Abstract
The Warburg effect indicates that cancer cells survive through glycolysis under aerobic conditions; as such, the topic of cancer metabolism has aroused interest. It is requisite to further explore cancer metabolism, as it helps to simultaneously explain the process of carcinogenesis and guide therapy. The flexible metabolism of cancer cells, which is the result of metabolic reprogramming, can meet the basic needs of cells, even in a nutrition-deficient environment. Glutamine is the most abundant non-essential amino acid in the circulation, and along with glucose, comprise the two basic nutrients of cancer cell metabolism. Glutamine is crucial in non-small cell lung cancer (NSCLC) cells and serves an important role in supporting cell growth, activating signal transduction and maintaining redox homeostasis. In this perspective, the present review aims to provide a new therapeutic strategy of NSCLC through inhibiting the metabolism of glutamine. This review not only summarizes the significance of glutamine metabolism in NSCLC cells, but also enumerates traditional glutamine inhibitors along with new targets. It also puts forward the concept of combination therapy and patient stratification with the aim of comprehensively showing the effect and prospect of targeted glutamine metabolism in NSCLC therapy. This review was completed by searching for keywords including ‘glutamine’, ‘NSCLC’ and ‘therapy’ on PubMed, and screening out articles.
Keywords: non-small cell lung cancer, glutamine metabolism, therapeutic strategies, Warburg effect, metabolism inhibitor
1. Introduction
Lung cancer is the second most common cancer in the world and remains the leading cause of cancer death proven by a high global diagnosis rate (11.4%) and mortality rate (18%). In 2020, there were 19.3 million new cases of cancer and 10 million cancer-associated deaths worldwide, and it was estimated that ~1.8 million of these individuals died of lung cancer (1). Among all subtypes of lung cancer, non-small cell lung cancer (NSCLC) accounts for 80–85%, of which ~40% cases are adenocarcinomas (AC), 25–30% cases are squamous cell carcinomas (SCCs) and 10–15% cases are large cell carcinomas (2). In the USA, the 5-year survival rate for NSCLC is ~26% (3). Since NSCLC is the most common type of lung cancer, research into the disease is necessary. The treatment of NSCLC includes traditional surgery, chemotherapy (including targeted drug therapy), radiotherapy and emerging immunotherapy for advanced tumors. In addition, some nanodrugs are at the research stage (4). The option of treatment mainly depends on, but not necessarily determined by, the stage of NSCLC (5). Despite these promising treatment options, the five-year survival rate of advanced NSCLC is still very low, particularly at stage IIIB, which is only 26% (6). Therefore, it is necessary to discover and explore new therapies.
It has been nearly a century since the first study on tumor metabolism, the Warburg effect, was published in 1924 (7). Research on tumor metabolism has burgeoned over the past decade. In the sense of exploring tumor metabolism, it not only elucidates the mechanism of tumorigenesis and progression, but is also conducive to diagnosis and treatment. The metabolism of cancer cells is both flexible and plastic. Through metabolic reprogramming, cancer cells can maintain cell vitality and growth, even under nutrition-deprived conditions or in an oxygen-deficient environment (8). Metabolic reprogramming is regarded as one of the emerging hallmarks of cancer (9). Glucose and glutamine are two major nutrients supporting survival and biosynthesis in tumor metabolism (10). The Warburg effect revealed the process of aerobic glycolysis in tumor cells, which is one of the most prominent features of tumor metabolism (11). Glutamine is a rich and versatile nutrient involved in energy generation, redox homeostasis, macromolecular synthesis and signal transduction (12). Based on the important role of glutamine in tumorigenesis and progression, it may be a promising direction of targeting its metabolic process to develop new clinical therapies.
A previous study indicated that NSCLC cells may use glutamine as a substrate through metabolic reprogramming to induce glutamine addiction, which is a promising therapeutic target (13). However, tumor metabolism is not a specific metabolic map, presenting challenges for targeting glutamine metabolism in cancer therapy (14). In this perspective, the present review combined the glutamine-dependent metabolism of NSCLC with the therapeutic strategy of targeting glutamine to provide new approaches for the treatment of NSCLC and to select those patients who may benefit the most from glutamine metabolism-targeted therapy, and ultimately to improve the overall survival rate and the long-term quality of life.
2. Metabolic reprogramming in NSCLC
It is widely accepted that metabolic programming is one of the hallmarks of cancer (15). Most normal cells use external stimuli to activate growth factor signals and to absorb a large amount of nutrients from the external environment for metabolism, whereas cancer cells maintain the activation of signal pathways through gene mutations (16). In this way, the metabolism of cancer cells is flexible and plastic, which provides the basis for metabolic reprogramming. The significance of metabolic reprogramming is to promote the growth and proliferation of tumor cells by generating energy, synthesizing necessary precursors and maintaining oxidative balance, especially under hypoxic and hypo-nutrient conditions (17). Furthermore, metabolic reprogramming plays an important role in malignant transformation, the progression of tumors and the resistance to antitumor therapy (18). In NSCLC cells, reactive oxygen species (ROS)-mediated metabolic reprogramming leads to oxidative phosphorylation, which results in cisplatin resistance (19). It has been shown that smoking can greatly increase the incidence of lung cancer partly due to the overexpression of enzymes related to glutamine metabolism, fatty acid degradation and lactate synthesis, resulting in mitochondrial metabolic reprogramming of NSCLC cells (20). Research on the metabolic reprogramming of cancer cells can be traced back to last century when the Warburg effect appeared. It has been shown that even in an aerobic environment, glucose is involved in the glycolytic pathway rather than in aerobic oxidation, revealing the metabolic reprogramming of glucose in cancer cells (11). The mechanism of metabolic reprogramming remains to be explored. Fundamentally, metabolic reprogramming is the consequence of oncogene or tumor suppressor gene mutation, including direct and indirect effects (21). KRAS mutations are among the most common mutations leading in NSCLC (22). NSCLC cells can become glutamine-dependent through a variety of metabolic reprogramming processes (23). For example, it activates autophagy to uptake glutamine through the degradation of cellular proteins (24). In addition, the increase in macropinocytosis also reflects the dependence on glutamine, which provides cells with a supply of amino acids, mainly glutamine (25). However, the process of KRAS-driven glutamine metabolism in NSCLC cells is different in vivo and in vitro (26). The LKB1 gene is also associated with altered metabolism in cells. LKB1 deletion promotes the expression of hypoxia-inducible factor (HIF)-1α and stabilizes it by regulating mTOR activity in NSCLC cells. This metabolic reprogramming increases the uptake and utilization of both glucose and glutamine, enhancing aerobic glycolysis and glutamine catabolism to promote cell growth (27,28). A previous study has shown that KRAS-mutant NSCLC cells lacking LBK1 usually also have Kelch-like ECH-associated protein 1 (KEAP1) gene mutations; these are known as KLK NSCLC cells. These three genes work corporately to promote metabolic reprogramming, making KLK NSCLC cells glutamine dependent and sensitive to glutamine inhibitors (29). Glutamine addiction occurs in c-Myc mutant NSCLC, where c-Myc is an oncogene that drives metabolic reprogramming. c-Myc can increase the expression of glutaminase (GLS) by inhibiting microRNA (miR)-23a/b to promote intracellular glutamine metabolism (30). However, metabolic reprogramming is not only determined by genes, but also by tissue specificity (14,31). In addition, long non-coding RNAs (lncRNAs) are inseparable from metabolic reprogramming. For example, the oncogenic lncRNA, Al355338, activates the EGFR/AKT signaling pathway by preventing enolase 1 from ubiquitination and degradation, making it stabilized, leading to metabolic reprogramming and increasing aerobic glycolysis (32). In NSCLC cells, lncRNA-AC020978 carries out metabolic reprogramming by regulating the pyruvate kinase M2/HIF-1α positive-feedback axis under the condition of hypoxia or glucose deficiency, which adapts the cells to the hypoxic environment and promotes glycolytic metabolism (33).
3. Glutamine metabolism in NSCLC
The Warburg effect demonstrated that cancer cells use glycolysis in an aerobic environment for rapid proliferation (11); since this discovery, the metabolic flexibility of tumors has attracted the interest of researchers. A number of studies have discussed the characteristics of tumor glucose metabolism and further verified the Warburg effect from different aspects. Glucose from the extracellular environment is not sufficient for tumor growth and proliferation; therefore, tumor cells use other sources of nutrients as well, glutamine being the most prominent (10). Characteristics of metabolism in NSCLC include increased glucose consumption and lactate production, as well as glutamine addiction (34).
