Abstract
Myocarditis and pericarditis have been reported after COVID‐19 vaccine administration in children and adolescents, raising the concern about their possible association with these vaccines. The objective was to explore the incidence, clinical presentation, and association of myocarditis and pericarditis with COVID‐19 vaccines in children and adolescents. We conducted a systematic literature search on three databases, that is, Cochrane, MEDLINE/PubMed, and EMBASE from inception till March 2022. A total of three case reports, four case series, and six observational studies were included in the review. For case reports and case series, the mean age of the patients was 17.4 years, with 96.9% being male. Chest pain (n = 31, 93.9%), fever (n = 18, 54.5%), myalgias (n = 15, 45.4%) and headache (n = 9, 27.2%) were the most common presentations. Out of 33 patients, 32 (96.9%) of patients received Pfizer‐BioNTech whereas only one (3.03%) received Moderna (mRNA 1273). Clinical investigations revealed ST elevation (n = 32, 97%), and elevated CRP (n = 9, 27.2%) and cardiac troponin (n = 29, 87.8%). The pooled incidence of myocarditis and pericarditis from observational studies was (0.00063%) and (0.000074%) %, respectively. Myocarditis and pericarditis in children and adolescents after the COVID‐19 vaccines were more prevalent among males and more commonly observed after the second dose of Pfizer. Though the overall incidence was low, however, the clinicians should consider myocarditis and pericarditis as probable diagnosis when encountering young patients, with a history of vaccine administration, presenting with suggestive findings.
Keywords: adolescents, children, COVID‐19 vaccination, myocarditis, pericarditis
1. INTRODUCTION
COVID‐19 was initially reported in December 2019 in Wuhan, China, and over 317 million global cases of COVID‐19 have been reported ever since. 1 To deal with this public health emergency of international concern (PHEIC), numerous expedited vaccination trials were conducted, which eventually led to the successful development of antiviral vaccines. The different types of coronavirus vaccines include mRNA‐based vaccines (i.e., Pfizer‐BioNTech, Moderna, Comirnaty), recombinant adenoviral vector vaccines (i.e., Johnson & Johnson/Janssen, Oxford‐AstraZeneca, and Sputnik V), and the inactivated whole viral vaccines (i.e., Sinovac Biotech and Sinopharm). 2 Mass immunization campaigns are currently underway across the globe, and our understanding of the virus, as well as the vaccine, is improving. More than 10 million doses of vaccines have been administered across the world and published literature has reported a significant reduction in severity of COVID‐19 infection, hospitalization, and mortality rates. 3 Postmarketing surveillance (Phase 4 trials) is an integral way to understand the various side effects of different types of vaccines, especially in case of expedited approvals. The results of Phases 2 and 3 trials have revealed various adverse effects of COVID‐19 vaccines which range from mild fever, fatigue, headache, muscle pain, and diarrhea to serious adverse effects such as myocarditis, pericarditis, thrombocytopenia, lymphadenopathy, bell's palsy, and cerebrovascular accident. 4 Among these adverse effects, several cases of myocarditis and pericarditis following COVID‐19 vaccine administration have also been reported around the world. 5
As of April 21, many cases of myocarditis and pericarditis in children and adolescents have been reported after administration of mRNA COVID‐19 vaccine, however, most of the cases were mild and self‐resolving with rare instances of hospital admission. 6 Myocarditis and pericarditis refers to the inflammation of myocardium and pericardium of heart respectively and commonly occurs as a consequence of viral infection. 7 , 8 , 9 It is usually a self‐limited condition that responds to conservative management without any long‐term sequelae but complications such as cardiomyopathy and heart failure have been reported. 10
The incidence of myocarditis in children is usually very low; accounting for 0.7% as reported in a large retrospective study. 11 Whereas, the incidence of myocarditis in adults is 1.5 million cases worldwide per year. 12 Viral infection has been reported to be the most common cause of myocarditis in children. 13 , 14 In the past, myocarditis has been reported as a side effect of live attenuated vaccines such as smallpox and influenza vaccines in children and adolescents. 15 The reporting of COVID‐19 vaccine‐related myocarditis and pericarditis cases are being investigated by safety agencies including the Centre of Disease Control and Prevention (CDC) in the United States and Pharmacovigilance Risk Assessment Committee (PRAC) in Europe. 16 Several published age, gender, and vaccine type‐based analyses have reported an increased risk among young males following mRNA vaccines such as Pfizer‐BioNTech and Moderna. 17 So far, it is still unclear if these results really reflect an increase in incidence or simply better reporting and recollection bias. The aim of this systematic review is to explore the incidence, clinical presentation, management, and association of myocarditis and pericarditis with the COVID‐19 vaccines in children and adolescents. To the best of our knowledge, this is the first systematic review on this topic with the aim of providing a comprehensive outline of available evidence regarding COVID‐19 vaccine‐associated myocarditis and pericarditis.
2. METHODS
The review has been registered on The International Prospective Register of Systematic Reviews (PROSPERO CRD 42021282961). The study was performed according to the Preferred Reporting Items for Systematic Reviews (PRISMA) guidelines. 18 A systematic literature search was conducted on the following three databases: Pubmed/MEDLINE, Cochrane, and EMBASE from inception till March 2022. No filter in terms of time, study design, language, country of publication, and so forth. was used to retrieve all the available literature. The complete search string for PubMed is given in Supporting Information: Table S1.
We considered only those studies which included the population of children and adolescents (from birth up to 19 years of age) who had received their first or second dose of COVID‐19 vaccine and had developed either myocarditis or pericarditis. Review articles, editorials, and those original articles that reported other side effects of vaccination but did not discuss myocarditis and pericarditis specifically, and articles in languages other than English were excluded from this review.
The search of three databases identified 242 articles. A total of 171 articles were removed due to duplication, and 96 articles were excluded due to irrelevance to the topic (Figure 1). After rigorous screening, 12 articles comprising three case series, four case reports, and six original studies were included in our review. 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 Identified studies were uploaded on Mendeley, and duplicates were removed. Initially, the articles were screened on the basis of title and abstract, after which full articles were reviewed. Articles were searched and extracted by two reviewers (M. F. and H. A. C) and a third investigator (M. H. A. K) was contacted to resolve any discrepancies.
Figure 1.
