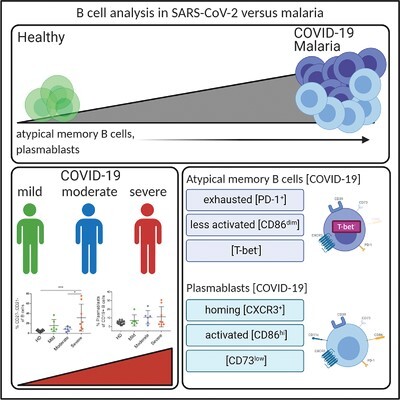
Abstract
B cells play a central role in antiviral and antiparasitic immunity, not only as producers of antibodies, but also as APCs and mediators of inflammation. In this study, we used 16-color flow cytometry analysis to investigate the frequency, differentiation, and activation status of peripheral B cells of patients with SARS-CoV-2 infection or acute Plasmodium falciparum malaria compared with the healthy individuals. As a main result, we observed an increase of the frequency of (CD27–, CD21–) atypical memory B cells and (CD19+, CD27+, CD38+) plasmablasts in malaria and COVID-19 patients. Additionally, CD86, PD-1, CXCR3, and CD39 expression was up-regulated, whereas CD73 was down-regulated on plasmablasts of COVID-19 and malaria patients compared with the bulk B cell population. In particular, there was a more pronounced loss of CD73+ B cells in malaria. The frequency of plasmablasts positively correlated with serum levels of CRP, IL-6, and LDH of COVID-19 patients. In the longitudinal course of COVID-19, a rapid normalization of the frequency of atypical memory B cells was observed. The role and function of plasmablasts and atypical memory B cells in COVID-19 and other acute infections remain to be further investigated. The role of B cells as either “driver or passenger” of hyperinflammation during COVID-19 needs to be clarified.
Keywords: CD19, CD73, CD39, hyperinflammation
Graphical Abstract
Plasmablasts and atypical memory B cells are more frequent in patients with COVID-19 and malaria than in healthy subjects.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first observed in the region Wuhan, Hubei Province, China in December 2019. Since then, this disease caused by the SARS-CoV-2 virus is now called coronavirus disease 2019 (COVID-19) and has emerged as a global health problem.1,2 COVID-19 is accompanied by fatigue, fever, and cough. In some cases, patients develop severe pneumonia and acute respiratory distress syndrome with high morbidity and mortality.3
SARS-CoV-2 has a tropism for angiotensin converting enzyme-2, which is expressed on multiple cell types including epithelial cells of the respiratory tract.4 Patients with severe COVID-19 have symptoms of systemic hyperinflammation and high serum levels of cytokines such as granulocyte-macrophage colony stimulating factor , TNF, IFN-γ, and IL-6, indicative of cytokine release syndrome.5 Additionally, this cytokine storm is associated with an increase in serum levels of C-reactive protein (CRP), lactate dehydrogenase (LDH), D-dimer, and ferritin.6–8
The central role of T cells and monocytes in the immune response to COVID-19 has been recognized.7,9,10 In contrast, knowledge about B cell immunology in COVID-19 is still very limited, although some alterations of the B cell compartment have been reported.11–13
However, it has already been shown that B cells are involved in the immune pathophysiology of various infectious diseases such as HIV and malaria.14–16 In these diseases, a change in B cell subset distribution toward elevated frequencies of atypical memory B cells and plasmablasts has been observed (see Methods section for a description of the B cell subset terminology used in this paper).17 Atypical memory B cells make up a heterogeneous population that is defined by an atypical memory/CD38– phenotype and that to a large extent overlaps with the population of CD27–, CD21– atypical memory cells.17 They contain B cells that express surface receptors with inhibitory potential like PD-1 and trafficking receptors like CD11c and CXCR3.18,19 Both populations are believed to be involved in the pathophysiology of malaria and HIV infection, and are also expanded in autoimmune diseases and immune deficiencies.16 Their composition and roles in different disease entities are currently subject of extensive investigation.20,21 First studies have shown that patients with primary antibody deficiency who are lacking B cells have a milder COVID-19 disease course than patients with dysfunctional B cells in common (but longer) variable immunodeficiency (CVID),22 indicating a potential role for B cells in the pathogenesis of COVID-19 infection.23
Furthermore, a particularly high proportion of B cells express the ectonucleotidases CD39 and CD73.24 In CVID and infectious diseases like HIV, a loss of CD73 is associated with a disturbance of the IgG class switch, the homing potential in lymph nodes and a disturbed conversion of adenosine monophosphate to adenosine.25–28
To date, there are few studies that have investigated the role and function of B cells in COVID-19.11–13,22
In the current study, samples of malaria-infected patients serve as an established immunologic comparator since a strong B cell immune response is a hallmark of malaria14,18,19 and both infections have a similar clinical presentation with severe systemic immune activation accompanied by lymphopenia and sometimes progressing to a sepsis-like status.29–31 In particular, the formation of high numbers of atypical memory B cells has been described for malaria,16,19 and high numbers of B cells with a plasmablast phenotype seem to be responsible for immune hyperactivation and nutrient deprivation.32 Recently, we have observed similarities between malaria and COVID-19 patients in the phenotypic patterns of T cells, in particular with regard to a transient increase of inhibitory receptors in the context of generalized acute immune activation and little is known whether these pattern also hold true for the B cell compartment.9 So far, the B cell response in COVID-19 has only been compared with the B cell phenotype of other noninfectious diseases like systemic lupus erythematodus.12
At the moment, it is not clear whether sterile immunity against SARS-CoV-2 can be achieved, whether the control of the virus is mainly T cell or B cell mediated,33 or whether previous infection with a common coronavirus strain leads to cross-immunity or to a misguided immune response as has been described in other infectious diseases such as dengue fever or hepatitis C infection.34,35 For this reason, further detailed studies about the B cell function in COVID-19 are needed.36
In this current study, we provide phenotypic data about the activation and differentiation status of peripheral B cells during acute COVID-19 infection compared with B cells in malaria and healthy individuals. The results of this explorative study suggest that the frequencies of atypical memory B cells and circulating plasmablasts correlate with disease severity, possibly contributing to the immune hyperactivation and dysregulation in COVID-19 pathophysiology.
Methods
Subjects
Cryopreserved PBMCs of SARS-CoV-2-infected patients (n = 20), P. falciparum-infected malaria patients (n = 12) as well as uninfected healthy controls (n = 15), all collected at the University Medical Center Hamburg–Eppendorf, were used for immunophenotypic staining. SARS-CoV-2 infection was confirmed by quantitative RT-PCR (qRT-PCR) of throat swab samples. Plasmodium falciparum infection was confirmed microscopically at the Bernhard-Nocht-Institute for Tropical Medicine. Thick blood film and thin blood smears were stained with 4% Giemsa and analyzed under oil immersion (original magnification ×100). Clinical characteristics and laboratory parameters of malaria patients and healthy donors (HDs) are shown in Table 2. All patients were hospitalized at the University Medical Center Hamburg-Eppendorf. Written consent was obtained from all study participants and the study was approved by the local ethics board of the Ärztekammer Hamburg (PV4238, PV4780, and PV7289).
TABLE 2.
