Abstract
Objective
Continuous physiological measurements during a laboratory-based exercise test can provide physiological biomarkers, such as heart rate (HR) and oxygen uptake (V̇O2) kinetics, that carry clinically relevant information. In contrast, it is not clear how continuous data generated by wearable devices during daily-life routines could provide meaningful biomarkers. We aimed to determine whether valid HR and V̇O2 kinetics can be obtained from measurements with wearable devices during outdoor walks in patients with chronic obstructive pulmonary disease (COPD).
Methods
HR (Polar Belt) and V̇O2(METAMAX3B) were measured during 93 physical activity transitions performed by eight patients with COPD during three different outdoor walks (ntr = 77) and a 6-minute walk test (ntr = 16). HR and V̇O2 kinetics were calculated every time a participant started a walk, finished a walk or walked upstairs. HR and V̇O2 kinetics were considered valid if the response magnitude and model fit were adequate, and model parameters were reliable.
Results
Continuous measurements with wearable devices provided valid HR kinetics when COPD patients started or finished (range 63%–100%) the different outdoor walks and valid V̇O2 kinetics when they finished (range 63%–100%) an outdoor walk. The amount of valid kinetics and kinetic model performance was comparable between outdoor walks and a laboratory-based exercise test (p > .05).
Conclusion
We envision that the presented approach could improve telemonitoring applications of patients with COPD by providing regular, unsupervised assessments of HR kinetics during daily-life routines. This could allow to early identify a decline in the patients’ dynamic physiological functioning, physical fitness and/or health status.
Keywords: Respiratory medicine, wearables personalised medicine, mHealth physiology, remote patient monitoring personalised medicine, exercise lifestyle, telehealth general
Introduction
Chronic obstructive pulmonary disease (COPD) is a highly prevalent non-communicable respiratory disease (around 11% of adults) that is characterised by persistent airflow limitation.1 COPD results in more than 3 million annual deaths (6% of all deaths worldwide), making it the third leading cause of death globally.2 Still, both prevalence and mortality rates are expected to further increase.2,3 The natural course of the disease is characterised by a progressive health decline, interspersed with sudden health deteriorations that worsen prognosis.1 Unfortunately, patients with COPD are only monitored sporadically (e.g., annually repeated spirometry),1 which precludes optimal control over the constant health changes during the progression of their disease.
Wearable devices that can continuously measure physiological variables over prolonged time periods could allow following up with patients with COPD more frequently.4–8 Some wearable devices, such as a chest strap, a wrist-worn device, or a smart shirt can be worn continuously during daily-life routines.9,10 Others, such as a wearable gas exchange analysis system, are impractical for long-term measurements and could only be used for short-term measurements (e.g., unsupervised training sessions).11 Despite the increasing popularity of wearable devices, it is not yet clear how the huge amounts of continuous data they generate can be optimally exploited to provide clinically relevant information.12
Alternatively, it is common and well-accepted to obtain clinical insights from continuous physiological measurements during exercise tests in clinical or laboratory settings.13 In this regard, the kinetics of HR and V̇O2 assessed during a standardised exercise test and extracted from kinetic models are well-established physiological biomarkers that describe the ability of the cardiovascular and respiratory systems to respond, in terms of speed and magnitude, to an abrupt transition in physical load.14,15 HR and V̇O2 kinetics are important indicators of cardiovascular, respiratory, and physical functioning in patients with COPD.15,16
We hypothesised that HR and V̇O2 kinetics can also be obtained outside of clinical or laboratory settings based on continuous physiological measurements with wearable devices during outdoor walks. These kinetics have to be valid (i.e., response magnitude and model fit have to be adequate, and model parameters have to be reliable)15 in order to provide accurate information about the dynamic cardiovascular and respiratory functioning of the considered patient. Consequently, we aimed to determine whether valid HR and V̇O2 kinetics can be obtained from measurements with wearable devices during outdoor walks in patients with COPD. As a secondary objective, we aimed to determine whether the amount of valid kinetics and kinetic model performance (assessed by model fit and standard errors of the parameter estimates) derived from these measurements were comparable between outdoor walks and a conventional, laboratory-based exercise test. We hypothesised that the following physical activity transitions during outdoor walks could induce a change in HR and V̇O2 that could in turn result in valid HR and V̇O2 kinetics: (i) starting a walk (transition from resting to walking), (ii) finishing a walk (transition from walking to resting), and (iii) walking upstairs (transition from walking flat to walking upstairs, representing the transition from a lower to a higher physical activity intensity).17
Methods
Study design and participants
Analyses were based on data from a study that aimed to develop and validate outdoor walking trails11 for subsequent use in a behavioural physical activity intervention (the Urban Training™ clinical trial).18 Ten clinically stable patients with COPD, diagnosed according to the American Thoracic Society and European Respiratory Society criteria,19 were recruited from the outpatient clinics of Hospital del Mar (Barcelona, Spain) and were invited to participate in a 4-day study. Exclusion criteria for participation were musculoskeletal limitation for walking, use of long-term oxygen therapy and intolerance of wearing a face mask.11 The study was approved by the Clinical Research Ethical Committee of Parc de Salut Mar (2011/4291/I), and all research was performed in accordance with relevant guidelines and regulations. Given the non-invasive nature of the tests and the high proportion of illiterate patients in the recruitment setting,20 oral informed consent (after providing the patients with standardised information on the study objectives and procedures) was considered appropriate. All patients provided oral informed consent before the start of the measurements.
Measurements
On the first day of study participation, data were collected from participants on demographics, weight, height and modified Medical Research Council dyspnoea grading scale.21 Exercise capacity was assessed by the 6-minute walk test (6MWT) conforming with the official guidelines, indicating the maximum distance that a patient can walk on a flat, hard surface in a period of 6 minutes.22 Postbronchodilator forced expiratory volume in 1 second and forced vital capacity were previously measured by forced spirometry following standardised procedures,23 and their values were extracted from medical records.
On three separate days, and in random order, the participants walked on three different outdoor trails (green, orange and red trail): the green trail was 1340 m long without any urban elements, the orange trail was 1400 m long with two upward staircases and two downward staircases, and the red trail was 1430 m long with two upward staircases, one upward ramp, one downward staircase and two downward ramps (see Appendix 1). Further details about the trails can be found in Arbillaga-Etxarri et al.11 The participants rested for at least 2 minutes before starting a walk (standing up) and for 5 minutes after finishing a walk (sitting down). Consequently, starting and finishing a walk represented a physical activity transition between resting and being active while walking upstairs represented a transition from a lower to a higher physical activity intensity. Participants were instructed to maintain a constant self-regulated walking speed and were allowed to stop to rest.
