Abstract
Background and Hypothesis
Psychotic Like Experiences (PLEs) are widely prevalent in children and adolescents and increase the risk of developing psychosis. Cortical gyrification characterizes brain development from in utero till about the first 2 years of life and can be measured in later years as static gyrification changes demonstrating neurodevelopment and dynamic gyrification changes reflecting brain maturation during adolescence. We hypothesized that PLEs would be associated with static cortical gyrification changes reflecting a neurodevelopmental abnormality.
Study Design
We studied 1252 adolescents recruited in the IMAGEN consortium. We used a longitudinal study design, with Magnetic Resonance Imaging measurements at age 14 years and age 19 years; measurement of PLEs using the Community Assessment of Psychic Experiences (CAPE) questionnaire at age 19 years; and clinical diagnoses at age 23 years.
Study Results
Our results show static gyrification changes in adolescents with elevated PLEs on 3 items of the CAPE—voice hearing, unusual experiences of receiving messages, and persecutory ideas—with lower cortical gyrification in fronto-temporal regions in the left hemisphere. This group also demonstrated dynamic gyrification changes with higher cortical gyrification in right parietal cortex in late adolescence; a finding that we replicated in an independent sample of patients with first-episode psychosis. Adolescents with high PLEs were also 5.6 times more likely to transition to psychosis in adulthood by age 23 years.
Conclusions
This is the largest study in adolescents that demonstrates fronto-temporal abnormality of cortical gyrification as a potential biomarker for vulnerability to PLEs and transition to psychosis.
Keywords: cortical gyrification, adolescence, psychotic like experiences, IMAGEN, MRI, psychosis
Introduction
Psychotic symptoms exist on a continuum, from Psychotic Like Experiences (PLE) at one end of the spectrum to clinical phenotypes such as schizophrenia at the other end. As the definition of PLEs varies,1 here we refer to PLEs as subtle, subclinical symptoms of psychosis (ie, in otherwise healthy, nontreatment seeking-individuals) that are present in the general population including perceptual disturbances, paranoid ideation, and “magical” thinking.2 PLEs offer a compelling model for subclinical psychosis because they are phenomenologically similar to psychotic illness, are heritable, share common genetic influences with depression and schizophrenia,3 and overlap with other risk factors for schizophrenia such as childhood trauma, victimization, and substance use.4 If PLEs arise from abnormalities of brain structure that predispose to dysfunction within cognitive and perceptual networks, then detecting such structural abnormalities may help understand the mechanisms underpinning psychosis and identify individuals at risk of developing psychosis.
PLEs are common in childhood, occurring in 17% of children aged 9–12 years,5 and have their highest prevalence in younger adolescents (21%–23%) compared to older adolescents (7%).6 Presence of PLEs in children aged 11 years may be a significant predictor of schizophreniform disorder at age 26 years.7 A large study of 3801 young adults reported that experience of hallucinations at age 14 years, especially in the presence of childhood behavior problems, was a predictor of delusional experiences and non-affective psychosis at age 21 years.8,9 Overall, adolescents with PLEs were 3.5 times more likely to transition to a clinical psychotic disorder in adulthood compared to adolescents with no PLEs10 and the association between PLEs and a diagnosable psychiatric disorder was shown to increase with age—where 57% of early adolescents (aged 11–13 years) and 80% of mid adolescents (aged 13–16 years) with reported psychotic symptoms had at least one diagnosis.6
Adolescence is marked by significant neurodevelopmental changes that may be reflected in structural changes in the brain. Structural brain abnormalities have been noted in adolescents experiencing PLEs11,12 with some of these structural changes being associated with genetic loading.3,13 Cortical gyrification is a developmental process that refers to the folding of the cortex into gyri and sulci. Gyrification increases rapidly in-utero until 2 years after birth and then remains relatively constant therefore offering a stable marker of neurodevelopmental changes.14 We refer to this stable type of gyrification as a result of early neurodevelopmental changes as “static” gyrification. There also may be further changes in cortical gyrification that occur later such as nonlinear decreases in gyrification15 although the processes that contribute to these later changes in gyrification are less well understood. Here we refer to this later maturational aspect of gyrification as “dynamic” gyrification. Abnormalities of gyrification may be associated with persistence of PLEs in adolescents and increased risk of developing psychosis.16
Only a handful of studies, mostly in young adults, have explored gyrification as a neurodevelopmental marker for risk of developing psychosis (see Appendix in Supplement). While the gyrification data presents a mixed picture, the main regional findings are alterations reported in the frontal, temporal, and parietal lobes, with some decreases in gyrification suggesting that an early neurodevelopmental change results in the persistence of psychotic symptoms16–18; and other studies reporting higher gyrification19,20 as well as no changes in gyrification.21,22 A recent review of these gyrification findings in ultra-high risk and early psychosis populations report higher gyrification in frontal, temporal, and parietal regions while there may be more significant decreases evident in the established disease.23 Another study suggests higher gyrification in early psychosis with less clear findings in those at high risk for psychosis.24 In these cross-sectional studies, the differentiation between static and dynamic gyrification changes and their implication in psychosis remain unclear.
Our aim was to study abnormalities in static and dynamic cortical gyrification in healthy adolescents experiencing PLEs compared to those not experiencing PLEs. We measured cortical gyrification from structural magnetic resonance imaging (MRI) scans collected at baseline (14 years) and follow-up (19 years) from 1252 adolescents that participated in the IMAGEN consortium.25 PLEs were assessed at follow-up (19 years) using the Community Assessment of Psychic Experiences (CAPE-42).26 We also investigated whether differences in cortical gyrification associated with PLEs were relevant to clinically diagnosed psychotic symptoms by comparing cortical gyrification in an independent sample of patients with first-episode psychosis and healthy controls (HC), and assessing how many high PLE adolescents transitioned to psychosis illness at a final follow-up phase (age 23 years).
