Abstract
Background: Cardiac conditions are a significant cause of maternal morbidity and mortality, significantly exacerbated during the hemodynamic demands of pregnancy. Mitral stenosis in pregnancy (MSp) is rare in the USA however, it has a high risk for maternal complications. Methods: We aim to outline the burden of MSp hospitalizations nationally. A retrospective review of HCUP/NIS data from 2002-2014 was conducted. Results: There were 2014 weighted discharges for both pregnancy and mitral stenosis (MS). Patients diagnosed with MS had a more considerable mean cost per discharge than the comparison group. Pulmonary Hypertension (PH), Atrial Arrhythmias (AA), Stroke, and Heart Failure (HF) were respectively reported in 25.71%, 7.14%, 0.95%, and 19.28% of the discharges. Our study identified a low incidence of MS in the US over the 12-year period; no deaths were identified. Conclusion: Our results substantiate MSp as a risk factor for PH, AA, HF, and stroke in pregnancy. Even though the mortality is low, it is essential that clinicians be aware of this diagnosis due to higher associated morbidity and costs.
Keywords: Mitral valve stenosis, pregnancy, epidemiology, United States
Introduction
Among pregnant women, cardiovascular conditions are a major cause of non-obstetric morbidity in high income countries with cardiovascular disease being in large proportion due to ischemic heart diseases, dilated cardiomyopathy, and cardiac valvular diseases [1,2].
There are currently no reports of mechanisms implicated in the de novo manifestations of MSp. Thereby, the Presence of MS in these patients its most commonly attributable to a prior history of sub-clinical MS whose symptoms are exacerbated during pregnancy. The most predominant cause of MS in females is rheumatic heart disease caused by rheumatic fever, which has a greater prevalence in low- and middle-income countries. In the USA and Canada, rheumatic disease accounts for less than 25% of pregnant women with heart disease [3,4]. In contrast, studies of patients from India, Senegal, Brazil, and Turkey suggest that rheumatic disease is present in 56-88% of pregnant women with heart disease [5-7].
In MS, the restricted mitral valve leaflets limit left ventricular filling, resulting in a greater trans mitral pressure gradient, and increased left atrial pressure. This will result in progressive left atrial enlargement. When combined with the hypercoagulable state and atrial irritability associated with pregnancy, this increases the prevalence of atrial arrhythmias, including atrial fibrillation with risk of thromboembolic stroke. Pulmonary hypertension and pulmonary edema due to elevated left heart pressures are also possible complications, contributing to the risk of poor perfusion and respiratory compromise in patients [8].
The hemodynamic changes caused by MS in addition to the normal hypervolemia and elevated cardiovascular demand during gestation increase the chance of maternal and fetal morbidity and mortality [9]. Specifically, there is an average 30-60% elevation in preload due to elevated plasma volume and subsequent cardiac output which continues to grow during gestation, requiring a compensatory elevation in heart rate to maintain perfusion [10]. In patients with MS, chronic morphological changes force a back pressure of blood from the left atrium into the lungs due to a stenotic outflow tract impeding optimal forward blood flow into the left ventricle. The elevated plasma volume collects in this setting, pooling in the left atrium and elevating backwards pressures which enforce increased pulmonary venous congestion and manifesting a greater severity of pulmonary hypertension as the heart rate increases [11,12]. Most reports of pregnancy related MS present with these worsening symptoms, noting that the pathology was pre-existing, and symptoms exacerbated during a pregnant state.
There are sparse data regarding the prevalence of MS and its associated risks for mothers and infants in the United States (USA). Most of the publications to date regarding this topic come from developing countries. Recent studies in developing countries suggest an incremental increase in the frequency of fetal and maternal complications tied to increasing severity of the MS. Moderate to severe cases of MS are associated with elevated fetal risks of stillbirth, neonatal death, and preterm birth. Maternal risk of mortality, pulmonary edema, and new/recurrent arrhythmias is also elevated [13]. The present research aim is to provide more information about this condition in the USA.
Methods
Data source
The National Inpatient Sample (NIS) is a database provided by the Health Care Utilization Project (HCUP) sponsored by the Agency for Healthcare Research and Quality (AHRQ). The NIS contains discharges from the State Inpatient Databases and is designed to represent hospitals and discharges at a national level using a random sampling of discharges stratified by US census region, urban or rural location, teaching status, ownership, and size. The data collected include demographic information and diagnostic and procedural codes from the patient’s hospital stay. One of the major strengths of the database is its size, with information about millions of patients each year. Limitations include the inability to assess causation or relation between different diagnoses, and dependence on the coding used during the stay. Only diagnoses that were coded during the hospitalization are reflected in the data.
The NIS-HUCP database contains about twenty percent of all US admissions. It provides an estimate of the totality of the US discharges based on the sample which are referred to as weighted discharges. As the database sample is 20% of all admissions, the weighted discharges are approximately 5 times more than measured number of discharges.