Glutamine metabolism
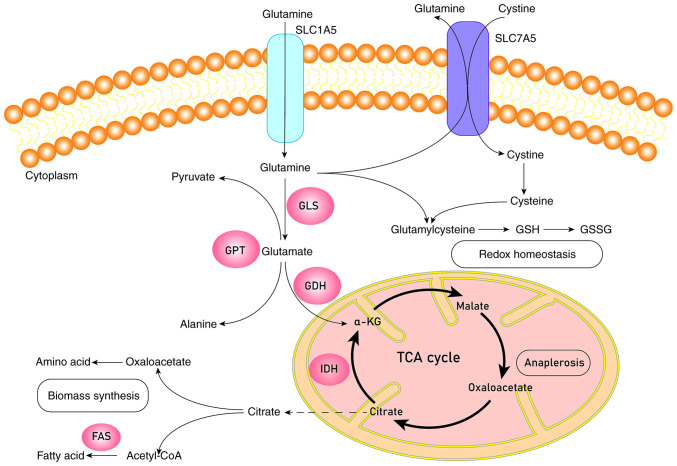
Glucose and glutamine are the two basic nutrients used by cancer cells (10). Although glutamine is a non-essential amino acid, it can accumulate from de novo synthesis and serve an indispensable role in cancer cell growth, signal transduction and maintaining redox homeostasis (35). Glutamine can be used as a nitrogen source to synthesize amino acids and nucleotides to promote cell growth, or as a carbon source to replenish the tricarboxylic acid (TCA) cycle when glucose levels are deficient (36). This process originates from the conversion of glutamine into glutamate through the catalysis of GLS, followed by the conversion of glutamate into α-ketoglutarate (α-KG) through catalysis by glutamine dehydrogenase (GDH) or transaminase (Fig. 1) (37). However, under hypoxia or mitochondrial dysfunction, α-KG is carbonylated to citrate, the substrate for fatty acid synthesis, indicating that glutamine actively participates in lipid synthesis (38). The enzymes involved in fatty acid synthesis mainly include citrate lyase, acetyl CoA carboxylase (ACC) and fatty acid synthase (FAS); the process is as follow: Citrate is converted by citrate lyase into acetyl-CoA, used for the synthesis of fatty acids, and oxaloacetate. Synthesis of malonyl-CoA from acetyl-CoA is catalyzed by ACC. Next, palmitic acid is synthesized through multiple processes of condensation-reduction-dehydration-reduction, catalyzed by FAS (39). Glutamine activates mTORC1 through a special mechanism that requires ADP ribosylation factor 1 and is independent of Rag GTPase to stimulate the lysosomal localization of mTORC1 and to exert its physiological functions, such as controlling cell growth, metabolism and autophagy (35). Glutamine participates in complex signaling pathways through this process and maintains redox homeostasis mainly by synthesizing glutathione (GSH), an antioxidant molecule, to resist ROS. When ROS accumulates at high levels, DNA, proteins and lipids will be degraded (40,41). GDH1 is another key enzyme involved in glutamine metabolism; it regulates redox homeostasis through activation of GSH peroxidase 1 (42).
Figure 1.
Schematic diagram of glutamine metabolism. Glutamine is transported into the cell by SLC1A5 or SLC7A5 and turns into glutamate catalyzed by transaminases or GLS. Glutamate will be catalyzed by GDH to generate α-KG. α-KG can enter into the TCA cycle to complete the process of anaplerosis or be catalyzed by IDH to produce citrate, which leaves mitochondria for the biomass synthesis of fatty acids and amino acids. Glutamine and cystine will synthesize glutathione through a series of catalytic processes to keep redox homeostasis. GLS, glutaminase; GPT, glutamic pyruvic transaminase; GDH, glutamate dehydrogenase; IDH, isocitrate dehydrogenase; FAS, fatty acid synthase; GSH, glutathione (reduced); GSSH, glutathione (oxidized); α-KG, α-ketoglutaric acid; TCA cycle, tricarboxylic acid cycle.
Glutamine metabolism has strong heterogeneity, reflected in its close relationship with the origin of the tumor tissue, oncogenes, and the tumor microenvironment (TME) (43). Studies have shown that the oncogene c-Myc inhibits miR-23a/b and, as a result, the expression of GLS is increased to promote the glutamine catabolism and to meet the needs of cancer cell growth and proliferation (44,45). In KRAS-driven NSCLC, glutamine has less contribution to TCA cycle than does glucose, meaning that the carbon in TCA cycle mainly comes from glucose rather than glutamine (26). Interestingly, it is shown that the mRNA levels of GLS and solute carrier family 1 member 5 (SLC1A5) increased in KRAS-driven NSCLC, suggesting that these cells are more dependent on glutamine rather than glucose metabolism (14). The seemingly contradictory results may be due to different TMEs and tumor histological subtypes, which further explains the complexity and heterogeneity of glutamine metabolism. The complex relationship between glutamine metabolism, tumor tissue and gene mutation are also reflected in whether the cancer cells catabolize glutamine to provide energy and nutrient substrates for cell growth and proliferation or if this leads to intracellular glutamine accumulation through de novo glutamine synthesis (31). For example, in MYC-induced liver cancer, glucose-derived synthesis of glutamine is suppressed by reducing the level of glutamine synthetase (GLUL), whereas upregulation of GLS leads to enhanced catabolism of glutamine. Conversely, in MET-driven liver cancer, glutamine synthesis is increased. In MYC-induced lung cancer, the mRNA expression levels of GLUL and GLS are upregulated simultaneously. However, the effect caused by GLUL overexpression overrides the effects of GLS upregulation, which leads to accumulation of glutamine in lung cancer cells (31). There are a number of key processes in glutamine metabolism. For example, glutamine enters cells through the SLC1A5 amino acid transporter and is converted into glutamate, catalyzed by the GLS enzyme. Glutamate is then converted into α-KG, which enters the process of TCA cycle anaplerosis either by transaminase or GDH and generates non-essential amino acids, such as alanine and aspartic acid (46). The enzymes and proteins involved in these key steps are potential oncotherapy targets.
Significance of glutamine metabolism in NSCLC
TCA cycle anaplerosis
The main purpose of glucose metabolism is to generate lactate, which can store a vast amount of carbon, resulting in a reduction of carbon sources entering the TCA cycle and a continuous flow of intermediate TCA cycle products into the cytoplasm for the synthesis of biological macromolecules, such as lipids, nucleotides and proteins; as such, glucose metabolism leads to TCA cycle cataplerosis (16,36). Glutamine provides a source of carbon to maintain the TCA cycle, which is used to produce sufficient ATP and metabolic derivatives (12). TCA cycle anaplerosis is the key to metabolism; through this process, cells can obtain substrates for the synthesis of various biological macromolecules, such as oxaloacetate and citrate (16,47).
Biomass synthesis
Cell proliferation requires the synthesis of a large amount of proteins, nucleic acids and lipids (11). Glutamine generates other non-essential amino acids through catalysis by transaminases. It is reported that ~50% of the non-essential amino acids in cells come from glutamine metabolism (48). Glutamine-derived α-KG is catalyzed by isocitrate dehydrogenases (IDHs) with the consumption of NADPH to produce citrate, which flows into the cytoplasm for lipid or nucleotide synthesis (49). Glutamine metabolism can produce a large amount of NADPH, providing reducing substances for the synthesis of lipids and nucleotides (50). Glutamine also provides a nitrogen source for nucleotide synthesis (34). The lipids in cells are mainly derived from glucose, followed by glutamine (36).
Activation of the mTOR signaling pathway
The mTOR signaling pathway promotes the anabolism of biological macromolecules, such as lipids, proteins and nucleotides, by integrating intracellular and extracellular signals, and inhibits autophagy to sustain cell survival (51). It has been shown that the activation of mTOR requires bidirectional transport of glutamine (52). Glutamine activates mTORC1 independently of rag GTPase and recruits it into the lysosome (53). Glutamine is regarded as a sensitive regulatory signal of mTORC1 complex, promoting the growth of cancer cells (13).
Metabolic process of glutamine in NSCLC
Glutamine metabolism in NSCLC involves related enzymes and transporters. One of the hallmarks of cancer is the reprogramming of energy metabolism for the regulation of metabolism-related enzymes and transporters at transcriptional or post-transcriptional level (54). For example, SLC15A is the primary transporter of glutamine entering into the cell. Statistical analysis revealed an association between SCL15A overexpression with poor prognosis in patients with NSCLC, making SLC15A a potentially important prognostic marker (55). SLC7A11, an antiporter of glutamate and other non-essential amino acids, is overexpressed in NSCLC; it may enhance the antioxidant stress ability of cancer cells and increase the consumption of glutamine, which is also known as glutamine addiction and is one of the characteristics of NSCLC metabolism (56). In addition, extracellular proteins are degraded through micropinocytosis to obtain glutamine for cell growth (48).
In BRAFV600E-driven lung adenocarcinoma, autophagy sustains mitochondrial glutamine metabolism, consistent with some ‘autophagy-addicted’ NSCLC cells (24). There is abnormal expression of a certain type of enzymes directly or indirectly related to glutamine metabolism in NSCLC cells. For example, GLS is a major enzyme involved in glutamine metabolism, in which it catalyzes the conversion of glutamine to glutamate. A number of oncogenes modulate glutamine metabolism by regulating the expression of GLS. For example, c-Myc increases the expression of GLS by inhibiting miR-23a/b to promote intracellular glutamine metabolism (30). Lactate can also stabilize HIF-2α to activate c-Myc in oxygenated/oxidative cancer cells, which indirectly enhances the expression of GLS (57). GLS1 is a rate-limiting enzyme of glutaminolysis in some glutamine-dependent tumor cells. GLS1 has two splice variants, of which KAC and GAC are the dominant isoforms in NSCLC (58). The ratio of GAC to KAC increases significantly in tumor cells, which has been shown to be due to the decrease of KAC expression. However, whether GAC expression is increased remains to be determined. Knockdown of GAC has greater effects on tumor growth inhibition compared to knockdown of KAC, thus, the targeted therapy of GAC may have broader prospects (58).
