Abstract
Background
Obesity is an important risk factor for heart failure (HF).
Hypothesis
Visceral adiposity index (VAI) is a simple metric for assessing obesity; however, the association between VAI and risk for HF has not been studied.
Methods
A cross‐sectional study involving 28 764 participants ≥18 years of age from the National Health and Nutrition Examination Survey (NHANES), 2009–2018, in the United States was performed. VAI was calculated using body mass index (BMI), waist circumference (WC), triglycerides (TG), and high‐density lipoprotein cholesterol. VAI was analyzed as a continuous and categorical variable to examine its association with HF. Subgroup analysis was also performed.
Results
The highest VAI (fourth quartile [Q4]) was found among males, BMI, systolic and diastolic blood pressure, WC, hypertension, diabetes, liver disease, coronary heart disease, smoking, total cholesterol, and TG. More participants in Q4 took β‐receptor blockers, angiotensin‐converting enzyme inhibitors/angiotensin II receptor blockers/angiotensin receptor‐neprilysin inhibitor, calcium channel blockers, and antidiabetic and antihyperlipidemic medications. Participants with HF exhibited greater VAI. A per‐unit increase in VAI resulted in a 4% increased risk for HF (odds ratio [OR] 1.04 [95% confidence interval (CI) 1.02–1.05]). After multivariable adjustment, compared with the lowest quartile, the OR for Q3 was 1.55 (95% CI 1.24–1.94). Subgroup analysis revealed no significant interactions between VAI and specific subgroups.
Conclusion
VAI was independently associated with the risk for HF. As a noninvasive index of visceral adiposity, VAI could be used for a “one shot” assessment of HF risk and may serve as a novel marker.
Keywords: heart failure, VAI, visceral adiposity index, visceral fat
1. INTRODUCTION
Heart failure (HF) refers to a group of complex clinical syndromes caused by many factors (myocardial dysfunction, valvular diseases, pathological changes in the pericardium and endocardium, and dysfunction of heart rhythm and conduction), 1 which leads to ventricular systolic or diastolic dysfunction. 2 In the United States, approximately 6.2 million individuals ≥20 years of age experience HF, with approximately 1 million newly diagnosed cases of HF annually, and the prevalence continues to rise. 3 , 4 Data from the European Society of Cardiology show that approximately 1% of patients with HF are <55 years of age and approximately 10% of those with HF are ≥70 years of age. 1 In developed countries, the incidence rate of HF may decrease after adjusting for age, which may reflect good management of cardiovascular disease (CVD); however, the overall incidence rate of HF is increasing due to aging. 1 This situation is similar to that observed in developing countries. According to the latest survey results of HF epidemiology released in 2019, the number of chronic HF cases in China is approximately 13.7 million, and its prevalence has increased by 44% in the past 15 years. 5
Approximately 29%–40% of patients with HF are overweight (body mass index [BMI] 25.0–29.9 kg/m2), and 30%–49% are obese (BMI ≥ 30 kg/m2). It is noteworthy that obesity is more common in patients with HF with preserved ejection fraction (HFpEF) than in those with HF with reduced ejection fraction (HFrEF), and >80% of HFpEF patients exhibit a BMI in the range of overweight or obesity. 6 , 7 However, the relationship between obesity and HF remains controversial. Dunlay et al. demonstrated that obesity, measured according to increased BMI, is a major risk factor for the development of HF. 8 However, some research appears to highlight the “obesity paradox,” 9 which means that overweight or grade 1 obesity can also result in a better survival rate for HF. In addition to BMI, visceral adipose tissue (VAT) is an indicator of obesity. Previous studies have shown that patients with HF, especially HFpEF, exhibit higher VAT. 10 , 11 , 12 These studies usually used computed tomography (CT) or magnetic resonance imaging (MRI) to evaluate VAT. The visceral adiposity index (VAI) was calculated using BMI, waist circumference (WC), triglycerides (TG), and high‐density lipoprotein cholesterol (HDL‐c). 13 Compared to CT or MRI, the calculation of VAI is easier, and more economical and convenient. Previous studies have shown that VAT is associated with diabetes, hyperuricemia, metabolic syndrome, hypertension, atherosclerosis, and vascular calcification. 13 , 14 , 15 , 16 , 17 , 18 However, to our knowledge, the association between VAI and HF has not been studied.
As such, the purpose of this study was to evaluate the association between VAI and HF among middle‐age and elderly participants of the US National Health and Nutrition Examination Survey (NHANES), 2009–2018.
2. METHODS
2.1. Study population
The NHANES is a nationally representative cross‐sectional study that enrolls participants through a stratified multistage probability and oversampling design that enables weighted analysis that represents the noninstitutionalized, civilian population of the United States (US). Data are released every 2‐year cycle. Each participant represents approximately 50 000 US citizens. All participants provided informed consent before participation, and ethics approval for the study was obtained from the Research Ethics Review Board at the National Centre for Health Statistics, which consisted of a physician, medical and health technicians, and dietary and health interviewers, who conducted surveys through interviews, health measurements, and laboratory tests. An advanced computer system collects and processes all NHANES data. Findings of this survey can be used to determine the prevalence of diseases and their risk factors.
