Abstract
Coffee pulp is an underutilized by-product of coffee industrial production rich in bioactive compounds such as phenolic compounds, caffeine, and dietary fiber. The widely known antioxidant, anti-inflammatory, anti-aging, antimicrobial and hepatoprotective health-promoting properties attributed to mentioned compounds enhance the use of coffee pulp as a bioactive food ingredient. Furthermore, the application of green sustainable extraction techniques pursuing highly efficient and selective extraction processes promotes this by-product exploitation in food science. Hence, this review gathers the available information relative to the impact of the extraction processes on the bioactive compound's recovery from coffee pulp, providing an overview of the most recent advances. An in-depth comparison workout between conventional and alternative extraction methods was performed to identify the most suitable techniques for coffee pulp valorization as functional ingredient until date. A critical discussion focused on advantages and drawbacks of the extraction methods applied to coffee pulp was included together a prospective of emerging extraction techniques.
Keywords: Coffee pulp, Green solvents, Advanced extraction techniques, Sustainable valorization, Phenolic compounds
Graphical abstract
Highlights
-
•
Extraction technologies for coffee pulp valorization were comprehensively reviewed.
-
•
Heat-assisted extraction is recommended for coffee pulp bioactive compounds recovery.
-
•
Combination of extractions techniques applied to coffee pulp is highly recommended.
-
•
Advantages and drawbacks must be considered when selecting the valorization strategy.
1. Introduction
Coffee is one of the most valued agricultural commodities due to its high commercialization rates for hot-water beverage brewing and other coffee-based food products manufacturing, besides being one of the leading products in fair trade (Ma et al., 2022). In 2020, the global green coffee production from more than 70 cultivation countries was estimated around 10.7 million tonnes (FAOSTAT, 2022). According to the International Coffee Organization, the annual coffee production is yearly increasing worldwide because of the growing demand by consumers (International Coffee Organization, 2020), mostly motivated by the health-promoting and functional claims of coffee food products. The commonly consumed coffee beverage is made from roasted beans however, other coffee plant parts which are currently neglected and wasted such as leaves, flowers, and trunks, could be commercially revalorized, i.e., for tobacco and perfumery applications (Lachenmeier et al., 2022). Furthermore, the industrial food production of the coffee cherry, including dry and wet processing, yields vast amounts of by-products, such as coffee husk, pulp, parchment and silverskin (Klingel et al., 2020), resulting nearby 50% of the fruit's dry weight. Such coffee by-products are prone to spoilage when a proper treatment and/or use is lacking and, added to the fact that they are difficult to be naturally degraded after being discarded, their production might result in a severe negative environmental impact. In this regard, recent studies toward improving the sustainability of the coffee by-products management systems, demonstrated that coffee by-products are superior resources to be revalorized in biorefinery, food and pharmaceutical industries (Bondam et al., 2022). Focusing on the value-added potential, topic of coffee pulp (CP, 26 items) in Web of Science Core Collection (from 1980 to 2020) has been less explored compared to silverskin (30 items) and spent coffee grounds (64 items) according to the scientometric analysis of coffee by-products previously reported (Durán-Aranguren et al., 2021).
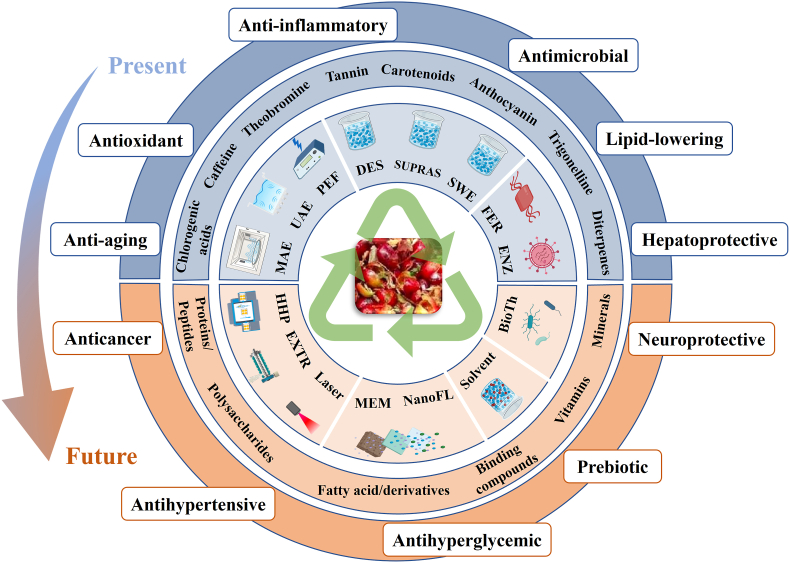
CP is the main by-product derived from the wet processing of coffee cherries, comprising approximately 40–50% of its fresh weight (Carmen et al., 2020). Considering that nowadays it is directly discarded or poured without pretreatment, CP may lead to the acidification of soil and rivers, seriously threatening the ecological environment and water system. Therefore, developing value-added products from CP supposes an effective green and sustainable strategy for diminishing landfill waste and effluent discharge. Traditional applications for CP valorization include the bioethanol production (Gurram et al., 2016), its use as animal feed (Maxiselly et al., 2022) or as culture medium for promoting the livestock and edible mushrooms growth (Rodríguez Frómeta et al., 2020). Moreover, CP is described as a low-cost and natural source of food components such as carbohydrates (45–89%), proteins (4–12%), lipids (1–2%) and minerals (6–10%), besides other interesting bioactive compounds such as phenolic compounds and caffeine (1.3%) (Klingel et al., 2020). Such chemical composition endows CP with the possibility of being used as a nutritional and/or functional emerging food ingredient. In fact, fiber-enriched breads (Rosas-Sánchez et al., 2021) and cookies (Moreno et al., 2019), sweet purees with fruity flavor (Buck et al., 2021) and cold beverages with high antioxidant capacity and consumer acceptance (Contreras-Oliva et al., 2022), were developed by incorporating CP into their formulation. Recently, based on traditional food declarations and applications from third countries, the European Food Safety Authority (EFSA) has assessed and considered to place CP (European Food Safety Authority, 2021a) or dried CP (European Food Safety Authority, 2021b) as novel food on the EU market. A promising alternative to the direct use of the whole CP has nowadays emerged focusing on the bioactive compound's recovery with potential health-benefits. Currently, CP's aqueous and organic solvent extracts have been proven to exert in vitro antioxidant (Cañas et al., 2022; Myo and Khat-udomkiri, 2022), antimicrobial (Khochapong et al., 2021) and anti-inflammatory properties (Magoni et al., 2018) as well as in vivo antidiabetic (Boonphang et al., 2021) and hepatoprotective activities (Ontawong et al., 2019a, Ontawong et al., 2019b) using rats as animal model, together with an improved metabolic syndrome in high-carbohydrate, high-fat diet-fed rats (Bhandarkar et al., 2021). Moreover, a daily consumption of CP extracts showed a deceleration in the skin aging process while improving the skin health (Tseng et al., 2022). Mentioned health-promoting effects were mainly attributed to the content of carotenoids, methylxanthines, and phenolic compounds, specially chlorogenic acids and procyanidins, determined in the extracts (Fig. 1). However, as is well-recognized, CP extracts derived from different coffee species and ripening stages varied in composition and antioxidant activities (Rodríguez-Durán et al., 2014). Moreover, the comprehensive revision of the scientific literature shows that the extraction efficiency and selectivity of bioactive compounds from CP and, in consequence, their respective biological activities, notably depend on the extraction method and conditions.
Fig. 1.
Representation of those advanced and sustainable extraction techniques explored until date to obtain enriched-bioactive fractions from coffee pulp (the upper half). It includes the bioactivities studies until date and the compounds pointed as responsible of such properties. Moreover, non-explored extraction techniques, bioactive compounds and health-promoting activities are proposed (the lower half). MAE: Microwave-Assisted Extraction, UAE: Ultrasound-Assisted Extraction, PEF: Pulsed Electric Field-Assisted Extraction, DES: Deep Eutectic Solvent, SUPRAS: Supramolecular Solvent, SWE: Subcritical Water Extraction, FER: Fermentation, ENZ: Enzymatic Treatment, HHP: High Hydrostatic Pressure; EXTR: Extrusion, MEM: Membrane, NanoFL: Nanofiltration, BioTh: Biotechnology.
Once raw material has been pre-treated, in example by dehydration, freeze-drying, grounding, etc., it is followed by an extraction procedure to separate the desired products from the matrix. Extraction methods include distillation, pressing, sublimation or solvent extraction, being the latest the most widely developed procedure for the recovery of bioactive compounds from CP. Solvent extraction is based on its penetration into the matrix followed by the solute dissolution and its further diffusion out the matrix to finally collects the solutes in the extraction media (Zhang et al., 2018). Conventional solvent extraction methods, such as maceration, percolation, or decoction with/without reflux methodologies by using aqueous, organic, acid solvents, or their combination including heating for the latest, are well-established and relatively easy to operate (Bondam et al., 2022). However, these procedures are high-priced with long processing times, achieving poor or moderate selectivity, and involving high energy and chemicals consumption (Mediani et al., 2022). Therefore, advanced techniques based on mass transfer improvement have been recently explored to achieve high efficiency and selectivity extraction rates while being environmentally friendly by using green alternative solvents, such as supramolecular solvent (SUPRAS) (Torres-Valenzuela et al., 2020) and deep eutectic solvents (DES) (Loukri et al., 2022), high-pressurized hot water (Punbusayakul et al., 2015) or supercritical carbon dioxide (SC–CO2) (Hernandez et al., 2009) (Fig. 1). Mentioned solvent extraction strategies can be considered chemical techniques since the success on the solute recovery is mostly based on the way the solvent surrounds and interacts with solute molecules (solvent solvation). Additionally, the use of assisted emerging techniques based on promoting the exposure of solute to solvent by cell wall weakening (named physicochemical techniques), such as microwave (Tran et al., 2022b), ultrasound (Myo and Khat-udomkiri, 2022) and pulsed electric field (Macías-Garbett et al., 2022) have been also explored. Besides, biochemical extraction approaches such as fermentation and enzymatic treatment mediated by, for example, lactic acid bacteria (Myo et al., 2021) or molds as Aspergillus spp. (Núñez Pérez et al., 2022) followed by solvent recovery, have gained growing interest during the last decades since they allow a better performance improving the extraction yield of certain target compounds from the CP (Fig. 2).
Fig. 2.
Schematic summary of solvent extraction techniques used for coffee pulp (CP) valorization in food science including the fundamentals of the extraction model and the main factors to be considered together with their respective advantages and disadvantages. S-L extraction: Solid-Liquid extraction, S-L ratio: Solid-Liquid ratio, MAE: Microwave-Assisted Extraction, UAE: Ultrasound-Assisted Extraction, PEF: Pulsed Electric Field-Assisted Extraction, DES: Deep Eutectic Solvent, SUPRAS: Supramolecular Solvent, SWE: Subcritical Water Extraction, T: Temperature, t: Time.
Regardless the extraction techniques, the recovery of bioactive compounds is driven by multiple factors, e.g., solvent polarity, extraction temperature and pressure, aeration, voltage, etc., that need to be investigated, optimized, and controlled to achieve the maximum diffusivity and solubility potential of each CP-solvent extraction system. Predictably, solvent type (e.g., water, alcohols, and their mixtures) and its concentration (e.g., ethanol aqueous solution) results the main factor for both conventional and assisted methods to reach high efficiency and selectivity extraction rates (Tran et al., 2020a). In addition, other experimental independent variables such as pH, time, solid-liquid (S-L) ratio, and particle size could enhance the mass transfer between phases, promoting the recovery of the target compounds (Zhang et al., 2018). Parameters to be considered for non-conventional extraction techniques, such as the power and frequency of ultrasound and microwave, and the voltage of the pulsed electric field, are also critical factors that affect the extraction efficiency (Macías-Garbett et al., 2022). Additionally, the selection of microorganisms and enzyme species, enzyme activity, and dosage were also pointed as relevant factors (Fig. 2) to be considered in biotechnology (Myo et al., 2021). Therefore, considering i) the dissimilar mechanisms of action of those abovementioned extraction methodologies for the recovery of bioactive fractions from CP, ii) the variables having influence on the results by extraction strategy, iii) the specific conditions tested and, iv) the type of outcomes provided by authors, it results necessary a thorough revision to integrate all scientific findings about the topic (Table 1).
Table 1.
