Abstract
Glia are as numerous in the brain as neurons and widely known to serve supportive roles such as structural scaffolding, extracellular ionic and neurotransmitter homeostasis, and metabolic support. However, over the past two decades, several lines of evidence indicate that astrocytes, which are a type of glia, play active roles in neural information processing. Astrocytes, although not electrically active, can exhibit a form of excitability by dynamic changes in intracellular calcium levels. They sense synaptic activity and release neuroactive substances, named gliotransmitters, that modulate neuronal activity and synaptic transmission in several brain areas, thus impacting animal behavior. This “dialogue” between astrocytes and neurons is embodied in the concept of the tripartite synapse that includes astrocytes as integral elements of synaptic function. Here, we review the recent work and discuss how astrocytes via calcium-mediated excitability modulate synaptic information processing at various spatial and time scales.
Keywords: astrocyte, gliotransmission, tripartite synapse, plasticity, calcium signaling
Introduction
Nervous systems throughout the animal kingdom vary in structure and complexity and are made up of neurons, specialized cells that can receive and transmit chemical or electrical signals, and glial cells, historically considered to only provide support functions to neurons. Glial cells were first described by Virchow in the 1850s as “nervenkitt” or nerve glue, implying a homogenous population of support cells holding them together (García-Marín et al., 2007). However, several different types of glia can be differentiated based on their different functions and morphology. Among them, there are microglia, oligodendrocytes, and astrocytes. The term astrocyte was coined by Michael von Lenhossek to describe star-shaped cells observed in histological brain specimens (Parpura and Verkhratsky, 2012). Subsequently, Camillo Golgi and Ramon y Cajal with the development of novel histological stains illustrated several astrocytes with their elaborated processes (García-Marín et al., 2007; Navarrete and Araque, 2014). Conventionally, two major classes of astrocytes have been distinguished in histological sections of the central nervous system (CNS) based on their morphology and distribution, the fibrous and protoplasmic astrocytes (Miller and Raff, 1984). The fibrous astrocytes are located mainly in white matter with few straight and long processes. Their processes are long (up to 300 μm), though much less elaborate as compared to protoplasmic astroglia. The protoplasmic astrocytes are mainly found in gray matter and are characterized by their extremely elaborate morphology with many branching processes yielding a “bushy” or “spongiform” appearance. Protoplasmic astrocytes extend their endfeet to blood vessels and enwrap them to form the glial limiting membrane, which is the outermost wall of the blood–brain barrier (BBB). More recently, the emergence of molecular approaches such as RNA-sequencing and proteomic analysis has revealed a much larger degree of astrocytic heterogeneity across various brain regions. Excellent reviews related to this topic can be found elsewhere (Zhang and Barres, 2010; Farmer and Murai, 2017; Miller, 2018; Xin and Bonci, 2018; Matias et al., 2019).
Astrocytes customarily have been identified using the intermediary filament protein Glial Fibrillary Acid Protein (GFAP) as a histological marker (Shehab et al., 1990; Zhang et al., 2019; Batiuk et al., 2020; Jurga et al., 2021). Other markers such as the enzyme glutamine synthetase or a Ca2+ binding peptide S100 have also been applied (Norenberg, 1979; Gonçalves et al., 2008). Transcriptome analysis of purified astrocytes identified novel molecular markers for astrocytes such as aldehyde dehydrogenase family 1 member L1 (Cahoy et al., 2008) or the transcription factor Sox9 (Sun et al., 2017).
Electrophysiologically, astrocytes are characterized by their lack of voltage-gated conductances, displaying a quasi-linear voltage-current relationship (Stevens and Wang, 1995). The expression of large amounts of inwardly rectifying potassium channels confers astrocytes with their characteristic low input resistance and membrane potential close to the equilibrium potential for transmembrane potassium. The principal potassium channels are the weakly inwardly rectifying Kir4.1 channels (Nwaobi et al., 2016) although other potassium channels such as the two-pore domain TWIK-1 and TREK-1 channels are also likely to be expressed in astrocytes (Zhou et al., 2009). Another major conductance found in astrocytes is the connexin channel such as connexin 43 which provides gap junctional coupling among astrocytes (Nagy and Rash, 2000). This gap junctional coupling allows the intercellular passive diffusion of endogenous signaling molecules, such as inositol (1,4,5)-triphosphate (IP3) (Leybaert et al., 1998), as well as glucose and its metabolites, glutamate, glutamine, and lactate (Medina et al., 1999). Therefore, astrocytes are considered to form a functional network of communicating cells.
Astrocytes also express various transporter proteins on the plasma membrane for the uptake of neurotransmitters. Transporters are vital for the normal CNS physiology by maintaining neurotransmitter homeostasis and modulating synaptic transmission. It is estimated that astrocytes remove about 80% of the glutamate released, whereas the remaining 20% is taken up by neurons (Parpura and Verkhratsky, 2012). Astrocytes remove extracellular glutamate by excitatory amino acid transporters (EAAT). Five types of EAATs are present in the human brain; the EAAT1 and EAAT2 are expressed almost exclusively in astrocytes (the rodent analogs are known as glutamate/aspartate transporter, GLAST, and glutamate transporter-1, GLT-1) (Murphy-Royal et al., 2017; Mahmoud et al., 2019).
Studies in the past few years have shown that astrocytes are spatially organized to form exquisite tridimensional structures (Gavrilov et al., 2018; Refaeli et al., 2021; Aten et al., 2022). Reconstruction of protoplasmic astrocyte assemblies in the rat hippocampus showed that astrocyte cell bodies are evenly spaced, and their processes overlap only minimally creating a “tiling” of astrocytes (Bushong et al., 2002; Ogata and Kosaka, 2002). This may be the case in some other brain regions as well (Halassa et al., 2007) though overlap of astrocyte territories have also been described (López-Hidalgo et al., 2016). Perhaps even more surprising is how a single astrocyte with its large territory and complex morphology can massively interact with a neuronal network. Indeed, a single astrocyte in the rat hippocampus is estimated to occupy a territory of 66,000 μm3 of neuropil and contact over 140,000 synapses (Bushong et al., 2002).
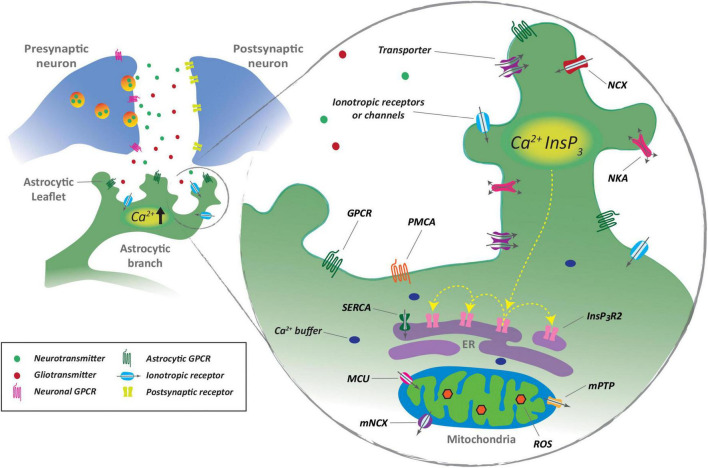
As discussed above, the role of astrocytes in promoting neurotransmitter clearance at synapses has long been recognized. A more unconventional role of astrocytes at synapses has emerged in the last three decades. The deployment of calcium imaging techniques in cultured cells and in brain slices provided evidence that when neurons communicate with each other they also signal to astrocytes. In turn, astrocytes respond to this neuronal signaling by releasing various neuroactive substances, mentioned in detailed in the section below, such as ATP, glutamate, D-serine, and GABA. Thus, the astrocytes form the third element at the synapses. Not only the information flows from presynaptic to postsynaptic elements but also streams to astrocytes that, in turn, regulate synaptic communication. This intimate morphological and functional association of astroglial processes in a synapse led to the conceptual term of a “tripartite synapse” (Figure 1).
FIGURE 1.
Synaptic regulation of astrocyte Ca2+ signaling. Astrocyte leaflets sense and respond to synaptic activity through neurotransmitter receptors and transmitter transporters. Ca2+ transients are triggered by Ca2+ entry and by Ca2+ release from the endoplasmic reticulum (ER) through inositol 1,4,5-triphosphate receptors (IP3R) after G-protein-coupled receptor (GPCR) activation. Mitochondria also participate in Ca2+ loci by action of mitochondrial permeability transition pore (mPTP) and mitochondria sodium/calcium exchanger (NCX). Ca2+ can be removed from the cell by action of Ca2+ ATPase (PCMA) or mobilized to the ER by sarcoplasmic/endoplasmic reticulum calcium ATPase (SERCA). NKA, Na+/K+ ATPase; InsP3, inositol 1,4,5-trisphosphate; MCU, mitochondria Ca2+ uniporter; ROS, reactive oxygen species.
Astrocytic Ca2+ excitability
The plasticity of neuronal connectivity requires dynamic cooperation between neurons and astrocytes (Allen and Eroglu, 2017). Astrocytes change their morphology and synaptic coverage to scale synaptic strength and modulate neuronal circuit activity (Gómez-Gonzalo et al., 2017; Verkhratsky and Nedergaard, 2018; Henneberger et al., 2020; Semyanov and Verkhratsky, 2021). Although not electrically excitable, astrocytes display complex intracellular Ca2+ pathways as a major component of astrocytic signaling. Interaction between synapses and astrocytic arborization promotes astrocyte Ca2+ events to modulate astrocyte neurotransmitter and K+ uptake, the release of neuroactive molecules (Wang et al., 2012; Zorec et al., 2012; Araque et al., 2014), and regulation of local blood flow (Petzold and Murthy, 2011; MacVicar and Newman, 2015). Astrocyte Ca2+ events manifest differentially in space and time within single astrocytes and across astrocytic networks (Semyanov et al., 2020). In soma and primary branches, Ca2+ events are primarily initiated by intracellular Ca2+ release from Ca2+ stores in the endoplasmic reticulum (ER) and mitochondria (Verkhratsky et al., 2018). Moreover, astrocytic Ca2+ transients mainly have also been suggested to occur by Ca2+ entry through the plasma membrane following Na+ increases during neurotransmitter uptake via the sodium/calcium exchanger (NCX) (Verkhratsky et al., 2018) or after activation of other ionotropic Ca2+ permeable receptors and transient receptor potential channels (Shigetomi et al., 2011, 2013; Shibasaki et al., 2014; Rakers and Petzold, 2017).