Data for the present study were derived from the 2009–2018 NHANES cycle. In this cohort, 49 693 participants completed the interviews. Participants who were <18 years of age (n = 19 341) and those with missing data regarding HF status (n = 1588) were excluded. Ultimately, data from 28 764 participants were included in this cross‐sectional study. Participants with HF were defined as those who answered yes to the question, “Has a doctor or other health professional ever told you that you had congestive HF?.” A detailed flow‐diagram illustrating participant selection is presented in Supporting Information: Figure 1. The National Center for Health Statistics Ethics Review Board reviewed and approved the NHANES protocol and all participants provided written informed consent before data collection.
2.2. VAI score
VAI score was calculated according to previously reported equations 13 :
For males,
For females,
NHANES researchers collected anthropometric data (i.e., height, weight, calculated BMI, and WC) and biochemical data (i.e., glycated hemoglobin, direct HDL‐c, and fasting TG) that were used to calculate VAI. A higher VAI score reflected a greater amount of estimated visceral adiposity.
2.3. Variables of interest
Potential covariates, including demographics, comorbidities, lifestyle variables, BMI, TG, TC, serum uric acid (UA), estimated glomerular filtration rate (eGFR), and markers of inflammation, were selected based on clinical relevance and statistical significance. The baseline characteristics of the participants, including demographics, comorbidities, and lifestyle information, were obtained using a questionnaire. BMI, WC, and other biochemical parameters were obtained from medical examinations and laboratory assessments performed at the mobile examination center. BMI was calculated as weight (kg) divided by height (m) squared (kg/m2). Hypertension was defined as self‐reported physician‐diagnosed hypertension, use of antihypertensive medications, or blood pressure measurement of 140/90 mmHg. 19 Diabetes mellitus was defined as self‐reported physician‐diagnosed diabetes, taking oral hypoglycemic agents or insulin, fasting glucose level of 126 mg/dl, or plasma glucose level of 200 mg/dl 2 h after an oral glucose tolerance test. 20 Participants with anemia were defined as those who answered yes to the question, “During the past 3 months, have you been on treatment for anemia?.” Participants with liver disease were defined as those who answered yes to the question, “Has a doctor or other health professional ever told you that you had any kind of liver condition?.” Participants with coronary heart disease were defined as those who answered yes to the question, “Has a doctor or other health professional ever told you that you had coronary heart disease?.” Participants with kidney disease were defined as those who answered yes to the question, “Have you ever been told by a doctor or other health professional that you had weak or failing kidneys? Do not include kidney stones, bladder infections, or incontinence?.” Participants with a history of heart attack were defined as those who answered yes to the question, “Has a doctor or other health professional ever told you that you had a heart attack (also called myocardial infarction)?.” Smokers were defined as those who had smoked at least 100 cigarettes in their lifetimes. eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration creatinine equation. 21 Dietary Inflammatory Index (DII) was calculated based on a 24 h dietary recall interview with each participant. 22 , 23 The inflammatory indicator was the neutrophil‐lymphocyte ratio (NLR). 24 , 25
2.4. Statistical analysis
According to the NHANES analytic guidelines, descriptive results are expressed as weighted mean ± standard error (SE) or median (first quartile, third quartile) (Q2 [Q1, Q3)] for continuous variables and frequency (weighted percentage) for categorical variables. The VAI was analyzed as a continuous and categorical variable (quartiles). Differences in VAI between groups (with and without HF) were tested using the Student's t‐test. Differences in characteristics between the groups were tested using the Student's t‐test for continuous variables and χ2 tests for categorical variables. The odds ratio (OR) and corresponding 95% confidence interval (CI) for HF per unit increase and each quartile, with the lowest quartile as the reference, was estimated using both univariate and multivariate logistic regression models. Tests for linear trends across the VAI categories were conducted using an independent ordinal variable (0, 1, 2, 3) in all models. In addition to the unadjusted model, potential covariates were progressively adjusted in the three models. Model 1 was adjusted for age, sex, and race; model 2 was additionally adjusted for hypertension, diabetes mellitus (DM), smoking, alcohol consumption, coronary heart disease (CHD), kidney disease, and liver disease; and model 3 was further adjusted for eGFR, systolic blood pressure (SBP), diastolic blood pressure (DBP), serum UA, albumin (Alb), hemoglobin (HGB), hematocrit (HCT), and NLR. The restricted cubic spline model was used for dose–response analysis. To explore whether the association between the VAI and HF was modified by sex, age, race, smoking status, and comorbidities, subgroup analyses was performed according to sex (male or female), age group (18–34, 35–54, 55–74 or ≥75 years of age), race, smoking (yes or no), hypertension (yes or no), diabetes (yes or no), CHD (yes or no), liver disease (yes or no), serum UA ( < 350 or ≥350 μmol/L), eGFR (<90 or ≥90 ml/min/1.73 m2), and Alb (<40 or ≥40 g/L), and examined the interactions between the stratified variables and VAI using likelihood ratio tests. The “nhanesR” package version 0.9.1.9 was used for data extraction and processing. Free statistics software version 1.4 and the statistical software package R 4.0.1 (R Foundation for Statistical Computing, Vienna, Austria) were used for all analyses. Differences with a two‐tailed p < .05 were considered to be statistically significant.
