Abstract
Objectives
Psychomotor slowing (PS) occurs in up to half of schizophrenia patients and is linked to poorer outcomes. As standard treatment fails to improve PS, novel approaches are needed. Here, we applied the RDoC framework using 3 units of analysis, ie, behavior, self-report, and physiology to test, whether patients with PS are different from patients without PS and controls.
Methods
Motor behavior was compared between 71 schizophrenia patients with PS, 25 without PS, and 42 healthy controls (HC) using 5 different measures: (1) for behavior, an expert rating scale: Motor score of the Salpêtrière Retardation Rating Scale, (2) for self-report, the International Physical Activity Questionnaire; and for physiology, (3) Actigraphy, which accounts for gross motor behavior, (4) Gait velocity, and (5) coin rotation task to assess manual dexterity.
Results
The ANCOVAs comparing the 3 groups revealed differences between patients with PS and HC in expert ratings, self-report, and instrumental measures (all P ≤ .001). Patients with PS also scored higher in expert ratings and had lower instrumental activity levels compared to patients without PS (all P ≤ .045). Instrumental activity levels correlated with an expert rating of PS (rho = −0.51, P-fdr corrected <.001) and classified similarly at 72% accuracy.
Conclusions
PS is characterized by slower gait, lower activity levels, and slower finger movements compared to HC. However, only actigraphy and observer ratings enable to clearly disentangle PS from non-PS patients. Actigraphy may become the standard assessment of PS in neuroimaging studies and clinical trials.
Keywords: schizophrenia, instrumental measures, motor abnormalities, psychomotor retardation, gait analysis, actigraphy
Introduction
Schizophrenia is a severe and potentially disabling mental disorder with a lifetime prevalence of 1%.1 Frequent symptoms of schizophrenia include hallucinations, delusions, disorganization, negative symptoms, but also motor abnormalities. One of these motor abnormalities is Psychomotor slowing (PS). PS refers to the slowing of both mental processes and physical activities. It encompasses slowness of thoughts as well as an observable decrease in movement initiation, amount, and velocity, visible in gross motor behavior such as gait, and in fine motor behavior such as writing, but also in facial expression.2–4 Whereas the slowing of motor behaviors is readily defined, the boundaries of the term “psychomotor” remain debated. On one hand, psychomotor includes the cognitive processes (ie, the prefix psycho) responsible for movements as well as the execution of movements (ie, the root word motor).2,3 But “psychomotor” is also used to describe how motor behavior, cognition and emotion interact on the neural level.5,6 The current report focuses on psychomotor behaviors, therefore, we are considering PS as combination of action planning and execution, knowing that multiple affective, and cognitive factors may interact.
PS is prevalent across all stages of schizophrenia, independent of the current medication dosage.3,7–9 PS is not only associated with poor cognition, lower functioning, higher levels of sedentary behavior, and cardio-metabolic risks in schizophrenia, but also with poor neuropsychological and functional outcomes across serious mental illnesses.10–17
Current treatment options for schizophrenia fall short of alleviating PS,2,3 calling for novel interventions, eg, noninvasive brain stimulation.18,19 These interventions will be informed by the pathobiology of PS. But studies on PS pathobiology require precise phenotyping and measurement, which the field is currently lacking. Particularly, the use of specific rating scales and objective instrumentation are recommended to study PS.20,21 But few studies have assessed PS outside neurocognitive tests with measures that have real-world face validity. In addition, prior studies on motor abnormalities in schizophrenia focused on syndromes that overlap with PS, such as neurological soft signs, negative symptoms, parkinsonism, or catatonia,3 while they measure broader constructs.22,23
Broad clinical rating scales often include a single item on “Motor Retardation,” which combines slowing of movements, speech, and thought. However, specificity and validity of single items have been challenged.24,25 Thus, specific expert rating scales, such as the Salpêtrière Retardation Rating Scale (SRRS) have been advocated.4,26,27 Instrumental measures of motor behavior such as actigraphy allow for continuous monitoring of physical activity in real-life.20,28 Lower activity as assessed by actigraphy was linked to more severe PS and negative symptoms in schizophrenia, while lower predictability of movement patterns was associated with positive symptom severity and disorganized behavior.25,27,29,30 Furthermore, actigraphy studies in psychosis linked sedentary behavior to illness chronicity, poor cognition, parkinsonism and catatonia.23,31–34 Gait analysis is another instrumental method to assess gross motor behavior, yet limited to the lab setting. For example, gait velocity is reduced in patients with schizophrenia compared to healthy controls (HC).35,36 Finally, fine motor behavior can be quantified with the coin rotation (CR) test, demonstrating reduced manual dexterity performance in schizophrenia vs controls.37–39 While there has been some work on PS in schizophrenia, the majority of studies limited the evaluation of PS to few measures of either gross or fine motor behavior, thus there is a lack of studies integrating multiple measures of PS.
PS can be conceptualized in the framework of the Research Domain Criteria (RDoc) initiative’s sensorimotor domain. RDoC aims at exploring these domains across multiple units of analysis, for example, genes, molecules, circuits, behavior, and self-report.40,41 This study aimed to understand the mechanisms of PS in schizophrenia within the RDoC framework. Thus, we aimed to provide extensive behavioral phenotyping of PS using 3 units of analysis: behavior (using expert ratings with SRRS), self-report, and physiology (using instrumental measures such as actigraphy, gait velocity, and CR) in a large sample of slowed patients with schizophrenia. To disentangle the behaviors specific to PS from general alterations in schizophrenia, we tested patients with schizophrenia and PS vs. patients with schizophrenia without PS vs. HC. We hypothesized that slowed patients with schizophrenia present impairments within all 3 units of analysis compared to non-slowed patients and HC, eg, slower gait, lower activity levels, and less self-reported activity. In addition, we hypothesized that within schizophrenia patients motor measures are correlating with each other; especially actigraphy activity levels should strongly correlate with expert ratings of PS. Finally, we will explore whether there are subgroups of patients with unique PS patterns.
Material and Method
Participants
We included the baseline data of the OCoPS-P study (Overcoming Psychomotor Slowing in Psychosis; ClinicalTrials.gov Identifier: NCT03921450), which is a prospective randomized clinical trial in patients with schizophrenia spectrum disorders (schizophrenia, schizoaffective, or schizophreniform disorders) according to DSM-5 (as assessed with the SCID). We analyzed the data of 71 patients with schizophrenia and PS according to the SRRS26 (SRRS, total score ≥15), 25 schizophrenia patients without PS (non-PS, SRRS score <15), and 42 HC (table 1).
Table 1.