Clinical and immunologic parameters of malaria patients and healthy donors
| Patient ID | Sex/Age | Days since start of symptoms | Initial parasitemia (%) | CRP (mg/l) | Hemoglobin (g/dl) | Thrombocytes (1000/μl) | Number of malaria episodes | Therapy |
|---|---|---|---|---|---|---|---|---|
| M1 | m 62 | 10 | <1 | 173 | 12.6 | 53 | Euratesim® | |
| M2 | m 49 | 6 | <1 | 110 | 14 | 115 | >1 | Euratesim® |
| M3 | m 62 | 2 | 2.4 | 118 | 12 | 123 | Atovaquon/Proguanil | |
| M4 | m 37 | 5 | <1 | 256 | 13.6 | 50 | Euratesim® | |
| M5 | m 72 | 10 | 18 | 169 | 10.8 | 106 | >1 | Arthemeter/Lumefantrin |
| M6 | w 47 | 14 | 10 | 170 | 10 | 91 | Atovaquon/Proguanil | |
| M7 | m 51 | 11 | 2.5 | 54 | 12.9 | 123 | Euartesim® | |
| M8 | m 68 | 21 | >1 | 51 | 10.9 | 155 | >1 | Atovaquon/Proguanil |
| M9 | m 71 | 3 | >1 | 60 | 11 | 114 | Atovaquon/Proguanil | |
| M10 | m 36 | > 14 | 2 | 58 | 10.5 | 155 | >1 | Artesunate/Lumefantrin |
| M11 | m 54 | 3 | <1 | 223 | 14.2 | 88 | Euratesim® | |
| M12 | w 52 | 5 | 3-4 | 56 | 12.1 | 50 | Euratesim® | |
| Mean | 55,1 | 8.7 | 3.7 | 124.8 | 12.05 | 101.9 | ||
| Median | 53 | 8 | 1.5 | 114 | 12.05 | 110 | ||
| HD1 | w 25 | |||||||
| HD2 | w 30 | |||||||
| HD3 | m 46 | |||||||
| HD4 | w 30 | |||||||
| HD5 | m 28 | |||||||
| HD6 | m 35 | |||||||
| HD7 | w 37 | |||||||
| HD8 | w 29 | |||||||
| HD9 | w 31 | |||||||
| HD10 | m 51 | |||||||
| HD11 | w 46 | |||||||
| HD12 | w 25 | |||||||
| HD13 | w 26 | |||||||
| HD14 | w 30 | |||||||
| HD15 | n.a. | |||||||
| Mean | 33.5 | |||||||
| Median | 30 |
n.a., not acquired; m, man; w, woman.
Intracellular staining and multiparametric flow cytometry
Intracellular as well as surface staining was performed as previously described.9 In total, we used 3 panels in this study. Panels 1 and 2 were exclusively surface panels. Panel 3 was the panel for the intracellular staining and included the staining of T-bet (Supplemental Fig. 1). In the FACS staining, the immunogolbulins IgM, IgD, and IgG were stained only on the surface. Cells were analyzed on a BD LSRFortessa (BD Bioscience). The following antibodies were used: BUV395 antiCD21 (Clone: B-Ig4, Catalog-number: 740228), BUV737 anti-CD39 (Clone: TU66, Catalog-number: 564726), BV421 anti-PD-1 (Clone: EH12.2H7, Catalog-number: 329920), BV510 anti-CD20 (Clone: 2H7, Catalog-number: 302340), BV605 anti-CD73 (Clone: AD2, Catalog-number: 344024), BV650 anti-CD24 (Clone: ML5, Catalog-number: 563710), BV711 anti-CD38 (Clone: HIT2, Catalog-number: 303528), BV785 anti-CD27 (Clone: O323, Catalog-number: 302831), FITC anti-IgM (Clone: MHM-88, Catalog-number: 314506), PerCp/Cy5.5 anti-IgD (Clone: IA6-2, Catalog-number: 348207), PerCp/Cy5.5. anti-IgG (Clone M1310G05, Catalog-number: 410710), PE anti-CD86 (Clone: IT2.2, Catalog-number: 305406), PE anti-CD138 (Clone MI15, Catalog-number 356504), PE/Dazzle™ anti-CD11c (Clone: 3.9., Catalog-number: 301641), PE/Cy7 CD39 (Clone: A1, Catalog-number: 328212), PE/Cy7 anti-CD19 (Clone: HIB19, Catalog-number: 302216), APC anti-CXCR3 (Clone: G025H7, Catalog-number: 353707), APC anti-T-bet (Clone: 4B10, Catalog-number 644814), Alexa Fluor® anti-CD19 (Clone: H1B19, Catalog-number: 302225), and Alexa Fluor® 700 anti-CD3 (Clone: OKT3, Catalog-number: 317340). BUV395 anti-CD21, BUV737 anti-CD39, and BV650 anti-CD24 were purchased from BD Bioscience (Heidelberg, Germany) and all other antibodies were purchased from BioLegend (Koblenz, Germany).
Classification of B cell subsets
Recognizing that there is no generally accepted scheme for the classification of B cell subsets, we have chosen to use the following terminology in this paper in accord with the gating strategy used. After exclusion of transitional B cells (defined as CD24+, CD38+), mature B cells were classified according to their expression of CD21 and CD27 into naïve (CD21+, CD27–), CD21+ memory (CD21+, CD27+), CD21–, CD27+ memory (CD21–, CD27+), and atypical memory (CD21–, CD27–) populations.16 In parallel to this classification, circulating plasmablasts were defined on the basis of their high coexpression of CD27 and CD38, and atypical memory B cells were defined by low expression of CD21 and absent expression of CD38.
Detection of SARS-CoV-2 antibodies
We used the WANTAI SARS-CoV-2 Ab Elisa Kit CE IVD from Szabo Scandic (Vienna, Austria) for the qualitative determination of the total amount of SARS-CoV-2-specific antibodies in the blood of patients, according to the manufacturer’s instructions.37–41 For the quantitative determination of SARS-CoV-2 antibodies, we used the LIAISON® SARS-CoV-2 IgM and LIAISON® SARS-CoV-2 S1/S2 IgG according to the manufacturer’s instruction.41
Statistical analysis
Cytometric data were analyzed using FlowJo version 10.6.2 software (Treestar, Ashland, OR). Statistical analysis was carried out using Prism 7.2 software (GraphPad Software, San Diego, CA). All results were checked for normal distribution using the D’Agostino-Pearson omnibus normality test or Shapiro–Wilk normality test. For the comparison of individual nonpaired samples, we used a parametric t-test or Mann–Whitney test. For paired analyses, we used a paired t-test or Wilcoxon matched-pairs signed rank test. For more than 2 groups, an ANOVA followed by Tukey’s multiple comparison test was used. For correlation analyses, a Pearson correlation for parametric and Spearman correlation for nonparametric data were performed. All data are expressed as mean values with sd, unless otherwise described in the text. P values of less than 0.05 were considered significant, where *, **, ***, and **** indicate P values < 0.05, < 0.01, < 0.001, and < 0.0001, respectively. Graphical abstract: The Illustration was created with the online software BioRender (San Francisco, CA). t-Distributed stochastic neighbor embedding (tSNE) analyses were performed on HDs, COVID-19, and malaria patients, using the tSNE plugin in FlowJo 10.6.2.42
Results
Clinical parameters of the study cohort
The clinical cohort consisted of 20 SARS-CoV-2 and 12 P. falciparum-infected patients and 15 HDs. All patients were hospitalized at the University Medical Center Hamburg Eppendorf. The patients infected with SARS-CoV-2 were classified into patients with mild (n = 6), moderate (n = 6), and severe (n = 8) disease course on the basis of clinical and laboratory parameters according to criteria of the World Health Organization43 (Supplemental Table 1). At the time of initial blood collection, none of the patients had been transferred to the intensive care unit. However, 3 COVID-19 patients were transferred to the intensive care unit during the course of their disease, and of these, 1 patient died from pulmonary artery embolism. Specimens were collected 10.5 ± 4.2 days on average after the onset of symptoms. The mean age of the COVID-19 patients was 56 years compared to 55.1 years of patients with malaria and 33.5 of HDs. Table 1 shows clinical laboratory parameters at the time of sample collection. As expected, the highest levels of IL-6, ferritin, and LDH were observed in patients with a severe COVID-19 course (Supplemental Fig. 2). In 5 patients, we were able to analyze longitudinal PBMC samples after resolution of the infection (defined as 2 consecutive nasopharyngeal swabs tested negative for SARS-CoV-2). Longitudinal samples were taken between days 24 and 27 after the primary infection, and 2 samples were taken on day 107 and 112 after the primary infection.
