Abstract
Background and hypothesis
Around 20% of people at clinical high risk (CHR) for psychosis later develop a psychotic disorder, but it is difficult to predict who this will be. We assessed the incidence of hearing speech (termed speech illusions [SIs]) in noise in CHR participants and examined whether this was associated with adverse clinical outcomes.
Study design
At baseline, 344 CHR participants and 67 healthy controls were presented with a computerized white noise task and asked whether they heard speech, and whether speech was neutral, affective, or whether they were uncertain about its valence. After 2 years, we assessed whether participants transitioned to psychosis, or remitted from the CHR state, and their functioning.
Study results
CHR participants had a lower sensitivity to the task. Logistic regression revealed that a bias towards hearing targets in stimuli was associated with remission status (OR = 0.21, P = 042). Conversely, hearing SIs with uncertain valence at baseline was associated with reduced likelihood of remission (OR = 7.72. P = .007). When we assessed only participants who did not take antipsychotic medication at baseline, the association between hearing SIs with uncertain valence at baseline and remission likelihood remained (OR = 7.61, P = .043) and this variable was additionally associated with a greater likelihood of transition to psychosis (OR = 5.34, P = .029).
Conclusions
In CHR individuals, a tendency to hear speech in noise, and uncertainty about the affective valence of this speech, is associated with adverse outcomes. This task could be used in a battery of cognitive markers to stratify CHR participants according to subsequent outcomes.
Keywords: signal-detection, white noise task, uncertainty, remission, transition
Introduction
Psychosis is often preceded by a clinical high risk (CHR) stage, characterized by attenuated psychotic symptoms and a reduction in functioning. Although 40%–50% remit from the CHR state within 2 years,1 more than half continue to experience symptoms and impairments in functioning2,3 and around 20% develop psychosis.4–6
People with hallucinatory experiences are at increased risk of developing psychosis.7,8 One way to experimentally assess proneness to psychotic experiences is to use the white noise task (WNT).9 In this computerized paradigm, participants are presented with white noise, with or without neutral speech, and asked whether they heard speech and if so, whether speech was neutral, positive, negative, or whether they were unsure about the valence of speech. The incidence of speech illusions (SIs)—speech heard on white noise-only trials—is increased in people who report hearing voices,10 is related to the level of familial risk for psychosis,9 and is associated with schizotypy in some9 but not all11 studies. However, to date, the frequency of SIs in CHR participants has not yet been compared to that in healthy controls (HC).
Data from the WNT can be analyzed within a signal detection theory (SDT) framework12 which describes the probabilistic processes of decision-making under conditions of uncertainty. According to SDT, on a given trial in which a target stimulus or noise may be presented, participants respond according to the value of an inner decision variable. If this reaches a certain criterion, the participant responds that a target is present; otherwise, the participant responds that it is not. Responses are categorized into hits (correctly detecting speech), false alarms (hearing speech when there is none), sensitivity (dʹ), and a bias towards responding “yes” or “no” (c).13,14 Healthy participants who report more hallucinatory experiences experience more false alarms, accompanied by a bias towards responding “yes,”15–17 but results are mixed as to whether this is associated with altered sensitivity on the WNT.12,17 The first aim of this study was to compare the incidence of SIs and SDT parameters of performance in a large sample of CHR participants and controls. We hypothesized that CHR participants would report SIs more frequently than healthy volunteers and would show reduced sensitivity and greater response bias.
Within CHR participants, impaired performance across a range of different cognitive tasks has been associated with adverse outcomes, including transition to psychosis,18–21 persistence of CHR symptoms,22 and low functioning.22 Whilst the perceived length of SI23 has been linked to transition to psychosis, the incidence of SIs, and associated SDT parameters, have not been associated with clinical outcome. The second aim of this study was to assess whether within a CHR cohort the incidence of SIs or associated SDT parameters at baseline is associated with transition to psychosis, persistence of symptoms, or decreased functioning, 2 years later.
Methods
Sample
Data were collected from 344 CHR participants and 67 HCs recruited as part of the EU-GEI high-risk study (European Network of National Networks studying Gene-Environment Interactions in Schizophrenia) (https://www.eu-gei.eu/),24 a naturalistic prospective multicentre study. Eleven sites contributed and from these sites, HCs were recruited from the general population.
Inclusion criteria for CHR participants were: Meet at least 1 criterion in the Comprehensive Assessment of At Risk Mental State (CAARMS4): (1) attenuated psychotic symptoms (subthreshold positive psychotic symptoms for at least 1 month in the previous year), (2) brief limited intermittent psychotic symptoms (an episode of frank psychotic symptoms that resolved in less than 1 week without treatment), and (3) vulnerability (a first-degree relative with a psychotic disorder or schizotypal personality disorder and a drop in functioning for at least 1 month in the previous year). Exclusion criteria for CHR participants were: An IQ lower than 60, a current or past psychotic disorder, or that symptoms could be explained by a disease or substance dependency. For CHR participants, the CAARMS was used to determine whether individuals met at least one of CHR criteria: Attenuated Psychosis Group, Vulnerability Group, or Brief Limited Intermittent Psychotic Symptoms Group. Exclusion criteria for all participants were: (1) past/present diagnosis of psychotic disorder, determined by CAARMS, and Structural Clinical Interview for DSM Disorders, (2) relevant symptoms explained by neurological disorder or drug/alcohol dependency, (3) contraindications to MRI scanning or unwillingness to provide blood/saliva sample (for the measures collected within the larger EU-GEI study); and (4) IQ estimate <60. HC participants did not meet CHR criteria. Typical age of participants was 18–35 years but not restricted due to variation between sites in the age at which persons are accepted by clinical services (table 1).
Table 1.