In contrast to neurons, astrocytes contain processes with distinct morphology and complement organelles that generate widely distributed Ca2+ loci that allow them to differentially respond to synaptic activity and integrate multiple synaptic inputs (Perea and Araque, 2005; Bernardinelli et al., 2014; Semyanov et al., 2020). Astrocytic branches are intermingled with neuronal structures and contain Ca2+ stores that can trigger and amplify Ca2+ events by activation of inositol-1,4,5-triphosphate receptors (IP3Rs). IP3Rs are synergistically modulated by IP3 and Ca2+ levels and further Ca2+-dependent phospholipase C activation, stimulating Ca2+ release from the ER (Foskett et al., 2007; Khakh and Sofroniew, 2015). Ca2+ levels can also reach the threshold for activation of IP3Rs by activation of plasmalemmal G-protein-coupled receptors (GPCRs) (Semyanov and Verkhratsky, 2021) and via increased diffusion of Ca2+ from multiple daughter leaflets (Semyanov, 2019). Intracellular Ca2+ amplification between clusters of IP3Rs can propagate Ca2+ waves within the astrocyte cell body and further astrocytic branches (Srinivasan et al., 2015; Semyanov and Verkhratsky, 2021). Ca2+ event generation in leaflets can be additionally enhanced by ER-independent release mechanisms, involving Ca2+ efflux from mitochondria, in response to the transient opening of permeability transition pores (Agarwal et al., 2017; Figure 1).
The analysis of the neurotransmitter-evoked astrocyte calcium dynamics has revealed that astrocytes integrate incoming synaptic information (Perea and Araque, 2005; Shigetomi et al., 2008). Indeed, synaptic action of excitatory or inhibitory neurotransmitters evoke non-linear calcium elevations and result in the control of the spatial propagation of the intracellular calcium signal within the astrocyte (Perea and Araque, 2005; Shigetomi et al., 2008; Mariotti et al., 2016; Durkee and Araque, 2019; Liu et al., 2022), which is indicative of synaptic information processing by astrocytes. The control of the spatial extent of the calcium signal may have important functional consequences, as it may regulate the spatial extention of the gliotransmitter release and the consequent synaptic regulation (Durkee and Araque, 2019). Moreover, converging Ca2+ signals from multiple daughter leaflets can be finally integrated by parent branches as a readout of local network activity (Lock et al., 2019). In some circumstances, propagating Ca2+ waves can spread through astrocytes and the astrocytic network to influence neuronal activity. This pathway has been suggested to guide information processing across neuronal networks (Tong et al., 2013; Semyanov and Verkhratsky, 2021). Ca2+ events are terminated by Ca2+ removal through the plasma membrane by Ca2+ ATPase (PCMA) or by uptake to Ca2+ stores by ER calcium ATPase (SERCA) (Bazargani and Attwell, 2016). Elongated mitochondria in astrocytic branchlets can also actively uptake intracellular Ca2+ by mitochondria Ca2+ uniporters (Zhang and Ding, 2018).
Heterogeneity of astrocytic Ca2+ signals
Astrocytic Ca2+ events can be classified as either spontaneous or neurotransmitter-evoked (Khakh and McCarthy, 2015; Semyanov et al., 2020). Spontaneous events are characterized by intrinsic Ca2+ fluctuations that can occur in the absence of external signals (Nett et al., 2002; Wang et al., 2006). These spontaneous Ca2+ oscillations persist even if neuronal firing or neuronal and astrocytic vesicular release is blocked (Wang et al., 2006; Sun et al., 2014). Even though the precise mechanisms mediating the triggering of spontaneous Ca2+ transients are not completely understood, it has been proposed that they can be the result of stochastic Ca2+ fluxes through simultaneous multiple pathways (Ding et al., 2018; Denizot et al., 2019). These mechanisms involve both entering Ca2+ from the extracellular space through Ca2+ permeable receptors, Ca2+ channels, and Na+/Ca2+ exchangers at the plasma membrane or intracellular Ca2+ stores through IP3Rs on the ER and mitochondrial permeability via transition pores (Rungta et al., 2016; Agarwal et al., 2017; Wu et al., 2019). The addition of small spatially determined Ca2+ events stimulates local cytosolic Ca2+ oscillations that can trigger Ca2+-dependent Ca2+ release via activation of IP3Rs, leading to amplification and propagation of Ca2+ events (Khakh and McCarthy, 2015). The magnitude of spontaneous Ca2+ activity can be influenced by the intrinsic activity of Gq GPCRs, which stimulates sufficient levels of IP3 to activate IP3Rs, or by focal points of elevated Ca2+ which acts as a co-agonist of IP3Rs. Ca2+ fluxes can be further strengthened or weakened depending on cellular energy states, changes in membrane potential, surface-to-volume ratio, and ER depletion (Khakh and McCarthy, 2015; Ding et al., 2018; Stobart et al., 2018). In soma and primary branches, intracellular Ca2+ waves will mobilize in a specific spatial path within the cell, depending on the proximity of ER IP3Rs, further distance from IP3Rs will terminate the cascade and buffer Ca2+ to basal levels (Denizot et al., 2019).
Astrocytic calcium signals in the soma and processes
Astrocytic Ca2+ signals are considered to rely mainly on the IP3R pathway, especially in the soma and primary branches, as genetic deletion of IP3R2, which is known to be enriched in astrocytes, reduces spontaneous Ca2+ oscillations with the complete abolition of Ca2+ signals in astrocytic soma. Residual Ca2+ activity in astrocyte processes, even if reduced, is still persistent in astrocytes of IP3R2–/– mice (Kanemaru et al., 2014), suggesting IP3R-independent Ca2+ release mechanisms, especially in processes (Patrushev et al., 2013). Such mechanisms involve low cytosolic Ca2+ elevations in mitochondria (Agarwal et al., 2017; Okubo et al., 2019) and transmembrane Ca2+ fluxes mediated by transient receptor potential ion channels (TRPA1), that contribute to the maintenance of basal Ca2+ levels within astrocytes (Shigetomi et al., 2010, 2011). Importantly, 80% of the astrocyte Ca2+ activity in vivo takes place in astrocytic ramifications, that account for 75% of astrocytic volume (Bindocci et al., 2017). Spatial restriction of spontaneous Ca2+ events has been reported in ex vivo and in vivo preparations. Such events occur predominantly in distal parts of astrocyte processes and do not propagate to the soma, thereby identifying autonomous functional domains called “microdomains” (Grosche et al., 1999; Lia et al., 2021). High-resolution imaging techniques have allowed a deeper understanding of the distinct properties and mechanisms underlying astrocyte somatic and microdomain Ca2+ activity. While somatic Ca2+ increases can be triggered by intense neuronal firing patterns, astrocytic processes also respond to local levels of synaptic activity, suggesting compartmentalized astrocyte neuronal communication integration. Microdomain Ca2+ oscillations are more frequently observed than somatic ones and occur asynchronously in various processes (Volterra et al., 2014). Microdomain Ca2+ events have been deferentially categorized based on their distinct properties, however, a rich diversity of Ca2+ signals are present within single astrocytes and are modulated by local brain environments in distinct brain areas (Shigetomi et al., 2013; Khakh and Sofroniew, 2015). Previous elegant classifications have distinguished microdomain Ca2+ activity in focal and expanded microdomains (Di Castro et al., 2011; Clarke and Barres, 2013). Focal microdomains, also later referred to in the field, as localized microdomains in branches and branchlets (Khakh and Sofroniew, 2015), depend largely on IP3R-dependent Ca2+ transients and seem to be independent of neuronal firing. A distinct hypothesis has suggested that these events could originate from spontaneous neurotransmitter release at neighboring synapses, potentially contributing to plastic adaptations at the tripartite synapse (Di Castro et al., 2011; Clarke and Barres, 2013).
On the other hand, expanded microdomains present different Ca2+ dynamics, compared to focal events, with larger amplitude, duration, and spatial extent, and are highly sensitive to surrounding neuronal firing. The increased magnitude of these Ca2+ events has been suggested to result from the synchronization of several autonomous microdomains and might represent a more coordinated Ca2+ response that could modulate gliotransmitter release probability (Di Castro et al., 2011; Panatier et al., 2011; Volterra et al., 2014).
Astrocytic calcium signaling in response to neuronal activity
Astrocytes sense, react and modify the extracellular transmitter homeostasis by responding in situ to neuronal activity. Ex vivo and in vivo examinations have provided strong evidence showing that neuronal inputs trigger astrocyte Ca2+ events by activation of multiple plasma membrane receptors (Nimmerjahn et al., 2004; Wang et al., 2006; Caudal et al., 2020; Figure 1). Engagement of distinct receptor arrays after neuronal input increases cytosolic IP3 levels and IP3R activation, promoting Ca2+ release from ER Ca2+ stores (Bazargani and Attwell, 2016). Additional Ca2+ entry to the cytosol and further triggering of Ca2+ transients can be observed after neuronal-mediated activation of ionotropic receptors, such as glutamate AMPA and NMDA (Saab et al., 2012), purinergic P2X (Abbracchio and Verderio, 2006), and nicotinic cholinergic receptors (Aryal et al., 2021) or after uptake of glutamate and GABA via Na+ influx via Na+/Ca2+ exchangers (Boddum et al., 2016; Brazhe et al., 2018; Rose et al., 2020). Evidence collected through the last decades has shown that astrocyte GPCR activation mainly leads to intracellular Ca2+ increases (Kofuji and Araque, 2021). Such a dynamic seems to oppose canonical responses observed in neuronal activation, as increases in astrocytic intracellular Ca2+ are triggered after activation of excitatory or inhibitory transmitter receptors (Mariotti et al., 2016; Perea et al., 2016) or other Gq, Gs, or Gi-coupled metabotropic receptors (Durkee and Araque, 2019; Yu et al., 2020). The consequences of GPCR-mediated increase in astrocytic Ca2+ are not fully characterized, however, exciting evidence has suggested that astrocytes can discriminate and integrate metabotropic signaling upstream of internal Ca2+ oscillations (Caudal et al., 2020). Different activation efficiencies of GPCRs exert equivalent (Shigetomi et al., 2008) or do not necessarily induce the release of gliotransmitters, contrary to the effects observed after Ca2+ uncaging or IP3 application (Wang et al., 2013).
