Abstract
Background:
Respiratory syncytial virus (RSV) is a poor inducer of antiviral interferon (IFN) responses which result in incomplete immunity and RSV disease. Several RSV proteins alter antiviral responses, including the non-structural proteins (NS1, NS2) and the major viral surface proteins, that is, fusion (F) and attachment (G) proteins. The G protein modifies the host immune response to infection linked in part through a CX3 C chemokine motif. Anti-G protein monoclonal antibodies (mAbs), that is, clones 3D3 and 2D10 that target the G protein CX3C chemokine motif can neutralize RSV and inhibit G protein-CX3CR1 mediated chemotaxis.
Objectives:
Determine how monoclonal antibodies against the RSV F and G proteins modify the type I and III IFN responses to RSV infection.
Design:
As the G protein CX3 C motif is implicated in IFN antagonism, we evaluated two mAbs that block G protein CX3C-CX3CR1 interaction and compared responses to isotype mAb control using a functional cellular assay and mouse model.
Methods:
Mouse lung epithelial cells (MLE-15 cells) and BALB/c mice were infected with RSV Line19 F following prophylactic mAb treatment. Cell supernatant or bronchoalveolar lavage fluid (BALF) were assayed for types I and III IFNs. Cells were interrogated for changes in IFN-related gene expression.
Results:
Treatment with an anti-G protein mAb (3D3) resulted in improved IFN responses compared with isotype control following infection with RSV, partially independently of neutralization, and this was linked to upregulated SOCS1 expression.
Conclusions:
These findings show that anti-G protein antibodies improve the protective early antiviral response, which has important implications for vaccine and therapeutic design.
Plain Language Summary
RSV is a leading cause of respiratory disease in infants and the elderly. The only Food and Drug Administration-approved prophylactic treatment is limited to an anti-F protein monoclonal antibody (mAb), that is, palivizumab which has modest efficacy against RSV disease. Accumulating evidence suggests that targeting the RSV attachment (G) protein may provide improved protection from RSV disease. It is known that the G protein is an IFN antagonist, and IFN has been shown to be protective against RSV disease. In this study, we compared IFN responses in mouse lung epithelial (MLE-15) cells and in mice infected with RSV Line19 F treated with anti-G protein or anti-F protein mAbs. The levels of type I and III IFNs were determined. Anti-G protein mAbs improved the levels of IFNs compared with isotype-treated controls. These findings support the concept that anti-G protein mAbs mediate improved IFN responses against RSV disease, which may enable improved treatment of RSV infections.
Keywords: F protein, G protein, IFN, ISG, Line19 F, mAb, monoclonal antibody, palivizumab, RSV
Introduction
Respiratory syncytial virus (RSV) is a leading cause of bronchiolitis in infants revealing some of the limitations in prophylactic measures, vaccines, and post-infection treatment options.1,2 Currently, the management of pediatric RSV infections is limited to prophylaxis with palivizumab (Synagis), which is directed against the RSV F protein.3,4 The RSV F and G proteins can induce neutralizing antibodies (Abs), and therefore are of interest for the development of vaccines and Ab therapeutics. Anti-F protein Abs neutralizes RSV infection but only partially protects from RSV disease that is in part mediated by modified host immune responses contributed by the G protein.5 The ability of Abs or monoclonal Abs (mAbs) to modify the antiviral immune responses is well understood. For example, it has been shown that mAbs directed to the RSV G protein can shift the adaptive immune response from Th2- to Th1-type leading to sustained and enhanced humoral and CD8+ T cell responses.6 It was shown that this effect was not Fc-dependent but instead due to the ability of the anti-G protein mAb to counteract the intrinsic immunosuppressive activity of the RSV G protein.7,8 Palivizumab has also been shown to modify immune responses. Specifically, palivizumab has been shown to decrease RSV-specific CD8+ IFNγ+ cells,9 and reduce the B cell activating factor of the TNF family (BAFF) similarly to anti-IFNβ antibodies.10
The G protein contains a CX3 C chemokine motif that interacts with CX3CR1 present on human airway epithelial cells, competes with CX3CL1 (fractalkine) for binding to CX3CR1, facilitates RSV infection, and may alter CX3CL1-mediated responses.11–14 Importantly, the G protein CX3 C motif can modify the host response to infection resulting in severe RSV disease.15 Virus membrane-bound and secreted forms of G protein and some G peptides can induce mucogenic Th2-biased responses.16,17 It has been shown that stimulation of human monocytes with a G protein peptide containing the CX3 C region suppressed secretion of interleukin 6 (IL-6) and inhibited innate immunity elicited by the virus and a lipopolysaccharide endotoxin.18 Moreover, the CX3 C motif in the G protein affects the adaptive immune response as indicated by diminished percentages of IFN-producing effector/memory T cells compared with a disrupted CX3 C region, that is, a CX4 C virus.19
Notably, the CX3 C motif has been shown to antagonize the early antiviral type I IFN response.20–22 Antiviral mAb therapies are typically characterized in terms of virus neutralization, but this efficacy measurement does not necessarily correlate with other protective characteristics. Clinically, lung viral load reduction has not been a reliable indicator of efficacy, for example, motavizumab and presatovir.23,24 Motavizumab is an affinity-matured derivative of palivizumab, and presatovir is an RSV fusion inhibitor (i.e. targeting the F protein). In contrast, mAbs targeting the G protein CX3 C motif improve the disease outcome following infection,25 and for some anti-G protein mAbs, the mAbs have shown to be superior to anti-F protein mAbs as determined by improved airway hyperresponsiveness and airway inflammation in mice.26 Specifically, anti-G protein mAbs targeting the CX3 C motif not only neutralize RSV, but also reduce features associated with disease in the mouse model including reducing bronchoalveolar lavage (BAL) cell infiltration, improving the breadth of Th1/Th2-cytokine responses, and improving lung disease pathology.27–29 Fc-dependent neutralization has been shown for anti-G CX3 C mAbs. Specifically, murine mAb 131-2G targeting the CX3 C motif and an F(ab’)2 of this mAb were compared in the murine RSV challenge model. The intact mAb neutralized RSV while the F(ab’)2 did not; however, treatment with F(ab’)2 resulted in similar reductions in proinflammatory responses to that seen with intact mAb.7 Importantly, anti-G mAbs can be poorly neutralizing in vitro.30 These data suggest that improvement of RSV disease using anti-G protein mAbs is in part independent of virus neutralization.
The induction of type I interferons (IFNα and IFNβ) has an important role early during RSV infection.31 Type I IFNs bind to their receptors IFNAR1 and IFNAR2 causing autophosphorylation and activation of JAKs (Tyk2 and JAK1).32 Type I IFNs are produced by most, if not all cells, including lung epithelial cells and have a range of direct and indirect effects on various cell types during virus infection.33–35 Type I IFNs are very effective in their antiviral functions providing selective pressure for RSV to modulate and suppress their expression in infected cells. The primary modulators of type I IFN response are the RSV NS1 and NS2 proteins.36–38 Due to their antiviral function, type I IFNs have been evaluated as a potential treatment for RSV, although a level of toxicity is a concern for human use and may prevent regulatory approval.31,39 IFNα2a and IFNα1b are distinct members of the IFNα family and have been investigated in humans to treat RSV. Previous studies examining IFNα2a in RSV-infected adults or infants did not indicate a reduction in viral titers or RSV disease with IFN treatment.40,41 However, a recent report indicated IFNα1b treatment improved RSV disease defined by cough, tachypnea, perilabial cyanosis, and moist rales in neonatal infants and reduced hospitalization time, although the latter was not statically significant.42 Importantly, there were no adverse reactions to IFNα1b treatment aside from two minor fevers suggesting IFNα1b treatment is a safe therapeutic option. A related study evaluated modalities of IFNα1b delivery in infants (<1 year of age) with RSV. The findings showed nebulized IFNα1b reduced cough and wheeze earlier than intramuscular injection of IFNα1b with no serious adverse reactions but with some mild to moderate safety observations.43 In mice, recombinant IFNα treatment before RSV infection protected against weight loss and reduced pathology scores.44 Together, these studies suggest a potential role for certain type I IFNs in treatment of RSV and underscore the importance of IFN antagonism mediated by RSV to prevent robust anti-viral responses. Improving endogenous antiviral responses via anti-G protein mAbs may improve immune responses without the need for exogenous cytokine treatment.