Continuous measurements of HR using a chest strap (Polar Belt with Polar RS800CX, Polar Electro, Finland) and V̇O2 using a face mask with a wearable gas exchange analysis system (METAMAX 3B, CORTEX, Germany) were performed during the three outdoor walks, as well as during the 6MWT, with a sampling time of 5 seconds. The Polar Belt and METAMAX 3B have been proven reliable for measuring HR9 and V̇O2,24 respectively. The unprocessed time series of HR and V̇O2 are freely available at https://dataverse.csuc.cat/dataset.xhtml?persistentId=doi:10.34810/data219.
Data preparation
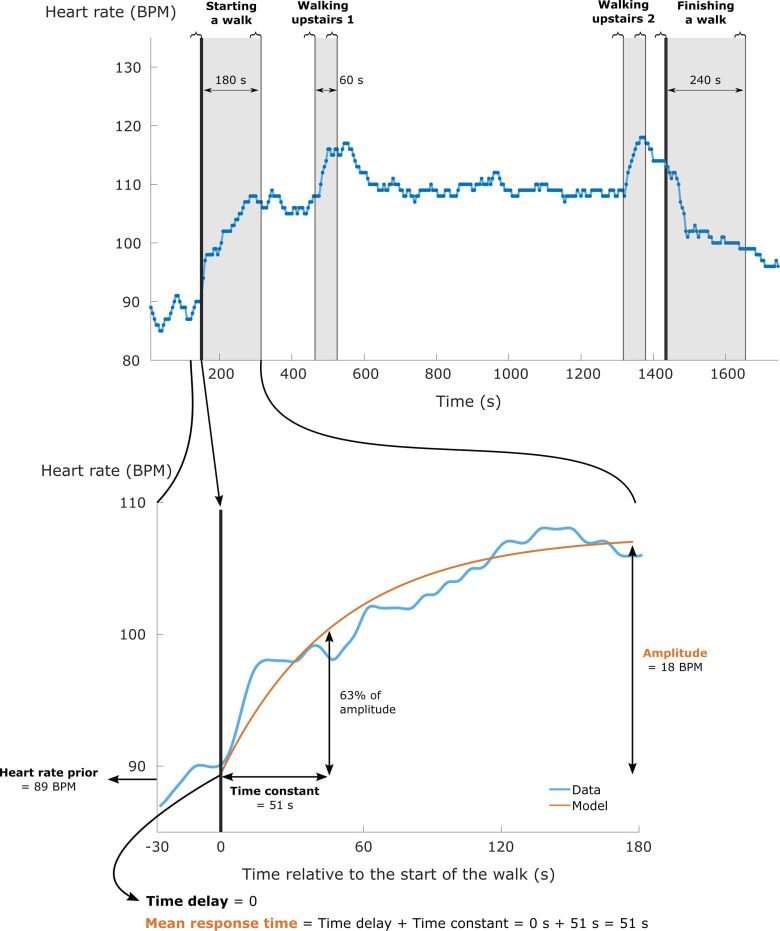
The HR and V̇O2 time series were resampled to a sampling time of 1 second (as commonly used for kinetic modelling of physiological variables)25–28 and smoothed using an integrated random walk smoothing algorithm.28 This smoothing algorithm was selected to preserve the dynamics of the original time series. Deviating values, identified as values deviating more than three standard deviations from the local mean (i.e., 31 seconds centred moving average), were removed. Then following this, HR and V̇O2 data were extracted in fixed time windows following each physical activity transition. The size of these time windows depended on the considered type of transition (i.e., starting a walk/test, finishing a walk/test or walking upstairs; Figure 1) and were independent of the walking speed. For starting a walk/test, a conventional time window of 180 seconds was used.15 For finishing a walk/test, a longer time window of 240 seconds was preferred due to the slow decrease of HR and V̇O2 after finishing a walk/test in these patients.29,30 For walking upstairs, a convenient time window of 60 seconds was selected due to the relatively short duration of walking upstairs (Figure 1). Only the data extracted from the considered time windows were used for kinetic modelling (Figure 1).
Figure 1.
The upper panel visualises the heart rate response of a subject walking on the orange trail. The start and the end of the walk are indicated by the two thick black lines. Light grey zones indicate the considered time windows for every physical activity transition. The curly braces above the figure represent the time periods that were used to calculate the magnitude of the heart rate response related to the different physical activity transitions. The lower panel zooms in on the considered time window when starting a walk to visualise the meaning of heart rate kinetics (e.g., mean response time and amplitude), as extracted from a kinetic model (orange line).
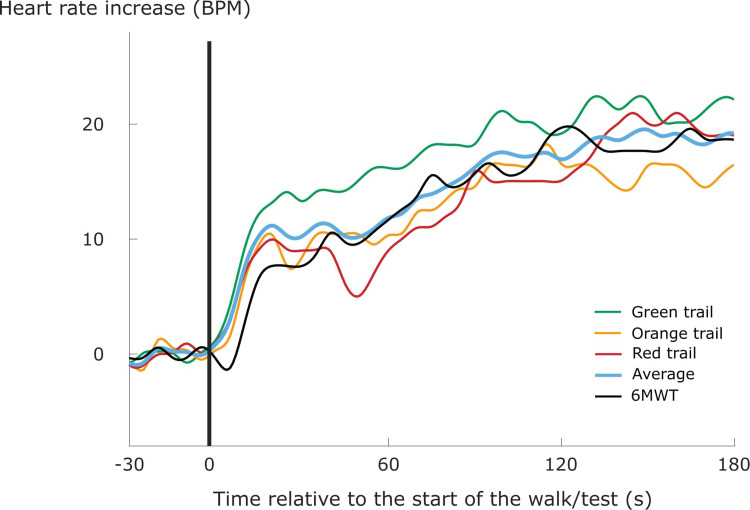
For every patient, we considered the HR and V̇O2 response following each transition during each walk/test separately, as well as an averaged response of all transitions of the same type (i.e., starting a walk, finishing a walk or walking upstairs) during the different outdoor walks (Figure 2). Previous literature suggests that the latter could improve model performance for kinetic modelling by improving signal-to-noise ratios.25,31,32 Specifically, three averaged responses were calculated for every patient: one for starting a walk with a time window of 180 seconds, one for finishing a walk with a time window of 240 seconds, and one for walking upstairs with a time window of 60 seconds. The average response for starting (or finishing) an outdoor walk was generated for every patient by taking the second-by-second mean of the three HR or V̇O2 responses when starting (or finishing) the three different outdoor walks (Figure 2). Similarly, an average response for walking upstairs was generated for every patient by taking the second-by-second mean of the four HR and V̇O2 responses when walking upstairs on the orange (two upward staircases) and red (two upward staircases) trails.