Sociodemographic Variables of the Participants Who Completed the White Noise Task, Split By Participant Group, Transition Status, and Remission Status
| Measure | Subject Group | Transition Status | Remission Status | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HC (N = 51) | CHR (N = 308) | P | T (N = 55) | NT (N = 253) | P | NR (N = 88) | R (N = 52) | P | |
| Age in years, M (SD) | 23.35 (3.99) 0 | 22.66 (4.92) 0 | .134 | 22.71 (4.95) 0 | 22.64 (4.92) 0 | .959 | 23.39 (5.34) 0 | 22.83 (4.59) 0 | .759 |
| Female sex, no (%) | 24 (47.05) 0 | 145 (47.08) 0 | 1.00 | 24 (43.63) 0 | 121 (47.83) 0 | .655 | 41 (46.59) 0 | 26 (50) 0 | .729 |
| Years in education, M (SD) | 16.35 (2.84) 0 | 14.48 (3.12) (29) | <.001 | 14.13 (3.49) 9 | 14.55 (3.04) 20 | .306 | 14.89 (3.36) 8 | 15.37 (2.58) 1 | .189 |
| WAIS IQ, mean (SD) | 110.25 (17.71) 0 | 98.65 (16.95) 20 | <.001 | 96.98 (13.64) 3 | 99.02 (17.60) 17 | .470 | 97.59 (14.92) 3 | 102.60 (14.22) 2 | .040 |
| Number of cigarettes per day, M (SD) | 6.69 (8.38) 25 | 10.81 (11.49) 117 | .030 | 2.58 (1.55) 17 | 11.14 (11.94) 94 | .414 | 10.23 (10.97) 32 | 8.65 (6.87) 21 | .817 |
| No. of alcoholic drinks per week, M (SD) | 6.21 (7.08) 8 | 7.7 (11.63) 99 | .750 | 8.95 (11.03) 18 | 7.43 (11.76) 81 | .281 | 8.38 (10.09) 25 | 5.97 (8.69) 14 | .135 |
| How often uses cannabis, M (SD) | 3.26 (1.19) 16 | 2.8 (1.56) 90 | .095 | 2.58 (1.55) 17 | 2.84 (1.56) 73 | .416 | 2.55 (1.55) 24 | 2.97 (1.48) 14 | .160 |
| Uses antipsychotic medication, no (%) | N/A | 23 (9.2) 57 | N/A | 9 (19.14) 6 | 14 (6.86) 49 | .020 | 10 (12.82) 10 | 1 (2.43) 11 | .095 |
| Baseline CAARMS positive score, M (SD) | N/A | 9.98 (4.05) 5 | N/A | 10.58 (3.56) 0 | 9.56 (4.14) 5 | .253 | 9.82 (4.11) 0 | 9.50 (3.64) 0 | .526 |
| Baseline CAARMS negative score, M (SD) | N/A | 7.01 (3.41) 9 | N/A | 7.45 (3.39) 0 | 6.91 (3.41) 9 | .374 | 7.51 (3.10) 1 | 7.69 (3.20) 0 | .956 |
| Baseline CAARMS total score, M (SD) | N/A | 49.15 (15.73) 62 | N/A | 52.43 (15.80) 8 | 48.38 (15.65) 54 | .169 | 50.45 (14.11) 13 | 48.81 (12.94) 3 | .548 |
| Baseline GAF average score, M (SD) | 87.14 (9.17) 1 | 55.5 (9.9) 26 | <.001 | 53.99 (9.98) 5 | 55.83 (9.87) 21 | .085 | 53.74 (9.61) 5 | 56.89 (8.08) 0 | .015 |
Note: HC, healthy controls; CHR, participants at clinical high-risk of psychosis; T, transition; NT, non-transition; NR, non-remission; R, remission; P, significance; SD, standard deviation; how often use cannabis, 1 = every day, 2 = more than once per week, 3 = a few times per month, 4 = a few times per year, 5=only once or twice; no, number; N/A, not applicable. Significant differences are in bold. Number of missing values for each variable is given in italics.
Instruments
Clinical Measures
Clinical measures were assessed at baseline and follow-up by assessors trained in the use of the CAARMS and the Global Assessment of Functioning Scale (GAF4,25,26). Participants undertook follow-up assessments at 24 months. Transition to psychosis was defined as the development of psychotic disorder using the CAARMS.4 Available clinical records were used to determine any diagnosis of a psychotic disorder when participants did not return for follow-up assessments. In CHR participants that could not be contacted at follow-up, the onset of psychosis was defined using information from clinical records. Remission was defined as a participant no longer meeting the criteria for the CHR state at follow-up. Those who transitioned at follow-up were classed as non-remitters. Level of functioning was assessed based on the GAF disability score, as in previous research.22
White Noise Task
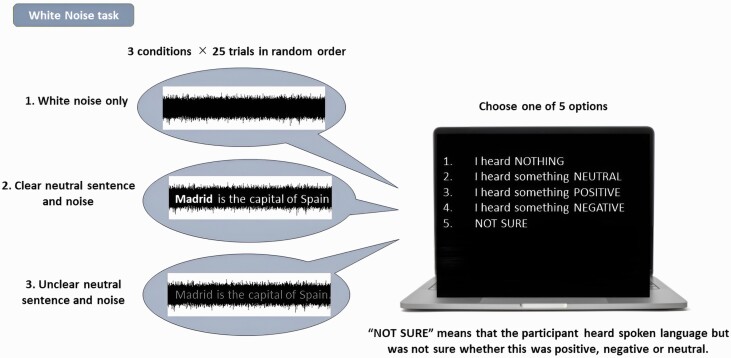
The WNT was used to assess the incidence of SIs heard in white noise9,11,27,28 (figure 1). Participants, wearing earphones, sat in a room with a trained experimenter and were presented with either: (1) white noise only, (2) white noise plus clearly audible neutral speech, and (3) white noise plus barely audible neutral speech. The neutral speech fragments had content such as: “Sport is good for health,” Speech was adapted to each country’s native language. Each fragment had a duration of 4.3 s and sound fragments were binaurally presented through headphones. Participants were presented with 25 trials of each condition, in random order, and were required to verbally endorse one of five possible responses per trial: (1) no speech heard, (2) heard speech saying something neutral, (3) heard speech saying something positive, or (4) heard speech saying something negative, and (5) heard speech, but uncertain if it was positive, negative, or neutral. A response of 2,3,4, or 5 during condition 1 (white noise only) was defined as a SI.
Fig. 1.
The white noise task. Participants were presented with one of three conditions whilst wearing earphones: (1) white noise only, (2) white noise plus clearly audible neutral speech, (3) white noise plus barely audible neutral speech and required to verbally endorse one of five possible responses per trial: (1) no speech heard; (2) heard speech saying something neutral; (3) heard speech saying something positive; (4) heard speech saying something negative; (5) heard speech, but uncertain if it was positive, negative, or neutral. A response of 2,3,4, or 5 during condition 1 (white noise only) was defined as a speech illusion.
IQ
A 15-minute version of the short-form Wechsler Adult Intelligence Scale, including Digit Symbol Coding, Block Design, Information, and Arithmetic subtests29,30 assessed intellectual function.
Groups for Analysis
Comparisons were made between the entire CHR sample and HC, and between CHR subgroups defined according to clinical outcomes: transition to psychosis (CHR-T vs CHR-NT) and remission from the CHR state (CHR-R vs CHR-NR).
Signal Detection Theory
The WNT was analyzed using a SDT framework.14 Conditions (2) and (3) (white noise with neutral speech) were treated as the target, and condition (1) (“white noise only”) was treated as the nontarget. Three parameters were calculated:
False alarms (F) (proportion of white-noise-only trials incorrectly identified as targets) (with a log-linear transformation to account for extreme values in the data31).
Sensitivity dʹ as a global measure of performance,31 calculated as the difference between the inverse normal distribution of the hit rate and the inverse normal distribution of the false alarm rate (NORMSINV(H)—NORMSINV(F) in excel).31
Response bias c, signifying a bias towards detecting a stimulus as a target (a lower value of c indicates a bias towards responding “yes”),31 calculated as the average of the inverse normal distribution of the hit rate and the inverse normal distribution of the false alarm rate ((NORMSINV(H) + NORMSINV(F))/2 in excel).31
Affective and Uncertain SIs
We assessed whether SIs were affectively salient, ie, when participants perceived speech as negative or positive, or whether there was uncertainty around SIs, ie, when participants were uncertain if the perceived speech was neutral or had either a negative or positive salience. SI scores were skewed, as expected, because participants expressed a low rate of false alarms. To address this, following previous work,9,27,28 performance was parameterized as 3 dichotomous variables according to responses during the white noise-only condition: Each participant was rated as 1 or 0 depending on whether they heard at least 2 SIs that they perceived as (1) neutral, (2) positive or negative, or (3) unsure whether they were neutral, positive, or negative. We included a threshold of at least 2 SIs per condition, to exclude possible erroneous attributions of SIs.27
Group Differences
Group differences between participant group (CHR vs HC), transition status and remission status on the incidence of SIs were assessed using Fisher’s exact tests, and on hit rate, false alarms, sensitivity (dʹ), and response bias (cʹ), using independent samples t tests. All tests used a significance threshold of P < .05 (two-tailed).
