Abstract
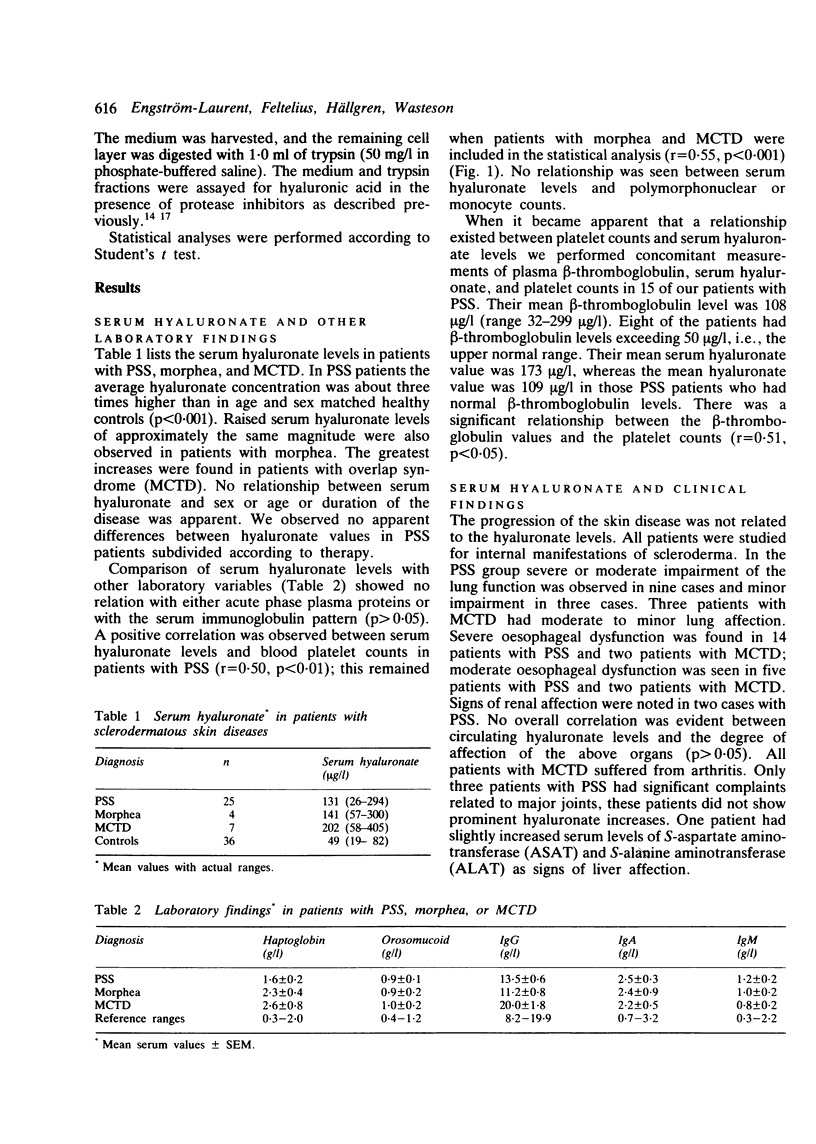
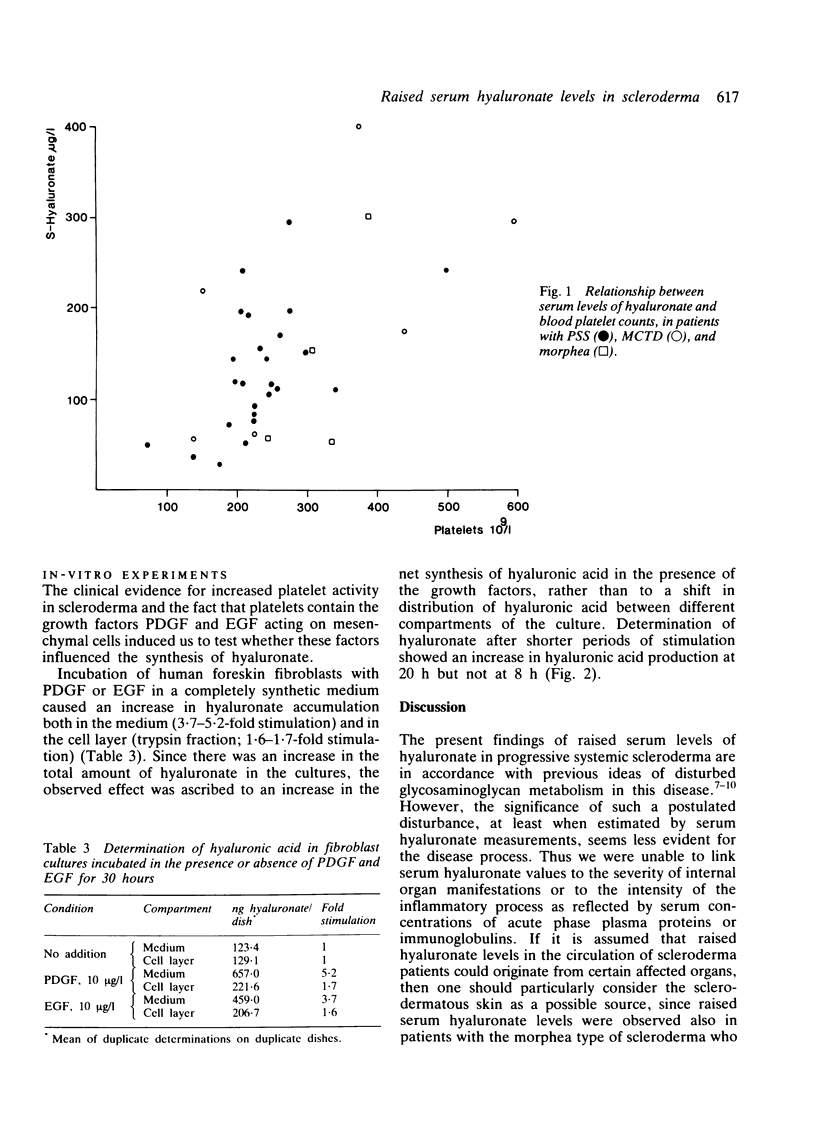
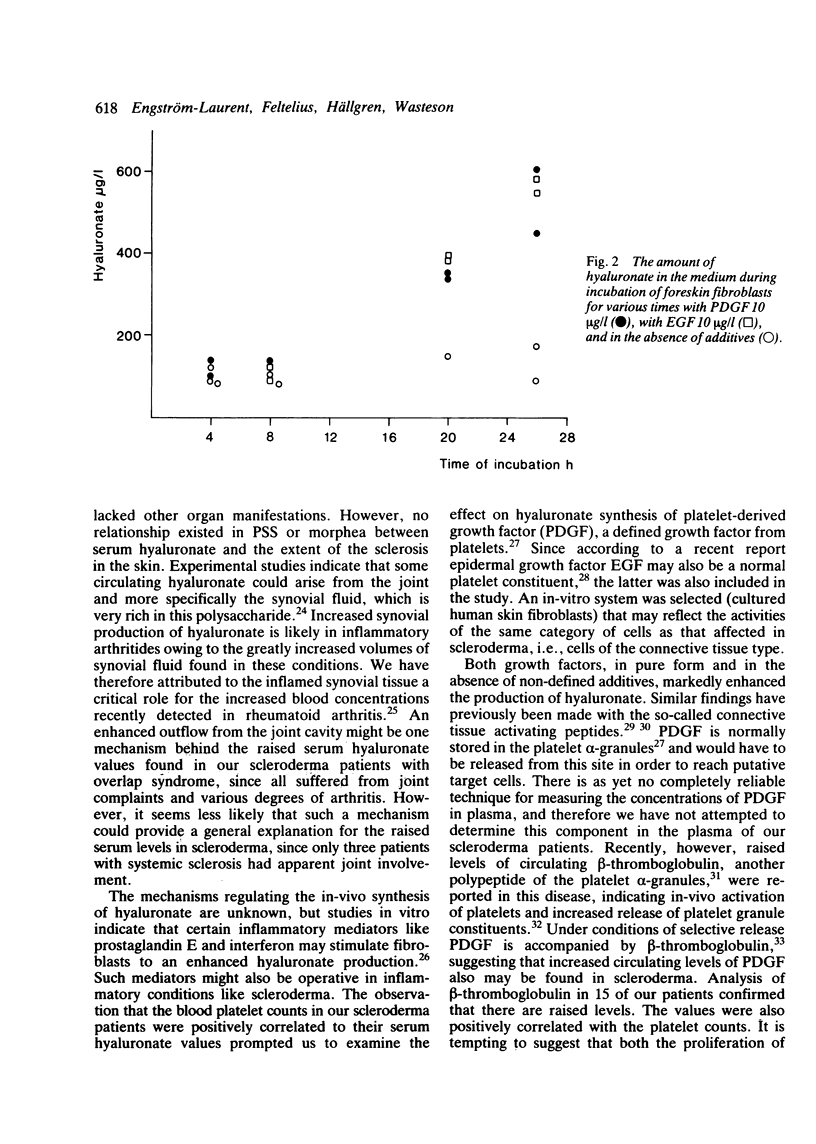
The circulating levels of hyaluronate were determined in 36 patients with scleroderma and in 36 control subjects matched for age and sex. The mean serum hyaluronate concentration in patients with progressive systemic sclerosis (n = 25) was 131 +/- 67 (SD) microgram/l and significantly greater (p less than 0.001) than that of the controls (mean level 49 +/- 21 (SD) microgram/l). Hyaluronate levels in patients with localised scleroderma (n = 4) were 141 +/- 47 (SEM) microgram/l and in patients with scleroderma-associated overlap syndromes (n = 7) 202 +/- 54 (SEM) microgram/l. The increase in serum hyaluronate probably reflected an enhanced synthesis or outflow of hyaluronate from the connective tissue, or both; it could not be explained by affection of the liver, which is the catabolic site of hyaluronate. The hyaluronate values were not related to certain serological indicators of inflammatory activity or to the extent of the skin lesions or the severity of internal organ manifestations. A positive correlation was noted between circulating platelet counts and hyaluronate levels (p less than 0.001). Plasma beta-thromboglobulin was measured in 15 of the patients with systemic sclerosis and found to correlate positively with platelet counts. Raised levels of beta-thromboglobulin were associated with the highest hyaluronate values. Platelet-derived growth factor, which stimulates connective tissue cells and is stored in the alpha-granules of platelets together with beta-thromboglobulin, was shown to enhance hyaluronate synthesis in fibroblast cultures. The results suggest an involvement in scleroderma of connective tissue activating substances released from platelets.