Highlights
-
•
Systematic review of longitudinal Shigella outcomes in children.
-
•
Shigella is associated with continued diarrhea and linear growth faltering.
-
•
There is a need for standardized measurement and reporting of Shigella outcomes.
Keywords: Shigella, Stunting, Enteric, Diarrhea, Growth faltering, Vaccine
Abstract
Objectives
We conducted a systematic review of the longitudinal consequences of Shigella infection in children to inform the value proposition for an effective vaccine.
Methods
We searched PubMed and Embase for studies published from January 01, 1980 to December 12, 2022 and conducted in low- and middle-income countries that included longitudinal follow-up after Shigella detection among children aged <5 years, irrespective of language. We collected data on all outcomes subsequent to Shigella detection, except mortality.
Results
Of 2627 papers identified, 52 met inclusion criteria. The median sample size of children aged <5 years was 66 (range 5-2172). Data were collected in 20 countries; 56% (n = 29) of the publications included Bangladesh. The most common outcomes related to diarrhea (n = 20), linear growth (n = 14), and the mean total cost of a Shigella episode (n = 4; range: $ 6.22-31.10). Among children with Shigella diarrhea, 2.9-61.1% developed persistent diarrhea (≥14 days); the persistence was significantly more likely among children who were malnourished, had bloody stool, or had multidrug-resistant Shigella. Cumulative Shigella infections over the first 2 years of life contributed to the greatest loss in length-for-age z-score.
Conclusion
We identified evidence that Shigella is associated with persistent diarrhea, linear growth faltering, and economic impact to the family.
Introduction
Shigella is a highly transmissible enteric pathogen, which causes an estimated 68,000 deaths in children aged <5 years each year [1] and is indirectly responsible for an additional 13,600 deaths from Shigella-associated linear growth faltering or stunting [2]. The mortality rates from Shigella have declined substantially over the last few decades due to the apparent disappearance of the highly virulent Shiga toxin-producing Shigella dysenteriae 1 serotype, measles vaccination, antibiotics, improvements in nutritional status, and economic development [3], [4], [5]. Despite these gains, antibiotic resistance to first and secondline antibiotics that have historically been effective in reducing disease severity, diarrhea duration, and pathogen excretion threatens the progress that has been made in reducing Shigella mortality [6].
In addition to its contribution to childhood mortality, Shigella is responsible for substantial morbidity among children aged <5 years. This gram-negative bacterium is often the leading cause of moderate-to-severe diarrhea (MSD) and is the leading cause of dysentery among children aged <5 years living in low- and middle-income countries (LMICs) [7,8]. The incidence of Shigella acute diarrhea ranges from 1 per 100 child-years to 75.1 per 100 child-years among children in LMICs [7,9,10]. Shigella infections, in the presence and absence of diarrhea, also contribute to linear growth faltering [11,12], likely through a mechanism involving environmental enteric dysfunction (EED) [9,13]. EED and linear growth faltering both have links to poor longer-term outcomes, including delayed cognitive development, poor school performance, and reduced economic potential [14], [15], [16]. Shigella infections also pose a significant financial burden on families and health systems due to the treatment/hospitalization cost of Shigella diarrhea [17,18] and from potential decreased economic/earning potential from the longer-term outcomes of Shigella [19].
Based on the clinical severity, disease burden, links to longer-term outcomes, and the emergence of antimicrobial resistance, Shigella is a priority for vaccine development in the target population of young children living in LMICs [20]. Vaccines targeting the most common Shigella flexneri serotypes and Shigella sonnei are in development [21,22]. As pediatric Shigella vaccines move toward licensure and policy makers consider vaccine introduction, there is a need to synthesize evidence on the long-term consequences of Shigella to aid global and country decision-making to support vaccine adoption [20,23]. We conducted a systematic review of the consequences of Shigella infection among children in LMICs to help characterize the potential value of a Shigella vaccine.
Methods
We conducted a systematic review following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [24] to identify literature on the consequences of Shigella infection in children aged <5 years in LMICs. We aimed to gather data on the breadth of sequelae attributable to Shigella infection among young children, including but not limited to diarrhea persistence, linear growth faltering, ponderal growth faltering, neurodevelopmental delay, economic impacts, immune response, and systemic and enteric inflammation. In addition to characterizing the evidence and direction of effect, we sought to identify evidence gaps that could be addressed in future research studies.
Search strategy and selection criteria
We searched PubMed and Embase for articles published from January 01, 1980 to December 12, 2022 that indicated longitudinal follow-up of children after detection of Shigella in fecal samples or blood by any laboratory method. We included terms that described LMICs, as well as the names of all countries categorized as LMICs by the World Bank in 2020 (see Appendix 1 for full search strings).
We included clinical trials and observational studies that followed up at least five children with Shigella detected for any duration beyond 1 hour, regardless of symptoms. We restricted to studies conducted in LMICs that reported outcome data for children aged <5 years (0-60 months) to focus on the population with the highest morbidity and mortality burden attributed to Shigella [1]. We excluded cross-sectional studies and outcomes that were assessed contemporaneously with Shigella detection. Conference abstracts were included if they met other inclusion criteria and contained outcome data. We translated non-English publications using DeepL Translator (Cologne, Germany) or Google Translate.
Two reviewers (FA, MD, or TL) independently screened the title and abstract of each article for eligibility using Covidence (Veritas Health Innovation, Melbourne, Australia). Any disagreements were resolved by a third reviewer (PP) or through group discussion and consensus. If a decision could not be made using the information available in the abstract or if no abstract was available, the article was passed to full-text review. The same methods (dual review and conflict resolution using Covidence) were used during full-text review. The review's International prospective register of systematic reviews registration number is CRD42021241169 (link).
Data analysis
The summary data were abstracted from full-text reports of included publications. We abstracted information on the original study design and methodology (e.g., length of follow-up, inclusion criteria), the location of study, the number of children and/or stools with Shigella detected, laboratory method of detection, Shigella species identified, co-infections, and funding source. For each outcome identified, we abstracted the method of measurement, time point of measurement or duration of follow-up, any adjustment variables, and the effect estimate. All longitudinal outcomes were abstracted except mortality because this outcome was recently summarized in a systematic review of case fatality rates for common diarrheal pathogens [25]. Clinical characteristics and outcomes reported only at medical presentation or study enrollment were not abstracted because it was not possible to determine temporality in relation to Shigella detection. Data from randomized trials were abstracted for each randomization arm; the measures of excess risk comparing randomization arms were not abstracted unless they compared children with and without Shigella detected.
Because all data in this review were treated as a cohort study (Shigella as the exposure), we did not feel it would be relevant to assess the risk of bias for the original study design (e.g., randomized control trial) nor would it be possible to uniformly apply a risk of bias assessment tool to the variety of designs included in this review because many questions are not suited to our included outcomes. Instead, we conducted a quality assessment of included studies using a modified version of a composite quality construct based on the Strengthening the Reporting of Observational Studies in Epidemiology statement [26], which was developed and implemented previously [27]. In this assessment, each article was awarded points (10 maximum) for satisfying components of the methods section of the Strengthening the Reporting of Observational Studies in Epidemiology statement checklist, which includes an assessment of efforts to address potential sources of bias (Appendix 2). A rating of ‘poor’ was assigned to articles with zero to four points, ‘fair’ with five to seven points, and ‘good’ with eight to 10 points. As part of our quality assessment, we reviewed information contained within a given publication, as well as the text of referenced articles as needed.
Data abstraction was performed by a single reviewer (FA, MD, or TL) and quality checks were performed on a random subset of the data (20%). The study data were collected and managed using Research Electronic Data Capture tools hosted at the University of Washington Institute of Translational Health Sciences [28,29]. We performed a descriptive summary of the study characteristics and longitudinal outcomes. The definitions of acute and persistent diarrhea were accepted from included studies, but the review adapted the distinction of <14 and ≥14 days, distinguishing the two as described in WHO diarrhea treatment guidelines [30]. We intended to conduct a meta-analysis for any outcomes that were reported consistently by more than two studies. Due to heterogeneity in the measurement methods, comparison groups, and follow-up duration, we report a narrative summary of the evidence for each outcome.
Results
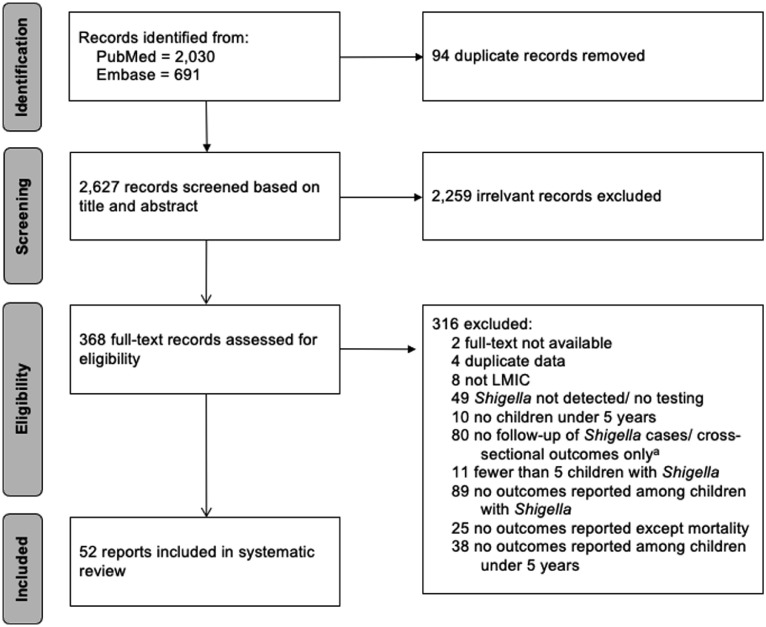
Our final search identified 2627 potentially eligible records from PubMed and Embase after deduplication (Figure 1). We completed the dual review of titles and abstracts passing 368 (14%) publications to full-text review, of which 52 met the inclusion criteria (Figure 1). The 316 studies excluded at full-text review are described in Appendix 3. The key characteristics of the 52 included articles are shown in Table 1 and summarized in Table 2. The data on Shigella outcomes were collected in 20 different countries; although 56% (n = 29) of the publications were from studies conducted at least partially in Bangladesh. There were 13 publications from studies conducted on the African continent. Five publications reported data from multiple countries either collected as part of the Etiology, Risk Factors, and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development (MAL-ED) cohort study (n = 3) [31] or the Global Enteric Multicenter Study (GEMS; n = 2) [32]. The study designs included cohort studies (n = 19), randomized trials (n = 13), disease surveillance (n = 11), and case-control studies (n = 9) (Table 2).
Figure 1.
Study selection (preferred reporting items for systematic reviews and meta-analyses [PRISMA] diagram).
aStudies that were excluded for “no follow-up of Shigella cases/cross-sectional outcomes only” include some studies that were longitudinal in nature, but presented outcomes cross-sectionally such that the likelihood of longitudinal outcomes given Shigella infection could not be determined (e.g., given all children with an outcome, the percent of children that had Shigella infection) either from direct interpretation of tables or through back calculations.
Abbreviations: LMIC, low– or middleincome country.
Table 1.