Association With Outcome
SDT Parameters
We applied a logistic regression model to each of the three SDT parameters (false alarm rate, sensitivity, or response bias) to assess whether each variable predicted transition or remission status in CHR participants. We also applied a linear regression model to each of the three SDT parameters to assess whether each variable predicted GAF disability score in CHR participants.
Dichotomous Variables
We applied a logistic regression model to each of the 3 dichotomous variables (heard 2 or more SIs, heard 2 or more positive/negative SIs, heard 2 or more SIs with uncertain valence) to assess whether each variable predicted transition or remission status in CHR participants. We also applied a linear regression model to each of the 3 dichotomous variables to assess whether each variable predicted GAF disability score in CHR participants.
All models used a significance threshold of P < .05.
All regression models were adjusted for site, age, sex, cannabis and tobacco use, IQ (shortened WAIS), and years in education, based on the previous work9,28 and on which variables showed group differences between HC and CHR participants (table 1).
Attrition Analysis
Due to numerous attritions at follow-up, we assessed group differences between participants who did or did not drop out at follow-up, to confirm that attritions were random and did not influence the assessment of association with outcome, using independent samples t tests with a significance threshold of P < .05 (two-tailed).
Effect of Antipsychotic Medication
We repeated the analyses to include only CHR participants who were not on antipsychotic medication at the baseline assessment.
Results
Demographics
All sociodemographic, clinical, and medication data categorized by group, transition, and remission status are summarized in table 1. Two years from baseline, 55 CHR participants (18% of the total sample) transitioned to psychosis (CHR-T) and 252 (82%) did not (CHR-NT), and 88 (63% of the remaining sample) had not remitted from the CHR state, and 52 (37%) had remitted. At baseline CHR participants had fewer years of education, lower IQ, smoked more cigarettes, and had a lower baseline GAF score than HC participants. Fewer CHR participants who transitioned to psychosis at 2 years were taking antipsychotic medication at baseline, and CHR participants who remitted from the CHR state had a higher IQ and a higher GAF average score at baseline. Of CHR participants who heard 2 or more SIs on the WNT, fewer were female, and participants who heard 2 or more affectively salient SIs at baseline had more years in education (supplementary table 4).
Group Differences on the WNT
Group differences on the WNT are summarized in table 2. HC participants (N = 51, M = 3.58) had a higher sensitivity dʹ than CHR participants (N = 308, M = 3.26) (P = .018). There were no other differences in the SDT parameters or the 3 dichotomous variables (heard 2 or more SIs, heard 2 or more affectively salient SIs, heard 2 or more SIs with uncertain valence) between HC and CHR participants, CHR-T (N = 55) and CHR-NT (N = 253) participants, or between CHR participants whose symptoms persisted at follow-up (CHR-NR) (N = 88) compared to CHR participants whose symptoms had resolved at follow-up (CHR-R) (N = 52) (table 2).
Table 2.
Participant Group and Outcome Differences on Signal Detection Theory Parameters Hit Rate, False Alarm Rate, d and c, and 3 Dichotomous Speech Illusion Variables “Heard 2 or More SI”, “Heard 2 or More Affective SI”, “Heard 2 or More Uncertain SI”. Significant differences are in bold. SI: Speech Illusion; no, Number
| Measure | Participant Group | Transition Status | Remission Status | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HC (N = 51) | CHR (N = 308) | P | T (N = 55) | NT (N = 253) | P | NR (N = 88) | R (N = 52) | P | |
| False alarm rate mean (SD) | 0.12 (0.16) | 0.14 (0.17) | .368 | 0.12 (0.12) | 0.14 (0.18) | .273 | 0.16 (0.17) | 0.12 (0.16) | .171 |
| dʹ mean (SD) | 3.58 (0.84) | 3.26 (1.01) | .018 | 3.29 (0.83) | 3.26 (1.04) | .803 | 3.19 (0.89) | 3.39 (0.87) | .200 |
| c mean (SD) | −0.35 (0.39) | -0.28 (0.51) | .290 | −0.27 (0.44) | −0.29 (0.53) | .743 | −0.38 (0.44) | −0.23 (0.46) | .064 |
| Heard SI, no (%) | 6 (11.8) | 39 (12.7) | 1.0 | 10 (18.2) | 29 (11.5) | .182 | 15 (17.0) | 5 (9.6) | .318 |
| Heard affective SI, no (%) | 1 (1.9) | 24 (7.8) | .229 | 6 (10.9) | 18 (7.1) | .402 | 9 (10.2) | 2 (3.8) | .212 |
| Heard SI with uncertain valence, no (%) | 18 (35.3) | 120 (39.0) | .645 | 23 (41.8) | 97 (38.3) | .649 | 42 (47.7) | 16 (30.7) | .053 |
Performance on the WNT Associated With Outcome
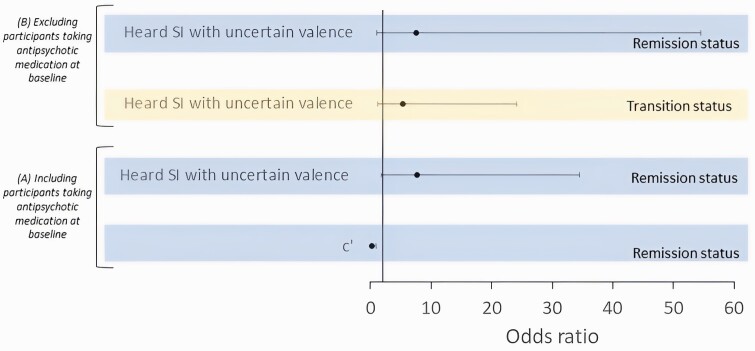
All results of the outcome regression models are summarized in table 3. In CHR participants, a lower value of c (a higher likelihood of hearing targets in stimuli) (OR = 0.21, P = .042) and hearing 2 or more SIs with uncertain valence at baseline (OR = 7.72, P = .007), were associated with non-remission at 2-year follow-up. See figure 2A for a figure summarizing the significant results. There were no other associations between task parameters and outcome measures.
Table 3.