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antonas K. N., Fraser J. R., Muirden K. D. Distribution of biologically labelled radioactive hyaluronic acid injected into joints. Ann Rheum Dis. 1973 Mar;32(2):103–111. doi: 10.1136/ard.32.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashey R. I., Millan A., Jimenez S. A. Increased biosynthesis of glycosaminoglycans by scleroderma fibroblasts in culture. Arthritis Rheum. 1984 Sep;27(9):1040–1045. doi: 10.1002/art.1780270911. [DOI] [PubMed] [Google Scholar]
- Cabral A., Castor C. W. Connective tissue activation. XXVII. The behavior of skin fibroblasts from patients with scleroderma. Arthritis Rheum. 1983 Nov;26(11):1362–1369. doi: 10.1002/art.1780261109. [DOI] [PubMed] [Google Scholar]
- Castor C. W., Ritchie J. C., Williams C. H., Jr, Scott M. E., Whitney S. L., Myers S. L., Sloan T. B., Anderson B. E. Connective tissue activation. XIV. Composition and actions of a human platelet autacoid mediator. Arthritis Rheum. 1979 Mar;22(3):260–272. doi: 10.1002/art.1780220308. [DOI] [PubMed] [Google Scholar]
- Chaves F. C., Rodrigo F. G., Franco M. L., Esteves J. Systemic sclerosis associated with auto-immune haemolytic anaemia. Br J Dermatol. 1970 Mar;82(3):298–302. doi: 10.1111/j.1365-2133.1970.tb12441.x. [DOI] [PubMed] [Google Scholar]
- Dahl L., Hopwood J. J., Laurent U. B., Lilja K., Tengblad A. The concentration of hyaluronate in amniotic fluid. Biochem Med. 1983 Dec;30(3):280–283. doi: 10.1016/0006-2944(83)90018-2. [DOI] [PubMed] [Google Scholar]
- Engström-Laurent A., Hällgren R. Circulating hyaluronate in rheumatoid arthritis: relationship to inflammatory activity and the effect of corticosteroid therapy. Ann Rheum Dis. 1985 Feb;44(2):83–88. doi: 10.1136/ard.44.2.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson S., Fraser J. R., Laurent T. C., Pertoft H., Smedsrød B. Endothelial cells are a site of uptake and degradation of hyaluronic acid in the liver. Exp Cell Res. 1983 Mar;144(1):223–228. doi: 10.1016/0014-4827(83)90458-5. [DOI] [PubMed] [Google Scholar]
