Abstract
A long-established strategy for transcription regulation is the tethering of transcription factors to cellular membranes. In contrast, the principal effectors of Hedgehog signaling, the Gli transcription factors, are regulated by microtubules in the primary cilium and the cytoplasm. How Gli is tethered to microtubules remains unclear. We uncover DNA mimicry by the ciliary kinesin Kif7 as a mechanism for the recruitment of Gli to microtubules, wherein the coiled-coil dimerization domain of Kif7, characterized by striking shape, size and charge similarity to DNA, forms a complex with the DNA-binding zinc fingers in Gli, thus revealing a mode of tethering a DNA-binding protein to the cytoskeleton. Gli increases the Kif7-microtubule affinity and consequently modulates the localization of both proteins to microtubules and the cilium tip. Thus, the kinesin-microtubule system is not a passive Gli tether but a regulatable platform tuned by the kinesin-transcription factor interaction. We re-tooled the unique coiled-coil-based Gli-Kif7 interaction for inhibiting the nuclear and cilium localization of Gli. This strategy can be potentially exploited for downregulating erroneously activated Gli in human cancers.
The tethering of transcription factors to membranes of cytoplasmic organelles or the plasma membrane, and their signal-dependent trafficking into the nucleus is a well-established mechanism for the precise regulation of gene repression and activation1. In recent years, the microtubule cytoskeleton has emerged as a scaffold for tethering transcription factors away from the nucleus for pathway repression2-4. However, the molecular and structural mechanisms by which the cytoskeleton regulates transcription factors remains poorly understood.
An essential developmental pathway with a strict requirement for the microtubule cytoskeleton is Hedgehog (Hh) signaling5-8. In vertebrates, this pathway has a unique dependence on the primary cilium9, a microtubule-based organelle. Central to regulation of the vertebrate Hh signaling is the accumulation of Gli proteins (Ci; Drosophila Melanogaster homolog) in the primary cilium. The Gli proteins consist of three isoforms: Gli1, a transcriptional activator10, and Gli2 and Gli3, dual-transcription factors characterized by an N-terminus repressor domain and a C-terminus trans-activator domain that are separated by DNA-binding zinc-finger domain and sites for proteolytic cleavage11,12. During Hh pathway repression, Gli2 and Gli3 are proteolytically processed into the shorter repressor form at the base of the cilium, with Gli3 acting as the major repressor13-15. Activation of the pathway results in increased accumulation of the full-length activator forms of Gli proteins at the distal cilium tip, with Gli2 acting as the major activator, which promotes the expression of Gli116-19. In vertebrates, defects in cilium assembly and architecture result in incorrect processing of the Gli proteins20,21. However, the mechanism of cilium-dependent Gli regulation, and the role of the microtubule cytoskeleton remain poorly understood.
The non-motile ciliary kinesin, Kif7 (ortholog of the Drosophila melanogaster Costal-2 kinesin-like protein and homolog of the motile ciliary kinesin Kif27), is a core Hh pathway component that provides the molecular link between the microtubule cytoskeleton and Gli regulation22-25. Loss of function mutations of Kif7 in zebrafish and mice embryos show defects in Hh-dependent developmental patterning22-24,26, and clinical mutations in Kif7 have been associated with severe ciliopathies27,28. When the Hh pathway is off, Kif7 is largely present at the base of the cilium, where it is thought to promote the formation of the repressor form of Gli. Upon pathway activation, both Kif7 and Gli accumulate at the tip of the primary cilium, a step required for Gli activation25. The precise mechanism for the localization of Kif7 to the base of the cilium is not known and multiple mechanisms have been suggested for its trafficking and cilium tip localization25,29-33. In the absence of Kif7, Gli can enter the cilium but is not correctly concentrated at its distal tip30. These observations together with the reported co-precipitation of Gli proteins with Kif7 in cell and embryo lysates23 have led to the proposal that microtubule-bound Kif7 establishes a ‘cilium-tip compartment’, a passive platform at the distal cilium tip, to which Gli is recruited25,30. Although current models assume a direct binding between Gli and Kif7, this has not been conclusively established and the molecular basis of their interaction remains elusive. Furthermore, it is unknown whether Kif7 binding is sufficient to tether the transcription factor to microtubules, and how the Kif7-Gli interaction contributes to the organization of the cilium tip compartment. The lack of mechanistic insights into these questions has precluded a deeper understanding of the role of the microtubule cytoskeleton and the primary cilium in Hh signaling. Here we report new structural and biochemical design principles by which the cytoskeleton can act as a cytoplasmic scaffold for transcription factor regulation.
Results
DNA structural mimicry underlies Gli-Kif7 interaction
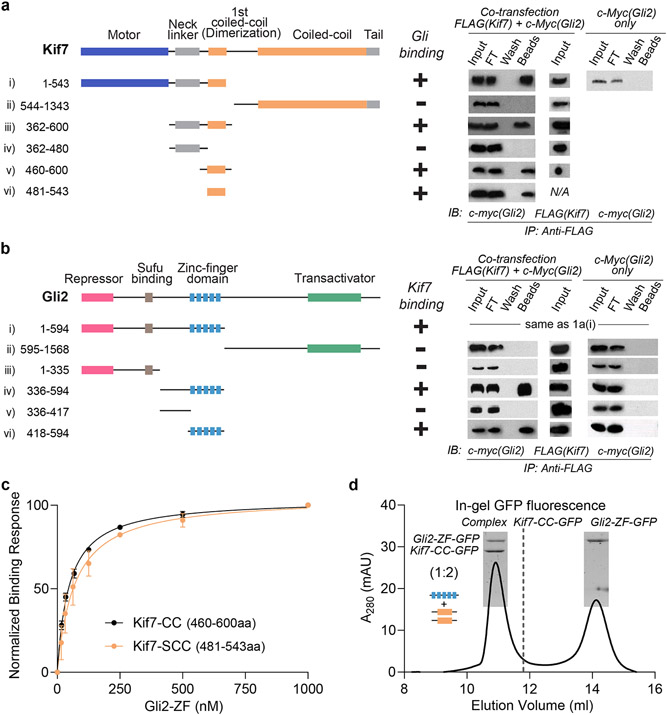
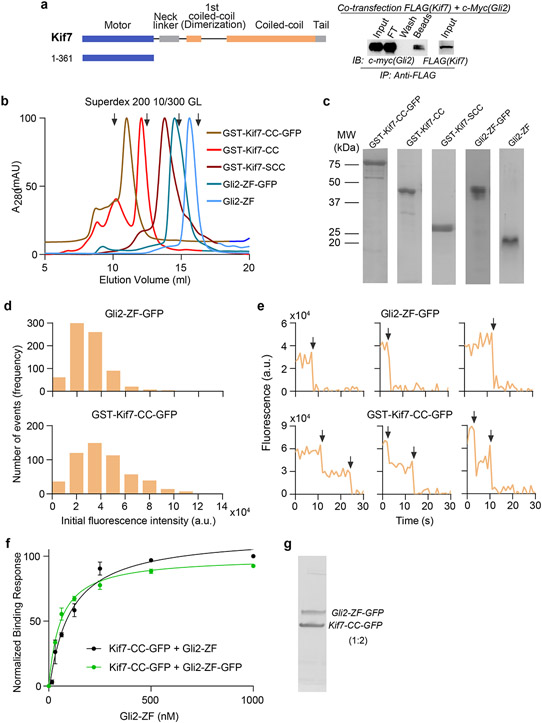
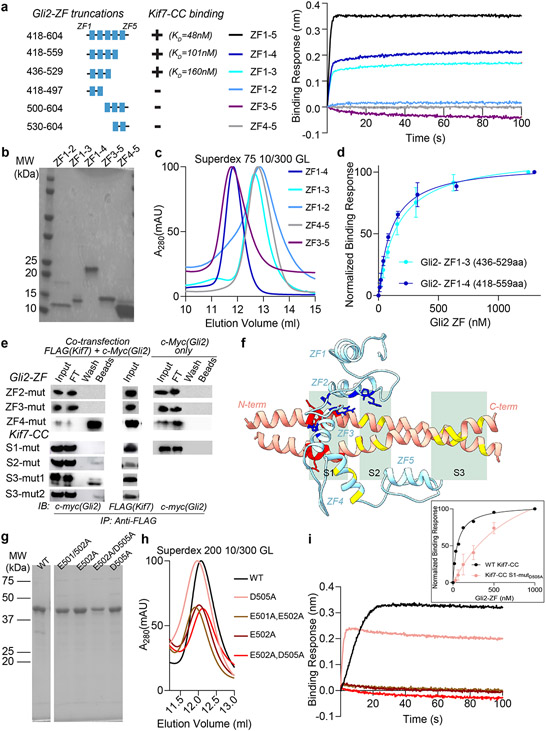
Gli proteins were shown to co-immunoprecipitate with Kif7 in pull-down assays from mouse embryo lysates23. However, biochemical characterization of Gli-Kif7 complexes has been challenging due to high proteolytic sensitivity and low solubility of these large (>150 kDa) multi-domain proteins. To overcome this technical challenge, we used a pull-down assay to map the interaction domains between Gli and Kif7. We observed that the DNA-binding zinc finger domain of Gli2 (418-594aa; Gli2-ZF) bound to the first coiled-coil dimerization domain of Kif7 (460-600aa; Kif7-CC) (Fig. 1a,b). In addition, a weak signal corresponding to interaction between monomeric Kif7 motor domain (1-361aa) and Gli2-ZF was observed (Extended Fig 1a).
Figure 1 ∣. DNA-binding domain of Gli2 directly binds the coiled-coil dimerization domain of Kif7.
a, Kif7 domains that interact with Gli2. Pull-down of c-Myc-Gli2(1-594aa) and FLAG-Kif7 constructs (i to vi) after co-transfection in Expi293F cells. (left) Domain architecture of full length Kif7 and deletion constructs used in this experiment. (right) Immunoprecipitation (IP) using anti-FLAG magnetic beads. Input (cell lysate), FT (flow through), wash and beads samples were immunoblotted (IB) with anti-c-myc antibody to detect Gli2. Transfection with c-Myc-Gli2 (1-594aa) alone was included as a negative control. b, Gli2 domains that interact with Kif7. Pull-down of c-Myc-Gli2 constructs (i to vi) with different FLAG-Kif7 constructs after co-transfection in Expi293F cells. Kif7(1-543aa) was used for i) & ii), Kif7(362-600aa) was used for iii) & iv) and Kif7(460-600aa) was used for v) & vi). (left) Domain architecture of full length Gli2 and deletion constructs used in this experiment. (right) Immunoprecipitation (IP) using anti-FLAG magnetic beads. Input (cell lysate), FT (flow through), wash and beads samples were immunoblotted (IB) with anti-c-myc antibody to detect Gli2. Transfection with each of the different c-Myc-Gli2 constructs alone were included as negative controls. All blots in a, and b, are representative images of a minimum of three repeats, where the +/− sign represents presence/absence of binding. c, Bio-Layer Interferometry (BLI) assay to quantitatively examine the binding affinity of Kif7 coiled-coil domain constructs; GST-Kif7-CC (460-600aa; black) & GST-Kif7-SCC (481-543aa; orange), to Gli2-ZF. Data represent mean +/− SD from three independent repeats. The plots of binding response versus Gli2-ZF concentration were fit to a Hill equation to determine equilibrium dissociation constants (Kd). For Kif7-CC (460-600aa): Kd = 48 ± 5nM, for Kif7-SCC (481-543aa): Kd = 69 ± 20nM. d, Chromatograms from size exclusion chromatography of a mixture of 125μM Kif7-CC-GFP and 750μM Gli2-ZF-GFP (Superdex 200 10/300 GL). In-gel GFP fluorescence analysis from SDS-PAGE of the complex (inset) used to determine stoichiometry indicated in parenthesis. Gel image is representative of a minimum of three repeats.
We first characterized the interaction between Kif7-CC and Gli2-ZF. Bio-layer interferometry (BLI)-based binding assays with purified recombinant proteins (Extended Data Fig. 1b,c) showed that the Kif7-CC:Gli2-ZF complex has a Kd of 48 ± 5 nM (Fig. 1c). We further narrowed the interaction to a 63 amino acid coiled-coil segment of Kif7-CC (481-543aa; Kif7-SCC; Kd of 69 ± 20 nM) (Fig. 1c). These data show that Kif7 uses its coiled-coil dimerization domain, and not the canonical C-terminal ‘cargo binding domain’ in kinesins, to bind Gli2 with high affinity, and the domain of the Gli2 transcription factor that mediates its interaction with DNA in the nucleus is repurposed to bind a kinesin in the cytoplasm.
How does the DNA binding domain of Gli bind the coiled-coil dimerization domain of Kif7? Single molecule fluorescence intensity and photobleaching analysis of the GFP-tagged proteins indicate that the GST-Kif7-CC is an obligate dimer and Gli2-ZF is a monomer (Extended Data Fig. 1d,e). We determined that the stoichiometry of the Kif7-CC-GFP:Gli2-ZF-GFP complex was 2:1; using in-gel GFP fluorescence and quantitative Western blot following size-exclusion chromatography (Fig. 1d and Extended Data Fig. 1f,g; see Methods). Thus, one molecule of monomeric Gli2-ZF binds to one Kif7-CC dimer.
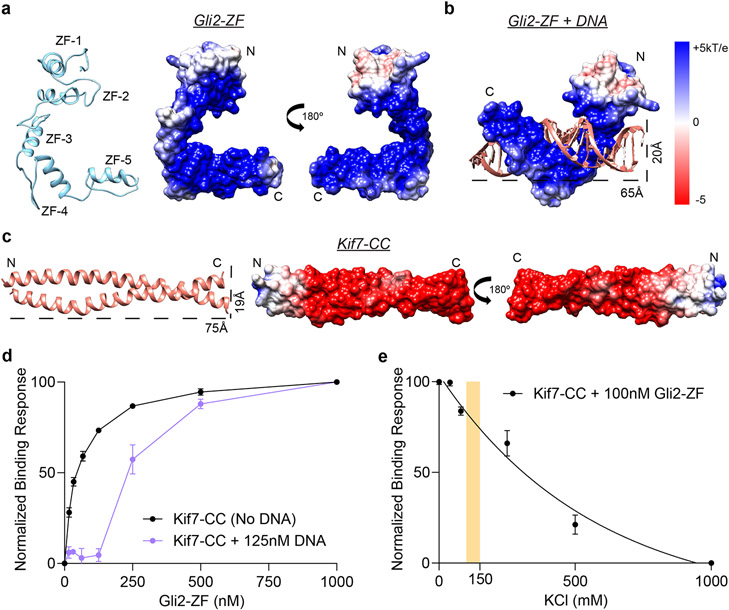
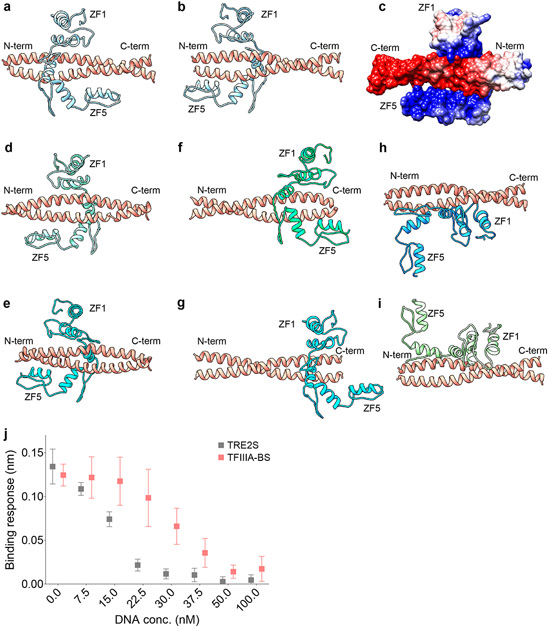
To gain insight into the structural basis of the Kif7-SCC:Gli2-ZF complex formation, we performed comparative homology modeling (Fig. 2a-c and Extended Data Fig. 2). The structure of Gli2-ZF domain was modelled using the X-ray structure of the zinc-finger domain of Gli1 isoform as a template (PDB: 2GLI, >95% sequence similarity)34 (Fig. 2a). The Kif7 coiled-coil domain was modeled as a homodimer with high confidence using X-ray structures of other coiled-coil domains as templates (Fig. 2c). The complex between Gli and DNA is characterized by the positively charged zinc-finger domain of Gli wrapping around the major groove of the rod-like DNA double-helix (Fig. 2b). Extensive electrostatic contacts exist between the negatively charged phosphate backbone of DNA and the second and third zinc-fingers in Gli34. As expected, the structural model of Gli2-ZF is very similar to Gli1-ZF (Fig. 2a,b). Analysis of the structural model of Kif7-SCC revealed two striking features. First, the overall size and shape of Kif7-SCC is similar to dsDNA. Second, analysis of the electrostatic surface potential of Kif7-SCC (pI = 4.48) suggests a negatively charged rod-shaped surface, similar to the DNA backbone (Fig. 2c). Structural models obtained by docking the Kif7-CC and Gli2-ZF as rigid bodies suggest that the positively charged interface of Gli2 zinc-fingers contact the negatively charged Kif7-SCC rod-like domain with some models closely resembling the Gli-DNA complex (Extended Data Fig. 2). The similarities in electrostatic surface charge complementarity, molecular dimensions, and mode of binding of Gli2-ZF with DNA and Kif7-SCC suggests that Kif7 may be a DNA molecular mimic for Gli binding in the cytoplasm.