Demographic and Clinical Data
| HC | PS | Non-PS | Group Comparison | |
|---|---|---|---|---|
| N | 42 | 71 | 25 | — |
| Age in years | 37.0 ± 12.7 | 36.5 ± 12.5 | 34.2 ± 12.3 | F (2, 135) = 0.42, P = .661 |
| Gender | 21M | 36M | 12M | X 2 (2, N = 138) = 0.05, P = .973 |
| Education in years | 16.1 ± 3.2 | 13.0 ± 2.4 | 12.8 ± 1.9 | F (2, 135) = 21.52, P < .001 |
| Duration of illness in years | — | 10.7 ± 10.3 | 7.9 ± 9.3 | t(94) = 1.2, P = .231 |
| Nr. of episodes | — | 5.1 ± 4.6 | 6.4 ± 12.0 | t(26.6) = −0.5, P = .605 |
| PANSS Total | — | 82.5 ± 19.1 | 66.2 ± 14.2 | t(94) = 3.9, P < .001 |
| PANSS Positive | — | 16.5 ± 5.8 | 16.9 ± 5.1 | t(94) = −0.3, P = .785 |
| PANSS Negative | — | 24.5 ± 6.6 | 15.8 ± 4.8 | t(94) = 6.0, P < .001 |
| PANSS Depression G6 | 2.6 ± 1.4 | 2.3 ± 1.3 | t(94) = 0.9, P = .383 | |
| Medication OLZ eq. in mg | — | 16.8 ± 11.0 | 14.6 ± 10.3 | t(94) = 0.9, P = .386 |
| UPDRS | — | 21.9 ± 12.6 | 8.8 ± 6.1 | t(84.9) = 6.8, P < .001 |
| BFCRS | — | 6.2 ± 4.7 | 1.3 ± 1.6 | t(93.98) = 7.6, P < .001 |
| AIMS | — | 1.0 ± 2.5 | 0.1 ± 0.3 | t(74.6) = 3.0, P = .004 |
Note: Displayed are mean ± sd of demographic and clinical variables for each of our 3 groups. Sd, Standard deviation; PANSS, Positive And Negative Syndrome Scale; OLZ eq., Olanzapine-equivalent (mg/day); UPDRS, Unified Parkinson Disease Rating Scale Part III; BFCRS, Bush-Francis Catatonia Rating Scale; AIMS, Abnormal Involuntary Movement Scale; M, male; HC, Healthy Controls; PS, Patients with Psychomotor Slowing; non-PS, Patients without Psychomotor Slowing
All patients were recruited at the in- and out-patient departments of the University Hospital of Psychiatry and Psychotherapy, Bern, Switzerland. Six patients (3 with PS and 3 without PS) were outpatients at the time of assessment. HC were recruited from the community by using flyers and word of mouth. They were age and gender-matched with patients. All participants provided written informed consent. The study protocol adhered to the declaration of Helsinki and was approved by the local ethics committee. General exclusion criteria were active substance dependence excluding nicotine, neurological disorders, which impacted motor behavior, and traumatic brain injury. Additional exclusion criteria for HC were history of any psychiatric disorder or any first degree relative with psychosis.
Measures
General Psychopathology.
We collected data on general symptom severity using the Positive And Negative Syndrome Scale (PANSS),24 parkinsonism, catatonia, and dyskinesia, using the Unified Parkinson’s Disease Rating Scale Part III (UPDRS), the Bush-Francis Catatonia Rating Scale (BFCRS) and the Abnormal Involuntary Movement Scale (AIMS) respectively.42–44 All patients were on antipsychotic medication and mean olanzapine equivalents (OLZ eq.) were calculated according to Leucht.45
Expert Ratings of Psychomotor Slowing.
We assessed PS using the 15-item SRRS which ranges from 0 to 60 and combines cognitive and pure motor symptoms.26 The SRRS total score was used as the classification criterion for PS with SRRS >15 as cutoff. However, as this report aims to provide behavioral phenotyping of PS, we extracted the items focusing exclusively on observable motor behavior in PS. The mSRRS is the sum of the pure motor items (items 1–5 and 15), with higher scores indicating more severe PS.4
Self-report of Physical Activity.
We assessed the activity during the past week by using the 7-item International Physical Activity Questionnaire (IPAQ). IPAQ has been used in epidemiological studies to calculate total physical activity and energy expenditure.46 While self-reported physical activity probably is reduced in subjects with PS, the IPAQ is not assessing PS specifically.
Physiology: Instrumental Measures of Psychomotor Slowing.
We assessed gross motor behavior using the tri-axial-accelerometer Move4 (movisens GmbH, Karlsruhe, Germany).28 Participants wore the wrist actiwatch on their non-dominant hand for 24h. The movement counts were stored in 60s intervals. Actigraphy data were processed using Movisens Software and Excel. We used the activity count during the wake phase, by subtracting the activity count during the night and nap times from the total activity count during 24h.47 Data were missing for 10 patients.
We measured gait velocity with the GAITRite® system (platinum GAITRite walkway, CIR Systems Inc., Sparta, NJ 07871; USA),48 using an 89cm × 701cm long pressure sensitive carpet connected to a computer. The active area was 61cm wide and 610cm long. A total of 20 040 sensors were placed 1.27cm apart with a scan rate of 120Hz (8.3). Here, we analyzed the individual averaged walking velocity while walking at self-selected speed during 4 trials (1 trial = 1 × length of the carpet). Data were missing for 1 participant.
Finally, fine motor skills were assessed with the CR task. Participants had to rotate a Swiss 50-Rappen coin, comparable to the size of a US dime with a diameter of 18.2mm, between thumb, index, and middle finger as fast as possible. They performed 3 trials of 10s with each hand. Video recordings of CR performance were scored blind to participant status. The first trial was a practice trial. The score of trials 2 and 3 was averaged. We calculated the CR score for each trial using the following validated formula: CR score = half turns − [(coin drops × 0.10) × half turns].49,50
Data Analysis
First, we compared the 3 groups PS, non-PS, and HC on demographic and clinical parameters with IBM SPSS Statistics (v28.0.0.0). Next, we used ANCOVAs to compare mSRRS, IPAQ, activity count, gait velocity, and CR between the three groups by controlling for age followed by post hoc tests between patients and controls with Sidak correction for multiple comparisons. As motor measures in patients are potentially associated with antipsychotic medication and illness severity (supplementary table S1A–C), we additionally run ANCOVAs to compare each measure between PS and non-PS patients using age, negative symptoms (assessed by PANSS negative) and medication (OLZ) as covariates. Furthermore, we conducted nonparametric partial correlations (Spearman) with age, medication and negative symptoms as covariates to explore potential associations of the different measures of PS in schizophrenia patients. For each measure, we controlled for multiple comparisons using the false discovery rate (FDR). Additionally, we ran binary logistic regressions to test the classification into PS and non-PS patients by using either activity level as a predictor or all instrumental motor measures (activity level, gait velocity, and both CR-Task performances) as predictors. Furthermore, we ran a k-means cluster analysis on the activity levels and compared the resulting groups (high vs low activity) on motor measures and psychopathology. Finally, to explore subgroups among PS patients, we performed a cluster analysis within the PS group on all instrumental measures and compared the resulting clusters regarding psychopathology, demographics, illness duration, medication, expert ratings of motor abnormalities, self-report, and instrumental assessments of PS. The missing values were replaced with the mean value of each measure.
Results
Demographic and Clinical Information
Patients with schizophrenia and PS (n = 71), without psychomotor slowing (non-PS, n = 25), and HC (n = 42) did not differ in age or gender. PS patients had more severe symptoms compared to non-PS patients, including negative symptoms, dyskinesia, parkinsonism, and catatonia. However, patient groups did not differ in current medication dosage, duration of illness, or PANSS depression ratings (table 1).
Group Comparison of Motor Assessments
Expert Ratings of Behavior.