- Fleischmajer R., Damiano V., Nedwich A. Scleroderma and the subcutaneous tissue. Science. 1971 Mar 12;171(3975):1019–1021. doi: 10.1126/science.171.3975.1019. [DOI] [PubMed] [Google Scholar]
- Fleischmajer R., Perlish J. S. Glycosaminoglycans in scleroderma and scleredema. J Invest Dermatol. 1972 Mar;58(3):129–132. doi: 10.1111/1523-1747.ep12538919. [DOI] [PubMed] [Google Scholar]
- Fraser J. R., Appelgren L. E., Laurent T. C. Tissue uptake of circulating hyaluronic acid. A whole body autoradiographic study. Cell Tissue Res. 1983;233(2):285–293. doi: 10.1007/BF00238296. [DOI] [PubMed] [Google Scholar]
- Fraser J. R., Laurent T. C., Engström-Laurent A., Laurent U. G. Elimination of hyaluronic acid from the blood stream in the human. Clin Exp Pharmacol Physiol. 1984 Jan-Feb;11(1):17–25. doi: 10.1111/j.1440-1681.1984.tb00235.x. [DOI] [PubMed] [Google Scholar]
- Fraser J. R., Laurent T. C., Pertoft H., Baxter E. Plasma clearance, tissue distribution and metabolism of hyaluronic acid injected intravenously in the rabbit. Biochem J. 1981 Nov 15;200(2):415–424. doi: 10.1042/bj2000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy K. H., Rosevear J. W., Sams W. M., Jr, Winkelmann R. K. Scleroderma and urinary excretion of acidic glycosaminoglycans. Mayo Clin Proc. 1971 Feb;46(2):119–127. [PubMed] [Google Scholar]
- Heinzerling R. H., Weyer R., Dziuba D. S., Belviso H. M., Burnham T. K. Elevated levels of antibodies to polyuridylic acid detected and quantitated in systemic scleroderma patients by solid phase radioimmunoassay. J Invest Dermatol. 1980 Sep;75(3):224–227. doi: 10.1111/1523-1747.ep12523196. [DOI] [PubMed] [Google Scholar]
- Johnsson A., Heldin C. H., Westermark B., Wasteson A. Platelet-derived growth factor: identification of constituent polypeptide chains. Biochem Biophys Res Commun. 1982 Jan 15;104(1):66–74. doi: 10.1016/0006-291x(82)91941-6. [DOI] [PubMed] [Google Scholar]