TABLE 1.
Clinical and immunologic parameters of COVID-19 patients
| Patient ID | WHO | Sex/Age | Days since start of symptoms | CT (cq) | CRP (mg/l) < 5 mg/l | IL-6 (ng/μl) < 7 ng/l | Procalcitonin (μg/l) < 0.5 μg/l | d-Dimers (mg/l) 0.21–0.52 mg/l | Ferritin (μg/l) 10–291 μg/l | LDH (U/l) 120–246U/l | Wantai | SARS-CoV-2 IgM positive > 1 | SARS-CoV-2 IgG positive > 15 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C19-01 | Severe | m 60 | 9 | 34.86 | 58 | 43.5 | 0.06 | 0.55 | 1972 | 390 | Negative | 0.1 | 3.8 |
| C19-02 | Mild | m 50 | 8 | 37.24 | 53 | 40.2 | n.a | 1.08 | 501 | 280 | Positive | 0.476 | 3.8 |
| C19-03 | Severe | m 77 | 6 | 28.29 | 97 | 63.3 | 0.04 | n.a | 1096 | 483 | Positive | 0.272 | 3.8 |
| C19-04 | Severe | m 58 | 13 | 32.75 | 61 | 21 | 0.09 | 2.43 | 2270 | 423 | Negative | 0.215 | 3.8 |
| C19-05 | Mild | m 57 | 5 | n.a. | 108 | 38.3 | n.a | 0.64 | 316 | 327 | Positive | n.a | n.a |
| C19-06 | Moderate | m 48 | 12 | neg. | 33 | 9.1 | 0.05 | n.a | 155 | 258 | Negative | 0.346 | 3.8 |
| C19-07 | Mild | m 75 | 10 | 27.51 | 56 | 33.5 | 0.02 | n.a | 564 | 243 | Positive | 1.32 | 5.25 |
| C19-08 | Mild | m 49 | 12 | 21.5 | 12 | 1.9 | 0.05 | n.a | 280 | 187 | Positive | 0.199 | 3.8 |
| C19-09 | Severe | m 63 | 11 | 25.1 | 133 | 125 | 0.08 | 23.05 | 1068 | 664 | Positive | 0.0898 | 3.8 |
| C19-10 | Moderate | m 45 | 9 | n.a. | 8 | 8.4 | n.a | n.a | n.a | 263 | Positive | 4.53 | 3.8 |
| C19-11 | Severe | m 66 | 10 | 24.9 | 171 | 106.5 | 0.31 | 35.20 | 1367 | 644 | Negative | 1.04 | 3.8 |
| C19-12 | Moderate | w 60 | 8 | 29.8 | 63 | 44.9 | 0.02 | 0.37 | 153 | 284 | Positive | 0.237 | 3.8 |
| C19-13 | Severe | w 71 | 9 | 32.09 | 104 | 47.2 | 0.16 | 2.98 | 2290 | 432 | Positive | 30.2 | 44.2 |
| C19-14 | Mild | w 38 | 4 | 28.41 | 6 | n.a | n.a | n.a | n.a | n.a | Negative | 0.369 | 3.8 |
| C19-15 | Moderate | w 51 | 11 | neg. | 244 | 46.4 | 1.91 | 1.15 | 2716 | 349 | Positive | 10.5 | 79.9 |
| C19-16 | Mild | m 39 | 22 | neg. | 46 | 4.7 | 0.05 | 1.07 | 249 | 240 | Positive | 5.29 | 55.3 |
| C19-17 | Severe | m 73 | 9 | 33.45 | 129 | 69.7 | 0.28 | 1.29 | 1714 | 685 | Positive | 2.37 | 3.8 |
| C19-18 | Moderate | m 33 | 12 | neg. | 306 | 63.7 | 0.22 | 2.57 | 1006.1 | 413 | Positive | 134 | 49.2 |
| C19-19 | Severe | m 52 | 20 | n.a. | 7 | 1.5 | 0.02 | 1.08 | 526 | 192 | Positive | 7.66 | 168 |
| C19-20 | Moderate | w 55 | 10 | neg. | 38 | 19.9 | 0.02 | 1.48 | 16.7 | 245 | n.a. | n.a | n.a |
| Mean | 56 | 10.5 | 29.66 | 86.65 | 41.51 | 0.2113 | 5.353 | 1014 | 368.5 | 11.07 | 24.86 | ||
| Median | 56 | 10 | 29.11 | 59.5 | 40.2 | 0.055 | 1.22 | 785.1 | 327 | 0.758 | 3.8 |
n.a., not acquired; neg, negative; m, man; w, woman.
