Abstract
The Mobile Health Technology for Improved Screening and Optimized Integrated Care in AF (mAFA-II) cluster-randomized trial showed that a mobile health (mHealth)-implemented ‘Atrial fibrillation Better Care’ (ABC) pathway approach reduced the risk of adverse events in atrial fibrillation (AF) patients. Whether this benefit can be applied to both males and females is unclear, especially given the suboptimal management and poorer cardiovascular outcomes in females with AF. In this post-hoc analysis, we performed a sex-stratified analysis of the mAFA-II trial. Between June 2018 and August 2019, adult AF patients were enrolled across 40 centers in China. The primary outcome was the composite of stroke, thromboembolism, all-cause death, and re-hospitalization. The effect of mAFA intervention according to sex was evaluated through adjusted Cox-regression models. Among the 3,324 patients enrolled in the trial, 2,062 (62.0%) patients were males (mean age: 67.5 ± 14.3 years; 1,021 allocated to mAFA intervention) and 1,262 (38.0%) were females (mean age: 70.2 ± 13.0; 625 allocated to mAFA intervention). A significant risk reduction of the primary composite outcome in patients allocated to mAFA intervention was observed in both males (adjusted hazard ratio [aHR] and 95% confidence interval [CI] 0.30 [0.17–0.52]) and females (aHR [95%CI] 0.50 [0.27–0.92]), without statistically significant interaction (p = 0.225). Sex-based interactions were observed for other secondary outcomes, including all-cause death (p = 0.026) and bleeding events (p = 0.032). A mHealth-technology implemented ABC pathway was similarly effective in reducing the risk of adverse clinical events both in male and female patients. Secondary outcomes showed greater benefits of mAFA intervention in men.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11739-022-03188-2.
Keywords: Atrial fibrillation, Integrated care, Women, Sex, Outcomes
Introduction
Atrial fibrillation (AF) is the most common atrial arrhythmia worldwide and is projected to affect 14 million patients in 2060 in Europe alone [1, 2]. Several characteristics influence the epidemiology and natural history of AF; among these, sex is one of the most investigated, with men showing a higher prevalence of AF [3], and women experiencing worse AF-related prognosis [4–6]. The higher thromboembolic risk of women is also reflected by the inclusion of female sex as a risk modifier in the CHA2DS2-VASc score, used to stratify thromboembolic risk in AF patients [4, 7, 8]
Female AF patients also show a higher burden of AF-related symptoms and a lower quality of life compared to males [9, 10]. Several reasons can explain this, including the lower adoption of rhythm control strategies in females [7], which influences the burden of symptoms reported.
The ‘Atrial Fibrillation Better Care’ (ABC) pathway has been proposed to streamline the implementation of a holistic and integrated care program and improve the prognosis of AF patients, based on three pillars [11]: A, anticoagulation/avoiding stroke; B, better symptom control, and C, optimization of cardiovascular comorbidities, including lifestyle changes. The ABC pathway has been already associated with the reduction of major outcomes in AF patients [12], and is currently recommended as a patient-centered approach by international guidelines [13, 14].
The Mobile Health Technology for Improved Screening and Optimized Integrated Care in AF (mAFA-II) trial evaluated the efficacy of a mobile health (mHealth)-implemented ABC pathway approach (mAFA intervention) in reducing the risk of adverse outcomes among AF patients [15, 16]. The primary analysis showed that mAFA intervention, compared to usual care, reduced the risk of the composite outcomes of ischemic stroke (IS) and systemic thromboembolism (TE), all-cause death, and re-hospitalization [15].
Nevertheless, it is unclear if the effect of mAFA intervention is consistent in both sexes; moreover, the importance of reporting sex-disaggregated data and sex-specific analyses in medical research has been repeatedly underlined, to improve our understanding of sex-based differences and ultimately lead to tailored and effective strategies in both females and males [17, 18]. In this post hoc ancillary analysis, we sought to evaluate the effectiveness of the mAFA intervention in male and female patients with AF, through a sex-stratified analysis of the mAFA-II trial.
Methods
A detailed description of the rationale, design, and primary results of the mAFA-II trial has been previously published and can be found elsewhere [15, 16]. In brief, the mAFA-II trial was a prospective, cluster-randomized multi-center trial that enrolled adults with AF (≥ 18 years old) across 40 centers in China, that were randomized in a 1:1 ratio to the mAFA intervention or usual care. Patients with mechanical prosthetic valves, those with moderate-to-severe mitral stenosis, and those unable to complete 1 year of follow-up for any reason were excluded. Between June 2018 and August 2019, 1,646 subjects with AF were allocated to mAFA intervention, while 1,678 AF patients were allocated to usual care. The study was approved by the Central Medical Ethic Committee of Chinese PLA General Hospital and by local institutional review boards, and was conducted in accordance with the Declaration of Helsinki and the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline; all the patients gave their written informed consent.