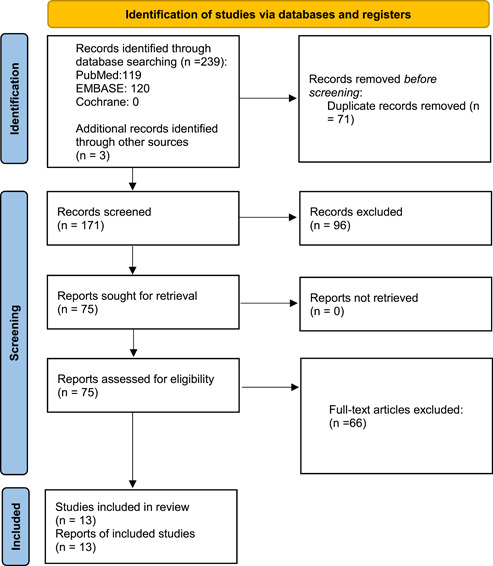
PRISMA flow chart. PRISMA, Preferred Reporting Items for Systematic Reviews.
Continuous variables were presented as means ± standard deviations, and categorical variables were presented as absolute values and percentages. Microsoft Excel was used to extract data and do calculations. Mendeley was used to add the references. The retrieved results of the case reports and case series are summarized in the form of two tables (Tables 1 and 2). One table focuses on the demographics, medical history, and outcomes, whereas the second is based on relevant medical investigations and diagnostic findings. The summary of included original articles (observational studies) has been delineated in Table 3. Table 3 consists of columns of study design, author and year of publication, country, sample size, age (range), gender, follow‐up, comparator group (if any), experimental group characteristics, outcomes (myocarditis or pericarditis or both), clinical features of the reported cases, and results (incidence, incidence rate ratio [IRR], cumulative incidence, risk difference [RD], rate ratio depending on data reported by respective study).
Table 1.
Demographics of patients with myocarditis and pericarditis after COVID‐19 vaccine
| Sr. No. | Doma in | References | Country reported | Number of patients | Age (years), gender (M/F) | Medical history | Type of vaccine administered | Myocarditis/pericarditis | Time between vaccine administration and development of myocarditis/pericarditis | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Case series | Marshall et al. 27 | USA | 7 | Patient no. 1 | 16, M | Not significant | Pfizer‐BioNTech | Myocarditis | 2 days after second dose |
| Patient no. 2 | 19, M | Not significant | Pfizer‐BioNTech | Myocarditis | 3 days after second dose | |||||
| Patient no. 3 | 17, M | Not significant | Pfizer‐BioNTech | Myopericarditis | 2 days after second dose | |||||
| Patient no. 4 | 18, M | Not significant | Pfizer‐BioNTech | Myocarditis | 3 days after second dose | |||||
| Patient no. 5 | 17, M | Not significant | Pfizer‐BioNTech | Myocarditis | 3 days after second dose | |||||
| Patient no. 6 | 16, M | Not significant | Pfizer‐BioNTech | Myocarditis | 3 days after second dose | |||||
| Patient no. 7 | 14, M | Not significant | Pfizer‐BioNTech | Myopericarditis | 2 days after second dose | |||||
| 2 | Case report | Minocha et al. 30 | USA | 1 | 17, M | Not significant | Pfizer‐BioNTech | Myocarditis | 2 days after second dose | |
| 3 | Case series | Dionne et al. 25 | USA | 15 | 12–18 (median = 15 years) 0.14 out of 15 were male | Not significant | Pfizer‐BioNTech | Myocarditis | 1 to 6 days after the second dose of the vaccine in all but 1 case | |
| 4 | Case series | Dickey et al. 24 | USA | 6 | Patient no. 1 | 35–40, M | Not significant | Pfizer‐BioNTech | Myocarditis | 4 days after second dose |
| Patient no. 2 | 16–20, M | Not significant | Pfizer‐BioNTech | Myocarditis | 3 days after second dose | |||||
| Patient no. 3 | 20–25, M | Not significant | Moderna | Myocarditis | 4 days after second dose | |||||
| Patient no. 4 | 20–25, M | Not significant | Pfizer‐BioNTech | Myocarditis | 2 days after second dose | |||||
| Patient no. 5 | 16–20, M | Not significant | Pfizer‐BioNTech | Myocarditis | 4 days after second dose | |||||
| Patient no. 6 | 16–20, M | Not significant | Pfizer‐BioNTech | Myocarditis | 3 days after second dose | |||||
| 5 | Case report | Isaak et al. 26 | USA | 1 | 15, M | Not significant | Pfizer‐BioNTech | Myocarditis | 1 day after second dose | |
| 6 | Case report | Watkins et al. 28 | USA | 1 | 20, M | Tobacco+, COVID+ history | Pfizer‐BioNTech | Myocarditis | 2 days after second dose | |
| 7 | Case series | Park et al. 29 | USA | 2 | Patient no. 1 | 15, M | Not significant | Pfizer‐BioNTech | Myocarditis | 3 days after first dose |
| Patient no. 2 | 16, M | Not significant | Pfizer‐BioNTech | Myocarditis | 2 days after second dose | |||||
Table 2.