Figure 2 ∣. The coiled-coil dimerization domain of Kif7 is a DNA structural mimic for Gli binding.
a, Structural model of Gli2-ZF (ribbon diagram; SWISS MODEL server: GMQE = 0.98, QMean = −5.14) and overall electrostatic surface representation of the model. b, Structural model of Gli2-ZF bound to DNA (based on Gli1-ZF-DNA crystal structure; PDB: 2GLI) included for comparison. c, Structural model of Kif7-CC (ribbon diagram; SWISS MODEL server: GMQE = 0.55, QMean = 1.84) and overall electrostatic surface representation of the model. Dotted lines represent molecular dimensions of Kif7-CC and DNA. d, Binding of Gli2-ZF and Kif7-CC in the absence (black) and presence of 125nM sequence-specific Gli2 target dsDNA (purple). Normalized binding response from the BLI measurements is plotted against Gli2-ZF concentration. Data represent mean +/− SD from three independent repeats. e, Effect of increasing ionic strength (KCl concentration) on the Kif7-CC and Gli2-ZF binding. Normalized binding response was measured at 100 nM Gli2-ZF using the BLI assay. Data represent mean +/− SD from three independent repeats. Yellow bar marks physiological ionic strength (~125-150mM KCl).
To test the structural model for the Kif7-CC:Gli2-ZF interaction we first examined whether Kif7-CC and DNA compete for the same binding site on Gli2. We measured the binding between Gli2-ZF and Kif7-CC in the presence of a Gli-specific target dsDNA (TRE-2S)35. In the presence of 125nM TRE-2S DNA, binding between Kif7-CC and Gli2-ZF could be detected only when Gli2-ZF concentration was above 125nM (Fig. 2d). This suggests that DNA-bound Gli2-ZF cannot bind Kif7-CC and that these interactions are mutually exclusive. In complementary experiments we examined the reduction in Kif7-CC and Gli2-ZF interaction with increasing concentrations of Gli target and non-target dsDNA. TFIIIA binding sequence was used as the Gli non-target sequence based on prior literature36. As expected, compared to TRE-2S, a greater concentration of the non-target DNA TFIIIA-BS is required to inhibit the Kif7-CC:Gli2-ZF interaction to similar extent (Extended Data Fig. 2j). Next, we conducted the BLI binding assay in buffers with increasing ionic strength and observed a systematic reduction in the measured binding response from Kif7-CC and Gli2-ZF binding, confirming the importance of electrostatic interactions in this complex (Fig. 2e).
Finally, we performed site-directed mutagenesis experiments to further confirm the structural model, and map the residues of Gli2-ZF and Kif7-CC required for complex formation. BLI binding assay with Gli2-ZF truncations showed that zinc-fingers 2 and 3 are necessary for Kif7-CC binding (Extended Data Fig. 3a-d). This is similar to the Gli-DNA interaction where ZF2 and ZF3 are critical for making contacts with the phosphate backbone of DNA34. Mutation of specific charged residues in Gli2 ZF-2 (H493A/R496A) and ZF-3 (H503A/R516A/K521A) abolished binding to Kif7-CC whereas those in ZF-4 (R550A/K552A/R556A) did not affect binding (Extended Data Fig. 3e,f). Interestingly, the residues H493 (ZF2), R516 (ZF3) and K512 (ZF3) also engage in phosphate backbone contacts with DNA34, suggesting that the same subset of amino acids interact with Kif7-CC. Analysis of the interaction surface on Kif7-CC revealed three patches of negatively charged amino acid residues (labeled as S1, S2 and S3 in Extended Data Fig. 3f) that are potential Gli2-ZF binding residues. Systematic mutation of residues in these patches revealed that while Kif7-CC S1 (E500A/E501A/E502A/D505A) mutant abolished binding to Gli2-ZF, the S2 (E511A/E515A) and S3 (E526A/E529A & E530A/R535A) mutants did not (Extended Data Fig. 3e,f). To further validate the interaction, we expressed and purified four recombinant S1 mutants (D505A, E501A/E502A, E502A, E502A/D505A). All four mutants elute similar to wild-type protein in size exclusion chromatography, indicating that they are properly folded (Extended Data Fig. 3g,h). Three of these mutants (E501A/E502A, E502A, E502A/D505A) showed no binding to Gli2-ZF while the fourth one (D505A), shows diminished binding (Extended Data Fig. 3i).
Taken together, the results from DNA competition assays, ionic strength-dependence and the mutagenesis studies support a DNA-mimicry-based mechanism, where the negatively-charged rod-like coiled-coil domain of Kif7 forms the Gli2 interaction site, with zinc-fingers 2 and 3 of Gli2 making key contacts, as seen previously in binding of Gli to DNA34.
A second Gli binding site on the Kif7 motor domain
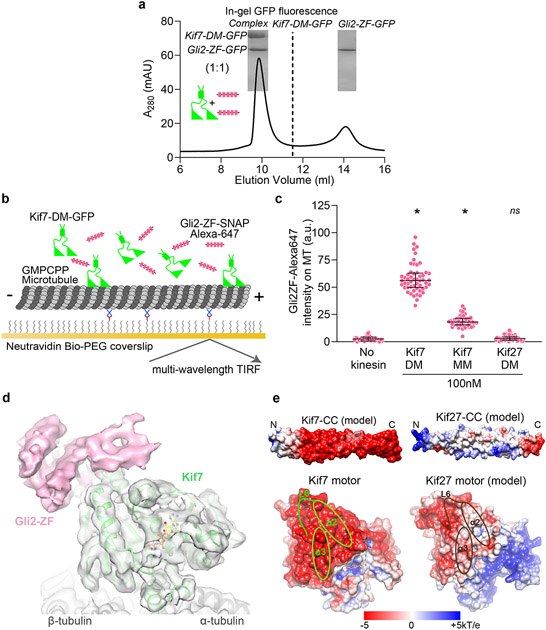
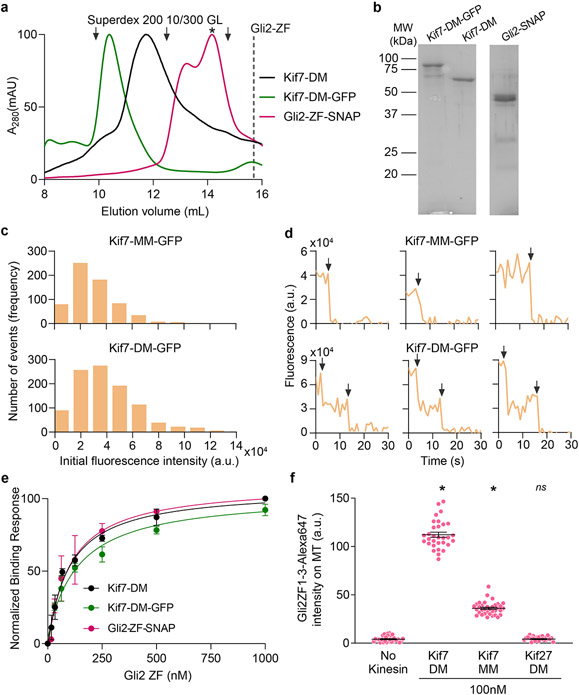
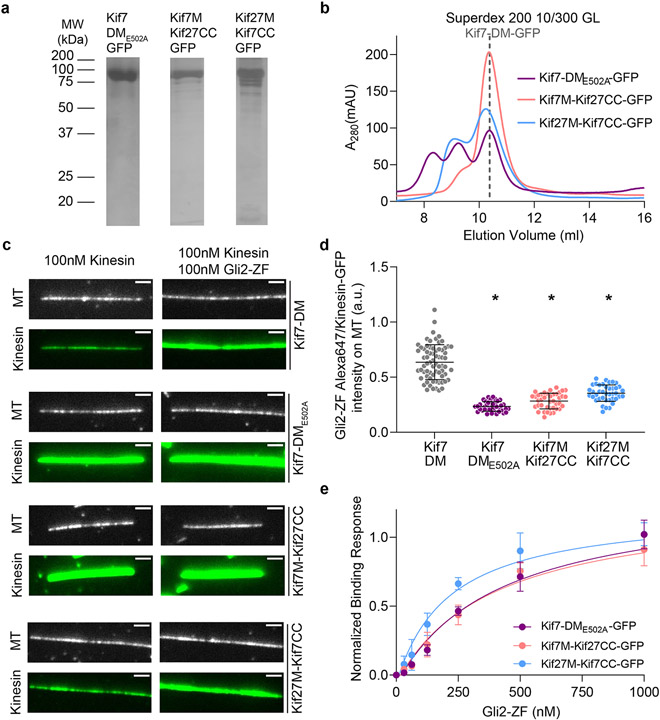
We characterized the interaction between Gli2-ZF and a dimeric Kif7 construct that includes the motor domain (1-543aa; Kif7-DM). The stoichiometry of Kif7-DM:Gli2-ZF complex in the absence of microtubules was 1:1, suggesting there are two molecules of Gli2-ZF bound per Kif7-DM (Fig. 3a and Extended Data Fig. 4a-e). Since one Gli2-ZF is bound to the coiled-coil dimer of Kif7, we hypothesized that the second Gli2-ZF molecule binds proximal to the Kif7 motor domains, as was also suggested by the weak signal observed in the pull-down assay using cell lysate (Extended Data Fig. 1a). However, we failed to detect any binding between the purified recombinant monomeric Kif7 motor domain (1-386aa, Kif7-MM) and Gli2-ZF at concentrations as high as 12μM Gli2-ZF in the BLI assay. The differences in the two experiments could arise from the presence of low levels of intact microtubules in the cell extracts. We therefore examined the recruitment of Gli2-ZF to GMPCPP-polymerized microtubules by Kif7 using an in vitro Total Internal Reflection Fluorescence microscopy (TIRFM) assay (Fig. 3b). We examined the microtubule localization of Alexa-647 labeled Gli2-ZF-SNAP in the presence of a monomeric Kif7 motor domain construct (1-386aa, Kif7-MM-GFP), which lacks the coiled-coil Gli interaction site, and compared it to dimeric Kif7 protein (1-543aa, Kif7-DM-GFP) which has both sites. Analysis of the Alexa-647 intensity revealed microtubule-bound Kif7-MM can bind Gli2-ZF, albeit to a lesser extent than Kif7-DM (Gli2-ZF enrichment over no kinesin control: ~3-fold with Kif7-MM, ~9-fold with Kif7-DM) (Fig. 3c). These data suggest that the motor domain of Kif7 likely forms a Gli-interaction site, but this interaction requires concentrating motors on microtubules or another mechanism such as dimerization, that brings motor domains in proximity.
Figure 3 ∣. Second Gli interaction site on Kif7 motor domain.
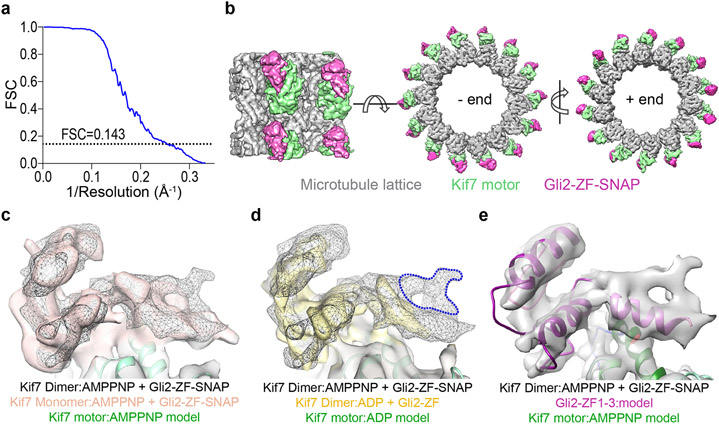
a, Chromatograms from size exclusion chromatography of a mixture of 125μM Kif7-DM-GFP and 750μM Gli2-ZF-GFP (Superdex 200 10/300 GL). In-gel GFP fluorescence analysis from SDS-PAGE of the complex (inset) used to determine stoichiometry indicated in parenthesis. Gel image is representative of a minimum of three repeats. b, Schematic of the in vitro total internal reflection fluorescence (TIRF) microscopy-based assay used to examine microtubule-localized Gli2-ZF and Kif7-DM. Fluorescently-labeled GMPCPP-stabilized microtubules (grey), immobilized on a PEG-treated glass coverslip (yellow) via neutravidin-biotin linkages (blue & brown respectively), were imaged after addition of Kif7-DM-GFP (green), Gli2-ZF-SNAP-Alexa 647 (pink) and 1mM ATP. c, Scatter plot of Gli2-ZF-Alexa647 intensity per pixel on microtubules (MT) represents recruitment of Gli on MT by Kif7-MM and Kif7-DM. The no kinesin condition was a control for non-specific binding of Gli2-ZF to MT, while Kif27-DM was included as a negative control for Gli2-ZF binding. Assay conditions: 100nM Gli2-ZF-SNAP-Alexa647, 100nM of kinesin. Data are presented as mean +/− SEM; N = 54 microtubules analysed over 3 independent experiments. One-way ANOVA (p < 0.0001) and post-hoc analysis (* indicates p<0.0001 in Dunnett’s multiple comparisons test) show statistically significant differences in Gli intensity compared to no kinesin control; ns is not significant (p=0.9253). d, Cryo-EM reconstruction of Kif7-DM bound to microtubules in the presence of Gli2-ZF-SNAP and AMPPNP. The structural model for Kif7 motor domain (green ribbon) bound to microtubule (grey ribbon) was fitted in the density and refined. In pink is the density corresponding to Gli2-SNAP and in yellow ball-and-stick model is AMPPNP. e, Comparison of electrostatic surface potential of coiled-coil and motor domains of Kif7 (PDB: 6 MLR) and Kif27. The structural models of Kif7-CC (SWISS MODEL server: GMQE=0.55, QMean=1.84), Kif27-CC (SWISS MODEL server: GMQE=0.51, QMean=0.84) and Kif27 motor domain (SWISS MODEL server: GMQE=0.47, QMean=−2.22) were obtained by homology modelling. Loop L6, helices α-2 and α-3 on the Kif7 motor domain that lie close to the Gli2-SNAP density are highlighted in green and the corresponding regions in the Kif27 motor domain model are highlighted in brown.