Neuronal influence on astrocytic activity can occur at individual synapses but also after diffusion of neuromodulators, such as dopamine, acetylcholine, serotonin, and noradrenaline, that modulate spatiotemporal spontaneous Ca2+ events that trigger new Ca2+ fluctuations (Takata et al., 2011; Ding et al., 2013; Jennings et al., 2017; Corkrum et al., 2020; Semyanov et al., 2020). Evidence collected during the last decades has suggested that the modulation of astrocyte intracellular Ca2+-induced by neuromodulators finely tunes K+ homeostasis and gliotransmitter release (Wang et al., 2012; Pacholko et al., 2020). By integrating the neuromodulatory effects, astrocytes act as crucial players in behavioral states. Neuromodulator effects have been especially evident in astrocyte Ca2+ network activity, as they influence astrocyte activity thresholds in response to local neuronal activity or depending on the brain’s vigilance state (Ding et al., 2013; Araque et al., 2014). Astrocyte Ca2+ events in leaflets and branchlets can also be triggered by gliotransmitters or other diffuse signals in the local environment, as well as by changes in partial pressures of CO2 and O2, osmotic pressure, pH, and temperature (Angelova et al., 2015; Turovsky et al., 2016; Kofuji and Araque, 2021; Semyanov and Verkhratsky, 2021). Astrocytic Ca2+ activity resulting from the interaction between astrocytic processes and synapses can trigger astrocyte morphological remodeling and gliotransmitter release, which feedback to neuronal network excitability and functioning (Kofuji and Araque, 2021).
Kinetics of astrocyte Ca2+ signals
Astrocyte Ca2+ signals in response to external stimulation present different temporal and spatial properties than neuronal activity. The timescale of astrocytic Ca2+ dynamics is generally much slower, with variable intervals between sensory stimulation and the onset of astrocytic Ca2+ event. Single action potentials that can last within a range of a few milliseconds differentiate from astrocytic Ca2+ events, as they can occur over durations of several hundred milliseconds to a few seconds (Paukert et al., 2014; Otsu et al., 2015). The differences in Ca2+ dynamics between neurons and astrocytes have raised the question of whether the astrocytic activity can be directly correlated to real-time information processing in the brain (Semyanov, 2019; Semyanov et al., 2020). Astrocyte information processing could potentially bridge information received by thousands of synapses belonging to different circuits and neurons and integrate the information in different spatial-temporal scales (Bushong et al., 2002; Perea and Araque, 2005; Halassa et al., 2007; Gordon et al., 2008). Indeed, recent evidence suggests that astrocytes could encode information by evoking specific time and spatial Ca2+ signal patterns, characterized by the different total area of appearance, number, and duration of Ca2+ events (Perea and Araque, 2005; Volterra et al., 2014; Nakayama et al., 2016; Wang et al., 2019).
Moreover, during information processing, astrocytes could incorporate not only, neuronal information, but also signals resulting from complex interactions with other non-neuronal cells and non-cellular elements part of the extracellular brain microenvironment (Volterra et al., 2014; Ribot et al., 2021; Semyanov and Verkhratsky, 2021). Further investigation is needed to elucidate the emerging complexity of mechanisms and dynamics mediating specific types of astrocytic Ca2+ patterns and astrocyte processing of information.
Calcium and gliotransmitter release from astrocytes
Since the coining of the term, “tripartite synapse,” researchers have been studying the extent that astrocytes actively communicate with neurons (Araque et al., 1998, 2014). One of the active mechanisms of astrocytes that impacts synaptic transmission is gliotransmission (Araque et al., 2014). Gliotransmission refers to the capacity of astrocytes to release neuroactive molecules that impact synaptic transmission or neuronal signaling (Araque et al., 2014; Volterra et al., 2014). Many of these signaling molecules include classic transmitters such as glutamate and GABA and amino acids like ATP/adenosine and d-serine. Even though the cellular and molecular mechanisms mediating gliotransmitter release are not completely understood, several studies have revealed both calcium-dependent and -independent release mechanisms (Guček et al., 2012; Li et al., 2013; Sloan and Barres, 2014; Figure 2).
FIGURE 2.
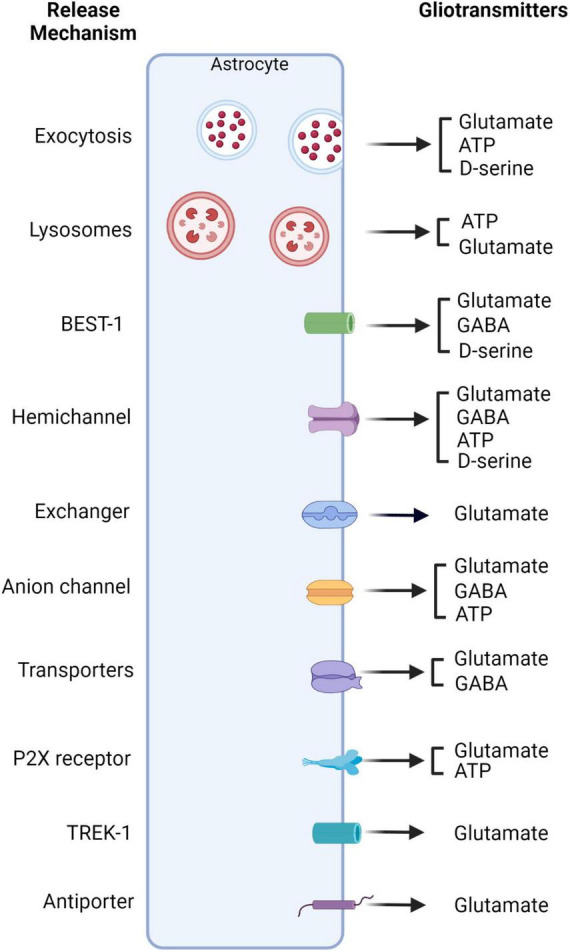
Schematic of Ca2+ dependent and independent gliotransmitter release. Astrocytes can release gliotransmitters through a variety of mechanisms dependent and independent of calcium. Glutamate has been shown to be released via a variety of mechanisms. These mechanisms include exocytosis, lysosomes, hemichannels, exchangers, anion channels, antiporters as well as channels such as TREK-1 and Bestropin-1 (BEST-1) (Araque et al., 2000; Montana et al., 2004; Zhang et al., 2004; Xu et al., 2007; Malarkey and Parpura, 2008; Yang et al., 2019; Okada et al., 2021). GABA on the other hand, has been shown to be released via BEST-1, hemichannels, as well as anion channels and transporters (Kozlov et al., 2006; Jiménez-González et al., 2011; Le Meur et al., 2012; Yoon and Lee, 2014; Christensen et al., 2018; Kwak et al., 2020). ATP can be released via hemichannels, exocytosis, anion channels, and lysosomes (Bezzi and Volterra, 2001; Fujii et al., 2017; Xiong et al., 2018). Lastly, D-serine has been shown to be released via exocytosis, BEST-1, and hemichannels (Wolosker et al., 1999; Martineau et al., 2013; Sild and Van Horn, 2013; Herman, 2018; Koh et al., 2022; Linsambarth et al., 2022; Park et al., 2022; Tapanes et al., 2022). Created with BioRender.com.
Glutamate
Calcium-dependent and -independent mechanisms for glutamate release from astrocytes have been proposed. These include (a) exocytosis from vesicles, (b) anion channel opening, (c) glutamate exchange via cystine-glutamate antiporter, (d) release from hemichannels, or (e) ionotropic purinergic receptors (Araque et al., 2000; Montana et al., 2004; Zhang et al., 2004; Malarkey and Parpura, 2008). Vesicular gliotransmitter release of glutamate has been supported by morphological and functional evidence. For example, it has been shown that astrocytes possess some of the proteins involved in exocytosis, including the soluble N-ethyl maleimide-sensitive fusion protein attachment protein receptor (SNARE) complex (Zhang et al., 2004), to control vesicle fusion. SNARE proteins, such as VAMP2 or VAMP3, Syntaxin 1, SNAP23, and synaptotagmin isoforms have been detected in astrocytes (Bohmbach et al., 2018; Mielnicka and Michaluk, 2021). Interestingly, the mechanisms involved in glutamate-mediated exocytosis have been highly debated in the last years (Li et al., 2008; Chai et al., 2017). Functionally, expression in astrocytes with the light chain of tetanus toxin that selectively cleaves the vesicle-associated SNARE protein potently inhibits the release of glutamate from astrocytes (Montana et al., 2004; Xu et al., 2007; Araque et al., 2014). However, complementary evidence has questioned the exact mechanisms involved in Ca2+-dependent glutamate exocytosis (Li et al., 2008; Chai et al., 2017). Deployment of a variety of experimental approaches revealed that fusion events from astrocytic vesicles following intracellular calcium increase occurs in a much slower time scale in comparison to neurons (Bezzi et al., 2004; Calì et al., 2008; Marchaland et al., 2008). While in neurons the fusion occurs in less than 0.5 ms following calcium increase, in astrocytes the exocytotic release takes place over two orders of magnitude slower (Bezzi et al., 2004; Calì et al., 2008; Marchaland et al., 2008; Südhof, 2012). The release of glutamate may also occur via the opening of glutamate-permeable, two-pore domain potassium channel TREK-1 or the opening of glutamate-permeable, calcium-activated bestrophin anion channel (Best1). Ultrastructural analyses demonstrate that TREK-1 is preferentially localized at cell body and processes, whereas Best1 is mostly found in microdomains of astrocytes near synapses (Woo et al., 2012). Recent evidence has also shown that activation of volume-regulated anion channels (VRAC) can lead to glutamate release. When this channel is activated by cell swelling, astrocytes in the hippocampus release glutamate (Yang et al., 2019). Lastly, glutamate may also be released via hemichannels which can be blocked by drugs targeting synaptic vesicle protein 2A (Okada et al., 2021).