3. RESULTS
3.1. Characteristics of the study population
Basic characteristics of the study population are summarized in Table 1. In total, 28 764 participants, with a weighted average age of 50 years, were included in this study. According to VAI quartile, participants with the highest VAI (i.e., Q4) had higher values in males, Mexican Americans, other Hispanics, non‐Hispanic whites, BMI, SBP, DBP, WC, hypertension, DM, liver diseases, CHD, smoking, TC, TG, and serum UA. More participants in Q4 took β‐receptor blockers, angiotensin‐converting enzyme inhibitors (ACEIs)/angiotensin II receptor blockers (ARBs)/angiotensin receptor‐neprilysin inhibitor (ARNI), calcium channel blockers (CCBs), antidiabetic medication, and antihyperlipidemic medications. The opposite patterns were observed for non‐Hispanic Blacks, alcohol, and HDL‐c. A comparison of the four groups revealed that sex, race, BMI, SBP, DBP, WC, alcohol, smoking, several diseases (hypertension, DM, HF, liver disease, CHD, heart attack, and kidney disease), relevant test results (TC, HDL‐C, TG, serum UA, eGFR, NLR, DII, lymphocytes (Lym), Alb, HGB, and HCT), and the utilization rate of medications (β‐receptor blocker, ACEI/ARB/ARNI, MRA, CCB, diuretic, antidiabetic, and antihyperlipidemic) were significantly different (p < .001).
Table 1.
Characteristics of the study participants
| Variables | All (n = 28764) | Q1 (n = 7191) | Q2 (n = 7191) | Q3 (n = 7191) | Q4 (n = 7191) | p |
|---|---|---|---|---|---|---|
| Age, years | 49 (34, 64) | 45 (30, 61) | 49 (34, 63) | 52 (36, 68) | 51 (38, 63) | <.001 |
| Gender, no.(%) | <.001 | |||||
| Male | 13910 (48.4) | 3661 (50.9) | 3354 (46.6) | 3343 (46.5) | 3552 (49.4) | |
| Female | 14854 (51.6) | 3530 (49.1) | 3837 (53.4) | 3848 (53.5) | 3639 (50.6) | |
| Race, no.(%) | <.001 | |||||
| Mexican American | 4157 (14.5) | 691 (9.6) | 1057 (14.7) | 1004 (14) | 1405 (19.5) | |
| Other Hispanic | 2994 (10.4) | 592 (8.2) | 762 (10.6) | 748 (10.4) | 892 (12.4) | |
| Non‐Hispanic White | 11266 (39.2) | 2671 (37.1) | 2829 (39.3) | 2700 (37.5) | 3066 (42.6) | |
| Non‐Hispanic Black | 6239 (21.7) | 2216 (30.8) | 1552 (21.6) | 1649 (22.9) | 822 (11.4) | |
| Other Race | 4108 (14.3) | 1021 (14.2) | 991 (13.8) | 1090 (15.2) | 1006 (14) | |
| BMI, kg/m2 | 28.5 (24.6, 32.5) | 25.1 (22.2, 29.1) | 28.0 (24.5, 32.5) | 29.3 (26.1, 32.3) | 30.4 (27.0, 34.9) | <.001 |
| SBP, mmHg | 124.0 (112.0, 132.0) | 118.0 (108.0, 130.0) | 122.0 (112.0, 134.0) | 124.3 (116.0, 132.0) | 124.3 (114.0, 134.0) | <.001 |
| DBP, mmHg | 70.2 (64.0, 78.0) | 70.0 (62.0, 76.0) | 70.2 (64.0, 78.0) | 70.2 (66.0, 76.0) | 72.0 (64.0, 80.0) | <.001 |
| WC, cm | 99.5 (89.0, 107.8) | 89.0 (80.3, 99.8) | 97.7 (88.5, 108.2) | 99.5 (97.1, 103.6) | 104.7 (95.9, 115.0) | <.001 |
| Hypertension, no.(%) | 11989 (41.7) | 2318 (32.2) | 2842 (39.5) | 3243 (45.1) | 3586 (49.9) | <.001 |
| Diabetes mellitus, no.(%) | 5470 (19.2) | 726 (10.2) | 1199 (16.9) | 1493 (21) | 2052 (28.8) | <.001 |
| HF, no.(%) | 958 (3.3) | 146 (2) | 189 (2.6) | 340 (4.7) | 283 (3.9) | <.001 |
| Anemia, no.(%) | 1292 (4.5) | 305 (4.2) | 304 (4.2) | 388 (5.4) | 295 (4.1) | <.001 |
| Liver disease, no.(%) | 1187 (4.1) | 216 (3) | 277 (3.9) | 295 (4.1) | 399 (5.5) | <.001 |
| CHD, no.(%) | 1173 (4.1) | 196 (2.7) | 263 (3.7) | 344 (4.8) | 370 (5.1) | <.001 |