We hypothesized that:
1) Adolescents with PLEs, with high CAPE scores, would show a neurodevelopmental abnormality as indexed by altered frontal and temporoparietal static cortical gyrification at baseline compared to those without PLEs.
2) Adolescents with PLEs, with high CAPE scores, would show a neurodevelopmental abnormality as evidenced by persistently altered frontal and temporoparietal static cortical gyrification from baseline to follow-up compared to adolescents without PLEs.
3) Altered static cortical gyrification in adolescents with PLEs would show clinical relevance to symptoms of psychosis as indexed by a) similarly altered static cortical gyrification in an independent sample of patients with early psychosis compared to HC, and b) a higher rate of transition to psychosis at follow-up in the adolescents experiencing elevated levels of PLEs.10
Methods
Participants
A total of 1252 adolescents were selected from the IMAGEN consortium25 based on availability of a complete behavioral and neuroimaging data set at ages 14 (baseline) and 19 years (follow-up) and additional behavioral data at age 23 years. Ethical approval had been obtained by the ethics research committee of the IMAGEN consortium.25 Access to the IMAGEN database was obtained after approval by the IMAGEN committee and deidentified data were downloaded. The present study included participants that were recruited between January 1, 2016 and January 1, 2017.
Sample Stratification
Participants were stratified into “high” and “low” PLE groups on the basis of scores of selected items from the CAPE questionnaire as described in our previous publication.26 The CAPE questionnaire is a 42-item questionnaire that has been shown to have good validity and reliability for measuring PLEs as reported in a recent meta-analysis of 111 studies.27,28 Briefly, two complementary stratifications were performed. First, high and low scorers were determined based on the upper and lower quartiles of the composite scores of items-5 (bizarre experiences), 7 (perceptual abnormalities), and 33 (persecutory ideas) from the CAPE questionnaire (hereon referred to as the “CAPE-3” sample). This stratification was based on 2 studies showing these 3 factors are most important for predicting poor outcomes in adolescents experiencing PLEs28,29 (see Supplementary Materials). Second, the upper and lower deciles of the total CAPE scores (hereon referred to as the “CAPE-42” sample) were used to determine high and low PLE scorers.
Measures of IQ and General Psychopathology
IQ was measured using the Wechsler Intelligence Scale for Children (WISC, IV),30 alcohol consumption using the Alcohol Use Disorders Identification Test (AUDIT),31,32 cannabis use using the Drug Abuse Screening Test (DAST),33 and depressive symptoms using the Adolescent Depression Rating Scale (ADRS).34 WISC scores were available only at baseline while CAPE, ADRS, AUDIT, and DAST scores were available only at follow-up.
MRI Acquisition
MRI data were acquired from the following participating centers for IMAGEN study: Institute of Psychiatry, University of Nottingham (UK); Charité University Berlin, Technical University Dresden, University Medical Centre Hamburg-Eppendorf, Central Institute of Mental Health Mannheim (Germany); National Institute of Health and medical Research (France); and Trinity College Dublin (Ireland) using 3T scanners from four different manufacturers namely Siemens, Philips, GE Healthcare and Bruker. A full description of the scanning protocols, cross-site standardization and quality checks, and pre-processing of resulting data are provided elsewhere.35
Cortical Gyrification
The cortical surface was extracted and local gyrification was measured across the whole brain using the CAT12 toolbox (http://dbm.neuro.uni-jena.de/cat/) in SPM12 software (http://www.fil.ion.ucl.ac.uk/spm, Wellcome Trust Centre for Neuroimaging), (see Supplementary Materials). This method for calculating a local gyrification index is based on the absolute mean curvature of the brain surface and was chosen as a simple measure that is sensitive to both amplitude and frequency of the folding pattern.36 CAT12 has also previously been used to explore gyrification in adults with PLEs.37 To ensure normal distribution of gyrification indices, smoothing was applied using a Gaussian kernel of 20 mm FWHM (the recommended CAT12 filter size) prior to statistical analyses. The results are displayed using the Desikan atlas.38
Statistical Analysis
Demographics, measures of IQ, and general psychopathology were analyzed using SPSS.39
All statistical analyses of neuroimaging data were performed using SPM12 (http://www.fil.ion.ucl.ac.uk/spm; Wellcome Trust Centre for Neuroimaging). A flexible factorial model was used with CAPE score grouping (ie CAPE-3 or CAPE-42) as the between-subjects factor (high and low scorers) and TIME as the within-subjects factor (baseline and follow-up visits) to explore the main effect of group, time, and the interaction between group and time.
Neuroimaging results were considered at a statistical cluster height threshold of P < .001 uncorrected, with the cluster extent of 50 vertices—similar to previous gyrification studies.40,41 Main effects reported at an uncorrected threshold were then subjected to more statistically stringent post-hoc tests using non-parametric methods such as Threshold Free Cluster Enhancement (TFCE) using a threshold of P < .001 and 5000 permutations.42
Independent Replication Sample
To examine whether the mechanism underlying PLEs was the same as that in clinical psychosis, we performed a cross-sectional replication analysis in an independent sample of 102 medicated patients with early psychosis (duration of illness <5 years) compared to 43 HC collected as part of the MUTRIPS study.43
Follow-up Diagnoses
Finally, we assessed the utility of the high and low CAPE-3 classification by examining how many adolescents in each group developed psychosis at a clinical follow-up at age 23 years. Clinical diagnoses were established using the Mini-International Neuropsychiatric Interview (M.I.N.I.).44
Results
Demographics
Demographics for each sample stratification are available in Table 1. Briefly, for the CAPE-3 sample stratification there was a total sample of 600 adolescents with 313 high CAPE-3 scorers and 287 low CAPE-3 scorers. There were no significant differences in age, IQ (WISC IV), alcohol use (AUDIT), and cannabis use (DAST) scores between the high and low CAPE-3 scorers. Only the ADRS scores differed between the 2 groups (Z = 9.7, P < .001) where low CAPE-3 scorers displayed higher depressive symptoms than high CAPE-3 scorers.