- Kahaleh M. B., Osborn I., Leroy E. C. Elevated levels of circulating platelet aggregates and beta-thromboglobulin in scleroderma. Ann Intern Med. 1982 May;96(5):610–613. doi: 10.7326/0003-4819-96-5-610. [DOI] [PubMed] [Google Scholar]
- Kaplan K. L., Broekman M. J., Chernoff A., Lesznik G. R., Drillings M. Platelet alpha-granule proteins: studies on release and subcellular localization. Blood. 1979 Apr;53(4):604–618. [PubMed] [Google Scholar]
- Laurent U. B. Hyaluronate in aqueous humour. Exp Eye Res. 1981 Aug;33(2):147–155. doi: 10.1016/s0014-4835(81)80063-2. [DOI] [PubMed] [Google Scholar]
- Laurent U. B., Tengblad A. Determination of hyaluronate in biological samples by a specific radioassay technique. Anal Biochem. 1980 Dec;109(2):386–394. doi: 10.1016/0003-2697(80)90665-x. [DOI] [PubMed] [Google Scholar]
- McKeehan W. L., Genereux D. P., Ham R. G. Assay and partial purification of factors from serum that control multiplication of human diploid fibroblasts. Biochem Biophys Res Commun. 1978 Feb 28;80(4):1013–1021. doi: 10.1016/0006-291x(78)91346-3. [DOI] [PubMed] [Google Scholar]
- McKeehan W. L., McKeehan K. A., Hammond S. L., Ham R. G. Improved medium for clonal growth of human diploid fibroblasts at low concentrations of serum protein. In Vitro. 1977 Jul;13(7):399–416. doi: 10.1007/BF02615100. [DOI] [PubMed] [Google Scholar]
- Moore S., Pepper D. S., Cash J. D. The isolation and characterisation of a platelet-specific beta-globulin (beta-thromboglobulin) and the detection of antiurokinase and antiplasmin released from thrombin-aggregated washed human platelets. Biochim Biophys Acta. 1975 Feb 27;379(2):360–369. doi: 10.1016/0005-2795(75)90143-9. [DOI] [PubMed] [Google Scholar]