Glutamate is converted into α-KG in two different pathways catalyzed by either transaminase accompanied by the production of other non-essential amino acids, or by GDH1 accompanied by generation of the reducing substance NADPH (12). GDH1 is the rate limiting enzyme for oxidative degradation of glutamate. The decrease of GDH1 activity limits the flow of carbon into TCA cycle (59). Glutamic-pyruvic transaminase (GPT) is a type of transaminase that converts glutamate into alanine through transamination. It was reported that the expression and distribution of GPT was significantly increased in NSCLC cells that were resistant to glutamine metabolic inhibitors (60). Other study showed that alanine metabolism can compensate for the intermediates of the TCA cycle and the metabolic derivatives during glutamine deprivation to maintain cell growth and survival (61). Pyruvate carboxylase catalyzes the conversion of pyruvate into oxaloacetate; it is highly expressed in early NSCLC cells and it selectively activates GLS. Previous study also have shown that knockdown of pyruvate carboxylase leads to inhibition of cell growth and the activity of the TCA cycle owing to the reduction of intermediates, lipid and nucleotide synthesis, and the imbalance of GSH which may lead to ROS excess (62). In NSCLC, the abundant expression of NADPH oxidase 4 induces GLS (glutamine catabolism) and GSH synthesis at the transcriptional and the post-transcriptional level; subsequently, GSH synthesis is increased leading to resistance of tumor cells to oxidative stress (63).
Metabolic heterogeneity of glutamine in NSCLC
Tumor metabolic heterogeneity
Although the Warburg effect describes the basic pattern of glucose metabolism in tumor cells, tumor heterogeneity indicates that metabolism is not a specific metabolic map (54). The association between tumor cell metabolism and the TME is one of the new characteristics of tumors (10). Specific tumor metabolism not only supports the growth and energy of cancer cells, but also serves a key role in the production of an immunosuppressive TME. Therefore, tumor metabolism is a way for cancer cells to escape antitumor immune responses. Glutamine metabolism not only promotes cell growth, but also creates a TME that benefits from tumor immune escape (64). Inhibition of glutamine metabolism can prevent tumor immune escape. Results of glutamine antagonist experiments revealed the previously unknown difference in metabolic plasticity between cancer cells and effector T cells, which can be used as a ‘metabolic checkpoint’ for tumor immunotherapy (65). Tumor stromal cells have flexible and adaptive metabolism involving the synthesis of glutamine using atypical carbon and nitrogen sources in a nutrient-deficient TME, supporting glutamine-dependent cell growth (66).
NSCLC metabolic heterogeneity
Glutamine metabolism in NSCLC is heterogeneous; there is higher glutamine consumption and metabolism in AC compared to SCC (14). EGFR and KRAS mutations are more common in AC cells with increased expression of GLS and SLC1A5 and decreased expression of GLUT (14). On the contrary, TP53 mutations are more common in SCC cells, suggesting that AC NSCLC cell lines mainly metabolize glutamine, whereas SCC cell lines mainly metabolize glucose (14). In cultured cells, glucose metabolism mainly produces lactate and glutamine mainly maintains the TCA cycle. However, in KRAS-driven NSCLC in vivo, production of lactate and progress of the TCA cycle is facilitated by glucose metabolism, whereas glutamine makes little contribution to the TCA cycle. The difference between in vivo and in vitro glucose metabolism implies the effect of the TME on NSCLC metabolism, even exceeding the gene phenotype (26). One of the internal factors that affect the heterogeneity of tumor metabolism includes provision of oxygen and substrates through different perfusion levels, exceeding the tumor genotype. In highly perfused NSCLC cells, lactate can be used as a substrate of respiratory chain, whereas in lowly perfused areas, lactate is produced and secreted through glycolysis (67).
4. Therapeutic targeting of glutamine metabolism in NSCLC
In addition to the classical metabolic substrate, glucose, glutamine is another important nutrient, making cancer cells glutamine-dependent by synthesizing biological macromolecules, supplementing TCA cycle substrates and maintaining redox balance. Simultaneously, it is also one of the hallmarks of NSCLC. Therefore, targeting the transport and metabolism of glutamine will become a promising therapeutic strategy for the treatment of NSCLC (34).
Glutamine targeted traditional therapy
The first step of glutamine influx or efflux is through the various glutamine transporters (68). SLC1A5 is the main amino acid transporter of glutamine influx. Overexpression of SLC1A5 was associated with poor prognosis of patients with NSCLC (69). Blocking its function can achieve an antitumor effect by suppressing the consumption of glutamine, inducing cell apoptosis through inherent pathways and preventing cell growth through oxidative stress, serving as an effective therapeutic target (55). L-γ-glutamyl-p-nitroanilide (GPNA), an inhibitor of SLC1A5, has shown potential antitumoral effects through the inhibition of glutamine influx and by acting as a competitive inhibitor of SLC7A5 [also known as human L-type amino acid transporter 1 (LAT1)], inhibiting the influx of essential amino acids (70). This inhibition may lead to changes in intracellular amino acid composition and mTORC1 activity (69,70). SLC7A5 is responsible for transporting glutamine out of the cells and transporting essential amino acids into the cells. A previous study has shown that, as a bidirectional transporter of glutamine, SLC1A5 cooperates with SLC7A5 and serves an important role in amino acid transportation, mTORC1 activation and regulation of cell growth (52). An inhibitor of SLC7A5, 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid (BCH), induces cancer cell apoptosis by preventing the activation of mTORC1 which is glutamine-dependent, therefore achieving its antitumor effect (71). SLC7A11 is a transporter of cysteine/glutamine, which is overexpressed in NSCLC. It synthesizes GSH by transporting cysteine to resist oxidative stress, and its overexpression induces metabolic dependency on glucose and glutamine synchronously, which is also a process of glutamine addiction in NSCLC (56,72). An SLC7A11 inhibitor, sulfasalazine, has shown its potential effect through the inhibition of GSH synthesis and the disturbance of the redox balance in KRAS-mutant lung AC (73).
Key enzymes of the glutamine metabolism process are promising therapeutic targets that have been widely studied, and some mature drugs have been used in clinical treatment (48). GLS is one of the most crucial enzymes that convert glutamine into glutamate to provide substrates for the TCA cycle. GLS and its isoforms have shown that it serves an irreplaceable role in glutamine metabolism, making it a potential therapeutic target (74). 6-Diazo-5-oxo-L-norleucine (DON), is a first-generation drug of glutamine analogues that can widely inhibit the enzymes used by glutamine, including GLS, transaminase and GLUL, and has therapeutic effects on patients with cancer. However, its clinical application is limited owing to oral toxicity, gastrointestinal toxicity, leukopenia and thrombocytopenia (75). More importantly, since glutamine is an important neurotransmitter, DON has a series of inevitable CNS-associated side effects (76). DON-derived drugs not only maintain their efficient therapeutic effect, but reduce toxicity and side effects, which are being investigated further (77). For example, the compound JHU083 not only inhibits tumor growth, but also enhances the effect of immunotherapy by improving the function and efficiency of immune cell responses, which is mainly exerted by CD8+ T cells (65). Bis-2-(5-phenylacetamido-1,3,4-thiadiazol-2-yl)ethyl sulfide (BPTES) is another GLS inhibitor that selectively inhibits GLS but does not inhibit KGA; however, the structure of BPTES, being different from that of either glutamine or glutamate, will not cause toxicity owing to interaction with other transporters or receptors (78,79). CB-839 (telaglenastat) is a potential and selective GLS inhibitor that shows a positive result in the treatment of triple-negative breast cancer and other glutamine-dependent cancers, such as NSCLC (80).
Combined therapeutic strategy
Surgery, chemotherapy, and radiotherapy are still the first-line treatment approaches for NSCLC; emerging immunotherapy and molecular-targeted therapy approaches also have hopeful prospects (5). However, all types of therapeutic strategies have their limitations and selectivity. Therefore, the combination of targeting glutamine metabolism with traditional chemotherapeutic drugs, such as cisplatin, paclitaxel, cyclophosphamide, gemcitabine and pemetrexed or the EGFR TKIs, including erlotinib and gefitinib, may be beneficial (19,80,81). Radiotherapy has great potential for the treatment of NSCLC, but not all patients can benefit from it. Recently, a study has shown that the GLS inhibitor CB-839 can improve the sensitivity of patients with NSCLC to radiotherapy by reducing the secretion of the intracellular antioxidant molecule GSH (82). Moreover, CB-839 can also be combined with some molecular-targeted therapeutic drugs to improve their antitumor effect. For example, the combination of CB-839 and selumetinib was shown to improve antitumoral effects in KRAS-driven NSCLC (83). The outcome of combined treatment is reflected by the redox stress caused by the decrease of mitochondrial membrane potential and the increase of the ROS levels, as well as the energy stress induced by the inhibition of glycolysis and glutamine metabolism. Meanwhile, combined treatment can inhibit the phosphorylation process of AKT, inducing autophagy, and eventually leading to cancer cell death (83). The combination of CB-839 and the EGFR inhibitor erlotinib, can lead to energy stress and metabolic risk through autophagy and activation of AMPK. In addition, the lower expression of MYC and HIF-1α, and their downstream targets GLUT1 and SLC1A5, indicate that the combination of these two drugs may block the metabolic process of two basic nutrients, glucose and glutamine, inducing cell death (84). MLN0128 (sapanisertib) is an antitumor drug that inhibits mTOR and glycolysis. However, GSK-3α/β, a central regulator in lung SCC cells, can promote the expression of GLS and glutamine metabolism by upregulating c-MYC and c-JUN to make cancer cells adapt to chronic mTOR and glycolysis inhibition (85). Therefore, CB-839 is used to block glutamine metabolism, avoid drug resistance and achieve unique antitumor effect due to simultaneous inhibition of glycolysis and glutamine metabolism (85).