GABA
GABA is an important neurotransmitter for neuronal inhibition. As neurons, astrocytes can also release GABA via transporters, anion channels, and gap junction channels (Yoon and Lee, 2014). In contrast to glutamate, GABA release from astrocytes has been reported to be mediated by distinct mechanisms, as the vesicular release of GABA seems unlikely, due to the lack of GABA-containing vesicles in astrocytes. Atypically, astrocytes synthesize GABA from the polyamine putrescine using monoamine oxidase B (Yoon and Lee, 2014). Early examples of GABA release from astrocytes have been found in the olfactory bulb, thalamus, and hippocampus (Kozlov et al., 2006; Jiménez-González et al., 2011; Le Meur et al., 2012). One of the major functional consequences of astrocyte-derived GABA is the tonic inhibition of various neuronal circuits. Various mechanisms of GABA release from astrocytes have been proposed. Calcium-dependent GABA release from astrocytes potentially involving the GABA transporter GAT has been reported in the dorsal root ganglia (Christensen et al., 2018). Other mechanisms for GABA release from astrocytes such as Best anion channels and gap Junction hemichannels have also been described. “Sniffer-patch” experiments have shown that the Best-1-mediated release of GABA is dependent on intracellular calcium and is triggered by GPCR activation. Tonic inhibition caused by GABA release via glial Best1 anion channels has been reported in the cerebellum and thalamus (Lee et al., 2010; Kwak et al., 2020). This mechanism has also been demonstrated in reactive astrocytes in the hippocampus (Pandit et al., 2020). Finally, gap junction hemichannels could be another route by which GABA can be released from astrocytes. GABA release via gap junction hemichannels is involved in the regulation of tonic GABA currents of neurons in cultured hippocampal neurons and acute hippocampal slices (Ransom et al., 2017).
ATP
ATP is a primary energy source in cells and also acts as an important messenger molecule through action on purinergic receptors. ATP plays an important role in calcium wave propagation in astrocytes (Bezzi and Volterra, 2001). Unlike the previously mentioned gliotransmitters, the mechanism for exocytosis was unclear in situ until recent years. This was due primarily to using indirect assays to measure quantal and non-quantal ATP release (Xiong et al., 2018). Many studies have examined calcium-dependent and independent mechanisms of ATP release. Evidence collected from mice conditionally expressing the SNARE domain of VAMP2 selectively in astrocytes (dn-SNARE mice), has shown Ca2+-dependent ATP release by astrocytes (Lalo et al., 2014). In addition, ATP release can be mediated by calcium-dependent lysosome exocytosis (Pangršič et al., 2007; Zhang et al., 2007). Lysosome exocytosis and ATP release occurred after mechanical stimulation in primary hippocampal astrocyte culture (Xiong et al., 2018) in a calcium-dependent manner (Lee et al., 2015). In addition, ATP can also be released via connexin 43 (Cx43) hemichannels and anion channels (Kang et al., 2008; Fujii et al., 2017).
D-serine
Astrocytes can produce and store D-serine in vesicles (Martineau et al., 2013; Sild and Van Horn, 2013). The enzyme, serine racemase converts L-serine to D-serine (Wolosker et al., 1999). Astrocytes play an important role in the serine shuttle by converting L-serine from glucose which can then supply to neurons (Herman, 2018). Ca2+ dependent vesicle release of D-serine has been demonstrated to modulate long-term potentiation (LTP) (Henneberger et al., 2010; Bergersen et al., 2012). Astrocytic glutamate activates on mGluRs and further activates LTP in cholinergic neurons (Navarrete et al., 2012). Moreover, astrocyte release of D-serine also leads to LTP modulating recognition memory (Robin et al., 2018). Glial D-serine is relevant for astrocytes across multiple species including Drosophila. In Drosophila, glial D-serine is required for thirst-directed behavior (Park et al., 2022). Many studies have shown that astrocytes can release D-serine under pathological conditions. For instance, preventing the release of d-serine from glia reduce synaptic damage after traumatic brain injury (Tapanes et al., 2022). Astroglial d-serine can also travel through Cx43 hemichannels. The form of release is particularly important for fear memories during fear conditioning. Blocking Cx43 in the basolateral amygdala impaired fear memory consolidation (Linsambarth et al., 2022). In addition, astrocytes can also release D-serine via Best1 channels. This has been shown to alter NMDA tone in the hippocampus (Koh et al., 2022).
Conclusion
The development of tools for visualization and manipulation of cell Ca2+ dynamics together with advances in imaging techniques have enabled the monitoring and modulation of astrocyte Ca2+ signaling in in vitro, ex vivo, and in vivo preparations (Li et al., 2013). Advanced optical imaging techniques, sensitive genetically encoded Ca2+ indicators (GECIs), and optogenetic and pharmacogenetic tools allow the selective measuring and activation of astrocyte Ca2+ signaling pathways to study astrocyte-neuron communication, mechanisms of gliotransmitter release, and role of astrocytes in physiology (Li et al., 2013; Semyanov et al., 2020). In particular, selective astrocyte GPCR activation has been useful to explore the functional role of astrocyte Ca2+ signaling in specific brain areas and astrocyte populations (Losi et al., 2017). A variety of experimental approaches are now available to increase astrocyte intracellular Ca2+ levels, such as light-gated glutamate receptor, channelrhodopsin-based effectors, melanopsin, optoXRs, and designer receptor exclusively activated by designer drugs (DREADDs) (Hirbec et al., 2020). In particular, Gq-GPCR and Gi-GPCR DREADDs have been widely used in the field, as they offer an opportunity for non-invasive and selective in vivo activation of astrocyte GPCR pathways after selective agonist administration (Losi et al., 2017). Even though there is a variety of tools to increase astrocyte Ca2+ signaling, till recently, IP3R2–/– mice and IP3 sponges (Agulhon et al., 2008; Petravicz et al., 2008) have been the only available options to achieve astrocyte Ca2+ selective attenuation. Recent studies have provided new tools to lessen intracellular Ca2+ release, such as activation of kappa-opioid receptor coupled to a Gi-GPCR selectively activated by salvinorin B (Vardy et al., 2015; Herrera Moro Chao et al., 2022) or by Cre-dependent expression of hPMCA2, a human plasma membrane Ca2+ ATPase pump that constitutively extrudes Ca2+ from astrocytes (Yu et al., 2018, 2021). Decreases in astrocyte intracellular Ca2+ levels have also been observed during neuropathology after astrocyte Gs-GPCR activation (Pham et al., 2021).
The evolving genetically targeted optical and pharmacological tools to modulate astrocytic Ca2+ signals have been of value in several studies in the field, showing that astrocyte function and astrocyte-neuron communication is heavily impacted during pathological conditions (Nedergaard et al., 2010; Nanclares et al., 2021; Herrera Moro Chao et al., 2022). Visualization of astrocyte Ca2+ by GECIs monitoring has shown that astrocytes become hyperactive in many neurological diseases such as traumatic brain injury, amyotrophic lateral sclerosis, epilepsy, and Alzheimer’s disease (AD) (Shigetomi et al., 2019). In addition, modified gliotransmitter release and synaptic transmission have been associated with the development of astrocyte hyperactivity and reactivity (Nedergaard et al., 2010; Nanclares et al., 2021; Herrera Moro Chao et al., 2022). In conclusion, further studies are essential for a precise understanding of the detailed mechanisms by which astrocyte-neuron communication mediates physiological outputs and how the dysregulation of this reciprocal communication affects the development of neuropathology. Tailoring novel molecular tools that specifically modulate astrocyte Ca2+ signaling pathways combined with advanced Ca2+ imaging techniques in vivo will further shed light on the complexity of astrocyte-neuron bidirectional communication and its impact on physiology.
Author contributions
JG, AA, PK, and DH drafted manuscript and approved the final version of the manuscript. All authors contributed to the article and approved the submitted version.