Characteristics of included publications (n = 52).
| Study | Country | Region | Study type | Datesb | Age rangec (months) | Population description | Primary Shigella detection method | # of children with Shigella | Diarrhea/ asymptomatic stools | Outcomes |
|---|---|---|---|---|---|---|---|---|---|---|
| Abu-Elyazeed et al.[40] | Egypt | Eastern Mediterranean | Cohort | 1995 - 1998 | 0-36 | Children in cohort without congenital abnormalities or hospitalization history | Culture | 101 | Diarrhea | Diarrhea, repeat Shigella infection(s) |
| Ahmed et al.[39] | Bangladesh | South-East Asia | Cohort | 1987 - 1989 | 0-59 | Children who were neighborhood contacts of Shigella cases and had diarrhea between 24 hours and 18 days of follow-up | Culture | 104 | Diarrhea | Diarrhea |
| Anders et al.[66] | Vietnam | South-East Asia | Cohort | 2009 - 2013 | 0-12 | Infants in birth cohort | qPCR | 108 | Diarrhea | Repeat Shigella infection(s) |
| Andersson et al.[62] | Tanzania | Africa | Cohort | Apr 2011 - Jul 2011 | 2-59 | Children with history of loose stools and fever | PCR | 42 | Diarrhea | Pathogen clearance |
| Ballard et al.[41] | Peru | The Americas | Case-control | Oct 2013 – May 2015 | 0-59 | Children seeking care for acute gastroenteritis and community controls | Culture | 23 | Diarrhea | Diarrhea |
| Baqui et al., [38] | Bangladesh | South-East Asia | Surveillance with case follow-up | May 1988 - Apr 1989 | 0-59 | Children in community-based cohort | Culture | Not specifieda | Diarrhea | Duration of Shigella excretion |
| Black et al.[43] | Bangladesh | South-East Asia | Cohort | Mar 1978 - Mar 1979 | 2-60 | Children in community-based cohort | Culture | Not specifieda | Diarrhea | Diarrhea |
| Black et al.[42] | Bangladesh | South-East Asia | Cohort | Mar 1978 - Mar 1979 | 2-48 | Children in community-based cohort | Culture | Not specifieda | Diarrhea | Diarrhea |
| Black et al.[49] | Bangladesh | South-East Asia | Cohort | Mar 1978 - Mar 1979 | 2-48 | Children in community-based cohort | Culture | 56 | Diarrhea | Linear growth, weight gain |
| Butler et al.[80] | Bangladesh | South-East Asia | Surveillance with case follow-up | Jul 1975 - Jun 1980 | 0-59 | Children admitted to hospital with confirmed Shigella infection | Culture | 2,172 | Diarrhea | Leukemoid reaction |
| Cravioto et al.[44] | Mexico | The Americas | Surveillance with case follow-up | Aug 1985 - Feb 1987 | 0-12 | Children in birth cohort | Culture | 11 | Both | Diarrhea |
| Das et al.[18] | Bangladesh | South-East Asia | Surveillance with case follow-up | Jan 2010 - Dec 2012 | 0-59 | All children with diarrhea in surveillance area at tertiary level hospital | Culture | 518 | Diarrhea | Economic outcomes |
| Das et al.[57] | Bangladesh | South-East Asia | Case-control | Dec 2007 – Mar 2011 | 0-59 | Children brought to health centers with MSD and community controls (enrolled in GEMS) | Culture | 591 | Diarrhea | Hospitalization, linear growth, ponderal growth, economic outcomes |
| Donowitz et al.[54] | Bangladesh | South-East Asia | Cohort | Jun 2014 - Mar 2016 | 0-24 | Children in birth cohort | qPCR | Not specifieda | Diarrhea | Linear growth, neurodevelopmental outcomes |
| Dutta et al.[81] | India | South-East Asia | Surveillance with case follow-up | Not specified | 0-59 | Children admitted to hospital with acute diarrhea or dysentery for <3 days | Culture | 46 | Diarrhea | Diarrhea |
| Dutta et al.[36] | India | South-East Asia | Surveillance with case follow-up | Jan 1985 - Dec 1988 | 6-59 | Children admitted to hospital with acute diarrhea or dysentery who did not receive antibiotics prior to hospitalization | Culture | 192 | Diarrhea | Diarrhea |
| Echeverria et al.[68] | Thailand | South-East Asia | Case-control | Not specified | 10-48 | Children with confirmed Shigella with fever, abdominal cramping, and bloody diarrhea | Culture | 19 | Diarrhea | Antibody response |
| Fujita et al.[82] | Kenya | Africa | Case-control | Sep 1986 - Aug 1987 | 12-59 | Children visiting health center with acute infectious diarrhea | Culture | 5 | Diarrhea | Stool pH/water content |
| Gaensbauer et al.[61] | Guatemala | The Americas | RCT | Mar 2015 - Jan 2016 | 6-35 | Children with moderate or severe diarrhea enrolled in an RCT of a nutritional product | PCR | 112 | Diarrhea | Pathogen clearance |
| George et al.[50] | Bangladesh | South-East Asia | Cohort | 2014 | 6-30 | A random subset of children enrolled in GEMS | qPCR | 71 | Both | Linear growth, ponderal growth/weight gain |
| Guh et al.[58] | China | Western-Pacific | Surveillance with case follow-up | Jan 2002- Dec 2002 | 0-59 | Children with diarrhea or dysentery and confirmed shigellosis presenting for healthcare | Culture | 55 | Diarrhea | Economic outcomes |
| Henry et al.[35] | Bangladesh | South-East Asia | Surveillance with case follow-up | Mar 1987 - Feb 1989 | 0-71 | Children in community-based cohort | Culture | 213 | Both | Diarrhea |
| Househam et al.[83] | South Africa | Africa | Cohort | Not specified | 1.5-12 | Children admitted to rehydration facility without associated parenteral infection | Culture | 31 | Diarrhea | Diarrhea |
| Huskins et al.[84] | Bangladesh | South-East Asia | Surveillance with case follow-up | Jan 1984 - Dec 1988 | 0-3 | Children hospitalized with confirmed Shigella infection | Culture | 159 | Diarrhea | Hospital discharge status |
| Huttly et al.[37] | Bangladesh | South-East Asia | Surveillance with case follow-up | Mar 1984 - Dec 1987 | 0-59 | Children in community-based environmental intervention trial | Culture | Not specifieda | Diarrhea | Diarrhea |
| Kabir et al.[56] | Bangladesh | South-East Asia | RCT | Not specified | 24-59 | Children from outpatient department with Shigella detected, treated for 5 days with effective antibiotic | Culture | 69 | Diarrhea | Linear growth, ponderal growth/weight gain |
| Kabir et al.[48] | Bangladesh | South-East Asia | RCT | Not specified | 24-59 | Children with bloody mucoid stools for <5 days enrolled in RCT (Kabir et al.[56] ) | Culture | 59 | Diarrhea | Diarrhea, linear growth, ponderal growth/weight gain, subsequent illness |
| Khan et al.[65] | Bangladesh | South-East Asia | Surveillance with case follow-up | 1973 - 1980 | 0-59 | Children with family member with Shigellosis | Culture | 132 | Diarrhea | Duration of Shigella excretion |
| Luoma et al.[51] | Malawi | Africa | Cohort | Feb 2011 – Aug 2012 | 18-24 | Seemingly healthy children participating in an extension to a nutrient supplement trial | qPCR | Not specifieda | Asymptomatic | Linear growth |
| Mazumder et al.[69] | Bangladesh | South-East Asia | RCT | Not specified | 12-48 | Malnourished children hospitalized with blood in stool for <72 hours | Culture | 23 | Diarrhea | Diarrhea, nutrient absorption |
| Mazumder et al.[45] | Bangladesh | South-East Asia | RCT | Not specified | 12-48 | Malnourished children with blood in stool for <96 hours | Culture | 75 | Diarrhea | Ponderal growth/weight gain |
| Mitra et al.[46] | Bangladesh | South-East Asia | Cohort | May 1995 - Dec 1995 | 5-60 | Children hospitalized with blood in stool and with no history of antibiotics or vitamin A supplementation | Culture | 66 | Diarrhea | Diarrhea, hospitalization, ponderal growth/weight gain, serum retinol concentration |
| Nasrin et al.[12] | Bangladesh, The Gambia, India, Kenya, Mali, Mozambique, Pakistan | South-East Asia, Africa | Case-control | 2007-2011 | 0-59 | Children with moderate-to-severe diarrhea enrolled in GEMS | Culture | Not specifieda | Diarrhea | Linear growth |
| Ndungo et al.[71] | Malawi | Africa | Cohort | Feb – Nov 2016 | 0-24 | Children enrolled in Malaria birth cohort study and sex- and age-matched controls | qPCR | 30 | Both | Microbiome composition |
| Perin et al.[85] | Bangladesh | South-East Asia | Case-control | 2014 - 2015 | 6-31 | Children in cohort | 16s sequencing | Not specifieda | Both | Linear growth, ponderal growth/weight gain |
| Platts-Mills et al.[55] | Tanzania | Africa | Cohort | Dec 2009 | 1-12 | Children in birth cohort with diarrhea | qPCR | 19 | Diarrhea | Linear growth |
| Platts-Mills et al.[86] | Bangladesh | South-East Asia | Case-control | 2009 - 2012 | 6-23 | Children participating in an intervention with WAZ <-2 (cases) and WAZ >-1 (controls) | qPCR | 139 | Diarrhea | Malnutrition |
| Platts-Mills et al.[8] | Niger | Africa | RCT | Oct 2014 – Dec 2017 | 0-23 | Children who received 3 doses of rotavirus vaccine or placebo without RCT protocol violation | qPCR | 147 | Diarrhea | Diarrhea |
| Rahman et al.[70] | Bangladesh | South-East Asia | RCT | Not specified | 6-35 | Children with bloody mucoid stools for <5 days and no history of potentially effective drugs | Culture | 66 | Diarrhea | Nutritional intake |
| Cruz et al.[67] | Guatemala | The Americas | Cohort | Not specified | 0-35 | Children in community-based cohort | Culture | 126 | Both | Diarrhea, repeat Shigella infection(s), nutritional intake |
| Rampengan et al.[47] | Indonesia | South-East Asia | Cohort | Jul 1974 - Jun 1976 | 0-59 | Children hospitalized with dysentery and confirmed Shigella infection | Culture | 46 | Diarrhea | Diarrhea, duration of fever, hospitalization |
| Raqib et al.[60] | Bangladesh | South-East Asia | RCT | Not specified | 12-59 | Moderately malnourished children with acute shigellosis | Culture | 56 | Diarrhea | Antibody response, EED, inflammation |
| Riewpaiboon et al.[17] | Thailand | South-East Asia | Case-control | May 2002 - Apr 2003 | 0-59 | Children presenting to health center with shigellosis | Culture | 130 | Diarrhea | Economic outcomes |
| Rodriguez et al.[63] | Mexico | The Americas | RCT | Jan 1987 - Jul 1988 | 2-59 | Children in RCT who visited hospital with bloody diarrhea <5 days and without history of potentially effective drugs | Culture | 35 | Diarrhea | Pathogen clearance |
| Rogawski et al.[11] | Bangladesh, Brazil, India, Nepal, Pakistan, Peru, South Africa, Tanzania | South-East Asia, Africa, the Americas | Cohort | 2009 - 2012 | 0-60 | Children in MAL-ED birth cohort: infants from singleton pregnancies without very low birth weight, congenital disease, or severe neonatal disease | qPCR | Not specifieda | Both | Linear growth, ponderal growth/weight gain |
| Rogawski McQuade et al.[9] | Bangladesh, Brazil, India, Nepal, Pakistan, Peru, South Africa, Tanzania | South-East Asia, Africa, the Americas | Cohort | 2009 - 2012 | 0-24 | Children in MAL-ED birth cohort | Culture | Not specifieda | Diarrhea | Diarrhea, fever in subsequent Shigella-attributable diarrhea episode, hospitalization |
| Rogawski McQuade et al.[52] | Brazil, South Africa, Tanzania | Africa, the Americas | Cohort | 2009-2012 | 0-24 | Children in MAL-ED birth cohort | qPCR | Not specifieda | Asymptomatic | Linear growth, neurodevelopmental outcomes |
| Roy et al.[33] | Bangladesh | South-East Asia | RCT | 1999 - 2002 | 12-59 | Moderately malnourished children with shigellosis dysentery | Culture | 56 | Diarrhea | Diarrhea, linear growth, ponderal growth/weight gain, subsequent illness |
| Schnee et al.[53] | Bangladesh | South-East Asia | RCT | 2011 - 2012 | 0-24 | Children in birth cohort with diarrhea | qPCR | Not specifieda | Diarrhea | Inflammation, linear growth |
| Taylor et al.[34] | Thailand | South-East Asia | RCT | Nov 1984 - Jan 1985 | 2-60 | Children in drug trial with diarrhea and fever, vomiting, or colic for <24 hours | Culture | 21 | Diarrhea | Diarrhea |
| Versloot et al.[64] | Malawi | Africa | RCT | Jan 2013 - Jul 2013 | 8-59 | Children in an RCT who were hospitalized for complicated severe acute malnutrition | PCR | 19 | Both | Pathogen clearance |
| Zimmermann et al.[59] | Bangladesh, The Gambia, India, Kenya, Mali, Mozambique, Pakistan | South-East Asia, Africa | Case-control | Dec 2007 - Mar 2011 | 0-59 | Children with acute diarrhea (any severity) enrolled in GEMS | Culture | 1,736 | Diarrhea | Economic outcomes |
Abbreviations: EED, environmental enteric dysfunction; GEMS, the Global Enteric Multicenter Study; MAL-ED, Etiology, Risk Factors, and Interactions of Enteric Infections and Malnutrition and the Consequences for Child Health and Development; RCT, randomized controlled trial; qPCR, quantitative polymerase chain reaction; MSD, moderate-to-severe diarrhea; WAZ, weight-for-age z-score.
