Abstract
Background and Hypothesis
To identify promising drug targets for psychiatric disorders, we applied Mendelian randomization (MR) design to systematically screen blood metabolome for potential mediators of psychiatric disorders and further predict target-mediated side effects.
Study Design
We selected 92 unique blood metabolites from 3 metabolome genome-wide association studies (GWASs) with totally 147 827 participants. Summary statistics for bipolar disorder (BIP), attention deficit hyperactivity disorder (ADHD), obsessive-compulsive disorder (OCD), major depressive disorder (MDD), schizophrenia (SCZ), panic disorder (PD), autistic spectrum disorder (ASD), and anorexia nervosa (AN) originated from the Psychiatric Genomics Consortium, involving 1 143 340 participants. Mendelian randomization (MR) analyses were conducted to estimate associations of blood metabolites with psychiatric disorders. Phenome-wide MR analysis was further performed to predict side effects mediated by metabolite-targeted interventions.
Results
Eight metabolites were identified associated with psychiatric disorders, including five established mediators: N-acetylornithine (BIP: OR, 0.72 [95% CI, 0.66–0.79]; SCZ: OR, 0.74 [0.64–0.84]), glycine (BIP: OR, 0.62 [0.50–0.77]), docosahexaenoic acid (MDD: OR, 0.96 [0.94–0.97]), 3-Hydroxybutyrate (MDD: OR, 1.14 [1.08–1.21]), butyrylcarnitine (SCZ: OR, 1.22 [1.12–1.32]); and three novel mediators: 1-arachidonoylglycerophosphocholine (1-arachidonoyl-GPC)(BIP: OR, 0.31 [0.23–0.41]), glycoproteins (BIP: OR, 0.94 [0.92–0.97]), sphingomyelins (AN: OR, 1.12 [1.06–1.19]). Phenome-wide MR analysis showed that all identified metabolites except for N-acetylornithine and 3-Hydroxybutyrate had additional effects on nonpsychiatric diseases, while glycine, 3-Hydroxybutyrate, N-acetylornithine, and butyrylcarnitine had no adverse side effects.
Conclusions
This MR study identified five established and three novel mediators for psychiatric disorders. N-acetylornithine, glycine, 3-Hydroxybutyrate, and butyrylcarnitine might be promising targets against psychiatric disorders with no predicted adverse side effects.
Keywords: psychiatric disorders, metabolites, Mendelian randomization, biomarkers, antipsychotics
Introduction
Psychiatric disorders are characterized by unhealthy and inflexible patterns of thoughts and behavior,1 mainly including bipolar disorder (BIP), attention deficit hyperactivity disorder (ADHD), obsessive-compulsive disorder (OCD; a common anxiety disorder), major depressive disorder (MDD), schizophrenia (SCZ), panic disorder (PD; the most severe form of anxiety disorder), autistic spectrum disorder (ASD), and anorexia nervosa (AN).2 In 2017, there were over 970 million patients with psychiatric disorders worldwide, and psychiatric disorders accounted for 14.39% of years lived with disability.2 Although a number of antipsychotics have been discovered, a considerable proportion of patients still had a poor response to treatment3 and experienced deleterious side effects.4 Based on the high attrition rate of drug development, it is imperative to explore the potential mediators of psychiatric disorders before being tested in clinical trials.5
Recently, accumulating evidence suggested that metabolic abnormalities were implicated in the pathophysiology of psychiatric disorders.6 Nelson et al. reported that genomics could help to elucidate the causal pathways linking risk factors and disease, and that drug with genetically supported targets would be twice as likely to reach market approval.5 Recent advancements in mass spectrometry and high-throughput genotyping enabled genome-wide association studies (GWASs) to comprehensively reveal genetic determinants of metabolome.7–9 Such developments offered an opportunity to accurately identify promising drug targets for psychiatric disorders by integrating genomic and metabolomics data through Mendelian randomization (MR) design.
MR is an emerging analytic approach using genetic variants associated with exposures as instrumental variables to estimate the potential causal effects of exposures on outcomes.10 Notably, alleles are randomly allocated during gamete formation, so MR is considered as a “natural” RCT, which can minimize the confounding and reverse-causality biases.11 In addition, genetic effects are lifelong, and datasets from psychiatric disorders GWASs with large sample sizes are available now.12–19 Therefore, MR studies could simulate large-scale and long-term clinical trials for the primary and secondary prevention of psychiatric disorders, eliminating the expensive cost and time-consuming follow-up process. MR design has been widely applied to identify the potential mediators of BIP, ADHD, MDD, SCZ, and ASD from the blood metabolome in the previous studies, but the potential side effects associated with metabolite-targeted intervention remained unclear.20 Of note, a phenome-wide MR (Phe-MR) analysis for drug-induced side effects could help to identify unexpected adverse effects and provide opportunities for drug repurposing prior to clinical testing.21 In addition, although the MR evidence for the role of blood metabolites in the etiology of some psychiatric disorders (e.g., OCD, PD, and AN) is limited, GWAS data for these psychiatric disorders have been published.14,17,19
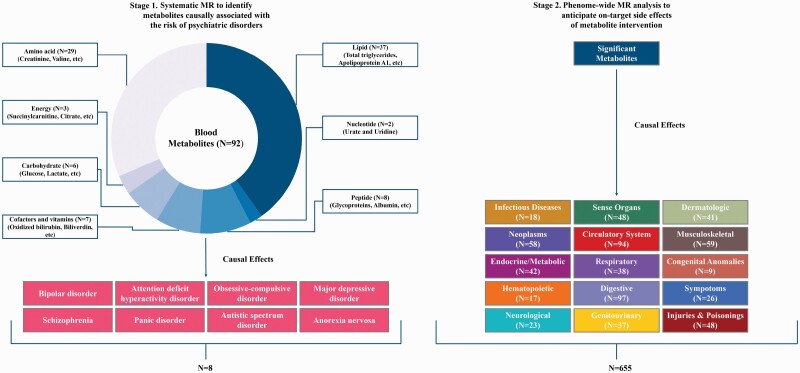
Herein, we conducted a two-stage MR study to identify promising drug targets for eight major psychiatric disorders, including BIP, ADHD, OCD, MDD, SCZ, PD, ASD, and AN. First, we systematically screened 92 circulating metabolites for the potential causal mediators of psychiatric disorders. Subsequently, we performed a Phe-MR analysis of 655 disease traits to predict on-target side effects of identified metabolites for a comprehensive appraisal of their clinical safety as intervention targets.
Methods
Study design
This two-stage MR study aimed to identify promising drug targets of psychiatric disorders from genetic perspective (figure 1). There were three core instrumental variable assumptions for MR study: (1) the genetic variants should be associated with the exposure; (2) the genetic variants should be independent of confounders; and (3) the genetic variants should influence the outcome only through the exposure. The summary-level data of the blood metabolome, psychiatric disorders, and 655 nonpsychiatric diseases utilized in the present study were derived from the publicly available GWASs based on the cohorts of predominately European ancestry.7–9,12–19,22 Details on the participant selection, genotyping and imputation steps of the population-based cohorts and the corresponding baseline characteristics have been reported previously.7–9,12–19,22 This MR study followed the guidelines of Strengthening the Reporting of Observational Studies in Epidemiology using Mendelian Randomization (STROBE-MR).
Figure 1.
Study design and conceptual workflow. This schematic representation revealed a 2-stage design of our Mendelian randomization (MR) study. First, we estimated the effects of 92 blood metabolites on the risk of 8 psychiatric disorders. Second, we explored a broad spectrum of target-mediated side effects associated with metabolite intervention in 655 nonpsychiatric diseases, involving 15 different International Classification of Disease (ICD)-9 chapters. At each stage, the Bonferroni-corrected P value threshold was set accounting for the corresponding number of metabolites and diseases analyzed.
Standard Protocol Approvals, Registrations, and Patient Consents
The protocol and data collection were approved by the ethics committee of the original GWASs, and written informed consent was obtained from each participant before data collection.
Data Source for Blood Metabolome
Summary-level data of the single nucleotide polymorphisms (SNPs) associated with the human metabolome originated from three large-scale GWASs with a combined total of 147 827 European participants (Table 1).7–9 In brief, Shin et al.7 analyzed 453 metabolic traits in 7824 participants with approximately 3 million SNPs from 2 cohorts; Kettunen et al.8 analyzed 123 metabolic traits in 24 925 participants with approximately 12 million SNPs from 14 cohorts; and Borges et al.9 analyzed 249 metabolic traits in 115 078 participants with approximately 12 million SNPs from UK Biobank (Table 1). Among these 825 metabolic traits, a total of 394 metabolic traits were excluded because they were overlapped metabolites, unknown metabolites, or non-metabolites (e.g., lipid concentrations in lipoproteins, relative proportion of lipids in lipoproteins, diameter for lipoproteins particles), and a total of 431 metabolites were retained.
Table 1.
