Abstract
Background and Hypotheses
Weight gain and adverse cardiometabolic effects often limit the clinical utility of olanzapine. In ENLIGHTEN-2, combining olanzapine with the opioid receptor antagonist samidorphan (OLZ/SAM) mitigated olanzapine-associated weight gain. These analyses tested the hypothesis that OLZ/SAM would be associated with reduced adverse cardiometabolic effects compared with olanzapine.
Study Design
This phase 3 double-blind study randomized adults with schizophrenia to OLZ/SAM or olanzapine for 24 weeks. Post hoc analyses assessed changes from baseline to week 24 in cardiometabolic risk parameters, including body mass index (BMI), risk of developing obesity (BMI ≥30 kg/m2) or metabolic syndrome, waist circumference, along with mean and potentially clinically significant changes in blood pressure, glucose, and lipids.
Results
After 24 weeks’ treatment, compared with olanzapine, OLZ/SAM was associated with smaller least-squares mean (LSM) changes from baseline in systolic blood pressure (LSM difference, −2.63 mm Hg; 95% CI: −4.78, −0.47), diastolic blood pressure (LSM difference, −0.75 mm Hg; 95% CI: −2.31, 0.80), and BMI (LSM difference, −0.65 kg/m2; 95% CI: −1.01, −0.28). OLZ/SAM treatment was also associated with reduced risk of shifting from normal blood pressure to stage 1/2 hypertension (odds ratio [OR], 0.48; 95% CI: 0.24, 0.96), becoming obese (OR, 0.52; 95% CI: 0.32, 0.82), and developing metabolic syndrome (OR, 0.55; 95% CI: 0.31, 0.99) compared with olanzapine. No treatment group differences were noted for risk of hyperglycemia or hyperlipidemia.
Conclusions
OLZ/SAM treatment was associated with lower risk of worsening cardiometabolic risk factors related to obesity, hypertension, and metabolic syndrome relative to olanzapine. NCT02694328, https://clinicaltrials.gov/ct2/show/NCT02694328.
Keywords: 3-carboxamido-4-hydroxynaltrexone, dyslipidemia, hyperglycemia, hypertension, metabolic syndrome, schizophrenia
Introduction
Serious mental illness (SMI), including schizophrenia and bipolar I disorder, is associated with increased cardiometabolic risk.1,2 This includes a higher risk for cardiovascular disease (in SMI) or stroke (in schizophrenia or bipolar disorder) compared with healthy matched controls.1,3,4 Many psychiatric medications have adverse cardiometabolic effects that can further exacerbate the risk of cardiometabolic morbidity and mortality in patients with SMI.1,5 Olanzapine is well-established as an effective antipsychotic for the treatment of schizophrenia and bipolar I disorder.6–10 In long-term studies, olanzapine is associated with decreased rates of hospitalization, increased rates of remission, and increased time on treatment compared with certain other antipsychotics.6,11,12 However, the clinical utility of olanzapine is often limited by the potential for substantial weight gain and associated adverse cardiometabolic effects.13–18 Specifically, olanzapine is associated with increased central adiposity and risk for developing diabetes mellitus, dyslipidemia, and metabolic syndrome,19–22 potentially adding to the greater risk for cardiovascular morbidity and mortality already observed in patients with SMI. Furthermore, weight gain and cardiometabolic side effects associated with olanzapine treatment23,24 may reduce medication adherence6 and lead to treatment switches,25 which, in turn, may increase the risk of relapse, hospitalization, and disease progression.25,26
Although the specific cause(s) of antipsychotic-associated weight gain remains unclear,1,27 past studies have focused on receptor interactions within serotoninergic, dopaminergic, histaminergic, adrenergic, cannabinoid, and muscarinic neurotransmitter pathways.28 Additionally, it is established that the endogenous opioid system plays a role in weight and metabolic regulation,29,30 and evidence of this effect from both nonclinical and clinical studies supports the rationale for targeting this system to mitigate weight-related side effects of antipsychotic treatment.30–34
A combination of olanzapine and the opioid receptor antagonist samidorphan35,36 (OLZ/SAM; Lybalvi, Alkermes, Inc.)37 was approved in the United States in May 2021 for the treatment of adults with schizophrenia as a maintenance monotherapy treatment, and for adults with bipolar I disorder, where it is approved for the acute treatment of manic or mixed episodes, either as a monotherapy or as an adjunct to lithium or valproate. Based on phase 2 and 3 clinical trials, this combination provides efficacy similar to that of olanzapine34,38,39 while mitigating olanzapine-associated weight gain in patients with schizophrenia.34,38 In the phase 3 ENLIGHTEN-2 study, OLZ/SAM was associated with a significantly lower mean percent change in body weight at 24 weeks compared with olanzapine as well as a 50% reduction in the likelihood of clinically significant weight gain of ≥10% from baseline at week 24 (the coprimary endpoints) and in weight gain of ≥7% from baseline at week 24 (the key secondary endpoint).34 Patients on OLZ/SAM also had significantly smaller increases in waist circumference at week 24 compared with olanzapine alone, a study measure serving as a proxy for central adiposity. Patients were less likely to experience an increase of ≥5 cm in waist circumference during treatment with OLZ/SAM, a threshold associated with increased mortality risk in males and females, regardless of body mass index (BMI).34 Despite these differences in weight gain and in waist circumference increases, changes in lipid and glycemic measures were generally small for patients treated with either OLZ/SAM or olanzapine, with no clinically meaningful differences between treatment groups.34
To better understand the potential benefits of OLZ/SAM compared with olanzapine, post hoc analyses were conducted to evaluate their respective effects across multiple cardiometabolic risk factors.40–42 Although mean between-group differences in lipid and glycemic parameters were not observed at 24 weeks, previously reported findings on weight and waist circumference suggest that differentiation on other measures of cardiometabolic risk could be detected within that 24-week time frame. We hypothesized that, compared with olanzapine alone, OLZ/SAM would be associated with reductions in some clinically relevant cardiometabolic risk factors, such as obesity, hypertension, and metabolic syndrome.