- Murray-Lyon I. M., Thompson R. P., Ansell I. D., Williams R. Scleroderma and primary biliary cirrhosis. Br Med J. 1970 Aug 1;3(5717):258–259. doi: 10.1136/bmj.3.5717.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton W. L., Nardo J. M. Vascular disease in progressive systemic sclerosis (scleroderma). Ann Intern Med. 1970 Aug;73(2):317–324. doi: 10.7326/0003-4819-73-2-317. [DOI] [PubMed] [Google Scholar]
- Oka Y., Orth D. N. Human plasma epidermal growth factor/beta-urogastrone is associated with blood platelets. J Clin Invest. 1983 Jul;72(1):249–259. doi: 10.1172/JCI110964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980 May;23(5):581–590. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- Sharp G. C., Irvin W. S., Tan E. M., Gould R. G., Holman H. R. Mixed connective tissue disease--an apparently distinct rheumatic disease syndrome associated with a specific antibody to an extractable nuclear antigen (ENA). Am J Med. 1972 Feb;52(2):148–159. doi: 10.1016/0002-9343(72)90064-2. [DOI] [PubMed] [Google Scholar]
- Sisson J. C., Castor C. W., Klavons J. A. Connective tissue activation. XVIII. Stimulation of hyaluronic acid synthetase activity. J Lab Clin Med. 1980 Aug;96(2):189–197. [PubMed] [Google Scholar]
- Uitto J., Ohlenschläger K., Lorenzen I. Solubility of skin collagen in normal human subjects and in patients with generalised scleroderma. Clin Chim Acta. 1971 Jan;31(1):13–18. doi: 10.1016/0009-8981(71)90356-1. [DOI] [PubMed] [Google Scholar]
- Winkelmann R. K., Carapeto F. J., Jordon R. E. Direct immunofluorescence in the diagnosis of scleroderma syndromes. Br J Dermatol. 1977 Mar;96(3):231–238. doi: 10.1111/j.1365-2133.1977.tb06130.x. [DOI] [PubMed] [Google Scholar]
- Yaron M., Yaron I., Wiletzki C., Zor U. Interrelationship between stimulation of prostaglandin E and hyaluronate production by poly (I) . poly (C) and interferon in synovial fibroblast culture. Arthritis Rheum. 1978 Jul-Aug;21(6):694–698. doi: 10.1002/art.1780210614. [DOI] [PubMed] [Google Scholar]


