Abstract
Fournier’s gangrene (FG) is a rare infectious disease with rapid disease progression and a high mortality rate. We report a case of a 61-year-old female with type 2 diabetes who developed FG caused by Actinomyces europaeus. A. europaeus is associated with abscesses, decubitus ulcers, and purulent urethritis. Although A. europaeus rarely causes FG as the main causative pathogen, we should still be alert to this pathogenic microorganism. To our knowledge, this is the first case report of FG caused by A. europaeus mono-infection, and it adds to the evidence that A. europaeus has the potential to cause necrotizing fasciitis.
Keywords: Fournier’s gangrene, Necrotizing fasciitis, Actinomyces europaeus
Key Summary Points
| Fournier’s gangrene is a rare infectious disease with a high mortality rate. |
| This is the first case report of FG caused by Actinomyces europaeus mono-infection. |
| Actinomyces europaeus has the potential to cause necrotizing fasciitis. |
Introduction
Fournier’s gangrene (FG) is a rapidly worsening, necrotizing infection of the soft tissues and fascia of the perineum and genital region. During FG, the superficial fascia and subcutaneous tissues cause necrosis, resulting in sepsis and even death [1]. Its common pathogens includes Group A Streptococcus, Bacteroides fragilis, Staphylococcus aureus, Clostridium species, Pseudomonas aeruginosa, Enterobacteriaceae, and others [1]. In extreme cases, FG can be caused by Actinomyces. Actinomycetes is a common genus of opportunistic pathogens found in the oral cavity, gastrointestinal tract, and genitourinary tract [2].
Actinomyces europaeus is one of the subspecies of Actinomyces. It was first isolated in humans in 1997 [2]. In 2019, the first case report on necrotizing fasciitis caused by A. europaeus and Actinotignum schaalii was published [3]. To our knowledge, this is the first case report of FG caused by A. europaeus mono-infection. This study was conducted following the 1964 Declaration of Helsinki and its subsequent amendments. Informed consent was obtained from the patient for being included in this case report.
Case Report
A 61-year-old female with a history of type 2 diabetes mellitus and hypertension presented with a soft, painless, 4 cm mass in the perineum without obvious inducement, on March 7 2022. The patient had a history of diabetes for 6 years and was taking metformin irregularly. She did not take hypoglycemic drugs nor monitor her blood glucose recently. However, on March 9, the lesions had spread to the left labia majora and mons pubis. The pain had affected her sleep and motion; she reported no fever or difficulty in urinating. She was managed at the local community hospital with infusion therapy (drug unknown) for painful swollen perineum, but she had no obvious improvement following the infusion therapy, and her symptoms further worsened with increased pain and enlarged lumps. The patient was then advised to present to our gynecological department by her primary care physician on March 15. On the day of presentation, she developed fever and cough with no known aggravating factors.
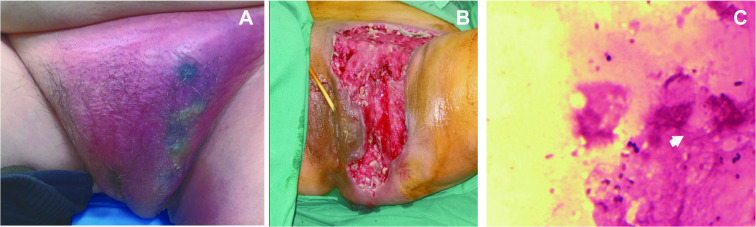
On admission, her vitals were stable, with a temperature of 37 °C, blood pressure of 112/70 mmHg, respiratory rate of 21 breaths per minute, heart rate of 96 beats per minute, and her random blood glucose level was 23.4 mmol/L. Physical examination showed there was extensive erythema in the left groin, the upper one-third of the inner side of the left thigh, the pubis, and the left labia majora with tenderness, edema, and skin necrosis (Fig. 1A). She underwent baseline laboratory investigations including routine bloods, pus and blood culture, and biochemical liver function tests (Table 1). After 3 days of bacterial culture, on March 20, bacterial culture of the pus detected Actinomyces europaeus (Fig. 1C), and blood cultures were negative. After a discussion with the dermatologist, the patient was initially diagnosed as FG according to her clinical manifestations on March 17. After the clinical diagnosis of FG was made, the patient underwent multiple staged debridement and graft skin closure surgery in the lithotomy position (Fig. 1B). On March 18, during the first operation, we found that the necrotic subcutaneous soft tissue extended from the lower abdomen to the anus, and the necrotizing fasciitis involved the perineum extending to both pelvic floor muscles and left hip joint. The necrotic tissue was excised and intraoperative wound cultures grew A. europaeus. The postoperative period remained uneventful and she showed improvement in her general condition.