Summary of the extraction methodologies for coffee pulp valorization as functional ingredient.
| Extraction method | Extraction solvent | Most suitable conditions | Target compounds | Biological activities | Reference |
|---|---|---|---|---|---|
| Solid-liquid extraction (S-L extraction) | Water | 1: 2 S-L ratio, undefined temperature | TPC (11.3 mg GAE/g extract), chlorogenic acid (12.2 mg/g extract), caffeine (1.6 mg/g extract), anthocyanin (11.4 mg C3G/g extract) | Antioxidant, antimicrobial | Khochapong et al. (2021) |
| Water | 1: 10 S-L ratio, RT, 16 h | TPC (33.0 mg GAE/g extract) | Antioxidant, anti-aging | Lonati et al. (2022) | |
| Hot water | 1: 5 S-L ratio, 92 °C, 2 min | TPC (3.5 mg GAE/g CP) | Antioxidant, antimicrobial | Duangjai et al. (2016) | |
| Hot water | 1: 10 S-L ratio, 85 °C, 15 min, particles <1.4 mm | TPC (9.2 mg GAE/g CP), chlorogenic acid (2.2 mg/g CP), caffeine (6.8 mg/g CP), gallic acid (0.73 mg/g CP), rutin (0.09 mg/g CP), protocatechuic acid (3.1 mg/g CP) | Antioxidant | Heeger et al. (2017) | |
| Hot water | 1: 100 S-L ratio, 100 °C, 60 min, shaking | TPC (23.4 mg GAE/g CP), TFC (19.2 mg CE/g CP), caffeine (3.9 mg/g CP), chlorogenic acid (3.5 mg/g CP) | Antioxidant | Tran et al. (2020a) | |
| Hot water | 100 °C, 10 min | TPC (23.3 mg GAE/g extract), chlorogenic acids (12.0 mg/g extract), caffeine (3.6 mg/g extract), catechin (0.26 mg/g extract), epicatechin (0.18 mg/g extract) | Hepatoprotective, lipid-lowering, antidiabetic, renoprotective | Ontawong et al. (2019a),Ontawong et al. (2019b),Boonphang et al. (2021) | |
| Hot water | Undefined temperature, 10 min | TPC (25.5 mg GAE/g extract), chlorogenic acid (6.5 mg/g extract) | Antioxidant, lipid- lowering | Ontawong et al. (2021) | |
| Aqueous HCl (1%) | 5: 12 S-L ratio, RT, 30 min, pH 1.8 | TPC (4.5 mg GAE/g CP), TFC (7.8 mg CE/g CP), chlorogenic acid (0.084 mg/g CP), tannin (0.94 mg CE/g CP), anthocyanin (0.004 mg C3G/g CP) | Antioxidant | Delgado et al. (2019) | |
| Aqueous HNO3 (0.1 M) | 1: 25 S-L ratio, boiling, 30 min | Pectin (14.6%) | Not validated | Reichembach and de Oliveira Petkowicz (2020) | |
| Aqueous methanol (80%) | 1: 4 S-L ratio | TPC (0.56 μg GAE/g CP), TFC (1.0 μg CE/g CP) | Antimicrobial | Sangta et al. (2021) | |
| Aqueous methanol (80%, acidified) | 1: 10 S-L ratio, 55 °C, 35 min, 100 rpm agitation | Hydroxycinnamates (25.5 mg/g CP) | Not validated | Rodríguez-Durán et al. (2014) | |
| Butylated hydroxytoluene methanol (1%) | 1: 20 S-L ratio, RT, 1 h, 100 rpm agitation | Phytoprostanes (654.6 ng/g CP), phytofurans (474.3 ng/g CP) | Not validated | Ruesgas-Ramón et al. (2019) | |
| Aqueous ethanol (50%) | 1: 40 S-L ratio, 70 °C, 60 min, 350 rpm agitation | TPC (45.0 mg GAE/g CP) | Antioxidant | Kusumocahyo et al. (2020) | |
| Ethyl acetate | 1: 5 S-L ratio, RT, 24 h, shaking | TPC (0.18 mg GAE/g CP), TFC (0.79 mg CE/g CP) | Antioxidant | Alkaltham et al. (2020) | |
| β-cyclodextrin | 9.25 mg/mL concentration, 1: 30 S-L ratio, 80 °C, 120 min | Caffeine (4.8 mg/g CP) | Antioxidant | Loukri et al. (2020) | |
| Deep eutectic solvent (DES) | Choline chloride: glycerol (1: 3 molecular ratio), 70% (w/v) concentration, 1: 47 S-L ratio, 55 °C, 120 min | Caffeine (4.9 mg/g CP) | Antioxidant | Loukri et al. (2022) | |
| Supramolecular solvents | Hexagonal inverted aggregates of octanoic acid: ethanol: water (5:24:71), 1: 4 S-L ratio, RT, 5 min | Caffeine (3.6 mg/g CP), protocatechuic acid (0.9 mg/g CP) | Antioxidant | Torres-Valenzuela et al. (2020) | |
| Subcritical water | Aqueous acetic acid (7%, v/v), 120 °C, 1500 psi pressure, 15 min | Cyanidin-3-rutinoside (30.4 μg/g CP) | Not validated | Punbusayakul et al. (2015) | |
| Fermentation | Water | Lactic acid bacteria, 30 °C, substrate: water: sugar (3: 10: 3), 5% starter, 72 h | TPC (3.3 mg GAE/g CP) | Antioxidant | Myo et al. (2021) |
| Aqueous methanol (80%, acidified) | Fungus Aspergillus tamarii | TPC (137.8 mg GAE/g CP), hydroxycinnamic acid (14.5 mg/g CP) | Antioxidant | Arellano-González et al. (2011) | |
| Methanol | Lactic acid bacteria, 31.8 °C, 4 m/s air flowrate, 66–68% humidity, 4.2 h; 1: 10 S-L ratio, 27 °C, 24 h | TPC (6.7%) | Antioxidant | Oktaviani et al. (2020) | |
| Acetone-methanol (7:3, v/v) | Yeast, 10% starter, 28 °C, 48 h | Carotenoids (16.4 mg/L) | Antimicrobial, antioxidant | Moreira et al. (2018) | |
| Aqueous ethanol (80%) | Actinomycetes, 10% (v/w) starter, 27 °C, 9 days; 1: 10 S-L ratio, 100 rpm agitation, 24 h | TPC (1.2 mg GAE/mL), anthocyanins (110.0 mg/mL), catechin (10.4 mg/mL) | Not validated | Kurniawati et al. (2016) | |
| Enzymatic extraction | Hot water | Viscozyme (1%, v/w), 1 h; 1: 10 S-L ratio, 90 °C, 10 min | TPC (12.2 mg GAE/g CP), TFC (5.5 mg CE/g CP), chlorogenic acid (2.2 mg/g CP), caffeine (2.4 mg/g CP), anthocyanins (0.18 mg/g CP) | Anti-diabetic, antioxidant | Patil et al. (2022) |
| Aqueous methanol (80%) | 1: 5 S-L ratio, 40 °C, 100 rpm agitation, 30 min | Chlorogenic acid (0.71 mg/g CP), caffeic acid (0.11 mg/g CP), ferulic acid (0.03 mg/g CP) | Not validated | Torres-Mancera et al. (2013) | |
| Ultrasound-assisted extraction (UAE) | Aqueous methanol (50%) | 1: 100 S-L ratio, 40 °C, 150 W, 30 min | TPC (14.4 mg GAE/g CP), TFC (13.2 CE/g CP), Caffeine (2.9 mg/g CP), proanthocyanidins (6.8 mg CE/g CP) | Antioxidant | Tran et al. (2020b) |
| Aqueous ethanol (50%) | 1: 16 S-L ratio, 30 °C, 15 min | TPC (23.2 mg GAE/g CP), TFC (25.1 mg quercetin/g CP) | Antioxidant, tyrosinase inhibitory | Chen et al. (2021) | |
| Aqueous ethanol (50%) | 1: 20 S-L ratio, 60 °C, 250 W, 35 min | TPC (46.7 mg GAE/g CP), TFC (36.4 mg CE/g CP), chlorogenic acid (7.9 mg/g CP), caffeine (5.7 mg/g CP) | Antioxidant | Tran et al. (2022b) | |
| Aqueous ethanol (70%) | 1: 10 S-L ratio, RT, 100 power, 37 Hz, 30 min | TPC (44.5 mg GAE/g extract) | Antioxidant, anti-inflammatory | Magoni et al. (2018) | |
| Aqueous propylene glycol (46.71%) | 1: 22 ratio, RT, 20 kHz, 8 min | TPC (9.3 mg GAE/g CP), TFC (58.8 mg quercetin/g CP), tannin (8.7 mg/g CP) | Antioxidant | Myo and Khat-udomkiri (2022) | |
| Deep eutectic solvent (DES) | Lactic acid: choline chloride (2:1 molecular ratio), 1: 5 S-L ratio, 200 W, 3 min | Chlorogenic acid (0.45 mg/g CP), caffeine (0.55 mg/g CP) | Not validated | Ruesgas-Ramón et al. (2020) | |
| Microwave-assisted extraction (MAE) | Aqueous ethanol (42.5%) | 1: 100 S-L ratio, 1000 W, RT, 85 min | TPC (38.7 mg GAE/g CP), TFC (27.0 mg CE/g CP), chlorogenic acid (7.0 mg/g CP), caffeine (5.5 mg/g CP) | Antioxidant | Tran et al. (2022a) |
| Aqueous ethanol (50%) | 1: 20 S-L ratio, 700 W, 70 min | TPC (20.9 mg GAE/g CP), TFC (18.8 mg CE/g CP), chlorogenic acid (2.3 mg/g CP), caffeine (3.3 mg/g CP) | Antioxidant | Tran et al. (2022b) | |
| UAE-MAE | Aqueous ethanol (30%) | UAE at 1: 12 S-L ratio, RT, 40 kHz, 20 min; MAE at 70 °C, 800 W, 5 min | TPC (328.9 mg/g extract), tannin (20.52 mg CE/g extract) | Not validated | González-González et al. (2022) |
| PEF-MAE | Water | PEF at 5 Hz, 18 kV (6 kV/cm) 5 min; MAE at 1: 10 S-L ratio, 10 min | TPC (14.4 mg GAE/g CP), TFC (3.1 mg rutin/g CP) | Antioxidant | Macías-Garbett et al. (2022) |
Abbreviations: S-L: solid-liquid, RT: room temperature, TPC: total phenolic content, TFC: total flavonoid content, GAE: gallic acid equivalent, CE: catechin equivalent, C3G: cyanidin-3-glucoside.
This review was conceived to provide an overview about the impact of the most advanced and sustainable extraction techniques on the health-promoting properties of CP as an emerging valorization strategy, through the study of the extraction efficiencies for bioactive compounds recovery offered by mentioned techniques. Their advantages and drawbacks compared to those known for conventional extraction techniques are also discussed, offering the most promising extraction techniques explored until date. Finally, the prospects of emerging extraction techniques which are expected to play a relevant role in the near future for CP revalorization, and the potential for food and pharmaceutical applications are highlighted. This work is a state-of-the-art about the use of alternative green solvents and sustainable techniques for the extraction of bioactive compounds from CP, at both laboratory and industry levels, based on healthy products and potential valorization practices.
2. Conventional solvent extraction methods
Conventional solvent extraction procedures (i.e., maceration or decoction) are widely practiced by food industry and research with the objective to achieve high recovery efficiencies of compounds under interest from a matrix. Such successful applicability is mostly due to the simple operating conditions besides the affordable technician formation and handling. Either liquid (e.g., fish oil) or solid matrices (e.g., coffee pulp) can be used to recover target bioactive compounds at atmospheric pressure by mixing or immersing the raw material in the selected solvent. If needed, heating and stirring can be also applied facilitating the solubility and diffusivity of compounds. The most frequently used conventional solvents are water, acidified aqueous solutions, and certain organic solvents such as methanol, ethanol, and acetone, which are employed as individual or combined extraction media (Bondam et al., 2022) (Table 1). In general terms, this process is considered trouble-free, guideless, and highly reproducible but has the disadvantage of high energy consumption, large extraction times and toxicity for some solvents, among others (Fig. 2).
2.1. Water extraction
Water is considered the safest, non-toxic, and greenest solvent for food production. The S-L extraction of CP using water is similar to the household process of brewing coffee. The simplest extraction process employing water was performed by Khochapong et al. (2021), who submitted the CP to a water extraction at the ratio of 1:2 (w/v) and room temperature under continuous homogenization using a blender, obtaining an extract enriched in phenolic compounds (11.3 mg GAE/g extract), chlorogenic acid (12.2 mg/g extract), caffeine content (1.6 mg/g extract), and monomeric anthocyanin content (11.4 mg/g extract) (Khochapong et al., 2021). Similarly, Magoni et al. (2018) also recovered higher phenolic compounds (33.0 mg/g extract) from CP at the ratio of 1:10 (w/v) and room temperature for 16 h, which might be attributed to the higher S-L ratio. Furthermore, the recovery of bioactive compounds from CP aqueous extracts was reported to be significantly affected by several factors (Fig. 2), including temperature, extraction time, S-L ratio, and particle size (Loukri et al., 2020). In this sense, Tran et al. (2020a) found that an increased extraction temperature would enhance the intermolecular contact, expediting the release of bioactive compounds. Indeed, the optimal conditions for the maximum recovery of total phenolic compounds (23.4 mg GAE/g CP), flavonoids (19.2 mg CE/g CP), caffeine (3.9 mg/g CP) and chlorogenic acids (3.5 mg/g CP) were found at 100 °C, 60 min and the ratio of sample to solvent 1:100 g/mL (Tran et al., 2020a). Such successful extraction yields induced by high extraction temperatures were supported by using hot water for 10 min yielding 10.2 mg GAE/g CP at 90 °C (Patil et al., 2022) and 25.5 mg GAE/g extract at undetailed temperature (Ontawong et al., 2021). Moreover, a notable high content of specific phenolic compounds obtained after 10 min with hot water has been reported i.e., 6.5 mg chlorogenic acid/g extract or 0.26 mg catechin/g extract and 0.18 mg epicatechin/g extract (Ontawong et al., 2019a, 2021). In addition, the appropriate particle size helps to extend the contact area between the hot water and the CP, thus boosting the leaching of bioactive substances. Heeger et al. (2017) suggested that CP material with a particle size lower than 1.4 mm yielded better results in terms of phenolic compounds recovery when extracting using hot water (85 °C), with an initial concentration of 1 g/mL for 15 min. Likewise, an increased acidity caused by the presence of strong acids (such as HCl) denatures the CP cell membranes, facilitating the release of substances covalently bound to the cell walls from the matrix, and ultimately improving the extraction efficiency of the bioactive compounds. In this regard, an aqueous solution of HCl 1% (pH 1.8) was applied to extract bioactive compounds from the CP at a ratio of 25: 60 (g/mL) in Caturra and Colombia varieties. The acidity increased the phenolic, flavonoid and tannin content of the extract by 49%–594% following reported data. The anthocyanins, which were not detected in the aqueous extracts at neutral pH, was found in considerable amounts (3.6–4.1 mg/100 g CP) in the acidic aqueous solution (Delgado et al., 2019). In addition, acidification of aqueous solutions is also commonly used for precipitation and extraction of pectin (Reichembach and de Oliveira Petkowicz, 2020) to achieve revalorization of carbohydrates from CP (Manasa et al., 2021).
Currently, natural alternatives with antioxidant properties are being widely pursued. The phenolic compounds and caffeine present in CP's aqueous extracts are proved to exert in vitro and in vivo antioxidant bioactivity (Fig. 3) (Cañas et al., 2021). Tran et al. (2020a) recovered phenolic antioxidants using water from Robusta dried CP, and indicated that phenolic, flavonoid, caffeine and chlorogenic acids content notably contributed to the 2,2′-azinobis-(3-ethylbenzthiazoline-6-sulphonate) (ABTS) and 1,1-diphenyl-2-picrylhydrazyl (DDPH) radical scavenging ability and ferric reducing antioxidant power (FRAP) (Tran et al., 2020a). Mentioned results relative to DPPH scavenging capacity supported similar findings previously reported (Magoni et al., 2018). Protocatechuic acid and gallic acid content experienced a high correlation with oxygen radical absorption capacity (ORAC, r = 0.78 and 0.86, respectively), and xanthonoids mangiferin and isomangiferin were also found to be highly positively correlated to DPPH (r = 0.95 and 0.93, respectively) in CP hot-water extract (Pua et al., 2021). The temperature and pH of the water extraction were proved to give a predominant impact on the biological activities. Thereby, the ABTS and DPPH radical scavenging abilities and FRAP value of the hot water extract were 2.1, 3.4 and 25.4 times higher than those observed at room temperature-water extract, respectively, which rationally might be attributed to the increased presence of phenolic compounds in the extracts (Fig. 3). Delgado et al. (2019) manifested that condensed tannins in acidified aqueous extract were better scavengers of free radicals compared to neutral pH extraction, with a significant correlation to ORAC values (r = 0.69). Nevertheless, coffee varieties must be considered when reporting antioxidant activities exerted by water extracts since high variability were also reported between Coffea arabica L. and Coffea canephora (Pua et al., 2021).
