Abstract
Objective
Sleep disturbance is a common nonmotor symptom associated with a decreased quality of life in patients with Parkinson's disease (PD). In this study, we evaluated the effects of zonisamide on motor and non-motor symptomology in patients with PD, especially with respect to objective sleep assessments conducted via polysomnography.
Methods
We conducted a 12-week, open-label study to assess the effects of zonisamide. The patients received 25 mg/day of zonisamide and underwent overnight polysomnography prior to and after 12 weeks of zonisamide treatment. They were assessed for their cognitive function (Mini-Mental State Examination and the Japanese version of the Montreal Cognitive Assessment), gait function (Timed Up-and-Go Test, 10-m Gait Walk Test), Parkinson's symptomology (Movement Disorder Society Revision of the Unified Parkinson's Disease Rating Scale parts 2 and 3), and self-reported sleep (Epworth Sleepiness Score, Parkinson's Disease Sleep Scale-2).
Results
Six patients completed the study. Polysomnographic data revealed a statistically significant increase in the percentage of time spent in sleep stage N2 (10.8%±9.2%, p=0.031) and a declining trend in the percentage of time spent in sleep stage N1 (-8.9%±12.7%, p=0.063). Although none of the patients had sleep stage N3 at baseline, 3 of the 6 patients experienced sleep stage N3 (1.1-5.4%) after 12 weeks of zonisamide treatment. The other polysomnographic parameters and clinical scores showed no statistically significant differences.
Conclusions
This preliminary study demonstrated that zonisamide improved objective sleep parameters measured by polysomnography in patients with PD.
Keywords: Parkinson's disease, zonisamide, polysomnography, sleep disturbances, sleep quality
Introduction
In addition to motor symptoms, various non-motor symptoms are frequently evident in patients with Parkinson's disease (PD). Sleep disturbance is one of the most common non-motor symptoms in patients with PD, and it is associated with a significantly decreased quality of life (1). Dopaminergic treatment can be less effective against non-motor symptoms than against motor symptoms, suggesting that sleep disorders can be difficult to manage in PD and that degeneration of non-dopaminergic neurons affects this symptomology.
Zonisamide has been approved for the treatment of patients with PD by numerous regulatory bodies, and it has been shown to improve the motor function without exacerbating dyskinesia or psychiatric symptomology (e.g. hallucinations) (2,3). Although several mechanisms, including dopaminergic and non-dopaminergic effects, have been reported as mediating the anti-Parkinsonian effects of zonisamide, the pharmacological mechanisms underlying these effects have not been fully elucidated. In a recent open-label study, zonisamide was shown to have a beneficial effect on ameliorating sleep problems in patients with PD, as assessed using self-administered questionnaires (4). However, to our knowledge, no prior studies have reported the efficacy of zonisamide in patients with PD using objective polysomnography sleep evaluations.
In this study, we evaluated the effects of zonisamide on motor and non-motor symptomology in patients with PD, especially with respect to objective sleep assessments conducted via polysomnography.
Materials and Methods
Study design and participants
This single-center, open-label study was conducted at the Saiseikai Matsuyama Hospital between March 2019 and June 2020. The inclusion criteria were as follows: (i) patients diagnosed with PD according to the UK Brain Bank Clinical Diagnostic Criteria (5), (ii) patients who had not previously received zonisamide, (iii) patients who had remained on the same anti-Parkinsonian medication for at least one month prior to study enrollment, and (iv) patients without dementia, defined as a Mini-Mental State Examination (MMSE) score of ≥24.
All patients underwent overnight polysomnography using a digital polygraph system (Alice PDx: Philips Respironics, Murrysville, USA) and were started on 25 mg/day of zonisamide the following day. Polysomnography was repeated after 12 weeks of zonisamide treatment. All digital files for overnight polysomnography were sent to the Philips Scoring Center (Tokyo, Japan) and scored by an investigator blinded to patient characteristics and identifiers. All polysomnographic records were staged and scored according to the American Academy of Sleep Medicine criteria. At baseline and after 12 weeks of zonisamide treatment, all patients were evaluated for their cognitive function using the MMSE and the Japanese version of the Montreal Cognitive Assessment (6), gait function using the Timed Up and Go test and 10-m walk test, and disease severity using the Movement Disorder Society-Sponsored Revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS, parts 2 and 3) (7). The Epworth Sleepiness Scale (ESS) (8) and Parkinson's Disease Sleep Scale-2 (PDSS-2) (9) were administered to obtain self-evaluations of sleep at baseline and after 2, 4, 8, and 12 weeks of zonisamide treatment. We also calculated the levodopa equivalent daily dose for each patient (10).