Clinical presentation, lab investigations, and diagnostic findings in patients with myocarditis and pericarditis after COVID‐19 vaccine
| Sr. no. | Doma in | References | Clinical features | ECG findings | Lab investigations | Treatment | Echocardiogram findings | Diagnostic criteria (CMR imaging) | Additional comments |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Case series | Marshall et al. 27 | Fatigue, poor appetite, fever of 38.3°C, and pain in the chest and both arms. | Atrioventric ular dissociation with junctional escape and ST elevation |
CRP = 12.3 mg/L, Troponin I = 2.59 ng/ml |
IVIg, IV methylprednisolone, oral prednisone, IV ketorolac | Normal | CMRI demonstrated apical and midchamber lateral wall subepicardial LGE. | Recovered and discharged |
| Acute, persistent chest pain, myalgias, fatigue, weakness, and subjective low‐fevers grade | Diffuse ST elevation | Troponin T = 232 ng/L, CRP = 6.7 mg/dl | IV ketorolac, colchicine, Aspirin | Echocardiogram was normal | CMR showed patchy, midwall LGE along the basal inferolateral wall segment. | Recovered and discharged | |||
| Chest pain. It worsened when lying flat and was associated with left arm pain and paresthesias | T wave abnormalities with diffuse ST elevation | Troponin I = 5.550 ng/ml, CRP = 25.3 mg/L | Ibuprofen | Normal | CMRI showed delayed enhancement at the LV subepicardial basal anterolateral segment and basal to mid‐ventricular inferolateral segments, consistent with myocardial necrosis, evidence of diffuse fibrosis on T1 weighted imaging, and myocardial edema on T2 mapping. | Recovered and discharged | |||
| Chest pain, malaise, arthralgia, myalgia, and subjective fever. It worsened when lying flat and was associated with left arm pain and paresthesias | ST elevation | Troponin T = 1.09 ng/ml, CRP = 12.7 mg/dl | IVIg, methylprednisolone, oral prednisone, Ibuprofen, Aspirin | Normal | CMRI demonstrated edema, hyperemia, and fibrosis. | Recovered and discharged | |||
| Chest pain, sore throat, headache, dry cough, and body aches. He also developed midsternal chest pain that was worse when lying flat and radiated to the left arm | ST‐elevation | Troponin T = 3.21 ng/ml, CRP = 18.1 mg/dl | IVIg. IV methylprednisolone, oral prednisone, Ibuprofen, Aspirin | Normal | CMRI demonstrated diffuse, nearly complete transmural LV free wall gadolinium enhancement. | Recovered and discharged | |||
| Midsternal Chest Pain, malaise, and subjective fever | ST‐segment elevation | Troponin T = 0.01 ng/ml, CRP = 1.8 mg/dl | IVIg, oral prednisone | Normal | LGE, diffuse myocardial edema. | Recovered and discharged | |||
| Pleuritic chest pain and shortness of breath | ST‐segment elevation |
CRP = 12.7 mg/dl, Troponin I = 0.02 ng/ml |
NSAID, famotidine, furosemide | Echocardiogram showed mildly depressed left and right ventricular systolic | LGE (subepicardial) involving mid and apical LV free wall, myocardial edema, hyperemia. | Recovered and discharged | |||
| 2 | Case report | Minocha et al. 30 | Sudden onset of severe, burning left‐sided chest pain that radiated to the left shoulder and the upper left arm. He reported that the chest pain worsened with exertion and movement | Diffuse ST‐segment elevations | Troponin = 2.3 ng/ml, CRP = 29 mg/L | NSAIDs | Not mentioned | CMR showed low normal LVEF (53%), trivial pericardial effusion, and subepicardial lGE. | Recovered and discharged |
| 3 | Case series | Dionne et al. 25 | Chest pain in all fever in 10 patients, myalgia in 8 patients, and headache in 6 patients. | Diffuse ST‐segment elevation present on admission in six patients and at some time during hospital admission in eight patients. Four patients had nonspecific ST‐segment changes. One patient had nonsustained ventricular tachycardia during hospital admission. | Troponin levels were elevated in all patients at admission (median, 0.25 ng/ml [range, 0.08– 3.15 ng/ml]) and peaked 0.1–2.3 days after admission. | Seven patients were treated with IVIg and methylprednisolone (1 mg/kg/dose twice a day, transitioned to prednisone at time of discharge) | Three patients had global LV systolic ventricular dysfunction (EF 44%, 49%, and 53%), one of whom also had regional wall motion abnormality at the apex. Two patients with systolic dysfunction had abnormal diastolic function indices, and one patient with borderline EF (55%) had evidence of diastolic dysfunction. Five patients had abnormal global longitudinal or global circumferential strain. | LGE = 12 patients, Systolic LV dysfunction = 3 patients, Findings consistent with myocarditis = 13 patients. | Recovered and discharged |
| 4 | Case series | Dickey et al. 24 | Positional and pleuritic chest and neck pain; chills; and myalgias | Sinus rhythm with inferolateral ST‐elevation | Troponin I (ng/ml) = 5.41 | Not mentioned | LVEF = 45% | Increased T2 signal and LGE in the midwall of the lateral segments in a patient who received their second SARS‐CoV‐2 vaccination 5 days earlier | Recovered and discharged |
| Pleuritic and positional chest pain; rhinorrhea; headache, fever | Sinus rhythm with diffuse ST‐elevation | Troponin I (ng/ml) = 38.3 | Not mentioned | LVEF = 53% | Increased T2 signal and LGE in the midwall and subepicardial layer throughout the left ventricle) in a patient who received their second SARS‐CoV‐2 vaccination 7 days earlier. | Recovered and discharged | |||
| Pleuritic and positional chest pain; chills; myalgias; and subjective fever | Sinus rhythm with diffuse ST‐elevation | Troponin I (ng/ml) = 18.94 | Not mentioned | LVEF = 58% | Increased T2 signal and LGE in the midwall and subepicardial layer of the mid‐posterolateral segment in a patient who received their second SARS‐CoV‐2 vaccination 6 days earlier. | Recovered and discharged | |||
| Nonpositional chest pain radiating to back; myalgia; malaise, fever | Sinus rhythm with diffuse ST‐elevation and PR depression; nonsustained ventricular tachycardia | Troponin I (ng/ml) = 13.4 | Not mentioned | LVEF = 48% | Not mentioned. | Recovered and discharged | |||
| Pleuritic and positional chest pain; headache | Sinus rhythm with nonspecific T wave abnormalities | Troponin I (ng/ml) = 5.21 | Not mentioned | LVEF = 46% | Not mentioned. | Recovered and discharged | |||
| Nonpositional chest pressure; myalgias | Ectopic atrial rhythm with diffuse ST‐elevation and PR depression | Troponin I (ng/ml) = 19.7 | Not mentioned | LVEF = 50% | Increased T2 signal and LGE in the subepicardial apical and apical lateral segments. | Recovered and discharged | |||
| 5 | Case report | Isaak et al. 26 | Fever, myalgia, and intermittent tachycardia | ST‐segment elevation in the left precordial lead | High‐sensitive cardiac troponin and C‐reactive protein levels were elevated (values not mentioned) | Not mentioned | Normal | Cardiac MRI at 1.5 T showed a normal LV size, a normal LVEF, and a small pericardial effusion. T2‐weighted short inversion time inversion recovery sequences displayed focal myocardial edema involving the lateral wall, most emphasized in the basal inferolateral segment. | Recovered and discharged |
| 6 | Case report | Watkins et al. 28 | Presented with midsternal chest pain that radiated to the left side, mild shortness of breath. | Diffuse concave ST segment elevations with PR depressions. | Troponin = 89 ng/L | Colchicine. Metoprolol, ibuprofen | LVEF = 59% | Bedside ultrasound revealed a small pericardial effusion without evidence of tamponade, which supported the diagnosis. CMR was positive for myocarditis. | Recovered and discharged |
| 7 | Case series | Park et al. 29 | Presented with acute onset, mid‐sternal, nonradiating chest pain associated with chest tightness | ST‐elevation and T wave inversion in lateral leads | Troponin T = 304 ng/L, CRP = 18.5 mg/L | Not mentioned | LVEF = 55%–60%, with basal inferior and basal inferolateral hypokinesis | CMR revealed LGE involving the basal inferior, basal to mid inferolateral, mid anterolateral, apical lateral, apical septal, and apical inferior wall segments in a subepicardial distribution pattern, consistent with myocarditis. | Recovered and discharged |
| Presented with acute onset, mid‐sternal, nonradiating chest pain associated with chest tightness | ST‐segment elevation in inferolateral leads, T wave inversion | Troponin T = 431 ng/L, CRP = 24.3 mg/L | Intravenous Ig | LVEF of 45% with moderate hypokinesis of the apex and apical septum | Lab findings. | Recovered and discharged |
Abbreviations: CMRI, cardiac magnetic resonance imaging; CRP, C‐reactive Protein; ECG, electrocardiogram; IV, intravenous; IVIg, intravenous immunoglobulins; LGE, late gadolinium enhancements; LVEF, left ventricular ejection fraction; NSAIDs, nonsteroidal anti‐inflammatory drugs.