To visualize the binding of Gli2-ZF to the Kif7 motor domain we obtained cryo-EM reconstructions of Kif7-DM bound to microtubules in the presence of Gli2-ZF-SNAP and the non-hydrolysable ATP analogue, AMP-PNP (3.9 Å resolution, Fig. 3d, Extended Data Fig. 5a,b and Supplementary Table 1-2). Comparisons of the cryo-EM reconstruction with a map of microtubule-bound Kif7 motor domain in the AMP-PNP state (PDB:6MLR, EMD:9141) showed that the motor domain adopts the same conformation while binding to microtubules in the presence and absence of Gli2-ZF (green in Fig. 3d). While no additional density was seen bound to the microtubule lattice, an extra region of density contacting the motor domain of Kif7 was observed in the presence of Gli2-ZF-SNAP (pink in Fig. 3d). Close inspection revealed that this extra density lies close to loop L6, helices α-2 and α-3 on the Kif7 motor domain, and away from the nucleotide-binding pocket as well as the microtubule-binding interface of the motor (Supplementary Video 1). We confirmed that the extra density originated from the Gli2-ZF protein by generating and comparing cryo-EM reconstructions of the following microtubule-bound complexes: (i) Kif7-MM in the AMP-PNP state with Gli2-ZF-SNAP at 4.8 Å resolution, and (ii) Kif7-DM in the ADP state with Gli2-ZF, at 4.3 Å resolution (Supplementary Table 1-2). The presence of extra density in the Kif7-MM:Gli2-SNAP map at the same position as in Kif7-DM:Gli2-SNAP map ruled out the contribution of Kif7-coiled-coil and neck-linker domains to the density (Extended Data Fig. 5c). Comparison of the Kif7-DM:Gli2-ZF and Kif7-DM:Gli2-SNAP maps confirmed that a large part of the extra density arose from Gli2-ZF and not SNAP (Extended Data Fig. 5d). Superposition of the cryo-EM maps obtained in the presence of Gli2-ZF and Gli2-ZF-SNAP was used to unambiguously assign the density corresponding to Gli2-ZF (Extended Data Fig. 5d). We built a structural model of the Gli2-ZF peptide backbone sequence (see Methods) into this extra density and were able to fit two complete and one partial zinc-fingers (Extended Data Fig. 5e and Supplementary Video 1). Since it is well established that under saturating motor concentrations needed for cryo-EM reconstructions, the density of only a single motor head can be resolved37,38, we do not expect to see the entire Gli2-ZF interaction site formed by both motor heads and the coiled-coil domain in a dimer. TIRFM assays with Alexa-647 labeled Gli2-ZF1-3-SNAP protein show that first three ZFs of Gli are sufficient to bind Kif7-MM (Extended Data Fig. 4f). Together these results confirm that in addition to the Kif7-CC, there is a second binding site for Gli2-ZF proximal to the Kif7 motor domain.
Kif27 is a close homolog of Kif7 and the two proteins are thought to arise from gene duplication39,40. Unlike Kif7, Kif27 plays a role in motile cilium biogenesis and is not thought to be involved in Hedgehog signaling41. We wondered if the structural determinants of Gli interaction sites are conserved between Kif7 and Kif27. We modelled the motor domain (1-398aa) and the coiled-coil dimerization domain (480-550aa) of Kif27 and compared their electrostatic surface potential with Kif7. Our analysis revealed that the electrostatic surface potential of Kif27-CC is overall neutral in contrast to Kif7-CC, and the motor domain regions of Kif27 (helix α-2, α-3 and loop L6) corresponding to the Gli2-ZF interaction site in Kif7, present a less negative surface potential (Fig. 3e). Consistent with this, Gli2-ZF is not recruited to microtubules by dimeric Kif27-DM-GFP (1-580aa) in TIRFM assays (Fig. 3c). The comparative structural analysis of Kif7 and Kif27 indicates a specific adaptation in Kif7 for Gli binding and Hedgehog signaling.
Synergistic microtubule binding of Kif7 and Gli in vitro
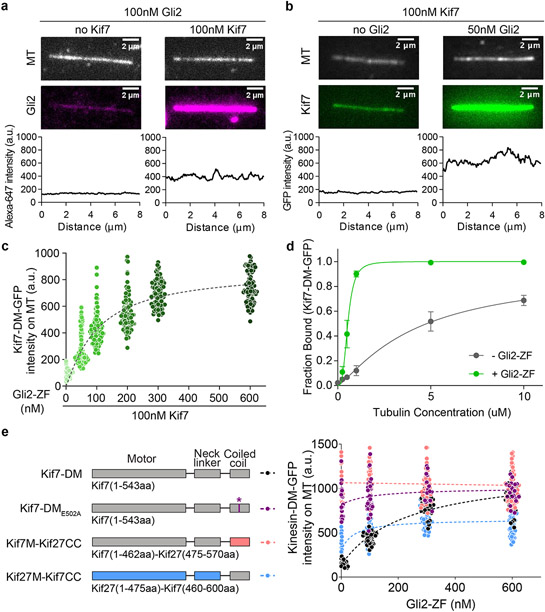
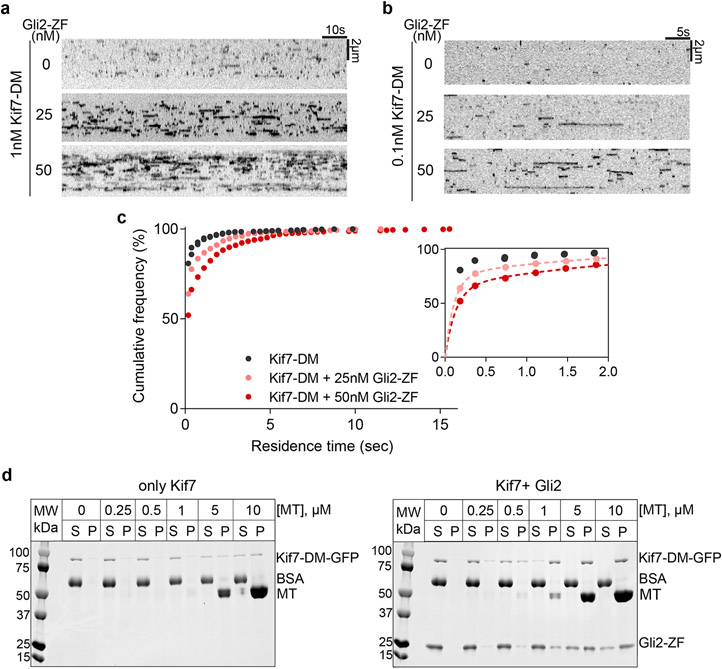
We examined if Gli altered the microtubule-binding of Kif7 by imaging the microtubule binding of Kif7-DM-GFP in the absence or presence of Alexa-647-labeled Gli2-ZF-SNAP using TIRFM. A 3-fold increase in Gli2-ZF-SNAP-Alexa-647 (100nM) fluorescence intensity on microtubules was observed in the presence of Kif7-DM (100nM) relative to experiments without Kif7-DM (Fig. 4a). The low levels of Alexa-647 fluorescence intensity on microtubules in the absence of Kif7 occurs from non-specific electrostatic interactions. Interestingly, analysis of Kif7 fluorescence intensity (100nM) revealed a >5-fold increase in Kif7-DM binding to microtubules in the presence of Gli2-ZF (50nM) (Fig. 4b). The intensity of Kif7-DM on microtubules increases with the solution concentration of Gli2-ZF until it reaches saturation (Fig. 4c). This is also consistent with the longer residence time of single molecules of Kif7-DM-GFP on microtubules with increasing Gli2-ZF concentrations (Extended Data Fig. 6a-c). We validated the findings from TIRF assays using a co-sedimentation assay, which showed that Kif7 has a higher microtubule binding affinity in the presence of Gli2-ZF (Kd = 0.54 ± 0.03 μM) compared to when Gli is absent (Kd = 4.23 ± 0.24 μM) (Fig. 4d and Extended Data Fig. 6d).
Figure 4 ∣. Gli2 ZF increases the microtubule-binding affinity of Kif7, thereby increasing its own recruitment on microtubules.
a, Representative images of microtubule(top) and Gli2-ZF-Alexa647(bottom) in the absence(left) or presence of Kif7-DM-GFP(right). The graphs show line scans of the corresponding Alexa647 fluorescence intensity. b, Representative images of microtubule(top) and Kif7-DM-GFP(bottom) in the absence(left) or presence of Gli2-ZF(right). The graphs show line scans of the corresponding GFP fluorescence. c, Scatter plot of Kif7-DM-GFP intensity per pixel on microtubules in the presence of increasing concentrations of Gli2-ZF. Assay conditions: 100nM Kif7-DM-GFP with 0, 50, 100, 200, 300 & 600nM Gli2-ZF. N = 127, 111, 103, 93, 87, 89 microtubules for the respective Gli2-ZF concentration. One-way ANOVA (p<0.0001) and post-hoc analysis (p<0.0001 in Dunnett’s multiple comparisons test) shows statistically significant differences among means at various Gli2-ZF concentrations compared to no Gli2-ZF. Data fit to dose response curve(dotted grey line) with half maximal response at ~160nM Gli2-ZF. d, Co-sedimentation assay curve analysed from gels in Extended Data Fig.6d, to quantitatively examine the microtubule binding affinity of Kif7DM-GFP in the absence(grey) and presence(blue) of Gli2-ZF. The plots of fraction of Kif7DM-GFP bound versus microtubule concentration were fit to Hill equation to determine the equilibrium dissociation constants (Kd). Data are presented as mean +/− SEM; N=3 biologically independent samples. For Kif7-DM-GFP – Gli2-ZF: Kd = 4.23 ± 0.24 μM (R2=0.93) and for Kif7-DM-GFP + Gli2-ZF: Kd = 0.54 ± 0.03 μM (R2=0.95). In the presence of Gli2-ZF, Kif7-DM-GFP shows co-operative binding to microtubules with a Hill co-efficient (h) of 3.2 ± 0.59. e, Schematics showing the domains swapped in the Kif7-Kif27 chimera proteins and the E502A mutant Kif7-DM. Scatter plot of Kinesin-GFP intensity per pixel on microtubules in the presence of increasing concentrations of Gli2-ZF. Assay conditions: 100nM of kinesin: Kif7-DM(black), Kif7-DME502A(purple), Kif7M-Kif27CC(red) and Kif27M-Kif7CC(blue), with 0, 100, 300 & 600nM Gli2-ZF. N = 40, 39, 40, 40 microtubules for each kinesin and respective Gli2-ZF concentrations. Two-way ANOVA (factor 1: Gli2-ZF concentration p<0.0001; factor 2: kinesin type p<0.0001 and their interaction p<0.0001) and post-hoc analysis (p<0.01 in Dunnett’s multiple comparisons test) shows statistically significant differences among means at various Gli2-ZF concentrations compared to Kif7-DM. Data were fit to dose response curve (dotted lines).
We wanted to determine the contribution of the two Gli interaction sites on Kif7 in regulating the Kif7-microtubule affinity. We first hypothesized that the binding of Gli2-ZF to the Kif7 motor domain may underlie the observed changes in the Kif7-microtubule binding. Therefore, we generated a chimera in which the Kif7 motor domains are intact, but the Kif7-CC is replaced by Kif27-CC (Kif7M-Kif27CC) (Fig. 4e and Extended Data Fig. 7a,b). To our surprise the Kif7M-Kif27CC chimera has constitutive higher binding to microtubules compared to Kif7-DM and the binding was independent of Gli concentration (Fig. 4e). To test this hypothesis further we generated a point mutation in Kif7-DM (E502A), which eliminates Gli binding to the coiled-coil domain of Kif7 (Extended Data Fig. 3i and 7a-b). Similar to Kif7M-Kif27CC, Kif7-DM(E502A) is a constitutive dimer and recruits lower levels of Gli2-ZF relative to wild-type Kif7-DM as expected for constructs lacking one Gli binding site (Extended Data Fig. 7c-e). We find that this mutant is identical to the Kif7M-Kif27CC chimera in terms of Gli-independent strong microtubule binding (Fig. 4e). Thus, counterintuitive to our expectation, the binding of Gli2-ZF to the Kif7-CC domain has a dominant role in regulating the Kif7-microtubule interaction. These data are consistent with a mechanism where the coiled-coil domain inhibits the Kif7-microtubule binding, and this inhibition is relieved by Gli.
To test if the inhibition by the coiled-coil is specific to the Kif7 motor domain, we generated a chimera where the motor and neck linker domain of Kif7 was replaced by that of Kif27 (Kif27M-Kif7CC). Similar to the other mutants described in this section, this chimera is dimeric and recruits lower levels of Gli compared to wild-type Kif7-DM (Extended Data Fig. 7). We found that the microtubule binding of Kif27M-Kif7CC chimera exhibited lower dependence on Gli concentrations (Fig. 4e and Extended Data Fig. 7). Together these data suggest that the inhibition of the Kif7-microtubule binding by the Kif7 coiled-coil domain is highest with the Kif7 motor domains in the Kif7-DM, and the complete alleviation of this inhibition by Gli sets the dynamic range of the Kif7-microtubule binding response to changes in Gli concentration.
Synergistic localization of Kif7 and Gli2 at the cilium tip
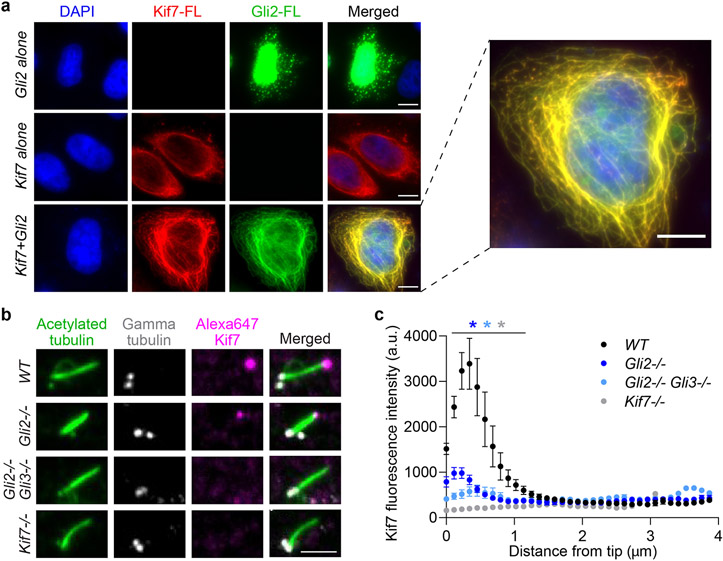
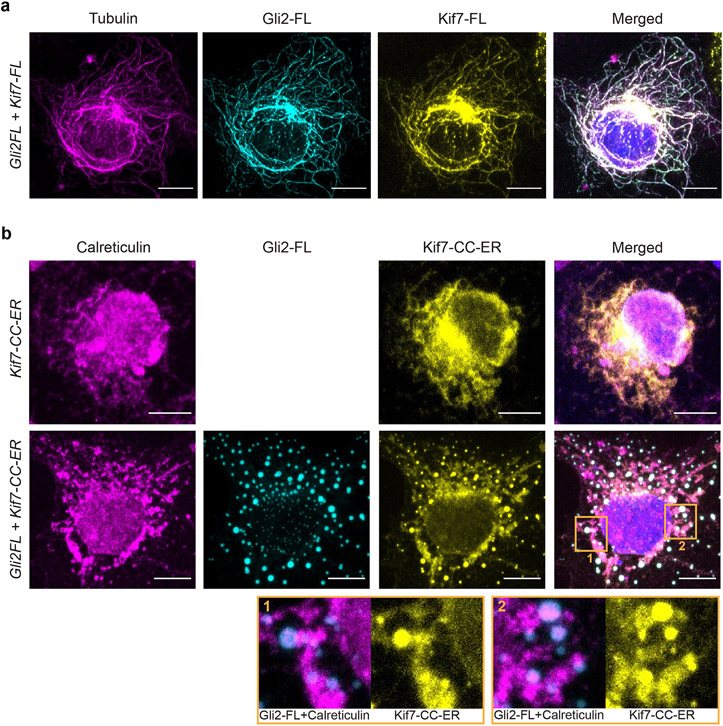
We examined the interaction between full-length Gli2 and Kif7 in the cellular context. When overexpressed individually in HeLa cells, Kif7-FL is exclusively localized in the cytoplasm and Gli2-FL is largely in the nucleus with a small fraction present as cytoplasmic puncta with no obvious microtubule localization. Co-transfection of Kif7-FL-mRuby with mNeonGreen-Gli2-FL revealed localization of both Kif7 and Gli2 along cytoplasmic microtubules (Fig. 5a & Extended Data Fig. 8a).