Descriptive analysis was used to assess the frequency of MSp and patient characteristics. The proportion (95% confidence interval) of MSp, as indicated by inclusion criteria diagnosis codes, is reported for the study period, 2002-2014.
Inclusion & exclusion criteria
Study group inclusion criteria consisted of the presence of at least one code for pregnancy and at least one code for MS. Comparison group inclusion criteria consisted of at least one code for pregnancy and the absence of at least one code for MS. All patients selected were >18 at the time of hospitalization, which was filtered for during data management prior to analysis. The database does not delineate between specific special populations (prisoners, homeless, at-risk populations) due to being deindentified and thereby these features were not considered as criteria for exclusion.
As ICD9 has multiple codes for MS, some of them combined with other valvopathies, we only included the codes exclusively for MS, thereby these other combinations were excluded from the study population.
Statistical analysis methodology
SAS 9.4 was used to perform all statistical analyses via the survey procedures, to account for discharge weights and sampling methodology of the NIS database A threshold of α=0.05 was used to determine statistical significance.
Descriptive tables were created for each variable with quantitative variables being represented as mean ± standard deviation and categorical variables represented as frequency (percent). Descriptive frequency tables were calculated for the variable’s: mortality, costs, and treatments by whether the patient had MS or not. No inference was done for any of these variables. Due to NIS confidentiality concerns, all cell counts that had a frequency less than 10 will be represented with a dash.
The prevalence of MSp was assessed by calculating a 95% confidence interval of the observed proportion. To assess the trend frequency over time, two two-sided Cochran-Armitage Trend Tests were performed to assess whether the frequency of MSp increased or decreased over time.
A Chi Square test was performed to assess whether the proportion of discharges that had at least one diagnostic code for MSp differs depending on hospital region. A Chi Square test was also performed to assess whether the pro portion of a particular co-diagnosis differed depending on whether the patients had MS. The co-diagnoses considered were heart failure, pulmonary hypertension, atrial arrhythmia, and stroke. Since four separate co-diagnoses were being tested, a Bonferroni multiplicity adjustment was implemented such that α=.05/4=0.0125 for each individual test.
Results
Baseline characteristics
The data available during the study period consisted of 100,790,900 discharges accounting for 482,872,274 weighted discharges. The data contain 7,380,599 pregnancy discharges representing a weighted total discharge count of 35,249,339 pregnancy discharges. There were 420 discharges that have a code for both pregnancy and MS which represents a total of 2014 weighted discharges with MSp (Figure 1).
Figure 1.
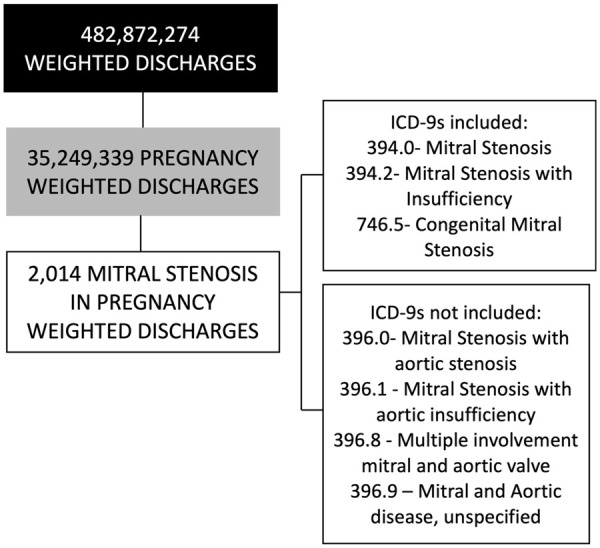
Size of the pregnant patients with mitral stenosis from the national inpatient sample between 2002-2014 that were used for analysis in this study.
Patient median age, length of stay, occurrence per US region, mortality, payer, costs, income, and ethnicity are summarized in the Table 1. None of the patients in MSp group died during the hospitalization. There was no significant evidence that the proportion of the discharges with MSp differs by region (P=0.11). A chi-Square test was performed revealed that non-white Americans were over-represented among pregnant patients with MS. Even though the lowest income quartile seems to be overrepresented in MS patients relative to the pregnant patients without MS, a chi-square test didn’t show statistical significance (Table 1).
Table 1.