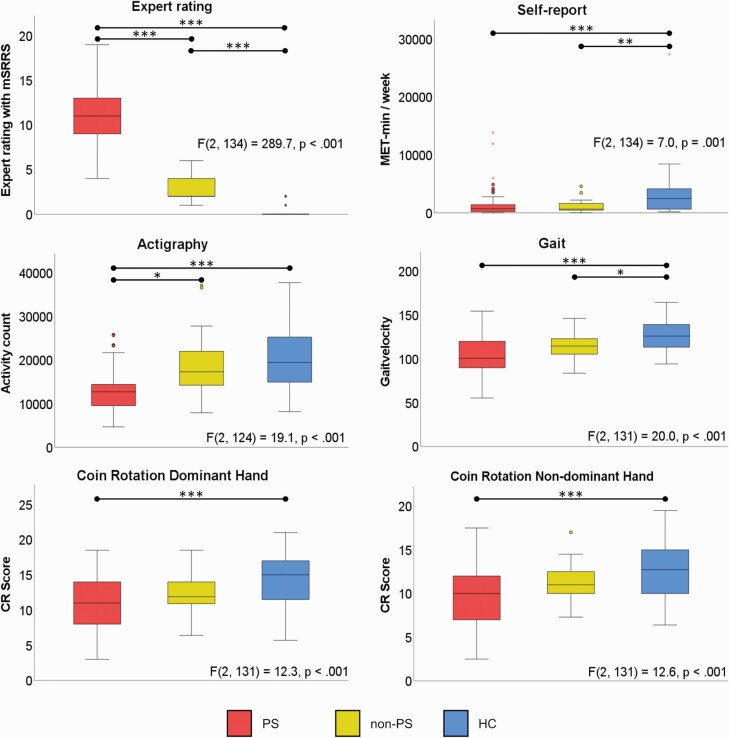
ANCOVAs indicated that PS had higher mSRRS scores than HC and non-PS (figure 1, table 2), reflecting the categorization of patients using the total SRRS cutoff.
Fig. 1.
Group comparison of motor assessments.
Note: ANCOVAs comparing PS, non-PS and HC; Post Hoc tests with Sidak correction for multiple comparison. PS: Patients with Psychomotor Slowing; non-PS: Patients without Psychomotor Slowing; HC: Healthy controls.
Table 2.
Group Comparison of Motor Assessments
| PS (mean ± sd) |
non-PS (mean ± sd) |
HC (mean ± sd) |
Main ANCOVA (controlling for age) |
Post Hocs | ANCOVA non-PS vs PS patients (controlling for age, medication & PANSS negative) |
|
|---|---|---|---|---|---|---|
| Expert rating, mSRRS | 10.7 ± 3.1 | 3.0 ± 1.5 | .2 ±.6 | F(2, 134) = 289.7, P < .001 |
PS vs HC: P < .001*** non-PS vs HC: P < .001*** |
F(1, 91) = 77.3, P < .001*** |
| Self-report, IPAQ | 1394 ± 2338 | 1150 ± 1099 | 3390 ± 4444 | F(2, 134) = 7.0, P = .001 |
PS vs HC: P = .003** non-PS vs HC: P = .010** |
F(1, 91) = 0.1, P = .784 |
| Actigraphy, counts/h | 13045 ± 5052 | 18793 ± 7644 | 20343 ± 7039 | F(2, 124) = 19.1, P < .001 |
PS vs HC: P < .001*** non-PS vs HC: P = .771 |
F(1, 81) = 4.1, P = .045*. |
| Gait velocity, m/s | 102.4 ± 22.3 | 114.5 ± 16.3 | 126.6 ± 17.6 | F(2, 131) = 20.0, P < .001 |
PS vs HC: P < .001*** non-PS vs HC: P = .039* |
F(1, 88) = 1.0, P = .314 |
| CR Dom | 11.0 ± 3.7 | 12.4 ± 3.1 | 14.4 ± 3.4 | F(2, 131) = 12.3, P < .001 |
PS vs HC: P < .001*** non-PS vs HC: P = .052 |
F(1, 88) = 0.04, P = .846 |
| CR non-Dom | 9.7 ± 3.2 | 11.1 ± 2.2 | 12.7 ± 3.3 | F(2, 131) = 12.6, P < .001 |
PS vs HC: P < .001*** non-PS vs HC: P = .121 |
F(1, 88) = 0.4, P = .550 |
Note: Post Hoc tests with Sidak correction for multiple comparisons. PS, Patients with Psychomotor Slowing; non-PS, Patients without Psychomotor Slowing; HC, Healthy controls.
Self-report.
HC reported more overall activity than either patient group. PS and non-PS did not differ in self-reported activity (figure 1, table 2).
Physiology: Instrumental Measures.
ANCOVAs indicated lower activity levels and slower gait in PS compared to HC, while no difference emerged between HC and non-PS regarding activity levels. PS demonstrated lower activity levels than non-PS, but no difference in gait velocity when controlling for medication and PANSS negative (figure 1, table 2).
Finally, HC presented superior fine motor performance than PS patients for both hands. No difference between PS and non-PS’s in CR performance was noted on either hand (figure 1, table 2).
Correlations Analyses in Schizophrenia Patients
The activity level measured with actigraphy correlated negatively with mSRRS (rho = −0.51, Pfdr_corr< .001), while activity levels correlated positively with all the other measures (all rho ≥ 0.22, Pfdr_corr ≤ .032). Self-paced gait velocity also correlated with the performance on the CR task with the dominant hand (rho = 0.32, Pfdr_corr = .01) and inversely correlated with mSRRS (rho = −0.23, Pfdr_corr = .065) at trend level. As expected, the performance of both hands on the CR task is highly correlated (rho = 0.74, Pfdr_corr < .001). The IPAQ was exclusively associated with actigraphy. Overall the strongest association was detected between activity levels (gold standard of instrumental PS assessment) and the mSRRS (expert rating of PS) (See figure 2 and supplementary table S3).
Fig. 2.
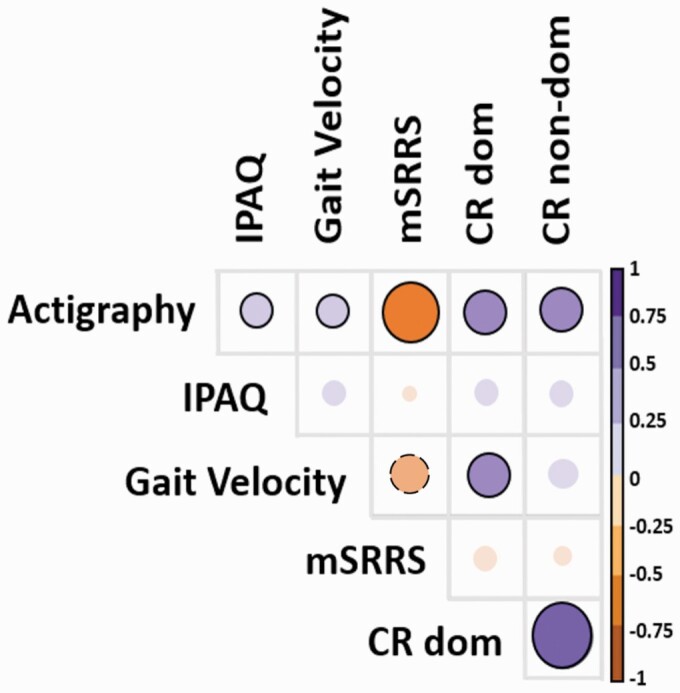
Partial correlations between measures.