The number of children with Shigella detected was not specified in some studies; see Appendix 4 for the # of Shigella-positive stools or diarrhea episodes attributable to Shigella, which were used to verify inclusion criteria of 5+ children with Shigella.
The months (if available) and years of participant enrollment.
The age range of enrolled children for whom outcomes were measured/reported.
Table 2.
Summary of included publications (n = 52).
| Publication characteristic | Number of publications | (%) |
|---|---|---|
| Geographic regiona | ||
| South-East Asia | 36 | 69% |
| Africa | 13 | 25% |
| The Americas | 8 | 15% |
| Western-Pacific | 1 | 2% |
| Eastern Mediterranean | 1 | 2% |
| Countrya | ||
| Bangladesh | 29 | 56% |
| India | 6 | 12% |
| Tanzania | 5 | 10% |
| Pakistan | 4 | 8% |
| South Africa | 4 | 8% |
| Malawi | 3 | 6% |
| Thailand | 3 | 6% |
| Other | 17 | 33% |
| Study type | ||
| Cohort | 19 | 37% |
| Randomized controlled trial | 13 | 25% |
| Surveillance (with case follow-up) | 11 | 21% |
| Case-control | 9 | 17% |
| Primary Shigella detection method | ||
| Culture | 37 | 71% |
| qPCR | 11 | 21% |
| PCR | 3 | 6% |
| 16S sequencing | 1 | 2% |
| Number of children with Shigella | ||
| Mean | 192 | |
| Median (range) | 66 (5-2,172) | |
| Publication date | ||
| 1980 to 1989 | 10 | 19% |
| 1990 to 1999 | 15 | 29% |
| 2000 to 2009 | 6 | 12% |
| 2010 to present | 21 | 40% |
| Reported outcomesa | ||
| Diarrhea-related outcomes | 20 | 38% |
| Linear growth | 14 | 27% |
| Other anthropometric measuresb | 10 | 19% |
| Economic outcomes | 5 | 10% |
| Pathogen clearance | 4 | 8% |
| Repeat Shigella infections | 4 | 8% |
| Systemic inflammation | 2 | 4% |
| Neurodevelopmental outcomes | 2 | 4% |
| Gut inflammation, environmental enteric dysfunction | 1 | 2% |
| Other outcomes | 16 | 31% |
| Quality score | ||
| Poor | 1 | 2% |
| Fair | 16 | 31% |
| Good | 35 | 67% |
Abbreviations: qPCR, quantitative polymerase chain reaction.
Categories are not mutually exclusive therefore percentages may exceed 100%
Includes ponderal growth, weight gain, underweight, malnutrition, etc.
Publications included a median of 66 children with Shigella, ranging from five to 2172 (Table 2). Of note, some of the included studies did not specify the number of children with Shigella but provided other information that made it possible to estimate the number of children with Shigella as being five or more (Appendix 4). Although most publications were among children with Shigella diarrhea only, nine (17%) publications also included Shigella detected in asymptomatic patients (Table 1). The study setting and initial inclusion criteria varied widely, such as malnourishment, current diarrhea, participation in birth and community-based cohorts or randomized controlled trials, admittance to hospitals, and presentation at health care facilities. Culture was the most common primary Shigella detection method (71%), followed by quantitative polymerase chain reaction (21%; Tables 1, 2). Most studies were rated ‘good’ quality (n = 35; 67%), followed by ‘fair’ quality (n = 16; 31%) and ‘poor’ quality (n = 1; 2%) (Appendix 5).
The most commonly reported outcomes of Shigella were related to diarrhea (n = 20) and linear growth (n = 14). Other anthropometric measures, such as ponderal growth (e.g., change in weight-for-height z-score [WHZ]) or weight gain (e.g., change in weight or weight-for-age z-score [WAZ]), were reported in 10 studies (Table 2). In each of these categories, fewer than three studies reported on the same outcome using a similar comparison group, thus precluding meta-analyses.
Diarrhea outcomes
There were three general categories of measurement among studies of diarrhea outcomes: duration of diarrhea measured continuously (n = 9); duration of diarrhea measured categorically (<7 days, 7-<14 days, ≥14 days) and presented as corresponding percentages, odds ratios (ORs), and risk ratios (n = 11); and characteristics of subsequent diarrhea episodes (both Shigella and unspecified) that occurred after diarrhea-free days (n = 3). The measurement details are summarized in Table 3.
Table 3.
Diarrhea outcomes, by measurement and follow-up duration.
| Measurement | Follow-up duration | Study | # with Shigella | Outcome measurement/ comparison groups | Effect measure |
|---|---|---|---|---|---|
| Acute diarrhea | |||||
| Proportion with diarrhea on Day X | |||||
| 3 days | Abu-Elyazeed et al.[40] | 101 | Percent of children with Shigella that had diarrheaa lasting 3 or more days, by serotype | All: 56%; S. flexneri: 53%; S. sonnei: 55%; S. dysenteriae: 61%; S. boydii: 50%; Mixed serogroups (1 case): 100% | |
| 4 days | Househam et al.[83] | 31 | Probability of acute diarrhea being self-limiting (less than 4 days of treatment in rehydration facility needed before discharge home and no past month or following months admissions to a rehydration facility) given Shigella present | 0.74; i.e., significantly higher (P <0.05) than when Shigella is not present | |
| 5 days | Abu-Elyazeed et al.[40] | 101 | Percent of children with Shigella that had diarrheaa lasting 5 days | 23% | |
| Relative proportion/odds of diarrhea on Day X (OR) | |||||
| 3 days | Abu-Elyazeed et al.[40] | 101 | Adjusted OR (95% CI) for Shigella diarrhea (as opposed to non-Shigella diarrhea) among children with illness duration of 3 or more days, adjusting for fever, vomiting, severe dehydration and bloody stool | 1.4 (95% CI: 1.0, 2.0) | |
| Prolonged diarrhea | |||||
| Proportion with diarrhea on Day X | |||||
| 7 days | Rogawski et al.[9] | Not specified | The percent of Shigella-attributable diarrhea episodes where prolonged diarrhea (7+ days) was present, by year of life | Year 1: 24.3%; Year 2: 17.7% | |
| 7 days | Rogawski et al.[9] | Not specified | The percent of Shigella-attributable diarrhea episodes where prolonged diarrhea was present (7+ days), by co-infection status | Shigella only: 19.5%; Viral co-etiology: 16.8%; Bacterial co-etiology: 17.9%; Parasitic co-etiology: 29.4% | |
| 7 days | Roy et al.[33] | 56 | Percent of children with Shigella dysentery at baseline who had not recovered by day 7 (defined as children who were 'three or fewer formed stools in a day, were afebrile, did not have visible blood or mucous in stools and did not have abdominal pain or tenderness) | Zinc: 11%; No zinc: 25% | |
| 7 days | Platts-Mills et al.[8] | 147 | Prevalence ratio (95% CI) for prolonged diarrhea (≥7 days) comparing children with diarrhea attributable to Shigella vs those not attributable to Shigella | 1.68 (95% CI: 0.99, 2.87) | |
| 7 days | Taylor et al.[34] | 21 | Proportion of children with Shigella diarrhea at baseline that still had diarrhea at day 7 | Erythromycin group: (3/8) 38%b; control group: (1/7) 14% | |
| Relative proportion/risk of diarrhea on Day X (RR) | |||||
| 7 days | Rogawski et al.[9] | Not specified | The site-adjusted risk ratio (95% CI) comparing the percent of Shigella-attributed episodes leading to prolonged diarrhea (7+ days) in the first year compared to the second year of life | 1.24 (95% CI: 0.88, 1.74) | |
| 7 days | Rogawski et al.[9] | Not specified | The site and age-adjusted risk ratios (95% CI) for prolonged diarrhea (7+ days) comparing Shigella episodes with co-etiologies to single etiology | Viral co-etiology: 1.15 (95% CI: 0.83, 1.60); Bacterial co-etiology: 1.18 (95% CI: 0.77, 1.80); RR for parasitic co-etiology not estimated due to small numbers | |
| Persistent diarrhea | |||||
| Proportion with diarrhea on Day X | |||||
| 14 days | Rogawski et al.[9] | Not specified | The percent of Shigella-attributable diarrhea episodes where persistent diarrhea (14+ days) was present, by year of life | Year 1: 5.6%; Year 2: 2.9% | |
| 14 days | Rogawski et al.[9] | Not specified | The percent of Shigella-attributable diarrhea episodes where persistent diarrhea (14+ days) was present, by co-etiology status (RRs not calculated due to small numbers of episodes) | Shigella only: 3.0%; Viral co-etiology: 4.1%; Bacterial co-etiology: 4.7%; Parasitic co-etiology: 0% | |
| 14 days | Henry et al.[35] | Not specified | Percent of Shigella episodes that had a duration of 14+ days | 14.9% (14/94) | |
| 14 days | Dutta et al.[36] | 192 | Percent of children who had diarrhea duration of 14+ days, by nutritional status | Well-nourished: 3.2%, Malnourished: 19.2%; p<0.001 | |
| 14 days | Dutta et al.[81] | 46 | Percent of Shigella diarrhea with duration of 14+ days, by serotype | S. flexneri: 44.8%; S. dysenteriae 1: 58.8% | |
| 14 days | Huttly et al.[37] | Not specified | Percent of Shigella episodes with diarrhea >14 days | 61.1% | |
| 1 month | Ahmed et al.[39] | 104 | Percent of Shigella diarrhea episodes that became persistent (14+ days) overall, and by presence of blood | Overall: 23% (24/104); bloody: 30%; nonbloody: 18.8%; p>0.05 | |
| 1 month | Ahmed et al.[39] | 104 | Percent of Shigella diarrhea episodes that became persistent (14+ days) by species | S. flexneri: 23.6%; S. dysenteriae 1: 26.3%; Other: 20.0%; p>0.05 | |
| 1 month | Ahmed et al.[39] | 104 | Percent of Shigella diarrhea episodes that became persistent (14+ days) among children with and without multiple antibiotic resistance (ampicillin, trimethoprim-sulfamethoxazole, and nalidixic acid) | With multiple antibiotic resistance: 66.7% (4/6); without: 20.4% (20/98); p<0.05 | |
| Relative risk of diarrhea on Day X (RR, OR) | |||||
| 14 days | Rogawski et al.[9] | Not specified | The site-adjusted risk ratio (95% CI) comparing the percent of Shigella-attributed episodes leading to persistent diarrhea (14+) in the first year compared to the second year of life | 1.32 (95% CI: 0.59, 2.93) | |
| 1 month | Ahmed et al.[39] | 104 | Age-adjusted RR (95% CI) of persistent diarrhea (14+ days) comparing Shigella-positive to Shigella-negative diarrhea episodes, overall and by the presence of blood | Overall: 1.83 (95% CI: 1.19, 2.81; P <0.01); Bloody diarrhea: 1.06 (95% CI: 0.60, 1.86; P >0.05); Nonbloody diarrhea: 2.31 (95% CI: 1.24, 4.30; P <0.01) | |
| 1 month | Ahmed et al.[39] | 104 | Age-adjusted RR (95% CI) for persistent diarrhea (14+ days) comparing children who have shigellosis with bloody diarrhea to children who have shigellosis with nonbloody diarrhea | 1.64 (95% CI: 0.82, 3.26) | |