Methods
Study Design
In the phase 3, double-blind ENLIGHTEN-2 study (NCT02694328), patients diagnosed with schizophrenia were randomized 1:1 to receive OLZ/SAM or olanzapine for 24 weeks. Treatment was initiated with OLZ/SAM 10/10 mg (olanzapine 10 mg/samidorphan 10 mg) or olanzapine 10 mg daily. The olanzapine dosage was increased to 20 mg daily (olanzapine 20 mg/samidorphan 10 mg [20/10 mg] or olanzapine 20 mg) beginning at week 2 but could be lowered to 10 mg daily for tolerability reasons at the end of week 2, 3, or 4 at the investigator’s discretion. Doses were fixed at week 4 and remained the same thereafter.34
Patients
Detailed eligibility criteria were reported previously.34 In short, stable outpatients aged 18 to 55 years with a primary diagnosis of schizophrenia and a BMI from 18 to 30 kg/m2 were enrolled. Patients were excluded if they had a history of treatment-resistant schizophrenia, had less than 1 year since the initial onset of symptoms, were antipsychotic treatment naïve, had active alcohol or substance use disorders (excluding nicotine), or any clinically significant or unstable medical illness (eg, diabetes mellitus, hypo- or hypertension, thyroid dysfunction, cardiac arrhythmia, cardiomyopathy, cardiac conduction defect, history of myocardial infarction or unstable angina within 6 months, and history of seizure disorder or brain tumor). There were a number of exclusion criteria based on baseline laboratory parameters, where total fasting cholesterol >280 mg/dL, fasting triglycerides >500 mg/dL, glycosylated hemoglobin (HbA1c) ≥6.0%, fasting plasma glucose ≥126 mg/dL, and/or a clinically significant electrocardiogram abnormality (ie, QT interval >450 ms for males and >470 ms for females, as corrected by the Fridericia formula) were exclusionary. All patients provided written informed consent to participate in the trial, which was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. The study protocol and all amendments were approved by institutional review boards at each study site.
Assessments
Body weight and waist circumference measures (both conducted in triplicate), vital signs, electrocardiograms, adverse events, fasting (≥8 h by self-report) metabolic laboratory parameters (triglycerides, cholesterol, glucose, and insulin), and HbA1c (fasting or nonfasting) were collected weekly through week 6 and then biweekly through week 24. Blood pressure was monitored after the patient had been supine for 5 min, preferably by automated measurement and using the same arm throughout the study.
Post Hoc Analysis of Cardiometabolic Risk
Post hoc analyses evaluated the mean change from baseline in BMI, waist circumference, and supine systolic and diastolic blood pressure at week 24. Additionally, blood pressure shifts from normal (<120/<80 mm Hg) or normal/elevated (120–129/<80 mm Hg) to stage 1 hypertension (130–139/80–89 mm Hg) or stage 2 hypertension (≥140/≥90 mm Hg) were evaluated at week 24. The proportion of patients who did not meet individual metabolic parameter criteria or the full definition for metabolic syndrome at baseline (defined as the presence of ≥3 of the criteria listed in supplementary table 143) but then went on to meet respective criteria for individual metabolic syndrome parameters or for the full metabolic syndrome at the last on-treatment assessment was also evaluated. Risk differences for shifts in metabolic laboratory parameters were based on sustained potentially clinically significant shifts (ie, the parameters met shift criteria at the last 2 on-treatment assessments).
Statistical Analyses
Least-squares mean (LSM) changes in continuous measures for OLZ/SAM and olanzapine were compared using an analysis of covariance model based on multiple imputation for missing data. For binary endpoints, including risk differences and odds ratios (ORs), a logistic regression model was used based on the same imputed data sets. Rubin’s rule was used to combine results by applying the logistic regression model on imputed data sets. The proportions of patients who developed metabolic syndrome or who met individual metabolic syndrome criteria were based on observed data at each patient’s last on-treatment assessment.
Results
Cardiometabolic effects were evaluated in a post hoc analysis of all patients who had at least one postbaseline weight assessment (n = 538).34 Baseline patient characteristics are shown in table 1. The final dose level of olanzapine was 20 mg for the majority of patients (78.8% and 80.4% of patients for OLZ/SAM and olanzapine, respectively), and the overall mean olanzapine dose level (time-weighted average over the study) was 16.8 mg for the OLZ/SAM group and 16.9 mg for the olanzapine group.34
Table 1.
Baseline Characteristics of Participants in the Post Hoc Analyses of Cardiometabolic Risk Factors in the ENLIGHTEN-2 Study
| Parameter | OLZ/SAM | Olanzapine |
|---|---|---|
| Randomized and received ≥1 dose, n | 274 | 276 |
| Completed, n (%) | 176 (64.2) | 176 (63.8) |
| Had ≥1 postbaseline weight assessment, n | 266 | 272 |
| Age (years), Mean (SD) | 40.3 (9.82) | 40.1 (10.05) |
| Male, n (%) | 188/266 (70.7) | 203/272 (74.6) |
| Race, n (%) | ||
| Black | 193/266 (72.6) | 190/272 (69.9) |
| White | 61/266 (22.9) | 64/272 (23.5) |
| BMI (kg/m2), Mean (SD) | 25.31 (3.1) | 25.48 (3.2) |
| BMI <30 kg/m2, n (%) | 266/266 (100.0) | 270/272 (99.3) |
| BMI ≥30 kg/m2, n (%) | 0/266 | 2/272 (0.7) |
| Body weight (kg), Mean (SD) | 77.00 (13.7) | 77.45 (13.5) |
| Waist circumference (cm), Mean (SD) | 90.8 (10.9) | 90.9 (10.6) |
| Waist circumference >80 cm (females) or >102 cm (males), n (%) | 75/265 (28.3) | 80/270 (29.6) |
| Metabolic syndrome, n (%) | 33/265 (12.5) | 23/270 (8.5) |
| Systolic blood pressure (mm Hg), Mean (SD)a | 121.4 (12.7) | 121.8 (11.9) |
| Diastolic blood pressure (mm Hg), Mean (SD)a | 77.2 (9.1) | 77.3 (9.6) |
| Hypertension, n (%) | ||
| Stage 1 (130–139/80–89 mm Hg) | 96/266 (36.1) | 86/272 (31.6) |
| Stage 2(≥140/≥90 mm Hg) | 35/266 (13.2) | 38/272 (14.0) |
| Plasma glucose (mg/dL), Mean (SD)b | 90.3 (11.6) | 91.4 (12.0) |
| HbA1c (%), Mean (SD)c | 5.40 (0.4) | 5.40 (0.4) |
| HOMA-IR, Mean (SD)d | 3.03 (6.4) | 2.88 (4.1) |
| Total cholesterol (mg/dL), Mean (SD)e | 183.4 (34.7) | 185.2 (37.3) |
| LDL cholesterol (mg/dL), Mean (SD)e | 109.6 (32.3) | 112.7 (34.0) |
| HDL cholesterol (mg/dL), Mean (SD)e | 62.4 (22.4) | 62.1 (21.0) |
| Triglycerides (mg/dL), Mean (SD)e | 114.4 (94.0) | 107.1 (62.1) |
aMeasured in supine position after 5 min at rest.