Table 1.
Demographics of the Participants
| CAPE-3 | Comparison Between CAPE-3 High and Low Scorers | CAPE-42 | Comparison Between CAPE-42 High and Low Scorers | Entire Sample | Correlation of CAPE Scores With Other Measures in the Entire Sample | |||
|---|---|---|---|---|---|---|---|---|
| No of participants (n) | 600 | 247 | 1252 | |||||
| Stratification | High CAPE-3 | Low CAPE-3 | High CAPE-42 | Low CAPE-42 | – | |||
| Stratification (n) | 313 | 287 | 123 | 124 | 1252 | |||
| Age (mean, SD) BL | 14.4, 0.4 | 14.4, 0.4 | 14.4, 0.4 | 14.4, 0.4 | 14.4, 0.4 | |||
| Age (mean, SD) FU | 18.9, 0.7 | 18.9, 0.7 | 18.9, 0.7 | 18.9, 0.7 | 18.9, 0.7 | |||
| Gender (M, F, NK) | 127, 180, 6 | 160, 123, 4 | 39, 84, 0 | 69, 55, 0 | 591, 651, 10 | |||
| Handedness (R, L, NK) | 263, 39, 11 | 239, 37, 11 | 107, 13, 3 | 104, 18, 2 | 1090, 141, 21 | |||
| WISC Verbal (mean, SD) BL | 111.2, 15.4 | 109.6, 15.1 | n.s | 112.2, 15.2 | 106.9, 15.2 | t (df = 238) = 2.65, P = .008 | 112.5, 14.7 | (r = 0.10, P < .001) |
| WISC performance (mean, SD) BL | 108.8, 14.9 | 106.5, 15.3 | n.s | 109.5, 14.9 | 105.1, 14.6 | t (df = 239) = 2.29, P = .023 | 108.9, 14.3 | (r = 0.094, P = .001). |
| ADRS (mean, SD) FU | 18.1, 2.5 | 19.7, 0.7 | (Z = 9.7, P < .001) | 16, 2.9 | 19.7, 0.6 | (z= 11, p<0.001) | 18.7, 2.1 | (ρ= -0.492, p<0.001 |
| AUDIT (mean, SD) FU | 5.9, 4.4 | 5.5, 4.2 | n.s | 7.4, 5.2 | 5.2, 3.7 | (z = −3.35, P = .001) | 5.6, 4.1 | (ρ = 0.079, P = .005) |
| DAST (mean, SD) FU | 1.1, 2.2 | 0.6, 1.2 | n.s | 1.7, 2.7 | 0.6, 1.0 | (z = −2.95, P = .003) | 0.9, 1.8 | (ρ = 0.097, P = .001) |
Note: n.s., not significant; Z, standardized score of nonparametric comparison; ρ, spearman correlation coefficient; r, Pearson’s correlation coefficient; n, number; SD, standard deviation; BL, baseline; FU, follow-up; M, male; F, female; R, right; L, left; NK, not known; ADRS, Adolescent Depression Rating Scale; AUDIT, Alcohol Use Disorders Identification Test; CAPE-42, Community Assessment of Psychic Experiences Questionnaire, 42 items instrument; DAST, Drug Abuse Screening Test for Cannabis; WISC, Wechsler Intelligence Scale for Children.
High Versus Low CAPE-3 Scorers
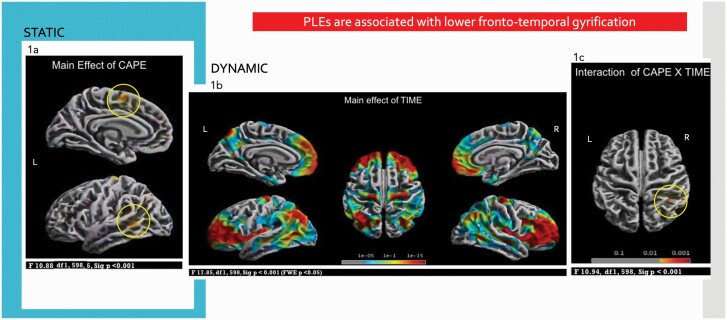
Using a flexible factorial model, there was a main effect of CAPE on (static) cortical gyrification in the banks of the left superior frontal gyrus, precentral gyrus, superior temporal sulcus, and middle temporal gyrus (F(1,598) = 10.88, P < .001). Post-hoc t-tests showed that the high scorers on the CAPE-3 had lower gyrification in these areas compared to low scorers (t(1,598) = 3.10, P < .001) (figure 1a) where effects in the left precentral gyrus and left middle temporal gyrus were the most robust (P < .001 TFCE). Adding gender and scanning site as covariates to the model did not provide additional findings.
Fig. 1.
PLEs in adolescence are associated with lower fronto-temporal gyrification. (a) Differences in STATIC gyrification in fronto-temporal areas in high CAPE-3 scorers compared to low CAPE-3 scorers. (b) Changes in DYNAMIC gyrification from mid (baseline, 14 years) to late adolescence (follow-up, 19 years) reflecting maturational processes, most pronounced in prefrontal areas. (c) CAPE-3 × TIME interaction showing changes in DYNAMIC gyrification where high CAPE-3 scorers showed higher gyrification in parietal areas at follow-up. Results reported at P < .001.
There was also a main effect of TIME (ie, across the whole CAPE-3 sample) where there were changes in (dynamic) cortical gyrification at follow-up, compared to baseline, in bilateral dorsal middle frontal, superior frontal, precentral, superior parietal, inferior parietal, precuneus, insula, and lingual gyrus; right postcentral, supramarginal, superior temporal, middle temporal, and entorhinal cortex. The observed changes in cortical gyrification were statistically significant (F(1,598) = 17.85, FWE corrected P < .05) and were most marked in prefrontal areas (figure 1b). Post hoc analysis (T(1,599) = 4.51, FWE corrected P < .05) confirmed increased gyrification at follow-up in the above areas and small areas of reduced gyrification in bilateral postcentral, superior parietal, and fusiform; and insula in right hemisphere.