| 1 month | Ahmed et al.[39] | 104 | Age-adjusted RR (95% CI) of persistent diarrhea (14+ days) with S. dysenteriae 1 and other Shigella serotypes, compared to risk of persistent diarrhea with S. flexneri | RRdys 1 vs flex: 1.25 (95% CI: 0.49, 3.18); RRother serotypes vs flex: 0.78 (95% CI: 0.34, 1.77) | |
| 1 month | Ahmed et al.[39] | 104 | Age-adjusted RR (95% CI) for persistent diarrhea (14+ days) comparing children with shigellosis with multiple antibiotic resistance (resistant to ampicillin, trimethoprim-sulfamethoxazole, and nalidixic acid) to children with shigellosis without multiple antibiotic resistance | 3.76 (95% CI: 1.51, 9.36) | |
| Mean/median duration of diarrhea | |||||
| 72 hours | Mazumder et al.[69] | 23 | Mean (SE) number of hours of Shigella dysentery in the intervention diet (higher protein and energy) and control diet groups | Control diet: 58 (7.9) hours; Test diet: 62 (9.8) hours | |
| Until 48 hrs symptom-free | Ballard et al.[41] | 23 | Mean (SD) duration among those with diarrhea | 6.8 (1.2) days | |
| 20 days | Black et al.[43] | 117 | Median, mean (SE), and range of duration in days of Shigella diarrhea episodes | Median: 7; Mean: 10.7 (1); Range: 1–20+ days | |
| 60 days | Black et al.[42] | Not specified | Mean duration (days) of Shigella diarrhea in highest and lowest weight-for-length Z score groups | Highest: 6.5 days; Lowest: 21.3 days | |
| 60 days | Black et al.[42] | Not specified | Mean (SE) duration in days of Shigella diarrhea by anthropometric group | Normal: 12.0 (3.1); Stunted: 13.8 (2.9); Stunted and wasted: 15.4 (4) | |
| 60 days | Black et al.[42] | Not specified | Mean (SE) duration in days of Shigella diarrhea among children <24 months by relative nutritional status | Weight-for-length ≥90%: 8.8 (2.3); 80-89%: 14.9 (3.1); ≤79%: 22.2 (5). Weight-for-age ≥75%: 11.5 (2.4); 60-74%: 16.1 (2.9); <60%: 15.1 (5.5). Length-for-age 90-94%: 13.9 (3); 85-89%: 16.8 (3.5); <85%: 11.2 (3.4); differences were not statistically significantly different |
|
| 6 months | Roy et al.[33] | 56 | Mean duration (days) of diarrhea episodes that occurred in the 6-month follow-up (95% CI) in the zinc group and the control group (no zinc supplementation) | Zinc: 9.8 (95% CI: 6.0, 15.9); No zinc: 7.1 (95% CI: 3.2, 12.6); P = 0.1 | |
| 12 months | Cravioto et al.[44] | 11 | Mean (SD) duration (days) of moderate-to-severe dysentery among children with Shigella | 5 (1) days | |
| Not specified | Roy et al.[33] | 56 | Median days to recovery (range) in the zinc group and the control group (no zinc supplementation) | Zinc: 2 (1–8); No zinc: 4 (1–8); P = 0.03 | |
| Not specified | Roy et al.[33] | 56 | Median days to disappearance from blood from stool (range) in the zinc group and the control group (no zinc supplementation) | Zinc: 2 (1–4); No zinc: 4 (2–5); P = 0.04 | |
| Not specified | Roy et al.[33] | 56 | Median days to disappearance from mucous from stool (range) in the zinc group and the control group (no zinc supplementation) | Zinc: 2 (1, 4); No zinc: 4 (1, 7); P = 0.04 | |
| Not specified | Roy et al.[33] | 56 | Median days to resolution of straining (range) in the zinc group and the control group (no zinc supplementation) | Zinc: 2 (1, 6); No zinc: 2 (1, 5); P = 0.5 | |
| Not specified | Mitra et al.[46] | 66 | Mean days (SD) until no visible blood in stool (days) | S. dysenteriae: 2.9 (1.8); Other Shigella: 0.8 (0.7) | |
| Not specified | Rampengan et al.[47] | 46 | Mean duration (days) of diarrhea during hospitalization | 5.8 days | |
| Not specified | Abu-Elyazeed et al.[40] | 101 | Mean duration (days) of illnessa | 4 days | |
| Subsequent diarrhea | |||||
| 6 months | Roy et al.[33] | 56 | Mean number of diarrhea episodes during the 6-month follow-up (95% CI) following an episode of Shigella diarrhea comparing children randomized to zinc group vs control group (no zinc supplementation) | Zinc: 2.2 (95% CI: 1.6, 4.1); No zinc: 3.3 (95% CI: 2.7, 4.1); P = 0.03 | |
| 6 months | Kabir et al.[48] | 59 | Number of diarrhea episodes per child in the 6-month follow-up period among children who received 14 days of high-protein diet and those who received standard-protein diet and the RR (95% CI) comparing standard to high protein. | High protein: 1.9 episodes/child; Standard-protein: 2.3 episodes/child; RR : 1.19 (95% CI: 0.76, 1.85) | |
| 2 years | Rogawski et al.[9] | Not specified | Among children who had more than one Shigella-attributable diarrhea episode, the percent of subsequent episodes that were severe (CODA score 4+) and the site and age-adjusted risk ratio for severe diarrhea comparing the first episode to subsequent episodes (95% CI) | 25.8%; RR: 1.08 (95% CI: 0.82, 1.41) | |
| 2 years | Rogawski et al.[9] | Not specified | Among children who had more than one Shigella-attributable diarrhea episode, the percent of subsequent episodes with blood and the site and age-adjusted risk ratio for bloody diarrhea comparing the first episode to subsequent episodes (95% CI) | 14.9%; RR: 0.81 (95% CI: 0.55, 1.20) | |
| 2 years | Rogawski et al.[9] | Not specified | Among children who had more than one Shigella-attributable diarrhea episode, the percent of subsequent episodes that were prolonged (7+ days) and the site and age-adjusted risk ratio for prolonged diarrhea comparing the first episode to subsequent episodes (95% CI) | 13.7%; RR: 1.13 (95% CI: 0.78, 1.64) | |
| 2 years | Rogawski et al.[9] | Not specified | Among children who had more than one Shigella-attributable diarrhea episode, the percent of subsequent episodes that were persistent (14+ days) and the site and age-adjusted risk ratio for persistent diarrhea comparing the first episode to subsequent episodes (95% CI) | 1.6%; RR: 1.75 (95% CI: 0.67, 4.59) | |
| 2 years | Rogawski et al.[9] | Not specified | Among children who had more than 1 Shigella-attributable diarrhea episode, the percent of subsequent episodes with high frequency (>6 loose stools in 24 hours) and the site and age-adjusted risk ratio comparing the first episode to subsequent episodes (95% CI) | 19%; RR: 1.21 (95% CI: 0.89, 1.63) | |
Abbreviations: CI, confidence interval; OR, odds ratio; RR, relative risk; SE, standard error; SEM, Standard error of the mean; CODA, a diarrheal severity score (Community Diarrhea).
"Illness" was presumed to mean diarrhea because stool samples were taken when diarrheal episodes were detected.
Briefly, based on three studies, between 11% and 25% of children with Shigella diarrhea went on to develop prolonged diarrhea (duration 7-<14 days) [9,33,34], with no statistically significant difference in risk by age (1 year vs 2 years) or co-infection status [9]. Six studies reported on persistent diarrhea (duration ≥14 days) and in these studies, 2.9-61.1% of children with Shigella diarrhea developed persistent diarrhea [9,[35], [36], [37], [38], [39]]. Two of these studies reported on risk factors of diarrhea persistence among Shigella diarrhea cases, with a statistically significantly higher likelihood of persistence among children who were malnourished (malnourished: 19.2% vs well-nourished: 3.2%) [36], had blood in stool (bloody: 30% vs nonbloody: 19%) [39], or had multidrug-resistant Shigella (multidrug resistant: 66% vs not multidrug resistant: 20%) [39]. Of note, a study comparing likelihood of persistent diarrhea between children with Shigella-positive diarrhea compared with Shigella-negative diarrhea found Shigella to be significantly associated with persistent diarrhea (relative risk: 1.83; 95% confidence interval [CI]: 1.91, 2.81) [39]. Similarly, another study reported a longer duration of diarrhea in children with Shigella diarrhea than those with other causes of diarrhea (OR of duration longer than 3 days: 1.4; 95% CI: 1.0-2.0) [40]. Across the studies, the continuously measured mean duration of diarrhea ranged from 2 to 22.2 days, with substantial variation by intervention status in trials and anthropometric groups [33,[40], [41], [42], [43], [44], [45], [46], [47]]. There was wide heterogeneity in the information presented on subsequent new diarrhea episodes (Table 3) [9,33,48].
Growth outcomes
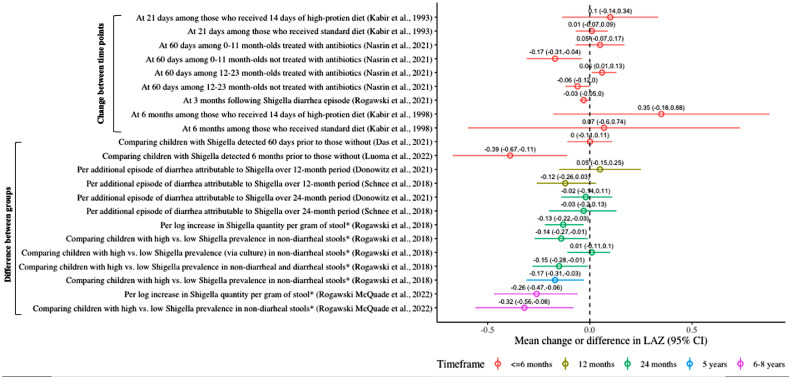
Six of 14 studies meeting the inclusion criteria found a statistically significant decrease in linear growth associated with Shigella in diarrheal [11,12,49,50] and nondiarrheal [11,51,52] stools (Table 4). There was substantial heterogeneity in measurement time points (ranging from 21 days to 8 years) and comparison groups (Table 4). Linear growth was commonly operationalized as the mean change in the length-for-age z-score (LAZ) between two time points (n = 3) or the difference in LAZ between two groups, defined by presence/absence of Shigella or high/low quantity of Shigella (n = 7). The effect estimates from these studies are summarized in Figure 2. The differences in LAZ comparing high with low Shigella prevalence in nondiarrheal stools ranged from -0.14 (95% CI: -0.27, -0.01) at 2 years to -0.32 (95% CI: -0.56, -0.08) at 6-8 years [11,52]; the mean differences in LAZ per attributable episode of Shigella diarrhea ranged from -0.12 (95% CI: -0.26, 0.03) [53] to 0.05 (95% CI: -0.15, 0.25) [54]. Two studies reported on the impact of Shigella diarrhea on linear growth at 3 months after diarrhea: one study found a statistically significant average loss of -0.03 (95% CI: -0.05, -0.00) in LAZ [11], whereas another study found no difference in the 3-month LAZ associated with Shigella quantity during the diarrheal episodes [55]. In GEMS, Shigella episodes not treated with antibiotics led to greater declines in linear growth than treated episodes among children aged <24 months [12]. Another study found that Malawian children with Shigella detected at age 18 months had, on average, 0.39 lower LAZ at 24 months than children without Shigella detected [51]. George et al. [18] found Shigella infection to be associated with a two-fold increase in the odds of stunting (defined as height-for-age z-score <-2) at 9 months of follow-up (OR: 2.01; 95% CI: 1.02, 3.93) [50], and Black et al. [7,8] reported a statistically significant association between the periods of Shigella diarrhea and change in height-for-age compared with a village standard between the beginning and end of the study period [49].
Table 4.