Logistic Regression Models Assessing the Association Between Transition Status/Remission Status and Linear Regression Models Assessing the Association Between Functional Outcome, With Signal Detection Theory Parameters Hit Rate, False Alarm Rate, d and c, and 3 Dichotomous Speech Illusion Variables “Heard 2 or More SI”, “Heard 2 or More Affective SI”, “Heard 2 or More Uncertain SI”. Each Row Signifies a Separate Model for Transition Status, Remission Status, and Functional Outcome. Significant Predictors are in Bold. All Models Were Adjusted for Age, Gender, Years in Education, IQ, Cigarettes Smoked Per Day, Site, and Cannabis Use, Replicating Previous Work9,29 and on Variables Showing Significant Differences at Baseline (table 1)
| Measure | Transition Status (N = 145) | Remission Status (N = 70) | Functional Outcome (N = 61) | |||||
|---|---|---|---|---|---|---|---|---|
| P | OR (95% CI) | P | OR (95% CI) | P | B | SE B | β | |
| False alarm rate | .237 | 7.45 (0.27, 207.59) | .279 | 10.59 (0.15, 759.81) | .107 | −19.84 | 12.08 | −0.23 |
| d' | .215 | 0.71 (0.42, 1.22) | .570 | 0.78 (0.34, 1.81) | .122 | 3.96 | 2.52 | 0.21 |
| C | .431 | 0.61 (0.17, 2.09) | .042 | 0.21 (0.05, 0.94) | .386 | 3.94 | 4.5 | 0.12 |
| Heard SI | .063 | 3.82 (0.93, 15.73) | .320 | 0.37 (0.05, 2.63) | .229 | −6.60 | 5.41 | −0.16 |
| Heard affective SI | .582 | 1.72 (0.25, 11.89) | .711 | 0.59 (0.04, 9.67) | .251 | −9.27 | 7.97 | −0.17 |
| Heard SI with uncertain valence | .059 | 2.87 (0.96, 8.58) | .007 | 7.72 (1.73, 34.49) | .099 | -−.88 | 4.09 | −0.23 |
Note: OR, odds ratio.
Fig. 2.
Associations between performance on the white noise task and outcomes in CHR participants 2 years later, (A) including participants taking antipsychotic medication at baseline, and (B) excluding participants taking antipsychotic medication at baseline.
Attrition Analysis
All results of the attrition analysis are summarized in supplementary table 1. CHR participants with follow-up data (N = 111) were at baseline older, had more years in education, and had a lower CAARMS positive score and a higher CAARMS negative score and a low CAARMS positive score than CHR participants without follow-up data (N = 197), and fewer CHR participants with follow-up data were taking antipsychotic medication at baseline than CHR participants without follow-up data.
Effect of Antipsychotic Medication
All results of the analysis which included only CHR participants who were not on antipsychotic medication at the baseline assessment are summarized in supplementary tables 2 and 3 (N = 46 HC and 228 CHR when excluding participants on antipsychotic medication [and participants whose data about antipsychotic medication was missing] compared to N = 51 HC and 308 CHR when including all participants). As in the analysis of all CHR participants (table 2), HC participants (N = 46, M = 3.53) had a higher sensitivity dʹ when compared to CHR participants who were taking antipsychotic medication (N = 228, M = 3.24) (P = .048), but there were no other group differences (supplementary table 2). As with the analysis in all CHR participants (table 3), in CHR participants who were not taking antipsychotic medication, hearing 2 or more SIs with uncertain valence at baseline was associated with non-remission from the CHR state at 2-year follow-up (OR = 7.61, P = .043). Unlike the analysis in all CHR participants (table 3), the association between c and non-remission at 2-year follow-up was not significant in CHR participants who were taking antipsychotic medication (supplementary table 3) (OR = 0.38, P = .277). A new finding was that hearing 2 or more SIs with uncertain valence at baseline was associated with transition to psychosis in CHR participants who were taking antipsychotic medication (supplementary table 3) (OR = 7.61, P = .029). See figure 2B for a figure summarizing the significant results There were no other associations between task parameters and outcome measures.
Discussion
The main findings of the study were that CHR participants had lower sensitivity (dʹ), and that CHR participants with a bias towards reporting hearing targets in stimuli, or who heard SIs with uncertain valence on the WNT at presentation, were less likely to be in remission at 2-year follow-up. This association between hearing SIs with uncertain valence and non-remission remained significant when assessing only CHR participants who were not taking antipsychotic medication at baseline. Furthermore, in this cohort, SIs with uncertain valence were also associated with transition to psychosis at follow-up.
Cognitive models propose that perception represents a compromise between top-down beliefs and bottom-up sensory information,32 and that psychosis symptoms,33 including hallucinations34 may result from an imbalance between these parameters. The finding that CHR participants had lower sensitivity on the WNT, and the associations between performance on the task and adverse outcomes at follow-up, is in line with this account. Our approach to assessing uncertainty on the WNT is in contrast to previous studies using this task, which strictly focused on affective salience.9,27,28 We examined uncertainty because decision-making under conditions of uncertainty is suboptimal in CHR participants,35–37 and is associated with psychotic-like symptoms.38 From a computational psychiatry perspective, this alteration can be attributed to the suboptimal precision-weighting of beliefs in the context of new information during perception.39 Interestingly, we found that within the CHR sample, SDT measures of performance were associated with worse clinical outcomes. SDT describes the probabilistic processes of decision-making under conditions of uncertainty.14 We are the first to assess an association between SDT parameters on the WNT and clinical outcome in CHR participants. Our analysis revealed that across all CHR participants, sensitivity (dʹ) is reduced, and in CHR participants whose symptoms persisted, a bias towards responding “yes” (c) predicted non-remission. This extends previous work showing that psychosis patients have worse general performance and make more false alarms than other psychiatric populations28 and evidence of an association between psychotic experiences and response bias.13 Together, these results suggest that psychosis symptoms are associated with altered uncertainty processing.13 It is notable that this association did not survive when assessing only CHR participants who were not taking antipsychotic medication at baseline (supplementary table 3). This result could be explained by a decrease in statistical power, given that 23% of CHR participants were removed in this second analysis, either because they were taking antipsychotic medication at baseline or because data about their medication use was missing. Another interpretation is that the association between response bias and non-remission in the main analysis was caused by CHR participants who were taking antipsychotic medication. This is possible given that some studies have found that cognitive impairments in psychosis are influenced by antipsychotic use.40,41
Feeling uncertain about the valence of SIs was linked to subsequent clinical outcomes in CHR participants. This link to uncertainty is potentially in line with evidence that beliefs about volatility are associated with a hippocampal-cerebellar network on a perceptual conditioning task,42 and remission from the CHR state is associated with normalization in hippocampal perfusion,43 which hints at a neural correlate for our behavioral results. Indeed, verbal memory in CHR participants is associated with hippocampal function,44 and verbal recall performance is also associated with remission in CHR participants.22,45 Generally, CHR participants show altered uncertainty processing as measured by salience tasks39 which are associated with transition to psychosis.46
In CHR participants not taking antipsychotic medication at baseline, we replicated the association between hearing SIs with uncertain valence and non-remission, and we also observed an association between hearing SIs with uncertain valence and transition to psychosis. This latter result is in line with a previous study that reported that the perceived length of SIs in multispeaker babble was associated with transition to psychosis in CHR participants,23 but only in medication-free participants. Similarly, our result was observed only in CHR participants not taking antipsychotic medication at baseline, suggesting that antipsychotic use confounded the association between SIs and transition to psychosis. Given replication of our results, the propensity to make uncertain responses on a cognitive task could offer a putative marker of clinical outcome in early psychosis, particularly in participants not taking antipsychotic medication, as would likely be the case at initial presentation. Future studies should investigate the association with outcomes using a battery of computational tasks which can offer individualized measures of uncertainty weighting, such as expected volatility, belief-updating,38 and learning rate.47,48 Task bases indicators of this type are likely to be cheaper and more practical to administer compared to neuroimaging procedures that may also have the potential to predict outcomes.