Note: The larger the circle the stronger the association, the ones with a black circle around are significant and the ones with a dotted black line around are at trend level.
Logistic regression analysis indicated that actigraphy levels had 72.1% accuracy in classifying patients with PS and non-PS compared to the expert rating (Wald Chi2 = 11.1, df = 1, P < .001). Accuracy is 72.3% when AL is combined with gait and CR for both hands, however, only AL contributes significantly to the model (Wald Chi2 = 8.7, df = 1 P = .003). Finally, we ran a cluster analysis on AL in all patients, which found 2 clusters (low AL n = 66, high AL n = 20). Low AL had higher ratings on PS, parkinsonism, negative symptoms, catatonia, and lower CR. But clusters did not differ in OLZ, positive symptoms, gait velocity, IPAQ, or dyskinesia (supplementary table S2).
Cluster Analysis Within PS
We performed k-means clustering to explore subgroups in PS. A 2 cluster solution was the most plausible from the data. One cluster (n = 42) included subjects with pronounced impairments in manual dexterity, lower activity, and slower gait, while the other cluster (n = 29) included subjects with less impairments in all instrumental measures (supplementary figure S1). table 3 demonstrates the clinical differences between the clusters with Cluster 1 having more psychotic symptoms, higher ratings of slowing, and more parkinsonism, whereas clusters were comparable on age, gender, medication, education, catatonia severity, and self-reported activity. The rigid cluster had specifically increased ratings of rigidity and bradykinesia in UPDRS single items (supplementary table S4).
Table 3.
Demographic and Clinical Data of the Clusters
| Highly Rigid and Slowed Patients | Less Rigid and Slowed Patients | Comparison | FDR-corr. P-value | |
|---|---|---|---|---|
| N | 42 | 29 | — | |
| Age in years | 37.3 ± 14.4 | 34.6 ± 11.0 | F(1, 69) = 0.8, P = .388 | .466 |
| Gender | 21M | 15M | X 2 (1, N = 71) = 0.02, P = .886 | .997 |
| Education in years | 13.0 ± 2.4 | 13.0 ± 2.4 | F(1, 69) =.001, P = .977 | .977 |
| Duration of illness in years | 12.6 ± 11.4 | 8.0 ± 7.9 | F(1, 69) = 3.5, P = .066 | .108 |
| Nr. of episodes | 6.2 ± 5.4 | 3.5 ± 2.6 | F(1, 69) = 6.2, P = .015 | .030* |
| SRRS total | 26.3 ± 6.7 | 22.0 ± 5.0 | F(1, 69) = 8.7, P = .004 | .013* |
| PANSS Total | 87.2 ± 21.6 | 75.7 ± 12.2 | F(1, 69) = 6.7, P = .012 | .026* |
| PANSS Positive | 17.6 ± 6.6 | 15.0 ± 4.1 | F(1, 69) = 3.5, P = .066 | .100 |
| PANSS Negative | 26.0 ± 7.2 | 22.2 ± 4.9 | F(1, 69) = 6.2, P =.015 | .027* |
| Medication OLZ eq. in mg | 17.7 ± 11.7 | 15.3 ± 9.7 | F(1, 69) = 0.8, P = .374 | .481 |
| UPDRS | 25.4 ± 12.1 | 16.9 ± 11.7 | F(1, 69) = 8.7, P = .004 | .015* |
| BFCRS | 5.9 ± 4.9 | 5.2 ± 4.3 | F(1, 69) = 2.2, P = .146 | .203 |
| mSRRS | 11.5 ± 3.1 | 9.6 ± 2.9 | F(1, 69) = 6.8, P = .011 | .029* |
| IPAQ | 1400.7 ± 2376.3 | 1382.0 ± 2324.5 | F(1, 69) = 0.001, P = .974 | >.9 |
| Actigraphy | 11313.6 ± 3584.4 | 15552.5 ± 5076.9 | F(1, 69) = 17.0, P < .001 | <.001*** |
| Gait velocity | 94.6 ± 20.1 | 113.6 ± 19.9 | F(1, 69) = 15.5, P < .001 | <.001*** |
| CR dominant hand | 8.6 ± 2.4 | 14.5 ± 1.8 | F(1, 69) = 125.9, P < .001 | <.001*** |
| CR non-dominant hand | 7.9 ± 2.4 | 12.2 ± 2.1 | F(1, 69) = 62.1, P < .001 | <.001*** |
Note: Displayed are mean ± sd of demographic and clinical variables for each cluster within the PS group. Sd, Standard deviation; SRRS, Salpêtrière Retardation Rating Scale; PANSS, Positive And Negative Syndrome Scale; OLZ eq., Olanzapine-equivalent (mg/day); UPDRS, Unified Parkinson Disease Rating Scale Part III; BFCRS, Bush-Francis Catatonia Rating Scale; mSRRS, motor score of SRRS; IPAQ, International Physical Activity Questionnaire; CR, Coin rotation task; M, male.
Discussion
This study aimed at exploring psychomotor slowing (PS) in psychosis from an RDoC perspective leveraging 3 units of analysis and testing PS against general impairments in schizophrenia spectrum disorders. Patients with PS differed from healthy subjects in expert ratings, self-report, and multiple instrumental measures. Patients with PS also scored higher in expert ratings and had lower instrumental activity levels compared to psychosis patients without PS. In patients, instrumental measures were strongly correlated to expert ratings of PS. Particularly, classification based on actigraphy provided similar results as classification by expert ratings. Finally, within PS patients we found 2 subgroups of severity, 1 of which is characterized by massively impaired manual dexterity and parkinsonism.
Multimodal Assessment of Psychomotor Slowing
We classified the patients with schizophrenia into PS (total SRRS ≥15) and non- (total SRRS <15) groups. This distinction is also reflected in the pure motor items as patients with PS had much higher mSRRS scores than patients without PS and HC. This focus on observable motor behavior is important. While SRRS total scores share 76% of the variance of mSRRS in patients, the group difference between patients with and without PS could also stem from the non-motor items of the SRRS. Thus, those identified as slow with the broad evaluation of PS (SRRS), are specifically slower in the mSRRS. However, the non-PS patients also had higher mSRRS scores than HC, indicating mild motor slowing, which confirms the frequent presence of motor abnormalities in schizophrenia in general.16,21,51,52
Both patients with and without PS report similar amounts of physical activity. This level of self-reported activity is lower than the one reported by HC suggesting this deficit might be related to schizophrenia. Multiple other factors next to PS could contribute to broadly lower self-reported activity in schizophrenia, eg, cognitive impairment or lack of active events in patients’ lives.53 Thus, self-report might not provide reliable estimates of physical activity, which is in line with a previous study.54 Concurrently, the IPAQ is not designed to measure PS and future self-report instruments might be better suited to capture PS. Most recently, the novel questionnaire for catatonia spectrum has been introduced, which had acceptable agreements with expert ratings.55
Next, instrumental measures tested gross motor behavior (physiology) using actigraphy and GAITRite. Patients with PS had lower activity levels than controls or patients without PS. Furthermore, PS patients walked slower than HC. These findings corroborate prior studies linking lower activity levels to slower processing speed, catatonia, parkinsonism, negative symptoms, and to deteriorating courses in schizophrenia spectrum disorders.23,25,27,30–33,56–58 However, no prior study compared activity levels between psychosis patients with PS and patients without PS. The current report suggests that activity levels measured by actigraphy might be a good marker to identify PS. In fact, accuracy was good compared to expert ratings and clustering according to activity levels provided similar results as the SRRS-based classification. Actigraphy requires instruments, but rater trainings and time for ratings can be saved. In line with previous studies, gait was slower in schizophrenia than in HC.35,36 However, gait velocity did not differ between patient groups.