Figure 5 ∣. Synergistic regulation of Gli-Kif7 promotes Kif7 localization to cytosolic microtubules and the distal cilium tip.
a, Localization of transfected mRuby-tagged full length Kif7 and mNeonGreen-tagged full length Gli2 in HeLa cells. Representative images are shown. DAPI staining was used to mark the nucleus. Overexpressed full length Gli2 shows predominant localization in the nucleus(row 1). Overexpressed full length Kif7 is cytoplasmic(row 2). Co-transfection with Kif7 and Gli2 shows localization of both proteins on microtubules in the cytoplasm(row 3) (100%; N=30 cells analysed over 3 independent experiments for each condition). Scale bars represent 10 μm. b, Cilium-tip localization of Kif7 in WT, Gli2−/−, Gli2−/−Gli3−/− and Kif7−/− MEFs. Representative immunofluorescent images of primary cilia are shown. Acetylated α-tubulin antibody was used to mark cilia, γ-tubulin antibody was used to mark centrioles (base of cilia) and Alexa-Fluor-647-labeled Kif7 antibody was used to measure Kif7 amounts in the cilia tips during Hh activation (+SAG). Scale bar represents 2 μm. c, Quantitative analysis of the levels of Kif7 in WT, Gli2−/−, Gli2−/−Gli3−/− and Kif7−/− MEFs. Line scans of cilia from the experiment in (b), was used to quantify distribution of Kif7 along the length of the cilia. Data represent mean and standard error from three independent repeats (N = 10 cilia for each genotype). Two-way ANOVA (factor 1: genotype p < 0.0001, factor 2: distance from tip p < 0.0001) and post-hoc analysis (* indicates p<0.0001 in Dunnett’s multiple comparison test) show statistically significant differences among Kif7 intensity in cilia tip of all genotypes compared to wild-type control.
Kif7 and Gli2 accumulate at the cilium tip in response to Hedgehog (Hh) pathway activation, and Kif7 is thought to form a stable platform for proper cilium-tip localization of Gli25,30. Contrary to this simple model, our findings suggest that the cilium localization of Kif7 may in turn be Gli-dependent. To examine this, we measured the amount of Kif7 at the cilium tip in Gli2−/− and Gli2−/−Gli3−/− cell lines (immortalized MEFs) compared to wild-type (WT) MEFs, upon stimulation with the Smoothened agonist SAG (Hh pathway agonist). We found that the amount of Kif7 at cilia tips upon Hh pathway activation depends on Gli. Gli2−/− and Gli2−/−Gli3−/− cells showed ~4- and 5-fold less Kif7 at the cilia tips compared to WT cells respectively (Fig. 5b,c). Thus, by modulating Kif7-microtubule binding, Gli acts as a master regulator of its own recruitment to the cilia tip for Hh pathway activation.
Together these findings suggest that the Kif7-Gli2 interaction is not only sufficient to recruit Gli onto microtubules, but it also positively regulates the microtubule-binding of Kif7 by increasing the amount of motor on microtubules and at the cilium tip. This positive regulation in turn increases the amount of Gli2 recruited to microtubules. Consequently, the interaction between Gli2 and Kif7 concentrates both proteins to higher levels on microtubules in a Gli-concentration dependent manner.
A tool for sequestrating Gli2 outside the nucleus
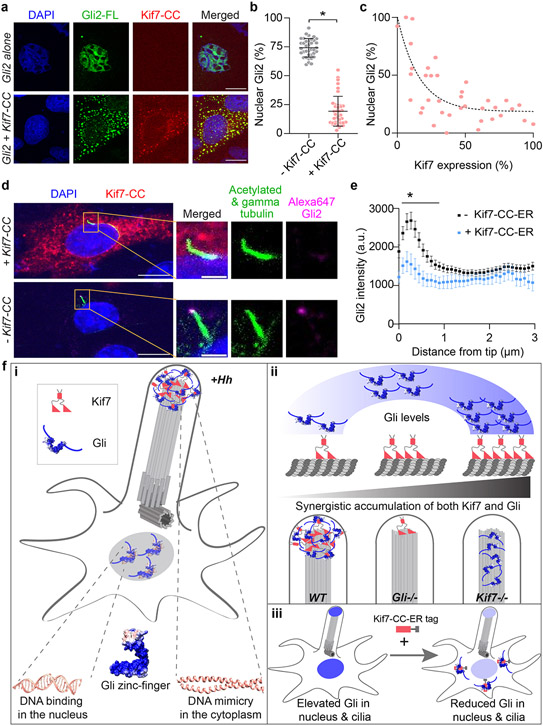
Given the high affinity of the DNA mimicking Kif7-CC domain for Gli2, we wondered if this domain could be retooled to sequester Gli away from the nucleus42-44. To test this possibility, we engineered the Kif7-CC to contain an endoplasmic reticulum (ER) retention tag and mRuby at the C-terminus, and expressed it along with full-length mNeonGreen-Gli2 from a single mRNA in a dual expression vector. The identical vector expressing mNeonGreen-Gli2 alone served as a control. These constructs were transfected in HeLa cells and the amount of Gli2 in the nucleus was measured as a percentage of total Gli expression levels using the mNeonGreen fluorescence intensity. The localization of mNeonGreen-Gli2 in control experiments was largely nuclear (>75%) whereas, in the presence of ER-localized Kif7-CC-mRuby the nuclear mNeonGreen-Gli2 decreased to <25% (Fig. 6a,b). Colocalization of fluorescent signals for Gli2, Kif7-CC and ER-marker calreticulin showed that ER-linked Kif7-CC-mRuby sequesters Gli2 in the cytoplasm (Extended Data Fig. 8b). The sequestration of Gli2 in the cytoplasm (and thus reduction in nucleus levels) depends on the level of Kif7-CC-mRuby expression in these cells (Fig. 6c).
Figure 6 ∣. Re-engineering the DNA-mimicking coiled-coil domain of Kif7 as a tool for sequestration of Gli away from the nucleus and cilia.
a, Localization of transfected mRuby-tagged Kif7-CC (460-600aa) with ER-retention tag and mNeonGreen-tagged full-length Gli2 (Gli2-FL) in HeLa cells. Overexpressed Gli2-FL shows predominant localization in the nucleus, as marked by DAPI(row 1). Co-transfection with Kif7-CC and Gli2-FL shows co-localization of Gli2 in the cytoplasm with Kif7-CC(row 2). Scale bars represent 10 μm. b, Percent of nucleus-localized Gli2 quantified from a, in the absence and presence of Kif7-CC-ER. Data represent mean +/− SD from three independent repeats (N=40 and N=38 cells treated with and without Kif7-CC-ER respectively, pooled from three independent experiments). c, Scatter plot of normalized nuclear Gli2 localization versus level of Kif7-CC expression. Normalization was done using cells with highest protein expression from the entire dataset analyzed. The dotted line represents fit to one-phase association. d, Representative immunofluorescent images of cilium-tip localization of endogenous Gli2 in NIH3T3 cell transfected with mRuby-tagged Kif7-CC (460-600aa) with ER-retention tag(top row) and untransfected cell from the same sample(bottom row). Scale bar in the left panel represents 10μm and in the cilium inset represents 2μm. e, Line scans of cilia from d, was used to quantify distribution of Gli2 along the length of the cilia. Data represent mean +/− SEM from three independent repeats (N=12 and N=37 cells treated with and without Kif7-CC-ER respectively, pooled from three independent experiments). Two-way ANOVA (factor 1: Kif7-CC-ER transfection p=0.04, factor 2: distance from cilia-tip p<0.0001) and post-hoc analysis (* indicates p<0.0001 in unpaired Student’s t-test, two-sided) shows statistically significant decrease in ciliary Gli2 intensity upon Kif7-CC-ER transfection compared to non-transfected control. f, Schematic of the biochemical basis and cellular implications of Kif7-Gli interaction. i)The zinc-finger domain of Gli that binds DNA in the nucleus is co-opted for binding the Kif7-CC out of the nucleus through DNA molecular mimicry. ii)The Kif7-CC acts as a regulatory domain necessary for the graded accumulation of both proteins on microtubules and at the cilium tip in a Gli-concentration responsive manner. iii) Kif7-CC peptide can be re-tooled as a reagent for sequestration of Gli away in the cytoplasm to decrease nuclear and ciliary Gli levels.
To test if the ER-tagged Kif7-CC could inhibit the cilium tip localization of Gli2 in response to Hedgehog pathway activation we transfected this construct into NIH3T3 cells. The cilium tip localization of Gli2 in Kif7-CC-ER transfected cells, induced with the Hedgehog pathway agonist SAG, was measured using a directly labeled Gli2 antibody that was further cleaned using Gli2−/− MEFs. Our results show that Kif7-CC-ER inhibits the levels of Gli2 at the cilia tips during pathway activation compared to non-transfected wild-type cells (Fig. 6d,e). These findings show that the Kif7-CC peptide can be re-engineered for sequestration of endogenous and over-expressed Gli in the cytoplasm, and inhibition of its nuclear and cilium localization.
Discussion
Our study reports the discovery of DNA-mimicry as a mechanism for regulating nuclear proteins in the cytoplasm of eukaryotes and provides critical insights into mechanisms that link the Hh signaling pathway and the microtubule cytoskeleton.
Coiled-coils are among the most ubiquitous structural folds in proteins45. While synthetic coiled-coils have been predicted to be suitable DNA mimics46, examples of their occurrence in endogenous proteins have been lacking. The Kif7-CC is characterized by a highly negatively charged rod-like structure that competes for the same binding sites on Gli2-ZF as DNA. These properties of Kif7-CC are consistent with that of a DNA-mimicking protein46-49. The zinc-finger domain of Gli2 binds DNA in the nucleus and is repurposed for Kif7-mediated cytoplasmic localization through the same structural design principle (Fig. 6fi). A handful of examples of DNA mimicking proteins described in eukaryotes are confined to the nucleus50,51 or function during mitosis52. Additionally, unlike reported DNA mimics that resemble bent/distorted/single stranded DNA, Kif7-CC mimics dsDNA50,51,53-55. While our mutagenesis studies were focused on charged amino-acids, additional interactions between zinc fingers of Gli2 and Kif7-CC may impart specificity and define the precise positioning of the coiled-coil relative to the Gli2-ZF (Extended Data Fig. 3f). Our observations raise the intriguing possibility that cytoskeletal proteins can be integral for binding and regulating nuclear proteins, especially transcription factors, in the cytoplasm, by mimicking some of the structural features of double stranded DNA.
We find that Gli binds to two unusual cargo-binding sites on Kif7 - the coiled-coil dimerization domain and the motor domain, and the Kif7-Gli interaction regulates the Kif7-microtubule affinity in a Gli dose-dependent manner (Fig. 6fii). Experiments with chimeras and mutant that specifically perturb Gli binding to either the coiled-coil or the motor domain in a Kif7-DM provide mechanistic insights into how Gli may regulate the Kif7-microtubule interaction. Our results suggest that in the absence of Gli, the coiled-coil domain of Kif7 may inhibit the Kif7-microtubule interaction. Gli binding to the coiled-coil interaction site on Kif7 alleviates this inhibition. The maximum dynamic range of the Kif7-microtubule binding response to Gli is only observed with wild-type Kif7-DM, as replacing the motor domain of Kif7 with that of Kif27 reduces the response. Thus, of the two sites on Kif7 that play a role in recruiting Gli, it is unexpectedly the Gli binding site further away from the motor domain that has a dominant role in Kif7-microtubule binding response. In general, cargo binding to the coiled-coil or the motor domains of kinesins is rare. Particularly, cargo-motor domain interactions typically have an inhibitory effect on the kinesin-microtubule binding and motility56-59, which is different from our observations with the Kif7-Gli interaction. It is noteworthy that the Drosophila homolog of Kif7, Costal2 is also proposed to have multiple binding sites for Ci60,61. It is possible that the multi-site interaction may be beneficial for concentrating low copy number transcription factors of the Hh pathway, on microtubules.
Our finding of positive feedback regulation implies that by modulating the levels of Kif7 at the cilium tip, Gli acts as a master regulator of its own recruitment to the cilia tip for its activation. Like other transcription factors, Gli is a low copy number and tightly regulated protein, hence the synergistic feedback between Kif7-Gli and Kif7-microtubule interactions is a mechanism that would be advantageous in rapidly concentrating Gli to the cilium tip upon Hh activation. A similar strategy may be used to concentrate and promote the formation of Gli repressor at the base of the cilium when the Hh pathway is off. Interestingly, in Drosophila, where the Hh pathway is cilium-independent, the Kif7 homolog Costal2 is proposed to tether the Gli homolog Ci to the cytoplasmic microtubules during pathway repression5. From an evolutionary perspective, our results indicate that despite the divergence in the utilization of cilium between vertebrates and invertebrates, the fundamental principles by which the microtubule cytoskeleton has been co-opted to regulate Hedgehog signal transduction remains conserved61. Our findings also validate that Kif7 and not Kif27 is the mammalian ortholog of Drosophila Costal2 protein that tethers Gli to microtubules.
Gli is the final downstream effector molecule of the Hh signaling pathway and it is therefore a target for regulation of pathway activity. Aberrant activation of Gli has been reported in multiple human cancers including basal cell carcinomas and glioblastomas through both Hh-dependent and independent mechanisms12,62,63. An inhibitor that directly targets Gli has therapeutic potential in various cancers with Gli overexpression irrespective of their etiology64. Our biochemical analyses of Kif7-Gli interaction and proof-of-principle sequestration experiments suggest that a small coiled-coil peptide from Kif7 can sequester Gli in the cytoplasm and restrict its entry into the nucleus (Fig. 6fiii). Currently, how Kif7 interferes with Gli nuclear trafficking is unknown. However, regardless of the precise mechanism, this general strategy can be exploited and developed further to downregulate Gli activity, as well as a tool for cell biological and optogenetic studies42-44. The structural analyses of the Kif7-Gli interaction opens the possibility of a new site on Gli that can be exploited for drug design. On a wider scale, it represents a possible strategy for inhibiting other zinc-finger proteins that play critical roles in gene regulation and may be erroneously activated in disease.
Methods
Pull-down assay
Plasmids for the pull-down assay were constructed by cloning human Kif7 and Gli2 sequences into pCMV-BICEP™-4 expression vector. FLAG-tagged Kif7 and c-myc-tagged Gli2 protein-coding sequences were cloned at MCS1 and MCS2 sites respectively. Point mutations were made using the Q5® Site-Directed Mutagenesis Kit (NEB). Expi293F™ cells were maintained in Expi293 Expression medium and cultured under 7% CO2, 93% air condition at 37°C with 220rpm shaking. Expi293 cells were transfected with plasmids using ExpiFectamine™ 293 Reagent, grown for 18-22 hours followed by addition of ExpiFectamine™ 293 Transfection Enhancer 1 and grown for another 24 hours. Cells were harvested by centrifugation at 2500xg for 5min, washed with phosphate buffered saline (PBS) and resuspended in lysis buffer (25mM Tris-HCl, 75mM NaCl, 1mM MgCl2, 0.5mM EDTA, 100μM ATP, 1mM DTT, 0.1% TritonX100 and Halt™ protease inhibitor cocktail; pH=7.5). Cells were lysed by a short sonication and the lysate was cleared by centrifugation at 125000xg for 30mins. Anti-FLAG M2 magnetic beads (Sigma Aldrich) pre-equilibrated in the lysis buffer was incubated with the cleared cell lysate (labeled ‘input’) for 1hr at 4°C. DynaMag-Spin was used to collect the supernatant labeled ‘flow through’ and the beads were washed with the buffer 5 times. The last supernatant from the washes was used as the ‘wash’ sample. The final ‘bead’ sample was prepared by resuspending the beads in ~50ul of buffer. The input, flow through, wash and bead samples were run on SDS-polyacrylamide gel and subjected to Western blotting. Binding was detected using anti-c-myc antibody (Sigma Aldrich; catalogue# C3956; source# 21190733; 1:1000 dilution) and the expression of the bait-protein was detected from the input sample with anti-FLAG antibody (Sigma Aldrich; catalogue# F3165; clone M2; batch# SLCD3990; 1:500 dilution).