Funding Statement
This work was funded by National Institutes of Health-NINDS (R01DA048822, R01MH119355, and 1F31NS124107-01A1 to JG).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Abbracchio M., Verderio C. (2006). Pathophysiological roles of P2 receptors in glial cells. Novartis Found Symp. 276 91–103. [PubMed] [Google Scholar]
- Agarwal A., Wu P., Hughes E., Fukaya M., Tischfield M., Langseth A., et al. (2017). Transient opening of the mitochondrial permeability transition pore induces microdomain calcium transients in astrocyte processes. Neuron 93 587–605.e7. 10.1016/j.neuron.2016.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agulhon C., Petravicz J., McMullen A., Sweger E., Minton S., Taves S., et al. (2008). What is the role of astrocyte calcium in neurophysiology? Neuron 59 932–946. 10.1016/j.neuron.2008.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen N., Eroglu C. (2017). Cell biology of astrocyte-synapse interactions. Neuron 96 697–708. 10.1016/j.neuron.2017.09.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angelova P., Kasymov V., Christie I., Sheikhbahaei S., Turovsky E., Marina N., et al. (2015). Functional oxygen sensitivity of astrocytes. J. Neurosci. 35 10460–10473. 10.1523/JNEUROSCI.0045-15.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A., Carmignoto G., Haydon P., Oliet S., Robitaille R., Volterra A. (2014). Gliotransmitters travel in time and space. Neuron 81 728–739. 10.1016/j.neuron.2014.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A., Li N., Doyle R., Haydon P. G. (2000). SNARE protein-dependent glutamate release from astrocytes. J. Neurosci. 20 666–673. 10.1523/JNEUROSCI.20-02-00666.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araque A., Sanzgiri R., Parpura V., Haydon P. (1998). Calcium elevation in astrocytes causes an NMDA receptor-dependent increase in the frequency of miniature synaptic currents in cultured hippocampal neurons. J. Neurosci. 18 6822–6829. 10.1523/JNEUROSCI.18-17-06822.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aryal S., Fu X., Sandin J., Neupane K., Lakes J., Grady M., et al. (2021). Nicotine induces morphological and functional changes in astrocytes via nicotinic receptor activity. Glia 69 2037–2053. 10.1002/glia.24011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aten S., Kiyoshi C., Arzola E., Patterson J., Taylor A., Du Y., et al. (2022). Ultrastructural view of astrocyte arborization, astrocyte-astrocyte and astrocyte-synapse contacts, intracellular vesicle-like structures, and mitochondrial network. Prog. Neurobiol. 213:102264. 10.1016/j.pneurobio.2022.102264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batiuk M., Martirosyan A., Wahis J., de Vin F., Marneffe C., Kusserow C., et al. (2020). Identification of region-specific astrocyte subtypes at single cell resolution. Nat. Commun. 11:1220. 10.1038/s41467-019-14198-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazargani N., Attwell D. (2016). Astrocyte calcium signaling: The third wave. Nat. Neurosci. 19 182–189. 10.1038/nn.4201 [DOI] [PubMed] [Google Scholar]
- Bergersen L., Morland C., Ormel L., Rinholm J., Larsson M., Wold J., et al. (2012). Immunogold detection of L-glutamate and D-serine in small synaptic-like microvesicles in adult hippocampal astrocytes. Cereb. Cortex 22 1690–1697. 10.1093/cercor/bhr254 [DOI] [PubMed] [Google Scholar]
- Bernardinelli Y., Randall J., Janett E., Nikonenko I., König S., Jones E., et al. (2014). Activity-dependent structural plasticity of perisynaptic astrocytic domains promotes excitatory synapse stability. Curr. Biol. 24 1679–1688. 10.1016/j.cub.2014.06.025 [DOI] [PubMed] [Google Scholar]
- Bezzi P., Gundersen V., Galbete J., Seifert G., Steinhäuser C., Pilati E., et al. (2004). Astrocytes contain a vesicular compartment that is competent for regulated exocytosis of glutamate. Nat. Neurosci. 7 613–620. 10.1038/nn1246 [DOI] [PubMed] [Google Scholar]
- Bezzi P., Volterra A. (2001). A neuron-glia signalling network in the active brain. Curr. Opin. Neurobiol. 11 387–394. 10.1016/s0959-4388(00)00223-3 [DOI] [PubMed] [Google Scholar]
- Bindocci E., Savtchouk I., Liaudet N., Becker D., Carriero G., Volterra A. (2017). Three-dimensional Ca2+ imaging advances understanding of astrocyte biology. Science 356 eaai8185. 10.1126/science.aai8185 [DOI] [PubMed] [Google Scholar]
- Boddum K., Jensen T., Magloire V., Kristiansen U., Rusakov D., Pavlov I., et al. (2016). Astrocytic GABA transporter activity modulates excitatory neurotransmission. Nat. Commun. 7:13572. 10.1038/ncomms13572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohmbach K., Schwarz M., Schoch S., Henneberger C. (2018). The structural and functional evidence for vesicular release from astrocytes in situ. Brain Res. Bull. 136 65–75. 10.1016/j.brainresbull.2017.01.015 [DOI] [PubMed] [Google Scholar]
- Brazhe A., Verisokin A., Verveyko D., Postnov D. (2018). Sodium-calcium exchanger can account for regenerative Ca2+ entry in thin astrocyte processes. Front. Cell Neurosci. 12:250. 10.3389/fncel.2018.00250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushong E., Martone M., Jones Y., Ellisman M. (2002). Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J. Neurosci. 22 183–192. 10.1523/JNEUROSCI.22-01-00183.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahoy J., Emery B., Kaushal A., Foo L., Zamanian J., Christopherson K., et al. (2008). A transcriptome database for astrocytes, neurons, and oligodendrocytes: A new resource for understanding brain development and function. J. Neurosci. 28 264–278. 10.1523/JNEUROSCI.4178-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calì C., Marchaland J., Regazzi R., Bezzi P. (2008). SDF 1-alpha (CXCL12) triggers glutamate exocytosis from astrocytes on a millisecond time scale: Imaging analysis at the single-vesicle level with TIRF microscopy. J. Neuroimmunol. 198 82–91. 10.1016/j.jneuroim.2008.04.015 [DOI] [PubMed] [Google Scholar]
- Caudal L., Gobbo D., Scheller A., Kirchhoff F. (2020). The Paradox of Astroglial Ca2 + Signals at the Interface of Excitation and Inhibition. Front. Cell Neurosci. 14:609947. 10.3389/fncel.2020.609947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai H., Diaz-Castro B., Shigetomi E., Monte E., Octeau J., Yu X., et al. (2017). Neural circuit-specialized astrocytes: Transcriptomic, proteomic, morphological, and functional evidence. Neuron 95 531–549.e9. 10.1016/j.neuron.2017.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen R., Delgado-Lezama R., Russo R., Lind B., Alcocer E., Rath M., et al. (2018). Spinal dorsal horn astrocytes release GABA in response to synaptic activation. J. Physiol. 596 4983–4994. 10.1113/JP276562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke L., Barres B. (2013). Emerging roles of astrocytes in neural circuit development. Nat. Rev. Neurosci. 14 311–321. 10.1038/nrn3484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corkrum M., Covelo A., Lines J., Bellocchio L., Pisansky M., Loke K., et al. (2020). Dopamine-evoked synaptic regulation in the nucleus accumbens requires astrocyte activity. Neuron 105 1036–1047.e5. 10.1016/j.neuron.2019.12.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denizot A., Arizono M., Nägerl U., Soula H., Berry H. (2019). Simulation of calcium signaling in fine astrocytic processes: Effect of spatial properties on spontaneous activity. PLoS Comput. Biol. 15:e1006795. 10.1371/journal.pcbi.1006795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Castro M., Chuquet J., Liaudet N., Bhaukaurally K., Santello M., Bouvier D., et al. (2011). Local Ca2+ detection and modulation of synaptic release by astrocytes. Nat. Neurosci. 14 1276–1284. 10.1038/nn.2929 [DOI] [PubMed] [Google Scholar]
- Ding F., O’Donnell J., Thrane A., Zeppenfeld D., Kang H., Xie L., et al. (2013). α1-Adrenergic receptors mediate coordinated Ca2+ signaling of cortical astrocytes in awake, behaving mice. Cell Calcium 54 387–394. 10.1016/j.ceca.2013.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X., Zhang X., Ji L. (2018). Contribution of calcium fluxes to astrocyte spontaneous calcium oscillations in deterministic and stochastic models. Appl. Math. Model. 55 371–382. [Google Scholar]
- Durkee C., Araque A. (2019). Diversity and specificity of astrocyte-neuron communication. Neuroscience 396 73–78. 10.1016/j.neuroscience.2018.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer W., Murai K. (2017). Resolving astrocyte heterogeneity in the CNS. Front. Cell Neurosci. 11:300. 10.3389/fncel.2017.00300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foskett J., White C., Cheung K., Mak D. (2007). Inositol trisphosphate receptor Ca2+ release channels. Physiol. Rev. 87 593–658. 10.1152/physrev.00035.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii Y., Maekawa S., Morita M. (2017). Astrocyte calcium waves propagate proximally by gap junction and distally by extracellular diffusion of ATP released from volume-regulated anion channels. Sci. Rep. 7:13115. 10.1038/s41598-017-13243-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Marín V., García-López P., Freire M. (2007). Cajal’s contributions to glia research. Trends Neurosci. 30 479–487. 10.1016/j.tins.2007.06.008 [DOI] [PubMed] [Google Scholar]
- Gavrilov N., Golyagina I., Brazhe A., Scimemi A., Turlapov V., Semyanov A. (2018). Astrocytic coverage of dendritic spines, dendritic shafts, and axonal boutons in hippocampal neuropil. Front. Cell Neurosci. 12:248. 10.3389/fncel.2018.00248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez-Gonzalo M., Martin-Fernandez M., Martínez-Murillo R., Mederos S., Hernández-Vivanco A., Jamison S., et al. (2017). Neuron-astrocyte signaling is preserved in the aging brain. Glia 65 569–580. 10.1002/glia.23112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves C., Leite M., Nardin P. (2008). Biological and methodological features of the measurement of S100B, a putative marker of brain injury. Clin. Biochem. 41 755–763. 10.1016/j.clinbiochem.2008.04.003 [DOI] [PubMed] [Google Scholar]