This study was approved by the Institutional Review Board for Clinical Research Ethics at Saiseikai Matsuyama Hospital and was conducted in accordance with the Declaration of Helsinki and its later amendments. Written informed consent was obtained from all study participants.
Statistical Analyses
Wilcoxon's signed-rank test was used to compare polysomnographic parameters and clinical scores at baseline and after 12 weeks of zonisamide treatment. Statistical significance was defined as a two-tailed p value of <0.05. All analyses were performed using the R statistical software program (version 4.0.3; The R Project for Statistical Computing, Vienna, Austria).
Results
The baseline clinical and demographic profiles of the enrolled patients are shown in Table 1. Six patients completed the study, excluding one patient who only underwent the initial polysomnography evaluation (and not a follow-up evaluation) due to concerns about coronavirus disease 2019 infection. Polysomnographic parameters and clinical scores (gait, cognitive function, sleep, and disease severity) at baseline and after 12 weeks of zonisamide treatment are summarized in Table 2.
Table 1.
Clinical Profiles of the Patients.
| PD (n=6) | |
|---|---|
| M/F | 3/3 |
| Age, years; range | 76.8±6.5; 69-87 |
| Disease duration, years | 5.3±3.6 |
| Hoehn and Yahr stage | 3.3±0.8 |
| Anti-parkinsonian medication, n (%) | |
| Levodopa | 6 (100.0) |
| Pramipexole | 1 (16.7) |
| Rotigotine | 2 (66.7) |
| Selegiline | 2 (66.7) |
| Amantadine | 2 (66.7) |
| Istradefylline | 2 (66.7) |
| Levodopa equivalent daily dose, mg/day | 415.7±173.6 |
Data are shown as mean±standard deviation or n (%).
PD: Parkinson’s disease, M: male, F: female
Table 2.
Results of Efficacy Analysis of Zonisamide.
| Baseline | Week 12 | p | |||||
|---|---|---|---|---|---|---|---|
| Polysomnographic sleep parameters | |||||||
| Stage N1, % | 31.9 | ± | 21.0 | 23.0 | ± | 22.9 | 0.063 |
| Stage N2, % | 56.5 | ± | 21.5 | 67.3 | ± | 18.9 | 0.031 |
| Stage N3, % | 0.0 | ± | 0.0 | 1.6 | ± | 2.2 | 0.25 |
| Stage REM, % | 11.6 | ± | 7.2 | 8.2 | ± | 7.0 | 0.219 |
| TST, min | 353.6 | ± | 84.6 | 313.3 | ± | 125.8 | 0.156 |
| WASO, min | 215.0 | ± | 73.2 | 200.7 | ± | 93.9 | 0.563 |
| Sleep efficacy, % | 62.0 | ± | 11.7 | 55.8 | ± | 19.9 | 0.219 |
| Arousal index, n/h | 24.0 | ± | 10.2 | 21.8 | ± | 11.7 | 0.563 |
| AHI, n/h | 17.0 | ± | 18.8 | 14.4 | ± | 23.6 | 0.219 |
| ODI, n/h | 11.1 | ± | 17.5 | 11.8 | ± | 23.8 | 0.438 |
| PLM index, n/h | 9.5 | ± | 13.9 | 8.8 | ± | 16.5 | 0.875 |
| TUG, s | 18.3 | ± | 12.6 | 16.5 | ± | 9.1 | 0.688 |
| 10-m walk, s | 11.9 | ± | 5.3 | 11.5 | ± | 4.8 | 0.563 |
| MMSE score | 27.5 | ± | 1.4 | 27.0 | ± | 2.3 | 0.625 |
| MoCA-J score | 22.2 | ± | 4.6 | 22.8 | ± | 2.8 | 0.625 |
| ESS score | 12.00 | ± | 5.83 | 11.83 | ± | 6.85 | 1.000 |
| PDSS-2 total score | 17.17 | ± | 7.19 | 15.17 | ± | 8.23 | 0.656 |
| MDS-UPDRS part 2 | 13.33 | ± | 6.41 | 13.50 | ± | 8.17 | 1.000 |
| MDS-UPDRS part 3 | 41.33 | ± | 11.22 | 36.83 | ± | 10.05 | 0.188 |
Data are shown as mean±standard deviation.