Murine lung epithelial (MLE-15) cells are an immortalized mouse lung type II epithelial cell line that portray RSV infection. MLE-15 cells maintain their differentiated phenotypes and functional characteristics45 and express surfactant proteins SP-A, SP-B, SP-C, and major histocompatibility complex (MHC) class I antigens.44,45 MLE-15 cells have been previously used to determine the role of the RSV G protein and NS1 and NS2 in IFN antagonism and interferon-stimulated gene (ISG) responses.46 Cells infected with a mutant RSV virus lacking the G gene (ΔG) exhibit increased levels of IFNβ compared with wild-type RSV A2 infection, and this suppression was linked to the induction of the suppressor of cytokine signaling (SOCS) proteins, that is, SOCS1 and SOCS3.46 The RSV G protein attenuated the type I IFN response comparably to whole virus. Interferon-stimulated gene 15 (ISG15) is a ubiquitin-like protein that is induced during early viral infection and functions to reduce viral replication and support the antiviral milieu.47 As a result of type I IFN antagonism, RSV G protein also negatively impacts ISG15 expression.
Type III IFNs comprise λ1 (IL29), λ2 (IL28A), λ3 (IL28B), and λ4 which were initially thought to be redundant type I IFNs.48 Our current understanding of type III IFNs has now shifted. While type I IFNs are ubiquitous, type III IFNs receptors are found on the epithelium of the respiratory and gastrointestinal tract, and type III IFNs induce a less inflammatory response compared with type I.49 The receptor complex is IL-10r/IFNLR which once activated, shares a similar downstream pathway as type I receptors. IFNL1 (IL29) and IFNL4 are pseudogenes in mice. Studies in mice evaluating IFNL2 (IL28A) and IFNL3 (IFN28B) genes have incomplete translation to human studies.50 While type I IFN is a protective cytokine in the context of RSV infection, less is known about type III IFN. RSV has been shown to induce an IFNλ1 response in well-differentiated primary pediatric bronchial epithelial cells (WD-PBECs), and treating these cells before infection with recombinant IFNλ1 reduced RSV titers.51 The G protein CX3 C chemokine motif has been implicated in reducing IFNλ responses as it has been shown that ablating this motif results in increased IFNλ in human airway epithelial (Calu-3) cells.52 The role of type III IFNs in RSV disease is unclear. One study correlated high levels of type III IFNs to infant hospitalization for severe RSV disease.53 However, a recent report indicated an age-independent role for protective type III responses.54 Thus, the G protein CX3C chemokine motif contributes to modified host responses including types I and III IFNs and contributes to disease.
Anti-G protein mAbs can block modified IFN responses. Recently, co-crystal structures of two broadly neutralizing human mAbs, that is, 3D3 and 2D10 were recently solved.55 The findings showed that the anti-G protein mAbs recognize distinct epitopes in the central conserved domain of the RSV G protein, neutralize RSV in vitro in the presence of complement, and block G protein CX3 C-CX3CR1 chemotaxis.55 mAb 3D3 has been studied in RSV challenge studies and shown to neutralize RSV in vivo and protect from disease.28 As the IFN antagonizing motif on RSV G protein is purported to be the CX3 C chemokine motif, we sought here to determine whether prophylactic anti-G protein mAbs which block the CX3 C chemokine motif are able to mitigate IFN antagonism and improve the IFN responses in cells and mice infected with Line19 F. This is a chimeric RSV A2 strain that causes high viral lung loads, the development of mucus, airway goblet cell hyperplasia, airway hyperreactivity, and increased breathing effort in mice.8,56 The findings from this study show that the anti-G protein CX3C mAb 3D3 augments type I and III IFN responses linked with SOCS1 expression, partially by a neutralization-independent mechanism by binding the CX3C motif of RSV G protein.
Methods
Cells and virus
MLE-15 (alveolar type II) cells were obtained from Jeffrey Whitsett at Cincinnati Children’s Hospital Medical Center and propagated and maintained in HITES media (RPMI-1640 (Gibco, Waltham, MA), 1x insulin, transferrin, selenium, 10nM hydrocortisone, 10nM B-estradiol, 10 mM HEPES, 2 mM L-glutamine (all from ThermoFisher, Waltham, MA), and 4% fetal bovine serum (FBS, Hyclone, Logan, UT). Vero E6 cells (CRL-1586) were maintained in DMEM (Gibco) supplemented with 10% fetal bovine serum.
Line19 F is an RSV A strain that was first isolated from an infant with respiratory illness.57 Mice infected with Line19 F develop RSV disease characterized by Th2-biased responses that are associated with increased goblet cell expansion and elevated IL-13 and MUC5AC levels when compared with Th1-type responses associated with RSV A2.58,59 Line 19 F, kindly provided by Larry Anderson (Emory University) was propagated in HEp-2 cells (CCL-23, ATCC) at a multiplicity of infection (MOI) of 0.01 as described.60 Viral titers were determined by plaque assay as previously described.61,62
Mice
Six-to-eight-week-old specific-pathogen-free female BALB/c mice (Jackson Laboratories, Bar Harbor, ME) were used in all experiments. Mice were housed in microisolator cages and fed sterilized water and food ad libitum. Mice were administered phosphate-buffered saline (PBS) or 1 mg/kg mAb 2D10 (provided by Rebecca Dubois, University of California, Santa Cruz), mAb 3D3 (Trellis Bioscience, Redwood City, CA), palivizumab (provided by Larry Anderson, Emory University), or isotype (hIgG1) control mAb (InVivoMab, BioXCell, Lebanon, NH) by i.p. injection 24 h prior to infection. For RSV infection, mice were anesthetized by intraperitoneal (i.p.) administration of Avertin (2% 2,2,2-tribromoethanol, Sigma-Aldrich St. Louis, MO) and challenged intranasally (i.n.) with 106 plaque-forming units (PFU) of Line19 F in serum-free minimal essential medium (MEM, Gibco). Five mice/group were sacrificed 24 hours post infection (hpi). Mice studies were performed according to a protocol approved by the University of Georgia Institutional Animal Care and Use Committee (A2022 04-023-Y1-A0, approval date 05/19/2022).
Plaque assay
To detect RSV infection in MLE-15 cells, Line19 F containing a mCherry marker (propagated as described above) was used to infect the MLE-15 cells at a MOI of 0.01.
Twenty-four hours post infection, the cells were analyzed on Cellomics ArrayScan (ThermoFisher). Fluorescent focus units (FFUs) were enumerated using HCS Cell Analysis Software (ThermoFisher). To quantitate lung viral titers, plaque assays were performed as previously described.63 Briefly, lungs were homogenized in 1 mL of sterile Dulbecco PBS per lung, and 10-fold serial dilutions in serum-free DMEM (Gibco) were added to 90% confluent Vero E6 cell monolayers in 24-well plates. After adsorption for 2 h at 37°C, cell monolayers were overlaid with 2% methylcellulose, incubated at 37°C for 6 days. Plaques were developed by immunostaining. Wells were blocked with blotto (5% nonfat dry milk) diluted in KPL Wash Buffer (SeraCare, Milford, MA) overnight at 4°C then incubated with anti-RSV mAb cocktail (clones 131-2G, 131-2A) diluted in blotto for 2 h at room temperature. Wells were washed with KPL wash buffer and incubated with 2° goat-anti-mouse conjugated to alkaline phosphatase (Invitrogen) for 2 h at room temperature. Wells were washed and plaques were developed with 1-Step NBT/BCIP (ThermoFisher) for 10 min at room temperature, rinsed with diH2O, and plaques were enumerated by dissection microscope.