bUpper reference value in an otherwise healthy population for fasting plasma glucose is 100 mg/dL.62
cUpper reference value in an otherwise healthy population for HbA1c concentration is 5.7%.62
dUpper reference value in an otherwise healthy population for HOMA-IR is 2.0.63
eOptimal lipid levels are <150 mg/dL total cholesterol, <100 mg/dL LDL cholesterol, and <150 mg/dL triglycerides; the reference range for HDL cholesterol varies by gender.64
BMI, body mass index; HbA1c, glycosylated hemoglobin; HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; LDL, low-density lipoprotein; OLZ/SAM, combination of olanzapine and samidorphan.
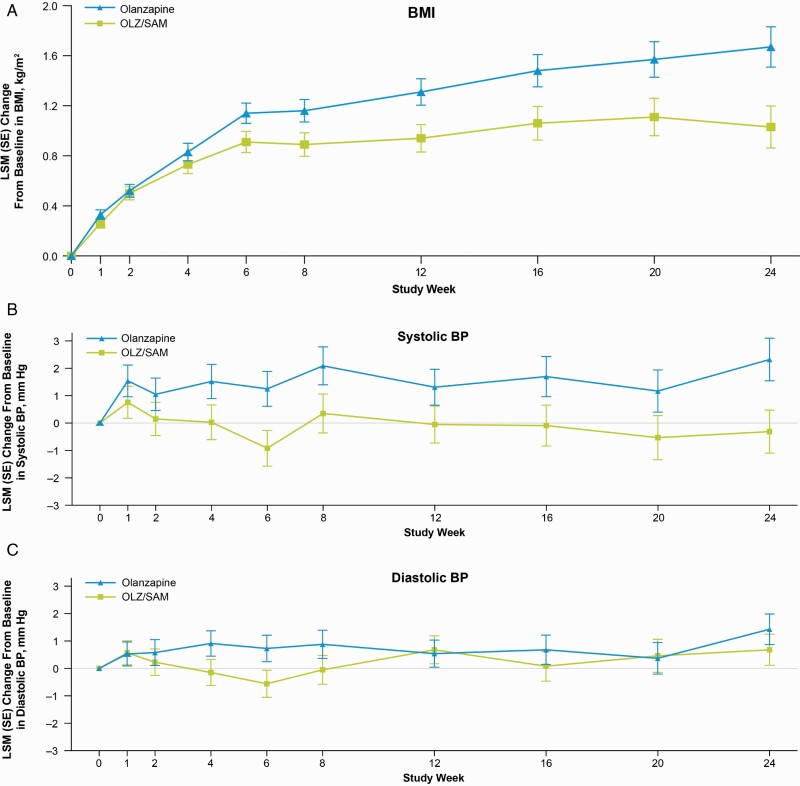
OLZ/SAM was associated with smaller increases in BMI from baseline to week 24 relative to olanzapine (LSM difference, −0.65 kg/m2; 95% CI, −1.01, −0.28) (figure 1A). Additionally, OLZ/SAM was associated with a smaller proportion of patients (15.1%) who met criteria for obesity (ie, BMI ≥30 kg/m2) at week 24 than was olanzapine (25.8%; OR, 0.52; 95% CI: 0.32, 0.82), with a number needed to treat (NNT) of 10.
Fig. 1.
By visit least-squares mean change from baseline in (A) body mass index,a (B) systolic blood pressure,b and (C) diastolic blood pressureb in ENLIGHTEN-2. aBased on an analysis of covariance approach using multiple imputation for missing post-baseline assessments. The model included treatment, race, and age group as factors and the baseline value as a covariate. LSM difference (95% CI) of OLZ/SAM versus olanzapine in BMI: −0.65 kg/m2 (−1.01, −0.28). bBased on an analysis of covariance or logistic regression approach using multiple imputation for missing post-baseline assessments. The model included treatment group as a factor and the baseline value as a covariate. LSM difference (95% CI) for OLZ/SAM versus olanzapine in BP: systolic BP: −2.63 mm Hg (−4.78, −0.47); diastolic BP: −0.75 mm Hg (−2.31, 0.80). BMI, body mass index; BP, blood pressure; HbA1c, glycosylated hemoglobin; LDL, low-density lipoprotein; LSM, least-squares mean; MetS, metabolic syndrome; OLZ/SAM, combination of olanzapine and samidorphan
At week 24, treatment with olanzapine was associated with an increase in supine systolic blood pressure (LSM [95% CI] change, 2.32 [0.79, 3.85] mm Hg), whereas OLZ/SAM was not (LSM [95% CI] change, −0.31 [−1.84, 1.23] mm Hg) (figure 1B). The LSM difference for OLZ/SAM versus olanzapine for change in supine systolic blood pressure at week 24 was −2.63 mm Hg (95% CI: −4.78, −0.47). Diastolic blood pressure increased in both treatment groups (LSM [95% CI] change of 0.68 [−0.43, 1.79] mm Hg for OLZ/SAM and 1.43 [0.33, 2.52] mm Hg for olanzapine), with an LSM difference for OLZ/SAM vs olanzapine of −0.75 mm Hg (95% CI: −2.31, 0.80) (figure 1C). At baseline, 49.2% in the OLZ/SAM treatment group and 45.6% in the olanzapine treatment group had blood pressure readings in the hypertensive range. Among those with normal blood pressure at baseline, the risk of shifting to stage 1/2 hypertension was reduced by approximately 50% with OLZ/SAM compared with olanzapine at week 24 (OR, 0.48; 95% CI: 0.24, 0.96) with an NNT of 7 (table 2). Among patients with normal/elevated blood pressure at baseline, the risk of shifting to stage 1 or 2 hypertension was reduced by approximately one third with OLZ/SAM compared with olanzapine at week 24 (OR, 0.66; 95% CI: 0.38, 1.17), with an NNT of 12.
Table 2.