Fig. 3.
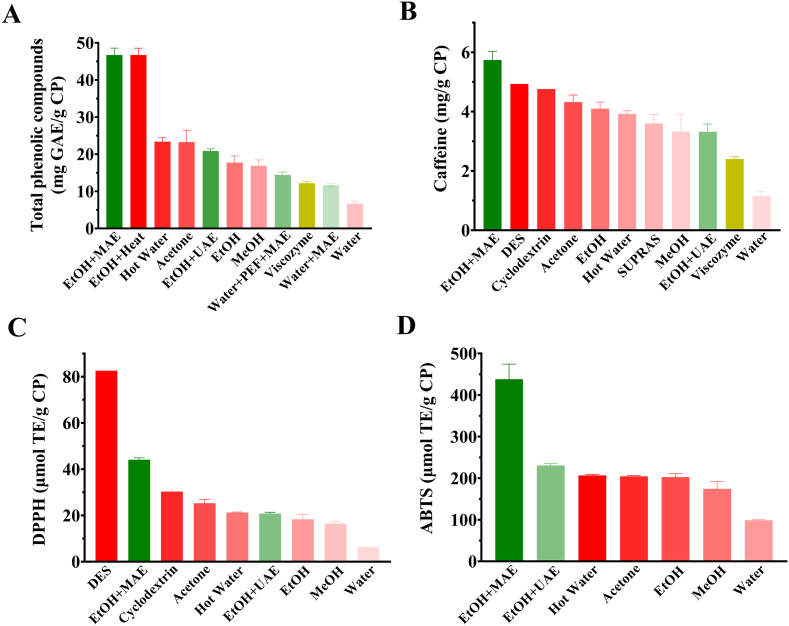
Ranking diagram of the extraction techniques applied to coffee pulp (CP) at optimal conditions in terms of total phenolic content (mg gallic acid equivalent/g CP) (A), caffeine content (mg/g CP) (B), DPPH (μmol Trolox equivalent/g CP) (C), and ABTS (μmol Trolox equivalent/g CP) (D). Only the studies providing the information needed to normalize the results for their appropriate comparison were included. According to the inclusion criteria, the plotted data were those reported information by Tran et al. (2020a); Tran et al. (2022a,b); Kusumocahyo et al. (2020); Patil et al. (2022); Macías-Garbett et al. (2022); Torres-Valenzuela et al. (2020); Loukri et al. (2020); Loukri et al. (2022). EtOH: Ethanol, MeOH: Methanol, MAE: Microwave-Assisted Extraction, UAE: Ultrasound-Assisted Extraction, PEF: Pulsed Electric Field-Assisted Extraction, DES: Deep Eutectic Solvent, SUPRAS: Supramolecular Solvent, SWE: Subcritical Water Extraction.
The antioxidants compounds contained in CP extracts can get into the lipid bilayer of the cytoplasmic membrane, modifying function, structure, and permeability, thus inhibiting the growth of microorganisms as reported Khochapong et al. (2021). This work showed that a CP aqueous extract enriched with antioxidant bioactive compounds also inhibited the growth of Escherichia coli TISTR 780 and Staphylococcus aureus TISTR 1466 at concentrations of 150 and 200 mg/mL (Khochapong et al., 2021). These results were supported by Duangjai et al. (2016) since a hot water extract obtained at 92 °C for 2 min (ratio of 1: 5) exhibited a superior growth inhibition for gram-positive (minimal inhibitory concentration, MIC, values of Staphylococcus aureus and Staphylococcus epidermidis were 37.5 and 4.7 mg/mL, respectively) rather than gram-negative bacteria (MIC values of Pseudomonas aeruginosa and Escherichia coli were 75.0 and 37.5 mg/mL respectively). These results were attributed to the relatively high content of quinic, malic and chlorogenic acids as well as the caffeine content in the CP extract (Duangjai et al., 2016). Moreover, aging-related diseases are often associated with excessive reactive oxygen species (ROS) production, breaking the neutralizing capacity of the endogenous antioxidant defense system. A notable anti-aging activity of CP extract, mainly due to the presence of phenolic compounds with antioxidant activity, was reported (Tseng et al., 2022). In this sense, it was suggested that procyanidins and quinic acid derivatives found in CP aqueous extracts might become a nutritional strategy to counteract age-related damage, pointing that an intake of 2 mg was effective (Lonati et al., 2022).
The hepatoprotective effect of CP hot water extract was also investigated by Ontawong and coworkers (2021). They found that CP hot-water extracts reduced the intracellular lipid content by stimulating triglyceride and cholesterol secretion while regulating the hepatic lipid metabolism and transporter protein-related genes in human hepatocyte cells (HepG2). The hepatoprotective effect of this extract was mostly attributed to chlorogenic acids and caffeine, which also exerted an anti-hepatic steatosis effect at 2.3 μg/mL and 0.8 μg/mL respectively in HepG2 cells. The in vivo hepatoprotective activity of this extract was further corroborated by using male Wistar rats fed with high-fat diet for 12 weeks. Supplementation of CP hot-water extract (1 g/kg body weight) could manage caloric intake, assist in controlling body weight and visceral fat mass in obese rats, and improve plasma metabolic parameters, insulin resistance and morphological characteristics of liver tissues. Mentioned extract was also able to modulate the expression levels of genes involved in hepatic lipid metabolism, ameliorating hepatic steatosis in obese rats induced by high-fat diet (Ontawong et al., 2019a). Moreover, the in vitro and in vivo lipid-lowering effect of CP hot-water extract was also studied using Caco-2 cells (50 mg/mL) and rat models (1 g/kg body weight) respectively. Results demonstrated a notable inhibition of the cholesterol transport by dietary mixed micelles through cell membrane and rat jejunal loops, and regulated Niemann-Pick C1-Like 1 (NPC1L1) protein and Liver X receptor alpha (LXRα) mRNA expression in Caco-2 cells and hypercholesterolemic rats (Ontawong et al., 2019b). CP hot water extract also significantly improved renal lipid content and lipid peroxidation in an experimental model of type 2 diabetes (T2D) and restored renal morphology and organic transport associated with T2D, thus its anti-diabetic and renal protective effects were also proved (Boonphang et al., 2021).
Although recovering bioactive compounds using aqueous extractions had been widely investigated, its use is still reduced to certain applications since some bioactive substances are easily degradable by environmental factors e.g., temperature, light exposure, etc. Additionally, the selectivity achieved by using water as extraction solvent usually results insufficient to recover target compounds, which could imply undesirable interactions and interferences on the pursued bioactivities. Some authors have suggested to overcome these limitations by using vigorous extraction media, such as organic solvents, instead of water.
2.2. Organic solvent extraction
Several organic solvents, within a wide range of polarities (i.e., from methanol to ethyl acetate), were employed to enhance the disruption of the CP matrix and, in consequence, increasing the release of bioactive compounds. Both extraction efficiency and selectivity will directly depend on the dissolving power of the selected extraction media to solubilize the compounds under interest. With the objective to optimize the extraction yields of certain group of compounds, e.g., phenolic compounds or methylxanthines besides others, several organic solvent aqueous mixtures are usually employed (Fig. 2).
It has been reported an extraction yield of 6.6% using 80% methanol as extraction solvent (1:4 w/v ratio) after a sample purification step using 95% dichloromethane and a total phenolic content of 0.56 μg/g CP with notable inhibitory activity against horticultural pathogens (Sangta et al., 2021). Nevertheless, different coffee varieties and ripening stages also showed different characteristics when extracted with 80% methanol aqueous solution. Indeed, the hexane-defatted CP using 1% (v/v) acetic acid for extraction solvent acidification, exhibited a maximum concentration of hydroxycinnamates (7.4–25.5 mg/g CP) at semiripe stage (Rodríguez-Durán et al., 2014). The acidification of methanol (MeOH/0.1% HCl (v/v)) was also successfully tested to obtain anthocyanins and carotenoids from arabica CP, as other phenolic compounds such as chlorogenic or mono- and di-caffeoylquinic acids (Esquivel et al., 2020). Moreover, an extract obtained using 1% (v/v) butylated hydroxytoluene methanol was abundant in phytoprostanes and phytofurans, known by their interesting biological properties such as anti-inflammatory and neuroprotection activities (Ruesgas-Ramón et al., 2019). Ethanol, with similar polarity to methanol, seems to be a promising candidate for the extraction of bioactive substances from CP (Fig. 3). Indeed, Magoni et al. (2018) reported slightly higher extraction yields from CP by using 30% ethanol compared to water (8.4% and 7.8%, respectively) after 16 h at room temperature. Lower values were observed when increasing the ethanol concentration from 30 to 100%, being 8.4% and 6.4% respectively. Similar trends were observed for total phenolic compounds content, yielding around average 36.0 mg GAE/g extract for both 30% and 70% ethanol, and 32.0 mg GAE/g extract for both 100% ethanol and water extractions. However, no significant differences on antioxidant bioactivity were detected for any of the extraction solutions tested (Magoni et al., 2018).
Less commonly used solvents for phytochemical recovery were also successfully explored e.g., propylene glycol, acetone and ethylacetate (Table 1). Then, propylene glycol (46%) was used for the extraction of phenolic, flavonoid and tannin compounds (8.5 mg GAE/g CP, 51.7 mg quercetin equivalent/g CP, and 7.8 mg tannic acid equivalent/g CP, respectively) from CP at a liquid-solid ratio of 22.2 mL/g for 24 h, resulting in significantly higher values compared to those obtained for ethanol and aqueous extractions (Myo & Khat-udomkiri, 2022). Moreover, acetone aqueous solution (70%) was suitable to obtain procyanidins from CP (Wong-Paz et al., 2021) and the use of ethyl acetate at room temperature for 24 h under shaken yielded up to 0.18 mg GAE/g CP (Alkaltham et al., 2020). Comparatively, Tran et al. (2020a) suggested extraction yield of phenolic compounds and caffeine content was ranked by acetone > ethanol > methanol, which were 2.5–3.5 and 2.9–3.7-fold higher than those of water extraction and comparable to those of hot water extraction (Fig. 3) (Tran et al., 2020a).
As reported for aqueous extracts, phytochemicals-enriched fractions obtained from CP using organic solvents typically exhibited bioactive properties such as antioxidant, anti-inflammatory or antimicrobial activities (Table 1). In this regard, Tran et al. (2020a) showed a prominent radical scavenging activity (ABTS methodology) of a 50% methanol aqueous solution, being positively correlated to proanthocyanidins content (r = 0.75) (Tran et al., 2020b). However, such antioxidant potential was reinforced by using acetone as extraction solvent, resulting the ABTS and DPPH scavenging abilities and FRAP determinations a 17.2%, 53.8%, 79.3% and 0.8%, 38.0%, 41.1% higher than those obtained from methanol and ethanol extracts, respectively. This work also claimed the higher extraction yields for phenolic compounds and caffeine, as well as antioxidant activities by using 50% solvent aqueous dilutions compare to pure solvents, being overall significantly higher than those observed for water extractions, and comparable to those from hot-water extraction (Fig. 3) (Tran et al., 2020a). Indeed, besides the solvent concentration, such bioactive potential seemed to be deeply influenced by the temperature and time of extraction. As example, a decreased IC50 of DPPH values was found for elevated extraction temperatures and times, mostly due to higher extraction efficiencies for the total phenolic content extractions (Kusumocahyo et al., 2020). Mentioned supremacy of the organic solvents over water extracts in terms of anti-inflammatory bioactivity was also reported. Thus, Magoni et al. (2018) compared the therapeutic effects of CP 70% ethanol and water extracts on in vitro inflammation cell culture model by using TNF-α-stimulated gastric epithelial cells (AGS cell line). Obtained results revealed that the ethanolic fraction was superior to aqueous extract at inhibiting IL-8 release (IC50 values of 61.5 μg/mL and 79.4 μg/mL, respectively). Meanwhile, the antimicrobial effect of CP organic extracts was also reported. The growth of Alternaria brassicicola, Pestalotiopsis sp. and Paramyrothecium breviseta fungi were inhibited when treated with 80% aqueous methanol CP extract enriched on caffeic acid and epigallocatechin gallate, displaying a significant bioactivity with inhibitory concentrations (IC50) of 0.09, 0.31 and 0.14 g/mL, respectively (Sangta et al., 2021).
Although organic solvent seems to offer higher extraction efficiencies to obtain bioactive compounds from CP, these extraction strategies might result cost-intensive when transferred to industry, besides being far from the paradigm of sustainable processes due to the polluted solvent by-products wasted to the environment (Bondam et al., 2022). In addition, bioactive extracts possibly suffer from toxic and harmful solvents residues, making them unfavorable for food applications (Gemechu, 2020).
In general terms, conventional extraction processes, using both water and organic solvents, are considered not effective regarding selectivity, energy, performance, yield and environmental impact (Alara et al., 2021). However, the abovementioned advantages and drawbacks of conventional S-L extractions must be considered for selecting the most suitable solvent to recover bioactive compounds from CP (Fig. 2). Nevertheless, to overcome the limitations previously mentioned, novel extraction strategies were explored pursuing the environmentally friendly and food grade character of water combined with the high extraction efficiency and selectivity of certain organic solvents (Choi and Verpoorte, 2019). Among them, the commonly known emerging green solvents e.g., deep eutectic solvents, were pointed as promising alternatives (Table 1).
3. Alternative chemical techniques
3.1. Extraction methods using emergent green solvents
Beyond the solvent's physicochemical characteristics (i.e., boiling point and polarity), their impact on human health and environmental sustainability should be considered. Therefore, agents like acetone and methanol, commonly used in scientific research focused on the extraction processes development for the recovery of bioactive compounds, are not desirable. In return, they were proposed to be substituted by emerging green solvent, such as deep eutectic solvents (DESs), supramolecular solvents (SUPRASs) and pressurized liquids (PLs) (Fig. 2).