Figure 2.
An average heart rate response for starting an outdoor walk (blue line) was generated by taking the second-by-second mean of the three resampled heart rate responses when starting the different outdoor walks (green, orange and red lines). The black line represents the heart rate response when starting a 6-minute walk test (6MWT). Heart rate values are displayed as the heart rate increase above the heart rate level prior to the start of the walk/test.
Kinetic modelling of HR and V̇O2 responses following a physical activity transition
Following data preparation, every separate and averaged HR (or V̇O2) response when starting a walk/test, finishing a walk/test and walking upstairs was separately described by a mono-exponential model. The following model was used to describe the HR responses during the considered time window of starting a walk/test and walking upstairs (Figure 1, lower panel):
where HRprior is the mean HR value of the last 30 seconds before the considered time window; time constant (TC) represents the time required for HR to reach 63% of the anticipated response amplitude; and time delay (TD) quantifies the potential delay in the onset of this increase (Figure 1, lower panel). Mean response time, which quantifies the overall speed of the HR increase, is the sum of TD and TC.14 The HR responses during the considered time window of finishing a walk/test were described by a similar mono-exponential model:
Identical models were used for describing V̇O2 responses. By convention, V̇O2 data during the first 20 seconds of the considered time window were omitted to exclude the cardio-dynamic phase of the V̇O2 response.28,33,34 Therefore, the TC of V̇O2 represents the time course of the fundamental component of the V̇O2 response, which is considered an estimate of the time course of muscle oxygen uptake.35,36 The model parameters (amplitude, TD, and TC) were estimated using non-linear least squares for both HR and V̇O2. Amplitude and mean response time of HR and V̇O2 are the main indicators of dynamic cardiovascular and respiratory functioning (Figure 1, lower panel).15
Kinetics validity and statistical analyses
Patient characteristics are presented as mean and standard deviation or as absolute values. The validity of the kinetics (primary objective) was separately tested for each type of physical activity transition (i.e., starting a walk, finishing a walk and walking upstairs), each type of walk (i.e., three different outdoor walks and the generated averaged response of the different outdoor walks), and each variable (i.e., HR and V̇O2) in three sequential steps.
First, the magnitude of each HR and V̇O2 response was calculated as the difference between the mean HR or V̇O2 during the last 30 seconds of the corresponding time window, and the mean HR or V̇O2 during the last 30 seconds before the onset of the physical activity transition (see curly braces in Figure 1).15 By analogy with the determination of this threshold in previous studies,15,37 an HR response < 5 beats per minute (i.e., 2.5 times the standard deviation of the natural HR fluctuations during rest, before the start of the 6MWT) and a V̇O2 response < 200 ml min−1 were considered too small.
Second, the model fits were evaluated by the normalised root-mean-squared error (NRMSE) value, calculated as the root mean square of the system model errors (i.e., the difference between the original and the modelled values) divided by the previously calculated response magnitude. A model was considered to have a poor fit if NRMSE ≥ 25%.15
Third, model parameters were considered unreliable if the mean response time ≥ 150 seconds (an indicator of a severely slowed response, which is rather linear in nature)15,28 or if the standard error of the TC estimate, expressed as a percentage of the actual TC value, was > 10%.31,38,39
The required amount of physical activity transitions to assess the proportion of valid HR and V̇O2 kinetics during outdoor walks was estimated as 73, based on a 95% confidence level, a precision of 10% and an expected proportion of valid models of 75%,15 using the GRANMO sample size and power calculator (https://www.imim.es/ofertadeserveis/software-public/granmo/). The available sample size of 77 physical activity transitions during outdoor walks exceeded this requirement.
To compare the validity of the kinetics obtained during the outdoor walks and during the 6MWT (secondary objective), we first determined the validity of the kinetics when starting and finishing a 6MWT following the same three steps as for the outdoor walks described above. Model performance (assessed by NRMSE and standard errors of the TC estimates) from models resulting in valid kinetics (i.e., models with an adequate HR or V̇O2 response magnitude, adequate model fit and reliable model parameters) and walking speeds of all walks/tests were compared between the different types of walks/test (i.e., outdoor walks on the green, orange, and red trail, the generated averaged response of the three outdoor walks, and the 6MWT) using one-way repeated measures ANOVA. If a statistically significant difference was detected, subsequent pair-wise repeated measures t-tests were performed. Statistical significance was accepted at the p < .05 level. All analyses were performed using Matlab 2019b (MathWorks Inc., Natick, Massachusetts, USA).
Results
Patient characteristics and physical activity transitions
Two out of the 10 recruited patients stopped walking before having finished the outdoor walks due to symptoms and were excluded from the present analyses. These two patients had the most severe impairments of exercise capacity (6-minute walking distance of 248 and 345 m) and the highest Modified Medical Research Council grading (grade 3 for both) of all recruited patients. Consequently, eight patients with COPD were included in the analyses (Table 1). Patients were mostly male (n = 7) and classified as moderate (n = 3), severe (n = 3), or very severe (n = 2) COPD based on the Global Initiative for Chronic Obstructive Lung Disease guidelines.1
Table 1.
Clinical characteristics, resting pulmonary function and kinetic parameters when starting/finishing a 6-minute walk test of participating patients with COPD, presented as mean (standard deviation).