Bronchioalveolar lavage (BAL)
Mice were anesthetized with Avertin and euthanized by exsanguination after severing the left axillary artery. BAL fluid (BALF) was harvested by lavaging the lungs 3x using PBS (Gibco). BALF was centrifuged for 10 min at 1000 g at 4°C to separate cells from BAL supernatant. Supernatants were stored at −80°C until cytokine evaluation. After collecting BALF, lungs were removed and homogenized using GentleMACS tubes (Miltenyi Biotech, Westphalia, Germany) in 1 mL DMEM (HyClone) and stored at −80°C as previously described.64
IFN assays
To determine the IFN response in vitro, RSV Line19 F (MOI = 1) was pre-incubated with 10 μg/mL of anti-RSV mAbs or isotype control for 1 h at 37°C. Following incubation, the Line19 F/mAb mixture, Line19 F alone, or media only components were overlaid onto confluent MLE-15 cells in a 96-well tissue culture plate (Corning, Corning, New York), and at 24 hpi, the cell-free supernatant was collected and stored at −80°C until evaluation by ELISA described below. Cells were stored at −20°C until evaluation by polymerase chain reaction (PCR) as described below.
ELISA
IFNβ and IFNλ2/λ3 capture ELISAs were performed according to the manufacturer’s instructions (R&D Systems, Minneapolis, MN). Briefly, the capture antibody was coated onto a high-binding ELISA plate (Corning) overnight. The following day, wells were washed 3x with KPL wash buffer [1X diluted in deionized water (SeraCare)] and blocked overnight at 4°C with 1% BSA/KPL buffer. BALF and cell supernatants were added to the plates and incubated overnight at 4°C. Twelve hours later, the wells were washed 3x with KPL wash buffer, and a biotinylated detection antibody was added for 2 h at room temperature. Wells were washed and incubated with streptavidin-HRP in the dark for 20 min at room temperature. Wells were washed 3x with KPL wash buffer and detected with One-Step TMB (ThermoFisher) and stopped with Stop Solution (ThermoFisher). Plates were read on a BioTek plate reader at OD450. A standard curve was generated to quantify protein concentrations using standards included in respective kits.
PCR
RNA was isolated by RNAzol RT (Molecular Research Center, Cincinnati, OH) as described by the manufacturer and quantified by NanoDrop (ThermoFisher). cDNA was synthesized by LunaScript RT SuperMix (New England Biolabs, Ipswich, MA) according to the manufacturer. RSV was detected in MLE-15 cells and mouse lungs using GoTaq 2X Probe Kit (Promega, Madison, WI). Standard curve was obtained using serial dilutions of known PFU of RSV RNA as previously described.64 Threshold cycles (Ct) values for each sample were converted to genome equivalents using the standard curve. To determine changes in gene expression, qPCR was performed using 2x Ultra-Brilliant III SYBR with low ROX (Agilent, Santa Clara, CA) using MX300 Real-Time PCR instrument (Agilent). The following primer sets were procured from Integrated DNA Technologies (IDT) (San Diego, CA) PrimeTime™ Pre-Designed Primers – ACTB, IFNA1, IFNB, IFNL3 (IL28b), ISG15, SOCS1, SOCS2, SOCS3, RSV M Gene (probe-based). Fold-changes in gene expression were determined using ΔΔCt method65 and normalized to ACTB.
Flow cytometry
MLE-15 cells were evaluated for the presence of the type III IFN receptor. Briefly, a single cell suspension of MLE-15 cells was washed in FACS buffer (0.8% BSA in PBS). The MLE-15 cells were blocked with 1 μg/106 cells of mouse Fc Block (anti-CD16/32, BD Biosciences, San Jose, CA) followed by incubation with rat anti-mIL-IL-10Rβ (R&D Systems) or isotype control for 1 h at 4°C. The MLE-15 cells were washed with FACS buffer and incubated with goat anti-rat AlexaFluor-488 (Thermofisher) for 45 min at 4°C. The expression of the type III IFN receptor were determined for > 20,000 events analyzed on a BD LSRII flow cytometer (BD Biosciences, Mountain View, CA), and data were analyzed using FlowJo software (TreeStar, Ashland, OR).
Statistics
MAb treatments were compared with isotype control–treated mice using a one-way analysis of variance (ANOVA) with Dunnett’s post hoc test. A p value ⩽0.05 was considered statistically significant. Data are shown as mean ± SEM. Cellular experiments were performed at least twice independently, with at least two independent assays with at least two replicates per assay. Mouse experiments were performed once (n = 5 mice/group), with at least two independent assays and at least two replicates per assay. All statistical analyses were performed using Prism 9 (GraphPad, San Diego, CA).
Results
Neutralization
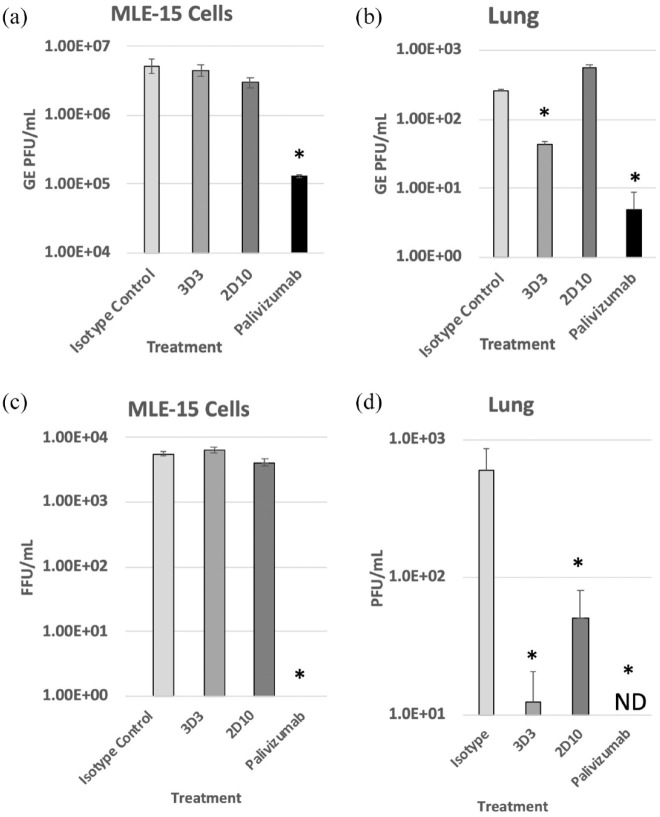
3D3 and 2D10 are human anti-RSV G protein mAbs that reduce CX3 C-mediated CX3CR1 chemotaxis. Importantly, anti-G protein mAbs neutralize virus in restricted cell types in vitro (e.g. primary human airway cell lines) and do not neutralize in immortalized cell lines without the addition of complement.15,66 Consistent with this function of anti-G protein mAbs, Figure 1 shows no decline in RSV M gene copies or FFUs in RSV-infected MLE-15 cells when treated with anti-G protein mAbs. Palivizumab, a known complement-independent neutralizing mAb, substantially reduced M gene transcripts and FFUs. Several studies have shown that anti-G protein mAbs are neutralizing in mice.27,67 At 24 hpi, mAb 3D3 and palivizumab treatments significantly (p < 0.05) reduced M gene copies and viral titers in the lungs compared with isotype control–treated mice. 2D10 significantly (p < 0.05) reduced RSV at 24 hpi by plaque assay. As mAbs 3D3 and 2D10 recognize opposing epitopes on the G protein CCD, their neutralization capacity in vivo may vary. These results indicate that modifications of IFN responses by anti-G protein mAbs are independent of neutralization in vitro.
Figure 1.
Anti-G protein mAbs are non-neutralizing in vitro but neutralize in mice. (a) RSV Line19 F was pre-incubated with 10 μg/mL mAb for 1 h at 37°C then added onto MLE-15 cells for 24 h. (b) BALB/c mice were i.p. treated prophylactically with 1 mg/kg of indicated mAb 24 h before i.n. infection with 106 PFU Line19 F. Twenty-four hours post infection, genome equivalent units (GEs) were determined in MLE-15 cells and homogenized mouse lung using a standard curve as described in materials and methods. (c) RSV Line19 F was pre-incubated with 10 μg/mL mAb for 1 h at 37°C then added onto MLE-15 cells for 24 h. After 24 h, plates were scanned using Cellomics Array Scan and fluorescent focus units (FFUs) were enumerated. (d) Viral titers in mouse lungs were determined by plaque assay. ND = not detected. * p < 0.05 as determined by one-way ANOVA with Dunnett’s post hoc test compared with isotype control. Experiments were individually repeated at least twice with technical replicates. Bars represent the mean ± SEM.