Fig. 1.
A, Representative images of gangrenous perineum; B postsurgical debridement; C, bacterial culture of the pus detected the growth of A. europaeus
Table 1.
Initial laboratory workup on admission
| Test | Results | Reference range |
|---|---|---|
| White blood cell count | 21.73 | 3.5–9.5 × 109/L |
| Neutrophils count | 19.51 | 1.8–6.3 × 109/L |
| C-reactive protein | 234.00 | 0–10 mg/L |
| D-dimer | 2.71 | 0–0.5 mg/L (FEU) |
| Fibrinogen level | 8.19 | 1.75–4.35 g/L |
| Human serum amyloid A | > 320.00 | 1–10 mg/L |
| Bilirubin direct | 8.93 | 0–4 μmol/L |
| Aspartate aminotransferase | 18 | 13–35 U/L |
| Alanine aminotransferase | 20 | 7–40 U/L |
| Bilirubin total | 28.99 | 0–21 μmol/L |
| Sodium | 130.90 | 137–147 mmol/L |
| Creatinine | 91.16 | 40–105 μmol/L |
| HbA1c | 11.2 | 4–6% |
During hospitalization, her antibiotic treatment plan was adjusted several times. On March 15, she was empirically given cefoperazone sulbactam and levo-ornidazole. On March 17, after the clinical diagnosis of FG was made, the antibiotic treatment plan was not changed. On March 18, after the first debridement, cefoperazone sulbactam 1.5 g twice daily was changed to 3 g three times daily. After the second debridement, the cefoperazone sulbactam/levo-ornidazole combination was changed to piperacillin sodium and tazobactam sodium 4.5 g three times daily.
Discussion
FG is a rare but fatal disease, with extremely high mortality. The annual incidence is 1.6 cases in 100,000 and the average mortality is 7.3% [4]. Recognized predisposing factors include advanced age, lowered immune function, diabetes mellitus, and decubitus ulcer [1]. Our patient had high risk factors for developing FG, including older age and having poorly controlled diabetes mellitus. FG is characterized by polymicrobial infection. However, our case is different. Bacterial smears of wound pus from our patients found Gram-positive cocci and Gram-negative bacilli, wound cultures showed A. europaeus, but blood cultures were negative. Thus, the microbiologists explained that the results of smears were normal flora, and only A. europaeus were cultured in an anaerobic environment, and that blood cultures in FG patients are usually negative. Therefore, we concluded that this is a special case of FG caused by A. europaeus mono-infection.
To our knowledge, this is the first case of A. europaeus as the primary organism causing FG. There are only two reports of A. europaeus causing necrotizing fasciitis, one of which was abdominal wall necrotizing fasciitis and the other was A. europaeus along with Actinotignum schaali causing necrotizing fasciitis [3, 5]. This case report reinforces the link between A. europaeus and necrotizing fasciitis. When A. european are cultured in specimens, we should be highly alert to necrotizing fasciitis and adjust antibiotics in a timely manner.
Early diagnosis, early use of effective antibiotics, and urgent surgical debridement are important for proper treatment of this disease. Wound cultures of FG often reveal polymicrobial infection of both aerobes and obligate anaerobes, but rarely show Actinomyces species. Generally, FG patients receive broad-spectrum antibiotics to cover as many bacterial species as possible. Then, antibiotics should be tailored according to Gram staining and cultures. Classical triple therapy involves generation cephalosporins or aminoglycosides, plus penicillin and metronidazole. Metronidazole and penicillin-based antibiotics are the most commonly used antimicrobials in the past two decades [4]. However, sometimes FG can be caused by atypical pathogens, such as Actinomyces. Arshan [6] reported a case of FG caused by Streptococcus anginosus, Actinomyces turicensis, and Peptoniphilus harei. Tongchun [7] reported that FG can be caused by Actinomyces turicensis, and Sección [8] reported that Actinomyces funkei, Fusobacterium gonidiaformans, and Clostridium hathewayi caused FG. These cases suggest that atypical pathogens, especially Actinomyces, should be considered as potential pathogens of FG. Therefore, in our case, A. europaeus particularly may be a suspicious organism causing necrotizing fasciitis. The results of Gram staining and cultures are significant for doctors to effectively manage these patients. However, cultivation of Actinomyces is quite difficult, and for this genus, metronidazole generally has poor activity, but β-lactam antimicrobial agents have good activity [9]. Steininger et al. recommended using β-lactam antimicrobial agents to treat Actinomyces [9].