The frequencies of CD27–, CD21– B cells and plasmablasts are elevated during acute COVID-19 and correlate with disease severity and laboratory parameters of inflammation
To characterize disease-associated changes in the B cell compartment, we collected peripheral blood samples from patients with COVID-19 (n = 20), patients with acute P. falciparum infection (n = 12), and uninfected controls (n = 15). The clinical and laboratory data are summarized in Tables 1 and 2. First, we analyzed the differentiation status of B cells (not including plasmablasts, see gating strategy in Supplemental Fig. 3), and compared the distribution of B cell subsets between the groups. Immature, transitional B cells were identified on the basis of high coexpression of CD24 and CD38, and the remaining mature B cells were classified into subpopulations on the basis of their expression of CD21 and CD27: naïve (CD27–, CD21+), CD21+ memory (CD27+, CD21+), CD27+, CD21– memory (CD27+, CD21–) and atypical memory B cells (CD27–, CD21–).16 Representative FACS plots for HDs, COVID-19, and malaria patients are shown in Figure 1A. We observed a dramatic relative increase of the frequency of atypical memory B cells in both COVID-19 and malaria patients compared with healthy (Fig. 1B). Because of their low expression of the B cell-defining markers CD19 and CD20, a separate gating strategy was used to identify circulating plasmablasts (Supplemental Fig. 4). Representative FACS plots for the plasmablasts of HDs, COVID-19, and malaria patients are shown in Figure 1C. We observed a significant increase of the frequency of plasmablasts in COVID-19 and malaria patients compared with the healthy controls (Fig. 1D). In contrast to malaria patients and healthy controls, COVID-19 patients showed a high variability of the frequency of their plasmablasts. We therefore investigated whether these changes of the B cell differentiation and phenotype were associated with disease severity and laboratory markers of inflammation. Indeed, atypical memory B cells were significantly enriched in severely ill patients in comparison with the moderately ill patients or healthy controls (Fig. 1E). A similar trend was observed for plasmablasts, but the values did not reach statistical significance most likely due to the low sample size (Fig. 1F). Infection with SARS-CoV-2 leads to systemic inflammation as evidenced by an increase in inflammation markers (e.g., CRP, IL-6, ferritin, LDH)6–8 (Supplemental Fig. 1). We observed positive correlations between the frequency of plasmablasts and the serum concentrations of IL-6, CRP, and LDH, and, similarly, between the frequency of atypical memory B cells and ferritin levels (Fig. 2A and B). Again, the patients with most severe courses of COVID-19 showed the highest values in terms of inflammation parameters (IL-6, LDH, and ferritin) and frequencies of plasmablasts and atypical memory B cells (Fig. 2A and B). However, there were no other significant correlations between B cell frequencies and other clinical laboratory parameters (Supplemental Fig. 5).
FIGURE 1.

Frequencies of CD27–, CD21– B cells and plasmablasts are elevated in acute COVID-19 and malaria compared with the healthy donors (HDs). (A) Representative FACS plots showing the frequency of transitional B cells (left panel) and the distribution of mature B cell subsets defined by CD21/CD27 (right panel) in HDs, COVID-19, and malaria patients. The panels are gated on CD19+, CD20+ cells, in the right panel transitional cells have been excluded (see Supplemental Fig. S3 for the detailed gating strategy). (B) Comparison of B cell subset distribution in HDs, COVID-19, and malaria patients. The pie charts show the mean frequency of transitional B cells (defined as CD24+, CD38+), naïve (CD21+, CD27–), CD21+ memory (CD21+, CD27+), CD21–, CD27+ memory(CD21–, CD27+) and atypical memory (CD21–, CD27–) populations in HDs, COVID-19, and malaria patients. (C) Representative FACS plots showing the frequency of plasmablasts (CD19+, CD27+, CD38+) in HDs, COVID-19, and malaria patients (see Supplemental Fig. S4 for the detailed gating strategy). (D) Frequency of plasmablasts in HDs and COVID-19 and malaria patients. (E and F) Comparison of the frequencies of atypical memory B cells (E) and plasmablasts (F) between mild, moderate, and severe COVID-19 patients. Data are shown as mean ± sd, where *, **, ***, and **** indicate P values <0.05, <0.01, <0.001, and <0.0001
FIGURE 2.

Frequency of plasmablasts and atypical memory B cells correlates with inflammation parameters. (A–D) Correlations between the frequency of plasmablasts and serum levels of IL-6 (A), CRP (B), and LDH (C), as well as the frequency of atypical memory B cells and serum levels of ferritin (D) in COVID-19 patients. Pearson correlation coefficients were applied for parametric data, respectively, and P values < 0.05 were considered significant
B cells during acute COVID-19 and malaria infection show a similar phenotype
In order to analyze the multidimensional phenotypic data of B cell populations, we performed a tSNE analysis. We identified specific clusters for healthy blood donors (green), COVID-19 (blue), and malaria patients (red) (Fig. 3A). Similarly, clusters representing CD19+, CD27+, CD38+ plasmablasts (Fig. 3B) and transitional, naïve, CD21+ memory, memory, and atypical memory B cells (Fig. 3C) were identified. Analysis of the expression levels of different markers of individual clusters (Fig. 3D) showed that plasmablasts expressed high levels of CD39, moderate levels of CD86, CXCR3, and low levels of CD73 in comparison with the other B cell populations. Not unexpectedly, the population of atypical memory cells (defined by lack of expression of CD21 and CD27) was fairly heterogeneous (Fig. 3B, red clusters). Clusters belonging to this population exhibited heterogeneous expression of functionally relevant markers such as CD39, CD73, CXCR3, CD86, or PD-1 (Fig. 3D), suggesting that this phenotypic population consists of several functionally different subsets. Separating atypical memory clusters into their groups of origin (HDs, COVID-19, and malaria patients) showed that in comparison with the HDs both disease groups were enriched for atypical memory populations of different functionality (Supplemental Fig. 6). For example, samples from COVID-19 patients contained expanded clusters with high expression of effector molecules such as CD39, CD86, and CXCR3 (red arrow), as well as clusters with higher expression of PD-1 and lower expression of CD86 and CXCR3 (green arrow) (Supplemental Fig. 6). We also evaluated the degree to which cells expressing the markers PD1, CD86, CXCR3, CD11c, CD39, and CD73 were enriched in atypical memory B cells (Fig. 3E) and plasmablasts (Fig. 3F) in comparison with the total B cells. Reflecting their heterogeneous nature, cells expressing the markers PD-1, CD86, CXCR3, and CD11c were significantly enriched in atypical memory B cells of malaria patients compared with the healthy controls. However, COVID-19 patients were more heterogeneous with regard to the expression of these markers, and enrichment of these populations could only be observed in individual patients and did not reach significance for the disease group as a whole. Since proinflammatory effector functions of CD21–, CD27– B cells have been linked to expression of the transcription factor T-bet,44 we analyzed expression of this transcription factor in atypical B cells from a cohort of 3 COVID-19 patients and 4 healthy controls (Supplemental Fig. 7). Intriguingly, expression of T-bet was reduced in atypical memory cells from COVID-19 patients as compared with the healthy controls.
FIGURE 3.

Phenotypic analysis of B cells in COVID-19 and malaria compared with the healthy donors. (A) A 2-dimensional map of CD19+ B cells concatenated from all samples (gray) was generated by t-SNE analysis. Samples from healthy donors (green), COVID-19 (blue), and malaria (red) patients were projected onto the total sample map. (B) Plasmablasts were identified by Boolean gating of concatenated CD19+ B cells from all samples and projected onto the t-SNE map (black). (C) Projection of subpopulations associated with B cell differentiation onto the t-SNE map: transitional (CD24+, CD38+; black), naïve (CD21+, CD27–; green), CD21+ memory (CD21+, CD27+; purple), classical memory (CD21–, CD27+, blue), and atypical memory (CD21–, CD27–, red) B cells were identified by Boolean gating of concatenated CD19+ B cells from all samples and projected onto the t-SNE map. (D) The expression levels (measured as mean fluorescence intensities) of the indicated surface molecules were projected onto the t-SNE map. (E) Frequency of cells expressing PD-1, CD86, CXCR3, CD11c, CD39, or CD73 among total CD19+, CD20+ B cells and atypical memory B cells (CD21–, CD27–) in healthy donors, COVID-19, and malaria patients. (F) Frequency of cells expressing PD-1, CD86, CXCR3, CD11c, CD39, or CD73 among total CD19+ B cells and plasmablasts in healthy donors, COVID-19, and malaria patients. Data are shown as mean ± sd, where *, **, ***, and **** indicate P values <0.05, <0.01, <0.001, and <0.0001
Among plasmablasts, the frequencies of cells expressing CD86+ and CXCR3+ were increased in both COVID-19 and malaria patients compared with the healthy controls, whereas the frequency of CD73+ plasmablasts was significantly decreased in malaria patients (Fig. 3F). Representative FACS plots for the analysis of PD-1, CD86, CXCR3, CD11c, CD39, and CD73 are shown in Supplemental Figure 8.