Type I IFN
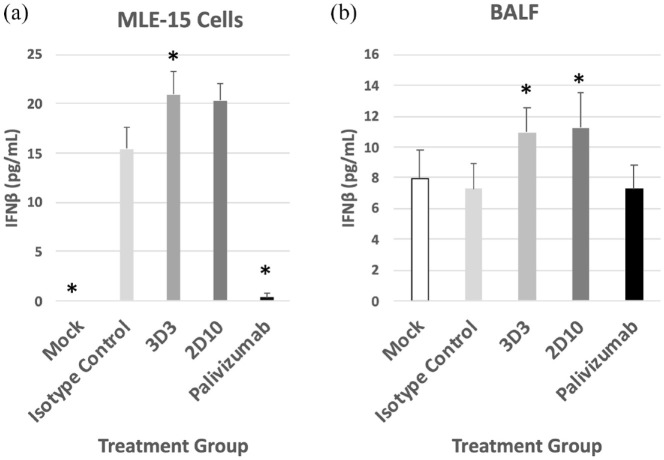
Line19 F (MOI = 1) was pre-incubated with 10 μg/mL of mAbs (3D3, 2D10, or palivizumab, or with isotype control hIgG1) diluted in tissue culture media for 1 h at 37°C.46 After 1 h, the Line19 F-mAb mixture, Line19 F alone, or media only components were overlaid onto confluent MLE-15 cells and responses were evaluated 24 hpi. Line19 F infection of MLE-15 cells led to substantial IFNβ compared with mock-infected MLE-15 cells (Figure 2(a)). Treatment of MLE-15 cells with mAbs 3D3 or 2D10 further increased IFNβ in RSV infected cells with 3D3 treatment significantly (p < 0.05) increasing IFNβ concentrations compared with isotype control. By contrast, palivizumab treatment significantly (p < 0.05) reduced IFNβ compared with isotype control–treated MLE-15 cells.
Figure 2.
RSV antibodies modify IFNβ responses. (a) RSV Line19 F was pre-incubated with 10 μg/mL mAb for 1 h at 37°C then added onto MLE-15 cells for 24 h and supernatant was assayed for IFNβ concentrations. (b) The BALB/c mice were i.p. treated prophylactically with 1 mg/kg of indicated mAb 24 h before i.n. infection with 106 PFU Line19 F. At 24 hpi, BALF was collected and assayed for IFNβ concentrations. Concentration of IFNβ was determined by ELISA and quantified using a standard curve of IFNβ. Bars represent the mean ± SEM of IFNβ (pg/mL). * p < 0.05 as determined by one-way ANOVA with Dunnett’s post hoc test compared with isotype control. For in vitro, bars represent the mean of three independent studies with technical replicates. For in vivo, bars represent mean of three independent assays with technical replicates from one experiment of 5 mice/group.
BALB/c mice are frequently used in RSV studies.68 To determine the outcome of anti-G protein or anti-F protein mAb treatment on IFNβ in vivo, BALB/c mice were treated with 1 mg/kg 3D3, 2D10, palivizumab, or isotype control mAbs for 24 h prior to Line19 F infection. Cell-free BAL supernatants were collected 24 hpi and assayed for IFNβ (Figure 2(b)). Consistent with previous in vivo findings,44 the results showed that Line19 F infection, like wild-type RSV, was a poor inducer of type I IFNs as there were no detectable differences in IFNβ between mock-infected and isotype-treated mice. In contrast, mice treated with mAbs 3D3 or 2D10 expressed significantly (p < 0.05) higher IFNβ in the BALF compared with isotype control, which suggests the G protein is an IFN antagonist consistent with previous reports. The differences in IFNβ expression between MLE-15 cells and BAL supernatants from mice during RSV infection likely reflect the biological features between the two models. For example, MLE-15 cells are a clonal cell line, whereas BAL supernatant is collected from a variety of BAL cell types including goblet cells, ciliated epithelial cells, basal cells, and lung immune cells. Moreover, there are other host factors expressed in vivo which are absent in cell lines.
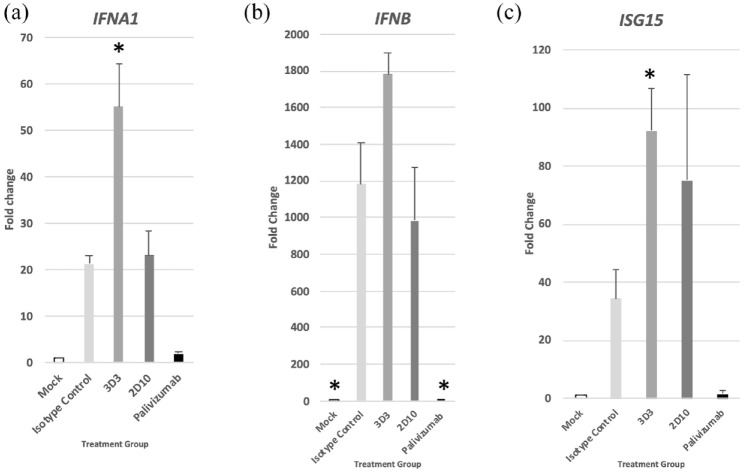
To better understand the type I IFN response, the expression of IFNα (IFNA1), IFNβ (IFNB), and ISG15 (a downstream effector) in MLE-15 cells were examined at 24 hpi (Figure 3) and the results normalized to uninfected, untreated cells. IFNA1, IFNB, and ISG15 genes were substantially upregulated following virus infection. IFNA1 (Figure 3(a)), IFNB (Figure 3(b)), and ISG15 (Figure 3(c)) were greatly increased in mAb 3D3-treated MLE-15 cells compared with isotype control treatment 24 hpi. By contrast, Palivizumab treatment significantly (p < 0.05) reduced IFNB expression. Taken together, the levels of IFNβ and type I gene expression suggest mAb 3D3 treatment improves the type I IFN response.
Figure 3.
RSV antibodies modify type I IFN gene expression. RSV was pre-incubated with 10 μg/mL mAb for 1 h at 37°C then added onto MLE-15 cells for 24 h. Twenty-four hours post infection, expression of (a) IFNA1, (b) IFNB, and (c) ISG15 genes was determined in MLE-15 cells. Bars represent the mean ± SEM of gene expression fold change using ΔΔCt PCR method normalized to ACTB expression and mock-infected cells. *p < 0.05 as determined by one-way ANOVA with Dunnett’s post hoc test compared with isotype control. Experiments were individually repeated at least twice with technical replicates.