Risk of Shifting From Normal or Normal/Elevated Blood Pressure Levels to Stage 1/2 Hypertension in ENLIGHTEN-2
| Shift Category | Patients Meeting Criteria, n/m (%) |
Odds Ratioa (95% CI) |
NNT | |
|---|---|---|---|---|
| OLZ/SAM | Olanzapine | |||
| Normal to stage 1/2 hypertensionb | 22/100 (21.6) |
41/112 (36.6) |
0.48 (0.24, 0.96) |
7 |
| Normal/elevated to stage 1/2 hypertensionb | 38/135 (28.2) |
55/148 (37.3) |
0.66 (0.38, 1.17) |
12 |
aOLZ/SAM was compared with olanzapine using a logistic regression model and a multiple imputation approach for missing data. The model included treatment, race (black or African American, non-black or African American) and age group (age <30 years, age ≥30 years), and treatment as factors and baseline value as covariate.
bBlood pressure definitions: normal, <120/<80 mm Hg; normal/elevated, ≥120 to ≤129/<80 mm Hg; stage 1 hypertension, ≥130 to ≤139/≥80 to ≤89 mm Hg; and stage 2 hypertension, ≥140/≥90 mm Hg.
n/m, number of responders who met criteria at week 24/number of participants who met baseline criteria; NNT, number needed to treat; OLZ/SAM, combination of olanzapine and samidorphan.
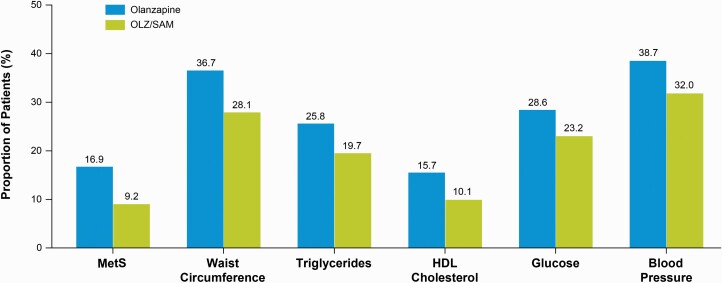
Overall, 33/265 (12.5%) of patients randomized to OLZ/SAM and 23/270 (8.5%) of patients randomized to olanzapine met criteria for metabolic syndrome at baseline. The proportion of patients without metabolic syndrome at baseline who went on to develop metabolic syndrome by the time of their last assessment was smaller in the OLZ/SAM group (21/228; 9.2%) than the olanzapine group (42/248; 16.9%). This translates to an approximate 45% reduction in the risk of developing metabolic syndrome with OLZ/SAM (OR, 0.55; 95% CI: 0.31, 0.99; NNT = 20) compared with olanzapine (figure 2). Additionally, among patients without metabolic syndrome at baseline, a numerically smaller proportion of patients treated with OLZ/SAM went on to meet any individual component criterion of metabolic syndrome compared with those treated with olanzapine, although the differences did not reach statistical significance (NNT range: 20–23). Among patients without metabolic syndrome at baseline, the proportions of patients with waist circumference >80 cm (for females) or >102 cm (for males) at their last assessment were numerically smaller with OLZ/SAM treatment (64/228 [28.1%]) than with olanzapine treatment (91/248 [36.7%]; OR, 0.76; 95% CI: 0.49, 1.19; NNT = 21).
Fig. 2.
Proportions of patients without metabolic syndrome at baseline who developed metabolic syndrome or any component criterion of metabolic syndrome.a,b, aOdds ratio (95% CI) for the development of MetS during treatment with OLZ/SAM vs olanzapine, at the last on-treatment assessment, in those without MetS at baseline: 0.55 (0.31, 0.99). bThe proportion of patients who developed metabolic syndrome or who developed individual metabolic syndrome parameter criteria were compared using a logistic regression model based on observed data at the patients’ last on-treatment assessments. The model included treatment, race (black, non-black), and age group (<30 years, ≥30 years) as factors, and baseline body mass index as covariate. HDL, high-density lipoprotein; MetS, metabolic syndrome; OLZ/SAM, combination of olanzapine and samidorphan.
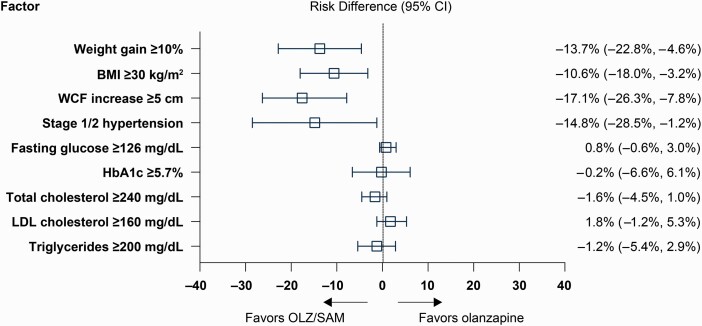
Risk differences for several factors known to be associated with increased cardiometabolic risk favored OLZ/SAM over olanzapine at week 24, including weight gain of ≥10% (absolute risk difference, −13.7%; 95% CI: −22.8%, −4.6%; NNT = 8), having a BMI ≥30 kg/m2 (−10.6%; 95% CI: −18.0%, −3.2%; NNT = 10), waist circumference increases of ≥5 cm (−17.1%; 95% CI: −26.3%, −7.8%; NNT = 6), and progression to stage 1/2 hypertension (−14.8%; 95% CI: −28.5%, −1.2%; NNT = 7) (figure 3). Mean changes in lipid and glycemic parameters between baseline and week 24 were generally small, as previously reported.34 No significant between-group LSM (SE) differences were observed for changes from baseline for HOMA-IR (LSM [SE] difference vs olanzapine, 0.08 [0.87]; 95% CI: −1.64, 1.80), insulin (LSM [SE] difference vs olanzapine, −0.68 [2.47]; 95% CI: −5.54, 4.18), or HbA1c (LSM [SE] difference vs olanzapine, −0.01 [0.03]; 95% CI: −0.06, 0.05) at week 24. No between-group differences were observed in the risk of developing sustained potentially clinically significant hyperglycemia (fasting glucose, ≥126 mg/dL and HbA1c ≥5.7%) or hyperlipidemia (total cholesterol ≥240 mg/dL, LDL cholesterol ≥160 mg/dL, and triglycerides ≥200 mg/dL) while on treatment (figure 3).
Fig. 3.