The frequency of CD73+ cells was reduced in all B cell subpopulations of malaria patients in comparison with the healthy controls with the exception of CD27+, CD21+ B cells (Supplemental Fig. 9).
In summary, we observed an increase of the frequencies of plasmablasts and atypical memory B cells in both COVID-19 and malaria patients compared with the healthy controls. Although this pattern was similar for both diseases, COVID-19 samples showed a greater heterogeneity together with stronger changes in individual patients.
Frequencies of atypical memory B cells decrease and frequencies of plasmablasts increase at longitudinal follow-up of COVID-19 patients
Longitudinal progression studies of COVID-19 patients have shown that T cells show high markers of coinhibitory molecules that quickly normalize after the resolution of infection.9 To investigate possible longer-lasting changes of the B cell compartment after COVID-19, we performed a longitudinal analysis of 5 patients. In 3 of them, follow-up samples were taken between days 24 and 27 after the primary infection, and 2 samples were taken on day 107 and 112 after the primary infection. The frequencies of atypical memory B cells and plasmablasts at time points T0 (acute infection) and T1 (follow-up) were compared. Representative FACS plots of acute and follow-up time points are shown in Figure 4A. We observed that atypical memory B cells that were expanded during the acute infection rapidly decreased in frequency after the viral infection was resolved (Fig. 4B). In contrast, the frequency of plasmablasts even increased over time (Fig. 4B). In fact, we detected a significant negative correlation between the time after manifestation of symptoms and the frequency of atypical memory B cells (Fig. 4C). When comparing the distribution of all subsets during acute infection with that after recovery, it was notable that the decrease in atypical memory B cells was mainly compensated by an increase in CD21+ memory B cells (Fig. 4D).
FIGURE 4.
Longitudinal analysis of B cells in COVID-19. (A) Representative FACS plots showing the frequencies of B cell subsets and plasmablasts during acute infection (T0) or at follow-up (T1). The plots were gated on CD19+, CD20+ B cells excluding transitional B cells and plasmablasts (left panel) or on CD19+ B cells (right panel, see gating strategy in Supplemental Figs. 3 and 4). (B) Frequency of atypical memory B cells (CD27–, CD21–) and plasmablasts of 5 individual COVID-19 patients during acute infection (T0) or at follow-up (T1). (C) Correlation between the frequency of atypical memory B cells and the number of days since the begin of symptoms. (D) Distribution of B cell subsets in COVID-19 patients during acute infection (T0) or at follow-up (T1). The pie charts show the mean frequencies of the indicated subsets in 5 patients. (E and F) Comparison of frequencies of plasmablasts and atypical memory B cells between COVID-19 patients who produced SARS-CoV-2 IgM or IgG antibodies at the time of sampling during acute infection. Data are shown as mean ± sd, ** indicates P < 0.01
However, no significant changes were observed over time when comparing the frequencies of PD-1+, CD86+, CD11c+, CXCR3+, CD39+, and CD73+ cells among atypical memory B cells or plasmablasts (Supplemental Fig. 10).
Since one of the central B cell functions is the production of antibodies, we investigated the production of SARS-CoV-2-specific IgM and IgG antibodies. We compared the frequencies of plasmablasts and atypical memory B cells between patients who had already developed a positive antibody response and those who had not yet produced antibodies. We observed that patients with low levels of plasmablasts or atypical memory B cells already produced specific IgG antibodies (P value [plasmablasts] = 0.0626, P value [CD27–, CD21– B cells] = 0.1630) (Fig. 4E and F). At the second longitudinal follow-up time point, in 4 of the 5 samples a positive SARS-CoV-2 IgG response was detectable (Supplemental Table 2).
These observations will need to be confirmed and extended in future studies with a larger cohort to understand whether the frequencies of plasmablasts and atypical memory B cells are associated with the amount or breadth of the antibody production against SARS-CoV-2.
Discussion
Considerable advances in the understanding of B cell differentiation pathways in general, and the B cell response to viral infections in particular, have recently been made that altogether highlight the complex functionality of B cells and their various interactions with other arms of the immune system.15,16,28 In addition to production of neutralizing antibodies, B cells are important APCs, modulate inflammation and the activation of different lymphocyte populations by cytokine secretion or the expression of ectoenzymes like CD39 and CD73.24,26–28
The main objective of our study was to characterize the phenotype of circulating B cell populations in COVID-19 patients in comparison with the patients with acute P. falciparum malaria, an infection in which the B cell response is traditionally considered to be an important factor in disease outcome.19,45,46
One of the main findings was a significant increase of atypical memory B cells and plasmablasts in the peripheral B cell compartment of both COVID-19 and malaria patients (Fig. 1B and D) that correlated with immune activation and disease severity in COVID-19 patients (Figs. 1E, F and 2A, B), in accordance with recent studies that describe a possible role of these cell populations in the immune pathophysiology of malaria infection.18,32,47,48 In our study, the frequency of plasmablasts correlated with the amount of circulating proinflammatory cytokines (IL-6, CRP, LDH; Fig. 2A, B, and C).
Interestingly, using a systems immunology approach, De Biasi et al.49 also describe an association between the increase of plasmablasts and the cytokine storm in patients with a severe COVID-19 course.
Our results are also consistent with a recent description of increased frequencies of plasmablasts and atypical memory B cells during COVID-19 by Kuri-Cervantes et al.50 and Mathew et al.13 The current study now adds data of detailed phenotypic analysis of these subpopulations demonstrating changes in homing potential, activation, and the extracellular adenosine metabolism of these B cell populations. Kaneko et al.51 already noted potential similarities of B cells in malaria and COVID-19 based on results of studies in a murine malaria model. Kaneko et al.51 could show that COVID-19 leads to a disturbance of the germinal center response, which has also been described in a different malaria mouse model by Ryg-Cornejo et al.52
Another unique aspect of this current study was the analysis of the expression pattern of the ectoenzymes CD39 and CD73 of B cells and describing in COVID-19 and malaria patients. These have been implicated as regulators of B cell differentiation, activation, and interaction with other lymphocyte subsets by converting proinflammatory ATP to adenosine.25–28,53
The majority of B cells coexpress CD39 and CD73.24 In HIV, it has been shown that a loss of CD73 expression on B cells is associated with a potential disturbance of class switching to IgG and increased immune activation.27,28 A disrupted adenosine pathway leads to immune hyperactivation and stimulation of monocytes and T cells.27 Among plasmablasts, cells with a low or negative CD73 expression were enriched. The combination of a high frequency of CD86+ and a low frequency of CD73+ cells among plasmablasts from COVID-19 and malaria patients is suggestive of a strong activating and proinflammatory role of these cells.