Characteristics of GWASs on the Blood Metabolome and Psychiatric Disorders.
| Phenotype | Cohort(s) | Sample size | Ethnicity |
|---|---|---|---|
| Metabolic trait (N = 453) | KORA; TwinsUK | 7,824 | German; British |
| Metabolic trait (N = 123) | EGCUT; ERF; FTC; FR97; COROGENE; GenMets; HBCS; KORA; LLS; NTR; NFBC 1966; PredictCVD; PROTE; YFS | 24,925 | Estonian; Dutch; Finnish; Finnish; Finnish; Finnish; Finnish; German; Dutch; Dutch; Finnish; Finnish; Estonian; Finnish |
| Metabolic trait (N = 249) | UK Biobank | 115,078 | British |
| BIP | BOMA-Australia; BOMA-Germany II; BOMA-Germany III; BOMA-Poland; BOMA-Spain; BOMA-Germany I; Trinity College Dublin; University of Edinburgh; FaST, TGEN1,2; French; BiGs: GAIN; BACCS; Nova Scotia, MGH i2b2 controls; ICCBD BDRN; Janssen, SAGE controls; Mayo Clinic; Pritzker Neuropsychiatric Disorders Research Consortium (U Michigan); Pfizer; BOMA-Romania; STEP2, MIGEN controls; STEP1; SWEBIC I Affy; SWEBIC I Illumina; TOP; TOP, additional samples on new platform; Dutch-UCLA; University College London; UMEA; ICCBD USC; WTCCC; Bulgarian trios; UK trios; Janssen; PsyCourse; BIGs/TGEN; Bipolar Depression Treatment Response Study; dutcha; gawlia; GUF-Bipolar; GPC; NeuRA-psych (including controls from The Australian Schizophrenia Research Bank); spsp3a; GEMS and EIMS; ukwa1a; usaw4a; usaw5a; iPSYCH; deCODE; Estonian Biobank; Nord-Trøndelag Health Study; UK Biobank; BOMA-Russia; ATLADIS; BIPGEN; BOMA-Romania II | 413,466 | Australian; German; German; Polish; Spanish; German; British; British; American; French; American; American; Canadian/American; British; American; American; American; American; Romanian; American; American; Swedish; Swedish; Norwegian; Norwegian; Dutch; British; Swedish; American; British; Bulgarian; British; East. European; German; American; German; Dutch; German; German; American; Australian; Spanish; Swedish; British; American; Canadian/American; Danish; Icelander; Estonian; Norwegian; British; Russian; Greek; Austrian; Romanian |
| ADHD | iPSYCH-ADHD, Denmark; Barcelona, Spain (from PGC); Beijing, China (from PGC); Bergen, Norway (from PGC); Cardiff, UK (from PGC); CHOP, USA (from PGC); Germany (from PGC); IMAGE-I (from PGC); IMAGE-II (from PGC); PUWMa (from PGC); Toronto, Canada (from PGC); Yale-Penn (from PGC) | 55,374 | Danish; Spanish; Han Chinese; Norwegian; British; American; German; European; European; Diverse (American); Canadian; American |
| OCD | IOCDFGC Ashkenazi Jewish; IOCDFGC Dutch; IOCDFGC European; IOCDFGC South African; IOCDFGC European Trios; OCGAS case/control; OCGAS Trios | 9,725 | European ancestry; European ancestry; European ancestry; European ancestry; European ancestry; European ancestry; European ancestry |
| MDD | UK Biobank; PGC_139k | 500,199 | British; European |
| SCZ | CLOZUK1; CardiffCOGS1; CLOZUK2; CardiffCOGS2; WTCCC2; Cardiff Controls; Generation Scotland; T1DGC; POBI; TWINSUK; QIMR; TEDS; GERAD | 35,802 | British; British; British; British; British; British; British; British; British; British; Australian; British; British |
| PD | Germany I; Germany II; Germany III; Sweden; Denmark; Estonia | 9,907 | German; German; German; Swedish; Danish; Estonian |
| ASD | iPSYCH-ASD | 46,350 | Danish |
| AN | CHOP/PFCG; GCAN/WTCCC3; GCAN/WTCCC3/IARC/CNG; ANGI-DK; ANGI-ANZ/QSkin; ANGI-US/PFCG; ANGI-SE (Riksät/SCÄ/LifeGene/other); UK Biobank | 72,517 | American/Canadian, British, German, Italian; Finnish, French, German, Norwegian, Swedish, Southern Italian, Greek, Dutch, Spanish, British, American/Canadian; Polish, Czech; Danish; Australian, New Zealander; American; Swedish, Danish; British |
Abbreviation: ADHD, attention deficit hyperactivity disorder; AN, anorexia nervosa; ANGI, Anorexia Nervosa Genetics Initiative; ASD, autistic spectrum disorder; ATLADIS, AThens Longitudinal Affective DIsorders Study; BACCS, Bipolar Association Case-Control Study; BDRN, Bipolar Disorder Research Network; BiGs, Bipolar Genome Study; BIP, bipolar disorder; BOMA, Bonn-Mannheim; CardiffCOGS, Cardiff Cognition in Schizophrenia; CHOP, Children’s Hospital of Philadelphia; CLOZUK, United Kingdom Clozapine Clinic; CNG, Centre National de Génotypage; COROGENE, Genetic Predisposition of Coronary Heart Disease in Patients Verified with Coronary Angiogram; EGCUT, Estonian Genome Center of University of Tartu Cohort; EIMS, Epidemiological Investigation of Multiple Sclerosis; ERF, Erasmus Rucphen Family Study; FR97, a subsample of FINRISK 1997; FTC, Finnish Twin Cohort; GAIN, Genetic Association Information Network; GCAN, Genetic Consortium for Anorexia Nervosa; GEMS, Genes and Environment in Multiple Sclerosis; GenMets, Genetics of METabolic Syndrome; GERAD, Genetic and Environmental Risk for Alzheimer’s Disease; GPC, Genomic Psychiatry Cohort; GUF, Goethe University Frankfurt; GWAS, genome-wide association study; HBCS, Helsinki Birth Cohort Study; IARC, International Agency for Research on Cancer; ICCBD, International Cohort Collection for Bipolar Disorder; IMAGE, International Multisite ADHD Genetics; IOCDFGC, International Obsessive Compulsive Disorder (OCD) Foundation Genetics Collaborative; iPSYCH, Integrative Psychiatric Research; KORA, Kooperative Health Research in the Region of Augsburg; LLS, Leiden Longevity Study; MDD, major depressive disorder; MGH, Massachusetts General Hospital; MIGEN, Myocardial Infarction Genetics Consortium; NFBC 1966, Northern Finland Birth Cohort 1966; NTR, Netherlands Twin Register; OCGAS, OCD Collaborative Genetics Association Study; PD, panic disorder; PFCG, Price Foundation Collaborative Group; PGC, Psychiatric Genomics Consortium; POBI, People of the British Isles; PredictCVD, FINRISK subsample of incident cardiovascular cases and controls; PROTE, EGCUT sub-cohort; PUWMa, Pfizer-funded study from University of Carlifornia, Los Angeles (UCLA), Washington University, and MGH; QIMR, Queensland Institute of Medical Research; QSkin, Sun and Health Study; Riksät, Swedish National Quality Register for Eating Disorders; SAGE, Study of ADHD, Genes and Environment; SCÄ, Stockholm Center for Eating Disorders; SCZ, schizophrenia; STEP, Systematic Treatment Enhancement Program for Bipolar Disorder; SWEBIC, Swedish Bipolar Collection; T1DGC, Type 1 Diabetes Genetics Consortium; TEDS, Twins Early Development Study; TGEN, Translational Genomics Research Institute; TOP, Thematically Organized Psychosis; WTCCC, Wellcome Trust Case-Control Consortium; YFS, The Cardiovascular Risk in Young Finns Study.
a Cohort whose sample name was not available was represented using the corresponding abbreviation in the original GWAS.
Data Source for Psychiatric Disorders
We obtained the summary genetic statistics of psychiatric disorders from the Psychiatric Genomics Consortium (Table 1), which included 8 predominantly European ancestry-based GWAS meta-analyses (BIP [41 917 cases and 371 549 controls],12 ADHD [20 183 cases and 35 191 controls],13 OCD [2688 cases and 7037 controls],14 MDD [170 756 cases and 329 443 controls],15 SCZ [11 260 cases and 24 542 controls],16 PD [2147 cases and 7760 controls],17 ASD [18 381 cases and 27 969 controls],18 and AN [16 992 cases and 55 525 controls]19) (available from Psychiatric Genomics Consortium website: https://www.med.unc.edu/pgc). Diagnostic criteria for these psychiatric disorders have been previously described in detail.12–19
Genetic Instruments of Blood Metabolome
In this MR study, SNPs that were identified to be associated with blood metabolites at genome-wide significance level (P value <5 × 10−8) in the published GWASs and were not in linkage disequilibrium (LD) with other SNPs (r2 < 0.1 within a clumping window of 500 kb) were used as genetic instruments for these metabolites. For SNPs exhibiting LD above a threshold of r2 = 0.1, we only selected the SNP with the lowest P value for association with the metabolite. Subsequently, we removed the genetic variants which were not available in GWAS datasets of psychiatric disorders to ensure that the included genetic variants could link blood metabolites to psychiatric disorders, and calculated the phenotypic variance of each metabolite explained by the genetic instruments. The metabolites with variance explained by genetic instruments less than 0.5% were removed due to insufficient statistical power for a valid causal inference.23 Furthermore, we excluded the metabolites associated with less than 3 SNPs to meet the minimum requirement of the number of SNP for some MR sensitivity analyses.24
Based on the above exclusion criteria, 339 of the 431 metabolites were further excluded. Finally, a total of 92 unique blood metabolites were retained for the present MR analyses (figure 1). An overview of the data concerning the SNPs used as genetic instruments in this MR study is listed in s-table S1, and further details are available in s-table S2. We calculated the F statistic to assess the strength of the genetic instruments for blood metabolites. An F statistic greater than 10 suggests a strong instrument.25
Statistical Analysis
In the main analysis, we adopted the inverse-variance weighted (IVW) MR method to estimate the associations between 92 blood metabolite and the risk of 8 psychiatric disorders, in which the SNP-psychiatric disorder estimate is regressed on the SNP-metabolite estimate and weighted by the inverse-variance of SNP-psychiatric disorder estimate, and the intercept is set to zero.10 We performed Cochran’s Q test to evaluate the heterogeneity among genetic variants used in the main analysis.26 A fixed-effect IVW model was used under the hypothesis of homogeneity; otherwise, a random-effect IVW model was used.