In this post hoc ancillary analysis, we evaluated the effect of mAFA intervention according to the sex of the participants.
mAFA intervention
The mAFA intervention group implemented the ABC pathway according to the following criteria:
‘A’ criterion: anticoagulation prescription according to regular assessment of thromboembolic and bleeding risk, with dose adjustment based on renal and liver function reassessment;
‘B’ criterion: regular monitoring of patient-reported symptoms (which were assessed according to the European Heart Rhythm Association classification), and management of symptoms that included antiarrhythmics and rhythm control treatments;
‘C’ criterion: active management and treatment optimization of concurrent comorbidities (e.g., hypertension management according to blood pressure monitoring, statin treatment in patients with vascular diseases, etc.). Patients were also provided with educational material and lifestyle recommendations.
Conversely, patients allocated to “usual care” were managed according to local practices.
Outcomes and follow-up
Follow-up was performed 6 and 12 months after the inclusion. Consistent with the primary trial analysis, the primary endpoint was the composite outcome of IS or systemic TE, all-cause death, and re-hospitalization. Other endpoints investigated were TE (defining as IS or other systemic TE), bleeding events (intracranial and/or extracranial), cardiovascular outcomes (recurrent AF, heart failure (HF), acute coronary syndrome), all-cause death, and re-hospitalization.
Statistical analysis
Baseline characteristics were reported as mean and standard deviation (SD) for normally distributed continuous variables, or median and interquartile range [IQR] for non-normally distributed continuous variables. Binary and categorical variables were reported as frequency and percentage.
For the purpose of this analysis, we analyzed the interactions between sex and the effect of mAFA intervention on the primary and secondary outcomes, using Cox proportional hazard models. All the models were adjusted for age, type of AF, comorbidities (hypertension, diabetes mellitus, CAD, history of HF, history of IS, peripheral artery disease (PAD)), previous AF treatments, and cluster effect. Survival curves were also reported for the risk of the primary composite outcome according to mAFA allocation and sex.
A two-sided p value < 0.05 was considered statistically significant. All statistical analyses were conducted using R 4.2.0 (R Foundation for Statistical Computing 2020, Vienna, Austria).
Results
Overall, 3,324 patients were enrolled in the mAFA-II trial (Fig. 1); of these, 2,062 (62.0%) were males (mean age ± SD: 67.5 ± 14.3), while 1,262 (38.0%) were females (mean age ± SD: 70.2 ± 13.0). Among males, 1,021 (49.5%) were allocated to mAFA intervention, while 625 females (49.5%) were allocated to mAFA intervention.
Fig. 1.
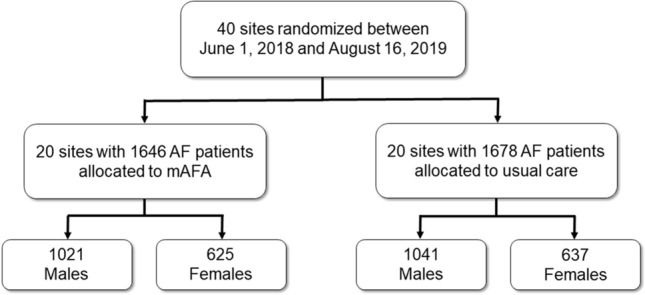
Flowchart of the mAFA-II trial. AF atrial fibrillation
Baseline characteristics according to mAFA allocation and sex are reported in Table 1. Compared to the usual care group, male patients in the mAFA intervention were younger and had a lower burden of comorbidities (prior IS, coronary artery disease (CAD), PAD, and prior brain bleeding); among females, those allocated to the mAFA intervention showed a higher prevalence of several comorbidities (HF, prior IS, PAD, and pulmonary hypertension) compared to the control group. Patients in the mAFA intervention were more likely to have received pharmacological cardioversion in both sexes compared to usual care. Treatments prescribed at the time of the enrollment are reported according to sex and allocation in Supplementary Materials, Table S1. In both sexes, patients allocated to mAFA intervention were more frequently treated with non-vitamin K antagonist oral anticoagulants (NOACs) (p < 0.001) and less likely to receive antiplatelet drugs.
Table 1.