It is well known that ATP production depends on cytosol NADP; NSCLC cells obtain ATP from cytosol NADH through the malate-aspartate shuttle system using glutamate, which is the catalysate of GLS1. GLS1 inhibitors, such as BPTES, reduce ATP production through glutamate deficiency, and finally inhibit cancer cell growth (86). Combined with the thymidylate synthase inhibitor 5-FU, GLS1 inhibitors have synthetic lethal effects (87). EGFR TKIs are targeted therapeutic drugs for patients with NSCLC with EGFR mutations; however, secondary drug resistance is the main reason for the limited antitumor effect (88). The expression of SLC1A5 increased after treatment with the EGFR TKI almonertinib (89), which may be related to secondary resistance since glutamine can activate the downstream signal of EGFR, such as mTOR. SLC1A5 inhibitors, such as GPNA, can enhance the antitumor effect of almonertinib independently of the presence of EGFR mutations, and the combination of these two drugs may be the reason for inducing apoptosis by inhibiting autophagy (89). The GAC inhibitor 968 can reverse the secondary resistance of erlotinib by preventing glutamine metabolism; their combination not only restricts glutamine and glycolytic metabolism, but also maximizes the antitumor effect and safety, which provides a direction for the treatment of patients with NSCLC resistant to EGFR TKIs (81).
In addition to targeting intracellular metabolic processes, the metabolism of cells in the TME has attracted the interest of researchers because the relationship between cell metabolism and TME is regarded as one of the emerging hallmarks of cancer (10). Cancer-associated fibroblasts (CAFs) are the most common cells in the TME; they exhibit metabolic flexibility and adaptability reflected by the synthesis of glutamine using atypical carbon and nitrogen sources in a nutrient-deprived TME to maintain the growth of glutamine dependent cells (90). The combination therapy between inhibiting glutamine synthesis in CAFs cells by targeting GLUL and blocking glutamine metabolism in tumor cells is a hopeful prospect (66). Glutamine metabolism supports the rapid growth of cancer cells and creates a TME conducive to tumor immune escape (66). The different phenotype induced by glutamine antagonists between cancer cells and T cells provide an opportunity for emerging immunotherapies (91). The function of glutamine antagonists in cancer cells is to induce intracellular hypoxia, acidosis and nutrient deprivation in cancer cells, whereas in effector T cells, glutamine antagonists promote intracellular antioxidant metabolism and induce transformation of T cells into long-lived, high-activity and high-memory subtypes; this is shown by the abnormal overexpression of activation markers, memory markers, anti-apoptotic proteins and transcription factors (65). Therefore, glutamine antagonists, such as DON and JHU038, may not only improve immunotherapy but also enhance inherent antitumor responses. Based on the data aforementioned, combined therapy is a promising strategy (92).
Targeting the sweet spot
Apart from the traditional targeted drugs that aim at inhibiting glutamine transporters and GLS, an increasing number of promising targets directly or indirectly involved in glutamine metabolic pathway have been studied, with the aims of achieving an antitumor effect by blocking the glutamine-dependent metabolism in tumor cells (61,93). Although using GLS as a target for immunotherapy is promising, there are still some limitations. GLS depends on glutamine metabolism and is a rate-limiting enzyme of some cancer cells, including hepatocellular carcinoma cells, colorectal cancer cells and NSCLC (86). The role of GLS has been widely studied and, until recently, selective inhibitors targeting GLS2 have been shown to induce autophagy and apoptosis by inhibiting the mTORC1 signaling pathway, making GLS2 a potential therapeutic target (92). Overexpression of GLS2 may contribute to drug resistance following treatment of breast cancer cells with the GLS inhibitor CB-839 (94). GPT2 is a key enzyme that catalyzes the reversible transformation of alanine and pyruvate. It was reported that the expression and distribution of GPT2 was significantly increased in patients with NSCLC who were resistant to glutamine metabolic inhibitors (61). A study also showed that, under glutamine-deficient conditions, alanine metabolism is involved in the production of TCA cycle intermediates and metabolic derivatives to maintain cell growth and survival. The GPT2 inhibitor L-cycloserine can effectively improve the sensitivity of NSCLC to GLS inhibitors (61).
Glutamine-dependency in tumor cells is partly determined by the process of TCA cycle anaplerosis. In MYC-transformed cells, the carbon provided by glutamine enters the TCA cycle mainly through transamination, which exposes the vulnerable points during metabolism and provides an opportunity for targeting the inhibition of transaminase (95). The therapeutic effect of the transaminase inhibitor aminooxyacetate further enhances the confidence of researchers, and the transaminase inhibitor may have promising development prospects (96). TCA cycle replenishment is a vital process in intracellular metabolism because it allows cells to obtain substrates from the TCA cycle for the synthesis of macromolecules. TCA replenishment involves the use of glutamine or oxaloacetate, which is the product of pyruvate under the catalysis of pyruvate carboxylase (PC) (16); thus, targeting the suppression of PC activity may also have a similar effect. Interestingly, researchers have shown that PC is highly expressed in early NSCLC cells, and knockdown of PC leads to reduced cell growth and proliferation and lower TCA cycle activity due to the reduction of compensatory intermediates, resulting in the reduction of lipid and nucleotide synthesis and the imbalance of GSH, which may lead to the excessive production of ROS (62). The function of glutamine in maintaining intracellular redox balance is mainly through GSH synthesis to combat ROS produced in cells. GDH1 is an essential enzyme that assists glutamine in maintaining intracellular redox balance by catalyzing the reversible oxidative deamination process of L-glutamine to α-KG. GDH1 regulates redox balance by controlling intracellular levels of α-KG and glutaminolysis (42). Targeting GDH1 disrupts intracellular redox homeostasis and suppresses the growth and proliferation of tumor cells. In NSCLC, the abundant expression of NOX4 induces the increase of GLS and GSH synthetase (GSS) both at mRNA and protein levels; NOX4 is the key enzyme catalyzing GSH synthesis, resulting in increased GSH synthesis, which is the basis for cancer cells to resist oxidative stress and gain oxidative resistance (63). Therefore, it is a promising therapy acting by accelerating the efflux of glutamine and selectively depriving GSH (97), and NOX4 may also be a novel therapeutic target.
Patient stratification and predictive markers
Tumor metabolism is not a specific metabolic map, which means that targeted metabolic therapy is not fixed (54). Therefore, the treatment of patients with NSCLC should be selective and specific to maximize the treatment effect, making patient stratification particularly important (98). Stratification mainly includes tumor histological subtypes and specific driver gene mutations. Owing to the metabolic heterogeneity of glutamine, we hypothesize that glutamine inhibitors may be more effective for the treatment of patients with either EGFR or KRAS mutations.
Among the Asian population, the proportion of EGFR mutations in lung AC ranges between 45 and 75% (99). For patients with EGFR mutations, EGFR-TKIs are the first-line treatment of choice. However, secondary drug resistance is an obstacle for achieving a satisfactory therapeutic effect, mainly due to the presence of EGFR T790M mutation, which represents a marker of secondary drug resistance and the amplification of MET gene expression (81). The combination of traditional EGFR-TKIs and glutamine inhibitors may provide a good solution to this problem. The issue of drug resistance could be prevented by combining gefitinib, a therapeutic drug for patients with NSCLC with EGFR mutations, and BCH, a SLC7A5 inhibitor that reduces the phosphorylation level of mTOR and its downstream molecules (93). Moreover, SLC7A5 inhibitors could also be used for the treatment of patients with NSCLC without EGFR mutations (93). The transcription factor p53 is a tumor suppressor, and mutations are often identified in lung SCC (14). GLS2 is a unique target gene of p53; it regulates glutamine metabolism, energy production and intracellular ROS levels (100). In NSCLC, p53 inhibits tumor growth by promoting pyroptosis (101). Moreover, p53 mutations are related to resistance of NSCLCs to EGFR TKIs (102). Hence, the status of p53 can be considered as a target molecule to improve the therapeutic effect of EGFR TKIs against NSCLC (102).
KRAS-driven cancer accounts for ~35% of lung AC cases (103). In NSCLC cells, simultaneous presence of mutations in KRAS, LKB1 and KEAP1 is possible and these are sensitive to GLS inhibitors due to glutamine dependency, making them promising targets for therapy in the future (29). Mutations in other genes, such as IDH, are rare in NSCLC, but these are often associated with smoking history and KRAS mutations (104). The prognosis of NSCLC is generally poor, but numerous studies have revealed that the expression levels of some molecules can be used as markers to predict the prognosis and therapeutic effect. For example, SLC1A5 is an independent marker of poor prognosis, particularly in patients with NSCLC AC who are undergoing surgery (105). The expression of SLC1A5 is significantly higher in non-AC cases and depends on a number of factors, including sex (particularly males) and presence of advanced tumors (105). Another transporter of glutamine is SLC7A11; its high expression is associated with KEAP1 mutations and abnormal expression of BAP1. These biological markers help identify tumors with high expression of SLC7A11, which may subsequently benefit patients with the combination of radiotherapy or chemotherapy and SLC7A11 inhibition (72). In KRAS-driven NSCLC, the expression of glutamine-dependent genes, especially glutamic-oxaloacetate transaminase 1 (GOT1) and malic enzyme 1 (ME1), is increased to maintain redox balance. A study has shown that ME1 and GOT1 are predictive markers of sensitivity to radiotherapy (106). Therefore, KRAS-driven cancer cells are sensitive to radiotherapy during glutamine deprivation (106). In addition to the markers mentioned above, the level of GPT2 can be used to identify those patients with NSCLC who will benefit the most from the treatment with GLS inhibitors (61). The purpose of patient stratification and biological predictive markers is to achieve individualized treatment as well as to obtain therapeutic effects and prognosis.