Type III IFN
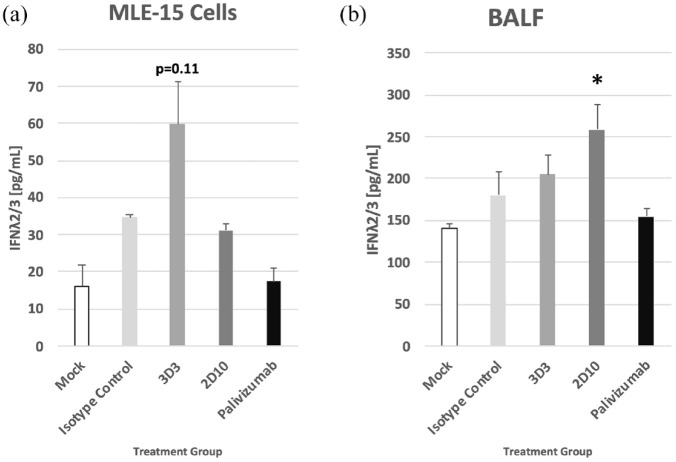
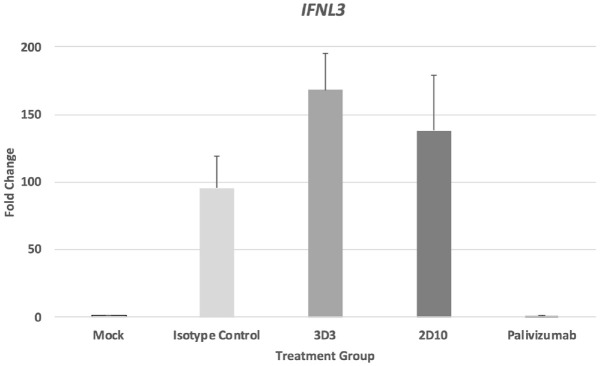
Previous studies evaluating palivizumab treatment showed treatment led to a reduction of type I IFN69 and type II IFN9 responses; however, there are inadequate findings for type III IFNs following RSV infection. To verify that the type III IFN receptor (IL-10Rβ) is expressed on MLE-15 cells, the cells were stained with antibody specific for the receptor or isotype control (Supplementary Figure 1). Consistent with other lung epithelial cell lines, MLE-15 cells robustly express the type III IFN receptor (>80% positive). In this study, MLE-15 cells had moderate increases of IFNλ2/3 following Line19 F infection (Figure 4(a)). However, treatment of Line19 F-infected MLE-15 cells with mAb 3D3 led to a substantial IFNλ2/3 compared with isotype control, although it was not statistically significant (p = 0.11). In cell-free BALF from Line19 F-infected BALB/c mice treated with mAbs, 2D10 treatment significantly (p < 0.05) enhanced the IFNλ2/3 compared with isotype control treatment (Figure 4(b)). IL28B (IFNL3) gene expression in MLE-15 cells was also determined (Figure 5). While mAb 3D3 treatment trended toward an increase in IFNL3 expression compared with isotype control–treated cells, this difference was not statistically significant. Taken together, these data show anti-G protein mAbs modify type III responses during RSV infection and help to highlight regulatory differences between types I and III IFNs.
Figure 4.
RSV antibodies modify IFNλ2/3 responses. (a) RSV Line19 F was pre-incubated with 10 μg/mL mAb for 1 h at 37°C, then added onto MLE-15 cells for 24 h, and supernatant was assayed for IFNλ2/3. (b) The BALB/c mice were i.p. treated prophylactically with 1 mg/kg of indicated mAb 24 h before i.n. infection with 106 PFU Line19 F. Twenty-four hours post infection, BALF was collected and assayed for IFNλ2/3. Concentration of IFNλ2/3 was determined by ELISA and quantified using a standard curve of IFNλ2/3. Bars represent the mean ± SEM of IFNλ2/3 (pg/mL). *p < 0.05 as determined by one-way ANOVA with Dunnett’s post hoc test compared with isotype control. For in vitro, bars represent the mean of three independent studies with technical replicates. For in vivo, bars represent mean of three independent assays with technical replicates from one experiment of 5 mice/group.
Figure 5.
RSV antibodies modify type III IFN gene expression. RSV Line19 F was pre-incubated with 10 μg/mL mAb for 1 h at 37°C then added onto MLE-15 cells for 24 h. At 24 hpi, the expression of IFNL3 genes was determined in MLE-15 cells. Bars represent the mean ± SEM of gene expression fold change using ΔΔCt PCR method normalized to ACTB expression and mock-infected cells. *p < 0.05 as determined by one-way ANOVA with Dunnett’s post hoc test compared with isotype control. Experiments were individually repeated at least twice with technical replicates.
SOCS
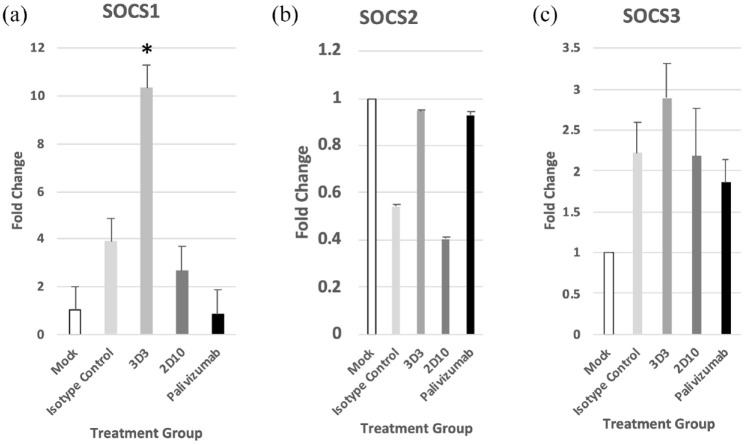
The suppressors of cytokine signaling (SOCS) family of proteins are important regulators of the type I IFN signaling and via negative feedback regulate the IFN signaling pathway. Members of the SOCS family are cytokine-inducible proteins that act in a classical negative-feedback loop to attenuate cytokine signal transduction.70 The SOCS family of proteins consist of eight cytokine-inducible SH2-containing protein (CIS)/SOCS family proteins, that is, CIS, SOCS1, SOCS2, SOCS3, SOCS4, SOCS5, SOCS6, and SOCS7; however, the predominant proteins are CIS, SOCS1, SOCS2, and SOCS3.71 Of the three SOCS genes examined in this study (i.e. SOCS1, SOCS2, and SOCS3), 3D3 mAb treatment of RSV infected cells resulted in a significant (p < 0.05) increase in SOCS1 (Figure 6(a)), while there were no significant differences with either SOCS2 (Figure 6(b)) or SOCS3 (Figure 6(c)) for any mAb treatment. These results complement a previous study in the MLE-15 cells that showed a considerable increase in SOCS1, SOCS3, and IFNβ in RSV strains lacking the G protein gene compared with wild-type RSV.46 Interestingly, an RSV deletion mutant virus, ΔNS1/2, was linked to SOCS1 while Δ G was linked to SOCS3 increased expression. These differences may be in part strain dependent. Taken together, these data demonstrate a role for anti-G protein CX3C-blocking mAbs in improving early IFN responses to RSV infection independently of virus neutralization.
Figure 6.
RSV antibodies modify the suppression of cytokine signaling (SOCS) gene expression. RSV Line19 F was pre-incubated with 10 μg/mL mAb for 1 h at 37°C, then added onto MLE-15 cells for 24 h. At 24 hpi, expression of (a) SOCS1, (b) SOCS2, and (c) SOCS3 genes was determined in MLE-15 cells. Bars represent the mean ± SEM of gene expression fold change using ΔΔCt PCR method normalized to ACTB expression and mock-infected cells. * p < 0.05 as determined by one-way ANOVA with Dunnett’s post hoc test compared with isotype control. Experiments were individually repeated at least twice with technical replicates.
Discussion
RSV is a leading cause of lower respiratory tract disease in the infant and elderly populations. There is no safe or approved RSV vaccine. The only approved prophylactic available in the United States is palivizumab, which is restricted to high-risk infants and modestly (~50%) reduces hospitalization.72 Moreover, palivizumab is not associated with the prevention of RSV-mediated asthma later in childhood; however, it has been demonstrated to reduce recurrent wheeze.73 Despite being identified > 70 years ago, there are still substantial gaps in understanding the host response to RSV. IFNs are canonical mediators of the antiviral response and are very important during RSV infection. Type I IFNs (IFNα/β) are generally expressed by plasmacytoid dendritic cells (pDCs),74 and their expression induces an intrinsic antiviral state in infected and neighboring cells that limit the spread of viral pathogens.75 Importantly, RSV is a poor inducer of type I IFNs, and young infants who are at the greatest risk for severe RSV disease have reduced IFN capacity following RSV infection.31,76,77 Type III IFNs function is similar to type I IFNs, but the antiviral effect is less inflammatory and serves mostly as a first-line defense against viruses in the respiratory and gut epithelia.78
RSV disease arises from both virus- and host-mediated activities. RSV infection damages the lung epithelial cell structure in the respiratory tract leading to the sloughing of multinucleated cells into the bronchiolar lumen.79 This cellular damage is particularly severe in infants whose bronchioles are sensitive to virus-mediated alterations to morphology.79 During lower respiratory tract infection (LRTI), RSV induces immune responses which can incidentally cause immunopathology.80 These responses include a Th2-skewed response mediated by G protein.81 IFNs are known to potentiate a Th1 response and reduce Th2 responses, and treatment with IFN has been shown to improve clinical RSV disease.41 However, RSV is a poor inducer of IFNs due to G protein along with NS1 and NS2 proteins.82,83 Blocking of the G protein CX3 C chemokine motif has been shown to increase type I and III responses IFN.19 Inducing CX3 C blocking Abs by G protein vaccination or treatment with anti-G protein mAb improve type I responses and reduce disease severity as evidenced by improving airway function and reducing lung inflammation, BAL cell influx, and mucus production.5,25,26,28,64 While some studies indicate IFN is useful for treating RSV, it is speculated that the safety profile may preclude widespread use particularly in the vulnerable populations affected by RSV, that is, the very young and very old.31
The data presented here build upon the accumulated evidence that IFNs are adversely affected by RSV G protein and that blocking the G protein CX3 C-CX3CR1 interaction improves these responses. Specifically, the results show that treatment with anti-G protein mAbs markedly improve type I and type III IFN responses. IFNβ and IFNλ2/3 protein concentrations were increased in Line19 F infected MLE-15 cells and in BALB/c mice when treated with mAbs 3D3 and/or 2D10. 3D3 treatment significantly increased IFN1A and ISG15 expression and IFNB and IFNL trended toward increased expression.