Demographic characteristics
| Males | Females | |||||
|---|---|---|---|---|---|---|
| Variables, n (%) | mAFA (n = 1021) | Usual care (n = 1041) |
p | mAFA (n = 625) | Usual care (n = 637) | p |
| Age, mean ± SD | 65.2 ± 15.1 | 69.7 ± 13.2 | < 0.001 | 69.7 ± 13.4 | 70.8 ± 12.5 | 0.119 |
| Current smoking | 140 (13.7) | 143 (13.7) | 1.000 | 19 (3.0) | 25 (3.9) | 0.482 |
| Hypertension | 530 (51.9) | 576 (55.3) | 0.130 | 378 (60.5) | 386 (60.6) | 1.000 |
| CAD | 373 (36.5) | 447 (42.9) | 0.003 | 262 (41.9) | 277 (43.5) | 0.614 |
| Diabetes mellitus | 220 (21.5) | 231 (22.2) | 0.764 | 161 (25.8) | 135 (21.2) | 0.065 |
| HF at baseline | 198 (19.4) | 231 (22.2) | 0.131 | 162 (25.9) | 123 (19.3) | 0.006 |
| Prior ischemic stroke | 90 (8.8) | 182 (17.5) | < 0.001 | 101 (16.2) | 50 (7.8) | < 0.001 |
| PAD | 89 (8.7) | 121 (11.6) | 0.035 | 83 (13.3) | 51 (8.0) | 0.003 |
| Renal dysfunction | 93 (9.1) | 121 (11.6) | 0.072 | 45 (7.2) | 51 (8.0) | 0.664 |
| Pulmonary hypertension | 32 (3.1) | 50 (4.8) | 0.068 | 55 (8.8) | 33 (5.2) | 0.016 |
| Liver dysfunction | 36 (3.5) | 34 (3.3) | 0.838 | 19 (3.0) | 14 (2.2) | 0.447 |
| Prior thromboembolism | 36 (3.5) | 43 (4.1) | 0.548 | 18 (2.9) | 16 (2.5) | 0.818 |
| Prior brain bleeding | 14 (1.4) | 31 (3.0) | 0.019 | 10 (1.6) | 7 (1.1) | 0.598 |
| Prior other bleeding | 29 (2.8) | 44 (4.2) | 0.113 | 25 (4.0) | 23 (3.6) | 0.830 |
| Dilated cardiomyopathy | 31 (3.0) | 50 (4.8) | 0.051 | 13 (2.1) | 11 (1.7) | 0.800 |
| Hyperthyroidism | 23 (2.3) | 28 (2.7) | 0.619 | 14 (2.2) | 23 (3.6) | 0.202 |
| Hypertrophic cardiomyopathy | 17 (1.7) | 23 (2.2) | 0.461 | 8 (1.3) | 6 (0.9) | 0.761 |
| Type of AF | < 0.001 | < 0.001 | ||||
| Unknown | 175 (17.2) | 64 (6.2) | 106 (17.2) | 49 (7.7) | ||
| New-onset AF | 119 (11.7) | 150 (14.4) | 76 (12.3) | 82 (12.9) | ||
| Paroxysmal AF | 419 (41.2) | 403 (38.8) | 254 (41.2) | 257 (40.3) | ||
| Persistent AF | 247 (24.3) | 290 (27.9) | 133 (21.6) | 158 (24.8) | ||
| Long-standing AF | 33 (3.2) | 61 (5.9) | 23 (3.7) | 40 (6.3) | ||
| Permanent AF | 23 (2.3) | 72 (6.9) | 25 (4.1) | 51 (8.0) | ||
| Prior AF treatment | ||||||
| Pharmacological cardioversion | 127 (12.4) | 99 (9.5) | 0.040 | 86 (13.8) | 56 (8.8) | 0.007 |
| Electrical cardioversion | 16 (1.6) | 28 (2.7) | 0.107 | 14 (2.2) | 7 (1.1) | 0.172 |
| AF ablation | 114 (11.2) | 107 (10.3) | 0.562 | 69 (11.0) | 66 (10.4) | 0.765 |
| Pacemaker | 47 (4.6) | 59 (5.7) | 0.320 | 29 (4.6) | 26 (4.1) | 0.728 |
| LAAO | 13 (1.3) | 25 (2.4) | 0.082 | 20 (3.2) | 5 (0.8) | 0.004 |
| Scores | ||||||
| CHA2DS2-VASc, median [IQR] | 2 [1–3] | 2 [1–3] | 0.045 | 3 [3–4] | 3 [2–4] | < 0.001 |
| HAS-BLED, median [IQR] | 1 [0–2] | 1 [1–2] | < 0.001 | 2 [1–2] | 1 [1, 2] | 0.122 |
CAD coronary artery disease, HF heart failure, IQR interquartile range, LAAO left atrial appendage occlusion, PAD peripheral artery disease, SD standard deviation; p values <0.05 are reported in bold.