REM: rapid eye movement, TST: total sleep time, WASO: wake time after sleep onset, AHI: apnea-hypopnea index, ODI: oxygen desaturation index, PLM: periodic limb movement, TUG: Timed Up and Go test, MMSE: Mini-Mental State Examination, MoCA-J: Japanese version of the Montreal Cognitive Assessment, ESS: Epworth Sleepiness Scale, PDSS-2: Parkinson’s Disease Sleep scale-2, MDS-UPDRS: Movement Disorder Society-Sponsored Revision of the Unified Parkinson’s disease Rating Scale
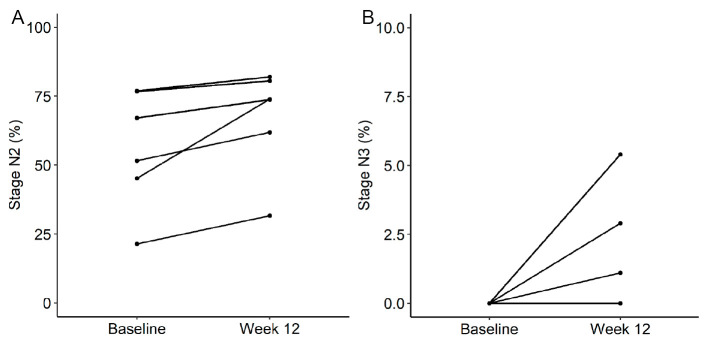
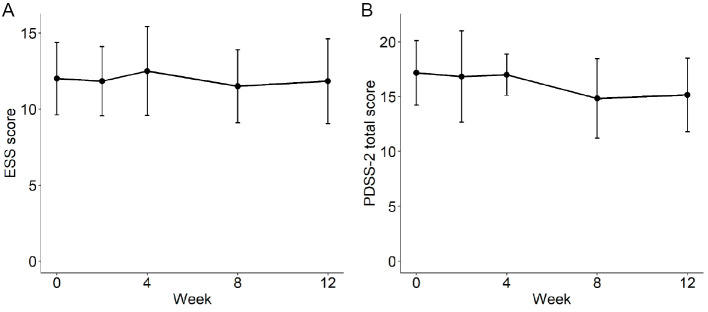
Polysomnographic data revealed a statistically significant increase in the percentage of time spent in sleep stage N2 [10.8±9.2% (mean±standard deviation), p=0.031] and a declining trend in the percentage of time spent in sleep stage N1 (-8.9%±12.7%; p=0.063). The percentage of time spent in sleep stage N2 increased in all six patients (Fig. 1A). Furthermore, although none of the patients had sleep stage N3 at baseline, 3 of the 6 experienced sleep stage N3 (1.1-5.4%) after 12 weeks of zonisamide treatment (Fig. 1B). The other polysomnographic parameters and clinical scores, including self-assessments of sleep (ESS and PDSS-2 total scores), showed no statistically significant differences between assessments conducted at baseline and after 12 weeks of zonisamide treatment (Table 2, Fig. 2).
Figure 1.
Mean changes in the percentage of time spent in sleep stages N2 (A) and N3 (B) between baseline and week 12 of zonisamide treatment.
Figure 2.
Mean changes in ESS (A) and PDSS-2 (B) scores between baseline and week 12 of zonisamide treatment. The error bars indicate standard errors of the mean. ESS: Epworth Sleepiness Score, PDSS-2: Parkinson’s disease sleep scale-2
Discussion
In this study, we compared polysomnographic parameters and other clinical scores prior to and after 12 weeks of zonisamide treatment. To our knowledge, this is the first study to evaluate the effects of zonisamide on objective sleep assessments using polysomnography in patients with PD.
Previous studies evaluating the effects of anti-Parkinsonian drugs on the sleep quality as assessed by polysomnography demonstrated that rotigotine improved the sleep efficacy (11) and rasagiline reduced the percentage of time spent in sleep stage N1, as well as the number of arousals and wake time after sleep onset in patients with PD (12). In contrast, dopaminergic medications, such as levodopa and cabergoline, were found to increase the number of arousals as well as the time spent in sleep stage N1 in de novo patients with PD (13), and pergolide was found to worsen actigraphic measures of sleep efficiency and sleep fragmentation in patients with PD (14).