| Heart attack, no.(%) | 1196 (4.2) | 202 (2.8) | 282 (3.9) | 356 (5) | 356 (5) | <.001 |
| Kidney disease, no.(%) | 997 (3.5) | 170 (2.4) | 202 (2.8) | 350 (4.9) | 275 (3.8) | <.001 |
| Alcohol, g/day | 0.0 (0.0, 15.4) | 0.0 (0.0, 15.4) | 0.0 (0.0, 15.4) | 15.4 (0.0, 15.4) | 0.0 (0.0, 15.4) | <.001 |
| Smoking, no.(%) | 12448 (43.3) | 2869 (39.9) | 3040 (42.3) | 3107 (43.2) | 3432 (47.7) | <.001 |
| TC, mmol/L | 5.0 (4.3, 5.5) | 4.6 (4.0, 5.3) | 4.8 (4.2, 5.5) | 5.0 (4.7, 5.1) | 5.2 (4.5, 5.9) | <.001 |
| HDL‐C, mmol/L | 1.4 (1.1, 1.6) | 1.7 (1.4, 2.0) | 1.4 (1.2, 1.6) | 1.4 (1.2, 1.4) | 1.0 (0.9, 1.2) | <.001 |
| TG, mmol/L | 1.5 (0.9, 2.0) | 0.7 (0.6, 0.9) | 1.2 (1.0, 1.4) | 1.7 (1.5, 1.7) | 2.7 (2.1, 3.5) | <.001 |
| Serum uric acid, mmol/L | 323.8 (267.7, 368.8) | 291.5 (243.9, 350.9) | 309.3 (261.7, 368.8) | 323.8 (309.3, 345.0) | 339.0 (279.6, 398.5) | <.001 |
| eGFR, ml/min/1.73 m2 | 94.0 (81.4, 109.4) | 100.0 (83.9, 114.8) | 96.6 (80.0, 111.7) | 94.0 (86.9, 97.6) | 94.2 (76.0, 108.2) | <.001 |
| NLR, % | 3.3 (2.8, 3.9) | 3.2 (2.7, 3.9) | 3.3 (2.8, 4.0) | 3.3 (3.1, 3.7) | 3.3 (2.8, 4.0) | <.001 |
| DII, mg/L | 0.9 (−0.1, 2.2) | 0.8 (−0.4, 2.1) | 1.0 (−0.3, 2.3) | 0.9 (0.0, 2.0) | 1.1 (−0.2, 2.3) | <.001 |
| Lym,109/L | 2.1 (1.7, 2.5) | 1.9 (1.5, 2.3) | 2.0 (1.6, 2.5) | 2.2 (1.9, 2.3) | 2.3 (1.8, 2.8) | <.001 |
| Alb, g/L | 42.2 (40.0, 44.0) | 43.0 (41.0, 45.0) | 42.0 (40.0, 45.0) | 42.2 (41.0, 43.0) | 42.0 (40.0, 44.0) | <.001 |
| HGB, g/L | 14.0 (13.1, 14.9) | 13.9 (12.9, 14.9) | 14.0 (13.0, 15.0) | 14.0 (13.4, 14.4) | 14.2 (13.2, 15.2) | <.001 |
| HCT, % | 41.3 (38.8, 43.9) | 41.2 (38.5, 43.9) | 41.3 (38.5, 44.2) | 41.3 (39.8, 42.5) | 41.9 (38.9, 44.7) | <.001 |
| Medications, no.(%) | ||||||
| β‐receptor blocker | 3208 (11.2) | 492 (6.8) | 697 (9.7) | 963 (13.4) | 1056 (14.7) | <.001 |
| ACEI/ARB/ARNI | 5843 (20.3) | 1046 (14.5) | 1404 (19.5) | 1558 (21.7) | 1835 (25.5) | <.001 |
| MRA | 235 (0.8) | 37 (0.5) | 48 (0.7) | 83 (1.2) | 67 (0.9) | <.001 |
| CCB | 2076 (7.2) | 404 (5.6) | 494 (6.9) | 586 (8.1) | 592 (8.2) | <.001 |
| Diuretic | 3719 (12.9) | 628 (8.7) | 840 (11.7) | 1133 (15.8) | 1118 (15.5) | <.001 |
| Antidiabetic | 3530 (12.3) | 440 (6.1) | 757 (10.5) | 1013 (14.1) | 1320 (18.4) | <.001 |
| Antihyperlipidemic | 5957 (20.7) | 1069 (14.9) | 1417 (19.7) | 1648 (22.9) | 1823 (25.4) | <.001 |
Abbreviations: ACEI, angiotensin converting enzyme inhibitor; Alb, albumin; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor‐neprilysin inhibitor; BMI, body mass index; CCB, calcium channel blocker; CHD, coronary heart disease; DBP, diastolic blood pressure; DII, dietary inflammatory index; eGFR, estimated glomerular filtration rate; HCT, hematocrit; HDL‐C, high‐density lipoprotein cholesterol; HF, heart failure; HGB, hemoglobin; Lym, lymphocytes; MRA, mineralocorticoid receptor antagonist; NLR, neutrophil‐lymphocyte ratio; SBP, systolic blood pressure; TC, total cholesterol; TG, triglycerides; WC, waist circumference.
3.2. Association between VAI and HF
Differences in VAI between the two groups with and without HF are shown in Figure 1. Results of analysis revealed that participants with HF exhibited a higher VAI than those without HF (p < .001).
Figure 1.
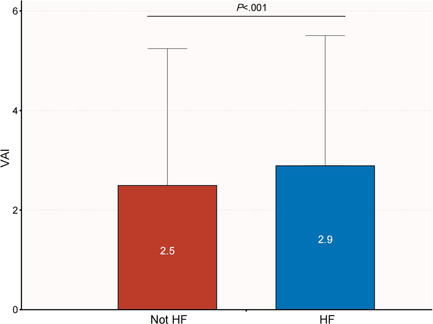
Comparison of VAI between patients with HF and non‐HF. HF, heart failure; VAI, visceral adiposity index.