General characteristics
| Characteristic | Pregnant with mitral stenosis | Pregnant without mitral stenosis |
|---|---|---|
| Age in years (IQR) | 31 (27-35) | 27 (22-32) |
| Median length of stay in days (IQR) | 3 (2-5) | 2 (2-5) |
| In hospital mortality | 0 | - |
| Median cost in USD (IQR) | 16,302 (9,447-31,777) | 9299 (5,691-18,444) |
| Ethnicity | ||
| - Caucasian | 32.48% | 51.10% |
| - African American | 18.52% | 16.75% |
| - Hispanic | 25.64% | 22.29% |
| - Asian/Pacific | 12.82% | 4.44% |
| - Native American | - | 0.78% |
| - Other | 9.4% | 4.63% |
| Median income per zipcode | ||
| - First quartile (lowest) | 25.93% | 27.4% |
| - Second quartile (2nd lowest) | 22.96% | 24.98% |
| - Third quartile (2nd higher) | 24.44% | 24.18% |
| - Forth quartile (higher) | 26.67% | 23.45% |
| region | ||
| - Northeast | 20.55% | 16.61% |
| - Midwest | 18.89% | 21.26% |
| - South | 33.89% | 38.43% |
| - West | 26.67% | 23.70% |
| Payer | ||
| - Medicare | - | 0.80% |
| - Medicaid | 198 (47.25%) | 44.82% |
| - Private insurance | 179 (42.72%) | 48.00% |
| - Self payer/Other | 31 (7.39%) | 6.16% |
| - No charge | - | 0.22% |
Co-occurrences and historical features
Co-occurrences (Table 2): There were 108 discharges (518 weighted discharges) or 25.71% of the discharges with MSp that also had a diagnostic code for Pulmonary Hypertension, compared to 0.04% of discharges with no MSp (OR 733.86 95% CI 538.10, 1000.17).
Table 2.
Co-occurrences
| Mitral stenosis group % of total (weighted discharges)* | Control group % of total (weighted discharges)** | OR/95% CI | |
|---|---|---|---|
| Pulmonary Hypertension | 25.71% (518) | 0.04% (16,624) | 733.86 95% CI (538.102, 1000.168) |
| Atrial arrhythmias | 4.28% (132) | 0.069% (24,558) | 100.92 95% CI (58.900, 172.938) |
| Stroke | 0.95% (19) | 0.058% (21,061) | 16.04 95% CI (4.633, 55.585) |
| Heart failure | 19.28% (384) | 0.22% (77,677) | 106.75 95% CI (77.207, 147.608) |
Number of mitral stenosis discharges-2014 patients.
Number of discharge without mitral stenosis-7,380,179 patients.
There were 28 discharges (132 weighted discharges) or 4.28% of the discharges with MSp that also had a diagnostic code for Atrial Arrhythmias, compared to 0.069% of discharges with no MSp (OR 100.92 95% CI 58.90, 172.94). Four discharges (19 weighted discharges) or 0.95% of the discharges with MSp that also had a diagnostic code for Stroke, compared to 0.058% of discharges with no MSp (OR 16.04 95% CI 4.63, 55.59). Eighty-one discharges (384 weighted discharges) or 19.28% of the discharges with MSp that also had a diagnostic code for Heart Failure, compared to 0.22% of discharges with no MSp (OR 106.754 95% CI 77.20, 147.60).
Figure 2 shows the proportion of discharges with MSp over time. A Cochran-Armitage Trend test was prevalence of having MSp has decreased overtime.
Figure 2.
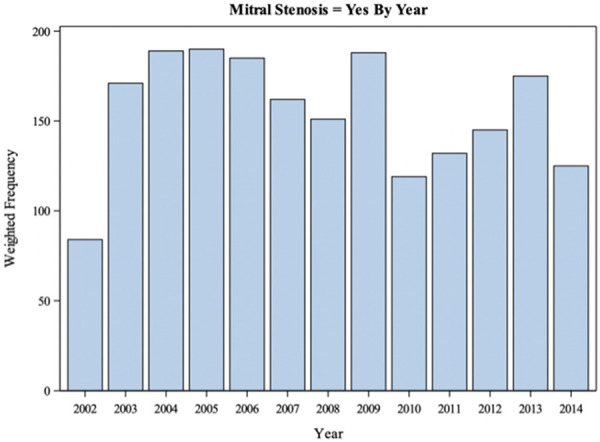
Total number of discharges with mitral stenosis in pregnancy codes over the period studied from the national inpatient sample. A Cochran-Armitage Trend test was performed and there is sufficient evidence to conclude that the prevalence of MSp decreases over time.
Procedural characteristics
The following four ICD-9 procedures were identified in the data set: Percutaneous Balloon Valvuloplasty, Mitral Valve Replacement with tissue graft, other replacement of mitral valve, closed heart valvotomy and open heart valvuloplasty without replacement. The data contained 104 weighted discharges with MSp that underwent one of the five procedures. Of those with MSp, 5.16% underwent one of these procedures. We also found 338 weighted discharges with those procedures in patients without MSp or 0.0009% of the patients without MSp (Table 3).
Table 3.
Procedures performed
| Procedure performed | Weighted frequency group with mitral stenosis* | Weighted frequency group without mitral stenosis** |
|---|---|---|
| Percutaneous valvuloplasty ICD9-33.96 | 2.58% (52) | 0.0002% (71) |
| Replacement of mitral valve with tissue graft ICD9-35.23 | 0.95% (20) | 0.0001% (53) |
| Other replacement of Mitral Valve ICD9-35.24 | 1.42% (27) | 0.0003% (105) |
| Closed heart valvotomy, mitral valve ICD9-35.02 | - | - |
| Open heart valvuloplasty of mitral valve without replacement ICD9-35.12 | 0% (0) | 0.0003% (109) |
Number of mitral stenosis discharges-2014 patients.
number of discharge without mitral stenosis-7,380,179 patients.