CAPE × TIME interaction on (dynamic) cortical gyrification was seen in right superior and inferior parietal lobules (F(1,598) = 10.94, P < .001) (figure 1c). Post-hoc independent sample t-tests for baseline and follow-up visits separately showed that high scorers had higher gyrification in these regions compared to low scorers at follow-up (t(1,598) = 3.10, P < .001) but not at baseline. This finding also survived the more stringent statistical threshold at P < .001 TFCE.
We performed additional analysis with the entire sample of 1252 adolescents and with CAPE-42 10th decile stratification which did not offer additional relevant information (see supplement) and both of these samples had significant confounding variables (see Table 1).
Independent Replication Sample
Demographics have been described in our previous article.43 The independent sample of patients with psychosis demonstrated higher gyrification in cuneus, caudal middle frontal in the left hemisphere and lingual/fusiform, lateral occipital/cuneus and superior parietal in the right hemisphere compared to HC (t(1,142) = 3.15, P < .001).
Follow-up Diagnoses
There were 1252 adolescents included in this study, of whom 756 had follow-up data at age 23 years and of these, 16 adolescents received a diagnosis of psychosis at follow-up (2.1%). There were 12 (75%) from the high CAPE-3 group (totaling 187), and 2 (12.5%) from the low CAPE-3 group (totaling 177); and 2 (12.5%) from the remaining adolescents (intermediate CAPE-3 group). The high CAPE-3 scorers were 5.6 times more likely to develop psychosis than low CAPE-3 scorers (relative risk ratio = (12/187)/(2/177) = 5.68).45 In comparison, using the CAPE-42 classification there were 6 (37.5%) that transitioned from the high CAPE-42 group, 9 (56.25%) from intermediate group and 1 (6%) from low CAPE-42 group. This supports the increased value of using the more specific CAPE-3 item scores to identify adolescents at increased risk of developing psychosis.
Discussion
Our longitudinal study presents a unique and powerful design exploring changes in gyrification during adolescence and differentiates between static and dynamic gyrification and their association with PLEs. We show that adolescents with high PLEs, especially with experiences of voice-hearing, receiving unusual messages and persecutory ideas (CAPE-3), have lower gyrification in fronto-temporal regions that persist through mid to late adolescence compared to adolescents with low PLEs thus reflecting static gyrification changes. Moreover, this high PLE group displayed higher gyrification in right superior and inferior parietal cortex at follow-up where cortical gyrification in these parietal regions was also altered (higher) in an independent sample of patients with early psychosis compared to controls, thus reflecting dynamic gyrification changes. The utility of using the more specific CAPE items (CAPE-3) to stratify participants was supported by the observation that 75% of the 16 adolescents that transitioned to clinical psychosis at age 23 years were in the high PLE group (relative risk ratio of 5.6). Together, this suggests that the neurodevelopmental abnormalities associated with high PLEs are clinically relevant to symptoms of psychosis.
Few studies have explored gyrification as a neurodevelopmental marker for risk of developing psychosis but indicate that an early neurodevelopmental insult results in an earlier plateau of cortical expansion which underlies persistence of psychotic symptoms.16–18 However, other studies have reported both increases19,20 and no changes in gyrification.21,22 Recently, Fonville et al reported that young adults with persistent PLEs (at age 18 and 20 years) showed lower gyrification in left temporal gyrus, but relatively intact white matter, suggesting early disturbances in cortical expansion underlying vulnerability to psychosis.46 Interestingly, Evermann et al. also showed that specific symptoms of PLEs (positive and depressive subscales) were associated with reductions in cortical gyrification in precuneus, right supramarginal, and temporal regions, but that cognition did not significantly mediate this relationship.37 While these studies advanced our understanding of PLEs, most are limited by their sample size and cross-sectional design. In addition, only a few have examined changes in cortical gyrification relating to PLEs in adolescents—a crucial period of neurodevelopment and for the emergence of psychiatric disorders.
The present findings showed that adolescents with elevated CAPE-3 scores showed lower gyrification in the banks of superior temporal sulcus, middle temporal gyrus, superior frontal gyrus, and precentral gyrus in the left hemisphere; that persisted from mid-adolescence to late adolescence thus reflecting static gyrification changes. This is in the context of very widespread changes in cortical dynamic gyrification in the entire CAPE-3 group over this period, most especially in prefrontal cortices, reflecting normal maturational processes between mid-adolescence to late-adolescence. Thus, this difference in the developmental trajectory of gyrification in the current study could reflect either a delay in this normal maturational increase, or a persistent reduction in gyrification. Lower gyrification in these areas among the higher scorers is consistent with a recent finding of lower gyrification in left temporal lobe in a cohort of young adults with persistent PLEs16 and lower cortical gyrification in schizophrenia.47,48 Disruption of left fronto-temporal connectivity has been associated with the genesis of positive psychotic symptoms, particularly hallucinations.49–51 Contemporary theories of hallucinatory experience suggest that deficits in sensory gating and language processing result in a “feedforward” aberrant information transmission leading to auditory hallucinations52; deficits in salience network deficits resulting in aberrant salience that could lead to both hallucinations52 and delusions.53 A recent cross-species study demonstrated that variation in cortical gyrification is related to underlying strength of white matter connections54; and changes in fronto-temporal white matter connectivity has been reported to be implicated in the etiology of psychotic symptoms.55 However, this effect was not evident in our replication study of patients with first-episode psychosis suggesting lower gyrification in these areas may reflect a marker of sub-clinical PLEs only.