We did not observe an association between SIs and functioning, in contrast with previous evidence linking with poorer functional outcomes in CHR participants with impaired performance on other cognitive tasks.22,49,50 This discrepancy could be because functional outcomes in the CHR participants in this study were associated with general cognitive functioning rather than probabilistic reasoning or uncertainty processing. Another explanation is that the rate of SIs on the WNT is low (only 13% of CHR participants reported hearing a SI in our task, similar to previous studies using this task27,28) which may have generated a floor effect in our data. Also, we did not observe an association between affectively salient SIs and outcome, despite previous findings that psychosis patients are more likely to hear affectively salient SIs.9,27,28 This hints that alterations in affective salience processing develop after changes in uncertainty processing associated with outcomes in CHR individuals.
Strengths of the study include its prospective design and a large sample size of CHR individuals who were mostly medication-naive. However, the correct hit rate on the WNT used was high (mean = 93%, SD = 15% across participants), suggesting that it may have been relatively easy for participants to detect true speech, and thus introduced a ceiling effect. We did not tailor detection thresholds for the task because, within a SDT analysis, it is assumed that all participants were exposed to the same stimuli. Although the hit rate was high, the false alarm rate (the variable of interest) was approximately the same as in other studies.51 Also, we only carried out a cross-sectional single assessment at baseline, and follow-up was only up to 2 years; it is possible that a more extensive longitudinal measure would provide a more sensitive insight into the association with outcome. Part of the CHR group were on antipsychotic medication. It should also be acknowledged that only 36% (N = 111) of the CHR participants had at least 1 outcome measure (remission vs non-remission, or functioning) at follow-up. Group comparisons showed that as well as being associated with CAARMS positive and negative scores, attrition was associated with a lower age, fewer years in education, and use of antipsychotic medication. We, therefore, repeated the analysis only assessing participants who were not using antipsychotic medication at baseline, and age and years in education were included as covariates in all analyses. It is also important to note that although we provided instructions that responding “not sure” on a trial indicates uncertainty about the valence of a SI, some participants may have misunderstood the task instructions, and their response “not sure” could indicate uncertainty as to whether speech was heard or not. Future studies should check that participants understand this instruction post-task. Furthermore, the WNT does not explicitly ask participants to employ top-down processing, rather relying on presentation of speech in some trials to induce an implicit expectation of speech. This type of expectation may differ from expectations induced explicitly, such as in tasks that show that hallucination-prone participants are more likely to hear SIs upon explicit instruction to employ auditory imagery.52,53 Finally, it is not clear whether susceptibility to SIs represents a novel marker for psychosis outcome, or whether it formalizes the link between hallucinatory experiences and psychosis risk which has already been observed in epidemiological studies in the general population.7,8
In conclusion, we show that in CHR participants, hearing SIs with uncertain valence was associated with non-remission from the CHR state and transition to psychosis. Uncertainty processing is altered in CHR participants, and our results suggest that this is also associated with future outcomes. Our findings relate to computational perspectives of psychosis which explain the disorder as an alteration in the precision-weighting of beliefs and new information. This could reflect a model in which early alterations in uncertainty processing are associated with prolonged subthreshold psychosis symptoms and a greater likelihood of developing psychosis or non-remission from the CHR state. Understanding these associations (and replication) could allow the incorporation of measures of uncertainty into predictive models to stratify individuals according to subsequent outcomes, and to tailor treatments to individuals.
Supplementary Material
Acknowledgment
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Contributor Information
Emily J Hird, Department of Psychosis Studies, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK.
Noriyuki Ohmuro, Department of Psychosis Studies, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK; Department of Psychiatry, Tohoku University Hospital, Sendai, Miyagi, Japan; Department of Psychiatry, Osaki Citizen Hospital, Osaki, Japan.
Paul Allen, Department of Psychosis Studies, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK; School of Psychology, Whitelands College, University of Roehampton, Holybourne Ave, London, SW15 4JD, UK.
Peter Moseley, Psychology Department, Northumbria University, College Lane, Newcastle-Upon-Tyne, NE1 8ST, UK.
Matthew J Kempton, Department of Psychosis Studies, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK.
Gemma Modinos, Department of Psychosis Studies, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK.
Gabriele Sachs, Department of Psychiatry and Psychotherapy, Medical University of Vienna, Vienna, Austria.
Mark van der Gaag, Faculty of Behavioural and Movement Sciences, Department of Clinical Psychology, VU University, van der Boechorststraat 1, 1081 BT Amsterdam, The Netherlands; EMGO Institute for Health and Care Research, VU University, van der Boechorststraat 1, 1081 BT Amsterdam, The Netherlands; Department of Psychosis Research, Parnassia Psychiatric Institute, Zoutkeetsingel 40, 2512 HN The Hague, The Netherlands.
Lieuwe de Haan, Department Early Psychosis, AMC, Academic Psychiatric Centre, Meibergdreef 5, 1105 AZ Amsterdam, The Netherlands; Arkin, Amsterdam, The Netherlands.
Ary Gadelha, LiNC - Lab Interdisciplinar Neurociências Clínicas, Depto Psiquiatria, Escola Paulista de Medicina, Universidade Federal de São Paulo – UNIFESP, São Paulo, Brazil.
Rodrigo Bressan, LiNC - Lab Interdisciplinar Neurociências Clínicas, Depto Psiquiatria, Escola Paulista de Medicina, Universidade Federal de São Paulo – UNIFESP, São Paulo, Brazil.
Neus Barrantes-Vidal, Departament de Psicologia Clínica i de la Salut (Universitat Autònoma de Barcelona), Fundació Sanitària Sant Pere Claver (Spain), Spanish Mental Health Research Network (CIBERSAM), Barcelona, Spain.
Stephan Ruhrmann, Department of Psychiatry and Psychotherapy, University of Cologne, Cologne, Germany.
Ana Catalan, Department of Psychosis Studies, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK; Psychiatry Department, Biocruces Bizkaia Health Research Institute, OSI Bilbao-Basurto, Facultad de Medicina y Odontología, University of the Basque Country UPV/EHU, Centro de Investigación en Red de Salud Mental (CIBERSAM), Instituto de Salud Carlos III, Plaza de Cruces 12, 48903 Barakaldo, Bizkaia, Spain.
Philip McGuire, Department of Psychosis Studies, Institute of Psychiatry, Psychology and Neuroscience, King’s College London, London, UK.