Protein Expression and Purification
The N-terminal fragment of Kif7 (Uniprot Q2M1P5) (Kif7-DM 1-543aa) was cloned into a pFastBac expression vector (Thermo) that included a tobacco etch virus (TEV) protease cleavable N-terminal 6x His-tag and SUMOstar solubilization tag. To design the Kif7-Kif27 chimeras, we performed homology modeling of the two kinesins using PROMALS 3D (UTSW). We complimented this with modeling Kif7 and Kif27 CC domains using COILS (EMBL) and PairCoil (MIT) for accurate coiled-coil substitution. The final sequences are: (1) Kif7M-Kif27-CC chimera is Kif7 1-462aa (motor) and Kif27 475-570aa (coiled-coil). (2) Kif27M-Kif7-CC chimera is Kif27 1-475aa (motor) and Kif7 460-600aa (coiled-coil). Dimeric Kif7 N-terminal constructs: Kif7-DM (1-543aa), Kif7-DME502A and the Kif7-Kif27 chimeric constructs were expressed in SF9 insect cell line using the Bac-to-Bac® Baculovirus Expressions System (Thermo) with cells grown in HyClone CCM3 SFM (GE Life Sciences) and expressed from P3 virus for 72H at 27°C. Monomeric Kif7 constructs (Kif7-MM 1-398aa) containing the motor domain was cloned into a modified pET-21-a expression vector with a TEV protease cleavable N terminal 6x His-tag. Kif7 monomers were expressed in BL21 (DE3) Rosetta (Millipore) E. coli at 18°C with 0.25mM IPTG for 18-20H. Dimeric and monomeric kinesin pellets containing the motor domain of Kif7 were lysed by short sonication in buffer A (50mM phosphate pH 8.0 300mM NaCl 5% glycerol, 1mM MgCl2 and 25mM imidazole) supplemented with 0.15% tween, 0.5% Igepal, 100μM ATP 2mM TCEP, 1mM PMSF, 75 U benzonase and 1X HALT (Thermo). Lysate was cleared by ultracentrifugation and supernatant was incubated with Ni-NTA for 1H. Resin was washed with buffer A supplemented with 20μM ATP and 0.5mM TCEP and eluted with 400mM imidazole with 100μM ATP. Peak fractions were pooled and, if needed, cleaved overnight by TEV (1/30 w/w) at 4°C. Proteins were further purified by size exclusion chromatography (Superdex 200 10/300GL) in 50mM HEPES pH 7.4 300mM NaCl 5% glycerol 5mM ß-Me 1mM MgCl2 and 100μM ATP and frozen in liquid nitrogen. Dimerization of a given construct was determinable by gel filtration profiles. The coiled-coil dimerization domain fragment of Kif7, (Kif7-CC 460-600aa) was cloned into a modified pGEX vector that contained a human rhinovirus (HRV) protease cleavable N-terminal GST-tag. Point mutants were generated by site-directed mutagenesis using In-Fusion HD cloning kit (Takara). The Kif7 SCC construct (aa488-540) was ordered as a gene-fragment (Genscript) and cloned into pETDuet-1 by In-Fusion HD Cloning. All Kif7-CCs and Kif7-SCC were expressed in BL21 (DE3) Rosetta (Millipore) E. coli at 18°C with 0.25mM IPTG for 18-20hr. The cell pellets were lysed by short sonication in buffer B (PBS pH 7.3, 5% glycerol) supplemented with .15% tween, 0.5% Igepal, 100μM ATP, 2mM TCEP, 1mM PMSF, 75 U benzonase and 1X HALT (Thermo). Lysate was cleared by ultracentrifugation and supernatant was incubated with GST-4B beads (GE Life Sciences) for 1H. Resin was washed with buffer B supplemented with 0.5mM TCEP and eluted with 10mM reduced glutathione in 40mM PIPES pH 7.3 150mM NaCl. Peak fractions were pooled and, if needed, cleaved overnight by GST-HRV-3C (1/40 w/w) at 4°C. Proteins were further purified by size exclusion chromatography (Superdex 200 10/300GL) in 40mM PIPES pH 7.3 150mM NaCl 5% glycerol 2mM TCEP and frozen in liquid nitrogen.
The zinc-finger domain of Gli2 (Uniprot P10070) (Gli2-ZF 418-604aa) was cloned into: (1) A modified pET-Duet-1 expression vector with an HRV protease cleavable N-terminal 6x His-tag. Truncations were made using the InFusion HD Cloning Kit (Takara). (2) A modified pET-Duet-1 vector with an HRV protease cleavable N-terminal 6x His-tag, and a C-terminal SNAP/GFP tag. Gli2-ZF protein was expressed in BL21 (DE3) Rosetta (Millipore) E. coli at 18°C with 0.25mM IPTG for 18-20hr. Gli2-ZF pellets were lysed by short sonication in buffer C (40mM PIPES pH 7.3 150mM NaCl) supplemented with .15% tween, 0.5% Igepal, 2mM TCEP, 1mM PMSF, 300 U benzonase and 1X HALT (Thermo). Lysate was cleared by ultracentrifugation and supernatant was incubated with Ni-NTA for 1H. Resin was washed with buffer A supplemented with 0.5mM TCEP and eluted with 400mM imidazole. Peak fractions were pooled and, if needed, cleaved overnight by GST-HRV-3C (1/40 w/w) at 4°C. Proteins were further purified by ion-exchange chromatography using a Heparin column; Gli2-ZF proteins are DNA binding and generally elute from the heparin column around 1M NaCl. Proteins were further purified by size exclusion chromatography (Superdex 75 10/300GL and Superdex 200 10/300GL) in 40mM PIPES pH 7.3 150mM NaCl 5% glycerol 2mM TCEP and frozen in liquid nitrogen. All proteins were greater than 95% pure and eluted as a single peak from the size exclusion chromatography column.
Bio-Layer Interferometry Assays
BLI experiments were performed in an Octet Red 96 instrument (ForteBio). Experiments were performed in an assay buffer containing 40mM TRIS pH7.3, 40mM KCl, 40μM ATP 1mM MgCl2, 0.12% tween and 0.5mM TCEP. GST-Kif7-CC protein was immobilized on an anti-GST biosensor chip at a concentration of 120nM. Unbound Kif7 was washed away with buffer, and the chips were then dipped into buffer with a range of Gli2-ZF concentrations from 0-1μM to allow for association of the two proteins. After 300 seconds, they were then moved back into a well with just buffer to allow dissociation for 400 seconds. Octet Data Analysis software was used to extract the binding response for BLI data. The responses were plotted as a function of protein concentration and fit to Hill curves using GraphPad Prism. The binding responses were normalized between 0 and 100 prior to curve fitting. The equilibrium response of each sensor from three independent repeats at each Gli2 concentration were normalized and plotted against Gli2 concentration and fitted to the following Hill function: Y=(Bmax*Xh)/(Kdh + Xh); where Bmax is the maximum specific binding, Kd is the ligand concentration needed to achieve a half-maximum binding at equilibrium and h is the Hill slope.
The DNA competition experiments (Fig. 2a) were performed in the same way with one notable exception: 125μM TRE-2S DNA (a Gli2 DNA binding target) was held constant along the Gli2 titration from 0-1 μM. For the DNA titration experiments, the Gli2-ZF concentration was kept constant at 125nM and titrated with an increasing concentration of Gli target dsDNA (TRE2S – 5’CCGGGAAGCCACCGGGAACCACCCA3’) and Gli non-target dsDNA (TFIIIA-BS – 5’AGGGGGGCTATAAAAGGGTG3’) from 0-100nM. The assays were also performed with HIS-tagged Kif7-DM, Kif7-DME502A and the Kif7-Kif27 chimeric proteins, requiring the use of an anti-penta-HIS biosensor chip, and referencing sensors to account for non-specific binding of Gli2 to the HIS sensor. A flipped assay, using HIS-tagged Gli2 on the sensor and a range of Kif7 concentrations in buffer, was also conducted as a control.
Kif7-Gli2 Complex Stoichiometry Determination
The Kif7-CC and Kif7-DM protein samples were not amenable to dynamic light scattering analysis. To determine the stoichiometry of the Kif7-Gli2 complexes, we purified GFP-tagged constructs of Kif7-DM, Kif7-CC, and Gli2-ZF. The Kif7 (Kif7-CC or Kif7-DM) and Gli2 were mixed at high concentration with Gli2-ZF in molar excess (125μM Kif7 and 750μM Gli2-ZF) and incubated for 15 minutes on ice. After incubation, 200μL of this mix was injected into a Superdex 200 10/300 GL column. Fractions that contained complexed protein were determined by elution volume shift in the gel filtration chromatogram. Complex containing fractions were heated at 50°C for 5 mins and run in SDS-PAGE and quantified in two ways: (1) Scanned the unstained SDS-PAGE gel at 488nm65. Since GFP is stable in SDS up to at least 0.5% w/v66 and SDS-PAGE uses 0.1%, the GFP fluorescence can be quantitatively analyzed on a regular SDS-PAGE gel with this sample preparation protocol65. The ratio of GFP fluorescence corresponding to the Kif7 and Gli bands was measured using an Amersham™ Typhoon Scanner and the intensity analyses was performed using ImageJ to obtain the stoichiometry. (2) Quantitative western blot analysis of the complex was performed using a directly labeled 488nM anti-GFP antibody (Santa Cruz Biotechnology; catalogue# sc-9996 AF488; clone B2; lot# C0316; 1:1000 dilution). The fluorescence intensity at 488 nm was quantified using an Amersham™ Typhoon Scanner and analyzed with ImageJ. The results from these two independent experiments were consistent with the stoichiometries reported in this study.
Structural modeling
Primary sequences of the zinc-finger domain of human Gli2 (UniprotKB P10070) (Gli2-ZF, 437-589aa), the coiled-coil domain of human Kif7 (UniprotKB Q2M1P5) (Kif7-SCC, 481-543aa), the coiled-coil domain of human Kif27 (UniprotKB Q86VH2) (Kif27-CC, 475-570aa) and the N-terminal motor domain of human Kif27 (UniprotKB Q86VH2) (Kif27M, 1-475aa) were retrieved from the database in FASTA format. A preliminary search for homologs to serve as templates for modeling was performed with BLAST67 and HHBlits68 against the SWISS-MODEL template library (SMTL). The following templates with the highest quality predictions (based on target-template alignment) were selected for homology modeling: (i) Gli1 zinc finger (PDB: 2GLI) for Gli2-ZF (ii) ROCK1 coiled-coil (PDB: 3O0Z) for Kif7-CC and Kif27-CC (iii) Kif7 motor domain (PDB: 6MLQ) for Kif27M. The 3D structure of these protein domains was built using ProMod3 in the SWISS-MODEL server69,70. The global and per-residue model quality was assessed using GMQE and QMEAN scoring function71. The structural models were visualized in UCSF Chimera72. Electrostatic potential of the surfaces were generated from the APBS server73. For obtaining the structural model of the Kif7-SCC:Gli2-ZF protein complex, the Gli2-ZF model was docked on the Kif7-CC model using an automated server, ClusPro 2.074, in which receptor was Kif7-SCC and Gli2-ZF was used as a ligand. Based on different desolvation and electrostatic potential, ClusPro 2.0 can differentiate thousands of conformations of the protein. The generated conformations were further categorized through clustering and 8 most fit structures (which are found to be closest to native structure from X-ray crystallography results) were considered. The interface areas in the structural model of the protein complex were calculated by the PDBsum webserver75. These contact residues were mutated to Ala for binding analysis to assess the predicted models.
Cryo-EM Sample Preparation
For grid preparation, all Kif7-Gli2 preparations were diluted using BRB80 (80 mM 1,4-piperazinediethanesulfonic acid [PIPES], pH 6.8, 1 mM MgCl2, 1 mM ethylene glycol tetraacetic acid [EGTA]). Porcine brain microtubules were prepared from frozen aliquots of 5mg/ml tubulin (Cytoskeleton, Denver, CO) in polymerization buffer (BRB80; 80 mM PIPES, pH 6.8, 1 mM EGTA, 2 mM MgCl2, 3mM GMP-CPP, 10% dimethyl sulfoxide (DMSO)) at 37° C for 2-3 hours. The polymerized microtubules were then incubated at room temperature for several hours or overnight before use. The following day or several hours later the microtubules were spun down on a bench top centrifuge at 18,000 x g for 10min to pellet. The pellet was resuspended in polymerization buffer without DMSO. The Kif7 dimer and monomer preparations were made by mixing the Kif7 with the Gli2 or Gli2-SNAP for 1 hour prior to incubation with the microtubules. Kif7-dimer (0.36 mg/ml) plus Gli2 (0.2mg/ml) in BRB plus 2mM ADP, 2mM MgCl2, Kif7-monomer (0.45mg/mL) or Kif7 dimer plus Gli2_SNAP (0.30mg/mL) plus BRB80 with 1mM AMPPNP and 1mM MgCl2.
All microtubule samples were prepared on 1.2/1.3 400-mesh grids (Electron Microscopy Services) Grids were glow-discharged before sample application. The cryo-samples were prepared using a manual plunger, which was placed in a homemade humidity chamber that varied between 80 and 90% relative humidity. A 4-μl amount of the microtubules at ~0.5 μM in BRB80 was allowed to absorb for 1 min, and then 4 μl of the different Kif7-dimer or monomer plus Gli2 with and without SNAP were incubated with microtubules on the grid. After a short incubation of 2 min, the sample was blotted (from the back side of the grid) and plunged into liquid ethane.
EM image acquisition and data processing
Images of frozen-hydrated Kif7-microtubule complexes (see Supplementary Fig. 1, Supplementary Table 1-2) were collected on a Titan Krios (FEI, Hillsboro, OR) operating at 300 keV or an Arctica (FEI, Hillsboro, OR) equipped with a K2 Summit direct electron detector (Gatan, Pleasanton, CA). The data were acquired using the Leginon automated data acquisition76,77. Image processing was performed within the Appion processing environment77,78. Movies were collected at a nominal magnification of 29000× and 36000x with a physical pixel size of 1.03 and 1.15 Å/pixel respectively. Movies were acquired using a dose rate of ~4.2 and 5.6 electrons/pixel/second over 9 and 8 seconds yielding a cumulative dose of ~36 and ~34 electrons/Å2(respectively). The MotionCor frame alignment program79,80 was used to motion-correct. Aligned images were used for CTF determination using CTFFIND481 and only micrographs yielding CC estimates better than 0.5 at 4 Å resolution were kept. Microtubule segments were manually selected, and overlapping segments were extracted with a spacing of 80 Å along the filament. Binned boxed segments (2.05Å/pixel, 240-pixel box size for the Krios data and 2.30Å/pixel, 192 pixel box size for the Arctica data) were then subjected to reference-free 2D classification using multivariate statistical analysis (MSA) and multi-reference alignment (MRA)80,82. Particles in classes that did not clearly show an 80Å layer line were excluded from further processing.
Cryo-EM 3D reconstruction
Undecorated 13,14- and 15-protofilament microtubule densities83 were used as initial models for all preliminary reconstructions. We used the IHRSR procedure84 for multimodel projection matching of microtubule specimens with various numbers of protofilaments85, using libraries from the EMAN2 image processing package86. After each round of projection matching, an asymmetric backprojection is generated of aligned segments, and the helical parameters (rise and twist) describing the monomeric tubulin lattice are calculated. These helical parameters are used to generate and average 13, 14 and 15 symmetry-related copies of the asymmetric reconstruction, and the resulting models were used for projection matching during the next round of refinement. The number of particles falling into the different helical families varied. Helical families that had enough segments were further refined. Final refinement of microtubule segment alignment parameters was performed in FREALIGN 87 without further refinement of helical parameters. The data is unbinned during refinement. The boxsize of the Krios data was binned from 480 to 380 reducing the pixel size from 1.03 to 1.3 Å/pixel. FSC curves were used to estimate the resolution of each reconstruction, using a cutoff of 0.143. To better display the high-resolution features of the 3D map shown, we applied a B-factor of 150 Å, using the program bfactor (http://grigoriefflab.janelia.org).
Model building in the EM map
The EM-derived structure of AMPPNP-Kif7 bound to microtubules (PDB: 6 MLR, EMD: 9141) was fit as a rigid body into the density for AMPPNP-Kif7 dimer bound to microtubules in the presence of Gli2-SNAP, using the ‘Fit in Map’ utility in UCSF Chimera 72. To locate the density corresponding to the Gli2 protein, cryo-EM maps for ADP-Kif7 dimer with Gli2, AMPPNP-Kif7 monomer with Gli2-SNAP and AMPPNP-Kif7 dimer with Gli2-SNAP were superposed via the Kif7 motor domains, and the minimal density common to all three maps was identified. To construct a structural model for Gli2 to fit the density, individual zinc fingers were first extracted from the crystal structure of a five-finger Gli1 in complex with DNA (PDB:2GLI). Next, various combinations of zinc fingers were fit into the minimal density corresponding to Gli2 and the best model was identified through its model-map correlation coefficient and visual inspection. Since the density corresponding to each zinc finger could be fit by any one of the five zinc-fingers of Gli2, the partial structure for Gli2 was modeled as a Poly-Ala sequence. Finally, flexible fitting for the structure comprising of Gli2 zinc fingers, AMPPNP-bound Kif7 motor and α-β tubulin heterodimer was performed with phenix.real_space_refine88. The final structure has a model-map correlation of 0.84 (calculated using the ‘Fit in Map’ utility), bond length RMSD of 0.008 Å and bond angle RMSD of 1.142°. There are no outliers in the Ramachandran map. Cryo-EM reconstructions and their associated models were superposed using the UCSF Chimera “Fit in map” tool72.