Cardiometabolic parameter risk differences with OLZ/SAM compared with olanzapine.a aThe odds ratio (95% CI) for developing obesity (BMI ≥30 kg/m2) with use of OLZ/SAM versus olanzapine was 0.52 (0.32, 0.82). Risk differences for shifts in metabolic laboratory parameters were based on sustained potentially clinically significant shifts (ie, the parameters met shift criteria at the last 2 on-treatment assessments). BMI, body mass index; HbA1c, glycosylated hemoglobin; LDL, low-density lipoprotein; OLZ/SAM, combination of olanzapine and samidorphan; WCF, waist circumference.
Discussion
In the current analyses, OLZ/SAM mitigated olanzapine-associated increases in BMI and supine blood pressure and reduced the risk of developing stage 1/2 hypertension, obesity, and metabolic syndrome—all well-established risk factors for cardiovascular morbidity and mortality.1,3,4 These post hoc analyses of the ENLIGHTEN-2 study build upon the primary results in which OLZ/SAM mitigated olanzapine-associated weight gain and increases in waist circumference.34 In ENLIGHTEN-2, olanzapine was associated with continued weight gain over the course of the 24-week study, whereas with OLZ/SAM, after an initial period of weight gain over the first 4 to 6 weeks of treatment, weight stabilized for the remainder of the treatment period.34 The significantly smaller increases in BMI and waist circumference and the reduced risk of obesity with OLZ/SAM vs olanzapine treatment are thus consistent with the pattern of weight gain mitigation observed in the primary analysis.34 Moreover, the magnitude of risk reduction is consistent with that previously observed in ENLIGHTEN-2 in which, based on the calculated ORs, the risks of gaining ≥10% or ≥7% of baseline body weight, or of having a waist circumference increase of ≥5 cm at week 24, were each reduced by about 50% with OLZ/SAM vs olanzapine.34 Similarly, in the current analyses, the risks of incident metabolic syndrome, obesity, or stage 1/2 hypertension were also reduced by about 50% with OLZ/SAM versus olanzapine.
Additionally, OLZ/SAM treatment was associated with reductions in cardiometabolic risk factors relative to olanzapine. At week 24, supine systolic blood pressure was 2.63 mm Hg lower in OLZ/SAM-treated patients than in olanzapine-treated patients, while supine diastolic blood pressure was generally similar between groups. These findings are consistent with experimental weight gain studies that suggested that even moderate weight gain (approximately 5% from baseline) can increase systolic blood pressure with minimal changes to diastolic blood pressure.44–46 Because increased systolic blood pressure correlates most substantially with central fat accumulation,44–46 the ability of OLZ/SAM to mitigate olanzapine-associated increases in waist circumference,34 a proxy measure of central adiposity, may explain the observed reduction in supine systolic blood pressure with minimal effects on diastolic blood pressure in the current study. While other potential mechanisms contributing to this difference have not been evaluated, evidence suggests a continuous relationship between blood pressure and risk of cardiovascular mortality, such that incremental reductions as small as 2 mm Hg in systolic blood pressure are associated with long-term reduction in risk of death from stroke and ischemic heart disease.41 Therefore, in reducing the risk of an increase in supine systolic blood pressure observed with olanzapine treatment, the stability in blood pressure observed with OLZ/SAM may result in longer-term, clinically meaningful morbidity and mortality benefits.
Antipsychotic-associated weight gain is a common17,18,21,22 and major concern for patients with schizophrenia for a host of psychological, biological, and mortality-related reasons. Weight gain often leads to central obesity, which, in turn, drives adverse metabolic consequences, such as hypertension, type 2 diabetes, and dyslipidemia.1,27 Epidemiologic studies have reported that body weight increases of as little as 1 to 5 kg raise the risk of cardiovascular disease and diabetes mellitus.47–49 Similarly, the risk of cardiovascular mortality increases exponentially as patients progress from being overweight to obese,50 and an increased waist circumference also increases cardiometabolic risk.51
Patients with schizophrenia are already known to be at increased risk for cardiovascular disease and cardiovascular-related mortality compared with the general population, contributing to a 15- to 20-year shorter life expectancy.52,53 Additionally, they have an increased prevalence of obesity, hyperglycemia, hypertension, and lipid abnormalities, leading to greater rates of metabolic syndrome compared with the general population.1,18,21,22,54–56 In the primary ENLIGHTEN-2 analyses, treatment with OLZ/SAM was associated with a reduced risk of 2 important cardiometabolic risk factors: significant weight gain and waist circumference increase of ≥5 cm.34 In the post hoc analyses, risk differences favoring OLZ/SAM over olanzapine were also observed with respect to the percentage of patients without obesity or hypertension at baseline who subsequently developed obesity or progressed to stage 1/2 hypertension, respectively, at week 24. Consistent with these findings, the proportion of patients who developed metabolic syndrome was reduced by nearly 50% for OLZ/SAM vs olanzapine, and fewer patients (among those who did not have metabolic syndrome at baseline) met any of the individual metabolic syndrome component criteria at study end. Interestingly, glycemic and lipid parameter changes were small and were similar between groups over the 24-week study,34 with no differences in the proportion of patients who developed hyperglycemia or hyperlipidemia.
The explanation for why OLZ/SAM and olanzapine differed in their effects on some cardiometabolic risk measures and not others is unknown. Data from previous studies suggest that the observed impact of samidorphan on olanzapine-associated weight gain and cardiometabolic dysregulation may be due to samidorphan’s effects on food reward centrally57 and insulin resistance peripherally.58 However, in the current study, measures of insulin levels and insulin resistance revealed no statistically significant differences between the olanzapine and OLZ/SAM groups, suggesting that other mechanisms may play a role. The lack of observed OLZ/SAM versus olanzapine differences in glycemic and lipid parameters may relate in part to the 24-week duration of the study, which would be sufficient to detect early, weight-independent effects on metabolic parameters59,60 but perhaps not long enough to detect potential weight-dependent changes and, even further, the impact of mitigating weight gain.
The analyses presented here are limited by their post hoc nature. Additionally, overall study limitations include the fact that nearly 40% of patients discontinued treatment early (like other 6-month studies of antipsychotics in schizophrenia61), with greater dropout in the olanzapine group than in the OLZ/SAM group. Further limitations include that self-reported fasting status was not independently confirmed and that enrollment was limited to patients younger than 55 years. Additionally, the restrictive BMI entry criterion of 18 to 30 kg/m2, and exclusion of patients with significant metabolic abnormalities, especially among patients with longstanding illness and antipsychotic treatment, could have enriched the population with patients less susceptible to antipsychotic-associated weight gain and metabolic dysregulation.34 Also, blood pressure measurements in ENLIGHTEN-2 may not have been conducted under the same rigor as in a dedicated hypertension study. Finally, the 24-week randomized double-blind treatment period versus olanzapine alone is a limitation when weight gain and increasing risk of adverse metabolic effects are documented to continue with life-long olanzapine therapy. Further prospective research in larger populations in real-world settings is needed to corroborate these findings.