EU-GEI High Risk Study:
Philip McGuire, Lucia R Valmaggia, Matthew J Kempton, Maria Calem, Stefania Tognin, Gemma Modinos, Lieuwe de Haan, Mark van der Gaag, Eva Velthorst, Tamar C Kraan, Daniella S van Dam, Nadine Burger, Barnaby Nelson, Patrick McGorry, Günter Paul Amminger, Christos Pantelis, Athena Politis, Joanne Goodall, Anita Riecher-Rössler, Stefan Borgwardt, Charlotte Rapp, Sarah Ittig, Erich Studerus, Renata Smieskova, Rodrigo Bressan, Ary Gadelha, Elisa Brietzke, Graccielle Asevedo, Elson Asevedo, Andre Zugman, Neus Barrantes-Vidal, Tecelli Domínguez-Martínez, Pilar Torrecilla, Thomas R Kwapil, Manel Monsonet, Lídia Hinojosa, Mathilde Kazes, Claire Daban, Julie Bourgin, Olivier Gay, Célia Mam-Lam-Fook, Marie-Odile Krebs, Dorte Nordholm, Lasse Randers, Kristine Krakauer, Louise Glenthøj, Birte Glenthøj, Merete Nordentoft, Stephan Ruhrmann, Dominika Gebhard, Julia Arnhold, Joachim Klosterkötter, Gabriele Sachs, Iris Lasser, Bernadette Winklbaur, Philippe A Delespaul, Bart P Rutten, and Jim van Os1
Funding
The European Network of National Schizophrenia Networks Studying Gene Environment Interactions (EU-GEI) Project is funded by grant agreement HEALTH-F2- 2010-241909 (Project EU-GEI) from the European Community’s Seventh Framework Programme. Additional support was provided by a Medical Research Council Fellowship to M Kempton (grant MR/J008915/1), and by the Ministerio de Ciencia, Innovación e Universidades to N Barrantes-Vidal (project PSI2017-87512-C2-1-R).
EU-GEI High-Risk Study Group
Philip McGuire1, Lucia R. Valmaggia2, Matthew J. Kempton1, Maria Calem1, Stefania Tognin1, Gemma Modinos1, Lieuwe de Haan3,4, Mark van der Gaag5,6, Eva Velthorst3,7, Tamar C. Kraan3, Daniella S. van Dam3, Nadine Burger6, Barnaby Nelson8, Patrick McGorry8, Günter Paul Amminger8, Christos Pantelis8, Athena Politis8, Joanne Goodall8, Anita Riecher-Rössler9, Stefan Borgwardt9, Charlotte Rapp9, Sarah Ittig9, Erich Studerus9, Renata Smieskova9, Rodrigo Bressan10, Ary Gadelha10, Elisa Brietzke11, Graccielle Asevedo10, Elson Asevedo10, Andre Zugman10, Neus Barrantes-Vidal12, Tecelli Domínguez-Martínez13, Pilar Torrecilla14, Thomas R. Kwapil15, Manel Monsonet14, Lídia Hinojosa14, Mathilde Kazes16, Claire Daban16, Julie Bourgin16, Olivier Gay16, Célia Mam-Lam-Fook16, Marie-Odile Krebs16, Dorte Nordholm17, Lasse Randers17, Kristine Krakauer17, Louise Glenthøj17, Birte Glenthøj18, Merete Nordentoft17, Stephan Ruhrmann19, Dominika Gebhard19, Julia Arnhold20, Joachim Klosterkötter19, Gabriele Sachs21, Iris Lasser21, Bernadette Winklbaur21, Philippe A. Delespaul22,23, Bart P. Rutten22, and Jim van Os122.
Affiliations of Group Author
1. Department of Psychosis Studies, Institute of Psychiatry, Psychology & Neuroscience, King’s College London, De Crespigny Park, Denmark 458 Hill, London, SE5 8AF, UK.
2. Department of Psychology, Institute of Psychiatry, Psychology & Neuroscience, King’s College London, De Crespigny Park, Denmark Hill, 456, London, SE5 8AF, UK.
3. Department Early Psychosis, AMC, Academic Psychiatric Centre, Meibergdreef 5, 1105 AZ Amsterdam, The Netherlands.
4. Arkin, Amsterdam, The Netherlands
5. Faculty of Behavioural and Movement Sciences, Department of Clinical Psychology and EMGO Institute for Health and Care Research, VU University, van der Boechorststraat 1, 1081 BT Amsterdam, The Netherlands.
6. Department of Psychosis Research, Parnassia Psychiatric Institute, Zoutkeetsingel 40, 2512 HN The Hague, The Netherlands.
7. Department of Psychiatry, Icahn School of Medicine at Mount Sinai, 1425 Madison Ave, New York, NY 10029, USA.
8. Centre for Youth Mental Health, University of Melbourne, 35 Poplar Road (Locked Bag 10), Parkville, Victoria 485 3052, Australia.
9. University Psychiatric Hospital, Wilhelm Klein-Strasse 27, CH-4002 Basel, Switzerland.
10. LiNC - Lab Interdisciplinar Neurociências Clínicas, Depto Psiquiatria, Escola Paulista de Medicina, Universidade Federal de São Paulo – UNIFESP, São Paulo, Brazil.
11. Depto Psiquiatria, Escola Paulista de Medicina, Universidade Federal de São Paulo – UNIFESP, São Paulo, Brazil.
12. Departament de Psicologia Clínica i de la Salut (Universitat Autònoma de Barcelona), Fundació Sanitària Sant Pere Claver (Spain), Spanish Mental Health Research Network (CIBERSAM), Barcelona, Spain.
13. CONACYT-Dirección de Investigaciones Epidemiológicas y Psicosociales, Instituto Nacional de Psiquiatría Ramón de la Fuente Muñiz (México), Mexico City, Mexico.
14. Departament de Psicologia Clínica i de la Salut (Universitat Autònoma de Barcelona), Barcelona, Spain.
15. Department of Psychology, University of Illinois at Urbana-Champaign, IL, USA.
16. University Paris Descartes, Hôpital Sainte-Anne, C’JAAD, Service HospitaloUniversitaire, Inserm U894, Institut de Psychiatrie (CNRS 3557) Paris, France
17. Mental Health Center Copenhagen and Center for Clinical Intervention and Neuropsychiatric Schizophrenia Research, CINS, Mental Health Center Glostrup, Mental Health Services in the Capital Region of Copenhagen, University of Copenhagen, Copenhagen, Denmark.
18. Centre for Neuropsychiatric Schizophrenia Research (CNSR) & Centre for Clinical Intervention and Neuropsychiatric Schizophrenia Research (CINS), Mental Health Centre Glostrup, University of Copenhagen, Glostrup, Denmark; EU-GEI WP5 Data agreement form v25.6.2018 6.