| Participants (n = 8) | |
|---|---|
| Clinical characteristics | |
| Male – female (n) | 7 – 1 |
| Age (years) | 64 (7) |
| Weight (kg) | 68 (14) |
| Height (m) | 1.66 (0.10) |
| Body mass index (kg·m−²) | 24.7 (4.3) |
| 6-Minute walking distance (m) | 486 (42) |
| 6-Minute walking distance (% predicted) | 83 (15) |
| Modified Medical Research Council grading: 1 – 2 – 3 (n) | 5 – 2 – 1 |
| Resting pulmonary function | |
| Forced expiratory volume in 1 second (FEV1; %predicted) | 44 (18) |
| Forced vital capacity (FVC; %predicted) | 68 (10) |
| FEV1/FVC (%) | 47 (15) |
| Kinetic parameters when starting a 6-minute walk test | |
| Heart rate amplitude (beats per minute) | 28 (11) |
| Heart rate mean response time (second) | 58 (41) |
| Oxygen uptake amplitude (ml min−1) | 834 (261) |
| Oxygen uptake mean response time (second) | 57 (14) |
| Kinetic parameters when finishing a 6-minute walk test | |
| Heart rate amplitude (beats per minute) | 32 (14) |
| Heart rate mean response time (second) | 99 (22) |
| Oxygen uptake amplitude (ml min−1) | 1127 (175) |
| Oxygen uptake mean response time (second) | 86 (32) |
All included patients performed the 6MWT and outdoor walks on the green, orange and red trails. The HR and V̇O2 responses could not be analysed for three upward staircases during the outdoor walks due to missing time indications, which precluded identifying the start of walking upstairs (number of transitions, ntr = 2), or because the participants took a rest right before starting to walk upstairs, which resulted in a physical activity transition from resting (instead of from walking flat) to walking upstairs (ntr = 1). As a result, 77 physical activity transitions during outdoor walks could be analysed: eight patients started three outdoor walks (ntr = 8 × 3 = 24), finished three outdoor walks (ntr = 8 × 3 = 24) and walked upstairs four times while the three previously described upward staircases were excluded (ntr = 8 × 4 − 3 = 29).
Kinetics validity and model performance
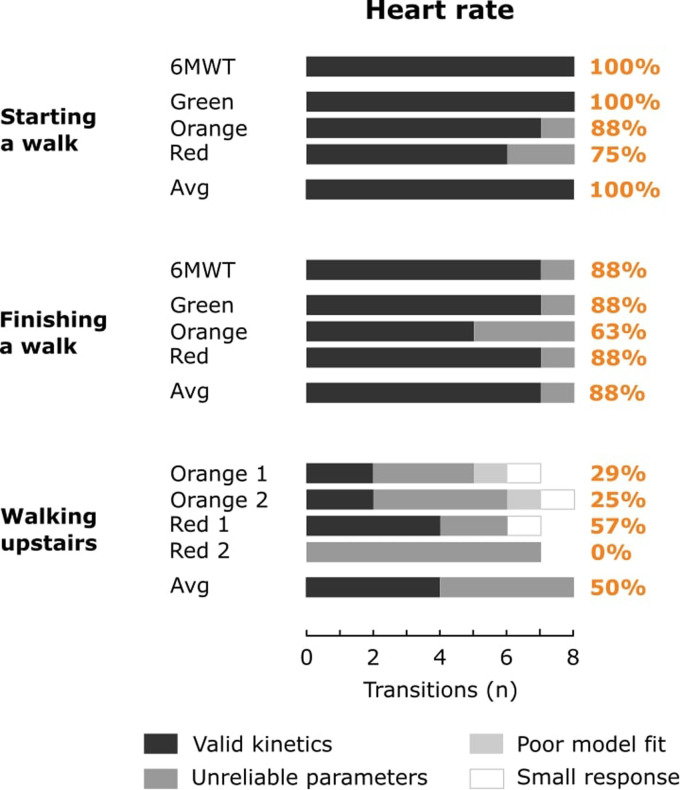
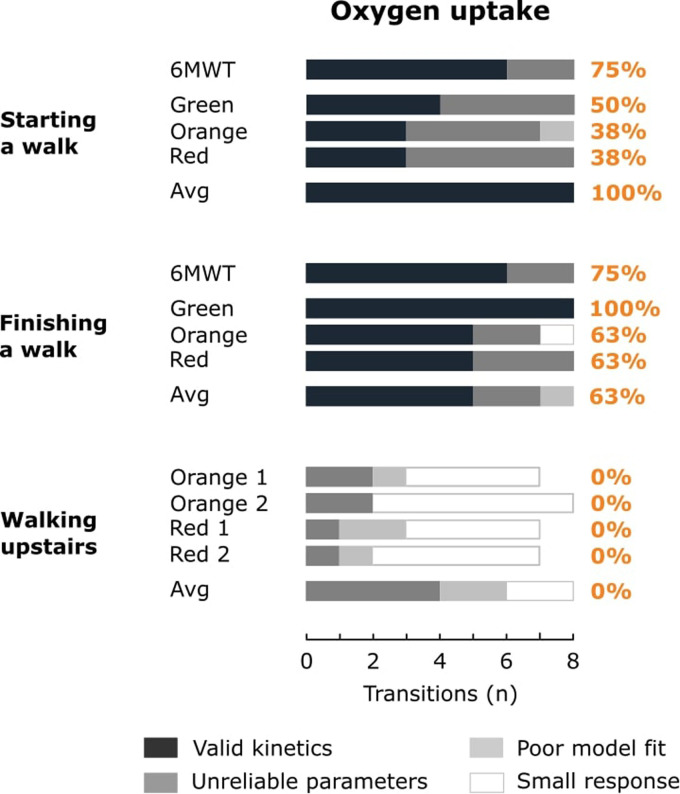
The absolute and relative amount of physical activity transitions resulting in a small HR or V̇O2 response, a poor model fit, unreliable model parameters or valid kinetics is shown in Figures 3 and 4 for HR and V̇O2, respectively. Overall, 62% and 36% of the 77 physical activity transitions during outdoor walks resulted in valid HR and V̇O2 kinetics, respectively. 79% and 54% of the 24 physical activity transitions within the averaged responses resulted in valid HR and V̇O2 kinetics, respectively.
Figure 3.
The bars represent the amount of physical activity transitions resulting in a small heart rate response, a poor model fit, unreliable model parameters or valid kinetics for the 6-minute walk test (6MWT), outdoor walks on the green, orange and red trail and the averaged response (Avg). Percentages indicate the relative amount of valid kinetics for the 6MWT, outdoor walks (green, orange and red) and the averaged response.
Figure 4.
The bars represent the amount of physical activity transitions resulting in a small oxygen uptake response, a poor model fit, unreliable model parameters or valid kinetics for the 6-minute walk test (6MWT), outdoor walks on the green, orange and red trail and the averaged response (Avg). Percentages indicate the relative amount of valid kinetics for the 6MWT, outdoor walks (green, orange and red) and the averaged response.
Most HR kinetics were valid when starting (range 75% to 100% for separate walks; 100% for the averaged response) or finishing (range 63% to 88% for separate walks; 88% for the averaged response) an outdoor walk (Figure 3). In contrast, HR responses < 5 beats per minute (ntr = 3), poor model fits (ntr = 2) or unreliable model parameters (ntr = 19) were observed for walking upstairs, resulting in only a limited valid HR kinetics for the separate outdoor walks (range 0% to 57%) and the averaged response (50%).
