-resistant Staphylococcus aureus. J Ethnopharmacol. (2000) 72(3):483–8. 10.1016/S0378-8741(00)00265-8. Available at: www.elsevier.com/locate/jethpharm [DOI] [PubMed] [Google Scholar]
- 232.Kadifkova Panovska T, Kulevanova S, Stefova M. In vitro antioxidant activity of some Teucrium species (Lamiaceae). Acta Pharm. (2005) 55:207–14. PMID: [PubMed] [Google Scholar]
- 233.Dapkevicius A, van Beek TA, Lelyveld GP, van Veldhuizen A, de Groot A, Linssen JPH, et al. Isolation and structure elucidation of radical scavengers from Thymus vulgaris leaves. J Nat Prod. (2002) 65(6):892–6. 10.1021/np010636j [DOI] [PubMed] [Google Scholar]
- 234.Ismaili H, Tortora S, Sosa S, Fkih-Tetouani S, Ilidrissi A, Loggia RD, et al. Topical anti-inflammatory activity of Thymus willdenowii. J Pharm Pharmacol. (2001) 53(12):1645–52. 10.1211/0022357011778250 [DOI] [PubMed] [Google Scholar]
- 235.Hu Y, Hou TT, Zhang QY, Xin HL, Zheng HC, Qin LP, et al. Evaluation of the estrogenic activity of the constituents in the fruits of Vitex rotundifolia L. For the potential treatment of premenstrual syndrome. J Pharm Pharmacol. (2007) 59(9):1307–12. 10.1211/jpp.59.9.0016 [DOI] [PubMed] [Google Scholar]
- 236.Shaiq Ali M, Saleem M, Ali Z, Uddin Ahmad V. Chemistry of Zataria multiflora (Lamiaceae). Phytochemistry. (2000) 55(8):933–6. 10.1016/S0031-9422(00)00249-1. Available at: www.elsevier.com/locate/phytochem [DOI] [PubMed] [Google Scholar]
- 237.Miñ J, Acevedo C, Moscatelli V, Ferraro G, Hnatyszyn O. Antinociceptive effect of the aqueous extract of Balbisia calycina. J Ethnopharmacol. (2002) 79(2):179–82. 10.1016/S0378-8741(01)00372-5. Available at: www.elsevier.com/locate/jethpharm [DOI] [PubMed] [Google Scholar]
- 238.Kamara BI, Brand DJ, Brandt EV, Joubert E. Phenolic metabolites from honeybush tea (Cyclopia subternata). J Agric Food Chem. (2004) 52(17):5391–5. 10.1021/jf040097z [DOI] [PubMed] [Google Scholar]
- 239.Pistelli L, Giachi I, Potenza D, Morelli I. A new isoflavone from Genista corsica. J Nat Prod. (2000) 63(4):504–6. 10.1021/np990282k [DOI] [PubMed] [Google Scholar]
- 240.Rauter AP, Martins A, Borges C, Ferreira J, Justino J, Bronze MR, et al. Liquid chromatography-diode array detection-electrospray ionisation mass spectrometry/nuclear magnetic resonance analyses of the anti-hyperglycemic flavonoid extract of Genista tenera: structure elucidation of a flavonoid-C-glycoside. J Chromatogr A. (2005) 1089(1–2):59–64. 10.1016/j.chroma.2005.06.046 [DOI] [PubMed] [Google Scholar]
- 241.Boué SM, Carter-Wientjes CH, Shih BY, Cleveland TE. Identification of flavone aglycones and glycosides in soybean pods by liquid chromatography-tandem mass spectrometry. J Chromatogr A. (2003) 991(1):61–8. 10.1016/S0021-9673(03)00209-7 [DOI] [PubMed] [Google Scholar]
- 242.Park K, Lee S, Min B, Lee K, Choi J, Chung S, et al. Inhibitory effect of luteolin 4-O-glucoside from Kummerowia striata and other flavonoids on interleukin-5 bioactivity. Planta Med. (1999) 65(5):457–9. 10.1055/s-2006-960812 [DOI] [PubMed] [Google Scholar]
- 243.Kowalska I, Stochmal A, Kapusta I, Janda B, Pizza C, Piacente S, et al. Flavonoids from barrel medic (Medicago truncatula) aerial parts. J Agric Food Chem. (2007) 55(7):2645–52. 10.1021/jf063635b [DOI] [PubMed] [Google Scholar]