19. Department of Psychiatry and Psychotherapy, University of Cologne, Cologne, Germany.
20. Psyberlin, Berlin, Germany.
21. Department of Psychiatry and Psychotherapy, Medical University of Vienna, Vienna, Austria.
22. Department of Psychiatry and Neuropsychology, School for Mental Health and Neuroscience, Maastricht University Medical Centre, PO Box 616, 6200 MD 464 Maastricht, The Netherlands
23. Mondriaan Mental Health Trust, PO Box 4436 CX Heerlen, The Netherlands
References
- 1. de Pablo GS, Soardo L, Cabras A, et al. Clinical outcomes in individuals at clinical high risk of psychosis who do not transition to psychosis: a meta-analysis. Epidemiol Psychiatr Sci. 2022;31:19–31. doi: 10.1017/S2045796021000639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Carrión RE, McLaughlin D, Goldberg TE, et al. Prediction of functional outcome in individuals at clinical high risk for psychosis. JAMA Psychiatry. 2013;70(11):1133–1142. doi: 10.1001/jamapsychiatry.2013.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. de Wit S, Wierenga LM, Oranje B, et al. Brain development in adolescents at ultra-high risk for psychosis: longitudinal changes related to resilience. Neuroimage Clin. 2016;12:542–549. doi: 10.1016/j.nicl.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yung AR, Yuen HP, McGorry PD, et al. Mapping the onset of psychosis: the comprehensive assessment of at-risk mental states. Aust N Z J Psychiatry. 2005;39(11–12):964–971. doi: 10.1111/j.1440-1614.2005.01714.x. [DOI] [PubMed] [Google Scholar]
- 5. Häfner H. Onset and early course as determinants of the further course of schizophrenia. Acta Psychiatr Scand Suppl. 2000;102(407):44–48. doi: 10.1034/j.1600-0447.2000.00008.x. [DOI] [PubMed] [Google Scholar]
- 6. Fusar-Poli P, Bonoldi I, Yung AR, et al. Predicting psychosis: meta-analysis of transition outcomes in individuals at high clinical risk. Arch Gen Psychiatry. 2012;69(3):220–229. doi: 10.1001/archgenpsychiatry.2011.1472. [DOI] [PubMed] [Google Scholar]
- 7. Hanssen M, Bak M, Bijl R, Vollebergh W, van Os J.. The incidence and outcome of subclinical psychotic experiences in the general population. Br J Clin Psychol. 2005;44(Pt 2):181–191. doi: 10.1348/014466505X29611. [DOI] [PubMed] [Google Scholar]
- 8. Krabbendam L, Myin-Germeys I, Bak M, van Os J.. Explaining transitions over the hypothesized psychosis continuum. Aust N Z J Psychiatry. 2005;39(3):180–186. doi: 10.1080/j.1440-1614.2005.01541.x. [DOI] [PubMed] [Google Scholar]
- 9. Galdos M, Simons C, Fernandez-Rivas A, et al. Affectively salient meaning in random noise: a task sensitive to psychosis liability. Schizophr Bull. 2011;37(6):1179–1186. doi: 10.1093/schbul/sbq029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Moseley P, Alderson-Day B, Common S, et al. Continuities and discontinuities in the cognitive mechanisms associated with clinical and nonclinical auditory verbal hallucinations. Clin Psychol Sci. 2022;10(4):21677026211059800. doi: 10.1177/21677026211059802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Artaza MG, Catalan A, Angosto V, et al. Can an experimental white noise task assess psychosis vulnerability in adult healthy controls? PLoS One. 2018;13(2):1–8. doi: 10.1371/journal.pone.0192373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brookwell ML, Bentall RP, Varese F.. Externalizing biases and hallucinations in source-monitoring, self-monitoring and signal detection studies: a meta-analytic review. Psychol Med. 2013;43(12):2465–2475. doi: 10.1017/S0033291712002760. [DOI] [PubMed] [Google Scholar]
- 13. Rossi R, Zammit S, Button KS, Munafò MR, Lewis G, David AS.. Psychotic experiences and working memory: a population-based study using signal-detection analysis. PLoS One. 2016;11(4):e0153148. doi: 10.1371/journal.pone.0153148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wickens TD. Elementary Signal Detection Theory. Oxford: Oxford University Press; 2001. [Google Scholar]
- 15. Barkus E, Smallman R, Royle N, Barkus C, Lewis S, Rushe T.. Auditory false perceptions are mediated by psychosis risk factors. Cognit Neuropsychiatry. 2011;16(4):289–302. doi: 10.1080/13546805.2010.530472. [DOI] [PubMed] [Google Scholar]
- 16. Varese F, Barkus E, Bentall RP.. Dissociation mediates the relationship between childhood trauma and hallucination-proneness. Psychol Med. 2012;42(5):1025–1036. doi: 10.1017/S0033291711001826. [DOI] [PubMed] [Google Scholar]
- 17. Moseley P, Aleman A, Allen P, et al. Correlates of hallucinatory experiences in the general population: an international multisite replication study. Psychol Sci. 2021;32(7):1024–1037. doi: 10.1177/0956797620985832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Catalan A, Salazar de Pablo G, Aymerich C, et al. Neurocognitive functioning in individuals at clinical high risk for psychosis: a systematic review and meta-analysis. JAMA Psychiatry. 2021;78(8):859–867. doi: 10.1001/jamapsychiatry.2021.1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hauser M, Zhang JP, Sheridan EM, et al. Neuropsychological test performance to enhance identification of subjects at clinical high risk for psychosis and to be most promising for predictive algorithms for conversion to psychosis: a meta-analysis. J Clin Psychiatry. 2017;78(1):e2812639–e2812e40. doi: 10.4088/JCP.15r10197. [DOI] [PubMed] [Google Scholar]
- 20. Bora E, Lin A, Wood SJ, Yung AR, McGorry PD, Pantelis C.. Cognitive deficits in youth with familial and clinical high risk to psychosis: a systematic review and meta-analysis. Acta Psychiatr Scand. 2014;130(1):1–15. doi: 10.1111/acps.12261. [DOI] [PubMed] [Google Scholar]
- 21. Fusar-Poli P, Deste G, Smieskova R, et al. Cognitive functioning in prodromal psychosis: a meta-analysis. Arch Gen Psychiatry. 2012;69(6):562–571. doi: 10.1001/archgenpsychiatry.2011.1592. [DOI] [PubMed] [Google Scholar]
- 22. Hedges EP, Dickson H, Tognin S, et al. Verbal memory performance predicts remission and functional outcome in people at clinical high-risk for psychosis. Schizophr Res Cogn. 2021;28:100222. doi: 10.1016/j.scog.2021.100222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hoffman RE, Woods SW, Hawkins KA, et al. Extracting spurious messages from noise and risk of schizophrenia-spectrum disorders in a prodromal population. Br J Psychiatry. 2007;191(4):355–356. [DOI] [PubMed] [Google Scholar]
- 24. European Network of National Networks studying Gene-Environment Interactions in Schizophrenia (EU-GEI); van Os J, Rutten BP, Myin-Germeys I, et al. Identifying gene-environment interactions in schizophrenia: contemporary challenges for integrated, large-scale investigations. Schizophr Bull. 2014;40(4):729–736. Accessed April 15, 2022. https://pubmed.ncbi.nlm.nih.gov/24860087/. [DOI] [PMC free article] [PubMed]
- 25. American Psychiatric Association. Multiaxial Assessment.DSM-IV-TR: Diagnostic and Statistical Manual of Mental Disorders. Washington, DC: American Psychiatric Association; 2002. [Google Scholar]