Table 3.
Characteristics of included cohort studies
| Sr. No. | Study design | References | Country reported | Sample size | Age (years) | Gender (M/F) | Follow‐up | Comparator | Experimental group characteristics (vaccine administered) | Outcome | Clinical features | Results |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Cohort study | Chua et al. 19 | Hong Kong | 178 163 | 12–17 | (89 806/88 357) | 4 months | N/A | Conmirnaty = 305 406 doses administered to 178 163 individuals |
Myocarditis = 16 Pericarditis = 2 Perimyocarditi s = 15 Total = 33 After second dose = 27 After first dose = 6 |
Chest pain = 33 Normal ECG = 6 Normal echocardiogram = 25 Normal CMRI = 7 Mild disease = 33 |
Incidence rate; myocarditis/pericarditis = 18.52 (95% CI, 11.67–29.09) per 100 000 persons For first dose = 3.37 (95% CI, 1.12–9.51) per 1000 persons Second dose = 21.22 (95% CI, 13.78– 32.28) per 100 000 persons Male = 32.29 (22.78–45.4) per 1000 persons Female = 4.53 (1.76–11.11) |
| 2 | Cohort study | Lai et al. 31 | Hong Kong, China | 252 399 | 12–18 | (138 319/136 565) | 28 days |
Unvaccinated N = 136 743 M/F = 68 747/67 996 First dose cohort: N = 136 743 Age: 14.15 ± 1.816 12 years = 28 907 13 years = 32 884 14 years = 23 134 15 years = 19 863 16 years = 11 674 17 years = 12 154 18 years = 8127 Second dose cohort: N = 118,300 Age: 14.426 ± 1.799 12 years = 18 235 13 years = 25 355 14 years = 22 885 15 years = 19 871 16 years = 11 677 17 years = 12 153 18 years = 8124 |
Vaccinated: N = 138 141 M/F = 69 572/68 569 First dose cohort: N = 138 141 Age: 14.17 ± 1.821 12 years = 28 008 13 years = 32 040 14 years = 23 324 15 years = 20 044 16 years = 11 951 17 years = 12 443 18 years = 8331 Second dose cohort: N = 119 664 Age: 14.440 ± 1.803 12 years = 18 300 13 years = 25 461 14 years = 23 091 15 years = 20 063 16 years = 11 954 17 years = 12 455 18 years = 8340 Vaccine administered: Pfizer‐BioNtech |
Total cases of Myocarditis: Vaccinated: (n = 38, 0.02%) Unvaccinated: (n = 2, 0.001%) After first dose: Vaccinated: (n = 8, 0.005%) Unvaccinated: (n = 1, 0.0007%) After second dose: Vaccinated: (n = 30, 0.02%) Unvaccinated: (n = 1, 0.0008%) |
N/A |
Overall Incidence rate of Myocarditis: IRR for first dose cohort: 9.15, 95% CI 1.14–73.16, p = 0.037) IRR for second dose cohort: 29.61 (95% CI 4.04–217.07, p = .0009) Incidence of myocarditis: For first dose: vaccinated = 2.91 (95% CI 1.26–5.73, p = .03) per 100 000 Persons unvaccinated = 0.35 (95% CI; 0.01–2.03) For second dose dose Vaccinated: 12.61 (95% CI 8.51–18.00) per 100 000 vaccinated persons Unvaccinated: 0.42 (95% CI 0.01–2.34) among the unvaccinated. |
| 3 | Cohort study | Nygaard et al. 21 | Denmark | 261 334 | 12–17 | 133 477/127 857 | N/A | N/A |
Vaccine administered: Pfizer‐BioNtech First dose: 261 334 Second dose: Not mentioned |
Total cases: N = 15 Male = (n = 13, 87%) Female = (n = 2, 13%) 12 (80%) patients had myocarditis(n = 10) or myopericarditis (n = 2) including 1 meeting the criteria for MIS‐C after vaccination. Pericarditis: (n = 3, 20%) |
Chest pain: (n = 15, 100%) Fever: (n = 13, 73%) Elevated CRP: (n = 12, 80%) Abnormal ECG: (n = 9, 60%) Abnormal CMRI: (n = 7, 46%) |
The incidence of myopericarditis: 97 males and 16 females per million equaling 1 of 10 000 males and 1 in 63 000 females. |
| 4 | Descriptive study | Oster et al. 20 | USA | 192 405 448 | 16–31 | N/A | N/A | N/A |
m‐RNA vaccine dose administered: 354 100 845 Time period between development of symptoms, median (IQR): 2 (1–3) |
Myocarditis: 1626 Pericarditis: 684 Myocarditis: BNT162b2 m‐RNA: Male = 1109 Female = 269 After dose 1 = 216 After dose 2 = 1066 After unknown dose = 103 m‐RNA 1273: Male = 447 Female = 156 After dose 1 = 174 After dose 2 = 390 Unknown Ddse = 42 Less than 30 years that met the criteria of diagnosis of myocarditis: Male = 1050 Female = 145 Greater than 30 years: Male = 284 Female = 146 Pericarditis: BNT162b2 m‐RNA: Male = 253 Female = 163 After dose 1 = 111 After dose 2 = 240 After unknown dose = 68 m‐RNA 1273: Male = 155 Female = 107 After dose 1 = 82 After dose 2 = 134 Unknown dose = 49 Less than 30 years pericarditis: N = 148 Greater than 30 years: N = 536 |
Chest pain = 727/817(89%) Shortness of breath 242/817; (30%) Elevated Troponin levels: 792/809, 98% Abnormal ECG: 569/794, 71% Decrease LVEF: 84/721, 11% Abnormal CMRI: 223/312, 71% |
Males comprised 82% (1334/1625) of the myocarditis case. Males aged 12–15 years = 70.7 (95% CI, 61.68–81.11) per million doses of the BNT162b2 vaccine. Males aged 16–17 years = 105.9 (95% CI, 91.65–122.27) per million doses of the BNT162b2 vaccine. Male aged 18–24 years = 52.4 (95% CI, 45.56–60.33) and 56.3 (95% CI, 47.08–67.34) per million doses of the BNT162b2 vaccine and the mRNA 1273 vaccine, respectively. |
| 5 | Retrospective Cohort | Witberg et al. 22 | Israel | 2 558 421 | 16–≥30 | 1 248 433/1 309 988 | 42 days | N/A | BNT162b2(Pfizer/BioNTech) = 2 558 421, all of the participants received first dose whereas 2 401 605 participants received second dose. |