Although the IVW method is a powerful option for causal inference, the main assumptions underlying IVW method are somewhat hard to meet in the presence of invalid instruments and pleiotropy. To assess the validity of the associations identified by the IVW method, we conducted a series of sensitivity analyses with different models. First, we adopted the penalized IVW method, which enabled us to penalize the SNPs with pleiotropy.27 Second, the MR-Robust Adjusted Profile Scoring (MR-RAPS) method was undertaken, in which method the MR estimates were robust to violations of key MR assumptions.28 Third, we utilized the maximum likelihood method to provide reliable results in presence of measurement error in SNP-exposure association.24 Fourth, we performed the MR-Egger regression to determine the potential pleiotropy through its intercept term.24
Phe-MR Analysis for Target-mediated Side Effects of Psychiatric Disorders-related Metabolites
We performed a Phe-MR analysis to explore the potential side effects of hypothetical interventions that reduced the risk of psychiatric disorders by targeting the identified metabolites. Summary statistics for 1403 disease traits were obtained from Zhou et al.’ GWAS with 408 961 white British participants and 28 million SNPs in the UK Biobank cohort.22 Disease traits were defined based on “PheCodes”, which is a system organized International Classification of Disease (ICD) codes into phenotypic outcomes, enabling investigators to perform systematic genetic analysis of multiple disease traits.22,23 To ensure the interpretability of the results, we only selected representative nonpsychiatric diseases to minimize the inherent redundancy between PheCodes. Moreover, sex-specific disease traits and disease traits with cases less than 500 had issues of data availability and statistical power, so we also excluded these disease traits. Finally, a total of 655 nonpsychiatric diseases were retained for the Phe-MR analysis (figure 1; s-table S3). According to the associations between metabolites and psychiatric disorders, Phe-MR findings are standardized to a change in metabolite level corresponding to a 10% reduction in the risk of psychiatric disorders, and the specific calculation formulas are available in Supplementary Methods. The standardized results in this manner enabled us to directly determine the direction of the side effects and compare their magnitude with the therapeutic effects of metabolite-targeted interventions in the prevention of psychiatric disorders.
All results for the outcomes are presented as odds ratios (ORs) and their 95% confidence intervals (CIs). In stage 1, an observed 2-sided P < 6.79 × 10−5 (Bonferroni-corrected significance threshold calculated as 0.05 divided by 736 [for 92 metabolites and 8 psychiatric disorders]) was considered as the statistically significant evidence for a causal inference. A 2-sided P < 0.05 in the MR-Egger regression method was considered as the suggestively significant evidence for potentially directional pleiotropy. All analyses were performed in R (version 3.4.3; R Development Core Team) with the packages gtx, MendelianRandomization, TwoSampleMR, ggplot2, ggrepel, grid, gridExtra, gtable, qqman, RColorBrewer, and RGraphics.
Data Availability
All summary statistics used in this two-stage Mendelian randomization are available online from each genome-wide association study. Statistical code is available on the request by directly contacting the corresponding author (email: zbzhu@suda.edu.cn).
Results
Strength of the Genetic Instruments for Blood Metabolites
A total of 92 unique blood metabolites were analyzed in the present study (s-table S1), and a specific description of genetic instruments for each blood metabolite is presented in Tables S2. The observed phenotypic variance explained by the genetic instruments ranged from 0.54% for acetone to 45.18% for butyrylcarnitine. The F statistics for the genetic instruments of the blood metabolites ranged from 32 to 460, suggesting that there was no instrument bias in this study (s-table S1).
Screening the Blood Metabolome for Potential Causal Mediators of Psychiatric Disorders
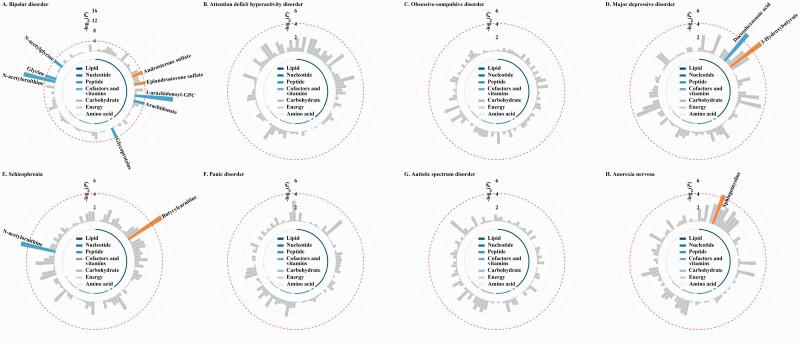
In the main IVW MR analysis of 92 blood metabolites and 8 psychiatric disorders, genetically determined low 1-arachidonoylglycerophosphocholine (1-arachidonoyl-GPC), arachidonate, glycoproteins, N-acetylornithine, glycine, N-acetylglycine, and docosahexaenoic acid, and genetically determined high androsterone sulfate, epiandrosterone sulfate, 3-Hydroxybutyrate, butyrylcarnitine, and sphingomyelins were significantly associated with increased risks of psychiatric disorders (figure 2 and s-table S4). Sensitivity analyses using the penalized IVW method, the MR-RAPS method, and the maximum likelihood method found the same associations identified in the main analysis, except for the attenuated associations of androsterone sulfate, epiandrosterone sulfate, arachidonate, and N-acetylglycine with BIP (s-table S5). The MR-Egger regression suggested that the genetic variants for these metabolites except for epiandrosterone sulfate had no directional pleiotropic effects on the risk of psychiatric disorders (all P > 0.05).
Figure 2.
Circular Manhattan plot illustrating the inverse-variance weighted estimates of the associations between blood metabolites and the risk of psychiatric disorders in this Mendelian randomization study. The red dashed line represents the Bonferroni-corrected significance threshold (P < 9.54 × 10−6), with significant metabolites were annotated with labels. The orange bars represent significant harmful mediators of psychiatric disorders, while the blue bars represent significant protective mediators of psychiatric disorders. According to the super-pathway listed in s-table S1, we grouped and color-coded the 92 blood metabolites. The detailed results are available in s-table S4. Abbreviation: 1-arachidonoyl-GPC, 1-arachidonoylglycerophosphocholine.
Overall, a total of 8 metabolites were identified as potential causal mediators for psychiatric disorders (table 2). Among these metabolites, per 1-SD increase in the genetically determined 1-arachidonoyl-GPC (BIP: OR = 0.31, 95% CI = 0.23–0.41, P = 3.52 × 10−16), glycoproteins (BIP: OR = 0.94, 95% CI = 0.92–0.97, P = 3.10 × 10−5), N-acetylornithine (BIP: OR = 0.72, 95% CI = 0.66–0.79, P = 1.08 × 10−13; SCZ: OR = 0.74, 95% CI = 0.64–0.84, P = 5.14 × 10−6), glycine (BIP: OR = 0.62, 95% CI = 0.50–0.77, P = 2.28 × 10−5), and docosahexaenoic acid (MDD: OR = 0.96, 95% CI = 0.94–0.97, P = 5.04 × 10−6) were associated with decreased risks of psychiatric disorders, while per 1-SD increase in genetically determined 3-Hydroxybutyrate (MDD: OR = 1.14, 95% CI = 1.08–1.21, P = 1.50 × 10−6), butyrylcarnitine (SCZ: OR = 1.22, 95% CI = 1.12–1.32, P = 1.10 × 10−6), and sphingomyelins (AN: OR = 1.12, 95% CI = 1.06–1.19, P = 2.34 × 10−5) were associated with increased risks of psychiatric disorders.
Table 2.
Significant Associations between Blood Metabolites and the Risk of Psychiatric Disorders in the MR Analysis.
| Metabolite | SNPs | IVW | Penalized IVW | MR-RAPS | Maximum likelihood | MR-Egger Intercept | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | P value | ||
| Bipolar disorder | ||||||||||
| 1-Arachidonoyl-GPC | 5 | 0.31 (0.23–0.41) | 3.52 × 10−16 | 0.31 (0.21–0.46) | 6.76 × 10−9 | 0.30 (0.23–0.41) | 9.55 × 10−15 | 0.30 (0.23–0.41) | 9.53 × 10−15 | 0.67 |
| Glycoproteins | 71 | 0.94 (0.92–0.97) | 3.10 × 10−5 | 0.94 (0.92–0.96) | 2.55 × 10−6 | 0.94 (0.92–0.96) | 9.97 × 10−8 | 0.94 (0.92–0.96) | 1.66 × 10–7 | 0.53 |
| N-acetylornithine | 9 | 0.72 (0.66–0.79) | 1.08 × 10−13 | 0.72 (0.66–0.79) | 1.08 × 10−13 | 0.72 (0.66–0.79) | 2.16 × 10−13 | 0.72 (0.66–0.78) | 1.96 × 10−13 | 0.15 |
| Glycine | 6 | 0.62 (0.50–0.77) | 2.28 × 10−5 | 0.62 (0.50–0.78) | 2.87 × 10−5 | 0.62 (0.50–0.78) | 2.74 × 10−5 | 0.62 (0.50–0.78) | 2.59 × 10−5 | 0.41 |
| Major depressive disorder | ||||||||||
| Docosahexaenoic acid | 133 | 0.96 (0.94–0.97) | 5.04 × 10−6 | 0.96 (0.95–0.98) | 4.13 × 10−5 | 0.96 (0.94–0.97) | 1.94 × 10−8 | 0.96 (0.94–0.97) | 2.57 × 10−8 | 0.94 |
| 3-Hydroxybutyrate | 25 | 1.14 (1.08–1.21) | 1.50 × 10−6 | 1.14 (1.08–1.21) | 6.76 × 10−6 | 1.15 (1.08–1.21) | 1.74 × 10−6 | 1.15 (1.09–1.21) | 1.25 × 10−6 | 0.55 |
| Schizophrenia | ||||||||||
| Butyrylcarnitine | 17 | 1.22 (1.12–1.32) | 1.10 × 10−6 | 1.22 (1.12–1.32) | 3.39 × 10−6 | 1.22 (1.12–1.32) | 1.18 × 10−6 | 1.22 (1.13–1.32) | 8.75 × 10−7 | 0.15 |
| N-acetylornithine | 9 | 0.74 (0.64–0.84) | 5.14 × 10−6 | 0.74 (0.64–0.84) | 5.14 × 10−6 | 0.73 (0.67–0.80) | 1.93 × 10−11 | 0.73 (0.67–0.80) | 2.49 × 10−11 | 0.41 |
| Anorexia nervosa | ||||||||||
| Sphingomyelins | 159 | 1.12 (1.06–1.19) | 2.34 × 10−5 | 1.13 (1.06–1.19) | 4.37 × 10−5 | 1.12 (1.07–1.19) | 2.25 × 10−5 | 1.12 (1.06–1.19) | 2.45 × 10−5 | 0.49 |
Odds ratios (ORs) with their 95% confidence intervals (CIs) represent the association estimates with the risk of psychiatric disorders of per 1-SD increase in 1-arachidonoylglycerophosphocholine (1-arachidonoyl-GPC), glycoproteins, N-acetylornithine, glycine, docosahexaenoic acid, 3-Hydroxybutyrate, butyrylcarnitine, and sphingomyelins levels, respectively. An observed 2-sided P < 6.79 × 10−5 was considered as statistically significant. A 2-sided P < .05 was considered as suggestive evidence for directional pleiotropy in the MR-Egger regression.