Risk of major outcomes according to mAFA intervention
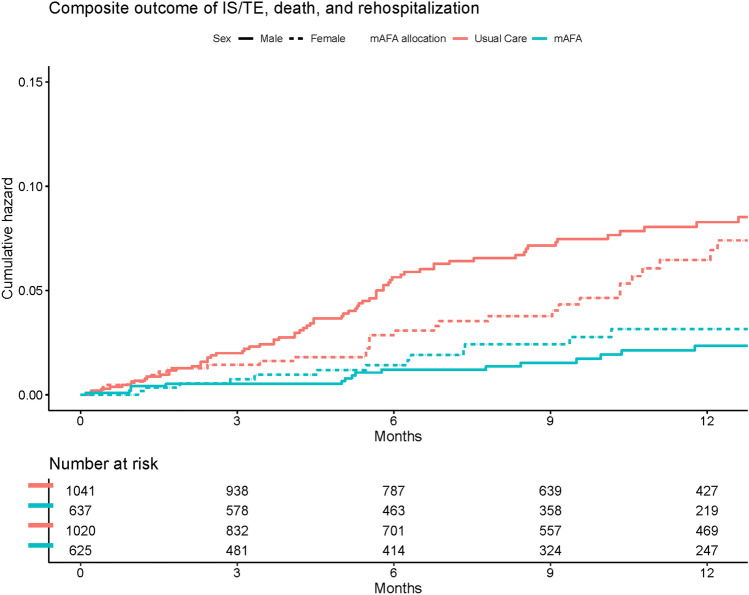
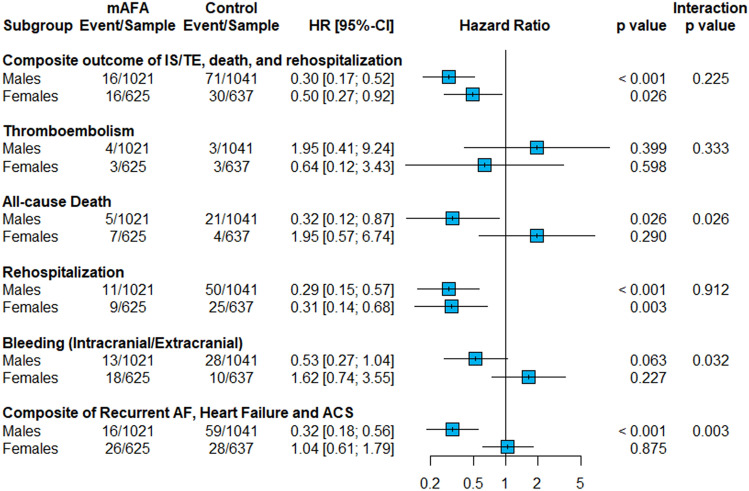
Survival curves for the primary composite outcome according to sex and mAFA allocation are reported in Fig. 2, while the results of the analysis on the interaction between sex and the effect of mAFA intervention on the risk of primary and secondary outcomes are reported in Fig. 3. Both male and female patients allocated to mAFA intervention showed a reduced risk of the primary composite outcome of IS/TE, all-cause death and re-hospitalization (aHR [95%CI] 0.30 [0.17–0.52] and 0.50 [0.27–0.92] for males and females, respectively), with no significant sex-based interaction (pint = 0.225). Among the exploratory secondary outcomes, a statistically significant sex-based interaction was observed for the all-cause death (pint = 0.026), bleeding events (pint = 0.032) and the composite outcome of recurrent AF, heart failure, and ACS (pint = 0.003); for all these outcomes, the effect of mAFA intervention in reducing the risk of the events was higher in male patients. No statistically significant interaction was observed for the risk of thromboembolism and re-hospitalizations alone.
Fig. 2.
Survival curves for the primary composite outcome, stratified by sex and mAFA allocation. p < 0.001 for males, p = 0.074 for females. mAFA intervention group = blue, control group = red, male sex = continuous line, female sex = dashed line. AF atrial fibrillation, IS ischemic stroke, TE thromboembolism
Fig. 3.