Discussion
NIS-HCUP is a large database with more than 480 million admissions over 12 years. Since MSp has a low prevalence in the USA, only 2,014 weighted cases were identified during the study period. Less than 0.01% of the patients with a diagnosis of pregnancy had an associated isolated MS diagnosis. Although we believe this population might be significantly larger since ICD9 with associated valvopathies were not included to have a more homogenous group. The prevalence of MS is higher in low and middle income countries, but it is also seen in high income countries [14] No large studies have been published on MSp in the USA and the incidence is not known, yet it has been well documented that incidence in females is far more common than in males with the onset generally happening between the third and fourth decades of life [15].
Incidence of Rheumatic heart disease has decreased in low- and middle-income countries as well as its complications such as MS, but the prevalence is still high [16]. Most recent reports have placed the incidence in emerging regions such as Africa at an estimated incidence of 35 cases per 100,00 are compared to estimated incidence of 1 case per 100,000 within the United States [15]. Even though we cannot determine incidence and prevalence of MSp with our data, our results show a down trending of hospitalizations with MSp from 2002 to 2013 in the USA. This may in part be due to a lower threshold, and easier access too, medications that prophylaxis for rheumatic heart disease at a younger age and prudent follow-ups options available for these patients. Despite this, it is important to consider that the database used for this study does not delineate from a single patient with multiple hospitalizations-leading to subsequent complications. This means that multiple hospitalizations may be from one patient but the proportion of this influence on our data is indeterminant but unlikely to comprise a meaningful impact.
Within the U.S. the most common cause of MS is still rheumatic heart disease, despite the access to prophylactic treatments. Rheumatic fever most commonly affects children from the ages of 5-15 years globally, becoming MUCH less common after that age with most patients affected at the lower threshold of that range [17]. The resulting attack of valvular tissue, which is due to the similarity of antigens on valves that the immune reaction during rheumatic fever is primed against, causing deformation and producing rigidity of valves. The resolution of this attack emerges chronic structural changes which manifest over 2-3 decades to become sufficiently permanent [18]. At this point, patients start to become symptomatic and are diagnosed. Thereby in females, the time taken for MS to manifest after rheumatic fever can emerge around the same time that pregnancy becomes likely. This relationship makes it imperative to secure proper prophylaxis for rheumatic fever during primary infection in childhood or as an adolescent. Furthermore, evaluating for any such history in newly pregnant patients may allow for possible suspicion to evaluation for MS before pregnancy related changes manifest symptoms.
Furthermore, understanding the possible intervention and screening options becomes imperative in females considering getting pregnant to be evaluated for a history of rheumatic heart disease of clinically silent valvular disease such as MS. Currently there exists no criteria or protocols for such evaluations. Other conditions including infective endocarditis, de novo calcification development on injured valves, and a result of insult from chronic autoimmune conditions that more commonly affect females [15] are also other documented causes. Yet the incidence of these is extremely rare and can be considered if a historical factor is recognized.
In patients with severe MS, increased left atrial pressure is transmitted retrograde into the pulmonary veins, resulting in pulmonary congestion and, in extreme cases, pulmonary hypertension (PH) [19]. Physiologic changes during pregnancy which include increases in blood volume, oxygen consumption and cardiac output and decreases in systemic vascular resistance and blood pressure [20]. These changes occurring in a patient with significant MS might contribute to right ventricular failure. In our study we found 25.7% of the discharges with some degree of pulmonary hypertension. Similar studies found Pulmonary Hypertension in 14-31% of the MSp patients [21,22]. Using the NIS data. we cannot establish the severity, or the burden placed on the body of the condition in our study group, yet it would be important to determine its lasting implications in this study group as PH in pregnant patients has been documented to be associated with morbidity with guidelines advising woman with PH against pregnancy in both national and international guidelines [23,24]. Although there were no reported mortalities in the patient population studied, MSp may have impacted the number and duration of hospitalizations. PH is also a known risk factor for hypercoagulable states due to vascular remodelling causing local impaired flow states promoting endothelial injury and inducing coagulative processes [25]. Within a pregnant state, whereby production of thrombogenic factors such as factor VII and fibrinogen increases, both can incite a greater risk for vascular comorbidities [26]. Within our study population, we did determine a greater proportional incidence of stroke in MS pregnant patients, but the NIS data is unable to delineate between embolic or thrombotic etiologies.