Abbreviations: IVW, inverse-variance weighted; MR-RAPS, Mendelian randomization robust adjusted profile score.
Phe-MR Analysis for the Associations between Identified Metabolites and 655 Nonpsychiatric Diseases
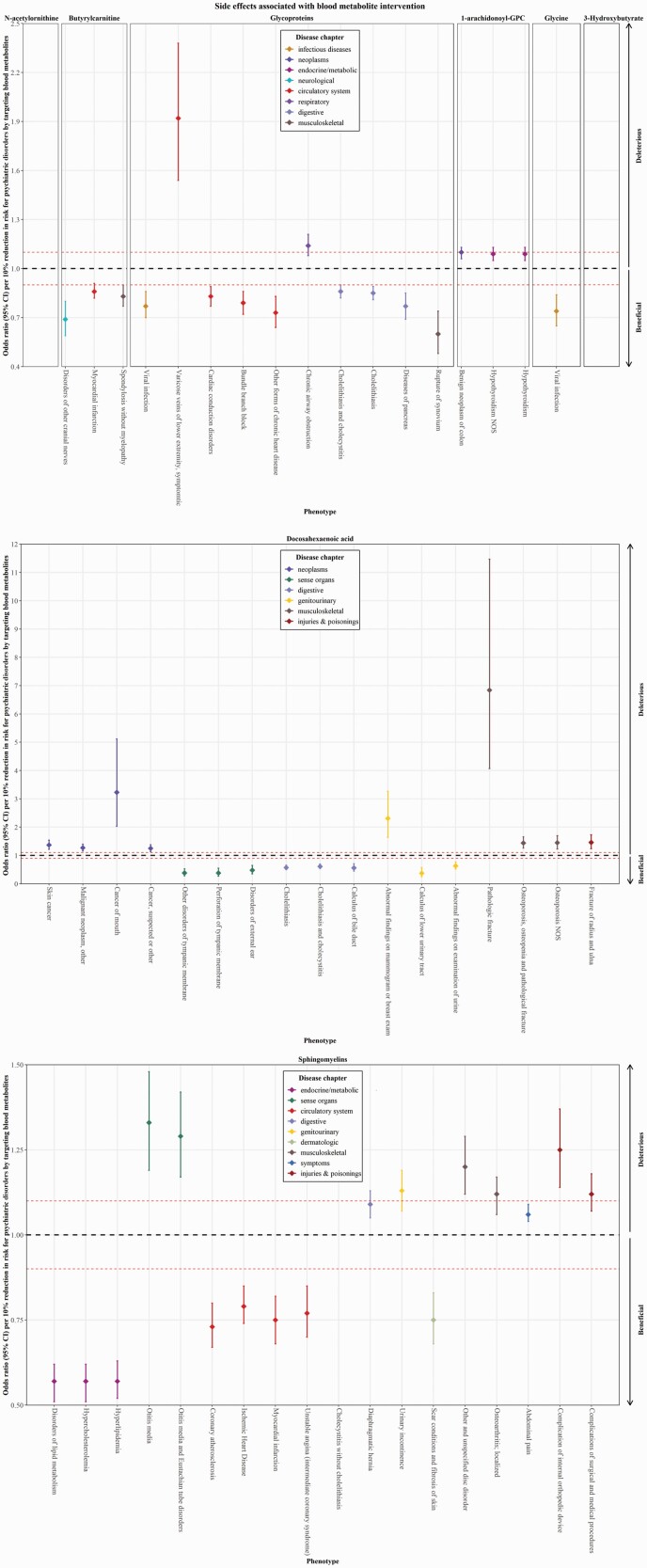
We further performed a Phe-MR analysis of 655 nonpsychiatric diseases to explore the potential side-effect profiles of targeting the identified metabolites. Notably, Phe-MR results were standardized to a 10% reduction in the risk of psychiatric disorders mediated by targeting a given metabolite, which can be interpreted as concomitant side effects expected to arise when each metabolite is targeted to prevent psychiatric disorders. Using the IVW method in the Phe-MR analysis, we found that 66 associations reached a Bonferroni-corrected significance threshold of P = 9.54 × 10−6 (0.05/5240 [8 metabolites*655 diseases]) (s-tables S6–S13; figure S1). Further sensitivity analyses robustly suggested 3 significant disease associations for 1-arachidonoyl-GPC, 10 significant disease associations for glycoproteins, 1 significant disease association for glycine, 18 significant disease associations for docosahexaenoic acid, 3 significant disease associations for butyrylcarnitine, and 22 significant disease associations for sphingomyelins, but none for N-acetylornithine and 3-Hydroxybutyrate (Table S14). In addition, the MR-Egger regression analysis found no directional pleiotropy for these significant associations, except for docosahexaenoic acid and sphingomyelins (s-table S14).
Overall, 6 out of 8 mediators of psychiatric disorders (1-arachidonoyl-GPC, glycoproteins, glycine, docosahexaenoic acid, butyrylcarnitine, and sphingomyelins) were identified to be associated with multiple diseases, involving 52 significant associations (table 3; figure 3). All side effects of interventions against glycine and butyrylcarnitine were protective, followed by a lower proportion against glycoproteins (80%), docosahexaenoic acid (47%), and sphingomyelins (44%), while the side effects of interventions against 1-arachidonoyl-GPC were all adverse (table S15). In terms of disease category, circulatory system was the most commonly affected system for the interventions against these metabolites (table S15). The most significant side effects of interventions against each metabolite was benign neoplasm of colon for 1-arachidonoyl-GPC (OR per 10% reduction in BIP risk = 1.10, 95% CI = 1.06–1.13, P = 4.47 × 10−10), cholelithiasis and cholecystitis for glycoproteins (OR per 10% reduction in BIP risk = 0.86, 95% CI = 0.82–0.90, P = 5.38 × 10−11), viral infection for glycine (OR per 10% reduction in BIP risk = 0.74, 95% CI = 0.65–0.84, P = 2.23 × 10−6), cholelithiasis for docosahexaenoic acid (OR per 10% reduction in MDD risk = 0.57, 95% CI = 0.50–0.66, P = 2.57 × 10−15), myocardial infarction for butyrylcarnitine (OR per 10% reduction in SCZ risk = 0.86, 95% CI = 0.82–0.91, P = 6.42 × 10−8), and disorders of lipid metabolism for sphingomyelins (OR per 10% reduction in AN risk = 0.57, 95% CI = 0.51–0.62, P = 2.67 × 10−30) (table 3).
Table 3.
Significant Associations Between Identified Metabolites and the Risk of Multiple Nonpsychiatric Diseases in the Phe-MR Analysis.
| PheCode | Outcome | SNPs | Disease chapter | IVW | Penalized IVW | MR-RAPS | Maximum likelihood | MR-Egger Intercept | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | OR (95% CI) | P value | P value | ||||
| 1-Arachidonoyl-GPC supplementation | ||||||||||||
| 208 | Benign neoplasm of colon | 5 | Neoplasms | 1.10 (1.06–1.13) | 4.47 × 10−10 | 1.10 (1.06–1.13) | 4.47 × 10−10 | 1.09 (1.06–1.13) | 1.88 × 10−9 | 1.09 (1.06–1.13) | 1.45 × 10−9 | 0.91 |
| 244.4 | Hypothyroidism NOS | 5 | Endocrine/metabolic | 1.09 (1.05–1.13) | 5.80 × 10−7 | 1.09 (1.05–1.13) | 6.34 × 10−6 | 1.09 (1.05–1.13) | 9.92 × 10−7 | 1.09 (1.05–1.13) | 9.29 × 10−7 | 0.87 |
| 244 | Hypothyroidism | 5 | Endocrine/metabolic | 1.09 (1.05–1.13) | 6.80 × 10−7 | 1.09 (1.05–1.13) | 2.00 × 10−6 | 1.09 (1.05–1.13) | 1.18 × 10−6 | 1.09 (1.05–1.13) | 1.08 × 10−6 | 0.90 |
| Glycoproteins supplementation | ||||||||||||
| 574 | Cholelithiasis and cholecystitis | 78 | Digestive | 0.86 (0.82–0.90) | 5.38 × 10−11 | 0.86 (0.83–0.90) | 2.57 × 10−11 | 0.86 (0.82–0.90) | 5.33 × 10−11 | 0.86 (0.82–0.90) | 8.24 × 10−11 | 0.30 |
| 574.1 | Cholelithiasis | 78 | Digestive | 0.85 (0.81–0.89) | 8.55 × 10−11 | 0.86 (0.82–0.90) | 3.70 × 10−11 | 0.85 (0.81–0.89) | 8.52 × 10−11 | 0.85 (0.81–0.89) | 1.36 × 10−10 | 0.52 |
| 454.11 | Varicose veins of lower extremity, symptomtic | 78 | Circulatory system | 1.92 (1.54–2.38) | 4.20 × 10−9 | 1.84 (1.50–2.26) | 4.20 × 10−9 | 1.90 (1.53–2.36) | 5.41 × 10−9 | 1.93 (1.55–2.40) | 3.83 × 10−9 | 0.21 |
| 426 | Cardiac conduction disorders | 78 | Circulatory system | 0.83 (0.77–0.89) | 3.20 × 10−8 | 0.84 (0.79–0.90) | 3.71 × 10−7 | 0.83 (0.78–0.89) | 3.22 × 10−8 | 0.83 (0.77–0.89) | 4.75 × 10−8 | 0.78 |
| 426.3 | Bundle branch block | 78 | Circulatory system | 0.79 (0.72–0.86) | 7.24 × 10−7 | 0.80 (0.73–0.88) | 1.31 × 10−6 | 0.79 (0.72–0.87) | 6.97 × 10−7 | 0.79 (0.72–0.87) | 1.01 × 10−6 | 0.88 |
| 577 | Diseases of pancreas | 78 | Digestive | 0.77 (0.69–0.85) | 9.47 × 10−7 | 0.78 (0.71–0.87) | 2.34 × 10−6 | 0.77 (0.69–0.85) | 9.84 × 10−7 | 0.77 (0.69–0.85) | 1.14 × 10−6 | 0.52 |
| 79 | Viral infection | 78 | Infectious diseases | 0.77 (0.70–0.86) | 1.04 × 10−6 | 0.79 (0.71–0.87) | 1.04 × 10−6 | 0.78 (0.70–0.86) | 1.16 × 10−6 | 0.77 (0.70–0.86) | 1.20 × 10−6 | 0.20 |
| 496 | Chronic airway obstruction | 78 | Respiratory | 1.14 (1.08–1.21) | 2.10 × 10−6 | 1.14 (1.08–1.20) | 1.22 × 10−6 | 1.14 (1.08–1.21) | 2.08 × 10−6 | 1.14 (1.08–1.21) | 2.08 × 10-6 | 0.54 |