Linear growth outcomes, by measurement and follow-up time frame.
| Outcome | Follow-up duration | Study | # with Shigella | Comparison groups | Effect measure |
|---|---|---|---|---|---|
| Mean change in LAZ between two time points | Mean △ in LAZ (95% CI) | ||||
| 21 days | Kabir et al.[56] | 69 | At 21 days compared to day 1 among those who received 14 days of high-protein diet | +0.1 (SD: 0.12) | |
| 21 days | Kabir et al.[56] | 69 | At 21 days compared to day 1 among those who received standard diet | +0.01 (SD: 0.04) | |
| ∼60 days (49-91) | Nasrin et al.[12] | 92 | At ∼60 days, among children 0-11 months, treated with antibiotic, adjusting for other pathogens | 0.05 (−0.07, 0.17) | |
| ∼60 days (49-91) | Nasrin et al.[12] | 72 | At ∼60 days, among children 0-11 months, not treated with antibiotic, adjusting for other pathogens | −0.17 (−0.31, −0.04) | |
| ∼60 days (49-91) | Nasrin et al.[12] | 282 | At ∼60 days, among children 12-23 months, treated with antibiotic, adjusting for other pathogens | 0.06 (0.009, 0.13) | |
| ∼60 days (49-91) | Nasrin et al.[12] | 159 | At ∼60 days, among children 12-23 months, not treated with antibiotic, adjusting for other pathogens | -0.06 (-0.12, 0.001) | |
| ∼60 days (49-91) | Nasrin et al.[12] | 396 | At ∼60 days, among children 24-59 months | Non-significant | |
| 3 months | Rogawski et al.[11] | NS; 1,469a | At 3 months following Shigella diarrhea episode | -0.03 (-0.05, -0.00) | |
| 6 months | Kabir et al.[48] | 59 | At 6 months compared to day 1 among those who received 14 days of high-protein diet | +0.35 (SD: 0.27) | |
| 6 months | Kabir et al.[48] | 59 | At 6 months compared to day 1 among those who received standard diet | +0.07 (SD: 0.34) | |
| Mean difference in LAZ | |||||
| ∼60 days (50-90) | Das et al.[57] | 591 | Comparing children with Shigella detected 60 days prior to those without, unadjusted | -0.11 (-0.21, -0.02) | |
| ∼60 days (50-90) | Das et al.[57] | 591 | Comparing children with Shigella detected 60 days prior to those without Shigella detected, adjusted for confounders, co-infections | 0.001 (-0.11, 0.11) | |
| 3 months | Platts-Mills et al.[55] | 19 | At 3 months post-diarrhea comparing high and low quantity of Shigella in diarrhea stools | “No specific pathogen quantity in diarrheal stools was significantly associated with poor growth” | |
| 6 months | Luoma et al.[51] | NS; 604a | At 24 months comparing children with Shigella detected at 18 months to those without Shigella detected | -0.39 (-0.67, -0.11) | |
| 12 months | Donowitz et al.[54] | NS; 250a | Per additional episode of diarrhea attributable to Shigella | +0.05 (-0.15, 0.25) | |
| 12 months | Schnee et al.[53] | NS; 125a | Per additional episode of diarrhea attributable to Shigella | -0.12 (-0.26, 0.03) | |
| 24 months | Donowitz et al.[54] | NS; 250a | Per additional episode of diarrhea attributable to Shigella | -0.02 (-0.14, 0.11) | |
| 24 months | Schnee et al.[53] | NS; 125a | Per additional episode of diarrhea attributable to Shigella | -0.03 (-0.20, 0.13) | |
| 24 months | Rogawski et al.[11] | NS; 1,469a | Comparing children with high (90th percentile) vs low (10th percentile) Shigella prevalence in nondiarrheal stools over 24-month period | -0.14 (-0.27, -0.01) | |
| 24 months | Rogawski et al.[11] | NS; 1,469a | Comparing children with high (90th percentile) vs low (10th percentile) Shigella prevalence in nondiarrheal stools (using culture instead of qPCR) | +0.01 (-0.11, 0.10) | |
| 24 months | Rogawski et al.[11] | NS; 1,469a | Comparing children with high (90th percentile) vs low (10th percentile) Shigella prevalence in nondiarrheal and diarrheal stools over 24-month period | -0.15 (-0.28, -0.01) | |
| 24 months | Rogawski et al.[11] | NS; 1,469a | Per one log increase in Shigella quantity (copy number) per gram of stool over 24-month period | -0.13 (-0.22, -0.03) | |
| 5 years | Rogawski et al.[11] | NS; 1,202a | Comparing children with high (90th percentile) vs low (10th percentile) Shigella prevalence in nondiarrheal stools over 24-month period | -0.17 (-0.31, -0.03) | |
| 6-8 years | Rogawski et al.[52] | NS; 451a | Per one log increase in Shigella quantity per gram of stool over 24-month period | -0.26 (-0.47, -0.06) | |
| 6-8 years | Rogawski et al.[52] | NS; 451a | Comparing children with high (90th percentile) vs low (10th percentile) Shigella prevalence in nondiarrheal stools over 24-month period | -0.32 (-0.56, -0.08) | |
| Risk of Stunting (HAZ <-2) | |||||
| 9 months | George et al.[50] | 71 | Comparing likelihood of stunting during follow-up among those with Shigella at baseline to those without, after adjusting for age, age, caregiver educational level, breastfeeding, and family size | OR: 2.01 (1.02, 3.93) | |
| HAZ | |||||
| 6 months | Kabir et al.[48] | 59 | Mean HAZ at 6 months among those who received a high-protein diet | 1.28 (SD: 1.15) | |
| 6 months | Kabir et al.[48] | 59 | Mean HAZ at 6 months among those who received a standard diet | -1.96 (SD: 1.43) | |
| Other linear growth measures | |||||
| 60 days | Roy et al.[33] | 56 | Mean linear growth per month (cm) among children with Shigella in zinc group | 0.58 cm | |
| 60 days | Roy et al.[33] | 56 | Mean linear growth per month (cm) among children with Shigella in control group | 0.65 cm | |
| 1 year | Black et al.[49] | 56 | Regression coefficient for Shigella on change in length (cm) or change in length status expressed as change in percentage of the village reference for age from the beginning to the end of the study period | Shigella coefficient had borderline significance (P = 0.07), but exact coefficient not reported | |
| 1 year | Black et al., [49] | 56 | Regression coefficient for Shigella on change in length status expressed as change in percentage of the village reference height-for-age from the beginning to the end of the study period | -0.083 (p<0.05) | |
| 1 year | Black et al.[49] | 56 | Regression coefficient for Shigella on change in length (cm) (adjusting for age and initial length) | -0.075 cm (p<0.05) | |
| 1 year | Black et al.[49] | 56 | Comparison of the percentage of expected linear growth rates (based on all village children) observed during periods of Shigella diarrhea compared to no diarrhea | “Periods with Shigella diarrhea had significantly lower growth rates” (P <0.01) | |
| 18 months | Perin et al.[85] | NS; 68a | Comparing children in the lowest tertile of change in HAZ to those in the highest tertile of change in HAZ | Average proportional abundance of Escherichia/ Shigella: 0.026 vs 0.030 | |
Abbreviations: CI, confidence interval; LAZ, length-for-age z-score; HAZ, height-for-age z-score; NS, not specified; OR, odds ratio; SD, standard deviation; SE, standard error
Represents the number of children enrolled in the study because the number with Shigella was not specified (results reported as Escherichia/ Shigella).
Figure 2.
Mean change or difference in LAZ by comparison group and duration of follow-up.
*Shigella prevalence or quantity was assessed over a 24-month period. “High” was defined as 90th percentile and “low” as 10th percentile.
Abbreviations: CI, confidence interval; LAZ, length-for-age z-score.
Additional anthropometric outcomes are summarized in Appendix 6. Seven studies assessed the ponderal growth and weight-for-age, four of which did not have a comparison group without Shigella infection nor with low levels of Shigella [45,46,48,56]. The MAL-ED study found no significant difference in mean WHZ or WAZ between children with high (90th percentile) and low (10th percentile) Shigella prevalence in nondiarrheal stools [11]. Two studies reported on children enrolled in the Bangladesh site of GEMS: one found children with Shigella infection had significantly lower WHZ (-0.11; 95% CI: -0.21, -0.001) than children who were Shigella-negative after 60 days of follow-up [57], whereas the other found no significant difference in the odds of wasting (WHZ <-2) or underweight (WAZ <-2) at the 9-month follow-up [50] (Appendix 6).
Cost of diarrhea episode
Five publications estimated the cost of a Shigella diarrhea episode (Table 5) [17,18,[57], [58], [59]]. In one of these studies, across seven sites, the mean total household out-of-pocket cost (including inpatient and outpatient medical costs, transportation, and prescriptions) was $10.61 (converted from local currency to 2012 US dollars), ranging from $4.92 in Mozambique to $17.18 in Mali [59]. This same study found no statistically significant difference in the cost between Shigella diarrhea and other pathogens. A study from China, which additionally included self-reported out-of-pocket expenses for overnight stays, estimated the mean cost to be $22 for children aged 0-1 year and $31 for 2-5 years, which represented 12% and 18% of the average monthly income, respectively [58]. One study from Bangladesh found Shigella episodes to cost an average of 5.7% (range <1-78%) of the household monthly income [18]. Although there was heterogeneity in measurement and adjustment factors across studies, a large proportion of costs were associated with hospitalization or inpatient care.
Table 5.
Economic outcomes.
| Outcome | Study | # with Shigella | Outcome measurement | Country | Effect measure(s) | |
|---|---|---|---|---|---|---|
| Cost of Shigella episode | Mean (SD) | Median | ||||
| Zimmerman et al.[59] | 1736 | Unadjusted, total household out-of-pocket costs (estimated by caregiver) including inpatient and outpatient medical costs, transportation, prescriptions (local currency converted to 2012 USD) | Seven combined | $10.61 (25.64) | $25.64 | |
| Same as above | Gambia | $7.95 (21.77) | $3.54 | |||
| Same as above | Mali | $17.18 (18.06) | $12.51 | |||
| Same as above | Mozambique | $4.92 (5.26) | $2.96 | |||
| Same as above | Kenya | $15.52 (8.71) | $13.87 | |||
| Same as above | India | $8.55 (8.51) | $5.27 | |||
| Same as above | Bangladesh | $11.17 (11.51) | $6.83 | |||
| Same as above | Pakistan | $8.68 (59.63) | $1.62 | |||
| Zimmerman et al.[59] | 1736 | Total household out-of-pocket costs (estimated by caregiver) after adjustment for co-pathogens, age group, and gender (local currency converted to 2012 USD) | Seven combined | $12.73 (95% CI 11.09, 14.37) | ||
| Riewpaiboon et al.[17] | 130 | Public treatment cost defined as cost of the visit, hospitalization, dispensing, drug, medical devices, and laboratory (2006 USD) | Thailand | $6.22 (95% CI 0.26, 12.19) | $3.20 | |
| Guh et al.[58] | 55 | Cost of illness by age group including self-reported out-of-pocket expenditures related to treatment and recovery, lab tests, medicines, treatment, and overnight stays (2002 PPP-adjusted USD) | China | Age 0-1 years: $22.00 (35.00) Age 2-5 years: $31.10 (71.10) |
||
| Das et al.[57] | 590 | Total household cost including direct and indirect medical costs (converted to current USD) | Bangladesh | $4.17 (3.64) | ||
| Das et al.[57] | 590 | Total household cost including direct and indirect medical costs by duration of hospital stay (converted to current USD) | Bangladesh | 1-3 days: $5.30; 4+ days: $8.95; p<0.001 |
||
| Das et al.[57] | 590 | Total household cost including direct and indirect medical costs by age group (converted to current USD) | Bangladesh | 0-11 months: $4.01; 12-23 months: $3.84; 24-50 months: $4.55; P = 0.080 | ||
| Cost of Shigella episode as percent of monthly household income | Mean (SD) | Median (range) | ||||
| Das et al.[18] | 518 | Total costs including drugs, consultations, and transportation before and after attending hospital measured as percent expenditure of monthly household income | Bangladesh | 5.74% (8.55) | 3.17% (0.06%-77.8%) | |
| Guh et al.[58] | 55 | Cost of illness by age group including lab tests, medicines, treatment, and overnight stays, as percent of average monthly household income (2002 PPP-adjusted income = $184/month) | China | Age 0-1 years: 12.0% Age 2-5 years: 16.9% |
||
Abbreviations: CI, confidence interval; SD, standard deviation; USD, U.S. dollars; PPP, purchasing power parity.