In a Canadian cohort study including 80 patients with MSp, pulmonary edema occurred in 26% of patients with mild MSp and 67% of patients with severe MSp. This was relatively well tolerated and responsive to medical therapy [21]. In a large international study, heart failure was reported in 21% of the patients [22]. About nineteen percent of the discharges with MSp in the NIS data had at least one code for heart failure or pulmonary edema which indicates a significant portion of MSp patients have experience sequala of MS. MS is known to cause heart failure, most commonly right sided heart failure, from chronic loss of forward flow due to the increased forward resistance emerging by the stenotic valve. The resultant congestion in vascular pathways within the pulmonary regions eventually causes great right sided pressures that can exhaust the chamber and reduce diastolic and systolic capacitance while also increased fluid pressures leading to transudative, and if damage is severe, exudative effusions which can manifest as flash pulmonary edema [27]. In pregnant patients, this effect is coupled with already increased vascular volume and greater demand from heart muscle to perfuse placental tissue, thereby potentially emerging as a likely risk-factor for acute HF symptoms and pulmonary edema. While this patient course was not specifically defined within the discharge data, it can be surmised from prior literature that a pregnant in our MS population is responsible for far greater proportion of HF rates observed over the study timeline.
Atrial arrhythmias can occur in MSp due to left atrial enlargement. Specifically, myocardial accommodations made during pregnancy that occur from greater vascular volume (as effective circulating volume can increase over 30%-50% from baseline) combined with a baseline larger pressure against a stenotic mitral valve occurring over an 8-9-month timeline can set the stage for conduction tissue rearrangement to occur as the left atrial chamber is enlarging to keep in pace. While rare, cardiac arrhythmias are documented to be a considerable contributor to pregnancy related cardiovascular complications, among which atrial arrhythmias are the most common [28]. Subsequently, when active arrhythmia episodes are present, they are also poorly tolerated. The loss of atrial contraction limits diastolic filling which increases risk for thrombus formation and embolic events, especially concerning as pregnancy already mediates a hypercoagulable state [29]. Our data confirms these consistencies with the literature as there are significantly increased risk of atrial arrhythmias and stroke in the MSp group, but the absolute numbers can be considered low. The overall low frequency might be explained by the heterogeneity of the severity of MS in our study population, which is not a recorded profile among our database samples but is a necessary question to inquire about specific etiological processes.
Among our Guidelines from AHA/ACC in 2014 recommend pre-pregnancy counselling and when indicated, interventions before pregnancy. Beta-blockers, most commonly metoprolol, for rate control are universally recommended with there being a temporal relationship favouring immediate administration to both events during pregnancy and in the post-partum period [30]. While we are not able to determine the pharmacological profile used for each hospitalization from these data, the significant proportional incidence within MSp patients does indicate a need to be informed about this intervention in these patients. Alongside rate control interventions, anticoagulant therapy should be initiated (or continued) given the combined risk of embolism from episodes alongside predisposition from pregnancy to induce a hypercoagulable state by mechanisms mentioned earlier. One important pharmacological decision-making process to be aware off is the use of diuretics in pregnant patients to target symptomatic pulmonary congestion, which as stated prior is a common sequala following stressors emerging from pregnancy changes over underlying MS [31]. While not specific teratogenic, diuretics function to reduce plasma volume, which can acutely reduce the burden of pulmonary edema but concurrently function to lower effective circulating volume-which is often reduced in MSp patients from the functional obstruction of a stenotic valve preventing adequate LV preload [32].
Procedural interventions are rarely indicated, but might be considered in symptomatic pregnant patients, refractory to medical therapy who have poor anatomy for PBMV [31]. Replacement of mitral valve with tissue graft (ICD9 35.23), other replacement of mitral valve (ICD9 35.24), closed heart valvotomy (ICD9 35.02) and open-heart surgery without valve replacement (ICD9 35.12) were performed in 20, 27, 5, and 0 of the weighted discharges or 0.95%, 1.42%, 0.24% and 0% of our patients, respectively. Percutaneous balloon mitral valvuloplasty (PBMV) is indicated when there is severe MSp, favourable valve morphology and a patient who remains symptomatic despite medical therapy [31]. When indicated, this procedure is usually well tolerated [33,34]. PBMV was performed in 2.58% of our patients.
Results from the Registry of Pregnancy and Cardiac Disease (ROPAC) published in 2018 registered 273 patients with MSp from 2008 to 2014 in an international cohort, most of them from middle income countries. Hospital admission occurred in 20% of the cases. Heart failure, atrial arrhythmias, thrombotic complications occurred in 21.61%, 4.02% and 0.36% respectively in this international study, these numbers are like our study 19.28%, 4.28% and 0.95% for the same complications [22].