Similarly, the assessment of fine motor behavior with CR-Task, revealed that slowed patients had a deficit in manual dexterity in both hands compared to HC. But CR-performance was not different between the PS and non-PS. CR performance was similar to previous studies in schizophrenia.38,39,50
The motor performance in fine and gross motor measures appears to follow a continuum with the HC on one end and the patients with PS on the other end, the patients without slowing showing an intermediate position. Still, CR and CR lacked a group difference when controlling for OLZ and negative symptoms, suggesting a general deficit in schizophrenia. In contrast, post hoc comparisons might have approached significance if the sample size was increased, as variance and means suggest. The RDoC motor domain calls for a dimensional assessment of motor abnormalities.40,41 Here, we demonstrate specific changes in psychosis patients with PS. However, the instrumental measures and self-report could be readily applied for dimensional assessments across various disorders.
We observed much variance in the PS group, calling for exploratory cluster analysis that identified two subgroups of severity in PS based on all instrumental measures. This distinction might result from different neural substrates. While motor abnormalities and especially PS seems to be associated with alteration of the entire motor circuitry,2,52,59,60 the patients with less motor impairments could present alterations only in the cortical motor network (primary motor and premotor areas) leading to abnormalities in movement execution, whereas the patients with severer impairment could present additional alterations in the cortico-subcortical circuits with the basal ganglia leading to abnormalities in both movement control and movement execution.3,61–64 Particularly, one PS cluster had higher ratings of rigidity items in the UPDRS. The severity of parkinsonism has been associated to alterations in cortical and subcortical structures in schizophrenia.65,66 Further neuroimaging studies will need to test whether PS clusters have distinct pathobiology. Eventually, specific noninvasive brain stimulation protocols might be needed for the 2 types of PS: one addressing cortical motor dysfunction, the other targeting the entire motor circuitry.18,19 First evidence suggests an amelioration of PS by inhibitory 1 Hz stimulation of the supplementary motor area (SMA).19
Correlation Within the Schizophrenia Patients
The actigraphically assessed activity level correlates with all the other measures, such as gait velocity, IPAQ, and manual dexterity, which is particularly true for expert ratings. Likewise, previous studies reported an association between activity levels and PS,27 or catatonia and parkinsonism.23,33,58 Furthermore, all motor measures assessing PS (except IPAQ) correlated with severity of catatonia and parkinsonism, which is in contrast to a prior report in which neither catatonia nor parkinsonism correlated with a single item measure of PS.67 However, in line with other reports, PS was strongly associated with negative symptom severity.4,23,67–69 Together, these results suggest that actigraphy could be considered as gold standard to evaluate PS as it is correlating with different domains of psychopathology.20,21,40 One could argue that if expert ratings and actigraphy are giving the same clinical information, then actigraphy that requires time and equipment might not be a good option in clinical settings. However, time to train clinicians using the scales and to perform assessments, is also costly. Moreover, actigraphy collects data continuously also beyond the clinical interview in the patients’ environment and therefore might be more informative than a few minutes interview with a clinician who relies on observation only. It may also help patients who struggle to come to regular visits or to provide sufficient information during the interview. From the logistic regression, we learned that actigraphy has 72% accuracy compared to the SRRS-based classification, suggesting that 30% of the classification can either not be explained by the behavioral measurement or that the expert rating scale did not classify correctly the patients; which would call for actigraphy.
Clinical Implications
Given the poor outcomes associated with PS and the lack of sufficient treatment options, the field should focus on developing and testing novel treatment approaches. To design neuroimaging studies exploring the pathobiology of PS, specific behavioral markers are required. Neuroimaging studies indicated that altered structural and functional connectivity within the motor network was linked to motor abnormalities assessed with wrist actigraphy.59,70 Especially the functional connectivity to the SMA as well as the activity of SMA is altered in schizophrenia patients with PS.2,59,71,72 These findings suggest that the SMA might be an ideal entry node to the motor system that can be modulated by noninvasive brain stimulation such as repetitive transcranial magnetic stimulation (rTMS).18 In fact, inhibitory rTMS over the SMA ameliorated PS in schizophrenia and depression.19 Further trials are on their way and maybe rTMS could become a novel treatment option for PS.
Limitations
The strength of the current report is multiple measures in a large group of schizophrenia patients with PS to explore psychomotor slowing in psychosis from an RDoC perspective with 3 units of analysis. Furthermore, this study tested the specificity of PS contrasting behaviors to patients without PS. However, there are also some limitations. First, the limited sample size of the non-slowed group has hampered the detection of smaller differences between patient groups. Furthermore, this is a cross-sectional analysis and future studies should test the longitudinal course of activity levels in slowed and non-slowed patients. In addition, current and past medication may impact PS measures. While we carefully controlled our analyses for age, current antipsychotic dosage, and negative symptoms, we could not control for total antipsychotic exposure. By trying to provide extensive phenotyping within the RdoC framework, we wanted to include self-report of PS, however, there is no questionnaire assessing self-reported PS, accordingly, we included a less specific self-report measure of physical activity, which taps into movement initiation, general motor activity, or sedentary behavior. Future research might benefit from the development of dedicated self-report instruments.55 Furthermore, in the current study, we focused on behavioral phenotyping of PS, and did not design the study to assess cognitive slowing. This important feature of PS should be included in future trials using cognitive assessments. In addition, PS observed in our sample might be associated with depression. The lack of a specific depression rating scale prevents us from fully exploring this question. However, the DSM-5 depression rating, as well as the PANSS G6 item, offer convergent information on depression. Using these two measures, we detected no effect of depression severity on the observable characteristics of PS (supplementary table 1, table S1a, and S5). Finally, our study might have selection bias, as we analyzed baseline data of a randomized controlled trial. Patients with PS had all agreed to participate in a 3-week trial with multiple assessments, limiting the generalizability of the findings.
Conclusion
PS manifests in slower gait, lower activity, and slower finger movements compared to HC. However, only actigraphy and observer ratings enable the identification of PS from non-PS patients. Actigraphy may become the standard assessment of PS in neuroimaging studies and clinical trials.
Supplementary Material
Acknowledgments
We would like to thank all the participants of the study.
Contributor Information
Niluja Nadesalingam, Translational Research Center, University Hospital of Psychiatry and Psychotherapy, University of Bern, Bern, Switzerland.