A previous study reported that zonisamide reduces PDSS-2 total scores after three months of treatment (4). The study found that the “tremor group” (defined as those with a score of 1 or higher on subitems 3.15, 3.16, and 3.17 of the MDS-UPDRS) had greater improvements in PDSS-2 scores than the non-tremor group. Although five of six patients enrolled in our study were categorized into the tremor group based on this definition, we found no evidence of statistically significant improvements in PDSS-2 total scores. This finding may have been influenced by the modest enrolled sample size in the current study. In addition, a cohort study based on claims data from the Japanese Diagnosis Procedure Combination hospital database revealed that the use of zonisamide was associated with a statistically significantly lower risk of insomnia than other evaluated anti-Parkinsonian drugs (ergot-derived dopamine agonist, anticholinergics, and droxidopa) (15).
The mechanisms underlying the effect of zonisamide on the sleep quality have not yet been fully clarified. A randomized placebo-controlled study showed that zonisamide reduced the apnea-hypopnea index in patients with obstructive sleep apnea (16). The study authors postulated that this mechanism was associated with the carbonic anhydrase inhibitory properties of zonisamide. The monoamine oxidase (MAO) inhibitory effects of zonisamide may also be involved in mediating the effects on the sleep quality. Zonisamide is known to inhibit MAO activity and decrease dopamine turnover (17). Furthermore, MAO inhibitors are reported to increase plasma melatonin levels (18), and a placebo-controlled study showed that melatonin treatment improved total sleep time as measured by actigraphy in patients with PD (19). Zonisamide has multiple mechanisms of action, including non-dopaminergic effects, which may positively impact sleep.
In this study, zonisamide treatment was found to statistically significantly increase the percentage of time spent in sleep stage N2. A recent meta-analysis demonstrated statistically significant reductions in the percentage of time spent in sleep stages N2 and N3, as well as an increase in the percentage of time spent in sleep stage N1, in patients with PD (20). In contrast, the current study showed that zonisamide treatment resulted in a non-statistically significant decreasing trend in the percentage of time spent in sleep stages N1, although these findings might have been influenced by the modest sample size and consequent lack of statistical power in the current study. Sleep stage N3, which was not detected in any of the enrolled patients prior to treatment, was found in three of the six patients after treatment.
A prior study evaluating motor skill learning demonstrated a statistically significant correlation between the total percentage of time spent in sleep stage N2 and overnight motor skill improvement in healthy subjects (21). Furthermore, in patients with PD taking dopaminergic drugs, working memory performance has been found to improve after nocturnal sleep intervals, and slow-wave sleep volume was positively correlated with the degree of improvement (22). Thus, we expect that zonisamide will help improve sleep disturbances and associated symptomology in patients with PD.
One limitation associated with this research is that we conducted a single-arm study with only a modest enrolled sample size. In addition, although zonisamide treatment was found to improve some polysomnographic sleep parameters, we did not detect any statistically significant differences in subjective evaluations (e.g., the ESS and PDSS-2). This may have been due to the insufficient statistical power of this preliminary investigation. Additional placebo-controlled studies with larger enrolled sample sizes will be necessary to confirm these results.
In conclusion, our study showed that zonisamide treatment improved the sleep quality as measured by polysomnography in patients with PD. The effects of zonisamide on sleep disturbance and the sleep quality in patients with PD should be explored further, as these findings were detected in a small, insufficiently powered preliminary investigation.
The authors state that they have no Conflict of Interest (COI).