Total Internal Reflection Fluorescence Microscopy Assays
In vitro TIRF-based microscopy experiments were carried out as described in Subramanian et al., 201389.
Microtubule binding assay: X-rhodamine/HiLyte 647 and biotin labeled microtubules were polymerized in the presence of GMPCPP, a non-hydrolysable GTP-analogue, and immobilized on a neutravidin coated glass coverslip. Coverslips were briefly incubated with casein to block non-specific surface binding before addition of 100nM kinesin and/or varying concentrations of Gli2-ZF in assay buffer and antifade reagent (25mMglucose, 40 mg/ml glucose oxidase, 35 mg/ml catalase and 0.5% β-mercaptoethanol). Images were acquired from multiple fields of the same chamber.
Single molecule assays:
Interaction of single Kif7-DM-GFP molecules with microtubules was imaged as above except with lower Kif7-DM-GFP (1 nM or 0.1 nM). Images were acquired at 0.37 s intervals on an ANDOR iXon Ultra EMCCD camera with EM gain set to 287. Tracking analysis was only performed with 0.1 nM Kif7-DM-GFP datasets due to high density of tracks in the 1 nM Kif7-DM-GFP data set.
Initial fluorescence intensity and step-photobleaching analysis was performed by immobilizing GFP-tagged protein non-specifically to a glass coverslip. After washing away unbound protein, BRB80-DTT buffer supplemented with OS mix was added to the chamber and images were acquired as above. Fluorescent spots were selected using Particle Tracker under Mosaic Fiji plugin and the average intensity of the first three frames was used to compute the initial intensity after background subtraction. A custom MATLAB code for this analysis is now freely available on GitHub (https://github.com/Peii39/Gli-Subramanian-Lab).
Co-sedimentation of Kif7 on GDP-Taxol Stabilized Microtubules
GDP-taxol stabilized microtubules were prepared from purified, pre-cleared bovine tubulin as described in Subramanian et al., 201090. Kif7-DM-GFP (1 μM) was incubated with microtubules (0–10 μM) in the presence of 1mM ATP, 2mM MgCl2, with or without 5μM Gli for 15 minutes at room temperature in 1x BRB80 supplemented with 0.1 mg/mL BSA and then subjected to sedimentation by ultracentrifugation at 90,000 x g for 10 minutes at 27°C in a TLA 120.1 rotor (Beckman Coulter). Pellets were subsequently resuspended and the amount of protein in each pellet and supernatant was analyzed by SDS-PAGE. Proteins were stained using Gel Code™ Blue Stain (Thermo Fischer Scientific) and scanned using GE Typhoon FLA 9000 Gel Scanner. Dissociation constants were calculated by fitting of the experimental data to a Hill equation using GraphPad Prism.
Kif7 and Gli2 antibody generation and labeling
Recombinant Kif7-CC (460-600aa) was used to produce antibodies in rabbits from Covance Inc. (Princeton, NJ). The Kif7 antiserum was affinity-purified using antigen conjugated CNBr-Sepharose resins. The cDNA sequence encoding 1053-1264 aa mouse Gli2 was cloned upstream of a polyhistidine stretch (His) tag seqeunce in pET23b (Novagen). Recombinant mGli2-His protein was expressed in BL21 (DE3) pLysS cells (Stratagene) and purified under denaturing condition (8 M urea) using the TALON metal affinity resin (Clontech), and subsequently used to produce antibodies in guinea pigs from Covance Inc. (Princeton, NJ). The Gli2 antiserum was affinity-purified using antigen conjugated CNBr-Sepharose resins. The Kif7 and Gli2 antibodies were further ‘cleaned’ by incubation with fixed Kif7−/− and Gli2−/− MEFs respectively to reduce non-specific staining in immunofluorescence experiments and labeled with Alex Fluor 647 dye using Molecular Probes kit.
Cell culture, transfections, and immunofluorescence
HeLa cells were obtained as a gift from Robert Kingston (Massachusetts General Hospital). COS7 (ATCC; CRL-1651) and NIH3T3 (BPS Biosciences; 60409) cells were purchased. Immortalized primary mouse embryonic fibroblast (MEF) cells (WT, Gli2−/−, Gli2−/−Gli3−/−, Kif7−/−) were obtained from Kathryn Anderson (Sloan Kettering Institute), Robert Lipinski (University of Wisconsin), Adrian Salic (Harvard Medical School), and Stephane Angers (University of Toronto). HeLa, NIH3T3 and MEF cells were maintained in high-glucose Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS), sodium pyruvate (1mM) and L-glutamine (2 mM). Cells were cultured on clean coverslips under the following conditions: 5% CO2, 95% air condition at 37°C.
HeLa and COS7 cells were transfected with plasmids using jetPrime transfection reagent and incubated for 18-22 hours. Cells were fixed using a 1:1 v/v mix of methanol and acetone for 10 minutes in −20C, washed with washing buffer (PBS + 0.05% Tween 20) and mounted on 25mmX75mm glass slides with ProLong Diamond Antifade Mountant with DAPI (Thermo Fisher). For immunostaining, cells were blocked for an hour at room temperature in blocking buffer (2% BSA+ PBS + 0.05% Tween 20) post fixation and probed overnight at 4C with the following specific primary antibodies (diluted in blocking buffer): Alexa Fluor 647nm labeled anti-α-tubulin antibody, (EMD Millipore; catalogue# 05-829-AF647; clone DM1alpha; 1:50 dilution), Alexa Fluor 647nm labeled anti-calreticulin antibody (Abcam; catalogue# ab196159; clone# EPR3924; 1:50 dilution). Z-stacks were acquired on an inverted Nikon confocal microscope using laser illumination source (405nm, 488nm, 561nm for DAPI, Gli2-Neongreen and Kif7 respectively) with a pinhole of 0.5.
For experiments with NIH3T3, cells were grown to ~70 % confluency and transfection with plasmids using jetPrime and incubated for 18-22 hours. For experiments with MEFs, cells were grown to complete confluency. Subsequently, both cell types were serum-starved with 0.2% FBS in DMEM to induce ciliogenesis for 24 hours, followed by treatment with 500nM SAG for 12-18 hours before being fixed for immunofluorescence. Cells were fixed using a 1:1 v/v mix of methanol and acetone for 10 minutes in −20C, washed with washing buffer (PBS+0.05% Tween 20) for 3 times, blocked with blocking buffer (PBS + 2%BSA; OmniPur BSA; EMD Millipore) for one hour at room temperature. Samples were probed overnight at 4C with the following specific primary antibodies (diluted in blocking buffer): Alexa Fluor 647nm labeled anti-Kif7 antibody (produced in-house; 1:10 dilution), Alexa Fluor 647nm-labeled anti-Gli2 antibody (produced in-house; 1:10 dilution), Cyanine3 or Alexa-488-labeled anti- γ-tubulin antibody (Thermo Fisher Scientific; catalogue# PA1-28043; 1:1000 dilution) and FITC-labeled anti-acetylated α-tubulin antibody (Santa Cruz Biotechnology; catalogue# sc-23950; clone# 6-11B-1; 1:500 dilution). Samples were mounted on a 25mmX75mm glass with ProLong Diamond Antifade Mountant (Thermo Fisher). Z-stacks were acquired on an inverted Nikon confocal microscope using laser illumination source (488nm, 561nm, 647nm) with a pinhole of 1. Z-projections were generated by sum of images from planes that included the entire cilium.
Image and Statistical Analysis
ImageJ was used to extract microtubule-associated GFP/Alexa 647 fluorescence intensities (Fig 4a-c). Average intensity per pixel was calculated using rectangular regions-of-interest with a width of 3 pixels and the length of each microtubule. Local background intensities were subtracted using regions-of-interest of the same area around the selected microtubule. Intensities were not analyzed for microtubules found at the edges of the camera’s field of view.
For single molecule residence time analysis, fluorescent spots along each microtubule were analyzed using the ImageJ tracking plugin TrackMate to measure the track durations of the single kinesin molecules. The cumulative frequency plots of residence time were fitted to the following bi-phasic association function model in GraphPad Prism:
SpanFast=(Plateau-Y0)*PercentFast*.01
SpanSlow=(Plateau-Y0)*(100-PercentFast)*.01
Y=Y0+ SpanFast*(1-exp(−KFast*X)) + SpanSlow*(1-exp(−KSlow*X))
And TauFast (reciprocal of KFast) represents lifetime of single Kif7-DM-GFP molecules and TauSlow (reciprocal of KSlow) are likely to represents lifetime of single Kif7-DM-GFP molecules bound by Gli2-ZF.
The cumulative frequency plot of residence time of Kif7-DM in the absence of Gli2-ZF was not fit since a high fraction of the events in this condition have a very short the residence times of 1-2 frames. This indicates that the true lifetime is lower than the time resolution of our experiment (0.37 s per frame). For the same reason, the TauFast values are likely to represent the upper limit for the lifetime of un-complexed Kif7-DM molecules.
All TIRFM data were analyzed using GraphPad Prism. “N” numbers in experiments refer to the unique number of microtubules or single kinesin molecules (as mentioned in the figure legends) used for the dataset. Details of fit parameters can be found in the corresponding figure legends.
ImageJ was used to analyze intensity of protein bands for stoichiometry determination. A rectangular box was drawn around the protein band of interest for intensity readout. Local background was subtracted using a 3-pixel radius around the region of interest.
ImageJ was used to determine Kif7 and Gli2 intensity in the cilia in Fig 5b-c and 6d-e. Alexa647 (corresponding to either Kif7 or Gli2) intensity profile was calculated along the cilium length from tip to base. Intensity profiles of different cilia were aligned by their tips and averaged, and integrated intensity profiles were plotted using GraphPad prism. Statistical details can be found in the results section and corresponding figure legends.
ImageJ was used to quantify cytoplasmic sequestration in Fig 6a-c. The nucleus boundary (including the nucleolus pattern) was identified by using automatic thresholding on DAPI channel (Otsu method). The threshold value was obtained from auto-thresholding the brightest frame of confocal z-stacks images. This threshold value was used to create binary masks (“DAPI Nucleus mask”) on individual frames of Z-stacks, which were used to define the boundary for measuring Gli2-neongreen signal within the nucleus. The nuclear Gli2-neongreen intensity was then integrated after background subtraction using the rolling ball method (r=100 pixels). Next, to create a mask to measure the Gli2-neongreen signal within the whole cell boundary an ImageJ macro (“Gli2 Nuclear Mask”) was applied that automatically determined the threshold value. Ideally, this threshold should select the same pixels within the nucleus in Gli2-neogreen channel as the “DAPI Nucleus mask” does in DAPI channel. This way, the number of pixels representing the Gli2 signals are not over-represented or under-represented. This “best-match” threshold was selected by finding the maximum value in XNOR operation between “Gli2 Nucleus mask” and “DAPI Nucleus mask”, i.e. the pixels selected to represent nucleus in Gli2 channel and those selected to represent nucleus in DAPI channel has the highest overlap. With the given threshold value, a binary mask over the whole cell area was created (“Gli2 whole cell mask”) and again the Gli2-neongreen intensity in the whole cell was integrated after background subtraction using the rolling ball method (r = 100 pixels). A MATLAB code for this analysis is now freely available on GitHub (https://github.com/Peii39/Gli-Subramanian-Lab).
For in vitro experiments or studies involving cells in culture, groups were attributed randomly between wells and slide chambers to avoid for well or slide positioning effect. Data collection and analyses were not performed blind to the conditions of the experiments.
For the lifetime distribution in Extended Data Fig. 6c, a single rate lining dissociation step was assumed. Consistent with that, the data fit well to a simple exponential lifetime distribution. For all other data individual data points have been shown and the distribution was assumed to be normal, but this was not formally tested.
No statistical methods were used to pre-determine sample sizes, but our sample sizes are similar to those reported in previous publications25,89-92.
Extended Data
Extended Data Fig. 1. Purification, stoichiometry analysis and binding of recombinant Kif7-CC and Gli2-ZF proteins.
a, Pull-down of c-Myc-Gli2 (418-594aa) and FLAG-Kif7 (1-361aa) after co-transfection in Expi293F cells. Input (cell-lysate), FT (flow-through), wash and anti-FLAG immunoprecipitated(IP) beads samples were immunoblotted(IB) with anti-c-myc antibody to detect Gli2. Immunoblot with anti-FLAG antibody was used to detect the expression of Kif7. The blot is representative of three independent repeats. b, Chromatograms from size exclusion chromatography of GST-Kif7-CC-GFP, GST-Kif7-CC, GST-Kif7-SCC, Gli2-ZF-GFP and Gli2-ZF on Superdex 200 10/300 GL. Arrows indicate elution volumes of standards: (left to right) 1-ferritin(440kDa), 2-aldolase(158kDa), 3-ovalbumin(44kDa) and 4-carbonic anhydrase(29kDa). c, SDS-PAGE of purified GST-Kif7-CC-GFP, GST-Kif7-CC, GST-Kif7-SCC, Gli2-ZF-GFP and Gli2-ZF. MW – molecular weight markers. The gels are representative images of a minimum of three repeats. d, Single molecule fluorescence intensity histograms of Gli2-ZF-GFP (Intensity = 3.0 x 104 ± 1.4 x 104, N = 746) and GST-tagged Kif7-CC-GFP (Intensity = 4.2 x 104 ± 1.9 x 104, N = 536). Intensities are reported as mean ± standard deviation. e, Single molecule photobleaching traces for Gli2-ZF-GFP (1strow) and GST-Kif7-CC-GFP (2ndrow). Background subtracted integrated fluorescence intensity versus time plots used for step photobleaching analysis. Photobleaching steps are indicated by arrows. f, BLI assay to quantitatively examine the binding affinity of Kif7-CC-GFP to Gli2-ZF (black) & Gli2-ZF-GFP (green). Data represent mean and standard deviation from three independent repeats. The plots of binding response versus Gli2-ZF concentration were fit to a Hill equation to determine equilibrium dissociation constants (Kd). For Kif7-CC-GFP + Gli2-ZF: Kd = 65 ± 22nM, for Kif7-CC-GFP + Gli2-ZF-GFP: Kd = 57 ± 9nM. [Note: GFP-tagged versions of proteins show a consistent increase in Kd that is within error range]. g, Western blot of peak complex fraction from size exclusion chromatography of Kif7-CC-GFP and Gli2-ZF-GFP (see Fig.1d). Stoichiometry of components in complex is indicated in parenthesis. The blot is representative of three independent repeats.
Extended Data Fig. 2. Structural models of Kif7-CC-Gli2-ZF protein complex and DNA competition.
High confidence structural models of the Kif7-CC-Gli2-ZF protein complex from docking analysis (ClusPro 2.0). Gli2-ZF model is represented in blue and green colored ribbon diagrams and Kif7-CC models represented in salmon colored ribbon diagrams in the different models. a-b, Structural models that seem most probable based on our experiments. c, Overall electrostatic surface representation of the complex in a represented on a scale from −5 (red) to 0 (white) to +5 kT/e (blue) d-g, Structural models that are less probable from mutagenesis experiments (specifically Kif7-CC S2-mut, S3-mut1 and S3-mut2, see Extended Data Fig.3e,f,i). h-i, Structural models that appear less probable based on DNA competition experiments (See Fig. 2d). j, Competitive inhibition of Kif7-CC and Gli2-ZF (125nM) binding response upon titration with Gli2 target DNA; TRE-2S (grey) and non-target DNA; TFIIIA-BS (red). Raw binding response from the BLI measurements is plotted against DNA concentration. Data represent mean and standard deviation from four independent repeats.