- 244.Kassem M, Mosharrafa SA, Saleh NAM, Abdel-Wahab SM. Two new flavonoids from Retama raetam. Fitoterapia. (2000) 71:649–54. 10.1016/S0367-326X(00)00224-0 [DOI] [PubMed] [Google Scholar]
- 245.Lopez-Lazaro M, Cordero-Martin C, Ayuso J. Flavonoids of Retama sphaerocarpa. Planta Med. (1999) 65:777–8. 10.1055/s-2006-960869 [DOI] [PubMed] [Google Scholar]
- 246.Mikhaeil BR, Badria FA, Maatooq GT, Amer MMA. Antioxidant and immunomodulatory constituents of henna leaves. Z Naturforsch. (2004) 59:468–76. 10.1515/znc-2004-7-803. Available at: http://www.znaturforsch.com [DOI] [PubMed] [Google Scholar]
- 247.Rauha J, Wolfender J, Salminen J, Pihlaja K, Hostettmann K, Vuorela H. Characterization of the polyphenolic composition of purple loosestrife (Lythrum salicaria). Z Naturforsch C J Biosci. (2001) 56:13–20. 10.1515/znc-2001-1-203. Available at: www.znaturforsch.com [DOI] [PubMed] [Google Scholar]
- 248.van Elswijk DA, Schobel UP, Lansky EP, Irth H, van der Greef J. Rapid dereplication of estrogenic compounds in pomegranate (Punica granatum) using on-line biochemical detection coupled to mass spectrometry. Phytochemistry. (2004) 65(2):233–41. 10.1016/j.phytochem.2003.07.001 [DOI] [PubMed] [Google Scholar]
- 249.Matlawska I, Sikorska M. Flavonoid compounds in the flowers of Abutilon indicum L. Sweet (Malvaceae). Acta Pol Pharm. (2002) 59(3):227–9. PMID: [PubMed] [Google Scholar]
- 250.Matlawska I. Flavonoid compounds in the flowers of Kitaibelia vitifolia willd. (Malvaceae). Acta Pol Pharm. (2001) 58(2):127–31. PMID: [PubMed] [Google Scholar]
- 251.Markham KR, Porter LJ. Isoscutellarein and hypolaetin 8-glucuronides from the liverwort marchantia berteroana. Phytochemistry. (1975) 14(4):109–1097. 10.1016/0031-9422(75)85194-6 [DOI] [Google Scholar]
- 252.Iwashina T, Kitajima J, Kato T, Tobe H. An analysis of flavonoid compounds in leaves of japonolirion (Petrosaviaceae). J Plant Res. (2005) 118(1):31–6. 10.1007/s10265-005-0194-6 [DOI] [PubMed] [Google Scholar]
- 253.Markham KR. A “flavone-polysaccharide” redefined as a mixture of 6-methoxyluteolin penta- and hexa-O-glycosides. Phytochemistry. (2003) 63(5):589–95. 10.1016/S0031-9422(03)00199-7 [DOI] [PubMed] [Google Scholar]
- 254.Tih AE, Ghogomu RT, Sondengam BL, Caux C, Bodo B. Minor biflavonoids from lophira alata leaves. J Nat Prod. (2006) 69(8):1206–8. 10.1021/np050169w [DOI] [PubMed] [Google Scholar]
- 255.Pieroni A, Pachaly P. An ethnopharmacological study on common privet (ligustrum vulgare) and phillyrea (phillyrea latifolia). Fitoterapia. (2000) 71:89–94. 10.1016/S0367-326X(00)00182-9 [DOI] [PubMed] [Google Scholar]
- 256.Lin YL, Shen CC, Huang YJ, Chang YY. Homoflavonoids from Ophioglossum petiolatum. J Nat Prod. (2005) 68(3):381–4. 10.1021/np0401819 [DOI] [PubMed] [Google Scholar]
- 257.Williams CA. The leaf flavonoids of the Orchidaceae. Phytochemistry. (1979) 18(5):803–13. 10.1016/0031-9422(79)80019-9 [DOI] [Google Scholar]
- 258.Mizokami H, Tomita-Yokotani K, Yoshitama K. Flavonoids in the leaves of oxalis corniculata and sequestration of the flavonoids in the wing scales of the pale grass blue butterfly, pseudozizeeria maha. J Plant Res. (2008) 121(1):133–6. 10.1007/s10265-007-0132-x [DOI] [PubMed] [Google Scholar]