Cox-regression analysis on the interaction between sex and mAFA intervention on the risk of primary and secondary outcomes. AF atrial fibrillation; HR hazard ratio, IS ischemic stroke, TE thromboembolism; *adjusted for age, type of AF, previous AF treatments, hypertension, diabetes mellitus, coronary artery disease, prior ischemic stroke, peripheral artery disease, chronic heart failure, and cluster factor
Discussion
In this ancillary analysis of the mAFA-II trial, our principal findings are as follows: (i) the mAFA intervention reduced the risk of the primary composite outcome of IS, TE, all-cause death, and re-hospitalization in both sexes; (ii) the magnitude of the risk reduction appeared higher among males; and (iii) a sex-based interaction was observed for some of the exploratory secondary outcomes, including all-cause death and bleeding events, with the effect of mAFA intervention being higher among male patients.
There has been growing interest in the potential sex-based differences in AF patients, from the pathophysiology and the accumulation of risk factors to the risk of adverse outcomes, including stroke [6, 19, 20]. Notwithstanding the under-representation of women in clinical trials [21], female AF patients experience a significant burden of symptoms [22, 23] and worse prognosis [6, 24], while also being often undertreated in clinical practice [23, 25]. Therefore, females represent one key group of AF patients with an unmet need for better management and improved outcomes.
Recent international guidelines [13, 14] have underlined the need for an integrated care approach to optimize the management of AF patients, and the ABC pathway has been proposed to streamline such a holistic bundle of care [11]. While the ABC pathway has been already proven effective in improving outcomes among AF patients [12, 26, 38], previous subgroups’ analysis of retrospective studies have suggested that potential sex-based differences may exist in the efficacy of the ABC pathway, with women potentially experiencing a lower magnitude of effect [27].
In this post hoc analysis from the mAFA-II trial, we showed how a mHealth-technology implemented ABC pathway consistently reduces the risk of the primary composite outcome of IS/TE, all-cause death, and re-hospitalizations in both sexes, without a statistically significant sex-based interaction. Nevertheless, we observed a trend toward a higher magnitude of risk reduction among male patients, and this finding was consistent with the analysis of the exploratory secondary outcomes, which showed a sex-based interaction for the effect of the ABC pathway on the risk of all-cause death, bleeding events, and the composite of non-fatal cardiovascular outcomes.
Several hypotheses may contribute to the results observed. First, in our study, male patients allocated to mAFA intervention were younger than those allocated to usual care, and with an overall lower burden of comorbidities at baseline, including CAD, PAD, and history of previous IS and bleeding events. On the other side, women allocated to mAFA showed higher prevalence of HF, PAD. and a history of IS. Taken together, while these imbalances in baseline characteristics can be explained by the cluster randomization design of the trial, they may have contributed to the higher magnitude of the effect observed among male patients compared to females allocated to mAFA intervention.
Moreover, both males and females allocated to mAFA intervention more frequently received OAC—and, specifically, NOACs—when compared to those who received usual care. The higher uptake of OAC can be seen both as a direct effect of the implementation of the mAFA intervention in these patients (with optimisation of stroke prevention as part of the mHealth-implemented ABC pathway), and one key determinants of the beneficial effects of mAFA in these patients. These findings reinforce the hypothesis that a holistic or integrated care approach improves management of AF patients, leading to better outcomes.
Nonetheless, it has already been shown that women may present with atypical symptoms of AF [28], which may contribute to challenges in the diagnosis and management; furthermore, several reports have identified female sex as being associated with less efficacy of rhythm control strategies in AF patients, including catheter ablation, and also higher rate of procedural complications [29–32]. Taken together, these data suggest that achievement of symptoms control may be more challenging in women than in men, and this may lead to worse quality of life and, ultimately, worse prognosis.
Finally, the role of social determinants of health in influencing the natural history and outcomes in AF is increasingly recognized [33, 34], and currently represents one of the most unmet needs in the management of AF patients. The detrimental impact of socioeconomic factors on cardiovascular outcomes, especially among women, has been already established [35]; moreover, women are disproportionately affected by social disparities and social deprivation, thus leading to overall low access to health resources, low quality of life, and worse prognosis [36]. Consistently, a recent study has shown how low educational status, low income, and living alone were all associated with a lower uptake of AF ablation after an incident AF diagnosis [37], further underlying how these issues are crucial—although often overlooked—in ensuring optimal management and prognosis in AF patients. Of note, the ABC pathway is not specifically designed to target the social determinants of health, and this may contribute to the findings observed in our study, especially considering how these issues disproportionately affect women.