V̇O2 kinetics were often valid when finishing an outdoor walk (range 63% to 100% for separate walks; 63% for the averaged response), but not when starting an outdoor walk (range 38% to 50% for separate walks; 100% for the averaged response; Figure 4). V̇O2 kinetics were never valid for walking upstairs, due to V̇O2 responses < 200 ml min−1 (ntr = 21), poor model fits (ntr = 6) or unreliable model parameters (ntr = 10). The low amount of valid kinetics for HR and V̇O2 when walking upstairs, and for V̇O2 when starting an outdoor walk, impeded the comparison of NRMSE values and standard errors of TC estimates between the different types of walks/test for these physical activity transitions (Table 2).
Table 2.
General characteristics, NRMSE values and standard errors of TC estimates for the different walks, presented as mean (standard deviation).
| 6-Minute walk test | Green trail | Orange trail | Red trail | Averaged response | |
|---|---|---|---|---|---|
| General characteristics | |||||
| Distance (m) | n. a. | 1340 | 1400 | 1430 | n. a. |
| Urban elements | 0 | 0 | 2× stairs up 2× stairs down |
2× stairs up 1× ramp up 1x stairs down 2× ramp down |
n. a. |
| Walking speed (km/h) | 4.5 (0.9)G,O,R | 4.5 (0.5)S,O,R | 4.2 (0.7)S,G | 4.1 (0.6)S,G | n. a. |
| Walking speed excluding periods of rest (km/h) | 4.6 (0.7)G,O,R | 4.5 (0.5)S,O,R | 4.2 (0.6)S,G | 4.2 (0.6)S,G | n. a. |
| Starting a walk/test | |||||
| HR – NRMSE (%) | 6.3 (2.1) | 8.0 (2.5) | 7.9 (1.3) | 8.2 (3.0) | 7.2 (1.6) |
| HR – standard error of TC estimates (% of TC value) | 4.9 (1.7) | 5.9 (1.1) | 6.0 (2.0) | 6.7 (1.6) | 5.7 (1.3) |
| V̇O2 – NRMSE (%) | 8.7 (3.3) | / | / | / | 7.6 (3.6) |
| V̇O2 – standard error of TC estimates (% of TC value) | 6.9 (2.4) | / | / | / | 6.7 (1.7) |
| Finishing a walk/test | |||||
| HR – NRMSE (%) | 5.8 (0.8) | 6.6 (2.5) | 8.5 (4.0) | 7.2 (3.0) | 5.5 (1.6) |
| HR – standard error of TC estimates (% of TC value) | 4.2 (0.7) | 4.9 (1.3) | 6.5 (3.1)R,A | 3.9 (1.2)O | 3.9 (1.1)O |
| V̇O2 – NRMSE (%) | 7.2 (3.2) | 7.0 (1.7) | 5.0 (1.9) | 6.7 (2.5) | 4.2 (1.4) |
| V̇O2 – standard error of TC estimates (% of TC value) | 4.8 (1.8) | 5.4 (2.2) | 3.4 (0.9) | 4.5 (1.6) | 2.8 (0.6) |
Only models resulting in valid kinetics were considered for the description of NRMSE values and standard errors of TC estimates. No values were shown for heart rate (HR) and oxygen uptake (V̇O2) when walking upstairs, or for V̇O2 when starting an outdoor walk, due to the low amount of valid kinetics. Superscripts S, G, O, R and A indicate statistically significant differences in values compared to values from the 6-minute walk test, outdoor walks on the green, orange and red trails, and averaged response, respectively (repeated measures t-test, p < .05). NRMSE: normalised root-mean-squared error; HR: heart rate; V̇O2: oxygen uptake; TC: time constant.
The relative amount of valid kinetics during outdoor walks was comparable to starting (HR: 100%; V̇O2: 75%) or finishing (HR: 88%; V̇O2: 75%) a conventional, laboratory-based 6MWT (Figures 3 and 4). No significant differences in NRMSE values or standard errors of TC estimates were found between the outdoor walks and the 6MWT (Table 2).
Discussion
The present study shows that: (1) valid HR kinetics in patients with COPD can be obtained from measurements with wearable devices when starting or finishing an outdoor walk, but less likely when transitioning from walking flat to walking upstairs; (2) measurements of HR resulted in more valid kinetics than measurements of V̇O2, as valid V̇O2 kinetics could only be obtained when finishing an outdoor walk; and (3) the amount of valid kinetics and kinetic model performance when starting/finishing an outdoor walk was comparable to starting/finishing a conventional, laboratory-based exercise test.
This is the first study to demonstrate that valid HR kinetics can be obtained from continuous measurements with wearable devices when performing physical activity transitions during outdoor walks. Joosen et al. calculated comparable biomarkers (i.e., model gain and TC of a first-order transfer function model with physical activity as the input variable and HR as the output variable) from measurements of HR and physical activity during daily-life routines of older adults in a semi-supervised home care setting, which were used to monitor the evolution of their physical fitness over a 9-week period.40 However, this study did not consider the validity of the calculated biomarkers. Similarly, previous laboratory-based studies seldom reported on the validity of the calculated kinetics in patients with COPD.15 Yet, the reduced physical capacity of these patients results in lower physiological response amplitudes and thus reduced signal-to-noise ratios for kinetic modelling,28,41 which emphasises the importance of evaluating the validity of kinetics in this population. This was illustrated by a study from 2020 showing that it might not be feasible to obtain valid kinetics for the most physically impaired patients with COPD, even when kinetics were obtained from a laboratory-based cycling test.15 The present study corroborates this notion, as the two patients with the lowest 6-minute walking distance of all recruited patients had to be excluded for kinetic modelling due to their inability to complete the outdoor walks. For the remaining patients, not every laboratory-based 6-minute walk test provided valid kinetics either.
Transitions from walking flat to walking upstairs did not result in valid kinetics, probably due to the relatively short duration of walking upstairs. To the best of our knowledge, it has not yet been empirically tested whether time windows shorter than 180 seconds could provide valid V̇O2 and HR kinetics. Considering that a mono-exponential curve has identical properties throughout the whole curve (i.e., a constant change rate, represented by the TC, and gain), it might be possible to obtain valid kinetic models using less than 180 seconds of data (i.e., before V̇O2 and HR reach steady state). Nevertheless, the results of the presented study corroborate the general notion that a 60 seconds time window is too short for mono-exponential modelling of V̇O2 and HR responses. More complex methodologies, such as transfer function models,28,40 frequency domain analysis,42 or a combination of machine learning and frequency domain analysis,43 might be required to quantify the dynamic HR and V̇O2 responses after physical activity transitions of very short duration, such as walking upstairs.