5. Conclusions
As the most abundant non-essential amino acid in the circulation, glutamine serves an indispensable role in the metabolism of some tumors. It has an important role in supporting cell growth and proliferation, activating signal transduction pathways, and maintaining redox balance. Since the publication of the Warburg effect, which proposed concept of aerobic glycolysis and glutamine addiction, there has been an increase in the number of studies on tumor metabolism. NSCLC cells are dependent on glutamine through the metabolic reprogramming caused by the mutation of oncogenes or tumor suppressor genes, which makes them unable to grow normally under glutamine deprivation. The metabolic reprogramming of tumor cells can promote cell growth and proliferation, as well as lead to the malignant progress of tumor and the emergence of drug resistance. Recently, targeting the key enzymes and transporters of glutamine metabolism with specific inhibitors showed pharmacological progress and has led to clinical trials, which revealed surprising results regarding treatment (summarized in Table I). However, the metabolic vulnerability of non-cancer cells and the metabolic plasticity of cancer cells in the TME should be taken into account. The metabolic reprogramming and flexibility of cancer cells may limit drug efficacy, and the impact on immune cells may limit the attack on cancer cells. Therefore, a better understanding of cancer cells and immune cell metabolism is required. Considering the glutamine dependence of NSCLC cells, CB-839, BPTES and DON are also increasingly used in treatment, but with mixed results. These drugs may be beneficial to some patients due to the important role of glutamine in cell growth. However, treatment is limited by the heterogeneity of NSCLC metabolism and not all patients may benefit. Therefore, we propose a therapy scheme including patient stratification and screening of special biological predictive markers. Patient stratification includes the classification of tissue and gene mutation subtypes, aiming to achieve individualized medical treatment and maximize the benefits for each patient. Biological predictive markers can be used to identify the most suitable treatment approach and make predictions regarding prognosis. Combined therapy strategies are a promising way to improve the effectiveness of treatment and may potentially be the leading treatment approach in the future. The combination of glutamine inhibitors with emerging immunotherapy or targeted-therapy drugs may not only improve their antitumor effect, but also may reduce drug resistance and increase sensitivity. In addition, new targets which are directly or indirectly associated with glutamine metabolism require further exploration.
Table I.
Summary of preclinical tools and clinical therapeutic drugs targeting different process of glutamine metabolism.
| First author/s, year | Class | Drug | Mechanism | Stage | Target | (Refs.) |
|---|---|---|---|---|---|---|
| Magill et al, 1957 | Glutamine analogue | DON | Widely inhibit the glutamine metabolic enzyme | Limited due to toxicity | Enzyme used by glutamine | (75) |
| Leone et al, 2019 | JHU083 | Pro-drug of DON | Preclinical compound tool | (65) | ||
| Robinson et al, 2007 | GLS inhibitors | BPTES | Inhibition of GLS | Preclinical tool | GLS | (78) |
| Gross et al, 2014 | CB-839 | Phase I and II | (80) | |||
| Caiola et al, 2020 | Transaminase inhibitors | L-cycloserine | Inhibition of TCA cycle | Preclinical tool | GPT2 | (61) |
| Moreadith and Lehninger, 1984 | AOA | anaplerosis | Preclinical tool | Aminobutyrate aminotransferase | (96) | |
| Hassanein et al, 2013 | Glutamine transporters inhibitors | GPNA | Inhibition of glutamine transport | Preclinical tool | SLC1A5 (also known as ASCT2) | (69) |
| Wise and Thompson, 2010 | BCH | Inhibition of essential amino acids | Preclinical tool | SLC7A5 (also known as LAT1) | (71) | |
| Hu et al, 2020 | Sulfasalazine | Inhibition of cysteine-glutamine transport | Phase II for breast cancer | SLC7A11 (also known as xCT) | (73) |
AOA, aminooxyacetate; ASCT2, amino acid transporter 2; BCH, 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid; BPTES, Bis-2-(5-phenylacetamido-1,3,4-thiadiazol-2-yl)ethyl sulfide; DON, 6-Diazo-5-oxo-L-norleucine; GLS, glutaminase; GPNA, L-γ-glutamyl-p-nitroanilide; GPT2, glutamic-pyruvic transaminase 2; LAT1, L-type amino acid transporter; SLC(X)A(Y), solute carrier family (X) member (Y); TCA, tricarboxylic acid.
This review described the identification of glutamine as a promising target for treatment by emphasizing its role in NSCLC cell metabolism. In addition to the traditional drugs targeting glutamine metabolic enzymes and transporters, new sweet spots, along with the concept of combination and individualized therapy, are also described. Inhibition of glutamine as a targeted therapy for NSCLC is promising, but still faces new challenges. Cancer and normal cells share many pathways, making selective inhibition particularly important. Future studies should not only consider glutamine metabolism in normal cells, but also target glutamine addiction in NSCLC cells, which will maximize the therapeutic effect and reduce side effects. Moreover, the heterogeneity of NSCLC metabolism and the difference in individual gene expression lead to the absence of a metabolic map suitable for everyone. 18F-flurodeoxyglucose provides useful information by tracking glucose metabolism in vivo based on the increase of glucose uptake and glycolysis by cancer cells (107). L-[5-11C]-glutamine may be used as an indicator for in vivoglutamine-dependent tumor metabolism (108). Therefore, the progress of glutamine-targeted therapy also depends on imaging technology.
Although the heterogeneity of cancer metabolism limits the application of metabolism-targeted drugs to some extent, glutamine-targeted therapy still has potential. Glutamine supplement treatment may be beneficial to prevent mucositis in patients receiving radiotherapy and chemotherapy. For example, glutamine can be used to prevent chemotherapeutic or radioactive esophagitis in patients with esophageal cancer (109). At present, metabolic tracking technologies, such as spectroscopy and PET/CT, have rapidly been developed. With the development of metabonomic research, the heterogeneity of cancer cell metabolism will be further explored. At the same time, the application of genetic testing and biomarker testing will help us choose the patients who would benefit the most from treatment.
Acknowledgements
Not applicable.
Funding Statement
This work was supported by the grants from The National Natural Science Foundation of China (grant no. 32170793, 82160133, 31960147 and 31760329), Jiangxi Provincial Natural Science Foundation (grant no. 20212BAB206086, 20212ACB216005, 20224ACB216013 and 20224BAB206007).
Availability of data and materials
Not applicable.