Overall, while confirming the efficacy of the ABC pathway in both sexes for the primary composite outcome, our findings are consistent with the previously reported trend of a potential sex-based difference in the efficacy of the ABC pathway [27]. These observations have several important clinical implications: first, the implementation of a mHealth technology-implemented ABC pathway to streamline an integrated care approach may help in reducing the health gap between male and female AF patients, otherwise wider, and therefore the implementation of this approach should be encouraged in both sexes. Notwithstanding, women may experience an attenuated effect of the ABC pathway for several reasons, including differences in the pathophysiology and clinical presentation of AF, and the higher impact of non-traditional risk factors (including social determinants of health), which require tailored strategies to reduce the risk of adverse outcomes in females. Specifically, increasing the awareness of the sex-based differences in the natural history of AF, and implementing strategies to tackle the social inequalities and barriers which may influence the access to care and prognosis of women, may represent two of the most crucial interventions that may reduce the inequalities between sexes. While the ABC pathway represents the cornerstone of treatment for AF patients, the combination of an integrated care approach with sex-tailored strategies may represent the key to further improving outcomes in AF patients, especially in females.
Strengths and limitations
Our study is the first analysis to provide a sex-stratified analysis on the efficacy of a mHealth-implemented ABC pathway, and will be particularly useful to inform sex-specific recommendations and guidance, especially given the urgent need for sex-disaggregated data in this scenario [21]. Furthermore, our results were largely consistent with the primary analysis of the trial, contributing to the reliability of our estimates.
Nonetheless, our study has some limitations. First, this was a post hoc analysis of a cluster-randomized trial and may lack statistical power for some of the outcomes investigated, and for the specific subgroups examined about which the trial was not originally powered. Second, there were some imbalances on the baseline characteristics in both males and females allocated to mAFA intervention vs. usual care. While this is compatible with the randomized cluster design of the trial, this may have contributed, at least partly, to the results observed. Third, we were unable to evaluate the role of the social determinants of health (SDOH) in determining the results observed. Further studies are required to evaluate the role of SDOH and the potential sex-based differences in this clinical context. Fourth, although we adjusted our analyses for several potential moderators, we cannot exclude the effect of unaccounted confounders on the findings observed.
Conclusion
In this post hoc analysis of the mAFA-II trial, we found that a mHealth-technology implemented ABC pathway was similarly effective in reducing the risk of adverse clinical events both in male and female patients. Secondary outcomes showed greater benefits of mAFA intervention in men.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful to all participants in the mAFA programme for their contribution.
Funding
This research was funded by the National Natural Science Foundation of China (82170309). This study was an investigator-initiated project, with limited funding by independent research and educational grants.
Data availability
Data supporting the current study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
GYHL has been consultant and speaker for BMS/Pfizer, Boehringer Ingelheim and Daiichi-Sankyo. No fees are directly received personally. All the disclosures happened outside the submitted work. All other authors have nothing to declare.
Ethical approval
The study was approved by the Central Medical Ethic Committee of Chinese PLA General Hospital and by local institutional review boards, and was conducted in accordance with the Declaration of Helsinki and the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Informed consent
All the patients gave their written informed consent.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Joint first authors Yutao Guo and Bernadette Corica.
References
- 1.Kornej J, Börschel CS, Benjamin EJ, Schnabel RB. Epidemiology of atrial fibrillation in the 21st century: novel methods and new insights. Circ Res. 2020;127:4–20. doi: 10.1161/CIRCRESAHA.120.316340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krijthe BP, Kunst A, Benjamin EJ, et al. Projections on the number of individuals with atrial fibrillation in the European union, from 2000 to 2060. Eur Heart J. 2013;34:2746–2751. doi: 10.1093/EURHEARTJ/EHT280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang J, Johnsen SP, Guo Y, Lip GYH. Epidemiology of atrial fibrillation: geographic/ecological risk factors, age, sex, genetics. Cardiac Electrophysiology Clinics. 2021;13:1–23. doi: 10.1016/J.CCEP.2020.10.010. [DOI] [PubMed] [Google Scholar]
