Abstract
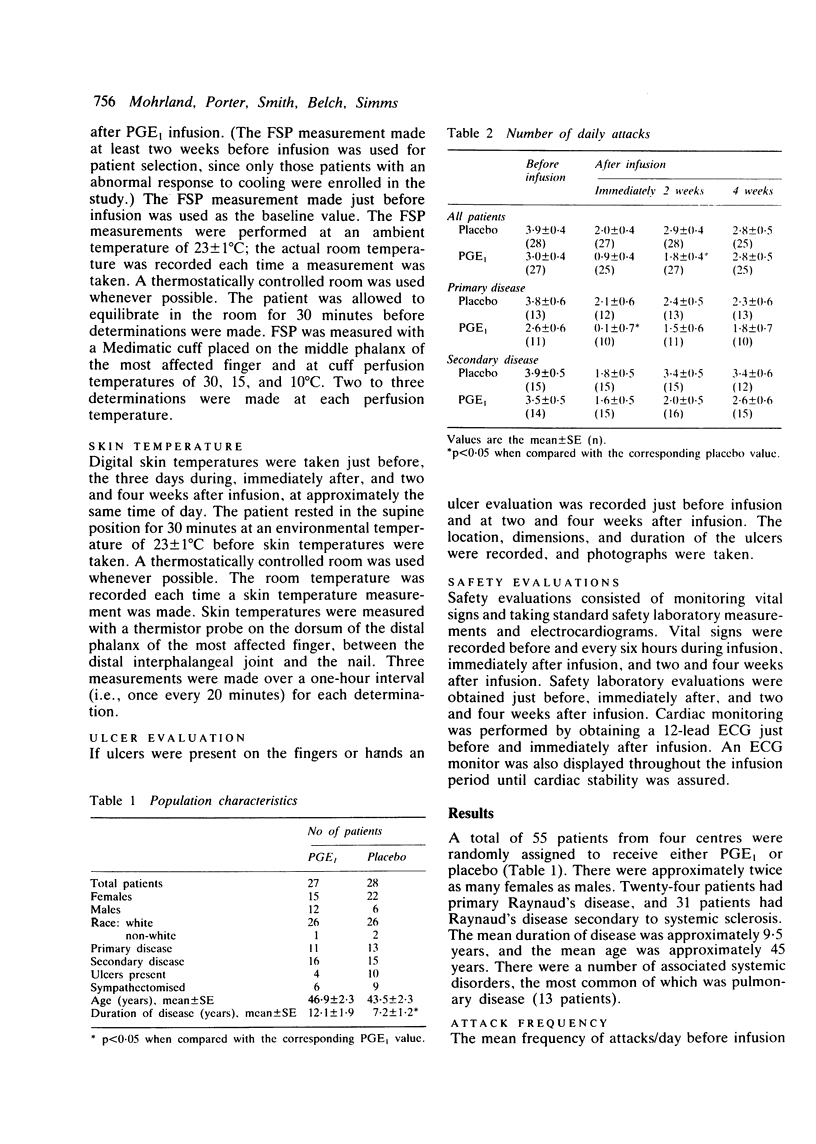
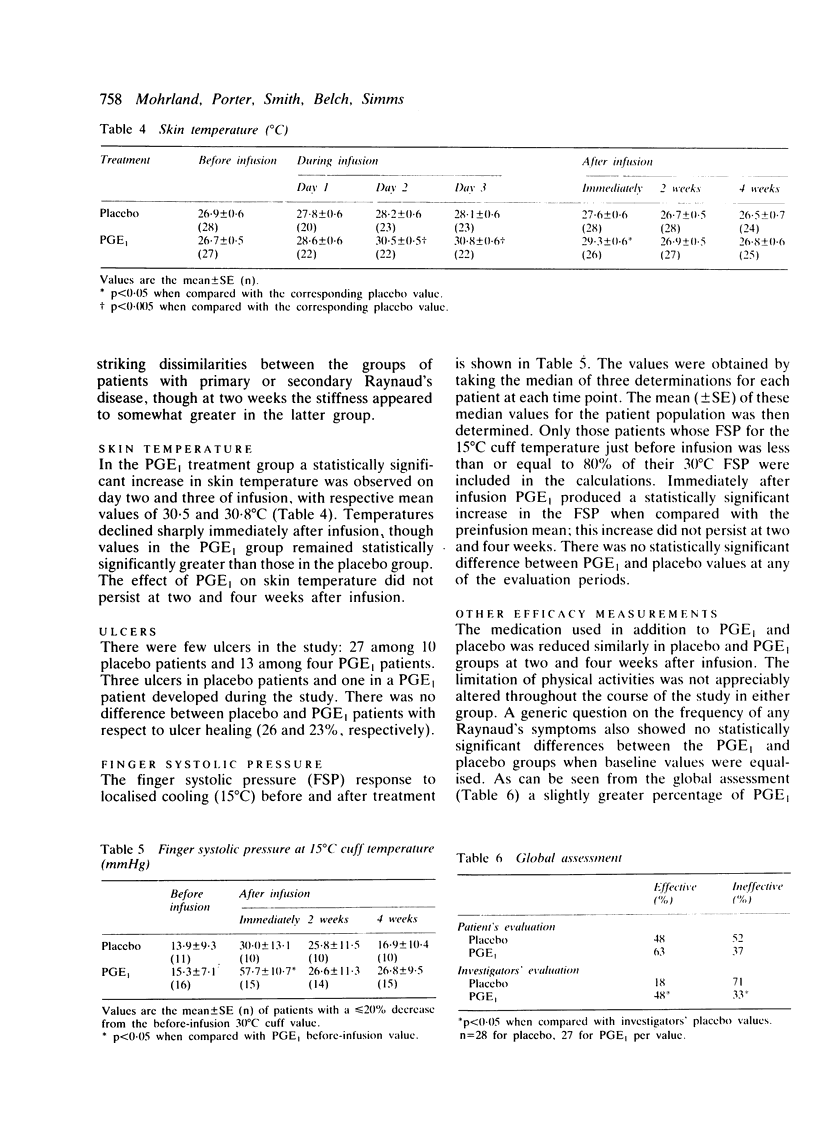
Prostaglandin E1 (alprostadil, Prostin VR Sterile Solution, PGE1) was evaluated in patients with Raynaud's syndrome in a multiclinic, placebo-controlled, double-blind study. A total of 55 patients with either primary Raynaud's disease or Raynaud's disease secondary to systemic sclerosis were randomly assigned to receive either PGE1 administered intravenously at 10 ng/kg/min for 72 hours or placebo administered in the same manner. The frequency and severity of Raynaud's attacks were then monitored for up to four weeks by use of in-clinic questionnaires and patients' daily diaries. Haemodynamic assessments included measurements of skin temperature and the finger systolic pressure response to localised digital cooling. Immediately after the infusion the overall symptoms in both the PGE1 and the placebo group showed marked improvement; by four weeks after infusion, in some cases, values had not returned to pretreatment levels. There was, however, no marked benefit of PGE1 treatment over that of placebo. Although PGE1 significantly increased skin temperature during and immediately after infusion, the effect did not persist at two- and four-week follow-up evaluations. The finger systolic pressure response to localised digital cooling (15 degrees C) increased more in the PGE1-treated group than in the placebo-treated group, but the difference was not statistically significant. There was no difference in ulcer healing between the two treatment groups. These results failed to substantiate earlier open-label reports that a 72-hour intravenous infusion of PGE1 in patients with Raynaud's syndrome produced significant clinical benefit.
Full text
PDF