3.1.1. Deep eutectic solvent (DES) extraction
DESs has been emerging since 2004 as a promising green alternative to conventional extractions because of their physicochemical properties i.e., high viscosity, non-inflammability, and low volatility, besides the chemical and thermal stability (Florindo et al., 2019; Mbous et al., 2017). They are defined as a homogeneous mixture of several solid substances at specific mixture ratio with a melting point lower than either of the individual components melting points, induced by the generation of intermolecular hydrogen bonds between hydrogen bond acceptors (HBA) and donors (HBD) (Choi and Verpoorte, 2019). DESs were recognized as cost-effective, highly efficient, and selective extraction media for target molecules from natural sources, besides their distinctive favorable sustainability (Saini et al., 2022). Moreover, such constituting compounds have been approved as food additives or supplements for human consumption because of their practically zero toxicity (Saini et al., 2022). Thereby, Ruesgas-Ramón et al. (2020) prepared six different DESs by using choline chloride, betaine, lactic acid, glycerol, and 1,4-butanediol to extract bioactive compounds from CP under heat-stirring assisted conditions for 1 h at 60 °C. Results exhibited the notable influence of extraction media composition on the recovery of phenolic compounds at both quantitative and qualitative levels. The mixture of choline chloride, lactic acid, and water (CCLA) at 1: 2: 1.5 mol ratio resulted the most efficient solvent for chlorogenic acid and caffeine recovery (4.1 and 3.9 mg/g CP, respectively) compared to the other tested DESs. Moreover, similar total phenolic content was found for CCLA DES (9.6 mg GAE/g CP) and a 70% ethanolic extraction following the described HPLC-MS quantification methodology, although a notably decrease of chlorogenic acid (25.5%) and caffeine (33.9%) content was noticed for CCLA DES (Ruesgas-Ramón et al., 2020). In addition, high concentrations of DES aqueous solution, composed by diluted mixtures of choline chloride and glycerol, were observed to impair the extraction efficiency of caffeine from CP, which was attributed to the reduced mass transfer caused by the higher densities of the concentrated eutectic mixtures. However, the solvent to CP ratio and temperature exerted an opposite effect, the higher values for both independent variables the higher caffeine yields. Finally, this scientific work also provided the most promising extraction conditions for caffeine recovery from CP using choline chloride-glycerol aqueous mixtures (40 mL/g liquid-solid ratio, 55 °C, and 70% DES concentration), resulting higher (4.9 mg/g) than those observed for water extraction (4.4 mg/g) and cyclodextrin extraction (4.7 mg/g) (Loukri et al., 2022).
DES not only improves the extraction yield but also enhances the biological activity of the obtained fractions by protecting the bioactive molecules. Apparently, the hydrogen bonding-induced interactions between DES and the phenolic compounds contributed to such bioactive compounds stabilization by reducing their free movement in the extraction media. Thus, it would involve shorter air exposures and ultimately, a safeguard of the molecules from oxidative degradation. This statement was provided by Loukri and coworkers (2020) following their findings relative to the antioxidant properties of DES extracts from CP. They observed that DPPH results generally increased with DES concentration (Fig. 3), DES-CP ratio, and temperature, establishing the optimal extraction conditions for bioactivity at 55 °C, 47 mL/g solvent-CP ratio and 70% DES concentration. Obtained results i.e., 82 μmol TE/g CP resulted superior to the obtained data from water, cyclodextrin, methanol, and ethanol extraction media by 54.9%, 55.0%, 83.4% and 88.5%, respectively.
Undoubtedly, DESs contribute to expand the opportunities range for achieving selective extractions of target bioactive compounds. However, as already pointed out by Ruesgas-Ramón and coworkers (2020), it is important to note the influence of the analytical methodology applied to the determined certain compounds in DES extracts e.g., total phenolic compounds by Folin-Ciocalteu methodology. Inconsistent results were found when comparing data from the latest colorimetric technique to those obtained by HPLC-MS quantification, which might be due to the analytical interferences exerted by the formation of insoluble salt precipitates induced by the choline chloride-based solvent (Ruesgas-Ramón et al., 2020). This limitation is one of the disadvantages recognized for DES (i.e., hindering analytics) however, other aspects must be considered such as the increased complexity of the reaction system, the large number combinations of HBA and HBD, the time-consuming relative to the recommended chemical characterization of the obtained DES prior carrying out the extraction process or the more arduous work to isolate the extracted compounds from the media when needed (Fig. 2).
3.1.2. Supramolecular solvent (SUPRAS) extraction
SUPRASs comprise green nano-structured solvents produced by the sequential self-assembly of amphiphilic colloidal suspensions triggered by external stimuli such as pH, temperature, or salt modifications (Musarurwa and Tavengwa, 2021). The phenomena behind the production of SUPRASs is based on the generation of reverse micelles or vesicles by the spontaneous self-aggregation of amphiphiles to reach a water-immiscible phase induced by external stimuli, causing the coacervation of an amphiphile-enriched liquid phase (SUPRASs phase) from the bulk solution (Musarurwa and Tavengwa, 2021; Rastegar et al., 2016). SUPRASs are renewable and low toxic solvents which allow easy and short extraction time as well as highly efficient and cost-effective extraction processes. These outstanding physico-chemical properties become them a desirable alternative to replace the use of organic solvents in the extraction processes of bioactive compounds (Fig. 2).
Notwithstanding such a promising SUPRASs prospect, its potential as extraction media to recover bioactive compounds from CP remains almost unexplored. Indeed, only one scientific work developed by Torres-Valenzuela et al. (2020) was published so far (Table 1). They recovered bioactive compounds from CP by SUPRAS using hexagonal inverted agglomerates of octanoic and decanoic acids (5% v/v) in several ethanol-water mixtures. Results revealed the dependent character of the extraction yields to the ethanol percentage, the SUPRAS composition and the solvent-sample ratio affected the extraction efficiency of the most abundant bioactive compounds i.e., caffeine and protocatechuic acid. Under the optimal conditions (4:1 v/w SUPRAS octanoic-sample ratio at RT for 5 min), the extraction yields of caffeine and protocatechuic acid were 3.6 mg/g, and 0.9 mg/g CP respectively, resulting 7–19 and 11-33-folds higher than those observed for conventional organic solvents (i.e., methanol, ethanol, acetone, and acetonitrile) under the same experimental conditions (Fig. 3). In addition, the DPPH and ABTS scavenging activities showed a linear correlation with SUPRAS concentration up to 15 g/L and 25 g/L respectively, concentrations at which the maximum antioxidant capacities were obtained, 45% for DPPH and 91% for ABTS.
The enhanced extraction efficiency claimed for SUPRAS could be explained by the presence of large regions with polarity differences in the supramolecular aggregates, which endows the possibility of extracting compounds within a large polarity range at high individual efficiencies e.g., from trigonelline to rutin (Torres-Valenzuela et al., 2020). Moreover, the high concentration of amphiphilic molecules provides numerous binding sites to interact with bioactive molecules by ion-ion, dipole-dipole, hydrogen bonding and dispersion, resulting crucial for their recovery the 3 and 4 hydrogen bond acceptors of caffeine and protocatechuic acid respectively (Torres-Valenzuela et al., 2020). Besides, SUPRAS is organized by coalescing droplets with discontinuities, creating a high surface area that facilitates mass transfer, and promotes extraction efficiency (Keddar et al., 2020). However, the further separation of compounds under interest and SUPRASs for food science applications as well as the solvent recovery after extraction are dilemmas that demand to be addressed. Fortunately, there is a wide variety of emerging extraction solvents and techniques available to be used for each specific application, together with those coming in near future. For example, using pressurized water and carbon dioxide is a prospective extraction technology which allows avoiding further recovery and separation steps.
3.2. Pressurized fluid extraction (PFE)
Pressurized fluid extraction is one of the several sample preparation technologies used to extract bioactive compounds from food matrices into a solvent under elevated temperatures and pressures, above room temperature and 0.1 MPa, respectively. Water, organic solvents and liquified gases (and their mixtures) are commonly used for PFEs. However, depending on the fluid combination and the pressure-temperature conditions, those fluids are placed at different areas of their respective phase diagrams. In this regard, low and high pressurized liquids (the latest commonly known as subcritical liquids) and supercritical fluids have been successfully employed as extraction media in food science. Pressurized liquid extraction (PLE) is based on increased the process temperature above the atmospheric boiling point while keeping the liquid state by applying high pressures. Meanwhile, supercritical fluid extraction (SFE) is based on turning the material state of the extraction media from either liquid or gas over the respective critical point. Both pressurized fluids strategies have shown encouraging results on bioactive compounds recovery (King, 2014; Mustafa and Turner, 2011). PFEs are usually time- and cost-saving, high selective and efficient while reducing the use of solvents compared to conventional and advanced solvent extraction techniques. Moreover, the extracts obtained could be non-toxic food grade fraction for PLE and even a solvent-free fractions for SFE using neat carbon dioxide.
3.2.1. Pressurized water extraction
Water is a highly polar solvent with an extensive hydrogen bonding structure and a high dielectric constant at room temperature under atmospheric pressure, resulting inefficient for moderate non-polar compounds extraction. However, high-pressurized hot water extraction, commonly known as subcritical water extraction (SWE), are characterized by a decreased surface tension, viscosity and dielectric constant while increasing the mass transfer diffusion rates. Therefore, highly efficient organic solvent-like extractions of low polar compounds will be achieved. Moreover, such improved extraction yields of SWE are also attained by the matrix surface disruption induced by high temperatures and pressures, significantly contributing to the mass transfer enhancement (Fig. 2). Nowadays, an accurate definition of SWE in terms of phase diagram location is still lack, however, subcritical state is widely considered as any situation above 100 °C and 0.1 MPa and bellow the critical point of water (374 °C and 22 MPa).
Under our knowledge (Table 1), the SWE application on CP for bioactive compounds recovery had been exclusively approached by Punbusayakul et al. (2015). They investigated the effect of temperature (65, 80, 105, 120, 135 and 155 °C) on the cyanidin-3-rutinoside anthocyanin extraction yield at 10.3 MPa for 15 min using acidified water with 7% acetic acid as solvent from CP. Results showed an improved extraction yields with the temperature until an inflexion point around 120 °C, reaching 30.4 μg/g CP (Punbusayakul et al., 2015). Given the limited reports of SWE focused on CP valorization, the study of both extraction efficiencies and biological activities compared to conventional solvent extractions failed to be achieved. However, the applicability of water under subcritical conditions had been widely investigated for other coffee by-products, especially for spent coffee grounds, where phenolic compounds were successfully extracted and tested as antioxidant compounds (Vandeponseele et al., 2020). Considering the green connotation of water together with the other previously mentioned advantages of SWE, it is strongly recommended to explore this technology for the valorization of CP in the near future.
3.2.2. Supercritical fluid extraction
Carbon dioxide is also considered green, non-toxic, and highly efficient solvent widely used as supercritical fluid, namely supercritical carbon dioxide (SC–CO2). SC-CO2 is a fluid state of carbon dioxide achieved by heating at or above its critical temperature (31.1 °C), and pressure (73.8 bar), featuring both liquid- and gaseous-like characteristics. The selectivity and efficiency of the extraction process can be easily tuned by modifying the temperature or pressure and time or solvent flow, respectively. Moreover, the operating conditions minimize the bioactive compounds degradation by avoiding light and air exposition, yielding higher quality extracts compared to other solvents and techniques (Vandeponseele et al., 2020). Unexpectedly, no studies about using SC-CO2 for CP's bioactive compounds extraction had been reported until date. However, it was successfully employed using coffee beans and other coffee by-products as raw material (e.g., spent coffee grounds and coffee husk), notably improving the recovery of antioxidant and antimicrobial bioactive compounds including caffeine, phenolic compounds, and sterols (Vandeponseele et al., 2020).
The combination of co-solvents (such as ethanol or ethyl lactate) with carbon dioxide at one phase supercritical state has been used to increase the polarity of the mixture and, in consequence, facilitating the extraction of polar molecules such as phenolic compounds (Coelho et al., 2020). For instance, the addition of ethanol promoted caffeine solubilization through intermolecular interactions such as hydrogen bonding, resulting in higher yields (de Marco et al., 2018). As far as we know, there is a single publication showing the results of using SC-CO2 with 10% t-butanol (v/v) at 15 MPa and 55 °C with the aim of esterifying chlorogenic acid from a CP aqueous extract but not pursuing bioactive compounds recovery (Hernandez et al., 2009). Therefore, considering the promising results obtained for coffee and other agro-industrial coffee by-products as raw materials for bioactive compounds recovery, as well as the feasibility of the industrial use of such technology as it is shown for coffee decaffeination, the use of neat carbon dioxide and its mixtures with food grade organic solvents at supercritical state must be explored.
4. Biochemical techniques: fermentation and enzyme-assisted extraction
Overall, the above-mentioned strategies can be gathered within chemical techniques group since the extraction efficiency and selectivity enhancement of bioactive compounds from CP is due to the chemical bonding and mass transfer improvement. However, fermentation and enzymatic treatment are also interesting biotechnological approaches (Fig. 2).
Fermentation involves the exploitation of microorganisms to consume substrates and accumulate desirable metabolites, which together with exogenous enzymes, are capable of breaking and weakening the cell walls, allowing the release of cell wall-linked and cellular compounds. Therefore, it was demonstrated that total phenolic compounds (12.2 mg GAE/g CP), flavonoids (5.5 mg catechin equivalents/g CP), anthocyanins (0.18 mg/g CP), chlorogenic acids (2.2 mg/g CP) and caffeine (2.4 mg/g CP, Fig. 3) from a CP hot water extract increased by 19.6%, 19.1%, 20.0%, 22.2%, 17.7%, respectively, after submitted to Viscozyme® L compared to non-treated samples (Patil et al., 2022). As expected, the type of enzymes and the solvent used for phytochemical recovery after treatment affected to the bioactive compound's recovery (Table 1). Indeed, a lower content of chlorogenic acid (0.71 mg/g) compared to Patil et al. (2022) data were obtained from an 80% methanol aqueous extraction of the enzyme-treated CP by using an enzymatic mixture from Aspergillus tamarii, Rhizomucor pusillus, and Trametes sp (Torres-Mancera et al., 2013).