Total cases of myocarditis = 54 cases of myocarditis by sex and age: Male sex = 51 Female sex = 3 Either sex, 16–29 years = 32 Either sex ≥ 30 years = 22 Male, 16–29 years = 31 Female, 16–29 years = 1 Male ≥30 years = 20 Female ≥30 years = 2 |
Mild myocarditis = 41(76%) Intermediate myocarditis = 12 (22%) LVD = 14(26%) chest pain = 44 (81%) Dyspnea = 3 (5.5%) Fever = 5 (9%) Pericardial effusion = 10 (18.5%) ECG changes = 38 (70%) Elevated Troponin T = 41 |
Cumulative Incidence (95% CI) for all cases of myocarditis: All vaccinated participants: 2.13 (1.6–2.70) Male sex = 4.12 (2.99–5.26) Female sex = 0.23 (0–0.49) Either sex, 16–29 years = 5.49 (3.59–7.39) Either sex ≥30 years = 1.13 (0.66–1.60) Male, 16–29 years = 10.69 (6.93–14.46) Female, 16–29 years = 0.34 (0–1) Male ≥30 years = 2.11 (1.19–3.04) Female ≥30 years = 0.20 (0–0.48) |
| 6 | Retrospective cohort study | Mevorach et al. 23 | Israel | 9 289 765 | 16–50 | 2 668 894/2 773 802 | 183 days | N/A |
BNT162b2 (Pfizer/BionTech) = 5 442 696. First dose: 5 442 696, second dose: 5 125 635 |
Myocarditis: 136, After first dose: 19 After second dose: 117 Male recipients: 16–19 years = 3 20–24 years = 5 25–29 years = 3 30–39 years=2 40–49 years=3 ≥50 years = 1 Female recipients: 16–19 years = 0 20–24 years = 0 25–29 years = 0 30–39 years = 0 40–49 years = 1 ≥50 years = 1 |
Mild myocarditis: 129 (90.9%) cases Chest pain = 129 (95%) Fever = 63 (46.7%) Dyspnea = 17 (12.5%) ECG changes = 93 (68%) Elevated Troponin I or T = 136 (100%) Elevated C‐reactive protein = 118 (86.7%) LGE = 48 (35%) |
Risk of myocarditis per 100 000 persons by age: Male recipients: 16–19 years = 1.34 20–24 years = 1.91 25–29 years = 1.22 30–39 years = 0.41 40–49 years = 0.65 ≥50 years = 0.10 Female recipients: 16–19 years = 0 20–24 years = 0 25–29 years = 0 30–39 years = 0 40‐49 years = 0.21 ≥50 years = 0.09 RD (95% CI) for myocarditis according to age and sex (21 days after first dose): 3.19 (2.37–4.02) Standardized Incidence ratio for myocarditis according to age, sex and dose: 5.34 (4.48–6.40), rate ratio of myocarditis within 30 days after second dose as compared to unvaccinated patients: 2.35 (1.10–5.02) |
Abbreviations: CMRI, cardiac magnetic resonance imaging; ECG, electrocardiogram; F, female; IRR, incidence rate ratio; LVEF, left ventricular ejection fraction; M, male; MIS‐C, multiple inflammatory syndrome in children; RD, risk difference.
The quality of the included case series and case reports was assessed by Joanna Briggs Institute Critical Appraisal Tool. 32 , 33 New‐Castle Ottawa scale was employed for the methodological assessment of observational studies. 34 Three reviewers (M. F., H. A. C., M. H. A. K.) first independently scored each article and then awarded a consensus score to each. The score reports are provided in Supporting Information: Tables S2–S4. Due to inconsistency and diversity in the reporting of outcomes, study design, and participant selection in the original articles, the results have been compiled in a qualitative manner, and a meta‐analysis was not conducted.
3. RESULTS
3.1. Case reports and case series
The data of 33 patients have been described in the case series and case reports (Tables 1 and 2). The mean age of the patients was 17.4 years (range 12–23 years). Thirty‐two (96.9%) patients were male whereas only 1 (3.03%) patient was female. Only one patient had a previous history of SARS‐CoV‐2 infection. Out of 33 patients, 32 (96.9%) of patients received Pfizer‐BioNTech whereas only 1 patient received Moderna (mRNA 1273). 93.9% (n = 31) had myocarditis following the COVID‐19 vaccine and two (6%) patients were diagnosed with myopericarditis. A total of 31 (93.9%) patients developed myocarditis or myopericarditis after the second dose and 2 (6%) developed the symptoms after the first dose. The mean time period between the development of symptoms and vaccine administration was 2.6 days for 18 patients and between 1 and 6 days for the 15 patients in a case series where individual data has not been presented.
In terms of clinical presentation, chest pain (n = 31, 93.9%), fever (n = 18, 54.5%), myalgias (n = 15, 45.4%), and headache (n = 9, 27.2%) were leading complaints. The treatment included intravenous immunoglobulins (n = 12, 36.4%), intravenous methylprednisolone (n = 10, 30.3%), oral prednisone (n = 11, 33.3%), and ibuprofen (n = 4, 12.1%) mainly. C‐reactive protein (CRP) values were reported in 11 (33.3%) patients out of which 9 (27.2%) patients had values greater than normal (normal < 10 mg/dl) and 29 (87.8%) patients presented with elevated serum troponin levels (normal range Troponin I = 0–0.04 ng/ml, Troponin T = 14–30 ng/ml). A total of 32 (97%) patients presented with ST‐segment elevation in ECG (electrocardiography) findings, 22 (66.7%) patients had normal left ventricular ejection fraction (LVEF) (range normal: 55–70, low normal: 50–55) and 11 (33.3%) patients had mildly reduced LVEF. Cardiac magnetic resonance (CMR) imaging findings were consistent with myocarditis in 30 (91%) patients.