Enteric and systemic inflammation
Three studies reported on the longitudinal markers of gut and/or systemic inflammatory response among children with Shigella (Appendix 7). In a study of children with Shigella treated with antibiotic therapy and randomly assigned to 14 days of zinc supplementation or control, there were no significant differences in concentrations of innate mediators (myeloperoxidase, superoxidase, nitrate) and cytokines (interleukin-2, interferon-γ) in stool or released from mitogen-stimulated mononuclear cells within or between treatment groups over 30 days of follow-up [60]. Stool interleukin-1ß concentrations and serum C-reactive protein levels significantly decreased at days seven and 30 in both groups [60]. Over 2 years of follow-up, Schnee et al. [45] found diarrhea attributable to Shigella to be associated with elevated C-reactive protein levels (increase of 0.24 [95% CI: 0.03, 0.49] per diarrhea episode).
Other outcomes
Two studies assessed neurodevelopmental outcomes but did not find statistically significant associations between the diarrhea episodes attributable to Shigella and neurodevelopmental scores for motor, language, or cognitive skills [54] or between Shigella prevalence in nondiarrheal stools and reasoning skills, phonemic fluency, or semantic fluency at age 6-8 years [52] (Appendix 7). Four studies assessed the proportion of children with Shigella who were no longer shedding pathogen at various time points (6, 14, or 31 days, or at clinical stabilization) overall [2,61], and/or stratified by antibiotic treatment [62,63] and/or nutritional status [62,64]. One study estimated the mean duration of Shigella excretion (4.1 days; range 1-12) [65] (Appendix 7). Four studies assessed the proportion of children who had repeat Shigella infections (ranging from 8% to 35%) [40,44,66,67]. Additional outcomes, including antigen-specific antibody response [60,68], duration of hospitalization [46,47], subsequent respiratory and febrile illnesses [33,48], nutritional intake [67,69,70], microbiome composition [71], and serum retinol [46], are summarized in Appendix 7.
Discussion
The World Health Organization recently articulated the need for evidence synthesis of long-term morbidities associated with key enteric pathogens, such as Shigella [23]. In this systematic review, we document the consequences of Shigella infection and disease in children aged <5 years living in LMICs. We found evidence that Shigella was associated with linear growth faltering and persistent diarrhea [9,11,12,16,39,51,52]. There was a substantial economic impact on families with children suffering from Shigella diarrhea [17,18,[57], [58], [59]]. Heterogeneity in measurement and presentation of outcomes and differences in comparison groups between studies prohibited quantitative synthesis of the data, highlighting the need for standardizing methods for characterizing and reporting on enteric pathogen sequelae.
Shigella is a well-known cause of diarrhea, with moderate and severe forms of diarrhea constituting a substantial financial burden on health care systems and families. Our systematic review added to this evidence base by highlighting the consequences of Shigella diarrhea. Notably, children with Shigella diarrhea had an average duration of illness of 2-22 days, with wide variation [33,40,41,43,44,46,47,49,69], and children with acute Shigella diarrhea were more likely to develop persistent diarrhea than children with acute diarrhea caused by other pathogens [39]. Longer diarrhea duration is associated with poorer health outcomes, including mortality, stunting, and wasting [72,73], and poses a greater burden on health care systems due to its increased need for facility-based care. In the few studies that included the economic consequences of Shigella, all were focused on the cost of Shigella diarrhea borne by families, which ranged from 1% to 78% of the monthly household income [18,58]. With Shigella infections likely having impact on a child's health, even in the absence of diarrhea, assigning an economic value to a Shigella vaccine will require additional data estimating the financial impact of Shigella sequelae beyond diarrhea.
We found Shigella to have modest and inconsistent effects on linear growth. Children who fall off their linear growth trajectories are at substantial risk for stunting, a precursor to poorer school performance, cognitive development, and reduced earning potential [74], [75], [76]. The greatest differences in LAZ were observed in the MAL-ED cohort study evaluating cumulative asymptomatic Shigella infections occurring over the first 24 months of life and their impact at 2, 5, and 6-8 years of life, with magnitudes ranging from -0.32 to -0.14 [11,52]. These magnitudes are expected to be high because they are comparing the extremes of Shigella infection burden: a high burden of attributable diarrhea episodes (90th percentile) with a low burden (10th percentile). To the best of our knowledge, there is no established threshold for what loss in LAZ translates to an increased risk of stunting or even more deleterious outcomes, such as impaired cognitive development and poor school performance. If infection rather than disease is primarily responsible for growth faltering from Shigella, then Shigella vaccines will need to induce sterilizing immunity to expect a growth benefit from the vaccine—a tall order for any vaccine. More realistically, and similar to the rotavirus vaccines, a Shigella vaccine will prevent more severe presentations of Shigella diarrhea [77]; we found Shigella diarrhea to have modest and inconsistently statistically significant effects on linear growth, which may be due to confounding by antibiotic use. Notably, the GEMS study found a statistically significant mean loss in LAZ of 0.06-0.17 after an untreated Shigella MSD episode in infants and toddlers, respectively [12], magnitudes of association consistent with studies of asymptomatic Shigella included in this review [11,52]. Shigella vaccine trials including linear growth as a secondary outcome, as has been suggested by recent study design consensus statements [20,78], will be best suited to estimate a causal association between Shigella and linear growth deficits.
One of the key pathways by which Shigella and other enteric pathogens are hypothesized to impact linear growth is through EED. EED is a syndrome characterized by inflammation and impaired function of the small intestine and has been associated with stunting among children [13]. The biomarkers of EED, such as myeloperoxidase, may be an intermediate marker of Shigella’s impact on linear growth, and therefore may be important targets for vaccine probe studies to estimate more quickly the impact of Shigella on growth. Although we only identified one study that looked at the biomarkers for EED longitudinally [60], we note that the indicators for EED may be measured cross-sectionally at the time of acute infection and such outcomes would not have met inclusion criteria. Therefore, our review of longitudinal consequences is not well suited to examine the cross-sectional associations between Shigella and EED.
This review was subject to several limitations in addition to those already discussed. To inform the value proposition for soon to be available Shigella vaccines, it was valuable to limit this review to outcomes reported among children aged <5 years with confirmed Shigella detection. However, excluding studies that did not disaggregate children with clinically compatible illness but without Shigella confirmation may have disproportionally excluded studies from certain time periods or settings with limited diagnostic capacity. For example, publications from the 1980s summarizing dysentery epidemics suspected to be caused by shigellosis rarely reported outcomes among the subgroup of children with culture-confirmed Shigella. In addition, some potentially relevant growth data by Lee and colleagues [79] were excluded because all reported results were aggregated with children aged >60 months. However, this study found a similar magnitude of change in linear growth (-0.081 cm) per Shigella diarrhea episode as another study included in this review, Black et al. [49], and thus, inclusion would not have changed our conclusions. Although the unpublished data were beyond the scope of this review, an individual-level reanalysis of included studies could provide valuable information on linear growth faltering associated with shigellosis. Finally, this review focused exclusively on LMICs, based on children living in these settings having the highest burden of Shigella morbidity and mortality.
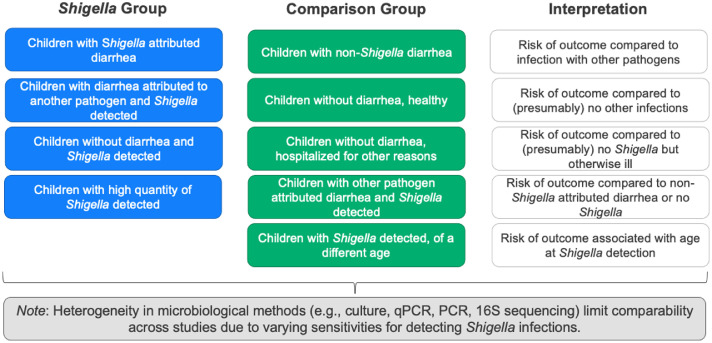
Our ability to meta-analyze these data was limited by substantial heterogeneity in comparison groups between studies and in how outcomes were measured. To illustrate the variability in comparison groups, we've summarized in Figure 3 some common ways children with Shigella were defined (in blue) and possible comparison groups (in green). The interpretation of the results is dependent on the combination of the two groups and may or may not be comparable between studies. In practice, it may not be possible to distinguish between the first two blue boxes (whether diarrhea is attributed to Shigella or to another pathogen) particularly in studies that do not test for multiple pathogens. Moreover, it was common for studies to not have any comparison group (particularly for diarrhea outcomes), which limits our ability to make conclusions regarding Shigella consequences, relative to other pathogens or to absence of diarrhea. The comparability of findings was further limited by study heterogeneity in pathogen confirmation techniques (e.g., varying sensitivity of polymerase chain reaction vs culture), assessment of costs, and adjustment for co-infections and confounding factors, including antibiotic use and differences in the standard of care over time and by setting. Furthermore, host factors, such as age, malnutrition, HIV, and measles, are all established risk factors for poor Shigella outcomes and although these characteristics were included in our descriptions of the studies, without a formal meta-analysis, we were unable to statistically assess their contribution to outcomes.
Figure 3.
Examples of heterogeneity in comparison groups for outcome measurement.
Abbreviations: qPCR, quantitative polymerase chain reaction.
The strengths of this systematic review include the wide time span of the reviewed literature and extensive breadth of the outcomes assessed. This review identified several evidence gaps, including lack of data on neurodevelopmental outcomes, relatively short follow-up periods for shigellosis, and limited geographic diversity in study locations. Future trials of Shigella-specific vaccines or treatments with long-term follow-up will ultimately be best positioned to document Shigella consequences.
Declaration of interests
The authors have no competing interests to declare.
Acknowledgments
Role of the funding source
This study was completed by the Strategic Analysis, Research, and Training (START) Center at the University of Washington. START is a collaborative effort with, and is supported by, the Bill and Melinda Gates Foundation (grant # OPP1155935). The funder of the study proposed the study design but had no role in data collection or data analysis. TL was supported, in part, by the University of Washington Biostatistics, Epidemiologic, and Bioinformatic Training in Environmental Health (BEBTEH) Training Grant (grant # NIEHS 5T32ES015459).
Ethical approval statement
Approval was not required. No human subjects were involved in this research.
Acknowledgments
The authors extend their thanks to the following who provided input on the search strategy and the systematic review protocol: Margaret Kosek, Karen Kotloff, Elizabeth Rogawski McQuade, Daniel Cohen, Nigel Cunliffe, James Platts-Mills, Mateusz Hasso-Agopsowicz, William Hausdorff, Karoun Bagamian, and Suzanne Scheele. We thank our colleagues at the START Center, especially Dr. Stephen Hawes and Jessie Seiler for their leadership and guidance on this project.
Author contributions
KV and CM conceived the idea and all authors developed the protocol for this review. TL, MD, and FA conducted the search; screened titles, abstracts, and full texts; and abstracted data for included articles, with final input from PP. All authors contributed to the development, reading, and approval the final version of the manuscript for publication.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijid.2023.01.034.