The enhanced recovery of bioactive compounds showed by enzyme activity was also demonstrated by an Aspergillus tamarii solid-state fermentation, since the free hydroxycinnamic acids content from a CP-fermented sample extracted by aqueous methanol followed by alkaline hydrolysis, significantly increased by 134%, suggesting a reduction of the phenolic compounds-cell wall covalent bounds (Arellano-González et al., 2011). Likewise, actinomycetes solid-state fermentation (Streptomyces exfoliatus 42 and Streptomyces costaricanus 45I-3) resulted a useful tool for releasing anthocyanins (110 mg/mL) and catechin (10.4 mg/mL) from CP using 80% aqueous ethanol as recovery media, being around 1.1- and 2.5-fold higher than results observed for spontaneous fermentation (Kurniawati et al., 2016). Moreover, solid-state fermentation by Rodothula mucilaginosa CCMA 0156 could also liberate carotenoids (16.4 mg/L) when submitted to a CP extract (obtained by S-L KOH 0.06% (w/v) and sterilization process) using acetone-methanol solution (7:3, v/v) for compounds recovery (Moreira et al., 2018). Lactic acid bacteria Lactobacillus plantarum TISTR 543 (Myo et al., 2021) and Leuconostoc pseudomesenteroides (Oktaviani et al., 2020) were also verified to release bioactive compounds from CP liquid-state fermentation using water as fermentation solvent, and solid-state fermentation using ethanol as recovery solvents, respectively. In addition to microorganism species, fermentation conditions such as time, temperature, aeration, and fermentation media to CP ratio are the key factors controlling the extraction yield (Table 1). Thus, Myo et al. (2021) found that the time of lactic acid bacteria (Lactobacillus plantarum TISTR 543) liquid-state fermentation (24–72 h) significantly promoted the total phenolic compounds and protocatechuic acid content in the aqueous fermentation fluid. The highest phenolic content (3.3 mg GAE/g CP) was achieved for a 3: 10: 3 substrate: water: sugar ratio and a 5% of starter (Myo et al., 2021). These findings were in concordance with those reported by Oktaviani et al. (2020), who found that the lactic acid bacteria (Leuconostoc pseudomesenteroides) solid-state fermentation of CP at 4.2 h and 31.8 °C, positively contributed to the phenolic content (6.7%) using methanol as recovery solvent. As above showed, fermentation and enzymatic treatment could certainly enhance the bioactive compounds recovery from CP but, unfortunately no comparison studies focused on the extraction efficiency between different treatments can be done due to the dissimilar procedures, microorganism species, incubation conditions and recovery solvents utilized (Table 1).
The health-promoting properties of enzyme-treated and fermented CP fractions are mainly focused on their antioxidant and antimicrobial biological activities (Table 1). Thus, anthocyanins, chlorogenic acid, total phenolic and flavonoid content showed a high correlation with the FRAP value of a Viscozyme ® L-treated hot water extraction, which resulted in 155.7 μmol/g, a 1.2-fold higher than non-treated extract (130.6 μmol/g) (Patil et al., 2022). In addition, the significant higher release of hydroxycinnamic acids after CP fermentation mediated by A. tamarii was also pointed as responsible of the enhanced ABTS scavenging data compared to non-fermented CP (Arellano-González et al., 2011). Moreover, the mentioned carotenoids release from pretreated CP by Rodothula mucilaginosa CCMA 0156 fermentation showed relevant DPPH results, and of linoleic acid oxidative protection (Moreira et al., 2018). Regarding the antioxidant capacity of Lactobacillus plantarum TISTR 543 fermented CP, it seemed that fermentation within optimal time (24 h) increased DPPH and ABTS scavenging activities and FRAP values by 30.2%, 23.1%, and 31.9%, respectively compared to non-fermented material, whereas the prolonged fermentation time resulted in a reduction in antioxidant activities of the CP extracts (Myo et al., 2021). Such phenomenon relative to the effect of the fermentation time over the antioxidant bioactivity was also observed by Oktaviani et al. (2020), since DPPH scavenging capacity of L. pseudomesenteroides-fermented CP fraction increased with time up to 4.2 h, likewise for temperature being 31.8 °C the optimal value. They also reported a 15% and 30% higher antioxidant activity for simultaneous aeration of CP fermentation than that observed by spontaneous fermentation and fresh CP respectively. Moreover, antimicrobial activity was also studied focusing on the growth inhibition of pathogenic bacteria, including Salmonella colorless, Escherichia coli, Staphylococcus aureus and Listeria monocytogenes, at a low MIC value between 0.61 and 1.20 mg/mL, resulting more effective than synthetic β-carotene (Moreira et al., 2018). Overall, a possible explanation for such as bioactivity improvements compared to untreated CP samples is that microorganisms and enzymes serve as a catalyst to enhance biological activities by modifying, degrading, or synthesizing novel natural compounds at the expense of CP substrate, namely biotransformation.
Despite such higher demonstrated recoveries, the extraction of bioactive compounds from CP via fermentation and enzymatic treatments routinely involve the use of organic solvents (Table 1), lacking the sustainability character fulfilled by other extraction techniques. Important to note the pretreatment of the raw material eventually needed prior fermentation which implies larger times, solvent- and chemicals-consuming and the presence of undesirable compounds in the recovered fraction. In consequence, the combination of fermented and enzymatic treatment, and the use of emerging green solvents described above (i.e., DES) for bioactive compounds recovery may result a promising approach of biological and chemical extraction techniques for achieving further sustainable extraction system. Additionally, the use of physicochemical techniques such as ultrasound, microwave and PEF could also be explored using CP fermented or enzyme-treated fractions as liquid or solid enriched-bioactive materials, although operational limitations must be carefully considered.
5. Physicochemical techniques
5.1. Ultrasound-assisted extraction (UAE)
The high-power UAE, above 20 kHz frequencies, is based on the ultrasound propagation effects in a S-L media due to the generation of acoustic cavitation bubbles. Original microbubbles will grow thought coalescence to finally collapse at compression phase generating hot-pressurized spots up to 4726 °C and 100 MPa (Kumar et al., 2021). Such extreme conditions lead to fragmentation, erosion, capillarity, sonoporation, shear forces and detexturization individual or combined phenomena occurrence (Chemat et al., 2017). Thus, cavitation bubbles explosion generates shockwaves and accelerated molecular collision which results in a matrix structure rupture. Such fragmentation enhances compounds solubility, meaning mass transfer, because of smaller particle sizes and higher surface areas. Moreover, higher extraction yields are also attributed to i) the material erosion due to bubbles implosion, ii) the capillary formation allowing the water absorption and rehydration of the matrix, iii) the generation of membrane pores (sonoporation) and iv) the local shear forces and turbulences, facilitating the diffusion of bioactive compounds into the extraction solvent (Chemat et al., 2017; Kumar et al., 2021).
UAE is commonly applied to assist the organic solvent extraction of bioactive compounds from CP (Table 1). For instance, Chen et al. (2021) showed a notable recovery of total phenolic (23.2 mg GAE/g CP) and total flavonoid (25.1 mg quercetin/g CP) using 50% ethanol as UAE solvent at 30 °C for 15 min. The beneficial effect of UAE on bioactive compounds recovery was also supported by Myo and Khat-udomkiri (2022), who reported an increased phenolic and tannin content using propylene glycol (9.3 mg GAE/g CP and 8.7 mg tannic acid/g CP) and ethanol (7.5 mg GAE/g CP and 7.1 mg tannic acid/g CP), resulting in a 4.2–9.3% and 9.9–11.8% higher content respectively compared to conventional solvent extraction procedures using the same solvents. Moreover, an exhaustive study related to the influence of extraction time and ultrasound power parameters on the total phenolic compounds, flavonoids, chlorogenic acid and caffeine extraction yields by 50% ethanol UAE-assisted extraction was also carried out (Fig. 3), being 20.9, 18.8, 2.3 and 3.3 mg/g, respectively at 250 W and 60 °C for 35 min (Tran et al., 2022b). Regardless the intrinsic differences associated to each extraction procedure, the higher efficiencies reported for conventional methods using 50% ethanol compared to non-diluted solvent (see section 2.2.) were also found for UAE assisted extraction as above showed. Additionally, a promising combination of UAE and lactic acid: choline chloride (2:1 mol ratio) DES was also reported by Ruesgas-Ramón et al. (2020), indicating that the extraction capacity of chlorogenic acid and caffeine from CP was 8.3% and around 37.5% respectively higher for UAE-assisted extraction at 200 W for 3min compared to heat-stirring conditions.
Mentioned higher content of phenolic compounds and caffeine from CP at UAE-assisted versus conventional extractions was reflected in an enhanced antioxidant activity (Fig. 3). Thus, the DPPH and ABTS radical scavenging activities and FRAP showed by CP UAE-assisted propylene glycol extracts significantly increased by 34.3%, 21.3% and 10.1%, respectively, compared to conventional solvent extraction, showing additionally a potent NIH/3T3 fibroblasts protection from oxidative stress induced by hydrogen peroxide (Myo and Khat-udomkiri, 2022). As reported by Tran et al. (2022b), the extraction conditions also influenced on the antioxidant activities of UAE CP extracts since the temperature and power significantly improved their bioactive properties up to ABTS, DPPH and FRAP values of 57.7, 5.2, 35.9 mg TE/g CP at optimal extraction conditions (60 °C for 35 min at a ratio of sample to solvent 5:100 g/mL and power of 250 W) (Tran et al., 2022b). Furthermore, it seems that the influence of extraction factors on extraction efficiency is interactive rather than independent. Myo and Khat-udomkiri (2022) verified that the interaction of extraction time and solvent concentration was positively correlated to ABTS results, while the interaction of solid to liquid ratio and solvent concentration was positively correlated to FRAP findings. However, the characteristics of the raw material using in UAE are supposed to be further deliberated, since a highly viscous material is likely to minimize the cavitation effect. In addition, the input energy differs from the selected UAE equipment (e.g., bath and probe), which may provide inconsistent results. A final separating step (e.g., centrifugation or filtration) after extraction to isolate solubilized bioactive compounds into solvent from the UAE extraction residues is also a drawback to be considered.
5.2. Microwave-assisted extraction (MAE)
MAE is considered one of the most promising technology to reach high extraction efficiencies in short times and reduced solvent amounts. It involves the oscillation of 1 mm to 1 m microwave wavelengths at frequencies ranging from 0.3 GHz to 300 GHz capable to penetrate the matrix (Pinto et al., 2021). The absorbed energy is converted into thermal energy enhancing the diffusion of target compounds out the CP matrix, while the breakage of hydrogen bonds promotes the dissolution of these substances into the extraction solution. The friction and collisions between ions and dipoles provoke the intracellular water evaporation resulting into a high-pressure environment that eventually gives rise to cell wall disruption and, in consequence, a further enhancement of the extraction yields (Fig. 2).
MAE has been successfully applied to a wide variety of food matrices for bioactive compounds recovery, especially small phenolic compounds such as phenolic acids and flavonoids (Aires, 2017; Alara et al., 2021). Regarding CP, Macías-Garbett et al. (2022) demonstrated that the MAE of CP (Arabe variety) using water as extraction media, improved the total phenolic and flavonoid content by 52.8% and 10.8% respectively compared to MAE-untreated samples, reaching up 10.7 mg GAE/g CP for total phenolic content and 0.24 mg rutin equivalents/g CP for flavonoids. Similar trend with slight differences was observed for Peñasco CP variety (Table 1). However, the recovery of caffeic acid by MAE determined by HPLC-MS was significantly higher for Peñasco (0.60 mg GAE/g CP) than Arabe (0.23 mg GAE/g CP) variety, highlighting the relevance of detailing the raw material origin when reporting scientific results (Macías-Garbett et al., 2022). Furthermore, Tran et al. (2022a) demonstrated the positive influence of extraction time and MAE power on the extraction yield using the response surface methodology as an optimization technique. The optimized extraction yields for total phenolic compounds, flavonoids, chlorogenic acid and caffeine (38.7, 27.0, 7.0 and 5.5 mg/g CP, respectively) were obtained by radiation a sample to solvent ratio of 1 g/100 mL using 42.5% ethanol as solvent and under a microwave power of 1000 W for 85 min (Tran et al., 2022a). A close similarity of extraction yields corresponding to total phenolic and abovementioned bioactive compounds were reported, it was 46.7 mg/g CP for total phenolic compounds, 36.4 mg/g CP for flavonoids, 7.9 mg/g CP for chlorogenic acid, 5.7 mg/g CP for caffeine submitting a 50% ethanol-CP mixture (ratio 20: 1 mL/g) to a MAE power of 700 W for 70 min (Tran et al., 2022b) (Table 1). Certainly, the extraction solvent selected for MAE procedures was recognized to be exceptionally correlated to the extraction success (Fig. 3) however, further studies are needed to detail the influence of this independent variable on the extraction yields of target compounds.
Consistently to the results observed for the bioactive compounds recovery, the MAE power and extraction time were pointed as the main factors with notably influence on the biological activities of the extracts (Fig. 3). In general terms, radiation time (10–70 min) positively contributed to the antioxidant capacity, whereas opposite trend was observed for the radiation power (400–900 W), which might be related to a decreased phenolic and flavonoid content. Even so, the optimized ABTS, DPPH and FRAP results (i.e., 57.7, 5.2, 35.9 mg TE/g CP) were over 47%, 57% and 52% higher than those obtained by UAE (Tran et al., 2020a). However, the positive effect of both extraction time and MAE power on the mentioned antioxidants measurements was observed by Tran et al. (2022a), who also reported a significant interaction between radiation time and solid to solvent ratio, reaching 88.0, 9.3, and 65.3 mg TE/g CP at the above detailed optimal conditions (Tran et al., 2022a). As expected, the promoting effect of MAE on the antioxidant activities of CP extracts is also related to the coffee variety, which is in concordance with previous reported information relative to the extraction yield efficiencies for some bioactive compounds i.e., caffeic acid. Indeed, more desirable values for DPPH and ABTS scavenging abilities and FRAP were obtained for Peñasco compared to Arabe CP.
These encouraging results point MAE as an excellent candidate for the extraction of certain bioactive compounds from CP. However, the selection of the MAE solvent must simultaneously fulfill a double criterion, a high affinity for the target compounds while being compatible to MAE extraction mechanism (it is the absorption ability). Unfortunately, the extraction selectivity is eventually compromised since some solvents i.e., hexane, does not meet the above-mentioned criteria (Table 1). Nevertheless, the combination of extraction techniques is also a viable strategy to minimize mentioned drawbacks. For example, a green process using ultrasound-microwave extraction system was developed for the promising recovery of 15 phenolic compounds from CP using water as solvent (González-González et al., 2022). Additionally, other combinations such as by pulsed electric field treatment followed by MAE was also proved to be an efficient method for the recovering of antioxidant compounds from CP.