We also observed a CAPE × TIME interaction where high CAPE-3 scorers showed an increase in gyrification in the right superior and inferior parietal lobules. The parietal changes over time may reflect the specific role of the parietal cortex in efficient multimodal integration, working memory, and self-monitoring.56 As the adolescents in our study did not have clinical intensity of symptoms as observed in the full-blown clinical illness in schizophrenia, but were experiencing PLEs, it is possible that these increases in gyrification in the inferior parietal lobule may reflect compensatory mechanisms supplementary to the more widely observed age-related maturational changes and hence changes in dynamic gyrification. This compensatory increase in gyrification may be secondary to lower fronto-temporal gyrification in high PLE adolescents that may reflect the consequent need for enhanced self-monitoring of self-generated material—a function of the left superior temporal gyrus that is often disrupted in psychotic patients.49–51 Indeed, this compensatory increase in gyrification is supported by data demonstrating higher parietal cortical gyrification in healthy adults was associated with better functional capacity57 while it was associated with persistent auditory hallucinations in schizophrenia.58 In addition, we observed higher gyrification in right parietal cortex in our independent sample of patients with first-episode psychosis compared to HC, thus replicating the changes of dynamic gyrification. This suggests a potential compensatory mechanism that spans across high-risk and clinical populations, supporting similar structural changes across a spectrum of psychotic experience. It is recognized that development during adolescence involves significant structural brain changes and cortical gyrification which may be influenced by underlying changes in gray matter volume, white matter integrity, brain volume, and surface area,59–61 referred to as dynamic gyrification. The changes in dynamic gyrification in parietal cortex which appears at late adolescence and also seen in those with first-episode psychosis may therefore reflect changes in shape and curvature related to cortical morphology/white matter pathology. It is known that persistent PLEs increase the risk of transition to psychosis,7–10 a finding that is replicated here (high CAPE-3 scorers were 5.6 times more likely to transition to psychosis than low CAPE-3 scorers). These abnormalities in developmental trajectory of gyrification may therefore underlie PLEs in adolescents and provide a structural marker for vulnerability to psychosis.
The exploratory stratification using the entire CAPE-42 replicated the higher gyrification in right superior parietal lobule but not the persistent reduction in gyrification as seen in the CAPE-3 high scorers. Of interest, although there was a strong correlation between CAPE-3 and CAPE-42 scores, and both were based on the same threshold on the positive subscale, our study indicates that stratification by CAPE-3 scores isolates a different subset of adolescents than when stratified by CAPE-42 scores. Indeed, there were no correlations between total CAPE-42 score and gyrification in the total sample and fewer adolescents that transitioned to psychosis were classified as experiencing high PLEs using the CAPE-42. This is not surprising because the CAPE 42 indexes a very broad range of experiences, with an increased number of nonspecific symptoms, while the CAPE-3 is focused on specific symptoms most proximal to clinical symptoms observed in psychotic illness. In our study, the high CAPE-3 scoring sample was not different on any confounding factors such as IQ, alcohol, or cannabis use. However, sub-clinical depressive symptoms (ADRS scores) were higher in the high versus low CAPE-3 scorers. Our data therefore support the value in stratifying adolescents according to CAPE-3 item scores and suggests that changes in gyrification are specific to these positive symptoms.
Limitations
First, PLEs, depression, and substance-use measures were available only at follow-up (age 19 years). It is unclear if some adolescents experienced PLEs at baseline or whether the lower gyrification in fronto-temporal regions contributed to PLEs at follow-up. The high and low CAPE-3 groups were relatively well matched for all key variables except for depressive symptoms. Additionally, although the main findings in the study relating to the CAPE-3 cohort are confirmed using robust non-parametric permutation methods (TFCE), most of the results are reported at a liberal statistical threshold (P < .001 uncorrected). This limitation may be a result of small effect sizes in cortical gyrification studies, heterogeneity between adolescents, and CAPE scores that were only measured at age 19 years follow-up—this reflects the difficulties in studying PLEs. Moreover, our analyses investigating transition to psychosis were restricted to the adolescents that were followed up at age 23 years. Although the percentage drop-out was similar between high (40% decrease) and low (38% decrease) CAPE-3 scorers, the large drop-out introduces (unavoidable) bias to the sample as the reasons for drop-out are unclear and may reflect worsening PLEs or other mental health issues. Future studies aiming to replicate the current findings are therefore needed. Finally, due to careful harmonization procedures conducted in advance between scanning sites, scanning site was controlled for in analyses using a commonly used regression approach to remove mean differences between sites. However more sophisticated harmonization procedures could be considered in future.
Conclusion
Our study demonstrates that adolescents with PLEs show increased rates of transition to psychosis accompanied by a neurodevelopmental brain structural abnormality of lower gyrification in fronto-temporal regions in the left hemisphere and increased gyrification in parietal regions in the right hemisphere. This was particularly in relation to positive PLEs such as hearing voices, bizarre experiences, and persecutory ideas, instead of negative PLEs such as blunted emotions. These neurodevelopmental abnormalities, especially in parietal cortex, are relevant to the clinical symptoms of psychosis and could offer a biomarker for identifying adolescents at high risk of transitioning to psychosis.
Supplementary Material
Acknowledgments
We are sincerely grateful to our collaborators in the IMAGEN consortium and our funding bodies.
Contributor Information
Raka Maitra, Department of Psychosis Studies, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, UK.
Charlotte M Horne, Department of Psychosis Studies, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, UK.
Owen O’Daly, Department of Neuroimaging, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, UK.
Evangelos Papanastasiou, Department of Psychosis Studies, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, UK; Therapeutic Area CNS, Boehringer Ingelheim International GmbH, Inghelheim, Germany.
Christian Gaser, Departments of Neurology, Jena University Hospital, Jena, Germany; Departments of Psychiatry and Psychotherapy, Jena University Hospital, Jena, Germany.