The relationship between VAI and HF as continuous and categorical variables is shown in Table 2. When VAI was analyzed as a continuous variable, a per unit increase in VAI resulted in a higher risk for HF in the univariate logistic regression model (OR 1.04 [95% CI 1.02–1.05]). The association remained statistically significant in all multivariate logistic regression models after adjusting for several covariates including sex, age, race, hypertension, DM, smoking, alcohol consumption, CHD, kidney disease, liver disease, eGFR, SBP, DBP, UA, Alb, HGB, HCT, and NLR (model 1, OR 1.05 [95% CI 1.03–1.07]; model 2, OR 1.02 [95% CI 1–1.05]; and model 3, OR 1.03 [95% CI 1–1.05]). When VAI was analyzed as a categorical variable, compared with the top VAI quartile, subjects in the third quartile (Q3) had the highest risk for HF (OR 1.55 [95% CI 1.24–1.94]), adjusting for age, sex, race, hypertension, DM, smoking, alcohol, CHD, kidney disease, liver disease, eGFR, SBP, DBP, UA, Alb, HGB, HCT, and NLR.
Table 2.
Multivariable‐adjusted ORs and 95% confidence intervals of the visceral adiposity index associated with heart failure
| Unadjusted | Model 1a | Model 2b | Model 3c | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | OR (95% CI) | p | |
| Continuous per unit increase | 1.04 (1.02~1.05) | <.001 | 1.05 (1.03~1.07) | <.001 | 1.02 (1~1.05) | .041 | 1.03 (1~1.05) | .031 |
| Quintilesd | ||||||||
| Q 1 | 1 (Ref) | 1 (Ref) | 1 (Ref) | 1 (Ref) | ||||
| Q 2 | 1.3 (1.05~1.62) | .018 | 1.26 (1.01~1.58) | .043 | 1.06 (0.83~1.34) | .641 | 1.01 (0.79~1.28) | .957 |
| Q 3 | 2.39 (1.97~2.92) | <.001 | 1.96 (1.6~2.4) | <.001 | 1.57 (1.26~1.95) | <.001 | 1.55 (1.24~1.94) | <.001 |
| Q 4 | 1.98 (1.61~2.42) | <.001 | 2.04 (1.65~2.51) | <.001 | 1.29 (1.02~1.62) | .032 | 1.19 (0.93~1.51) | .16 |
| p for trend | <.001 | <.001 | .002 | .014 | ||||
Abbreviations: Alb, albumin; CHD, coronary heart disease; CI, confidence interval; DBP, diastolic blood pressure; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HCT, hematocrit; HGB, hemoglobin; NLR, neutrophil‐lymphocyte ratio; SBP, systolic blood pressure; UA, serum uric acid.
Model 1 adjusted for gender, age and race.
Model 2 further adjusted for hypertension, DM, smoker, alcohol, CHD, kidney disease and liver disease.
Model 3 further adjusted for eGFR, SBP, DBP, UA, Alb, HGB, HCT, and NLR.
The quintile cutoff values of the Visceral Adiposity index are 1.093, 2.041, and 2.676.
There was a linear relationship between VAI and the OR for HF in Model 3 (p for nonlinearity, .151), which used the restricted cubic spline model (Figure 2).
Figure 2.
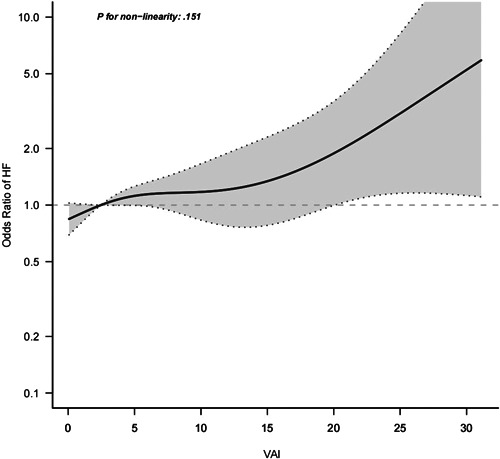
Relationship between VAI and the odds ratio of HF. HF, heart failure; VAI, Visceral adiposity index.
3.3. Subgroup analysis
Sex, age, race, smoking status, hypertension, DM, CHD, liver disease, serum UA, and eGFR were used as stratification variables to observe the effect size trend, and a Forrest plot of data was generated (Figure 3). All associations were positive in the different subgroups, except for Mexican Americans and participants without hypertension. There were no significant interactions between VAI and sex, age, race, smoking status, hypertension, DM, CHD, liver disease, serum UA, eGFR, or albumin.
Figure 3.
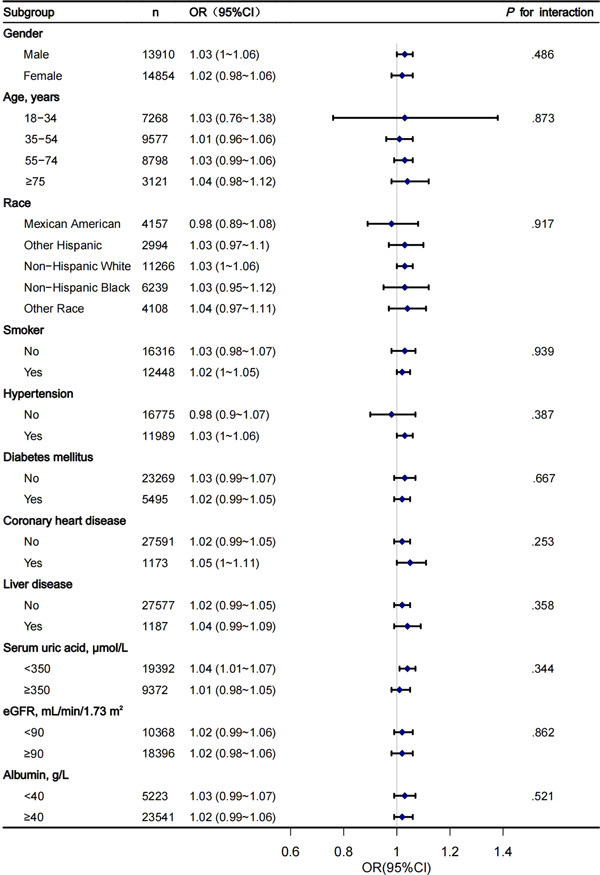
Subgroup analysis of the association between VAI and HF. CI, confidence interval; eGFR, estimated glomerular filtration rate; HF, heart failure; OR, odds ratio; VAI, Visceral adiposity index.