- 4.Friberg L, Benson L, Rosenqvist M, Lip GYH. Assessment of female sex as a risk factor in atrial fibrillation in Sweden: nationwide retrospective cohort study. BMJ. 2012;344:e3522–e3522. doi: 10.1136/bmj.e3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Friberg J, Scharling H, Gadsbøll N, et al. Comparison of the impact of atrial fibrillation on the risk of stroke and cardiovascular death in women versus men (the Copenhagen city heart study) Am J Cardiol. 2004;94:889–894. doi: 10.1016/J.AMJCARD.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 6.Marzona I, Proietti M, Farcomeni A, et al. Sex differences in stroke and major adverse clinical events in patients with atrial fibrillation: a systematic review and meta-analysis of 993,600 patients. Int J Cardiol. 2018;269:182–191. doi: 10.1016/J.IJCARD.2018.07.044. [DOI] [PubMed] [Google Scholar]
- 7.Dagres N, Nieuwlaat R, Vardas PE, et al. Gender-related differences in presentation, treatment, and outcome of patients with atrial fibrillation in Europe: a report from the Euro heart survey on atrial fibrillation. J Am Coll Cardiol. 2007;49:572–577. doi: 10.1016/J.JACC.2006.10.047. [DOI] [PubMed] [Google Scholar]
- 8.Lip GYH, Nieuwlaat R, Pisters R, et al. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/CHEST.09-1584. [DOI] [PubMed] [Google Scholar]
- 9.Brundel BJJM, Ai X, Hills MT, et al. Atrial fibrillation. Nat Rev Dis Primers. 2022 doi: 10.1038/s41572-022-00347-9. [DOI] [PubMed] [Google Scholar]
- 10.von Eisenhart RA, Hutt F, Baumert J, et al. Depressed mood amplifies heart-related symptoms in persistent and paroxysmal atrial fibrillation patients: a longitudinal analysis—data from the German competence network on atrial fibrillation. Europace. 2015;17:1354–1362. doi: 10.1093/europace/euv018. [DOI] [PubMed] [Google Scholar]
- 11.Lip GYH. The ABC pathway: an integrated approach to improve AF management. Nat Rev Cardiol. 2017;14:627–628. doi: 10.1038/NRCARDIO.2017.153. [DOI] [PubMed] [Google Scholar]
- 12.Romiti GF, Pastori D, Rivera-Caravaca JM, et al. Adherence to the “Atrial Fibrillation Better care” pathway in patients with atrial fibrillation: impact on clinical outcomes-a systematic review and meta-analysis of 285,000 patients. Thromb Haemost. 2022;122:406–414. doi: 10.1055/A-1515-9630. [DOI] [PubMed] [Google Scholar]
- 13.Hindricks G, Potpara T, Dagres N, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association for cardio-thoracic surgery (EACTS): the task force for the diagnosis and management of atrial fibrillation of the Europea. Eur Heart J. 2021;42:373–498. doi: 10.1093/EURHEARTJ/EHAA612. [DOI] [PubMed] [Google Scholar]
- 14.Chao TF, Joung B, Takahashi Y, et al. Focused update consensus guidelines of the Asia Pacific heart rhythm society on stroke prevention in atrial fibrillation: executive summary. Thromb Haemost. 2022;122:20–47. doi: 10.1055/S-0041-1739411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo Y, Lane DA, Wang L, et al. Mobile health technology to improve care for patients With atrial fibrillation. J Am Coll Cardiol. 2020;75:1523–1534. doi: 10.1016/J.JACC.2020.01.052. [DOI] [PubMed] [Google Scholar]
- 16.Guo Y, Lane DA, Wang L, et al. Mobile Health (mHealth) technology for improved screening, patient involvement and optimising integrated care in atrial fibrillation: the mAFA (mAF-App) II randomised trial. Inter J Clinical Practice. 2019 doi: 10.1111/ijcp.13352. [DOI] [PubMed] [Google Scholar]
- 17.Avery E, Clark J. Sex-related reporting in randomised controlled trials in medical journals. The Lancet. 2016;388:2839–2840. doi: 10.1016/S0140-6736(16)32393-5. [DOI] [PubMed] [Google Scholar]
- 18.(2022) Raising the bar on sex and gender reporting in research. Nature Medicine 28 (6) 1099–1099. 10.1038/s41591-022-01860-w [DOI] [PubMed]
- 19.Kloosterman M, Crijns HJGM, Investigators for the RI et al. Sex-related differences in risk factors, outcome, and quality of life in patients with permanent atrial fibrillation: results from the RACE II study. EP Europace. 2020;22:1619–1627. doi: 10.1093/EUROPACE/EUZ300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Odening KE, Deiß S, Dilling-Boer D, et al. Mechanisms of sex differences in atrial fibrillation: role of hormones and differences in electrophysiology, structure, function, and remodelling. EP Europace. 2019;21:366–376. doi: 10.1093/EUROPACE/EUY215. [DOI] [PubMed] [Google Scholar]