Stéphanie Lefebvre, Translational Research Center, University Hospital of Psychiatry and Psychotherapy, University of Bern, Bern, Switzerland.
Danai Alexaki, Translational Research Center, University Hospital of Psychiatry and Psychotherapy, University of Bern, Bern, Switzerland; Klinik Sonnenhalde AG Psychiatrie und Psychotherapie, Basel, Switzerland.
Daniel Baumann Gama, Translational Research Center, University Hospital of Psychiatry and Psychotherapy, University of Bern, Bern, Switzerland.
Florian Wüthrich, Translational Research Center, University Hospital of Psychiatry and Psychotherapy, University of Bern, Bern, Switzerland.
Alexandra Kyrou, Translational Research Center, University Hospital of Psychiatry and Psychotherapy, University of Bern, Bern, Switzerland.
Hassen Kerkeni, Department of Neurology, Inselspital University Hospital Bern, Bern, Switzerland.
Roger Kalla, Department of Neurology, Inselspital University Hospital Bern, Bern, Switzerland.
Sebastian Walther, Translational Research Center, University Hospital of Psychiatry and Psychotherapy, University of Bern, Bern, Switzerland.
Funding
This work was supported by the Swiss National Science Foundation (#182469 to SW). The funding source had no further role in study design; in the collection, analysis, and interpretation of data; in writing of the report; and in the decision to submit the paper for publication.
Disclosure Statement
SW has received honoraria from Lundbeck, Mepha, and Neurolite. NN, SL, DA, DB, FW, AK, HK, and RK reported no biomedical financial interests or potential conflicts of interest.
References
- 1. McCutcheon RA, Reis Marques T, Howes OD.. Schizophrenia - An overview. JAMA Psychiatry. 2020;77(2):201–210. doi: 10.1001/jamapsychiatry.2019.3360. [DOI] [PubMed] [Google Scholar]
- 2. Osborne KJ, Walther S, Shankman SA, Mittal VA.. Psychomotor slowing in Schizophrenia: Implications for endophenotype and biomarker development. Biomarkers Neuropsychiatry. 2020;2:100016. doi: 10.1016/j.bionps.2020.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morrens M, Hulstijn W, Sabbe B.. Psychomotor slowing in schizophrenia. In: Schizophrenia Bulletin. Schizophr Bull. 2007;33:1038–1053. doi: 10.1093/schbul/sbl051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Docx L, Morrens M, Bervoets C, et al. Parsing the components of the psychomotor syndrome in schizophrenia. Acta Psychiatr Scand. 2012;126(4):256–265. doi: 10.1111/j.1600-0447.2012.01846.x. [DOI] [PubMed] [Google Scholar]
- 5. Northoff G, Hirjak D, Wolf RC, Magioncalda P, Martino M.. All roads lead to the motor cortex: psychomotor mechanisms and their biochemical modulation in psychiatric disorders. Mol Psychiatry. 2021;26:92–102. doi: 10.1038/s41380-020-0814-5. [DOI] [PubMed] [Google Scholar]
- 6. Hirjak D, Kubera KM, Wolf RC, Northoff G.. Going back to Kahlbaum’s psychomotor (and GABAergic) origins: is catatonia more than just a motor and dopaminergic syndrome? Schizophr Bull. 2020;46(2):272–285. doi: 10.1093/schbul/sbz074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aas M, Dazzan P, Mondelli V, Melle I, Murray RM, Pariante CM.. A systematic review of cognitive function in first-episode psychosis, including a discussion on childhood trauma, stress, and inflammation. Front Psychiatry. 2014;4:1–13. doi: 10.3389/fpsyt.2013.00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kelleher I, Murtagh A, Clarke MC, Murphy J, Rawdon C, Cannon M.. Neurocognitive performance of a community-based sample of young people at putative ultra high risk for psychosis: support for the processing speed hypothesis. Cogn Neuropsychiatry. 2013;18(1-2):9–25. doi: 10.1080/13546805.2012.682363. [DOI] [PubMed] [Google Scholar]
- 9. Fatouros-Bergman H, Cervenka S, Flyckt L, Edman G, Farde L.. Meta-analysis of cognitive performance in drug-naïve patients with schizophrenia. Schizophr Res. 2014;158(1-3):156–162. doi: 10.1016/j.schres.2014.06.034. [DOI] [PubMed] [Google Scholar]
- 10. Stubbs B, Chen LJ, Chung MS, Ku PW.. Physical activity ameliorates the association between sedentary behavior and cardiometabolic risk among inpatients with schizophrenia: a comparison versus controls using accelerometry. Compr Psychiatry. 2017;74:144–150. doi: 10.1016/j.comppsych.2017.01.010. [DOI] [PubMed] [Google Scholar]
- 11. Stubbs B, Ku PW, Chung MS, Chen LJ.. Relationship between objectively measured sedentary behavior and cognitive performance in patients with schizophrenia vs controls. Schizophr Bull. 2017;43(3):566–574. doi: 10.1093/schbul/sbw126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee RSC, Hermens DF, Naismith SL, et al. Neuropsychological and functional outcomes in recent-onset major depression, bipolar disorder and schizophrenia-spectrum disorders: a longitudinal cohort study. Transl Psychiatry. 2015;5(4):e555–e555. doi: 10.1038/tp.2015.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dean DJ, Walther S, Bernard JA, Mittal VA.. Motor clusters reveal differences in risk for psychosis, cognitive functioning, and thalamocortical connectivity: evidence for vulnerability subtypes. Clin Psychol Sci. 2018;6(5):721–734. doi: 10.1177/2167702618773759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bodén R, Abrahamsson T, Holm G, Borg J.. Psychomotor and cognitive deficits as predictors of 5-year outcome in first-episode schizophrenia. Nord J Psychiatry. 2014;68(4):282–288. doi: 10.3109/08039488.2013.830771. [DOI] [PubMed] [Google Scholar]
- 15. Martin-Sierra A, Vancampfort D, Probst M, et al. Walking capacity is associated with health related quality of life and physical activity level in patients with schizophrenia: a preliminary report. Actas Esp Psiquiatr. 2011;39(4):211–216. [PubMed] [Google Scholar]
- 16. Nadesalingam N, Chapellier V, Lefebvre S, et al. Motor abnormalities are associated with poor social and functional outcomes in schizophrenia. Compr Psychiatry. 2022;115:152307. doi: 10.1016/j.comppsych.2022.152307. [DOI] [PubMed] [Google Scholar]
- 17. Putzhammer A, Perfahl M, Pfeiff L, Hajak G.. Correlation of subjective well-being in schizophrenic patients with gait parameters, expert-rated motor disturbances, and psychopathological status. Pharmacopsychiatry. 2005;38(3):132–138. doi: 10.1055/s-2005-864125. [DOI] [PubMed] [Google Scholar]
- 18. Lefebvre S, Pavlidou A, Walther S.. What is the potential of neurostimulation in the treatment of motor symptoms in schizophrenia? Expert Rev Neurother. 2020;20(7):697–706. doi: 10.1080/14737175.2020.1775586. [DOI] [PubMed] [Google Scholar]