Measurements of HR resulted in more valid kinetics than measurements of V̇O2, as valid V̇O2 kinetics could only be obtained when finishing, but not when starting, an outdoor walk. This is most likely due to the lower relative magnitude of fluctuations (i.e., ‘noise’) in HR compared to fluctuations in V̇O2, resulting in higher signal-to-noise ratios for HR.25,31 From a practical perspective, HR measurements with a chest strap are also more convenient and less costly than measurements of V̇O2 that require the use of a face mask. Moreover, reliable HR measurements from wrist-worn devices could most likely replace HR measurements from a chest strap,9 allowing to obtain HR kinetics during outdoor walks in a very user-friendly manner. Still, V̇O2 measurements using a face mask with a wearable gas exchange analysis system could be used more sporadically, or as an alternative approach when a fully equipped laboratory setting is not available. Nevertheless, all results combined indicate that the proposed methodology is particularly interesting for obtaining HR kinetics when patients with COPD start or finish an outdoor walk.
Until now, HR and V̇O2 kinetics have mainly been assessed during laboratory-based exercise tests, such as standardised cycling or walking tests.14,15,44 The present study demonstrated that the amount of valid kinetics and kinetic model performance when starting/finishing an outdoor walk was comparable to starting/finishing a conventional, laboratory-based exercise test (i.e., the 6MWT). As expected,25,31,32 there were indications that an averaged response of multiple physical activity transitions could result in a higher amount of valid kinetics than modelling the HR and V̇O2 response during a single exercise test (especially for V̇O2 when starting an outdoor walk). However, it is not clear yet if the generation of an averaged response mainly improves kinetic model performance, or if it rather leads to a loss of information by decreasing the frequency with which these biomarkers can be assessed.
Wearable devices are already extensively used by the general population and will provide unique opportunities for a more personalised approach in respiratory medicine.7 Wearable devices are currently often used to measure physical activity (e.g., steps per day, time spent in moderate-to-vigorous physical activity),45 and they have been incorporated in motivational interventions to increase physical activity levels of patients with COPD.18 Although wearable devices can continuously measure many other variables besides physical activity (such as HR and V̇O2), it is not yet clear how these huge amounts of time series data can be interpreted or exploited for clinical decision-making.12 We envision that the approach presented in the current study could be used to leverage the high-resolution time series data obtained from wearable devices for improving future telemonitoring applications for patients with COPD. Regular, unsupervised assessments of HR kinetics obtained from wearable devices during daily-life routines of patients with COPD could be incorporated into telemonitoring systems to closely monitor the progression of their dynamic cardiovascular functioning over time, on top of the conventional monitoring of static physiological resting values. Regular assessments of these well-established biomarkers could then allow us to early identify a decline in dynamic physiological functioning, induced by acute (e.g., exacerbation onset) or chronic (e.g., physical deconditioning) stressors, which could in turn be indicative of a general decline in physical fitness or health status.40,46 It is important to highlight that the envisioned purpose of the presented approach (and telemonitoring in general) is not to replace controlled, laboratory-based testing, but to complement it with additional, more frequent assessments of these well-established physiological biomarkers during the daily-life routines of patients with COPD. A benefit of focussing on outdoor walks, as compared to indoor daily-life activities, is that these walks include physical activity transitions of a sufficiently long duration to allow for developing valid mono-exponential kinetic models. Performing these outdoor walks in a group could facilitate the adoption of the telemonitoring system.47 Repeatedly walking on the same outdoor trail could furthermore reduce any location-dependent bias in the calculated biomarkers. Calculations of non-model-based physiological biomarkers (e.g., HR recovery)30,48 and non-physiological prognostic markers (e.g., self-selected walking speed)49 from wearable device data could additionally be incorporated into these systems to enable regular, comprehensive assessments of different health aspects.50 This way, the huge amounts of data generated by wearable devices could be translated into a variety of clinically meaningful biomarkers. We envision that incorporating regular assessments of these biomarkers into telemonitoring applications could enable close monitoring of different aspects of the disease progression in patients with COPD. This approach could be especially valuable in locations with limited access to high technological medical care. It could furthermore be assumed that the presented approach will have wider applicability besides patients with COPD.
The presented study has some limitations. First, the small sample size may have limited statistical comparisons between the different types of walks/tests related to the secondary objective. Hence, the small sample size might have contributed to the absence of statistically significant differences in the amount of valid kinetics and kinetic model performance between the outdoor walks and the 6MWT. Furthermore, our results should be interpreted with caution for female patients with COPD, as most participants were male. Second, the calculation of HR and V̇O2 kinetics when starting a walk requires a constant walking speed during the first 3 minutes of the walk. Although participants in the current study were asked to maintain a constant walking speed, momentaneous walking speed was not quantitatively assessed. Furthermore, there were statistically significant differences in average walking speed between the different types of walks/test, but there were no indications that this affected model performance. Third, the duration of walking upstairs was relatively short and variable. Hence, the presented results do not preclude the possibility that valid kinetics could still be obtained from walking upstairs on alternative stair designs, such as multiple or longer flights of stairs. Fourth, the comparison between the outdoor walks and the laboratory-based exercise test focussed on comparing the validity of the kinetics, and not the kinetic parameter values (e.g., mean response time values), because these values can vary between days51 and data were collected on separate days.
Strengths of this study include the novelty of the approach (deploying techniques from the field of exercise physiology to improve telemonitoring applications) and the in-depth analyses of kinetics validity. The inclusion of patients with COPD in different stages of the disease allowed testing the presented approach in a broad range of disease severities. Moreover, the currently used dataset was collected during a study that did not intend to examine HR and V̇O2 kinetics,11 which suggests that the proposed methodology can easily be adopted in other studies that are based on different types of wearable devices, walking trails or patient populations (implying external validity).
Conclusions
This study indicates that continuous measurements with wearable devices can provide valid HR kinetics when starting or finishing an outdoor walk, and valid V̇O2 kinetics when finishing an outdoor walk, in patients with COPD. The amount of valid kinetics and kinetic model performance was comparable between outdoor walks and a conventional, laboratory-based 6MWT. We envision that the presented approach could improve future telemonitoring applications of patients with COPD by providing regular, unsupervised assessments of HR kinetics during daily-life routines. This could allow us to early identify a decline in the patients’ dynamic physiological functioning, induced by acute or chronic stressors, which could in turn be indicative of a general decline in physical fitness or health status.