- 21.Alipour P, Azizi Z, Norris CM, et al. Representation of women in atrial fibrillation clinical practice guidelines. Can J Cardiol. 2022;38:729–735. doi: 10.1016/J.CJCA.2021.12.017. [DOI] [PubMed] [Google Scholar]
- 22.Ikemura N, Kohsaka S, Kimura T, et al. Assessment of sex differences in the initial symptom burden, applied treatment strategy, and quality of life in japanese patients with atrial fibrillation. JAMA Netw Open. 2019 doi: 10.1001/JAMANETWORKOPEN.2019.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schnabel RB, Pecen L, Ojeda FM, et al. Gender differences in clinical presentation and 1-year outcomes in atrial fibrillation. Heart. 2017;103:1024–1030. doi: 10.1136/HEARTJNL-2016-310406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ko D, Rahman F, Schnabel RB, et al. Atrial fibrillation in women: epidemiology, pathophysiology, presentation, and prognosis. Nat Rev Cardiol. 2016;13:321–332. doi: 10.1038/NRCARDIO.2016.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hsu JC, Maddox TM, Kennedy K, et al. Aspirin instead of oral anticoagulant prescription in atrial fibrillation patients at risk for stroke. J Am Coll Cardiol. 2016;67:2913–2923. doi: 10.1016/J.JACC.2016.03.581. [DOI] [PubMed] [Google Scholar]
- 26.Proietti M, Romiti GF, Olshansky B, et al. Improved outcomes by integrated care of anticoagulated patients with atrial fibrillation using the simple abc (atrial fibrillation better care) pathway. Am J Med. 2018;131:1359–1366.e6. doi: 10.1016/J.AMJMED.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 27.Pastori D, Menichelli D, Violi F, et al. The atrial fibrillation better care (abc) pathway and cardiac complications in atrial fibrillation: a potential sex-based difference. the ATHERO-AF study. Eur J Intern Med. 2021;85:80–85. doi: 10.1016/J.EJIM.2020.12.011. [DOI] [PubMed] [Google Scholar]
- 28.Scheuermeyer FX, Mackay M, Christenson J et al. (2015) There Are sex differences in the demographics and risk profiles of emergency department (ED) patients with atrial fibrillation and flutter but no apparent differences in ED management or outcomes. Acad Emerg Med 22 (9) 1067–1075. 10.1111/acem.12750 [DOI] [PubMed]
- 29.Elayi CS, Darrat Y, Suffredini JM, et al. Sex differences in complications of catheter ablation for atrial fibrillation: results on 85,977 patients. J Interv Card Electrophysiol. 2018;53:333–339. doi: 10.1007/s10840-018-0416-1. [DOI] [PubMed] [Google Scholar]
- 30.Hermida A, Burtin J, Kubala M, et al. Sex differences in the outcomes of cryoablation for atrial fibrillation. Front Cardiovasc Med. 2022 doi: 10.3389/fcvm.2022.893553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuck K-H, Brugada J, Fürnkranz A, et al (2018) impact of female sex on clinical outcomes in the fire and ice trial of catheter ablation for atrial fibrillation. Circ: Arrhythmia and Electrophysiology 10.1161/CIRCEP.118.006204 [DOI] [PubMed]
- 32.Kaiser DW, Fan J, Schmitt S, et al. Gender differences in clinical outcomes after catheter ablation of atrial fibrillation. JACC Clin Electrophysiol. 2016;2:703–710. doi: 10.1016/j.jacep.2016.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.LaRosa AR, Claxton J, O’Neal WT, et al. Association of household income and adverse outcomes in patients with atrial fibrillation. Heart. 2020;106:1679–1685. doi: 10.1136/heartjnl-2019-316065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Essien UR, Kornej J, Johnson AE, et al. Social determinants of atrial fibrillation. Nat Rev Cardiol. 2021;18:763–773. doi: 10.1038/s41569-021-00561-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lindley KJ, Aggarwal NR, Briller JE, et al. Socioeconomic determinants of health and cardiovascular outcomes in women. J Am Coll Cardiol. 2021;78:1919–1929. doi: 10.1016/j.jacc.2021.09.011. [DOI] [PubMed] [Google Scholar]
- 36.Schultz WM, Kelli HM, Lisko JC, et al. Socioeconomic status and cardiovascular outcomes. Circulation. 2018;137:2166–2178. doi: 10.1161/CIRCULATIONAHA.117.029652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vinter N, Calvert P, Kronborg MB, et al. Social determinants of health and catheter ablation after an incident diagnosis of atrial fibrillation: a Danish nationwide cohort study. Eur Heart J Quality Care Clinical Outcomes. 2022 doi: 10.1093/ehjqcco/qcac038. [DOI] [PubMed] [Google Scholar]
- 38.Romiti GF, Proietti M, Bonini N, et al. Adherence to the Atrial Fibrillation Better Care (ABC) pathway and the risk of major outcomes in patients with atrial fibrillation: A post-hoc analysis from the prospective GLORIA-AF Registry. eClinicalMedicine. 2023;55:101757. doi: 10.1016/j.eclinm.2022.101757. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the current study are available from the corresponding author upon reasonable request.