Indeed, the phenotype of B cells in general, and the changes of the frequency and phenotype of plasmablasts observed in the current study can be seen in the context of the general hyperinflammation observed in COVID-19 patients. Zhou et al.7 showed evidence that the cytokine storm in COVID-19 is linked to a disturbed TH1 and monocyte response where TH1 cells produce IFN-y and IL-6 to activate monocytes that are significantly involved in the tissue damage in the lung. The increased plasmablast population with low expression of CD73 in COVID-19 patients may also lead to an increased activation of monocytes.27
In malaria patients, we observed a significantly lower CD73 expression of B cells, whereas we did not see this to the same degree in COVID-19 patients (Fig. 3F and Supplemental Fig. 9). The reason for this finding is not clear. In addition to low levels of CD73 expression, we observed a significantly lower CD39 expression resulting in high frequencies of CD39/CD73 double negative B cells in malaria (Supplemental Fig. 9). It has been shown that CD73, in addition to its already described functions, is involved in the homing process of B cells into the lymph nodes.26 It is tempting to speculate that the particular decrease of CD73 on B cells in malaria may contribute to an altered central homing potential and support the hypothesis of infiltration of these B cells into inflammatory tissue via the receptors CXCR3 and CD11c.26 We have previously described a similar effect for B cells in HIV, where the loss of CD73 on B cells is associated with reduced adenosine production and an impaired class-switch.28 Understanding the role of CD73 on B cells during acute malaria infection may provide clues as to why B cells are not able to build up a sufficient immune memory against P. falciparum.
Several case reports in COVID-19 have indicated a potentially harmful role for B cells in the acute immune response to SARS-CoV-2 infection: Quinti et al.22 showed that children with primary antibody deficiencies who completely lack B cells showed a milder course of SARS-CoV-2 infection than CVID patients who have only slightly dysfunctional B cells.
Plasmablasts were enriched for cells expressing PD-1, CD86, or CXCR3 compared with the bulk population of B cells in both patient groups as well as in HDs (Fig. 3F). CD86 and CXCR3 exert positively activating effects by facilitating T cell activation and the migration of cells into inflamed tissues, respectively, whereas PD-1 has inhibitory effects. We observed an additional enrichment of cells expressing CD86 or CXCR3 in plasmablasts from COVID-19 and malaria patients in comparison with the healthy controls (Fig. 3F), suggesting that plasmablasts likely play an activating role in the immune response of these diseases. However, this does not exclude that the expansion of plasmablasts may have negative consequences for the establishment of long-term immunologic memory, as it has been shown that early expansion of plasmablasts in malaria inhibits germinal center formation and memory B cell formation.32
In addition to the accumulation of plasmablasts, there was also a strong increase of the frequency of atypical memory B cells in the blood of COVID-19 and malaria patients. Atypical memory B cells, defined as mature CD19+, CD20+ B cells neither expressing CD21 nor CD27, constitute a heterogeneous population with yet ill-defined roles in disease. Cells with this phenotype have been called by various names, including tissue-like memory cells15 and age-associated B cells.44 The population overlaps—to a large extent—with the (also heterogeneous) population termed CD21low B cells, defined as CD21low/CD38– cells, that is enriched in patients with autoimmune diseases and some primary immune deficiencies.54 Due to its heterogeneity, this phenotypic population has been associated with widely contrasting functional states, including anergy/exhaustion,55 or effector cells in protective or pathogenic immune responses.44 In malaria, atypical memory B cells have been described as functionally impaired B cells with inhibitory potential,19 but have also been associated with a strong TH1 response.47,56 In HIV infection, a subpopulation of these cells lacking the Fc receptor-like protein 5 (FCRL5) had a dysfunctional phenotype linked to up-regulation of PD-1,57 while atypical CD21–, CD27– and CD21–, CD27+ memory B cells in HIV also could produce IL-6.58 We observed that atypical memory B cells from malaria patients were enriched for cells expressing markers associated with effector functions, such as CXCR3, CD86, and CD11c, but also cells expressing the inhibitory receptor PD-1 (Fig. 3E), suggesting that subpopulations of cells with this phenotype may play different roles in this disease. By contrast, we did not find significant enrichment of effector or inhibitory markers in atypical memory cells from COVID-19 patients. Proinflammatory effector functions of CD21–, CD27– B cells have been linked to expression of the transcription factor T-bet. Our preliminary finding that expression of T-bet was reduced in atypical memory B cells from COVID-19 patients (Supplemental Fig. 7) suggests that this cell population is not a major driver of inflammation in this disease but is more likely to be inhibitory or dysfunctional in the regulation of T cell responses, as has been observed in malaria infection.16 This view is supported by our finding of a reduction of atypical memory B cells upon recovery from COVID-19, together with a negative correlation between their frequency and the time since the onset of symptoms. As it has been shown in HIV infection that atypical memory B cells can produce IL-6, functional studies should investigate whether this population contributes to the hyperinflammation and disease severity of COVID-19 through secretion of IL-6.58
Major limitations of this descriptive study include the small cohort size. In future studies, it will be important to further understand the functional role of the B cell populations, assess whether the expansion of the 2 B cell populations described in this study is antigen driven or merely a byproduct of immune hyperactivation, as well as to directly test the importance of adenosine signaling for their function. In addition, the breadth, avidity, and neutralizing efficacy of the SARS-CoV-2 antibody B cell response should be assessed. However, future studies need to compare the B-cell phenotype and functional immune response with other acute viral respiratory infections such as influenza.
In this pilot study, we performed a phenotypic characterization of circulating B cells in patients infected with SARS-CoV-2 or P. falciparum. The main findings regarding B cell differentiation and phenotype in COVID-19 and malaria are summarized in Figure 5. In particular, we found significant increases in the frequency of plasmablasts (COVID-19 patients showed a high variability of the frequency of their plasmablasts) and atypical memory B cells in COVID-19 patients that correlated with serum levels of CRP, IL-6 and ferritin as markers of disease severity, suggesting that monitoring these B cell populations may be additional useful prognostic markers of COVID-19 outcome. Here, we present the first study, to our knowledge, that showed reduced frequencies of CD73+ plasmablasts and atypical memory B cells in malaria and COVID-19 patients. It is tempting to speculate that low expression of the ectonucleotidase CD73 of B cells may contribute to the hyperinflammatory pathophysiology of COVID-19.5,7,28
FIGURE 5.

B cell differentiation and phenotype in COVID-19 and malaria patients. Plasmablasts and atypical memory B cells are more frequent in patients with COVID-19 and malaria than in healthy subjects, and severe courses of COVID-19 are associated with a higher frequency of atypical memory B cells. Illustration created with the online software BioRender (San Francisco, CA)
Authorship
N.H.W., F.B., F.H., and J.S.Z.W. contributed with the conception and experimental design. N.H.W., P.A., and S.S. conducted the experiments and acquired the data. M.K., C.B., M.M.A., and J.S.Z.W. helped with recruiting the patients and collecting the clinical data. M.L. and F.H. performed the antibody testing and clinical diagnostics. N.H.W. and J.S.Z.W. did the writing of the first draft of the manuscript. All authors had important contributions toward the study and performed the proofreading of the paper. F.H. and Z.S.Z.W. contributed equally to this work.