- 19. Walther S, Alexaki D, Schoretsanitis G, et al. Inhibitory repetitive transcranial magnetic stimulation to treat psychomotor slowing: a transdiagnostic, mechanism-based Randomized Double-Blind Controlled Trial. Schizophr Bull Open. 2020;1(1). doi: 10.1093/schizbullopen/sgaa020. [DOI] [Google Scholar]
- 20. van Harten PN, Walther S, Kent JS, Sponheim SR, Mittal VA.. The clinical and prognostic value of motor abnormalities in psychosis, and the importance of instrumental assessment. Neurosci Biobehav Rev. 2017;80:476–487. doi: 10.1016/j.neubiorev.2017.06.007. [DOI] [PubMed] [Google Scholar]
- 21. Walther S, van Harten PN, Waddington JL, et al. Movement disorder and sensorimotor abnormalities in schizophrenia and other psychoses - European consensus on assessment and perspectives. Eur Neuropsychopharmacol. 2020;38:25–39. doi: 10.1016/j.euroneuro.2020.07.003. [DOI] [PubMed] [Google Scholar]
- 22. Walther S, Mittal VA.. Motor behavior is relevant for understanding mechanism, bolstering prediction, and improving treatment: a transdiagnostic perspective. Schizophr Bull. Published online February 5, 2022;48:741–748. doi: 10.1093/schbul/sbac003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Walther S, Vladimirova I, Alexaki D, et al. Low physical activity is associated with two hypokinetic motor abnormalities in psychosis. J Psychiatr Res. 2022;146:258–263. doi: 10.1016/j.jpsychires.2021.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kay SR, Fiszbein A, Opler LA.. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13(2):261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- 25. Walther S, Koschorke P, Horn H, Strik W.. Objectively measured motor activity in schizophrenia challenges the validity of expert ratings. Psychiatry Res. 2009;169(3):187–190. doi: 10.1016/j.psychres.2008.06.020. [DOI] [PubMed] [Google Scholar]
- 26. Widlöcher D, Ghozlan A.. The measurement of retardation in depression. In: Hindmarch I, Stonier PD, eds. Human Psychopharmacology: Measures and Methods. Vol. 2. New York: Wiley; 1989. [Google Scholar]
- 27. Docx L, Sabbe B, Provinciael P, Merckx N, Morrens M.. Quantitative psychomotor dysfunction in schizophrenia: a loss of drive, impaired movement execution or both? Neuropsychobiology. 2013;68(4):221–227. doi: 10.1159/000355293. [DOI] [PubMed] [Google Scholar]
- 28. Wee ZY, Yong SWL, Chew QH, Guan C, Lee TS, Sim K.. Actigraphy studies and clinical and biobehavioural correlates in schizophrenia: a systematic review. J Neural Transm. 2019;126(5):531–558. doi: 10.1007/s00702-019-01993-2. [DOI] [PubMed] [Google Scholar]
- 29. Walther S, Ramseyer F, Horn H, Strik W, Tschacher W.. Less structured movement patterns predict severity of positive syndrome, excitement, and disorganization. Schizophr Bull. 2014;40(3):585–591. doi: 10.1093/schbul/sbt038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kluge A, Kirschner M, Hager OM, et al. Combining actigraphy, ecological momentary assessment and neuroimaging to study apathy in patients with schizophrenia. Schizophr Res. 2018;195:176–182. doi: 10.1016/j.schres.2017.09.034. [DOI] [PubMed] [Google Scholar]
- 31. Walther S, Stegmayer K, Horn H, Razavi N, Müller TJ, Strik W.. Physical activity in schizophrenia is higher in the first episode than in subsequent ones. Front Psychiatry. 2015;6. doi: 10.3389/fpsyt.2014.00191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Chen LJ, Steptoe A, Chung MS, Ku PW.. Association between actigraphy-derived physical activity and cognitive performance in patients with schizophrenia. Psychol Med. 2016;46(11):2375–2384. doi: 10.1017/S0033291716000921. [DOI] [PubMed] [Google Scholar]
- 33. Pieters LE, Deenik J, Tenback DE, Van Oort J, Van Harten PN.. Exploring the relationship between movement disorders and physical activity in patients with Schizophrenia: an actigraphy study. Schizophr Bull. 2021;47(4):906–914. doi: 10.1093/schbul/sbab028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mittal VA, Bernard JA, Strauss GP, Walther S.. New Insights into sedentary behavior highlight the need to revisit the way we see motor symptoms in psychosis. Schizophr Bull. 2021;47(4):877–879. doi: 10.1093/schbul/sbab057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Putzhammer A, Heindl B, Broll K, Pfeiff L, Perfahl M, Hajak G.. Spatial and temporal parameters of gait disturbances in schizophrenic patients. Schizophr Res. 2004;69(2-3):159–166. doi: 10.1016/S0920-9964(03)00090-2. [DOI] [PubMed] [Google Scholar]
- 36. Presta V, Paraboschi F, Marsella F, et al. Posture and gait in the early course of schizophrenia. PLoS One. 2021;16:e0245661. doi: 10.1371/journal.pone.0245661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gebhardt A, Vanbellingen T, Baronti F, Kersten B, Bohlhalter S.. Poor dopaminergic response of impaired dexterity in Parkinson’s disease: Bradykinesia or limb kinetic apraxia? Mov Disord. 2008;23(12):1701–1706. doi: 10.1002/mds.22199. [DOI] [PubMed] [Google Scholar]
- 38. Walther S, Kunz M, Müller M, et al. Single session transcranial magnetic stimulation ameliorates hand gesture deficits in schizophrenia. Schizophr Bull. 2020;46(2):286–293. doi: 10.1093/schbul/sbz078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Schäppi L, Stegmayer K, Viher PV, Walther S.. Distinct associations of motor domains in relatives of schizophrenia patients-different pathways to motor abnormalities in schizophrenia? Front Psychiatry. 2018;9. doi: 10.3389/fpsyt.2018.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Walther S, Bernard JA, Mittal VA, Shankman SA.. The utility of an RDoC motor domain to understand psychomotor symptoms in depression. Psychol Med. 2019;49(2):212–216. doi: 10.1017/S0033291718003033. [DOI] [PubMed] [Google Scholar]
- 41. Sanislow CA, Ferrante M, Pacheco J, Rudorfer MV, Morris SE.. Advancing translational research using NIMH research domain criteria and computational methods. Neuron. 2019;101(5):779–782. doi: 10.1016/j.neuron.2019.02.024. [DOI] [PubMed] [Google Scholar]
- 42. Fahn S; Elton RM of the UDC. The unified Parkinson’s disease rating scale. Recent Dev Park Dis. 1987;2:153–163; 293–304. [Google Scholar]
- 43. Bush G, Fink M, Petrides G, Dowling F, Francis A, Catatonia I.. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93(2):129–136. doi: 10.1111/j.1600-0447.1996.tb09814.x. [DOI] [PubMed] [Google Scholar]
- 44. Guy W. ECDEU Assessment Manual for Psychopharmacology: Revised. Rockville: US Department of Health, Education and Welfare; 1976. [Google Scholar]
- 45. Leucht S, Samara M, Heres S, et al. Dose equivalents for second-generation antipsychotic drugs: the classical mean dose method. Schizophr Bull. 2015;41(6):1397–1402. doi: 10.1093/schbul/sbv037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-Country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. [DOI] [PubMed] [Google Scholar]