Authors' contributions
LZ, QZha and QZhu wrote the manuscript; YZ and YL revised the manuscript and XH revised and confirmed the final version of the manuscript. All authors read and approved the final version of the manuscript. Data authentication is not applicable.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Schabath MB, Cote ML. Cancer progress and priorities: Lung cancer. Cancer Epidemiol Biomarkers Prev. 2019;28:1563–1579. doi: 10.1158/1055-9965.EPI-19-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ganti AK, Klein AB, Cotarla I, Seal B, Chou E. Update of incidence, prevalence, survival, and initial treatment in patients with non-small cell lung cancer in the US. JAMA Oncol. 2021;7:1824–1832. doi: 10.1001/jamaoncol.2021.4932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou Y, Du Q, Zhao Q, Zhang M, Qin X, Jiang Y, Luan Y. A heme-regulatable chemodynamic nanodrug harnessing transcription factor Bach1 against lung cancer metastasis. J Colloid Interface Sci. 2022;610:698–708. doi: 10.1016/j.jcis.2021.11.124. [DOI] [PubMed] [Google Scholar]
- 5.Duma N, Santana-Davila R, Molina JR. Non-Small cell lung cancer: Epidemiology, screening, diagnosis, and treatment. Mayo Clin Proc. 2019;94:1623–1640. doi: 10.1016/j.mayocp.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 6.Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P, Mitchell A, Bolejack V, et al. The IASLC Lung Cancer Staging Project: Proposals for Revision of the TNM Stage Groupings in the Forthcoming (Eighth) Edition of the TNM Classification for Lung Cancer. J Thorac Oncol. 2016;11:39–51. doi: 10.1016/j.jtho.2015.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Otto AM. Warburg effect(s)-a biographical sketch of Otto Warburg and his impacts on tumor metabolism. Cancer Metab. 2016;4:5. doi: 10.1186/s40170-016-0145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boroughs LK, DeBerardinis RJ. Metabolic pathways promoting cancer cell survival and growth. Nat Cell Biol. 2015;17:351–359. doi: 10.1038/ncb3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Pavlova NN, Thompson CB. The emerging hallmarks of cancer metabolism. Cell Metab. 2016;23:27–47. doi: 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hensley CT, Wasti AT, DeBerardinis RJ. Glutamine and cancer: Cell biology, physiology, and clinical opportunities. J Clin Invest. 2013;123:3678–3684. doi: 10.1172/JCI69600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohamed A, Deng X, Khuri FR, Owonikoko TK. Altered glutamine metabolism and therapeutic opportunities for lung cancer. Clin Lung Cancer. 2014;15:7–15. doi: 10.1016/j.cllc.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meijer TWH, Looijen-Salamon MG, Lok J, van den Heuvel M, Tops B, Kaanders JHAM, Span PN, Bussink J. Glucose and glutamine metabolism in relation to mutational status in NSCLC histological subtypes. Thorac Cancer. 2019;10:2289–2299. doi: 10.1111/1759-7714.13226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kroemer G, Pouyssegur J. Tumor cell metabolism: Cancer's Achilles' heel. Cancer Cell. 2008;13:472–482. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 16.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: Metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 17.DeBerardinis RJ, Chandel NS. Fundamentals of cancer metabolism. Sci Adv. 2016;2:e1600200. doi: 10.1126/sciadv.1600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshida GJ. Metabolic reprogramming: The emerging concept and associated therapeutic strategies. J Exp Clin Cancer Res. 2015;34:111. doi: 10.1186/s13046-015-0221-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cruz-Bermudez A, Laza-Briviesca R, Vicente-Blanco RJ, García-Grande A, Coronado MJ, Laine-Menéndez S, Palacios-Zambrano S, Moreno-Villa MR, Ruiz-Valdepeñas AM, Lendinez C, et al. Cisplatin resistance involves a metabolic reprogramming through ROS and PGC-1α in NSCLC which can be overcome by OXPHOS inhibition. Free Radic Biol Med. 2019;135:167–181. doi: 10.1016/j.freeradbiomed.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 20.Solanki HS, Babu N, Jain AP, Bhat MY, Puttamallesh VN, Advani J, Raja R, Mangalaparthi KK, Kumar MM, Prasad TSK, et al. Cigarette smoke induces mitochondrial metabolic reprogramming in lung cells. Mitochondrion. 2018;40:58–70. doi: 10.1016/j.mito.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 21.Pavlova NN, Zhu J, Thompson CB. The hallmarks of cancer metabolism: Still emerging. Cell Metab. 2022;34:355–377. doi: 10.1016/j.cmet.2022.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Judd J, Abdel Karim N, Khan H, Naqash AR, Baca Y, Xiu J, VanderWalde AM, Mamdani H, Raez LE, Nagasaka M, et al. Characterization of KRAS mutation subtypes in non-small cell lung cancer. Mol Cancer Ther. 2021;20:2577–2584. doi: 10.1158/1535-7163.MCT-21-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawada K, Toda K, Sakai Y. Targeting metabolic reprogramming in KRAS-driven cancers. Int J Clin Oncol. 2017;22:651–659. doi: 10.1007/s10147-017-1156-4. [DOI] [PubMed] [Google Scholar]
- 24.Strohecker AM, Guo JY, Karsli-Uzunbas G, Price SM, Chen GJ, Mathew R, McMahon M, White E. Autophagy sustains mitochondrial glutamine metabolism and growth of BrafV600E-driven lung tumors. Cancer Discov. 2013;3:1272–1285. doi: 10.1158/2159-8290.CD-13-0397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Commisso C, Davidson SM, Soydaner-Azeloglu RG, Parker SJ, Kamphorst JJ, Hackett S, Grabocka E, Nofal M, Drebin JA, Thompson CB, et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013;497:633–637. doi: 10.1038/nature12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Davidson SM, Papagiannakopoulos T, Olenchock BA, Heyman JE, Keibler MA, Luengo A, Bauer MR, Jha AK, O'Brien JP, Pierce KA, et al. Environment impacts the metabolic dependencies of ras-driven non-small cell lung cancer. Cell Metab. 2016;23:517–528. doi: 10.1016/j.cmet.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dowling CM, Zhang H, Chonghaile TN, Wong KK. Shining a light on metabolic vulnerabilities in non-small cell lung cancer. Biochim Biophys Acta Rev Cancer. 2021;1875:188462. doi: 10.1016/j.bbcan.2020.188462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Faubert B, Vincent EE, Griss T, Samborska B, Izreig S, Svensson RU, Mamer OA, Avizonis D, Shackelford DB, Shaw RJ, Jones RG. Loss of the tumor suppressor LKB1 promotes metabolic reprogramming of cancer cells via HIF-1α. Proc Natl Acad Sci USA. 2014;111:2554–2559. doi: 10.1073/pnas.1312570111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Galan-Cobo A, Sitthideatphaiboon P, Qu X, Poteete A, Pisegna MA, Tong P, Chen PH, Boroughs LK, Rodriguez MLM, Zhang W, et al. LKB1 and KEAP1/NRF2 pathways cooperatively promote metabolic reprogramming with enhanced glutamine dependence in KRAS-Mutant lung adenocarcinoma. Cancer Res. 2019;79:3251–3267. doi: 10.1158/0008-5472.CAN-18-3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T, Zeller KI, De Marzo AM, Van Eyk JE, Mendell JT, Dang CV. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458:762–765. doi: 10.1038/nature07823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuneva MO, Fan TW, Allen TD, Higashi RM, Ferraris DV, Tsukamoto T, Matés JM, Alonso FJ, Wang C, Seo Y, et al. The metabolic profile of tumors depends on both the responsible genetic lesion and tissue type. Cell Metab. 2012;15:157–170. doi: 10.1016/j.cmet.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hua Q, Wang D, Zhao L, Hong Z, Ni K, Shi Y, Liu Z, Mi B. AL355338 acts as an oncogenic lncRNA by interacting with protein ENO1 to regulate EGFR/AKT pathway in NSCLC. Cancer Cell Int. 2021;21:525. doi: 10.1186/s12935-021-02232-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hua Q, Mi B, Xu F, Wen J, Zhao L, Liu J, Huang G. Hypoxia-induced lncRNA-AC020978 promotes proliferation and glycolytic metabolism of non-small cell lung cancer by regulating PKM2/HIF-1α axis. Theranostics. 2020;10:4762–4778. doi: 10.7150/thno.43839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vanhove K, Derveaux E, Graulus GJ, Mesotten L, Thomeer M, Noben JP, Guedens W, Adriaensens P. Glutamine addiction and therapeutic strategies in lung cancer. Int J Mol Sci. 2019;20:252. doi: 10.3390/ijms20020252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi YK, Park KG. Targeting glutamine metabolism for cancer treatment. Biomol Ther (Seoul) 2018;26:19–28. doi: 10.4062/biomolther.2017.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeBerardinis RJ, Mancuso A, Daikhin E, Nissim I, Yudkoff M, Wehrli S, Thompson CB. Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc Natl Acad Sci USA. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiao D, Zeng L, Yao K, Kong X, Wu G, Yin Y. The glutamine-alpha-ketoglutarate (AKG) metabolism and its nutritional implications. Amino Acids. 2016;48:2067–2080. doi: 10.1007/s00726-016-2254-8. [DOI] [PubMed] [Google Scholar]
- 38.Metallo CM, Gameiro PA, Bell EL, Mattaini KR, Yang J, Hiller K, Jewell CM, Johnson ZR, Irvine DJ, Guarente L, et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature. 2011;481:380–384. doi: 10.1038/nature10602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kuhajda FP. Fatty acid synthase and cancer: New application of an old pathway. Cancer Res. 2006;66:5977–5980. doi: 10.1158/0008-5472.CAN-05-4673. [DOI] [PubMed] [Google Scholar]
