INTRODUCTION
Dyspnea is the subjective experience of breathing discomfort and affects the quality of life in patients with serious, life-limiting illness.1 2 Dyspnoea is also one of the most distressing symptoms that necessitates visits to the emergency department (ED) in the last six months of life, and it is increasingly ranked the highest in the last two weeks of life.1 In addition to disease-oriented treatments, an adjunct opioid-based treatment for palliation of dyspnoea improves quality of life and functions significantly in patients with advanced respiratory illnesses.1 2 Despite the strong clinical evidence and many national organisations recommending the use of systemic opioids (grade 1B) as an adjunct therapy for relieving dyspnoea in patients with advanced terminal illnesses and refractory dyspnoea at the end of life,2 the utilisation by emergency clinicians is unknown. This study aims to determine the acceptability of this protocol by emergency clinicians and to gain information to improve the protocol implementation.
METHODS
Due to the pressing need to relieve dyspnea with COVID-19, the palliative symptom management experts in our institution reviewed the current evidence and designed the protocol for acute dyspnoea in the ED, which provided the recommended prescription for all patients who experience any severity of dyspnoea (online supplemental file 1). This study took place in the ED of an academic medical centre. The participants included attending physicians, resident physicians and physician assistants, who provided initial care for patients with moderate to severe dyspnoea in the ED. This included patients ≥18 years old with shortness of breath or a respiratory rate >20/min, and at least one of the following clinical signs of dyspnoea: agitation, lethargy, use of accessory muscles, heart rate >120/min, oxygen saturation in room air <90%. Patients who were intubated or in the setting of a traumatic injury were not eligible. Emergency clinicians were recruited consecutively between September and October 2021. A trained researcher who was also an emergency physician identified patients in the triage area who met the criteria and then reached out to the responding emergency clinicians. When clinically appropriate, the researcher handed over the protocol to remind the participants of the protocol’s existence, asked for the consent and then asked to complete our electronic acceptability surveys within seven days of the clinical encounter. The survey consisted of clinicians’ self-reports of the use of the palliative dyspnoea protocol in corresponding patients, general clinical indications for using and not using, and suggestions for improvement.
The primary outcome was the acceptability rated on a 5-point Likert scale. Acceptability was prespecified as a combination of ‘very helpful’ and ‘somewhat helpful’. We hypothesized that the protocol is more than 50% acceptable for use by practicing emergency clinicians. The secondary outcome was clinical assessment of dyspnoea severity, the Respiratory Distress Observation Scale (RDOS),3 at triage and 2–4 hours after. The descriptive statistics and chi-squared test were used to illustrate the demographics. Two-tailed Wilcoxon matched-pairs signed-rank test was used to compare the severity of dyspnoea. Logistic regression analysis was used to determine associations between the variables. All levels of significance were set at p<0.05.
RESULTS
The acceptability survey was completed by 35 of 46 emergency clinicians (76% response rate). The majority of the participants were female (54%), 30–39 years old (49%), attending physicians (40%) and had less than six years of clinical experience (54%) (online supplemental file 2). The participants rated the protocol as 43% acceptable whereas 57% ‘neutral’. No participant rated ‘somewhat unhelpful’ or ‘very unhelpful’. The most common reasons for using the palliative dyspnoea protocol were end of life (86%) and respiratory failure from advanced malignancy (86%)(online supplemental file 3) The most cited concern for avoiding the protocol was opioid side effects, while the least was opioid dependency (figure 1). The most suggested improvements were clarity of indication (51%), application of the dyspnea assessment scale (29%), and the clarity of the treatment end point (23%) online supplemental file 4. The compared RDOS at triage and follow-up were shown in online supplemental file 5. Among the participants, four clinicians (11%) actually used the protocol and prescribed adjunct opioid treatment for dyspnoea relief, as shown in online supplemental file 6.