Notably, we detected differences between mAbs 3D3 and 2D10 in their ability to affect IFN responses, specifically between type I and type III IFN responses during RSV infection, and neutralization-independent modifications of IFN. Treatment with mAb 3D3 increased type I IFN responses in MLE-15 cells compared with mAb 2D10 where mAb 3D3 treatment resulted in significantly (p < 0.05) increased expression of IFN1A and ISG15 and increases of IFNβ. However, in the mouse model, only treatment with mAb 2D10 significantly (p < 0.05) increased IFNλ in the BALF, while in vitro only mAb 3D3 treatment trended (p = 0.11) toward increasing IFNλ in the BALF. Neither mAb treatment significantly improved IFNL expression compared with isotype control. We also observed improved IFN responses in mice treated with anti-G protein mAbs having various neutralization abilities. Only mAb 3D3 treatment significantly (p < 0.05) increased SOCS1 expression suggesting these IFN modifications are linked to SOCS1. RSV has been well documented for modifying IFN responses by SOCS,84 but the results presented here provide the first evidence to suggest a role for G protein CX3 C in SOCS1 regulation. Interestingly, polymorphisms of SOCS1 may lead to asthma,85 and SOCS1 is a regulator of CX3CR1 expression.86
Here, we describe a role for RSV G protein in negatively modifying host IFN responses in a neutralization-independent mechanism. As 3D3 and 2D10 bind the G protein CCD and prevent CX3 C chemotaxis, we postulate blocking these epitopes rescue IFN responses. Increased IFN is associated with protection against severe RSV disease thus describing one of the protective mechanisms of blocking G protein CX3C. Since the anti-G protein mAbs are also effective at reducing viral load, the additional benefit from improved IFN responses does not compromise that important activity.
Supplemental Material
Supplemental material, sj-docx-1-tai-10.1177_20499361231161157 for Anti-G protein antibodies targeting the RSV G protein CX3C chemokine region improve the interferon response by Harrison C. Bergeron, Lawrence M. Kauvar and Ralph A. Tripp in Therapeutic Advances in Infectious Disease
Acknowledgments
We thank Rebecca Dubois and Ana Nuñez Castrejon (University of California Santa Cruz) for the 2D10 mAb. We thank Larry Anderson (Emory University) for palivizumab.
Footnotes
ORCID iD: Harrison C. Bergeron  https://orcid.org/0000-0002-1329-0209
https://orcid.org/0000-0002-1329-0209
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Harrison C. Bergeron, Department of Infectious Diseases, College of Veterinary Medicine, University of Georgia, Athens, GA, USA
Lawrence M. Kauvar, Trellis Bioscience, Redwood City, CA, USA
Ralph A. Tripp, Department of Infectious Diseases, College of Veterinary Medicine, University of Georgia, Athens, GA 30605, USA.
Declarations
Ethics approval and consent to participate: Mice studies were performed in compliance with all national and institutional guidelines and guidelines from the Human Care and Use of Laboratory Animals (American Association for Laboratory Animal Science) and performed according to a protocol approved by the University of Georgia Institutional Animal Care and Use Committee (IACUC) (A2022 04-023-Y1-A0, approval date 05/19/2022). All efforts were made to minimize animal pain and discomfort.
Consent for publication: Not applicable.
Author contributions: Harrison C. Bergeron: Conceptualization; Data curation; Formal analysis; Methodology; Writing – original draft; Writing – review & editing.
Lawrence M. Kauvar: Funding acquisition; Resources; Writing – review & editing.
Ralph A. Tripp: Funding acquisition; Methodology; Project administration; Resources; Supervision; Visualization; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding was in part provided by Trellis Bioscience, the Georgia Research Alliance, and included support from NIAID SBIR grants 5R44AI122360 and 2R44AI106077.
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: LMK is the founder and chief scientific officer of Trellis Bioscience.
Availability of data and materials: Data and materials are available upon reasonable request.
References
- 1. Boyoglu-Barnum S, Tripp RA. Up-to-date role of biologics in the management of respiratory syncytial virus. Expert Opin Biol Ther 2020; 20: 1073–1082. [DOI] [PubMed] [Google Scholar]
- 2. Dalziel SR, Haskell L, O’Brien S, et al. Bronchiolitis. Lancet 2022; 400: 392–406. [DOI] [PubMed] [Google Scholar]
- 3. Georgescu G, Chemaly RF. Palivizumab: where to from here? Expert Opin Biol Ther 2009; 9: 139–147. [DOI] [PubMed] [Google Scholar]
- 4. Bergeron HC, Tripp RA. Emerging small and large molecule therapeutics for respiratory syncytial virus. Expert Opin Investig Drugs 2020; 29: 285–294. [DOI] [PubMed] [Google Scholar]
- 5. Tripp RA, Power UF, Openshaw PJM, et al. Respiratory syncytial virus: targeting the G protein provides a new approach for an old problem. J Virol 2018; 92: e01302-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Boyoglu-Barnum S, Chirkova T, Todd SO, et al. Prophylaxis with a respiratory syncytial virus (RSV) anti-G protein monoclonal antibody shifts the adaptive immune response to RSV rA2-line19F infection from Th2 to Th1 in BALB/c mice. J Virol 2014; 88: 10569–10583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miao C, Radu GU, Caidi H, et al. Treatment with respiratory syncytial virus G glycoprotein monoclonal antibody or F(ab’)2 components mediates reduced pulmonary inflammation in mice. J Gen Virol 2009; 90: 1119–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Boyoglu-Barnum S, Gaston KA, Todd SO, et al. A respiratory syncytial virus (RSV) anti-G protein F(ab’)2 monoclonal antibody suppresses mucous production and breathing effort in RSV rA2-line19F-infected BALB/c mice. J Virol 2013; 87: 10955–10967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kruijsen D, Bakkers MJ, van Uden NO, et al. Serum antibodies critically affect virus-specific CD4+/CD8+ T cell balance during respiratory syncytial virus infections. J Immunol 2010; 185: 6489–6498. [DOI] [PubMed] [Google Scholar]
- 10. McNamara PS, Fonceca AM, Howarth D, et al. Respiratory syncytial virus infection of airway epithelial cells, in vivo and in vitro, supports pulmonary antibody responses by inducing expression of the B cell differentiation factor BAFF. Thorax 2013; 68: 76–81. [DOI] [PubMed] [Google Scholar]
- 11. Tripp RA, Jones LP, Haynes LM, et al. CX3C chemokine mimicry by respiratory syncytial virus G glycoprotein. Nat Immunol 2001; 2: 732–738. [DOI] [PubMed] [Google Scholar]
- 12. Jeong KI, Piepenhagen PA, Kishko M, et al. CX3CR1 is expressed in differentiated human ciliated airway cells and co-localizes with respiratory syncytial virus on cilia in a G protein-dependent manner. PLoS ONE 2015; 10: e0130517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Johnson SM, McNally BA, Ioannidis I, et al. Respiratory syncytial virus uses CX3CR1 as a receptor on primary human airway epithelial cultures. Plos Pathog 2015; 11: e1005318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Green G, Johnson SM, Costello H, et al. CX3CR1 is a receptor for human respiratory syncytial virus in cotton rats. J Virol 2021; 95: e0001021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Anderson LJ, Jadhao SJ, Paden CR, et al. Functional features of the respiratory syncytial virus G protein. Viruses 2021; 13: 1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tripp RA, Moore D, Jones L, et al. Respiratory syncytial virus G and/or SH protein alters Th1 cytokines, natural killer cells, and neutrophils responding to pulmonary infection in BALB/c mice. J Virol 1999; 73: 7099–7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harcourt J, Alvarez R, Jones LP, et al. Respiratory syncytial virus G protein and G protein CX3C motif adversely affect CX3CR1+ T cell responses. J Immunol 2006; 176: 1600–1608. [DOI] [PubMed] [Google Scholar]