Supplementary Material
Supporting information
Acknowledgments
We thank the patients who participated in this study and Silke Kummer and Robin Woost for their technical help. We also thank the attendings of the Institute for Infectiology in the University Medical Center Hamburg Eppendorf for the help with patient recruitment, Eun-Seong Kim for critical discussion, and Marissa Herrmann, Sophie Pflüger, and Marcel Seungsu Woo for collecting the clinical data.
The authors are grateful for the fundings from DFG SFB841 (N.H.W., S.S., J.S.Z.W., and M.L.), DFG SFB1328 (P.A., J.S.Z.W., and F.H.), and DZIF (J.S.Z.W., M.L., C.B., and M.M.A.).
Abbreviations
- CRP
C-reactive protein
- CVID
common variable immunodeficiency
- FCRL5
Fc receptor-like protein 5
- HD
healthy donor
- LDH
lactate dehydrogenase
- qRT-PCR
quantitative RT-PCR
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- tSNE
t-distributed stochastic neighbor embedding
Contributor Information
Nils H Wildner, Department of Medicine, Section Infectious Diseases, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
Parimah Ahmadi, Department of Medicine, Section Infectious Diseases, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
Sophia Schulte, Department of Medicine, Section Infectious Diseases, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
Franziska Brauneck, Department of Medicine, Center for Oncology, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
Matin Kohsar, Department of Medicine, Section Infectious Diseases, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
Marc Lütgehetmann, Institute of Medical Microbiology, Virology and Hygiene, Center for Diagnostics, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
Claudia Beisel, Department of Medicine, Section Infectious Diseases, University Medical Center Hamburg-Eppendorf, Hamburg, Germany; German Center for Infection Research (DZIF), Partner Site Hamburg-Lübeck-Borstel-Riems, Hamburg, Germany.
Marylyn M Addo, Department of Medicine, Section Infectious Diseases, University Medical Center Hamburg-Eppendorf, Hamburg, Germany; German Center for Infection Research (DZIF), Partner Site Hamburg-Lübeck-Borstel-Riems, Hamburg, Germany.
Friedrich Haag, Institute of Immunology, Center for Diagnostics, University Medical Center Hamburg-Eppendorf, Hamburg, Germany.
Julian Schulze zur Wiesch, Department of Medicine, Section Infectious Diseases, University Medical Center Hamburg-Eppendorf, Hamburg, Germany; German Center for Infection Research (DZIF), Partner Site Hamburg-Lübeck-Borstel-Riems, Hamburg, Germany.
Disclosures
The authors declare no conflicts of interest.
References
- Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199-1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LQ, Huang T, Wang YQ, et al. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J Med Virol. 2020;92:577-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271-280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Jiang M, Chen X, et al. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J Leukoc Biol. 2020;108:17-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Wu Z, Li JW, et al. The cytokine release syndrome (CRS) of severe COVID-19 and Interleukin-6 receptor (IL-6R) antagonist Tocilizumab may be the key to reduce the mortality. Int J Antimicrob Agents. 2020;55:105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Fu B, Zheng X, et al. Pathogenic T cells and inflammatory monocytes incite inflammatory storm in severe COVID-19 patients. Natl Sci Rev. 2020. 10.1093/nsr/nwaa041. Epub ahead of print March. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann M, Schulte S, Wildner NH, et al. Analysis of co-inhibitory receptor expression in COVID-19 infection compared to acute plasmodium falciparum malaria: lAG-3 and TIM-3 correlate with t cell activation and course of disease. Front Immunol. 2020;11:1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi P, Hartjen P, Kohsar M, et al. Defining the CD39/CD73 axis in SARS-CoV-2 infection: the CD73- phenotype identifies polyfunctional cytotoxic lymphocytes. Cells. 2020;9. 10.3390/cells9081750. Epub ahead of print July 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thevarajan I, Nguyen THO, Koutsakos M, et al. Breadth of concomitant immune responses prior to patient recovery: a case report of non-severe COVID-19. Nat Med. 2020;26:453-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff MC, Ramonell RP, Nguyen DC, et al. Extrafollicular B cell responses correlate with neutralizing antibodies and morbidity in COVID-19. Nat Immunol. 2020:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew D, Giles JR, Baxter AE, et al. Deep immune profiling of COVID-19 patients reveals distinct immunotypes with therapeutic implications. Science (80-). 2020;369:eabc8511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silveira ELV, Dominguez MR, Soares IS. To B or not to B: understanding B cell responses in the development of malaria infection. Front Immunol. 2018;9:2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moir S, Fauci AS. B-cell responses to HIV infection. Immunol Rev. 2017;275:33-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal S, Obeng-Adjei N, Moir S, et al. Atypical memory B cells in human chronic infectious diseases: an interim report. Cell Immunol. 2017;321:18-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braddom AE, Batugedara G, Bol S, et al. Potential functions of atypical memory B cells in plasmodium-exposed individuals. Int J Parasitol. 2020. 10.1016/j.ijpara.2020.08.003. Epub ahead of print September 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss GE, Crompton PD, Li S, et al. Atypical memory B cells are greatly expanded in individuals living in a malaria-endemic area. J Immunol. 2009;183:2176-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portugal S, Tipton CM, Sohn H, et al. Malaria-associated atypical memory B cells exhibit markedly reduced B cell receptor signaling and effector function. Elife. 2015;4. 10.7554/eLife.07218. Epub ahead of print May 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JL, Rosenthal RL, Knox JJ, et al. The transcription factor T-bet resolves memory B cell subsets with distinct tissue distributions and antibody specificities in mice and humans. Immunity. 2020;52:842-855.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phalke S, Aviszus K, Rubtsova K, et al. Age associated B cells appear in patients with granulomatous lung diseases. Am J Respir Crit Care Med. 2020. 10.1164/rccm.201911-2151oc. Epub ahead of print June 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinti I, Lougaris V, Milito C, et al. A possible role for B cells in COVID-19?: lesson from patients with Agammaglobulinemia. J Allergy Clin Immunol. 2020. 10.1016/j.jaci.2020.04.013. Epub ahead of print April. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malsy J, Veletzky L, Heide J, et al. Sustained response after remdesivir and convalescent plasma therapy in a B-cell depleted patient with protracted COVID-19. Clin Infect Dis. 2020. 10.1093/cid/ciaa1637. Epub ahead of print October 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saze Z, Schuler PJ, Hong CS, et al. Adenosine production by human B cells and B cell-mediated suppression of activated T cells. Blood. 2013;122:9-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schena F, Volpi S, Faliti CE, et al. Dependence of Immunoglobulin class switch recombination in B cells on vesicular release of ATP and CD73 ectonucleotidase activity. Cell Rep. 2013;3:1824-1831. [DOI] [PubMed] [Google Scholar]