- 40.Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov. 2013;12:931–947. doi: 10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- 41.Harris IS, Treloar AE, Inoue S, Sasaki M, Gorrini C, Lee KC, Yung KY, Brenner D, Knobbe-Thomsen CB, Cox MA, et al. Glutathione and thioredoxin antioxidant pathways synergize to drive cancer initiation and progression. Cancer Cell. 2015;27:211–222. doi: 10.1016/j.ccell.2015.01.009. [DOI] [PubMed] [Google Scholar]
- 42.Jin L, Li D, Alesi GN, Fan J, Kang HB, Lu Z, Boggon TJ, Jin P, Yi H, Wright ER, et al. Glutamate dehydrogenase 1 signals through antioxidant glutathione peroxidase 1 to regulate redox homeostasis and tumor growth. Cancer Cell. 2015;27:257–270. doi: 10.1016/j.ccell.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cluntun AA, Lukey MJ, Cerione RA, Locasale JW. Glutamine metabolism in cancer: Understanding the heterogeneity. Trends Cancer. 2017;3:169–180. doi: 10.1016/j.trecan.2017.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu W, Le A, Hancock C, Lane AN, Dang CV, Fan TW, Phang JM. Reprogramming of proline and glutamine metabolism contributes to the proliferative and metabolic responses regulated by oncogenic transcription factor c-MYC. Proc Natl Acad Sci USA. 2012;109:8983–8988. doi: 10.1073/pnas.1203244109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kerr EM, Gaude E, Turrell FK, Frezza C, Martins CP. Mutant Kras copy number defines metabolic reprogramming and therapeutic susceptibilities. Nature. 2016;531:110–113. doi: 10.1038/nature16967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jin L, Alesi GN, Kang S. Glutaminolysis as a target for cancer therapy. Oncogene. 2016;35:3619–3625. doi: 10.1038/onc.2015.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.DeBerardinis RJ, Cheng T. Q's next: The diverse functions of glutamine in metabolism, cell biology and cancer. Oncogene. 2010;29:313–324. doi: 10.1038/onc.2009.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Altman BJ, Stine ZE, Dang CV. From Krebs to clinic: Glutamine metabolism to cancer therapy. Nat Rev Cancer. 2016;16:619–634. doi: 10.1038/nrc.2016.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoo HC, Yu YC, Sung Y, Han JM. Glutamine reliance in cell metabolism. Exp Mol Med. 2020;52:1496–1516. doi: 10.1038/s12276-020-00504-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Alberghina L, Gaglio D. Redox control of glutamine utilization in cancer. Cell Death Dis. 2014;5:e1561. doi: 10.1038/cddis.2014.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149:274–293. doi: 10.1016/j.cell.2012.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nicklin P, Bergman P, Zhang B, Triantafellow E, Wang H, Nyfeler B, Yang H, Hild M, Kung C, Wilson C, et al. Bidirectional transport of amino acids regulates mTOR and autophagy. Cell. 2009;136:521–534. doi: 10.1016/j.cell.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jewell JL, Kim YC, Russell RC, Yu FX, Park HW, Plouffe SW, Tagliabracci VS, Guan KL. Metabolism. Differential regulation of mTORC1 by leucine and glutamine. Science. 2015;347:194–198. doi: 10.1126/science.1259472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Strickaert A, Saiselet M, Dom G, De Deken X, Dumont JE, Feron O, Sonveaux P, Maenhaut C. Cancer heterogeneity is not compatible with one unique cancer cell metabolic map. Oncogene. 2017;36:2637–2642. doi: 10.1038/onc.2016.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hassanein M, Qian J, Hoeksema MD, Wang J, Jacobovitz M, Ji X, Harris FT, Harris BK, Boyd KL, Chen H, et al. Targeting SLC1a5-mediated glutamine dependence in non-small cell lung cancer. Int J Cancer. 2015;137:1587–1597. doi: 10.1002/ijc.29535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Santarpia M, Aguilar A, Chaib I, Cardona AF, Fancelli S, Laguia F, Bracht JWP, Cao P, Molina-Vila MA, Karachaliou N, Rosell R. Non-Small-cell lung cancer signaling pathways, metabolism, and PD-1/PD-L1 Antibodies. Cancers (Basel) 2020;12:1475. doi: 10.3390/cancers12061475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perez-Escuredo J, Dadhich RK, Dhup S, Cacace A, Van Hée VF, De Saedeleer CJ, Sboarina M, Rodriguez F, Fontenille MJ, Brisson L, et al. Lactate promotes glutamine uptake and metabolism in oxidative cancer cells. Cell Cycle. 2016;15:72–83. doi: 10.1080/15384101.2015.1120930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van den Heuvel AP, Jing J, Wooster RF, Bachman KE. Analysis of glutamine dependency in non-small cell lung cancer: GLS1 splice variant GAC is essential for cancer cell growth. Cancer Biol Ther. 2012;13:1185–1194. doi: 10.4161/cbt.21348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang C, Sudderth J, Dang T, Bachoo RM, McDonald JG, DeBerardinis RJ. Glioblastoma cells require glutamate dehydrogenase to survive impairments of glucose metabolism or Akt signaling. Cancer Res. 2009;69:7986–7993. doi: 10.1158/0008-5472.CAN-09-2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen SL, Xue N, Wu MT, Chen H, He X, Li JP, Liu WL, Dai SQ. Influence of preoperative serum aspartate aminotransferase (AST) level on the prognosis of patients with non-small cell lung cancer. Int J Mol Sci. 2016;17:1474. doi: 10.3390/ijms17091474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Caiola E, Colombo M, Sestito G, Lupi M, Marabese M, Pastorelli R, Broggini M, Brunelli L. Glutaminase inhibition on NSCLC depends on extracellular alanine exploitation. Cells. 2020;9:1766. doi: 10.3390/cells9081766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sellers K, Fox MP, Bousamra M, II, Slone SP, Higashi RM, Miller DM, Wang Y, Yan J, Yuneva MO, Deshpande R, et al. Pyruvate carboxylase is critical for non-small-cell lung cancer proliferation. J Clin Invest. 2015;125:687–698. doi: 10.1172/JCI72873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zeng C, Wu Q, Wang J, Yao B, Ma L, Yang Z, Li J, Liu B. NOX4 supports glycolysis and promotes glutamine metabolism in non-small cell lung cancer cells. Free Radic Biol Med. 2016;101:236–248. doi: 10.1016/j.freeradbiomed.2016.10.500. [DOI] [PubMed] [Google Scholar]
- 64.Oh MH, Sun IH, Zhao L, Leone RD, Sun IM, Xu W, Collins SL, Tam AJ, Blosser RL, Patel CH, et al. Targeting glutamine metabolism enhances tumor-specific immunity by modulating suppressive myeloid cells. J Clin Invest. 2020;130:3865–3884. doi: 10.1172/JCI131859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leone RD, Zhao L, Englert JM, Sun IM, Oh MH, Sun IH, Arwood ML, Bettencourt IA, Patel CH, Wen J, et al. Glutamine blockade induces divergent metabolic programs to overcome tumor immune evasion. Science. 2019;366:1013–1021. doi: 10.1126/science.aav2588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang L, Achreja A, Yeung TL, Mangala LS, Jiang D, Han C, Baddour J, Marini JC, Ni J, Nakahara R, et al. Targeting stromal glutamine synthetase in tumors disrupts tumor microenvironment-regulated cancer cell growth. Cell Metab. 2016;24:685–700. doi: 10.1016/j.cmet.2016.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hensley CT, Faubert B, Yuan Q, Lev-Cohain N, Jin E, Kim J, Jiang L, Ko B, Skelton R, Loudat L, et al. Metabolic heterogeneity in human lung tumors. Cell. 2016;164:681–694. doi: 10.1016/j.cell.2015.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bhutia YD, Ganapathy V. Glutamine transporters in mammalian cells and their functions in physiology and cancer. Biochim Biophys Acta. 2016;1863:2531–2539. doi: 10.1016/j.bbamcr.2015.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hassanein M, Hoeksema MD, Shiota M, Qian J, Harris BK, Chen H, Clark JE, Alborn WE, Eisenberg R, Massion PP. SLC1A5 mediates glutamine transport required for lung cancer cell growth and survival. Clin Cancer Res. 2013;19:560–570. doi: 10.1158/1078-0432.CCR-12-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chiu M, Sabino C, Taurino G, Bianchi MG, Andreoli R, Giuliani N, Bussolati O. GPNA inhibits the sodium-independent transport system L for neutral amino acids. Amino Acids. 2017;49:1365–1372. doi: 10.1007/s00726-017-2436-z. [DOI] [PubMed] [Google Scholar]