There are few published studies of hospital costs in patients with MSp. The mean hospital cost in our study was $16,302 US dollars, 76% more than the comparative group (without MS) for a similar length of stay [35]. The described complications, the need for expensive procedures and the close follow up required for this group of patients may explain the high cost of hospitalization when compared to pregnant patients without MSp. Furthermore, MSp patients likely required the involvement of multiple cardiovascular teams working in conjunction with obstetrics, especially more when procedural accommodations were necessary, which may establish a relatively uncommon team dynamic that may contribute to greater baseline costs. Despite this increased cost, having a consistent LOS between each group indicates that the dynamic of cardiovascular teams and obstetrics teams working together was likely incredibly effective over a national scope and many not have served as a barrier for effective care. Literature documentation of such a team dynamic is relatively scarce but may provide effective contributions, even if the incidence of MSp has nationally remained consistently low. An important paradigm regarding costs to also consider through is that these data only reflect the hospital stay yet these patients are likely to require specialized antenatal and prenatal care at tertiary referral centres, particularly with the involvement of specialized cardiac services for which can be surmised that the actual difference is cost is even higher.
Even though MSp is associated with higher morbidity, particularly with changes exacerbating PH and serving as an arrhythmogenic factors, the mortality in these patients are still low with no recent reported deaths in North America. In our study, the number of deaths were zero which stands consistent with other reports of the same population in the literature. In a Sri Lankan study, however, MSp was responsible for a third of the cases of maternal mortality due to cardiovascular conditions [36]. We believe that better access to medical care in a healthcare system with advanced speciality care may explain the difference in outcomes when compared to low- and middle-income countries.
Limitations
The NIS-HCUP database doesn’t provide individual patient information, so we must rely on ICD9 codes added during the hospitalization. The ICD9 is often nonspecific in many medical conditions. Our study was limited by:
- Multiple obstetrics ICD9’s had to be used to determine whether the hospital stay was pregnancy related, as a lack of a specific designation for this patient population may contribute to a subset of patients with greater hospitalization burden which were not delineated in our analysis.
- ICD9 does not have specific codes for different severities therefore we cannot determine severity of the MS in our population.
- ICD9 has codes for MS combined with other valvular pathologies such as aortic stenosis and aortic insufficiency such as 396.0, 396.1, 396.8 and 396.9. As we could not determine MS severity based only on the ICD9 code and combined valvopathies can be completely different than isolated mitral stenosis, we decided to not include those codes to have a more homogenous group. Due to this choice, we might have missed patients with severe MSp as we did identify MS procedures done in the group without MSp.
- We excluded codes for non-specific mitral disease such as 394.9, 396.9 that may have included disorders other than mitral stenosis such as mitral valve prolapse or varying degrees of mitral regurgitation. Many pregnant patients with mitral stenosis may have been categorized with these codes.
- Based on the number of mitral valve procedures performed in pregnant patients who were not in our study group we estimate in total that our study identified approximately 40% of the pregnant patients with MS.
- To protect the privacy of patients the NIS database does not allow detection of cells with fewer than ten patients. With low numbers of MSp patients we cannot make definitive conclusions about several outcomes. Specifically, no deaths were detected by our study however up to 9 deaths could occur before our study would detect them.
Conclusion
This study provides additional information about demographics of patients with MSp in the USA. It’s a rare condition in the USA with low mortality, but with a high morbidity due to complications such as atrial arrythmias, heart failure, pulmonary hypertension, and stroke. Pre-pregnancy counselling should be mandatory for patients with known valvular heart disease. If diagnosed during pregnancy MS should be treated in a tertiary centre where the described cardiac interventions can be performed when indicated.
Disclosure of conflict of interest
None.
References