3.2. Observational studies
A total of 204 945 530 participants were included in the observational studies, with the greatest number of participants from the United States (n = 192 405 448, 99.6%) (Table 3). Out of the 8 715 498 participants included in five observational studies where baseline characteristics of participants according to gender type was available, 49.1% (n = 4 278 929) participants were male and 50.9% (n = 4 436 569) were female. Most of the participants were given mRNA vaccine (n = 200 806 040, 97.9%). Comirnaty (COVID‐19 vaccine) was administered to 178 163 (0.086%) participants
A total of 2586 (0.0012%) participants reported outcomes out of which 1464 (0.00071%) fell under the category of ≤29 years age group. Out of these 1464 patients (≤29 years) 1294 (0.00063%) developed myocarditis, 153(0.000074%) developed pericarditis and 17 (0.0000083%) presented with myopericarditis. Overall, most of the patients reported myocarditis (n = 1880, 0.00092%). The remaining developed pericarditis (n = 689, 0.00036%) and myopericarditis or perimyocarditis (n = 17, 0.0000083%). Out of 2586 patients, majority presented with chest pain (n = 948, 36.6%) and shortness of breath (n = 263, 10.1%). On investigations, 736 (28.4%) patients showed abnormal ECGs, 969 (37.4%) showed elevated troponin levels, and 256 (9.8%) presented with abnormal CMRI. The mean follow‐up period of four studies was 94 days. Only one study included a controlled arm, that is, an unvaccinated group. In the study by Lai et al., the overall incidence of myocarditis was higher for the vaccinated group as compared to unvaccinated group for both the first dose and second dose cohort, and there was a statistically significant risk of myocarditis with BNT162b2, especially after the second dose (first‐dose cohort: IRR = 9.15, 95% confidence interval [CI] 1.14–73.16; second‐dose cohort: IRR = 29.61, 95% CI 4.04–217.07). A pictorial summary of major findings in our study and the proposed pathogenesis of myocarditis and pericarditis after COVID‐19 vaccination in children and adolescents is presented in Figure 2.
Figure 2.
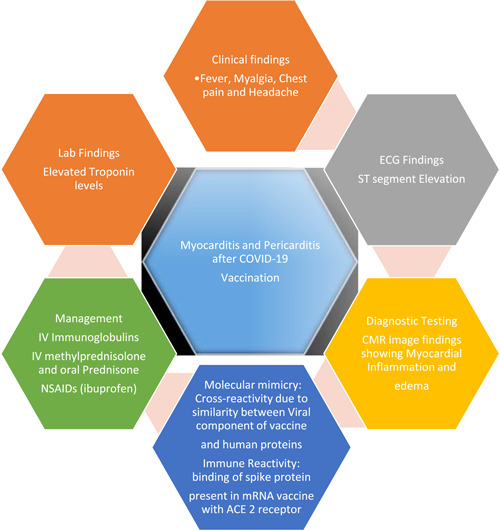
Summary of myocarditis and pericarditis after COVID‐19 vaccination
4. DISCUSSIONS
This systematic review analyzed and synthesized evidence from case reports, case series, and original articles regarding the development of myocarditis and pericarditis following COVID‐19 vaccination in children and adolescents. The incidence of myocarditis and pericarditis was higher among younger males than females and patients receiving a second dose of vaccine were more likely to develop these complications. 97% of the reported cases received Pfizer‐BioNTech (BNT162b2) indicating the association of myocarditis and pericarditis with mRNA vaccines. This greater risk being only reported with Pfizer‐BioNTech may be due to the fact that it is currently the only vaccination that has obtained complete FDA approval for use in adolescents. 30 Chest pain, myalgias, and headache were the most common presentations. 31 CMR imaging revealed myocardial inflammation and edema in the majority of patients thus confirming the diagnosis of myocarditis according to Lake Lousie criteria. 32 Majority of the patients had no significant history and all of the patients had a good recovery and were discharged.
Previously, myocarditis has been reported as a side effect of other vaccines such as the smallpox vaccine. 15 However, the smallpox vaccine differs from the COVID‐19 vaccine in its composition and induced action. The exact mechanism behind the development of myocarditis and pericarditis following the COVID‐19 vaccine has not been defined yet. Molecular mimicry has been hypothesized to be a possible pathology behind this condition according to which the similarity between the viral component of vaccines and specific human proteins can lead to immune cross‐reactivity. 35 A second proposed mechanism is immune reactivity, which describes the binding of SARS‐CoV‐2 spike protein encoded by mRNA vaccine with angiotensin‐converting enzyme 2 receptor thus resulting in myocardial damage. 36 There is a possibility that IgG antibodies produced in response to vaccine‐induced spike protein may cross‐react with myocardial contractile protein. Hajjo et al. 37 has highlighted the central signaling role of IFN‐gamma and TNF‐alpha in both myocarditis and viral disease maps.
Since most of the cases had a mild presentation, the diagnosis was made on the basis of edema and myocardial inflammation observed in CMR imaging. The presence of edema and decreased ejection fraction are indicators of reversible damage in CMR evaluation of early myocarditis and can hence play a role in determining the prognosis and functional recovery of patients. It also sheds light on the possible underlying mechanism and etiology behind this condition. CMR findings revealed a close temporal relationship between a clinical and CMR picture of myocarditis and vaccination; however, these observations alone are not enough to prove whether COVID‐19 immunization caused myocarditis or not. Only large‐scale studies with advanced imaging techniques can provide any possible causative association. 38
The increased prevalence among males can be related to the differences in hormone signaling hence indicating its involvement in the pathophysiology of COVID‐19 vaccine‐related myocarditis. 39 There is a decrease in cell‐mediated immune response in females owing to the inhibitory effect of estrogen on pro‐inflammatory T‐cells, whereas, in males, testosterone stimulates a more aggressive T‐helper‐1 cell type immune response by inhibiting anti‐inflammatory cells. 40 The investigations regarding gender and age disparities in postvaccine myocarditis have revealed that the levels of pro‐inflammatory cytokines such as TNF‐alpha and IFN‐gamma fluctuate between men and women during puberty and then decline later in life, implying hormonal impacts. This corresponds to the findings of the increased incidence of postvaccine myocarditis in adolescents and young adults. 37
The most important considerations for parents to have confidence in getting their children immunized are vaccine efficacy and safety. The reporting of myocarditis and pericarditis in children and adolescents in large clinical trials of vaccines has remained low. 16 This can be attributed to the fact that there was limited recruitment from this age group in the clinical trials and also the rarity of this condition in this age group. The Phase 2/3 clinical trial of Pfizer included only 2260 from 12 to 15 years and 754 participants from the age group 16 to 17 years as compared to adults (43 661 participants enrolled in Phase 3 trials). 41 , 42
NSAIDs, colchicine, and IVIG remained the most frequently opted treatments for myocarditis and pericarditis following COVID‐19 vaccination which aligns with the current guidelines for the management of viral myocarditis. 43 Because the pathophysiology of cardiac dysfunction in myocarditis is caused by a maladaptive hyperimmune response driven by a viral infection, medication aimed at modifying the immune response has been proposed as a possible treatment option, and the same treatment plan has been opted for COVID‐19 vaccine‐associated myocarditis. The majority of the patients presented with ST‐elevation, CRP, and troponin levels raised and preserved ventricular ejection fraction thus indicating a mild presentation of this condition.