- Gordon G., Choi H., Rungta R., Ellis-Davies G., MacVicar B. (2008). Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature 456 745–749. 10.1038/nature07525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosche J., Matyash V., Möller T., Verkhratsky A., Reichenbach A., Kettenmann H. (1999). Microdomains for neuron-glia interaction: Parallel fiber signaling to Bergmann glial cells. Nat. Neurosci. 2 139–143. 10.1038/5692 [DOI] [PubMed] [Google Scholar]
- Guček A., Vardjan N., Zorec R. (2012). Exocytosis in astrocytes: Transmitter release and membrane signal regulation. Neurochem. Res. 37 2351–2363. 10.1007/s11064-012-0773-6 [DOI] [PubMed] [Google Scholar]
- Halassa M., Fellin T., Takano H., Dong J., Haydon P. (2007). Synaptic islands defined by the territory of a single astrocyte. J. Neurosci. 27 6473–6477. 10.1523/JNEUROSCI.1419-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneberger C., Bard L., Panatier A., Reynolds J., Kopach O., Medvedev N., et al. (2020). LTP induction boosts glutamate spillover by driving withdrawal of perisynaptic astroglia. Neuron 108 919–936.e11. 10.1016/j.neuron.2020.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneberger C., Papouin T., Oliet S., Rusakov D. (2010). Long-term potentiation depends on release of D-serine from astrocytes. Nature 463 232–236. 10.1038/nature08673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman W. (2018). “The Neurobiology of d-Serine Signaling,” in Apprentices to Genius: A tribute to Solomon H. Snyder, eds Coyle J., Pasternak G. (Cambridge, MA: Academic Press; ). [Google Scholar]
- Herrera Moro Chao D., Kirchner M., Pham C., Foppen E., Denis R., Castel J., et al. (2022). Hypothalamic astrocytes control systemic glucose metabolism and energy balance. Cell Metab. 34 1532–1547.e6. 10.1016/j.cmet.2022.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirbec H., Déglon N., Foo L., Goshen I., Grutzendler J., Hangen E., et al. (2020). Emerging technologies to study glial cells. Glia 68 1692–1728. 10.1002/glia.23780 [DOI] [PubMed] [Google Scholar]
- Jennings A., Tyurikova O., Bard L., Zheng K., Semyanov A., Henneberger C., et al. (2017). Dopamine elevates and lowers astroglial Ca2+ through distinct pathways depending on local synaptic circuitry. Glia 65 447–459. 10.1002/glia.23103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez-González C., Pirttimaki T., Cope D., Parri H. (2011). Non-neuronal, slow GABA signalling in the ventrobasal thalamus targets δ-subunit-containing GABA(A) receptors. Eur. J. Neurosci. 33 1471–1482. 10.1111/j.1460-9568.2011.07645.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurga A., Paleczna M., Kadluczka J., Kuter K. (2021). Beyond the GFAP-Astrocyte protein markers in the Brain. Biomolecules 11:1361. 10.3390/biom11091361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemaru K., Sekiya H., Xu M., Satoh K., Kitajima N., Yoshida K., et al. (2014). In vivo visualization of subtle, transient, and local activity of astrocytes using an ultrasensitive Ca(2+) indicator. Cell Rep. 8 311–318. 10.1016/j.celrep.2014.05.056 [DOI] [PubMed] [Google Scholar]
- Kang J., Kang N., Lovatt D., Torres A., Zhao Z., Lin J., et al. (2008). Connexin 43 hemichannels are permeable to ATP. J. Neurosci. 28 4702–4711. 10.1523/JNEUROSCI.5048-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh B., McCarthy K. (2015). Astrocyte calcium signaling: From observations to functions and the challenges therein. Cold Spring Harb. Perspect. Biol. 7:a020404. 10.1101/cshperspect.a020404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khakh B., Sofroniew M. (2015). Diversity of astrocyte functions and phenotypes in neural circuits. Nat. Neurosci. 18 942–952. 10.1038/nn.4043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofuji P., Araque A. (2021). G-Protein-Coupled Receptors in Astrocyte-Neuron Communication. Neuroscience 456 71–84. 10.1016/j.neuroscience.2020.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh W., Park M., Chun Y., Lee J., Shim H., Park M., et al. (2022). Astrocytes Render Memory Flexible by Releasing D-Serine and Regulating NMDA Receptor Tone in the Hippocampus. Biol. Psychiatry 91 740–752. 10.1016/j.biopsych.2021.10.012 [DOI] [PubMed] [Google Scholar]
- Kozlov A., Angulo M., Audinat E., Charpak S. (2006). Target cell-specific modulation of neuronal activity by astrocytes. Proc. Natl. Acad. Sci. U.S.A. 103 10058–10063. 10.1073/pnas.0603741103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak H., Koh W., Kim S., Song K., Shin J., Lee J., et al. (2020). Astrocytes control sensory acuity via tonic inhibition in the thalamus. Neuron 108 691–706.e10. 10.1016/j.neuron.2020.08.013 [DOI] [PubMed] [Google Scholar]
- Lalo U., Palygin O., Rasooli-Nejad S., Andrew J., Haydon P., Pankratov Y. (2014). Exocytosis of ATP from astrocytes modulates phasic and tonic inhibition in the neocortex. PLoS Biol. 12:e1001747. 10.1371/journal.pbio.1001747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Meur K., Mendizabal-Zubiaga J., Grandes P., Audinat E. (2012). GABA release by hippocampal astrocytes. Front. Comput. Neurosci. 6:59. 10.3389/fncom.2012.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Chun Y., Han K., Lee J., Woo D., Lee C. (2015). Ca(2+) entry is required for mechanical stimulation-induced ATP Release from Astrocyte. Exp. Neurobiol. 24 17–23. 10.5607/en.2015.24.1.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Yoon B., Berglund K., Oh S., Park H., Shin H., et al. (2010). Channel-mediated tonic GABA release from glia. Science 330 790–796. 10.1126/science.1184334 [DOI] [PubMed] [Google Scholar]
- Leybaert L., Paemeleire K., Strahonja A., Sanderson M. (1998). Inositol-trisphosphate-dependent intercellular calcium signaling in and between astrocytes and endothelial cells. Glia 24 398–407. [PubMed] [Google Scholar]
- Li D., Agulhon C., Schmidt E., Oheim M., Ropert N. (2013). New tools for investigating astrocyte-to-neuron communication. Front. Cell Neurosci. 7:193. 10.3389/fncel.2013.00193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D., Ropert N., Koulakoff A., Giaume C., Oheim M. (2008). Lysosomes are the major vesicular compartment undergoing Ca2+-regulated exocytosis from cortical astrocytes. J. Neurosci. 28 7648–7658. 10.1523/JNEUROSCI.0744-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lia A., Henriques V., Zonta M., Chiavegato A., Carmignoto G., Gómez-Gonzalo M., et al. (2021). Calcium signals in astrocyte microdomains, a decade of great advances. Front. Cell Neurosci. 15:673433. 10.3389/fncel.2021.673433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linsambarth S., Carvajal F., Moraga-Amaro R., Mendez L., Tamburini G., Jimenez I., et al. (2022). Astroglial gliotransmitters released via Cx43 hemichannels regulate NMDAR-dependent transmission and short-term fear memory in the basolateral amygdala. FASEB J. 36:e22134. 10.1096/fj.202100798RR [DOI] [PubMed] [Google Scholar]
- Liu J., Feng X., Wang Y., Xia X., Zheng J. (2022). Astrocytes: GABAceptive and GABAergic Cells in the Brain. Front. Cell Neurosci. 16:892497. 10.3389/fncel.2022.892497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock J., Smith I., Parker I. (2019). Spatial-temporal patterning of Ca2+ signals by the subcellular distribution of IP3 and IP3 receptors. Semin. Cell Dev. Biol. 94 3–10. 10.1016/j.semcdb.2019.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Hidalgo M., Hoover W., Schummers J. (2016). Spatial organization of astrocytes in ferret visual cortex. J. Comp. Neurol. 524 3561–3576. 10.1002/cne.24015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losi G., Mariotti L., Sessolo M., Carmignoto G. (2017). New Tools to Study Astrocyte Ca2+ Signal Dynamics in Brain Networks In Vivo. Front. Cell Neurosci. 11:134. 10.3389/fncel.2017.00134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacVicar B., Newman E. (2015). Astrocyte regulation of blood flow in the brain. Cold Spring Harb. Perspect. Biol. 7:a020388. 10.1101/cshperspect.a020388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud S., Gharagozloo M., Simard C., Gris D. (2019). Astrocytes maintain glutamate homeostasis in the CNS by controlling the balance between glutamate uptake and release. Cells 8:184. 10.3390/cells8020184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malarkey E., Parpura V. (2008). Mechanisms of glutamate release from astrocytes. Neurochem. Int. 52 142–154. 10.1016/j.neuint.2007.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchaland J., Calì C., Voglmaier S., Li H., Regazzi R., Edwards R., et al. (2008). Fast subplasma membrane Ca2+ transients control exo-endocytosis of synaptic-like microvesicles in astrocytes. J. Neurosci. 28 9122–9132. 10.1523/JNEUROSCI.0040-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariotti L., Losi G., Sessolo M., Marcon I., Carmignoto G. (2016). The inhibitory neurotransmitter GABA evokes long-lasting Ca(2+) oscillations in cortical astrocytes. Glia 64 363–373. 10.1002/glia.22933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau M., Shi T., Puyal J., Knolhoff A., Dulong J., Gasnier B., et al. (2013). Storage and uptake of D-serine into astrocytic synaptic-like vesicles specify gliotransmission. J. Neurosci. 33 3413–3423. 10.1523/JNEUROSCI.3497-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matias I., Morgado J., Gomes F. (2019). Astrocyte heterogeneity: Impact to brain aging and disease. Front. Aging Neurosci. 11:59. 10.3389/fnagi.2019.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina J., Giaume C., Tabernero A. (1999). Metabolic coupling and the role played by astrocytes in energy distribution and homeostasis. Adv. Exp. Med. Biol. 468 361–371. 10.1007/978-1-4615-4685-6_28 [DOI] [PubMed] [Google Scholar]
- Mielnicka A., Michaluk P. (2021). Exocytosis in astrocytes. Biomolecules 11:1367. 10.3390/biom11091367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R., Raff M. (1984). Fibrous and protoplasmic astrocytes are biochemically and developmentally distinct. J. Neurosci. 4 585–592. 10.1523/JNEUROSCI.04-02-00585.1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. (2018). Astrocyte heterogeneity in the adult central nervous system. Front. Cell Neurosci. 12:401. 10.3389/fncel.2018.00401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montana V., Ni Y., Sunjara V., Hua X., Parpura V. (2004). Vesicular glutamate transporter-dependent glutamate release from astrocytes. J. Neurosci. 24 2633–2642. 10.1523/JNEUROSCI.3770-03.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Royal C., Dupuis J., Groc L., Oliet S. (2017). Astroglial glutamate transporters in the brain: Regulating neurotransmitter homeostasis and synaptic transmission. J. Neurosci. Res. 95 2140–2151. 10.1002/jnr.24029 [DOI] [PubMed] [Google Scholar]