Figure 1.
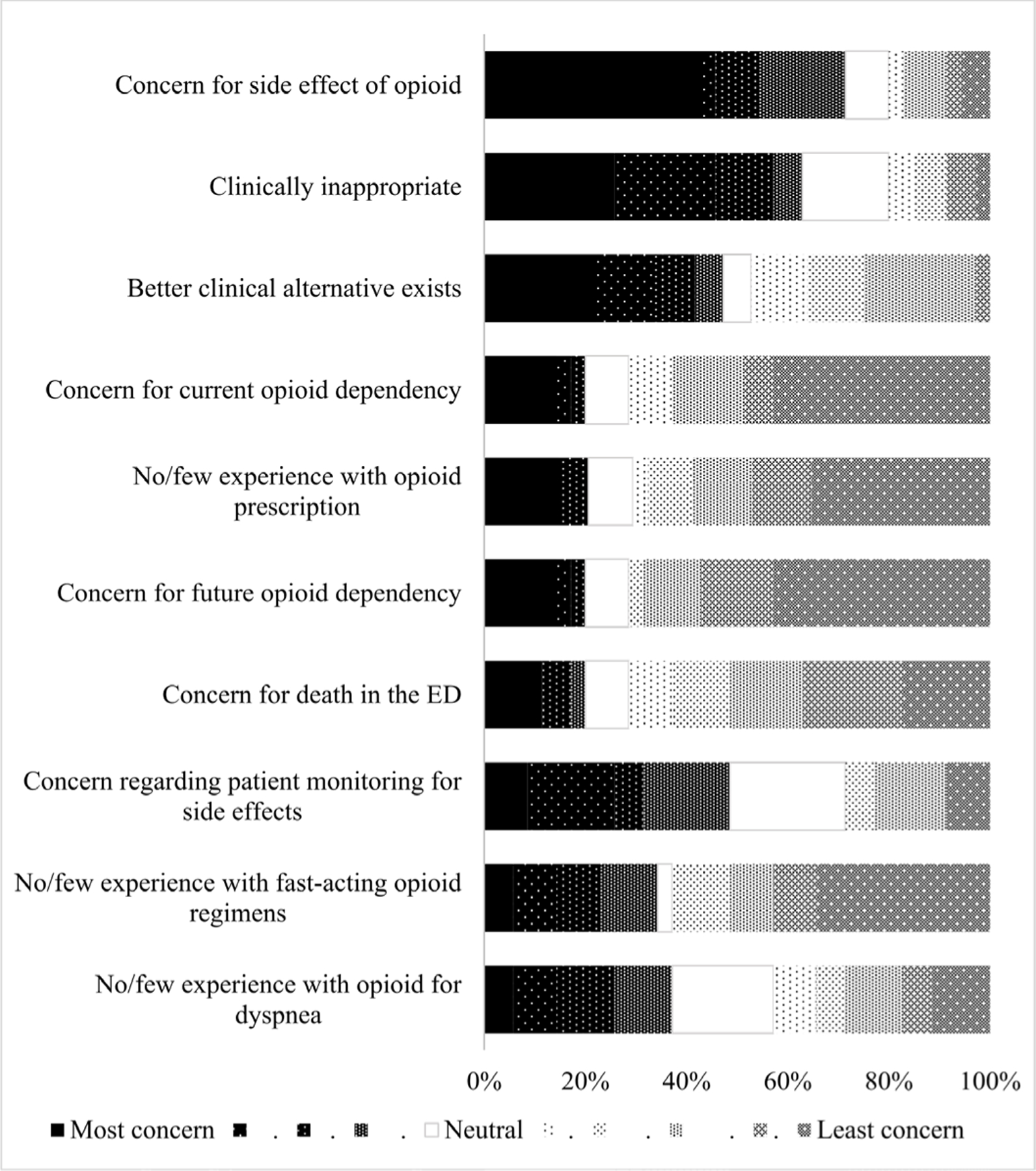
The reasons to not use the palliative dyspnoea protocol in the ED (the most and least concerning). ED, emergency department.
DISCUSSION
Nearly half of the participating clinicians reported that the palliative dyspnoea protocol was acceptable for use in the ED, while only 11% of the participants prescribed adjunct opioid treatments. The most common indications for using the protocol were ‘end of life’ and ‘advanced malignancy’. Opioid side effects were the most concerning reason to avoid the use of the protocol. To improve the implementation of this protocol, specifying the indications, and monitoring parameters and endpoints are required. These findings were consistent with prior studies. Most general doctors were aware of and willing to prescribe opioids for refractory dyspnoea, which is common for patients who are at the end of life and have malignancy.4 Other chronic cardiopulmonary illnesses were less common.4 A significant difference was reported in opioid prescriptions for dyspnoea in advanced chronic obstructive pulmonary disease among palliative medicine (75%) and respiratory doctors (41%).5 Concerns regarding opioid side effects were also similar to prior studies.5 A previous study reported that routinely assessing the severity of dyspnoea, measuring it and documenting it could improve dyspnoea management.4 Because of the recently introduced protocol in the COVID-19 crisis at the time of our study, participants indicated that they were informed of the protocol but were unfamiliar with it.
This preliminary study was limited by the small sample size and the broad inclusion criteria, and was conducted at a single academic medical centre. The term ‘palliative,’ which appears in the protocol’s title, could have negative connotations for its utilisation. A social desirability bias may result from one researcher handing out the survey. The RDOS has only been validated in non-ED settings. However, given the high prevalence of dyspnoea and the prior strength of evidence for the palliative dyspnoea protocol, all the findings of this study were beneficial in pointing out suggestions for further study into dyspnoea in the ED.
CONCLUSIONS
The emergency clinicians found the palliative dyspnoea treatment protocol acceptable mainly in situations of end of life and advanced malignancy. Implementation suggestions were identified.
Supplementary Material
Acknowledgments
Funding
The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Footnotes
Competing interests None declared.
Patient consent for publication Not required.
Ethics approval This study involves human participants but was determined to be exempt from the review by our institutional review board. Participants gave informed consent to participate in the study before taking part.
Provenance and peer review Not commissioned; internally peer reviewed.
Supplemental material This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Additional supplemental material is published online only. To view, please visit the journal online (http://dx.doi.org/10.1136/spcare-2022-003959).
REFERENCES
- 1.Hsu H-S, Wu T-H, Lin C-Y, et al. Enhanced home palliative care could reduce emergency department visits due to non-organic dyspnea among cancer patients: a retrospective cohort study. BMC Palliat Care 2021;20:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dudgeon D Assessment and management of dyspnea in palliative care. UpToDate Available: https://www.uptodate.com/contents/assessment-and-management-of-dyspnea-in-palliativecare [Accessed 30 Nov 2021].
- 3.Zhuang Q, Yang GM, Neo SH-S, et al. Validity, reliability, and diagnostic accuracy of the respiratory distress observation scale for assessment of dyspnea in adult palliative care patients. J Pain Symptom Manage 2019;57:304–10. [DOI] [PubMed] [Google Scholar]
- 4.Stefan MS, DH A, Mularski RA Dyspnea assessment and management survey. J. Hosp. Med 2015;11:724–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smallwood N, Currow D, Booth S, et al. Differing approaches to managing the chronic breathlessness syndrome in advanced COPD: a Multi-National survey of specialists. COPD 2018;15:294–302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