| 414 | Other forms of chronic heart disease | 78 | Circulatory system | 0.73 (0.64–0.83) | 2.66 × 10−6 | 0.74 (0.65–0.84) | 3.71 × 10−6 | 0.73 (0.65–0.84) | 2.69 × 10−6 | 0.73 (0.64–0.84) | 3.40 × 10−6 | 0.37 |
| 727.5 | Rupture of synovium | 78 | Musculoskeletal | 0.60 (0.48–0.74) | 5.59 × 10−6 | 0.61 (0.50–0.76) | 7.19 × 10−6 | 0.60 (0.48–0.74) | 5.35 × 10−6 | 0.60 (0.48–0.75) | 7.75 × 10−6 | 0.32 |
| Glycine supplementation | ||||||||||||
| 79 | Viral infection | 9 | Infectious diseases | 0.74 (0.65–0.84) | 2.23 × 10−6 | 0.74 (0.65–0.84) | 4.77 × 10−6 | 0.74 (0.65–0.84) | 2.77 × 10−6 | 0.74 (0.65–0.84) | 2.60 × 10−6 | 0.20 |
| Docosahexaenoic acid supplementation | ||||||||||||
| 574.1 | Cholelithiasis | 152 | Digestive | 0.57 (0.50–0.66) | 2.57 × 10−15 | 0.52 (0.45–0.59) | 1.49 × 10−21 | 0.57 (0.51–0.63) | 4.35 × 10−26 | 0.57 (0.52–0.64) | 3.78 × 10−25 | 0.69 |
| 574 | Cholelithiasis and cholecystitis | 152 | Digestive | 0.61 (0.54–0.70) | 6.61 × 10−13 | 0.57 (0.51–0.64) | 1.40 × 10−19 | 0.61 (0.56–0.67) | 2.28 × 10−23 | 0.62 (0.56–0.68) | 1.76 × 10−22 | 0.25 |
| 743 | Osteoporosis, osteopenia and pathological fracture | 152 | Musculoskeletal | 1.44 (1.26–1.66) | 1.61 × 10−7 | 1.57 (1.31–1.87) | 7.86 × 10−7 | 1.44 (1.26–1.65) | 1.55 × 10−7 | 1.45 (1.26–1.66) | 1.50 × 10−7 | 0.63 |
| 172 | Skin cancer | 152 | Neoplasms | 1.37 (1.22–1.54) | 2.33 × 10−7 | 1.49 (1.29–1.71) | 4.84 × 10−8 | 1.37 (1.23–1.52) | 2.98 × 10−9 | 1.37 (1.24–1.52) | 3.27 × 10−9 | 0.08 |
| 195.1 | Malignant neoplasm, other | 152 | Neoplasms | 1.27 (1.16–1.40) | 5.54 × 10−7 | 1.34 (1.19–1.51) | 9.39 × 10−7 | 1.27 (1.16–1.40) | 5.57 × 10−7 | 1.27 (1.16–1.40) | 5.09 × 10−7 | 0.05 |
| 195 | Cancer, suspected or other | 152 | Neoplasms | 1.25 (1.14–1.38) | 1.45 × 10−6 | 1.32 (1.17–1.48) | 3.63 × 10−6 | 1.25 (1.14–1.38) | 1.44 × 10−6 | 1.26 (1.15–1.38) | 1.34 × 10−6 | 0.10 |
| 743.2 | Pathologic fracture | 152 | Musculoskeletal | 6.84 (4.07–11.47) | 3.43 × 10−13 | 9.90 (5.25–18.66) | 1.34 × 10−12 | 6.79 (4.05–11.38) | 3.95 × 10−13 | 6.88 (4.09–11.56) | 3.35 × 10−13 | 0.63 |
| 145 | Cancer of mouth | 152 | Neoplasms | 3.23 (2.03–5.12) | 6.79 × 10−7 | 4.34 (2.47–7.64) | 3.57 × 10−7 | 3.21 (2.03–5.10) | 7.05 × 10−7 | 3.23 (2.03–5.13) | 6.98 × 10−7 | 0.43 |
| 594.2 | Calculus of lower urinary tract | 152 | Genitourinary | 0.37 (0.25-0.57) | 5.16 × 10−6 | 0.30 (0.18–0.50) | 3.83 × 10−6 | 0.38 (0.25–0.57) | 5.27 × 10−6 | 0.37 (0.24–0.57) | 4.35 × 10−6 | 0.45 |
| 574.2 | Calculus of bile duct | 152 | Digestive | 0.56 (0.45–0.71) | 1.12 × 10−6 | 0.50 (0.37–0.66) | 1.76 × 10−6 | 0.57 (0.45–0.71) | 1.13 × 10−6 | 0.57 (0.45–0.71) | 1.36 × 10−6 | 0.21 |
| 611 | Abnormal findings on mammogram or breast exam | 152 | Genitourinary | 2.31 (1.64–3.27) | 2.00 × 10−6 | 2.96 (2.02–4.35) | 2.85 × 10−8 | 2.31 (1.72–3.11) | 2.88 × 10−8 | 2.32 (1.72–3.13) | 3.10 × 10−8 | 0.74 |
| 380 | Disorders of external ear | 152 | sense Organs | 0.48 (0.35–0.65) | 2.34 × 10−6 | 0.40 (0.28–0.59) | 2.34 × 10−6 | 0.48 (0.35–0.65) | 2.42 × 10−6 | 0.48 (0.35–0.65) | 2.61 × 10−6 | 0.63 |
| 384 | Other disorders of tympanic membrane | 152 | Sense organs | 0.38 (0.28–0.53) | 3.45 × 10−9 | 0.31 (0.21–0.46) | 1.18 × 10−8 | 0.38 (0.28–0.53) | 3.46 × 10−9 | 0.38 (0.28–0.53) | 3.60 × 10−9 | 0.82 |
| 384.4 | Perforation of tympanic membrane | 152 | Sense organs | 0.38 (0.27–0.55) | 2.14 × 10−7 | 0.30 (0.19–0.47) | 1.30 × 10−7 | 0.38 (0.27–0.55) | 2.22 × 10−7 | 0.38 (0.26–0.55) | 1.90 × 10−7 | 0.75 |
| 598 | Abnormal findings on examination of urine | 152 | Genitourinary | 0.63 (0.52–0.77) | 5.33 × 10−6 | 0.56 (0.44–0.71) | 2.90 × 10−6 | 0.63 (0.52–0.77) | 5.43 × 10−6 | 0.63 (0.52–0.77) | 6.01 × 10−6 | 0.72 |
| 803.2 | Fracture of radius and ulna | 152 | Injuries & Poisonings | 1.46 (1.24–1.73) | 6.27 × 10−6 | 1.63 (1.33–1.99) | 2.36 × 10−6 | 1.46 (1.24–1.72) | 6.33 × 10−6 | 1.46 (1.24–1.73) | 6.24 × 10−6 | 0.67 |
| 743.11 | Osteoporosis NOS | 152 | Musculoskeletal | 1.45 (1.23–1.70) | 6.44 × 10−6 | 1.62 (1.32–1.97) | 2.27 × 10−6 | 1.45 (1.23–1.70) | 6.32 × 10−6 | 1.45 (1.23–1.70) | 6.27 × 10−6 | 0.95 |
| Butyrylcarnitine inhibition | ||||||||||||
| 411.2 | Myocardial infarction | 25 | Circulatory system | 0.86 (0.82–0.91) | 6.42 × 10−8 | 0.86 (0.81–0.92) | 1.06 × 10−6 | 0.86 (0.82–0.91) | 5.75 × 10−8 | 0.86 (0.82–0.91) | 3.79 × 10−8 | 0.30 |
| 352 | Disorders of other cranial nerves | 25 | Neurological | 0.69 (0.59–0.80) | 8.97 × 10−7 | 0.69 (0.59–0.80) | 8.97 × 10−7 | 0.69 (0.59–0.80) | 9.76 × 10−7 | 0.69 (0.60–0.80) | 8.80 × 10−7 | 0.86 |
| 721.1 | Spondylosis without myelopathy | 25 | Musculoskeletal | 0.83 (0.77–0.90) | 6.13 × 10−6 | 0.83 (0.77–0.90) | 6.13 × 10−6 | 0.83 (0.77–0.90) | 6.44 × 10−6 | 0.83 (0.77–0.90) | 4.37 × 10−6 | 0.88 |
| Sphingomyelins inhibition | ||||||||||||
| 272 | Disorders of lipid metabolism | 185 | Endocrine/metabolic | 0.57 (0.51–0.62) | 2.67 × 10−30 | 0.65 (0.62–0.69) | 1.53 × 10−71 | 0.53 (0.52–0.55) | 1.00 × 10−99 | 0.55 (0.53–0.57) | 1.00 × 10−99 | 0.21 |
| 272.11 | Hypercholesterolemia | 185 | Endocrine/metabolic | 0.57 (0.51–0.62) | 3.01 × 10−30 | 0.67 (0.64–0.71) | 6.04 × 10−58 | 0.53 (0.52–0.55) | 1.00 × 10−99 | 0.54 (0.52–0.56) | 1.00 × 10−99 | 0.25 |
| 272.1 | Hyperlipidemia | 185 | Endocrine/metabolic | 0.57 (0.52–0.63) | 4.27 × 10−30 | 0.66 (0.63–0.69) | 4.62 × 10−69 | 0.53 (0.52–0.55) | 1.00 × 10−99 | 0.54 (0.53–0.56) | 1.00 × 10−99 | 0.21 |
| 411.4 | Coronary atherosclerosis | 185 | Circulatory system | 0.73 (0.67–0.80) | 1.33 × 10−11 | 0.75 (0.71–0.79) | 2.75 × 10−27 | 0.72 (0.69–0.75) | 5.56 × 10−73 | 0.72 (0.70–0.75) | 1.40 × 10−63 | 0.08 |
| 411 | Ischemic Heart Disease | 185 | Circulatory system | 0.79 (0.74–0.85) | 2.46 × 10−10 | 0.79 (0.75–0.82) | 1.31 × 10−30 | 0.79 (0.76–0.81) | 1.97 × 10−59 | 0.79 (0.77–0.81) | 8.80 × 10−52 | 0.06 |