Acknowledgements
The authors would like to thank the patients who participated in the study for their time and commitment.
Appendix 1 Green, orange and red urban trails. Walking happened on sidewalks, so patients were not required to walk on sand.
Green, orangeand red urban trails. Walking happened on sidewalks, so patients were not required to walk on sand.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: Joren Buekers acknowledges the support of the European Respiratory Society – ERS Long-Term Research Fellowship 2020. ISGlobal acknowledges support from the Spanish Ministry of Science and Innovation through the ‘Centro de Excelencia Severo Ochoa 2019–2023’ Program (CEX2018-000806-S) and support from the Generalitat de Catalunya through the CERCA Program.
Ethical approval: The study was approved by the Clinical Research Ethical Committee of Parc de Salut Mar (2011/4291/I).
Guarantor: JB.
Contributorship: Ane Arbillaga-Etxarri, Judith Garcia-Aymerich, Elena Gimeno-Santos and David Donaire-Gonzalez were involved in the conception, design and data collection of the original study. Joren Buekers performed the main data analyses and wrote the first draft of this manuscript. All authors were involved in the refinement of data analyses, interpretation of the results and editing of the manuscript. All authors read and approved the final manuscript.
ORCID iDs: Joren Buekers https://orcid.org/0000-0003-4866-5741
David Donaire-Gonzalez https://orcid.org/0000-0003-2337-1712
References
- 1.Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management, and prevention of Chronic Obstructive Pulmonary Disease - 2021 Report. 2021; 1–164.
- 2.World Health Organization. The top 10 causes of death, https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed 27 October 2021).
- 3.Adeloye D, Chua S, Lee C, et al. Global and regional estimates of COPD prevalence: systematic review and meta-analysis. J Glob Health 2015; 5: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buekers J, Theunis J, De Boever P, et al. Wearable finger pulse oximetry for continuous oxygen saturation measurements during daily home routines of patients with chronic obstructive pulmonary disease (COPD) over one week: observational study. JMIR mHealth uHealth 2019; 7: e12866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buekers J, De Boever P, Vaes AW, et al. Oxygen saturation measurements in telemonitoring of patients with COPD: a systematic review. Expert Rev Respir Med 2018; 12: 113–123. [DOI] [PubMed] [Google Scholar]
- 6.Al Rajeh AM, Aldabayan YS, Aldhahir A, et al. Once daily versus overnight and symptom versus physiological monitoring to detect exacerbations of chronic obstructive pulmonary disease: pilot randomized controlled trial. JMIR mHealth uHealth 2020; 8: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aliverti A. Wearable technology: role in respiratory health and disease. Breathe 2017; 13: e27–e36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santos CD, Santos AF, das Neves RC, et al. Telemonitoring of daily activities compared to the six-minute walk test further completes the puzzle of oximetry-guided interventions. Sci Rep 2021; 11: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuller D, Colwell E, Low J, et al. Reliability and validity of commercially available wearable devices for measuring steps, energy expenditure, and heart rate: systematic review. JMIR mHealth uHealth 2020; 8: 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brickwood KJ, Williams AD, Watson G, et al. Older adults’ experiences of using a wearable activity tracker with health professional feedback over a 12-month randomised controlled trial. Digit Heal 2020; 6: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arbillaga-Etxarri A, Torrent-Pallicer J, Gimeno-Santos E, et al. Validation of walking trails for the Urban TrainingTM of chronic obstructive pulmonary disease patients. PLoS One 2016; 11: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terry NP. Mobile health: assessing the barriers. Chest 2015; 147: 1429–1434. [DOI] [PubMed] [Google Scholar]
- 13.Palange P, Laveneziana P, Neder JA, et al. Clinical exercise testing (European Respiratory Society Monograph). Sheffield: European Respiratory Society, 2018. [Google Scholar]
- 14.Poole DC, Jones AM. Oxygen uptake kinetics. Compr Physiol 2012; 2: 933–996. [DOI] [PubMed] [Google Scholar]
- 15.Buekers J, Aerts J-M, Theunis J, et al. Kinetic analyses as a tool to examine physiological exercise responses in a large sample of patients with COPD. J Appl Physiol 2020; 128: 813–821. [DOI] [PubMed] [Google Scholar]
- 16.Casaburi R, Porszasz J, Burns MR, et al. Physiologic benefits of exercise training in rehabilitation of patients with severe chronic obstructive pulmonary disease. Am J Respir Crit Care Med 1997; 155: 1541–1551. [DOI] [PubMed] [Google Scholar]
- 17.Ainsworth BE, Haskell WL, Herrmann SD, et al. Compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc 2011; 43: 1575–1581. [DOI] [PubMed] [Google Scholar]
- 18.Arbillaga-Etxarri A, Gimeno-Santos E, Barberan-Garcia A, et al. Long-term efficacy and effectiveness of a behavioural and community-based exercise intervention (Urban Training) to increase physical activity in patients with COPD: a randomised controlled trial. Eur Respir J 2018; 52: 1800063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med 2011; 155: 179–191. [DOI] [PubMed] [Google Scholar]
- 20.Arbillaga-Etxarri A, Gimeno-Santos E, Barberan-Garcia A, et al. Socio-environmental correlates of physical activity in patients with chronic obstructive pulmonary disease (COPD). Thorax 2017; 72: 796–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fletcher C. Standardised questionnaire on respiratory symptoms: a statement prepared and approved by the MRC Committee on the Aetiology of Chronic Bronchitis (MRC breathlessness score). Br Med J 1960; 2: 1665.13688719 [Google Scholar]
- 22.American Thoracic Society. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166: 111–117. [DOI] [PubMed] [Google Scholar]
- 23.Miller MR, Hankinson J, Brusasco V, et al. Standardisation of spirometry. Eur Respir J 2005; 26: 319–338. [DOI] [PubMed] [Google Scholar]