5.3. Pulsed electric field (PEF)-assisted extraction
PEF assisted extraction is carried out by applying intermittent short pulses (μs-ms) of low-intensity electric fields (0.5–5.0 kV/cm) on raw material, which modifies the cell membrane potential increasing its permeability and decreasing the selectivity for the entry of extracellular components (Bocker and Silva, 2022). Such membrane potential forms hydrophilic pores (electroporation) through which internal components are easily released, enhancing mass transfer of target compounds (Ranjha et al., 2021). To date, studies focused on the extraction of bioactive compounds from CP by PEF had not been reported. However, as previously mentioned, Macías-Garbett et al. (2022) developed a green extraction system by combining PEF-assisted extraction and MAE with the aim to explore the effect of PEF pretreatment on CP MAE fractions (Table 1). Results showed that total phenolic, flavonoid and caffeic acid content determined in MAE fractions were further increased after PEF pretreatment by 14.9–23.6%, 6.0–29.1%, and 23.1–30.0%, respectively, for both Peñasco and Arabe coffee varieties. Likewise, PEF pretreatment also improved the antioxidant activities of MAE extracts, which also varied depending on coffee varieties. Indeed, the DPPH results of fractions obtained by PEF-MAE treatment increased by 5.8% and 8.5% for Arabe and Peñasco CP varieties, respectively. Similarly, the ABTS results exhibited a 2.4% and 13.4% of increase for both mentioned CP varieties, whereas a slight decrease of FRAP were observed. Such reduced FRAP values were attributed to formation of phenolic compounds-based complexes (e.g., glycosylated molecules) blocking their binding sites needed to exert the scavenging activity (Macías-Garbett et al., 2022).
PEF could become a powerful green extraction process characterized by low time-, solvent- and energy-consumption yielding both highly efficient and selective extraction recoveries of target bioactive compounds (Fig. 2). The applicability of PEF is almost unexplored although one of the main advantages in terms of sustainability and circular bioeconomy is the straightforward use of agricultural by-products such as CP avoiding pretreatments like a grounding step. Nevertheless, the optimization of the extraction process must be performed by considering factors such as electric field strength, energy input, number and width of pulses, temperature, and time.
6. Applicability of current extraction techniques used for CP's bioactive compounds recovery
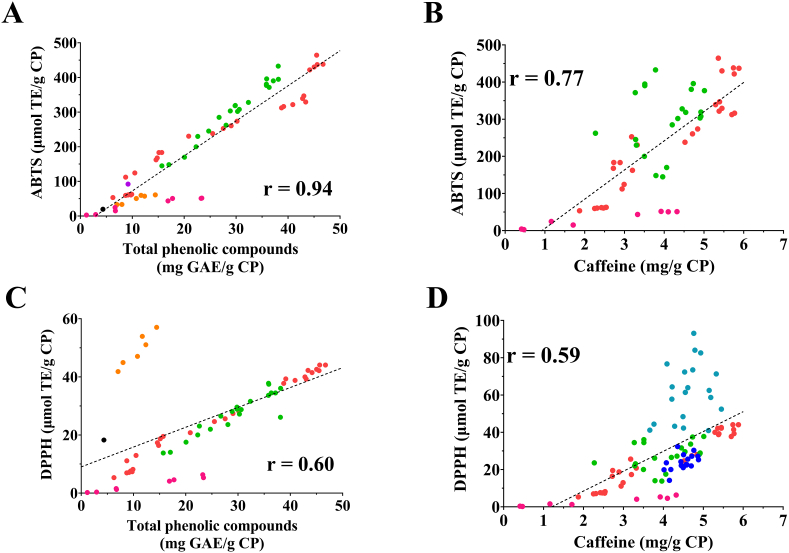
The CP valorization as a source of bioactive compounds is being supported by notable emerging experimental work. CP's phenolic compounds and caffeine content have attracted the scientific interest due to this by-product valorization as antioxidant source material. When gathering all results reported by food science researchers and regardless the extraction technique employed, a very strong correlation (r = 0.94, p < 0.05) was found between the content of phenolic compounds and its antioxidant activity determined by ABTS scavenging methodology in the obtained extracts, being moderate but not significant (r = 0.77, p > 0.05) for caffeine (Fig. 4A and B). However, no differences were found for total phenolic compounds and caffeine using DPPH antioxidant methodology, resulting in fair-moderate correlations, r = 0.60 (p < 0.01) and r = 0.59 (p < 0.05), respectively (Fig. 4C and D). Nevertheless, the dominance of ABTS over DPPH methodology when analyzing complex plant-based matrices (Floegel et al., 2011) reveals the undoubtedly suitability of CP as an excellent source of antioxidant compounds when making a conscious decision of the extraction techniques to be used.
Fig. 4.
Correlation of the most studied bioactive compounds from coffee pulp (CP) and their antioxidant activity, corresponding to total phenolic compounds (A) or caffeine (B)versus ABTS radical scavenging activity and total phenolic compounds (C) or caffeine (D)versus DPPH. Colored circles represent the study providing the data being, purple for Heeger et al. (2017), black for Myo et al. (2021), green for Tran et al. (2022a), red for Tran et al. (2022b), pink for Tran et al. (2020a), orange for Macías-Garbett et al. (2022), dark blue for Loukri et al. (2020) and light blue for Loukri et al. (2022). Results were normalized before plotting using the available data provided by authors therefore, studies missing the information needed for recalculation were excluded. Pearson's correlation coefficients (r) were obtained by SPSS v24 software (p = 0.02 for A, p = 0.05 for B, p = 0.005 for C and p = 0.04 for D). (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article).
Overall, the conventional solvent techniques for the extraction of bioactive compounds from CP are well-established and frequently referred to as standard methods in both research and industry fields (Alara et al., 2021). Using water is preferred as the safest conventional solvent extraction approach to obtain bioactive compounds-enriched fractions from CP (Table 1). However, limited extraction efficiency and selectivity together with the eventually depletion of bioactive compounds due to hydrolysis and oxidation during extraction, are the main drawbacks widely perceived in these processes. A more target extraction process (highly selective) is pursued when using organic solvents compared to aqueous extractions. However, the environmental risk generated by the consumption of massive amounts of organic solvents in the extraction process is not favorable to the nowadays pursued sustainability paradigm (Fig. 2). Although ethanol is considered a food grade solvent, other extraction media such as methanol or acetone are rejected within food science applications owing to their possible toxicity (Bondam et al., 2022). Regardless the solvent polarity, the efficiency of the conventional solvent systems is frequently enhanced by facilitating mass transfer from solid to liquid phases based on heating (e.g., hot water extraction) and stirring (e.g., heat-assisted stirring extraction). Nevertheless, the degradation of thermolabile compounds and the large energy consumption are also major disadvantages that need to be considered.
Emerging green solvents, which are affordable and low-toxic while allowing highly efficient and selective recovery processes, have become promising alternatives to conventional solvent extraction (Table 1). Furthermore, these green solvents can protect bioactive substances from deterioration or functionality loss, bringing huge benefits for the further use of high-sensitive compounds as food ingredients. Thus, DES is proposed to reduce the movement of phenolic compounds between molecules through hydrogen bindings and, in consequence, reducing its exposure to air and ultimately, reducing the oxidative degradation of the bioactive substances (Choi and Verpoorte, 2019). The intermolecular interactions induced by supramolecular solvents can also generate highly stable assemblies to protect such bioactive compounds from damage (Vandeponseele et al., 2020). DES and SUPRAS are promising solvents to achieve desirable physicochemical properties for the extraction of target compounds from CP by compiling the composition of the solvent. However, despite some exceptions, a subsequent treatment of the sample after the extraction must be considered to isolate the bioactive fraction from DES/SUPRASs depending on possible analytical interferences occurrence and the further extract applications i.e., for cell culturing assays. Moreover, strict conditions for their production define the purpose of such promising extraction strategies at industry. Nevertheless, we encourage to further investigate the extraction potential of such solvents for food science application since we deeply believe that most of the mentioned drawbacks will be overcome with scientific knowledge generation. The abovementioned final step for compounds recovery is avoided by using pressurized fluid extractions i.e., pressurized hot water and supercritical fluid extractions. They are considered as inexpensive and time-saving extraction techniques with from low solvent consumption to solvent-free character while keeping non-toxic and high selective and efficient extraction processes. Surprisingly, very few works focusing on the recovery of bioactive compounds using PFE can be found in the literature. The high selectivity rates that can be reached by using PFE, specially by supercritical fluid extraction, broaden the valorization strategies for CP. In addition, the high chance of further industrial implementation, e.g., the extraction of caffeine from CP using supercritical carbon dioxide, should be reason enough to further research.
Additionally, innovative green techniques such as UAE, MAE and PEF are well considered to be exceptionally promising for sustainable revalorization of CP due to their distinctive characteristics and robustness (Table 1). The predominant efficiency of these techniques basically involves the disruption of plant tissues (e.g., cell membranes and walls) and the breakdown of linked components (e.g., lignins and celluloses) induced by exogenous energy (Singh and Yadav, 2022). However, these techniques require sophisticated extraction equipment, such as ultrasonic probe and bath, and controlled condition fermenters, which renders the extraction process more technically challenging and costly. In addition, most of these extraction techniques are still at laboratory or pilot scale levels with insufficient technology maturity. As example, some commercial ultrasonic probe and bath systems may not be as efficient as in laboratory experiment (Mediani et al., 2022). Consequently, the adaptability of bioactive compounds extraction from CP on an industrialized scale, which is a further critical barrier for achieving commercial-scale application of these techniques, requires to be validated by extensive investigations. Similar mechanisms involved breakdown of tissues and linked components, have been achieved through the biochemical approaches using solid- and liquid-state fermentation and enzymatic treatment. The hydrolytic action induced by enzymes or fermenting microorganisms causes biodegradation of tissue-linked components, contributing to the prominent efficiency of bioactive compounds recovery from CP.
Although green techniques are initially selected because their sustainability, the genuine influence of a specific extraction technique must be carefully analyzed since, occasionally, the observed extraction efficiency enhancement is outweighed by simple solvent changes. As shown in Fig. 3, extraction performed using both organic solvents and DES is far more efficient for total phenolic compounds and caffeine recovery than aqueous UAE, MAE and enzymatic treatments. Moreover, the highest DPPH and ABTS scavenging activities (Fig. 3) were also obtained with DES and ethanol as extraction solvents. Therefore, the choice of solvent is normally the first parameter to be determined for extraction of bioactive compounds from CP. On the other hand, thermal process-assisted extraction tends to be more effective than non-thermal process-assisted extraction, demonstrated by the higher extraction yields and bioactivities observed for MAE, heat-assisted extraction and hot water extractions compared to those from UAE and enzymatic treatment (Fig. 2). Finally, following the available information and considering the advantages and drawbacks of these techniques, we recommend the sequential and/or simultaneous combination of green emerging solvent, physicochemical and biochemical techniques, as the most promising strategy for the valorization of CP directed to obtained bioactive compounds-enriched fractions for their further use at food, nutraceutical, or pharma industries. For instance, some of these combinations could be i) a pretreated-UAE CP easily fermented by microorganisms due to cell wall broking, ii) the sequential use of MAE followed by pressurized fluid extraction or, iii) DES and PFE simultaneous combination to achieve enhanced extraction yields and bioactivities.
7. Future perspectives
Unlike other coffee agroindustry by-products, CP has not been so well-explored until date while large amounts are yearly generated, and its composition is similar to coffee beans in qualitative terms. Further research is highly recommended to promote the valorization of CP as source of bioactive compounds for food science applications, for instance:
-
i)
The study of other relevant compounds, such as polysaccharides, peptides, and phospholipids, as these compounds had been isolated and characterized from other coffee by-products.
-
ii)
A wide-ranging evaluation of the CP bioactive potential by studying the so far unexplored properties such as antihyperglycaemic, antihypertensive, anticarcinogenic, neuroprotective, and prebiotic activities, at both in vitro and in vivo experimentation, as the effectiveness of coffee consumption in these diseases had been validated.
-
iii)
The exploration of green extraction techniques potentially suitable such as high hydrostatic pressure, high voltage fields, and nanofiltration, since these techniques had been successfully applied to other coffee by-products.
-
iv)
The use of more advanced features i.e., computational tools, to model the physicochemical properties of solvents and extraction techniques. In example, for the composition-dependent DES and SPURAS properties or the density and polarity prediction of pressurized liquids by varying temperature and pressure. These tools are conducive to a time-saving screening process and contributed to a selective extraction of target compounds in the future.
-
v)
A comprehensive study about the applicability of obtained antioxidant-enriched extracts from CP as ingredients to promote food safety and quality, providing more stability and shelf life by inhibiting the production of free radicals and the oxidation of nutrients in different food products due to presence of antioxidant compounds.
8. Conclusion
CP is an underutilized by-product of coffee industrial production highly rich in bioactive compounds i.e., phenolic compounds, caffeine, etc., serving as raw material for the obtention of bioactive compounds-enriched fractions. Indeed, it has been shown the notably health-promoting benefits of CP components including antioxidant, anti-inflammatory, anti-aging, antimicrobial and hepatoprotective activities among others. The global aim to develop sustainable and circular production systems has notably increased the investigation of CP valorization for food science applications. Although further research is high endorsed several environmentally friendly extraction strategies using DES, SUPRAS, subcritical water and SC-CO2 as solvents, have been pointed candidates to overcome the drawbacks relative to conventional solvent extraction methods i.e., the use of organic solvents, the high energy consumption, the polluting residues. Other physicochemical extraction techniques such as UAE, MAE or PEF-assisted extraction are alternatives to conventional methods due to their high sustainability, efficiency, safety, and reproducibility. Nevertheless, the sequential or simultaneous combination of several extraction techniques, as observed for MAE-PEF extraction system, allows to take advantage of their respective strengths to achieve the most suitable valorization strategy of coffee pulp. However, the further perspective of the scientific knowledge transferring to industry must be present when selecting an extraction technique so, other parameters such as easily handling or reproducible data acquisition must be also considered.
Funding sources
This work was supported by the Spanish State Plan for Scientific, Technical and Innovation Research (2021–2023) (PEICTI) within the framework of the Spanish Recovery, Transformation and Resilience Plan thought the call Strategic Projects Oriented to the Ecological Transition and to the Digital Transition (TED2021-129262A-I00). MAMC thanks the Excellence Line for University Teaching Staff within the Multiannual Agreement between the Community of Madrid and the UAM (2019–2023). SH is grateful to the China Scholarship Council (CSC) for awarding him with a pre-doctoral fellowship (CSC 202208360052) to develop his Ph.D. at UAM. MMT also thanks to the Investigo Program 2022 supported by the Spanish Recovery, Transformation and Resilience-NextgenerationEU.