4. DISCUSSION
Using data from a representative national sample of middle‐age and elderly populations in the US, we found that VAI was associated with HF after adjustment for other covariates, exhibiting a near linear dose–response relationship. Subgroup analysis makes it possible to better understand VAI and HF in different populations, suggesting that the direction of relationships between the VAI and HF in different subgroups was consistent with that in the total study population.
The overall prevalence of HF in our study was approximately 2.4%, which is consistent with previously published data. 3 , 4 Previous studies have reported an association between obesity and HF, 26 , 27 , 28 and determined that obesity is the main risk factor for hypertension, CVD, and left ventricular hypertrophy (LVH), which are strong risk factors for the development of HF. 29 , 30 In the Framingham Heart Study, which included 5881 participants, after adjusting for some risk factors, each unit increase in BMI increased the incidence of HF by 5% in males and 7% in females, and the risk for HF increased across the BMI range. 26 The Physicians’ Health Study, which included 21 094 males (mean age, 53 years) without known CHD at baseline, demonstrated that every 1 kg/m2 increase in BMI was associated with an 11% increase in HF risk, and obese participants had a 180% increase in HF risk. 31 A study involving 59 178 Finnish participants 25–74 years of age who had no HF at baseline reported that the multivariable adjusted risk ratio for HF was highest in the high BMI group (>30 kg/m2) among men and women, and abdominal obesity was associated with a greater risk for HF in men and women. 28
However, obesity includes both overall and abdominal obesity. Different obesity phenotypes may lead to different incidence, mortality, and treatment outcomes of HF. 32 BMI can be used as an indicator of overall obesity; however, for individuals with simple abdominal obesity, BMI may not be the best indicator, and there is even “normal weight obesity” in the population. Even for normal‐weight individuals, the risk for CVD may be higher among those with high WC. WC, an indicator of abdominal fat, is associated with cardiac metabolic diseases and CVD and can predict mortality. 33 , 34 Therefore, other indicators are needed to evaluate abdominal obesity, among which VAT is one. Rao et al. demonstrated that VAT was independently associated with hospitalization for HFpEF in individuals without baseline CVD. 35 Selvaraj et al. suggested that patients with HFpEF had significantly increased pericardial and subcutaneous fat thicknesses compared to patients without HF. 12 Sorimachi et al. reported that female HFpEF patients had a higher VAT, and the accumulation of excess VAT played an important role in the pathophysiology of female HFpEF patients. 11 In these studies, the VAT was measured using abdominal CT or MRI. These methods are accurate but have high cost and low efficiency, and are rarely used in clinics. VAI can be calculated by measuring WC, height, weight, and TG and HDL‐c levels in the blood. The clinical operation is simple and the data are easy to obtain. Previous studies have concluded that VAI is associated with diabetes, hyperuricemia, metabolic syndrome, hypertension, atherosclerosis, and vascular calcification. 13 , 14 , 15 , 16 , 17 , 18 However, to the best of our knowledge, results from previous studies investigating the correlation between VAI and HF are limited. A cohort study of 116 patients 35–80 years of age, who were hospitalized for aggravated HF between 2011 and 2013, demonstrated that VAI may be a good predictor of mortality in patients with ischemic heart failure, and that patients with higher VAI had a better survival prognosis. 36 However, this study only examined the relationship between VAI and mortality in patients with ischemic heart failure, and did not study the relationship between VAI and the prevalence of HF.
As expected, there was a linear relationship between VAI and the OR for HF. In terms of pathophysiological mechanism, VAT can induce cardiomyocyte hypertrophy, lead to myocardial fibrosis, and activate inflammatory pathways related to macrophage infiltration and cytokine gene expression. Excessive VAT accumulation may lead to higher circulating blood volume and more local and systemic atherogenic inflammatory factors. It may also increase the risk for stroke, increase heart wall pressure and myocardial injury, lead to left ventricular remodeling and, eventually, cause HF. 37 , 38 , 39
To the best of our knowledge, this is the first study to examine the association between VAI and HF in a large and representative national sample of adults in the US. Our study had the advantages of rigorous study protocols and quality controls, a large representative sample, and available data on many vital covariates by integrating the NHANES data. Nevertheless, this study had some limitations. First, the NHANES does not collect echocardiography and N‐terminal pro brain natriuretic peptide (NT‐proBNP) data from participants. Participants with HF were defined as those with self‐reported physician‐diagnosed HF. The same situation also occurs in hypertension, DM, anemia, liver disease, CHD, kidney disease, and a history of heart attack. Second, this was a cross‐sectional study that did not include follow‐up data. The changes in VAI and the risk for HF over time are unclear. Our study design did not permit identification of a causal association between VAI and HF during the study period. Third, it did not distinguish between the types of HF in participants and could not evaluate whether VAI has a different relationship with different types of HF.