- Nagy J., Rash J. (2000). Connexins and gap junctions of astrocytes and oligodendrocytes in the CNS. Brain Res. Brain Res. Rev. 32 29–44. 10.1016/s0165-0173(99)00066-1 [DOI] [PubMed] [Google Scholar]
- Nakayama R., Sasaki T., Tanaka K., Ikegaya Y. (2016). Subcellular calcium dynamics during juvenile development in mouse hippocampal astrocytes. Eur. J. Neurosci. 43 923–932. 10.1111/ejn.13188 [DOI] [PubMed] [Google Scholar]
- Nanclares C., Baraibar A., Araque A., Kofuji P. (2021). Dysregulation of astrocyte-neuronal communication in Alzheimer’s disease. Int. J. Mol. Sci. 22:7887. 10.3390/ijms22157887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete M., Araque A. (2014). The Cajal school and the physiological role of astrocytes: A way of thinking. Front. Neuroanat. 8:33. 10.3389/fnana.2014.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarrete M., Perea G., Fernandez de Sevilla D., Gómez-Gonzalo M., Núñez A., Martín E. (2012). Astrocytes mediate in vivo cholinergic-induced synaptic plasticity. PLoS Biol. 10:e1001259. 10.1371/journal.pbio.1001259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard M., Rodríguez J., Verkhratsky A. (2010). Glial calcium and diseases of the nervous system. Cell Calcium 47 140–149. 10.1016/j.ceca.2009.11.010 [DOI] [PubMed] [Google Scholar]
- Nett W., Oloff S., McCarthy K. (2002). Hippocampal astrocytes in situ exhibit calcium oscillations that occur independent of neuronal activity. J. Neurophysiol. 87 528–537. 10.1152/jn.00268.2001 [DOI] [PubMed] [Google Scholar]
- Nimmerjahn A., Kirchhoff F., Kerr J., Helmchen F. (2004). Sulforhodamine 101 as a specific marker of astroglia in the neocortex in vivo. Nat. Methods 1 31–37. 10.1038/nmeth706 [DOI] [PubMed] [Google Scholar]
- Norenberg M. (1979). Distribution of glutamine synthetase in the rat central nervous system. J. Histochem. Cytochem. 27 756–762. 10.1177/27.3.39099 [DOI] [PubMed] [Google Scholar]
- Nwaobi S., Cuddapah V., Patterson K., Randolph A., Olsen M. (2016). The role of glial-specific Kir4.1 in normal and pathological states of the CNS. Acta Neuropathol. 132 1–21. 10.1007/s00401-016-1553-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogata K., Kosaka T. (2002). Structural and quantitative analysis of astrocytes in the mouse hippocampus. Neuroscience 113 221–233. 10.1016/s0306-4522(02)00041-6 [DOI] [PubMed] [Google Scholar]
- Okada M., Fukuyama K., Shiroyama T., Ueda Y. (2021). Brivaracetam prevents astroglial l-glutamate release associated with hemichannel through modulation of synaptic vesicle protein. Biomed. Pharmacother. 138:111462. 10.1016/j.biopha.2021.111462 [DOI] [PubMed] [Google Scholar]
- Okubo Y., Kanemaru K., Suzuki J., Kobayashi K., Hirose K., Iino M. (2019). Inositol 1,4,5-trisphosphate receptor type 2-independent Ca2+ release from the endoplasmic reticulum in astrocytes. Glia 67 113–124. 10.1002/glia.23531 [DOI] [PubMed] [Google Scholar]
- Otsu Y., Couchman K., Lyons D., Collot M., Agarwal A., Mallet J., et al. (2015). Calcium dynamics in astrocyte processes during neurovascular coupling. Nat. Neurosci. 18 210–218. 10.1038/nn.3906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacholko A., Wotton C., Bekar L. (2020). Astrocytes-the ultimate effectors of long-range neuromodulatory networks? Front. Cell Neurosci. 14:581075. 10.3389/fncel.2020.581075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panatier A., Vallée J., Haber M., Murai K., Lacaille J., Robitaille R. (2011). Astrocytes are endogenous regulators of basal transmission at central synapses. Cell 146 785–798. 10.1016/j.cell.2011.07.022 [DOI] [PubMed] [Google Scholar]
- Pandit S., Neupane C., Woo J., Sharma R., Nam M., Lee G., et al. (2020). Bestrophin1-mediated tonic GABA release from reactive astrocytes prevents the development of seizure-prone network in kainate-injected hippocampi. Glia 68 1065–1080. 10.1002/glia.23762 [DOI] [PubMed] [Google Scholar]
- Pangršič T., Potokar M., Stenovec M., Kreft M., Fabbretti E., Nistri A., et al. (2007). Exocytotic release of ATP from cultured astrocytes. J. Biol. Chem. 282 28749–28758. 10.1074/jbc.M700290200 [DOI] [PubMed] [Google Scholar]
- Park A., Croset V., Otto N., Agarwal D., Treiber C., Meschi E., et al. (2022). Gliotransmission of D-serine promotes thirst-directed behaviors in Drosophila. Curr. Biol. 32 3952–3970.e8. 10.1016/j.cub.2022.07.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parpura V., Verkhratsky A. (2012). Astrocytes revisited: Concise historic outlook on glutamate homeostasis and signaling. Croat. Med. J. 53 518–528. 10.3325/cmj.2012.53.518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrushev I., Gavrilov N., Turlapov V., Semyanov A. (2013). Subcellular location of astrocytic calcium stores favors extrasynaptic neuron-astrocyte communication. Cell Calcium 54 343–349. 10.1016/j.ceca.2013.08.003 [DOI] [PubMed] [Google Scholar]
- Paukert M., Agarwal A., Cha J., Doze V., Kang J., Bergles D. (2014). Norepinephrine controls astroglial responsiveness to local circuit activity. Neuron 82 1263–1270. 10.1016/j.neuron.2014.04.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perea G., Araque A. (2005). Glial calcium signaling and neuron-glia communication. Cell Calcium 38 375–382. 10.1016/j.ceca.2005.06.015 [DOI] [PubMed] [Google Scholar]
- Perea G., Gómez R., Mederos S., Covelo A., Ballesteros J., Schlosser L., et al. (2016). Activity-dependent switch of GABAergic inhibition into glutamatergic excitation in astrocyte-neuron networks. eLife 5:e20362. 10.7554/eLife.20362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petravicz J., Fiacco T., McCarthy K. (2008). Loss of IP3 receptor-dependent Ca2+ increases in hippocampal astrocytes does not affect baseline CA1 pyramidal neuron synaptic activity. J. Neurosci. 28 4967–4973. 10.1523/JNEUROSCI.5572-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold G., Murthy V. (2011). Role of astrocytes in neurovascular coupling. Neuron 71 782–797. 10.1016/j.neuron.2011.08.009 [DOI] [PubMed] [Google Scholar]
- Pham C., Hérault K., Oheim M., Maldera S., Vialou V., Cauli B., et al. (2021). Astrocytes respond to a neurotoxic Aβ fragment with state-dependent Ca2+ alteration and multiphasic transmitter release. Acta Neuropathol. Commun. 9:44. 10.1186/s40478-021-01146-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakers C., Petzold G. (2017). Astrocytic calcium release mediates peri-infarct depolarizations in a rodent stroke model. J. Clin. Invest. 127 511–516. 10.1172/JCI89354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransom C., Ye Z., Spain W., Richerson G. (2017). Modulation of Tonic GABA currents by anion channel and connexin hemichannel antagonists. Neurochem. Res. 42 2551–2559. 10.1007/s11064-017-2246-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Refaeli R., Doron A., Benmelech-Chovav A., Groysman M., Kreisel T., Loewenstein Y., et al. (2021). Features of hippocampal astrocytic domains and their spatial relation to excitatory and inhibitory neurons. Glia 69 2378–2390. 10.1002/glia.24044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribot J., Breton R., Calvo C., Moulard J., Ezan P., Zapata J., et al. (2021). Astrocytes close the mouse critical period for visual plasticity. Science 373 77–81. 10.1126/science.abf5273 [DOI] [PubMed] [Google Scholar]
- Robin L., Oliveira da Cruz J., Langlais V., Martin-Fernandez M., Metna-Laurent M., Busquets-Garcia A., et al. (2018). Astroglial CB1 Receptors Determine Synaptic D-Serine Availability to Enable Recognition Memory. Neuron 98 935–944.e5. 10.1016/j.neuron.2018.04.034 [DOI] [PubMed] [Google Scholar]
- Rose C., Ziemens D., Verkhratsky A. (2020). On the special role of NCX in astrocytes: Translating Na+-transients into intracellular Ca2+ signals. Cell Calcium 86:102154. 10.1016/j.ceca.2019.102154 [DOI] [PubMed] [Google Scholar]
- Rungta R., Bernier L., Dissing-Olesen L., Groten C., LeDue J., Ko R., et al. (2016). Ca2+ transients in astrocyte fine processes occur via Ca2+ influx in the adult mouse hippocampus. Glia 64 2093–2103. 10.1002/glia.23042 [DOI] [PubMed] [Google Scholar]
- Saab A., Neumeyer A., Jahn H., Cupido A., Šimek A., Boele H., et al. (2012). Bergmann glial AMPA receptors are required for fine motor coordination. Science. 337 749–753. 10.1126/science.1221140 [DOI] [PubMed] [Google Scholar]
- Semyanov A. (2019). Spatiotemporal pattern of calcium activity in astrocytic network. Cell Calcium 78 15–25. 10.1016/j.ceca.2018.12.007 [DOI] [PubMed] [Google Scholar]
- Semyanov A., Henneberger C., Agarwal A. (2020). Making sense of astrocytic calcium signals - from acquisition to interpretation. Nat. Rev. Neurosci. 21 551–564. 10.1038/s41583-020-0361-8 [DOI] [PubMed] [Google Scholar]
- Semyanov A., Verkhratsky A. (2021). Astrocytic processes: From tripartite synapses to the active milieu. Trends Neurosci. 44 781–792. 10.1016/j.tins.2021.07.006 [DOI] [PubMed] [Google Scholar]
- Shehab S., Cronly-Dillon J., Nona S., Stafford C. (1990). Preferential histochemical staining of protoplasmic and fibrous astrocytes in rat CNS with GFAP antibodies using different fixatives. Brain Res. 518 347–352. 10.1016/0006-8993(90)90996-o [DOI] [PubMed] [Google Scholar]