CRediT authorship contribution statement
Shuai Hu: Writing – original draft, Writing – review & editing, Data gathering, Formal analysis, Figures, Conceptualization, All authors have read and agreed to the published version of the manuscript. Alicia Gil-Ramírez: Conceptualization, Supervision, Writing – review & editing, Project administration, Funding acquisition, All authors have read and agreed to the published version of the manuscript. María Martín-Trueba: Writing – review & editing, Data gathering, Formal analysis, All authors have read and agreed to the published version of the manuscript. Vanesa Benítez: Writing – review & editing, Project administration, Funding acquisition, All authors have read and agreed to the published version of the manuscript. Yolanda Aguilera: Writing – review & editing, All authors have read and agreed to the published version of the manuscript. María A. Martín-Cabrejas: Conceptualization, Supervision, Writing – review & editing, All authors have read and agreed to the published version of the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Handling Editor: Dr. Maria Corradini
Contributor Information
Alicia Gil-Ramírez, Email: alicia.gil@uam.es.
María A. Martín-Cabrejas, Email: maria.martin@uam.es.
Data availability
No data was used for the research described in the article.
References
- Aires A. Phenolic Compounds - Natural Sources. Importance and Applications; 2017. Phenolics in foods: extraction, analysis and measurements; pp. 61–88. [DOI] [Google Scholar]
- Alara O.R., Abdurahman N.H., Ukaegbu C.I. Extraction of phenolic compounds: a review. Curr. Res. Food Sci. 2021;4:200–214. doi: 10.1016/j.crfs.2021.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkaltham M.S., Salamatullah A., Hayat K. Determination of coffee fruit antioxidants cultivated in Saudi Arabia under different drying conditions. J. Food Meas. Char. 2020;14:1306–1313. doi: 10.1007/s11694-020-00378-4. [DOI] [Google Scholar]
- Arellano-González M.A., Ascención Ramírez-Coronel M., Torres-Mancera M.T., Pérez-Morales G.G., Saucedo-Castañeda G. Antioxidant activity of fermented and nonfermented coffee (Coffea arabica) pulp extracts. Food Technol. Biotechnol. 2011;49:374–378. https://hrcak.srce.hr/71068 [Google Scholar]
- Bhandarkar N.S., Mouatt P., Majzoub M.E., Thomas T., Brown L., Panchal S.K. Coffee pulp, a by-product of coffee production, modulates gut microbiota and improves metabolic syndrome in high-carbohydrate, high-fat diet-fed rats. Pathogens. 2021;10:1369. doi: 10.3390/pathogens10111369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocker R., Silva E.K. Pulsed electric field assisted extraction of natural food pigments and colorings from plant matrices. Food Chem. X. 2022;15 doi: 10.1016/j.fochx.2022.100398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondam A.F., Diolinda da Silveira D., Pozzada dos Santos J., Hoffmann J.F. Phenolic compounds from coffee by-products: extraction and application in the food and pharmaceutical industries. Trends Food Sci. Technol. 2022;123:172–186. doi: 10.1016/j.tifs.2022.03.013. [DOI] [Google Scholar]
- Boonphang O., Ontawong A., Pasachan T., Phatsara M., Duangjai A., Amornlerdpison D., Jinakote M., Srimaroeng C. Antidiabetic and renoprotective effects of Coffea arabica pulp aqueous extract through preserving organic cation transport system mediated oxidative stress pathway in experimental type 2 diabetic rats. Molecules. 2021;26:1907. doi: 10.3390/molecules26071907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck N., Wohlt D., Winter A.R., Ortner E. Aroma-active compounds in Robusta coffee pulp puree—evaluation of physicochemical and sensory properties. Molecules. 2021;26:3925. doi: 10.3390/molecules26133925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cañas S., Rebollo-Hernanz M., Cano-Muñoz P., Aguilera Y., Benítez V., Braojos C., Gila-Díaz A., Rodríguez-Rodríguez P., Cobeta I.M., Pablo Á.L.L. de, González M., del C., Arribas S.M., Martin-Cabrejas M.A. Critical evaluation of coffee pulp as an innovative antioxidant dietary fiber ingredient: nutritional value, functional properties, and acute and sub-chronic toxicity. Proceedings. 2021;70:65. doi: 10.3390/foods_2020-07623. [DOI] [Google Scholar]
- Cañas S., Rebollo-Hernanz M., Braojos C., Benítez V., Ferreras-Charro R., Dueñas M., Aguilera Y., Martín-Cabrejas M.A. Understanding the gastrointestinal behavior of the coffee pulp phenolic compounds under simulated conditions. Antioxidants. 2022;11:1818. doi: 10.3390/antiox11091818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmen M.T., Lorena Z.C., Alexander V.A., Amandio V., Raúl S. Coffee pulp: an industrial by-product with uses in agriculture, nutrition and biotechnology. Rev. Agric. Sci. 2020;8:323–342. doi: 10.7831/ras.8.0_323. [DOI] [Google Scholar]
- Chemat F., Rombaut N., Sicaire A.G., Meullemiestre A., Fabiano-Tixier A.S., Abert-Vian M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017;34:540–560. doi: 10.1016/j.ultsonch.2016.06.035. [DOI] [PubMed] [Google Scholar]
- Chen C.Y., Shih C.H., Lin T.C., Zheng J.H., Hsu C.C., Chen K.M., Lin Y.S., Wu C.T. Antioxidation and tyrosinase inhibitory ability of coffee pulp extract by ethanol. J. Chem. 2021 doi: 10.1155/2021/8649618. 2021. [DOI] [Google Scholar]
- Choi Y.H., Verpoorte R. Green solvents for the extraction of bioactive compounds from natural products using ionic liquids and deep eutectic solvents. Curr. Opin. Food Sci. 2019;26:87–93. doi: 10.1016/j.cofs.2019.04.003. [DOI] [Google Scholar]
- Coelho J.P., Filipe R.M., Paula Robalo M., Boyadzhieva S., Cholakov G.S., Stateva R.P. Supercritical CO2 extraction of spent coffee grounds. Influence of co-solvents and characterization of the extracts. J. Supercrit. Fluids. 2020;161 doi: 10.1016/j.supflu.2020.104825. [DOI] [Google Scholar]
- Contreras-Oliva A., Uscanga-Sosa D.P., González-Rios O., Morales-Ramos V. The use of coffee (Coffea arabica L.) pulp in the preparation of a beverage with antioxidant properties. Int. Food Res. J. 2022;29:274–282. doi: 10.47836/ifrj.29.2.06. [DOI] [Google Scholar]
- de Marco I., Riemma S., Iannone R. Life cycle assessment of supercritical CO2 extraction of caffeine from coffee beans. J. Supercrit. Fluids. 2018;133:393–400. doi: 10.1016/j.supflu.2017.11.005. [DOI] [Google Scholar]
- Delgado S.R., Arbelaez A.F.A., Rojano B. Antioxidant capacity, bioactive compounds in coffee pulp and implementation in the production of infusions. Acta Sci. Pol. Technol. Aliment. 2019;18:235–248. doi: 10.17306/J.AFS.2019.0663. [DOI] [PubMed] [Google Scholar]
- Duangjai A., Suphrom N., Wungrath J., Ontawong A., Nuengchamnong N., Yosboonruang A. Comparison of antioxidant, antimicrobial activities and chemical profiles of three coffee (Coffea arabica L.) pulp aqueous extracts. Integr. Med. Res. 2016;5:324–331. doi: 10.1016/j.imr.2016.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durán-Aranguren D.D., Robledo S., Gomez-Restrepo E., Valencia J.W.A., Tarazona N.A. Scientometric overview of coffee by-products and their applications. Molecules. 2021;26:7605. doi: 10.3390/molecules26247605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquivel P., Viñas M., Steingass C.B., Gruschwitz M., Guevara E., Carle R., Schweiggert R.M., Jiménez V.M. Coffee (Coffea arabica L.) by-products as a source of carotenoids and phenolic compounds—evaluation of varieties with different peel color. Front. Sustain. Food Syst. 2020;4 doi: 10.3389/fsufs.2020.590597. [DOI] [Google Scholar]
- European Food Safety Authority Technical report on the notification of cherry pulp from Coffea arabica L. and Coffea canephora Pierre ex A. Froehner as a traditional. 2021. food from a third country following Article 14 of Regulation (EU) 2015/2283. ESFA support. publ. 18. [DOI]
- European Food Safety Authority Technical report on the notification of dried cherry pulp from Coffea arabica L. and Coffea canephora Pierre ex A. Froehner as a traditional food from a third country pursuant to Article 14 of Regulation. 2021. (EU) 2015/2283. ESFA support. publ. 18. [DOI]
- FAOSTAT . FAO; 2022. Statistics Division. Crop Statistic of Green Coffee.https://www.fao.org/faostat/en/#data/QCL [Google Scholar]
- Floegel A., Kim D.O., Chung S.J., Koo S.I., Chun O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011;24:1043–1048. doi: 10.1016/j.jfca.2011.01.008. [DOI] [Google Scholar]
- Florindo C., Lima F., Ribeiro B.D., Marrucho I.M. Deep eutectic solvents: overcoming 21st century challenges. Curr. Opin. Green Sustain. Chem. 2019;18:31–36. doi: 10.1016/j.cogsc.2018.12.003. [DOI] [Google Scholar]
- Gemechu F.G. Embracing nutritional qualities, biological activities and technological properties of coffee byproducts in functional food formulation. Trends Food Sci. Technol. 2020;104:235–261. doi: 10.1016/j.tifs.2020.08.005. [DOI] [Google Scholar]
- González-González G.M., Palomo-Ligas L., Nery-Flores S.D., Ascacio-Valdés J.A., Sáenz-Galindo A., Flores-Gallegos A.C., Zakaria Z.A., Aguilar C.N., Rodríguez-Herrera R. Coffee pulp as a source for polyphenols extraction using ultrasound, microwave, and green solvents. Environ. Qual. Manag. 2022;32:451–461. doi: 10.1002/tqem.21903. [DOI] [Google Scholar]
- Gurram R., Al-Shannag M., Knapp S., Das T., Singsaas E., Alkasrawi M. Technical possibilities of bioethanol production from coffee pulp: a renewable feedstock. Clean Technol. Environ. Policy. 2016;18:269–278. doi: 10.1007/s10098-015-1015-9. [DOI] [Google Scholar]
- Heeger A., Kosińska-Cagnazzo A., Cantergiani E., Andlauer W. Bioactives of coffee cherry pulp and its utilisation for production of Cascara beverage. Food Chem. 2017;221:969–975. doi: 10.1016/j.foodchem.2016.11.067. [DOI] [PubMed] [Google Scholar]
- Hernandez C.E., Chen H.H., Chang C.I., Huang T.C. Direct lipase-catalyzed lipophilization of chlorogenic acid from coffee pulp in supercritical carbon dioxide. Ind. Crop. Prod. 2009;30:359–365. doi: 10.1016/j.indcrop.2009.07.004. [DOI] [Google Scholar]
- International Coffee Organization World coffee consumption. 2020. https://www.ico.org/prices/new-consumption-table.pdf ICO.