IMAGEN list of authors:
Tobias Banaschewski, Gareth J Barker, Arun L W Bokde, Sylvane Desrivières, Herta Flor, Antoine Grigis, Hugh Garavan, Penny Gowland, Andreas Heinz, Rüdiger Brühl, Jean-Luc Martinot, Marie-Laure Paillère Martinot, Eric Artiges, Frauke Nees, Dimitri Papadopoulos Orfanos, Herve Lemaitre, Tomáš Paus, Luise Poustka, Sarah Hohmann, Sabina Millenet, Juliane H Fröhner, Lauren Robinson, Michael N Smolka, Henrik Walter, Jeanne M Winterer, Robert Whelan, Erin Burke Quinlan, Alex Ing, and Gunter Schumann
IMAGEN Consortium:
Funding
This work received support from the following sources: the European Union-funded FP6 Integrated Project IMAGEN (Reinforcement-related behaviour in normal brain function and psychopathology) (LSHM-CT- 2007-037286), the Horizon 2020 funded ERC Advanced Grant ‘STRATIFY’ (Brain network based stratification of reinforcement-related disorders) (695313), Human Brain Project (HBP SGA 2, 785907, and HBP SGA 3, 945539), the Medical Research Council Grant 'c-VEDA’ (Consortium on Vulnerability to Externalizing Disorders and Addictions) (MR/N000390/1), the National Institute of Health (NIH) (R01DA049238, A decentralized macro and micro gene-by-environment interaction analysis of substance use behavior and its brain biomarkers), the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London, the Bundesministeriumfür Bildung und Forschung (BMBF grants 01GS08152; 01EV0711; Forschungsnetz AERIAL 01EE1406A, 01EE1406B; Forschungsnetz IMAC-Mind 01GL1745B), the Deutsche Forschungsgemeinschaft (DFG grants SM 80/7-2, SFB 940, TRR 265, NE 1383/14-1), the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany´s Excellence Strategy – EXC-2049 – 390688087, the Medical Research Foundation and Medical Research Council (grants MR/R00465X/1 and MR/S020306/1), the National Institutes of Health (NIH) funded ENIGMA (grants 5U54EB020403-05 and 1R56AG058854-01). Further support was provided by grants from: - the ANR (ANR-12-SAMA-0004, AAPG2019 - GeBra), the Eranet Neuron (AF12-NEUR0008-01 - WM2NA; and ANR-18-NEUR00002-01 - ADORe), the Fondation de France (00081242), the Fondation pour la Recherche Médicale (DPA20140629802), the Mission Interministérielle de Lutte-contre-les-Drogues-et-les-Conduites-Addictives (MILDECA), the Assistance-Publique-Hôpitaux-de-Paris and INSERM (interface grant), Paris Sud University IDEX 2012, the Fondation de l’Avenir (grant AP-RM-17-013 ), the Fédération pour la Recherche sur le Cerveau; the National Institutes of Health, Science Foundation Ireland (16/ERCD/3797), USA (Axon, Testosterone and Mental Health during Adolescence; RO1 MH085772-01A1), and by NIH Consortium grant U54 EB020403, supported by a cross-NIH alliance that funds Big Data to Knowledge Centres of Excellence, the National Institute for Health Research (NIHR) Biomedical Research Centre (BRC) at South London and Maudsley NHS Foundation Trust (SLaM) and King’s College London (KCL).
Conflict of Interest
Dr Banaschewski served in an advisory or consultancy role for ADHS digital, Infectopharm, Lundbeck, Medice, Neurim Pharmaceuticals, Oberberg GmbH, Roche, and Takeda. He received conference support or speaker’s fee by Medice and Takeda. He received royalities from Hogrefe, Kohlhammer, CIP Medien, Oxford University Press; the present work is unrelated to these relationships.Dr Barker has received honoraria from General Electric Healthcare for teaching on scanner programming courses. Dr Poustka served in an advisory or consultancy role for Roche and Viforpharm and received speaker’s fee by Shire. She received royalties from Hogrefe, Kohlhammer and Schattauer. Dr Papanastasiou is an employee of Boehringer Ingelheim; the present work is unrelated to this relationship. The present work is unrelated to the above grants and relationships. The other authors report no biomedical financial interests or potential conflicts of interest.
References
- 1. Hinterbuchinger B, Mossaheb N.. Psychotic-like experiences: a challenge in definition and assessment. Front Psychiatry. 2021;12:582392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fusar-Poli P, Borgwardt S, Bechdolf A, et al. The psychosis high-risk state: a comprehensive state-of-the-art review. JAMA Psychiatry. 2013;70(1):107–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pain O, Dudbridge F, Cardno AG, et al. Genome-wide analysis of adolescent psychotic-like experiences shows genetic overlap with psychiatric disorders. Am J Med Genet. 2018;177(4):416–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mackie CJ, Castellanos-Ryan N, Conrod PJ.. Developmental trajectories of psychotic-like experiences across adolescence: impact of victimization and substance use. Psychol Med. 2011;41(1):47–58. [DOI] [PubMed] [Google Scholar]
- 5. Kelleher I, Connor D, Clarke MC, Devlin N, Harley M, Cannon M.. Prevalence of psychotic symptoms in childhood and adolescence: a systematic review and meta-analysis of population-based studies. Psychol Med. 2012;42(9):1857–1863. [DOI] [PubMed] [Google Scholar]
- 6. Kelleher I, Keeley H, Corcoran P, et al. Clinicopathological significance of psychotic experiences in non-psychotic young people: evidence from four population-based studies. Br J Psychiatry. 2012;201(1):26–32. [DOI] [PubMed] [Google Scholar]
- 7. Poulton R, Caspi A, Moffitt TE, Cannon M, Murray R, Harrington H.. Children’s self-reported psychotic symptoms and adult schizophreniform disorder: a 15-year longitudinal study. Arch Gen Psychiatry. 2000;57(11):1053–1058. [DOI] [PubMed] [Google Scholar]
- 8. Scott J, Martin G, Welham J, et al. Psychopathology during childhood and adolescence predicts delusional-like experiences in adults: a 21-year birth cohort study. Am J Psychiatry. 2009;166(5):567–574. [DOI] [PubMed] [Google Scholar]