Extended Data Fig. 3. Site-directed mutagenesis to validate the structural model of the Kif7-CC:Gli2-ZF complex.
a, Summary of Gli2-ZF truncations binding to Kif7-CC (left). Data is representative of raw-trace from the association step in BLI assay (right) from a minimum of three repeats. b, SDS-PAGE of purified Gli2-ZF truncation proteins. Gels are representative images from three repeats. c, Chromatograms from size-exclusion chromatography of Gli2-ZF truncation proteins on Superdex 75 10/300GL. d, BLI binding response of Kif7-CC with Gli2 truncation constructs: ZF1-3 and ZF1-4. Data represent mean +/− SD from three independent repeats. The binding response plots were fit to a Hill equation to determine equilibrium dissociation constants(Kd). For Gli2-ZF1-4: Kd = 103±15nM and Gli2-ZF1-4: Kd = 160±40nM. e, Pull-down experiment with c-Myc-Gli2(418-594aa) and FLAG-Kif7-CC(460-600aa) mutant proteins in Expi293F cells. Input (cell lysate), FT (flow through), wash and anti-FLAG immunoprecipitated(IP) beads samples were immunoblotted(IB) with anti-c-Myc antibody to detect Gli2. The blots are representative images of a minimum of three repeats. Gli2-ZF mutants: ZF2-mut(H493A/R496A), ZF3-mut(H503A/R516A/K521A), ZF4-mut(R550A/K552A/R556A). Kif7-CC mutants: S1-mut(E500A/E501A/E502A/D505A), S2-mut(E511A/E515A), S3-mut1(E526A/E529A) and S3-mut2(E530A/R535A). f, Structural model of Kif7-SCC:Gli2-ZF protein-complex. In dark blue are Gli2-ZF residues when mutated to alanine abolish Kif7-CC binding; in red are Kif7-CC residues when mutated to alanine abolish Gli2-ZF binding; in yellow are residues when mutated to alanine did not abolish binding; in green are sections(S1,S2,S3) represent 3 patches of Kif7-CC residues predicted as potential Gli-binding sites by PDBsum analysis. g, SDS-PAGE of purified Kif7-CC-S1 point mutant proteins. Gels are representative from three repeats. h, Chromatograms from size-exclusion chromatography of Kif7-CC S1 point mutant proteins on Superdex 200 10/300GL. i, Raw traces from the association step in the BLI assay performed with Kif7-CC S1 point mutant proteins: D505A, E501A/E502A, E502A, E502A/D505A, with Gli2-ZF. Inset, Binding response in BLI assay for D505A Kif7-CC mutant to Gli2-ZF. Data represent mean +/− SD from three independent repeats.
Extended Data Fig. 4. Purification and stoichiometry analysis of recombinant Kif7-DM Kif7-MM and Gli2-ZF-SNAP proteins.
a, Chromatograms from size-exclusion chromatography of Kif7-DM(black), Kif7-DM-GFP(green) and Gli2-ZF-SNAP(magenta, *represents peak used for experiments) on Superdex 200 10/300GL. Dotted line indicates elution volume of Gli2-ZF. Arrows indicate elution volumes of the following standards: (left to right) 1-ferritin(440kDa), 2-aldolase(158kDa) and 3-ovalbumin(44kDa). b, SDS-PAGE of purified Kif7-DM, Kif7-DM-GFP and Gli2-ZF-SNAP. Gels are representative images from three repeats. c, Fluorescence intensity histograms of Kif7-MM-GFP (Intensity = 3.0 x 104 ± 1.7 x 104, N=648) and Kif7-DM-GFP (Intensity = 4.0 x 104 ± 2.0 x 104, N=1015). Intensities are reported as mean +/− SD. d, Single molecule photobleaching traces for Kif7-MM-GFP(top) and Kif7-DM-GFP(bottom). Photobleaching steps are indicated by arrows. e, BLI assay to quantitatively examine binding affinity of Kif7-DM(black) and Kif7-DM-GFP(green) to Gli2-ZF, & Kif7-DM to Gli2-ZF-SNAP(maroon). Data represent mean +/− SD from three independent repeats. The plots of binding response versus Gli2-ZF concentration were fit to a Hill equation to determine equilibrium dissociation constants (Kd). For Kif7-DM + Gli2-ZF: Kd = 99 ± 25nM, for Kif7-DM + Gli2-ZF-SNAP: Kd = 99 ± 23nM, for Kif7-DM-GFP + Gli2-ZF: Kd = 138 ± 41nM, for [Note: GFP-tagged versions of proteins show a consistent increase in Kd that is within error range]. f, Scatter plot of Gli2-ZF-1-3-Alexa647 intensity per pixel on microtubules(MT) represents recruitment of Gli on MT by Kif7-MM and Kif7-DM. The no kinesin condition was a control for non-specific binding of Gli2-ZF1-3 to MT, and Kif27-DM was included as a negative control for Gli binding. Assay conditions: 100nM Gli2-ZF-1-3-SNAP-Alexa647 with 100nM of kinesin in each case. Data are presented as mean +/− SEM; N=32 microtubules analysed over 3 independent experiments for each condition. One-way ANOVA (p<0.0001) and post-hoc analysis (*indicates p<0.0001 in Dunnett’s multiple comparisons test) show statistically significant differences in Gli intensity compared to no kinesin control; ns is not significant (p=0.9425).
Extended Data Fig. 5. Cryo-EM maps of Kif7 and Gli2-ZF on microtubules.
a, Fourier shell correlation (FSC) curve for Kif7-DM bound to GMPCPP microtubules in the presence of AMPPNP and Gli2-ZF-SNAP. b, Cryo-EM reconstruction of dimeric Kif7 motor domain (green) in complex with AMPPNP bound on the GMPCPP-microtubule lattice (grey) in the presence of Gli2-ZF-SNAP (pink), shown in two orientations, and when viewed from the – end and +end of the microtubule. Density of Gli2-ZF-SNAP is seen only attached to the Kif7 motor domain and no extra density is seen along the microtubule lattice. c, EM reconstructions of AMPPNP-bound Kif7-DM with Gli2-ZF-SNAP (black mesh) and AMPPNP-bound Kif7-MM with Gli2-ZF-SNAP (orange) superposed via the Kif7 motor domain. Structural model for AMPPNP-bound Kif7 motor (green) is shown. d, EM reconstructions of AMPPNP-bound Kif7-DM with Gli2-ZF-SNAP (black mesh) and ADP-bound Kif7-DM with Gli2-ZF (yellow) superposed via the Kif7 motor domain. Structural model for AMPPNP-bound Kif7 motor (green) is shown. Blue dotted region indicates density seen only with Gli2-ZF-SNAP and not with Gli2-ZF (thereby likely corresponding to part of SNAP peptide). e, Cryo-EM reconstructions of AMPPNP-bound Kif7-DM with Gli2-ZF-SNAP. Structural models for AMPPNP-bound Kif7 motor (green) and Gli2-ZF consisting of 2 complete and one partial zinc finger (magenta) are shown. Helix α-2 (dark green) and loop L2 (blue) of Kif7 make contacts with the density for Gli2-ZF-SNAP. See also Supplementary Video 1.
Extended Data Fig. 6. Single molecule residence time analysis and microtubule co-sedimentation assay with Kif7-DM-GFP in the presence of Gli2-ZF.
a, Representative kymographs from assays to visualize single molecules of Kif7-DM-GFP (1nM) on X-rhodamine labeled microtubules with increasing Gli2-ZF concentrations (0, 25 & 50 nM). b, Representative kymographs from assays to visualize single molecules of Kif7-DM-GFP (0.1nM) on X-rhodamine labeled microtubules with increasing Gli2-ZF concentrations (0, 25 & 50 nM). c, Cumulative frequency distribution plots of Kif7-DM-GFP residence time on microtubules from analysis of 0.1 nM Kif7-DM-GFP data set with increasing Gli2-ZF concentrations. Numbers of observed events at different Gli2-ZF concentrations: 0 nM, 1191; 25 nM, 576; 50 nM, 817. Inset shows expanded view of the same data. Dotted lines represent bi-phasic association functions fitted to the 25nM and 50nM Gli data sets. [Parameters from fit: (i) For 25 nM Gli; R2 = 0.99; TauFast = 0.11 s; TauSlow = 1.64 s; (ii) For 50 nM Gli; R2 = 0.99; TauFast = 0.13 s; TauSlow = 2.05 s]. See Methods for details. d, SDS-PAGE analysis of 1 μM Kif7DM-GFP co-sedimentation on GDP-taxol stabilized microtubules (0 – 10 μM) in the presence of 1 mM ATP, 2mM MgCl2, with or without 5 μM Gli2-ZF, and the analysis of 5 μM Gli2-ZF non-specific co-sedimention on microtubules in the absence of Kif7 (S: Supernatant, unbound fraction. P: Pellet, bound fraction. BSA: Bovine Serum Albumin, gel loading control). The gels are representative images of a minimum of three repeats.
Extended Data Fig. 7. Purification and binding of recombinant dimeric Kif7 mutant and chimera proteins to Gli2-ZF in solution and on microtubules.
a, SDS-PAGE of purified Kif7-DME502A-GFP, Kif7M-Kif27CC-GFP and Kif27M-Kif7CC-GFP. MW – molecular weight markers. The gels are representative images of a minimum of three repeats. b, Chromatograms from size exclusion chromatography of Kif7-DME502A-GFP (purple), Kif7M-Kif27CC-GFP (red) and Kif27M-Kif7CC-GFP (blue) on Superdex 200 10/300 GL. Dotted line indicates elution volume of Kif7-DM-GFP on the same column. c, Representative images of microtubule (MT) and Kinesin-DM-GFP (Kinesin) in the absence (left) or presence of Gli2-ZF (right, 100nM). Fluorescence intensities were scaled similarly for images. Scale bars represent 2μm. d, Recruitment of Gli to microtubules (MT) by Kif7-DM-GFP (grey), Kif7-DME502A-GFP (purple), Kif7M-Kif27CC-GFP (red) and Kif27M-Kif7CC-GFP (blue). Scatter plot shows the ratio of Gli2-ZF-Alexa647 intensity to Kinesin GFP intensity on microtubules. Assay conditions: 100nM Gli2-ZF-SNAP-Alexa647 with 100nM of kinesin in each case. Data are presented as mean +/− SD; N = 43 microtubules analysed over 3 independent experiments for each condition. One-way ANOVA (p < 0.0001) and post-hoc analysis (* indicates p < 0.0001 in Dunnett’s multiple comparisons test) show statistically significant differences in Gli intensity compared to Kif7-DM-GFP control. e, BLI assay to quantitatively examine the binding affinity of Kif7-DME502A-GFP (purple), Kif7M-Kif27CC-GFP (red) and Kif27M-Kif7CC-GFP (blue) to Gli2-ZF. Data represent mean and standard deviation from three independent repeats. The plots of binding response versus Gli2-ZF concentration were fit to a Hill equation to determine equilibrium dissociation constants (Kd). For Kif7-DME502A-GFP: Kd = 414 ± 103nM, for Kif7M-Kif27CC-GFP: Kd = 397 ± 88nM and for Kif27M-Kif7CC-GFP: Kd = 217 ± 70nM.
Extended Data Fig. 8. Immunofluores cence of microtubules and ER-marker calreticulin in cellular localization experiments.
a, Localization of co-transfected mRuby-tagged full length Kif7 and mNeonGreen-tagged full length Gli2 on microtubules. b, Localization of co-transfected mRuby-tagged Kif7-CC (460-600aa) with ER retention tag, mNeonGreen-tagged full length Gli2 and ER-marker calreticulin in COS7 cells. Kif7-CC alone shows localization at the endoplasmic reticulum in the cytoplasm. DAPI staining was used to mark the nucleus. Gli2 is sequestered in the cytoplasm and shows co-localization with Kif7-CC both on the endoplasmic reticulum and in cytoplasmic puncta as seen in the zoomed insets (1 & 2). Scale bars represent 10 μm. The images are representative of three independent repeats.
Supplementary Material
Supplementary Video 1 Cryo-EM reconstructions of AMPPNP-bound Kif7-DM with Gli2-ZF-SNAP. Structural models for AMPPNP-bound Kif7 motor (green) and Gli2-ZF consisting of 2 complete and one partial zinc finger (magenta) are shown.
Acknowledgements
We thank Dr. Robert E. Kingston (Massachusetts General Hospital), Dr. Kathryn V. Anderson (Sloan Kettering Institute), Dr. Robert Lipinski (University of Wisconsin), Dr. Adrian Salic (Harvard Medical School), and Dr. Stephane Angers (University of Toronto) for kindly providing us with the HeLa and MEF (WT, Gli2−/−, Gli2−/−Gli3−/−, Kif7−/−) cell lines. We also thank Dr. Mu He (University of California San Francisco) for valuable discussions. R.S. was supported through the Pew Biomedical Scholars program, the American Cancer Society (Ellison Foundation Research Scholar) and the Smith Family Award for Excellence in Biomedical Research. R.A.M was supported through NIH R37GM052468.
Footnotes
Competing Interests Statement
Massachusetts General Hospital filed a provisional patent application (Application No. 63/089,770) covering use of peptides inhibitors of Glioma-associated oncogene derived from the coiled-coil dimerization domain of Kif7 that lists R.S., F.H., C.F. and Q.Y. as inventors. The remaining authors declare no competing interests.
Data availability
Source data are provided with this paper. The authors declare that all relevant data supporting the findings of this study are available within the paper and its Supplementary Information files. Structural data that support the findings of this study have been deposited in the publicly available repository, Protein Data Bank under Accession number 7RX0. Previously published protein primary sequences that were re-analysed here are available under UniprotKB with accession codes human Gli2 (P10070), the coiled-coil domain of human Kif7 (Q2M1P5), the coiled-coil domain of human Kif27 (Q86VH2), and the N-terminal motor domain of human Kif27 (Q86VH2). Previously published protein structures that were re-analyzed here are available under the Research Collaboratory for Structural Bioinformatics Protein Data Bank under accessions Gli1 zinc finger (PDB: 2GLI), ROCK1 coiled-coil (PDB: 3O0Z), and Kif7 motor domain (PDB: 6MLQ). The EM-derived structure of AMPPNP-Kif7 bound to microtubules is available at the PDB and Electron Microscopy Data Bank (PDB: 6 MLR, EMD: 9141).
Code availability
All custom codes used for analyses of the data are freely available on GitHub (https://github.com/Peii39/Gli-Subramanian-Lab).