- 18. Polack FP, Irusta PM, Hoffman SJ, et al. The cysteine-rich region of respiratory syncytial virus attachment protein inhibits innate immunity elicited by the virus and endotoxin. Proc Natl Acad Sci USA 2005; 102: 8996–9001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chirkova T, Boyoglu-Barnum S, Gaston KA, et al. Respiratory syncytial virus G protein CX3C motif impairs human airway epithelial and immune cell responses. J Virol 2013; 87: 13466–13479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oshansky CM, Krunkosky TM, Barber J, et al. Respiratory syncytial virus proteins modulate suppressors of cytokine signaling 1 and 3 and the type I interferon response to infection by a toll-like receptor pathway. Viral Immunol 2009; 22: 147–161. [DOI] [PubMed] [Google Scholar]
- 21. Shingai M, Azuma M, Ebihara T, et al. Soluble G protein of respiratory syncytial virus inhibits toll-like receptor 3/4-mediated IFN-beta induction. Int Immunol 2008; 20: 1169–1180. [DOI] [PubMed] [Google Scholar]
- 22. Becker Y. Respiratory syncytial virus (RSV)-induced allergy may be controlled by IL-4 and CX3C fractalkine antagonists and CpG ODN as adjuvant: hypothesis and implications for treatment. Virus Genes 2006; 33: 253–264. [DOI] [PubMed] [Google Scholar]
- 23. Porter DP, Guo Y, Perry J, et al. Assessment of drug resistance during phase 2b clinical trials of presatovir in adults naturally infected with respiratory syncytial virus. Antimicrob Agents Chemother 2020; 64: e02312-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Weisman LE. Motavizumab, a second-generation humanized mAb for the prevention of respiratory syncytial virus infection in high-risk populations. Curr Opin Mol Ther 2009; 11: 208–218. [PubMed] [Google Scholar]
- 25. Kauvar LM, Harcourt JL, Haynes LM, et al. Therapeutic targeting of respiratory syncytial virus G-protein. Immunotherapy 2010; 2: 655–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boyoglu-Barnum S, Todd SO, Chirkova T, et al. An anti-G protein monoclonal antibody treats RSV disease more effectively than an anti-F monoclonal antibody in BALB/c mice. Virology 2015; 483: 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Radu GU, Caidi H, Miao C, et al. Prophylactic treatment with a G glycoprotein monoclonal antibody reduces pulmonary inflammation in respiratory syncytial virus (RSV)-challenged naive and formalin-inactivated RSV-immunized BALB/c mice. J Virol 2010; 84: 9632–9636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Caidi H, Miao C, Thornburg NJ, et al. Anti-respiratory syncytial virus (RSV) G monoclonal antibodies reduce lung inflammation and viral lung titers when delivered therapeutically in a BALB/c mouse model. Antiviral Res 2018; 154: 149–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Haynes LM, Caidi H, Radu GU, et al. Therapeutic monoclonal antibody treatment targeting respiratory syncytial virus (RSV) G protein mediates viral clearance and reduces the pathogenesis of RSV infection in BALB/c mice. J Infect Dis 2009; 200: 439–447. [DOI] [PubMed] [Google Scholar]
- 30. Bergeron HC, Tripp RA. RSV replication, transmission, and disease are influenced by the RSV G protein. Viruses 2022; 14: 2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hijano DR, Vu LD, Kauvar LM, et al. Role of type I interferon (IFN) in the respiratory syncytial virus (RSV) immune response and disease severity. Front Immunol 2019; 10: 566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ivashkiv LB, Donlin LT. Regulation of type I interferon responses. Nat Rev Immunol 2014; 14: 36–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McNab F, Mayer-Barber K, Sher A, et al. Type I interferons in infectious disease. Nat Rev Immunol 2015; 15: 87–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kishimoto K, Wilder CL, Buchanan J, et al. High dose IFN-beta activates GAF to enhance expression of ISGF3 target genes in MLE12 epithelial cells. Front Immunol 2021; 12: 651254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Peng L, Oganesyan V, Wu H, et al. Molecular basis for antagonistic activity of anifrolumab, an anti-interferon-alpha receptor 1 antibody. mAbs 2015; 7: 428–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sedeyn K, Schepens B, Saelens X. Respiratory syncytial virus nonstructural proteins 1 and 2: exceptional disrupters of innate immune responses. PLoS Pathog 2019; 15: e1007984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thornhill EM, Verhoeven D. Respiratory syncytial virus’s non-structural proteins: masters of interference. Front Cell Infect Microbiol 2020; 10: 225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Spann KM, Tran KC, Chi B, et al. Suppression of the induction of alpha, beta, and lambda interferons by the NS1 and NS2 proteins of human respiratory syncytial virus in human epithelial cells and macrophages [corrected]. J Virol 2004; 78: 4363–4369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dubois J, Hershon L, Carmant L, et al. Toxicity profile of interferon alfa-2b in children: a prospective evaluation. J Pediatr 1999; 135: 782–785. [DOI] [PubMed] [Google Scholar]
- 40. Chipps BE, Sullivan WF, Portnoy JM. Alpha-2A-interferon for treatment of bronchiolitis caused by respiratory syncytial virus. Pediatr Infect Dis J 1993; 12: 653–658. [DOI] [PubMed] [Google Scholar]
- 41. Sung RY, Yin J, Oppenheimer SJ, et al. Treatment of respiratory syncytial virus infection with recombinant interferon alfa-2a. Arch Dis Child 1993; 69: 440–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. He L, Yang L, Zhang H, et al. Efficacy and safety of interferon on neonates with respiratory syncytial virus pneumonia. Exp Ther Med 2020; 20: 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Chen L, Shi M, Deng Q, et al. A multi-center randomized prospective study on the treatment of infant bronchiolitis with interferon alpha1b nebulization. PLoS ONE 2020; 15: e0228391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Guerrero-Plata A, Baron S, Poast JS, et al. Activity and regulation of alpha interferon in respiratory syncytial virus and human metapneumovirus experimental infections. J Virol 2005; 79: 10190–10199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wikenheiser KA, Vorbroker DK, Rice WR, et al. Production of immortalized distal respiratory epithelial cell lines from surfactant protein C/simian virus 40 large tumor antigen transgenic mice. Proc Natl Acad Sci USA 1993; 90: 11029–11033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Moore EC, Barber J, Tripp RA. Respiratory syncytial virus (RSV) attachment and nonstructural proteins modify the type I interferon response associated with suppressor of cytokine signaling (SOCS) proteins and IFN-stimulated gene-15 (ISG15). Virol J 2008; 5: 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Perng YC, Lenschow DJ. ISG15 in antiviral immunity and beyond. Nat Rev Microbiol 2018; 16: 423–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Lazear HM, Schoggins JW, Diamond MS. Shared and distinct functions of type I and type III interferons. Immunity 2019; 50: 907–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mesev EV, LeDesma RA, Ploss A. Decoding type I and III interferon signalling during viral infection. Nat Microbiol 2019; 4: 914–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Peterson ST, Kennedy EA, Brigleb PH, et al. Disruption of type III interferon (IFN) genes Ifnl2 and Ifnl3 recapitulates loss of the type III IFN receptor in the mucosal antiviral response. J Virol 2019; 93: e01073-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Villenave R, Broadbent L, Douglas I, et al. Induction and antagonism of antiviral responses in respiratory syncytial virus-infected pediatric airway epithelium. J Virol 2015; 89: 12309–12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bakre AA, Harcourt JL, Haynes LM, et al. The central conserved region (CCR) of respiratory syncytial virus (RSV) G protein modulates host miRNA expression and alters the cellular