- 259.Wang X, Cheng C, Sun Q, Li F, Liu J, Zheng C. Isolation and purification of four flavonoid constituents from the flowers of paeonia suffruticosa by high-speed counter-current chromatography. J Chromatogr A. (2005) 1075(1–2):127–31. 10.1016/j.chroma.2005.04.017 [DOI] [PubMed] [Google Scholar]
- 260.Hillenbrand M, Zapp J, Becker H. Depsides from the petals of Papaver rhoeas. Planta Med. (2004) 70(4):380–2. 10.1055/s-2004-818956 [DOI] [PubMed] [Google Scholar]
- 261.Ferreres F, Sousa C, Valentão P, Andrade PB, Seabra RM, Gil-Izquierdo Á. New C-deoxyhexosyl flavones and antioxidant properties of Passiflora edulis leaf extract. J Agric Food Chem. (2007) 55(25):10187–93. 10.1021/jf072119y [DOI] [PubMed] [Google Scholar]
- 262.Pereira M, Campos DE, Filho VC, Zanoni R, Silva DA, Yunes RA, et al. Evaluation of antifungal activity of Piper solmsianum C. DC. Var. solmsianum (Piperaceae). Biol Pharm Bull. (2005) 28(8):1527–30. 10.1248/bpb.28.1527 [DOI] [PubMed] [Google Scholar]
- 263.Domínguez M, Marín JC, Esquivel B, Céspedes CL. Pensteminoside, an unusual catalpol-type iridoid from penstemon gentianoides HBK (Plantaginaceae). Phytochemistry. (2007) 68(13):1762–6. 10.1016/j.phytochem.2007.03.035 [DOI] [PubMed] [Google Scholar]
- 264.Fleer H, Verspohl EJ. Antispasmodic activity of an extract from Plantago lanceolata L. And some isolated compounds. Phytomedicine. (2007) 14(6):409–15. 10.1016/j.phymed.2006.05.006 [DOI] [PubMed] [Google Scholar]
- 265.Davey MP, Bryant DN, Cummins I, Ashenden TW, Gates P, Baxter R, et al. Effects of elevated CO2 on the vasculature and phenolic secondary metabolism of Plantago maritima. Phytochemistry. (2004) 65(15):2197–204. 10.1016/j.phytochem.2004.06.016 [DOI] [PubMed] [Google Scholar]
- 266.Cheel J, Theoduloz C, Rodríguez J, Schmeda-Hirschmann G. Free radical scavengers and antioxidants from lemongrass (Cymbopogon citratus (DC. Stapf.). J Agric Food Chem. (2005) 53(7):2511–7. 10.1021/jf0479766 [DOI] [PubMed] [Google Scholar]
- 267.Sartelet H, Aguie-Aguie G, Martiny L, Haye B. Flavonoids extracted from fonio millet (Digitaria exilis) reveal potent antithyroid properties. Nutrition. (1996) 12(2):100–6. 10.1016/0899-9007(96)90707-8 [DOI] [PubMed] [Google Scholar]
- 268.Watanabe M. Antioxidative phenolic compounds from Japanese barnyard millet (Echinochloa utilis) grains. J Agric Food Chem. (1999) 47(11):4500–5. 10.1021/jf990498s [DOI] [PubMed] [Google Scholar]
- 269.Wang GJ, Chen YM, Wang TM, Lee CK, Chen KJ, Lee TH. Flavonoids with iNOS inhibitory activity from Pogonatherum crinitum. J Ethnopharmacol. (2008) 118(1):71–8. 10.1016/j.jep.2008.03.005 [DOI] [PubMed] [Google Scholar]
- 270.Colombo R, Yariwake JH, Queiroz EF, Ndjoko K, Hostettmann K. On-line identification of further flavone C- and O-glycosides from sugarcane (Saccharum officinarum L., gramineae) by HPLC-UV-MS. Phytochem Anal. (2006) 17(5):337–43. 10.1002/pca.923 [DOI] [PubMed] [Google Scholar]
- 271.Murakami S, Takahara H, Shiraiwa M. Purification and characterization of three neutral extracellular isoperoxidases from rye leaves. Phytochemistry. (2007) 68(6):777–84. 10.1016/j.phytochem.2006.11.015 [DOI] [PubMed] [Google Scholar]
- 272.Kwon YS, Kim EY, Kim J, Kim K, Kim CM. Antioxidant constituents from Setaria viridis. Arch Pharm Res. (2002) 25(3):300–5. 10.1007/BF02976630. Available at: http://apr.psk.or.kr [DOI] [PubMed] [Google Scholar]