| 411.2 | Myocardial infarction | 185 | Circulatory system | 0.75 (0.68–0.82) | 1.30 × 10−9 | 0.75 (0.71–0.79) | 4.28 × 10−24 | 0.74 (0.71–0.78) | 2.60 × 10−37 | 0.75 (0.71–0.78) | 3.64 × 10−34 | 0.20 |
| 785 | Abdominal pain | 185 | Symptoms | 1.06 (1.04–1.09) | 1.14 × 10−6 | 1.06 (1.03–1.08) | 3.50 × 10−6 | 1.06 (1.04–1.09) | 1.23 × 10−6 | 1.06 (1.04–1.09) | 1.34 × 10−6 | 0.77 |
| 550.2 | Diaphragmatic hernia | 185 | Digestive | 1.09 (1.05–1.13) | 4.84 × 10−6 | 1.08 (1.05–1.12) | 1.41 × 10−6 | 1.09 (1.06–1.12) | 2.46 × 10−8 | 1.09 (1.06–1.12) | 4.28 × 10−8 | 0.57 |
| 599.4 | Urinary incontinence | 185 | Genitourinary | 1.13 (1.07–1.19) | 4.96 × 10−6 | 1.12 (1.07–1.18) | 7.90 × 10−6 | 1.13 (1.07–1.19) | 4.86 × 10−6 | 1.13 (1.07–1.19) | 5.20 × 10−6 | 0.70 |
| 740.1 | Osteoarthritis; localized | 185 | Musculoskeletal | 1.12 (1.06–1.17) | 6.32 × 10−6 | 1.10 (1.05–1.14) | 5.73 × 10−6 | 1.12 (1.08–1.16) | 2.90 × 10−9 | 1.12 (1.08–1.16) | 6.50 × 10−9 | 0.21 |
| 1011 | Complications of surgical and medical procedures | 185 | Injuries & poisonings | 1.12 (1.07–1.18) | 7.34 × 10−6 | 1.12 (1.07–1.17) | 4.21 × 10−6 | 1.12 (1.07–1.18) | 7.41 × 10−6 | 1.12 (1.07–1.18) | 7.16 × 10−6 | 0.99 |
| 701.2 | Scar conditions and fibrosis of skin | 185 | Dermatologic | 0.75 (0.68–0.83) | 6.77 × 10−9 | 0.76 (0.69–0.84) | 1.34 × 10−8 | 0.75 (0.68–0.83) | 7.19 × 10−9 | 0.75 (0.68–0.83) | 7.42 × 10−9 | 0.26 |
| 381.1 | Otitis media | 185 | Sense organs | 1.33 (1.19–1.48) | 4.42 × 10−7 | 1.31 (1.18–1.46) | 7.41 × 10−7 | 1.33 (1.19–1.48) | 4.46 × 10−7 | 1.33 (1.19–1.49) | 4.49 × 10−7 | 0.24 |
| 381 | Otitis media and Eustachian tube disorders | 185 | Sense organs | 1.29 (1.17–1.42) | 5.12 × 10−7 | 1.28 (1.16–1.41) | 5.12 × 10−7 | 1.29 (1.17–1.42) | 5.35 × 10−7 | 1.29 (1.17–1.43) | 4.93 × 10−7 | 0.09 |
| 574.3 | Cholecystitis without cholelithiasis | 185 | Digestive | 1.40 (1.23–1.60) | 5.36 × 10−7 | 1.36 (1.24–1.49) | 2.05 × 10−11 | 1.40 (1.28–1.54) | 1.26 × 10−13 | 1.40 (1.28–1.53) | 4.88 × 10−13 | 0.98 |
| 411.1 | Unstable angina (intermediate coronary syndrome) | 185 | Circulatory system | 0.77 (0.70–0.85) | 1.65 × 10−7 | 0.77 (0.71–0.84) | 7.48 × 10−10 | 0.77 (0.72–0.82) | 3.11 × 10−15 | 0.77 (0.72–0.82) | 1.18 × 10−14 | 0.34 |
| 722.9 | Other and unspecified disc disorder | 185 | Musculoskeletal | 1.20 (1.12–1.29) | 6.34 × 10−7 | 1.18 (1.10–1.27) | 2.43 × 10−6 | 1.20 (1.12–1.29) | 6.27 × 10−7 | 1.20 (1.12–1.29) | 6.33 × 10−7 | 0.75 |
| 858 | Complication of internal orthopedic device | 185 | Injuries & poisonings | 1.25 (1.14-1.37) | 2.38 × 10−6 | 1.24 (1.13–1.35) | 2.22 × 10−6 | 1.25 (1.15–1.36) | 1.82 × 10−7 | 1.25 (1.15–1.36) | 2.08 × 10−7 | 0.38 |
Odds ratios (ORs) with their 95% confidence intervals (CIs) represent the effect estimates on the risk of multiple non-psychiatric diseases of per 10% reduction in risk for psychiatric disorders by targeting 1-arachidonoylglycerophosphocholine (1-arachidonoyl-GPC), glycoproteins, glycine, docosahexaenoic acid, butyrylcarnitine, and sphingomyelins, respectively. An observed 2-sided P < 9.54 × 10-6 was considered as statistically significant. A 2-sided P<0.05 was considered as suggestive evidence for directional pleiotropy in the MR-Egger regression.
Abbreviations: IVW, inverse-variance weighted; MR-RAPS, Mendelian randomization robust adjusted profile score; Phe-MR, phenome-wide Mendelian randomization.
Figure 3.
Potential on-target side effects of identified metabolites using phenome–wide Mendelian randomization analysis. Odds ratios (ORs) with the 95% confidence intervals (CIs) represent the effects on the risk of multiple non-psychiatric diseases of per 10% reduction in risk for psychiatric disorders associated with intervention targeting 1-arachidonoylglycerophosphocholine (1-arachidonoyl-GPC), glycoproteins, N-acetylornithine, glycine, docosahexaenoic acid, 3-Hydroxybutyrate, butyrylcarnitine, and sphingomyelins, respectively. Associations above the horizontal black midline represent deleterious side effects, while these below the horizontal black midline represent beneficial side effects. The horizontal red line (OR = 1.10) represents the point at which decreased risk of psychiatric disorders is counterbalanced by an equal increase in decrease risk.
Discussion
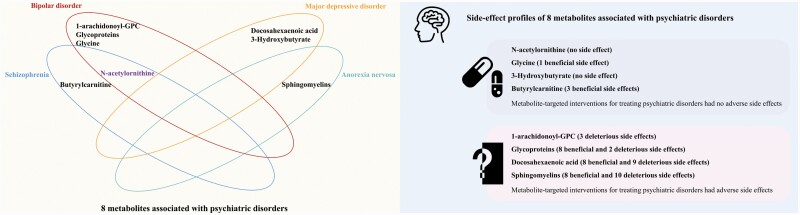
This is the first systematic MR study to screen human blood metabolome for the potential mediators of psychiatric disorders and comprehensively assess their potential on-target clinical side effects. Among the 92 blood metabolites, we identified 8 metabolites as potential causal mediators of psychiatric disorders, including 1-arachidonoyl-GPC, glycoproteins, N-acetylornithine, glycine, docosahexaenoic acid, 3-Hydroxybutyrate, butyrylcarnitine, and sphingomyelins (figure 4). Specifically, 1-arachidonoyl-GPC, glycoproteins, N-acetylornithine, glycine, and docosahexaenoic acid had protective effects, while 3-Hydroxybutyrate, butyrylcarnitine, and sphingomyelins had adverse effects on psychiatric disorders. We further performed a Phe-MR analysis to explore the on-target side effects associated with potential treatment of psychiatric disorders via interventions of the identified metabolites. In the Phe-MR analysis, we found that all identified metabolites except for N-acetylornithine and 3-Hydroxybutyrate could mediate a series of nonpsychiatric diseases, while glycine, 3-Hydroxybutyrate, N-acetylornithine, and butyrylcarnitine had no adverse side effects (figure 4).
Figure 4.
Blood metabolites associated with risk of psychiatric disorders and a comprehensive assessment of their potential on-target clinical side effects. Abbreviation: 1-arachidonoyl-GPC, 1-arachidonoylglycerophosphocholine.