- Keddar M.N., Ballesteros-Gómez A., Amiali M., Siles J.A., Zerrouki D., Martín M.A., Rubio S. Efficient extraction of hydrophilic and lipophilic antioxidants from microalgae with supramolecular solvents. Separ. Purif. Technol. 2020;251 doi: 10.1016/j.seppur.2020.117327. [DOI] [Google Scholar]
- Khochapong W., Ketnawa S., Ogawa Y., Punbusayakul N. Effect of in vitro digestion on bioactive compounds, antioxidant and antimicrobial activities of coffee (Coffea arabica L.) pulp aqueous extract. Food Chem. 2021;348 doi: 10.1016/j.foodchem.2021.129094. [DOI] [PubMed] [Google Scholar]
- King J.W. Modern supercritical fluid technology for food applications. Annu. Rev. Food Sci. Technol. 2014;5:215–238. doi: 10.1146/annurev-food-030713-092447. [DOI] [PubMed] [Google Scholar]
- Klingel T., Kremer J.I., Gottstein V., de Rezende T.R., Schwarz S., Lachenmeier D.W. A review of coffee by-products including leaf, flower, cherry, husk, silver skin, and spent grounds as novel foods within the European Union. Foods. 2020;9:665. doi: 10.3390/foods9050665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar K., Srivastav S., Sharanagat V.S. Ultrasound assisted extraction (UAE) of bioactive compounds from fruit and vegetable processing by-products: a review. Ultrason. Sonochem. 2021;70 doi: 10.1016/j.ultsonch.2020.105325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurniawati N., Meryandini A., Sunarti T.C. Introduction of actinomycetes starter on coffee fruits fermentation to enhance quality of coffee pulp. Emir. J. Food Agric. 2016;28:188–195. doi: 10.9755/ejfa.2015-05-192. [DOI] [Google Scholar]
- Lachenmeier D.W., Rajcic de Rezende T., Schwarz S. An update on sustainable valorization of coffee by-products as novel foods within the European Union. Biol. Life Sci. Forum. 2022;6:37. doi: 10.3390/foods2021-10969. [DOI] [Google Scholar]
- Lonati E., Carrozzini T., Bruni I., Mena P., Botto L., Cazzaniga E., del Rio D., Labra M., Palestini P., Bulbarelli A. Coffee-derived phenolic compounds activate Nrf2 antioxidant pathway in I/R injury in vitro model: a nutritional approach preventing age related-damages. Molecules. 2022;27:1049. doi: 10.3390/molecules27031049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loukri A., Tsitlakidou P., Goula A., Assimopoulou A.N., Kontogiannopoulos K.N., Mourtzinos I. Green extracts from coffee pulp and their application in the development of innovative brews. Appl. Sci. 2020;10:1–13. doi: 10.3390/app10196982. [DOI] [Google Scholar]
- Loukri A., Sarafera C., Goula A.M., Gardikis K., Mourtzinos I. Green extraction of caffeine from coffee pulp using a deep eutectic solvent (DES) Appl. Food Res. 2022;2 doi: 10.1016/j.afres.2022.100176. [DOI] [Google Scholar]
- Ma J., Li J., He H., Jin X., Cesarino I., Zeng W., Li Z. Characterization of sensory properties of Yunnan coffee. Curr. Res. Food Sci. 2022;5:1205–1215. doi: 10.1016/j.crfs.2022.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macías-Garbett R., Sosa-Hernández J.E., Iqbal H.M.N., Contreras-Esquivel J.C., Chen W.N., Melchor-Martínez E.M., Parra-Saldívar R. Combined pulsed electric field and microwave-assisted extraction as a green method for the recovery of antioxidant compounds with electroactive potential from coffee agro-waste. Plants. 2022;11:2362. doi: 10.3390/plants11182362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magoni C., Bruni I., Guzzetti L., Dell'Agli M., Sangiovanni E., Piazza S., Regonesi M.E., Maldini M., Spezzano R., Caruso D., Labra M. Valorizing coffee pulp by-products as anti-inflammatory ingredient of food supplements acting on IL-8 release. Food Res. Int. 2018;112:129–135. doi: 10.1016/j.foodres.2018.06.026. [DOI] [PubMed] [Google Scholar]
- Manasa V., Padmanabhan A., Anu Appaiah K.A. Utilization of coffee pulp waste for rapid recovery of pectin and polyphenols for sustainable material recycle. Waste Manage. (Tucson, Ariz.) 2021;120:762–771. doi: 10.1016/j.wasman.2020.10.045. [DOI] [PubMed] [Google Scholar]
- Maxiselly Y., Chiarawipa R., Somnuk K., Hamchara P., Cherdthong A., Suntara C., Prachumchai R., Chanjula P. Digestibility, blood parameters, rumen fermentation, hematology, and nitrogen balance of goats after receiving supplemental coffee cherry pulp as a source of phytochemical nutrients. Vet. Sci. 2022;9:532. doi: 10.3390/vetsci9100532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mbous Y.P., Hayyan M., Hayyan A., Wong W.F., Hashim M.A., Looi C.Y. Applications of deep eutectic solvents in biotechnology and bioengineering—Promises and challenges. Biotechnol. Adv. 2017;35:105–134. doi: 10.1016/j.biotechadv.2016.11.006. [DOI] [PubMed] [Google Scholar]
- Mediani A., Kamal N., Lee S.Y., Abas F., Farag M.A. Green extraction methods for isolation of bioactive substances from coffee seed and spent. Separ. Purif. Rev. 2022:1–19. doi: 10.1080/15422119.2022.2027444. [DOI] [Google Scholar]
- Moreira M.D., Melo M.M., Coimbra J.M., Reis K.C., dos Schwan R.F., Silva C.F. Solid coffee waste as alternative to produce carotenoids with antioxidant and antimicrobial activities. Waste Manage. (Tucson, Ariz.) 2018;82:93–99. doi: 10.1016/j.wasman.2018.10.017. [DOI] [PubMed] [Google Scholar]
- Moreno J., Cozzano S., Mercedes Pérez A., Arcia P., Curutchet A. Coffee pulp waste as a functional ingredient: effect on salty cookies quality. J. Food Nutr. Res. 2019;7:632–638. doi: 10.12691/jfnr-7-9-2. [DOI] [Google Scholar]
- Musarurwa H., Tavengwa N.T. Supramolecular solvent-based micro-extraction of pesticides in food and environmental samples. Talanta. 2021;223 doi: 10.1016/j.talanta.2020.121515. [DOI] [PubMed] [Google Scholar]
- Mustafa A., Turner C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: a review. Anal. Chim. Acta. 2011;703:8–18. doi: 10.1016/j.aca.2011.07.018. [DOI] [PubMed] [Google Scholar]
- Myo H., Nantarat N., Khat-Udomkiri N. Changes in bioactive compounds of coffee pulp through fermentation-based biotransformation using Lactobacillus plantarum TISTR 543 and its antioxidant activities. Fermentation. 2021;7:292. doi: 10.3390/fermentation7040292. [DOI] [Google Scholar]
- Myo H., Khat-udomkiri N. Optimization of ultrasound-assisted extraction of bioactive compounds from coffee pulp using propylene glycol as a solvent and their antioxidant activities. Ultrason. Sonochem. 2022;89 doi: 10.1016/j.ultsonch.2022.106127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Núñez Pérez J., Chávez Arias B.S., de la Vega Quintero J.C., Zárate Baca S., Pais-Chanfrau J.M. Multi-objective statistical optimization of pectinolytic enzymes production by an Aspergillus sp. on dehydrated coffee residues in solid-state fermentation. Fermentation. 2022;8:170. doi: 10.3390/fermentation8040170. [DOI] [Google Scholar]
- Oktaviani L., Astuti D.I., Rosmiati M., Abduh M.Y. Fermentation of coffee pulp using indigenous lactic acid bacteria with simultaneous aeration to produce cascara with a high antioxidant activity. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e04462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ontawong A., Boonphang O., Pasachan T., Duangjai A., Pongchaidecha A., Phatsara M., Jinakote M., Amornlerdpison D., Srimaroeng C. Hepatoprotective effect of coffee pulp aqueous extract combined with simvastatin against hepatic steatosis in high-fat diet-induced obese rats. J. Funct.Foods. 2019;54:568–577. doi: 10.1016/j.jff.2019.02.011. [DOI] [Google Scholar]
- Ontawong A., Duangjai A., Muanprasat C., Pasachan T., Pongchaidecha A., Amornlerdpison D., Srimaroeng C. Lipid-lowering effects of Coffea arabica pulp aqueous extract in Caco-2 cells and hypercholesterolemic rats. Phytomedicine. 2019;52:187–197. doi: 10.1016/j.phymed.2018.06.021. [DOI] [PubMed] [Google Scholar]
- Ontawong A., Pasachan T., Trisuwan K., Soodvilai S., Duangjai A., Pongchaidecha A., Amornlerdpison D., Srimaroeng C. Coffea arabica pulp aqueous extract attenuates oxidative stress and hepatic lipid accumulation in HepG2 cells. J. Herb. Med. 2021;29 doi: 10.1016/j.hermed.2021.100465. [DOI] [Google Scholar]
- Patil S., Pimpley V., Warudkar K., Murthy P.S. Valorisation of coffee pulp for development of innovative probiotic beverage using Kefir: physicochemical, antioxidant, sensory analysis and shelf life studies. Waste Biomass Valorization. 2022;13:905–916. doi: 10.1007/s12649-021-01554-3. [DOI] [Google Scholar]
- Pinto D., Silva A.M., Freitas V., Vallverdú-Queralt A., Delerue-Matos C., Rodrigues F. Microwave-assisted extraction as a green technology approach to recover polyphenols from Castanea sativa shells. ACS Food Sci. Technol. 2021;1:229–241. doi: 10.1021/acsfoodscitech.0c00055. [DOI] [Google Scholar]
- Pua A., Choo W.X.D., Goh R.M.V., Liu S.Q., Cornuz M., Ee K.H., Sun J., Lassabliere B., Yu B. A systematic study of key odourants, non-volatile compounds, and antioxidant capacity of cascara (dried Coffea arabica pulp) LWT (Lebensm.-Wiss. & Technol.) 2021;138 doi: 10.1016/j.lwt.2020.110630. [DOI] [Google Scholar]
- Punbusayakul N., Saim N., Abdul Haiyee Z., Sarnkhuankaew W., Osman R. Subcritical water extraction (SWE) of anthocyanin from Arabica coffee pulp. Sci. Lett. 2015;8:34–40. doi: 10.2174/22102892013040100176. [DOI] [Google Scholar]
- Ranjha M.M.A.N., Kanwal R., Shafique B., Arshad R.N., Irfan S., Kieliszek M., Kowalczewski P.Ł., Irfan M., Khalid M.Z., Roobab U., Aadil R.M. A critical review on pulsed electric field: a novel technology for the extraction of phytoconstituents. Molecules. 2021;26:4893. doi: 10.3390/molecules26164893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastegar A., Alahabadi A., Esrafili A., Rezai Z., Hosseini-Bandegharaei A., Nazari S. Application of supramolecular solvent-based dispersive liquid-liquid microextraction for trace monitoring of lead in food samples. Anal. Methods. 2016;8:5533–5539. doi: 10.1039/c6ay01463a. [DOI] [Google Scholar]
- Reichembach L.H., de Oliveira Petkowicz C.L. Extraction and characterization of a pectin from coffee (Coffea arabica L.) pulp with gelling properties. Carbohydr. Polym. 2020;245 doi: 10.1016/j.carbpol.2020.116473. [DOI] [PubMed] [Google Scholar]
- Rodríguez Frómeta R.A., Sánchez J.L., Ros García J.M. Evaluation of coffee pulp as substrate for polygalacturonase production in solid state fermentation. Emir. J. Food Agric. 2020;32:117–124. doi: 10.9755/ejfa.2020.v32.i2.2068. [DOI] [Google Scholar]
- Rodríguez-Durán L.v., Ramírez-Coronel M.A., Aranda-Delgado E., Nampoothiri K.M., Favela-Torres E., Aguilar C.N., Saucedo-Castañeda G. Soluble and bound hydroxycinnamates in coffee pulp (Coffea arabica) from seven cultivars at three ripening stages. J. Agric. Food Chem. 2014;62:7869–7876. doi: 10.1021/jf5014956. [DOI] [PubMed] [Google Scholar]
- Rosas-Sánchez G.A., Hernández-Estrada Z.J., Suárez-Quiroz M.L., González-Ríos O., Rayas-Duarte P. Coffee cherry pulp by-product as a potential fiber source for bread production: a fundamental and empirical rheological approach. Foods. 2021;10:742. doi: 10.3390/foods10040742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruesgas-Ramón M., Figueroa-Espinoza M.C., Durand E., Suárez-Quiroz M.L., González-Ríos O., Rocher A., Reversat G., Vercauteren J., Oger C., Galano J.M., Durand T., Vigor C. Identification and quantification of phytoprostanes and phytofurans of coffee and cocoa by- and co-products. Food Funct. 2019;10:6882–6891. doi: 10.1039/c9fo01528k. [DOI] [PubMed] [Google Scholar]
- Ruesgas-Ramón M., Suárez-Quiroz M.L., González-Ríos O., Baréa B., Cazals G., Figueroa-Espinoza M.C., Durand E. Biomolecules extraction from coffee and cocoa by- and co-products using deep eutectic solvents. J. Sci. Food Agric. 2020;100:81–91. doi: 10.1002/jsfa.9996. [DOI] [PubMed] [Google Scholar]
- Saini A., Kumar A., Panesar P.S., Thakur A. Potential of deep eutectic solvents in the extraction of value‐added compounds from agro‐industrial by‐products. Appl. Food Res. 2022;2 doi: 10.1016/j.afres.2022.100211. [DOI] [Google Scholar]
- Sangta J., Wongkaew M., Tangpao T., Withee P., Haituk S., Arjin C., Sringarm K., Hongsibsong S., Sutan K., Pusadee T., Sommano S.R., Cheewangkoon R. Recovery of polyphenolic fraction from arabica coffee pulp and its antifungal applications. Plants. 2021;10:1422. doi: 10.3390/plants10071422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N., Yadav S.S. A review on health benefits of phenolics derived from dietary spices. Curr. Res. Food Sci. 2022;5:1508–1523. doi: 10.1016/j.crfs.2022.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Mancera M.T., Baqueiro-Peña I., Figueroa-Montero A., Rodríguez-Serrano G., González-Zamora E., Favela-Torres E., Saucedo-Castañeda G. Biotransformation and improved enzymatic extraction of chlorogenic acid from coffee pulp by filamentous fungi. Biotechnol. Prog. 2013;29:337–345. doi: 10.1002/btpr.1696. [DOI] [PubMed] [Google Scholar]
- Torres-Valenzuela L.S., Ballesteros-Gómez A., Rubio S. Supramolecular solvent extraction of bioactives from coffee cherry pulp. J. Food Eng. 2020;278 doi: 10.1016/j.jfoodeng.2020.109933. [DOI] [Google Scholar]
- Tran T.M.K., Akanbi T., Kirkman T., Nguyen M.H., Vuong Q. van. Optimal aqueous extraction conditions as a green technique for recovery of phenolic antioxidants from Robusta dried coffee pulp. Eur. J. Eng. Technol. Res. 2020;5:1069–1074. doi: 10.24018/ejers.2020.5.9.2116. [DOI] [Google Scholar]
- Tran T.M.K., Kirkman T., Nguyen M., van Vuong Q. Effects of drying on physical properties, phenolic compounds and antioxidant capacity of Robusta wet coffee pulp (Coffea canephora) Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e04498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran T.M.K., Akanbi T., Kirkman T., Nguyen M., Vuong Q. van. Maximising recovery of bioactive compounds from coffee pulp waste using microwave-assisted extraction. Eur. J. Eng. Technol. Res. 2022;7:2812. doi: 10.24018/ejeng.2022.7.2.2812. [DOI] [Google Scholar]
- Tran T.M.K., Akanbi T.O., Kirkman T., Nguyen M.H., Vuong Q. van. Recovery of phenolic compounds and antioxidants from coffee pulp (Coffea canephora) waste using ultrasound and microwave-assisted extraction. Processes. 2022;10:1011. doi: 10.3390/pr10051011. [DOI] [Google Scholar]
- Tseng Y.P., Liu C., Chan L.P., Liang C.H. Coffee pulp supplement affects antioxidant status and favors anti-aging of skin in healthy subjects. J. Cosmet. Dermatol. 2022;21:2189–2199. doi: 10.1111/jocd.14341. [DOI] [PubMed] [Google Scholar]
- Vandeponseele A., Draye M., Piot C., Chatel G. Subcritical water and supercritical carbon dioxide: efficient and selective eco-compatible solvents for coffee and coffee by-products valorization. Green Chem. 2020;22:8544–8571. doi: 10.1039/d0gc03146a. [DOI] [Google Scholar]
- Wong-Paz J.E., Guyot S., Aguilar-Zárate P., Muñiz-Márquez D.B., Contreras-Esquivel J.C., Aguilar C.N. Structural characterization of native and oxidized procyanidins (condensed tannins) from coffee pulp (Coffea arabica) using phloroglucinolysis and thioglycolysis-HPLC-ESI-MS. Food Chem. 2021;340 doi: 10.1016/j.foodchem.2020.127830. [DOI] [PubMed] [Google Scholar]
- Zhang Q.W., Lin L.G., Ye W.C. Techniques for extraction and isolation of natural products: a comprehensive review. Chin. Med. 2018;13:1–26. doi: 10.1186/s13020-018-0177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.