- 273.Cavaliere C, Foglia P, Pastorini E, Samperi R, Laganà A. Identification and mass spectrometric characterization of glycosylated flavonoids in Triticum durum plants by high-performance liquid chromatography with tandem mass spectrometry. Rapid Commun Mass Spectrom. (2005) 19(21):3143–58. 10.1002/rcm.2185 [DOI] [PubMed] [Google Scholar]
- 274.Dietrych-Szostak D, Oleszek W. Effect of processing on the flavonoid content in buckwheat (Fagopyrum esculentum moench) grain. J Agric Food Chem. (1999) 47(10):4384–7. 10.1021/jf990121m [DOI] [PubMed] [Google Scholar]
- 275.Park CW, Kim MH, Park JH, Park CW. Flavonoid chemistry of Fallopia section Fallopia (Polygonaceae). Biochem Syst Ecol. (2000) 28:433–41. 10.1016/S0305-1978(99)00084-8 [DOI] [PubMed] [Google Scholar]
- 276.Waridel P, Wolfender JL, Lachavanne JB, Hostettmann K. Identification of the polar constituents of Potamogeton species by HPLC-UV with post-column derivatization, HPLC-MS n and HPLC-NMR, and isolation of a new ent-labdane diglycoside. Phytochemistry. (2004) 65(16):2401–10. 10.1016/j.phytochem.2004.06.031 [DOI] [PubMed] [Google Scholar]
- 277.Chen SB, Gao GY, Leung HW, Yeung HW, Yang JS, Xiao PG. Aquiledine and isoaquiledine, novel flavonoid alkaloids from Aquilegia ecalcarata. J Nat Prod. (2001) 64(1):85–7. 10.1021/np000256i [DOI] [PubMed] [Google Scholar]
- 278.Tomczyk M, Gudej J, Sochacki M. Flavonoids from Ficaria verna huds. Z Naturforsch. (2002) 57:440–4. 10.1515/znc-2002-5-606. Available at: www.znaturforsch.com [DOI] [PubMed] [Google Scholar]
- 279.Ringl A, Prinz S, Huefner A, Kurzmann M, Kopp B. Chemosystematic value of flavonoids from Crataegus x macrocarpa (Rosaceae) with special emphasis on (R)-and (S)-eriodictyol-7-O-glucuronide and luteolin-7-O-glucuronide. Chem Biodivers. (2007) 4(2):154–62. 10.1002/cbdv.200790020 [DOI] [PubMed] [Google Scholar]
- 280.Xue P, Zhao Y, Wang B, Liang H. Simultaneous determination of seven flavonoids in ptentilla multifida HPLC. J Chromatogr Sci. (2007) 45(4):216–9. 10.1093/chromsci/45.4.216 [DOI] [PubMed] [Google Scholar]
- 281.Lin CF, Ni CL, Huang YL, Sheu SJ, Chen CC. Lignans and anthraquinones from the fruits of morinda citrifolia. Nat Prod Res. (2007) 21(13):1199–204. 10.1080/14786410601132451 [DOI] [PubMed] [Google Scholar]
- 282.Cimanga RK, Kambu K, Tona L, Hermans N, Apers S, Totté J, et al. Cytotoxicity and in vitro susceptibility of entamoeba histolytica to morinda morindoides leaf extracts and its isolated constituents. J Ethnopharmacol. (2006) 107(1):83–90. 10.1016/j.jep.2006.02.010 [DOI] [PubMed] [Google Scholar]
- 283.Gattuso G, Caristi C, Gargiulli C, Bellocco E, Toscano G, Leuzzi U. Flavonoid glycosides in bergamot juice (Citrus bergamia risso). J Agric Food Chem. (2006) 54(11):3929–35. 10.1021/jf060348z [DOI] [PubMed] [Google Scholar]
- 284.Min YS, Bai KL, Yim SH, Lee YJ, Song HJ, Kim JH, et al. The effect of luteolin-7-O-13-D-glucuronopyranoside on gastritis and esophagitis in rats. Arch Pharm Res. (2006) 29(6):484–9. 10.1007/BF02969421. Available at: http://apr.psk.or.kr [DOI] [PubMed] [Google Scholar]
- 285.Hoffmann-Bohm K, Lotter H, Seligmann O, Wagner H. Antihepatotoxic C-glycosylflavones from the leaves of allophyllus edulis var. edulis and gracilis. Planta Med. (1992) 58(6):544–8. 10.1055/s-2006-961546 [DOI] [PubMed] [Google Scholar]