Among the 8 mediators of psychiatric disorders, 5 metabolites have been identified in previous epidemiological studies. Glycine is a member of neurotransmitter, and glycine supplementation could improve brain morphology without neurotoxic effects.29 In a previous clinical trial, high-dose adjuvant glycine was reported to be able to augment the efficacy of olanzapine and risperidone in the treatment of SCZ.30 Our study further extended this information to BIP and suggested that glycine supplementation was associated with a decreased risk of BIP. In addition, our Phe-MR analysis also confirmed the previously reported antiviral activity of glycine.31 Docosahexaenoic acid, an omega-3 poly-unsaturated fatty acid, has been widely studied with respect to its role in the inhibition of neuroinflammation.32 Some previous randomized clinical trials had demonstrated that docosahexaenoic acid had extensive health benefits, including the treatment of MDD.33 However, in case of excessive supplementation, docosahexaenoic acid may adversely impact multiple diseases via its oxidation products, such as 4-hydroxy-2-hexenal.34 In the present systematic MR study, we confirmed these associations of docosahexaenoic acid with the risk of MDD and some non-psychiatric diseases from genetic perspective. 3-Hydroxybutyrate is a ketone body managing emotional system in the brain via energy metabolism.35 Recently, a multicenter pilot analysis of plasma metabolome suggested that 3-Hydroxybutyrate levels were positively associated with the severity of depression and suicidal ideation.35 Similarly, we found that genetically determined high 3-Hydroxybutyrate levels were associated with an increased risk of MDD, and lowering 3-Hydroxybutyrate for treating MDD had no side effects on the other nonpsychiatric diseases. Butyrylcarnitine belongs to acylcarnitines, which is a family of prooxidative compounds generated with incomplete fatty acid β-oxidation.36 In a recent mass spectrometry analysis of plasma acylcarnitine profiles, butyrylcarnitine level was found higher in patients with SCZ than that in healthy individuals.37 By combining genetics and metabolomics, our findings supported a positive association between butyrylcarnitine and the risk of SCZ from the perspective of causality. Further Phe-MR analysis also suggested a protective effect of lowering butyrylcarnitine levels on the risk of 3 nonpsychiatric diseases. N-Acetylornithineis is an important intermediate in arginine metabolism,38 and a previous MR study had showed beneficial effects of N-acetylornithine on both BIP and SCZ.20 Using stricter criteria for genetic instruments selection in the present study, we further confirmed the inverse associations of N-acetylornithine levels with the risk of BIP and SCZ. These shared associations might be attributed to the common genetic etiology between BIP and SCZ.39 In addition, our Phe-MR analysis also found no side effects of N-acetylornithine supplementation for treating BIP and SCZ. Taken together, glycine, 3-Hydroxybutyrate, and N-acetylornithine can serve as promising drug targets for psychiatric disorders without predicted adverse side effects.
In the present study, we also identified 3 novel mediators for psychiatric disorders, including 1-arachidonoyl-GPC, glycoproteins, and sphingomyelins. Although the biological role of these metabolites in central nervous system was not well defined, inflammation might be the potential pathophysiological mechanism underlying the associations between these metabolites and psychiatric disorders.40 1-arachidonoyl-GPC is an important lysophosphatidylcholine, which can suppress CXCR3-mediated T cell migration to inflamed microenvironments.41,42 In the present study, genetically determined higher 1-arachidonoyl-GPC levels were associated with a lower risk of BIP. However, based on the Phe-MR analysis of 655 diseases, 1-arachidonoyl-GPC supplementation appeared to be deleterious for thyroid gland and colon neoplasms. Glycoproteins are molecules that comprise protein and carbohydrate chains, and play a critical role in relieving inflammation.43 For example, as major glycoproteins, progranulin44 and fetuin-A45 were implicated in neuroprotection by inhibiting proinflammatory cytokine production. In this MR study, we revealed a beneficial role of glycoproteins in the etiology of BIP. Moreover, our Phe-MR analysis indicated that glycoproteins supplementation had additional benefits on multiple systems, but it could also lead to increased risks of varicose veins of lower extremity and chronic airway obstruction. Sphingomyelins are dominant sphingolipids in cell membranes, acting as both constructive components and signal carriers in physiological processes. Accumulating experimental evidence had suggested the integral role of sphingomyelins cycle in the proinflammatory process (e.g., Nuclear factor kappa B signaling pathway),46 and downregulation of sphingomyelin synthases was able to reduce microglial inflammation in mice.47 In our MR and Phe-MR analyses, higher sphingomyelins levels were shown to be significantly associated with an increased risk of AN, while lowering sphingomyelins for treating AN had a series of adverse side effects. Therefore, interventions targeting 1-arachidonoyl-GPC, glycoproteins and sphingomyelin for preventing and treating psychiatric disorders should be applied with a comprehensive consideration of the therapeutic effects and target-mediated side effects.
Our study has important public health and clinical implications. Psychiatric disorders are a major global public health challenge today, while the high-risk criteria appears to be insufficient to predict the onset of first-episode psychiatric disorders.48 Therefore, it is of public health importance to identify novel biomarkers for better monitoring high-risk individuals and improving the early prevention of psychiatric disorders. From the findings of our study, 1-arachidonoyl-GPC, glycoproteins, N-acetylornithine, glycine, docosahexaenoic acid, 3-Hydroxybutyrate, butyrylcarnitine, and sphingomyelins are potential predictive biomarkers for psychiatric disorders. Moreover, the misdiagnosis rate of psychiatric disorders is approximately 40%,49 so further studies are warranted to assess the clinical value of these biomarkers in differential diagnosis of psychiatric disorders.
On the other hand, the current available treatment approach for psychiatric disorders mainly includes psychotherapy and pharmacotherapy.1 As the most commonly used antipsychotics, atypical (second generation) antipsychotics still cause a series of adverse side effects, such as diabetes, hyperlipidemia, and myocarditis.4 In addition, preliminary tests are recommended in clinical practice before taking certain antipsychotic drugs to ascertain the appropriate dose due to the interindividual heterogeneity of the antipsychotic response.3 Given the limited therapeutic options currently available for psychiatric disorders, it is of clinical interest for psychiatrists to develop novel intervention strategies in the management of psychiatric disorders by targeting N-acetylornithine, glycine, 3-Hydroxybutyrate, and butyrylcarnitine. Further clinical trials are warranted to verify our findings and assess the effective dose of each promising drug target for psychiatric disorders.
The present study has several strengths. Firstly, based on genomics and metabolomics data, this systematic MR study provided new insight into the potential causal mediators of BIP, ADHD, OCD, MDD, SCZ, PD, ASD, and AN. Secondly, based on the multi-cohort setting of the original GWASs, we could make a valid causal inference among large-scale populations with a high statistical power. Finally, we conducted the Phe-MR analysis to comprehensively assess side-effect profiles of interventions against our identified metabolites, which could further help inform drug target prioritization in the drug development and clinical trials. However, our study also has certain limitations. First, this MR study was based on the summary-level data on the blood metabolome, while psychiatric disorders were mainly caused by brain lesions. Further studies are warranted to analyze metabolite changes in cerebrospinal fluid to identify additional promising biomarkers and drug targets for psychiatric disorders. Second, the present study was conducted in individuals of predominantly European descent, which minimized the population stratification bias but restricted the interethnic extrapolation of our findings. Further studies conducted in non-European individuals are needed to confirm our findings. Third, patients in PheCode system were diagnosed in hospital, so the disease traits with low rates of hospital admission may be poorly represented. Finally, there might be an overlap between participants in the GWASs, which may lead to weak instrument bias. Although the F statistics suggested that there was no instrument bias in this MR study, further MR studies based on the independent cohorts without participant overlap are needed to better understand the role of blood metabolites in the etiology of psychiatric disorders.
In this systematic MR analysis, 8 mediators were identified for psychiatric disorders, including 5 established metabolites (N-acetylornithine, glycine, docosahexaenoic acid, 3-Hydroxybutyrate, and butyrylcarnitine) and 3 novel metabolites (1-arachidonoyl-GPC, glycoproteins, and sphingomyelins). Side-effect profiles were characterized to help inform drug target prioritization. N-acetylornithine, glycine, 3-Hydroxybutyrate, and butyrylcarnitine might be promising targets against psychiatric disorders with no predicted adverse side effects.
Supplementary Material
Acknowledgement
We thank the investigators of Psychiatric Genomics Consortium, UK Biobank, and the 3 European-descent GWASs of blood metabolome for making their results publicly available. Full acknowledgement and funding statements for each of these resources are available via the relevant cited reports.
Contributor Information
Yiming Jia, Department of Epidemiology, School of Public Health and Jiangsu Key Laboratory of Preventive and Translational Medicine for Geriatric Diseases, Medical College of Soochow University, Suzhou, China.
Li Hui, Research Center of Biological Psychiatry, The Affiliated Guangji Hospital of Soochow University, Suzhou, China.
Lulu Sun, Department of Epidemiology, School of Public Health and Jiangsu Key Laboratory of Preventive and Translational Medicine for Geriatric Diseases, Medical College of Soochow University, Suzhou, China.
Daoxia Guo, Department of Epidemiology, School of Public Health and Jiangsu Key Laboratory of Preventive and Translational Medicine for Geriatric Diseases, Medical College of Soochow University, Suzhou, China; School of Nursing, Medical College of Soochow University, Suzhou, China.
Mengyao Shi, Department of Epidemiology, School of Public Health and Jiangsu Key Laboratory of Preventive and Translational Medicine for Geriatric Diseases, Medical College of Soochow University, Suzhou, China.
Kaixin Zhang, Department of Epidemiology, School of Public Health and Jiangsu Key Laboratory of Preventive and Translational Medicine for Geriatric Diseases, Medical College of Soochow University, Suzhou, China.
Pinni Yang, Department of Epidemiology, School of Public Health and Jiangsu Key Laboratory of Preventive and Translational Medicine for Geriatric Diseases, Medical College of Soochow University, Suzhou, China.
Yu Wang, Department of Epidemiology, School of Public Health and Jiangsu Key Laboratory of Preventive and Translational Medicine for Geriatric Diseases, Medical College of Soochow University, Suzhou, China.
Fanghua Liu, Department of Epidemiology, School of Public Health and Jiangsu Key Laboratory of Preventive and Translational Medicine for Geriatric Diseases, Medical College of Soochow University, Suzhou, China.
Ouxi Shen, Department of Occupational Health, Suzhou Industrial Park Center for Disease Control and Prevention, Suzhou, China.
Zhengbao Zhu, Department of Epidemiology, School of Public Health and Jiangsu Key Laboratory of Preventive and Translational Medicine for Geriatric Diseases, Medical College of Soochow University, Suzhou, China.
Author contributions
The study was conceived and designed by YJ, LH and ZZ. YJ, LH and ZZ coordinated the study. YJ, LH, LS, DG, MS, KZ, PY, YW, FL, OS and ZZ contributed to data collection. YJ and LH performed the statistical analysis and prepared the first draft of manuscript. ZZ revised the paper and helped to write the final draft of manuscript. ZZ is guarantor.