Though the published literature emphasizes a possible association of COVID‐19 vaccine and myocarditis, the incidence is too small to provide a causal association. Based on available data, the short time span between vaccine administration and development of myocarditis and pericarditis, and the elevated incidence in younger males does suggest a temporal relationship, however, due to the poorly understood mechanism behind this and lack of experimental studies it is difficult to provide a cause‐effect association. 3 , 44
Healthcare workers and physicians working with young patients can benefit from the data synthesized in this review and remain updated regarding this association along with its diagnostic modalities and management. Since the majority of the cases were reported after the administration of Pfizer, this raises the concern in the emergency of approval of this vaccine for adolescents and children.
The authors would like to acknowledge a few limitations in the review. Firstly, since there has been no large‐scale clinical trial conducted so far to assess myocarditis/pericarditis associated with COVID‐19 vaccines, this review is based on case reports, case series, and observational studies only. Second, a major proportion of included participants was from original articles, and we did not have access to individual‐level data which imposes another limitation to the devised substantiation. Additionally, we lack sufficient data to support the findings for the population under the age of 12 years. Moreover, due to mild presentation and good recovery, there is a probability that a number of cases might have gone unreported which imposes a limitation in associating the development of myocarditis and pericarditis with the COVID‐19 vaccine. Lastly, a possible publication bias can also exist due to the rarity of this condition.
5. CONCLUSION
Myocarditis and pericarditis in children and adolescents after the COVID‐19 vaccine were more prevalent among males and after the second dose of Pfizer. Clinical investigations revealed ST‐segment elevation, CRP, and troponin elevation, and the presence of edema and myocardial damage on CMR imaging. NSAIDs and IVIG were the most commonly opted treatment choices, and all of the cases had a good recovery and were discharged. The cell‐mediated immune responses to vaccination components can cross‐react with heart cells, causing myocardial and pericardial inflammation. However, the exact pathophysiology behind this phenomenon remains unknown. Although the overall incidence is low, however, the clinicians should consider myocarditis and pericarditis as a probable diagnosis when encountering young patients with a history of vaccine administration and presenting with suggestive findings.
AUTHOR CONTRIBUTIONS
Maurish Fatima, Muhammad H. A. Khan, and Huzaifa A. Cheema: Conceptualization; methodology; writing – original draft preparation. Muhammad S. Ali, Muhammad W. Murad, Aleena Ahmed, and Sarya Swed: Data extraction; writing; critical appraisal. Amna Nisar, Aleena Ahmed, and Muhammad Osama: Data extraction; writing. Muhammad A. U. Rehman, Hareem Farooq, Usman A. Akbar, and Huzaifa A. Cheema: Writing – reviewing and editing.
Supporting information
Supplementary information.
Fatima M, Khan MHA, Ali MS, et al. Development of myocarditis and pericarditis after COVID‐19 vaccination in children and adolescents: a systematic review. Clin Cardiol. 2023;46:243‐259. 10.1002/clc.23965
DATA AVAILABILITY STATEMENT
The data underlying this article are available from the authors on reasonable request.
REFERENCES
- 1.COVID Live—Coronavirus Statistics—Worldometer [Internet]. 2022. https://www.worldometers.info/coronavirus/
- 2. Cai C, Peng Y, Shen E, et al. A comprehensive analysis of the efficacy and safety of COVID‐19 vaccines. Mol Ther. 2021;29(9):2794‐2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Morgan MC, Atri L, Harrell S, Al‐Jaroudi W, Berman A. COVID‐19 vaccine‐associated myocarditis. World J Cardiol. 2022;14(7):382‐391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barda N, Dagan N, Ben‐Shlomo Y, et al. Safety of the BNT162b2 mRNA Covid‐19 vaccine in a nationwide setting. N Engl J Med. 2021;385(12):1078‐1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mei R, Raschi E, Forcesi E, Diemberger I, De Ponti F, Poluzzi E. Myocarditis and pericarditis after immunization: gaining insights through the vaccine adverse event reporting system. Int J Cardiol. 2018;273:183‐186. [DOI] [PubMed] [Google Scholar]
- 6. Das BB, Moskowitz WB, Taylor MB, Palmer A. Myocarditis and pericarditis following mRNA COVID‐19 vaccination: what do we know so far? Children. 2021;8(7):607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cooper LT Jr. Myocarditis. N Eng J Med. 2009;360(15):1526‐1538. 10.1056/NEJMra0800028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Snyder MJ, Bepko J, White M. Acute pericarditis: diagnosis and management. Am Fam Physician. 2014;89(7):553‐560. [PubMed] [Google Scholar]
- 9. Tunuguntla H, Jeewa A, Denfield SW. Acute myocarditis and pericarditis in children. Pediatr Rev. 2019;40(1):14‐25. [DOI] [PubMed] [Google Scholar]
- 10. Shakuntala P, Sumitra V. Acute myocarditis. Indian J Pract Pediatr. 2021;10(2):65‐73. [Google Scholar]
- 11. Checcucci E, Piramide F, Pecoraro A, et al. The vaccine journey for COVID‐19: a comprehensive systematic review of current clinical trials in humans. Panminerva Med. 2020;64(1):72‐79. https://pubmed.ncbi.nlm.nih.gov/32456404/ [DOI] [PubMed] [Google Scholar]
- 12. Kang M, An J. Viral Myocarditis. StatPearls; 2022. https://www.ncbi.nlm.nih.gov/books/NBK459259/ [PubMed] [Google Scholar]
- 13. Vasudeva R, Bhatt P, Lilje C, et al. Trends in acute myocarditis related pediatric hospitalizations in the United States, 2007‐2016. Am J Cardiol. 2021;149:95‐102. [DOI] [PubMed] [Google Scholar]
- 14. Putschoegl A, Auerbach S. Diagnosis, evaluation, and treatment of myocarditis in children. Pediatr Clin North Am. 2020;67(5):855‐874. [DOI] [PubMed] [Google Scholar]
- 15. Halsell JS, et al. Myopericarditis following smallpox vaccination among vaccinia‐naive US military personnel. JAMA. 2003;289(24):3283‐3289. [DOI] [PubMed] [Google Scholar]