5. CONCLUSION
Results of the present study revealed that VAI was independently associated with the risk for HF. More simply stated, noninvasive scores of visceral adiposity permitted a simple noninvasive “one shot” assessment of HF risk(s). In view of the increasing prevalence and enormous health burden of HF, individuals with high VAI warrant greater attention to prevent HF. As such, its potential use as a novel marker of HF risk merits further investigation.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Supporting information.
ACKNOWLEDGMENT
The work was funded by the National Natural Science Foundation of China (81900444, 81873516, 82170463); the National Key Research and Development Program of China (2021YFF0501404, 2021YFF0501403); the Natural Science Foundation of Shandong Province (ZR2019PH030, ZR2019BH052, ZR2020QH007); China International Medical Foundation (Z‐2016‐23‐2001‐01, Z‐2019‐42‐1908‐2) and the Clinical Research Center of Shandong University (2020SDUCRCA009).
Zhang X, Sun Y, Li Y, et al. Association between visceral adiposity index and heart failure: a cross‐sectional study. Clin Cardiol. 2023;46:310‐319. 10.1002/clc.23976
Xinyu Zhang and Yijun Sun are co‐first authors.
Contributor Information
Huixia Lu, Email: luhuixia@sdu.edu.cn.
Xiaoping Ji, Email: jixiaoping@sdu.edu.cn.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in the National Health and Nutrition Examination Survey (NHANES) at https://www.cdc.gov/nchs/nhanes/index.htm.
REFERENCES
- 1. McDonagh TA, Metra M, Adamo M, et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021;42(36):3599‐3726. [DOI] [PubMed] [Google Scholar]
- 2. Heart Failure Group of Chinese Society of Cardiology of Chinese Medical A, Chinese Heart Failure Association of Chinese Medical Doctor A, Editorial Board of Chinese Journal of C . Chinese guidelines for the diagnosis and treatment of heart failure 2018. Zhonghua Xin Xue Guan Bing Za Zhi. 2018;46(10):760‐789. [DOI] [PubMed] [Google Scholar]
- 3. Benjamin EJ, Muntner P, Alonso A, et al. Heart disease and stroke statistics‐2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56‐e528. [DOI] [PubMed] [Google Scholar]
- 4. Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics‐2017 update: a report from the American Heart Association. Circulation. 2017;135(10):e146‐e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hao G, Wang X, Chen Z, et al. Prevalence of heart failure and left ventricular dysfunction in China: the China Hypertension Survey, 2012–2015. Eur J Heart Fail. 2019;21(11):1329‐1337. [DOI] [PubMed] [Google Scholar]
- 6. Lewis GA, Schelbert EB, Williams SG, et al. Biological phenotypes of heart failure with preserved ejection fraction. JACC. 2017;70(17):2186‐2200. [DOI] [PubMed] [Google Scholar]
- 7. Ather S, Chan W, Bozkurt B, et al. Impact of noncardiac comorbidities on morbidity and mortality in a predominantly male population with heart failure and preserved versus reduced ejection fraction. JACC. 2012;59(11):998‐1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dunlay SM, Roger VL, Redfield MM. Epidemiology of heart failure with preserved ejection fraction. Nat Rev Cardiol. 2017;14(10):591‐602. [DOI] [PubMed] [Google Scholar]
- 9. Donataccio MP, Vanzo A, Bosello O. Obesity paradox and heart failure. Eat Weight Disord. 2021;26(6):1697‐1707. [DOI] [PubMed] [Google Scholar]
- 10. Rao VN, Fudim M, Mentz RJ, Michos ED, Felker GM. Regional adiposity and heart failure with preserved ejection fraction. Eur J Heart Fail. 2020;22(9):1540‐1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sorimachi H, Obokata M, Takahashi N, et al. Pathophysiologic importance of visceral adipose tissue in women with heart failure and preserved ejection fraction. Eur Heart J. 2021;42(16):1595‐1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Selvaraj S, Kim J, Ansari BA, et al. Body composition, natriuretic peptides, and adverse outcomes in heart failure with preserved and reduced ejection fraction. JACC Cardiovasc Imaging. 2021;14(1):203‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bagyura Z, Kiss L, Lux Á, et al. Association between coronary atherosclerosis and visceral adiposity index. Nutr Metab Cardiovasc Dis. 2020;30(5):796‐803. [DOI] [PubMed] [Google Scholar]
- 14. Nusrianto R, Tahapary DL, Soewondo P. Visceral adiposity index as a predictor for type 2 diabetes mellitus in Asian population: a systematic review. Diabetes Metab Syndr. 2019;13(2):1231‐1235. [DOI] [PubMed] [Google Scholar]
- 15. Huang X, Jiang X, Wang L, et al. Visceral adipose accumulation increased the risk of hyperuricemia among middle‐aged and elderly adults: a population‐based study. J Transl Med. 2019;17(1):341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jung JY, Ryoo J‐H, Oh C‐M, et al. Visceral adiposity index and longitudinal risk of incident metabolic syndrome: Korean genome and epidemiology study (KoGES). Endocr J. 2020;67(1):45‐52. [DOI] [PubMed] [Google Scholar]