- 286.Kong LD, Wolfender JL, Cheng CH, Hostettmann K, Xiang Tan R. Xanthine oxidase inhibitors from brandisia hancel. Planta Med. (1999) 65(8):744–6. 10.1055/s-2006-960854 [DOI] [PubMed] [Google Scholar]
- 287.Gonzalez AG, Breton JL, Navarro E, Trujillo J, Boada J, Rodriguez R. Phytochemical study of isoplexis chalcantha. Planta Med. (1985) 51(1):9–11. 10.1055/s-2007-969379 [DOI] [PubMed] [Google Scholar]
- 288.Hiremath S, Hanumantha Rae S. Antifertility efficacy of the plant etriga lutea (scrophulariacae) on rats. Contraception. (1990) 42(4):467–477. 10.1016/0010-7824(90)90053-X [DOI] [PubMed] [Google Scholar]
- 289.Hiremath SP, Badami S, Hunasagatta SK, Patil SB. Antifertility and hormonal properties of flavones of striga orobanchioides. Eur J Pharmacol. (2000) 391:193–7. 10.1016/S0014-2999(99)00723-2. Available at: www.elsevier.nlrlocaterejphar [DOI] [PubMed] [Google Scholar]
- 290.Shindo K, Saito E, Sekiya M, Matsui T, Koike Y. Antioxidative activity of the flower of Torenia fournieri. J Nat Med. (2008) 62(2):247–8. 10.1007/s11418-007-0207-y [DOI] [PubMed] [Google Scholar]
- 291.Saracoglu I, Varel M, Harput US, Nagatsu A. Acylated flavonoids and phenol glycosides from Veronica thymoides subsp. pseudocinerea. Phytochemistry. (2004) 65(16):2379–85. 10.1016/j.phytochem.2004.05.011 [DOI] [PubMed] [Google Scholar]
- 292.Loizzo MR, Said A, Tundis R, Rashed K, Statti GA, Hufner A, et al. Inhibition of angiotensin converting enzyme (ACE) by flavonoids isolated from Ailanthus excelsa (roxb) (Simaroubaceae). Phytother Res. (2007) 21(27):32–6. 10.1002/ptr.2008. Available at: www.interscience.wiley.com [DOI] [PubMed] [Google Scholar]
- 293.Sánchez-Rabaneda F, Jáuregui O, Casals I, Andrés-Lacueva C, Izquierdo-Pulido M, Lamuela-Raventós RM. Liquid chromatographic/electrospray ionization tandem mass spectrometric study of the phenolic composition of cocoa (Theobroma cacao). J Mass Spectrom. (2003) 38(1):35–42. 10.1002/jms.395 [DOI] [PubMed] [Google Scholar]
- 294.Park BY, Min BS, Oh SR, Kim JH, Bae KH, Lee HK. Isolation of flavonoids, a biscoumarin and an amide from the flower buds of Daphne genkwa and the evaluation of their anti-complement activity. Phytother Res. (2006) 20:610–3. 10.1002/ptr.1915. Available at: www.interscience.wiley.com [DOI] [PubMed] [Google Scholar]
- 295.Nowik W. HPLC-PDA characterisation of Daphne gnidium L. (thymemeacea) dyeing extracts using two different C-18 stationary phases. J Sep Sci. (2005) 28(13):1595–600. 10.1002/jssc.200401770 [DOI] [PubMed] [Google Scholar]
- 296.Ferrari J, Terreaux C, Sahpaz S, Msonthi JD, Wolfender JL, Hostettmann K. Benzophenone glycosides from Gnidia involucrata. Phytochemistry. (2000) 54(8):883–9. 10.1016/S0031-9422(00)00046-7. Available at: www.elsevier.com/locate/phytochem [DOI] [PubMed] [Google Scholar]
- 297.Zhao J, Pawar RS, Ali Z, Khan IA. Phytochemical investigation of turnera diffusa. J Nat Prod. (2007) 70(2):289–92. 10.1021/np060253r [DOI] [PubMed] [Google Scholar]
- 298.Kim JS, Kim JC, Shim SH, Lee EJ, Jin W, Bae K, et al. Chemical constituents of the root of Dystaenia takeshimana and their anti-inflammatory activity. Arch Pharm Res. (2006) 29(8):617–23. 10.1007/BF02968244. Available at: http://apr.psk.or.kr [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.