- 16. Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA‐1273 SARS‐CoV‐2 vaccine. N Engl J Med. 2021;384(5):403‐416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID‐19 mRNA vaccines. Circulation. 2021;144(6):471‐484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Page MJ, Mckenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ, 372:n71. https://www.bmj.com/content/372/bmj.n71.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chua GT, Kwan MYW, Chui CSL, et al. Epidemiology of acute myocarditis/pericarditis in Hong Kong adolescents following Comirnaty vaccination. Clin Infect Dis. 2021;75(4):673‐681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Oster ME, Shay DK, Su JR, et al. Myocarditis cases reported after mRNA‐based COVID‐19 vaccination in the US from December 2020 to August 2021. JAMA. 2022;327(4):331‐340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nygaard U, Holm M, Bohnstedt C, et al. Population‐based incidence of myopericarditis after COVID‐19 vaccination in Danish adolescents. Pediatr Infect Dis J. 2022;41(1):e25‐e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Witberg G, Barda N, Hoss S, et al. Myocarditis after Covid‐19 vaccination in a large health care organization. N Engl J Med. 2021;385:2132‐139. http://www.ncbi.nlm.nih.gov/pubmed/34614329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mevorach D, Anis E, Cedar N, et al. Myocarditis after BNT162b2 mRNA vaccine against Covid‐19 in Israel. N Engl J Med. 2021;385:2140‐2149. https://www.nejm.org/doi/full/10.1056/NEJMoa2109730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dickey JB, Albert E, Badr M, et al. A series of patients with myocarditis following SARS‐CoV‐2 vaccination with mRNA‐1279 and BNT162b2. JACC Cardiovasc Imaging. 2021;14(9):1862‐1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dionne A, Sperotto F, Chamberlain S, et al. Association of myocarditis with BNT162b2 messenger RNA COVID‐19 vaccine in a case series of children. JAMA Cardiol. 2021;6:1446‐1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Isaak A, Feisst A, Luetkens JA. Myocarditis following COVID‐19 vaccination. Radiology. 2021;301(1):E378‐E379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Marshall M, Ferguson ID, Lewis P, et al. Symptomatic acute myocarditis in 7 adolescents after Pfizer‐BioNTech COVID‐19 vaccination. Pediatrics. 2021;148(3):e2021052478. [DOI] [PubMed] [Google Scholar]
- 28. Watkins K, Griffin G, Septaric K, Simon EL. Myocarditis after BNT162b2 vaccination in a healthy male. Am J Emerg Med. 2021;50:815. http://www.ncbi.nlm.nih.gov/pubmed/34229940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Park J, Brekke DR, Bratincsak A. Self‐limited myocarditis presenting with chest pain and ST segment elevation in adolescents after vaccination with the BNT162b2 mRNA vaccine. Cardiol Young. 2021;32:146‐149. [DOI] [PubMed] [Google Scholar]
- 30. Minocha PK, Better D, Singh RK, Hoque T. Recurrence of acute myocarditis temporally associated with receipt of the mRNA coronavirus disease 2019 (COVID‐19) vaccine in a male adolescent. J Pediatr. 2021;238:321‐323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lai FTT, Chua GT, Chan EWW, et al. Adverse events of special interest following the use of BNT162b2 in adolescents: a population‐based retrospective cohort study. Emerg Microbes Infect. 2022;11(1):885‐893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Munn Z, Barker TH, Moola S, et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth. 2020;18(10):2127‐2133. https://pubmed.ncbi.nlm.nih.gov/33038125/ [DOI] [PubMed] [Google Scholar]
- 33. Aromataris E, Munn Z, eds. JBI manual for evidence synthesis. JBI; 2020. Available from https://synthesismanual.jbi.global [Google Scholar]
- 34. Gierisch JM, Beadles C, Shapiro A, et al. Newcastle‐Ottawa Scale Coding Manual for Cohort Studies. 2014. https://www.ncbi.nlm.nih.gov/books/NBK299087/ [Google Scholar]
- 35. Segal Y, Shoenfeld Y. Vaccine‐induced autoimmunity: the role of molecular mimicry and immune crossreaction. Cell Mol Immunol. 2018;15(6):586‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kyriakidis NC, López‐Cortés A, González EV, Grimaldos AB, Prado EO. SARS‐CoV‐2 vaccines strategies: a comprehensive review of phase 3 candidates. NPJ Vaccines. 2021;6(1):28. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7900244/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hajjo R, Sabbah DA, Bardaweel SK, Tropsha A. Shedding the light on post‐vaccine myocarditis and pericarditis in covid‐19 and non‐covid‐19 vaccine recipients. Vaccines (Basel). 2021;9(10):1186. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8541143/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Friedrich MG, Marcotte F. Cardiac magnetic resonance assessment of myocarditis. Circ Cardiovasc Imaging. 2013;6(5):833‐839. [DOI] [PubMed] [Google Scholar]
- 39. Fatima M, Cheema HA, Khan MHA, et al. Development of myocarditis and pericarditis after COVID‐19 vaccination in adult population: a systematic review. Ann Med Surg (Lond). 2022;76:103486. https://pubmed.ncbi.nlm.nih.gov/35291413/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Heymans S, Eriksson U, Lehtonen J, Cooper LT. The quest for new approaches in myocarditis and inflammatory cardiomyopathy. J Am Coll Cardiol. 2016;68(21):2348‐2364. [DOI] [PubMed] [Google Scholar]
- 41. Pfizer. Pfizer‐BioNTech Announce Positive Topline Results of Pivotal COVID‐19 Vaccine Study in Adolescents. Pfizer; 2022. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-biontech-announce-positive-topline-results-pivotal [Google Scholar]
- 42. Pfizer. Pfizer and BioNTech Conclude Phase 3 Study of COVID‐19 Vaccine Candidate, Meeting All Primary Efficacy Endpoints. Pfizer; 2022. https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-conclude-phase-3-study-covid-19-vaccine [Google Scholar]
- 43. Salah HM, Mehta JL. COVID‐19 vaccine and myocarditis. Am J Cardiol. 2021;157:146‐148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hendren NS, Carter S, Grodin JL. Severe COVID‐19 vaccine associated myocarditis: zebra or unicorn? Int J Cardiol. 2021;343:197‐198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information.
Data Availability Statement
The data underlying this article are available from the authors on reasonable request.