References
- 1. Zhang Y, Zhao JH, Huang DY, et al. Multiple comorbid sleep disorders adversely affect quality of life in Parkinson's disease patients. NPJ Parkinson's Dis 6: 25, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Murata M, Hasegawa K, Kanazawa I; Japan Zonisamide on PD Study Group. Zonisamide improves motor function in Parkinson disease: a randomized, double-blind study. Neurology 68: 45-50, 2007. [DOI] [PubMed] [Google Scholar]
- 3. Murata M, Hasegawa K, Kanazawa I, et al. Zonisamide improves wearing-off in Parkinson's disease: a randomized, double-blind study. Mov Disord 30: 1343-1350, 2015. [DOI] [PubMed] [Google Scholar]
- 4. Suzuki K, Fujita H, Matsubara T, et al. Zonisamide effects on sleep problems and depressive symptoms in Parkinson's disease. Brain Behav 11: e02026, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55: 181-184, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fujiwara Y, Suzuki H, Yasunaga M, et al. Brief screening tool for mild cognitive impairment in older Japanese: validation of the Japanese version of the Montreal Cognitive Assessment. Geriatr Gerontol Int 10: 225-232, 2010. [DOI] [PubMed] [Google Scholar]
- 7. Kashihara K, Kondo T, Mizuno Y, et al. Official Japanese version of the Movement Disorder Society-Unified Parkinson's Disease Rating Scale: validation against the original English version. Mov Disord Clin Pract 1: 200-212, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Takegami M, Suzukamo Y, Wakita T, et al. Development of a Japanese version of the Epworth Sleepiness Scale (JESS) based on item response theory. Sleep Med 10: 556-565, 2009. [DOI] [PubMed] [Google Scholar]
- 9. Suzuki K, Miyamoto M, Miyamoto T, et al. Nocturnal disturbances and restlessness in Parkinson's disease: using the Japanese version of the Parkinson's disease sleep scale-2. J Neurol Sci 318: 76-81, 2012. [DOI] [PubMed] [Google Scholar]
- 10. Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord 25: 2649-2653, 2010. [DOI] [PubMed] [Google Scholar]
- 11. Pierantozzi M, Placidi F, Liguori C, et al. Rotigotine may improve sleep architecture in Parkinson's disease: a double-blind, randomized, placebo-controlled polysomnographic study. Sleep Med 21: 140-144, 2016. [DOI] [PubMed] [Google Scholar]
- 12. Schrempf W, Fauser M, Wienecke M, et al. Rasagiline improves polysomnographic sleep parameters in patients with Parkinson's disease: a double-blind, baseline-controlled trial. Eur J Neurol 25: 672-679, 2018. [DOI] [PubMed] [Google Scholar]
- 13. Brunner H, Wetter TC, Hogl B, Yassouridis A, Trenkwalder C, Friess E. Microstructure of the non-rapid eye movement sleep electroencephalogram in patients with newly diagnosed Parkinson's disease: effects of dopaminergic treatment. Mov Disord 17: 928-933, 2002. [DOI] [PubMed] [Google Scholar]
- 14. Comella CL, Morrissey M, Janko K. Nocturnal activity with nighttime pergolide in Parkinson disease: a controlled study using actigraphy. Neurology 64: 1450-1451, 2005. [DOI] [PubMed] [Google Scholar]
- 15. Iwaki H, Tagawa M, Iwasaki K, Kawakami K, Nomoto M. Comparison of zonisamide with non-levodopa, anti-Parkinson's disease drugs in the incidence of Parkinson's disease-relevant symptoms. J Neurol Sci 402: 145-152, 2019. [DOI] [PubMed] [Google Scholar]
- 16. Eskandari D, Zou D, Karimi M, Stenlof K, Grote L, Hedner J. Zonisamide reduces obstructive sleep apnoea: a randomised placebo-controlled study. Eur Respir J 44: 140-149, 2014. [DOI] [PubMed] [Google Scholar]
- 17. Uemura MT, Asano T, Hikawa R, Yamakado H, Takahashi R. Zonisamide inhibits monoamine oxidase and enhances motor performance and social activity. Neurosci Res 124: 25-32, 2017. [DOI] [PubMed] [Google Scholar]
- 18. Murphy DL, Tamarkin L, Sunderland T, Garrick NA, Cohen RM. Human plasma melatonin is elevated during treatment with the monoamine oxidase inhibitors clorgyline and tranylcypromine but not deprenyl. Psychiatry Res 17: 119-127, 1986. [DOI] [PubMed] [Google Scholar]
- 19. Dowling GA, Mastick J, Colling E, Carter JH, Singer CM, Aminoff MJ. Melatonin for sleep disturbances in Parkinson's disease. Sleep Med 6: 459-466, 2005. [DOI] [PubMed] [Google Scholar]
- 20. Zhang Y, Ren R, Sanford LD, et al. Sleep in Parkinson's disease: a systematic review and meta-analysis of polysomnographic findings. Sleep Med Rev 51: 101281, 2020. [DOI] [PubMed] [Google Scholar]
- 21. Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron 35: 205-211, 2002. [DOI] [PubMed] [Google Scholar]
- 22. Scullin MK, Trotti LM, Wilson AG, Greer SA, Bliwise DL. Nocturnal sleep enhances working memory training in Parkinson's disease but not Lewy body dementia. Brain 135: 2789-2797, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]