Appendix. Supplementary materials
References
- 1.Khalil IA, Troeger C, Blacker BF, Rao PC, Brown A, Atherly DE, et al. Morbidity and mortality due to Shigella and enterotoxigenic Escherichia coli diarrhoea: the Global Burden of Disease Study 1990–2016. Lancet Infect Dis. 2018;18:1229–1240. doi: 10.1016/S1473-3099(18)30475-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson JD, 4th, Bagamian KH, Muhib F, Amaya MP, Laytner LA, Wierzba T, et al. Burden of enterotoxigenic Escherichia coli and Shigella non-fatal diarrhoeal infections in 79 low-income and lower middle-income countries: a modelling analysis. Lancet Glob Health. 2019;7:e321–e330. doi: 10.1016/S2214-109X(18)30483-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koster FT, Curlin GC, Aziz KM, Haque A. Synergistic impact of measles and diarrhoea on nutrition and mortality in Bangladesh. Bull World Health Organ. 1981;59:901–908. [PMC free article] [PubMed] [Google Scholar]
- 4.Khatun F, Faruque ASG, Koeck JL, Olliaro P, Millet P, Paris N, et al. Changing species distribution and antimicrobial susceptibility pattern of Shigella over a 29-year period (1980–2008) Epidemiol Infect. 2011;139:446–452. doi: 10.1017/S0950268810001093. [DOI] [PubMed] [Google Scholar]
- 5.Levine MM, Kotloff KL, Barry EM, Pasetti MF, Sztein MB. Clinical trials of Shigella vaccines: two steps forward and one step back on a long, hard road. Nat Rev Microbiol. 2007;5:540–553. doi: 10.1038/nrmicro1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Christopher PR, David KV, John SM, Sankarapandian V. Antibiotic therapy for Shigella dysentery. Cochrane Database Syst Rev. 2010;2010 doi: 10.1002/14651858.CD006784.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu J, Platts-Mills JA, Juma J, Kabir F, Nkeze J, Okoi C, et al. Use of quantitative molecular diagnostic methods to identify causes of diarrhoea in children: a reanalysis of the GEMS case-control study. Lancet. 2016;388:1291–1301. doi: 10.1016/S0140-6736(16)31529-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Platts-Mills JA, Houpt ER, Liu J, Zhang J, Guindo O, Sayinzoga-Makombe N, et al. Etiology and incidence of moderate-to-severe diarrhea in young children in Niger. J Pediatr Infect Dis Soc. 2021;10:1062–1070. doi: 10.1093/jpids/piab080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rogawski McQuade ET, Shaheen F, Kabir F, Rizvi A, Platts-Mills JA, Aziz F, et al. Epidemiology of Shigella infections and diarrhea in the first two years of life using culture-independent diagnostics in 8 low-resource settings. PLoS Negl Trop Dis. 2020;14 doi: 10.1371/journal.pntd.0008536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kotloff KL, Nataro JP, Blackwelder WC, Nasrin D, Farag TH, Panchalingam S, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet. 2013;382:209–222. doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 11.Rogawski ET, Liu J, Platts-Mills JA, Kabir F, Lertsethtakarn P, Siguas M, et al. Use of quantitative molecular diagnostic methods to investigate the effect of enteropathogen infections on linear growth in children in low-resource settings: longitudinal analysis of results from the MAL-ED cohort study. Lancet Glob Health. 2018;6:e1319. doi: 10.1016/S2214-109X(18)30351-6. –28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nasrin D, Blackwelder WC, Sommerfelt H, Wu Y, Farag TH, Panchalingam S, et al. Pathogens associated with linear growth faltering in children with diarrhea and impact of antibiotic treatment: the Global Enteric Multicenter Study. J Infect Dis. 2021;224:S848–S855. doi: 10.1093/infdis/jiab434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tickell KD, Atlas HE, Walson JL. Environmental enteric dysfunction: a review of potential mechanisms, consequences and management strategies. BMC Med. 2019;17:181. doi: 10.1186/s12916-019-1417-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lorntz B, Soares AM, Moore SR, Pinkerton R, Gansneder B, Bovbjerg VE, et al. Early childhood diarrhea predicts impaired school performance. Pediatr Infect Dis J. 2006;25:513–520. doi: 10.1097/01.inf.0000219524.64448.90. [DOI] [PubMed] [Google Scholar]
- 15.Soni A, Fahey N, Bhutta ZA, Li W, Frazier JA, Moore Simas T, et al. Early childhood undernutrition, preadolescent physical growth, and cognitive achievement in India: a population-based cohort study. PLoS Med. 2021;18 doi: 10.1371/journal.pmed.1003838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.George CM, Perin J, Kuhl J, Williams C, Coglianese N, Thomas ED, et al. Linear growth faltering is associated with subsequent adverse child cognitive developmental outcomes in the Democratic Republic of the Congo (REDUCE program) Am J Trop Med Hyg. 2021;106:356–360. doi: 10.4269/ajtmh.21-0502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riewpaiboon A, Youngkong S, Sreshthaputra N, Stewart JF, Samosornsuk S, Chaicumpa W, et al. A cost function analysis of shigellosis in Thailand. Value Health. 2008;11:S75–S83. doi: 10.1111/j.1524-4733.2008.00370.x. [DOI] [PubMed] [Google Scholar]
- 18.Das J, Das SK, Ahmed S, Ferdous F, Farzana FD, Sarker MHR, et al. Determinants of percent expenditure of household income due to childhood diarrhoea in rural Bangladesh. Epidemiol Infect. 2015;143:2700–2706. doi: 10.1017/S0950268814003781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shekar M, Dayton Eberwein JD, Kakietek J. The costs of stunting in South Asia and the benefits of public investments in nutrition. Matern Child Nutr. 2016;12:186–195. doi: 10.1111/mcn.12281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Health Organization . World Health Organization; Geneva: 2021. WHO preferred product characteristics for vaccines against Shigella. editor. [Google Scholar]
- 21.MacLennan CA, Talaat KR, Kaminski RW, Cohen D, Riddle MS, Giersing BK. Critical needs in advancing Shigella vaccines for global health. J Infect Dis. 2022;225:1500–1503. doi: 10.1093/infdis/jiab462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mani S, Wierzba T, Walker RI. Status of vaccine research and development for Shigella. Vaccine. 2016;34:2887–2894. doi: 10.1016/j.vaccine.2016.02.075. [DOI] [PubMed] [Google Scholar]
- 23.Hasso-Agopsowicz M, Lopman BA, Lanata CF, Rogawski McQuade ET, Kang G, Prudden HJ, et al. World Health Organization Expert Working Group: Recommendations for assessing morbidity associated with enteric pathogens. Vaccine. 2021;39:7521–7525. doi: 10.1016/j.vaccine.2021.11.033. S0264-410X(21)01478-X. [DOI] [PubMed] [Google Scholar]
- 24.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. PLoS Med. 2021;18 doi: 10.1371/journal.pmed.1003583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Asare EO, Hergott D, Seiler J, Morgan B, Archer H, Wiyeh AB, et al. Case fatality risk of diarrhoeal pathogens: a systematic review and meta-analysis. Int J Epidemiol. 2022;51:1469–1480. doi: 10.1093/ije/dyac098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. PLoS Med. 2007;4:e296. doi: 10.1371/journal.pmed.0040296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ganguly E, Sharma PK, Bunker CH. Prevelance and risk factors of diarrhea morbidity among under-five children in India: a systematic review and meta-analysis. Indian J Child Health. 2015;02:152–160. doi: 10.32677/IJCH.2015.v02.i04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research Electronic Data Capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World HealthOrganization . 4th rev. World Health Organization; Geneva: 2005. The Treatment of diarrhoea: a manual for physicians and other senior health workers. editor. [Google Scholar]
- 31.MAL-ED Network. Investigators The MAL-ED study: a multinational and multidisciplinary approach to understand the relationship between enteric pathogens, malnutrition, gut physiology, physical growth, cognitive development, and immune responses in infants and children up to 2 years of age in resource-poor environments. Clin Infect Dis. 2014;59:S193–S206. doi: 10.1093/cid/ciu653. [DOI] [PubMed] [Google Scholar]
- 32.Kotloff KL, Blackwelder WC, Nasrin D, Nataro JP, Farag TH, van Eijk A, et al. The Global Enteric Multicenter Study (GEMS) of diarrheal disease in infants and young children in developing countries: epidemiologic and clinical methods of the case/control study. Clin Infect Dis. 2012;55:S232–S245. doi: 10.1093/cid/cis753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roy SK, Raqib R, Khatun W, Azim T, Chowdhury R, Fuchs GJ, et al. Zinc supplementation in the management of shigellosis in malnourished children in Bangladesh. Eur J Clin Nutr. 2008;62:849–855. doi: 10.1038/sj.ejcn.1602795. [DOI] [PubMed] [Google Scholar]
- 34.Taylor DN, Blaser MJ, Echeverria P, Pitarangsi C, Bodhidatta L, Wang WL. Erythromycin-resistant Campylobacter infections in Thailand. Antimicrob Agents Chemother. 1987;31:438–442. doi: 10.1128/AAC.31.3.438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henry FJ, Udoy AS, Wanke CA, Aziz KM. Epidemiology of persistent diarrhea and etiologic agents in Mirzapur, Bangladesh. Acta Paediatr Suppl. 1992;381:27–31. doi: 10.1111/j.1651-2227.1992.tb12368.x. [DOI] [PubMed] [Google Scholar]
- 36.Dutta P, Bhattacharya SK, Sen D, Bhattacharya MK, Mitra U, Rasaily R, et al. Shigellosis in children: a prospective hospital based study. Indian Pediatr. 1992;29:1125–1130. [PubMed] [Google Scholar]
- 37.Huttly SR, Hoque BA, Aziz KM, Hasan KZ, Patwary MY, Rahaman MM, et al. Persistent diarrhoea in a rural area of Bangladesh: a community-based longitudinal study. Int J Epidemiol. 1989;18:964–969. doi: 10.1093/ije/18.4.964. [DOI] [PubMed] [Google Scholar]
- 38.Baqui AH, Yunus MD, Zaman K, Mitra AK, Hossain KM. Surveillance of patients attending a rural diarrhoea treatment centre in Bangladesh. Trop Geogr Med. 1991;43:17–22. [PubMed] [Google Scholar]
- 39.Ahmed F, Ansaruzzaman M, Haque E, Rao MR, Clemens JD. Epidemiology of postshigellosis persistent diarrhea in young children. Pediatr Infect Dis J. 2001;20:525–530. doi: 10.1097/00006454-200105000-00011. [DOI] [PubMed] [Google Scholar]
- 40.Abu-Elyazeed RR, Wierzba TF, Frenck RW, Putnam SD, Rao MR, Savarino SJ, et al. Epidemiology of Shigella-associated diarrhea in rural Egyptian children. Am J Trop Med Hyg. 2004;71:367–372. doi: 10.4269/ajtmh.2004.71.367. [DOI] [PubMed] [Google Scholar]
- 41.Ballard SB, Requena D, Mayta H, Sanchez GJ, Oyola-Lozada MG, Colquechagua Aliaga FD, et al. Enteropathogen changes after rotavirus vaccine scale-up. Pediatrics. 2022;149 doi: 10.1542/peds.2020-049884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Black RE, Brown KH, Becker S. Malnutrition is a determining factor in diarrheal duration, but not incidence, among young children in a longitudinal study in rural Bangladesh. Am J Clin Nutr. 1984;39:87–94. doi: 10.1093/ajcn/39.1.87. [DOI] [PubMed] [Google Scholar]
- 43.Black RE, Brown KH, Becker S, Alim AR, Huq I. Longitudinal studies of infectious diseases and physical growth of children in rural Bangladesh. II. Incidence of diarrhea and association with known pathogens. Am J Epidemiol. 1982;115:315–324. doi: 10.1093/oxfordjournals.aje.a113308. [DOI] [PubMed] [Google Scholar]
- 44.Cravioto A, Reyes RE, Trujillo F, Uribe F, Navarro A, De La, Roca JM, et al. Risk of diarrhea during the first year of life associated with initial and subsequent colonization by specific enteropathogens. Am J Epidemiol. 1990;131:886–904. doi: 10.1093/oxfordjournals.aje.a115579. [DOI] [PubMed] [Google Scholar]