- 17. Fan Y, He D, Liu S, Qiao Y, Gao H, Xin L. Association between visceral adipose index and risk of hypertension in a middle‐aged and elderly Chinese population. Nutr Metab Cardiovasc Dis. 2021;31(8):2358‐2365. [DOI] [PubMed] [Google Scholar]
- 18. Son DH, Ha HS, Lee HS, et al. Association of the new visceral adiposity index with coronary artery calcification and arterial stiffness in Korean population. Nutr Metab Cardiovasc Dis. 2021;31(6):1774‐1781. [DOI] [PubMed] [Google Scholar]
- 19. Fryar CD, Ostchega Y, Hales CM, Zhang G, Kruszon‐Moran D. Hypertension prevalence and control among adults: United States, 2015‐2016. NCHS Data Brief. 2017;(289):1‐8. [PubMed] [Google Scholar]
- 20. McClure ST, Schlechter H, Oh S, et al. Dietary intake of adults with and without diabetes: results from NHANES 2013‐2016. BMJ Open Diabetes Res Care. 2020;8(1):e001681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604‐612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gojanovic M, Holloway‐Kew KL, Hyde NK, et al. The dietary inflammatory index is associated with low muscle mass and low muscle function in older Australians. Nutrients. 2021;13(4):1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liu Z, Liu H, Deng Q, et al. Association between dietary inflammatory index and heart failure: results from NHANES (1999–2018). Front Cardiovasc Med. 2021;8:702489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Martens P, Verluyten L, Van de Broek H, et al. Determinants of maximal dose titration of sacubitril/valsartan in clinical practice. Acta Cardiol. 2021;76(1):20‐29. [DOI] [PubMed] [Google Scholar]
- 25. Adamstein NH, MacFadyen JG, Rose LM, et al. The neutrophil‐lymphocyte ratio and incident atherosclerotic events: analyses from five contemporary randomized trials. Eur Heart J. 2021;42(9):896‐903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kenchaiah S, Evans JC, Levy D, et al. Obesity and the risk of heart failure. N Engl J Med. 2002;347(5):305‐313. [DOI] [PubMed] [Google Scholar]
- 27. Bozkurt B, Aguilar D, Deswal A, et al. Contributory risk and management of comorbidities of hypertension, obesity, diabetes mellitus, hyperlipidemia, and metabolic syndrome in chronic heart failure: a scientific statement from the American Heart Association. Circulation. 2016;134(23):e535‐e578. [DOI] [PubMed] [Google Scholar]
- 28. Hu G, Jousilahti P, Antikainen R, Katzmarzyk PT, Tuomilehto J. Joint effects of physical activity, body mass index, waist circumference, and waist‐to‐hip ratio on the risk of heart failure. Circulation. 2010;121(2):237‐244. [DOI] [PubMed] [Google Scholar]
- 29. Lavie CJ, Laddu D, Arena R, Ortega FB, Alpert MA, Kushner RF. Reprint of: healthy weight and obesity prevention. JACC. 2018;72(23 pt B):3027‐3052. [DOI] [PubMed] [Google Scholar]
- 30. Elagizi A, Kachur S, Lavie CJ, et al. An overview and update on obesity and the obesity paradox in cardiovascular diseases. Prog Cardiovasc Dis. 2018;61(2):142‐150. [DOI] [PubMed] [Google Scholar]
- 31. Kenchaiah S, Sesso HD, Gaziano JM. Body mass index and vigorous physical activity and the risk of heart failure among men. Circulation. 2009;119(1):44‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Alagiakrishnan K, Banach M, Ahmed A, Aronow WS. Complex relationship of obesity and obesity paradox in heart failure—higher risk of developing heart failure and better outcomes in established heart failure. Ann Med. 2016;48(8):603‐613. [DOI] [PubMed] [Google Scholar]
- 33. Piché M‐E, Poirier P, Lemieux I, Després JP. Overview of epidemiology and contribution of obesity and body fat distribution to cardiovascular disease: an update. Prog Cardiovasc Dis. 2018;61(2):103‐113. [DOI] [PubMed] [Google Scholar]
- 34. Sahakyan KR, Somers VK, Rodriguez‐Escudero JP, et al. Normal‐weight central obesity: implications for total and cardiovascular mortality. Ann Intern Med. 2015;163(11):827‐835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rao VN, Zhao D, Allison MA, et al. Adiposity and incident heart failure and its subtypes. JACC Heart Fail. 2018;6(12):999‐1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Vogel P, Stein A, Marcadenti A. Visceral adiposity index and prognosis among patients with ischemic heart failure. Sao Paulo Med J. 2016;134(3):211‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Murase T, Hattori T, Ohtake M, et al. Cardiac remodeling and diastolic dysfunction in DahlS.Z‐Lepr(fa)/Lepr(fa) rats: a new animal model of metabolic syndrome. Hypertension Res. 2012;35(2):186‐193. [DOI] [PubMed] [Google Scholar]
- 38. Rodriguez Flores M, Aguilar Salinas C, Piché M‐E, Auclair A, Poirier P. Effect of bariatric surgery on heart failure. Expert Rev Cardiovasc Ther. 2017;15(8):567‐579. [DOI] [PubMed] [Google Scholar]
- 39. Neeland IJ, Gupta S, Ayers CR, et al. Relation of regional fat distribution to left ventricular structure and function. Circ Cardiovasc Imaging. 2013;6(5):800‐807. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.
Data Availability Statement
The data that support the findings of this study are openly available in the National Health and Nutrition Examination Survey (NHANES) at https://www.cdc.gov/nchs/nhanes/index.htm.