In conclusion, these post hoc analyses build on earlier findings from ENLIGHTEN-2, where OLZ/SAM mitigated olanzapine-associated weight gain. Patients with schizophrenia were less likely to experience worsening of certain cardiometabolic risk factors related to obesity, hypertension, and metabolic syndrome when treated with OLZ/SAM compared with olanzapine.
Supplementary Material
Acknowledgments
The authors thank the ALK3831-A303 Study Group, as well as Mark S. Todtenkopf, PhD, of Alkermes, Inc., who assisted in the preparation and proofreading of the manuscript. Medical writing and editorial support were provided by Laurie LaRusso, MS, and John H. Simmons, MD, of Peloton Advantage, LLC, an OPEN Health company, and funded by Alkermes, Inc. C.U.C. has served as a consultant and/or advisor to or has received honoraria from AbbVie, Acadia, Alkermes, Allergan, Angelini, Aristo, Axsome, Cardio Diagnostics, CNX Therapeutics, Compass Pathways, Damitsa, Gedeon Richter, Hikma, Holmusk, Intra-Cellular Therapies, Janssen/Johnson & Johnson, Karuna, LB Pharma, Lundbeck, MedAvante-ProPhase, MedinCell, Medscape, Merck, MindPax, Mitsubishi Tanabe Pharma, Mylan, Neurocrine, Noven, Otsuka, Pfizer, Pharmabrain, Recordati, Relmada, Reviva, Rovi, Seqirus, Servier, SK Life Science, Sumitomo Dainippon, Sunovion, Sun Pharma, Supernus, Takeda, Teva, and Viatris. He provided expert testimony for Janssen and Otsuka. He served on a Data Safety Monitoring Board for Lundbeck, Relmada, Reviva, Rovi, and Teva. He has received grant support from Janssen and Takeda. He received royalties from UpToDate and is also a stock option holder of Cardio Diagnostics, MindPax, and LB Pharma. E.S. has received consulting fees from Alkermes. C.G., L.D., S.A., A.D.S., Y.J., S.Y., and C.H. are or were employees of Alkermes, Inc., and may own stock/options in the company. D.M. is an employee of Alkermes Pharma Ireland Ltd and may own stock/options in the company.
Contributor Information
Christoph U Correll, Department of Psychiatry, Zucker Hillside Hospital, Northwell Health, Glen Oaks, NY, USA; Department of Psychiatry and Molecular Medicine, Donald and Barbara Zucker School of Medicine at Hofstra/Northwell, Hempstead, NY, USA; Department of Child and Adolescent Psychiatry, Charité Universitätsmedizin, Berlin, Germany.
Evan Stein, Metabolic & Atherosclerosis Research Center, Cincinnati, OH, USA.
Christine Graham, Alkermes, Inc., Waltham, MA, USA.
Lauren DiPetrillo, Alkermes, Inc., Waltham, MA, USA.
Sarah Akerman, Alkermes, Inc., Waltham, MA, USA.
Arielle D Stanford, Alkermes, Inc., Waltham, MA, USA.
Ying Jiang, Alkermes, Inc., Waltham, MA, USA.
Sergey Yagoda, Alkermes, Inc., Waltham, MA, USA.
David McDonnell, Alkermes Pharma Ireland Ltd., Dublin, Ireland.
Craig Hopkinson, Alkermes, Inc., Waltham, MA, USA.
Funding
This work was supported by Alkermes, Inc.
References
- 1. Firth J, Siddiqi N, Koyanagi A, et al. The Lancet Psychiatry Commission: a blueprint for protecting physical health in people with mental illness. Lancet Psychiatry. 2019;6(8):675–712. [DOI] [PubMed] [Google Scholar]
- 2. Pillinger T, D’Ambrosio E, McCutcheon R, Howes OD.. Is psychosis a multisystem disorder? A meta-review of central nervous system, immune, cardiometabolic, and endocrine alterations in first-episode psychosis and perspective on potential models. Mol Psychiatry. 2019;24(6):776–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Han TS, Lean ME.. A clinical perspective of obesity, metabolic syndrome and cardiovascular disease. JRSM Cardiovasc Dis. 2016;5:2048004016633371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Correll CU, Solmi M, Veronese N, et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: a large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry. 2017;16(2):163–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Correll CU, Detraux J, De Lepeleire J, De Hert M.. Effects of antipsychotics, antidepressants and mood stabilizers on risk for physical diseases in people with schizophrenia, depression and bipolar disorder. World Psychiatry. 2015;14(2):119–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353(12):1209–1223. [DOI] [PubMed] [Google Scholar]
- 7. Huhn M, Nikolakopoulou A, Schneider-Thoma J, et al. Comparative efficacy and tolerability of 32 oral antipsychotics for the acute treatment of adults with multi-episode schizophrenia: a systematic review and network meta-analysis. Lancet. 2019;394(10202):939–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kishimoto T, Hagi K, Nitta M, Kane JM, Correll CU.. Long-term effectiveness of oral second-generation antipsychotics in patients with schizophrenia and related disorders: a systematic review and meta-analysis of direct head-to-head comparisons. World Psychiatry. 2019;18(2):208–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cipriani A, Barbui C, Salanti G, et al. Comparative efficacy and acceptability of antimanic drugs in acute mania: a multiple-treatments meta-analysis. Lancet. 2011;378(9799):1306–1315. [DOI] [PubMed] [Google Scholar]
- 10. Yildiz A, Nikodem M, Vieta E, Correll CU, Baldessarini RJ.. A network meta-analysis on comparative efficacy and all-cause discontinuation of antimanic treatments in acute bipolar mania. Psychol Med. 2015;45(2):299–317. [DOI] [PubMed] [Google Scholar]
- 11. Haro JM, Suarez D, Novick D, Brown J, Usall J, Naber D.. Three-year antipsychotic effectiveness in the outpatient care of schizophrenia: observational versus randomized studies results. Eur Neuropsychopharmacol. 2007;17(4):235–244. [DOI] [PubMed] [Google Scholar]
- 12. Kahn RS, Fleischhacker WW, Boter H, et al. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet. 2008;371(9618):1085–1097. [DOI] [PubMed] [Google Scholar]
- 13. Berkowitz RL, Patel U, Ni Q, Parks JJ, Docherty JP.. The impact of the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) on prescribing practices: an analysis of data from a large midwestern state. J Clin Psychiatry. 2012;73(4):498–503. [DOI] [PubMed] [Google Scholar]