- 1.Emmanuel Y, Thorne SA. Heart disease in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2015;29:579–597. doi: 10.1016/j.bpobgyn.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 2.Lima NA, de Castro RL Jr, Huffman C, Diaz M, Lima CCV, Schauer M, Linares ST, Melgar TA. Hospital admissions for aortic stenosis in pregnancy in the United States-a thirteen year analysis. Am J Cardiovasc Dis. 2020;10:398–404. [PMC free article] [PubMed] [Google Scholar]
- 3.Siu SC, Sermer M, Colman JM, Alvarez AN, Mercier LA, Morton BC, Kells CM, Bergin ML, Kiess MC, Marcotte F, Taylor DA, Gordon EP, Spears JC, Tam JW, Amankwah KS, Smallhorn JF, Farine D, Sorensen S Cardiac Disease in Pregnancy (CARPREG) Investigators. Prospective multicenter study of pregnancy outcomes in women with heart disease. Circulation. 2001;104:515–521. doi: 10.1161/hc3001.093437. [DOI] [PubMed] [Google Scholar]
- 4.Kuklina E, Callaghan W. Chronic heart disease and severe obstetric morbidity among hospitalisations for pregnancy in the USA: 1995-2006. BJOG. 2011;118:345–352. doi: 10.1111/j.1471-0528.2010.02743.x. [DOI] [PubMed] [Google Scholar]
- 5.Avila WS, Rossi EG, Ramires JA, Grinberg M, Bortolotto MR, Zugaib M, da Luz PL. Pregnancy in patients with heart disease: experience with 1,000 cases. Clin Cardiol. 2003;26:135–142. doi: 10.1002/clc.4960260308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diao M, Kane A, Ndiaye MB, Mbaye A, Bodian M, Dia MM, Sarr M, Kane A, Monsuez JJ, Ba SA. Pregnancy in women with heart disease in sub-Saharan Africa. Arch Cardiovasc Dis. 2011;104:370–374. doi: 10.1016/j.acvd.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 7.Baghel J, Keepanasseril A, Pillai AA, Mondal N, Jeganathan Y, Kundra P. Prediction of adverse cardiac events in pregnant women with valvular rheumatic heart disease. Heart. 2020;106:1400–1406. doi: 10.1136/heartjnl-2020-316648. [DOI] [PubMed] [Google Scholar]
- 8.Iftikhar SF, Biswas M. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022. Cardiac disease in pregnancy. [PubMed] [Google Scholar]
- 9.Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Bärwolf C, Levang OW, Tornos P, Vanoverschelde JL, Vermeer F, Boersma E, Ravaud P, Vahanian A. A prospective survey of patients with valvular heart disease in Europe: the euro heart survey on valvular heart disease. Eur Heart J. 2003;24:1231–1243. doi: 10.1016/s0195-668x(03)00201-x. [DOI] [PubMed] [Google Scholar]
- 10.Robson SC, Hunter S, Boys RJ, Dunlop W. Serial study of factors influencing changes in cardiac output during human pregnancy. Am J Physiol. 1989;256:H1060–H1065. doi: 10.1152/ajpheart.1989.256.4.H1060. [DOI] [PubMed] [Google Scholar]
- 11.Canobbio MM, Warnes CA, Aboulhosn J, Connolly HM, Khanna A, Koos BJ, Mital S, Rose C, Silversides C, Stout K American Heart Association Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; Council on Functional Genomics and Translational Biology; and Council on Quality of Care and Outcomes Research. Management of pregnancy in patients with complex congenital heart disease: a scientific statement for healthcare professionals from the American Heart Association. Circulation. 2017;135:e50–e87. doi: 10.1161/CIR.0000000000000458. [DOI] [PubMed] [Google Scholar]
- 12.Lewey J, Andrade L, Levine LD. Valvular heart disease in pregnancy. Cardiol Clin. 2021;39:151–161. doi: 10.1016/j.ccl.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ducas RA, Javier DA, D’Souza R, Silversides CK, Tsang W. Pregnancy outcomes in women with significant valve disease: a systematic review and meta-analysis. Heart. 2020;106:512–519. doi: 10.1136/heartjnl-2019-315859. [DOI] [PubMed] [Google Scholar]
- 14.Chandrashekhar Y, Westaby S, Narula J. Mitral stenosis. Lancet. 2009;374:1271–1283. doi: 10.1016/S0140-6736(09)60994-6. [DOI] [PubMed] [Google Scholar]
- 15.Shah SN, Sharma S. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022. Mitral stenosis. [Google Scholar]
- 16.Shah B, Sharma M, Kumar R, Brahmadathan KN, Abraham VJ, Tandon R. Rheumatic heart disease: progress and challenges in India. Indian J Pediatr. 2013;80(Suppl 1):S77–86. doi: 10.1007/s12098-012-0853-2. [DOI] [PubMed] [Google Scholar]
- 17.Zaman MM, Rouf MA, Haque S, Khan LR, Chowdhury NA, Razzaque SA, Yoshiike N, Tanaka H. Does rheumatic fever occur usually between the ages of 5 and 15 years? Int J Cardiol. 1998;66:17–21. doi: 10.1016/s0167-5273(98)00140-5. [DOI] [PubMed] [Google Scholar]
- 18.Dass C, Kanmanthareddy A. StatPearls. Treasure Island (FL): StatPearls Publishing Copyright © 2022, StatPearls Publishing LLC.; 2022. Rheumatic heart disease. [Google Scholar]
- 19.Pessel C, Bonanno C. Valve disease in pregnancy. Semin Perinatol. 2014;38:273–284. doi: 10.1053/j.semperi.2014.04.016. [DOI] [PubMed] [Google Scholar]