- Shibasaki K., Ikenaka K., Tamalu F., Tominaga M., Ishizaki Y. (2014). A novel subtype of astrocytes expressing TRPV4 (transient receptor potential vanilloid 4) regulates neuronal excitability via release of gliotransmitters. J. Biol. Chem. 289 14470–14480. 10.1074/jbc.M114.557132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E., Bowser D., Sofroniew M., Khakh B. (2008). Two forms of astrocyte calcium excitability have distinct effects on NMDA receptor-mediated slow inward currents in pyramidal neurons. J. Neurosci. 28 6659–6663. 10.1523/JNEUROSCI.1717-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E., Bushong E., Haustein M., Tong X., Jackson-Weaver O., Kracun S., et al. (2013). Imaging calcium microdomains within entire astrocyte territories and endfeet with GCaMPs expressed using adeno-associated viruses. J. Gen. Physiol. 141 633–647. 10.1085/jgp.201210949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E., Kracun S., Sofroniew M., Khakh B. S. (2010). A genetically targeted optical sensor to monitor calcium signals in astrocyte processes. Nat. Neurosci. 13 759–766. 10.1038/nn.2557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E., Saito K., Sano F., Koizumi S. (2019). Aberrant Calcium Signals in Reactive Astrocytes: A Key Process in Neurological Disorders. Int. J. Mol. Sci. 20:996. 10.3390/ijms20040996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E., Tong X., Kwan K., Corey D., Khakh B. (2011). TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat. Neurosci. 15 70–80. 10.1038/nn.3000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sild M., Van Horn M. (2013). Astrocytes use a novel transporter to fill gliotransmitter vesicles with D-serine: Evidence for vesicular synergy. J. Neurosci. 33 10193–10194. 10.1523/JNEUROSCI.1665-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan S., Barres B. (2014). Looks can be deceiving: Reconsidering the evidence for gliotransmission. Neuron 84 1112–1115. 10.1016/j.neuron.2014.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan R., Huang B., Venugopal S., Johnston A., Chai H., Zeng H., et al. (2015). Ca(2+) signaling in astrocytes from Ip3r2(-/-) mice in brain slices and during startle responses in vivo. Nat. Neurosci. 18 708–717. 10.1038/nn.4001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens C., Wang Y. (1995). Facilitation and depression at single central synapses. Neuron 14 795–802. 10.1016/0896-6273(95)90223-6 [DOI] [PubMed] [Google Scholar]
- Stobart J., Ferrari K., Barrett M., Glück C., Stobart M., Zuend M., et al. (2018). Cortical circuit activity evokes rapid astrocyte calcium signals on a similar timescale to neurons. Neuron 98 726–735.e4. 10.1016/j.neuron.2018.03.050 [DOI] [PubMed] [Google Scholar]
- Südhof T. (2012). Calcium control of neurotransmitter release. Cold Spring Harb. Perspect. Biol. 4:a011353. 10.1101/cshperspect.a011353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M., Devaraju P., Xie A., Holman I., Samones E., Murphy T., et al. (2014). Astrocyte calcium microdomains are inhibited by bafilomycin A1 and cannot be replicated by low-level Schaffer collateral stimulation in situ. Cell Calcium 55 1–16. 10.1016/j.ceca.2013.10.004 [DOI] [PubMed] [Google Scholar]
- Sun W., Cornwell A., Li J., Peng S., Osorio M., Aalling N., et al. (2017). SOX9 is an astrocyte-specific nuclear marker in the adult brain outside the neurogenic regions. J. Neurosci. 37 4493–4507. 10.1523/JNEUROSCI.3199-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takata N., Mishima T., Hisatsune C., Nagai T., Ebisui E., Mikoshiba K., et al. (2011). Astrocyte calcium signaling transforms cholinergic modulation to cortical plasticity in vivo. J. Neurosci. 31 18155–18165. 10.1523/JNEUROSCI.5289-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapanes S., Arizanovska D., Díaz M., Folorunso O., Harvey T., Brown S., et al. (2022). Inhibition of glial D-serine release rescues synaptic damage after brain injury. Glia 70 1133–1152. 10.1002/glia.24161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong X., Shigetomi E., Looger L., Khakh B. (2013). Genetically encoded calcium indicators and astrocyte calcium microdomains. Neuroscientist 19 274–291. 10.1177/1073858412468794 [DOI] [PubMed] [Google Scholar]
- Turovsky E., Theparambil S., Kasymov V., Deitmer J., Del Arroyo A., Ackland G., et al. (2016). Mechanisms of CO2/H+ Sensitivity of Astrocytes. J. Neurosci. 36 10750–10758. 10.1523/JNEUROSCI.1281-16.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardy E., Robinson J., Li C., Olsen R., DiBerto J., Giguere P., et al. (2015). A New DREADD facilitates the multiplexed chemogenetic interrogation of behavior. Neuron 86 936–946. 10.1016/j.neuron.2015.03.065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A., Nedergaard M. (2018). Physiology of astroglia. Physiol. Rev. 98 239–389. 10.1152/physrev.00042.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A., Trebak M., Perocchi F., Khananshvili D., Sekler I. (2018). Crosslink between calcium and sodium signalling. Exp. Physiol. 103 157–169. 10.1113/EP086534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volterra A., Liaudet N., Savtchouk I. (2014). Astrocyte Ca2+ signalling: An unexpected complexity. Nat. Rev. Neurosci. 15 327–335. 10.1038/nrn3725 [DOI] [PubMed] [Google Scholar]
- Wang F., Smith N., Xu Q., Fujita T., Baba A., Matsuda T., et al. (2012). Astrocytes modulate neural network activity by Ca2+-dependent uptake of extracellular K+. Sci. Signal. 5 ra26. 10.1126/scisignal.2002334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Smith N., Xu Q., Goldman S., Peng W., Huang J., et al. (2013). Photolysis of caged Ca2+ but not receptor-mediated Ca2+ signaling triggers astrocytic glutamate release. J. Neurosci. 33 17404–17412. 10.1523/JNEUROSCI.2178-13.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Lou N., Xu Q., Tian G., Peng W., Han X., et al. (2006). Astrocytic Ca2+ signaling evoked by sensory stimulation in vivo. Nat. Neurosci. 9 816–823. 10.1038/nn1703 [DOI] [PubMed] [Google Scholar]
- Wang Y., DelRosso N., Vaidyanathan T., Cahill M., Reitman M., Pittolo S., et al. (2019). Accurate quantification of astrocyte and neurotransmitter fluorescence dynamics for single-cell and population-level physiology. Nat. Neurosci. 22 1936–1944. 10.1038/s41593-019-0492-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolosker H., Blackshaw S., Snyder S. (1999). Serine racemase: A glial enzyme synthesizing D-serine to regulate glutamate-N-methyl-D-aspartate neurotransmission. Proc. Natl. Acad. Sci. U.S.A. 96 13409–13414. 10.1073/pnas.96.23.13409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo D., Han K., Shim J., Yoon B., Kim E., Bae J., et al. (2012). TREK-1 and Best1 channels mediate fast and slow glutamate release in astrocytes upon GPCR activation. Cell 151 25–40. 10.1016/j.cell.2012.09.005 [DOI] [PubMed] [Google Scholar]
- Wu Y., Gordleeva S., Tang X., Shih P., Dembitskaya Y., Semyanov A. (2019). Morphological profile determines the frequency of spontaneous calcium events in astrocytic processes. Glia 67 246–262. 10.1002/glia.23537 [DOI] [PubMed] [Google Scholar]
- Xin W., Bonci A. (2018). Functional astrocyte heterogeneity and implications for their role in shaping neurotransmission. Front. Cell Neurosci. 12:141. 10.3389/fncel.2018.00141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y., Teng S., Zheng L., Sun S., Li J., Guo N., et al. (2018). Stretch-induced Ca2+ independent ATP release in hippocampal astrocytes. J. Physiol. 596 1931–1947. 10.1113/JP275805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Peng H., Kang N., Zhao Z., Lin J., Stanton P., et al. (2007). Glutamate-induced exocytosis of glutamate from astrocytes. J. Biol. Chem. 282 24185–24197. 10.1074/jbc.M700452200 [DOI] [PubMed] [Google Scholar]
- Yang J., Vitery M., Chen J., Osei-Owusu J., Chu J., Qiu Z. (2019). Glutamate-Releasing SWELL1 channel in astrocytes modulates synaptic transmission and promotes brain damage in stroke. Neuron 102 813–827.e6. 10.1016/j.neuron.2019.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon B., Lee C. (2014). GABA as a rising gliotransmitter. Front. Neural Circuits 8:141. 10.3389/fncir.2014.00141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Moye S., Khakh B. (2021). Local and CNS-Wide astrocyte intracellular calcium signaling attenuation in vivo with calexflox mice. J. Neurosci. 41 4556–4574. 10.1523/JNEUROSCI.0085-21.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Nagai J., Khakh B. (2020). Improved tools to study astrocytes. Nat. Rev. Neurosci. 21 121–138. 10.1038/s41583-020-0264-8 [DOI] [PubMed] [Google Scholar]
- Yu X., Taylor A., Nagai J., Golshani P., Evans C., Coppola G., et al. (2018). Reducing astrocyte calcium signaling in vivo alters striatal microcircuits and causes repetitive behavior. Neuron 99 1170–1187.e9. 10.1016/j.neuron.2018.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N., Ding S. (2018). Imaging of Mitochondrial and Cytosolic Ca2+ Signals in Cultured Astrocytes. Curr. Protoc. Neurosci. 82 2.29.1–2.29.11. 10.1002/cpns.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Pangrsic T., Kreft M., Krzan M., Li N., Sul J., et al. (2004). Fusion-related release of glutamate from astrocytes. J. Biol. Chem. 279 12724–12733. 10.1074/jbc.M312845200 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Barres B. (2010). Astrocyte heterogeneity: An underappreciated topic in neurobiology. Curr. Opin. Neurobiol. 20 588–594. 10.1016/j.conb.2010.06.005 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Chen G., Zhou W., Song A., Xu T., Luo Q., et al. (2007). Regulated ATP release from astrocytes through lysosome exocytosis. Nat. Cell Biol. 9 945–953. 10.1038/ncb1620 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Ma Z., Zou W., Guo H., Liu M., Ma Y., et al. (2019). The appropriate marker for astrocytes: Comparing the distribution and expression of three astrocytic markers in different mouse cerebral regions. Biomed. Res. Int. 2019:9605265. 10.1155/2019/9605265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou M., Xu G., Xie M., Zhang X., Schools G., Ma L., et al. (2009). TWIK-1 and TREK-1 are potassium channels contributing significantly to astrocyte passive conductance in rat hippocampal slices. J. Neurosci. 29 8551–8564. 10.1523/JNEUROSCI.5784-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorec R., Araque A., Carmignoto G., Haydon P., Verkhratsky A., Parpura V. (2012). Astroglial excitability and gliotransmission: An appraisal of Ca2+ as a signalling route. ASN Neuro 4:e00080. 10.1042/AN20110061 [DOI] [PMC free article] [PubMed] [Google Scholar]