- 45.Mazumder RN, Hoque SS, Ashraf H, Kabir I, Wahed MA. Early feeding of an energy dense diet during acute shigellosis enhances growth in malnourished children. J Nutr. 1997;127:51–54. doi: 10.1093/jn/127.1.51. [DOI] [PubMed] [Google Scholar]
- 46.Mitra AK, Alvarez JO, Wahed MA, Fuchs GJ, Stephensen CB. Predictors of serum retinol in children with shigellosis. Am J Clin Nutr. 1998;68:1088–1094. doi: 10.1093/ajcn/68.5.1088. [DOI] [PubMed] [Google Scholar]
- 47.Rampengan TH, Ongkie AS, Wantania JM, Munir M. Bacillary dysentery in children below five years of age at the general hospital, Manado. Paediatr Indones. 1982;22:222–226. [PubMed] [Google Scholar]
- 48.Kabir I, Rahman MM, Haider R, Mazumder RN, Khaled MA, Mahalanabis D. Increased height gain of children fed a high-protein diet during convalescence from shigellosis: a six-month follow-UP study. J Nutr. 1998;128:1688–1691. doi: 10.1093/jn/128.10.1688. [DOI] [PubMed] [Google Scholar]
- 49.Black RE, Brown KH, Becker S. Effects of diarrhea associated with specific enteropathogens on the growth of children in rural Bangladesh. Pediatrics. 1984;73:799–805. doi: 10.1542/peds.73.6.799. [DOI] [PubMed] [Google Scholar]
- 50.George CM, Burrowes V, Perin J, Oldja L, Biswas S, Sack D, et al. Enteric infections in young children are associated with environmental enteropathy and impaired growth. Trop Med Int Health. 2018;23:26–33. doi: 10.1111/tmi.13002. [DOI] [PubMed] [Google Scholar]
- 51.Luoma J, Adubra L, Ashorn P, Ashorn U, Bendabenda J, Dewey KG, et al. Association between asymptomatic infections and linear growth in 18–24-month-old Malawian children. Matern Child Nutr. 2023;19:e13417. doi: 10.1111/mcn.13417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rogawski McQuade ET, Scharf RJ, Svensen E, Huggins A, Maphula A, Bayo E, et al. Impact of Shigella infections and inflammation early in life on child growth and school-aged cognitive outcomes: findings from three birth cohorts over eight years. PLoS Negl Trop Dis. 2022;16 doi: 10.1371/journal.pntd.0010722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schnee AE, Haque R, Taniuchi M, Uddin MJ, Alam MM, Liu J, et al. Identification of etiology-specific diarrhea associated with linear growth faltering in Bangladeshi infants. Am J Epidemiol. 2018;187:2210–2218. doi: 10.1093/aje/kwy106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Donowitz JR, Drew J, Taniuchi M, Platts-Mills JA, Alam M, Ferdous T, et al. Diarrheal pathogens associated with growth and neurodevelopment. Clin Infect Dis. 2021;73:e683–e691. doi: 10.1093/cid/ciaa1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Platts-Mills JA, Gratz J, Mduma E, Svensen E, Amour C, Liu J, et al. Association between stool enteropathogen quantity and disease in Tanzanian children using TaqMan array cards: a nested case-control study. Am J Trop Med Hyg. 2014;90:133–138. doi: 10.4269/ajtmh.13-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kabir I, Malek MA, Mazumder RN, Rahman MM, Mahalanabis D. Rapid catch-up growth of children fed a high-protein diet during convalescence from shigellosis. Am J Clin Nutr. 1993;57:441–445. doi: 10.1093/ajcn/57.3.441. [DOI] [PubMed] [Google Scholar]
- 57.Das R, Haque MA, Chisti MJ, Faruque ASG, Ahmed T. Associated factors, post infection child growth, and household cost of invasive enteritis among under 5 children in Bangladesh. Sci Rep. 2021;11:12738. doi: 10.1038/s41598-021-92132-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guh S, Xingbao C, Poulos C, Qi Z, Jianwen C, von Seidlein L, et al. Comparison of cost-of-illness with willingness-to-pay estimates to avoid shigellosis: evidence from China. Health Policy Plan. 2008;23:125–136. doi: 10.1093/heapol/czm047. [DOI] [PubMed] [Google Scholar]
- 59.Zimmermann M, Kotloff K, Nasrin D, Roose A, Levine MM, Rheingans R, et al. Household costs of diarrhea by etiology in 7 countries, the Global Enterics Mulitcenter Study (GEMS) Open Forum Infect Dis. 2019;6:ofz150. doi: 10.1093/ofid/ofz150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raqib R, Roy SK, Rahman MJ, Azim T, Ameer SS, Chisti J, et al. Effect of zinc supplementation on immune and inflammatory responses in pediatric patients with shigellosis. Am J Clin Nutr. 2004;79:444–450. doi: 10.1093/ajcn/79.3.444. [DOI] [PubMed] [Google Scholar]
- 61.Gaensbauer JT, Lamb M, Calvimontes DM, Asturias EJ, Kamidani S, Contreras-Roldan IL, et al. Identification of enteropathogens by multiplex PCR among rural and urban Guatemalan children with acute diarrhea. Am J Trop Med Hyg. 2019;101:534–540. doi: 10.4269/ajtmh.18-0962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Andersson ME, Elfving K, Shakely D, Nilsson S, Msellem M, Trollfors B, et al. Rapid clearance and frequent reinfection with enteric pathogens among children with acute diarrhea in Zanzibar. Clin Infect Dis. 2017;65:1371–1377. doi: 10.1093/cid/cix500. [DOI] [PubMed] [Google Scholar]
- 63.Rodriguez RS, Chavez AZ, Galindo E. A randomized, controlled, single-blind study comparing furazolidone with trimethoprim-sulfamethoxazole in the empirical treatment of acute invasive diarrhea. Scand J Gastroenterol Suppl. 1989;169:47–53. doi: 10.3109/00365528909091332. [DOI] [PubMed] [Google Scholar]
- 64.Versloot CJ, Attia S, Bourdon C, Richardson SE, Potani I, Bandsma RHJ, et al. Intestinal pathogen clearance in children with severe acute malnutrition is unrelated to inpatient morbidity. Clin Nutr ESPEN. 2018;24:109–113. doi: 10.1016/j.clnesp.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 65.Khan MU, Shahidullah M, Ahmed WU, Barua DK, Begum T, et al. Changes in the trend of shigellosis in Dhaka: family study on secondary infection, clinical manifestation and sensitivity pattern: 1980. Trans R Soc Trop Med Hyg. 1984;78:151–156. doi: 10.1016/0035-9203(84)90262-1. [DOI] [PubMed] [Google Scholar]
- 66.Anders KL, Thompson CN, Thuy NT, Nguyet NM, Tu le TP, Dung TT, et al. The epidemiology and aetiology of diarrhoeal disease in infancy in southern Vietnam: a birth cohort study. Int J Infect Dis. 2015;35:3–10. doi: 10.1016/j.ijid.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ramiro Cruz J, Cano F, Bartlett AV, Méndez H. Infection, diarrhea, and dysentery caused by Shigella species and Campylobacter jejuni among Guatemalan rural children. Pediatr Infect Dis J. 1994;13:216–223. doi: 10.1097/00006454-199403000-00010. [DOI] [PubMed] [Google Scholar]
- 68.Echeverria P, Hanchalay S, Taylor DN. Serological response to plasmid-encoded antigens in children and adults with shigellosis. Diagn Microbiol Infect Dis. 1988;10:75–80. doi: 10.1016/0732-8893(88)90043-0. [DOI] [PubMed] [Google Scholar]
- 69.Mazumder RN, Kabir I, Rahman MM, Khatun M, Mahalanabis D. Absorption of macronutrients from a calorie-dense diet in malnourished children during acute shigellosis. J Pediatr Gastroenterol Nutr. 1996;23:24–28. doi: 10.1097/00005176-199607000-00005. [DOI] [PubMed] [Google Scholar]
- 70.Rahman MM, Mazumder RN, Ali M, Mahalanabis D. Role of amylase-treated, energy-dense liquid diet in the nutritional management of acute shigellosis in children: a controlled clinical trial. Acta Paediatr. 1995;84:867–872. doi: 10.1111/j.1651-2227.1995.tb13782.x. [DOI] [PubMed] [Google Scholar]
- 71.Ndungo E, Holm JB, Gama S, Buchwald AG, Tennant SM, Laufer MK, et al. Dynamics of the gut microbiome in Shigella-infected children during the first two years of life. mSystems. 2022;7 doi: 10.1128/msystems.00442-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Checkley W, Buckley G, Gilman RH, Assis AM, Guerrant RL, Morris SS, et al. Multi-country analysis of the effects of diarrhoea on childhood stunting. Int J Epidemiol. 2008;37:816–830. doi: 10.1093/ije/dyn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moore SR, Lima NL, Soares AM, Oriá RB, Pinkerton RC, Barrett LJ, et al. Prolonged episodes of acute diarrhea reduce growth and increase risk of persistent diarrhea in children. Gastroenterology. 2010;139:1156–1164. doi: 10.1053/j.gastro.2010.05.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, de Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382:427–451. doi: 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- 75.Guerrant RL, DeBoer MD, Moore SR, Scharf RJ, Lima AAM. The impoverished gut–a triple burden of diarrhoea, stunting and chronic disease. Nat Rev Gastroenterol Hepatol. 2013;10:220–229. doi: 10.1038/nrgastro.2012.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Black RE, Allen LH, Bhutta ZA, Caulfield LE, de Onis M, Ezzati M, et al. Maternal and child undernutrition: global and regional exposures and health consequences. Lancet. 2008;371:243–260. doi: 10.1016/S0140-6736(07)61690-0. [DOI] [PubMed] [Google Scholar]
- 77.Talaat KR, Alaimo C, Martin P, Bourgeois AL, Dreyer AM, Kaminski RW, et al. Human challenge study with a Shigella bioconjugate vaccine: analyses of clinical efficacy and correlate of protection. EBiomedicine. 2021;66 doi: 10.1016/j.ebiom.2021.103310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pavlinac PB, Rogawski McQuade ET, Platts-Mills JA, Kotloff KL, Deal C, Giersing BK, et al. Pivotal Shigella vaccine efficacy trials-study design considerations from a Shigella vaccine trial design working group. Vaccines. 2022;10:489. doi: 10.3390/vaccines10040489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee G, Paredes Olortegui M, Peñataro Yori P, Black RE, Caulfield L, Banda Chavez C, et al. Effects of Shigella-, Campylobacter- and ETEC-associated diarrhea on childhood growth. Pediatr Infect Dis J. 2014;33:1004–1009. doi: 10.1097/INF.0000000000000351. [DOI] [PubMed] [Google Scholar]
- 80.Butler T, Islam MR, Bardhan PK. The leukemoid reaction in shigellosis. Am J Dis Child. 1984;138:162–165. doi: 10.1001/archpedi.1984.02140400044010. [DOI] [PubMed] [Google Scholar]
- 81.Dutta P, Lahiri M, Sen D, Pal SC. Prospective hospital based study on persistent diarrhoea. Gut. 1991;32:787–790. doi: 10.1136/gut.32.7.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fujita K, Kaku M, Yanagase Y, Ezaki T, Furuse K, Ozawa A, et al. Physicochemical characteristics and flora of diarrhoeal and recovery faeces in children with acute gastro-enteritis in Kenya. Ann Trop Paediatr. 1990;10:339–345. doi: 10.1080/02724936.1990.11747455. [DOI] [PubMed] [Google Scholar]
- 83.Househam KC, Bowie DC, Mann MD, Bowie MD. Factors influencing the duration of acute diarrheal disease in infancy. J Pediatr Gastroenterol Nutr. 1990;10:37–40. doi: 10.1097/00005176-199001000-00007. [DOI] [PubMed] [Google Scholar]
- 84.Huskins WC, Griffiths JK, Faruque ASG, Bennish ML. Shigellosis in neonates and young infants. J Pediatr. 1994;125:14–22. doi: 10.1016/S0022-3476(94)70115-6. [DOI] [PubMed] [Google Scholar]
- 85.Perin J, Burrowes V, Almeida M, Ahmed S, Haque R, Parvin T, et al. A retrospective case-control study of the relationship between the gut microbiota, enteropathy, and child growth. Am J Trop Med Hyg. 2020;103:520–527. doi: 10.4269/ajtmh.19-0761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Platts-Mills JA, Taniuchi M, Uddin MJ, Sobuz SU, Mahfuz M, Gaffar SA, et al. Association between enteropathogens and malnutrition in children aged 6–23 mo in Bangladesh: a case-control study. Am J Clin Nutr. 2017;105:1132–1138. doi: 10.3945/ajcn.116.138800. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.