References
- 1.Liu Y, Li P, Fan L & Wu M The nuclear transportation routes of membrane-bound transcription factors. Cell Communication and Signaling 16, 12 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dong C, Li Z, Alvarez R Jr, Feng X-H & Goldschmidt-Clermont PJ Microtubule binding to Smads may regulate TGFβ activity. Molecular cell 5, 27–34 (2000). [DOI] [PubMed] [Google Scholar]
- 3.Ziegelbauer J et al. Transcription factor MIZ-1 is regulated via microtubule association. Molecular cell 8, 339–349 (2001). [DOI] [PubMed] [Google Scholar]
- 4.Batut J, Howell M & Hill CS Kinesin-mediated transport of Smad2 is required for signaling in response to TGF-β ligands. Developmental cell 12, 261–274 (2007). [DOI] [PubMed] [Google Scholar]
- 5.Robbins DJ et al. Hedgehog elicits signal transduction by means of a large complex containing the kinesin-related protein costal2. Cell 90, 225–234 (1997). [DOI] [PubMed] [Google Scholar]
- 6.Sisson JC, Ho KS, Suyama K & Scott MP Costal2, a novel kinesin-related protein in the Hedgehog signaling pathway. Cell 90, 235–245 (1997). [DOI] [PubMed] [Google Scholar]
- 7.Wilson CW & Chuang P-T Mechanism and evolution of cytosolic Hedgehog signal transduction. Development 137, 2079–2094 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ingham PW, Nakano Y & Seger C Mechanisms and functions of Hedgehog signalling across the metazoa. Nature Reviews Genetics 12, 393–406 (2011). [DOI] [PubMed] [Google Scholar]
- 9.Huangfu D et al. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 426, 83–87 (2003). [DOI] [PubMed] [Google Scholar]
- 10.Lee J, Platt KA, Censullo P & Ruiz i Altaba A Gli1 is a target of Sonic hedgehog that induces ventral neural tube development. Development 124, 2537–2552 (1997). [DOI] [PubMed] [Google Scholar]
- 11.Hui C-C, Slusarski D, Platt KA, Holmgren R & Joyner AL Expression of three mouse homologs of the Drosophila segment polarity gene cubitus interruptus, Gli, Gli-2, and Gli-3, in ectoderm-and mesoderm-derived tissues suggests multiple roles during postimplantation development. Developmental biology 162, 402–413 (1994). [DOI] [PubMed] [Google Scholar]
- 12.Hui C.-c. & Angers S Gli proteins in development and disease. Annual review of cell and developmental biology 27, 513–537 (2011). [DOI] [PubMed] [Google Scholar]
- 13.Sasaki H, Nishizaki Y, Hui C.-c., Nakafuku M & Kondoh H Regulation of Gli2 and Gli3 activities by an amino-terminal repression domain: implication of Gli2 and Gli3 as primary mediators of Shh signaling. Development 126, 3915–3924 (1999). [DOI] [PubMed] [Google Scholar]
- 14.Wang B, Fallon JF & Beachy PA Hedgehog-regulated processing of Gli3 produces an anterior/posterior repressor gradient in the developing vertebrate limb. Cell 100, 423–434 (2000). [DOI] [PubMed] [Google Scholar]
- 15.Wen X et al. Kinetics of hedgehog-dependent full-length Gli3 accumulation in primary cilia and subsequent degradation. Molecular and cellular biology 30, 1910–1922 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matise MP, Epstein DJ, Park HL, Platt KA & Joyner AL Gli2 is required for induction of floor plate and adjacent cells, but not most ventral neurons in the mouse central nervous system. Development 125, 2759–2770 (1998). [DOI] [PubMed] [Google Scholar]
- 17.Ding Q et al. Diminished Sonic hedgehog signaling and lack of floor plate differentiation in Gli2 mutant mice. Development 125, 2533–2543 (1998). [DOI] [PubMed] [Google Scholar]
- 18.Kim J, Kato M & Beachy PA Gli2 trafficking links Hedgehog-dependent activation of Smoothened in the primary cilium to transcriptional activation in the nucleus. Proceedings of the National Academy of Sciences 106, 21666–21671 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zeng H, Jia J & Liu A Coordinated translocation of mammalian Gli proteins and suppressor of fused to the primary cilium. PloS one 5, e15900 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Caspary T, Larkins CE & Anderson KV The graded response to Sonic Hedgehog depends on cilia architecture. Developmental cell 12, 767–778 (2007). [DOI] [PubMed] [Google Scholar]
- 21.Goetz SC & Anderson KV The primary cilium: a signalling centre during vertebrate development. Nature Reviews Genetics 11, 331–344 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liem KF, He M, Ocbina PJR & Anderson KV Mouse Kif7/Costal2 is a cilia-associated protein that regulates Sonic hedgehog signaling. Proceedings of the National Academy of Sciences 106, 13377–13382 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheung HO-L et al. The kinesin protein Kif7 is a critical regulator of Gli transcription factors in mammalian hedgehog signaling. Science signaling 2, ra29–ra29 (2009). [DOI] [PubMed] [Google Scholar]
- 24.Endoh-Yamagami S et al. The mammalian Cos2 homolog Kif7 plays an essential role in modulating Hh signal transduction during development. Current biology 19, 1320–1326 (2009). [DOI] [PubMed] [Google Scholar]
- 25.He M et al. The kinesin-4 protein Kif7 regulates mammalian Hedgehog signalling by organizing the cilium tip compartment. Nature cell biology 16, 663–672 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maurya AK et al. Positive and negative regulation of Gli activity by Kif7 in the zebrafish embryo. PLoS Genet 9, e1003955 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dafinger C et al. Mutations in KIF7 link Joubert syndrome with Sonic Hedgehog signaling and microtubule dynamics. The Journal of clinical investigation 121, 2662–2667 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Putoux A et al. KIF7 mutations cause fetal hydrolethalus and acrocallosal syndromes. Nature genetics 43, 601–606 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blasius TL et al. Sequences in the stalk domain regulate autoinhibition and ciliary tip localization of the immotile kinesin-4 KIF7. Journal of Cell Science (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu YC et al. The PPFIA1-PP2A protein complex promotes trafficking of Kif7 to the ciliary tip and Hedgehog signaling. Science signaling 7, ra117–ra117 (2014). [DOI] [PubMed] [Google Scholar]
- 31.Schwarz N et al. Arl3 and RP2 regulate the trafficking of ciliary tip kinesins. Human molecular genetics 26, 2480–2492 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye F, Nager AR & Nachury MV BBSome trains remove activated GPCRs from cilia by enabling passage through the transition zone. Journal of Cell Biology 217, 1847–1868 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eguether T, Cordelieres FP & Pazour GJ Intraflagellar transport is deeply integrated in hedgehog signaling. Molecular biology of the cell 29, 1178–1189 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pavletich NP & Pabo CO Crystal structure of a five-finger GLI-DNA complex: new perspectives on zinc fingers. Science 261, 1701–1707 (1993). [DOI] [PubMed] [Google Scholar]
- 35.Dan S, Tanimura A & Yoshida M Interaction of Gli2 with CREB protein on DNA elements in the long terminal repeat of human T-cell leukemia virus type 1 is responsible for transcriptional activation by tax protein. Journal of virology 73, 3258–3263 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kinzler KW & Vogelstein B The GLI gene encodes a nuclear protein which binds specific sequences in the human genome. Molecular and cellular biology 10, 634–642 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoenger A et al. A new look at the microtubule binding patterns of dimeric kinesins. Journal of molecular biology 297, 1087–1103 (2000). [DOI] [PubMed] [Google Scholar]
- 38.Hirose K et al. Structural comparison of dimeric Eg5, Neurospora kinesin (Nkin) and Ncd head–Nkin neck chimera with conventional kinesin. The EMBO Journal 19, 5308–5314 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katoh Y & Katoh M KIF27 is one of orthologs for Drosophila Costal-2. International journal of oncology 25, 1875–1880 (2004). [PubMed] [Google Scholar]
- 40.Katoh Y & Katoh M Characterization of KIF7 gene in silico. International journal of oncology 25, 1881–1886 (2004). [PubMed] [Google Scholar]
- 41.He M, Agbu S & Anderson KV Microtubule motors drive hedgehog signaling in primary cilia. Trends in cell biology 27, 110–125 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Araghi RR et al. Iterative optimization yields Mcl-1–targeting stapled peptides with selective cytotoxicity to Mcl-1–dependent cancer cells. Proceedings of the National Academy of Sciences 115, E886–E895 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Araghi RR & Keating AE Designing helical peptide inhibitors of protein–protein interactions. Current opinion in structural biology 39, 27–38 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Foight GW, Chen TS, Richman D & Keating AE in Modeling Peptide-Protein Interactions 213–232 (Springer, 2017). [Google Scholar]
- 45.Truebestein L & Leonard TA Coiled-coils: The long and short of it. Bioessays 38, 903–916(2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yöksel D, Bianco PR & Kumar K De novo design of protein mimics of B-DNA. Molecular BioSystems 12, 169–177 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tsonis PA & Dwivedi B Molecular mimicry: structural camouflage of proteins and nucleic acids. Biochimica et Biophysica Acta (BBA)-Molecular Cell Research 1783, 177–187 (2008). [DOI] [PubMed] [Google Scholar]
- 48.Wang HC, Chou CC, Hsu KC, Lee CH & Wang AHJ New paradigm of functional regulation by DNA mimic proteins: recent updates. IUBMB life 71, 539–548 (2019). [DOI] [PubMed] [Google Scholar]
- 49.Wang H-C, Ho C-H, Hsu K-C, Yang J-M & Wang AH-J DNA mimic proteins: functions, structures, and bioinformatic analysis. Biochemistry 53, 2865–2874 (2014). [DOI] [PubMed] [Google Scholar]
- 50.Bochkareva E et al. Single-stranded DNA mimicry in the p53 transactivation domain interaction with replication protein A. Proceedings of the National Academy of Sciences 102, 15412–15417(2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu D et al. Solution structure of a TBP–TAFII230 complex: protein mimicry of the minor groove surface of the TATA box unwound by TBP. Cell 94, 573–583 (1998). [DOI] [PubMed] [Google Scholar]
- 52.Adriaans IE et al. MKLP2 Is a Motile Kinesin that Transports the Chromosomal Passenger Complex during Anaphase. Current Biology (2020). [DOI] [PubMed] [Google Scholar]
- 53.Ferry L et al. Methylation of DNA ligase 1 by G9a/GLP recruits UHRF1 to replicating DNA and regulates DNA methylation. Molecular cell 67, 550–565. e555 (2017). [DOI] [PubMed] [Google Scholar]
- 54.Kino T, Hurt DE, Ichijo T, Nader N & Chrousos GP Noncoding RNA gas5 is a growth arrest–and starvation-associated repressor of the glucocorticoid receptor. Science signaling 3, ra8–ra8 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu L, Yin M, Wang M & Wang Y Phage AcrIIA2 DNA mimicry: structural basis of the CRISPR and anti-CRISPR arms race. Molecular cell 73, 611–620. e613 (2019). [DOI] [PubMed] [Google Scholar]
- 56.Kaan HYK, Hackney DD & Kozielski F The structure of the kinesin-1 motor-tail complex reveals the mechanism of autoinhibition. Science 333, 883–885 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lee PL, Ohlson MB & Pfeffer SR The Rab6-regulated KIF1C kinesin motor domain contributes to Golgi organization. Elife 4, e06029 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kevenaar JT et al. Kinesin-binding protein controls microtubule dynamics and cargo trafficking by regulating kinesin motor activity. Current Biology 26, 849–861 (2016). [DOI] [PubMed] [Google Scholar]
- 59.Ren J et al. Coiled-coil 1-mediated fastening of the neck and motor domains for kinesin-3 autoinhibition. Proceedings of the National Academy of Sciences 115, E11933–E11942 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang G & Jiang J Multiple Cos2/Ci interactions regulate Ci subcellular localization through microtubule dependent and independent mechanisms. Developmental biology 268, 493–505 (2004). [DOI] [PubMed] [Google Scholar]
- 61.Zhou Q & Kalderon D Costal 2 interactions with Cubitus interruptus (Ci) underlying Hedgehog-regulated Ci processing. Developmental biology 348, 47–57 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Matise MP & Joyner AL Gli genes in development and cancer. Oncogene 18, 7852–7859(1999). [DOI] [PubMed] [Google Scholar]
- 63.i Altaba AR, Sánchez P & Dahmane N Gli and hedgehog in cancer: tumours, embryos and stem cells. Nature Reviews Cancer 2, 361–372 (2002). [DOI] [PubMed] [Google Scholar]
- 64.Peer E, Tesanovic S & Aberger F Next-generation Hedgehog/GLI pathway inhibitors for cancer therapy. Cancers 11, 538 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bird LE et al. Green fluorescent protein-based expression screening of membrane proteins in Escherichia coli. Journal of visualized experiments: JoVE (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saeed IA & Ashraf SS Denaturation studies reveal significant differences between GFP and blue fluorescent protein. International journal of biological macromolecules 45, 236–241 (2009). [DOI] [PubMed] [Google Scholar]
- 67.Camacho C et al. BLAST+: architecture and applications. BMC bioinformatics 10, 421 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Remmert M, Biegert A, Hauser A & Söding J HHblits: lightning-fast iterative protein sequence searching by HMM-HMM alignment. Nature methods 9, 173–175 (2012). [DOI] [PubMed] [Google Scholar]
- 69.Guex N, Peitsch MC & Schwede T Automated comparative protein structure modeling with SWISS-MODEL and Swiss-PdbViewer: A historical perspective. Electrophoresis 30, S162–S173 (2009). [DOI] [PubMed] [Google Scholar]
- 70.Waterhouse A et al. SWISS-MODEL: homology modelling of protein structures and complexes. Nucleic acids research 46, W296–W303 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Benkert P, Biasini M & Schwede T Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics 27, 343–350 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pettersen EF et al. UCSF Chimera--a visualization system for exploratory research and analysis. J Comput Chem 25, 1605–1612, doi: 10.1002/jcc.20084 (2004). [DOI] [PubMed] [Google Scholar]
- 73.Jurrus E et al. Improvements to the APBS biomolecular solvation software suite. Protein Sci 27, 112–128, doi: 10.1002/pro.3280 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kozakov D et al. The ClusPro web server for protein–protein docking. Nature protocols 12, 255 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De Beer TA, Berka K, Thornton JM & Laskowski RA PDBsum additions. Nucleic acids research 42, D292–D296 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Suloway C et al. Automated molecular microscopy: the new Leginon system. J Struct Biol 151, 41–60, doi: 10.1016/j.jsb.2005.03.010 (2005). [DOI] [PubMed] [Google Scholar]
- 77.Wilson-Kubalek EM, Cheeseman IM & Milligan RA Structural comparison of the Caenorhabditis elegans and human Ndc80 complexes bound to microtubules reveals distinct binding behavior. Mol Biol Cell 27, 1197–1203, doi: 10.1091/mbc.E15-12-0858 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lander GC et al. Appion: an integrated, database-driven pipeline to facilitate EM image processing. J Struct Biol 166, 95–102 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Li X et al. Electron counting and beam-induced motion correction enable near-atomic-resolution single-particle cryo-EM. Nature methods 10, 584–590 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hirschi M et al. Cryo-electron microscopy structure of the lysosomal calcium-permeable channel TRPML3. Nature 550, 411–414, doi: 10.1038/nature24055 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rohou A & Grigorieff N CTFFIND4: Fast and accurate defocus estimation from electron micrographs. J Struct Biol 192, 216–221, doi: 10.1016/j.jsb.2015.08.008 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ogura T, Iwasaki K & Sato C Topology representing network enables highly accurate classification of protein images taken by cryo electron-microscope without masking. J Struct Biol 143, 185–200 (2003). [DOI] [PubMed] [Google Scholar]
- 83.Sui H & Downing KH Structural basis of interprotofilament interaction and lateral deformation of microtubules. Structure 18, 1022–1031, doi: 10.1016/j.str.2010.05.010 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Egelman EH The iterative helical real space reconstruction method: surmounting the problems posed by real polymers. J Struct Biol 157, 83–94, doi: 10.1016/j.jsb.2006.05.015 (2007). [DOI] [PubMed] [Google Scholar]
- 85.Alushin GM et al. High-resolution microtubule structures reveal the structural transitions in alphabeta-tubulin upon GTP hydrolysis. Cell 157, 1117–1129, doi: 10.1016/j.cell.2014.03.053 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tang G et al. EMAN2: an extensible image processing suite for electron microscopy. J Struct Biol 157, 38–46, doi: 10.1016/j.jsb.2006.05.009 (2007). [DOI] [PubMed] [Google Scholar]
- 87.Grigorieff N FREALIGN: high-resolution refinement of single particle structures. J Struct Biol 157, 117–125, doi: 10.1016/j.jsb.2006.05.004 (2007). [DOI] [PubMed] [Google Scholar]
- 88.Afonine PV, Headd JJ, Terwilliger TC & Adams PD New tool: phenix.real_space_refine. Computational Crystallography Newsletter 4, 43–44 (2013). [Google Scholar]
- 89.Subramanian R, Ti S-C, Tan L, Darst SA & Kapoor TM Marking and measuring single microtubules by PRC1 and kinesin-4. Cell 154, 377–390 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Subramanian R et al. Insights into antiparallel microtubule crosslinking by PRC1, a conserved nonmotor microtubule binding protein. Cell 142, 433–443 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jiang S et al. Interplay between the kinesin and tubulin mechanochemical cycles underlies microtubule tip tracking by the non-motile ciliary kinesin Kif7. Developmental cell 49, 711–730. e718 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Subramanian R & Gelles J Two distinct modes of processive kinesin movement in mixtures of ATP and AMP-PNP. The Journal of general physiology 130, 445–455 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Video 1 Cryo-EM reconstructions of AMPPNP-bound Kif7-DM with Gli2-ZF-SNAP. Structural models for AMPPNP-bound Kif7 motor (green) and Gli2-ZF consisting of 2 complete and one partial zinc finger (magenta) are shown.
Data Availability Statement
Source data are provided with this paper. The authors declare that all relevant data supporting the findings of this study are available within the paper and its Supplementary Information files. Structural data that support the findings of this study have been deposited in the publicly available repository, Protein Data Bank under Accession number 7RX0. Previously published protein primary sequences that were re-analysed here are available under UniprotKB with accession codes human Gli2 (P10070), the coiled-coil domain of human Kif7 (Q2M1P5), the coiled-coil domain of human Kif27 (Q86VH2), and the N-terminal motor domain of human Kif27 (Q86VH2). Previously published protein structures that were re-analyzed here are available under the Research Collaboratory for Structural Bioinformatics Protein Data Bank under accessions Gli1 zinc finger (PDB: 2GLI), ROCK1 coiled-coil (PDB: 3O0Z), and Kif7 motor domain (PDB: 6MLQ). The EM-derived structure of AMPPNP-Kif7 bound to microtubules is available at the PDB and Electron Microscopy Data Bank (PDB: 6 MLR, EMD: 9141).
All custom codes used for analyses of the data are freely available on GitHub (https://github.com/Peii39/Gli-Subramanian-Lab).