- 71.Wise DR, Thompson CB. Glutamine addiction: A new therapeutic target in cancer. Trends Biochem Sci. 2010;35:427–433. doi: 10.1016/j.tibs.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Koppula P, Zhuang L, Gan B. Cystine transporter SLC7A11/xCT in cancer: Ferroptosis, nutrient dependency, and cancer therapy. Protein Cell. 2021;12:599–620. doi: 10.1007/s13238-020-00789-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hu K, Li K, Lv J, Feng J, Chen J, Wu H, Cheng F, Jiang W, Wang J, Pei H, et al. Suppression of the SLC7A11/glutathione axis causes synthetic lethality in KRAS-mutant lung adenocarcinoma. J Clin Invest. 2020;130:1752–1766. doi: 10.1172/JCI124049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Katt WP, Cerione RA. Glutaminase regulation in cancer cells: A druggable chain of events. Drug Discov Today. 2014;19:450–457. doi: 10.1016/j.drudis.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Magill GB, Myers WP, Reilly HC, Putnam RC, Magill JW, Sykes MP, Escher GC, Karnofsky DA, Burchenal JH. Pharmacological and initial therapeutic observations on 6-diazo-5-oxo-1-norleucine (DON) in human neoplastic disease. Cancer. 1957;10:1138–1150. doi: 10.1002/1097-0142(195711/12)10:6<1138::AID-CNCR2820100608>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 76.Dang CV, Le A, Gao P. MYC-induced cancer cell energy metabolism and therapeutic opportunities. Clin Cancer Res. 2009;15:6479–6483. doi: 10.1158/1078-0432.CCR-09-0889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lemberg KM, Vornov JJ, Rais R, Slusher BS. We're Not ‘DON’ Yet: Optimal dosing and prodrug delivery of 6-Diazo-5-oxo-L-norleucine. Mol Cancer Ther. 2018;17:1824–1832. doi: 10.1158/1535-7163.MCT-17-1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Robinson MM, McBryant SJ, Tsukamoto T, Rojas C, Ferraris DV, Hamilton SK, Hansen JC, Curthoys NP. Novel mechanism of inhibition of rat kidney-type glutaminase by bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide (BPTES) Biochem J. 2007;406:407–414. doi: 10.1042/BJ20070039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shukla K, Ferraris DV, Thomas AG, Stathis M, Duvall B, Delahanty G, Alt J, Rais R, Rojas C, Gao P, et al. Design, synthesis, and pharmacological evaluation of bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide 3 (BPTES) analogs as glutaminase inhibitors. J Med Chem. 2012;55:10551–10563. doi: 10.1021/jm301191p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gross MI, Demo SD, Dennison JB, Chen L, Chernov-Rogan T, Goyal B, Janes JR, Laidig GJ, Lewis ER, Li J, et al. Antitumor activity of the glutaminase inhibitor CB-839 in triple-negative breast cancer. Mol Cancer Ther. 2014;13:890–901. doi: 10.1158/1535-7163.MCT-13-0870. [DOI] [PubMed] [Google Scholar]
- 81.Xie C, Jin J, Bao X, Zhan WH, Han TY, Gan M, Zhang C, Wang J. Inhibition of mitochondrial glutaminase activity reverses acquired erlotinib resistance in non-small cell lung cancer. Oncotarget. 2016;7:610–621. doi: 10.18632/oncotarget.6311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Boysen G, Jamshidi-Parsian A, Davis MA, Siegel ER, Simecka CM, Kore RA, Dings RPM, Griffin RJ. Glutaminase inhibitor CB-839 increases radiation sensitivity of lung tumor cells and human lung tumor xenografts in mice. Int J Radiat Biol. 2019;95:436–442. doi: 10.1080/09553002.2018.1558299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xia M, Li X, Diao Y, Du B, Li Y. Targeted inhibition of glutamine metabolism enhances the antitumor effect of selumetinib in KRAS-mutant NSCLC. Transl Oncol. 2021;14:100920. doi: 10.1016/j.tranon.2020.100920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Momcilovic M, Bailey ST, Lee JT, Fishbein MC, Magyar C, Braas D, Graeber T, Jackson NJ, Czernin J, Emberley E, et al. Targeted Inhibition of EGFR and glutaminase induces metabolic crisis in EGFR mutant lung cancer. Cell Rep. 2017;18:601–610. doi: 10.1016/j.celrep.2016.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Momcilovic M, Bailey ST, Lee JT, Fishbein MC, Braas D, Go J, Graeber TG, Parlati F, Demo S, Li R, et al. The GSK3 signaling axis regulates adaptive glutamine metabolism in lung squamous cell carcinoma. Cancer Cell. 2018;33:905–921. doi: 10.1016/j.ccell.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yu W, Yang X, Zhang Q, Sun L, Yuan S, Xin Y. Targeting GLS1 to cancer therapy through glutamine metabolism. Clin Transl Oncol. 2021;23:2253–2268. doi: 10.1007/s12094-021-02645-2. [DOI] [PubMed] [Google Scholar]
- 87.Lee JS, Kang JH, Lee SH, Hong D, Son J, Hong KM, Song J, Kim SY. Dual targeting of glutaminase 1 and thymidylate synthase elicits death synergistically in NSCLC. Cell Death Dis. 2016;7:e2511. doi: 10.1038/cddis.2016.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yoneda K, Imanishi N, Ichiki Y, Tanaka F. Treatment of non-small cell lung cancer with EGFR-mutations. J UOEH. 2019;41:153–163. doi: 10.7888/juoeh.41.153. [DOI] [PubMed] [Google Scholar]
- 89.Liu Y, Ge X, Pang J, Zhang Y, Zhang H, Wu H, Fan F, Liu H. Restricting glutamine uptake enhances NSCLC Sensitivity to Third-Generation EGFR-TKI Almonertinib. Front Pharmacol. 2021;12:671328. doi: 10.3389/fphar.2021.671328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sazeides C, Le A. Metabolic relationship between cancer-associated fibroblasts and cancer cells. Adv Exp Med Biol. 2018;1063:149–165. doi: 10.1007/978-3-319-77736-8_11. [DOI] [PubMed] [Google Scholar]
- 91.Cerezo M, Rocchi S. Cancer cell metabolic reprogramming: A keystone for the response to immunotherapy. Cell Death Dis. 2020;11:964. doi: 10.1038/s41419-020-03175-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee YZ, Yang CW, Chang HY, Hsu HY, Chen IS, Chang HS, Lee CH, Lee JC, Kumar CR, Qiu YQ, et al. Discovery of selective inhibitors of Glutaminase-2, which inhibit mTORC1, activate autophagy and inhibit proliferation in cancer cells. Oncotarget. 2014;5:6087–6101. doi: 10.18632/oncotarget.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Imai H, Kaira K, Oriuchi N, Shimizu K, Tominaga H, Yanagitani N, Sunaga N, Ishizuka T, Nagamori S, Promchan K, et al. Inhibition of L-type amino acid transporter 1 has antitumor activity in non-small cell lung cancer. Anticancer Res. 2010;30:4819–4828. [PubMed] [Google Scholar]
- 94.Lukey MJ, Cluntun AA, Katt WP, Lin MJ, Druso JE, Ramachandran S, Erickson JW, Le HH, Wang ZE, Blank B, et al. Liver-Type Glutaminase GLS2 is a druggable metabolic node in luminal-subtype breast cancer. Cell Rep. 2019;29:76–88. doi: 10.1016/j.celrep.2019.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wise DR, DeBerardinis RJ, Mancuso A, Sayed N, Zhang XY, Pfeiffer HK, Nissim I, Daikhin E, Yudkoff M, McMahon SB, Thompson CB. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc Natl Acad Sci USA. 2008;105:18782–18787. doi: 10.1073/pnas.0810199105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Moreadith RW, Lehninger AL. The pathways of glutamate and glutamine oxidation by tumor cell mitochondria. Role of mitochondrial NAD(P)+-dependent malic enzyme. J Biol Chem. 1984;259:6215–6221. doi: 10.1016/S0021-9258(20)82128-0. [DOI] [PubMed] [Google Scholar]
- 97.Estrela JM, Ortega A, Obrador E. Glutathione in cancer biology and therapy. Crit Rev Clin Lab Sci. 2006;43:143–181. doi: 10.1080/10408360500523878. [DOI] [PubMed] [Google Scholar]
- 98.Yang WH, Qiu Y, Stamatatos O, Janowitz T, Lukey MJ. Enhancing the efficacy of glutamine metabolism inhibitors in cancer therapy. Trends Cancer. 2021;7:790–804. doi: 10.1016/j.trecan.2021.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Clay TD, Russell PA, Do H, Sundararajan V, Conron M, Wright GM, Dobrovic A, Moore MM, McLachlan SA. Associations between the IASLC/ATS/ERS lung adenocarcinoma classification and EGFR and KRAS mutations. Pathology. 2016;48:17–24. doi: 10.1016/j.pathol.2015.11.002. [DOI] [PubMed] [Google Scholar]
- 100.Suzuki S, Tanaka T, Poyurovsky MV, Nagano H, Mayama T, Ohkubo S, Lokshin M, Hosokawa H, Nakayama T, Suzuki Y, et al. Phosphate-activated glutaminase (GLS2), a p53-inducible regulator of glutamine metabolism and reactive oxygen species. Proc Natl Acad Sci USA. 2010;107:7461–7466. doi: 10.1073/pnas.1002459107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhang T, Li Y, Zhu R, Song P, Wei Y, Liang T, Xu G. Transcription Factor p53 suppresses tumor growth by prompting pyroptosis in non-small-cell lung cancer. Oxid Med Cell Longev. 2019;2019:8746895. doi: 10.1155/2019/8746895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jung S, Kim DH, Choi YJ, Kim SY, Park H, Lee H, Choi CM, Sung YH, Lee JC, Rho JK. Contribution of p53 in sensitivity to EGFR tyrosine kinase inhibitors in non-small cell lung cancer. Sci Rep. 2021;11:19667. doi: 10.1038/s41598-021-99267-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rekhtman N, Ang DC, Riely GJ, Ladanyi M, Moreira AL. KRAS mutations are associated with solid growth pattern and tumor-infiltrating leukocytes in lung adenocarcinoma. Mod Pathol. 2013;26:1307–1319. doi: 10.1038/modpathol.2013.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Toth LN, de Abreu FB, Tafe LJ. Non-small cell lung cancers with isocitrate dehydrogenase 1 or 2 (IDH1/2) mutations. Hum Pathol. 2018;78:138–143. doi: 10.1016/j.humpath.2018.04.014. [DOI] [PubMed] [Google Scholar]
- 105.Shimizu K, Kaira K, Tomizawa Y, Sunaga N, Kawashima O, Oriuchi N, Tominaga H, Nagamori S, Kanai Y, Yamada M, et al. ASC amino-acid transporter 2 (ASCT2) as a novel prognostic marker in non-small cell lung cancer. Br J Cancer. 2014;110:2030–2039. doi: 10.1038/bjc.2014.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chakrabarti G. Mutant KRAS associated malic enzyme 1 expression is a predictive marker for radiation therapy response in non-small cell lung cancer. Radiat Oncol. 2015;10:145. doi: 10.1186/s13014-015-0457-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Almuhaideb A, Papathanasiou N, Bomanji J. 18F-FDG PET/CT imaging in oncology. Ann Saudi Med. 2011;31:3–13. doi: 10.4103/0256-4947.75771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Qu W, Oya S, Lieberman BP, Ploessl K, Wang L, Wise DR, Divgi CR, Chodosh LA, Thompson CB, Kung HF. Preparation and characterization of L-[5-11C]-glutamine for metabolic imaging of tumors. J Nucl Med. 2012;53:98–105. doi: 10.2967/jnumed.111.093831. [DOI] [PubMed] [Google Scholar]
- 109.Anderson PM, Lalla RV. Glutamine for amelioration of radiation and chemotherapy associated mucositis during cancer therapy. Nutrients. 2020;12:1675. doi: 10.3390/nu12061675. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.