- 9. Welham J, Scott J, Williams G, et al. Emotional and behavioural antecedents of young adults who screen positive for non-affective psychosis: a 21-year birth cohort study. Psychol Med. 2009;39(4):625–634. [DOI] [PubMed] [Google Scholar]
- 10. Kaymaz N, Drukker M, Lieb R, et al. Do subthreshold psychotic experiences predict clinical outcomes in unselected non-help-seeking population-based samples? A systematic review and meta-analysis, enriched with new results. Psychol Med. 2012;42(11):2239–2253. [DOI] [PubMed] [Google Scholar]
- 11. DeRosse P, Ikuta T, Karlsgodt KH, et al. White matter abnormalities associated with subsyndromal psychotic-like symptoms predict later social competence in children and adolescents. Schizophr Bull. 2017;43(1):152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Drakesmith M, Dutt A, Fonville L, et al. Mediation of developmental risk factors for psychosis by white matter microstructure in young adults with psychotic experiences. JAMA Psychiatry. 2016;73(4):396–406. [DOI] [PubMed] [Google Scholar]
- 13. Nesvag R, Reichborn-Kjennerud T, Gillespie NA, et al. Genetic and environmental contributions to the association between cannabis use and psychotic-like experiences in young adult twins. Schizophr Bull. 2017;43(3):644–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Armstrong E, Schleicher A, Omran H, Curtis M, Zilles K.. The ontogeny of human gyrification. Cereb Cortex. 1995;5(1):56–63. [DOI] [PubMed] [Google Scholar]
- 15. Zilles K, Armstrong E, Schleicher A, Kretschmann HJ.. The human pattern of gyrification in the cerebral cortex. Anat Embryol (Berl). 1988;179(2):173–179. [DOI] [PubMed] [Google Scholar]
- 16. Fonville L, Drakesmith M, Zammit S, Lewis G, Jones DK, David AS.. MRI indices of cortical development in young people with psychotic experiences: influence of genetic risk and persistence of symptoms. Schizophr Bull. 2019;45(1):169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Wit S, Ziermans TB, Nieuwenhuis M, et al. Individual prediction of long-term outcome in adolescents at ultra-high risk for psychosis: applying machine learning techniques to brain imaging data. Hum Brain Mapp. 2017;38(2):704–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Damme KSF, Gupta T, Nusslock R, Bernard JA, Orr JM, Mittal VA.. Cortical morphometry in the psychosis risk period: a comprehensive perspective of surface features. Biol Psychiatry Cogn Neurosci Neuroimaging. 2019;4(5):434–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harris JM, Whalley H, Yates S, Miller P, Johnstone EC, Lawrie SM.. Abnormal cortical folding in high-risk individuals: a predictor of the development of schizophrenia? Biol Psychiatry. 2004;56(3):182–189. [DOI] [PubMed] [Google Scholar]
- 20. Sasabayashi D, Takayanagi Y, Takahashi T, et al. Increased occipital gyrification and development of psychotic disorders in individuals with an at-risk mental state: a multicenter study. Biol Psychiatry. 2017;82(10):737–745. [DOI] [PubMed] [Google Scholar]
- 21. Bakker G, Caan MW, Vingerhoets WA, et al. Cortical morphology differences in subjects at increased vulnerability for developing a psychotic disorder: a comparison between subjects with ultra-high risk and 22q11.2 deletion syndrome. PLoS One. 2016;11(11):e0159928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Padula MC, Schaer M, Armando M, et al. Cortical morphology development in patients with 22q11.2 deletion syndrome at ultra-high risk of psychosis. Psychol Med. 2018;48(14):2375–2383. [DOI] [PubMed] [Google Scholar]
- 23. Matsuda Y, Ohi K.. Cortical gyrification in schizophrenia: current perspectives. Neuropsychiatr Dis Treat. 2018;14:1861–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sasabayashi D, Takahashi T, Takayanagi Y, Suzuki M.. Anomalous brain gyrification patterns in major psychiatric disorders: a systematic review and transdiagnostic integration. Transl Psychiatry. 2021;11(1):176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Welcome to the IMAGENStudy . https://imagen-europe.com/. Accessed January 1, 2018.
- 26. Mossaheb N, Becker J, Schaefer MR, et al. The Community Assessment of Psychic Experience (CAPE) questionnaire as a screening-instrument in the detection of individuals at ultra-high risk for psychosis. Schizophr Res. 2012;141(2-3):210–214. [DOI] [PubMed] [Google Scholar]
- 27. Konings M, Bak M, Hanssen M, van Os J, Krabbendam L.. Validity and reliability of the CAPE: a self-report instrument for the measurement of psychotic experiences in the general population. Acta Psychiatr Scand. 2006;114(1):55–61. [DOI] [PubMed] [Google Scholar]
- 28. Mark W, Toulopoulou T.. Psychometric properties of “community assessment of psychic experiences”: review and meta-analyses. Schizophr Bull. 2016;42(1):34–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yung AR, Nelson B, Baker K, Buckby JA, Baksheev G, Cosgrave EM.. Psychotic-like experiences in a community sample of adolescents: implications for the continuum model of psychosis and prediction of schizophrenia. Aust N Z J Psychiatry. 2009;43(2):118–128. [DOI] [PubMed] [Google Scholar]
- 30. Wechsler D. Wechsler Intelligence Scale for Children. 4th ed. San Antonio, TX: Psychological Corporation; 2003. [Google Scholar]
- 31. Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M.. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption--II. Addiction. 1993;88(6):791–804. [DOI] [PubMed] [Google Scholar]
- 32. Bohn MJ, Babor TF, Kranzler HR.. The Alcohol Use Disorders Identification Test (AUDIT): validation of a screening instrument for use in medical settings. J Stud Alcohol. 1995;56(4):423–432. [DOI] [PubMed] [Google Scholar]
- 33. Skinner HA. The drug abuse screening test. Addict Behav. 1982;7(4):363–371. [DOI] [PubMed] [Google Scholar]