response to infection. Vaccines 2017; 5: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Selvaggi C, Pierangeli A, Fabiani M, et al. Interferon lambda 1-3 expression in infants hospitalized for RSV or HRV associated bronchiolitis. J Infect 2014; 68: 467–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Taveras J, Garcia-Maurino C, Moore-Clingenpeel M, et al. Type-III interferons, viral loads, age, and disease severity in young children with respiratory syncytial virus infection. J Inf Dis 2022; 227: 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Fedechkin SO, George NL, Wolff JT, et al. Structures of respiratory syncytial virus G antigen bound to broadly neutralizing antibodies. Sci Immunol 2018; 3: eaar3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hotard AL, Lee S, Currier MG, et al. Identification of residues in the human respiratory syncytial virus fusion protein that modulate fusion activity and pathogenesis. J Virol 2015; 89: 512–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Herlocher ML, Ewasyshyn M, Sambhara S, et al. Immunological properties of plaque purified strains of live attenuated respiratory syncytial virus (RSV) for human vaccine. Vaccine 1999; 17: 172–181. [DOI] [PubMed] [Google Scholar]
- 58. Lukacs NW, Moore ML, Rudd BD, et al. Differential immune responses and pulmonary pathophysiology are induced by two different strains of respiratory syncytial virus. Am J Pathol 2006; 169: 977–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Moore ML, Peebles RS., Jr. Respiratory syncytial virus disease mechanisms implicated by human, animal model, and in vitro data facilitate vaccine strategies and new therapeutics. Pharmacol Ther 2006; 112: 405–424. [DOI] [PubMed] [Google Scholar]
- 60. Moore ML, Chi MH, Luongo C, et al. A chimeric A2 strain of respiratory syncytial virus (RSV) with the fusion protein of RSV strain line 19 exhibits enhanced viral load, mucus, and airway dysfunction. J Virol 2009; 83: 4185–4194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Jorquera PA, Oakley KE, Powell TJ, et al. Layer-by-layer nanoparticle vaccines carrying the G protein CX3C motif protect against RSV infection and disease. Vaccines 2015; 3: 829–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Jorquera PA, Choi Y, Oakley KE, et al. Nanoparticle vaccines encompassing the respiratory syncytial virus (RSV) G protein CX3C chemokine motif induce robust immunity protecting from challenge and disease. PLoS ONE 2013; 8: e74905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Murray J, Bergeron HC, Jones LP, et al. Probenecid inhibits respiratory syncytial virus (RSV) replication. Viruses 2022; 14: 912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Bergeron HC, Murray J, Nunez Castrejon AM, et al. Respiratory syncytial virus (RSV) G protein vaccines with central conserved domain mutations induce CX3C-CX3CR1 blocking antibodies. Viruses 2021; 13: 352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001; 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 66. Kishko M, Catalan J, Swanson K, et al. Evaluation of the respiratory syncytial virus G-directed neutralizing antibody response in the human airway epithelial cell model. Virology 2020; 550: 21–26. [DOI] [PubMed] [Google Scholar]
- 67. Andersson C, Liljeström P, Ståhl S, et al. Protection against respiratory syncytial virus (RSV) elicited in mice by plasmid DNA immunisation encoding a secreted RSV G protein-derived antigen. FEMS Immunol Med Microbiol 2000; 29: 247–253. [DOI] [PubMed] [Google Scholar]
- 68. Altamirano-Lagos MJ, Díaz FE, Mansilla MA, et al. Current animal models for understanding the pathology caused by the respiratory syncytial virus. Front Microbiol 2019; 10: 873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Schijf MA, Lukens MV, Kruijsen D, et al. Respiratory syncytial virus induced type I IFN production by pDC is regulated by RSV-infected airway epithelial cells, RSV-exposed monocytes and virus specific antibodies. PLoS ONE 2013; 8: e81695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Alexander WS. Suppressors of cytokine signalling (SOCS) in the immune system. Nat Rev Immunol 2002; 2: 410–416. [DOI] [PubMed] [Google Scholar]
- 71. Linossi EM, Babon JJ, Hilton DJ, et al. Suppression of cytokine signaling: the SOCS perspective. Cytokine Growth Factor Rev 2013; 24: 241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Homaira N, Rawlinson W, Snelling TL, et al. Effectiveness of palivizumab in preventing RSV hospitalization in high risk children: a real-world perspective. Int J Pediatr 2014; 2014: 571609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Mochizuki H, Kusuda S, Okada K, et al. Palivizumab prophylaxis in preterm infants and subsequent recurrent wheezing. Six-year follow-up study. Am J Resp Crit Care Med 2017; 196: 29–38. [DOI] [PubMed] [Google Scholar]
- 74. Fitzgerald-Bocarsly P, Dai J, Singh S. Plasmacytoid dendritic cells and type I IFN: 50 years of convergent history. Cytokine Growth Factor Rev 2008; 19: 3–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Platanias LC. Mechanisms of type-I- and type-II-interferon-mediated signalling. Nat Rev Immunol 2005; 5: 375–386. [DOI] [PubMed] [Google Scholar]
- 76. Cormier SA, Shrestha B, Saravia J, et al. Limited type I interferons and plasmacytoid dendritic cells during neonatal respiratory syncytial virus infection permit immunopathogenesis upon reinfection. J Virol 2014; 88: 9350–9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wang H, Peters N, Schwarze J. Plasmacytoid dendritic cells limit viral replication, pulmonary inflammation, and airway hyperresponsiveness in respiratory syncytial virus infection. J Immunol 2006; 177: 6263–6270. [DOI] [PubMed] [Google Scholar]
- 78. Ali S, Mann-Nüttel R, Schulze A, et al. Sources of type I interferons in infectious immunity: plasmacytoid dendritic cells not always in the driver’s seat. Front Immunol 2019; 10: 778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Pickles RJ, DeVincenzo JP. Respiratory syncytial virus (RSV) and its propensity for causing bronchiolitis. J Pathol 2015; 235: 266–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bergeron HC, Tripp RA. Immunopathology of RSV: an updated review. Viruses 2021; 13: 2478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Becker Y. Respiratory syncytial virus (RSV) evades the human adaptive immune system by skewing the Th1/Th2 cytokine balance toward increased levels of Th2 cytokines and IgE, markers of allergy – a review. Virus Genes 2006; 33: 235–252. [DOI] [PubMed] [Google Scholar]
- 82. Lo MS, Brazas RM, Holtzman MJ. Respiratory syncytial virus nonstructural proteins NS1 and NS2 mediate inhibition of Stat2 expression and alpha/beta interferon responsiveness. J Virol 2005; 79: 9315–9319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Russell CD, Unger SA, Walton M, et al. The human immune response to respiratory syncytial virus infection. Clin Microbiol Rev 2017; 30: 481–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hashimoto K, Ishibashi K, Ishioka K, et al. RSV replication is attenuated by counteracting expression of the suppressor of cytokine signaling (SOCS) molecules. Virology 2009; 391: 162–170. [DOI] [PubMed] [Google Scholar]
- 85. Harada M, Nakashima K, Hirota T, et al. Functional polymorphism in the suppressor of cytokine signaling 1 gene associated with adult asthma. Am J Respir Cell Mol Biol 2007; 36: 491–496. [DOI] [PubMed] [Google Scholar]
- 86. Schuett J, Kreutz J, Grote K, et al. Suppressor of cytokine signaling 1 is involved in gene regulation which controls the survival of Ly6C(low) monocytes in mice. Cell Physiol Biochem 2019; 52: 336–353. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tai-10.1177_20499361231161157 for Anti-G protein antibodies targeting the RSV G protein CX3C chemokine region improve the interferon response by Harrison C. Bergeron, Lawrence M. Kauvar and Ralph A. Tripp in Therapeutic Advances in Infectious Disease