- Takedachi M, Qu D, Ebisuno Y, et al. CD73-generated adenosine restricts lymphocyte migration into draining lymph nodes. J Immunol. 2008;180:6288-6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang W-X, Huang H-H, Huang L, et al. Skewed CD39/CD73/adenosine pathway in B cells is associated with innate immune hyperactivation in chronic HIV-1 infection. Transl Med Commun. 2019;4:4. [Google Scholar]
- Kim E-S, Ackermann C, Tóth I, et al. Down-regulation of CD73 on B cells of patients with viremic HIV correlates with B cell activation and disease progression. J Leukoc Biol. 2017;101:1263-1271. [DOI] [PubMed] [Google Scholar]
- Auma MA, Siedner MJ, Nyehangane D, et al. Malaria is an uncommon cause of adult sepsis in south-western Uganda. Malar J. 2013;12:146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Zhou X, Qiu Y, et al. Clinical characteristics of 82 cases of death from COVID-19. PLoS One. 2020;15:e0235458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Tu L, Zhu P, et al. Clinical features of 85 fatal cases of COVID-19 from Wuhan: a retrospective observational study. Am J Respir Crit Care Med. 2020;201. 10.1164/rccm.202003-0543OC. Epub ahead of print April 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijay R, Guthmiller JJ, Sturtz AJ, et al. Infection-induced plasmablasts are a nutrient sink that impairs humoral immunity to malaria. Nat Immunol. 2020;21:790-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo MS, Steins D, Häußler V, et al. Control of SARS-CoV-2 infection in rituximab-treated neuroimmunological patients. J Neurol. 2020. 10.1007/s00415-020-10046-8. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze zur Wiesch J, Lauer GM, Timm J, et al. Immunologic evidence for lack of heterologous protection following resolution of HCV in patients with non-genotype 1 infection. Blood. 2007;110: 1559-1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead SB, Rohanasuphot S, Sangkawibha N. Original antigenic sin in dengue. Am J Trop Med Hyg. 1983;32:154-156. [DOI] [PubMed] [Google Scholar]
- Nguyen-Contant P, Embong AK, Kanagaiah P, et al. S protein-reactive IgG and memory B cell production after human SARS-CoV-2 infection includes broad reactivity to the S2 subunit. MBio. 2020;11. 10.1128/mBio.01991-20. Epub ahead of print September 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plebani M, Padoan A, Negrini D, et al. Diagnostic performances and thresholds: the key to harmonization in serological SARS-CoV-2 assays?. Clin Chim Acta. 2020. 2020.05.22.20106328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caini S, Bellerba F, Corso F, et al. Meta-analysis of diagnostic performance of serological tests for SARS-CoV-2 antibodies up to 25 April 2020 and public health implications. Eurosurveillance. 2020;25:2000980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GeurtsvanKessel CH, Okba NMA, Igloi Z, et al. An evaluation of COVID-19 serological assays informs future diagnostics and exposure assessment. Nat Commun. 2020;11:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou B, Li T-D, Zheng S-F, et al. Serology characteristics of SARS-CoV-2 infection since exposure and post symptom onset. Eur Respir J. 2020. 10.1183/13993003.00763-2020. Epub ahead of print May 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflüger LS, Bannasch JH, Brehm TT, et al. Clinical evaluation of five different automated SARS-CoV-2 serology assays in a cohort of hospitalized COVID-19 patients. J Clin Virol. 2020;130:104549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Wang Y, Zhu L. Automatic clustering method of flow cytometry data based on t-distributed stochastic neighbor embedding. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2018;35:697-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. Clinical management of COVID-19. Clinical management of COVID-19 Available from: https://www.who.int/publications/i/item/clinical-management-of-covid-19. Accessed September 24, 2020.
- Rubtsova K, Rubtsov A V, Cancro MP, et al. Age-associated B cells: a T-bet–dependent effector with roles in protective and pathogenic immunity. J Immunol. 2015;195:1933-1937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muellenbeck MF, Ueberheide B, Amulic B, et al. Atypical and classical memory B cells produce plasmodium falciparum neutralizing antibodies. J Exp Med. 2013;210:389-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen DS, Obeng-Adjei N, Ly A, et al. Emerging concepts in T follicular helper cell responses to malaria. Int J Parasitol. 2017;47:105-110. [DOI] [PubMed] [Google Scholar]
- Obeng-Adjei N, Portugal S, Holla P, et al. Malaria-induced interferon-γ drives the expansion of Tbethiatypical memory B cells. PLoS Pathog. 2017;13. 10.1371/journal.ppat.1006576. Epub ahead of print September 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migot F, Chougnet C, Henzel D, et al. Anti-malaria antibody-producing B cell frequencies in adults after a Plasmodium falciparum outbreak in Madagascar. Clin Exp Immunol. 2008;102:529-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Biasi S, Lo Tartaro D, Meschiari M, et al. Expansion of plasmablasts and loss of memory B cells in peripheral blood from COVID-19 patients with pneumonia. Eur J Immunol. 2020;50:1283-1294. [DOI] [PubMed] [Google Scholar]
- Kuri-Cervantes L, Pampena MB, Meng W, et al. Comprehensive mapping of immune perturbations associated with severe COVID-19. Sci Immunol. 2020;5. 10.1126/sciimmunol.abd7114. Epub ahead of print July 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko N, Kuo H-H, Boucau J, et al. Loss of Bcl-6-expressing T follicular helper cells and germinal centers in COVID-19. Cell. 2020;183:143-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryg-Cornejo V, Ioannidis LJ, Ly A, et al. Severe malaria infections impair germinal center responses by inhibiting T follicular helper cell differentiation. Cell Rep. 2016;14:68-81. [DOI] [PubMed] [Google Scholar]
- Conter LJ, Song E, Shlomchik MJ, et al. CD73 expression is dynamically regulated in the germinal center and bone marrow plasma cells are diminished in its absence. PLoS One. 2014;9. 10.1371/journal.pone.0092009. Epub ahead of print March 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehr C, Eibel H, Masilamani M, et al. A new CD21 low B cell population in the peripheral blood of patients with SLE. Clin Immunol. 2004;113:161-171. [DOI] [PubMed] [Google Scholar]
- Del Padre M, Minafò YA, Marrapodi R, et al. Rheumatoid factor-producing CD21low anergic clonal B-cells in essential mixed cryoglobulinaemia: a model for autoantigen-driven pathogenesis of infectious and non-infectious cryoglobulinaemias. Clin Exp Rheumatol. 2020;38:139-147. [PubMed] [Google Scholar]
- Scholzen A, Teirlinck AC, Bijker EM, et al. BAFF and BAFF receptor levels correlate with B cell subset activation and redistribution in controlled human malaria infection. J Immunol. 2014;192:3719-3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Borrego F, Nagata S, et al. Fc receptor–like 5 expression distinguishes two distinct subsets of human circulating tissue–like memory B cells. J Immunol. 2016;196:4064-4074. [DOI] [PubMed] [Google Scholar]
- Siewe B, Nipper AJ, Sohn H, et al. FcRL4 expression identifies a pro-inflammatory B cell subset in viremic HIV-infected subjects. Front Immunol. 2017;8. 10.3389/fimmu.2017.01339. Epub ahead of print October 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information