- 34. Revah-Levy A, Birmaher B, Gasquet I, Falissard B.. The Adolescent Depression Rating Scale (ADRS): a validation study. BMC Psychiatry. 2007;7(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Schumann G, Loth E, Banaschewski T, et al. The IMAGEN study: reinforcement-related behaviour in normal brain function and psychopathology. Mol Psychiatry. 2010;15:1128–1139. [DOI] [PubMed] [Google Scholar]
- 36. Luders E, Thompson PM, Narr KL, Toga AW, Jancke L, Gaser C.. A curvature-based approach to estimate local gyrification on the cortical surface. Neuroimage. 2006;29(4):1224–1230. [DOI] [PubMed] [Google Scholar]
- 37. Evermann U, Gaser C, Besteher B, Langbein K, Nenadić I.. Cortical gyrification, psychotic-like experiences, and cognitive performance in nonclinical subjects. Schizophr Bull. 2020;46(6):1524–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Desikan RS, Segonne F, Fischl B, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. [DOI] [PubMed] [Google Scholar]
- 39. IBM. IBM SPSS Statistics for Macintosh. Version 25.0 ed. Armonk, NY: IBM Corp.; 2017. [Google Scholar]
- 40. Caverzasi E, Battistella G, Chu SA, et al. Gyrification abnormalities in presymptomatic c9orf72 expansion carriers. J Neurol Neurosurg Psychiatry. 2019;90(9):1005–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Lee SE, Sias AC, Mandelli ML, et al. Network degeneration and dysfunction in presymptomatic C9ORF72 expansion carriers. Neuroimage Clin. 2017;14:286–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Smith SM, Nichols TE.. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44(1):83–98. [DOI] [PubMed] [Google Scholar]
- 43. Thomas M, Szentgyorgyi T, Vanes LD, et al. Cognitive performance in early, treatment-resistant psychosis patients: could cognitive control play a role in persistent symptoms? Psychiatry Res. 2021;295:113607. [DOI] [PubMed] [Google Scholar]
- 44. Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33;quiz 34. [PubMed] [Google Scholar]
- 45. Andrade C. Understanding relative risk, odds ratio, and related terms: as simple as it can get. J Clin Psychiatry. 2015;76(7):e857–e861. [DOI] [PubMed] [Google Scholar]
- 46. Fonville L, Drakesmith M, Zammit S, Lewis G, Jones DK, David AS.. MRI indices of cortical development in young people with psychotic experiences: influence of genetic risk and persistence of symptoms. Schizophr Bull. 2019;45(1):169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. White T, Hilgetag CC.. Gyrification and neural connectivity in schizophrenia. Dev Psychopathol. 2011;23(1):339–352. [DOI] [PubMed] [Google Scholar]
- 48. Cao B, Mwangi B, Passos IC, et al. Lifespan gyrification trajectories of human brain in healthy individuals and patients with major psychiatric disorders. Sci Rep. 2017;7(1):511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Simons CJ, Tracy DK, Sanghera KK, et al. Functional magnetic resonance imaging of inner speech in schizophrenia. Biol Psychiatry. 2010;67(3):232–237. [DOI] [PubMed] [Google Scholar]
- 50. Shergill SS, Brammer MJ, Fukuda R, Williams SC, Murray RM, McGuire PK.. Engagement of brain areas implicated in processing inner speech in people with auditory hallucinations. Br J Psychiatry. 2003;182:525–531. [DOI] [PubMed] [Google Scholar]
- 51. Shergill SS, Brammer MJ, Fukuda R, et al. Modulation of activity in temporal cortex during generation of inner speech. Hum Brain Mapp. 2002;16(4):219–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tracy DK, Shergill SS.. Mechanisms underlying auditory hallucinations-understanding perception without stimulus. Brain Sci. 2013;3(2):642–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. So SH, Chau AKC, їPeters ER, Swendsen J, Garety PA, Kapur S.. Moment-to-moment associations between negative affect, aberrant salience, and paranoia. Cogn Neuropsychiatry. 2018;23(5):299–306. [DOI] [PubMed] [Google Scholar]
- 54. Van Essen DC, Donahue CJ, Coalson TS, Kennedy H, Hayashi T, Glasser MF.. Cerebral cortical folding, parcellation, and connectivity in humans, nonhuman primates, and mice. Proc Natl Acad Sci USA. 2019;116(52):26173–26180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Jones DK, Catani M, Pierpaoli C, et al. Age effects on diffusion tensor magnetic resonance imaging tractography measures of frontal cortex connections in schizophrenia. Hum Brain Mapp. 2006;27(3):230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ribolsi M, Lisi G, Di Lorenzo G, et al. Perceptual pseudoneglect in schizophrenia: candidate endophenotype and the role of the right parietal cortex. Schizophr Bull. 2013;39(3):601–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Green S, Blackmon K, Thesen T, et al. Parieto-frontal gyrification and working memory in healthy adults. Brain Imaging Behav. 2018;12(2):303–308. [DOI] [PubMed] [Google Scholar]
- 58. Kubera KM, Thomann PA, Hirjak D, et al. Cortical folding abnormalities in patients with schizophrenia who have persistent auditory verbal hallucinations. Eur Neuropsychopharmacol. 2018;28(2):297–306. [DOI] [PubMed] [Google Scholar]
- 59. Gautam P, Anstey KJ, Wen W, Sachdev PS, Cherbuin N.. Cortical gyrification and its relationships with cortical volume, cortical thickness, and cognitive performance in healthy mid-life adults. Behav Brain Res. 2015;287:331–339. [DOI] [PubMed] [Google Scholar]
- 60. Schultz CC, Wagner G, Schachtzabel C, et al. Increased white matter radial diffusivity is associated with prefrontal cortical folding deficits in schizophrenia. Psychiatry Res Neuroimaging. 2017;261:91–95. [DOI] [PubMed] [Google Scholar]
- 61. Toro R, Perron M, Pike B, et al. Brain size and folding of the human cerebral cortex. Cereb Cortex. 2008;18(10):2352–2357. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.