- 24.Macfarlane DJ, Wong P. Validity, reliability and stability of the portable Cortex Metamax 3B gas analysis system. Eur J Appl Physiol 2012; 112: 2539–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benson AP, Bowen TS, Ferguson C, et al. Data collection, handling, and fitting strategies to optimize accuracy and precision of oxygen uptake kinetics estimation from breath-by-breath measurements. J Appl Physiol 2017; 123: 227–242. [DOI] [PubMed] [Google Scholar]
- 26.Berton DC, Barbosa PB, Takara LS, et al. Bronchodilators accelerate the dynamics of muscle O2 delivery and utilisation during exercise in COPD. Thorax 2010; 65: 588–593. [DOI] [PubMed] [Google Scholar]
- 27.Faisal A, Zoumot Z, Shah PL, et al. Effective bronchoscopic lung volume reduction accelerates exercise oxygen uptake kinetics in emphysema. Chest 2016; 149: 435–446. [DOI] [PubMed] [Google Scholar]
- 28.Buekers J, Theunis J, Peña Fernández A, et al. Box-Jenkins transfer function modelling for reliable determination of VO2 kinetics in patients with COPD. Appl Sci 2019; 9: 1822. [Google Scholar]
- 29.Baty F, Ritz C, Van Gestel A, et al. Modeling the oxygen uptake kinetics during exercise testing of patients with chronic obstructive pulmonary diseases using nonlinear mixed models. BMC Med Res Methodol 2016; 16: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Delgado-Ortiz L, Arbillaga-Etxarri A, Rodríguez-Chiaradía DA, et al. Physical activity and cardiac autonomic dysfunction in patients with chronic obstructive pulmonary disease: a cross-sectional analysis. Ann Phys Rehabil Med 2021; 65: 101501. [DOI] [PubMed] [Google Scholar]
- 31.Lamarra N, Whipp BJ, Ward SA, et al. Effect of interbreath fluctuations on characterizing exercise gas exchange kinetics. J Appl Physiol 1987; 62: 2003–2012. [DOI] [PubMed] [Google Scholar]
- 32.Markovitz GH, Sayre JW, Storer TW, et al. On issues of confidence in determining the time constant for oxygen uptake kinetics. Br J Sports Med 2004; 38: 553–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nery LE, Wasserman K, Andrews JD, et al. Ventilatory and gas exchange kinetics during exercise in chronic airways obstruction. J Appl Physiol 1982; 53: 1594–1602. [DOI] [PubMed] [Google Scholar]
- 34.Siqueira ACB, Borghi-Silva A, Bravo DM, et al. Effects of hyperoxia on the dynamics of skeletal muscle oxygenation at the onset of heavy-intensity exercise in patients with COPD. Respir Physiol Neurobiol 2010; 172: 8–14. [DOI] [PubMed] [Google Scholar]
- 35.Rossiter HB, Ward SA, Doyle VL, et al. Inferences from pulmonary O2 uptake with respect to intramuscular [phosphocreatine] kinetics during moderate exercise in humans. J Physiol 1999; 518: 921–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barstow TJ, Mole PA. Simulation of pulmonary O2 uptake during exercise transients in humans. J Appl Physiol 1987; 63: 2253–2261. [DOI] [PubMed] [Google Scholar]
- 37.Buekers J, De Boever P, Theunis J, et al. Physiological changes differ between responders and nonresponders to pulmonary rehabilitation in COPD. Med Sci Sport Exerc 2021; 53: 1125–1133. [DOI] [PubMed] [Google Scholar]
- 38.Spencer MD, Murias JM, Lamb HP, et al. Are the parameters of VO2, heart rate and muscle deoxygenation kinetics affected by serial moderate-intensity exercise transitions in a single day? Eur J Appl Physiol 2011; 111: 591–600. [DOI] [PubMed] [Google Scholar]
- 39.Keir DA, Murias JM, Paterson DH, et al. Breath-by-breath pulmonary O2 uptake kinetics: effect of data processing on confidence in estimating model parameters. Exp Physiol 2014; 99: 1511–1522. [DOI] [PubMed] [Google Scholar]
- 40.Joosen P, Piette D, Buekers J, et al. A smartphone-based solution to monitor daily physical activity in a care home. J Telemed Telecare 2018; 25: 611–622. [DOI] [PubMed] [Google Scholar]
- 41.Casaburi R, Barstow TJ, Robinson T, et al. Influence of work rate on ventilatory and gas exchange kinetics. J Appl Physiol 1989; 67: 547–555. [DOI] [PubMed] [Google Scholar]
- 42.Hughson RL, Winter DA, Patla AE, et al. Investigation of V̇O2 kinetics in humans with pseudorandom binary sequence work rate change. J Appl Physiol 1990; 68: 796–801. [DOI] [PubMed] [Google Scholar]
- 43.Beltrame T, Amelard R, Wong A, et al. Extracting aerobic system dynamics during unsupervised activities of daily living using wearable sensor machine learning models. J Appl Physiol 2018; 124: 473–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baty F, van Gestel AJ, Kern L, et al. Oxygen uptake recovery kinetics after the 6-minute walk test in patients with chronic obstructive pulmonary disease. Respiration 2016; 92: 371–379. [DOI] [PubMed] [Google Scholar]
- 45.Demeyer H, Mohan D, Burtin C, et al. Chronic obstructive pulmonary diseases: objectively measured physical activity in patients with COPD: recommendations from an international task force on physical activity. J COPD Found 2021; 8: 528–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schiepek G, Strunk G. The identification of critical fluctuations and phase transitions in short term and coarse-grained time series-a method for the real-time monitoring of human change processes. Biol Cybern 2010; 102: 197–207. [DOI] [PubMed] [Google Scholar]
- 47.Slevin P, Kessie T, Cullen J, et al. A qualitative study of chronic obstructive pulmonary disease patient perceptions of the barriers and facilitators to adopting digital health technology. Digit Heal 2019; 5: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lacasse M, Maltais F, Poirier P, et al. Post-exercise heart rate recovery and mortality in chronic obstructive pulmonary disease. Respir Med 2005; 99: 877–886. [DOI] [PubMed] [Google Scholar]
- 49.Fritz S, Lusardi M. White paper: walking speed: the sixth vital sign. J Geriatr Phys Ther Epub ahead of print 2009; 32: 46–49. 10.1519/00139143-200932020-00002 [DOI] [PubMed] [Google Scholar]
- 50.Angelucci A, Aliverti A. Telemonitoring systems for respiratory patients: technological aspects. Pulmonology 2020; 26: 221–232. [DOI] [PubMed] [Google Scholar]
- 51.Kemps HMC, De Vries WR, Hoogeveen AR, et al. Reproducibility of onset and recovery oxygen uptake kinetics in moderately impaired patients with chronic heart failure. Eur J Appl Physiol 2007; 100: 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]