Funding
This study was supported by the National Natural Science Foundation of China (grant: 82103917 and 82103921), the Natural Science Research Project of Jiangsu Provincial Higher Education (grant: 21KJB330006), and a Project of the Priority Academic Program Development of Jiangsu Higher Education Institutions, China. The funders had no role in the design and conduct of the study, collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Ethical approval
This study is based on publicly available summarized data. The protocol and data collection were approved by the ethics committee of each genome-wide association study.
Consent to participate
Written informed consent was obtained from each participant of previously published GWASs before data collection.
Conflicts of interest
The authors report no conflicts of interest.
References
- 1. Leichsenring F, Steinert C, Rabung S, Ioannidis JPA.. The efficacy of psychotherapies and pharmacotherapies for mental disorders in adults: an umbrella review and meta-analytic evaluation of recent meta-analyses. World Psychiatry 2022;21(1):133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. James SL, Abate D, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. The Lancet 2018;392(10159):1789–1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Haddad PM, Correll CU.. The acute efficacy of antipsychotics in schizophrenia: a review of recent meta-analyses. Ther Adv Psychopharmacol 2018;8(11):303–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ucok A, Gaebel W.. Side effects of atypical antipsychotics: a brief overview. World Psychiatry 2008;7(1):58–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nelson MR, Tipney H, Painter JL, et al. The support of human genetic evidence for approved drug indications. Nat Genet. 2015;47(8):856–860. [DOI] [PubMed] [Google Scholar]
- 6. Oresic M, Tang J, Seppanen-Laakso T, et al. Metabolome in schizophrenia and other psychotic disorders: a general population-based study. Genome Med. 2011;3(3):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shin SY, Fauman EB, Petersen AK, et al. An atlas of genetic influences on human blood metabolites. Nat Genet. 2014;46(6):543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kettunen J, Demirkan A, Wurtz P, et al. Genome-wide study for circulating metabolites identifies 62 loci and reveals novel systemic effects of LPA. Nat Commun. 2016;7:11122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nightingale Health and UK Biobank announces major initiative to analyse half a million blood samples to facilitate global medical research. https://www.ukbiobank.ac.uk/learn-more-about-uk-biobank/news/nightingale-health-and-uk-biobank-announces-major-initiative-to-analyse-half-a-million-blood-samples-to-facilitate-global-medical-research. 2018. Accessed October 23th 2021.
- 10. Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G.. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27(8):1133–1163. [DOI] [PubMed] [Google Scholar]
- 11. Hemani G, Bowden J, Davey Smith G.. Evaluating the potential role of pleiotropy in Mendelian randomization studies. Hum Mol Genet. 2018;27(R2):R195–R208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mullins N, Forstner AJ, O’Connell KS, et al. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nat Genet. 2021;53(6):817–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Demontis D, Walters RK, Martin J, et al. Discovery of the first genome-wide significant risk loci for attention deficit/hyperactivity disorder. Nat Genet. 2019;51(1):63–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. International Obsessive Compulsive Disorder Foundation Genetics C, Studies OCDCGA. Revealing the complex genetic architecture of obsessive-compulsive disorder using meta-analysis. Mol Psychiatry. 2018;23(5):1181–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Howard DM, Adams MJ, Clarke TK, et al. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci. 2019;22(3):343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pardinas AF, Holmans P, Pocklington AJ, et al. Common schizophrenia alleles are enriched in mutation-intolerant genes and in regions under strong background selection. Nat Genet. 2018;50(3):381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Forstner AJ, Awasthi S, Wolf C, et al. Genome-wide association study of panic disorder reveals genetic overlap with neuroticism and depression. Mol Psychiatry. 2021;26(8):4179–4190. [DOI] [PubMed] [Google Scholar]
- 18. Grove J, Ripke S, Als TD, et al. Identification of common genetic risk variants for autism spectrum disorder. Nat Genet. 2019;51(3):431–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Watson HJ, Yilmaz Z, Thornton LM, et al. Genome-wide association study identifies eight risk loci and implicates metabo-psychiatric origins for anorexia nervosa. Nat Genet. 2019;51(8):1207–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang J, Yan B, Zhao B, et al. Assessing the causal effects of human serum metabolites on 5 major psychiatric disorders. Schizophr Bull. 2020;46(4):804–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bennett DA, Holmes MV.. Mendelian randomisation in cardiovascular research: an introduction for clinicians. Heart 2017;103(18):1400–1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhou W, Nielsen JB, Fritsche LG, et al. Efficiently controlling for case-control imbalance and sample relatedness in large-scale genetic association studies. Nat Genet. 2018;50(9):1335–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chong M, Sjaarda J, Pigeyre M, et al. Novel drug targets for ischemic stroke identified through Mendelian randomization analysis of the blood proteome. Circulation 2019;140(10):819–830. [DOI] [PubMed] [Google Scholar]
- 24. Hemani G, Zheng J, Elsworth B, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife 2018;7:e34408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brion MJ, Shakhbazov K, Visscher PM.. Calculating statistical power in Mendelian randomization studies. Int J Epidemiol. 2013;42(5):1497–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bowden J, Davey Smith G, Haycock PC, Burgess S.. Consistent estimation in Mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40(4):304–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Burgess S, Butterworth A, Thompson SG.. Robust instrumental variable methods using multiple candidate instruments with application to Mendelian randomization. arXiv 2016:1606.03729. [Google Scholar]
- 28. Zhao Q, Wang J, Hemani G, Bowden J, DS S. Statistical inference in two-sample summary data Mendelian Randomization using robust adjusted profile score. arXiv 2019:1801.09652. [Google Scholar]
- 29. Shoham S, Javitt DC, Heresco-Levy U.. Chronic high-dose glycine nutrition: effects on rat brain cell morphology. Biol Psychiatry. 2001;49(10):876–885. [DOI] [PubMed] [Google Scholar]
- 30. Heresco-Levy U, Ermilov M, Lichtenberg P, Bar G, Javitt DC.. High-Dose Glycine Added to Olanzapine and Risperidone for the Treatment of Schizophrenia. Biol Psychiatry. 2004;55(2):165–171. [DOI] [PubMed] [Google Scholar]
- 31. Habibi S, Joshi PU, Mi X, Heldt CL, Minerick AR.. Changes in membrane dielectric properties of porcine kidney cells provide insight into the antiviral activity of glycine. Langmuir 2020;36(29):8344–8356. [DOI] [PubMed] [Google Scholar]
- 32. Butler MJ, Deems NP, Muscat S, Butt CM, Belury MA, Barrientos RM.. Dietary DHA prevents cognitive impairment and inflammatory gene expression in aged male rats fed a diet enriched with refined carbohydrates. Brain Behav Immun. 2021;98:198–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ghasemi Fard S, Wang F, Sinclair AJ, Elliott G, Turchini GM.. How does high DHA fish oil affect health? A systematic review of evidence. Crit Rev Food Sci Nutr. 2019;59(11):1684–1727. [DOI] [PubMed] [Google Scholar]
- 34. Shoeb M, Ansari NH, Srivastava SK, Ramana KV.. 4-Hydroxynonenal in the pathogenesis and progression of human diseases. Curr Med Chem. 2014;21(2):230–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Setoyama D, Kato TA, Hashimoto R, et al. Plasma metabolites predict severity of depression and suicidal ideation in psychiatric patients-a multicenter pilot analysis. PLoS One. 2016;11(12):e0165267e0165267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Aguer C, McCoin CS, Knotts TA, et al. Acylcarnitines: potential implications for skeletal muscle insulin resistance. FASEB J. 2015;29(1):336–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cao B, Wang D, Pan Z, et al. Characterizing acyl-carnitine biosignatures for schizophrenia: a longitudinal pre- and post-treatment study. Transl Psychiatry. 2019;9(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Armstrong MD. Nδ-acetylornithine and S-methylcysteine in blood plasma. Biochimica et Biophysica Acta (BBA) - General Subjects 1979;587(4):638–642. [DOI] [PubMed] [Google Scholar]
- 39. Lichtenstein P, Yip BH, Björk C, et al. Common genetic determinants of schizophrenia and bipolar disorder in Swedish families: a population-based study. The Lancet 2009;373(9659):234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Najjar S, Pearlman DM, Alper K, Najjar A, Devinsky O.. Neuroinflammation and psychiatric illness. J Neuroinflammation. 2013;10:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. de Groot P, Nikolic T, Pellegrini S, et al. Faecal microbiota transplantation halts progression of human new-onset type 1 diabetes in a randomised controlled trial. Gut 2021;70(1):92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kurachi M, Kurachi J, Suenaga F, et al. Chemokine receptor CXCR3 facilitates CD8(+) T cell differentiation into short-lived effector cells leading to memory degeneration. J Exp Med. 2011;208(8):1605–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gabay C, Kushner I.. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340(6):448–454. [DOI] [PubMed] [Google Scholar]
- 44. Menzel L, Kleber L, Friedrich C, et al. Progranulin protects against exaggerated axonal injury and astrogliosis following traumatic brain injury. Glia 2017;65(2):278–292. [DOI] [PubMed] [Google Scholar]
- 45. Heinen MC, Babler A, Weis J, et al. Fetuin-A protein distribution in mature inflamed and ischemic brain tissue. PLoS One. 2018;13(11):e0206597e0206597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nixon GF. Sphingolipids in inflammation: pathological implications and potential therapeutic targets. Br J Pharmacol. 2009;158(4):982–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yang Y, Hu F, Yang G, Meng Q.. Lack of sphingomyelin synthase 2 reduces cerebral ischemia/reperfusion injury by inhibiting microglial inflammation in mice. Exp Ther Med 2020;20(6):241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Fusar-Poli P, Bonoldi I, Yung AR, et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69(3):220–229. [DOI] [PubMed] [Google Scholar]
- 49. Ayano G, Demelash S, Yohannes Z, et al. Misdiagnosis, detection rate, and associated factors of severe psychiatric disorders in specialized psychiatry centers in Ethiopia. Ann Gen Psychiatry 2021;20(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All summary statistics used in this two-stage Mendelian randomization are available online from each genome-wide association study. Statistical code is available on the request by directly contacting the corresponding author (email: zbzhu@suda.edu.cn).