- 26. Pedersen G, Karterud S.. The symptom and function dimensions of the Global Assessment of Functioning (GAF) scale. Compr Psychiatry. 2012;53(3):292–298. doi: 10.1016/j.comppsych.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 27. Catalan A, Simons CJP, Bustamante S, et al. Novel evidence that attributing affectively salient signal to random noise is associated with psychosis. PLoS One. 2014;9(7):e102520. doi: 10.1371/journal.pone.0102520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Catalan A, de Artaza MG, Fernández-Rivas A, et al. Affectively salient signal to random noise might be used to identify psychosis vulnerability in severe mental disorders. Eur Psychiatry. 2018;49:37–42. doi: 10.1016/j.eurpsy.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 29. Blyler CR, Gold JM, Iannone VN, Buchanan RW.. Short form of the WAIS-III for use with patients with schizophrenia. Schizophr Res. 2000;46(2):209–215. doi: 10.1016/S0920-9964(00)00017-7. [DOI] [PubMed] [Google Scholar]
- 30. Velthorst E, Levine SZ, Henquet C, et al. To cut a short test even shorter: reliability and validity of a brief assessment of intellectual ability in Schizophrenia—a control-case family study. Cognit Neuropsychiatry. 2013;18(6):574–593. doi: 10.1080/13546805.2012.731390. [DOI] [PubMed] [Google Scholar]
- 31. Stanislaw H, Todorov N.. Calculation of signal detection theory measures. Behav Res Methods Instrum Comput. 1999;31(1):137–149. doi: 10.3758/BF03207704. [DOI] [PubMed] [Google Scholar]
- 32. Friston K. Does predictive coding have a future? Nat Neurosci. 2018;21(8):1019–1021. doi: 10.1038/s41593-018-0200-7. [DOI] [PubMed] [Google Scholar]
- 33. Sterzer P, Adams RA, Fletcher P, et al. The predictive coding account of psychosis. Biol Psychiatry. 2018;84(9):634–643. doi: 10.1016/j.biopsych.2018.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Corlett PR, Horga G, Fletcher PC, Alderson-Day B, Schmack K, Powers AR.. Hallucinations and strong priors. Trends Cogn Sci. 2019;23(2):114–127. doi: 10.1016/j.tics.2018.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Johns LC, Allen P, Valli I, et al. Impaired verbal self-monitoring in individuals at high risk of psychosis. Psychol Med. 2010;40(9):1433–1442. doi: 10.1017/S0033291709991991. [DOI] [PubMed] [Google Scholar]
- 36. Roiser JP, Howes OD, Chaddock CA, Joyce EM, McGuire P.. Neural and behavioral correlates of aberrant salience in individuals at risk for psychosis. Schizophr Bull. 2013;39(6):1328–1336. doi: 10.1093/schbul/sbs147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Broome MR, Johns LC, Valli I, et al. Delusion formation and reasoning biases in those at clinical high risk for psychosis. Br J Psychiatry. 2007;191(S51):s38–s42. doi: 10.1192/bjp.191.51.s38. [DOI] [PubMed] [Google Scholar]
- 38. Reed EJ, Uddenberg S, Suthaharan P, et al. Paranoia as a deficit in non-social belief updating. eLife. 2020;9:1–55. doi: 10.7554/eLife.56345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Howes OD, Hird EJ, Adams RA, Corlett PR, McGuire P.. Aberrant salience, information processing, and dopaminergic signaling in people at clinical high risk for psychosis. Biol Psychiatry. 2020;88(4):304–314. doi: 10.1016/j.biopsych.2020.03.012. [DOI] [PubMed] [Google Scholar]
- 40. Élie D, Poirier M, Chianetta J, Durand M, Grégoire C, Grignon S.. Cognitive effects of antipsychotic dosage and polypharmacy: a study with the BACS in patients with schizophrenia and schizoaffective disorder. J Psychopharmacol. 2010;24(7):1037–1044. doi: 10.1177/0269881108100777. [DOI] [PubMed] [Google Scholar]
- 41. Albert N, Randers L, Allott K, et al. Cognitive functioning following discontinuation of antipsychotic medication. A naturalistic sub-group analysis from the OPUS II trial. Psychol Med. 2019;49(7):1138–1147. doi: 10.1017/S0033291718001836. [DOI] [PubMed] [Google Scholar]
- 42. Powers AR, Mathys C, Corlett PR.. Pavlovian conditioning–induced hallucinations result from overweighting of perceptual priors. Science. 2017;357(6351):596–600. doi: 10.1126/science.aan3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Allen P, Chaddock CA, Egerton A, et al. Resting hyperperfusion of the hippocampus, midbrain, and basal ganglia in people at high risk for psychosis. Am J Psychiatry. 2016;173(4):392–399. doi: 10.1176/appi.ajp.2015.15040485. [DOI] [PubMed] [Google Scholar]
- 44. Allen P, Seal ML, Valli I, et al. Altered prefrontal and hippocampal function during verbal encoding and recognition in people with prodromal symptoms of psychosis. Schizophr Bull. 2011;37(4):746–756. doi: 10.1093/schbul/sbp113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Simon AE, Grädel M, Cattapan-Ludewig K, et al. Cognitive functioning in at-risk mental states for psychosis and 2-year clinical outcome. Schizophr Res. 2012;142(1):108–115. doi: 10.1016/j.schres.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 46. Catalan A, Tognin S, Kempton MJ, et al. Relationship between jumping to conclusions and clinical outcomes in people at clinical high-risk for psychosis. Psychol Med. 2022;52(8):1569–1577. doi: 10.1017/S0033291720003396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ermakova AO, Knolle F, Justicia A, et al. Abnormal reward prediction-error signalling in antipsychotic naive individuals with first-episode psychosis or clinical risk for psychosis. Neuropsychopharmacology. 2018;43(8):1691–1699. doi: 10.1038/s41386-018-0056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Murray GK, Cheng F, Clark L, et al. Reinforcement and reversal learning in first-episode psychosis. Schizophr Bull. 2008;34(5):848–855. doi: 10.1093/schbul/sbn078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Carrión RE, Goldberg TE, McLaughlin D, Auther AM, Correll CU, Cornblatt BA.. Impact of neurocognition on social and role functioning in individuals at clinical high risk for psychosis. Am J Psychiatry. 2011;168(8):806–813. doi: 10.1176/appi.ajp.2011.10081209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fujioka M, Kirihara K, Koshiyama D, et al. Mismatch negativity predicts remission and neurocognitive function in individuals at ultra-high risk for psychosis. Front Psychiatry. 2020;11:770. doi: 10.3389/fpsyt.2020.00770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Catalan A, Gonzalez de Artaza M, Fernández-Rivas A, et al. Affectively salient signal to random noise might be used to identify psychosis vulnerability in severe mental disorders. Eur Psychiatry. 2018;49:37–42. doi: 10.1016/j.eurpsy.2017.12.008. [DOI] [PubMed] [Google Scholar]
- 52. Moseley P, Smailes D, Ellison A, Fernyhough C.. The effect of auditory verbal imagery on signal detection in hallucination-prone individuals. Cognition. 2016;146:206–216. doi: 10.1016/j.cognition.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vercammen A, Aleman A.. Semantic expectations can induce false perceptions in hallucination-prone individuals. Schizophr Bull. 2010;36(1):151–156. doi: 10.1093/schbul/sbn063. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.