- 20.Olsson KM, Channick R. Pregnancy in pulmonary arterial hypertension. Eur Respir Rev. 2016;25:431–437. doi: 10.1183/16000617.0079-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silversides CK, Colman JM, Sermer M, Siu SC. Cardiac risk in pregnant women with rheumatic mitral stenosis. Am J Cardiol. 2003;91:1382–1385. doi: 10.1016/s0002-9149(03)00339-4. [DOI] [PubMed] [Google Scholar]
- 22.van Hagen IM, Thorne SA, Taha N, Youssef G, Elnagar A, Gabriel H, ElRakshy Y, Iung B, Johnson MR, Hall R, Roos-Hesselink JW ROPAC Investigators and EORP Team. Pregnancy outcomes in women with rheumatic mitral valve disease: results from the registry of pregnancy and cardiac disease. Circulation. 2018;137:806–816. doi: 10.1161/CIRCULATIONAHA.117.032561. [DOI] [PubMed] [Google Scholar]
- 23.Regitz-Zagrosek V, Blomstrom Lundqvist C, Borghi C, Cifkova R, Ferreira R, Foidart JM, Gibbs JS, Gohlke-Baerwolf C, Gorenek B, Iung B, Kirby M, Maas AH, Morais J, Nihoyannopoulos P, Pieper PG, Presbitero P, Roos-Hesselink JW, Schaufelberger M, Seeland U, Torracca L ESC Committee for Practice Guidelines. ESC Guidelines on the management of cardiovascular diseases during pregnancy: the task force on the management of cardiovascular diseases during pregnancy of the European Society of Cardiology (ESC) Eur Heart J. 2011;32:3147–3197. doi: 10.1093/eurheartj/ehr218. [DOI] [PubMed] [Google Scholar]
- 24.Pieper PG. Pre-pregnancy risk assessment and counselling of the cardiac patient. Neth Heart J. 2011;19:477–481. doi: 10.1007/s12471-011-0188-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bazan IS, Fares WH. Hypercoagulability in pulmonary hypertension. Clin Chest Med. 2018;39:595–603. doi: 10.1016/j.ccm.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka KA, Bharadwaj S, Hasan S, Judd M, Abuelkasem E, Henderson RA, Chow JH, Williams B, Mazzeffi MA, Crimmins SD, Malinow AM. Elevated fibrinogen, von Willebrand factor, and factor VIII confer resistance to dilutional coagulopathy and activated protein C in normal pregnant women. Br J Anaesth. 2019;122:751–759. doi: 10.1016/j.bja.2019.02.012. [DOI] [PubMed] [Google Scholar]
- 27.Musuku SR, Pani S, Cagino J. Acute right ventricular failure postintubation in a mitral stenosis patient. J Cardiovasc Echogr. 2018;28:48–50. doi: 10.4103/jcecho.jcecho_27_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Enriquez AD, Economy KE, Tedrow UB. Contemporary management of arrhythmias during pregnancy. Circ Arrhythm Electrophysiol. 2014;7:961–967. doi: 10.1161/CIRCEP.114.001517. [DOI] [PubMed] [Google Scholar]
- 29.Maganti K, Rigolin VH, Sarano ME, Bonow RO. Valvular heart disease: diagnosis and management. Mayo Clin Proc. 2010;85:483–500. doi: 10.4065/mcp.2009.0706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ishibashi K, Aiba T, Kamiya C, Miyazaki A, Sakaguchi H, Wada M, Nakajima I, Miyamoto K, Okamura H, Noda T, Yamauchi T, Itoh H, Ohno S, Motomura H, Ogawa Y, Goto H, Minami T, Yagihara N, Watanabe H, Hasegawa K, Terasawa A, Mikami H, Ogino K, Nakano Y, Imashiro S, Fukushima Y, Tsuzuki Y, Asakura K, Yoshimatsu J, Shiraishi I, Kamakura S, Miyamoto Y, Yasuda S, Akasaka T, Horie M, Shimizu W, Kusano K. Arrhythmia risk and β-blocker therapy in pregnant women with long QT syndrome. Heart. 2017;103:1374–1379. doi: 10.1136/heartjnl-2016-310617. [DOI] [PubMed] [Google Scholar]
- 31.Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, O’Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM 3rd, Thomas JD ACC/AHA Task Force Members. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:2440–2492. doi: 10.1161/CIR.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 32.Al-Balas M, Bozzo P, Einarson A. Use of diuretics during pregnancy. Can Fam Physician. 2009;55:44–45. [PMC free article] [PubMed] [Google Scholar]
- 33.Lima NA, Santos GS, Lima CCV, Schauer MD. Percutaneous mitral balloon valvulotomy in pregnant women with mitral stenosis from rheumatic heart disease: a series of cases. J Obstet Gynaecol. 2020;40:1169–1170. doi: 10.1080/01443615.2019.1693525. [DOI] [PubMed] [Google Scholar]
- 34.Vinayakumar D, Vinod GV, Madhavan S, Krishnan MN. Maternal and fetal outcomes in pregnant women undergoing balloon mitral valvotomy for rheumatic mitral stenosis. Indian Heart J. 2016;68:780–782. doi: 10.1016/j.ihj.2016.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Torio CM, Moore BJ. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs. Rockville (MD): Agency for Healthcare Research and Quality (US); 2006. National inpatient hospital costs: the most expensive conditions by payer, 2013. [PubMed] [Google Scholar]
- 36.Haththotuwa HR, Attygalle D, Jayatilleka AC, Karunaratna V, Thorne SA. Maternal mortality due to cardiac disease in Sri Lanka. Int J Gynaecol Obstet. 2009;104:194–198. doi: 10.1016/j.ijgo.2008.10.031. [DOI] [PubMed] [Google Scholar]