- 14. Ketter TA, Haupt DW.. Perceptions of weight gain and bipolar pharmacotherapy: results of a 2005 survey of physicians in clinical practice. Curr Med Res Opin. 2006;22(12):2345–2353. [DOI] [PubMed] [Google Scholar]
- 15. Zhang Y, Wang Q, Reynolds GP, et al. Metabolic effects of 7 antipsychotics on patients with schizophrenia: a short-term, randomized, open-label, multicenter, pharmacologic trial. J Clin Psychiatry. 2020;81(3):19m–12785. [DOI] [PubMed] [Google Scholar]
- 16. Citrome L, Holt RI, Walker DJ, Hoffmann VP.. Weight gain and changes in metabolic variables following olanzapine treatment in schizophrenia and bipolar disorder. Clin Drug Investig. 2011;31(7):455–482. [DOI] [PubMed] [Google Scholar]
- 17. Pillinger T, McCutcheon RA, Vano L, et al. Comparative effects of 18 antipsychotics on metabolic function in patients with schizophrenia, predictors of metabolic dysregulation, and association with psychopathology: a systematic review and network meta-analysis. Lancet Psychiatry. 2020;7(1):64–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. De Hert M, Detraux J, van Winkel R, Yu W, Correll CU.. Metabolic and cardiovascular adverse effects associated with antipsychotic drugs. Nat Rev Endocrinol. 2012;8(2):114–126. [DOI] [PubMed] [Google Scholar]
- 19. Baker RA, Pikalov A, Tran QV, Kremenets T, Arani RB, Doraiswamy PM.. Atypical antipsychotic drugs and diabetes mellitus in the US Food and Drug Administration Adverse Event database: a systematic Bayesian signal detection analysis. Psychopharmacol Bull. 2009;42(1):11–31. [PubMed] [Google Scholar]
- 20. Moisan J, Gregoire JP, Gaudet M, Cooper D.. Exploring the risk of diabetes mellitus and dyslipidemia among ambulatory users of atypical antipsychotics: a population-based comparison of risperidone and olanzapine. Pharmacoepidemiol Drug Saf. 2005;14(6):427–436. [DOI] [PubMed] [Google Scholar]
- 21. Vancampfort D, Correll CU, Galling B, et al. Diabetes mellitus in people with schizophrenia, bipolar disorder and major depressive disorder: a systematic review and large scale meta-analysis. World Psychiatry. 2016;15(2):166–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vancampfort D, Stubbs B, Mitchell AJ, et al. Risk of metabolic syndrome and its components in people with schizophrenia and related psychotic disorders, bipolar disorder and major depressive disorder: a systematic review and meta-analysis. World Psychiatry. 2015;14(3):339–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kinon BJ, Basson BR, Gilmore JA, Tollefson GD.. Long-term olanzapine treatment: weight change and weight-related health factors in schizophrenia. J Clin Psychiatry. 2001;62(2):92–100. [PubMed] [Google Scholar]
- 24. Schoemaker J, Naber D, Vrijland P, Panagides J, Emsley R.. Long-term assessment of asenapine vs. olanzapine in patients with schizophrenia or schizoaffective disorder. Pharmacopsychiatry. 2010;43(4):138–146. [DOI] [PubMed] [Google Scholar]
- 25. Dayabandara M, Hanwella R, Ratnatunga S, Seneviratne S, Suraweera C, de Silva VA.. Antipsychotic-associated weight gain: management strategies and impact on treatment adherence. Neuropsychiatr Dis Treat. 2017;13:2231–2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hasan A, Falkai P, Wobrock T, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of schizophrenia, part 2: update 2012 on the long-term treatment of schizophrenia and management of antipsychotic-induced side effects. World J Biol Psychiatry. 2013;14(1):2–44. [DOI] [PubMed] [Google Scholar]
- 27. Correll CU, Lencz T, Malhotra AK.. Antipsychotic drugs and obesity. Trends Mol Med. 2011;17(2):97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Roerig JL, Steffen KJ, Mitchell JE.. Atypical antipsychotic-induced weight gain: insights into mechanisms of action. CNS Drugs. 2011;25(12):1035–1059. [DOI] [PubMed] [Google Scholar]
- 29. Bodnar RJ. Endogenous opioid modulation of food intake and body weight: Implications for opioid influences upon motivation and addiction. Peptides. 2019;116:42–62. [DOI] [PubMed] [Google Scholar]
- 30. Czyzyk TA, Nogueiras R, Lockwood JF, et al. kappa-Opioid receptors control the metabolic response to a high-energy diet in mice. FASEB J. 2010;24(4):1151–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Czyzyk TA, Romero-Pico A, Pintar J, et al. Mice lacking delta-opioid receptors resist the development of diet-induced obesity. FASEB J. 2012;26(8):3483–3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Tudurí E, Beiroa D, Stegbauer J, et al. Acute stimulation of brain mu opioid receptors inhibits glucose-stimulated insulin secretion via sympathetic innervation. Neuropharmacology. 2016;110(Pt A):322–332. [DOI] [PubMed] [Google Scholar]
- 33. Tabarin A, Diz-Chaves Y, Carmona Mdel C, et al. Resistance to diet-induced obesity in mu-opioid receptor-deficient mice: evidence for a “thrifty gene.” Diabetes. 2005;54(12):3510–3516. [DOI] [PubMed] [Google Scholar]
- 34. Correll CU, Newcomer JW, Silverman B, et al. Effects of olanzapine combined with samidorphan on weight gain in schizophrenia: a 24-week phase 3 study. Am J Psychiatry. 2020;177(12):1168–1178. [DOI] [PubMed] [Google Scholar]
- 35. Bidlack JM, Knapp BI, Deaver DR, et al. In vitro pharmacological characterization of buprenorphine, samidorphan, and combinations being developed as an adjunctive treatment of major depressive disorder. J Pharmacol Exp Ther. 2018;367(2):267–281. [DOI] [PubMed] [Google Scholar]
- 36. Shram MJ, Silverman B, Ehrich E, Sellers EM, Turncliff R.. Use of remifentanil in a novel clinical paradigm to characterize onset and duration of opioid blockade by samidorphan, a potent mu-receptor antagonist. J Clin Psychopharmacol. 2015;35(3):242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lybalvi [package insert]. Waltham, MA: Alkermes, Inc.; 2021. [Google Scholar]