Conclusions
Here we report a rare case of FG induced by Actinomyces europaeus, which was effectively treated by early aggressive debridements, broad-spectrum antibiotics, and skin transplantation. FG mainly caused by A. europaeus is seldom reported, so it is very difficult to correctly identify. It ought to be regarded as a suspected causative agent to avoid missed diagnosis.
Declarations
Funding
No funding or sponsorship was received for this study or publication of this article. The Rapid Service Fee was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Shurong Zhang and Yunkai Xie assisted in drafting and revising the manuscript. Yonghui Zou and Rongtao Cui performed the surgery together. Yunkai Xie and Guoyu Jin searched all the cases and made the analysis. All authors read and approved the manuscript for publication.
Disclosures
Shurong Zhang, Yunkai Xie, Yanqiu Wang, Guoyu Jin, Rongtao Cui and Yonghui Zou have nothing to disclose.
Compliance with Ethics Guidelines
Compliance with Ethics Guideline. This study was conducted following the 1964 Declaration of Helsinki and its subsequent amendments. Informed consent was obtained from the patient for being included in this case report.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hysong AA, Posey SL, Blum DM, et al. Necrotizing fasciitis: pillaging the acute phase response. J Bone Joint Surgery-Am Vol. 2020;102(6):526–537. doi: 10.2106/jbjs.19.00591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Funke G, Alvarez N, Pascual C, et al. Actinomyces europaeus sp. nov, isolated from human clinical specimens. Int J Systematic Bacteriol. 1997;47(3):687–92. 10.1099/00207713-47-3-687 [DOI] [PubMed]
- 3.Kus NJ, Kim BJ, Ross HM. A case report of necrotizing fasciitis with growth of Actinomyces europaeus and Actinotignum schaalii. J Surg Case Reports. 2019(10). 10.1093/jscr/rjz286 [DOI] [PMC free article] [PubMed]
- 4.Bowen D, Juliebo-Jones P, Somani BK. Global outcomes and lessons learned in the management of Fournier's gangrene from high-volume centres: findings from a literature review over the last two decades. World J Urol. 2022;40(10):2399–2410. doi: 10.1007/s00345-022-04139-4. [DOI] [PubMed] [Google Scholar]
- 5.Allen N, James G, Jain Y. A rare case of abdominal wall necrotizing fasciitis caused by Actinomyces europaeus-a novel pathogen. J Surg Case Reports. 2021;2021(12). 10.1093/jscr/rjab533 [DOI] [PMC free article] [PubMed]
- 6.Khan A, Gidda H, Murphy N, et al. An Unusual Bacterial Etiology of Fournier's Gangrene in an Immunocompetent Patient. Cureus J Med Sci. 2022;14(7). 10.7759/cureus.26616 [DOI] [PMC free article] [PubMed]
- 7.Mao T-c, Zhou X, Tian M-n, Zhang Y-m, Wang S-l. A rare case of male Fournier's gangrene with mixed Actinomyces turicensis infection. Bmc Urol. 2022;22(1). 10.1186/s12894-022-00975-z [DOI] [PMC free article] [PubMed]
- 8.Tena D, Losa C, Jose Medina-Pascual M, Antonio S-N. Fournier's gangrene caused by Actinomyces funkei, Fusobacterium gonidiaformans and Clostridium hathewayi. Anaerobe. 2014;27:14–16. doi: 10.1016/j.anaerobe.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Steininger C, Willinger B. Resistance patterns in clinical isolates of pathogenic Actinomyces species. J Antimicrob Chemother. 2016;71(2):422–427. doi: 10.1093/jac/dkv347. [DOI] [PubMed] [Google Scholar]