- 47. Walther S, Horn H, Razavi N, Koschorke P, Müller TJ, Strik W.. Quantitative motor activity differentiates schizophrenia subtypes. Neuropsychobiology. 2009;60(2):80–86. doi: 10.1159/000236448. [DOI] [PubMed] [Google Scholar]
- 48. Webster KE, Wittwer JE, Feller JA.. Validity of the GAITRite® walkway system for the measurement of averaged and individual step parameters of gait. Gait Posture. 2005;22(4):317–321. doi: 10.1016/j.gaitpost.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 49. Barkemeyer CA, Maria MPS, Browndyke JN, Callon EB, Dunn AM.. The coin rotation task: a convenient and sensitive measure of fine motor control. Arch Clin Neuropsychol. 1998;13(1):18–18. doi: 10.1093/arclin/13.1.18a. [DOI] [Google Scholar]
- 50. Walther S, Vanbellingen T, Müri R, Strik W, Bohlhalter S.. Impaired pantomime in schizophrenia: association with frontal lobe function. Cortex. 2013;49(2):520–527. doi: 10.1016/j.cortex.2011.12.008. [DOI] [PubMed] [Google Scholar]
- 51. Walther S, Mittal VA.. Motor system pathology in psychosis. Curr Psychiatry Rep. 2017;19(12). doi: 10.1007/s11920-017-0856-9. [DOI] [PubMed] [Google Scholar]
- 52. Walther S, Strik W.. Motor symptoms and schizophrenia. Neuropsychobiology. 2012;66(2):77–92. doi: 10.1159/000339456. [DOI] [PubMed] [Google Scholar]
- 53. Reichenberg A, Weiser M, Caspi A, et al. Premorbid intellectual functioning and risk of schizophrenia and spectrum disorders. J Clin Exp Neuropsychol. 2006;28(2):193–207. doi: 10.1080/13803390500360372. [DOI] [PubMed] [Google Scholar]
- 54. Duncan MJ, Arbour-Nicitopoulos K, Subramanieapillai M, Remington G, Faulkner G.. Revisiting the International Physical Activity Questionnaire (IPAQ): assessing physical activity among individuals with schizophrenia. Schizophr Res. 2017;179:2–7. doi: 10.1016/j.schres.2016.09.010. [DOI] [PubMed] [Google Scholar]
- 55. Dell’Osso L, Amatori G, Cappelli A, et al. Catatonia spectrum: validation of a questionnaire investigating catatonia spectrum. Front Psychiatry. 2022;13:1005. doi: 10.3389/fpsyt.2022.913286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Umbricht D, Cheng WY, Lipsmeier F, Bamdadian A, Lindemann M.. Deep learning-based human activity recognition for continuous activity and gesture monitoring for schizophrenia patients with negative symptoms. Front Psychiatry. 2020;11. doi: 10.3389/fpsyt.2020.574375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Servaas MN, Kos C, Gravel N, et al. Rigidity in motor behavior and brain functioning in patients with schizophrenia and high levels of apathy. Schizophr Bull. 2019;45(3):542–551. doi: 10.1093/schbul/sby108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. von Känel S, Nadesalingam N, Alexaki D, et al. Measuring catatonia motor behavior with objective instrumentation. Front Psychiatry. 2022;13. doi: 10.3389/fpsyt.2022.880747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Walther S, Stegmayer K, Federspiel A, Bohlhalter S, Wiest R, Viher PV.. Aberrant hyperconnectivity in the motor system at rest is linked to motor abnormalities in schizophrenia spectrum disorders. Schizophr Bull. 2017;43(5):982–992. doi: 10.1093/schbul/sbx091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Walther S. Psychomotor symptoms of schizophrenia map on the cerebral motor circuit. Psychiatry Res. 2015;233(3):293–298. doi: 10.1016/j.pscychresns.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 61. Walther S, Federspiel A, Horn H, et al. Resting state cerebral blood flow and objective motor activity reveal basal ganglia dysfunction in schizophrenia. Psychiatry Res. 2011;192(2):117–124. doi: 10.1016/j.pscychresns.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 62. Walther S, Federspiel A, Horn H, et al. Alterations of white matter integrity related to motor activity in schizophrenia. Neurobiol Dis. 2011;42(3):276–283. doi: 10.1016/j.nbd.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 63. Hirjak D, Thomann PA, Kubera KM, Wolf ND, Sambataro F, Wolf RC.. Motor dysfunction within the schizophrenia-spectrum: a dimensional step towards an underappreciated domain. Schizophr Res. 2015;169(1-3):217–233. doi: 10.1016/j.schres.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 64. Hirjak D, Kubera KM, Thomann PA, Wolf RC.. Motor dysfunction as an intermediate phenotype across schizophrenia and other psychotic disorders: progress and perspectives. Schizophr Res. 2018;200:26–34. doi: 10.1016/j.schres.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 65. Fritze S, Harneit A, Waddington JL, et al. Structural alterations in brainstem, basal ganglia and thalamus associated with parkinsonism in schizophrenia spectrum disorders. Eur Arch Psychiatry Clin Neurosci. 2021;271(8):1455–1464. doi: 10.1007/s00406-021-01270-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wolf RC, Rashidi M, Fritze S, et al. A neural signature of parkinsonism in patients with schizophrenia spectrum disorders: a multimodal mri study using parallel ICA. Schizophr Bull. 2020;46(4):999–1008. doi: 10.1093/schbul/sbaa007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Fritze S, Sambataro F, Kubera KM, et al. Characterizing the sensorimotor domain in schizophrenia spectrum disorders. Eur Arch Psychiatry Clin Neurosci. 2022;272:1097–1108. doi: 10.1007/s00406-021-01354-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. McKenna PJ, Mortimer AM, Lund CE.. The motor disorders of severe psychiatric illness: a conflict of paradigms. Br J Psychiatry. 1988;152:863–864. doi: 10.1192/bjp.152.6.863. [DOI] [PubMed] [Google Scholar]
- 69. Peralta V, Basterra V, Campos MS, De Jalón EG, Moreno-Izco L, Cuesta MJ.. Characterization of spontaneous Parkinsonism in drug-naïve patients with nonaffective psychotic disorders. Eur Arch Psychiatry Clin Neurosci. 2012;262(2):131–138. doi: 10.1007/s00406-011-0219-1. [DOI] [PubMed] [Google Scholar]
- 70. Walther S, Federspiel A, Horn H, et al. White matter integrity associated with volitional motor activity. Neuroreport. 2010;21(5):381–385. doi: 10.1097/WNR.0b013e328337ca29. [DOI] [PubMed] [Google Scholar]
- 71. Walther S, Schäppi L, Federspiel A, et al. Resting-state hyperperfusion of the supplementary motor area in catatonia. Schizophr Bull. 2017;43(5):972–981. doi: 10.1093/schbul/sbw140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Foucher JR, Zhang YF, Roser M, et al. A double dissociation between two psychotic phenotypes: periodic catatonia and cataphasia. Prog Neuro-Psychopharmacol Biol Psychiatry. 2018;86:363–369. doi: 10.1016/j.pnpbp.2018.03.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.