- 38. Martin WF, Correll CU, Weiden PJ, et al. Mitigation of olanzapine-induced weight gain with samidorphan, an opioid antagonist: a randomized double-blind phase 2 study in patients with schizophrenia. Am J Psychiatry. 2019;176(6):457–467. [DOI] [PubMed] [Google Scholar]
- 39. Potkin SG, Kunovac J, Silverman BL, et al. Efficacy and safety of a combination of olanzapine and samidorphan in adult patients with an acute exacerbation of schizophrenia: outcomes from the randomized, phase 3 ENLIGHTEN-1 study. J Clin Psychiatry. 2020;81(2):19m–12769. [DOI] [PubMed] [Google Scholar]
- 40. Cerhan JR, Moore SC, Jacobs EJ, et al. A pooled analysis of waist circumference and mortality in 650,000 adults. Mayo Clin Proc. 2014;89(3):335–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R.. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet. 2002;360(9349):1903–1913. [DOI] [PubMed] [Google Scholar]
- 42. McNeill AM, Rosamond WD, Girman CJ, et al. The metabolic syndrome and 11-year risk of incident cardiovascular disease in the atherosclerosis risk in communities study. Diabetes Care. 2005;28(2):385–390. [DOI] [PubMed] [Google Scholar]
- 43. Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Circulation. 2005;112(17):2735–2752. [DOI] [PubMed] [Google Scholar]
- 44. Covassin N, Sert-Kuniyoshi FH, Singh P, et al. Experimental weight gain increases ambulatory blood pressure in healthy subjects: implications of visceral fat accumulation. Mayo Clin Proc. 2018;93(5):618–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gentile CL, Orr JS, Davy BM, Davy KP.. Modest weight gain is associated with sympathetic neural activation in nonobese humans. Am J Physiol Regul Integr Comp Physiol. 2007;292(5):R1834–R1838. [DOI] [PubMed] [Google Scholar]
- 46. Orr JS, Gentile CL, Davy BM, Davy KP.. Large artery stiffening with weight gain in humans: role of visceral fat accumulation. Hypertension. 2008;51(6):1519–1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zheng Y, Manson JE, Yuan C, et al. Associations of weight gain from early to middle adulthood with major health outcomes later in life. JAMA. 2017;318(3):255–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Choi S, Kim K, Kim SM, et al. Association of obesity or weight change with coronary heart disease among young adults in South Korea. JAMA Intern Med. 2018;178(8):1060–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Larsson SC, Bäck M, Rees JMB, Mason AM, Burgess S.. Body mass index and body composition in relation to 14 cardiovascular conditions in UK Biobank: a Mendelian randomization study. Eur Heart J. 2020;41(2):221–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ebbert JO, Elrashidi MY, Jensen MD.. Managing overweight and obesity in adults to reduce cardiovascular disease risk. Curr Atheroscler Rep. 2014;16(10):445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Neeland IJ, Ross R, Després JP, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol. 2019;7(9):715–725. [DOI] [PubMed] [Google Scholar]
- 52. Ignaszewski MJ, Yip A, Fitzpatrick S.. Schizophrenia and coronary artery disease. B C Med J. 2015;57(4):154–157. [Google Scholar]
- 53. Ringen PA, Engh JA, Birkenaes AB, Dieset I, Andreassen OA.. Increased mortality in schizophrenia due to cardiovascular disease: a non-systematic review of epidemiology, possible causes, and interventions. Front Psychiatry. 2014;5:137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cohen D, Stolk RP, Grobbee DE, Gispen-de Wied CC.. Hyperglycemia and diabetes in patients with schizophrenia or schizoaffective disorders. Diabetes Care. 2006;29(4):786–791. [DOI] [PubMed] [Google Scholar]
- 55. Dixon L, Weiden P, Delahanty J, et al. Prevalence and correlates of diabetes in national schizophrenia samples. Schizophr Bull. 2000;26(4):903–912. [DOI] [PubMed] [Google Scholar]
- 56. McEvoy JP, Meyer JM, Goff DC, et al. Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res. 2005;80(1):19–32. [DOI] [PubMed] [Google Scholar]
- 57. Cunningham JI, Eyerman DJ, Todtenkopf MS, et al. Samidorphan mitigates olanzapine-induced weight gain and metabolic dysfunction in rats and nonhuman primates. J Psychopharmacol. 2019;33(10):1303–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Toledo FGS, Martin WF, Morrow L, et al. Insulin and glucose metabolism with olanzapine and a combination of olanzapine and samidorphan: exploratory phase 1 results in healthy volunteers. Neuropsychopharmacology. 2022;47(3):696–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Teff KL, Rickels MR, Grudziak J, Fuller C, Nguyen HL, Rickels K.. Antipsychotic-induced insulin resistance and postprandial hormonal dysregulation independent of weight gain or psychiatric disease. Diabetes. 2013;62(9):3232–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Rickels MR, Perez EM, Peleckis AJ, et al. Contribution of parasympathetic muscarinic augmentation of insulin secretion to olanzapine-induced hyperinsulinemia. Am J Physiol Endocrinol Metab. 2018;315(2):E250–E257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Breier A, Berg PH, Thakore JH, et al. Olanzapine versus ziprasidone: results of a 28-week double-blind study in patients with schizophrenia. Am J Psychiatry. 2005;162(10):1879–1887. [DOI] [PubMed] [Google Scholar]
- 62. American Diabetes Association. Standards of medical care in diabetes—2022. Diabetes Care. 2022;45(suppl 1):S1–S264. [DOI] [PubMed] [Google Scholar]
- 63. Salgado AL, Carvalho L, Oliveira AC, Santos VN, Vieira JG, Parise ER.. Insulin resistance index (HOMA-IR) in the differentiation of patients with non-alcoholic fatty liver disease and healthy individuals. Arq Gastroenterol. 2010;47(2):165–169. [DOI] [PubMed] [Google Scholar]
- 64. Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol. Circulation. 2019;139(25):e1082–e1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.